- 1Department of Clinical and Experimental Medicine, Dermatology, University of Messina, Messina, Italy
- 2Department of Clinical and Experimental Medicine, Pharmacology, University of Messina, Messina, Italy
- 3Department of Dermatology, University of Modena and Reggio Emilia, Modena, Italy
- 4Department of Human Pathology in Adult and Developmental Age “Gaetano Barresi, Pediatry”, University of Messina, Messina, Italy
Among benign vascular tumors of infancy, hemangiomas are the commonest, affecting approximately 5–10% of one-year-old children. They are derived from a benign proliferation of vascular endothelial cells (VECs) in the mesoderm and may arise anywhere on the body around 1–2 weeks after birth. Infantile hemangiomas (IHs) are characterized by an early proliferative phase in the first year followed by a spontaneous progressive regression within the following 5 years or longer. IH prevalence is estimated to be 5%–10% in one-year-old children and commonly affects female, Caucasian and low-birth weight infants. Although most of them spontaneously regress, approximately 10% requires treatment to prevent complications due to the site of occurrence such as bleeding, ulceration, cosmetically disfigurement, functional impairment, or life-threatening complications. For over 30 years, steroids have represented the first-line treatment for IHs, but recently topical or systemic β-blockers are increasingly being used and recognized as effective and safe. A search for “Cutaneous infantile hemangioma” [All Fields] AND “Treatment” [All Fields] was performed by using PubMed and EMBASE databases. Treatment of IHs with labeled drugs, such as oral propranolol, but also with off-label drugs, such as topical β-blockers, including topical timolol and carteolol, steroids, itraconazole or sirolimus, with a focus on formulations types and adverse events were described in our review. We also discussed the benefits of pulsed dye laser and the treatment of IHs with involvement of central nervous system, namely the PHACE and LUMBAR syndrome.
Introduction
Infantile hemangiomas (IHs) are the commonest vascular tumors of the children (Munden et al., 2014; Püttgen, 2014). The lesions arise from the benign proliferation of vascular endothelial cells (VECs) in the mesoderm occurring anywhere on the body, more frequently on the head or face 1–2 weeks after birth (Hoornweg et al., 2012). The course of hemangiomas is characterized by a proliferative phase followed by a plateau and a regression phase. During the first year (proliferative phase), IH grow rapidly and may also ulcerate, bleed, or become infected. This phase is followed by gradual spontaneous involution (regression phase) over the next 1–5 years or longer (Chang et al., 2008; Yanes et al., 2016).
IHs can be classified by general appearance: 1) superficial, located in the upper dermis 2) deep, extending to subcutaneous fascia, 3) mixed (Hoornweg et al., 2012) and the diagnosis is predominantly clinical. The estimated incidence of IHs is 1.1–2.6% in newborns, while IH prevalence is estimated to be 5%–10% in one-year-old children and commonly affects female, Caucasian and low-birth weight infants (Püttgen, 2014; Wang et al., 2017; Price et al., 2018). The use of drugs in first-degree relatives increase IHs risk (Li J. et al., 2011). Other predisposing factors associated with IHs are represented by old maternal age, placenta previa and pre-eclampsia (Bauland et al., 2010).
Materials and Methods
PubMed (https://ncbi.nlm.nih.gov/PubMed) and EMBASE databases were checked by using the string “Cutaneous infantile hemangioma” [All Fields] AND “Treatment” [All Fields]. Only papers written in English language, concerning humans and with 5 years’ time limits were included. The references retrieved were critically examined to select those pertinent, thus reporting the type of the selected articles (review, retrospective cohort study, clinical trial, case series, research studies, case reports). The reference lists of these papers were also examined to find other relevant articles, which were eventually included if appropriate.
Results
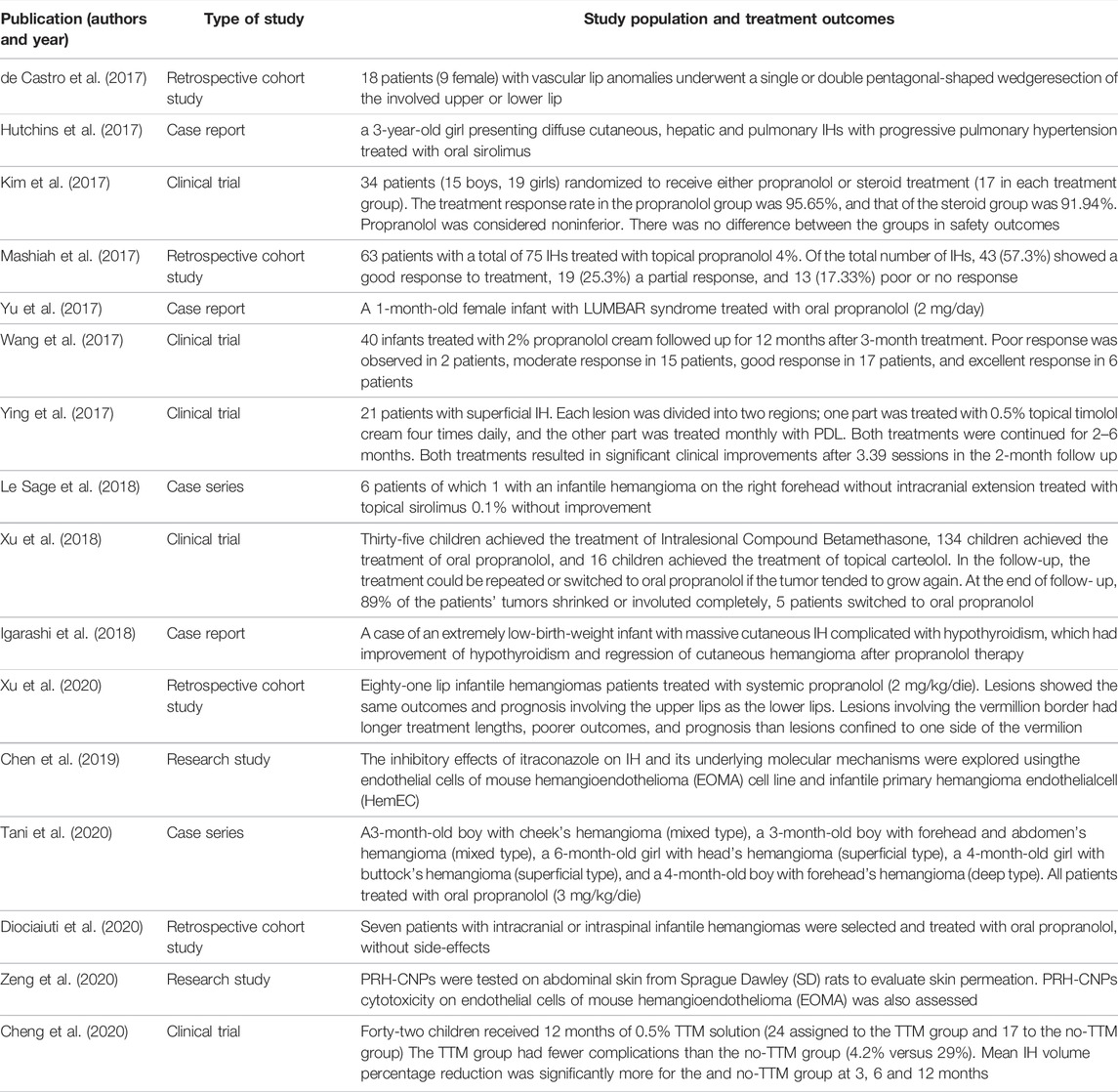
As of 21 October 2021, we found 23 articles of interest. Among these, seven reviews, four retrospective cohort studies, five clinical trials, 2 case series, two research studies and 3 case reports were selected. In Table 1 are summarized the following features of each article: first author, year of publication, type of study, study population and strength level.
Treatment OF IHs
This is the case of a particular rare, self-limited disease featured by multiple cutaneous IHs without markable visceral lesions: benign neonatal hemangiomatosis (BNH). BNH lesions spontaneously subside within 4 months of onset or within the second year of life. In an infant with multiple (>5) cutaneous hemangiomas, it is important to differentiate BNH from diffuse neonatal hemangiomatosis (DNH), which is marked by multiple cutaneous and visceral hemangiomas with mortality ranging from 60% to 81% (Korekawa et al., 2020).
Given that most IHs spontaneously regress, they do not require any treatment, so periodic follow-up is sufficient (Schwartz et al., 2009).
Generally, complications are mild but some IHs can dramatically grow and leave residual cutaneous modifications after the involution phase, including telangiectasia, atrophy, scarring and skin laxity (Bauland et al., 2011; Léauté-Labrèze et al., 2017). Approximately 10% requires treatment to prevent distressing complications due to the site of occurrence such as bleeding, ulceration, visual impairment, eating disorder, airway obstruction, lifelong disfigurement, congestive heart failure, or bowel obstruction (Frieden et al., 2005; Hemangioma Investigator Group et al., 2007; Cheng and Friedlander, 2016; Novoa et al., 2019). In the next paragraphs, we’ll report the last 5-years experiences with propranolol, the first line therapy for IHs treatment, and, in particular, with off-label drugs, such as topical β-blockers, including topical timolol and carteolol, steroids, itraconazole or sirolimus.
Steroids
For over 30 years, steroids have been used as the first-line therapy for IHs (Zarem and Edgerton, 1967; Folkman, 1984) thanks to their possible antiangiogenic effect. Corticosteroids can be orally, intravenously, intramuscularly, and topically administered. However, their systemic use may lead to various complications including Cushing-like manifestations, adrenal suppression, gastroesophageal reflux and growth disorders, even though such complications are linked with long-term and high dose therapy (George et al., 2004). Topical administration is advantageous, safe with fast response, and is extensively used for small tumors (Yuan et al., 2015). A significant lesion reduction (85.7% response rate) was observed in the study of Xu et al. (2018): 35 out of 185 IHs patients (mean age 3.9 months), presenting small-size hemangiomas which were raised and <3 cm X 3 cm, were treated with intralesional administration of betamethasone (one or two injection of Diprospan 1 ml/ampoule).
Oral Propranolol
Oral Propranolol Versus Steroids
Some positive effects of propranolol use for the treatment of IH have been reported. Differently from Zimmermann et al. (2010), the clinical trial lead by Kim et al. (2017) showed non-inferiority of the therapeutic effects of propranolol compared with steroids, with a response rate of 95.65% in the propranolol-treated group and of 91.94% in the steroids-treated one; therefore, no statistically significant difference between the two groups (3.71%) was observed, thus demonstrating that propranolol shows safe outcomes and effectiveness if compared with steroids (Kim et al., 2017).
Propranolol: Mechanism of Action
The therapeutic effect of oral propranolol administration in IHs was incidentally discovered in 2008 (Léauté-Labrèze et al., 2008). Propranolol is a nonselective beta-blocker that acts on both β-1 and β-2 adrenergic receptors. Several studies demonstrated that the natural course of IHs can be shortened by propranolol-based therapy, which also suppress lesion’s proliferation (Chen et al., 2013; Tan et al., 2015). As its mechanism of action has not been fully understood, it has been suggested that propranolol suppresses both vascular endothelial growth factor (VEGF) (Tang et al., 2015) and vascular endothelial growth factor receptor-2 (VEGFR-2) expression (Lamy et al., 2010). Tani et al. (2020) measured the serum cytokine concentrations of five patients with IH before and during the treatment with propranolol. The authors observed a significant reduction of the platelet-derived growth factor-BB (PDGF-BB) during treatment. This report suggested that PDGF-BB may be involved in propranolol effects and might be considered as a potential marker of the therapeutic effect.
Propranolol as First Line Treatment
Oral propranolol actually represents the first line approach for the pharmacological treatment of IHs (Hoeger et al., 2015). Oral beta-blocker are typically indicated in case of functional impairment (e.g., periocular IH causing amblyopia, nasal IH causing nose deformity, lip IH leading to feeding difficulties, and auricular IH causing deafness) and to avoid life-threatening complications due to lesion’s locations (e.g., respiratory distress caused by lung IH, airways obstruction caused by subglottic IH, hepatic dysfunction and heart failure subsequent to large cutaneous IH) (Wedgeworth et al., 2016).
In the study conducted by Xu et al. (2018), 134 out of 185 patients received treatment with oral propranolol (1.5 mg/kg/day) and the response rate was 91.7%. The effective dose of oral propranolol is between 1 and 3 mg/kg/day (Léauté-Labrèze et al., 2015).
Oral propranolol may be administered also in cases of IHs and comorbidities, such as hypothyroidism because of the hypotensive effects. Hypothyroidism has been also reported in association with hepatic IHs (Igarashi et al., 2018). Generally, the cells of hepatic IHs lead to thyroid hormone inactivating enzyme type-3 iodothyronine deiodinase (D3) overexpression, causing rapid degradation of thyroid hormones with consequent hypothyroidism (Simsek et al., 2018). Igarashi et al. (2018) reported a case of an extremely low-birth-weight infant with extensive cutaneous IH associated with hypothyroidism. The Authors observed an improvement of hypothyroidism and regression of cutaneous hemangioma following propranolol therapy, thus providing evidence for its effectiveness.
Adverse Events
The potential propranolol-related AEs generally comprise hypoglycemia, bradycardia, hypotension, bronchospasm, and electrolyte disturbance, even if the overall risk of AEs outbreaks is relatively low, in particular when used at low doses (Hoeger et al., 2015; Tan et al., 2015).
Topical Propranolol
Topical Versus Oral Propranolol
Topical propranolol seems to be less effective but safer than oral administration propranolol and particularly helpful in patients that present small superficial hemangiomas, where the aesthetic or asymptomatic impact did not require oral propranolol treatment (Baselga et al., 2016; Zaher et al., 2013). A systematic review identified 12 studies published between 2012 and 2017 and reported that the administration of topical propranolol was the first-line therapy for IHs in over 600 patients, which did not show any systemic side effect. Propranolol preparations comprehend creams, unguents and gels prepared by galenic formulations with a concentration of propranolol ranging from 0.5% to 5%; treatment duration ranged from 2 weeks to 16.5 months. Overall, the initiation of topical propranolol led to lesion’s improvement in 90% of cases, reducing lesion’s size of at least 50% in 59% of IHs (Price et al., 2018).
Topical Propranolol Formulations
Casiraghi et al. (2016) showed that hydrophilic preparations, such as cream or gel, guarantees higher levels of propranolol permeation than hydrophobic ointments. Mashiah et al. (2017) Fare clic o toccare qui per immettere il testo. observed that 43 out of 65 patients (57.3%), with a total of 75 IHs, had a good response to the treatment with propranolol 4% gel. They observed minor local side effects, namely irritation, redness, and scaling of the treated area, in only two cases but did not observe systemic adverse effects (Mashiah et al., 2017).
Wang et al. (2017) treated proliferating IHs evaluating the effectiveness and the safety of 2% propranolol cream. In two patients the treatment response was graded scale 1, in 15 patients scale 2, in 17 patients scale 3, and in 6 patients scale 4. The Authors did not observe significant differences in location/size-related outcomes.
Transdermal Propranolol
Several in vitro studies have been carried out to improve transdermal delivery of propranolol in order to reach deep IHs. Zeng et al. (2020) tested propranolol hydrochloride-loaded cubic nanoparticles (PRH-CNPs) on the abdominal skin obtained from Sprague Dawley rats and on endothelial cells of mouse hemangioendothelioma (EOMA). Smaller-sized PRH-CNPs demonstrated enhanced skin permeation towards EOMA cells when compared with the PRH solution.
Chopra et al. (2019) described the effects of a preparation composed by amorphous melts of propranolol incorporated into transdermal patches. The amorphous melts of propranolol were prepared using ionic liquids (ILs), which may be used as formulation additives, replacing oil or water, and may enhance transdermal penetration. In addition to a significant improvement in propranolol transdermal permeability from its amorphous melts, Chopra et al. (2019) observed also a reduction of skin irritation.
Topical Timolol
Topical Timolol Versus Oral Propranolol
Recently, timolol, a non-selective topical beta-blocker, was studies as a valid and safe option for the treatment of superficial, localized, small and uncomplicated IHs with less systemic absorption as well as absence of adverse effects (Khan et al., 2017).
According to Novoa et al. (2019), oral propranolol (1.0 mg/kg tablet once a day) and topical timolol maleate (0.5% eye drops twice a day) may equally produce a 50% or greater decrease in hemangioma diameter at 24 weeks (low-quality evidence). Although topical timolol was found as an effective treatment for superficial IHs, Khan et al. (2017) advised against its use when systemic treatment is justified by the anatomical location or size of the hemangioma that could lead to hepatic or cardiac impairment.
Adverse Events
Eczema, ulcers, skin rashes, desquamation, and erythema are frequent adverse effects of topical timolol (Filoni et al., 2021). A large recent study in over 700 superficial IHs reported that 3.9% of patients treated with oral propranolol (2 mg/kg/day) presented systemic adverse events (AEs) while topical timolol treatment (0.5% hydrogel, three times daily) did not cause AEs (Wu et al., 2018).
High-Risk Areas
Timolol is superior to watchful waiting in infants with superficial IH in high-risk areas. Cheng et al. (2020) studied infants of <1-year-old (within 13-month) which presented superficial IHs in high-risk areas. The IHs were smaller than 2 cm. Patients who received timolol showed significantly fewer complications than the control group, in which watchful waiting has been performed (4.2% versus 29%).
Lip represents a high-risk region for ulceration in IHs patients (Cheng and Friedlander, 2016). Xu et al. (2020) demonstrated that topical timolol administration was not effective for lip IHs compared to oral beta-blockers. Although very efficient and safe, therapy with oral propranolol is often not sufficient for IHs of lips and most cases needed additional therapy after systemic propranolol. A retrospective study showed that a pentagonal wedge resection of the segmental lip IHs is also an effective procedure for the treatment of lip IHs as well as for other vascular lip anomalies (de Castro et al., 2017). Other well-known surgical procedures for lip IHs include wedge resections and elliptical excisions (Zide et al., 1997) or rectangular block excision technique (Li W. Y. et al., 2011).
Topical Carteolol
Carteolol is another nonselective beta-blocker, which shared with propranolol similar mechanisms of action; superficial and small IHs were successfully treated with topical carteolol. Xu et al. (2018) demonstrated a reduction of tumors and a response rate of 75% following treatment with carteolol. In addition, patients that showed complications, such as erythema and scarring, recovered without concern. This study suggested that topical carteolol is an effective, safe and noninvasive therapeutic alternative for IHs. Moreover, carteolol showed few complications. Head and neck hemangiomas, such as periorbital or cervical IHs, as well as localized and superficial hemangiomas, are particularly suitable for this therapy.
Itraconazole
A single study by Chen et al. (2019) tested the effects of itraconazole on endothelial cells of mouse hemangioendothelioma (EOMA) cell line and infantile primary hemangioma endothelial cells (HemEC). Itraconazole blocked cellular proliferation in a dose-dependent manner and caused apoptosis in both cell lines. Moreover, itraconazole reduced vascular endothelial cell angiogenesis of HemEC and inhibited the expression of platelet-derived growth factor D (PDGF-D), thus reducing the PI3K/Akt/mTOR signaling, which is involved in the IHs pathogenesis. Gastrointestinal and liver disorders may appear as intraconazole side effects. Moreover, a possible interaction with any other concurrent medications should be considered (Chen et al., 2019).
Sirolimus
Sirolimus is an inhibitor of mammalian target of rapamycin (mTOR), which is involved in the regulation of cell cycle and, therefore, of the vascular endothelial proliferation. Hutchins et al. (2017) observed a 3-year-old girl presenting diffuse cutaneous, hepatic and pulmonary IHs with progressive pulmonary hypertension which was treated with oral sirolimus (0.8 mg/m2 per dose twice daily). During the treatment, improvement of hepatic lesions and pulmonary hypertension was noted but the child died because of development of unexpected severe hypoglycemia. The authors, advocating the safety of sirolimus, however recommended its use in complicated cases with multi-organ involvement.
Le Sage et al. (2018) observed no improvement in patients with infantile hemangioma treated with sirolimus. The authors claimed that the absence of a lymphatic component in IHs may explain the low effectiveness of topical sirolimus in these lesions: this would explain its useful treatment for cutaneous manifestations of lymphatic malformations.
Pulsed Dye Laser
Pulsed dye laser (PDL) is widely used for the treatment of IHs but its use is controversial. Ying et al. (2017) observed that both timolol maleate 0.5% cream and 595-nm pulsed dye laser (PDL) resulted in a significant clinical improvement of superficial IH during the proliferating phase.
The association of PDL with beta-blockers for the treatment of superficial and mixed IHs have appeared superior to PDL in efficacy and cost benefit (Asilian et al., 2015; Reddy et al., 2013). According to Valdebran et al. (2017), lasers are recommended for the treatment of superficial and thin IHs, whereas oral propranolol is mandatory for the therapy of deep IHs affecting the airways or obstructing the visual field. Finally, combined therapies may improve the outcome of mixed IHs and refractory superficial IHs (Valdebran et al., 2017).
Infantile Hemangiomas With Involvement of Central Nervous System: PHACE and LUMBAR Syndrome
PHACE syndrome is characterized by a large (>5 cm) facial hemangioma which is associated with several congenital anomalies (Judd et al., 2007).
Instead, in LUMBAR syndrome a segmental IH in the lower body region is associated with urogenital, bony, anorectal, arterial anomalies and mielopathy (Golabi et al., 2014).
The risk of hidden arterial anomalies in LUMBAR syndrome requires a careful investigation before starting treatment with oral propranolol (Johnson and Smidt, 2014; Yu et al., 2017). In a recent European multicenter observational retrospective study, seven infants with large or segmental cutaneous IHs, involving the head, neck, lumbar or sacral area, were screened using MRI for PHACES or LUMBAR syndromes revealing intracranial or intraspinal IH. All patients underwent oral propranolol treatment for 6–14 months. All CNS lesions responded to treatment and five patients had a complete or almost complete resolution of the cutaneous IHs, thus demonstrating that propranolol can pass the blood-brain barrier. This study suggest that oral propranolol should be considered the first-line approach for intra-CNS IHs to avoid possible complications (Diociaiuti et al., 2020).
Conclusion
The treatment of infantile hemangiomas is challenging even today. In our review, we described the last 5-year experiences with propranolol, the first line therapy for IHs treatment, and, in particular, with off-label drugs, such as topical β-blockers, including topical timolol and carteolol, steroids, itraconazole or sirolimus. Currently, oral propranolol is used in the majority of cases, but topical β-blockers can be preferred in superficial and uncomplicated forms. Oral and topical β-blockers have changed the prognosis of IHs, but some parents and physicians are reluctant to systemic therapies because of the risk of adverse events. Some alternative therapies are emerging, but data are not still enough to evaluate their efficacy and safety.
The need to lower as much as possible the risk of adverse events in pediatric population drives the search of other therapies than oral propranolol or steroids. We hope that further studies may confirm the safety of current therapies for IHs and expand the range of alternatives.
Author Contributions
ML: Conceptualization, data curation, writing–original draft, first authorship; AD: Methodology, writing–review and editing, first authorship; DL: Conceptualization, writing–review and editing; IN: Validation, writing–review and editing; BF: Methodology, supervision; LF: Investigation, validation; VF: Investigation, validation; SV: Methodology, data curation; SF: Supervision, methodology, validation; VM: Conceptualization, investigation, supervision, writing–review and editing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor FS is currently organizing a Research Topic with the author DA.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Asilian, A., Mokhtari, F., Kamali, A. S., Abtahi-Naeini, B., Nilforoushzadeh, M. A., and Mostafaie, S. (2015). Pulsed Dye Laser and Topical Timolol Gel versus Pulse Dye Laser in Treatment of Infantile Hemangioma: A Double-Blind Randomized Controlled Trial. Adv. Biomed. Res. 4, 257. doi:10.4103/2277-9175.170682
Baselga, E., Roe, E., Coulie, J., Muñoz, F. Z., Boon, L. M., McCuaig, C., et al. (2016). Risk Factors for Degree and Type of Sequelae after Involution of Untreated Hemangiomas of Infancy. JAMA Dermatol. 152, 1239–1243. doi:10.1001/jamadermatol.2016.2905
Bauland, C. G., Lüning, T. H., Smit, J. M., Zeebregts, C. J., and Spauwen, P. H. (2011). Untreated Hemangiomas: Growth Pattern and Residual Lesions. Plast. Reconstr. Surg. 127, 1643–1648. doi:10.1097/PRS.0b013e318208d2ac
Bauland, C. G., Smit, J. M., Bartelink, L. R., Zondervan, H. A., and Spauwen, P. H. (2010). Hemangioma in the Newborn: Increased Incidence after Chorionic Villus Sampling. Prenat. Diagn. 30, 913–917. doi:10.1002/pd.2562
Casiraghi, A., Musazzi, U. M., Rocco, P., Franzè, S., and Minghetti, P. (2016). Topical Treatment of Infantile Haemangiomas: A Comparative Study on the Selection of a Semi-solid Vehicle. Skin. Pharmacol. Physiol. 29, 210–219. doi:10.1159/000447672
Chang, L. C., Haggstrom, A. N., Drolet, B. A., Baselga, E., Chamlin, S. L., Garzon, M. C., et al. (2008). Growth Characteristics of Infantile Hemangiomas: Implications for Management. Pediatrics 122, 360–367. doi:10.1542/peds.2007-2767
Chen, S., Zhuang, K., Sun, K., Yang, Q., Ran, X., Xu, X., et al. (2019). Itraconazole Induces Regression of Infantile Hemangioma via Downregulation of the Platelet-Derived Growth Factor-D/PI3K/Akt/mTOR Pathway. J. Invest. Dermatol. 139, 1574–1582. doi:10.1016/j.jid.2018.12.028
Chen, T. S., Eichenfield, L. F., and Friedlander, S. F. (2013). Infantile Hemangiomas: An Update on Pathogenesis and Therapy. Pediatrics 131, 99–108. doi:10.1542/peds.2012-1128
Cheng, C. E., and Friedlander, S. F. (2016). Infantile Hemangiomas, Complications and Treatments. Semin. Cutan. Med. Surg. 35, 108–116. doi:10.12788/j.sder.2016.050
Cheng, J. W. C. H., Lam, Y. Y., Fung, G. P. G., Sin, C., Luk, D. C. K., Chan, B. H. B., et al. (2020). Randomised Controlled Trial: Can Topical Timolol Maleate Prevent Complications for Small Superficial Infantile Haemangiomata in High-Risk Areas? Pediatr. Res. 88, 756–760. doi:10.1038/s41390-020-0917-3
Chopra, H., Kumar, P., and Singh, I. (2019). Ionic Liquid-Based Transdermal Delivery of Propranolol: a Patent Evaluation of US2018/0169033A1. Pharm. Pat. Anal. 8, 203–209. doi:10.4155/ppa-2019-0018
de Castro, D. K., Ng, Z. Y., Holzer, P. W., Waner, M., Cetrulo, C. L., and Fay, A. (2017). One-Stage Supramaximal Full-Thickness Wedge Resection of Vascular Lip Anomalies. J. Oral Maxillofac. Surg. 75, 2449–2455. doi:10.1016/j.joms.2017.03.020
Diociaiuti, A., Carnevale, C., Torres, E. B., Léauté-Labrèze, C., Neri, I., Rotunno, R., et al. (2020). Cutaneous Infantile Haemangiomas with Intracranial and Intraspinal Involvement: A European Multicentre Experience and Review. Acta Derm. Venereol. 100, adv00255. doi:10.2340/00015555-3608
Filoni, A., Ambrogio, F., de Marco, A., Pacifico, A., and Bonamonte, D. (2021). Topical Beta-Blockers in Dermatologic Therapy. Dermatol. Ther. 34, e15016. doi:10.1111/dth.15016
Folkman, J. (1984). Toward a New Understanding of Vascular Proliferative Disease in Children. Pediatrics 74, 850–856. doi:10.1542/peds.74.5.850
Frieden, I. J., Haggstrom, A. N., Drolet, B. A., Mancini, A. J., Friedlander, S. F., Boon, L., et al. (2005). “Infantile Hemangiomas: Current Knowledge, Future Directions. Proceedings of a Research Workshop on Infantile Hemangiomas,” in Proceedings of a research workshop on infantile hemangiomas, Bethesda, Maryland, USA, April 7-9, 2005, 383–406. doi:10.1111/j.1525-1470.2005.00102.x
George, M. E., Sharma, V., Jacobson, J., Simon, S., and Nopper, A. J. (2004). Adverse Effects of Systemic Glucocorticosteroid Therapy in Infants with Hemangiomas. Arch. Dermatol 140, 963–969. doi:10.1001/archderm.140.8.963
Golabi, M., An, A. C., Lopez, C., Lee, L., Kwong, M., and Hall, B. D. (2014). A New Case of a LUMBAR Syndrome. Am. J. Med. Genet. A 164A, 204–207. doi:10.1002/ajmg.a.36215
Hemangioma Investigator Group Haggstrom, A. N., Haggstrom, A. N., Drolet, B. A., Baselga, E., Chamlin, S. L., Garzon, M. C., et al. (2007). Prospective Study of Infantile Hemangiomas: Demographic, Prenatal, and Perinatal Characteristics. J. Pediatr. 150, 291–294. doi:10.1016/j.jpeds.2006.12.003
Hoeger, P. H., Harper, J. I., Baselga, E., Bonnet, D., Boon, L. M., Ciofi Degli Atti, M., et al. (2015). Treatment of Infantile Haemangiomas: Recommendations of a European Expert Group. Eur. J. Pediatr. 174, 855–865. doi:10.1007/s00431-015-2570-0
Hoornweg, M. J., Smeulders, M. J., Ubbink, D. T., and van der Horst, C. M. (2012). The Prevalence and Risk Factors of Infantile Haemangiomas: a Case-Control Study in the Dutch Population. Paediatr. Perinat. Epidemiol. 26, 156–162. doi:10.1111/j.1365-3016.2011.01214.x
Hutchins, K. K., Ross, R. D., Kobayashi, D., Martin, A., and Rajpurkar, M. (2017). Treatment of Refractory Infantile Hemangiomas and Pulmonary Hypertension with Sirolimus in a Pediatric Patient. J. Pediatr. Hematol. Oncol. 39, e391. doi:10.1097/MPH.0000000000000961
Igarashi, A., Hata, I., Yuasa, M., Okuno, T., and Ohshima, Y. (2018). A Case of an Infant with Extremely Low Birth Weight and Hypothyroidism Associated with Massive Cutaneous Infantile Hemangioma. J. Pediatr. Endocrinol. Metab. 31, 1377–1380. doi:10.1515/jpem-2018-0369
Johnson, E. F., and Smidt, A. C. (2014). Not just a Diaper Rash: LUMBAR Syndrome. J. Pediatr. 164, 208–209. doi:10.1016/j.jpeds.2013.08.045
Judd, C. D., Chapman, P. R., Koch, B., and Shea, C. J. (2007). Intracranial Infantile Hemangiomas Associated with PHACE Syndrome. AJNR Am. J. Neuroradiol. 28, 25–29.
Khan, M., Boyce, A., Prieto-Merino, D., Svensson, Å., Wedgeworth, E., and Flohr, C. (2017). The Role of Topical Timolol in the Treatment of Infantile Hemangiomas: A Systematic Review and Meta-Analysis. Acta Derm. Venereol. 97, 1167–1171. doi:10.2340/00015555-2681
Kim, K. H., Choi, T. H., Choi, Y., Park, Y. W., Hong, K. Y., Kim, D. Y., et al. (2017). Comparison of Efficacy and Safety between Propranolol and Steroid for Infantile Hemangioma: A Randomized Clinical Trial. JAMA Dermatol. 153, 529–536. doi:10.1001/jamadermatol.2017.0250
Korekawa, A., Nakajima, K., Nakano, H., and Sawamura, D. (2020). Benign Neonatal Hemangiomatosis with Early Regression of Skin Lesions: A Case Report and Review of the Published Work. J. Dermatol. 47, 911–916. doi:10.1111/1346-8138.15413
Lamy, S., Lachambre, M. P., Lord-Dufour, S., and Béliveau, R. (2010). Propranolol Suppresses Angiogenesis In Vitro: Inhibition of Proliferation, Migration, and Differentiation of Endothelial Cells. Vasc. Pharmacol. 53, 200–208. doi:10.1016/j.vph.2010.08.002
le Sage, S., David, M., Dubois, J., Powell, J., McCuaig, C. C., Théorêt, Y., et al. (2018). Efficacy and Absorption of Topical Sirolimus for the Treatment of Vascular Anomalies in Children: A Case Series. Pediatr. Dermatol. 35, 472–477. doi:10.1111/pde.13547
Léauté-Labrèze, C., Dumas de la Roque, E., Hubiche, T., Boralevi, F., Thambo, J. B., and Taïeb, A. (2008). Propranolol for Severe Hemangiomas of Infancy. N. Engl. J. Med. 358, 2649–2651. doi:10.1056/NEJMc0708819
Léauté-Labrèze, C., Hoeger, P., Mazereeuw-Hautier, J., Guibaud, L., Baselga, E., Posiunas, G., et al. (2015). A Randomized, Controlled Trial of Oral Propranolol in Infantile Hemangioma. N. Engl. J. Med. 372, 735–746. doi:10.1056/NEJMoa1404710
Léauté-Labrèze, C., Harper, J. I., and Hoeger, P. H. (2017). Infantile Haemangioma. Lancet 390. doi:10.1016/S0140-6736(16)00645-0
Li, J., Chen, X., Zhao, S., Hu, X., Chen, C., Ouyang, F., et al. (2011). Demographic and Clinical Characteristics and Risk Factors for Infantile Hemangioma: a Chinese Case-Control Study. Arch. Dermatol. 147, 1049–1056. doi:10.1001/archdermatol.2011.122
Li, W. Y., Chaudhry, O., and Reinisch, J. F. (2011). Guide to Early Surgical Management of Lip Hemangiomas Based on Our Experience of 214 Cases. Plast. Reconstr. Surg. 128, 1117–1124. doi:10.1097/PRS.0b013e31822b6908
Mashiah, J., Kutz, A., Rabia, S. H., Ilan, E. B., Goldberg, I., Sprecher, E., et al. (2017). Assessment of the Effectiveness of Topical Propranolol 4% Gel for Infantile Hemangiomas. Int. J. Dermatol. 56, 148–153. doi:10.1111/ijd.13517
Munden, A., Butschek, R., Tom, W. L., Marshall, J. S., Poeltler, D. M., Krohne, S. E., et al. (2014). Prospective Study of Infantile Haemangiomas: Incidence, Clinical Characteristics and Association with Placental Anomalies. Br. J. Dermatol. 170, 907–913. doi:10.1111/bjd.12804
Novoa, M., Baselga, E., Beltran, S., Giraldo, L., Shahbaz, A., Pardo-Hernandez, H., et al. (2019). Interventions for Infantile Haemangiomas of the Skin: Abridged Cochrane Systematic Review and GRADE Assessments. Br. J. Dermatol. 180, 527–533. doi:10.1111/bjd.17407
Price, A., Rai, S., Mcleod, R. W. J., Birchall, J. C., and Elhassan, H. A. (2018). Topical Propranolol for Infantile Haemangiomas: a Systematic Review. J. Eur. Acad. Dermatol. Venereol. 32, 2083–2089. doi:10.1111/jdv.14963
Püttgen, K. B. (2014). Diagnosis and Management of Infantile Hemangiomas. Pediatr. Clin. North Am. 61, 383–402. doi:10.1016/j.pcl.2013.11.010
Schwartz, R. A., Sidor, M. I., Musumeci, M. L., Lin, R. L., and Micali, G. (2009). Infantile Haemangiomas: a Challenge in Paediatric Dermatology. J. Eur. Acad. Dermatol. Venereol. 24, 631–638. doi:10.1111/j.1468-3083.2010.03650.x
Simsek, E., Demiral, M., and Gundoğdu, E. (2018). Severe Consumptive Hypothyroidism Caused by Multiple Infantile Hepatic Haemangiomas. J. Pediatr. Endocrinol. Metab. 31, 823–827. doi:10.1515/jpem-2018-0055
Tan, C. E., Itinteang, T., Leadbitter, P., Marsh, R., and Tan, S. T. (2015). Low-dose Propranolol Regimen for Infantile Haemangioma. J. Paediatr. Child. Health 51, 419–424. doi:10.1111/jpc.12720
Tang, Y.-j., Zhang, Z.-z., Chen, S.-q., Chen, S.-m., Li, C.-j., Chen, J.-w., et al. (2015). Effect of Topical Propranolol Gel on Plasma Renin, Angiotensin Ii and Vascular Endothelial Growth Factor in Superficial Infantile Hemangiomas. J. Huazhong Univ. Sci. Technol. Med. Sci.] 35, 759–762. doi:10.1007/s11596-015-1503-5
Tani, S., Kunimoto, K., Inaba, Y., Mikita, N., Kaminaka, C., Kanazawa, N., et al. (2020). Change of Serum Cytokine Profiles by Propranolol Treatment in Patients with Infantile Hemangioma. Drug Discov. Ther. 14, 89–92. doi:10.5582/ddt.2020.03014
Valdebran, M., Martin, B., and Kelly, K. M. (2017). State-of-the-art Lasers and Light Treatments for Vascular Lesions: from Red Faces to Vascular Malformations. Semin. Cutan. Med. Surg. 36, 207–212. doi:10.12788/j.sder.2017.044
Wang, Y., Zhang, X., Yang, Y., Zhang, J., Yang, Y., and Lu, Y. (2017). Efficacy and Safety of 2% Topical Propranolol Cream for the Treatment of Proliferating Infantile Strawberry Hemangiomas. Indian J. Pediatr. 84, 425–429. doi:10.1007/s12098-017-2303-7
Wedgeworth, E., Glover, M., Irvine, A. D., Neri, I., Baselga, E., Clayton, T. H., et al. (2016). Propranolol in the Treatment of Infantile Haemangiomas: Lessons from the European Propranolol in the Treatment of Complicated Haemangiomas (PITCH) Taskforce Survey. Br. J. Dermatol. 174, 594–601. doi:10.1111/bjd.14233
Wu, H. W., Wang, X., Zhang, L., Zheng, J. W., Liu, C., and Wang, Y. A. (2018). Topical Timolol vs. Oral Propranolol for the Treatment of Superficial Infantile Hemangiomas. Front. Oncol. 8, 605. doi:10.3389/fonc.2018.00605
Xu, M. N., Zhang, M., Xu, Y., Wang, M., and Yuan, S. M. (2018). Individualized Treatment for Infantile Hemangioma. J. Craniofac. Surg. 29, 1876–1879. doi:10.1097/SCS.0000000000004745
Xu, P., Yu, Q., Huang, H., Zhang, W., and Li, W. (2020). Lip Infantile Hemangiomas Involving the Vermillion Border Have Worse Outcomes and Prognosis to Oral Propranolol Than Lesions Confined to One Side of the Vermillion. J. Oral Maxillofac. Surg. 78, 446–454. doi:10.1016/j.joms.2019.09.010
Yanes, D. A., Pearson, G. D., and Witman, P. M. (2016). Infantile Hemangiomas of the Lip: Patterns, Outcomes, and Implications. Pediatr. Dermatol. 33, 511–517. doi:10.1111/pde.12928
Ying, H., Zou, Y., Yu, W., Qiu, Y., Ma, G., Chang, L., et al. (2017). Prospective, Open-Label, Rater-Blinded and Self-Controlled Pilot Study of the Treatment of Proliferating Superficial Infantile Hemangiomas with 0.5% Topical Timolol Cream versus 595-nm Pulsed Dye Laser. J. Dermatol. 44, 660–665. doi:10.1111/1346-8138.13747
Yu, X., Zhang, J., Wu, Z., Liu, M., Chen, R., Gu, Y., et al. (2017). LUMBAR Syndrome: A Case Manifesting as Cutaneous Infantile Hemangiomas of the Lower Extremity, Perineum and Gluteal Region, and a Review of Published Work. J. Dermatol. 44, 808–812. doi:10.1111/1346-8138.13763
Yuan, S. M., Zhang, M., Guo, Y., Cui, L., Hong, Z. J., and Jiang, H. Q. (2015). Intralesional Injection of Diprospan Is Effective for Infantile Hemangioma. J. Craniofac. Surg. 26, 422–424. doi:10.1097/SCS.0000000000001274
Zarem, H. A., and Edgerton, M. T. (1967). Induced Resolution of Cavernous Hemangiomas Following Prednisolone Therapy. Plast. Reconstr. Surg. 39, 76–83. doi:10.1097/00006534-196701000-00010
Zeng, L., Tao, C., Liu, Z., Zhang, J., Zhang, M., Zhang, J., et al. (2020). Preparation and Evaluation of Cubic Nanoparticles for Improved Transdermal Delivery of Propranolol Hydrochloride. AAPS PharmSciTech 21, 266. doi:10.1208/s12249-020-01809-7
Zide, B. M., Glat, P. M., Stile, F. L., and Longaker, M. T. (1997). Vascular Lip Enlargement: Part I. Hemangiomas-Ttenets of Therapy. Plast. Reconstr. Surg. 100, 1664–1673. doi:10.1097/00006534-199712000-00004
Keywords: infantile hemangioma, steroids, propranolol, timolol, carteolol, itraconazole, pulsed dye laser, segmental hemangioma
Citation: Macca L, Altavilla D, Di Bartolomeo L, Irrera N, Borgia F, Li Pomi F, Vaccaro F, Squadrito V, Squadrito F and Vaccaro M (2022) Update on Treatment of Infantile Hemangiomas: What’s New in the Last Five Years?. Front. Pharmacol. 13:879602. doi: 10.3389/fphar.2022.879602
Received: 19 February 2022; Accepted: 05 May 2022;
Published: 26 May 2022.
Edited by:
Francesco Salvo, Université de Bordeaux, FranceReviewed by:
Anant D. Patil, Padmashree Dr. D.Y. Patil University, IndiaCopyright © 2022 Macca, Altavilla, Di Bartolomeo, Irrera, Borgia, Li Pomi, Vaccaro, Squadrito, Squadrito and Vaccaro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luca Di Bartolomeo, lucadibartolomeo@live.it
†These authors have contributed equally to this work and share first authorship
 Laura Macca
Laura Macca Domenica Altavilla
Domenica Altavilla Luca Di Bartolomeo
Luca Di Bartolomeo Natasha Irrera
Natasha Irrera Francesco Borgia
Francesco Borgia Federica Li Pomi
Federica Li Pomi Federico Vaccaro
Federico Vaccaro Violetta Squadrito
Violetta Squadrito Francesco Squadrito
Francesco Squadrito Mario Vaccaro
Mario Vaccaro