- 1Department of Pharmacy, The Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University, Changzhou, China
- 2Department of Pharmacy, Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research, The Affiliated Cancer Hospital of Nanjing Medical University, Nanjing, China
- 3Department of Clinical Pharmacy, School of Pharmacy, Nanjing Medical University, Nanjing, China
The ultimate goal of cancer treatment is to kill cancer cells, based on the use of various therapeutic agents, such as chemotherapy, radiotherapy, or targeted therapy drugs. Most drugs exert their therapeutic effects on cancer by targeting apoptosis. However, alterations in apoptosis-related molecules and thus assisting cells to evade death, eventually lead to tumor cell resistance to therapeutic drugs. The increased incidence of non-apoptotic cell death modes such as induced autophagy, mitotic catastrophe, senescence, and necrosis is beneficial to overcoming multidrug resistance mediated by apoptosis resistance in tumor cells. Therefore, investigating the function and mechanism of drug-induced non-apoptotic cell death modes has positive implications for the development of new anti-cancer drugs and therapeutic strategies. Phytochemicals show strong potential as an alternative or complementary medicine for alleviating various types of cancer. Quercetin is a flavonoid compound widely found in the daily diet that demonstrates a significant role in inhibiting numerous human cancers. In addition to direct pro-tumor cell apoptosis, both in vivo and in vitro experiments have shown that quercetin exerts anti-tumor properties by triggering diverse non-apoptotic cell death modes. This review summarized the current status of research on the molecular mechanisms and targets through which quercetin-mediated non-apoptotic mode of cancer cell death, including autophagic cell death, senescence, mitotic catastrophe, ferroptosis, necroptosis, etc.
1 Introduction
Even with advances in medical technology and the development of anti-cancer drugs, treatment for most cancers remains a lingering problem. According to GLOBOCAN, approximately 19.3 million patients will be newly diagnosed with cancer and almost 10 million cancer patients died occurred in 2020 worldwide, while the global cancer burden is expected to reach 28.4 million cases in 2040 (Sung et al., 2021). Mainstream cancer therapies include radiotherapy, chemotherapy (anti-cancer drugs), surgery, and new anti-tumor technologies such as immunotherapy and targeted cancer therapy (Furue, 2003; Baskar et al., 2012; Vanneman and Dranoff, 2012). The ultimate goal of all these therapies is to regulate the survival or death of cancer cells, and anti-cancer drugs can kill clonogenic malignant cells by regulating various cell death modes, such as apoptosis, autophagy, senescence, mitotic catastrophe, ferroptosis, necroptosis, etc. (Galluzzi et al., 2018; Sun et al., 2022).
Apoptosis is a programmed cell death (PCD) process that occurs following the stimulation of cells by various death signals, which is characterized by caspase-dependent, cellular contraction, and the formation of apoptotic body (Su et al., 2020). Currently, the majority of chemotherapeutic drugs inhibit the growth of cancer cells by inducing apoptosis, thus providing treatment for various malignancies. However, the inherent apoptotic resistance of cancer cells or the occurrence of a series of pro-survival mutations occurs during the malignant transformation rendering them resistant to apoptosis, which is the main cause of radioresistance and chemoresistance in most cancers (Liu et al., 2010; Carneiro and El-Deiry, 2020; Cao et al., 2021). An early study has demonstrated that the sensitivity of chemotherapeutic drug-induced cell death via apoptosis depends on the activation of caspases, such as cytarabine, doxorubicin, and methotrexate, whereas the inactivation of caspases leads to drug resistance in cancer cells (Los et al., 1997). As a key regulator of the mitochondrial apoptotic pathway, overexpression of members of B-cell leukemia/lymphoma-2 (BCL-2) family proteins inhibit apoptosis both in normal cells and tumor cells, which is another drug resistance factor (Kapoor et al., 2020). Overall, most anti-tumor drugs exert their anticancer activity by targeting cancer cells through apoptosis, and apoptosis defective, manifested by mutations, deletions, and/or overexpression of pro-apoptotic genes, contribute to the development of acquired therapy resistance of cancer cells to chemotherapeutic agents. Hence, it is essential to develop adjuvant or alternative drugs that target the non-apoptotic cell death modes.
Natural products are an essential source of anticancer lead molecules due to their multi-targeting efficacy and low toxicity, especially flavonoids (Yang H. et al., 2022a; Liao et al., 2022). As a flavonoid with various biological activities, quercetin is abundantly present in plants, fruits, and vegetables, mainly in the form of glycosides, such as onions, apples, blueberries, cauliflower, etc. (Batiha et al., 2020) (Figure 1). Considering its anti-inflammatory and antioxidant abilities as well as the modulating effects on tumor microenvironment, quercetin has been added to functional foods as a dietary supplement for the prevention and/or treatment of diverse diseases such as cancer (Li Y. et al., 2016b; Andres et al., 2018; Reyes-Avendaño et al., 2022). Numerous in vivo and in vitro research have found that quercetin induces apoptosis in different cancer cell lines and exhibits anti-tumor properties (Hashemzaei et al., 2017; Khorsandi et al., 2017; Safi et al., 2021). Nevertheless, recent studies suggest that quercetin may also kill cancer cells via several different mechanisms. The current paper summarizes the literature on the regulation of diverse cancer cell death modes and mechanisms following cancer treatment with quercetin, rather than apoptosis.
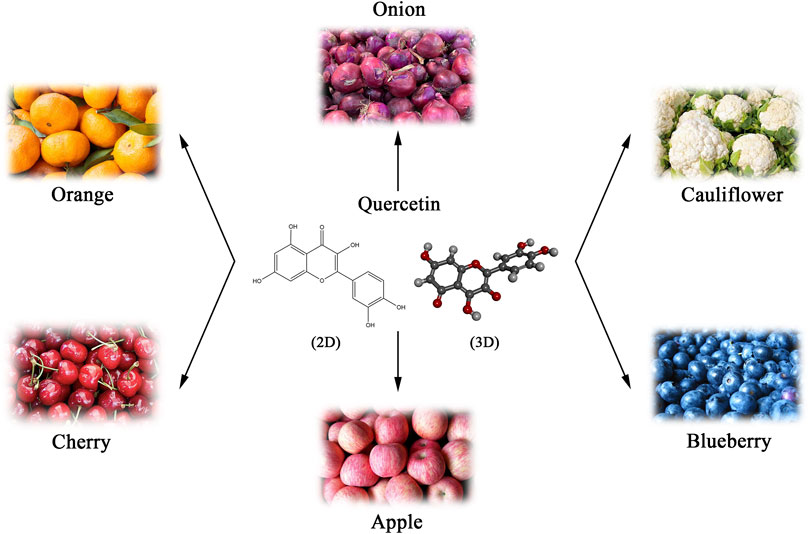
FIGURE 1. Natural sources and chemical structure (Skeletal formulas and 3D stick representations) of quercetin.
2 Background information on quercetin
Quercetin, a member of the flavonoid family, with the chemical name of 3,5,7-trihydroxy-2-(3,4-dihydroxy phenyl)-4-Hchromen-4-one (Li Y. et al., 2016b). As a derivative of phenyl benzoyl ketone, quercetin consists of two benzene rings (A and B rings) linked by an oxygenated pyrene ring (C ring), with the flavonoid structure C6 (A ring)-C3 (C ring)-C6 (B ring) as the basic backbone (Figure 1). It is structurally evident that the flavonol skeletal framework of quercetin has five hydroxyl groups located on the 3,3′, 4′, 5 and 7 carbons, therefore it is also known as pentahydroxyflavonol. The relative substitution of various functional groups on the flavonol molecule is the main cause and effect of the wide range of pharmacological activities of quercetin and its metabolites (Khan et al., 2016). For instance, the substitution pattern of the A and B rings of quercetin and the presence and amount of free hydroxyl groups in their backbone are known to be key to the perceived free radical scavenging potential of quercetin (Nabavi et al., 2012). The sugars, lipids, alcohols and a sulphate groups are all able to conjugate to quercetin via the O-glycosidic bond, resulting in the formation of its derivatives (Williams and Grayer, 2004). Numerous studies have confirmed that prenylated quercetin analogs show powerful potential in antibacterial properties (Cushnie and Lamb, 2005; Wang et al., 2018). Furthermore, it was found that the natural form of quercetin in the form of a sulphate conjugate had significant anticoagulant activity (Guglielmone et al., 2020). Hence, the investigation and development of functional group substitutions for the different biological effects of quercetin analogues is of considerable and far-reaching importance.
As the most popular dietary flavonoid, quercetin has the wealthiest resources (Figure 1), with β-glucoside being the main form of quercetin that exists in the diet. In the gastrointestinal tract, glycosides are highly dependent on microbially-derived β-glucosidases for hydrolyzing to the aglycon before their absorption and transportation take place (Zhao L. et al., 2022a). In intestinal epithelial cells, quercetin is extensively metabolized to quercetin-3- and quercetin-7-glucuronide. Afterward, they are rapidly metabolized in the liver to methyl, glucuronides, or sulfate conjugates, the forms of quercetin present in circulation (Terao et al., 2011). Notably, the highly lipophilic nature of quercetin determines its relatively low water solubility and bioavailability. Consequently, it is imperative to investigate the absorption, distribution, metabolism, and excretion (ADME) of quercetin, which contributes to understanding its bio-transfer in vivo. Yin et al. investigated the pharmacokinetics of quercetin. After oral administration of quercetin (50 mg/kg) to rats (n = 5), the pharmacokinetic analysis showed that quercetin peaked after 1 h. The mean plasma concentration (CMax) of quercetin was 7.47 ± 2.63 μg/mL, and the average area under the plasma concentration-time curve extrapolated to infinitive time (AUC0-∞) and elimination half-life (t1/2) was 2,590.5 ± 987.9 mg/L*min and 437.3 ± 54.3 min, respectively (Yin et al., 2019). The distribution of quercetin in the tissues of rats fed with 0.2% quercetin diet for 11 weeks was observed, and its concentration distribution was in the following order: lung > testis > kidney > thymus > heart > liver > brown fat > bone > brain > spleen (de Boer et al., 2005). In addition, researchers exposed pigs to a high-dose quercetin diet [500 mg/kg/day)] for 3 days. The results showed that the distribution of quercetin concentration is ranked by: liver > kidney > brain > heart > spleen. 3-hydroxyphenylacetic acid, benzoic acid, and hippuric acid are the main excretion products of quercetin, the majority of which are excreted through urine and feces, however, when taken in a high amount of quercetin, the lungs are also one of the organs of clearance (Walle et al., 2001; Guo and Bruno, 2015). The safety of quercetin as a dietary supplement also requires consideration. In a double-blind, placebo-controlled crossover trial, the overweight or obese volunteers were administered a relatively low dose of quercetin (150 mg/day) for 6 weeks. The results of blood biochemical tests showed that the parameters of biomarkers of liver and kidney function (alanine transaminase, g-glutamyl-transpeptidase, aspartate transaminase, creatinine, etc.) were within normal limits, indicating no adverse effects of quercetin (Egert et al., 2009). Moreover, continuous administration of quercetin (250–5,000 mg) for 28 days showed no exacerbation of liver enzyme (aspartate transaminase and alanine transaminase) levels in patients with chronic hepatitis C (Lu et al., 2016). However, data related to the safety evaluation of long-term, high-dose quercetin supplementation are still limited. Hence, more in-depth exploration of these issues is needed in future intervention research.
As research progresses, quercetin has received increasing attention for its nutritional and therapeutic potential, benefiting from its wide range of biological activities (Yang et al., 2020). Numerous in vivo studies have demonstrated the strong potential of quercetin for anti-diabetes and its complications, together with antioxidant, anti-inflammatory, anti-viral, and significant neurological and cardiovascular-related benefits (Patel et al., 2018; Shi et al., 2019; Yang et al., 2019; Di Petrillo et al., 2022; Zhang et al., 2022). Moreover, growing evidence suggests that in addition to apoptosis, quercetin also exerts anti-tumor effects via multiple signaling pathways that induce non-apoptotic cancer cell death modes, making it a promising natural product for the prevention and nutritional management of cancer (Rauf et al., 2018; Tang et al., 2020).
3 Therapeutic mechanisms of quercetin targeting non-apoptotic cell death patterns in cancer
Different tumor therapy may induce cancer cell death via diverse mechanisms. As a common mode of cell death caused by impaired cytogenetic content, conventional cancer therapy usually evokes cell death by inducing apoptosis, however, the inherent and/or acquired apoptotic resistance of cancer cells persists being a major hindrance to the efficacy of chemotherapy (Farhat et al., 2014; Murray et al., 2014; Fox and Storey, 2015). Chemotherapeutic drugs are also known to kill cancer cells via other different mechanisms, including autophagic cell death, mitotic catastrophe, senescence, ferroptosis, necroptosis, etc. (Galluzzi et al., 2018; Sun et al., 2022). Quercetin induces cell death via different mechanisms, the most common of which is triggering apoptosis. However, it has been suggested that some other mechanisms are also possible factors for quercetin to trigger cancer-killing (Tang et al., 2020). With the sustained exploration of cell death mechanisms, targeting non-apoptotic cell death modes has emerged as a potentially new mechanism of cell death induced by cancer therapies, which may complement or replace apoptosis-induced cancer cell therapy (Denisenko et al., 2016; Li et al., 2017). This section reviews the various mechanisms of non-apoptotic cell death induced following cancer therapy with quercetin based on the literature conducted over the past 10 years (Tables 1, 2).
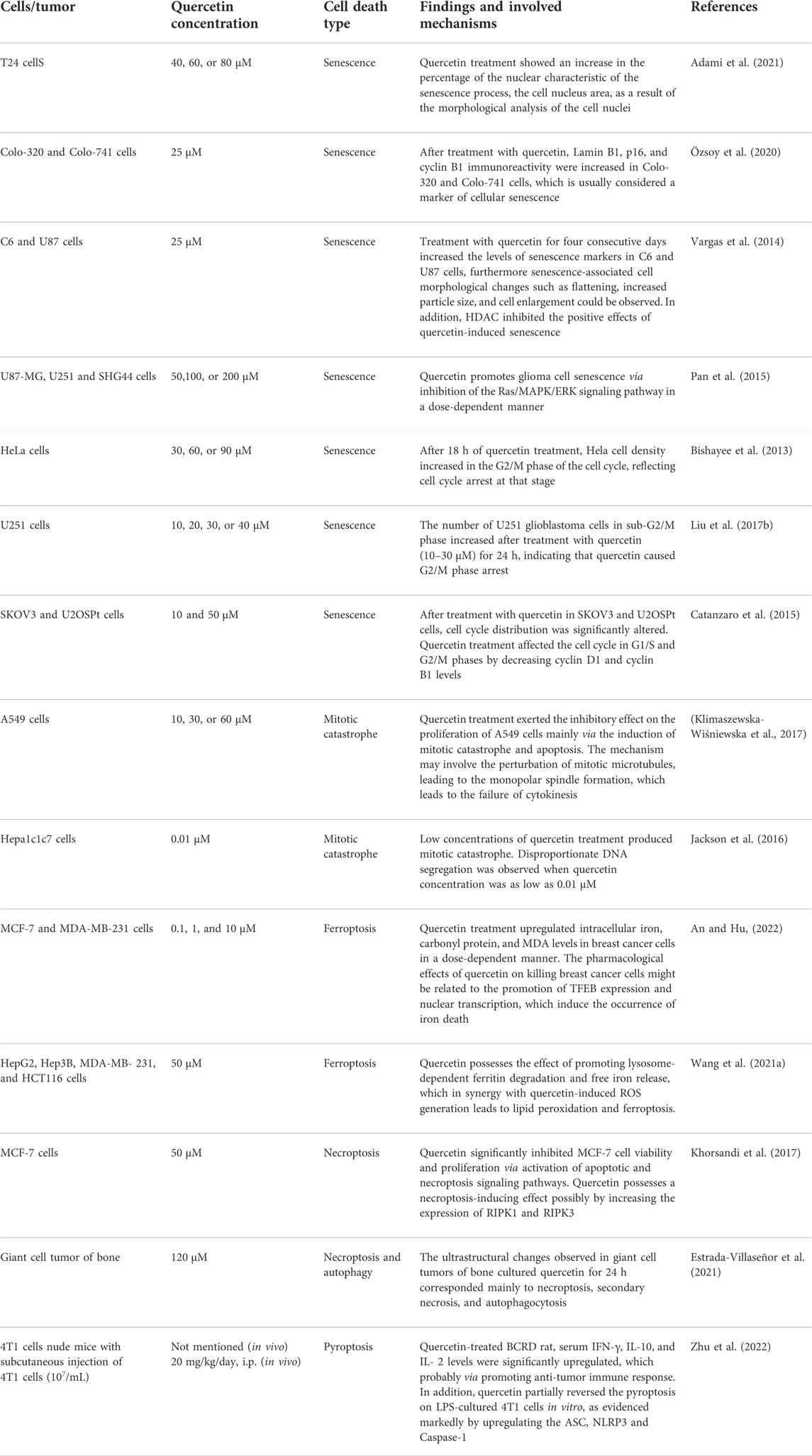
TABLE 2. Summarized mechanisms of non-apoptotic death of cancer cells following treatment with quercetin.
3.1 Quercetin and autophagy
Autophagy is a type II programmed cell death that prevents tumor initiation and suppresses cancer progression in early tumorigenesis. As a key regulator of cellular metabolism during starvation, autophagy normally protects cells from stressors like nutrient deprivation. (Kimmelman and White, 2017). Stimulation of the tumor microenvironment usually triggers autophagy, such as nutrient deprivation, reactive oxygen species (ROS), hypoxia, and pathogen invasion (Su et al., 2015). Typically, autophagy passes through distinct stages including induction of autophagy, nucleation of the autophagosome, expansion, and elongation of autophagosomal membranes, closure, and fusion of autophagosomes with lysosomal membranes, and degradation and recirculation of intracapsular products (Li et al., 2020). During cancer cell survival, autophagy plays dichotomous role, exerting dynamic tumor-suppressive or tumor-promoting effects at different stages or settings (White, 2015; Levy et al., 2017). Although autophagy promotes tumorigenesis, abundant evidence indicates that the triggering of autophagy may limit tumor progression and improve response to cancer therapy (White, 2015; Amaravadi et al., 2019). In addition to the cytoprotective and cytotoxic forms of autophagy, Gewirtz DA proposed in a review published in 2014 that autophagy is actually populated by at least two additional players, a nonprotective form of autophagy and a cytostatic form of autophagy (Gewirtz, 2014). As a survival response, cytoprotective autophagy enables tumor cells to evade apoptotic signals and become resistant to chemotherapy and radiotherapy. When cytoprotective autophagy is blocked, it can enhance the sensitivity of tumor cells to chemotherapy and increase apoptosis in cancer cells (Ulasov et al., 2020; Xu et al., 2022). For this reason, multiple clinical trials are currently being conducted for treating cancer by targeting cytoprotective forms of autophagy using autophagy inhibitors (e.g., chloroquine or hydroxychloroquine) in combination with various conventional therapeutic modalities tto achieve improved efficacy (Gewirtz, 2014). Although autophagy promotes tumorigenesis, abundant evidence indicates that the triggering of autophagy may limit tumor progression and improve response to cancer therapy (White, 2015; Amaravadi et al., 2019). Cytotoxic autophagy promotes tumor cell death, either by killing its own cells or by acting as a precursor to apoptosis (Sui et al., 2013; Gewirtz, 2014). Generally, the key to distinguish between cytotoxic autophagy and cytoprotective autophagy is to observe the sensitivity of tumor cells to the therapeutic modality. Besides, Thorburn A and Gewirtz DA observed induction of another form of autophagy by radiation, which would be termed ‘‘nonprotective’’, whose inhibition is neither affect cell proliferation nor apoptosis (Bristol et al., 2013). In a particular tumor cell line, the manner of treatment may determine whether autophagy is cytoprotective or nonprotective (Gewirtz, 2016). In 2014, Gewirtz DA proposed for the first time a novel form of autophagy, namely cytostatic autophagy (Sharma et al., 2014). This study noted that combined treatment with vitamin D (or a vitamin D analogue, EB 1089) and radiation resulted in more pronounced growth inhibition in non-small cell lung cancer cells than radiation alone, as well as greater sensitivity to radiation. In addition, they identified that radiation-induced conversion of cytoprotective autophagy to cytostatic autophagy. Considering that cytostatic autophagy usually mediates cell growth inhibition, this form of autophagy may affect the effectiveness of chemotherapy and/or radiotherapy targeting tumor cell growth arrest. Unfortunately, there is no well-defined biochemical or molecular signature that distinguishes these forms from each other.
Quercetin has been demonstrated to promote and control the regulation of autophagy in different types of cancers. Treatment of Burkitt lymphoma cell lines with quercetin, LC3Ⅰ was converted to LC3Ⅱ, which is commonly considered a biomarker of autophagy, suggesting that quercetin induced a complete autophagic flux (Granato et al., 2016). The same findings were obtained in HL-60 xenograft mice, where quercetin administration induced the conversion of LC3-I to LC3-II and activated autophagy proteins, indicating that quercetin treatment triggered the autophagic process and thus anti-tumor growth (Calgarotto et al., 2018). In addition to upregulating the LC3-II/I ratio in a dose-dependent manner, a large number of double membranes were observed in quercetin-treated glioma cell lines (Bi et al., 2016). In SH-SY5Y cells, quercetin also induced autophagy by upregulating LC3II. This study also revealed that quercetin restored organelle endoplasmic reticulum homeostasis and alleviated the cytotoxic damage induced to Cu by regulating the autophagic pathway (Chakraborty et al., 2022). Moreover, the highest number of cellular autophagic vacuoles was observed in HeLa cells treated with 50 μM quercetin, but the number decreased instead at higher doses (Wang et al., 2016). In lung cancer cells, quercetin treatment also significantly increased Beclin 1 protein expression and the number of autophagic vacuoles and autophagosomes (Guo et al., 2021). Contrasting results were observed in human glioblastoma cell lines, where quercetin treatment increased the formation of autophagic lysosomal vesicles but had no effect on the expression of Beclin-1 (Kim et al., 2013). Mammalian target of rapamycin protein (mTOR) is one of the key proteins regulating the autophagic process, causing phosphorylation and inactivation of autophagy protein (ATG), thus inhibition of mTOR leads to upregulation of ATG and initiation of the autophagic process (Kim et al., 2011; Kim and Guan, 2015). In an in vivo and in vitro study, quercetin was used to treat breast cancer. This study indicated that quercetin induced cellular autophagy by inactivating the protein kinase B (Akt)-mTOR pathway, while the use of Akt-mTOR pathway inducers and autophagy inhibitors further confirmed the involvement of the Akt-mTOR pathway in quercetin-induced autophagy (Jia et al., 2018). Similarly, autophagosomes and autophagolysosomes were significantly increased in hepatocellular carcinoma (HCC) cells after quercetin treatment. By using pathway-specific inhibitors or activators, it is suggested that quercetin stimulates autophagy by inactivating the AKT/mTOR pathway and activating the MAPK pathway (Ji et al., 2019). In addition, quercetin significantly induced cellular autophagy and enhanced gemcitabine-induced cytotoxicity by inhibiting the phosphatidylinositol-3-kinase (PI3K)/AKT/mTOR axis in pancreatic cancer cells (Lan et al., 2019). In addition, quercetin may also induce autophagy in cancer cells through other mechanisms. Wu et al. found that quercetin treatment induced cellular autophagy by upregulating LC3 expression and downregulating p62 expression in a time-dependent manner. These effects were at least partially dependent on quercetin downregulation of Janus kinase 2 (JAK2) and signal transducer and activator of transcription 3 (STAT3) activation (Wu et al., 2019). In primary ovarian cancer (OC) cells, quercetin treatment activates the p-STAT3/Bcl-2 axis followed by induction of protective autophagy (Liu, Gong, et al., 2017). In the study of quercetin-induced osteosarcoma cell death, quercetin promoted the expression of autophagy-related genes through activation of NUPR1 gene activity, which subsequently triggered excessive autophagy in cancer cells (Wu et al., 2020). There are complex interactions between autophagy and apoptosis. A study confirmed that quercetin significantly enhanced tumor necrosis factor (TNF)-related apoptosis-induced ligand-mediated lung cancer cell death through activation of autophagy (Moon et al., 2015) (Figure 2).
Interestingly, the combination of quercetin with other drugs can improve the antitumor efficacy of quercetin by inhibiting or inducing autophagy. Granat et al. demonstrated that quercetin treatment induced a complete autophagic flux in the primary effusion lymphoma (PEL) cell lines. Furthermore, further accumulation of the lipidated form of the autophagy marker LC3 (LC3-II) was observed in quercetin combined with vesicular proton pump inhibitor-treated BC3, BCBL1, and BC1 cells compared to the single treatments (Granato et al., 2017). Another study demonstrated that quercetin-induced autophagy reduced its therapeutic effect on gastric cancer (GC) cells, and treatment with quercetin combined with miR-143 agonist, an inhibitor of autophagy in GC cells targeting GABARAPL1, could improve the antitumor efficacy of quercetin (Du et al., 2015). In addition, quercetin combined with soluble epoxide hydrolase inhibitor (t-AUCB) promotes cell death by inducing autophagy blockade, which may be a potential strategy for the treatment of glioblastoma (Li J. et al., 2016a).
3.2 Quercetin and senescence
Cellular senescence, a permanent state of cell cycle arrest due to various cancer-induced stresses, inhibits cancer by irreversibly preventing cell proliferation and is one of the protective mechanisms against cancer in addition to apoptosis (Collado et al., 2007; Calcinotto et al., 2019). Cellular stress, DNA damage, and oncogene activation are among the stimuli that cause cellular senescence (Ma et al., 2018; Jochems et al., 2021). Besides the cell cycle arrest, the morphological features that accompany cellular senescence include cellular enlargement, flattening, vacuolization, and occasionally multinucleation or increased nuclear occupancy. However, these changes are usually only observed during cellular senescence in vitro cultures, while in vivo senescent cells maintain normal morphologically determined tissue structure (Collado and Serrano, 2006; Muñoz-Espín and Serrano, 2014; Bernadotte et al., 2016). In cultured cells and/or tissues, the detection of a collection of biomarkers is used to define senescence. The histochemical assay for β-galactosidase activity is the most widely used assay for senescence. Common mediators of senescence, including p16, ARF, p53, p21, p15, and p27, are also typical biomarkers of senescence (Muñoz-Espín and Serrano, 2014). In addition, alterations in Lamin B1 levels are a common feature of many types of senescence (Lukášová et al., 2018; Radspieler et al., 2019). Generally, senescence is considered as an effective anti-tumor mechanism through which cancer cells proliferate and inhibit malignant progression. Furthermore, senescence is one of the physiological tumor suppressor mechanisms that limit the progression of tumors from benign tumor lesions to malignant ones. As a consequence of these effects, it has cancer suppressive potential, while senescence-associated secretory phenotype (SASP) plays an important role in the pathophysiological role of senescent cells (Wyld et al., 2020; Özsoy Gökbilen et al., 2022). However, the signaling pathway of senescence is also a key effector of radiotherapy and chemotherapy injury, which may lead to reduced recovery in patients receiving anticancer therapy and may result in cancer recurrence. On the other hand, growing evidence indicates that senescent cells may induce proliferative pathology in cancer, moreover, SASP factors may also trigger epithelial-mesenchymal transition (EMT) in premalignant epithelial cells (Liu and Hornsby, 2007; Kuilman et al., 2008). Therefore, the use of senolytic agents to remove senescent cells may play a key role in preventing cancer recurrence.
It was found that quercetin can induce senescence in cancer cells. In quercetin-treated T24 bladder cancer cells, an increase in the percentage of nuclei during cellular senescence was observed by nuclear morphometric analysis (NMA) (Adami et al., 2021). Furthermore, the immunoreactivity of Lamin B1, p16, and cyclin B1, markers of cellular senescence, was also increased in Colo-320 and Colo-741 cells after treatment with quercetin (Özsoy et al., 2020). Pan et al. found that the mechanism by which quercetin promotes cellular senescence may be via inhibition of the Ras/MAPK/ERK signaling pathway in a dose-dependent manner (Pan et al., 2015). In another study, treating C6 and U87 cells with quercetin for 4 consecutive days elevated levels of cellular senescence markers, furthermore senescence-associated cell morphological changes such as flattening, increased particle size, and cell enlargement could be observed. The researchers also found that histone deacetylase (HDAC) played a key role in quercetin-induced senescence. HDAC inhibitors significantly enhanced quercetin-induced senescence in human and rat glioma cell lines (Vargas et al., 2014). Quercetin triggers cellular senescence probably by increasing the expression of tumor suppressor gene p53 and cell cycle protein-dependent kinase (CDK) and cyclin B1 inhibitors p21, p27, thus inducing cell cycle arrest in G1 and G2/M phases (Tang et al., 2017; Özsoy Gökbilen et al., 2022). A study in 2013 showed that quercetin treatment for 18 h increased Hela cell density in the G2/M phase of the cell cycle, reflecting cell cycle arrest at this stage (Bishayee et al., 2013). Moreover, Liu et al. confirmed the same finding in different cell lines. After quercetin treatment for 24 h, the number of U251 glioblastoma cells in the sub-G2/M phase increased, indicating that quercetin induced G2/M phase arrest and thus inhibited U251 cell proliferation (Liu, Tang, et al., 2017). Cantanzaro et al. designed a study to investigate the factors by which quercetin affects the G1/S and G2/M cell cycles. The results showed that quercetin treatment on SKOV3 and U2OSPt cells for 48 h significantly altered the cell cycle distribution, possibly affecting the G1/S and G2/M phases of the cell cycle by decreasing the levels of cyclin D1 and cyclin B1 (Catanzaro et al., 2015).
Quercetin is usually not as powerful as targeted senolytics agents, but quercetin in combination with other senolytic agents can be more effective in removing senescent cells. Zhu Y et al. first reported a senolytic cocktail (Dasatinib combined with quercetin) that effectively killed senescent cells with the help of small interfering RNA (Zhu et al., 2015). Subsequently, in two open-label Phase I pilot studies, researchers demonstrated for the first time that senolytic agent (Dasatinib combined with quercetin) significantly reduces human senescent cell burden and provided preliminary evidence that senolytic agents may alleviate physical dysfunction in patients with idiopathic pulmonary fibrosis (IPF) (Hickson et al., 2019; Justice et al., 2019). Notably, some chemotherapeutic agents that exert their antitumor effects via the induction of cancer cell senescence also trigger cellular senescence in normal cells, which drives the development of a malignant phenotype in residual living tumor cells (Demaria et al., 2017; Bruni et al., 2019). Quercetin has been demonstrated to reduce chemotherapeutic drug-induced aging in combination with anticancer drugs. Recent studies have established that quercetin pre-treatment can prevent doxorubicin-induced senescence in normal cells by reducing the number of senescent cells and the production of SASP factors (Bientinesi et al., 2022). Furthermore, quercetin pre-treatment may also protect normal cells from doxorubicin treatment-induced ROS damage, by increasing cellular antioxidant defense. In another study, a quercetin derivative (quercetin-3-O-β-D-glucuronide) also showed a good inhibitory effect on cellular senescence in doxorubicin-treated HDFs and HUVECs cells (Yang et al., 2014). Considering that cancer cells may use senescence as an escape strategy from cancer treatment, the use of quercetin may selectively remove spontaneous cancer cells previously induced by chemotherapy and/or radiotherapy. Therefore, the use of some phytochemicals as senolytic agents or protectors, such as quercetin, is probably useful for overcoming tumor resistance (Figure 3).
FIGURE 3. Modulation of senescence and mitotic catastrophe in cancer cells by quercetin (By Figdraw).
3.3 Quercetin and mitotic catastrophe
Mitotic catastrophe is another critical non-apoptotic mechanism of cancer cells, defined by the Nomenclature Committee on Cell Death (NCCD) in 2012, which usually occurs after massive damage to DNA following ROS generation (Galluzzi et al., 2012). Cells undergoing mitotic catastrophe are often in partnership with other mechanisms of cell death, such as autophagy, senescence, or, necroptosis (Eom et al., 2005; Zhao W. et al., 2022b; Egorshina et al., 2022). Recent studies have found that the therapeutic efficiency of anticancer modalities such as radiotherapy and chemotherapy can be reinforced by stimulating mitotic catastrophe, which is also a promising way to overcome multidrug resistance (Pérès et al., 2015; Bai et al., 2017). In addition, ionizing radiation (IR) can also trigger immune cell mitotic catastrophe (Adjemian et al., 2020). Hence, the stimulation of mitotic catastrophe constitutes a new direction for tumor therapy. However, very few studies have evaluated the effects of quercetin on mitotic mutations, and the available studies have only found that quercetin induced mitotic mutations, but precisely little has been done to characterize exactly what signals are involved. Jackson et al. characterized the effect of different concentrations of quercetin on mitotic mutagenesis in Hepa1c1c7 cells. The results demonstrated that aberrant mitotic images were observed at concentrations as low as 0.01 μM quercetin, showing disproportionate DNA segregation (Jackson et al., 2016). Sufficient evidence documented that such mitotic abnormalities may eventually lead to mitotic catastrophes (Zhu et al., 2005). Thus, it appears that utilizing lower doses of quercetin to trigger mitotic catastrophes would significantly limit the side effects of the administration of large doses of quercetin anti-tumor. In another study, to investigate whether quercetin can induce growth inhibition in A549 cells by altering the cell cycle, image cytometric analysis of cellular DNA content was used to assess the effect of quercetin on cell cycle distribution. It was found that quercetin treatment dose-dependently resulted in a decrease in cell cycle distribution in G0/G1-phase accompanied by an increase in cell cycle distribution in G2/M-phase in A549 cells (Kobayashi et al., 2008). Moreover, the researchers attribute the failure of cytokinesis to quercetin-induced mitotic catastrophe, with monopolar spindle formation caused by perturbation of mitotic microtubules as a possible mechanism. However, the mechanism of quercetin in regulating mitotic catastrophe needs further elucidation (Figure 3).
3.4 Quercetin and ferroptosis
Ferroptosis, a novel mode of cell death first introduced in 2012, depends on intracellular iron and differ from apoptosis, necroptosis, and autophagy in morphology and biochemistry (Dixon et al., 2012; Lei et al., 2022). Ferroptosis is mainly characterized by iron ion accumulation and ROS-induced lipid peroxidation, which morphologically induces marked mitochondrial contraction, increased membrane density, and reduction or disappearance of mitochondrial cristae (Xie et al., 2016; Yang and Stockwell, 2016). The important role of ferroptosis in oxidative stress, iron metabolism, inflammation, and amino acid metabolism has been convincingly established (Galaris et al., 2019; Sun et al., 2020; Yang J. et al., 2022b). Correspondingly, ferroptosis involves multiple physiological and pathological processes, including hematological disorders, ischemia-reperfusion injury, renal injury, and particularly tumor inhibition (Mou et al., 2019; Chen et al., 2021; Li et al., 2021; Zhao et al., 2021).
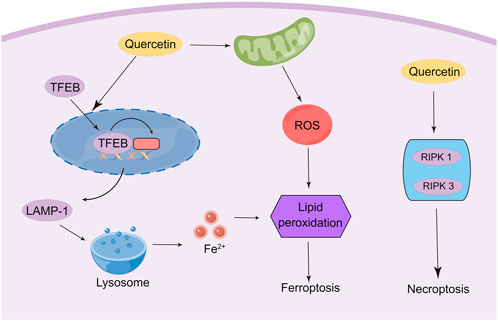
Recently, it has been found that quercetin exerts anti-tumor effects via triggering ferroptosis to induce cancer cell death. The activation of ferroptosis involves multiple signaling pathways. Recent research has identified a new ferroptosis-inducing pathway stimulated by autophagy, namely autophagy-dependent ferroptosis, which is selective autophagy that contributes to ferroptosis (Pierzynowska et al., 2021). During autophagy, the macromolecules in need of degradation are engulfed by phagophores, followed by acid hydrolase digestion of their contents by the lysosome. Transcription factor EB (TFEB) is the master gene for lysosomal biogenesis and autophagy (Settembre et al., 2011; Medina et al., 2015). These findings enlighten us that lysosomal storage diseases (LSD) and related molecular pathogenesis may involve the regulation of ferroptosis. Wang et al. found that the induction of cell death by quercetin could be reversed by lysosomal inhibitors and knockdown of the TFEB, which indicated the involvement of lysosomes in quercetin-induced cell death (Wang Z. X. et al., 2021b). In addition, quercetin promotes ferritin degradation, free iron release, and lipid peroxidation, which was induced by promoting nuclear TFEB and transcriptional activation of lysosomal genes that induce lysosomal activation and inducing ROS production. The synergistic effect of both together leads to iron death. Interestingly, another research also demonstrated that TFEB-mediated lysosomal activation plays an important role in quercetin-induced ferroptosis (An and Hu, 2022). In breast cancer cells, quercetin treatment induced the onset of ferroptosis by promoting TFEB expression and nuclear transcription. Further mechanistic studies showed that the degradation of ferritin and release of ferric ions were regulated by the lysosome-related gene LAMP-1, which was up-regulated due to the high expression of TFEB in the nucleus. Notably, quercetin exhibits a therapeutic effect in several non-tumor disease models due to its significant antioxidant activity characterized by reduced malondialdehyde (MDA) and lipid ROS levels and increased glutathione (GSH) levels, which contradicts the induction of ferroptosis in tumor cells (Wang Y. et al., 2021a; Jiang et al., 2022). ROS is a double-edged sword, and the heterogeneity of tumor cells and non-tumor cells leads to their different responses to ROS. Therefore, quercetin may exhibit opposite abilities in regulating ROS in different cells. Thus, the mechanisms and signaling pathways of quercetin regulation of ferroptosis for cancer treatment still need to be further elucidated (Figure 4).
3.5 Quercetin and necroptosis
As another important mechanism of cancer cell death, necroptosis was initially found to be an alternative to apoptosis following the involvement of death domain receptors (Degterev et al., 2005). Triggering necrosis in cancer cells is a promising way to avoid the failure of cancer chemotherapy due to apoptosis resistance by bypassing the apoptotic pathway to induce cancer cell death. As such, the molecular mechanisms of necroptosis have been well investigated, which depends critically on receptor-interacting serine-threonine kinase 1 (RIPK1), RIPK3, and mixed lineage kinase domain-like (MLKL), regardless of the upstream trigger (Hu et al., 2022; Yan et al., 2022). Currently, only limited experiments have shown the induction of necroptosis in cancer cells by quercetin. A study was conducted to observe the ultrastructural changes of quercetin on giant cell tumor of bone (GCTB) cells. The researchers demonstrated that, in addition to autophagy, quercetin treatment affected all histological components of necroptosis and secondary necroptosis by increasing the expression of RIPK1 (Estrada-Villaseñor et al., 2021). Another study reported that quercetin treatment of MCF-7 breast cancer cells depended on multiple cell death pathways, which mainly involve necroptosis (Khorsandi et al., 2017). This study indicated that quercetin induced necroptosis mainly through increasing the expression of RIPK1 and RIPK3. However, other studies indicated that quercetin treatment inhibits M1 macrophage/microglia polarization after spinal cord injury by inhibiting signal transducer and activator of transcription-1 (STAT1) and nuclear factor kappa-B (NF-κB) pathways, which ultimately results in partial alleviation of necrosis of Oligodendrocytes (Fan et al., 2019). The contradictory findings suggest that quercetin may possess the ability to specifically identify and kill tumor cells, which results in opposed effects in tumor cells and non-tumor cells. However, numerous types of research are still needed to confirm this conjecture (Figure 4).
3.6 Quercetin and pyroptosis
Pyroptosis, programmed cell death in the form of inflammation, is mediated by the gasdermin family (GSDMs) (Kovacs and Miao, 2017). In the canonical pathway of pyroptosis, certain inflammasomes drive cysteinyl aspartate specific proteinase-1 (Caspase-1) activation, leading to cleavage of gasdermin D (GSDMD) and activation of the inactive cytokines like interleukin-18 (IL-18) and interleukin-1beta (IL-1β), ultimately triggering pyroptosis (He et al., 2015; Schneider et al., 2017). Recent evidence suggests that pyroptosis induces a strong inflammatory response and shows a strong tumor regression effect (Fang et al., 2020). Currently, quercetin has not been reported to possess anti-tumor effects through the regulation of pyroptosis. However, it has been investigated that the specific role of quercetin in breast cancer-related depression (BCRD), in which the inhibition of pyroptosis and promotion of immune response are the main mechanisms for effectively mitigating the progression of BCRD (Zhu et al., 2022). In this study, quercetin partially reversed the pyroptosis on LPS-cultured 4T1 cells in vitro, as evidenced markedly by upregulating the card structural domain, NLR family pyrin structural domain (NLRP3), and Caspase-1. In addition, quercetin also promoted an anti-tumor immune response in xenograft mice. Quercetin treatment significantly upregulated the levels of interferon-gamma (IFN-γ), interleukin-10 (IL-10), and interleukin-2 (IL-2) in BCRD Mice. However, the modulation pathways of pyroptosis, especially the immunological effects, remain to be further elucidated after the treatment of cancer with quercetin.
4 Summary and future perspective
In summary, this paper reviews the progress of pharmacological research on the non-apoptotic cell death modes induced by quercetin in cancer cells in the last decade. Various cancer cell death modes are interrelated, and regulators of different death modes may crosstalk each other, causing shifts between modes and even accelerating or alleviating tumor cell death. The crosstalk between these cell death mechanisms is complex. For example, apoptosis, autophagy and necrosis are interrelated. Autophagy may promote or antagonize apoptosis through multiple mechanisms, as many regulators, including the mTOR kinase pathway, Beclin 1, caspases, and p53, are involved in both autophagy and apoptosis (Nikoletopoulou et al., 2013; Gali-Muhtasib et al., 2015). Furthermore, a genetic relationship exists between autophagy and aging. On the one hand, the aging program controls the activation of autophagy, on the other hand, different types of autophagy regulation can act through either an anti- or pro-senescence mechanism (Kang and Elledge, 2016). Most anti-cancer drugs exert their efficacy in killing cancer cells mainly by inducing apoptosis, however, apoptosis-resistant cancer cells are often present in the advanced stages of tumor formation and metastasis. Fortunately, some plant-derived components may induce the death of cancer cells resistant to apoptotic stimuli through other non-apoptotic mechanisms. Emerging evidence indicates that quercetin indirectly kills cancer cells by promoting the modes of apoptosis, mitotic catastrophe, senescence, ferroptosis, necroptosis, and pyroptosis. Correspondingly, the induction of each non-apoptotic cancer cell death mode by quercetin is dependent on the up- or down-regulation of some survival-related mediators, such as mTOR, AKT, p53, p21, p15, p27, RIPK1, RIPK3, NLRP3, etc. In addition to exerting anticancer effects through the regulation of epigenetics, the sensitivity of tumor cells to chemotherapeutic agents could be enhanced synergistically by quercetin (Bądziul et al., 2014; Kedhari Sundaram et al., 2019; Zhai et al., 2021). Surprisingly, the experiments conducted by Kovacovicova et al. suggested that the combination of dasatinib and quercetin did not synergistically increase the antitumor efficacy of adriamycin or remove adriamycin-induced HCC senescent cells, and even dasatinib + quercetin alone shown acute pro-tumorigenic effects (Kovacovicova et al., 2018). Given the comprehensive and complex pharmacological treatment strategy for oncology patients with underlying diseases, the use of quercetin needs to be carefully considered. Besides, several studies have found that the efficacy of quercetin for inducing cellular autophagy is strongly correlated with the concentration of administration, with the advantage of lower concentrations being more pronounced (Kim et al., 2013).
However, the current research still has some objective limitations, and many issues affecting the development of quercetin as a drug remain to be solved. Firstly, the low bioavailability, poor stability and weak tumor-targeted biodistribution of quercetin extremely limit its application as an anti-tumor drug. To address these challenges, researchers developed some strategies to increase the bioavailability of quercetin. As a carrier for quercetin, chitosan helps quercetin release into the target site in a sustained and controlled state through various epithelial systems, thus enhancing cellular uptake (Rashedi et al., 2019; Elsayed et al., 2021). Lipid nanoparticles are also effective in enhancing the bioavailability of quercetin (Lou et al., 2016; Ren et al., 2017; Chen et al., 2020). Hence, the development of novel carriers and overlays for quercetin to enhance its bioavailability and targeted tumor effect is a critical research direction in the future. Secondly, most of the currently available studies on the pharmacological effects of quercetin in the non-apoptotic mode of induction of cancer cells are dominated by animal and cellular experiments, whereas large-sample, multicenter randomized controlled clinical trials are still needed to explore its true efficacy on tumors, including side effects. Thirdly, considering that the appropriate transfer of drug doses from animal models to humans is important in the development of new drugs, we recommend that additional investigations must be conducted to determine the appropriate and most effective doses for human use. Last but not least, the effects of quercetin on cell death mechanisms such as mitotic catastrophe, ferroptosis, necroptosis, and pyroptosis need to be fully investigated in terms of network pharmacology and genomics.
Author contributions
DS and JW contributed to the conception of the review. HY and SX wrote the manuscript. DS and JW reviewed the entire draft. The rest of the authors edited the figures and tables. All authors reviewed the manuscript and agreed to be accountable for the content of the work.
Funding
The work was supported by the Young Talent Development Plan of Changzhou Health Commission (CZQM2020076, CZQM2022012), the Major Science and Technology Project of Changzhou Health Commission (ZD201911).
Acknowledgments
We also thank Figdraw (www.figdraw.com) for the assistance in creating diagram.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ATG, Autophagy protein; BCL-2, B-cell leukemia/lymphoma-2; CDK, Cycle protein-dependent kinase; EMT, Epithelial-mesenchymal transition; GSDMD, Gasdermin D; GSDMs, Gasdermin family; GC, Gastric cancer; GCTB, Giant cell tumor of bone; GSH, Glutathione; HCC, Hepatocellular carcinoma; HDAC, Histone deacetylase; IPF, Idiopathic pulmonary fibrosis; IFN-γ, Interferon-gamma; IL-1β, Interleukin-1beta; IL-2, Interleukin-2; IL-10, Interleukin-10; IL-18, Interleukin-18; IR, Ionizing radiation; JAK2, Janus kinase 2; MDA, Malondialdehyde; mTOR, Mammalian target of rapamycin protein; MLKL, Mixed lineage kinase domain-like; NF-κB, Nuclear factor kappa-B; NLRP3, NLR family pyrin structural domain; NMA, nuclear morphometric analysis; OC, Ovarian cancer; PI3K, Phosphatidylinositol-3-kinase; PCD, Programmed cell death; Akt, Protein kinase B; ROS, Reactive oxygen species; RIPK1, Receptor-interacting serine-threonine kinase 1; SASP, Senescence-associated secretory phenotype; STAT1, Signal transducer and activator of transcription-1; STAT3, Signal transducer and activator of transcription 3; TFEB, Transcription factor EB; TNF, Tumor necrosis factor.
References
Adami, B. S., Diz, F. M., Oliveira Gonçalves, G. P., Reghelin, C. K., Scherer, M., Dutra, A. P., et al. (2021). Morphological and mechanical changes induced by quercetin in human T24 bladder cancer cells. Micron 151, 103152. doi:10.1016/j.micron.2021.103152
Adjemian, S., Oltean, T., Martens, S., Wiernicki, B., Goossens, V., Vanden Berghe, T., et al. (2020). Ionizing radiation results in a mixture of cellular outcomes including mitotic catastrophe, senescence, methuosis, and iron-dependent cell death. Cell Death Dis. 11 (11), 1003. doi:10.1038/s41419-020-03209-y
Amaravadi, R. K., Kimmelman, A. C., and Debnath, J. (2019). Targeting autophagy in cancer: Recent advances and future directions. Cancer Discov. 9 (9), 1167–1181. doi:10.1158/2159-8290.cd-19-0292
An, S., and Hu, M. (2022). Quercetin promotes TFEB nuclear translocation and activates lysosomal degradation of ferritin to induce ferroptosis in breast cancer cells. Comput. Intell. Neurosci. 2022, 5299218. doi:10.1155/2022/5299218
Andres, S., Pevny, S., Ziegenhagen, R., Bakhiya, N., Schäfer, B., Hirsch-Ernst, K. I., et al. (2018). Safety aspects of the use of quercetin as a dietary supplement. Mol. Nutr. Food Res. 62 (1), 1700447. doi:10.1002/mnfr.201700447
Bądziul, D., Jakubowicz-Gil, J., Paduch, R., Głowniak, K., and Gawron, A. (2014). Combined treatment with quercetin and imperatorin as a potent strategy for killing HeLa and Hep-2 cells. Mol. Cell. Biochem. 392 (1-2), 213–227. doi:10.1007/s11010-014-2032-4
Bai, Z., Gao, M., Zhang, H., Guan, Q., Xu, J., Li, Y., et al. (2017). BZML, a novel colchicine binding site inhibitor, overcomes multidrug resistance in A549/Taxol cells by inhibiting P-gp function and inducing mitotic catastrophe. Cancer Lett. 402, 81–92. doi:10.1016/j.canlet.2017.05.016
Baskar, R., Lee, K. A., Yeo, R., and Yeoh, K. W. (2012). Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 9 (3), 193–199. doi:10.7150/ijms.3635
Batiha, G. E., Beshbishy, A. M., Ikram, M., Mulla, Z. S., El-Hack, M. E. A., Taha, A. E., et al. (2020). The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods 9 (3), 374. doi:10.3390/foods9030374
Bernadotte, A., Mikhelson, V. M., and Spivak, I. M. (2016). Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY) 8 (1), 3–11. doi:10.18632/aging.100871
Bi, Y., Shen, C., Li, C., Liu, Y., Gao, D., Shi, C., et al. (2016). Inhibition of autophagy induced by quercetin at a late stage enhances cytotoxic effects on glioma cells. Tumour Biol. 37 (3), 3549–3560. doi:10.1007/s13277-015-4125-4
Bientinesi, E., Lulli, M., Becatti, M., Ristori, S., Margheri, F., and Monti, D. (2022). Doxorubicin-induced senescence in normal fibroblasts promotes in vitro tumour cell growth and invasiveness: The role of Quercetin in modulating these processes. Mech. Ageing Dev. 206, 111689. doi:10.1016/j.mad.2022.111689
Bishayee, K., Ghosh, S., Mukherjee, A., Sadhukhan, R., Mondal, J., and Khuda-Bukhsh, A. R. (2013). Quercetin induces cytochrome-c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: Signal cascade and drug-DNA interaction. Cell Prolif. 46 (2), 153–163. doi:10.1111/cpr.12017
Bristol, M. L., Emery, S. M., Maycotte, P., Thorburn, A., Chakradeo, S., and Gewirtz, D. A. (2013). Autophagy inhibition for chemosensitization and radiosensitization in cancer: Do the preclinical data support this therapeutic strategy? J. Pharmacol. Exp. Ther. 344 (3), 544–552. doi:10.1124/jpet.112.199802
Bruni, E., Cazzetta, V., Donadon, M., Cimino, M., Torzilli, G., Spata, G., et al. (2019). Chemotherapy accelerates immune-senescence and functional impairments of Vδ2(pos) T cells in elderly patients affected by liver metastatic colorectal cancer. J. Immunother. Cancer 7 (1), 347. doi:10.1186/s40425-019-0825-4
Calcinotto, A., Kohli, J., Zagato, E., Pellegrini, L., Demaria, M., and Alimonti, A. (2019). Cellular senescence: Aging, cancer, and injury. Physiol. Rev. 99 (2), 1047–1078. doi:10.1152/physrev.00020.2018
Calgarotto, A. K., Maso, V., Junior, G. C. F., Nowill, A. E., Filho, P. L., Vassallo, J., et al. (2018). Antitumor activities of Quercetin and Green Tea in xenografts of human leukemia HL60 cells. Sci. Rep. 8 (1), 3459. doi:10.1038/s41598-018-21516-5
Cao, L., Zhao, S., Yang, Q., Shi, Z., Liu, J., Pan, T., et al. (2021). Chidamide combined with doxorubicin induced p53-driven cell cycle arrest and cell apoptosis reverse multidrug resistance of breast cancer. Front. Oncol. 11, 614458. doi:10.3389/fonc.2021.614458
Carneiro, B. A., and El-Deiry, W. S. (2020). Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 17 (7), 395–417. doi:10.1038/s41571-020-0341-y
Catanzaro, D., Ragazzi, E., Vianello, C., Caparrotta, L., and Montopoli, M. (2015). Effect of quercetin on cell cycle and cyclin expression in ovarian carcinoma and osteosarcoma cell lines. Nat. Prod. Commun. 10 (8), 1934578X1501000–1368. doi:10.1177/1934578x1501000813
Chakraborty, J., Pakrashi, S., Sarbajna, A., Dutta, M., and Bandyopadhyay, J. (2022). Quercetin attenuates copper-induced apoptotic cell death and endoplasmic reticulum stress in SH-SY5Y cells by autophagic modulation. Biol. Trace Elem. Res. 200, 5022–5041. doi:10.1007/s12011-022-03093-x
Chen, S. Q., Wang, C., Song, Y. Q., Tao, S., Yu, F. Y., Lou, H. Y., et al. (2020). Quercetin covalently linked lipid nanoparticles: Multifaceted killing effect on tumor cells. ACS Omega 5 (46), 30274–30281. doi:10.1021/acsomega.0c04795
Chen, Y., Fan, H., Wang, S., Tang, G., Zhai, C., and Shen, L. (2021). Ferroptosis: A novel therapeutic target for ischemia-reperfusion injury. Front. Cell Dev. Biol. 9, 688605. doi:10.3389/fcell.2021.688605
Collado, M., Blasco, M. A., and Serrano, M. (2007). Cellular senescence in cancer and aging. Cell 130 (2), 223–233. doi:10.1016/j.cell.2007.07.003
Collado, M., and Serrano, M. (2006). The power and the promise of oncogene-induced senescence markers. Nat. Rev. Cancer 6 (6), 472–476. doi:10.1038/nrc1884
Cushnie, T. P., and Lamb, A. J. (2005). Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 26 (5), 343–356. doi:10.1016/j.ijantimicag.2005.09.002
de Boer, V. C., Dihal, A. A., van der Woude, H., Arts, I. C., Wolffram, S., Alink, G. M., et al. (2005). Tissue distribution of quercetin in rats and pigs. J. Nutr. 135 (7), 1718–1725. doi:10.1093/jn/135.7.1718
Degterev, A., Huang, Z., Boyce, M., Li, Y., Jagtap, P., Mizushima, N., et al. (2005). Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1 (2), 112–119. doi:10.1038/nchembio711
Demaria, M., O'Leary, M. N., Chang, J., Shao, L., Liu, S., Alimirah, F., et al. (2017). Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7 (2), 165–176. doi:10.1158/2159-8290.cd-16-0241
Denisenko, T. V., Sorokina, I. V., Gogvadze, V., and Zhivotovsky, B. (2016). Mitotic catastrophe and cancer drug resistance: A link that must to be broken. Drug resist. updat. 24, 1–12. doi:10.1016/j.drup.2015.11.002
Di Petrillo, A., Orrù, G., Fais, A., and Fantini, M. C. (2022). Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 36 (1), 266–278. doi:10.1002/ptr.7309
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 149 (5), 1060–1072. doi:10.1016/j.cell.2012.03.042
Du, F., Feng, Y., Fang, J., and Yang, M. (2015). MicroRNA-143 enhances chemosensitivity of Quercetin through autophagy inhibition via target GABARAPL1 in gastric cancer cells. Biomed. Pharmacother. 74, 169–177. doi:10.1016/j.biopha.2015.08.005
Egert, S., Bosy-Westphal, A., Seiberl, J., Kürbitz, C., Settler, U., Plachta-Danielzik, S., et al. (2009). Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 102 (7), 1065–1074. doi:10.1017/s0007114509359127
Egorshina, A. Y., Zamaraev, A. V., Kaminskyy, V. O., Radygina, T. V., Zhivotovsky, B., and Kopeina, G. S. (2022). Necroptosis as a novel facet of mitotic catastrophe. Int. J. Mol. Sci. 23 (7), 3733. doi:10.3390/ijms23073733
Elsayed, A. M., Sherif, N. M., Hassan, N. S., Althobaiti, F., Hanafy, N. A. N., and Sahyon, H. A. (2021). Novel quercetin encapsulated chitosan functionalized copper oxide nanoparticles as anti-breast cancer agent via regulating p53 in rat model. Int. J. Biol. Macromol. 185, 134–152. doi:10.1016/j.ijbiomac.2021.06.085
Eom, Y. W., Kim, M. A., Park, S. S., Goo, M. J., Kwon, H. J., Sohn, S., et al. (2005). Two distinct modes of cell death induced by doxorubicin: Apoptosis and cell death through mitotic catastrophe accompanied by senescence-like phenotype. Oncogene 24 (30), 4765–4777. doi:10.1038/sj.onc.1208627
Estrada-Villaseñor, E., Delgado-Cedillo, A., Hernández-Pérez, A., Meneses, A., Olivos Meza, A., Hidalgo-Bravo, A., et al. (2021). Ultrastructural changes in giant cell tumor of bone cultured cells exposed to quercetin. Ultrastruct. Pathol. 45 (6), 335–345. doi:10.1080/01913123.2021.1979704
Fan, H., Tang, H. B., Shan, L. Q., Liu, S. C., Huang, D. G., Chen, X., et al. (2019). Quercetin prevents necroptosis of oligodendrocytes by inhibiting macrophages/microglia polarization to M1 phenotype after spinal cord injury in rats. J. Neuroinflammation 16 (1), 206. doi:10.1186/s12974-019-1613-2
Fang, Y., Tian, S., Pan, Y., Li, W., Wang, Q., Tang, Y., et al. (2020). Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 121, 109595. doi:10.1016/j.biopha.2019.109595
Farhat, M., Poissonnier, A., Hamze, A., Ouk-Martin, C., Brion, J. D., Alami, M., et al. (2014). Reversion of apoptotic resistance of TP53-mutated Burkitt lymphoma B-cells to spindle poisons by exogenous activation of JNK and p38 MAP kinases. Cell Death Dis. 5 (5), e1201. doi:10.1038/cddis.2014.150
Fox, J. L., and Storey, A. (2015). BMX negatively regulates BAK function, thereby increasing apoptotic resistance to chemotherapeutic drugs. Cancer Res. 75 (7), 1345–1355. doi:10.1158/0008-5472.can-14-1340
Furue, H. (2003). Chemotherapy cancer treatment during the past sixty years. Gan Kagaku Ryoho. 30 (10), 1404–1411.
Galaris, D., Barbouti, A., and Pantopoulos, K. (2019). Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta. Mol. Cell Res. 1866 (12), 118535. doi:10.1016/j.bbamcr.2019.118535
Gali-Muhtasib, H., Hmadi, R., Kareh, M., Tohme, R., and Darwiche, N. (2015). Cell death mechanisms of plant-derived anticancer drugs: Beyond apoptosis. Apoptosis 20 (12), 1531–1562. doi:10.1007/s10495-015-1169-2
Galluzzi, L., Vitale, I., Aaronson, S. A., Abrams, J. M., Adam, D., Agostinis, P., et al. (2018). Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 25 (3), 486–541. doi:10.1038/s41418-017-0012-4
Galluzzi, L., Vitale, I., Abrams, J. M., Alnemri, E. S., Baehrecke, E. H., Blagosklonny, M. V., et al. (2012). Molecular definitions of cell death subroutines: Recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 19 (1), 107–120. doi:10.1038/cdd.2011.96
Gewirtz, D. A. (2016). The challenge of developing autophagy inhibition as a therapeutic strategy. Cancer Res. 76 (19), 5610–5614. doi:10.1158/0008-5472.can-16-0722
Gewirtz, D. A. (2014). The four faces of autophagy: Implications for cancer therapy. Cancer Res. 74 (3), 647–651. doi:10.1158/0008-5472.can-13-2966
Granato, M., Rizzello, C., Gilardini Montani, M. S., Cuomo, L., Vitillo, M., Santarelli, R., et al. (2017). Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways. J. Nutr. Biochem. 41, 124–136. doi:10.1016/j.jnutbio.2016.12.011
Granato, M., Rizzello, C., Romeo, M. A., Yadav, S., Santarelli, R., D'Orazi, G., et al. (2016). Concomitant reduction of c-Myc expression and PI3K/AKT/mTOR signaling by quercetin induces a strong cytotoxic effect against Burkitt's lymphoma. Int. J. Biochem. Cell Biol. 79, 393–400. doi:10.1016/j.biocel.2016.09.006
Guglielmone, H. A., Agnese, A. M., Nuñez-Montoya, S. C., Cabrera, J. L., and Cuadra, G. R. (2020). Antithrombotic "in vivo" effects of quercetin 3, 7, 3', 4'-tetrasulfate isolated from Flaveria bidentis in an experimental thrombosis model in mice. Thromb. Res. 195, 190–192. doi:10.1016/j.thromres.2020.07.040
Guo, H., Ding, H., Tang, X., Liang, M., Li, S., Zhang, J., et al. (2021). Quercetin induces pro-apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thorac. Cancer 12 (9), 1415–1422. doi:10.1111/1759-7714.13925
Guo, Y., and Bruno, R. S. (2015). Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 26 (3), 201–210. doi:10.1016/j.jnutbio.2014.10.008
Hashemzaei, M., Delarami Far, A., Yari, A., Heravi, R. E., Tabrizian, K., Taghdisi, S. M., et al. (2017). Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 38 (2), 819–828. doi:10.3892/or.2017.5766
He, W. T., Wan, H., Hu, L., Chen, P., Wang, X., Huang, Z., et al. (2015). Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 25 (12), 1285–1298. doi:10.1038/cr.2015.139
Hickson, L. J., Langhi Prata, L. G. P., Bobart, S. A., Evans, T. K., Giorgadze, N., Hashmi, S. K., et al. (2019). Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456. doi:10.1016/j.ebiom.2019.08.069
Hu, B., Gao, J., Shi, J., Zhang, F., Shi, C., Wen, P., et al. (2022). Necroptosis throws novel insights on patient classification and treatment strategies for hepatocellular carcinoma. Front. Immunol. 13, 970117. doi:10.3389/fimmu.2022.970117
Jackson, S. J., Singletary, K. W., Murphy, L. L., Venema, R. C., and Young, A. J. (2016). Phytonutrients differentially stimulate NAD(P)H:quinone oxidoreductase, inhibit proliferation, and trigger mitotic catastrophe in Hepa1c1c7 cells. J. Med. Food 19 (1), 47–53. doi:10.1089/jmf.2015.0079
Ji, Y., Li, L., Ma, Y. X., Li, W. T., Li, L., Zhu, H. Z., et al. (2019). Quercetin inhibits growth of hepatocellular carcinoma by apoptosis induction in part via autophagy stimulation in mice. J. Nutr. Biochem. 69, 108–119. doi:10.1016/j.jnutbio.2019.03.018
Jia, L., Huang, S., Yin, X., Zan, Y., Guo, Y., and Han, L. (2018). Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci. 208, 123–130. doi:10.1016/j.lfs.2018.07.027
Jiang, J. J., Zhang, G. F., Zheng, J. Y., Sun, J. H., and Ding, S. B. (2022). Targeting mitochondrial ROS-mediated ferroptosis by quercetin alleviates high-fat diet-induced hepatic lipotoxicity. Front. Pharmacol. 13, 876550. doi:10.3389/fphar.2022.876550
Jochems, F., Thijssen, B., De Conti, G., Jansen, R., Pogacar, Z., Groot, K., et al. (2021). The cancer SENESCopedia: A delineation of cancer cell senescence. Cell Rep. 36 (4), 109441. doi:10.1016/j.celrep.2021.109441
Justice, J. N., Nambiar, A. M., Tchkonia, T., LeBrasseur, N. K., Pascual, R., Hashmi, S. K., et al. (2019). Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563. doi:10.1016/j.ebiom.2018.12.052
Kang, C., and Elledge, S. J. (2016). How autophagy both activates and inhibits cellular senescence. Autophagy 12 (5), 898–899. doi:10.1080/15548627.2015.1121361
Kapoor, I., Bodo, J., Hill, B. T., Hsi, E. D., and Almasan, A. (2020). Targeting BCL-2 in B-cell malignancies and overcoming therapeutic resistance. Cell Death Dis. 11 (11), 941. doi:10.1038/s41419-020-03144-y
Kedhari Sundaram, M., Hussain, A., Haque, S., Raina, R., and Afroze, N. (2019). Quercetin modifies 5'CpG promoter methylation and reactivates various tumor suppressor genes by modulating epigenetic marks in human cervical cancer cells. J. Cell. Biochem. 120 (10), 18357–18369. doi:10.1002/jcb.29147
Khan, F., Niaz, K., Maqbool, F., Ismail Hassan, F., Abdollahi, M., Nagulapalli Venkata, K. C., et al. (2016). Molecular targets underlying the anticancer effects of quercetin: An update. Nutrients 8 (9), E529. doi:10.3390/nu8090529
Khorsandi, L., Orazizadeh, M., Niazvand, F., Abbaspour, M. R., Mansouri, E., and Khodadadi, A. (2017). Quercetin induces apoptosis and necroptosis in MCF-7 breast cancer cells. Bratisl. Lek. Listy 118 (2), 123–128. doi:10.4149/bll_2017_025
Kim, H., Moon, J. Y., Ahn, K. S., and Cho, S. K. (2013). Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxid. Med. Cell. Longev. 2013, 596496. doi:10.1155/2013/596496
Kim, J., Kundu, M., Viollet, B., and Guan, K. L. (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13 (2), 132–141. doi:10.1038/ncb2152
Kim, Y. C., and Guan, K. L. (2015). mTOR: a pharmacologic target for autophagy regulation. J. Clin. Invest. 125 (1), 25–32. doi:10.1172/jci73939
Kimmelman, A. C., and White, E. (2017). Autophagy and tumor metabolism. Cell Metab. 25 (5), 1037–1043. doi:10.1016/j.cmet.2017.04.004
Klimaszewska-Wiśniewska, A., Hałas-Wiśniewska, M., Izdebska, M., Gagat, M., Grzanka, A., and Grzanka, D. (2017). Antiproliferative and antimetastatic action of quercetin on A549 non-small cell lung cancer cells through its effect on the cytoskeleton. Acta Histochemica 119 (2), 99–112. doi:10.1016/j.acthis.2016.11.003
Kobayashi, S., Tanabe, S., Sugiyama, M., and Konishi, Y. (2008). Transepithelial transport of hesperetin and hesperidin in intestinal Caco-2 cell monolayers. Biochim. Biophys. Acta 1778 (1), 33–41. doi:10.1016/j.bbamem.2007.08.020
Kovacovicova, K., Skolnaja, M., Heinmaa, M., Mistrik, M., Pata, P., Pata, I., et al. (2018). Senolytic cocktail Dasatinib+Quercetin (D+Q) does not enhance the efficacy of senescence-inducing chemotherapy in liver cancer. Front. Oncol. 8, 459. doi:10.3389/fonc.2018.00459
Kovacs, S. B., and Miao, E. A. (2017). Gasdermins: Effectors of pyroptosis. Trends Cell Biol. 27 (9), 673–684. doi:10.1016/j.tcb.2017.05.005
Kuilman, T., Michaloglou, C., Vredeveld, L. C., Douma, S., van Doorn, R., Desmet, C. J., et al. (2008). Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133 (6), 1019–1031. doi:10.1016/j.cell.2008.03.039
Lan, C. Y., Chen, S. Y., Kuo, C. W., Lu, C. C., and Yen, G. C. (2019). Quercetin facilitates cell death and chemosensitivity through RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J. Food Drug Anal. 27 (4), 887–896. doi:10.1016/j.jfda.2019.07.001
Lei, G., Zhuang, L., and Gan, B. (2022). Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 22 (7), 381–396. doi:10.1038/s41568-022-00459-0
Levy, J. M. M., Towers, C. G., and Thorburn, A. (2017). Targeting autophagy in cancer. Nat. Rev. Cancer 17 (9), 528–542. doi:10.1038/nrc.2017.53
Li, D., Liu, B., Fan, Y., Liu, M., Han, B., Meng, Y., et al. (2021). Nuciferine protects against folic acid-induced acute kidney injury by inhibiting ferroptosis. Br. J. Pharmacol. 178 (5), 1182–1199. doi:10.1111/bph.15364
Li, J., Tang, C., Li, L., Li, R., and Fan, Y. (2016a). Quercetin blocks t-AUCB-induced autophagy by Hsp27 and Atg7 inhibition in glioblastoma cells in vitro. J. Neurooncol. 129 (1), 39–45. doi:10.1007/s11060-016-2149-2
Li, X., He, S., and Ma, B. (2020). Autophagy and autophagy-related proteins in cancer. Mol. Cancer 19 (1), 12. doi:10.1186/s12943-020-1138-4
Li, Y. J., Lei, Y. H., Yao, N., Wang, C. R., Hu, N., Ye, W. C., et al. (2017). Autophagy and multidrug resistance in cancer. Chin. J. Cancer 36 (1), 52. doi:10.1186/s40880-017-0219-2
Li, Y., Yao, J., Han, C., Yang, J., Chaudhry, M. T., Wang, S., et al. (2016b). Quercetin, inflammation and immunity. Nutrients 8 (3), 167. doi:10.3390/nu8030167
Liao, X., Zhuang, X., Liang, C., Li, J., Flaumenhaft, R., Yuan, C., et al. (2022). Flavonoids as protein disulfide isomerase inhibitors: Key molecular and structural features for the interaction. J. Agric. Food Chem. 70 (14), 4475–4483. doi:10.1021/acs.jafc.1c07994
Liu, D., and Hornsby, P. J. (2007). Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 67 (7), 3117–3126. doi:10.1158/0008-5472.can-06-3452
Liu, L., Zhang, H., Sun, L., Gao, Y., Jin, H., Liang, S., et al. (2010). ERK/MAPK activation involves hypoxia-induced MGr1-Ag/37LRP expression and contributes to apoptosis resistance in gastric cancer. Int. J. Cancer 127 (4), 820–829. doi:10.1002/ijc.25098
Liu, Y., Gong, W., Yang, Z. Y., Zhou, X. S., Gong, C., Zhang, T. R., et al. (2017a). Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis 22 (4), 544–557. doi:10.1007/s10495-016-1334-2
Liu, Y., Tang, Z. G., Lin, Y., Qu, X. G., Lv, W., Wang, G. B., et al. (2017b). Effects of quercetin on proliferation and migration of human glioblastoma U251 cells. Biomed. Pharmacother. 92, 33–38. doi:10.1016/j.biopha.2017.05.044
Los, M., Herr, I., Friesen, C., Fulda, S., Schulze-Osthoff, K., and Debatin, K. M. (1997). Cross-resistance of CD95- and drug-induced apoptosis as a consequence of deficient activation of caspases (ICE/Ced-3 proteases). Blood 90 (8), 3118–3129. doi:10.1182/blood.v90.8.3118
Lou, M., Zhang, L. N., Ji, P. G., Feng, F. Q., Liu, J. H., Yang, C., et al. (2016). Quercetin nanoparticles induced autophagy and apoptosis through AKT/ERK/Caspase-3 signaling pathway in human neuroglioma cells: In vitro and in vivo. Biomed. Pharmacother. 84, 1–9. doi:10.1016/j.biopha.2016.08.055
Lu, N. T., Crespi, C. M., Liu, N. M., Vu, J. Q., Ahmadieh, Y., Wu, S., et al. (2016). A phase I dose escalation study demonstrates quercetin safety and explores potential for bioflavonoid antivirals in patients with chronic hepatitis C. Phytother. Res. 30 (1), 160–168. doi:10.1002/ptr.5518
Lukášová, E., Kovařík, A., and Kozubek, S. (2018). Consequences of Lamin B1 and Lamin B receptor downregulation in senescence. Cells 7 (2), E11. doi:10.3390/cells7020011
Ma, C., Wang, F., Han, B., Zhong, X., Si, F., Ye, J., et al. (2018). SALL1 functions as a tumor suppressor in breast cancer by regulating cancer cell senescence and metastasis through the NuRD complex. Mol. Cancer 17 (1), 78. doi:10.1186/s12943-018-0824-y
Medina, D. L., Di Paola, S., Peluso, I., Armani, A., De Stefani, D., Venditti, R., et al. (2015). Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 17 (3), 288–299. doi:10.1038/ncb3114
Moon, J. H., Eo, S. K., Lee, J. H., and Park, S. Y. (2015). Quercetin-induced autophagy flux enhances TRAIL-mediated tumor cell death. Oncol. Rep. 34 (1), 375–381. doi:10.3892/or.2015.3991
Mou, Y., Wang, J., Wu, J., He, D., Zhang, C., Duan, C., et al. (2019). Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 12 (1), 34. doi:10.1186/s13045-019-0720-y
Muñoz-Espín, D., and Serrano, M. (2014). Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 15 (7), 482–496. doi:10.1038/nrm3823
Murray, M. E., Gavile, C. M., Nair, J. R., Koorella, C., Carlson, L. M., Buac, D., et al. (2014). CD28-mediated pro-survival signaling induces chemotherapeutic resistance in multiple myeloma. Blood 123 (24), 3770–3779. doi:10.1182/blood-2013-10-530964
Nabavi, S. F., Nabavi, S. M., Mirzaei, M., and Moghaddam, A. H. (2012). Protective effect of quercetin against sodium fluoride induced oxidative stress in rat's heart. Food Funct. 3 (4), 437–441. doi:10.1039/c2fo10264a
Nikoletopoulou, V., Markaki, M., Palikaras, K., and Tavernarakis, N. (2013). Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 1833 (12), 3448–3459. doi:10.1016/j.bbamcr.2013.06.001
Özsoy Gökbilen, S., Becer, E., and Vatansever, H. S. (2022). Senescence-mediated anticancer effects of quercetin. Nutr. Res. 104, 82–90. doi:10.1016/j.nutres.2022.04.007
Özsoy, S., Becer, E., Kabadayı, H., Vatansever, H. S., and Yücecan, S. (2020). Quercetin-mediated apoptosis and cellular senescence in human colon cancer. Anticancer. Agents Med. Chem. 20 (11), 1387–1396. doi:10.2174/1871520620666200408082026
Pan, H. C., Jiang, Q., Yu, Y., Mei, J. P., Cui, Y. K., and Zhao, W. J. (2015). Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. Neurochem. Int. 80, 60–71. doi:10.1016/j.neuint.2014.12.001
Patel, R. V., Mistry, B. M., Shinde, S. K., Syed, R., Singh, V., and Shin, H. S. (2018). Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 155, 889–904. doi:10.1016/j.ejmech.2018.06.053
Pérès, E. A., Gérault, A. N., Valable, S., Roussel, S., Toutain, J., Divoux, D., et al. (2015). Silencing erythropoietin receptor on glioma cells reinforces efficacy of temozolomide and X-rays through senescence and mitotic catastrophe. Oncotarget 6 (4), 2101–2119. doi:10.18632/oncotarget.2937
Pierzynowska, K., Rintz, E., Gaffke, L., and Węgrzyn, G. (2021). Ferroptosis and its modulation by autophagy in light of the pathogenesis of lysosomal storage diseases. Cells 10 (2), 365. doi:10.3390/cells10020365
Radspieler, M. M., Schindeldecker, M., Stenzel, P., Försch, S., Tagscherer, K. E., Herpel, E., et al. (2019). Lamin-B1 is a senescence-associated biomarker in clear-cell renal cell carcinoma. Oncol. Lett. 18 (3), 2654–2660. doi:10.3892/ol.2019.10593
Rashedi, J., Ghorbanihaghjo, A., Asgharzadeh, M., and Baradaran, B. (2019). Chitosan and quercetin: Potential hand in hand encountering tumors in oral delivery system. Curr. Pharm. Des. 25 (28), 3074–3086. doi:10.2174/1381612825666190829144508
Rauf, A., Imran, M., Khan, I. A., Ur-Rehman, M., Gilani, S. A., Mehmood, Z., et al. (2018). Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 32 (11), 2109–2130. doi:10.1002/ptr.6155
Ren, K. W., Li, Y. H., Wu, G., Ren, J. Z., Lu, H. B., Li, Z. M., et al. (2017). Quercetin nanoparticles display antitumor activity via proliferation inhibition and apoptosis induction in liver cancer cells. Int. J. Oncol. 50 (4), 1299–1311. doi:10.3892/ijo.2017.3886
Reyes-Avendaño, I., Reyes-Jiménez, E., González-García, K., Pérez-Figueroa, D. C., Baltiérrez-Hoyos, R., Tapia-Pastrana, G., et al. (2022). Quercetin regulates key components of the cellular microenvironment during early hepatocarcinogenesis. Antioxidants (Basel) 11 (2), 358. doi:10.3390/antiox11020358
Safi, A., Heidarian, E., and Ahmadi, R. (2021). Quercetin synergistically enhances the anticancer efficacy of docetaxel through induction of apoptosis and modulation of PI3K/AKT, MAPK/ERK, and JAK/STAT3 signaling pathways in MDA-MB-231 breast cancer cell line. Int. J. Mol. Cell. Med. 10 (1), 11–22. doi:10.22088/ijmcm.bums.10.1.11
Schneider, K. S., Groß, C. J., Dreier, R. F., Saller, B. S., Mishra, R., Gorka, O., et al. (2017). The inflammasome drives GSDMD-independent secondary pyroptosis and IL-1 release in the absence of caspase-1 protease activity. Cell Rep. 21 (13), 3846–3859. doi:10.1016/j.celrep.2017.12.018
Settembre, C., Di Malta, C., Polito, V. A., Garcia Arencibia, M., Vetrini, F., Erdin, S., et al. (2011). TFEB links autophagy to lysosomal biogenesis. Science 332 (6036), 1429–1433. doi:10.1126/science.1204592
Sharma, K., Goehe, R. W., Di, X., Hicks, M. A., Torti, S. V., Torti, F. M., et al. (2014). A novel cytostatic form of autophagy in sensitization of non-small cell lung cancer cells to radiation by vitamin D and the vitamin D analog, EB 1089. Autophagy 10 (12), 2346–2361. doi:10.4161/15548627.2014.993283
Shi, G. J., Li, Y., Cao, Q. H., Wu, H. X., Tang, X. Y., Gao, X. H., et al. (2019). In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomed. Pharmacother. 109, 1085–1099. doi:10.1016/j.biopha.2018.10.130
Su, D., Wang, W., Wu, X., Li, M., Yan, X., Hua, Z., et al. (2020). Meriolin1 induces cell cycle arrest, apoptosis, autophagy and targeting the Akt/MAPKs pathways in human neuroblastoma SH-SY5Y cells. J. Pharm. Pharmacol. 72 (4), 561–574. doi:10.1111/jphp.13224
Su, Z., Yang, Z., Xu, Y., Chen, Y., and Yu, Q. (2015). Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 14, 48. doi:10.1186/s12943-015-0321-5
Sui, X., Chen, R., Wang, Z., Huang, Z., Kong, N., Zhang, M., et al. (2013). Autophagy and chemotherapy resistance: A promising therapeutic target for cancer treatment. Cell Death Dis. 4 (10), e838. doi:10.1038/cddis.2013.350
Sun, M., Liu, D., Yuan, Y., Dan, J., Jia, S., Luo, Y., et al. (2022). Indole hydrazide compound IHZ-1 induces apoptosis and autophagy via activation of ROS/JNK pathway in hepatocellular carcinoma. Front. Oncol. 12, 811747. doi:10.3389/fonc.2022.811747
Sun, Y., Chen, P., Zhai, B., Zhang, M., Xiang, Y., Fang, J., et al. (2020). The emerging role of ferroptosis in inflammation. Biomed. Pharmacother. 127, 110108. doi:10.1016/j.biopha.2020.110108
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tang, Q., Ji, F., Wang, J., Guo, L., Li, Y., and Bao, Y. (2017). Quercetin exerts synergetic anti-cancer activity with 10-hydroxy camptothecin. Eur. J. Pharm. Sci. 109, 223–232. doi:10.1016/j.ejps.2017.08.013
Tang, S. M., Deng, X. T., Zhou, J., Li, Q. P., Ge, X. X., and Miao, L. (2020). Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 121, 109604. doi:10.1016/j.biopha.2019.109604
Terao, J., Murota, K., and Kawai, Y. (2011). Conjugated quercetin glucuronides as bioactive metabolites and precursors of aglycone in vivo. Food Funct. 2 (1), 11–17. doi:10.1039/c0fo00106f
Ulasov, I., Fares, J., Timashev, P., and Lesniak, M. S. (2020). Editing cytoprotective autophagy in glioma: An unfulfilled potential for therapy. Trends Mol. Med. 26 (3), 252–262. doi:10.1016/j.molmed.2019.11.001
Vanneman, M., and Dranoff, G. (2012). Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 12 (4), 237–251. doi:10.1038/nrc3237
Vargas, J. E., Filippi-Chiela, E. C., Suhre, T., Kipper, F. C., Bonatto, D., and Lenz, G. (2014). Inhibition of HDAC increases the senescence induced by natural polyphenols in glioma cells. Biochem. Cell Biol. 92 (4), 297–304. doi:10.1139/bcb-2014-0022
Walle, T., Walle, U. K., and Halushka, P. V. (2001). Carbon dioxide is the major metabolite of quercetin in humans. J. Nutr. 131 (10), 2648–2652. doi:10.1093/jn/131.10.2648
Wang, S., Yao, J., Zhou, B., Yang, J., Chaudry, M. T., Wang, M., et al. (2018). Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot. 81 (1), 68–78. doi:10.4315/0362-028x.jfp-17-214
Wang, Y., Quan, F., Cao, Q., Lin, Y., Yue, C., Bi, R., et al. (2021a). Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 28, 231–243. doi:10.1016/j.jare.2020.07.007
Wang, Y., Zhang, W., Lv, Q., Zhang, J., and Zhu, D. (2016). The critical role of quercetin in autophagy and apoptosis in HeLa cells. Tumour Biol. 37 (1), 925–929. doi:10.1007/s13277-015-3890-4
Wang, Z. X., Ma, J., Li, X. Y., Wu, Y., Shi, H., Chen, Y., et al. (2021b). Quercetin induces p53-independent cancer cell death through lysosome activation by the transcription factor EB and Reactive Oxygen Species-dependent ferroptosis. Br. J. Pharmacol. 178 (5), 1133–1148. doi:10.1111/bph.15350
White, E. (2015). The role for autophagy in cancer. J. Clin. Invest. 125 (1), 42–46. doi:10.1172/jci73941
Williams, C. A., and Grayer, R. J. (2004). Anthocyanins and other flavonoids. Nat. Prod. Rep. 21 (4), 539–573. doi:10.1039/b311404j
Wu, B., Zeng, W., Ouyang, W., Xu, Q., Chen, J., Wang, B., et al. (2020). Quercetin induced NUPR1-dependent autophagic cell death by disturbing reactive oxygen species homeostasis in osteosarcoma cells. J. Clin. Biochem. Nutr. 67 (2), 137–145. doi:10.3164/jcbn.19-121
Wu, L., Li, J., Liu, T., Li, S., Feng, J., Yu, Q., et al. (2019). Quercetin shows anti-tumor effect in hepatocellular carcinoma LM3 cells by abrogating JAK2/STAT3 signaling pathway. Cancer Med. 8 (10), 4806–4820. doi:10.1002/cam4.2388
Wyld, L., Bellantuono, I., Tchkonia, T., Morgan, J., Turner, O., Foss, F., et al. (2020). Senescence and cancer: A review of clinical implications of senescence and senotherapies. Cancers (Basel) 12 (8), E2134. doi:10.3390/cancers12082134
Xie, Y., Hou, W., Song, X., Yu, Y., Huang, J., Sun, X., et al. (2016). Ferroptosis: Process and function. Cell Death Differ. 23 (3), 369–379. doi:10.1038/cdd.2015.158
Xu, J., Elshazly, A. M., and Gewirtz, D. A. (2022). The cytoprotective, cytotoxic and nonprotective functional forms of autophagy induced by microtubule poisons in tumor cells-implications for autophagy modulation as a therapeutic strategy. Biomedicines 10 (7), 1632. doi:10.3390/biomedicines10071632
Yan, J., Wan, P., Choksi, S., and Liu, Z. G. (2022). Necroptosis and tumor progression. Trends Cancer 8 (1), 21–27. doi:10.1016/j.trecan.2021.09.003
Yang, D., Wang, T., Long, M., and Li, P. (2020). Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxid. Med. Cell. Longev. 2020, 8825387. doi:10.1155/2020/8825387
Yang, H. H., Hwangbo, K., Zheng, M. S., Cho, J. H., Son, J. K., Kim, H. Y., et al. (2014). Quercetin-3-O-β-D-glucuronide isolated from Polygonum aviculare inhibits cellular senescence in human primary cells. Arch. Pharm. Res. 37 (9), 1219–1233. doi:10.1007/s12272-014-0344-2
Yang, H., Wang, Y., Xu, S., Ren, J., Tang, L., Gong, J., et al. (2022a). Hesperetin, a promising treatment option for diabetes and related complications: A literature review. J. Agric. Food Chem. 70 (28), 8582–8592. doi:10.1021/acs.jafc.2c03257
Yang, H., Yang, T., Heng, C., Zhou, Y., Jiang, Z., Qian, X., et al. (2019). Quercetin improves nonalcoholic fatty liver by ameliorating inflammation, oxidative stress, and lipid metabolism in db/db mice. Phytother. Res. 33 (12), 3140–3152. doi:10.1002/ptr.6486
Yang, J., Dai, X., Xu, H., Tang, Q., and Bi, F. (2022b). Regulation of ferroptosis by amino acid metabolism in cancer. Int. J. Biol. Sci. 18 (4), 1695–1705. doi:10.7150/ijbs.64982
Yang, W. S., and Stockwell, B. R. (2016). Ferroptosis: Death by lipid peroxidation. Trends Cell Biol. 26 (3), 165–176. doi:10.1016/j.tcb.2015.10.014
Yin, H., Ma, J., Han, J., Li, M., and Shang, J. (2019). Pharmacokinetic comparison of quercetin, isoquercitrin, and quercetin-3-O-β-D-glucuronide in rats by HPLC-MS. PeerJ 7, e6665. doi:10.7717/peerj.6665
Zhai, K., Mazurakova, A., Koklesova, L., Kubatka, P., and Büsselberg, D. (2021). Flavonoids synergistically enhance the anti-glioblastoma effects of chemotherapeutic drugs. Biomolecules 11 (12), 1841. doi:10.3390/biom11121841
Zhang, L., Ma, J., Yang, F., Li, S., Ma, W., Chang, X., et al. (2022). Neuroprotective effects of quercetin on ischemic stroke: A literature review. Front. Pharmacol. 13, 854249. doi:10.3389/fphar.2022.854249
Zhao, L., Zhao, H., Zhao, Y., Sui, M., Liu, J., Li, P., et al. (2022a). Role of ginseng, quercetin, and tea in enhancing chemotherapeutic efficacy of colorectal cancer. Front. Med. (Lausanne) 9, 939424. doi:10.3389/fmed.2022.939424
Zhao, W., Wang, Q., Li, L., Xie, C., Wu, Y., Gautam, M., et al. (2022b). SIRT1 regulates mitotic catastrophe via autophagy and BubR1 signaling. Mol. Cell. Biochem. doi:10.1007/s11010-022-04470-9
Zhao, Y., Huang, Z., and Peng, H. (2021). Molecular mechanisms of ferroptosis and its roles in hematologic malignancies. Front. Oncol. 11, 743006. doi:10.3389/fonc.2021.743006
Zhu, J., Beattie, E. C., Yang, Y., Wang, H. J., Seo, J. Y., and Yang, L. X. (2005). Centrosome impairments and consequent cytokinesis defects are possible mechanisms of taxane drugs. Anticancer Res. 25 (3b), 1919–1925.
Zhu, Q., Yang, L., Yang, H., Han, Y., Chen, Y., and He, Y. (2022). Quercetin alleviates the progression of breast cancer-related depression via inhibiting the pyroptosis and promoting the immune response. Mediat. Inflamm. 2022, 8011988. doi:10.1155/2022/8011988
Keywords: quercetin, non-apoptotic cancer cell death, autophagy, senescence, mitotic catastrophe
Citation: Yang H, Xu S, Tang L, Gong J, Fang H, Wei J and Su D (2022) Targeting of non-apoptotic cancer cell death mechanisms by quercetin: Implications in cancer therapy. Front. Pharmacol. 13:1043056. doi: 10.3389/fphar.2022.1043056
Received: 13 September 2022; Accepted: 07 November 2022;
Published: 16 November 2022.
Edited by:
Jingwen Xu, Guangdong Pharmaceutical University, ChinaReviewed by:
Stefania Moccia, National Research Council (CNR), ItalyJun Yin, China Pharmaceutical University, China
Copyright © 2022 Yang, Xu, Tang, Gong, Fang, Wei and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jifu Wei, weijifu@njmu.edu.cn; Dan Su, bjj4461@163.com
†These authors have contributed equally to this work
 Hao Yang
Hao Yang Shan Xu1†
Shan Xu1† Jinhong Gong
Jinhong Gong Jifu Wei
Jifu Wei