- 1College of Pharmacy, Chengdu University of Traditional Chinese Medicine, Sichuan, China
- 2College of Ethnomedicine, Chengdu University of Traditional Chinese Medicine, Sichuan, China
Dengzhanxixin (DZXX), the dried whole plant of Erigeron breviscapus (Vaniot) Hand.-Mazz., belonging to Compositae and first published in Materia Medica of South Yunnan by Lan Mao in the Ming Dynasty (1368 AD–1644 AD), is included in Medicinal Materials and Decoction Pieces of the 2020 edition of the Pharmacopeia of the People’s Republic of China. Its main chemical components are flavonoids that mainly include flavonoid, flavonols, dihydroflavones, flavonol glycosides, flavonoid glycosides, coffee acyl compounds, and other substances, such as volatile oil compounds, coumarins, aromatic acids, pentacyclic terpenoids, phytosterols, and xanthones. Among them, scutellarin and 1,5-dicoffeoylquininic acid are the main active components of DZXX. DZXX has pharmacological effects, such as improving cerebral and cerebrovascular ischemia, increasing blood flow, inhibiting platelet aggregation, promoting antithrombotic formation, improving microcirculation, reducing blood viscosity, protecting optic nerves, exhibiting anti-inflammatory properties, scavenging free radicals, and eliciting antioxidant activities. It is widely used in the treatment of cardiovascular and cerebrovascular ischemic diseases, kidney diseases, liver diseases, diabetic complications, and glaucoma. Pharmacokinetic studies have shown that the active components of DZXX have a low bioavailability and a high elimination rate in vivo. Nevertheless, its utilization can be improved through liposome preparation and combination with other drugs. Acute and subacute toxicity studies have shown that DZXX is a safe medicinal material widely used in clinical settings. However, its target and drug action mechanism are unclear because of the complexity of its composition. In this paper, the clinical application and pharmacological toxicology of DZXX are reviewed to provide a reference for further studying its active components and action mechanism.
Introduction
Dengzhanxixin (DZXX), the dried grass of Erigeron breviscapus (Vaniot) Hand.-Mazz. belonging to Compositae, is a perennial herb, 5–50 cm tall, with woody rhizomes and thick rhizomes. The stem is upright, with a few branches in the middle, and the whole plant is covered with multicellular short bristles or mixed with glandular hairs. The leaves are mainly concentrated at the base and are in the shape of a rosette. The leaves are obovate-lanceolate or wide-spoon-shaped. The base is half embracing, and the upper part is often reduced to a small bracteole with no petiole. The flower head is solitary at the top of the stem or branch, and the involucre is hemispherical. DZXX is mainly distributed in Yunnan, Guangxi, Guizhou, and other places. It is a characteristic medicinal material of Yunnan, and is listed as the four major cardio-cerebral vascular medicines along with ginkgo, panax notoginseng and salvia. DZXX is commonly used in Miao nationality, Yi, Tibetan, Dai, and other ethnic groups and was first published in the Ming Dynasty Lanmao’s Materia Medica of South Yunnan (about the 14th to 15th century). It has been used to treat ischemic cerebrovascular diseases, such as paralysis; it is included in the “Medicinal Materials and Decoction Pieces” of the 2020 edition of the Pharmacy of the People’s Republic of China. In the 1930s, the whole herb of the Yunnan Miao herb DZXX was used to treat stroke and hemiplegia. In the 1960s, under the impetus of the Chinese medicine movement, Luo San of Yunnan Miao Medicine cut the whole plant during the flowering period of DZXX at an altitude of 1,000–1,400 m, mixed with eggs and water and steamed for 15 min before taking it. The effect of treating cerebral palsy is remarkable. Since it was introduced to the world, it has been extensively applied and studied. It has officially entered the ranks of national legal medicinal materials, and a series of unilateral Chinese medicine preparations has been developed, used as raw materials, and applied in clinical practice. In 1994, DZXX was listed as a high-technology product of the National Torch Program and became an essential Chinese medicine for emergency treatment in Chinese medicine hospitals across the country. In 2000, it was listed as a national protected variety of Chinese medicine and prescription drug (Yang, 2014). Such drugs are administered to treat diseases such as hemiplegia, coronary heart disease, cerebral thrombosis, rheumatism, and microcirculation disorders, and they have a wide range of treatment, a definite curative effect, and minimal side effects. Especially for the treatment of cardiovascular and cerebrovascular diseases and their sequelae, the curative effect is more obvious, the therapeutic effect is more than 95% (Tao, 2016).
Since the inclusion of the “Yunnan Pharmaceutical Standards” in 1997, according to incomplete statistics, the annual purchase of DZXX, which is widely used as a raw material in the pharmaceutical industry in Yunnan Province, has reached more than 1 million kg, mainly from wild DZXX resources. The total amounts of privately collected wild resources were 300, 400, 1,000, 1,100, 1,200, and 1,000 tons in 1995, 1996, 1997, 1998, 1999, and 2000, respectively. The drug product based on the total flavonoids of DZXX requires about 1.5 million kg of raw materials. For breviscapine tablets, breviscapine injection, and other patented medicines based on breviscapine, the demand for raw materials is about 1.2 million kg. DZXX injection produced on the basis of phenolic acid compounds, such as scutellarin and total caffeic acid esters, requires about 400,000 kg of raw materials for medicinal materials, and the total demand is about 3.1 million kg. The domestic market demand for DZXX continues to increase by 15–20% per year. At present, the annual amount of wild DZXX available in Yunnan Province does not exceed 500,000 kg. With several years of collection, wild DZXX resources have become scarce (Zhang et al., 2013; Tao, 2016). At present, the wild resources of DZXX have been exhausted, and artificial cultivation of DZXX has become the only way to maintain the sustainable development of the medicine industry of DZXX. The survey shows that the stock of DZXX resources from Yunnan Province is only about 800–1,000 tons, and the supply can only meet 18.5% of the market demand (Wang N. et al., 2012). Now Yunnan has mastered the mature technology of planting breviscapine, and the planting scale of breviscapine in the province has reached 10,000 Mu (6.67 square kilometers). The output of medicinal materials reached 4,000 tons, from the serious gap in market demand to the resource demand that can guarantee the development of the breviscapine industry. At the same time, in view of the current artificial cultivation of mixed sources, poor stability, difficulty in seedling breeding, low seed production efficiency, lag in the development of high-quality and high-yield standardized planting technology, and serious pests and diseases, Yunnan Province has established a technical system for seed production and floating seedlings of DZXX and cultivate a professional planting technical team. Promote large-scale, standardized and industrialized production of Erigeron breviscapus.
The 2017 China Dengzhanhua Industry Development Report shows that in 2015, the sales of cardio-cerebrovascular Chinese patent medicines made from DZXX in Yunnan accounted for 1.71% of the national public medical institution cardio-cerebrovascular Chinese patent medicine market, compared with other categories such as Danhong injection, there is still a large market space. According to investigations and studies, the average treatment course of breviscapine-related preparations used in hospitals is 7–18 days. Most of them are used in elderly patients, mainly for patients with cerebral infarction and coronary heart disease and angina pectoris. At the same time, the medication indications and instructions are in line with the high rate. The dosages are all within the scope of the instructions, and the incidence of adverse events is less than 1%. A few adverse reactions occur mostly within half an hour. Most of them are immediate and mild, mainly middle-aged and elderly people, mainly manifested as skin allergies and headaches., Abdominal pain, the adverse reaction may be related to the use of super-indications and combined medication (Gao C. et al., 2007; Gao Z.et al., 2008; Ji et al., 2009; Li YY. et al., 2015; Xiang et al., 2018).
DZXX has become a common drug in the clinical treatment of cardiovascular and cerebrovascular related diseases. With the deepening of system, organ, and molecular levels, the scope of its clinical applications is expanding. This paper searches the published literature, using DZXX as key word; the search scope includes CNKI, Wanfang Database, Web of science database, Springer Link foreign language journal database and other databases. In this paper, the clinical application and pharmacological toxicology of DZXX and its related preparations are reviewed to clarify the main treatment-related mechanism of DZXX and its preparation and to provide ideas for further research.
Chemical Composition and Preparation of DZXX
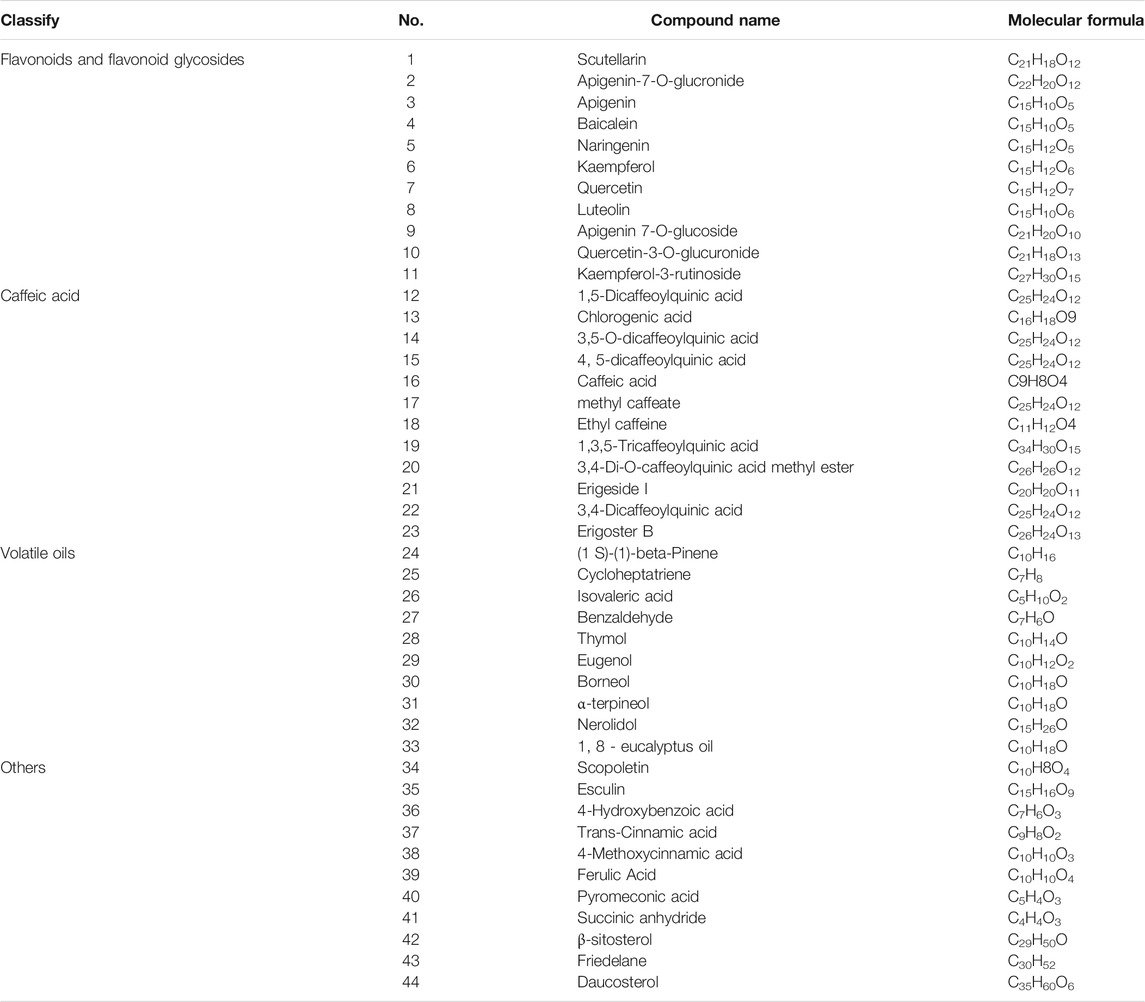
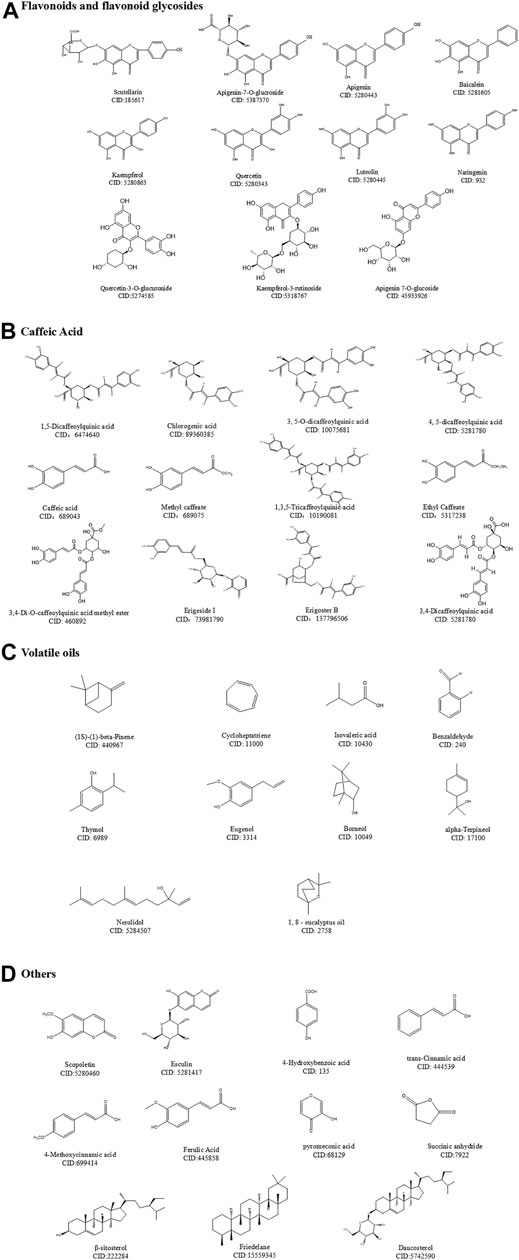
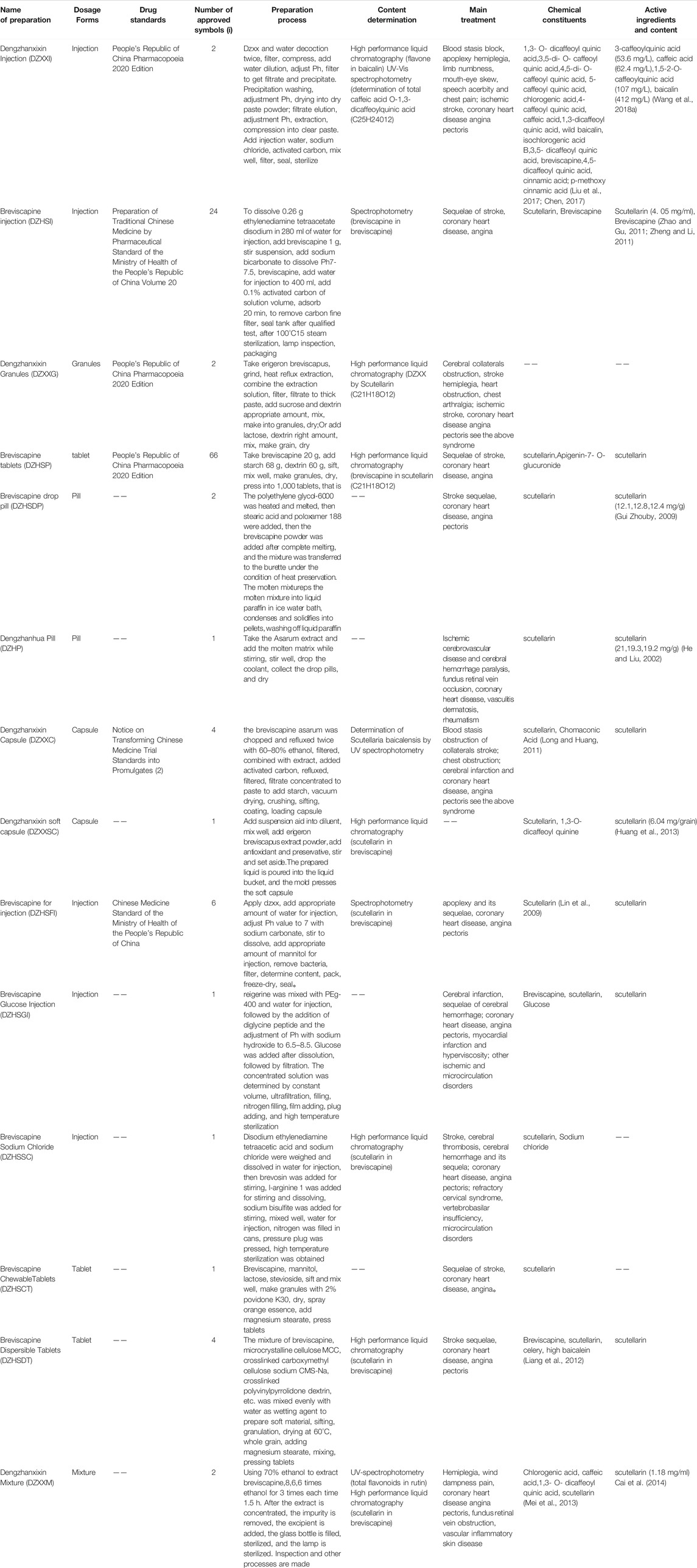
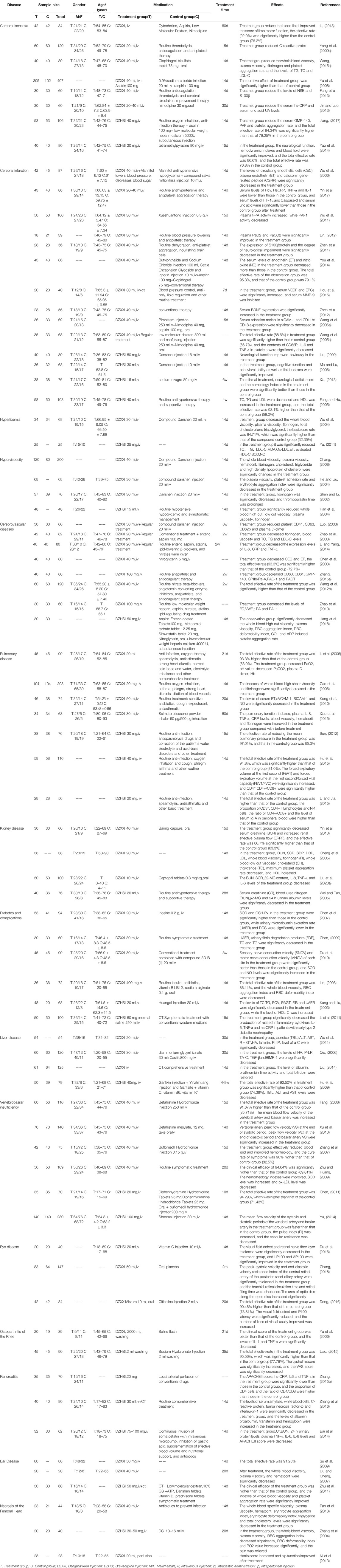
At present, 25 kinds of flavonoids, 46 kinds of caffeoyl compounds, 78 kinds of volatile oils, and nearly 40 kinds of other compounds, including coumarin, pentacyclic triterpenes, aromatic acids, phytosterols, and oxythracrone, are isolated from DZXX (Zhang et al., 2000a; Zhang et al., 2000b; Guo et al., 2019). Table 1 and Figure 1 below show some familiar structural formulas. The chemical structure painted by ChemDraw Software.
Flavonoids and Flavonoid Glycosides
Flavonoids and their glycosides are the main components of DZXX, which are mainly composed of flavonoids, flavonols, dihydroflavonoids, flavonol glycosides and flavonoid glycosides. Among them, scutellarin is considered to be the main active ingredient in flavonoids, and it is also the most studied ingredient in DZXX (Guo et al., 2019). See the Table 1 and Figure 1A below for details.
Caffeic Acid
DZXX caffeoyl compounds mostly exist in the form of a combination of the nucleus and different numbers of caffeic acid, in the form of quinic acid (CQA), 2,7-anhydro-3-deoxy-2-octylpyrrolidone acid (CDOA), 2, 7-anhydro-2-octylpyrrolidone acid (COA), 1- (2'-γ-pyrone) four types as main. The caffeoylquinic acid compounds in DZXX are mostly, and the main active ingredient is 1,5-diCQA. Table 1 and Figure 1B below show some of the caffeoyl (Guo et al., 2019). See the Table and Figure below for details.
Volatile Oils
Volatile oil compounds are mostly long-chain fatty acids, cyclics, long-chain fatty alkanes and other compounds. Table 1 and Figure 1C below show some of the volatile oils (Guo et al., 2019). See the Table and Figure below for details.
Others
Dzxx also contains coumarins, aromatic acids, pentacyclic triterpenes, phytosterols, xanthones and other compounds. The following Table 1 and Figure 1D below show some of the other compounds (Zhang et al., 2000a; Zhang et al., 2000b; Guo et al., 2019). See the Table and Figure below for details.
DZXX has been developed into numerous formulations, involving injections, granules, tablets, dispersible tablets, capsules, mixtures, extracts, and dripping pills. The number of formulations is nearly 20 products. According to the State Food and Drug Administration (Medical Products Administration, 2020), currently marketed single-prescription DZXX preparations are mainly oral and injectable. Their raw materials include breviscapine (3 approval numbers) and DZXX extract (2 approval numbers), and most of them are preparations with scutellarin as raw materials, oral preparations include breviscapine tablets (DZHSP) (66 approval number), DZXX granules (2 approval number), breviscapine dispersion tablets (4 approval document numbers), breviscapine chewable tablets (1 approval document number), Dengzhanhua dripping pills (1 approval document number), breviscapine dripping pills (2 approval document numbers). Injection preparations are available DZXX injection (DZXXI) (2 approval document numbers), breviscapine injection (DZHSI) (24 approval document numbers), breviscapine for injection (6 approval document numbers), breviscapine sodium chloride injection (1 approval document), breviscapine glucose injection (1 approval number). There are less preparations using DZXX extract as raw materials, mainly oral DZXX mixture (2 approval number), DZXX capsules (4 approval document number), and DZXX soft capsule (1 approval document number).
DZXXI was approved by China Food and Drug Administration in 2005 and listed in the Chinese Pharmacopoeia. DZXX injection is a sterile aqueous solution (injection) made by extracting phenolic components from E. breviscapus. The analysis of HPLC charts and ultraviolet spectra shows that the main component of DZXX includes caffeoyl derivatives (or caffeic acid analogs) and contains a small amount of scutellarin and other flavonoids with a pH between 5.5 and 7.5 (Yang et al., 2005). Flavonoids are represented by scutellarin, which is recognized as the main active ingredient of DZXX injection. Phenolic acids include caffeic acid, chlorogenic acid, and dicaffeoylquinic acid series isomers (Zhang et al., 2002; Zhou L. et al., 2011). DZXX injection is prepared by water decoction, alcohol precipitation (80% ethanol concentration), ethyl acetate extraction, water solubility (pH 8–8.5), activated carbon treatment, and liquid preparation (Yang et al., 2005). In addition, the extraction rate of DZXX after pulverization increases by about 0.17 percentage points (a relative increase of about 15%); however, the whole herb is fed more often because of difficulty in filtrating the components and no difference in extracting from the whole herb or cutting into sections. Flavonoid glycosides in DZXX are more polar, and ethyl acetate cannot be used to completely extract flavonoid glycosides. A certain amount of ethanol can be added to adjust the polarity of extracts and improve the extraction rate (Tang et al., 2002). According to the first edition of the Pharmacopoeia of the People’s Republic of China in 2020, the quality standards of scutellarin for flavonoids and 1,3-O-dicaffeoyl quinic acid for total phenolic acids in DZXX injection are established. Each 1 ml contains flavonoids based on scutellarin (C21H18O12) and should be equivalent to 0.40–0.60 mg. The total caffeic acid content per milliliter is calculated as 1,3-O-dicaffeoylquinic acid (C25H24O12), which should be 2.0–3.0 mg.
Breviscapine injection is a sterile aqueous solution made of flavonoid gluconate glycosides extracted from the whole plant of DZXX and extracted flavonoids. The main component of this solution is scutellarin, a small amount of apigenin-7-O-β-D-glucuronide, and other flavonoid glycosides, but it has no caffeoyl derivatives; its pH is 6.3–8.3 (Tian et al., 2014). The drug is included in Volume 20 of the Prescriptions of Traditional Chinese Medicines of the Ministry of Health of the People’s Republic of China. The quality standard of breviscapine injection has been established on the basis of scutellarin. Its content is determined through spectrophotometry. Breviscapine is based on scutellarin, and it should be 95.0–105.0% of the amount of specimens. Studies have found that the pH of breviscapine injection is about 6.8. When Na2HPO4 is used as a cosolvent, the color, pH, and drug content of the drug solution become more stable. In addition, the combination of EDTA-2Na and NaHSO3 as an antioxidant is more conducive to improving the stability of the drug; when the amount of added sodium bisulfite is 0.2%, the color of the injection is light, and its stability is better (Wang et al., 2010).
Experimental studies have found that scutellarin is easily soluble in water, methanol, and ethanol below 90% concentration, slightly soluble in absolute ethanol and acetone, and hardly soluble in ethyl acetate. Most caffeoyl derivatives are easily soluble in water, ethanol, and water. Ethanol, methanol, and acetone are soluble in ethyl acetate but hardly soluble in chloroform. Other DZXX preparations, such as DZXX capsules, Dengzhanhua granules, and Yimaikang tablets, are all water extracts or 60–80% ethanol extracts, so they contain both flavonoids and caffeoyl derivatives (Yang et al., 2005). Some details about DZXX series preparations are presented in Table 2.
Clinical Applications
Cerebrovascular Diseases
Cerebral Ischemia
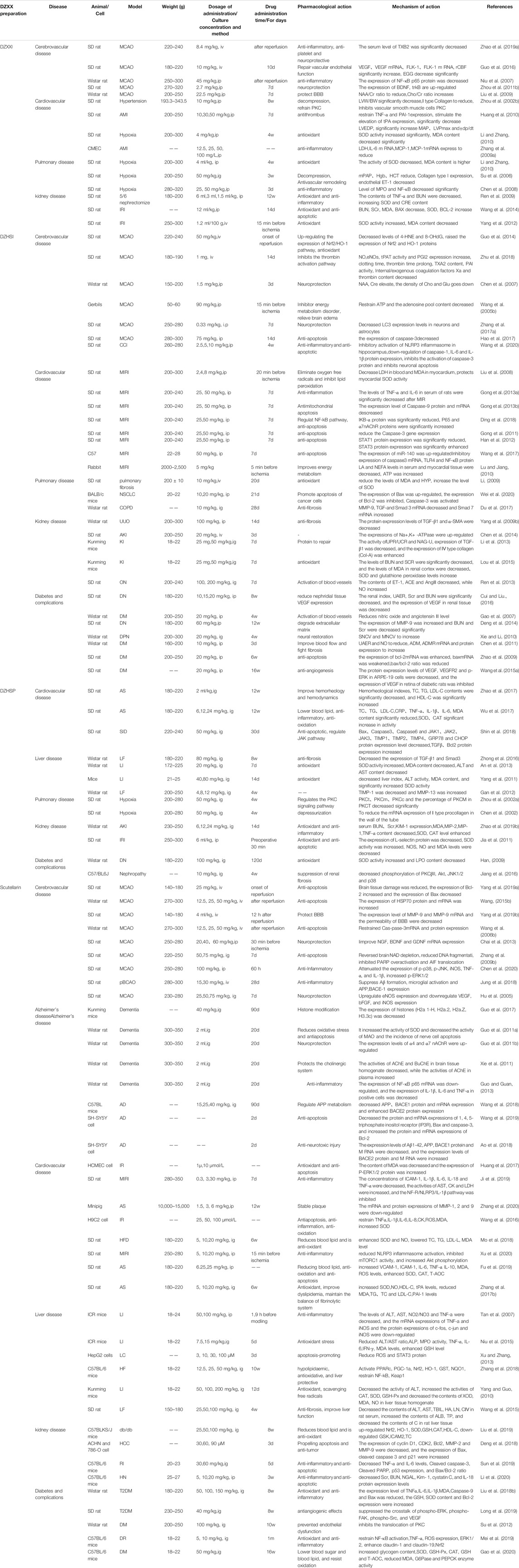
Li divided 84 patients with ischemic stroke into a control group and an observation group. The control group was treated with modern medical treatment and given citicoline, aspirin, low-molecular dextran, and nimodipine. The observation group was treated with DZXX injection. After 60 days, DZXX injection improved the patient’s blood lipid index and blood viscosity, and significantly improved the prognosis of stroke patients. (Wang and Guo, 2011; Li, 2018). Yang Nan divided 120 patients with initial ischemic stroke and recurrent ischemic stroke into a control group and a breviscapine injection treatment group. Their results reveal that DZXX injection can effectively reduce plasma C-reactive protein and inhibit its pro-inflammatory effects, indicating that breviscapine can promote the stability of cerebral atherosclerotic plaques through anti-inflammatory activities and improve the prognosis of stroke (Yang, 2009). In another clinical study, breviscapine injection reduced plasma alpha granule membrane protein (GMP-140), platelet activating factor (PAF) and platelet aggregation rate in patients with cerebral ischemia, and improved transient ischemic attack (TIA) patients with platelet activity index, the total effective rate of treatment reached 94.34%, and the total incidence of adverse events was lower than that of the conventional treatment group (Jiang, 2017). A large number of clinical data indicate that DZXX preparations can improve patients’ cerebral ischemia and improve neuromotor function by anti-inflammatory, anti-thrombotic, improving blood rheology, and protecting nerves. The detailed study of DZXX and its related preparations in the treatment of patients with cerebral ischemia is shown in Table 3.
Cerebral Infarction
Wu divided 87 patients with cerebral infarction into a control group and an observation group. The control group was treated with compound Danshen injection, and the observation group was treated with DZXXI. After 14 days, DZXXI reduced plasma nitric oxide (NO) and plasma circulating endothelial cells (CEC) and endothelin (ET) levels in patients with cerebral infarction, improved the vascular endothelial function of patients with cerebral infarction, increased blood flow, and markedly improved microcirculation and nerve function (Wu et al., 2006). Another study also found that DZXXI can increase the expression of vascular endothelial growth factor (VEGF), endothelial progenitor cells (EPCs), brain-derived neurotrophic factor (BDNF), reduce serum adhesion molecules and platelet inflammatory factors, promote angiogenesis, and antiplatelet Gather to improve cerebral infarction (Zhen et al., 2012; Hou et al., 2015). In addition, breviscapine injection can also reduce the plasma tissue-type plasminogen activator (t-PA) in patients with cerebral infarction, improve blood lipids, and improve cerebral infarction (Zhang et al., 2008). Clinical data show that DZXX preparations are more effective than conventional antihypertensive, lipid-lowering, anticoagulant and other traditional Chinese medicine treatments. DZXX preparations can improve the patient’s blood rheology and endothelial cell function, reduce blood lipids and inflammation, and improve the prognosis of patients with cerebral infarction. The detailed study of DZXX and its related preparations in the treatment of patients with cerebral infarction is shown in Table 3.
Hyperlipidemia
68 patients with hyperlipidemia-related cerebral infarction were randomly divided into DZXXI group and compound Danshen injection group, and intravenous drip. After 14 days, the basic cure rate in the DZXXI group was 64.71%, which was significantly higher than the 32.35% in the compound Danshen group (p < 0.01). After treatment, the whole blood specific viscosity, plasma specific viscosity, fibrinogen, total cholesterol, and three Acylglycerol is significantly lower than before treatment (p < 0.05) (Wu et al., 2004). In clinical treatments, breviscapine injection has obvious curative effects on hyperlipidemia. After treatment, patients’ TC, TG, LDL-C, MDA, oxidized low-density lipoprotein Ox-LDL, and plasma endothelin (ET) significantly decrease. By contrast HDL-C, SOD, and NO increase to varying degrees. DZHSI improves hyperlipidemia by scavenging oxygen free radicals, lowering blood lipids and other activities, enhancing the activity of antioxidant enzymes in patients with hyperlipidemia, reducing lipid peroxidation damage, and protecting patients’ vascular endothelial function (Yu, 2011). The detailed study of DZXX preparations in the treatment of patients with hyperlipidemia is shown in Table 3.
Hyperviscosity
DZXX injection (observation group) and compound Danshen injection (control group) were used to treat patients with hyperviscosity. After the treatment, the results of DZXX injection reveal that whole blood viscosity, plasma viscosity, hematocrit, fibrinogen, cholesterol, triglyceride, and high-density lipoprotein cholesterol levels significantly improve (p < 0.05). The results of the study showed that the observation of HCT, Fib, TC, TG and HDL-C in observation group were significantly improved without adverse reactions compared with before treatment and after treatment (Chang, 2008). Therefore, DZXX injection can prevent and treat hyperviscosity by improving blood rheology, reducing fibrinogen, enhancing blood lipids, and prolonging the time of partial thromboplastin activity (Shen and Li, 2002). After 48 hospitalized patients with hyperviscosity were treated with DZHSI, the whole blood, high-shear viscosity, low-shear viscosity, plasma viscosity, fibrinogen, and other factors are significantly lower than the initial values (p < 0.01). This result indicates that the treatment can reduce blood viscosity, increase tissue perfusion flow, and effectively prevent a series of pathophysiological changes induced by hyperviscosity (Han et al., 2004). The detailed study of DZXX preparations in the treatment of hyperviscosity patients is shown in Table 3.
Cardiovascular Diseases
Luo divided 60 patients with unstable angina pectoris into a treatment group and a control group. They were treated with DZXXI and compound Danshen injection. After 14 days, the clinical efficacy, electrocardiogram changes, and hemorheology of the patients in the DZXXI treatment group were all improved. The measured values of platelet CD41, CD63, CD62P and plasma D-dimer were significantly lower than the level before treatment, and the degree of improvement was more obvious than that of the control group, suggesting that DZXXI can improve angina pectoris by anti-platelet activation and improve coagulation and fibrinolysis activity (Luo, 2003). Other studies have also found that DZXXI can reduce the blood lipid level of patients with angina pectoris, improve lipid metabolism, reduce the expression levels of IL-6, CRP and TNF-α in the serum of patients, improve inflammation, and inhibit the development of angina pectoris (Zhao et al. (2008); Li and Yang, 2014). In addition, DZHSI can also inhibit platelet aggregation and internal coagulation function, activate the fibrinolytic system, promote fibrin degradation, interfere with related molecules before thrombosis, inhibit thrombosis, and thereby improve angina pectoris (Zhao et al., 2010). More clinical studies The data shows that compared with patients with angina pectoris who are given nitrate, anticoagulant, lipid-regulating western medicine and other traditional Chinese medicine compound injections, the symptoms of angina pectoris in patients treated with DZXX preparations are more improved, and the treatment efficiency is higher. The detailed study of DZXX preparations in the treatment of patients with angina pectoris is shown in Table 3.
Pulmonary Diseases
DZXXI can significantly improve the clinical symptoms of patients with acute exacerbation of pulmonary heart disease, increase PaO2, decrease PaCO2, increase pH, significantly decrease plasma D-dimer and Hb (Li et al., 2006), and reduce plasma IL-8 and TNF-α, CRP, blood viscosity, hematocrit, and fibrinogen levels. The mechanism of action of DZXX injection may be related to reducing blood viscosity, cytoinflammatory factor levels, and blood hyperviscosity in patients with COPD, correcting heart failure, respiratory failure, and other factors, ensuring a stable lung function for elderly patients with moderate to severe COPD, and decreasing the number of acute exacerbations (Cao et al., 2006; Xiao et al., 2015). Other researchs also found that conventional treatment combined with adjuvant breviscapine injection reduced the level of serum endothelin (ET), soluble vascular cell adhesion molecule-1 (sVCAM-1), and soluble intercellular adhesion molecule-1 (sICAM-1) in patients with cor pulmonale, increase NO level and improve cardiac output (CO) and cardiac index (CI) (Kong et al., 2010). And In another study, it was found that DZHSI can enhance the body’s immune function and improve the therapeutic effect of patients with COPD (Li and Jia, 2015). These results indicate that breviscapine changes the vascular endothelial function of patients with chronic pulmonary heart disease, improves blood hypercoagulability and blood viscosity, and reduces the specific volume of blood cells. Consequently, this treatment reduces endothelial cell damage, restores the balance of vasoactive factors, minimizes platelet aggregation, lowers the occurrence of thrombosis, and improves heart function and clinical efficacy during decompensation (Kong et al., 2010). Detailed study of DZXX preparations in the treatment of pulmonary disease is shown in Table 3.
Kidney Diseases
A number of clinical treatments have shown that both DZXXI and scutellarin injection mainly reduce serum creatinine (Scr), blood urea nitrogen (BUN), 24 h urine total protein (24 h UTP) and microglobulin (B2M) content, and Increase the creatinine clearance rate (Ccr) to play a therapeutic role and improve the patient’s renal function (Wu et al., 2018). In addition, it can also improve kidney lipid metabolism, blood rheology indexes and inflammatory factor levels to improve renal function (Cheng et al., 2005). The detailed study of breviscapine and its related preparations in the treatment of patients with nephropathy is shown in Table 3.
Diabetes Complications
Patients with stage IV diabetic nephropathy were divided into treatment and control groups. The treatment group was injected with 20 ml of DZXX, and the control group was administered with 0.2 g of inosine once a day for 2 weeks and 1 course. The results show that DZXXI can significantly reduce proteinuria in patients with diabetes. DZXXI can reduce the urinary microalbumin excretion rate (UAER), increase SOD and GSH-Px activities, inhibit reactive oxygen species production and membrane lipid peroxidation, remove reactive oxygen species, increase tissue antioxidant enzyme activities, decrease ROS production through MAPK and JAK-STAT channels, reduce the thickening of the basement membrane of the diabetic glomerulus and the proliferation of the mesangial matrix, and delay glomerular sclerosis (Chen, 2009; Cheng et al., 2007). Kang used breviscapine injection to treat diabetic nephropathy and found that the urine albumin excretion rate of patients significantly decreases, and the hematocrit, platelet aggregation rate, and plasma fibrinogen significantly improve. These results suggest that breviscapine may reduce urine albumin in patients with diabetes, alleviate patients’ microcirculation disorders, and reduce blood viscosity (Kang and Liu, 2003). After 45 patients with diabetic peripheral neuropathy were treated with DZXXI, sensory nerve conduction velocity (SNCV) and motor nerve conduction velocity (MNCV) were restored, and the levels of SOD and NO were improved. By expanding blood vessels, removing oxygen free radicals, improving blood rheology indexes, and improving clinical efficacy (Du et al., 2019). The detailed study of DZXX preparation in the treatment of diabetic patients is shown in Table 3.
Liver Diseases
DZXXI has a good curative effect on patients with chronic hepatitis B hepatic fibrosis. In particular, it reduces the serum indices of liver fibrosis especially the hepatic fibrosis indices hyaluronic acid (HA) and laminin (LN), improves the liver function and B-ultrasound imaging indices of the patients (Wu Y. H. et al., 2011), restores albumin level and prothrombin time activity (PTA), improves ALT, AST, and ALB and decreases the total bilirubin level of hyperbilirubinemia (Liu, 2014) Therefore, DZXX injection likely dilates small arteries, improves liver microcirculation and metabolism, and eliminates harmful substances, thereby improving liver function and reducing liver fibrosis (Wu D. F. et al., 2011). The detailed study of DZXX preparation in the treatment of liver disease is shown in Table 3.
Vertebrobasilar Insufficiency
The 116 patients with vertebrobasilar artery insufficiency were randomly divided into a treatment group of 60 cases and a control group of 56 cases. The treatment group received intravenous infusion of DZXXI, and the control group received intravenous infusion of betahistine hydrochloride injection. Observed after 15 days of treatment, the total effective rate of the treatment group was 91.67%, and the total effective rate of the control group was 85.71%. The average blood flow velocity of the vertebrobasilar artery of the two groups was significantly improved, and the average blood flow velocity of the vertebral artery and the basilar artery were increased in the treatment group compared with the control group (p < 0.05) (Fang, 2008). Clinically, DZXX preparations can resist thrombosis, improve blood viscosity and microcirculation, and increase local blood supply. See Table 3 for detailed research.
Eye Diseases
DZXXI can effectively improve the ocular hemodynamics of open-angle glaucoma patients with controlled intraocular pressure after selective laser trabeculoplasty (SLT), and improve the flow velocity and resistance index of the short posterior ciliary artery and central retinal artery), shorten the arm-retinal circulation time (A-CT) and retinal filling time (A-VT), increase the thickness of the retinal optic nerve fiber layer (RNFL), increase the area of the optic disc, improve the blood circulation of the optic disc, and improve the RNFL and optic disc edge Area, so as to protect the function of the optic nerve. The clinical studies are shown in Table 3.
Osteoarthritis of the Knee
DZXXI is used clinically to treat knee osteoarthritis, which can effectively reduce cytokines in synovial fluid, remove pathogenic factors in joints, relieve pain and improve knee joint function, and improve the clinical symptoms and function of patients with knee osteoarthritis due to blood stasis block. The clinical studies are shown in Table 3.
Pancreatitis
Clinically, breviscapine injection can effectively improve the nutritional status of patients with acute pancreatitis, increase the levels of albumin, prealbumin, transferrin, and hemoglobin, and reduce serum amylase, white blood cells, c-reactive protein, and tumor necrosis factor-α and interleukin-1 levels, improve immunity, and help patients recover (Zhang et al., 2016). Breviscapine injection can also reduce blood creatinine (Cr), urea nitrogen (BUN), 24 h urine protein levels, improve kidney function and inflammatory factors to improve pancreatitis symptoms (Bai et al., 2014). The clinical studies are shown in Table 3.
Ear Diseases
Thirty patients with sudden deafness were treated with breviscapine injection. After 14 days, breviscapine injection was 70% effective, which was higher than 56.7% in the control group. Breviscapine injection significantly improves the blood viscosity of patients, resists platelet aggregation, improves clinical efficacy, and also greatly reduces the rate of deafness (Zhu et al., 2011). The clinical studies are shown in Table 3.
Necrosis of the Femoral Head
Twenty-eight patients with femoral head necrosis (osteonecrosis of the femoral head, ONFH) were treated with DZXX injection perfusion in the internal circumflex femoral artery. Imaging showed that small branch vessels increase and elongate, the femoral head and neck vessels increase, and the area enlarges. The average Harris score before the treatment of the hip joint function was 64.9 ± 3.6, and the average after treatment was 76.3 ± 5.2. The difference was statistically significant (p < 0.05). The pain of the hip joint was reduced, the walking distance was increased, and the claudication was reduced. There were no obvious complications during follow-up (Ni et al., 2013). The clinical studies are shown in Table 3.
Pharmacological Effects
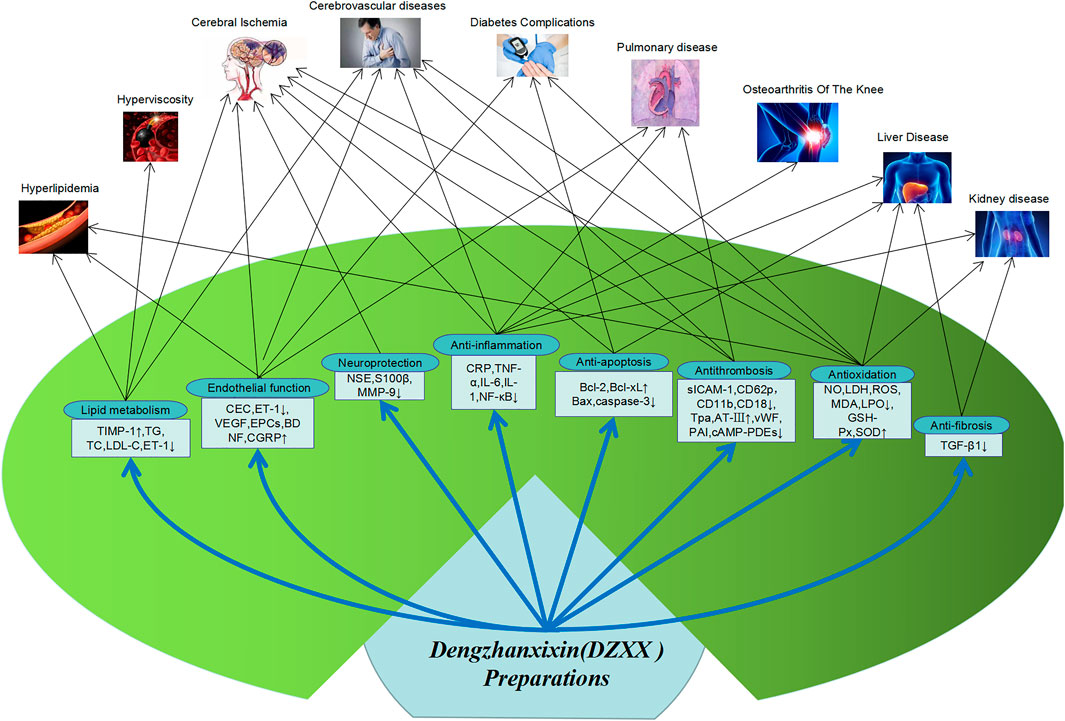
Looking at the literature, it is found that DZXXI, DZHSI, and DZHSP are the most commonly used DZXX preparations in pharmacological research. Therefore, the pharmacological researches on these three preparations and their main active ingredients are reviewed. And Main pharmacological action and action mechanism of DZXX preparation show in Figure 2.
Dengzhanxixin Injection (DZXXI)
Cerebrovascular Diseases
Combined with clinical experimental data, it is found that DZXXI can reduce blood lipids, blood viscosity and serum adhesion molecules in the treatment of cerebrovascular diseases, thereby reducing platelet aggregation, increasing blood flow in brain tissue, improving blood supply, and reducing inflammatory factors TNF-α, IL-6 and the apoptotic factor caspase-3, thereby reducing the damage after cerebral ischemia and cerebral infarction (Wang JL. et al., 2005; Yin et al., 2017), and also improving hyperlipidemia and hyperviscosity. In the rat MCAO model, it was found that DZXXI up-regulated the expression of vascular endothelial growth factor (VEGF) and VEGF receptor Flk-1, induced angiogenesis in ischemic brain tissue, promoted damaged vascular endothelial repair, reduced EEG pathological spikes, increased the regional cerebral blood flow (rCBF) in the ischemic area (Guo et al., 2016). This may also be related to the significant reduction in NF-κB expression, inhibiting the formation or release of inflammatory mediators, and reducing nerve excitability, eliminating abnormal discharge after DZXXI treatment of cerebral ischemia and reperfusion in rats, thereby improving microcirculation, increasing oxygen supply to brain tissue, and reducing reperfusion injury after cerebral ischemia (Niu et al., 2007). In clinical and animal experiments, it has been found that DZXXI also upregulates brain-derived neurotrophic factor (BDNF), inhibits delayed neuron necrosis, and reduces cell apoptosis (Zhou Y. R. et al., 2011). In addition, in Liu’s research, it was found that DZXX may improve cell energy balance by regulating neuronal metabolism, maintain BBB stability after cerebral ischemia in rats, reduce brain edema, and play a role in brain protection (Liu et al., 2009). Related pharmacological studies are shown in Table 4.
Cardiovascular Diseases
Combined clinical trials have found that DZXXI can also improve heart blood supply and angina pectoris by reducing adhesion molecules and platelet aggregation, improving blood viscosity and blood lipids. In addition, in animal and cell experiments, it has been found that DZXXI can reduce the release of inflammatory factors IL-6 and MCP-1 in cardiac microvascular endothelial cells (CMEC) induced by TNF-α stimulation, and protect vascular endothelial cells by inhibiting inflammation (Zhang et al., 2009). It was also found in the rat model of myocardial infarction that DZXXI can directly inhibit the overexpression of TNF-α, regulate the balance of PAI-1 and tPA, slow down thrombosis, and improve the hemodynamics of the heart after infarction (Huang et al., 2010). DZXXI can also reduce the content of MDA in the heart and lung tissues of hypoxic rats, increase the level of SOD, and improve heart function by scavenging oxygen free radicals (Li and Zhang, 2010). Related pharmacological studies are shown in Table 4.
Pulmonary Diseases
In the COPD rat model test, it was found that DZXXI can reduce rat hematocrit, reduce the expression of pulmonary arteriole type collagen and endothelin 1 (ET-1), reduce pulmonary vascular resistance, thereby inhibiting pulmonary vasoconstriction and remodeling, relieving pulmonary hypertension and preventing the formation of hypoxic pulmonary hypertension (Zhang et al., 2009), which is also consistent with the previous literature reports of clinical therapeutic effects. DZXX can improve the proportion of blood flow in the lungs and increase the partial pressure of arterial blood oxygen, improve hypoxemia, reduce hematocrit, and reduce pulmonary vascular resistance (Li et al., 2006; Xiao et al., 2015). In addition, DZXXI can also reduce the content of MDA, increase the expression of SOD, improve lung hypoxia and improve lung function through anti-oxidation (Li and Zhang, 2010). Animal experiments have also found that DZXXI can reduce neutrophil infiltration in lung tissue and inhibit inflammatory damage by inhibiting NF-κB activation. This is also in line with the reduction of TNF-α, IL-8, CPR and other inflammatory factors in patients with COPD in clinical trials (Chen et al., 2008). Related pharmacological studies are shown in Table 4.
Kidney Diseases
Consistent with clinical treatment data, DZXXI also effectively reduces the animal’s BUN and Scr levels in the treatment of animal kidney disease models and improves renal function. A number of experimental data show that DZXXI can reduce the level of TNF-α, increase the activity of Bcl-2 and SOD, reduce the content of BAX and MDA in the animal kidney injury and renal insufficiency model. DZXXI protects the kidney by reducing kidney inflammatory factors, scavenging oxygen free radicals, and reducing renal tissue cell apoptosis. Related pharmacological studies are shown in Table 4.
Others
Eye Diseases
DZXXI can promote the recovery of optic nerve axoplasmic transport block in acute experimental high intraocular pressure rats. After 20 days of administration, it was found that axoplasmic transport was partially restored, and the number of retinal ganglion cells (RGCs) increased, thereby protecting the optic nerve (Li Q. C. et al., 2007). In addition, DZXXI was intraperitoneally administered to glaucoma rats. After 7 days, it was found that the density in RGCs and the thickness of layers in retina increased, which had a certain protective effect on experimental glaucoma in rats (Zhu et al., 2000; Li Q. C. et al., 2007). Related pharmacological studies are shown in the Table 4.
Diabetes Complications
Studies have also found that injecting DZXX into the vitreous of diabetic mice can increase the GAP-43 protein and promote the regeneration of the optic nerve in diabetic mice (Tian et al., 2020). In addition, combined with previous clinical studies, DZXXI increases the activity of SOD and GSH-Px in the renal tissues of patients with diabetic nephropathy and reduces the production of related inflammatory factors IL-6, TNF-α and hs-CRP, inhibit the production of reactive oxygen species and membrane lipid peroxidation and reduce renal inflammation, thereby delaying glomerular sclerosis (Cheng et al., 2007; Li et al., 2011). In addition, studies have found that DZXXI can also up-regulate the protein expression of MMP-9 in kidney tissue to achieve the purpose of treating diabetic nephropathy (Wang and Li, 2006). Related pharmacological studies are shown in the Table 4.
Breviscapine Injection (DZHSI)
Cerebrovascular Diseases
In an MCAO (Middle cerebral artery occlusion) rat model, DZHSI can inhibit neuron-specific enolase levels, 4-hydroxy-2-nonenal and 8-hydroxy-2-deoxyguanosine and increase the expression levels of nuclear factor red sample 2 related factor (Nrf2) and heme oxygenase-1 (HO-1) protein. These results suggest that DZHSI may increase the expression of the Nrf2/HO-1 pathway and play a role in the treatment of cerebral ischemia-reperfusion injury (Guo et al., 2014). DZHSI can also prevent the activation of protein kinase C (PKC) induced by cerebral ischemia and reperfusion, reduce calcium overload, and decrease the volume of ischemic infarction (Chen and Dong, 1998; Shuai and Dong, 1998). DZHSI is used in advance on forebrain ischemia–reperfusion model gerbils, and results reveal that pre-administration before ischemia can significantly inhibit the decrease in hippocampal ATP and adenylate pool content caused by cerebral ischemia–reperfusion and reduce the water content of the brain cortex, the mechanism of action may be related to the reduction of energy metabolism disorders and cerebral edema caused by cerebral ischemia and reperfusion (Wang J. G. et al., 2005). DZHSI can also inhibit the production and release of interleukins and other inflammatory factors and caspase and other apoptotic factors, inhibit the activation of NLRP3 inflammasomes in hippocampus, and reduce the pathological damage and apoptosis of ischemic neurons (Hao et al., 2017; Wang et al., 2020). Related pharmacological studies are shown in the Table 4.
Cardiovascular Diseases
The clinical treatment results of DZHSI also show that it can improve blood viscosity and blood lipids in patients with angina pectoris, reduce the expression of prethrombotic molecular markers, reduce the platelet aggregation rate, and protect vascular endothelial cells. Animal experiments have found that high-fat feeding combined with the intravenous injection of calf serum albumin can be used to establish an atherosclerosis model in rabbit. Treatment with DZXXI can increase plasma vasodilator, HDL-C, TIMP-1 levels, reduce TC, TG, LDL-C, vasoconstrictor (ET-1), and MMP-9 levels, decrease plaque lesions, and delay plaque progression. These results indicate that DZXX injection can regulate lipid metabolism, may improve vascular endothelial functions, and adjust the balance of MMP-9/TIMP-1, thereby reducing atherosclerotic plaque (Cai et al., 2017). It was found that in animal models of myocardial ischemia, DZHSI mainly reduces myocardial cell apoptosis and relieves myocardial ischemia by down-regulating apoptotic factors such as caspase 3 and STAT1. In addition, DZHSI can also reduce the myocardial injury of ischemia-reperfusion rats by inhibiting inflammatory factors such as TNF-α (Han et al., 2012; Wang et al., 2017). Related pharmacological studies are shown in the Table 4.
Pulmonary Diseases
DZHSI can inhibit the increase in bronchial wall thickness and collagen fiber thickness in COPD model rats, reduce MMP-9, TGF-and Smad3 mRNA levels in lung tissue in COPD rats, raise Smad7 mRNA levels and improve fibrosis in lung tissue, thus delaying or improving the disease progression of COPD airway remodeling (Du et al., 2017). In addition, DZHSI can also improve lung tissue fibrosis by increasing lung tissue SOD content, reducing MDA level, and removing oxygen free radicals in lung tissue (Li, 2009). At the same time, DZHSI can reduce the content of Bcl-2 in lung cancer cells and increase the expression of Bax and Caspase 3 to promote the apoptosis of non-small cell lung cancer A549 CELL and play an anti-cancer effect (Wei et al., 2020). Related pharmacological studies are shown in the Table 4.
Kidney Diseases
In the rat model of kidney injury, after DZHSI administration, the levels of BUN, Scr, and MDA are reduced, SOD activity is increased, and kidney injury is improved through anti-oxidation (Lou et al., 2015). This is consistent with the results of clinical trials. Experimental studies have found that DZHSI can affect vasoactive substances in renal tissues, down-regulate the expression of transforming growth factor (TGF)-β1 and α-SMA proteins, increase the level of type IV collagen, and reduce renal damage due to renal fibrosis (Li et al., 2013). Related pharmacological studies are shown in the Table 4.
Diabetes and Complications
DZHSI reduces the expression of VEGF, the levels of nitric oxide and angiotensin Ⅱ in the kidney tissue of diabetic nephropathy rat models, protects vascular endothelial cells, activates blood vessels and improves renal hemodynamics (Gao Y. et al., 2007; Cui and Liu, 2016). In addition, studies have also found that DZHSI improves renal cell apoptosis through its anti-apoptotic effect. Other studies have shown that DZHSI can inhibitthe expression of MMP-9 and reduce the deposition of the mesangial matrix, thereby blocking the occurrence and development of diabetic nephropathy (Deng et al., 2014). Zhao found that DZHSI inhibited renal cell apoptosis by affecting the expression of apoptosis-related genes bcl-2 and bax, thus exerting a renal protective effect (Zhao et al., 2009). Similarly, for retinopathy caused by diabetes, DZHSI can also delay the course of diabetic retinopathy by reducing the expression of VEGF in the rat retina (Wang Y. H. et al., 2015). Related pharmacological studies are shown in the Table 4.
Liver Diseases
In vivo and in vitro experiments have demonstrated that breviscapine significantly reduces ALT, AST, and hydroxyproline levels in a dose-dependent manner. Breviscapine inactivates CCl4 and LPS-induced MAPK (p38, ERK1/2 and JNK) signals and Toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) signaling pathway, downregulates the expression and chemokine secretion of inflammatory factors, such as TNF-α, IL-6, IL-1β protein and MCP-1 factor, enhances Bcl2 levels, reduces bcl2-related X protein, apoptotic protease activator 1, caspase 3, and PARP activities, and decreases apoptosis levels. Breviscapine can also block CCl4-induced oxidative stress by reducing ROS production, improving antioxidants, and blocking mitogen-activated protein kinase pathways; by contrast, it can induce CCl4-induced acute liver injury and LPS induction by inhibiting inflammation and apoptosis of L02 cells, elicit a protective effect on apoptosis, and improve the histological changes and collagen deposition induced by CCl4 in mice (Liu Y. et al., 2018). According to histopathological analysis, DZHSI can reduce the levels of the liver enzymes aspartate and alanine aminotransferase, decrease MDA levels, and increase the SOD activity. Western blot and RT-q polymerase chain reaction have shown that breviscapine pretreatment can reduce the expression of mitochondrial fusion protein 2 (mfn2), caspase-3, and cytoplasmic cytochrome c protein. Therefore, breviscapine pretreatment may reduce lipid peroxidation, inhibit oxidative stress, and inhibit the protein and mRNA expression of Mfn2 to achieve a protective effect against liver ischemia–reperfusion injury (Lin et al., 2016; Bao et al., 2018).
Breviscapine Pills (DZHSP)
Cardiovascular Diseases
Similarly, in pharmacological experiments, it was found that DZHSP mainly exerts anti-inflammatory effects by lowering blood lipids, changing hemodynamics, reducing interleukins, tumor necrosis factor and other inflammatory factors, increasing the activity of SOD in the body, and reducing caspase and other apoptotic factors, thereby exerting anti-oxidation, anti-apoptosis of cardiomyocytes, improve symptoms of cardiovascular disease (Shi et al., 2016; Wu et al., 2017). See the Table 4 for more research.
Liver Diseases
Give DZHSP intragastric treatment to rats with ischemia-reperfusion liver injury. The degeneration and necrosis of hepatocytes are reduced, ALT and AST are significantly reduced, and SOD activity is significantly increased (An et al., 2013). DZHSP improves the body’s elimination of free radicals through anti-oxidation and enhancement of SOD activity, and ability to protect liver function and reduce liver damage. For liver transplantation donor liver, DZHSP pretreatment can significantly inhibit inflammation-related factors and apoptosis-related pathways and protect microcirculation endothelial cells to reduce ischemia-reperfusion injury after liver transplantation in rats (Zhong et al., 2016). See the Table 4 for specific research.
Pulmonary Diseases
DZHSP reduces the pulmonary artery pressure in rats with chronic hypoxic pulmonary hypertension, inhibits the proliferation of pulmonary arterioles media smooth muscle cells and the production and accumulation of pulmonary artery wall collagen, reduces the activation and expression of PKC, and blocks various PKC signaling pathways thereby Inhibit chronic hypoxic pulmonary hypertension and pulmonary vascular remodeling. See the Table 4 for specific research.
Kidney Diseases
The animal model of kidney injury was treated with DZHSP and found that DZHSP inhibits reactive oxygen species (ROS) and kidney injury molecule-1 (KIM-1), reduces the expression of TNF-α, MCP-1, MIP-2 inflammatory factors, inhibits inflammatory cells chemotaxis and activation, reduce the level of selectin expression, inhibit oxidative stress, reduce lipid peroxidation and free radical damage, thereby reducing renal ischemia-reperfusion injury (Jia et al., 2011; Zhao et al., 2019). See the Table 4 for specific research.
Diabetes and Complications
It can also be found in diabetic nephropathy models that DZHSP can reduce renal fibrosis and tubular damage, improve the expression of fibrosis markers of diabetic nephropathy, reduce proteinuria and serum creatinine, and phosphorylate PKCβII/Akt/JNK1/2/p38 signaling pathway Inhibit renal fibrosis (Jiang et al., 2016). See the table for specific research. After diabetic retinopathy (DR) rats were treated with DZHSP intragastrically for 120 days, the lipid peroxide level in the retina was significantly reduced, and the superoxide dismutate activity was significantly increased, suggesting that DZHSP may enhance the antioxidant capacity and Inhibit retinal cell apoptosis to improve retinopathy (Han, 2009). See the Table 4 for specific research.
Scutellarin
As the main active ingredient studied in DZXX preparations, scutellarin has multiple effects such as anti-oxidation, anti-free radical, anti-coagulation, anti-inflammatory, anti-apoptosis and anti-fibrosis. See Table 4 for the pharmacological studies.
Pharmacological experimental studies have shown that in the MCAO rat model, scutellarin inhibits the release of TNF-α, IL-1β and other inflammatory factors, reduces the expression of MMP-9, Caspase 3, increases the expression of anti-apoptotic factor Bcl-2, and prevents Extracellular matrix damage and blood-brain barrier destruction, inhibit cell inflammation and apoptosis, promote the recovery of nerve function, and improve cerebrovascular diseases (Wang L. Z. et al., 2006; Yang et al., 2019a; Chen et al., 2020).
Taking cognitive dysfunction rats as the research object, it was found that scutellarin can significantly improve their learning and memory decline. Further research found that scutellarin reduces the content of MDA in the hippocampus, increases the level of SOD, and reduces the inflammation Factors NF-κB, TNF-α, IL-6 expression, reduce the beta amyloid precursor protein (APP) in brain tissue, reduce the generation of free radicals, inhibit neuronal toxicity, reduce cell apoptosis and inflammation, thereby improving recognition Cognitive dysfunction (Guo et al., 2011a; Guo et al., 2011b; Guo and Guan, (2013); Wang Y. J. et al., 2018).
It has also been found in animals and cell models of myocardial ischemia that scutellarin mainly improves dyslipidemia, increases tPA levels against platelet aggregation, increases fibrinolytic activity, reduces the expression of MMPs, stabilizes heart plaques, and reduces the expression of inflammatory factors in cardiomyocytes Scutellarin can also reduce ROS and MDA content, increase SOD activity, increase cell viability and mitochondrial membrane potential, inhibit myocardial inflammation, apoptosis and oxidative stress, and reduce myocardial cell damage.
In an animal model of lipopolysaccharide-mediated liver cell injury, scutellarin significantly increases the activities of SOD, GSH-Px, and CAT in liver tissues, reduces the content of XOD, MDA, and NO, and resists lipid peroxidation, reduces the production of lipid peroxide, enhances the body’s ability to scavenging free radicals, expands blood vessels, improves liver hemoperfusion and protects the liver (Yang and Guo, (2010)). In addition, scutellarin can also reduce the content of TNF-α, IL-6, IFN-γ inflammatory factors to improve liver inflammatory response and reduce liver damage (Niu et al., 2015). And scutellarin was found to reduce the expression of apoptotic factor Stat3 in liver cancer cells, and inhibit the metastasis of liver cancer cells by inhibiting the STAT3/Girdin/Akt signaling pathway (Xu and Zhang, 2013).
Scutellarin can inhibit the proliferation, migration and invasion of renal cancer cells (ACHN, 786-O) in a dose-dependent manner in vivo and in vitro, and induce their apoptosis, and significantly reduce cyclin D1 (cyclin D1) and cyclin-dependent kinases (CDK1), Bcl-2, MMP-2, MMP-9 and other key protein expression, enhance the expression of Bax, cleaved Caspase-3 and p21, induce cancer cell apoptosis, and also increase PTEN by inhibiting the P13K/AKT/mTOR pathway, partially inhibit the proliferation and invasion of renal cell carcinoma (Deng et al., 2018). In addition, scutellarin can also reduce the levels of TNF-α and IL-6 in kidney tissue to exert anti-inflammatory effects, up-regulate the expression of nuclear factor red-like 2 related factor 2 (Nrf2), and increase heme oxygenase 1 (HO-1), regulate the Nrf2/HO-1 signaling pathway to play a role in lowering blood sugar and renal protection (Liu et al., 2019; Sun et al., 2019).
In diabetic animals and cell models, it was found that scutellarin can reduce the blood sugar level of diabetic rats by improving inflammation and anti-oxidation. In addition, scutellarin up-regulates the expression of Nrf2 and promotes the expression of HO-1, SOD, CAT in the kidney, which may inhibit the oxidative damage of the kidney through the Nrf2/HO-1 signaling pathway and improve diabetic nephropathy (Liu T. et al., 2018; Mei et al., 2019; Gao et al., 2020). Scutellarin can reduce the expression of NF-κB, TNF-α, ERK1/2, reduce retinal damage caused by the activation of microglia during the development of diabetic retinopathy, and can also inhibit VEGF and its downstream protein p-ERK, phosphorylate focal adhesions Kinase (p-FAK), phosphorylated tyrosine protein kinase (p-Src), inhibits the angiogenesis of diabetic retinopathy by down-regulating vascular endothelial growth factor/ERK/FAK/Src pathway signals, and improves microvascular dysfunction (Mei et al., 2019).
Pharmacokinetics
Studies on the pharmacokinetics and absolute bioavailability of scutellarin in dogs have shown that after the intravenous administration of scutellarin, it is metabolized, is excreted rapidly, and has a short elimination half-life; its oral administration is almost not absorbed, and the absolute bioavailability is only 0.2–0.75% (Liu et al., 2002). The main reasons for the low oral bioavailability of scutellarin are low solubility in gastrointestinal fluid, poor membrane permeability, first-pass metabolism in the gastrointestinal tract, and efflux of transport proteins. The average bioavailabilities of 1,5-dicaffeoylquinic acid (1,5-DCQA) in dogs and rats are only 3.50 and 0.52%, respectively. The causes of low bioavailability of caffeic acid esters were intestinal metabolism, poor self-absorption and efflux of transporters (Xia, 2016).
After the oral and intravenous administration of breviscapine, the drug-time curve has a multipeak phenomenon. Further experiments should confirm whether the multi-peak phenomenon is related to the liver and intestinal circulation. The in vivo process of total caffeic acid esters in DZXX injection conforms to the two-compartment model of intravenous injection. At the same time, the drug–time curve shows a multipeak phenomenon. Because scutellarin in DZXX injection is also an effective ingredient for promoting blood circulation and removing blood stasis, whether the pharmacokinetic behavior of the two ingredients influences each other needs to be further explored (Zhang et al., 2005; Li et al., 2007b; Li et al., 2007c). Studies have shown that scutellarin is mainly absorbed in the intestinal tract via passive diffusion, and absorption is linearly related to concentration in the range of 50–400 μg/ml, and absorption is not affected by pH at pH 6.0–7.4. Ge Qinghua et al. found that intravenous administration of 90 mg or 1.8 g of scutellarin in beagle dogs is rapidly eliminated in the body after intravenous administration, whereas the absolute bioavailability of oral administration is only 0.40 ± 0.19% (Ding and Jiang, 2003; Ju et al., 2005). Rats are intragastrically given 80 mg/kg scutellarin after 4, 8, and 12 h after administration; the content of scutellarin in the kidney is the highest, followed by the heart, liver and brain. After scutellarin is injected into the blood, the half-life of the distribution phase is very short, i.e., 1.3 min in rabbits. Domestic dogs are only 7 min (Jiang et al., 2003). Rats are given the same amount of scutellarin aglycone and scutellarin via gavage. Scutellarin aglycone is easily absorbed through oral administration. The relative bioavailability of scutellarin aglycone is 301.8% compared with that of scutellarin (Che et al., 2006). In addition, the half-life (t1/2) and residence time (MRT) of caffeic acid in breviscapine injection in rats were significantly higher than that of caffeic acid monomer alone. Certain components in breviscapine injection are different from caffeic acid interacts, prolonging the time of caffeic acid in rats (Dai et al., 2013). Aspirin injection combined with DZXX injection in rat tail vein, T1/2β of coffee increased significantly, the clearance rate of CL decreased, and the area under the plasma concentration–time curve AUC (0–t) and the surface volume of distribution (Vd) increased, indicating that aspirin can slow down the metabolic process of caffeic acid in the body (Dai et al., 2014). After the intravenous injection of scutellarin liposomes in beagle dogs, the blood concentration is greatly increased, the pharmacokinetic properties of the scutellarin original drug are significantly improved, and it has a sustained release effect (Lv et al., 2006)..
In a PK-PD study, after the MCAO model of rats, a one-time intravenous bolus of DZXXI (5 ml/kg/day), from 5 min to 50 h after cerebral ischemia, can reduce cerebral infarction rate in MCAO rats. The onset of T is cerebral ischemia for 5 min, the duration of T is 48 h, Tmax is 24 h, T1/2 is 21.84 h, and Emax is 11.71%. DZXXI reduces cerebral infarction rate in MCAO model rats with fast onset and long maintenance time. The reduction has the characteristics of quick onset and long maintenance time and provides a reference for specific drug screening, optimal dosing regimen, and clinical rational use of ischemic stroke. The peak of drug influence lags the peak of plasma concentration, and the effect of reducing the cerebral infarction rate is negatively correlated with the average blood concentration of the seven chemical components at 5–10 min of cerebral ischemia and positively correlated at 10 min–6 h; in addition, the time-quantity relationship of seven chemical components was negatively correlated. The pharmacokinetic values of the seven chemical components in Dengzhanhua injection were the highest 5 min after cerebral ischemia. The lowest detectable values of baicalin and isochlorogenic acid B appeared after 6 h of cerebral ischemia and could not be detected after 8 h. The lowest detectable value of 5-caffeinyl quinic acid and 4,5-bisphenol quinic acid appeared at the 3rd hour of cerebral ischemia and no longer visible at the 4th hour. The lowest detectable values of 4-caffeinylquinic acid, 3,5-bisphenol-quinic acid, and chlorogenic acid were observed 2 h after cerebral ischemia and no longer detected after 3 h (Liu G. et al., 2020).
Toxicological Research and Safety Evaluation
From the records of traditional literature to the acute and subacute toxicity experiments of this product, DZXX has low toxicity and is a safe medicinal material. Subacute toxicity test shows that scutellarin (mainly containing scutellarin B and scutellarin A) has no effect on blood, liver and kidney functions, and no substantial changes in organs (Wang Z. et al., 2012).
The acute toxicity test of DZXX injection shows that the mice developed abnormal mental behavior and breathing, followed by behavioral disorders, convulsions, and death after a single intravenous or intraperitoneal injection of E. breviscapus injection. Female and male intravenous bolus injections of DZXX injection measured with the Bliss method had LD50 of 1,676.75 and 1,744.76 mg kg−1, respectively, and no significant difference were detected. Long-term toxicity test observe that the rats intraperitoneally injected with 480 mg kg−1 DZXX once a day for 2 months, its body weight increased slowly, and pathological examination found that some renal tubular epithelium in the cortex of renal tissue had mild turbid swelling. The weight gain of rats was slow, and pathological examination shows that some renal tubular epithelia in the inner cortex of renal tissues had mild turbid swelling, but 120 and 30 mg kg−1 dose groups did not cause drug damage and delayed toxicity after drug treatment was terminated. The results of the long-term toxicity test of DZXX injection in beagle dogs show that animals in the 160 mg kg−1 dose group of DZXX injection were continuously administered intravenously for 60 days, and drug damage and reactions occurred after drug withdrawal. Blood biochemical examination revealed that treatment for 30 days increases creatinine levels. Some animals in the 40 mg kg dose group also had drug reactions during the administration, and their blood biochemical examination indicated that the total protein content increased after administration. The dose of 10 mg kg had no significant effect on mental behavior, blood and urine biochemical examinations (Guo and Li, 2012).
A safety test study on breviscapine injection has shown that breviscapine was intravenously injected into the abdominal cavity of guinea pigs for three consecutive times for 14 and 21 days after the administration, and no allergic reaction was found. with the rabbit ear margin intravenous injection of DZXX injection 1 time/day for three consecutive days, no obvious irritation was observed at the injection site. At the same time, hemolytic test showed that breviscapine injection had no hemolysis and agglutination. The rabbits were injected intramuscularly with breviscapine injection. No obvious hyperemia, redness, and swelling were observed on the surface of the muscle. The rabbits had normal activities and no obvious abnormal reactions. After execution, the order of muscle irritation at the administration site was level 0; the rabbits were continuously injected with the drug in the ear vein once a day. After the administration for 5 days, no abnormal reaction was observed in the ear veins. The microscopic examination of the tissue section revealed that the endothelium of the ear veins was intact and smooth. No inflammatory reaction was found in the wall of the ear vein and no blood column formation in the lumen (Liang and Su, 2014).
The rats were continuously administered through the intraperitoneal injection of E. breviscapus for 90 days. The drug was discontinued every 7 days for 14 days. The main manifestations were fluctuations in blood routine indicators and prolonged PT and aPTT. The high-dose group of 20 crude drugs/g and the middle-dose group of 10 crude drugs/g in all female and male rats had lower HCT (p < 0.01 or p < 0.05) and decreased in a dose-dependent manner. WBC, YLM, and MID decreased (p < 0.01), but they were similar to the values after 90 days of administration, and no abnormality was detected in body weight and liver function, which was presumed to be the performance of the drug’s blood-activating and stasis-removing effect. Indicators can be restored after the drug administration was terminated. No obvious delayed toxicity was observed, and the safe dose range was 20 g/kg and below (Wu et al., 2007).
The acute toxicity test of compound Dengzhanhua dripping pills was performed on mice once a day and observed for 7 days. Within 7 days after the administration, the physiological conditions of the mice were normal, their weight increased, and no death occurred. In a long-term toxicity test, rats were intragastrically treated once per day at doses of 0.9, 0.45, and 0.225 g/kg for 10 weeks of continuous administration and withdrawal for 2 weeks, the body weight, blood biochemical examination, organ coefficient, organ tissue structure, and other aspects of rats in each group were normal, and no obvious specific pathological changes related to drug toxicity were found (Wan et al., 2003).
The oral toxicity of DZXX extract is relatively small. When a mouse is given 80 g kg−1 through gavage once, no death was observed within 3 days; LD50 (ip) = 13.14 ± 5.43 was calculated with the simplified probability unit method (g kg−1), LD50 (iv) = (10.02 ± 1.55) g kg−1 (Wang and Wang, 1985; Liang and Su, 2014).
The ADRs of DZXX-related preparations mainly include allergic reactions, nervous system reactions, digestive system reactions, cardiovascular system reactions, respiratory system reactions, and blood system reactions. ADR symptoms include rash, chills, fever, and shortness of breath, palpitation, headache, lower extremity edema, elevated blood pressure, and abdominal pain. The top 10 ADR symptoms are pruritus, rash, dizziness, chills, palpitation, headache, fever, suffocation, nausea, and flush. The most common systems and organs affected by ADRs are damage to the skin and its accessory organs. Men have more adverse reactions than women. The most severe ADR occurs in elderly patients aged 60 years and older (Li Y. et al., 2015).
Conclusion
According to the China Cardiovascular Disease Report 2018, the current number of cases of cardiovascular diseases in China is estimated to be 290 million, including stroke 13 million, coronary heart disease 11 million, pulmonary heart disease 5 million, heart failure 4.5 million, rheumatic heart disease 2.5 million, congenital heart disease 2 million, hypertension 245 million. Cardiovascular and cerebrovascular diseases are currently the number one cause of death among Chinese residents. The incidence increases and tends to affect younger individuals. The mortality rate in rural areas is higher than that in urban areas. In 2016, the death rate of cardiovascular and cerebrovascular diseases in rural areas was 309.33/100,000. Of these cases, the death rate of heart disease was 151.18/100,000. In urban areas, the death rate of cardiovascular and cerebrovascular diseases was 265.11/100,000. Of these cases, the death rate of heart disease was 138.70/100,000. Hypertension, hyperlipidemia, diabetes, obesity, and elevated blood uric acid are the main risk factors of cardiovascular and cerebrovascular diseases. With the increase in the number of patients with cardiovascular and cerebrovascular diseases, the demand for drugs increases yearly. The sales of cardiovascular drugs in China are second only to anticold and gastrointestinal drugs, ranking third. The amount of sales of cardiocerebral vascular drugs in China has shown an increasing trend, that is, it increased from 60.93 billion yuan in 2013 to 83.44 billion yuan in 2017, with a compound annual growth rate of 8.2%. Therefore, the pharmaceutical market’s demand for DZXX raw materials and biological drugs has also increased (Zhou, 2006; Hu et al., 2019). According to reports, in 2019, the sales revenue of traditional Chinese medicine preparations composed of DZXX as a raw material was about 3 billion yuan.
At present, the preparation of marketed DZXX varies. For example, breviscapine granules are extracted with alcohol, and DZXX injection is extracted with water. The chemical compositions of similar products from different manufacturers differ, and different batches of the same pharmaceutical company also have significant differences. Such differences are unreasonable. In addition, chemical fingerprint analysis via HPLC-DAD and other techniques has revealed that the chemical composition spectrum is also significantly different. Although DZXX and breviscapine injections differ in terms of preparation processes and chemical compositions, their clinical functions and indications are the same. It can be inferred that scutellarin is one of the common substance base, but it also suggests that there are still a large number of invalid components in different preparations in theory, and it is necessary to further explore the basis of pharmacodynamic substances to provide guarantee for the improvement of prescription and technology. Therefore, for the different preparations of E. breviscapus included in the national drug standards, the rationality of existing traditional Chinese medicine preparations should be studied in terms of their curative effect or efficacy. Improvement plans should be carried out on the premise of focusing on the material basis of drug effects. The preparation process and quality standards of related preparations should be standardized, some of the preparations should be eliminated on the basis of effectiveness, and the preparations of different processes should be compared and examined to find a more comprehensive material basis for using original medicinal materials. Based on HPLC and other techniques, the quality control methods of fingerprinting and determination of pharmacodynamic components from medicinal materials from medicinal materials, intermediate raw materials to preparations, and qualitative and quantitative studies of flavonoids, caffeoyl and other chemical components in DZXX related preparations were carried out to ensure the homogeneity, safety and stability of product quality, and to further improve the quality control level of breviscapine asarum and lay the foundation for the entry of breviscapine asarum into the international market (Wang, 2009; Zhou, 2006).
The bioavailability of oral preparations of DZXX medicinal components is low. Their injections have a short half-life and are rapidly eliminated from the body. They are also associated with poor patient compliance and inconvenient use. Therefore, the development of a drug delivery system with simple preparation process, high drug loading, improved oral absorption, and improved bioavailability of baicalin and total caffeic acid ester as main pharmacodynamic components of breviscapine is the main breakthrough point in future work, and is also the key link to give full play to the advantages of breviscapine in the treatment of cardiovascular and cerebrovascular diseases. Studies on new formulations of E. breviscapus have focused on the same aspects of DZXX preparations. Most of them have explored the bioavailability of flavonoids but have rarely investigated the simultaneous improvement of the main effective components of breviscapine. Coffee acyl components occupy a large proportion of DZXX, but the present research on light lamps mainly focuses on flavonoids, coffee acyl components also have good activity. Research has shown that 3,5-di-O-caffeoyl quinic acid can increase serum SOD, GSH-Px, NOS activities and reduce MDA content in MCAO rats to increase BBB permeability, and improve cerebral ischemia. Studies on the bioavailability of baicalin and total caffeic acid esters, the mechanism of action, pharmacodynamics, and structure-activity relationships of the active ingredients of DZXX should be performed in detail (Sheng et al., 2016). Furthermore, research on new formulations is insufficient at cellular and molecular levels, at the same time; there are still many deficiencies in the study of animal and human for pharmacokinetics. Therefore, studies on the drug release characteristics, transport in the body, absorption kinetics, bioavailability, and efficacy of E. breviscapus should be conducted (Xia, 2016).
DZXX has complex chemical components and extensive pharmacological effects. It has the characteristics of multiple components, multiple targets, and overall regulation when it exerts its drug effects. Studies on the effect of DZXX on some metabolites in rats with ischemic brain injury have verified and explained the traditional mechanism, but studies have yet to discover new biomarkers and mechanism of action. Recent studies were based on the BBB, and they involved the use of the ROS/RNS-MMP-TJ signaling pathway as the entry point to explore the molecular mechanism of DZXX injection that protects the BBB damage caused by cerebral ischemia in rats. However, mechanisms and pathways in diseases treated with DZXX remain unclear. Therefore, methods such as network pharmacology and HPLC should be applied to study the distribution of the active ingredients of DZXX-related preparations, therapeutic targets, and signal pathways, to determine and clarify their chemical composition, to investigate their mechanism of action and regulation, and to provide a scientific theoretical basis for clinically applying DZXX-related preparations.
Author Contributions
RW conceived the study. YL was responsible for organizing the literature and drawing chemical formulas. MX, KF, and LW reviewed and summarized the literatures. YZ was in charge of consulting the literature. RW wrote the manuscript and drew the figures. ZW supervised the study and gave final approval of the version to be published. The final version of the manuscript was read and approved by all authors.
Funding
This work was supported by the national natural science foundation of China (NO.81102895), the special scientific research fund for doctoral programs of higher education in China’s ministry of education (NO.20115132120007), the key project of Sichuan province applied and basic research program (NO.2016JY0017), the first batch of young Chinese medicine research special program of Sichuan provincial Chinese medicine administration (NO. 2016Q051).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
An, Y., Yang, N. J., Li, Q., Wang, S., Zhong, X. H., Zhang, Y. Z., et al. (2013). Protective Effect of Breviscapine Preconditioning on Hepatic Injury Induced by Limb Ischemia Reperfusion in Rats. J. Pract. Med. 29 (04), 543–545. doi:10.3969/j.issn.1006-5725.2013.04.013
Ao, J. W., Wang, Y. J., Yu, Y. N., and Guo, L. L. (2018). Influence of Scutellarin on Generation of β-amyloid Protein in SH-Sy5y Cells. Mod. Prev. Med. 45 (08), 1466–1470.
Bai, Y., Cui, D. X., Xie, S. R., Lv, Z., and Han, X. R. (2014). Clinical Study of Breviscapine in the Treatment of Severe Acute Pancreatitis. Mil. Med. Sci. (7), 567–568. doi:10.7644/j.issn.1674-9960.2014.07.026
Bao, Z., Chen, W., Pan, F., Peng, B., and Gong, J. (2018). Role of Mitofusin 2 in the Protective Effect of Breviscapine against Hepatic Ischemia/reperfusion Injury in Rats. Exp. Ther. Med. 15 (4), 3582–3588. doi:10.3892/etm.2018.5834
Cai, H. R., Huang, Y. P., Yuan, K., Zhao, S., Li, Z. S., Zeng, J., et al. (2017). The Effects of Dengzhanxixin Injection on Lipid Metabolism, Vascular Endothelial Function and MMP-9/TIMP-1 in Atherosclerosis Rabbit Models. J. Clin. Cardiol. 33 (10), 1000–1003.
Cai, L. Z., Feng, C., Luo, S. S., Li, J., and Rao, G. X. (2014). Determination of Scutellarin in Dengzhanxixin Mixture (Sugar-free) by HPLC. J. Yunnan Univ. Traditional Chin. Med. 37 (6), 5–8.
Cao, R. B., He, L. H., and He, X. Z. (2006). Curative Effects of Eriseron Breviscapus Injection upon Acutely Aggravating Stage of Cor Pulmonale. China J. Mod. Med. 16 (10), 1560–1563. doi:10.3969/j.issn.1005-8982.2006.10.038
Chai, L., Guo, H., Li, H., Wang, S., Wang, Y. L., Shi, F., et al. (2013). Scutellarin and Caffeic Acid Ester Fraction, Active Components of Dengzhanxixin Injection, Upregulate Neurotrophins Synthesis and Release in Hypoxia/reoxygenation Rat Astrocytes. J. Ethnopharmacol 150 (1), 100–107. doi:10.1016/j.jep.2013.08.011
Chang, Y. (2008). Observation of the Curative Effect of Dengzhan Xixin Injection in the Treatment of Hyperviscosity. Shandong Med. Journa 48 (22), 54–55. doi:10.3969/j.issn.1002-266X.2008.22.024
Chang, Y. (2018). Protective Effect of Dengzhan Xixin Injection on Optic Nerve in Open Angle Glaucoma Patients with Controlled Intraocular Pressure after Selective Laser Trabeculoplasty. China Pharmaceuticals 27 (21), 49–51. doi:10.3969/j.issn.1006-4931.2018.21.015
Che, Q. M., Chen, Y., Pan, L. Y., and He, H. (2006). Scutellarein’s Pharmacokinetics in Rats. Chin. J. New Drugs 15 (18), 1557. doi:10.3321/j.issn:1003-3734.2006.18.013
Chen, B. L., Duan, M. K., Wang, T., Ma, L. M., and Rao, X. G. (2008). Effects of erigeron Breviscapine on Nuclear Factor-Κb Expression Following. Chin. J. Pathophysiology 24 (6), 1218–1221. doi:10.3321/j.issn:1000-4718.2008.06.035
Chen, H. L., Jia, W. J., Li, H. E., Han, H., Li, F., Zhang, X. L., et al. (2020). Scutellarin Exerts Anti-inflammatory Effects in Activated Microglia/Brain Macrophage in Cerebral Ischemia and in Activated BV-2 Microglia through Regulation of MAPKs Signaling Pathway. Neuromolecular Med. 22 (2), 264–277. doi:10.1007/s12017-019-08582-2
Chen, K., and Dong, W. (1998). Study on Effect of Erigeron Injection in Prevention and Treatment of Cerebral Ischemic Injury. Zhongguo Zhong Xi Yi Jie He Za Zhi 18 (11), 684–686.
Chen, S. X., Zhou, H., Wang, L. X., Xie, Y. P., Chen, Y. F., Wang, Q. J., et al. (2002). Effect of Breviscapine on Pulmonary Arterial Pressure and Pulmonary Arterial wall Collagen of Chronic Hypoxic Rats. Shanghai Med. J. (06), 363–365. doi:10.3969/j.issn.0253-9934.2002.06.015
Chen, W., Du, M., and Xu, X. (2017). An Explicit Closed-form Analytical Solution for European Options under the CGMY Model. Commun. Nonlinear Sci. Numer. Simulation 42 (24), 285–297. doi:10.3969/j.issn.1007-7103.2017.24.19310.1016/j.cnsns.2016.05.026
Chen, W. D. (2009). Effect of Dengzhanxixin injection on urinary microalbumin excretion rate in patients with early diabetic nephropathy. Chin. J. Integr. Trad. Western Nephrol. 10 (11), 996–997. doi:10.3969/j.issn.1009-587X.2009.11.018
Chen, W. X., Liu, H., Liao, W. J., Yue, Y., and Deng, F. (2007). Effects of Herba Erigerontis on Metabolites in Cerebral Ischemia Rats. Chin. J. Rehabil. Theor. Pract. (01), 29–31. doi:10.3969/j.issn.1006-9771.2007.01.008
Chen, X. X., Hu, R., Wang, Y. B., and Chen, J. H. (2003). Dengzhanxixin Injection in the Treatment of Unstable Angina Pectoris and its Effect on Plasma Endothelin. Guangdong Med. J. 24 (1), 77–78. doi:10.3969/j.issn.1001-9448.2003.01.041
Chen, X. Y. (2011). Breviscapine Injection Combined with Treatment of Vertebral-Basal Artery Ischemic Vertigo in 35 Cases. J. Emerg. Traditional Chin. Med. (9), 1500–1501. doi:10.3969/j.issn.1004-745X.2011.09.079
Chen, Y. S., Liu, H. Q., Gao, Y., and Qin, W. (2011). The Effect of Breviscapine on the Level of Adrenomedullin and its Receptor Expression in the Kidney of Diabetic Rats. Lishizhen Med. Materia Med. Res. 22 (01), 98–99. doi:10.3969/j.issn.1008-0805.2011.01.044
Chen, Z. J., Shi, L., Chen, X., and Jiang, X. F. (2014). Effects of Breviscapine on the Na+,K+-ATPase Activity in Kidney Tissues of Rats with Contrast-Induced Acute Kidney Injury. Guangdong Med. J. 35 (07), 978–981. doi:10.13820/j.cnki.gdyx.2014.07.005
Cheng, H., Liu, H. Y., and Qiu, C. J. (2007). Effect of Breviscapin Injection on Reactive Oxygen Species of Diabetic Nephropathy. China Pharmacist 10 (8), 762–764. doi:10.3969/j.issn.1008-049X.2007.08.012
Cheng, S. P., Liu, J. L., and Yuan, J. (2005). The Effect of Dengzhanxixin Injection on Hemorheology and Renal Function in Patients with Chronic Renal Failure. Pract. Geriatr. 19 (4), 215–216. doi:10.3969/j.issn.1003-9198.2005.04.020
Cui, X. L., and Liu, X. J. (2016). Effects of Breviscapine Injection on Expressions of VEGF in Kindey of Diabetic Rats. Pharmacol. Clin. Chin. Materia Med. 32 (01), 145–149. doi:10.13412/j.cnki.zyyl.2016.01.041
Dai, G. L., Ma, S. T., Liu, S. J., Cheng, X. G., Zang, Y. X., Ju, W. Z., et al. (2013). Study on Determination of Caffeic Acid, Chlorogenic Acid in Rat Plasma and Their Pharmacokinetics with LC-MS/MS. Zhongguo Zhong Yao Za Zhi 38 (21), 3753–3757. doi:10.4268/cjcmm20132129
Dai, G. L., Liu, S. J., Li, C. Y., Wu, L., Ma, S. T., Ju, W. Z., et al. (2014). Pharmacokinetic Effect of Aspirin on Caffeic Acid in Dengzhanxixin Injection. Chin. Pharm acological Bull. 30 (4), 570–574. doi:10.3969/j.issn.1001-1978.2014.04.028
Deng, L. P., Le, Y. W., Huang, X. Q., and Shen, M. J. (2014). Effect of Breviscapine on Expression of Renal Tissue MMP-9 in Rats with Diabetic Nephropathy. China Trop. Med. 14 (04), 401–403. doi:10.13604/j.cnki.46-1064/r.2014.04.030
Deng, W., Han, W., Fan, T., Wang, X., Cheng, Z., Wan, B., et al. (2018). Scutellarin Inhibits Human Renal Cancer Cell Proliferation and Migration via Upregulation of PTEN. Biomed. Pharmacother. 107, 1505–1513. doi:10.1016/j.biopha.2018.08.127
Ding, D., Jiao, L. H., Wang, X. C., Liu, Z. Y., Fan, L. H., and Li, Q. H. (2018). Influence of Breviscapine on Myocardial Apoptosis and NF-kB Pathway Signaling Molecules (α7nAChR, P65 and IkB-α) in Rats with Myocardial Ischemia-Reperfusion Injury. Chin. J. Evidence-Based Cardiovasc. Med. 10 (12), 1480–1483+1487. doi:10.3969/j.issn.1674-4055.2018.12.11
Ding, J. S., and Zhang, J. S. (2003). Absorption of Breviscapine in Small Intestine of Rat. J. China Pharm. Univ. 34 (1), 65–69. doi:10.3321/j.issn:1000-5048.2003.01.017
Dong, J. Y. (2016). Clinical Observation of Dengzhanxixin Mistura Combined with Citicoline in Treatment of Glaucoma Optic Atrophy. Drugs & Clinic 31 (3), 354–357. doi:10.7501/j.issn.1674-5515.2016.03.020
Du, B., Du, R., and Wu, H. Q. (2016). The Effect of Breviscapine on the Visual Function of Primary Open-Angle Patients in the Middle and Late Stages of Glaucoma Filtering Surgery. Mod. J. Integrated Traditional Chin. West. Med. 25 (24), 2709–2710. doi:10.3969/j.issn.1008-8849.2016.24.032
Du, F., He, G., and Chen, D. G. (2017). Effects of Breviscapine on Airway Remodeling in Rats with Chronic Obstructive Pulmonary Disease Model. Hebei J. Traditional Chin. Med. 39 (07), 1069–1073. doi:10.3969/j.issn.1002-2619.2017.07.027
Du, F. Z., Niu, R. K., and Zhu, K. J. (2019). The Clinical Effect of Dengzhan Xixin Injection in the Treatment of Diabetic Peripheral Neuropathy. Guangdong Med. J. 40 (5), 740–742. doi:10.3969/j.issn.1001-9448.2019.05.030
Fang, X., Jin, S., and Bao, Y. C. (2013). The Neuroprotective Effect of Dengzhanxixn Injection on Patients with Transient Ischemic Attack. Clin. J. Traditional Chin. Med. 25 (11), 990–991. doi:10.16448/j.cjtcm.2013.11.043
Fang, Z. J. (2008). Clinical Observation of Dengzhanxixin Injection in the Treatment of Vertebrobasilar Blood Supply Insufficiency in 60 Cases. China Foreign Med. Treat. 27 (16), 7–8. doi:10.3969/j.issn.1674-0742.2008.16.005
Feng, Z. W., and Hu, Z. C. (2005).Observation of the Curative Effect of Breviscapine Injection in the Treatment of Acute Cerebral Infarction. J. Guangxi Med. Univ., 438–439. doi:10.16190/j.cnki.45-1211/r.2005.03.058
Fu, Y., Sun, S., Sun, H., Peng, J., Ma, X., Bao, L., et al. (2019). Scutellarin Exerts Protective Effects against Atherosclerosis in Rats by Regulating the Hippo-Foxo3a and PI3K/AKT Signaling Pathways. J. Cel Physiol 234 (10), 18131–18145. doi:10.1002/jcp.28446
Gan, H. Z., He, Q., Ai, Q. B., Zhang, Y. J., Zhang, S. B., Chen, Y. J., et al. (2012). Breviscapine Pretreatment of Donor Liver Reduces Early Ischemia-Reperfusion Injury after Liver Transplantation in Rats. Chin. J. Organ. Transplant. (01), 44–47. doi:10.3760/cma.j.issn.0254-1785.2012.01.012
Gao, C., Zhao, Z. G., Xu, C. M., Wang, R. L., Li, D. K., Xue, L. N., et al. (2007a). Investigation and Analysis of the Usage of DengZhanxixin and DengZhanhuaSU Injections in Inpatients Covered by Beijing Medical Innsurence System. Clin. Medication J. (02), 42–46. doi:10.3969/j.issn.1672-3384.2007.02.014
Gao, L., Tang, H., Zeng, Q., Tang, T., Chen, M., and Pu, P. (2020). The Anti-insulin Resistance Effect of Scutellarin May Be Related to Antioxidant Stress and AMPKα Activation in Diabetic Mice. Obes. Res. Clin. Pract. 14 (4), 368–374. doi:10.1016/j.orcp.2020.06.005
Gao, Y., Du, F., Shen, D. B., Yu, D. Q., and Liu, B. (2007b). Effects of Br Eviscapine on the Levels of Nitrogen Monoxidum and Angiotensin Ⅱ in the R Enal Cortex of Early Diabetic Rats. Chin. J. Tissue Eng. Res. (38), 7597–7600. doi:10.3321/j.issn:1673-8225.2007.38.035
Gao, Z., Jia, Y., Xu, C. M., Li, T., Cheng, X. S., Wang, L. W., et al. (2008). Clinical Investigation of 200 Cases of DengZhanxixin Injection in Beijing Tiantan Hospital Capital Medicine, 37–38.
Gong, M. Y., Du, C., and Su, L. (2013). The Effect of Breviscapine on the Expression of Caspase-9 Protein and mRNA in Myocardial Ischemia-Reperfusion Rats. Chin. J. Gerontol. 33 (02), 347–349. doi:10.3969/j.issn.1005-9202.2013.02.046
Gong, M. Y., Du, C., and Yuan, B. Y. (2013). Effects of Breviscapine on Serum TNF-α and IL-6 in Rats with Myocardial Ischemia Reperfusion. Lishizhen Med. Materia Med. Res. 24 (07), 1615–1616. doi:10.3969/j.issn.1008-0805.2013.07.031
Gong, M. Y., Zhou, X. H., Zhang, L., and Chen, Y. J. (2011). Inhibition of Breviscapine on Cardiomyocytes Apoptosis Induced by Ischemia-Reperfusion Injury in Rat. J. Basic Chin. Med. 17 (05), 517–519.
Gui, S. Y., and ZhoubY, Q. (2009). Determination of Scutellarin in Breviscapine Dropping Pills by Reversed-phase High Performance Liquid Chromatography. China J. Chin. Materia Med. 29 (4), 94–95. doi:10.3321/j.issn:1001-5302.2004.04.028
Guo, C., Zhu, Y., Weng, Y., Wang, S., Guan, Y., Wei, G., et al. (2014). Therapeutic Time Window and Underlying Therapeutic Mechanism of Breviscapine Injection against Cerebral Ischemia/reperfusion Injury in Rats. J. Ethnopharmacol 151 (1), 660–666. doi:10.1016/j.jep.2013.11.026
Guo, L. L., Deng, J., Wang, Y. L., Huang, Y., and Guan, Z. Z. (2011a). Influence of Scutellarin on Oxidative Stress and Neuronal Apoptosis of Rats with Dementia. Zhong Yao Cai 34 (02), 237–241. doi:10.13863/j.issn1001-4454.2011.02.024
Guo, L. L., Wang, Y. L., Huang, Y., and Guan, Z. Z. (2011b). Effect of Scutellarin on Expressions of Nicotinic Acetylcholine Receptor Protein and mRNA in the Brains of Dementia Rats. Zhongguo Zhong Xi Yi Jie He Za Zhi 31 (06), 789–793.
Guo, L. L., and Guan, Z. Z. (2013). Intervention of Scutellarin on Neuro-Inflammatory Reaction in the Brains of Rats with Dementia. Chin. J. Exp. Traditional Med. Formulae 19 (15), 186–190. doi:10.11653/syfj2013150186
Guo, L. L., Wang, Y. J., and Yu Y, N. (2017). Influence of Scutellarin on Protein Expression Spectrum in Dementia Mice Brain Tissue. J. Chin. Med. Mater. 40 (08), 1917–1924. doi:10.13863/j.issn1001-4454.2017.08
Guo, T., and Li, Y. Y. (2012). Progresses on Pharmacological and Toxicological Effects of Dengzhanxixin Injection. Zhongguo Zhong Yao Za Zhi 37 (17), 2820–2823. doi:10.4268/cjcmm20121838
Guo, X., Lin, S., Wu, L. M., and Tian, X. H. (2019). Progress in Studies on Chemical Constituents and Pharmacological Action of Erigeron Breviscapus. Chin. Traditional Patent Med. 41 (02), 393–402. doi:10.3969/j.issn.1001-1528.2019.02.030
Guo, X. L., Zhang, H. B., Chen, W., and Jiang, X. L. (2016). Research of Fleabane Raising VEGF after Focal Cerebral Ischemia and Reperfusion in Rats. J. Emerg. Traditional Chin. Med. 25 (06), 985–987+1000. doi:10.3969/j.issn.1004-745X.2016.06.012
Han, D. (2009). The Protective Effect of Breviscapine on Retinal Oxidative Stress and Retinal Cell Apoptosis in Diabetic Rats. Chin. J. Med. Guide 11 (09), 1537–1538+1541. doi:10.3969/j.issn.1009-0959.2009.09.054
Han, F. W., Gong, M. Y., and Yang, J. (2012). Effects of Breviscapine on Myocardial Apoptosis and STAT Expression in Rats with Ischemia-Reperfusion. Shandong Med. J. 52 (01), 39–40. doi:10.3969/j.issn.1002-266X.2012.01.017
Han, Y. L., Wang, Q., and Jiao, C. Y. (2004). Observation of Curative Effect of Breviscapine Injection on Hyperviscosity. Chin. J. Pract. Nervous Dis. 7 (5), 64–65. doi:10.3969/j.issn.1673-5110.2004.05.064
Hao, P. D., Wu, Q. J., Wang, S. M., Yang, M. F., and Sun, B. L. (2017). Protective Effect of Breviscapine on Cerebral Ischemia/reperfusion Injury in Rats. Chin. J. Geriatr. Heart Brain Vessel Dis. 19 (07), 749–754. doi:10.3969/j.issn.1009-0126.2017.07.018
He, S., and Liu, X. M. (2004). The Effect of Dengzhanxixin Injection on Hyperviscosity. Med. Inf. 17 (6), 339. doi:10.3969/j.issn.1006-1959.2004.06.036
He, Y. Z., and Liu, X. Y. (2002). Study on the Determination of Breviscapine Dripping Pills. Chin. Traditional Herbal Drugs 33 (6), 42–65. doi:10.7501/j.issn.0253-2670.2002.6.261
Hou, L. B., Qiao, L. J., and Guo, J. W. (2015). Effect of Erigeron Breviscap Us Injection on VEGFMMP-9 and EPCs in Patients with Acute Cerebral Infarction of Blood Stasis Pattern. Chin. Traditional Patent Med. 37 (11), 2373–2378. doi:10.3969/j.issn.1001-1528.2015.11.008
Hu, H. M., Xu, Y., Yan, P., Li, M., and Lu, H. D. (2015). Clinical Efficacy of Breviscapine in Combination with Roflumilast in the Treatment of Chronic Obstructive Pulmonary Disease and its Effects on Immunological Function. Chin. J. Clin. Pharmacol. (17), 1706–1708. doi:10.13699/j.cnki.1001-6821.2015.17.002
Hu, S. S., Gao, R. L., Su., Li. L., Zhu, M. L., Wang, W., Wang, Y. J., et al. (2019). Summary of the 2018 Report on Cardiovascular Diseases in China. Chin. Circ. J. 34 (3), 209–220.
Hu, X. M., Zhou, M. M., Hu, X. M., and Zeng, F. D. (2005). Neuroprotective Effects of Scutellarin on Rat Neuronal Damage Induced by Cerebral Ischemia/reperfusion. Acta Pharmacol. Sin 26 (12), 1454–1459. doi:10.1111/j.1745-7254.2005.0023910.1111/j.1745-7254.2005.00239.x
Hu, Y. S., Liao, J., Liu, S. F., and Luo, J. F. (2009). Effects of Breviscapine Injection on Hyperbilirubinemia Caused by Viral Hepatitis. Chin. J. Exp. Clin. Infect. Dis. 3 (3), 298–301. doi:10.3969/j.issn.1674-1358.2009.03.011
Huang, K., Guo, J. W., Chen, J. M., Qiu, G. Q., Deng, Z. J., Li, L. M., et al. (2010). Effects of Erigeron Breviscapus Injection on TNF-α, PAI-1 and tPA in Rats with Acute Myocardial Infarction. J. Chin. Med. Mater. 33 (10), 1592–1595. doi:10.13863/j.issn1001-4454.2010.10.016
Huang, X., Li, X. X., Yu, Z., Yang, W. M., and Li, L. (2017). Scutellarin Antagonizes Ischemia-Reperfusion Injury to Human Cardiac Microvascular Endothelial Cells by Increasing ERK Phosphorylation. J. Med. Postgraduates 30 (09), 912–916. doi:10.16571/j.cnki.1008-8199.2017.09.004
Huang, X. Y., Wan, S. L., Ding, Y., Zheng, J. F., and Li, W. L. (2013). Study on Quality Standard for Dengzhan Xixin Soft Capsules. China Pharmacist 16 (9), 1288–1289.
Jack Li, J. (2009). Smiles Rearrangement. Proceeding Clin. Med. 18 (19), 511–514. doi:10.3969/j.issn.1671-8631.2009.07.01410.1007/978-3-642-01053-8_238
Ji, L. W., Zhang, Y. T., Lin, X., Zhou, B., Ni, Q., Hu, X., et al. (2009). Analysis of the Use of Deng-Zhan-Xi-Xin Injection in Beijing Hospital. Chin. J. Clin. Pharmacol. 25 (04), 355–356. doi:10.3969/j.issn.1001-6821.2009.04.017
Ji, Y. B., MaX, Y., Chen, R. C., Yang, L. P., Sun, G. B., and Sun, X. B. (2019). Protective Effect of Scutellarin on Myocardial Ischemia/Reperfusion Injury and its Mechanism. Chin. Pharmacol. Bull. 35 (05), 648–653. doi:10.3969/j.issn.1001-1978.2019.05.013
Jia, J. H., Zhou, L. Z., Chen, S. X., and Pan, W. (2011). Protective Effects of Breviscapine on Renal Ischemia-Reperfusion Injury in Rats. Jiangsu Med. J. 37 (05), 506–508. doi:10.19460/j.cnki.0253-3685.2011.05.004
Jiang, D. P. (2017). Effects of Breviscapine Injection on Platelet Activity Indexes in Patients with Transient Ischemic Attack. China Med. Eng. 25 (12), 50–52. doi:10.19338/j.issn.1672-2019.2017.12.016
Jiang, W., Li, Z., Zhao, W., Chen, H., Wu, Y., Wang, Y., et al. (2016). Breviscapine Attenuatted Contrast Medium-Induced Nephropathy via PKC/Akt/MAPK Signalling in Diabetic Mice. Am. J. Transl Res. 8 (2), 329–341.
Jiang, X. H., Li, S. H., Lan, K., Yang, J. Y., and Zhou, J. (2003). Study on the Pharmacokinetics of Scutellarin in Dogs. Yao Xue Xue Bao 38 (5), 371–373. doi:10.3321/j.issn:0513-4870.2003.05.013
Jiang, Y. S., Liang, G. S., and Lv, H. J. (2018). Efficacy of Breviscapine Injection in the Treatment of Unstable Angina Pectoris Caused by Coronary. Med. Innovation China 15 (4), 76–79. doi:10.3969/j.issn.1674-4985.2018.04.022
Jin, Z. Y., and Luo, Y. Y. (2013). Erigeron Injection in Treatment of Ischemic Stroke and its Influence on Serum Hs-CRP and UA Levels. Chin. Arch. Traditional Chin. Med. 31 (06), 1390–1392. doi:10.13193/j.archtcm.2013.06.176.jinzhy.032
Ju, W. Z., Zhang, J., and Tan, H. S. (2005). Determination of Scutellarin in Human Plasma by LC-MS Method and its Clinical Pharmacokinetics in Chinese Healthy Volunteers. Chin. J. Clin. Pharmacol. Ther. 10 (3), 298–301. doi:10.3969/j.issn.1009-2501.2005.03.011
Kang, S. Q., and Liu, J. Y. (2003). Effect of Breviscapine on Urinary Micro-albumine in Patients with Diabetes Mellitus Type 2. Zhongguo Zhong Xi Yi Jie He Za Zhi 23 (06), 458–459. doi:10.3321/j.issn:1003-5370.2003.06.019
Kong, Q. F., Wang, J. H., He, M., Zhao, M., Gao, R. H., and Wang, J. (2010). Impacts of Breviscapine Injection on Vascular Endothelia Function and Heart Function in Chronic Cor Pulmonale Decompensation. World J. Integrated Traditional West. Med. 5 (8), 692–695. doi:10.3969/j.issn.1673-6613.2010.08.016
Li, G., Guan, C., Xu, L., Wang, L., Yang, C., Zhao, L., et al. (2020). Scutellarin Ameliorates Renal Injury via Increasing CCN1 Expression and Suppressing NLRP3 Inflammasome Activation in Hyperuricemic Mice. Front. Pharmacol. 11, 584942. doi:10.3389/fphar.2020.584942
Li, J. H., and Zhang, C. (2010). Effects of Herba Erigerontis Injection on SOD Activity and MDA Content in Heart and Lung Tissues of Rats Treated by Chronic Hypoxia. Med. J. Natl. Defending Forces Southwest China 20 (08), 825–826. doi:10.3969/j.issn.1004-0188.2010.08.005
Li, J., Wu, L. Y., and Wang, C. Y. (2011). Influence of Breviscapine Injection to Correlated Inflammatory Cytokines in Patients with Type 2 Early Diabetic Nephropathy. Pharmacol. Clin. Chin. Materia Med. 27 (3), 110–112. doi:10.13412/j.cnki.zyyl.2011.03.044
Li, Q. C., Li, Y. J., Zhang, H., and Li, S. F. (2007a). The Protective Effect of Herba Erigeromtis against Glaucoma of SD Rats. J. Binzhou Med. Univ. 30 (5), 330–332. doi:10.3969/j.issn.1001-9510.2007.05.004
Li, R., and Jia, J. (2015). Observation of the Curative Effect of Breviscapine in the Treatment of Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Mod. J. Integrated Traditional Chin. West. Med. (25), 2796–2798. doi:10.3969/j.issn.1008-8849.2015.25
Li, T., and Yang, L. (2014). The Clinical Research of DZXX Injection in the Treatment of Unstable Angina Pectoris. Chin. Foreign Med. Res. (32), 20–21. doi:10.14033/j.cnki.cfmr.2014.32.01222
Li, W. J., Tao, C. G., and Peng, C. (2007b). Pharmacokinetic of Scutellarin after Iv Erigeron Injection in SD Rats. West China J. Pharm. Sci. 22 (05), 520–522. doi:10.3969/j.issn.1006-0103.2007.05.015
Li, W. J., Tao, C. G., and Peng, C. (2007c). The Pharmacokinetic Study of Caffeic Acid Esters after Iv Erigeron Injection in Rats. Pharmacol. Clin. Chin. Materia Med. 23 (02), 26–28. doi:10.3969/j.issn.1001-859X.2007.02.015
Li, X. (2018). Clinical Effect of Dengzhanxixin Injection on Patients with Ischemic Stroke. Chin. J. Gerontol. 38 (16), 3850–3851. doi:10.3969/j.issn.1005-9202.2018.16.004
Li, X. P., Ma, F., Zhang, Y. P., and Ren, L. (2013). Effect of Breviscapine on the Expressions of TGF-Β1 and Col-Ⅳ in Mice with Renal Impairment. China Pharm. 24 (03), 213–215.
Li, Y., Lin, G., Xie, Y., Zhang, W., and Guo, T. (2015b). Postmarketing Evaluation on the Safety and Effectiveness of Deng-Zhanxixin Injection Made from Dengzhanxixin (Herba Erigerontis Breviscapi). J. Tradit Chin. Med. 35 (1), 99–103. doi:10.1016/s0254-6272(15)30015-7
Li, Y. Y., Lei, L., and Xie, Y. M. (2015a). A Postmarketing Surveillance Study on 31,724 Patients Using Dengzhan Xixin Injection in Hospital. Zhongguo Zhong Yao Za Zhi 40 (24), 4757–4761. doi:10.4268/cjcmm20152407
Li, Z. W., Chen, Q., and Cai, F. Q. (2006). Observation on the Curative Effect of Dengzhanxixin Injection in the Treatment of Acute Exacerbation of Chronic Cor Pulmonale. Guangdong Med. J. 27 (5), 757–758. doi:10.3969/j.issn.1001-9448.2006.05.077
Liang, J. N., Huang, X. Y., Long, H. Y., Xia, X. H., and Li, W. L. (2012). HPLC Fringerprint Analysis on Breviscarpin Dispersive in Tablets and Breviscapine Material. Chin. J. Exp. Traditional Med. Formulae 18 (10), 77–80. doi:10.3969/j.issn.1005-9903.2012.10.022
Liang, Y. J., and Su, S. L. (2014). Safety Evaluation of Dengzhanhuasu Injection. China Hwalth Care Nutr. 24 (4), 1913–1914.
Liao, C. Z. (2015). Breviscapine for Treating Knee Osteoarthritis in 45 Cases. China Pharmaceuticals (8), 88–89.
Lin, W. P., Ding, T., Wu, M. D., and Zhou, H. P. (2009). Study on Compatibility Stability of Breviscapine for Injection with Common Infusion. Herald Med. 28 (2), 260–261. doi:10.3870/yydb.2009.02.059
Lin, Y. Z., Lu, Z. Y., Liang, X. H., Li, K., Peng, B., and Gong, J. (2016). Effect of Breviscapine against Hepatic Ischemia Reperfusion Injury. J. Surg. Res. 203 (203), 268–274. doi:10.1016/j.jss.2016.02.013
Lin, Z. H. (2008). Clinical Observation of 36 Cases of Diabetic Foot Treated with Conventional Therapy Combined with Dengzhanhuaxixin Injection. J. New Chin. Med. 40 (8), 25–26. doi:10.3969/j.issn.0256-7415.2008.08.015
Lin, Z. H. (2012). The Effect of Dengzhanxixin Injection on Plasma PaO2and PaCO2in Patients with Acute Cerebral Infarction. Guangdong Med. J. 33 (15), 2339–2340. doi:10.13820/j.cnki.gdyx.2012.15.005
Liu, F., Liu, Z. G., and Guo, X. L. (2020a). Effect of Erigeron Breviscapus Combined with Captopril on Renal Function in Patients with Acute Glomerulonephritis. 22(04),25–28. doi:10.3969/j.issn.1008-987x.2020.04.06
Liu, G., Tang, G., Liang, W., Wang, Z., Xu, W., Fan, G., et al. (2020b). PK-PD Correlation of Erigeron Breviscapus Injection in the Treatment of Cerebral Ischemia-Reperfusion Injury Model Rats. J. Mol. Neurosci. 1–23. doi:10.1007/s12031-020-01651-3
Liu, G. L., Xu, W. L., and Wang, Z. (2017). Simultaneous Determination of Ten Constituents in DengzhanXixin Injection by HPLC. Chin. Traditional Patent Med. 39 (12), 2521–2524.
Liu, H., Liao, W., Wei, L., and Lei, H. (2009). Effects of Dengzhan Xixin on Blood-Brain Barrier Permeability and Metabolites after Cerebral Ischemia-Reperfusion Injuries. Zhongguo Zhong Yao Za Zhi 34 (02), 208–211. doi:10.3321/j.issn:1001-5302.2009.02.023
Liu, H., and Cheng, W. H. (2007). Changes of Hemorheology in Patients with Sudden Deafness before and after Treatment with Dengzhanxixin Injection. Guangdong Med. J. 28 (11), 1866. doi:10.3969/j.issn.1001-9448.2007.11.069
Liu, H., Yang, X. L., and Xu, H. B. (2002). Advances in Studies on Erigeron Breviscapus. Chin. Traditional Herbal Drugs 33 (6), 566. doi:10.7501/j.issn.0253-2670.2002.6.282
Liu, Q. H. (2014). Treatment of Chronic Hepatitis B by Breviscapine-Asarum Injection. Chin. Med. Mod. Distance Edu. China 12 (2), 18–19. doi:10.3969/j.issn.1672-2779.2014.02.009
Liu, T., Li, W., Zhao, J. M., and Luan, X. (2018b). The Effect of Scutellarin on Cell Apoptosis in Type 2 Diabetic Rats. Pharmacol. Clin. Chin. Materia Med. 34 (01), 70–73. doi:10.13412/j.cnki.zyyl.2018.01.017
Liu, X. J., Wang, X. N., Ma, Y., Zhao, J., and Qi, Z. M. (2008). Effects of Breviscapine Injection on Myocardial Ischemia-Reperfusion Arrhythmia in Rats. Pharmacol. Clin. Chin. Materia Med. (01), 33–34. doi:10.3969/j.issn.1001-859X.2008.01.016
Liu, Y., Wang, J., Zhang, X., Wang, L., Hao, T., Cheng, Y., et al. (2019). Scutellarin Exerts Hypoglycemic and Renal Protective Effects in Db/db Mice via the Nrf2/HO-1 Signaling Pathway. Oxid Med. Cel Longev 2019, 1354345. doi:10.1155/2019/1354345
Liu, Y., Wen, P. H., Zhang, X. X., Dai, Y., and He, Q. (2018a). Breviscapine Ameliorates CCl4-induced L-iver I-njury in M-ice through I-nhibiting I-nflammatory A-poptotic R-esponse and ROS G-enerationammatory A-poptotic R-esponse and ROS G-eneration. Int. J. Mol. Med. 42 (2), 755–768. doi:10.3892/ijmm.2018.3651
Lo, W. L., Guo, J. X., Ping, Q. N., Li, J., Zhao, C. W., and Zhang, L. (2006). Pharmacokinetics of Breviscapine Liposomes Following Intravenous Injection in Beagle Dogs. Yao Xue Xue Bao 41 (1), 24–29. doi:10.3321/j.issn:0513-4870.2006.01.005
Long, H. Y., and Huang, X. Y. (2011). Determination of Pyromeconic Acid in Dengzhan Xixin Jiaonang by HPLC. Cent. South Pharm. 9 (3), 188–190. doi:10.3969/j.issn.1672-2981.2011.03.009
Long, L., Li, Y., Yu, S., Li, X., Hu, Y., Long, T., et al. (2019). Scutellarin Prevents Angiogenesis in Diabetic Retinopathy by Downregulating VEGF/ERK/FAK/Src Pathway Signaling. J. Diabetes Res. 2019, 1–17. doi:10.1155/2019/48754212019
Lou, X. Y., Cheng, J. L., and Zhang, B. (2015). Therapeutic Effect and Mechanism of Breviscapine on Cisplatin-Induced Nephrotoxicity in Mice. Asian Pac. J. Trop. Med. 8 (10), 873–877. doi:10.1016/j.apjtm.2015.09.017
Lu, J. P., and Jiang, L. (2010). Protective Effect of Breviscapine on Myocardial Ischemia Reperfusion Injury in Rabbits. J. Traditional Chin. Med. 51 (11), 1031–1032+1037. doi:10.13288/j.11-2166/r.2010.11.038
Luo, Z. R. (2003). Effect of Dengzhan Xixin Injection on Early Carotid Atherosclerosis in Patients with Unstable Angina Pectoris. Guangdong Med. J. 24 (1), 9–80.
Medical Products Administration (2020). available at: http://app1.nmpa.gov.cn/data_nmpa/face3/base.jsp?tableId=25&tableName=TABLE25&title=%B9%FA%B2%FA%D2%A9%C6%B7&bcId=152904713761213296322795806604 (Accessed May 15, 2020).
Mei, X., Zhang, T., Ouyang, H., Lu, B., Wang, Z., and Ji, L. (2019). Scutellarin Alleviates Blood-Retina-Barrier Oxidative Stress Injury Initiated by Activated Microglia Cells during the Development of Diabetic Retinopathy. Biochem. Pharmacol. 159, 82–95. doi:10.1016/j.bcp.2018.11.011
Mei, Y., Feng, C., Li, J., Zhu, G. H., and Su, S. (2013). Simultaneous Determination of the Contents of 4 Active Ingredients in Dengzhanxixin Mixture by HPLC. J. Chin. Med. Mater. 36 (4), 665–667.
Mo, J., Yang, R., Li, F., Zhang, X., He, B., Zhang, Y., et al. (2018). Scutellarin Protects against Vascular Endothelial Dysfunction and Prevents Atherosclerosis via Antioxidation. Phytomedicine 42, 66–74. doi:10.1016/j.phymed.2018.03.021
Ni, Z. J., Ye, F. S., Tang, X. K., and Tong, P. J. (2013). Observation on the Short-Term Curative Effect of Dengzhanxixin Injection in the Treatment of Femoral Head Necrosis through Internal Circumflex Femoral Artery. Zhejiang Clin. Med. J. 15 (4), 486–488. doi:10.3969/j.issn.1008-7664.2013.04.024
Niu, C., Sheng, Y., Yang, R., Lu, B., Bai, Q., Ji, L., et al. (2015). Scutellarin Protects against the Liver Injury Induced by Diosbulbin B in Mice and its Mechanism. J. Ethnopharmacol 164, 301–308. doi:10.1016/j.jep.2015.02.031
Niu, Y. L., Jiang, Y., Tian, Z. Q., and Miao, Q. (2007). The Effect of Breviscapine on the Expression of NF-Κb after Cerebral Ischemia and Reperfusion in Rats. Guangdong Med. J. (09), 1408–1409. doi:10.3969/j.issn.1001-9448.2007.09.015
Pan, R. G., Teng, J. Z., Zhu, J. L., Ou, Z. X., Han, J., and Wang, D. W. (2018). Clinical Observation on the Effect of Dengzhan Xixin Injection on Hemorheology and Blood Lipid after Decompression of the Heart of Femoral Head Necrosis. Mod. J. Integrated Traditional Chin. West. Med. 27 (1), 64–66. doi:10.3969/j.issn.1008-8849.2018.01
Pengyue, Z., Tao, G., Hongyun, H., Liqiang, Y., and Yihao, D. (2017a). Breviscapine Confers a Neuroprotective Efficacy against Transient Focal Cerebral Ischemia by Attenuating Neuronal and Astrocytic Autophagy in the Penumbra. Biomed. Pharmacother. 90, 69–76. doi:10.1016/j.biopha.2017.03.039
Qiu, M. (2006). The Effect of Breviscapine on TIMP-1 Serum Liver Fibrosis Indexes in Patients with Chronic Hepatitis B. Chin. J. Integrated Traditional West. Med. Digestion 14 (3), 188–190. doi:10.3969/j.issn.1671-038X.2006.03.015
Ren, L. W., Zhang, Y. Z., Wang, W. R., He, H. D., Yu, Y., and Wu, H. Y. (2009). Experimental Study of Dengzhanxixin Injection in the Treatment of Rats with Experimental Study of Dengzhanxixin Injection in the Treatment of Rats with. Chin. Arch. Traditional Chin. Med. 27 (11), 2275–2277. doi:10.13193/j.archtcm.2009.11.36.renlw.065
Ren, L., Zhang, Z. G., Li, D. H., Ma, T. J., and Lou, X. Y. (2013). Effect of Breviscapine on Vascular Active Matter in Renal Tissue of Rats with Obstructive Nephropathy. Chin. J. Exp. Traditional Med. Formulae 19 (10), 221–225. doi:10.11653/syfj2013100221
Shen, W. Z., and Li, G. C. (2002). Effects of Dengzhanxixin Injection on Hyperviscosity Syndrome. J. Chin. Microcirc. 6 (4), 222–223.
Sheng, Y. M., Tang, S. W., Zhang, J., and Zhang, Y. (2016). Study of 3,5-Di-O-Caffeoyl Quinic Acid on Penetrating the Blood-Brain Barrier In Vitro and Inhibiting Cerebral Ischemical Reperfusion Injury in Rats. Pharmacol. Clin. Chin. Materia Med. 32 (06), 26–29. doi:10.13412/j.cnki.zyyl.2016.06.008
Shi, J., Zeng, A. N., and Xiong, D. G. (2016). The Mechanism of Breviscapine in Improving Cardiac Function in Rats with Stress Cardiomyopathy. Chin. J. Gerontol. 36 (15), 3646–3648. doi:10.3969/j.issn.1005-9202.2016.15.016
Shin, J. W., Kweon, K. J., Kim, D. K., Kim, P., Jeon, T. D., Maeng, S., et al. (2018). Scutellarin Ameliorates Learning and Memory Deficit via Suppressing β-Amyloid Formation and Microglial Activation in Rats with Chronic Cerebral Hypoperfusion. Am. J. Chin. Med. 46 (6), 1203–1223. doi:10.1142/S0192415X18500635
Shuai, J., and Dong, W. W. (1998). Experimental Research of PKC Inhibitor, erigeron Breviscapus on the Ischemic/reperfusional Brain Injury. Chin. Pharmacol. Bull. 14 (4), 75–77.
Su, F., Song, J. S., Zhao, M. J., and Xie, J. H. (2009). OMC. Promesses et marchandages. Hainan Med. J. 20 (7), 252–253. doi:10.3969/j.issn.1003-6350.2009.07.13110.3917/scpo.jacqu.2009.01.0252
Su, Q. L., Cheng, D. Y., Zheng, B. X., Xia, X. Q., Yang, L., and Chen, X. J. (2006). Preventive Effect of Breviscapine on Hypoxic Pulmonary Hypertension in Rats. J. Sichuan Univ. (Medical Sciences) (06), 951–954. doi:10.3969/j.issn.1672-173X.2006.06.033
Su, Y., Liu, W., Ma, L., Liu, X., Liu, Z., and Zhu, B. (2012). Scutellarin Inhibits Translocation of Protein Kinase C in Diabetic Thoracic Aorta of the Rat. Clin. Exp. Pharmacol. Physiol. 39 (2), 136–140. doi:10.1111/j.1440-1681.2011.0564510.1111/j.1440-1681.2011.05645.x
Sun, C. Y., Nie, J., Zheng, Z. L., Zhao, J., Wu, L. M., Zhu, Y., et al. (2019). Renoprotective Effect of Scutellarin on Cisplatin-Induced Renal Injury in Mice: Impact on Inflammation, Apoptosis, and Autophagy. Biomed. Pharmacother. 112, 108647. doi:10.1016/j.biopha.2019.108647
Sun, J. H. (2012). Effect of Breviscapine Injection on Hypoxemia-Related Pulmonary Hypertension in High Altitude Area. Asia-Pacific Traditional Med. 8 (7), 167–168. doi:10.3969/j.issn.1673-2197.2012.07.098
Tan, Z. H., Yu, L. H., Wei, H. L., and Liu, G. T. (2007). The Protective Action of Scutellarin against Immunological Liver Injury Induced by Concanavalin A and its Effect on Pro-inflammatory Cytokines in Mice. J. Pharm. Pharmacol. 59 (1), 115–121. doi:10.1211/jpp.59.1.0015
Tang, L. P., Gao, Y. L., and Rao, G. X. (2002). Study on Extraction Procedure of Breviscapin Injection. Chin. J. Ethnomedicine Ethnopharmacy 58 (05), 302–310. doi:10.3969/j.issn.1007-8517.2002.05.035
Tao, L. (2016). Analysis on Current Situation of Erigeron Breviscapus Production in Jiucheng Town and Suggestions on Industrialization Development. Friends of farmers getting rich, 20–21. doi:10.3969/j.issn.1003-1650.2016.02.019
Tian, L. H., Zhao, L. Z., Gu, J., Cai, J., and Yu, L. (2014). Breviscapine Listed on Progress of New Varieties and Dosage Form Research. Zhongguo Zhong Yao Za Zhi 39 (19), 3719–3722. doi:10.4268/cjcmm20141911
Tian, R., Zhou, J., Tian, C. W., Gao, X., and Zhao, M. R. (2020). Effect of Intravitreal Injection of erigeron Breviscapus on Optic Nerve Regeneration in Diabetic Mice and Related Mechanisms. J. Clin. Exp. Med. 19 (20), 2136–2139.
Wan, L., Li, S. F., Xiong, Z. M., and Liang, B. (2003). Study on Toxicity of Compound Dengzhanhua Dripping Pills. J. GuiZhou Univ. Traditional Chin. Med. 25 (62), 61–63. doi:10.3969/j.issn.1002-1108.2003.02.048
Wang, B., Ma, Y. H., Hu, Q. R., Tian, L. Y., Zhang, H. X., Ruan, Y., et al. (2014). Study of Protective Effect of erigeron Injection on Renal Ischemia-Reperfusion Injury. Chin. J. Biochem. Pharmaceuticals 34 (01), 38–40.
Wang, D., Zhai, C. S., and Sun, X. M. (2012b). Dengzhanxixin Injection in the Treatment of Unstable Angina Pectoris and its Effect on Hypersensitivity C-Reactive Protein. J. Emerg. Traditional Chin. Med. 21 (08), 1354–1355. doi:10.3969/j.issn.1004-745X.2012.08.106
Wang, J. G., Chen, Q., and Zeng, Y. M. (2005b). Effects of Breviscapine Parenteral Solution on Energy Metabolism and Cerebral Edema after Cerebral Ischemia-Reperfusion in Gerbils. Chin. J. Tissue Eng. Res. (45), 177–179.
Wang, J. L., Gu, W., and Tan, F. (2005a). Effect of erigeron Injection on Platelet Level of CD62p and Serum Content of TNF-Alpha and IL-6 in Patients with Acute Cerebral Infarction. Zhongguo Zhong Xi Yi Jie He Za Zhi 25 (04), 324–326. doi:10.3321/j.issn:1003-5370.2005.04.009
Wang, J. L., Tan, F., Gu, W., Huang, B., Wu, H. K., and Huang, T. (2006a). Effect of Erigeron Injection on Expresions of Adhesion Molecules CD11b/CD18 and Solution Cell Adhesion Molecule-1 in Peripheral Blood of Patients with Acute Cerebral Infarction (ACI). Int. Med. Health Guidance News (19), 4–6. doi:10.3760/cma.j.issn.1007-1245.2006.19.001
Wang, J. N., Yang, L. Q., and Deng, Y. B. (2015a). Effect of Scutellarin on VEGF Expression in Human Retinal Pigment Epithelial Cells and Retinas of Diabetic Rats. Chin. J. Pathophysiology 31 (05), 900–905. doi:10.3969/j.issn.1000-4718.2015.05.023
Wang, J. P., and Wang, Y. M. (1985). Study on Pharmacological Effects of Dengzhanhua. Chin. Traditional Patent Med. 7 (12), 251.
Wang, L. Z. (2015b). Effects of Scutellarin on the Expression of Heat Shock Protein 70 in the Ischemic Side Brain Tissue of Rats with Focal Cerebral Ischemia-Reperfusion Injury. Chin. J. Gerontol. 35 (08), 2182–2184. doi:10.3969/j.issn.1005-9202.2015.08.081
Wang, L. Z., Liu, Z. P., Wang, J. Y., Liu, P., and Zhang, X. M. (2006b). Protective Effect and Mechanisms of Scutellarin on Focal Brain Ischemia-Reperfusion Injury in Rats. J. Shandong Univ. (Health Sciences) (02), 127–130. doi:10.3969/j.issn.1671-7554.2006.02.005
Wang, N., Yang, Z. X., and Yang, S. Y. (2012a). Review and prospect of Research and Development of Erigeron Breviscapus. Yunnan J. Traditional Chin. Med. Materia Med. 33 (05), 69–72. doi:10.3969/j.issn.1007-2349.2012.05.043
Wang, S. B., Fu, M., and Mei, Z. N. (2010). Optimization of the Formulation of Breviscapine Injection. J. South-Central U niversity Nationalities(Nat Sci Edition) 29 (3), 37–39. doi:10.3969/j.issn.1672-4321.2010.03.010
Wang, S. H., Sun, F. F., Xue, W., Jiang, Q., and Dong, L. Y. (2020). Breviscapine Regulates the Activation of NLRP3 Inflammasome to Inhibit Pyroptosis and Apoptosis of Neurons during Chronic Cerebral Ischemia in Rats. Acta Universitatis Medicinalis Anhui 55 (09), 1321–1326. doi:10.19405/j.cnki.issn1000-1492.2020.09.002
Wang, W. W., Zhang, H. M., Wang, L., and Bao, T. H. (2017). Scutellarin Protects against Myocardial Ischemia-Reperfusion Injury by Upregulating miR-140 Expression. Nat. Product. Res. Dev. 29 (02), 299–303+328. doi:10.16333/j.1001-6880.2017.2.021
Wang, X. F., and Li, Y. (2006). Effects of Dengzhanxixin on the Expression and Activity of MMP-9 in Kidney Tissue of Diabetic Rats. Chin. J. Integrated Traditional West. Nephrol. 7 (11), 644–645. doi:10.3969/j.issn.1009-587X.2006.11.009
Wang, X. H. (2015a). Effect of Dengzhanxixin Injection on Cerebral Blood Circulation in Patients with Ischemic Stroke. J. New Chin. Med. 47 (06), 23–24. doi:10.13457/j.cnki.jncm.2015.06.011
Wang, X. M., Wang, Y. F., Pan, G. X., and Yang, J. (2018a). HPLC Determination of Four Active Components in Dengzhanxixin Injection. Zhongguo Zhong Yao Za Zhi 33 (14), 1681–1683.
Wang, Y. G., and Guo, W. P. (2011). Effects of Dengzhanxixin Injection on Hemorheology Indexes and Blood Lipid Levels in Patients with Ischemic Stroke. Mod. J. Integrated Traditional Chin. West. Med. 20 (33), 4260. doi:10.3969/j.issn.1008-8849.2011.33.052
Wang, Y. H., Geng, L., and Li, H. (2015b). China Journal of Chinese Materia Medica. China J. Chin. Materia Med. 40 (10), 1999–2003. doi:10.4268/cjcmm20151029
Wang, Y. J., Ao, J. W., Guo, L. L., and Yu, Y. N. (2018b). Effect of Scutellarin on Expression of Aβ Generation Pathway-Related Protein in Brain Tissues of Model Mice with Alzheimer′s Disease. Shandong Med. J. 58 (26), 5–8. doi:10.3969/j.issn.1002-266X.2018.26.002
Wang, Y. J., Ao, J. W., Yu, Y. N., and Guo, L. L. (2019). Scutellarin Inhibits β-amyloid Induced Neuronal Apoptosis by IP3R-Ca2 + Pathway. Chin. J. Hosp. Pharm. 39 (6), 561–565. doi:10.13286/j.cnki.chinhosppharmacyj.2019.06.05
Wang, Z., Yu, J., Wu, J., Qi, F., Wang, H., Wang, Z., et al. (2016). Scutellarin Protects Cardiomyocyte Ischemia-Reperfusion Injury by Reducing Apoptosis and Oxidative Stress. Life Sci. 157 (157), 200–207. doi:10.1016/j.lfs.2016.01.018
Wang, Z., Qu, W., and Liang, J. Y. (2012c). Advances in Studies on Erigeron Breuiscapus. Strait Pharm. J. 24 (6), 1–8. doi:10.3969/j.issn.1006-3765.2012.06.001
Wei, L., and Tan, J. (2005). Clinical Observation on Breviscapine in Treating Hypertension Patients Complicated with Micro-albuminuria of Renal Impairment. Chin. J. Integr. Med. 11 (1), 31–33. doi:10.1007/BF02835745
Wei, W. T., Chen, S., Wang, L., and Zeng, J. (2020). Breviscapine Induced the Apoptosis of Non-small Cell Lung Cancer A549 Cells. Chin. J. Clin. Pharmacol. Ther. 25 (06), 618–624. doi:10.12092/j.issn.1009-2501.2020.06.003
Wu, C. J., Tang, B., Xue, F., Shi, Y. P., Zhong, H., and Chen, K. (2007).Long-term Toxicity Test of Dengzhanxixin for Injection in Rats. In The 7th Academic Conference of Chinese Medicine Experimental Pharmacology Branch of Chinese Society of Chinese Medicine.
Wu, D. F., Chen, X., and Qin, Y. C. (2011b). Clinical Observation on 54 Cases of Liver Cirrhosis Treated by Dengzhan Xixin Injection. Med. Inf. 24 (5), 145–146.
Wu, L. Y., Liu, M., and Fang, Z. Y. (2018). Combined therapy of hypertensive nephropathy with breviscapine injection and antihypertensive drugs: a systematic review and a meta-analysis. Evid Based Compl. Alternat. Med. 7, 2958717. doi:10.1155/2018/2958717
Wu, S. X., Chen, X. G., and Li, S. M. (2004). Observation of Efficacy of Dengzhan Xixin Injection in. Treating Hyperlipidemia-related Cereb. Infarction 17 (3), 223–224. doi:10.3969/j.issn.1004-1648.2004.03.024
Wu, Y. B., Wu, Y. H., Zhuang, W. D., and Zheng, X. (2006). Influences of Dengzhan X Ix in Injection on Vascular Endothelial Function in Patients with Acute Cerebral Infarction. Chin. J. Integrated Traditional West. Med. Intensive Crit. Care (01), 6–8. doi:10.3321/j.issn:1008-9691.2006.01.003
Wu, Y. H., Cao, L. P., Zhu, G. Q., Shao, Y., and Huang, Y. (2011a). Effect of Dengzhanxixin Injection on the Plasma Levels of T-PA and PAI-1 in Patients with Acute Cerebral Infarction. Guangdong Med. J. 32 (10), 1345–1346. doi:10.13820/j.cnki.gdyx.2011.10.004
Wu, Y. L., Zhao, T., and Li, M. (2017). Protective Effects and Mechanism of Breviscapine on Atherosclerosis Rats. Glob. Traditional Chin. Med. 10 (06), 669–673. doi:10.3969/j.issn.1674-1749.2017.06.007
Xia, L. (2016). Research Progress on the Chemical Constituents and Preparations of Dengzhanxixin. China Pharm. 27 (1), 111–113. doi:10.6039/j.issn.1001-0408.2016.01.36
Xia, X. L. (2013). Clinical Value Research on Dengzhanhuasu Injection Intreatmen to Felderly Patients with Cerebral in Farction. J. Med. Forum 34 (03), 13–14+17.
Xiang, J. J., Yao, S. Y., and Xie, Y. M. (2018). The Safety Research on Dengzhanxixin Injection. Guangdong Med. J. 39 (03), 458–462. doi:10.13820/j.cnki.gdyx.20180209.010
Xiao, M. S., Cheng, Y. H., and Luo, F. Y. (2015). The Application of Dengzhanxixin Injection in the Treatment of Moderate to Severe Chronic Obstructive Pulmonary Disease in the Stable Stage of the Elderly. Guangdong Med. J. (10), 1605–1607. doi:10.13820/j.cnki.gdyx.2015.10.041
Xie, X. G., and Li, Q. X. (2010). Breviscapine Regulates Nerve Conduction in Diabetic Rats. Chin. J. Public Health 26 (11), 1415–1416.
Xie, X. G., Wu, Q., and Sun, X. X. (2011). The Intervention Effect of Breviscapine on the Learning and Memory Ability of Rats with Vascular Dementia. Chin. J. Gerontol. 31 (13), 2498–2499. doi:10.3969/j.issn.1005-9202.2011.13.051
Xu, H., and Zhang, S. (2013). Scutellarin-Induced Apoptosis in HepG2 Hepatocellular Carcinoma Cells via a STAT3 Pathway. Phytother Res. 27 (10), 1524–1528. doi:10.1002/ptr.4892
Xu, L. J., Chen, R. C., Ma, X. Y., Zhu, Y., Sun, G. B., and Sun, X. B. (2020). Scutellarin Protects against Myocardial Ischemia-Reperfusion Injury by Suppressing NLRP3 Inflammasome Activation. Phytomedicine 68, 153169. doi:10.1016/j.phymed.2020.153169
Xu, Z. J., Cai, Y. L., Chen, H. Z., Wang, Y. X., and Xu, G. C. (2010). Dengzhanxixin Combined with Betahistine in the Treatment of Vertebrobasilar Artery Insufficiency in 70 Cases. Guangdong Med. J. 31 (1), 112–113. doi:10.3969/j.issn.1001-9448.2010.01.045
Yang, C. D. (2014). Current Situation and Countermeasures of Erigeron Breviscapus Industry Development. Yunnan Agric. (02), 56–57.
Yang, M., Zhang, Y., Chen, B. C., Yang, Y. R., Xia, P., Xiao, L. R., et al. (2009b). Protective Effects of Breviscapine on Renal Interstitial Fibrosis Induced by Unilateral Ureteral Obstruction in Rats. Med. J. Chin. People's Liberation Army 34 (10), 1206–1210. doi:10.3321/j.issn:0577-7402.2009.10.014
Yang, N., Zhang, Z. Q., and Wang, B. G. (2009a). Effect of Dengzhanxixin Injection on Plasma C-Reactive Protein in Patients with Acute Ischemic Stroke. J. Emerg. Traditional Chin. Med. 18 (2), 219–220. doi:10.3969/j.issn.1004-745X.2009.02.033
Yang, S. Y., Zhong, X. H., Zhang, Y. Z., and Zhao, L. W. (2011). Protective Effect of Breviscapine on Hepatic Injury Induced by Anti-tubercular Drugs in Mice and its Mechanism. Chin. Pharm. J. 46 (16), 1242–1244.
Yang, T. J., He, C. H., Cao, X. G., Ren, J. K., Chen, X. B., and Wang, Y. (2012). Protective Effects of Eirgeron Breviscapus( Vant) Hand-Mazz on Renal Cold Ischemia and Reperfusion Injury in Rats. J. Zhengzhou University(Medical Sciences) 47 (02), 225–228. doi:10.3969/j.issn.1671-6825.2012.02.026
Yang, W. Y., Zhang, Y., and Zhang, J. (2005). Comparing Erigerontis Injection with Breviscapine Tablet on the Clinical and Chemical Constituents. West China J. Pharm. Sci. 20 (04), 298–300. doi:10.3969/j.issn.1006-0103.2005.04.006
Yang, X. F., and Guo, J. J. (2010). Mechanism of the Protection of Scutellarin against Immunological Liver Injury Induced by BCG+LPS in Mice. Lishizhen Med. Materia Med. Res. 21 (08), 1849–1851. doi:10.3969/j.issn.1008-0805.2010.08.001
Yang, Y. C., Wang, R. F., Chen, X. J., Gao, S. H., Zhang, X. L., and Ren, Z. C. (2019b). Effects and Mechanisms of Scutellarin on Cerebral Ischemia-Reperfusion Injury in Rats with Hyperlipemia. Chin. J. Anat. 42 (03), 262–266. doi:10.3969/j.issn.1001-1633.2019.03.010
Yang, Y. C., Wang, R. F., Chen, X. J., Zhang, X. L., Gao, S. H., and Ren, Z. C. (2019a). Expression of Bcl-2 and Bax after Cerebral Ischemia-Reperfusion Injury in Rats with Hyperlipemia and the Effect of Scutellarin. Acta Anatomica Sinica 50 (04), 431–437. doi:10.16098/j.issn.0529-1356.2019.04.005
Yao, J., Liu, Y. M., and Zhang, H. F. (2014). Observation of Therapeutic Effect of Breviscapine Injection in Treating Acute Ischemic Apoplexy with Phlegm-Heat Blocking Collateral Syndrome. Guiding J. Traditional Chin. Med. Pharm. 20 (02), 54–56. doi:10.13862/j.cnki.cn43-1446/r.2014.02.022
Yin, W. W., Chen, S., Bi, K., Lu, B., and Zhang, H. Y. (2017). Study on Effect of Lamps Asarum Injection on Acute Cerebral Infarction Patients with Serum HIF-1α, Caspase-3 and Blood Uric Acidlevels. Liaoning J. Traditional Chin. Med. 44 (05), 996–998. doi:10.13192/j.issn.1000-1719.2017.05.033
Yin, Z. G., Xiao, J., Jiang, Y. P., Guan, J. G., and Huang, L. (2010). Study on the Effect of Dengzhan Xixin Injection on Renal Effective Plasma Flow in Patients with Chronic Renal Failure. Lishizhen Med. Materia Med. Res. 21 (2), 390–391. doi:10.3969/j.issn.1008-0805.2010.02.063
You, M. Y., Cai, Y. H., Wang, S. J., and Zhao, Y. (2014). The Effect of Erigeron Breviscapus Injection on Endothelin and Nitric Oxide Levels of Senior Patients with Cerebral Infarction. J. Guizhou Med. Univ. 39 (03), 333–335+339. doi:10.19367/j.cnki.1000-2707.2014.03.014
Yu, B. D., Wei, J. Y., and Sun, H. X. (2008). Clinical Effect of Dengzhanxixin Injection in Treating Ischemic Cerebrovascular Disease. China Med. Herald (31), 54–55. doi:10.3969/j.issn.1673-7210.2008.31.032
Yu, C. L. (2014). Posters. Clin. Oral Impl. Res. 25 (5), 612. doi:10.13429/j.cnki.cjcr.2014.05.04610.1111/clr.12458_585
Yu, T., Li, Q., and Wang, B. F. (2006). Clinical Observation on Treating 20 Cases of Osteoarthritis of Knee Joint by Washing with Dengzhanxixin Injection. Guiding J. Traditional Chin. Med. Pharmacol. 12 (5), 42–43. doi:10.3969/j.issn.1672-951X.2006.05.02054
Yu, X. P. (2011). Lamps to Spend Grain Injection Hyperlipidemia Patient Treatment Function and its Mechanism of Clinical Observation. Pract. Clin. J. Integrated Traditional Chin. West. Med. 11 (3), 20–21. doi:10.3969/j.issn.1671-4040.2011.03.012
Zhang, B. Y. (2015a). To Investigate the Effect of Erigeron Injection on Platelet Activation in Patients with Coronary Heart Disease and Angina Pectoris. China Cont. Med. Edu. 7 (27), 205–206. doi:10.3969/j.issn.1674-9308.2015.27.143
Zhang, C. H. (2015b). Effect of Continuous Intraarterial Breviscapine Infusion on Geriatric Severe Acute Pancreatitis. Chin. Traditional Patent Med. 37 (8), 1669–1672. doi:10.3969/j.issn.1001-1528.2015.08.008
Zhang, H. F., Hu, X. M., Wang, L. X., Xu, S. Q., and Zeng, F. D. (2009b). Protective Effects of Scutellarin against Cerebral Ischemia in Rats: Evidence for Inhibition of the Apoptosis-Inducing Factor Pathway. Planta Med. 75 (2), 121–126. doi:10.1055/s-0028-1088368
Zhang, H., Wang, X. Y., Liu, Y., Chai, L. J., Wang, H., Zhang, B. L., et al. (2009a). Effects of Dengzhan Xixin Injection on Inflammatory Cytokine Production in Rat Cardiac Microvascular Cells Induced by Tumor Necrosis Factor. Chin. Pharm. J. 44 (23), 1791–1795. doi:10.3321/j.issn:1001-2494.2009.23.008
Zhang, H. Y., Ping, Q. N., Guo, J. X., and Cao, F. (2005). Pharmacokinetics of Breviscapine and its Beta-Cyclodextrin Complex in Rats. Yao Xue Xue Bao 40 (6), 563–567. doi:10.3321/j.issn:0513-4870.2005.06.018
Zhang, L., Bai, L. Y., Mu, K. X., Zhang, X. C., Yang, R. H., and He, B. (2020). Effects of Scutellarin on Expressions of MMP-1, 2, 9 in AS Plaques of Experimental Atherosclerotic Minipigs. Pharmacol. Clin. Chin. Materia Med. 36 (06), 91–97. doi:10.13412/j.cnki.zyyl.2020.06.012
Zhang, L. Y., Liu, Z. Y., Chen, W. D., Li, C. P., Fu, G. F., and Hu, M. Q. (2008). Effect of Dengzhanhuasu injection on acute cerebral infraction and its influence on PAI-1. Chin. J. Integr. Med. Cardio-Cerebrovasc. Dis. 7, 786–787. doi:10.3969/j.issn.1672-1349.2008.07.016
Zhang, M., Kang, X. D., and Liu, Y. (2016). Effects of breViscapine Injection on Nutritional Status and Immune Function of Patients with Acute Pancreatitis. Mod. J. Integrated Traditional Chin. West. Med. 25 (31), 3437–3439. doi:10.3969/j.issn.1008-8849.2016.31.006
Zhang, W., Yang, S. C., Zhang, G. H., and Su, B. (2013). Research on Situation and Countermeasure of Erigeron Breviscapus Plant Production. Zhongguo Zhong Yao Za Zhi 38 (14), 2227–2230. doi:10.4268/cjcmm20131401
Zhang, W. D., Chen, W. S., Kong, D. Y., Li, H. T., Wang, Y. H., and Liu, W. Y. (2000b). Studies on the Chemical Constituents of Erigeron Breviscapus. Chin. Traditional Herbal Drugs 35 (8), 514–516. doi:10.3321/j.issn:1001-2494.2000.08.005
Zhang, W. D., Chen, W. S., Wang, Y. H., Yang, G. J., Kong, D. Y., and Li, H. T. (2000a). Studies on the Flavone Glycosides from the Extract of Erigeron Breviscapus. Chin. Traditional Herbal Drugs 31 (8), 565–566. doi:10.3321/j.issn:0253-2670.2000.08.002
Zhang, W. D., Thi Bang Tam, H. A., and Chen, W. S. (2002). Studies on the Structure and Activity of Phenylic Compounds from Erigeron Breviscapus. Chin. Pharm. J. 37 (8), 579.
Zhang, X., Ji, R., Sun, H., Peng, J., Ma, X., Wang, C., et al. (2018). Scutellarin Ameliorates Nonalcoholic Fatty Liver Disease through the PPARγ/PGC-1α-Nrf2 Pathway. Free Radic. Res. 52 (2), 198–211. doi:10.1080/10715762.2017.1422602
Zhang, X. C., Shen, Z. Q., Yang, R. H., Guo, X. B., Yang, H. L., Luo, W. X., et al. (2017b). Prevention Effect of Scutellarin on Experimental Atherosclerotic Rats. Pharmacol. Clin. Chin. Materia Med. 33 (02), 59–63. doi:10.13412/j.cnki.zyyl.2017.02.017
Zhang, X., Duan, J., and Zhu, K. H. (2004). Study on the Inhibitory Effect of Breviscapine Injection on Early Avascular Necrosis of Femoral Head. Lishizhen Med. Materia Med. Res. 15 (9), 575–576. doi:10.3969/j.issn.1008-0805.2004.09.018
Zhang, X. M. (2007). Dengzhanxixin Injection in the Treatment of Vertebral-Basal Artery Insufficiency. Guangdong Med. J. 28 (3), 470–472. doi:10.3969/j.issn.1001-9448.2007.03.06510.1080/03091900701727955
Zhao, H. Y., Cao, T. H., and Chen, Y. N. (2008). The Effect of Dengzhanxixin Injection on Blood Lipid Levels in Patients with Coronary Heart Disease and Angina Pectoris. China Pract. Med. 3 (28), 101–102. doi:10.3969/j.issn.1673-7555.2008.28.076
Zhao, J., Lv, C., Wu, Q., Zeng, H., Guo, X., Yang, J., et al. (2019a). Computational Systems Pharmacology Reveals an Antiplatelet and Neuroprotective Mechanism of Deng-Zhan-Xi-Xin Injection in the Treatment of Ischemic Stroke. Pharmacol. Res. 147, 104365. doi:10.1016/j.phrs.2019.104365
Zhao, J. G., Liu, J. Z., Qin, J. H., and Zhang, H. (2010). Breviscapine Injection in the Treatment of Unstable Angina Pectoris and its Effect on Prothrombotic Molecular Markers. Chin. J. Integr. Med. Cardio-Cerebrovascular Dis. 8 (04), 396–397. doi:10.3969/j.issn.1672-1349.2010.04.008
Zhao, L. Y., Hu, H. Y., and Ren, L. B. (2019b). Protective Effect and Mechanism Study of Breviscapine on Acute Kidney Injury Induced by Gentamicin. Tianjin J. Traditional Chin. Med. 36 (06), 603–607. doi:10.11656/j.issn.1672-1519.2019.06.21
Zhao, M., and Gu, J. F. (2011). Determination of Scutellarin in Breviscapine Injection by HPLC. Guizhou Med. J. 35 (04), 360. doi:10.3969/j.ISSN.1000-744X.2011.04.032
Zhao, T., Wu, Y. L., and Li, M. (2017). The Effects and Mechanism of Breviscapine on Atherosclerosis in Rats. J. Cardiovasc. Pulm. Dis. 36 (03), 226–229. doi:10.3969/j.issn.1007-5062.2017.03.020
Zhao, Y., Yang, Q. P., Xiao, H., Zhao, C., Liu, H., and Song, D. P. (2009). The Effect of erigeron Breviscapus on Apoptosis and Expression of Bax,bcl-2 in Kidney of Diabetic Rats. Chin. J. Diabetes 17 (11), 864–867. doi:10.3969/j.issn.1006-6187.2009.11.02110.11569/wcjd.v17.i23.2409
Zhen, J., Kong, M., Li, Z. D., Xu, X. H., Luo, S. J., and Geng, J. H. (2011). Effect of Dengzhan Xixin Injection on Serum S100β Protein Expression and Nerve Function Recovery in Patients with Acute Cerebral Infarction. J. Chin. Med. Mater. 34 (10), 1642–1644. doi:10.13863/j.issn1001-4454.2011.10.007
Zhen, J., Lai, J. Y., Dong, J., Kong, M., Luo, S. J., and Li, Z. D. (2012). The Clinical Study of Dengzhanxixin Injection Intervention in Serum BDNF Expression in Patients with Acute Cerebral Infarction. J. Chin. Med. Mater. 35 (04), 670–672. doi:10.13863/j.issn1001-4454.2012.04.011
Zheng, J. F., and Li, W. L. (2011). HPLC Determination of Scutellarin and its Related Substances in Breviscapine Injection. Chin. J. Pharm. Anal. 31 (1), 34–38.
Zhong, H. X., Zhang, Y. Z., Sun, Y. M., Yang, N. J., Qi, L., Zhao, D. H., et al. (2016). Effects of Breviscapine on the Expression of TGF-Β1 and Smad3 in Liver Tissues of Rats with Liver Fibrosis. Chin. J. Vet. Med. 52 (12), 105–107+56.
Zhou, H., Chen, S. X., Wang, L. X., Xie, Y. P., Chen, Y. F., Wang, Q. J., et al. (2002a). Effect of Breviscapine on Protein Kinase C of Chronic Hypoxic Rats. Chin. Pharmacol. Bull. (01), 39–42. doi:10.3321/j.issn:1001-1978.2002.01.012
Zhou, J. Z., Lei, H., Chen, Y. Z., and Li, F. Q. (2002b). Effect of Erigeron Breviscapus Injection on Ventricular and Vascular Remodeling in Spontaneous Hypertension Rats. Zhongguo Zhong Xi Yi Jie He Za Zhi 22 (02), 122–125. doi:10.3321/j.issn:1003-5370.2002.02.013
Zhou, L., Xie, L. Y., Xu, J., Ju, W. Z., Zhang, J., and Tan, H. S. (2011a). Simultaneous Determination of Six Major Compounds in Dengzhanxixin Injection by HPLC. Chin. J. Exp. Traditional Med. Formulae 17 (21), 78–81. doi:10.3969/j.issn.1005-9903.2011.21.023
Zhou, S. J. (2006). Pharmacody Progress and Clinical Evaluation of Dengzhan Xixin Prepartion, Eval. Anal. Drug use hospitals China 6, 22–25. doi:10.3969/j.issn.1672-2124.2006.01.007
Zhou, Y. R., He, L., Zhang, Z., Zeng, Z., Gao, L., Shen, F. W., et al. (2011b). Effect of Breviscapine with BDNF and TrkB in Cerebral Ischemic Rat. Med. J. West China 23 (04), 615–616. doi:10.3969/j.issn.1672-3511.2011.04.006
Zhu, A. P., Wang, L., and Li, Q. (2018). Effect of Breviscapine Injection on Cerebral Vascular Endothelial Function in Rats with Cerebral Ischemia/Reperfusion Injury. J. Emerg. Traditional Chin. Med. 27 (09), 1552–1555+1559. doi:10.3969/j.issn.1004-745X.2018.09.012
Zhu, R. G., Zhou, Z. H., and Huang, C. Y. (2011). Observation and Analysis of the Clinical Efficacy of Breviscapine Injection in the Treatment of Sudden Deafness. China Mod. Doctor 49 (10), 37–38. doi:10.3969/j.issn.1673-9701.2011.10.018
Zhu, Y., Jiang, Y., Liu, Z., Luo, X., and Wu, Z. (2000). The Affect of Erigeron Breviscapus (Vant.) Hand-Mazz on Axoplasmic Transport of Optic Nerve in Rats with Experimentally Elevated Intraocular Pressure. Zhonghua Yan Ke Za Zhi 36 (04), 289–318. doi:10.3760/j:issn:0412-4081.2000.04.014
Keywords: Erigeron breviscapus, breviscapine, scutellarin, clinical application, pharmacology
Citation: Wu R, Liang Y, Xu M, Fu K, Zhang Y, Wu L and Wang Z (2021) Advances in Chemical Constituents, Clinical Applications, Pharmacology, Pharmacokinetics and Toxicology of Erigeron breviscapus. Front. Pharmacol. 12:656335. doi: 10.3389/fphar.2021.656335
Received: 20 January 2021; Accepted: 06 July 2021;
Published: 02 September 2021.
Edited by:
Karl Tsim, Hong Kong University of Science and Technology, Hong Kong, SAR ChinaReviewed by:
Xi rui He, Zunyi Medical University, ChinaShengchao Yang, Yunnan Agricultural University, China
Copyright © 2021 Wu, Liang, Xu, Fu, Zhang, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhang Wang, wangzhangcqcd@cdutcm.edu.cn
 Ruixia Wu1
Ruixia Wu1 Zhang Wang
Zhang Wang