- 1Chongqing Key Laboratory of Traditional Chinese Medicine for Prevention and Cure of Metabolic Diseases, College of Traditional Chinese Medicine, Chongqing Medical University, Chongqing, China
- 2Chongqing Key Laboratory for Pharmaceutical Metabolism Research, The Key Laboratory of Biochemistry and Molecular Pharmacology, College of Pharmacy, Chongqing Medical University, Chongqing, China
- 3School of Safety Engineering, Chongqing University of Science and Technology, Chongqing, China
- 4Department of Anesthesiology, The First Affiliated Hospital, Chongqing Medical University, Chongqing, China
- 5Radiation Oncology Center, Chongqing University Cancer Hospital and Chongqing Cancer Institute and Chongqing Cancer Hospital, Chongqing, China
Overview: The treatment of chronic renal failure (CRF) with traditional Chinese medicine has attracted much attention, but its mechanism is not clear. Network pharmacology is an effective strategy for exploring the interaction mechanisms between Chinese herbs and diseases, however, it still needs to be validated in cell and/or animal experiments due to its virtual screening characteristics. Herein, the anti-CRF mechanism of the Fushengong decoction (FSGD) was investigated using a dual-dimension network pharmacological strategy combined with in vivo experiment.
Methods: The traditional Chinese medicine systems pharmacology (TCMSP) database (https://tcmspw.com) and UHPLC-MS/MS technology were used to identify the effective compounds of FSGD in theory and practice, such as quercetin, formononetin, and pachymic acid. The putative targets of FSGD and CRF were obtained from the Swisstarget prediction platform and the Genecards database, respectively. The common target pathways between FSGD and CRF were got from the dual-dimension network pharmacology analysis, which integrated the cross-common targets from the TCMSP components-Swisstarget-Genecards-Venn platform analysis in theory, and the UHPLC-MS/MS identified effective ingredients-Swisstarget screening, such as TNF and PI3K/AKT. Furthermore, system molecular determinations were used to prove the dual-dimension network pharmacology study through CRF rat models, which were constructed using adenine and treated with FSGD for 4 weeks.
Results: A total of 121 and 9 effective compounds were obtained from the TCMSP database and UHPLC-MS/MS, respectively. After dual-dimension network pharmacology analysis, the possible mechanism of PTEN/PI3K/AKT/NF-κB pathway was found for FSGD in CRF. In vivo experiments indicated that FSGD can play a role in protecting renal function and reducing fibrosis by regulating the PTEN/PI3K/AKT/NF-κB pathway. These findings provide a reference for FSGD in CRF.
Conclusion: Based on the theoretical and practical dual-dimension network pharmacology analysis for FSGD in CRF, the possible molecular mechanism of PTEN/PI3K/AKT/NF-κB was successfully predicted, and these results were verified by in vivo experiments. In this study, the dual-dimension network pharmacology was used to interpret the key signal pathway for FSGD in CRF, which also proved to be a smart strategy for the study of effective substances and pharmacology in FSGD.
1 Introduction
Chronic renal failure (CRF) is a disease caused by many causes of progressive loss of renal function that eventually produce renal failure (Mehta et al., 2020). The prevalence rate in developed and developing countries is as high as 10.8–16.0% (Yuan et al., 2019). Recently, CRF has become a major public healthcare problem that cannot be ignored because of its critical condition, high morbidity, and mortality. It has been shown that the pathogenesis of CRF may be closely related to genetic factors, hemodynamic changes, inflammatory factors, and oxidative stress, etc (Ullah and Basile, 2019). The main treatment methods for CRF are kidney transplantation and hemodialysis, but the curative effect is often poor.
Traditional Chinese medicine (TCM) has received great attention for improving the quality of life and survival rate of patients with CRF (Wang et al., 2012). The Fushengong decoction (FSGD, Supplementary Table S1) was summarized by the master and professor of Chinese medicine, Ziguang Guo, who added and deducted some herbs in the traditional Chinese medicines (TCM) formula “Liuwei Dihuang Pill” with 60 years of clinical experience. Its functions are to tonify the kidney, promote blood circulation, and reduce turbidity to improve renal function and renal fibrosis (Yang et al., 2017). In our previous study, FSGD can significantly lowered the expression levels of α-SMA and TGF-β1 to reduce renal fibrosis (Wang et al., 2016; Tong et al., 2020). Although the clinical application of FSGD in CRF has been verified, the mechanism of FSGD in treating CRF remains unclear because of the complexity of its components and targets. Therefore, further mechanism study of FSGD in CRF is very valuable.
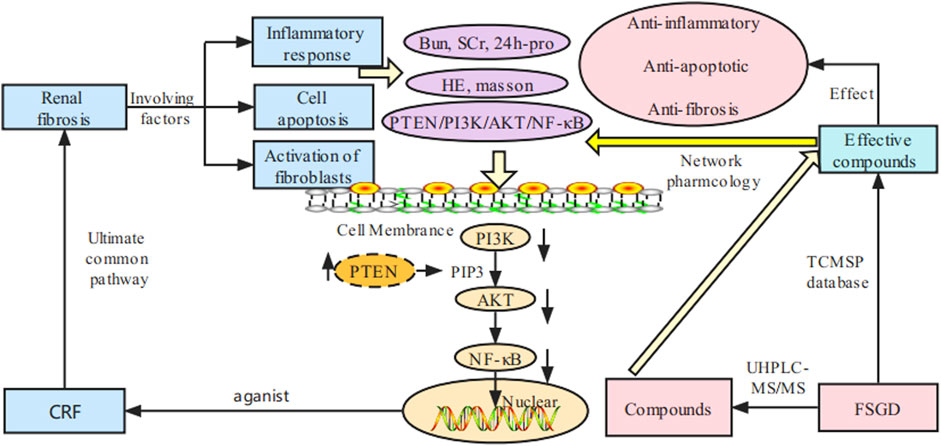
Network pharmacology is an effective strategy for exploring the interaction mechanisms between chinese herbs and diseases. Because of the complexity of components and multi-targets in TCM, network pharmacology has the advantages of predicting the potential mechanism of prescription action in the treatment of diseases through compound-target-disease interaction network and bioinformatics analysis, which is consistent with the holistic perspective of TCM (Wang X et al., 2020). However, it still needs to be validated in cell and/or animal experiments due to its virtual screening characteristics. To explore the anti-fibrosis mechanisms of FSGD on CRF, dual-dimension network pharmacology of the effective components of the database and the compounds detected by UHPLC-MS/MS were used to predict candidate compounds and mechanisms of FSGD in the treatment of CRF. And in vivo experiment was conducted to further confirm the pathway. An overview of this study is showed in Figure 1 by BioRender (https://biorender.com/).
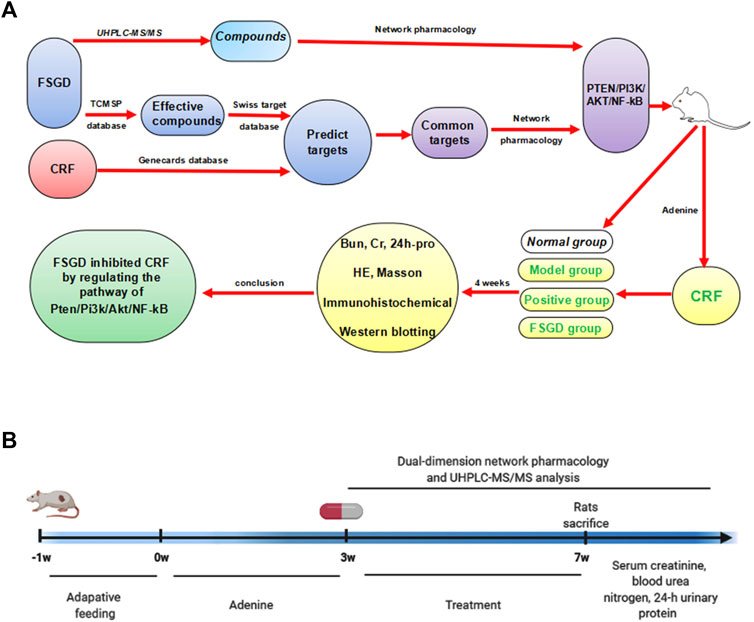
FIGURE 1. The study schematic illustration of the mechanism for FSGD in CRF. (A) Process of experiments. (B) Timeline of experiments.
2 Materials and Methods
2.1 Materials
Niaoduqing granules (NDQ, Z20073256) were obtained from kangchen pharmaceutical Co., Ltd., and 0.5% adenine was purchased from Jiangsu Nantong trofi feed company (Jiangsu China); serum creatinine (Cr), serum urea nitrogen (BUN), and urine protein detection kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Antibodies against PTEN (48756), PI3K (48848), p-PI3K (11508), AKT (48888), p-AKT (11054) and NF-κB (48498) were obtained from Signalway antibody (United States).
2.2 Preparation of Fushengong Decoction
FSGD is composed of huangqi (Astragalus mongholicus Bunge), dihuang [Rehmannia glutinosa (Gaertn.) DC.], shanyao (Dioscorea oppositifolia L.), shanzhuyu (Cornus officinalis Siebold & Zucc.), cheqian (Plantago asiatica L.), niuxi (Achyranthes bidentata Blume), mudanpi (Paeonia × suffruticosa Andrews), zexie [Alisma plantago-aquatica subsp. orientale (Sam.) Sam.], fuling [Poria Cocos (Schw.) Wolf], cangzhu [Atractylodes lancea (Thunb.) DC.], duzhong (Eucommia ulmoides Oliv.), shuizhi (Hirudo Whitman) and huangbai (Phellodendron chinense C. K. Schneid.). The herbal information and composition ratio are shown in Supplementary Table S1. FSGD herbs were selected according to the “Chinese pharmacopoeia” 2020 edition. All herbs are purchased from the famous Tongjun Pavilion in China. These herbs were identified by professor Xuekuan Huang of Chongqing Medical University and preserved in the Chongqing Key Laboratory of Traditional Chinese Medicine for Prevention and Cure of Metabolic Diseases. Combined with the surface area conversion between rats and humans (6.3) during daily dosage of adults, the daily dose of rats was calculated to be 8 g/kg. In the last 7 years, low, medium, and high doses of FSGD was evaluated for the treatment of adenine-induced CRF in rats, respectively. Our previous experiments showed that 8 g/kg is the reasonable dose for the treatment of CRF (Wang et al., 2016; Tong et al., 2020). According to the effective therapy (8 g/kg) and the preparation of traditional decoction, FSGD was soaked for 30 min with purified water and boiled three times every 30 min. The boiling liquid was collected, filtered, concentrated to crude drug (1 g/ml), and stored at 4°C for use (Wang et al., 2016; Yang et al., 2017; Tong et al., 2020). Part of the FSGD decoction was stored at −80°C for further analysis.
2.3 UPLC-QTOF-MS Analysis
The samples of FSGD were thawed in ice water, vortexed for 30 s, centrifuged at 12,000 rpm at 4°C for 15 min. A 300 μl aliquot of sample was precisely transferred to an eppendorf tube. After the addition of 1,000 μl extracting solution (methanol: water = 4:1, v/v, including internal standard concentration is 10 μg/ml), all samples were vortexed for 30 s, sonicated for 5 min in an ice-water bath, incubated at −40°C for 1 h, and centrifuged at 12,000 rpm at 4°C for 15 min. A 500 μl of the supernatant was passed through a 0.22 μm filter membrane and then transferred to ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) analysis.
LC-MS/MS analysis was performed on a Agilent ultra-high performance liquid chromatography 1290 UPLC system with a Waters UPLC BEH C18 column (1.7 μm × 2.1 mm × 100 mm). The column temperature was set at 55°C and the sample injection volume was set at 5 μl. The flow rate was set at 0.5 ml/min. The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The multi-step linear elution gradient program was as follows: 0–11 min (85–25% A), 11–12 min (25–2% A), 12–14 min (2–2% A), 14–14.1 min (2–85% A), 14.1–15 min (85–85% A), 15–16 min (85–85% A). An Q Exactive Focus mass spectrometer coupled with an Xcalibur software was employed to obtain the MS and MS/MS data with the IDA acquisition mode. During each acquisition cycle, the mass range was from 100 to 1,500, and the top three of every cycle were screened and the corresponding MS/MS data were further acquired. Sheath gas flow rate: 45 Arb, Aux gas flow rate: 15 Arb, Capillary temperature: 400°C, Full MS resolution: 70000, MS/MS resolution: 17500, Collision energy: 15/30/45 in NCE mode, Spray Voltage: 4.0 kV (positive) or −3.6 kV (negative). Mass spectra were imported raw using XCMS software. Materials identification of peaks containing MSMS data was performed using the secondary mass spectrometry database (Shanghai BIOTREE biotech Co., Ltd.) and the corresponding cleavage law matching method.
2.4 Animal Experiments
Eight-week-old male Sprague-Dawley rats (200 ± 20 g) were purchased from the Laboratory Animal Center of Chongqing Medical University and kept in a specific-pathogen-free level at the center (SCXK 2018-0003). The animal study was reviewed and approved by the Ethics Committee of Chongqing Medical University. After 1 week of adaptive feeding, all rats were randomly divided into four groups (n = 8), normal group, model group, NDQ group, and FSGD group. NDQ was used as the positive drug, which is widely used in clinical practice and is a commonly used drug for CRF (Zheng et al., 2017). The normal group was fed a regular diet, while the other group was fed a diet supplemented with adenine by gavage at a dose of 0.5% adenine to induce the CRF model for 3 weeks according to our previous method (Wang et al., 2016; Yang et al., 2017; Tong et al., 2020). After 3 weeks, CRF mice were randomly divided into three groups (n = 8). Rats in the normal and model groups were treated with physiological saline, FSGD (8 g/kg) and NDQ (5 g/kg), respectively, by gavage for 4 weeks. At the end of the experimental period, all rats were fasted for 12 h and sacrificed. Blood samples were then collected and centrifuged at 3500 rpm for 10 min, and the serum was collected and stored at −80°C until further analyses.
2.5 Network Pharmacology Analysis
2.5.1 Identification of the Theoretical Active Compounds and Protein Targets of FSGD
FSGD effective compounds were collected from the traditional Chinese medicine systems pharmacology (TCMSP) database (http://lsp.nwsuaf.edu.cn) using the ADME filter method, and the main parameters included oral bioavailability (OB) and drug-likeness (DL). The effective compounds were defined based on the values of OB of ≥30%, DL of ≥0.18 and half-life (HL) ≥4 (Su et al., 2007). The compounds with CRF treatment were identified as potential compounds in FSGD via text mining. The CAS number of each compound was uploaded to the pubchem platform (https://pubchem.ncbi.nlm.nih.gov/) to obtain its 2D structure, and the swiss target prediction platform (http://www.swisstargetprediction.ch/) was used to identify compound-related targets, zero probability, and repeated targets were removed. Moreover, the human gene symbol corresponding to the protein target name was standardized using the uniProt database (https://www.uniprot.org/).
2.5.2 CRF Related Target Prediction
Target genes associated with CRF were acquired from genecards (https://www.Genecards.org/). The targets common in FSGD and CRF were identified by the venn platform and used for further analysis (http://bioinformatics.psb.ugent.be/webtools/Venn/).
2.5.3 GO and KEGG Pathway Enrichment Analysis
To obtain the potential signaling pathway associated with FSGD in the treatment of CRF, KEGG analysis was conducted by david (https://david.ncifcrf.gov), and GO analysis was conducted using metascape (http://metascape.org/).
2.6 Detection of Metabolic and Biochemical Indicators
Serum Cr, BUN and 24 h urine protein level were assessed according to the manufacturer’s instructions. Then, 24 h urinary protein quantification was calculated based on urine volume and urinary protein concentration.
2.7 Histological and Immunohistochemical Assays
According to the manufacturer’s instructions, kidney tissues were fixed in 4% paraformaldehyde for 24 h, dehydrated, embedded in paraffin, and cut into 4 μm sections for hematoxylin-eosin (HE) imaging, Masson staining, and immunohistochemical (IHC) experiments. All the sections were analyzed by microscopy (BX53, Olympus Corporation, Japan). The cumulative optical density was collected and calculated using Image-Pro Plus software (Media Cybernetics, United States).
2.7.1 Hematoxylin-Eosin Staining
The paraffin sections were dewaxed to water, dip-stained with hematoxylin staining solution for 4 min, flushed with distilled water, dehydrated with gradient alcohol, and then stained with eosin staining solution for 5 min, dehydrated and sealed.
2.7.2 Masson Staining
The sections were soaked overnight with 2.5% potassium dichromate mordant, stained with ferric hematoxylin staining for 2 min, rinsed with distilled water, differentiated with 1% hydrochloric acid alcohol for 30 s, rinsed with distilled water, transferred into lichun red acidic fuchsin dye for 6 min. And the lichun red sections were rinsed with distilled water, soaked in 1% phosphomolybdic acid for 50 s, rinsed with distilled water, dyed with 2.5% aniline blue for 20 s, rinsed and differentiated with 1% glacial acetic acid. Xylene transparent, gum sealed sheet. After sealing, the slides were examined and analyzed under microscope.
2.7.3 Immunohistochemical Experiments
The sections were incubated with citrate antigen retrieval solution for 20 min at 95°C, and primary antibody [PTEN (1:100), PI3K (1:100), AKT (1:100), and p-AKT (1:100)] and secondary antibody was incubated with these sections for overnight and 50 min, respectively. The cumulative optical density was collected and calculated with Image-Pro Plus software (Media Cybernetics, United States).
2.8 Western Blotting Analysis
Protein expression levels were detected using antibodies against PTEN (1:5,000), PI3K (1:2,000), p-PI3K (1:500), AKT (1:5,000), p-AKT (1:1,000) and NF-κB (1:2,000). GAPDH (1:5,000) was used as a control. Target proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. These membranes with target proteins were then blocked with 5% skim milk for 1.5 h and incubated with primary and secondary antibodies. Enhanced chemiluminescence reagents were added to the membrane and target proteins were visualized with a chemiluminescence imaging system (Odyssey Fc, LI-COR Biosciences, United States).
2.9 Statistical Analysis
Statistical analyses were performed on the SPSS 20.0 software (IBM, United States), and using one-way analysis of variance (ANOVA) followed by a least significant difference (LSD) test or Dunnett’s T3 test. All experiments in this study were independently repeated thrice to ensure reliable results. Values are expressed as mean ± standard deviation.
3 Results
3.1 Information of the Theoretical Active Compounds and Related Targets of FSGD
A total of 121 FSGD compounds were retrieved from the database of TCMSP. OB ≥ 30% and DL ≥ 0.18 were recognized as compound screening criteria. Then, the eligible compounds were used for further analysis, and 893 putative targets were predicted in FSGD.
3.2 CRF Target Prediction and the Common Targets
A total of 8596 CRF targets were obtained from the genecards database. The higher relevance score indicated a better correlation between the gene and CRF. The 1,115 targets’ scores were more than two-fold of the average value, which was considered as the value of candidate therapeutic targets. After comparing the targets of FSGD with those of CRF, 192 matched targets were identified, such as EGFR, TNF and AKT1, and a venn diagram was plotted (Figure 2A). The first-dimension common targets were regarded as the potential therapeutic targets for FSGD against CRF.
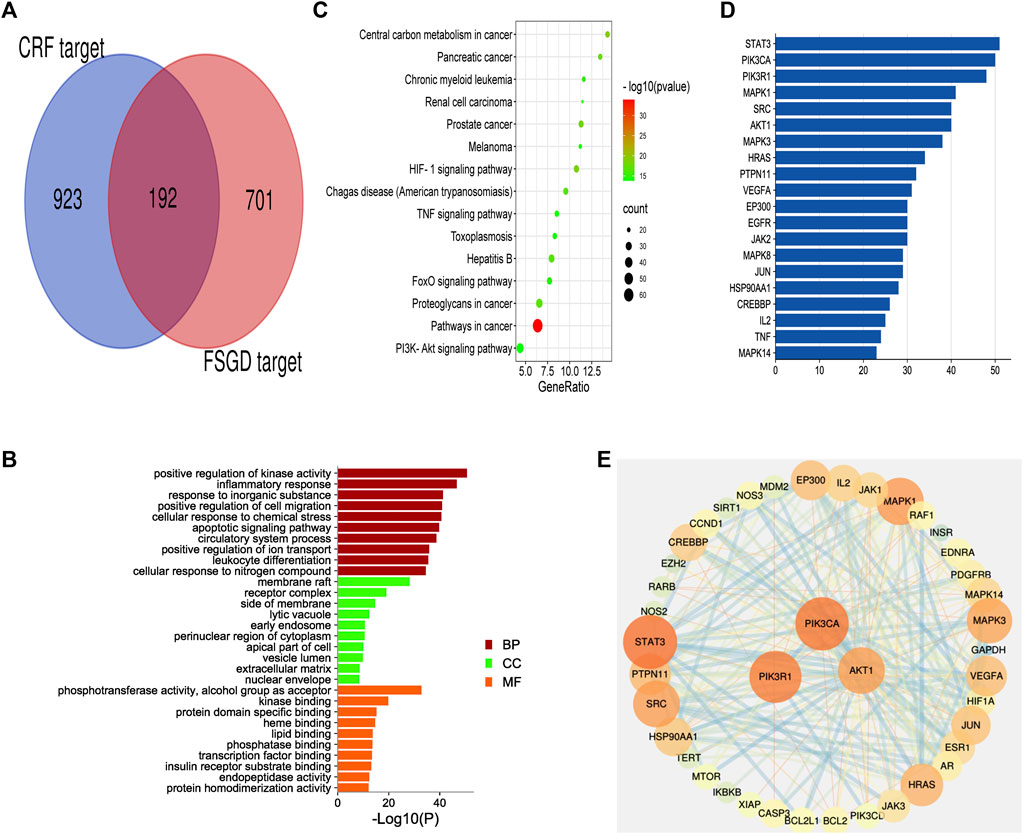
FIGURE 2. The first dimension-network pharmacology study of FSGD in CRF. (A) Venn diagram of common targets in FSGD and CRF. (B) GO enrichment analysis. X-axis represents the different values of −log10(p). Y-axis represents the name of biological processes. (C) The top 15 KEGG pathway enrichment dot-plot diagram. X-axis represents the ratio of enriched target genes/background genes. Y-axis represents the term of enriched pathways. The sizes of the dots indicate the number of target genes in a certain pathway, and the colors of the dots reflect the different values of −log10(p). (D) The construction of protein-protein interaction network. Edges represent protein-protein interactions, edge thickness indicates the strength of data support, the larger the node, the higher the degree value. These circles represent the targets that interact with AKT1. (E) The common targets-bar plot diagram. X-axis represents the number of nodes connected and Y-axis represents the target gene symbol.
3.3 GO and KEGG Enrichment Analysis
The common target genes were analyzed online using the bioinformatics software metascape, and the molecular function (MF), cell components (CC) and biological processes (BP) of these genes were analyzed by GO analysis (Figure 2B). After GO analysis, inflammatory response, apoptotic signaling pathway and extracellular matrix were found to be involved in the process of CRF (Taewon et al., 2016). The KEGG pathway enrichment analysis was performed by enhio (http://www.ehbio.com/) (p ≤ 0.05) (Figure 2C). Among the top 15 signaling pathways, PTEN/PI3K/AKT/NF-κB was involved in cell apoptosis and proliferation, inflammation, and fibrosis in the process of CRF (Song et al., 2020). Previous studies have shown that FSGD can improve renal function and inhibit renal fibrosis in CRF rats through the ACE-Ang II-AT1R axis in the RAS system, while RAS is closely related to the PI3K/AKT pathway in the liver (Molinaro et al., 2019; Xu J et al., 2021; Xu K et al., 2021). Based on the network pharmacological analysis and previous experimental studies, we speculate that the mechanism of FSGD in the treatment of CRF may be through the regulating of the PTEN/PI3K/AKT/NF-κB signaling pathway (Supplementary Figure S1).
3.4 The First-Dimension Network Visualization for the Theoretical Active Ingredients for FSGD in CRF
To further verify our conjecture, a string platform was employed to explore the interaction among the common 192 targets, and a PPI network was constructed by cytoscape3.7.2 (Supplementary Figure S2). This network comprised 168 nodes (ABCG2, ABCC1, PON1, CETP, PSEN1. GAA were concealed as disconnected nodes in the network) and 874 edges, with an average degree value of 10.40. The higher density connections representing the hub genes in network clusters of FSGD in CRF. STAT3, PIK3CA, PIK3R1, MAPK1, SRC and AKT1 were the top six target genes in the PPI network (Supplementary Table S2). PIK3CA, PIK3R1 and AKT1 were related to the PTEN/PI3K/AKT/NF-κB pathway, and AKT1 was a key gene, its targets are shown in Figure 2D, and the hunt hub genes are obtained in Figure 2E.
3.5 The Second-Dimension Network Pharmacological Analysis of Effective Compounds Obtained by UHPLC-MS/MS
To identify the major chemical components, the FSGD samples were analyzed using UHPLC-MS/MS. The total positive (Figure 3A) and negative (Figure 3B) ion chromatograms of FSGD demonstrated the chemical composition of all compounds, and we found that 40 compounds were found in FSGD (Supplementary Table S3). Nine candidate compounds were screened using the previous screening criteria, and their targets were predicted (Figure 3C), and the network pharmacology analysis was then carried out. PPI results show that AKT1was the top target gene and the central gene of the network. In addition, the results of GO, KEGG, and PPI all involved in the pathway of PTEN/PI3K/AKT/NF-κB (Figures 4A–D).
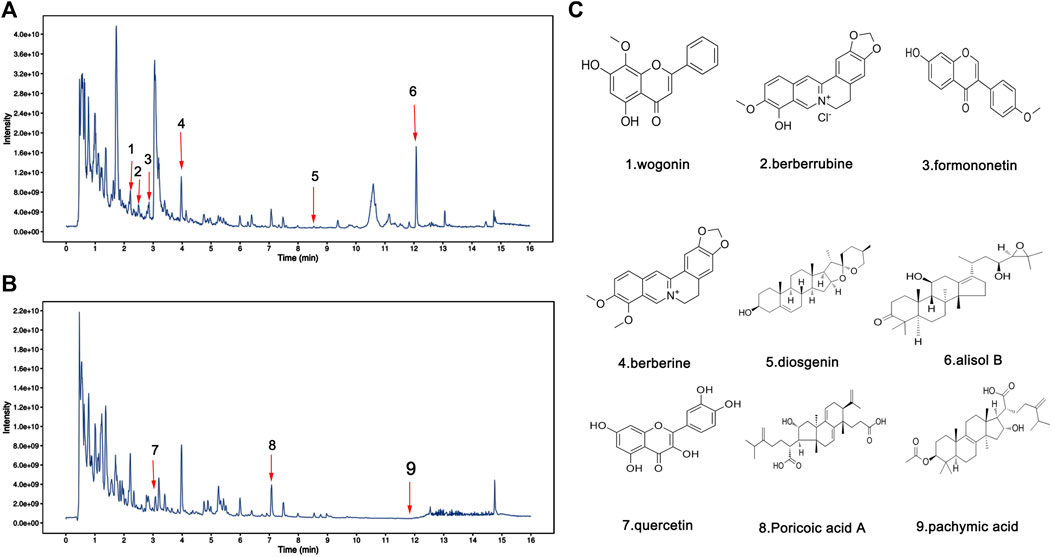
FIGURE 3. Identification of chemical components in water extract solutions of FSGD by ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC–MS/MS). Total ion chromatography in positive (A) and negative (B) ion modes for FSGD samples as shown. The number corresponds to the compound on the left. (C) Molecular structure of constituents.
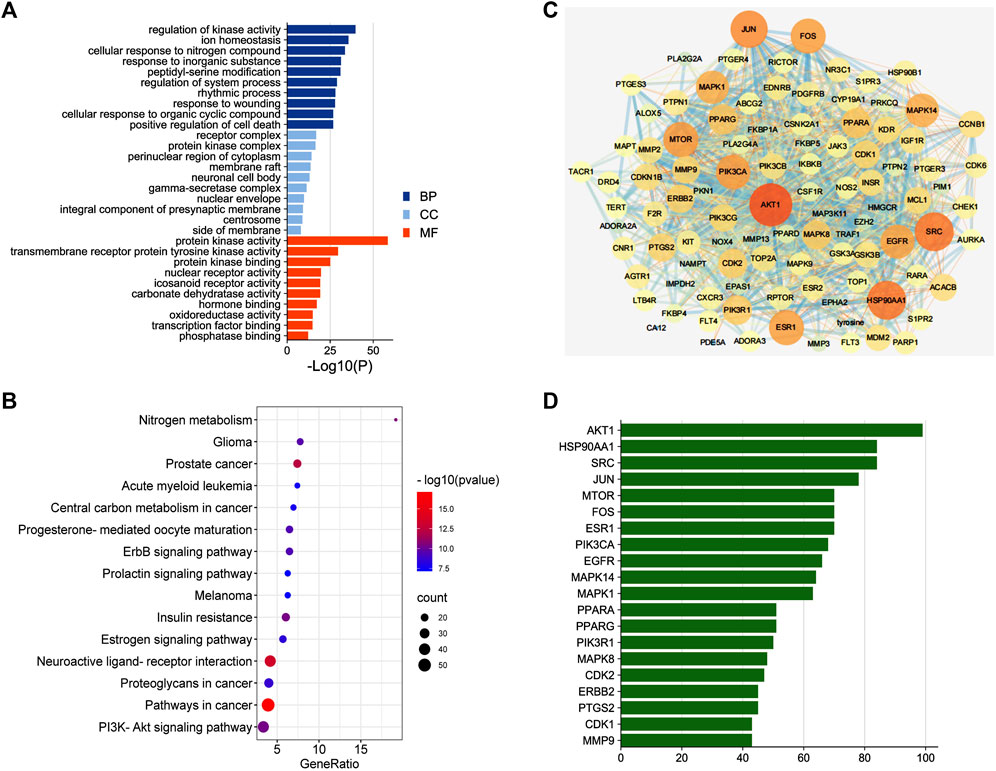
FIGURE 4. The second dimension-network pharmacology study of the detected ingredients of FSGD in CRF. (A) GO enrichment analysis. (B) The top 15 KEGG pathway enrichment dot-plot diagram. (C) The construction of protein-protein interaction network. (D) Common Targets-bar plot diagram.
3.6 FSGD Protects Renal Function
Change in serum biochemical parameters are related to kidney toxicity parameters in CRF (Firouzi and Haghighatdoost, 2018). Renal failure causes a decline in glomerular filtration function and further lead to the increase of the serum Cr and Bun (Cai et al., 2018). The 24-h urinary protein quantification, serum Cr, and BUN levels were used to examine the kidney function. Compared with the control, the 24-h urine protein quantification, serum Cr, and BUN of the model rats were significantly increased (p < 0.01), which indicated that the CRF rat model was established successfully in our experiment. However, FSGD and NDQ significantly reduced these CRF indicators (p < 0.05), and the improvement in the FSGD group was obvious (Figures 5A–C). These results show that FSGD can improve CRF symptoms and renal function, which is in line with previous research (Yang et al., 2017; Xu J et al., 2021; Xu K et al., 2021; Tong et al., 2020).

FIGURE 5. Effects of FSGD on renal pathological injury and renal fibrosis. (A–C) Effects of FSGD on serum concentrations of BUN CR and 24-h urinary protein. (D) Renal pathological changes were analyzed by hematoxylin-eosin and Masson staining was used to analyze collagen fibers in the kidney (×400). (E) The collagen fibers deposition was statistically analyzed with ImageJ software. The data are expressed as: compared with the normal group; Data were presented as the means ± SD. *p < 0.05, **p < 0.01; versus normal group; ##p < 0.01, #p < 0.05, compared with the model group, respectively.
3.7 FSGD Alleviates Renal Pathological Injury and Renal Fibrosis
In this study, we evaluated the pathological changes in the kidney from different perspectives by HE (Figure 5D). In contrast to the normal group, the pathological morphology of the kidney in the model group was significantly changed, the renal tubules were enlarged or atrophied, many inflammatory cells were infiltrated, and the lumen was blocked by adenine crystal deposition. Meanwhile, NDQ and FSGD alleviate the above pathological injuries. In masson staining, red color indicates muscle fibers and blue indicates collagen fibers. Compared with the control, a significant accumulation of collagen fibers was found in the kidney tissue of the model group (p < 0.01); instead, FSGD significantly improved renal fibrosis (p < 0.05, Figure 5E).
3.8 Effects of FSGD on PI3K/AKT Pathways in CRF Model
The results showed that the expression of PTEN was significantly decreased in the model group by immunohistochemical determination (p < 0.01), but FSGD significantly increased the expression of PTEN (p < 0.05, Figures 6A,B), and which indicated that the PTEN is mainly regulated by the PI3K/AKT pathways. After immunohistochemistry analysis, the expression of PI3K, AKT, and p-AKT in the kidney are both significantly increased in model group (p < 0.01), FSGD and NDQ significantly upregulated the expression of PTEN and reduced the expression of PI3K, AKT, and p-AKT by inhibiting the PI3K/AKT pathway (p < 0.05, Figures 6C–E). Zheng et al. have shown that L-Carnitine can eliminate tacrolimus-induced PI3K/AKT activation and PTEN inhibition and counteract oxidative stress-mediated programmed cell death (Zheng et al., 2021). So, L-Carnitine could reduce the renal injury caused by TAC, our research consistent with the previous studies (Zheng et al., 2021).
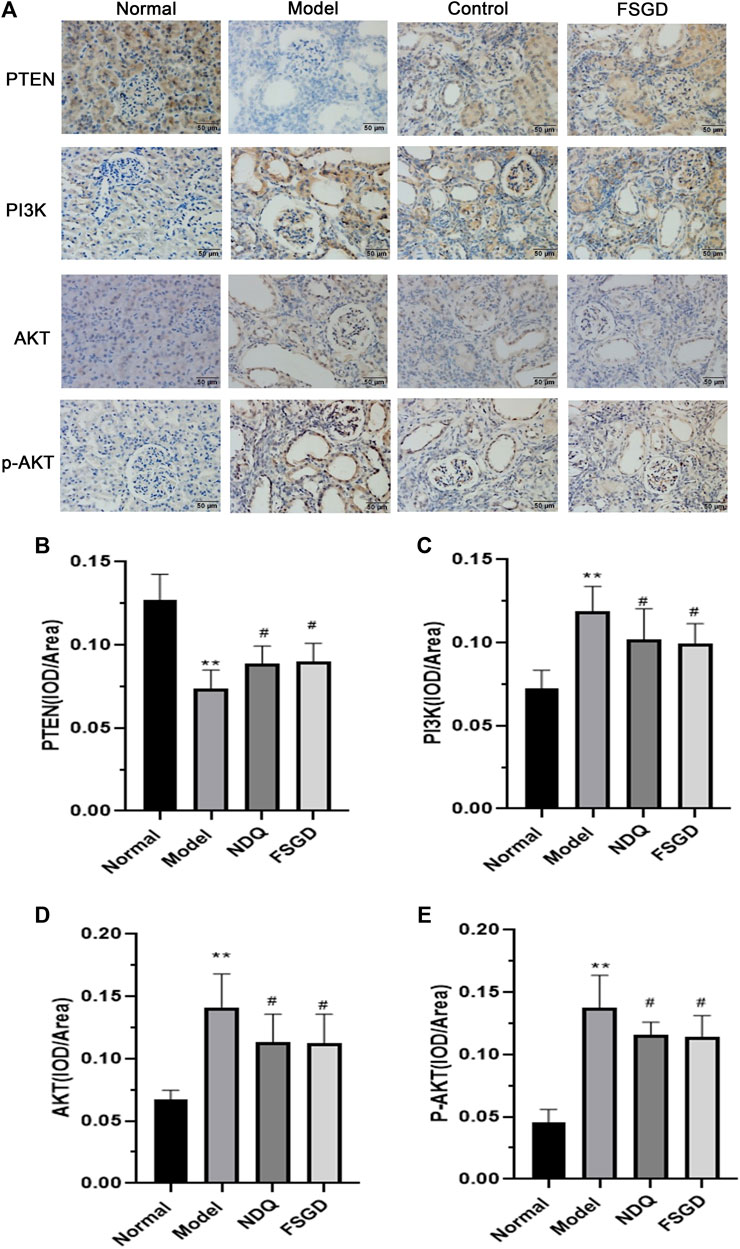
FIGURE 6. Effects of FSGD on PI3K/AKT pathway by immunohistochemistry. (A) The immunohistochemistry staining of PI3K/AKT pathway. (B–E) Image-Pro Plus software was used to statistically analyze the immunohistochemical staining results of PTEN, PI3K, AKT and p-AKT, respectively. The data are expressed as: compared with the normal group; *p < 0.05, **p < 0.01; versus normal group; ##p < 0.01, #p < 0.05, compared with the model group, respectively.
To confirm our hypothesis, the key factor PTEN and its upstream factors PI3K and p-PI3K in the kidney were detected using western blotting. The expression of PTEN was significantly decreased in the model group (p < 0.01), but it significantly increased after FSGD and NDQ treatment (p < 0.05, Figures 7A–D), compared with the modern group. The expressions of PI3K and p-PI3K were significantly increased in the model group, (p < 0.01), but significantly decreased after FSGD and NDQ treatment (p < 0.05, Figures 8A–D), compared with the model group. These similar results can be found in previous study (Haitao et al., 2021; Huo et al., 2021).
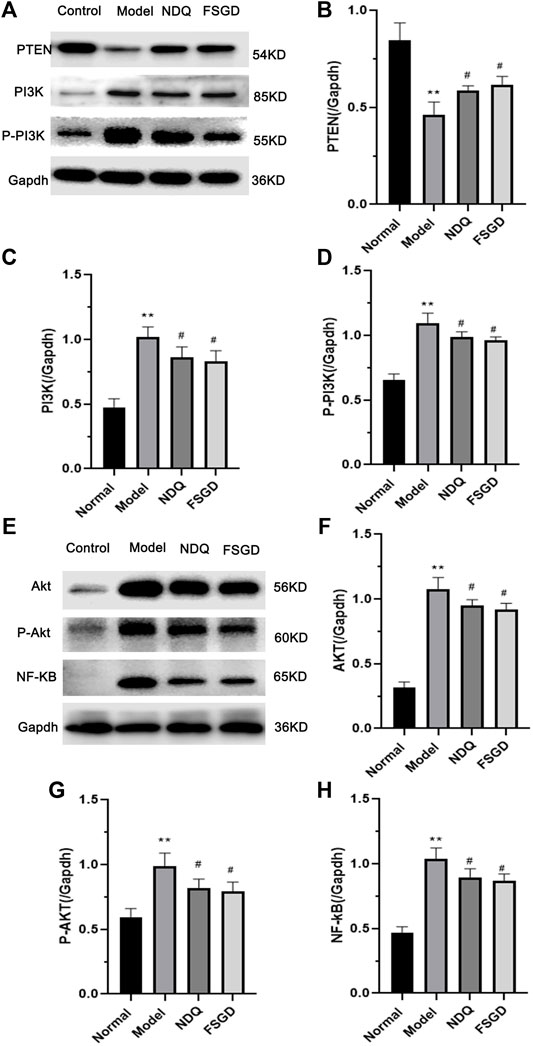
FIGURE 7. The protein analysis of Pi3k/Akt pathway for FSGD in CRF. (A,E) Western blotting of PTEN, PI3K, p-PI3K, AKT, p-AKT and NF-κB in the kidney. [(B–D) and (F–H)] The relative expression concentration analysis for PTEN, PI3K, p-PI3K, AKT, p-AKT and NF-κB in the kidney. Compared with the normal group, *p < 0.05, **p < 0.01; versus normal group; ##p < 0.01, #p < 0.05, compared with the model group, respectively.
To further explain the mechanism of FSGD on the PTEN/PI3K/AKT/NF-κB pathway, the downstream factors AKT, p-AKT, and NF-κB were measured because these factors play an important role in renal fibrosis. Compared with the normal group, AKT, p-AKT, and NF-κB were significantly increased in the model group (p < 0.01), indicating that the PTEN/PI3K/AKT/NF-κB pathway was activated in the kidneys of CRF rats, while the expressions of AKT, p-AKT, and NF-κB were significantly decreased after FSGD and NDQ treatment (p < 0.05, Figures 8E–H). The result of our study is the same as the earlier conclusion (Ba et al., 2020).
4 Discussion
TCM therapy has become increasing popular in China and other Asian countries for refractory diseases (Peng et al., 2017; Pan et al., 2019), and network pharmacology is a new strategy to explore the mechanism of drug treatment of diseases (Li et al., 2020). In our study, the candidate FSGD compounds and targets against CRF were predicted using network pharmacology, which yielded 121 effective compounds and 192 targets. However, this has only based on the database analysis and has not been verified by experiments. Therefore, we verified the authenticity and feasibility of two-dimensional network drug prediction through experiments. An overview of this study is shown in Figure 8.
The results indicated that the main FSGD compounds involved in the treatment of CRF were quercetin, formononetin, and poria acid. The previous study found that quercetin could reduce epithelial to mesenchymal transition (EMT), extracellular matrix (ECM) deposition, and cell proliferation to exert anti-fibrosis effects in NRK-52E cells treated with TGF- β1 and in renal obstruction rats through the overactive Hedgehog pathway (Liu et al., 2019). Formononetin and pachymic acid have anti-inflammatory and antioxidant effects (Feng et al., 2020; Oza and Kulkarni, 2020). In a word, these compounds have been shown to exert anti-inflammatory, anti-fibrosis, and antioxidant effects in CRF treatment, which could play a positive role (Ullah and Basile, 2019). And these selected compounds in this study can lay the foundation for the development of new drugs to treat CRF in the future. This study, like a beacon before dawn, points out the direction for our follow-up research to treat CRF as soon as possible.
Further analysis showed that AKT1, EGFR, and TNF may be potential targets for FSGD in the treatment of CRF, and the PPI network indicated that AKT1 was one of the central genes related to 40 highly valuable targets and is closely related to fibrosis, and it may be a new target for CRF (Lin et al., 2020). AKT1 can promote EGFR ubiquitination and degradation to prevent renal intertubule fibrosis (Zhu et al., 2020). Neutralizing inflammatory cytokines (TNF) can reduce the level of FGF23 in animal models of chronic kidney disease (CKD) and high levels of FGF23 are associated with increased cardiovascular morbidity and mortality in patients with CKD (Egli-Spichtig et al., 2019). Therefore, TNF can be used as a potential therapeutic target for CRF, thereby reducing mortality in patients with CRF. Moreover, the KEGG results suggest that the PTEN/PI3K/AKT/NF-κB signal pathway may be a potential mechanism of FSGD in the treatment of CRF, and the inhibition of the PTEN/PI3K/AKT/NF-κB pathway can reduce inflammation, anti-apoptosis and reduce fibrosis in 60 diabetic C57BL/6 mice (Song et al., 2021). And then, we obtained the effective components of FSGD by mass spectrometry and carried out the second-dimension network pharmacological analysis, and the results were consistent with the results of theoretical network pharmacology analysis. Therefore, we speculate that PTEN/PI3K/AKT/NF-κB pathway is an important mechanism of FSGD in the treatment of CRF.
Renal fibrosis is the ultimate common pathway of progressive nephropathy, characterized by excessive accumulation and deposition of the renal interstitium and glomerular extracellular matrix, which ultimately leads to end-stage renal failure (Du et al., 2020). Renal fibrosis is a pathological process driven by multiple factors, including inflammatory response, immune cell apoptosis, proliferation, and activation of fibroblasts (Taewon et al., 2016). Evidence that PI3K/AKT/PTEN signaling pathway is involved in the pathogenesis of many renal diseases, and the PI3K/AKT/PTEN signal axis links oxidative stress with programmed cell death (Zheng et al., 2021). The inhibition of the over-activation of PI3K/AKT signaling pathway can protect the integrity of the podocyte skeleton and foot process in rats through low renal pathological injury and reduced urinary protein (Lin et al., 2019). And PTEN/PI3K/AKT pathway can also reduce the expression of collagen I (COL I), fibronectin (FN) and α-smooth muscle actin (α-SMA) mediated by fibrotic factor TGF-β1, reduce inflammation and oxidative stress in the kidney, and thereby inhibit renal interstitial fibrosis (Song et al., 2020). Furthermore, PTEN can regulate endogenous factor transforming growth factor TGF-β to reduce the accumulation of ECM (Tang et al., 2020) and EMT (Liu B et al., 2018). These findings show that PTEN is closely related to renal tissue fibrosis (Wang Y et al., 2020). In this study, two-dimensional network pharmacological analysis was used to predict that PTEN/PI3K/AKT/NF-κB is an important mechanism of FSGD in the treatment of CRF. David et al have shown that uremia can lead to significant disturbances in the AKT system. Some studies have shown that there are disturbances in total AKT and phosphorylated AKT in uremic rats. The consequences of AKT activation vary greatly depending on the activation pathway, the duration of activation and the specific subtypes affected. The results of acute and chronic activation of AKT pathway are different. Chronic AKT activation is harmful, and chronic overexpression of AKT1 in LVH can lead to cardiac fibrosis associated with AKT1 (Matsui et al., 2002; Nagoshi et al., 2005; Shioi et al., 2002; Li et al., 2007; Semple et al., 2011). It was found that the expression of total AKT and total PI3K protein increased in the kidneys of CRF rats, which may be related to the complex pathogenesis and pathway of PTEN in CRF, which is also consistent with the previous studies (Gao et al., 2021). In addition, PTEN can inhibit the downstream AKT and reduce the recruitment of AKT-mediated monocytes to injured kidney, as a negative regulator of PI3K (Haddadi et al., 2018; Sasaki et al., 2021). The lower expressions of PI3K/AKT inhibit the downstream NF-κB signal transduction, which could reduce the production of inflammatory factors and various chemokines in renal fiber formation and prevent the transformation of fibroblasts into myofibroblasts with stronger secretion and proliferation ability to improve kidney injury and reduce renal fibrosis and glomerular sclerosis, etc. (Liu X et al., 2018; He et al., 2019). Wang et al have founded that PI3K, p-PI3K, AKT, and p-AKT increased significantly in the rat model of ulcerative colitis with yang deficiency of spleen and kidney, while their express levels decreased significantly after drug intervention (Wang et al., 2021). And some researchers found that NF-κB is highly activated in inflammation (Amini-Farsani et al., 2021; Ilchovska and Barrow, 2021). And NF-κB is the downstream of the PTEN/PI3K/AKT pathway (Supplementary Figure S1). Compared with the normal group, the NF-κB protein expression in the model group was significantly increased. The expression of NF-κB protein was significantly reduced after drug intervention. Our results are consistent with the above reported (Wang et al., 2021). But the study of the PTEN/PI3K/AKT/NF-κB pathway was extremely rare in the CRF. Therefore, this study explored the mechanism of FSGD against renal fibrosis in rats with CRF.
In our study, to improve the predication accuracy of network pharmacology, the first-dimensional network pharmacology is based on the theoretical prediction of the mechanism of drug action on the disease, and the second-dimensional network pharmacology is based on the actual potential effective compounds in prescription on CRF for network analysis with the UHPLC-MS/MS identification technology. And then, the GO results of both network pharmacology show that it is closely related to protein kinase, and many studies have also shown that protein kinase plays an important role in CRF (Yang et al., 2019; Wada et al., 2020). At the same time, two-dimensional network pharmacology KEGG results show that PTEN/PI3K/AKT/NF-κB pathway plays an important role in CRF (Song et al., 2020). PPI results show that AKT is a key gene in protein interaction. Studies have shown that AKT is the key node of PTEN/PI3K/AKT/NF-κB signaling pathway (Xu J et al., 2021; Xu K. et al., 2021). In addition, inflammatory response, hormones, and apoptosis are involved in the process of CRF (Li H et al., 2021) (Wang et al., 2019; Yazgan et al., 2021). HIF-1 signaling pathway, ErbB signaling pathway, estrogen signaling pathway and other pathways need to be further studied to find new targets for the treatment of CRF. Two-dimensional network pharmacology combines theory with practice to successfully predict the important mechanism of FSGD acting on CRF, which lays a solid foundation for follow-up molecular experiments. Recently, many literatures have also shown that many new drugs and new targets for the treatment of diseases have been developed based on network pharmacology (Kibble et al., 2015; Li R et al., 2021). Based on the two-dimensional network pharmacology, we also found that there are many components and targets to treat CRF. These findings provide a new direction for the drug development. In this study, we conducted further dual-dimensional network pharmacology analysis on the possible mechanism of diseases and prescription of Chinese medicine and found that the PI3K/AKT pathway is very important for FSGD to treat CRF. Combined with animal experiments, we found that FSGD significantly enhanced renal function and improved renal fibrosis to produce renal function recovery and renal tissue repair. Immunohistochemical and western blot analyses showed that FSGD can regulate the PTEN/PI3K/AKT/NF-κB signaling pathway through increasing PTEN expression and inhibiting the activation of PTEN/PI3K/AKT/NF-κB, compared with the model rats. Moreover, the results showed that the treatment effect of FSGD formula was better than that of the positive drug NDQ.
In conclusion, this work systematically identified the compounds and studied the mechanisms of FSGD in the treatment of CRF using dual-dimensional network pharmacology and in vivo experiments. Our strategy found that the effect of FSGD on reducing renal fibrosis and renal injury in CRF rats may be related to the inhibition of the PTEN/PI3K/AKT/NF-κB signaling pathway. Two-dimensional network pharmacology is a new strategy for exploring the mechanism of complex diseases and drug multi-targets, which can help us to find the most probable mechanism of drug treatment more accurately and provide a more sufficient theoretical basis for further experiments. More experiments are needed to provide more evidence in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by Chongqing Medical University.
Author Contributions
HL and XH performed experiments, data analysis, and wrote the manuscript. MW and KX assisted in the experiments, MW assisted in data analysis, BZ, SY, QP, LR, PL, and LT assisted in writing manuscript. XH, CY, and YP conceived and designed the study and supervised the experiments. All authors have reviewed and approved it for publication.
Funding
This work was supported by the Chongqing Health and Family Planning Commission, Chongqing Science and Technology Commission (ZY201802149), Chongqing Technical Innovation and Application Development Special Project (cstc2019jscxmsxmX0119), Discipline Talents Program of School of Pharmacy and Innovate Start-up Project in Chongqing Medicine University (YXY2021XSZ04), the National Natural Science Foundation of China (81202896) and the Natural Science Foundation Project of CQ CSTC (cstc2018jcyjAX0460).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks for all institutions that provided the funding. We also thanks for the Editage (www.editage.cn) language editing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.807651/full#supplementary-material
Abbreviations
BUN, urea nitrogen; Cr, creatinine; CRF, chronic renal failure; DL, drug-likeness; FSGD, Fushengong decoction; NDQ, Niaoduqing; OB, oral bioavailability; TCM, traditional Chinese medicine; TCMSP, traditional Chinese medicine systems pharmacology.
References
Amini-Farsani, Z., Yadollahi-Farsani, M., Arab, S., Forouzanfar, F., Yadollahi, M., and Asgharzade, S. (2021). Prediction and Analysis of microRNAs Involved in COVID-19 Inflammatory Processes Associated with the NF-kB and JAK/STAT Signaling Pathways. Int. Immunopharmacol 100, 108071. doi:10.1016/j.intimp.2021.108071
Ba, Y., Hu, G., Wang, L., Li, Y., Ai, Z., and Li, Y. (2020). Experimental Study on the Effect of Different Decocting Time of Shudahuang (Prepared Rhubarb) on Chronic Renal Failure. Chin. Arch. Traditional Chin. Med. 38, 6–9+259. doi:10.13422/j.cnki.syfjx.20202436
Cai, H., Su, S., Li, Y., Zeng, H., Zhu, Z., and Guo, J., (2018). Protective Effects of Salvia Miltiorrhiza on Adenine-Induced Chronic Renal Failure by Regulating the Metabolic Profiling and Modulating the NADPH oxidase/ROS/ERK and TGF-β/Smad Signaling Pathways. J. Ethnopharmacol. 212, 153–165. doi:10.1016/j.jep.2017.09.021
Du, Y., Liu, P., Chen, Z., He, Y., Zhang, B., Dai, G., et al. (2020). PTEN Improve Renal Fibrosis In Vitro and In Vivo through Inhibiting FAK/AKT Signaling Pathway. J. Cel Biochem 120 (10), 17887–17897. doi:10.1002/jcb.29057
Egli-Spichtig, D., Imenez Silva, P. H., Glaudemans, B., Gehring, N., Bettoni, C., Zhang, M., et al. (2019). Tumor Necrosis Factor Stimulates Fibroblast Growth Factor 23 Levels in Chronic Kidney Disease and Non-renal Inflammation. Kidney Int. 96 (4), 890–905. doi:10.1016/j.kint.2019.04.009
Feng, Z., Shi, H., Liang, B., Ge, T., Cai, M., Liu, F., et al. (2020). Bioinformatics and Experimental Findings Reveal the Therapeutic Actions and Targets of Pachymic Acid against Cystitis Glandularis. Biofactors 47 (4), 665–673. doi:10.1002/biof.1734
Firouzi, S., and Haghighatdoost, F. (2018). The Effects of Prebiotic, Probiotic, and Synbiotic Supplementation on Blood Parameters of Renal Function: A Systematic Review and Meta-Analysis of Clinical Trials. Nutrition 51, 104–113. doi:10.1016/j.nut.2018.01.007
Gao, F., Wang, Z., Yang, B., and Tan, J. (2021). Mechanism of Jiawei Shengjiang Powder in the Treatment of Membranous Nephropathy Based on Network Pharmacology. Pharmacol. Clin. Chin. Materia Med. 1, 1–18. doi:10.13412/j.cnki.zyyl.20210202.003
Haddadi, N., Lin, Y., Travis, G., Simpson, A., Nassif, N., and McGowan, E. (2018). PTEN/PTENP1: 'Regulating the Regulator of RTK-dependent PI3K/Akt Signalling', New Targets for Cancer Therapy. Mol. Cancer 17 (1), 37. doi:10.1186/s12943-018-0803-3
Haitao, T., Duanhua, M., Yuanyuan, L., Shuifu, T., Ying, L., Gangyi, C., et al. (2021). Quercetin Alleviates Chronic Renal Failure by Targeting the PI3k/Akt Pathway. Bioengineered 12, 6538–6558. doi:10.1080/21655979.2021.1973877
He, X., Jiang, H., Gao, F., Liang, S., Wei, M., and Chen, L. (2019). Indoxyl Sulfate-Induced Calcification of Vascular Smooth Muscle Cells via the PI3K/Akt/NF-Κb Signaling Pathway. Microsc. Res. Tech. 82 (12), 2000–2006. doi:10.1002/jemt.23369
Huo, C., Wang, L., Wang, Q., Yang, Y., and Chen, B. (2021). Hydroxysafflor Yellow A Inhibits the Viability and Migration of Vascular Smooth Muscle Cells Induced by Serum from Rats with Chronic Renal Failure via Inactivation of the PI3K/Akt Signaling Pathway. Exp. Ther. Med. 22, 850. doi:10.3892/etm.2021.10282
Ilchovska, D., and Barrow, D. (2021). An Overview of the NF-kB Mechanism of Pathophysiology in Rheumatoid Arthritis, Investigation of the NF-kB Ligand RANKL and Related Nutritional Interventions. Autoimmun. Rev. 20, 102741. doi:10.1016/j.autrev.2020.102741
Kibble, M., Saarinen, N., Tang, J., Wennerberg, K., Mäkelä, S., and Aittokallio, T. (2015). Network Pharmacology Applications to Map the Unexplored Target Space and Therapeutic Potential of Natural Products. Nat. Prod. Rep. 32, 1249–1266. doi:10.1039/c5np00005j
Li, H., Feng, Y., Sun, W., Kong, Y., and Jia, L. (2021). Antioxidation, Anti-inflammation and Anti-fibrosis Effect of Phosphorylated Polysaccharides from Pleurotus Djamor Mycelia on Adenine-Induced Chronic Renal Failure Mice. Int. J. Biol. Macromol 170, 652–663. doi:10.1016/j.ijbiomac.2020.12.159
Li, L., Dai, W., Li, W., Zhang, Y., Wu, Y., Guan, C., et al. (2020). Integrated Network Pharmacology and Metabonomics to Reveal the Myocardial Protection Effect of Huang-Lian-Jie-Du-Tang on Myocardial Ischemia. Front. Pharmacol. 11, 589175. doi:10.3389/fphar.2020.589175
Li, R., Li, Y., Liang, X., Yang, L., Su, M., and Lai, K. (2021). Network Pharmacology and Bioinformatics Analyses Identify Intersection Genes of Niacin and COVID-19 as Potential Therapeutic Targets. Brief Bioinform 22, 1279–1290. doi:10.1093/bib/bbaa300
Li, Y., Takemura, G., Okada, H., Miyata, S., Maruyama, R., Esaki, M., et al. (2007). Molecular Signaling Mediated by Angiotensin II Type 1A Receptor Blockade Leading to Attenuation of Renal Dysfunction-Associated Heart Failure. J. Card. Fail. 13, 155–162. doi:10.1016/j.cardfail.2006.11.005
Lin, J., Ouyang, H., Liang, C. L., Zhao, Z. D., Wang, X. G., and Zhou, J. Y. (2019). Effects of Total Glucosides of Paeony on Kidney protection and Autophagy in Rats with Membranous Nephropathy. Chin. Med. New Drugs Clin. Pharmacol. 30 (09), 1025–1031. doi:10.19378/j.issn.1003-9783.2019.09.002
Lin, Y., Chen, Y., Chen, Y., Ta, A., Lee, H., MacGregor, G., et al. (2020). Tubular Mitochondrial AKT1 Is Activated during Ischemia Reperfusion Injury and Has a Critical Role in Predisposition to Chronic Kidney Disease. Kidney Int. 99 (4), 870–884. doi:10.1016/j.kint.2020.10.038
Liu B, B., Tang, T., Lv, L., and Lan, H. (2018). Renal Tubule Injury: a Driving Force toward Chronic Kidney Disease. Kidney Int. 93 (3), 568–579. doi:10.1016/j.kint.2017.09.033
Liu X, X., Zhang, Y., Shi, M., Wang, Y., Zhang, F., Yan, R., et al. (2018). Notch1 Regulates PTEN Expression to Exacerbate Renal Tubulointerstitial Fibrosis in Diabetic Nephropathy by Inhibiting Autophagy via Interactions with Hes1. Biochem. Biophys. Res. Commun. 497 (4), 1110–1116. doi:10.1016/j.bbrc.2018.02.187
Liu, X., Sun, N., Mo, N., Lu, S., Song, E., Ren, C., et al. (2019). Quercetin Inhibits Kidney Fibrosis and the Epithelial to Mesenchymal Transition of the Renal Tubular System Involving Suppression of the Sonic Hedgehog Signaling Pathway. Food Funct. 10 (6), 3782–3797. doi:10.1039/c9fo00373h
Matsui, T., Li, L., Wu, J., Cook, S., Nagoshi, T., and Picard, M. H. (2002). Phenotypic Spectrum Caused by Transgenic Overexpression of Activated Akt in the Heart. J. Biol. Chem. 277, 22896–22901. doi:10.1074/jbc.M200347200
Mehta, R., Cai, X., Lee, J., Xie, D., Wang, X., Scialla, J., et al. (2020). CRIC Study Investigators. Serial Fibroblast Growth Factor 23 Measurements and Risk of Requirement for Kidney Replacement Therapy: The CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 75 (6), 908–918. doi:10.1053/j.ajkd.2019.09.009
Molinaro, A., Becattini, B., Mazzoli, A., Bleve, A., Radici, L., Maxvall, I., et al. (2019). Insulin-Driven PI3K-AKT Signaling in the Hepatocyte Is Mediated by Redundant PI3Kα and PI3Kβ Activities and Is Promoted by RAS. Cell Metab 29 (6), 1400–1409. e5. doi:10.1016/j.cmet.2019.03.010
Nagoshi, T., Matsui, T., Aoyama, T., Leri, A., Anversa, P., and Li, L. (2005). PI3K Rescues the Detrimental Effects of Chronic Akt Activation in the Heart during Ischemia/reperfusion Injury. J. Clin. Invest. 115, 2128–2138. doi:10.1172/JCI23073
Oza, M., and Kulkarni, Y. (2020). Formononetin Attenuates Kidney Damage in Type 2 Diabetic Rats. Life Sci. 219, 109–121. doi:10.1016/j.lfs.2019.01.013
Pan, H., Xiao, Y., Wang, W., Ren, R., Leung, E. L-H., and Liu, L. (2019). Traditional Chinese Medicine as a Treatment for Rheumatoid Arthritis: From Empirical Practice to Evidence-Based Therapy. Engineering 5 (5), 895–906. doi:10.1016/j.eng.2019.01.018
Peng, Y., Zhao, Z., Liu, T., Li, X., Hu, X., Wei, X., et al. (2017). Smart Human-Serum-Albumin-As2O3 Nanodrug with Self-Amplified Folate Receptor-Targeting Ability for Chronic Myeloid Leukemia Treatment. Angew. Chem. Int. Ed. Engl. 56 (36), 10845–10849. doi:10.1002/anie.201701366
Sasaki, K., Terker, A., Pan, Y., Li, Z., Cao, S., Wang, Y., et al. (2021). Deletion of Myeloid Interferon Regulatory Factor 4 (Irf4) in Mouse Model Protects against Kidney Fibrosis after Ischemic Injury by Decreased Macrophage Recruitment and Activation. J. Am. Soc. Nephrol. 32 (5), 1037–1052. doi:10.1681/ASN.2020071010
Semple, D., Smith, K., Bhandari, S., and Anne-Marie, S. (2011). Uremic Cardiomyopathy and Insulin Resistance: a Critical Role for Akt? J. Am. Soc. Nephrol. 22, 207–215. doi:10.1681/ASN.2009090900
Shioi, T., McMullen, J., Kang, P., Douglas, P., Obata, T., and Franke, T. (2002). Akt/protein Kinase B Promotes Organ Growth in Transgenic Mice. Mol. Cel Biol 22, 2799–2809. doi:10.1128/mcb.22.8.2799-2809.2002
Song, Y., Liu, W., Tang, K., Zang, J., Li, D., and Gao, H. (2020). Mangiferin Alleviates Renal Interstitial Fibrosis in Streptozotocin-Induced Diabetic Mice through Regulating the PTEN/PI3K/Akt Signaling Pathway. J. Diabetes Res. 2020, 9481720. doi:10.1155/2020/9481720
Song, Y., Liu, W., Zhao, Y., Zang, J., and Gao, H. (2021). Ochratoxin A Induces Human Kidney Tubular Epithelial Cell Apoptosis through Regulating Lipid raft/PTEN/AKT Signaling Pathway. Environ. Toxicol. 36 (9), 1880–1885. doi:10.1002/tox.23308
Su, X., Kong, L., Lei, X., Hu, L., Ye, M., and Zou, H. (2007). Biological Fingerprinting Analysis of Traditional Chinese Medicines with Targeting ADME/Tox Property for Screening of Bioactive Compounds by Chromatographic and MS Methods. Mini Rev. Med. Chem. 7, 87–98. doi:10.2174/138955707779317830
Taewon, K., Youngjung, K., Changseob, S., Hyuntae, K., Sera, P., Meeyoung, L., et al. (2016). Elsholtzia Ciliata (Thunb.) Hylander Attenuates Renal Inflammation and Interstitial Fibrosis via Regulation of TGF-SS and Smad3 Expression on Unilateral Ureteral Obstruction Rat Model. Phytomedicine 23 (4), 331–339. doi:10.1016/j.phymed.2016.01.013
Tang, J., Goldschmeding, R., Samarakoon, R., and Higgins, P. J. (2020). Protein Phosphatase Mg2+/Mn2+ dependent-1A and PTEN Deregulation in Renal Fibrosis: Novel Mechanisms and Co-dependency of Expression. FASEB J. 34 (2), 2641–2656. doi:10.1096/fj.201902015RR
Tong, P., Huang, X., Shen, Q., Zhang, Y., and Xu, K. (2020). Effect of Fushengong Detection on p38MAPK Signal Pathway of Rats with Chronic Renal Failure. Chin. J. Exp. Traditional Med. Formulae 14, 105–110. doi:10.13422/j.cnki.syfjx.20201440
Ullah, M. M., and Basile, D. P. (2019). Role of Renal Hypoxia in the Progression from Acute Kidney Injury to Chronic Kidney Disease. Semin. Nephrol. 39 (6), 567–580. doi:10.1016/j.semnephrol.2019.10.006
Wada, Y., Kondo, M., Sakairi, K., Nagashima, A., Tokita, K., Tominaga, H., et al. (2020). Renoprotective Effects of a Novel Receptor-Interacting Protein Kinase 2 Inhibitor, AS3334034, in Uninephrectomized Adriamycin-Induced Chronic Kidney Disease Rats. J. Pharmacol. Exp. Ther. 374, 428–437. doi:10.1124/jpet.120.265678
Wang, L., Huang, X., Lei, W., Yang, Y., and Liu, L. (2016). Effect of Fushengong Detection on the Expression of α-SMA in Kidney of Rats with Chronic Renal Failure. Chin. J. Clin. Pharmacol. Ther. 21, 33–37.
Wang, M., Yang, L., Yang, J., Zhou, Y., and Wang, C. (2019). Magnesium Lithospermate B Attenuates Renal Injury in 5/6 Renal Ablation/infarction Rats by Mitochondrial Pathway of Apoptosis. Biomed. Pharmacother. 118, 109316. doi:10.1016/j.biopha.2019.109316
Wang, X., Xue, N., Zhao, S., Shi, Y., Ding, X., and Fang, Y. (2020). Upregulation of miR-382 Contributes to Renal Fibrosis Secondary to Aristolochic Acid-Induced Kidney Injury via PTEN Signaling Pathway. Cell Death Dis 11 (8), 620. doi:10.1038/s41419-020-02876-1
Wang, Y., He, L. Q., Sun, W., Lu, Y., Wang, X. Q., Zhang, P. Q., et al. (2012). Optimized Project of Traditional Chinese Medicine in Treating Chronic Kidney Disease Stage 3: a Multicenter Double-Blinded Randomized Controlled Trial. J. Ethnopharmacol 139 (3), 757–764. doi:10.1016/j.jep.2011.12.009
Wang, Y., Liu, R., and Zhu, X. (2021). Immunohistochemical Effect of Sishen Pill on PI3K/Akt/mTOR Signal Pathway in colon Tissue of Rats with Ulcerative Colitis of Spleen Kidney Yang Deficiency. Acta Laboratorium Animalis Scientia Sinica 29, 42–48. doi:10.3969/j.issn.1005-4847.2021.01.006
Wang, Y., Yang, S., Zhong, K., Jiang, T., Zhang, M., Kwan, H., et al. (2020). Network Pharmacology-Based Strategy for the Investigation of the Anti-obesity Effects of an Ethanolic Extract of Zanthoxylum Bungeanum Maxim. Front. Pharmacol. 11, 572387. doi:10.3389/fphar.2020.572387
Xu J, J., Yu, X., Martin, T., Bansal, A., Cheung, K., Lubin, A., et al. (2021). AKT Degradation Selectively Inhibits the Growth of PI3K/PTEN Pathway-Mutant Cancers with Wild-type KRAS and BRAF by Destabilizing Aurora Kinase B. Cancer Discov. 1, 2159–8290. doi:10.1158/2159-8290.CD-20-0815
Xu K, K., Huang, X., Shen, Q., Zhang, Y., Luo, H., Tian, J., et al. (2021). Effects of Fushengong Prescreption on the "Regulation-Antagonism" Effect of ACE-Angⅱ-At1r and ACE2-Ang (1-7)-MASR axis in Chronic Renal Failure Rats. Chin. J. Exp. Formulas 27 (05), 62–69. doi:10.13422/j.cnki.syfjx.20210544
Yang, L., Gong, N., Zhang, Q., Ma, Y., and Zhou, H. (2019). Apparent Correlations between AMPK Expression and Brain Inflammatory Response and Neurological Function Factors in Rats with Chronic Renal Failure. J. Mol. Neurosci. 68, 204–213. doi:10.1007/s12031-019-01299-8
Yang, Y., Wei, J., Huang, X., Wu, M., Lv, Z., Tong, P., et al. (2017). iTRAQ-Based Proteomics of Chronic Renal Failure Rats after FuShengong Decoction Treatment Reveals Haptoglobin and Alpha-1-Antitrypsin as Potential Biomarkers. Evidence-Based Complementary and Alternative Medicine. eCAM (Evid Based Complement Alternat Med) 2017, 1480514. doi:10.1155/2017/1480514
Yazgan, B., Avcı, F., Memi, G., and Tastekin, E. (2021). Inflammatory Response and Matrix Metalloproteinases in Chronic Kidney Failure: Modulation by Adropin and Spexin. Exp. Biol. Med. (Maywood). 246, 1917–1927. doi:10.1177/15353702211012417
Yuan, Q., Wang, J., Peng, Z., Zhou, Q., Xiao, X., Xie, Y., et al. (2019). C-STRIDE Study Group. Neutrophil-To-Lymphocyte Ratio and Incident End-Stage Renal Disease in Chinese Patients with Chronic Kidney Disease: Results from the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). J. Transl Med. 17 (1), 86. doi:10.1186/s12967-019-1808-4
Zheng, H., Zhang, H., Zhu, C., Li, H., Cui, S., Jin, J., et al. (2021). L-Carnitine Protects against Tacrolimus-Induced Renal Injury by Attenuating Programmed Cell Death via PI3K/AKT/PTEN Signaling. Acta Pharmacol. Sin 42, 77–87. doi:10.1038/s41401-020-0449-8
Zheng, Y., Cai, G., He, L., Lin, H., Cheng, X., Wang, N., et al. (2017). Efficacy and Safety of Niaoduqing Particles for Delaying Moderate-To-Severe Renal Dysfunction: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Clinical Study. Chin. Med. J. 130, 2402–2409. doi:10.4103/0366-6999.216407
Keywords: Fushengong decoction, chronic renal failure, dual-dimension network pharmacology, UHPLC-MS/MS, PTEN/PI3K/AKT/NF-κB
Citation: Luo H, Wang M, Xu K, Peng Q, Zou B, Yin S, Yu C, Ren L, Li P, Tang L, Peng Y and Huang X (2022) Effect of Fushengong Decoction on PTEN/PI3K/AKT/NF-κB Pathway in Rats With Chronic Renal Failure via Dual-Dimension Network Pharmacology Strategy. Front. Pharmacol. 13:807651. doi: 10.3389/fphar.2022.807651
Received: 02 November 2021; Accepted: 28 February 2022;
Published: 15 March 2022.
Edited by:
Xuezhong Zhou, Beijing Jiaotong University, ChinaReviewed by:
Juan Du, The Chinese University of Hong Kong, Shenzhen, ChinaEswar Shankar, The Ohio State University, United States
Copyright © 2022 Luo, Wang, Xu, Peng, Zou, Yin, Yu, Ren, Li, Tang, Peng and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuekuan Huang, xkhuang2002@cqmu.edu.cn; Yongbo Peng, pengyongbo2000@126.com
 Hongyu Luo
Hongyu Luo Munan Wang1
Munan Wang1 Yongbo Peng
Yongbo Peng Xuekuan Huang
Xuekuan Huang