- 1Department of Pediatric Surgery, Children’s Hospital of Nanjing Medical University, Nanjing, China
- 2Department of Pediatrics, Huai’an Maternal And Child Health Care center, Huai’an, China
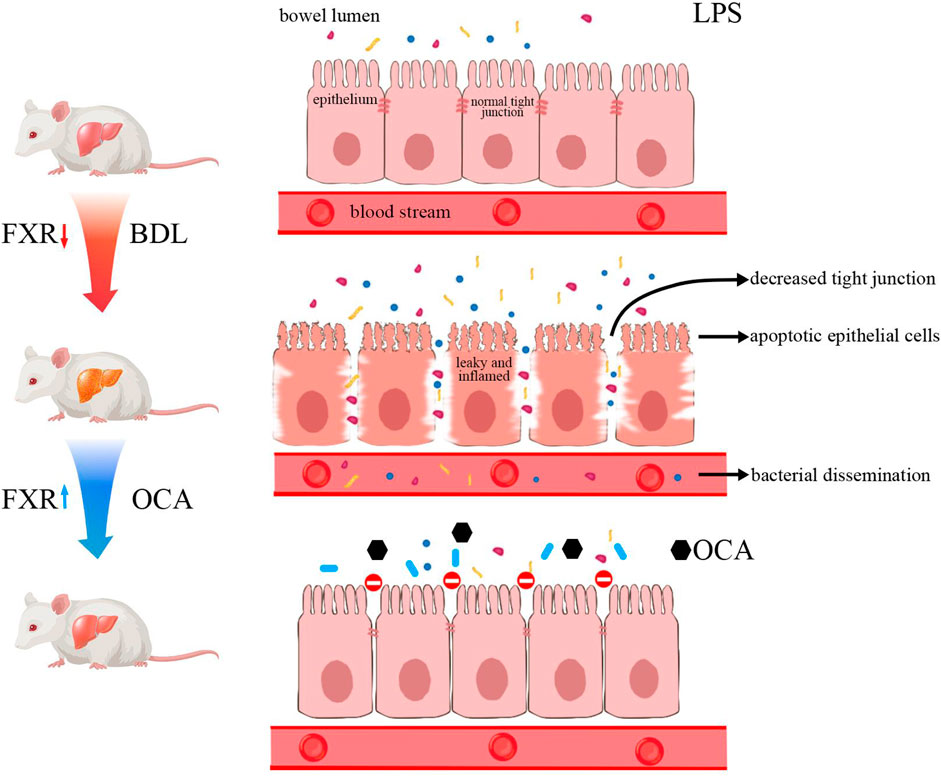
Background: The farnesoid X receptor (FXR) is a key factor regulating hepatic bile acid synthesis and enterohepatic circulation. Repression of bile acid synthesis by the FXR is a potential strategy for treating cholestatic liver disease. However, the role of intestinal FXR on the intestinal barrier and intestinal microbiota needs further investigation.
Materials: Intestinal tissues were collected from patients with biliary atresia or without hepatobiliary disease. Then, intestinal mRNA levels of FXR-related molecules were determined. To investigate the effect of FXR activation, bile-duct-ligation rats were treated with obeticholic acid [OCA (5 mg/kg/day)] or vehicle (0.5% methyl cellulose) per oral gavage for 14 days. The mRNA levels of intestinal FXR, SHP, TNF-α, FGF15 and bile acid transporter levels were determined. In addition, the intestinal permeability, morphologic changes, and composition of the intestinal microbiota were evaluated. Gut Microbiome was determined by 16S rDNA MiSeq sequencing, and functional profiling of microbial communities was predicted with BugBase and PICRUSt2. Finally, the role of OCA in injured intestinal epithelial cell apoptosis and proliferation was examined by pretreatment with lipopolysaccharide (LPS) in Caco-2 cells.
Results: The downstream of the FXR in ileum tissues was inhibited in biliary obstruction. Activation of the FXR signaling pathway by OCA significantly reduced liver fibrosis and intestinal inflammation, improved intestinal microbiota, and protected intestinal mucosa in BDL rats. OCA also altered the functional capacities of ileum microbiota in BDL rats. Significant differences existed between the controls and BDL rats, which were attenuated by OCA in the alpha diversity analysis. Principal coordinates analysis showed that microbial communities in BDL rats clustered separately from controls, and OCA treatment attenuated the distinction. Bugbase and PICRUSt2 analysis showed that OCA changed the composition and structure of the intestinal microbiota and improved the metabolic function of the intestinal microbiota by increasing the relative abundance of beneficial bacteria and reducing the relative abundance of harmful bacteria. Moreover, OCA reduced the apoptosis induced by LPS in Caco-2 cells.
Conclusion: The FXR agonist, OCA, activates the intestinal FXR signaling pathway and improves the composition and structure of the intestinal microbiota and intestinal barrier in BDL rats.
The FXR agonist, OCA, activates the intestinal FXR signaling pathway and improves the composition and structure of the intestinal microbiota and intestinal barrier in BDL rats.
Introduction
Biliary obstruction is a pathologic condition of intrahepatic cholestasis caused by partial or complete obstruction of the intra- and extra-hepatic bile ducts that restricts bile flow into the intestinal tract. Biliary atresia (BA) is the most common cause of biliary obstruction in newborns. The most common cause of BA is inflammation and fibrosis of extrahepatic bile ducts, which reduces the passage of bile into the intestine (Asai et al., 2015; Wang et al., 2020). The reduction of bile acids in the intestine results in apoptosis of intestinal epithelial cells and atrophy of the intestinal mucous (Assimakopoulos et al., 2004). When the intestinal mucosal barrier is injured, the intestinal bacteria and endotoxin enter the blood, resulting in bacteremia and endotoxemia, respectively (Sorribas et al., 2019).
Bile acids are produced from cholesterol through oxidation reactions in hepatocytes, then conjugated with glycine and taurine. The bile acid enterohepatic circulation is strictly regulated by a large number of enzymes and transporters, of which the most important regulatory factor is the farnesoid X receptor (FXR) (Gonzalez et al., 2017). Bile acids in the body are natural ligands of the FXR (Cariello et al., 2018), including the primary bile acids [chenodeoxycholic acid (CDCA) and cholic acid (CA)], the secondary bile acids [deoxycholic acid (DCA) and lithocholic acid (LCA)], and their conjugates with taurine and glycine (Makishima et al., 1999; Trabelsi et al., 2017). The FXR also plays an important role in the reabsorption of bile acids by regulating the bile acid transporter in the ileum (Trauner and Fuchs, 2022). Activation of the FXR pathway in the ileum influences expression of the apical sodium-BA transporter (ASBT), intestinal BA-binding protein (I-BABP), and organic solute and steroid transporter alpha-beta (OSTα/β), thus, increasing bile acid reabsorption into the blood (Dawson, 2011). In addition, intestinal FXR increases the expression of FGF19, a hormone secreted into the portal blood and transported to the liver to suppress CYP7A1 expression (Kong et al., 2012; Liu et al., 2020). Therefore, the FXR has an important effect on regulating bile acid synthesis and enterohepatic circulation.
The intestinal microbiota is closely related to bile acid metabolism via interaction with bile acids (Fiorucci and Distrutti, 2015; Schneider et al., 2018). Accumulation of bile acids in the liver leads to hepatocyte apoptosis and changes in the diversity of the intestinal microbiota (Wang et al., 2020). Bile acids and bacteria have a two-way regulation relationship; specifically, bile acids affect the survival and growth of bacteria, while bacteria regulate the consistency of intestinal bile acids (Stojancevic et al., 2012). The mechanism underlying bile acid toxicity on bacteria is multifactorial, and includes membrane effects, DNA damage, RNA structure changes, and protein denaturation (Assimakopoulos et al., 2007). Patients with BA do not have normal bile acids in the intestinal tract, which has a significant impact on the intestinal microbiota (Song et al., 2021). In mice, the intestinal microbiota not only regulates secondary bile acid metabolism but also regulates bile acid metabolism by reducing the level of T-βMCA, which improves intestinal FGF15 production and reduces BA synthesis (Sayin et al., 2013). Gut microbial dysbiosis results in endotoxin translocation into the portal vein, where activation of the NLRP3 inflammasome contributes to increased liver injury (Keitel et al., 2019). Besides, transferring the intestinal microbiota of cholestatic mice to healthy mice leads to severe liver damage (Kwong and Puri, 2021). Therefore, regulation of the intestinal microbiota is a potential treatment for liver disease secondary to BA.
The regulatory function of hepatic and intestinal FXR on bile acid metabolism has been extensively studied, but the role of intestinal FXR on the intestine has not been established. The activation of FXR has been proved to increase the expression of the tight-junction proteins claudin-1 and occluding in bacterial translocation (Verbeke et al., 2015). The present study determined the influence of the FXR agonist, obeticholic acid (OCA), in protecting the intestinal barrier in rats with obstructive jaundice by blocking the entry of bile into the intestine, excluding the indirect effect of bile on the intestine, then determining the effect of intestinal FXR on the intestine. We showed that OCA partly restored the enterohepatic circulation by increasing FGF19 expression. In addition, we demonstrated that the FXR has a protective effect on cholestatic liver injury and improves intestinal epithelial cell apoptosis in BDL rats, thus providing a new potential target for clinical prevention of intestinal mucosal barrier injury in patients with obstructive jaundice.
Materials and Methods
Tissues and Gut Microbial Collection
The current study involved 24 infants without hepatobiliary disease and 16 BA patients at the Children’s Hospital of Nanjing Medical University from November 2017 to December 2020. All BA patients were diagnosed based on intraoperative cholangiograms and pathologic evaluation of liver biopsies at the Children’s Hospital of Nanjing Medical University. Tissue samples were collected from BA patients undergoing the Kasai procedure. Matched controls were derived from patients without liver failure or malignancies, and were confirmed to not have BA or other congenital malformations. No study subjects were recently treated with antibiotics. All tissue samples were immediately frozen in liquid nitrogen and stored at −80°C. We acquired written informed consent from the subjects or their legal guardians. Ethics approval was given by the Research Ethics Committee of the Children’s Hospital of Nanjing Medical University.
Animals Experiment Design
Animals were randomized into three groups and fed a regular diet. For the bile duct ligation (BDL) model, rats were anesthetized with chloral hydrate, then the bile duct was exposed and ligated with two non-resorbable surgical sutures. Rats that underwent BDL were divided into two groups (n = 6–7). After the procedure, rats received the treatment with vehicle (0.5% methylcellulose) or OCA (5 mg/kg/day dissolved in 0.5% methylcellulose) by daily gavage for 2 weeks. Two weeks later, all animals were sacrificed under anesthesia. Ileum content, the ileum, serum, and fecal samples were collected and immediately stored at −80°C.
Serum Measurements
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using an automated bioanalyzer (Thermo Scientific Indiko Plus, Wuhan, China). The serum lipopolysaccharide (LPS) levels were determined using enzyme-linked immunosorbent assay (ELISA) kits (RayBiotech, Inc., United States).
Intestinal Gene Expression
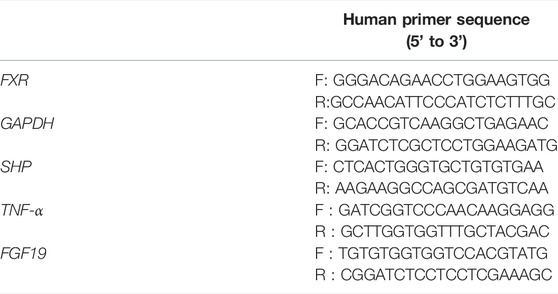
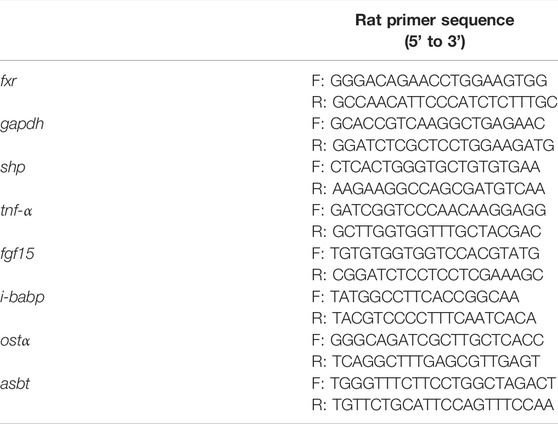
Ileum tissues (3–5 cm) were collected at 0.5–1.5 cm proximal to the ileocecal flap for gene expression studies. The process of tissue homogenates, RNA extraction and real-time quantitative polymerase chain reaction followed the protocol. The primer sequence is shown in Table 1, 2.
Western Blot Analysis
The frozen tissues and cells were lysed on ice with RIPA buffer (Solarbio). The expression levels of target proteins in the ileum were detected by respective primary antibodies. For protein density, each band was determined and then quantified by using Image J software. Primary antibodies involved in this study includes: anti-cleaved caspase-3 (1:2000, Cell Signaling Technology), anti-PCNA (1:1,000, Santa Cruz), anti-β-actin (1:1,000, Santa Cruz).
Gut Microbiota Sequencing and Microbial Analysis
Genomic DNA was extracted from fecal samples using a E. Z.N.A. ®Stool DNA Kit (D4015, Omega, Inc., United States) according to manufacturer’s instructions. Amplicons of the V3–V4 region of the 16S rDNA gene were conducted by using a 341F/805R primer pair. Sequencing was carried out on a NovaSeq PE250 platform. Sequence data analyses were mainly conductd by using Quantitative Insights Into Microbial Ecology2 (QIIME2) and R packages (v3.5.2). For dereplication, feature table and feature sequence were obtained by DADA2. The raw data of 16Sr DNA gene sequencing and metabolomic sequencing quality control in each sample are provided in the supplementary materials. Bugbase was used for the predictions of the functional profile of a microbial community based on 16S rDNA sequence data. Based on Kyoto Encyclopedia of Genes and Genomes (KEGG) functional pathways, PICRUSt2 was used to analyze the metabolic networks of ileum microbiota, with an emphasis on the enriched pathways.
Cell Culture and Treatments
The human intestinal epithelial cell line, Caco-2, was cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 0.1 mg/ml of streptomycin, 100 U/mL of penicillin, and 2 mmol/L L-glutamine. Caco-2 cells were incubated in a humid atmosphere (5% CO2 and 95% air) at 37°C. Caco-2 cells were treated with LPS (100 μg/ml) for 24 h to induce intestinal epithelial injury followed with the FXR agonists, OCA (10 μM) an additional 24 h.
Cell Viability Assay
Cell viability was detected with a CCK8 kit (Beyotime, Nantong, China). After the above treatment, cells were cultivated in 96-well microplates at a concentration of 1 cells/well × 104 cells/well and incubated in medium containing 5% FBS for 12 h. CCK-8 (10 μl/well) was then added to the wells and 96-well microplates were incubated at 37°C for 1–4 h. The absorbance at 450 nm was measured using a Tecan Infinite M200 multimode microplate reader (Tecan, Mechelen, Belgium).
Cell Apoptosis Analysis
The TdT-mediated dUTP nick-end labeling (TUNEL) assay was used in rat ileum sections to measure apoptosis of intestinal epithelial cells according to the manufacturer’s instructions (Beyotime, Shanghai, China). To detect Caco-2 cell apoptosis, cells were harvested and stained with the Annexin V-FITC/propidium iodide kit (KeyGen Biotech, Nanjing, China) following the manufacturer’s instructions. Apoptosis rates were analyzed using FlowJo V7 software.
Statistical Analysis
All statistical analyses are conducted using GraphPad Prism (version 8; GraphPad Software Inc., San Diego, CA, United States). Data are presented as the mean ± SEM. Statistical comparisons were made using the one-way analysis of variance (ANOVA) with tukey’s post hoc test or double-sided Student’s t-test where appropriate. p value < 0.05 was considered significant. The qualitative data represent three independent experiments.
Results
Clinical Information Analysis
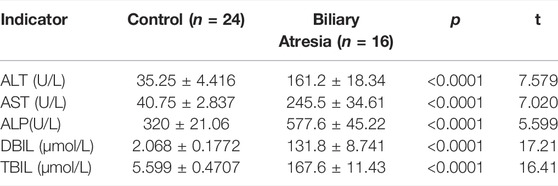
Clinical information, including age, gender, and biochemical indicators, was obtained from 16 BA patients and 24 healthy controls. The mean ages of BA patients and controls were 55.63 ± 6.11 and 70.81 ± 20.25 days, respectively; there was no significant difference in mean age between the BA patients and healthy controls (Table 3). The serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) direct bilirubin (DBIL), and total bilirubin (TBIL) levels were significantly increased in the BA group (Table 4).
The Farnesoid X Receptor-FGF19 Signaling Pathway and Intestinal Barrier Are Impaired in Patients With Biliary Atresia
First, FXR-related molecule expression in the 16 BA and 24 control samples was determined. The FXR was highly expressed in BA patients, while the small heterodimer partner (SHP) and FGF19 were significantly lower than in the control group (Figures 1A–C). The FXR-FGF19 signaling pathway was damaged in biliary obstruction, which might be involved in the development of BA. We also evaluated the apoptosis, proliferation and inflammatory response in intestinal tissues. The protein level of cleaved caspase-3 was greatly increased in BA samples (Figures 1D,E). Meanwhile, the expression levels of PCNA was significantly decreased in BA (Figures 1F,G). Serum LPS and intestinal TNF-α were significantly increased in BA patients (Figures 1H,I).
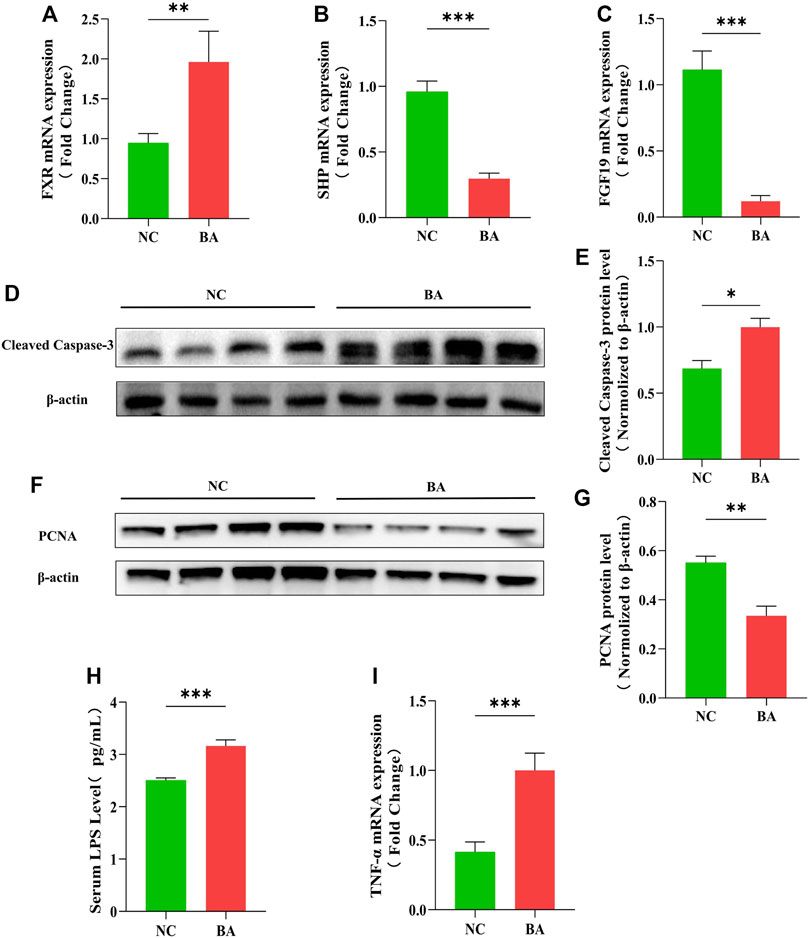
FIGURE 1. The FXR-FGF19 signaling pathway and intestinal barrier are impaired in patients with biliary atresia. (A–C) The expression of intestinal FXR, SHP, and FGF19 mRNA in BA tissues (n = 16) and controls (n = 24). (D–G) The levels of PCNA and cleaved caspase-3 protein expression in BA tissues and controls. (H) Serum LPS detected by ELISA. (I) Intestinal expression of TNF-α mRNA. The data are expressed as the mean ± SEM. (*p < 0.05, **p < 0.01, ***p < 0.001).
Obeticholic Acid Modulated Farnesoid X Receptor Signaling Pathway and Attenuated Intestinal Injury in Bile Duct Ligation Rats
To gain further insight into the effect of OCA, we determined the expression of FXR-related molecules and the impact on BDL rat intestines. HE staining revealed impaired intestinal mucosal architecture in BDL rats, in which intestinal villi were short, thick, and edematous, as shown by the red arrow. The mucosal injury was significantly decreased in BDL rats treated with OCA (Figure 2A). The serum and intestinal level of LPS were significantly increased in BDL rats compared with the control group, whereas OCA caused a marked decrease in the serum levels of these markers (Figures 2B,C). Intestinal TNF-α was also significantly increased in BDL rats, which was reduced by OCA (Figure 2D). Then, we examined the ileum FXR signaling pathway in the BDL rats and the changes produced by OCA. Expression of FXR in BDL rats ileum was higher than controls (Figure 2E). However, the reduced activity of the FXR signaling pathway was indicated by the decreased ileum expression of the FXR target gene, such as SHP and FGF15. OCA effectively modulated the ileum FXR signaling pathway in BDL rats (Figures 2F,G). Furthermore, lack of bile acid in ileum contributde to the decreased trend of bile acid transporter mRNA expression, and FXR activation partially reversed this change (Figures 2H–J).
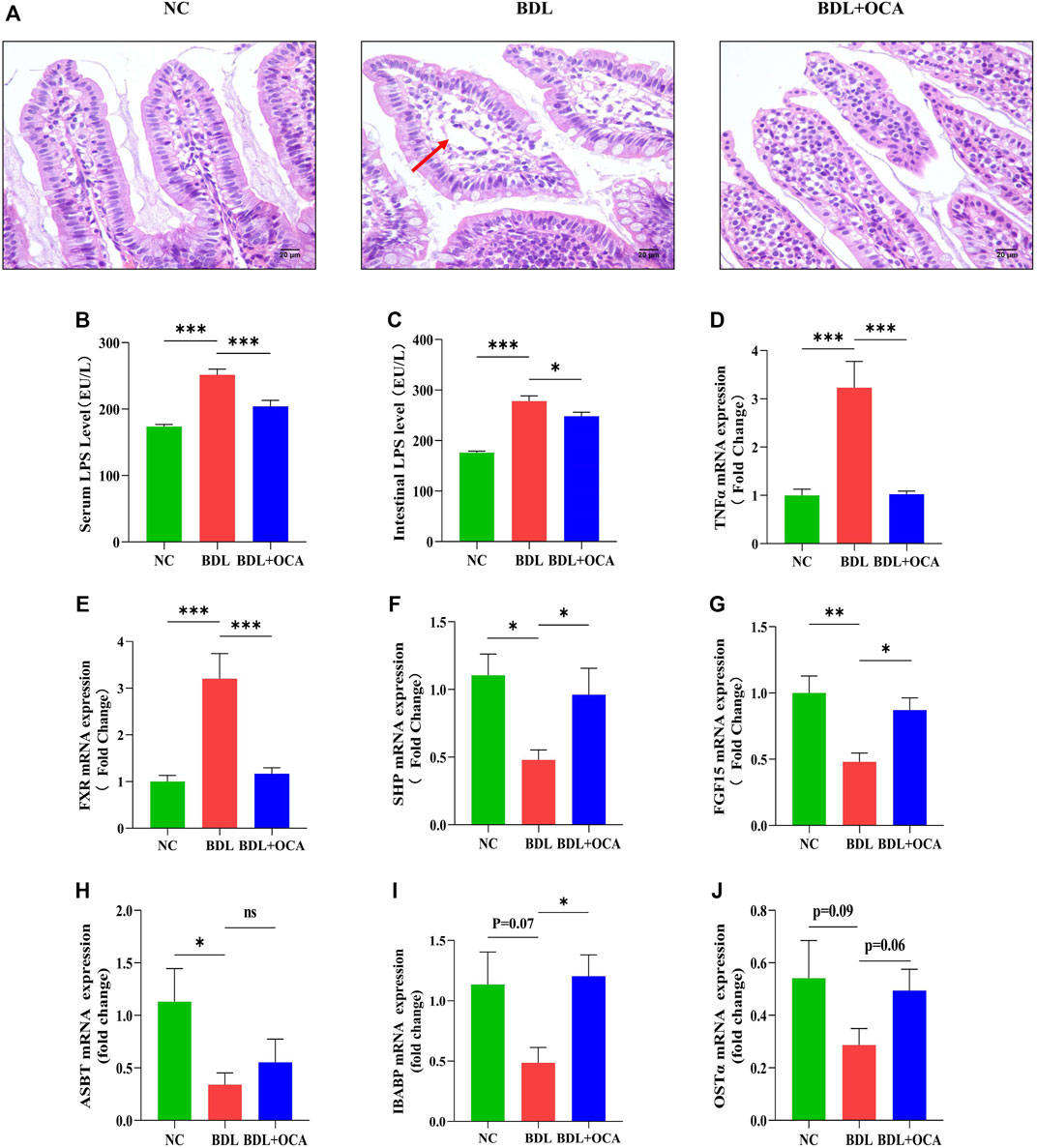
FIGURE 2. OCA modulates the FXR signaling pathway and attenuates intestinal injury in BDL rats. (A) Representative images of intestinal sections stained with hematoxylin and eosin. (B,C) Serum and intestinal content of LPS detected by ELISA. (D) Intestinal mRNA expression of TNF-α. (E–G) Ileum expression of FXR, SHP, and FGF15 mRNA. (H–J) The bile acid transporter mRNA expression in ileum. The data are expressed as the mean ± SEM (n = 7–9). (OCA [5 mg/kg/day]) (*p < 0.05, **p < 0.01, ***p < 0.001)
OCA attenuated BDL-induced apoptosis of ileum epithelial cells.
Previous studies have shown that bile acids stimulate intestinal epithelial proliferation (Yasuda et al., 2007). A lack of bile acid in the intestinal lumen is mainly attributed to impaired cell proliferation and increased apoptosis (Assimakopoulos et al., 2007). To determine whether the OCA-mediated protective effect against BDL-induced disruption of intestinal barrier function was due to an inhibition of cell apoptosis, we performed the TUNEL assay in ileum sections. Compared with controls, intestinal epithelial cell apoptosis was significantly increased in BDL rats, as shown by the white arrow (Figure 3A). The number of apoptotic cells was clearly greater than controls (Figure 3B). As expected, intestinal epithelial cell apoptosis was markedly inhibited by OCA. Similar results were observed in a Western blot analysis, the levels of cleaved caspase-3 protein detected by western blot were increased in BDL rats compared with controls, and OCA attenuated BDL-induced upregulation of cleaved caspase-3 (Figures 3C,D). Consistent with these results, Treatment with OCA increased the expression of the PCNA in BDL rats (Figures 3E,F).
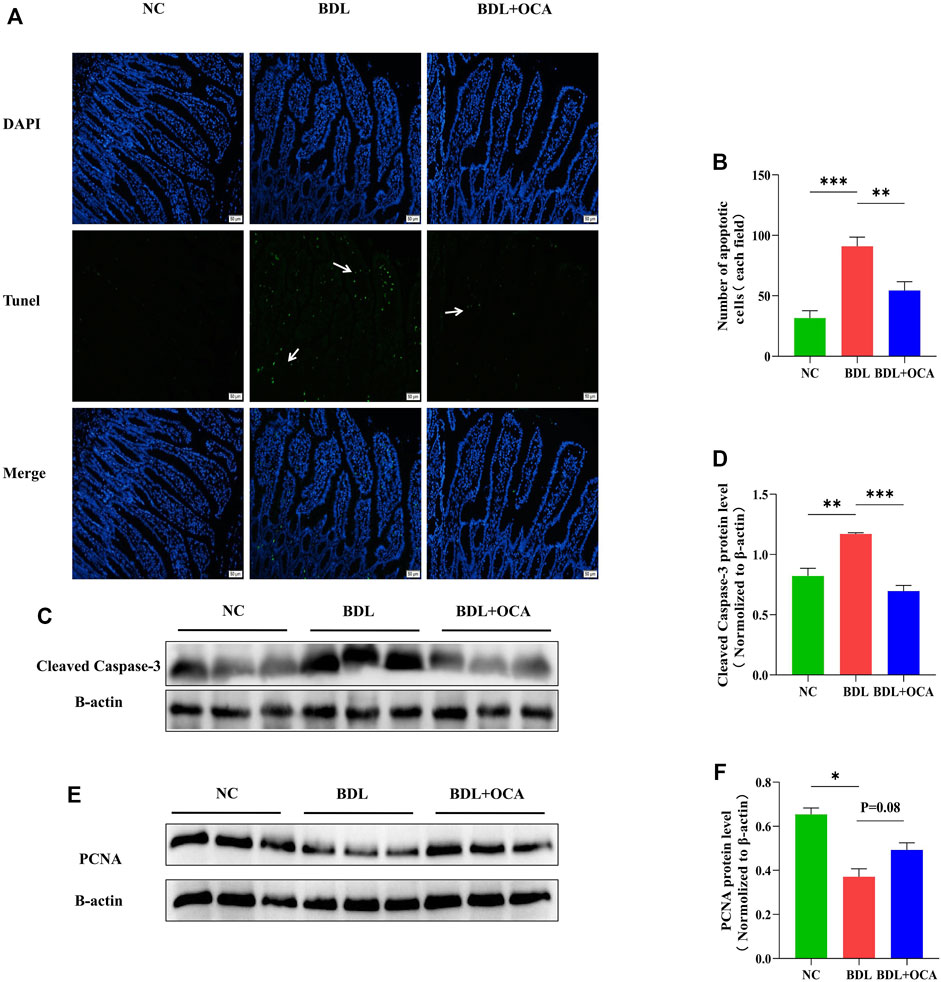
FIGURE 3. OCA attenuates BDL-induced apoptosis of ileum epithelial cells. (A) TUNEL assay of small ileum sections. (B) The number of TUNEL-positive cells in the intestinal epithelium is shown. (C,D) Western blots of cleaved caspase-3 in ileum tissues from rats. (E,F) The protein expression level of PCNA in three groups. Bar graphs represent the mean ± SEM (n = 7–9). (OCA [5 mg/kg/day]) (*p < 0.05, **p < 0.01, ***p < 0.001)
Obeticholic Acid Ameliorated the Dysbiosis of the Ileum Microbiota in Bile Duct Ligation Rats
To determine the mechanism involved in OCA-mediated protection against BDL-induced intestinal injury, we assessed the effects of BDL and OCA on microbiota composition in the ileum by amplifying and analyzing amplicons from the V3-V4 region of the 16S rDNA gene. A Venn diagram showed 75 features in the controls, 678 features in BDL rats, and 93 features in BDL rats treated with OCA (Figure 4A). The Shannon and Chao1 index was calculated to assess α-diversity in bacterial diversity. Significant differences existed between the controls and BDL rats, which were attenuated by OCA (Figures 4B,C). Principal coordinates analysis showed that microbial communities in BDL rats clustered separately from controls, and OCA treatment attenuated the distinction (Figure 4D). Differences in the microbiota community composition, ordered by relative abundance in the samples, were observed at the phylum and genus levels (Figures 4E,F).
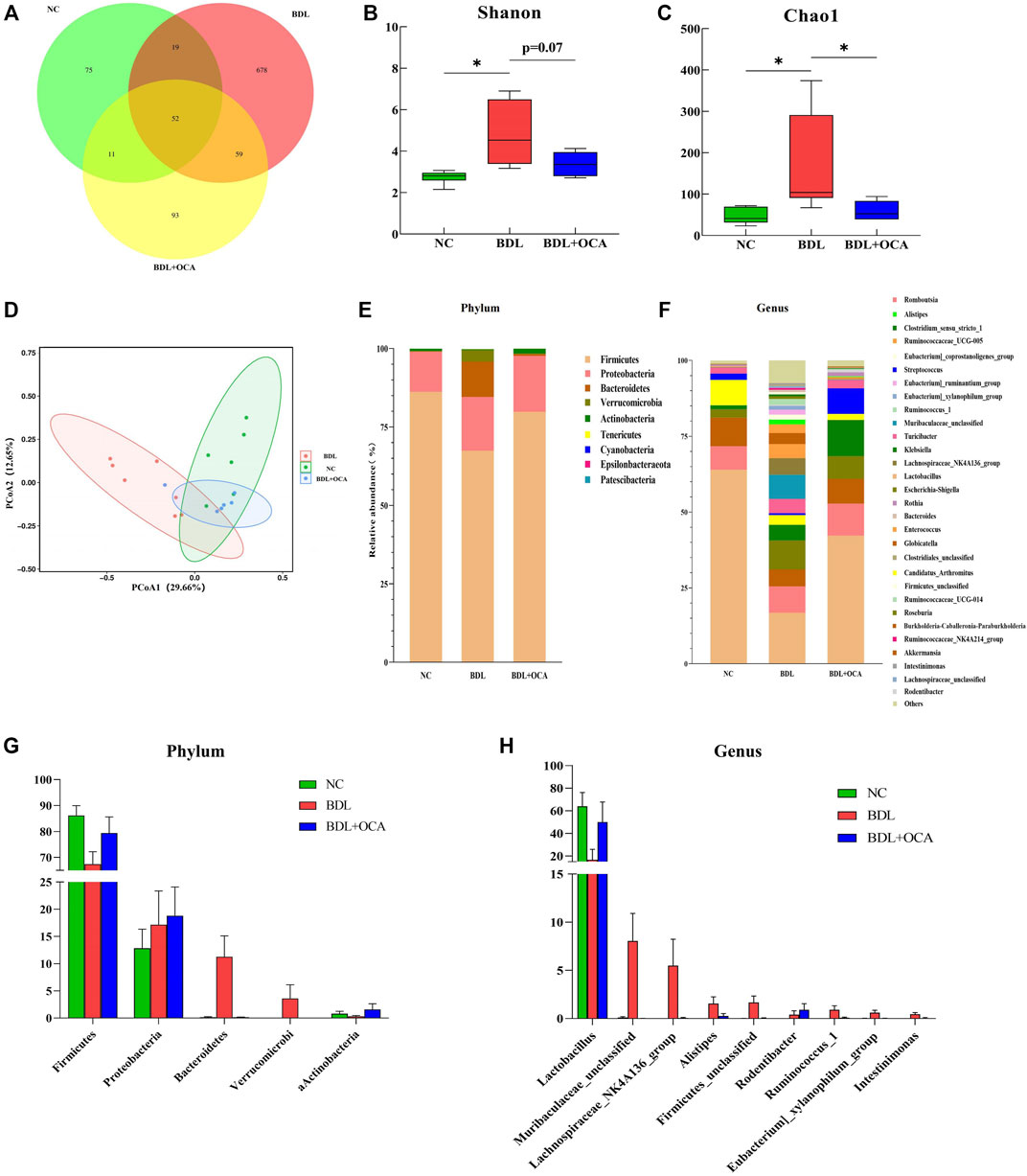
FIGURE 4. OCA ameliorates dysbiosis of the ileum microbiota in BDL rats. (A) Venn diagram showing exclusive features per group. (B,C) Alpha-diversity analysis of ileum microbiota (Shannon and Chao1 index). (D) Differences of ileum microbial β-diversity. Beta-diversity was analyzed by principal coordinates using weighted Unifrac distances. (E,F) Composition of ileum microbiota at the phylum and genus levels. (G,H) Bacterial phyla and genera in ileum microbiota that were significantly changed in three groups and corresponding relative abundance. The data are expressed as the mean ± SEM (n = 6–7). (OCA [5 mg/kg/day]) (*p < 0.05, **p < 0.01, ***p < 0.001).
As shown in Figure 4G, BDL rats had a reduced relative abundance of phylum Firmicutes that harbor bacteria with high bile salt hydrolase activity, which was increased by OCA treatment (Jones et al., 2008). In contrast, Bacteroidetes, a major bacterial phylum harboring bacteria with low bile salt hydrolase (BSH) activity, was significantly increased in BDL rats and attenuated by OCA treatment. At the genus level, the abundance of Lactobacillus was significantly decreased, and the abundance of the Lachnospiraceae NK4A136 group, Alistipes, Eubacterium_ruminantium_group, Intestinimonas, Ruminococcus_1, and Ruminococcaceae_NK4A214_group was significantly increased in BDL rats; however, OCA changed the unique enteric microbiome of BDL rats (Figure 4H). Compared with BDL rats, OCA-treated rats had fewer Lachnospiraceae_NK4A136_group, Eubacterium_ruminantium_group, Ruminococcus_1, and Intestinimonas, and more abundance of Lactobacillus.
Obeticholic Acid Altered the Functional Capacities of Ileum Microbiota in Bile Duct Ligation Rats
Four potential phenotypes (anaerobes, containing mobile elements, gram-negatives, and potentially pathogenic) were predicted to be significantly different in the three groups using BugBase. At the microbial community level, gene functions related to anaerobes, gram-negatives, and potentially pathogenic were increased in BDL rats. OCA treatment reduced the enrichment of gram-negatives and potentially pathogenic, possibly attributed to a decrease in the abundance of Bacteroidetes (Figure 5A). In addition, we gained functional prediction of the fecal bacteria through PICRUSt2 based on a KEGG database. The primary identified pathway of ileum microbiota was related to metabolism (Figure 5B), which was significantly decreased in BDL rats; however, OCA treatment improved enrichment of metabolism compared to BDL rats.
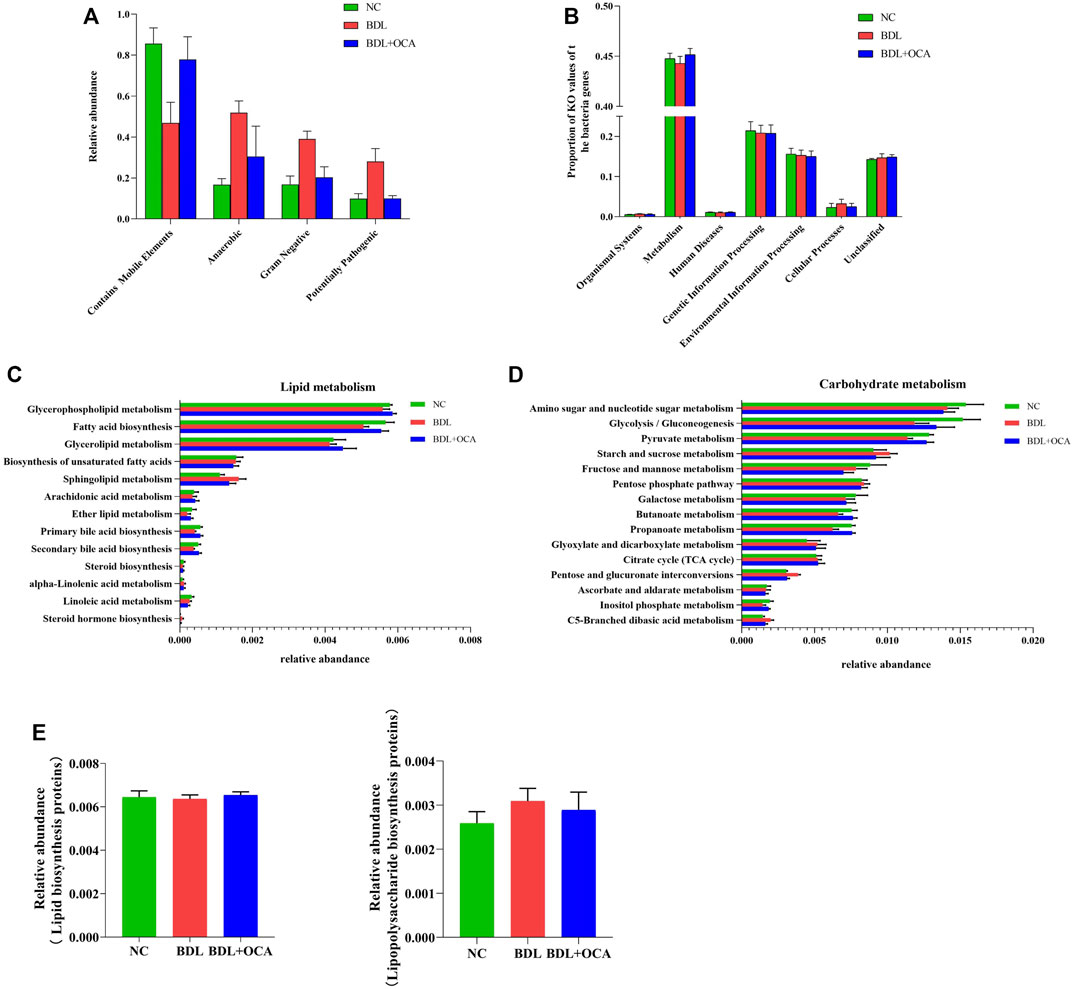
FIGURE 5. Obeticholic acid ameliorated the dysbiosis of the ileum microbiota in bile duct ligation rats. (A) Relative abundances of four potential phenotypes predicted by BugBase. (B) Predicted functional profiles in all groups. (C,D) Relative abundance of the subordinate pathways of lipid and carbohydrate metabolism. (E) Comparison of gene sets associated with lipopolysaccharide biosynthesis and lipopolysaccharide biosynthesis proteins. The data are expressed as the mean ± SEM (n = 6–7). [OCA (5 mg/kg/day)].
Furthermore, the relative abundances of ileum microbiota involved in primary and secondary bile acid biosynthesis-associated lipid metabolism were lower in BDL rats than controls, which may have been associated with lower bile acid levels (Figure 5C). Compared with BDL rats, OCA-treated BDL rats had an increased abundance of primary and secondary bile acid biosynthesis. The relative abundances of ileum microbiota related to sphingolipid metabolism and steroid hormone biosynthesis, which are involved in lipid metabolism, and were more significant in BDL rats than in other groups (Figure 5C). Subordinate pathways of carbohydrate metabolism were also analyzed and compared. Pyruvate metabolism, glycolysis/gluconeogenesis, an amino sugar, and nucleotide sugar metabolism were enriched in three groups of ileum microbiota (Figure 5D); however, there was an insignificant difference between the three cognitive groups in lipopolysaccharide biosynthesis and lipopolysaccharide biosynthesis proteins (Figure 5E).
Obeticholic Acid Protected Against Lipopolysaccharide-Induced Apoptosis in Vitro
The presence of bile acid in the intestine contributes to a normal gut barrier function by promoting intestinal epithelial cell proliferation. In biliary obstruction, intestinal mucosal injury is mainly due to more LPS caused by intestinal flora disorder (Assimakopoulos et al., 2007; Luo et al., 2022). To further confirm the effect of OCA on the proliferation and apoptosis of intestinal mucosal epithelial cells, Caco2 cells were incubated with LPS and OCA in vitro. OCA promoted the proliferation of Caco-2 cells based on concentration (Figure 6A). OCA improved the inhibitory effect of LPS on cell viability and cell proliferation rates (Figure 6B). OCA gradually reduced the released LDH in Caco-2 cells incubated with LPS as the OCA concentration increased (Figure 6C). Next, treatment of OCA significantly prevented apoptosis of Caco-2 cells incubated with LPS (Figure 6D-F). In addition, we determined whether OCA directly activates FXR by RT-PCR. OCA restored LPS-suppressed FXR expression in Caco-2 cells (Figures 6G–I).
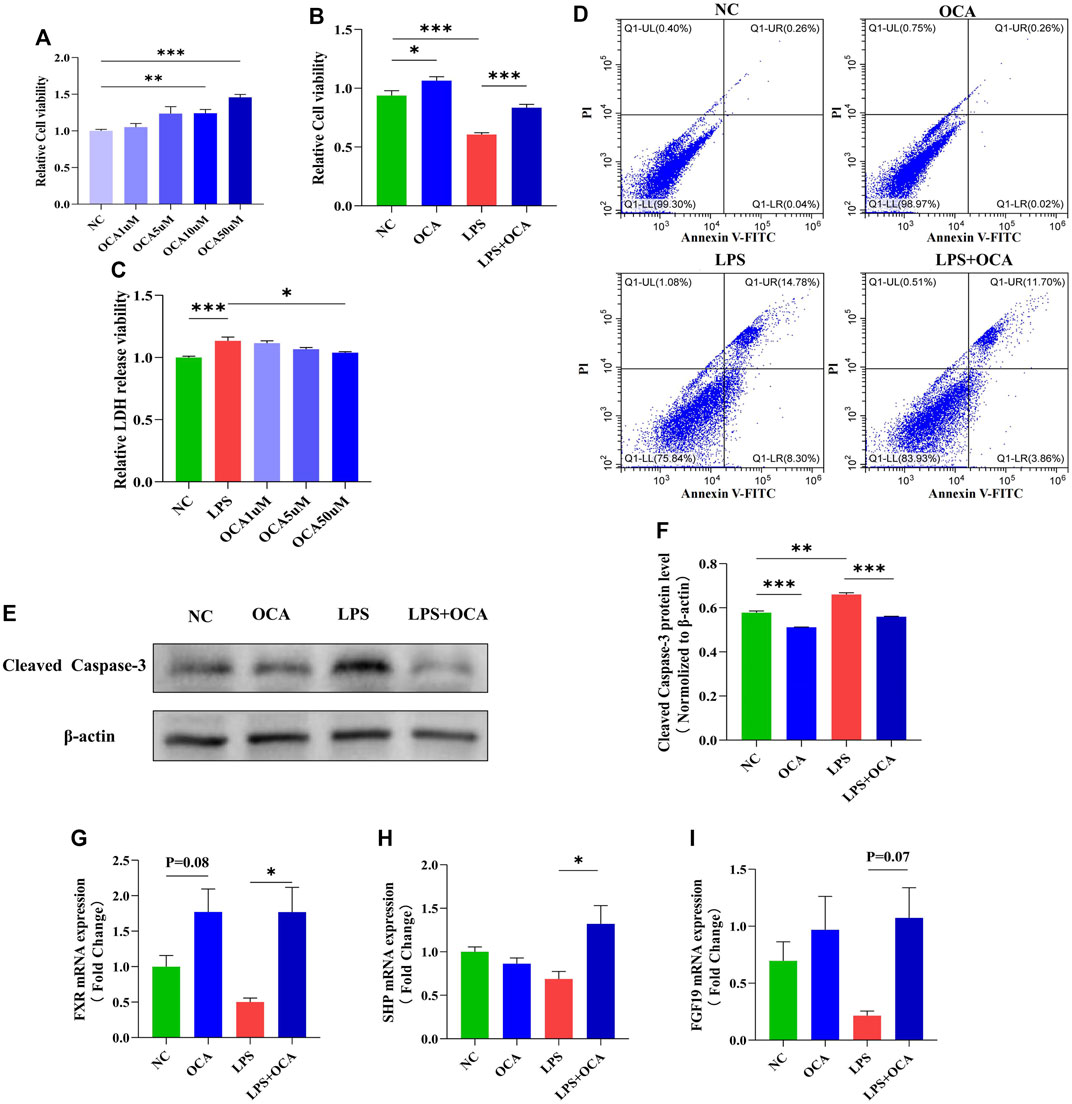
FIGURE 6. OCA protected against LPS-induced apoptosis in vitro. (A,B) cell viability evaluated by CCK8 in the Caco-2 cell line. (C) LDH released in the supernatant of cultured cells was measured at the indicated time. (D) Cell apoptosis assay detected by flow cytometry in the Caco-2 cell line. (E,F) The level of cleaved caspase-3 protein expression in the Caco-2 cell line. (G–I) Real-time PCR analysis of FXR and its target genes FGF-19 and SHP. (OCA [10 μM], LPS [100 μg/ml]) (*p < 0.05, **p < 0.01, ***p < 0.001)
Discussion
Because biliary obstruction not only causes inflammation and fibrosis of the liver, biliary obstruction can also cause damage to the intestinal barrier, leading to endotoxemia. Therefore, protecting the intestinal mucosal barrier during biliary obstruction is an important treatment strategy. Indeed, our study showed that intestinal permeability, inflammation, and intestinal epithelial apoptosis was increased during biliary obstruction.
It is well-accepted that the FXR regulates the synthesis, transport, and enterohepatic circulation of bile acids by modulating the expression of related genes in the liver and small intestine (Liu et al., 2020). The main finding of this study showed that the relationship between the FXR pathway and the intestinal mucosal barrier during biliary obstruction. In BDL rats, the intestinal FXR signaling pathway was inhibited after bile duct ligation, and the restoration of the FXR pathway improved intestinal barrier damage and intestinal microbiota imbalance. This finding provides new insight into the role of the FXR pathway in intestinal barrier injury.
Protection of the activated FXR pathway on intestinal barrier integrity was associated with reduced liver fibrogenesis. Hepatic accumulation of bile acid plays a pivotal role in the apoptosis of hepatocytes and bile duct hyperplasia, and eventually leads to liver fibrosis and cirrhosis in biliary obstruction (Fickert and Wagner, 2017). The decrease in intestinal bile leads to a deficiency in intestinal FXR activity, which weakens the inhibitory effect of CYP7A1 and leads to increased bile acid production and aggravation of liver fibrosis (Liu et al., 2020). In agreement with previous studies (Liu et al., 2020; Tsai et al., 2020; Modica et al., 2012), our results showed that OCA reduced liver fibrosis in rats with cholestasis by BDL (Supplementary Figure S1). The mechanisms underlying improved hepatic fibrosis by OCA remain unclear, although there are several explanations. Previous research has shown that bile acid activates intestinal FXR and elevates FGF15/19, which binds the FGF receptor and the β-Klotho complex on the surface of hepatocytes to inhibit CYP7A1 expression (Hartmann et al., 2018; Lee et al., 2018). In our study, biliary obstruction reduced intestinal bile acid concentration, leading to a significant reduction of SHP and FGF15 expression. Moreover, OCA significantly promoted the expression of SHP and FGF15, which inhibited CYP7A1 expression and decreased the production of bile acids. In addition, mitigation of liver fibrosis caused by OCA may be achieved by regulating the intestinal microbiota and restoring the intestinal barrier, thereby improving the “gut-liver axis” circulation, reducing liver inflammation, and ultimately alleviating liver fibrosis. Previous studies have shown that antibiotics which improve liver function in cholestatic patients indicate an active role of the microbiome in mediating liver injury during cholestasis (Tabibian et al., 2013). In previous study, decreased LPS production from the gut by OCA treatment activated TLR-4 and TLR-9, thus promoting inflammation, steatosis, and fibrosis, which might have contributed to reduced liver fibrosis (Lichtman et al., 1990).
The presence of bile in the intestine promotes intestinal epithelial cell proliferation and protects against apoptotic cell death, which contributes to the integrity of the intestinal barrier (Keitel and Häussinger, 2011). With the absence or reduction of bile in the intestine, the intestinal mucosal barrier is damaged, and the intestinal permeability is increased so that pathogenic bacteria and LPS translocate into the blood, causing bacteremia and endotoxemia (Iida et al., 2009; Wang et al., 2010). More LPS production facilitates liver and intestinal mucosal barrier damage through the LPS-TLR4 signaling pathway (An et al., 2022). Activation of the FXR pathway or the FXR agonists protects the mucosal integrity by regulating the expression of downstream genes related to mucosa protection and defense against inflammation in rodents (Vavassori et al., 2009; Gadaleta et al., 2011). Interestingly, in the case of bile duct obstruction, adding OCA can activate the FXR like bile acid, promoting the proliferation of intestinal epithelial cells and preventing cell apoptosis. Moreover, similar results were demonstrated in vitro, in which OCA attenuated LPS-induced apoptosis of Caco-2 cells. In the other hand, the increase of LPS disruptes bile acid metabolism (Xiong et al., 2017; Zhang et al., 2020), and contributes to the damage of liver and intestinal barrier. In the study of Sai Wang et al., LPS inhibited the expression of FXR signaling pathway in mouse primary hepatocytes (Wang et al., 2022), and we also found that OCA actived the FXR signaling pathway in lps-incubated Caco-2 cells, which may be involved in the mechanisms affecting the proliferation and apoptosis of Caco-2 cells. Our results extend the reparative effect of OCA on impaired intestinal barrier function; however, we only detected the expression of FXR-related genes, the mechanism by which the FXR promoted intestinal epithelial proliferation and inhibited apoptotic cell death warrants further study.
Microbiota and intestinal mucosal barrier are mutually influenced. Normal microbiota applies trophic effects on the intestinal mucosa, which display a significant role in mucosal protection and epithelial regeneration. Recently, the role of microbiota in maintaining the intestinal barrier has increasingly attracted more research attention. Probiotics and fecal microbiota transplantation are widely used in intestinal inflammatory diseases (Al-Sadi et al., 2021; Dou et al., 2021). The PICRUST2 analysis showed no difference in functional microbiota profiles of the LPS biosynthesis pathway among the three groups. These results implied that the elevation of plasma LPS in the BDL group and BA patients were primarily attributable to intestinal barrier dysfunction and microbiota dysbiosis, which has been associated with an increase in the levels of Gram-negative microbiota. Our results suggest that increased LPS produced by intestinal dysbiosis might damage intestinal barrier function, in which OCA treatment improved the intestinal barrier in biliary obstruction by modulating microbiota. In response to OCA, the increased bacterial load was normalized and intestinal dysbiosis improved in BDL rats, which showed that the beneficial effects of OCA on intestinal epithelial apoptosis in biliary obstruction are likely due to remodeling of intestinal microbiota. BSH is a principal enzyme that stimulates the “gateway” reaction in the bacterial metabolism of conjugated bile acid to produce deconjugated bile acid. OCA treatment rectifies the rising relative abundance of Bacteroidetes and the decreased relative richness of Firmicutes at the phylum level, which increases enrichment of the gut microbiota with BSH-containing phyla. Furthermore, Lactobacillus plays a key role in maintaining the intestinal mucosal barrier by increasing immunoglobulin secretion (Liu et al., 2021; Niu et al., 2021; Yang et al., 2021; Zhang et al., 2021). Our results showed that OCA treatment exhibited more abundance of Lactobacillus, thereby protecting against an impaired intestinal mucosal barrier in obstructive jaundice.
Intestinal microbiota displays various potential phenotypes and functions in response to changes in a different environment. Against colonization of potentially harmful bacteria in the intestine is a major function for normal intestinal microbiota (Dai and Walker, 1999). We observed that the OCA treatment significantly decreased the high abundance of potentially pathogenic and gram-negative microflora in the BDL group, mostly attributed to the change in the abundance of Bacteroidetes. LPS derived from gram-negative bacteria can cause inflammatory responses and liver injuries (Rietschel et al., 1994; Machida et al., 2006; Aloman et al., 2007). This result is consistent with the level of LPS and TNF-α in the current study. In addition, analysis of KEGG pathway enrichment showed that OCA treatment greatly influences genes belonging to lipid metabolism pathways, including steroid hormone biosynthesis, sphingolipid metabolism, and primary and secondary bile acid biosynthesis.
Furthermore, it was concluded that the changed pathways in lipid metabolism were associated with biliary obstruction and OCA treatment. Biliary obstruction decreased bile acid metabolism-related genes, which were thought to be involved with inhibition of the FXR pathway. OCA treatment greatly stimulated activity of the FXR pathway, increasing the expression of bile acid metabolism-related genes. Recent studies have shown that intestinal FXR plays an indispensable role in glycolipid metabolism regulation, and intestinal-specific FXR agonists or antagonists participate in glucose and lipid metabolism regulation in vivo (Jiang et al., 2015a; Jiang et al., 2015b; Grundy, 2016). Our results also demonstrated that OCA attenuated the relative abundances of the genes involved in carbohydrate metabolism in biliary obstruction. The results of KEGG metabolic pathway analysis suggested that OCA had beneficial effects on BDL rats, which may be closely related to effective regulation of microbial metabolic pathways.
In general, OCA improved mucosal barrier function, down-regulated the concentrations of TNF-α and LPS, decreased the richness and diversity of the gut microbiota in the ileum, and reversed metabolic disorders.
Conclusion
Our study indicated that the active FXR pathway by OCA had beneficial effects on the intestinal barrier in BDL rats. Specifically, OCA changed the composition and structure of the intestinal microbiota and improved the metabolic function of the intestinal microbiota by increasing the relative abundance of beneficial bacteria and reducing the relative abundance of harmful bacteria. Moreover, OCA promoted the recovery of the intestinal barrier function of BDL rats by downregulating the levels of inflammatory cytokines and LPS. Therefore, this research provides a theoretical and application basis for developing the FXR pathway as a functional target to alleviate intestinal barrier injury in biliary obstruction.
Data Availability Statement
The data presented in the study are deposited in the SRA of the NCBI (https://www.ncbi.nlm.nih.gov/sra), accession number SRR18791516-SRR18791534.
Ethics Statement
The studies involving human participants were reviewed and approved by the medical ethic committee of Nanjing Children’s Hospital of Nanjing Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. The animal study was reviewed and approved by Institutional Animal Care and Use Committee of NMU and Nanjing medical University.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was funded by the Top Talents of Jiangsu Provincial Health Committee’s Six One Project, China (LGY2020019); General Project of Nanjing Health Commission (YKK20121); the Jiangsu Provincial Key Research and Development Program, China (BE2017609); and Key Project of Science and Technology Development of Nanjing health committee, China (ZKX18037).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.906452/full#supplementary-material
Abbreviations
FXR, farnesoid X receptor; OCA, Obeticholic acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; BDL, bile duct ligation; TNF-α, tumor necrosis factor-alpha; FGF15/19, fibroblast growth factor 15/19; SHP, small heterodimer partner; LPS, lipopolysaccharide; SHP, small heterodimer partner; PCNA, Proliferating Cell Nuclear Antigen; T-βMCA, taurine-β-muricholic acid; ASBT, Apical Sodium-BA transporter; I-BABP, Induction of intestinal BA-binding protein; OSTα, Organic Solute and Steroid Transporter Alpha; CYP7A1, Cholesterol 7-alpha Hydroxy-lase.
References
Al-Sadi, R., Dharmaprakash, V., Nighot, P., Guo, S., Nighot, M., Do, T., et al. (2021). Bifidobacterium Bifidum Enhances the Intestinal Epithelial Tight Junction Barrier and Protects against Intestinal Inflammation by Targeting the Toll-like Receptor-2 Pathway in an NF-κb-independent Manner. Int. J. Mol. Sci. 22 (15), 8070. doi:10.3390/ijms22158070
Aloman, C., Gehring, S., Wintermeyer, P., Kuzushita, N., and Wands, J. R. (2007). Chronic Ethanol Consumption Impairs Cellular Immune Responses against HCV NS5 Protein Due to Dendritic Cell Dysfunction. Gastroenterology 132 (2), 698–708. doi:10.1053/j.gastro.2006.11.016
An, L., Wirth, U., Koch, D., Schirren, M., Drefs, M., Koliogiannis, D., et al. (2022). The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J. Gastrointest. Surg. 26 (3), 671–683. doi:10.1007/s11605-021-05188-7
Asai, A., Miethke, A., and Bezerra, J. A. (2015). Pathogenesis of Biliary Atresia: Defining Biology to Understand Clinical Phenotypes. Nat. Rev. Gastroenterol. Hepatol. 12 (6), 342–352. doi:10.1038/nrgastro.2015.74
Assimakopoulos, S. F., Scopa, C. D., and Vagianos, C. E. (2007). Pathophysiology of Increased Intestinal Permeability in Obstructive Jaundice. World J. Gastroenterol. 13 (48), 6458–6464. doi:10.3748/wjg.v13.i48.6458
Assimakopoulos, S. F., Vagianos, C. E., Patsoukis, N., Georgiou, C., Nikolopoulou, V., and Scopa, C. D. (2004). Evidence for Intestinal Oxidative Stress in Obstructive Jaundice-Induced Gut Barrier Dysfunction in Rats. Acta Physiol. Scand. 180 (2), 177–185. doi:10.1046/j.0001-6772.2003.01229.x
Cariello, M., Piccinin, E., Garcia-Irigoyen, O., Sabbà, C., and Moschetta, A. (2018). Nuclear Receptor FXR, Bile Acids and Liver Damage: Introducing the Progressive Familial Intrahepatic Cholestasis with FXR Mutations. Biochim. Biophys. Acta Mol. Basis Dis. 1864 (4 Pt B), 1308–1318. doi:10.1016/j.bbadis.2017.09.019
Dai, D., and Walker, W. A. (1999). Protective Nutrients and Bacterial Colonization in the Immature Human Gut. Adv. Pediatr. 46, 353–382.
Dawson, P. A. (2011). Role of the Intestinal Bile Acid Transporters in Bile Acid and Drug Disposition. Handb. Exp. Pharmacol. 201, 169–203. doi:10.1007/978-3-642-14541-4_4
Dou, X., Qiao, L., Chang, J., Yan, S., Song, X., Chen, Y., et al. (2021). Lactobacillus Casei ATCC 393 and It's Metabolites Alleviate Dextran Sulphate Sodium-Induced Ulcerative Colitis in Mice through the NLRP3-(Caspase-1)/IL-1β Pathway. Food Funct. 12 (23), 12022–12035. doi:10.1039/d1fo02405a
Fickert, P., and Wagner, M. (2017). Biliary Bile Acids in Hepatobiliary Injury - what Is the Link? J. Hepatol. 67 (3), 619–631. doi:10.1016/j.jhep.2017.04.026
Fiorucci, S., and Distrutti, E. (2015). Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol. Med. 21 (11), 702–714. doi:10.1016/j.molmed.2015.09.001
Gadaleta, R. M., Van Erpecum, K. J., Oldenburg, B., Willemsen, E. C., Renooij, W., Murzilli, S., et al. (2011). Farnesoid X Receptor Activation Inhibits Inflammation and Preserves the Intestinal Barrier in Inflammatory Bowel Disease. Gut 60 (4), 463–472. doi:10.1136/gut.2010.212159
Gonzalez, F. J., Jiang, C., Xie, C., and Patterson, A. D. (2017). Intestinal Farnesoid X Receptor Signaling Modulates Metabolic Disease. Dig. Dis. 35 (3), 178–184. doi:10.1159/000450908
Grundy, S. M. (2016). Metabolic Syndrome Update. Trends Cardiovasc Med. 26 (4), 364–373. doi:10.1016/j.tcm.2015.10.004
Hartmann, P., Hochrath, K., Horvath, A., Chen, P., Seebauer, C. T., Llorente, C., et al. (2018). Modulation of the Intestinal Bile Acid/farnesoid X Receptor/fibroblast Growth Factor 15 axis Improves Alcoholic Liver Disease in Mice. Hepatology 67 (6), 2150–2166. doi:10.1002/hep.29676
Iida, A., Yoshidome, H., Shida, T., Kimura, F., Shimizu, H., Ohtsuka, M., et al. (2009). Does Prolonged Biliary Obstructive Jaundice Sensitize the Liver to Endotoxemia? Shock 31 (4), 397–403. doi:10.1097/SHK.0b013e31818349ea
Jiang, C., Xie, C., Li, F., Zhang, L., Nichols, R. G., Krausz, K. W., et al. (2015a). Intestinal Farnesoid X Receptor Signaling Promotes Nonalcoholic Fatty Liver Disease. J. Clin. Invest. 125 (1), 386–402. doi:10.1172/JCI76738
Jiang, C., Xie, C., Lv, Y., Li, J., Krausz, K. W., Shi, J., et al. (2015b). Intestine-selective Farnesoid X Receptor Inhibition Improves Obesity-Related Metabolic Dysfunction. Nat. Commun. 6, 10166. doi:10.1038/ncomms10166
Jones, B. V., Begley, M., Hill, C., Gahan, C. G., and Marchesi, J. R. (2008). Functional and Comparative Metagenomic Analysis of Bile Salt Hydrolase Activity in the Human Gut Microbiome. Proc. Natl. Acad. Sci. U. S. A. 105 (36), 13580–13585. doi:10.1073/pnas.0804437105
Keitel, V., Dröge, C., and Häussinger, D. (2019). Targeting FXR in Cholestasis. Handb. Exp. Pharmacol. 256, 299–324. doi:10.1007/164_2019_231
Keitel, V., and Häussinger, D. (2011). TGR5 in the Biliary Tree. Dig. Dis. 29 (1), 45–47. doi:10.1159/000324127
Kong, B., Wang, L., Chiang, J. Y., Zhang, Y., Klaassen, C. D., and Guo, G. L. (2012). Mechanism of Tissue-specific Farnesoid X Receptor in Suppressing the Expression of Genes in Bile-Acid Synthesis in Mice. Hepatology 56 (3), 1034–1043. doi:10.1002/hep.25740
Kwong, E. K., and Puri, P. (2021). Gut Microbiome Changes in Nonalcoholic Fatty Liver Disease & Alcoholic Liver Disease. Transl. Gastroenterol. Hepatol. 6, 3. doi:10.21037/tgh.2020.02.18
Lee, S., Choi, J., Mohanty, J., Sousa, L. P., Tome, F., Pardon, E., et al. (2018). Structures of β-klotho Reveal a 'zip Code'-like Mechanism for Endocrine FGF Signalling. Nature 553 (7689), 501–505. doi:10.1038/nature25010
Lichtman, S. N., Sartor, R. B., Keku, J., and Schwab, J. H. (1990). Hepatic Inflammation in Rats with Experimental Small Intestinal Bacterial Overgrowth. Gastroenterology 98 (2), 414–423. doi:10.1016/0016-5085(90)90833-m
Liu, Y., Chen, K., Li, F., Gu, Z., Liu, Q., He, L., et al. (2020). Probiotic Lactobacillus Rhamnosus GG Prevents Liver Fibrosis through Inhibiting Hepatic Bile Acid Synthesis and Enhancing Bile Acid Excretion in Mice. Hepatology 71 (6), 2050–2066. doi:10.1002/hep.30975
Liu, Y., Zheng, S., Cui, J., Guo, T., Zhang, J., and Li, B. (2021). Alleviative Effects of Exopolysaccharide Produced by Lactobacillus Helveticus KLDS1.8701 on Dextran Sulfate Sodium-Induced Colitis in Mice. Microorganisms 9 (10), 2086. doi:10.3390/microorganisms9102086
Luo, Q., Shi, R., Liu, Y., Huang, L., Chen, W., and Wang, C. (2022). Histamine Causes Pyroptosis of Liver by Regulating Gut-Liver Axis in Mice. Int. J. Mol. Sci. 23 (7), 3710. doi:10.3390/ijms23073710
Machida, K., Cheng, K. T., Sung, V. M., Levine, A. M., Foung, S., and Lai, M. M. (2006). Hepatitis C Virus Induces Toll-like Receptor 4 Expression, Leading to Enhanced Production of Beta Interferon and Interleukin-6. J. Virol. 80 (2), 866–874. doi:10.1128/JVI.80.2.866-874.2006
Makishima, M., Okamoto, A. Y., Repa, J. J., Tu, H., Learned, R. M., Luk, A., et al. (1999). Identification of a Nuclear Receptor for Bile Acids. Science 284 (5418), 1362–1365. doi:10.1126/science.284.5418.1362
Modica, S., Petruzzelli, M., Bellafante, E., Murzilli, S., Salvatore, L., Celli, N., et al. (2012). Selective Activation of Nuclear Bile Acid Receptor FXR in the Intestine Protects Mice against Cholestasis. Gastroenterology 142 (2), 355–365. e1-4. doi:10.1053/j.gastro.2011.10.028
Niu, H., Zhou, X., Gong, P., Jiao, Y., Zhang, J., Wu, Y., et al. (2021). Effect of Lactobacillus Rhamnosus MN-431 Producing Indole Derivatives on Complementary Feeding-Induced Diarrhea Rat Pups through the Enhancement of the Intestinal Barrier Function[J]. Mol. Nutr. Food Res. 2021, e2100619. doi:10.1002/mnfr.202100619
Rietschel, E. T., Kirikae, T., Schade, F. U., Mamat, U., Schmidt, G., Loppnow, H., et al. (1994). Bacterial Endotoxin: Molecular Relationships of Structure to Activity and Function. Faseb J. 8 (2), 217–225. doi:10.1096/fasebj.8.2.8119492
Sayin, S. I., Wahlström, A., Felin, J., Jäntti, S., Marschall, H. U., Bamberg, K., et al. (2013). Gut Microbiota Regulates Bile Acid Metabolism by Reducing the Levels of Tauro-Beta-Muricholic Acid, a Naturally Occurring FXR Antagonist. Cell Metab. 17 (2), 225–235. doi:10.1016/j.cmet.2013.01.003
Schneider, K. M., Albers, S., and Trautwein, C. (2018). Role of Bile Acids in the Gut-Liver axis. J. Hepatol. 68 (5), 1083–1085. doi:10.1016/j.jhep.2017.11.025
Song, W., Sun, L. Y., Zhu, Z. J., Wei, L., Qu, W., Zeng, Z. G., et al. (2021). Association of Gut Microbiota and Metabolites with Disease Progression in Children with Biliary Atresia. Front. Immunol. 12, 698900. doi:10.3389/fimmu.2021.698900
Sorribas, M., Jakob, M. O., Yilmaz, B., Li, H., Stutz, D., Noser, Y., et al. (2019). FXR Modulates the Gut-Vascular Barrier by Regulating the Entry Sites for Bacterial Translocation in Experimental Cirrhosis. J. Hepatol. 71 (6), 1126–1140. doi:10.1016/j.jhep.2019.06.017
Stojancevic, M., Stankov, K., and Mikov, M. (2012). The Impact of Farnesoid X Receptor Activation on Intestinal Permeability in Inflammatory Bowel Disease. Can. J. Gastroenterol. 26 (9), 631–637. doi:10.1155/2012/538452
Tabibian, J. H., Talwalkar, J. A., and Lindor, K. D. (2013). Role of the Microbiota and Antibiotics in Primary Sclerosing Cholangitis. Biomed. Res. Int. 2013, 389537. doi:10.1155/2013/389537
Trabelsi, M. S., Lestavel, S., Staels, B., and Collet, X. (2017). Intestinal Bile Acid Receptors Are Key Regulators of Glucose Homeostasis. Proc. Nutr. Soc. 76 (3), 192–202. doi:10.1017/S0029665116002834
Trauner, M., and Fuchs, C. D. (2022). Novel Therapeutic Targets for Cholestatic and Fatty Liver Disease. Gut 71 (1), 194–209. doi:10.1136/gutjnl-2021-324305
Tsai, Y. L., Liu, C. W., Hsu, C. F., Huang, C. C., Lin, M. W., Huang, S. F., et al. (2020). Obeticholic Acid Ameliorates Hepatorenal Syndrome in Ascitic Cirrhotic Rats by Down-Regulating the Renal 8-Iso-Pgf2α-Activated COX-TXA2 Pathway. Clin. Sci. 134 (15), 2055–2073. doi:10.1042/CS20200452
Vavassori, P., Mencarelli, A., Renga, B., Distrutti, E., and Fiorucci, S. (2009). The Bile Acid Receptor FXR Is a Modulator of Intestinal Innate Immunity. J. Immunol. 183 (10), 6251–6261. doi:10.4049/jimmunol.0803978
Verbeke, L., Farre, R., Verbinnen, B., Covens, K., Vanuytsel, T., Verhaegen, J., et al. (2015). The FXR Agonist Obeticholic Acid Prevents Gut Barrier Dysfunction and Bacterial Translocation in Cholestatic Rats. Am. J. Pathol. 185 (2), 409–419. doi:10.1016/j.ajpath.2014.10.009
Wang, J., Qian, T., Jiang, J., Yang, Y., Shen, Z., Huang, Y., et al. (2020). Gut Microbial Profile in Biliary Atresia: a Case-Control Study. J. Gastroenterol. Hepatol. 35 (2), 334–342. doi:10.1111/jgh.14777
Wang, N., Yu, H., Ma, J., Wu, W., Zhao, D., Shi, X., et al. (2010). Evidence for Tight Junction Protein Disruption in Intestinal Mucosa of Malignant Obstructive Jaundice Patients. Scand. J. Gastroenterol. 45 (2), 191–199. doi:10.3109/00365520903406701
Wang, S., Feng, R., Wang, S. S., Liu, H., Shao, C., Li, Y., et al. (2022). FOXA2 Prevents Hyperbilirubinaemia in Acute Liver Failure by Maintaining Apical MRP2 Expression Gut Published Online First. doi:10.1136/gutjnl-2022-326987
Xiong, X., Ren, Y., Cui, Y., Li, R., Wang, C., and Zhang, Y. (2017). Obeticholic Acid Protects Mice against Lipopolysaccharide-Induced Liver Injury and Inflammation. Biomed. Pharmacother. 96, 1292–1298. doi:10.1016/j.biopha.2017.11.083
Yang, K. M., Zhu, C., Wang, L., Cao, S. T., Yang, X. F., Gao, K. G., et al. (2021). Early Supplementation with Lactobacillus Plantarum in Liquid Diet Modulates Intestinal Innate Immunity through Toll-like Receptor 4-mediated Mitogen-Activated Protein Kinase Signaling Pathways in Young Piglets Challenged with Escherichia coli K88. J. Anim. Sci. 99 (6), skab128. doi:10.1093/jas/skab128
Yasuda, H., Hirata, S., Inoue, K., Mashima, H., Ohnishi, H., and Yoshiba, M. (2007). Involvement of Membrane-type Bile Acid Receptor M-BAR/TGR5 in Bile Acid-Induced Activation of Epidermal Growth Factor Receptor and Mitogen-Activated Protein Kinases in Gastric Carcinoma Cells. Biochem. Biophys. Res. Commun. 354 (1), 154–159. doi:10.1016/j.bbrc.2006.12.168
Zhang, C., Gan, Y., Lv, J. W., Qin, M. Q., Hu, W. R., Liu, Z. B., et al. (2020). The Protective Effect of Obeticholic Acid on Lipopolysaccharide-Induced Disorder of Maternal Bile Acid Metabolism in Pregnant Mice. Int. Immunopharmacol. 83, 106442. doi:10.1016/j.intimp.2020.106442
Keywords: FXR, biliary atresia, intestinal microbiota, obeticholic acid, bile duct ligation
Citation: Yan M, Hou L, Cai Y, Wang H, Ma Y, Geng Q, Jiang W and Tang W (2022) Effects of Intestinal FXR-Related Molecules on Intestinal Mucosal Barriers in Biliary Tract Obstruction. Front. Pharmacol. 13:906452. doi: 10.3389/fphar.2022.906452
Received: 28 March 2022; Accepted: 18 May 2022;
Published: 13 June 2022.
Edited by:
Laura Grasa, University of Zaragoza, SpainReviewed by:
Takeshi Susukida, University of Toyama, JapanRodrigo M. Florentino, University of Pittsburgh, United States
Copyright © 2022 Yan, Hou, Cai, Wang, Ma, Geng, Jiang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiwei Jiang, wwjiang@njmu.edu.cn; Weibing Tang, twbcn@njmu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Meng Yan
Meng Yan Li Hou
Li Hou Yaoyao Cai1
Yaoyao Cai1 Weiwei Jiang
Weiwei Jiang Weibing Tang
Weibing Tang