- 1The Ritchie Centre, Hudson Institute of Medical Research, Clayton, VIC, Australia
- 2Department of Obstetrics and Gynaecology, Monash University, Melbourne, VIC, Australia
- 3Monash Biomedicine Discovery Institute, Monash University, Melbourne, VIC, Australia
- 4Centre for Inflammatory Diseases, School of Clinical Sciences, Monash University, Melbourne, VIC, Australia
- 5Gastroenterology and Hepatology Unit, Monash Health, Melbourne, VIC, Australia
The development of tissue fibrosis in the context of a wound-healing response to injury is common to many chronic diseases. Unregulated or persistent fibrogenesis may lead to structural and functional changes in organs that increase the risk of significant morbidity and mortality. We will explore the natural history, epidemiology, and pathogenesis of fibrotic disease affecting the lungs, kidneys, and liver as dysfunction of these organs is responsible for a substantial proportion of global mortality. For many patients with end-stage disease, organ transplantation is the only effective therapy to prolong life. However, not all patients are candidates for the major surgical interventions and life-long immunosuppression required for a successful outcome and donor organs may not be available to meet the clinical need. We will provide an overview of the latest treatment strategies for these conditions and will focus on stem or progenitor cell-based therapies for which there is substantial pre-clinical evidence based on animal models as well as early phase clinical trials of cell-based therapy in man.
Introduction
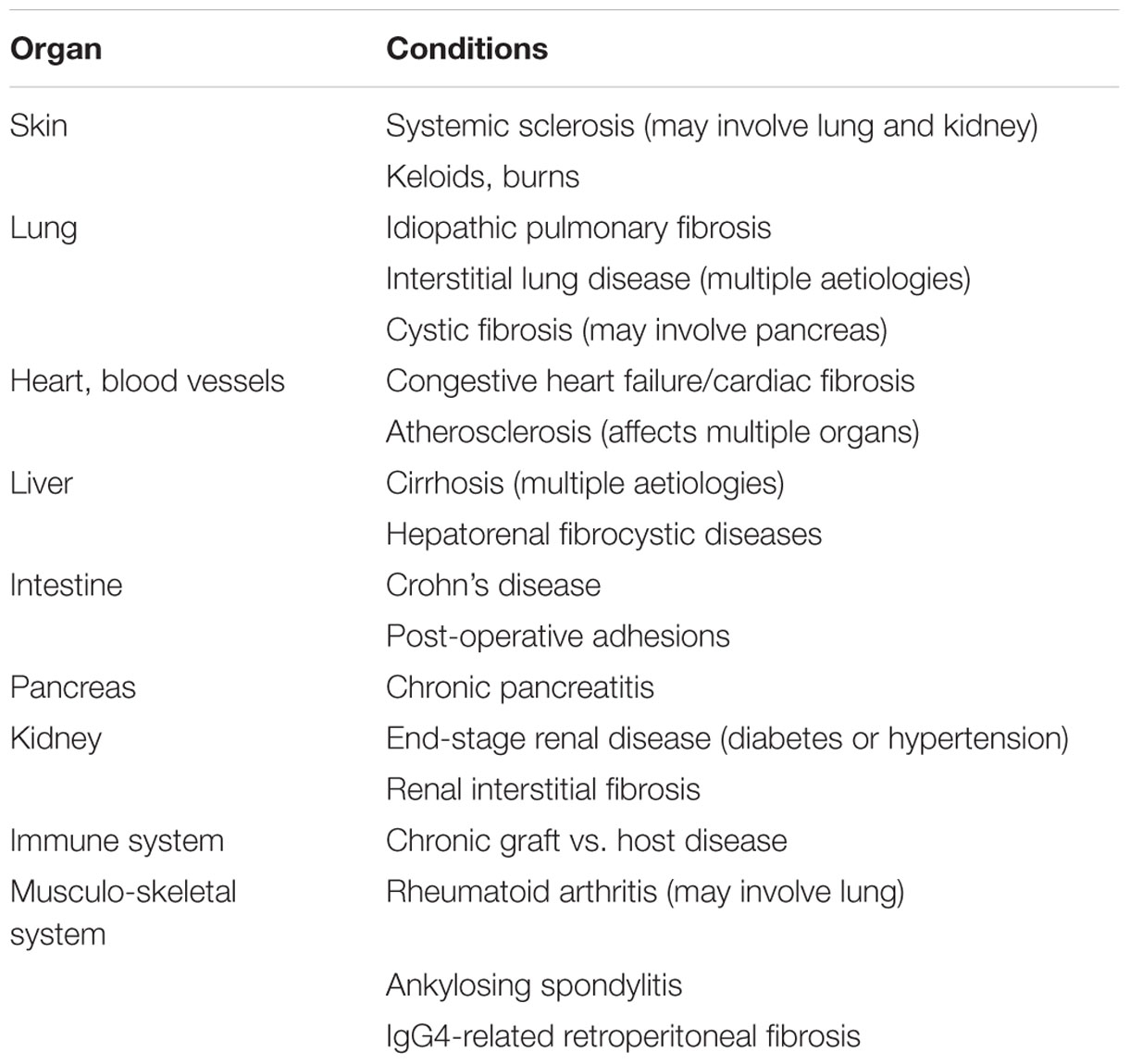
An appropriate response to injury is required for homeostasis. While injury may take many forms, the repair response is typically generic. An understanding of aberrant wound repair has direct relevance to human disease given that organ fibrosis has been estimated to contribute to 45% of all-cause human mortality (Wynn, 2004). While large, this statistic should not be surprising given the significance of fibrosis in chronic diseases affecting multiple organs (Table 1). Despite an extensive understanding of fibrogenesis in response to injury, no effective anti-fibrotic therapies are currently available. The highly conserved wound healing response is also highly redundant with multiple overlapping pathways suggesting that inhibition of a single candidate molecule or pathway is insufficient and new approaches are required. Based on this notion, cell-based therapies with the potential to alter multiple therapeutic targets are gaining popularity. A broad discussion of all stem cell types is beyond the focus of this mini-review. We will concentrate on mesenchymal stem cells (MSCs), which form the largest experience in cell therapy, as well as our work with placental stem cells.
Lung Fibrosis
Epidemiology, Burden of Disease, and Natural History
Pulmonary fibrosis is a family of over 200 chronic lung diseases stemming from multiple underlying causes including autoimmune diseases such as scleroderma and rheumatoid arthritis. Pulmonary fibrosis may be a consequence of environmental exposure to inhaled dust, bacteria, or molds, but can also arise following exposure to cancer treatments such as radiation therapy or chemotherapy using bleomycin or methotrexate. However, idiopathic pulmonary fibrosis (IPF), a type of pulmonary fibrosis where the cause is unknown occurs in 3–9 per 100,000 people annually based on conservative estimates from Europe and North America (Hutchinson et al., 2015). The incidence of IPF is increasing globally, comparable to many cancers (Hutchinson et al., 2015). A low incidence of IPF in some countries may reflect exclusion of milder cases or inconsistent classification. The severity of reported disease appears to be greater in East Asia, where the majority of cases were recorded as “unspecified interstitial lung disease” rather than IPF (Munakata et al., 1994; Ohno et al., 2008; Lai et al., 2012; Han et al., 2013).
Current Clinical Management
The clinical progression of IPF is often slow and gradual but an accelerated decline has been reported in some patients, associated with episodes of acute respiratory exacerbations. The median survival rates are historically poor at 2–3 years, with 5-year survival ranging between 30 and 50% (Bjoraker et al., 1998; Mapel et al., 1998; Rudd et al., 2007; Raghu et al., 2011). To date, lung transplantation remains the only intervention with proven benefit. Corticosteroid use is discouraged due to the association between steroid use and survival rates following acute exacerbations (Papiris et al., 2015).
While drugs such as nintedanib and pirfenidone appear to reduce disease progression, widespread usage is unlikely due to their high cost and conflicting data surrounding clinical efficacy. Currently, the proposed use of pirfenidone is to bridge between diagnosis and lung transplantation (Delanote et al., 2016). Nintedanib has also been found to prevent disease progression, and both drugs are comparable in terms of their estimated costs and health-related quality of life benefits (Rinciog et al., 2017). However, neither is curative and their cost is high (£100,000 per QALY). Thus, there is a need to identify alternative therapies.
Pathophysiology
Historically, IPF was believed to be an inflammatory disorder that progresses to fibrosis. The failure of anti-inflammatory and immunosuppressive therapeutic strategies triggered the need for reassessment (Selman et al., 2001; Raghu et al., 2012). The current consensus is that IPF is a consequence of multiple interacting genetic and environmental risk factors, with repeated damage and premature aging of alveolar epithelial cells (AECs) in genetically susceptible individuals (Wells and Maher, 2017). One robust genetic linkages to IPF is MUC5B polymorphism; however, the role of this gene in IPF pathogenesis remains undefined (Conti et al., 2016; Nakano et al., 2016). Unsurprisingly, the prototypic pro-fibrotic transforming growth factor-β (TGFβ) plays a central role in IPF, and while its function is well described, the source of excess TGFβ and activation of its latent form are poorly understood. A recent study by Froese et al. (2016) uncovered a role for mechanotransduction in TGFβ activation, unique to fibrotic lungs, suggesting that the physical stiffness of IPF lungs and mechanical forces applied to fibrotic lungs may contribute to disease perpetuation. Premature aging, telomere shortening, and alveolar senescence are also thought to contribute to IPF pathogenesis. Telomere dysfunction in AECs but not collagen-producing cells is responsible for age-related lung fibrosis (Naikawadi et al., 2016). When telomere dysfunction was conditionally induced in type 2 AECs (AEC2) in mice, an AEC2-induced cytokine response was detected and when challenged with bleomycin, a 100% mortality rate was observed, supporting the critical role of telomere function in AEC2 for alveolar repair (Alder et al., 2015). Given the role of AEC2 as alveolar progenitor cells, Adler et al. concluded that alveolar stem cell failure might contribute to lung fibrosis. These observations have led some to postulate that a regenerative approach is required (Chambers and Hopkins, 2013).
Cell Therapies for IPF
To date there are six Phase I/II clinical trials (ClinicalTrials.gov) using stem cells for IPF, predominantly allogeneic bone marrow-derived MSCs (NCT01919827, NCT02594839, and NCT02013700). However, placenta and adipose tissue-derived MSCs have also been tested (NCT01385644 and NCT02135380). Interest in MSC-based therapies is attributed to their reported immunomodulatory and anti-fibrotic properties exerted through paracrine mediators. For example, there is recent evidence that MSC can reduce ER stress, thereby improving survival and function of AEC2 through the release of hepatocyte growth factor (Nita et al., 2017). One current clinical trial is aimed at a specific subset (p63+/Krt5+) of the patient’s own lung stem cells (NCT02745184), with the purpose of encouraging cell engraftment and restoring the lost p63+/Krt5+ distal airway stem cells in the fibrotic lung (Zuo et al., 2015). The outcomes of two trials have been published. Allogeneic placental MSCs given at a dose of 1 or 2 × 106 cells/kg body weight were well tolerated in moderate to severe IPF (Chambers et al., 2014). Similarly, a single infusion of 20, 100, or 200 million allogeneic bone marrow MSCs was well tolerated by patients with mild to moderate IPF (Glassberg et al., 2016). While these safety outcomes are encouraging, clinical efficacy remains to be determined.
Kidney Fibrosis
Epidemiology and Pathogenesis of Fibrosis in Kidney Disease
The epidemic of chronic kidney disease (CKD) and end-stage renal failure (ESRF) is a crisis for global healthcare. There is urgent need for new therapeutic options considering the high morbidity of dialysis, extensive healthcare costs, and donor-kidney shortages. Known risk factors for CKD include age, hypertension, obesity, and diabetes (McMahon et al., 2014).
Regardless of etiology, the common end-point of kidney injury is fibrosis leading to CKD development (Samarakoon et al., 2012). An excessive inflammatory and fibrotic response to injury results in decreased renal function as the renal tubules are damaged by scar tissue (Hewitson, 2009). Following initial renal injury, endogenous kidney cells release pro-inflammatory chemokines (Balasubramanian, 2013) that recruit inflammatory cells, activating fibroblasts, and causing tubular dilation (Meran and Steadman, 2011). The recruited immune cells release further inflammatory cytokines including those from the TGFβ superfamily and mitogen-activated protein kinases (MAPK/ERK) that activate fibrotic genes through SMAD signaling (Chevalier et al., 2010), leading to interstitial fibrosis and extracellular matrix accumulation. While inflammation and the TGFβ pathway are essential for normal kidney development and homeostasis, unopposed expression results in a harmful cycle of injury as seen in CKD (Schnaper et al., 2009).
Potential of Cell-Based Therapies for Kidney Disease
Stem or progenitor cell therapies offer a strategy for modulating CKD progression by suppressing multiple pathogenic pathways and promoting pro-regenerative mechanisms. MSCs are pursued as a therapeutic tool as they are immunomodulatory, easily obtainable from bone marrow, and can be expanded in culture for use in the clinic (Yagi et al., 2010). MSCs elicit endogenous repair through paracrine and/or endocrine mechanisms that modulate the immune response, ultimately allowing for cellular replacement. In pre-clinical studies we have demonstrated that MSCs have immunomodulatory properties, and secrete anti-inflammatory cytokines that promote inhibition of pro-inflammatory cytokines (Wise et al., 2014; Huuskes et al., 2015; Wise et al., 2016). MSCs have been used in experimental and clinical settings to improve diabetes and diabetic complications including kidney fibrosis. Recent clinical trials show that MSCs are safe and well tolerated in diabetes (Skyler et al., 2015); however, the diabetic microenvironment and/or comorbidities alter the quality or efficacy of MSCs following transplantation. Further mechanistic studies are needed to understand how MSCs protect against fibrotic injury and to improve efficacy following cell transplantation to overcome the transient clinical benefits that observed to date.
Endothelial progenitor cells (EPCs) also have therapeutic potential. EPCs can be mobilized from the bone marrow and adventitial tissue surrounding endothelial cells (ECs), and home toward sites of injury. There, they influence the release of vasoactive substances or directly differentiate into mature ECs to regenerate damaged endothelium. Diabetes-related EPC dysfunction is closely linked to the impaired healing response experienced by many patients with diabetic CKD. Circulating EPCs are low in type 2 diabetic patients and the loss of function of these cells may contribute to the vasodegenerative changes observed in diabetic micro- and macrovasculature disease (Schatteman et al., 2000). Therefore, harnessing the vascular reparative properties of EPCs represents a novel treatment for therapeutic revascularization and vascular repair for CKD patients with diabetes.
Challenges to Reverse Kidney Fibrosis to Promote Repair
A growing number of clinical trials show that MSCs are safe and well tolerated in diabetes (Skyler et al., 2015). The exogenous application of angiogenic-stimulating EPCs has shown promise for treatment of kidney failure, heart disease, and diabetes including retinopathy (Stitt et al., 2013). Both MSCs and EPCs mediate their effects largely through paracrine signaling and therefore require microenvironments that support optimal cell engraftment and proliferation. However, impediments in clinical translation occur due to low cell survival rates following transplantation that limit therapeutic efficacy (Chevalier et al., 2010). In particular, the fibrosis and chronic inflammation hamper cell survival and limit the cell integration into host tissue. Modulation and removal of the fibrotic lesion is therefore crucial to facilitate cell integration. In addition, the low number of transplanted cells retained at the site of injury also hampers stem cell efficacy.
To overcome these limitations, we recently reported a bimodal attack by combining MSC therapy and relaxin (RLX) to combat kidney fibrosis progression and aid in MSC survival (Huuskes et al., 2015). Combined MSCs and RLX administration in an obstructive nephropathy model significantly ameliorated kidney fibrosis, reduced macrophage infiltration, myofibroblast proliferation, and upregulated active MMP-2 compared to either therapy alone. This suggested that rather than inhibiting collagen accumulation, combination therapy induced significant collagen degradation. We provide evidence that RLX may influence MSCs in vivo creating a more favorable environment for MSC-mediated repair (Huuskes et al., 2015). Targeting fibrosis resolution and limiting vascular damage may also be beneficial through combination therapy, as kidney function is dependent on adequate organ perfusion.
Liver Fibrosis
Epidemiology, Burden of Disease, and Natural History
Globally in 2013, cirrhosis was the 6th cause of life years lost in developed countries; ranging from 5th in Europe and central Asia, to 9th in southeast Asia and Oceania. In the United States, cirrhosis was the 12th leading cause of death overall and the 5th in adults aged 45–54 years (Heron, 2012). Common causes of chronic injury leading to cirrhosis include non-alcoholic steatohepatitis (NASH), alcohol use, and viral hepatitis.
Hepatic fibrosis will progress to cirrhosis in many patients unless the cause of injury is removed. Progressive hepatocyte loss and subsequent disruption of the hepatic vasculature by unregulated ECM expansion result in liver insufficiency characterized by jaundice, coagulopathy, and hypoalbuminemia. Portal hypertension leads to ascites, variceal hemorrhage, and hepatic encephalopathy. The onset of any of these conditions defines hepatic decompensation, which has a significantly higher 1-year mortality than compensated cirrhosis, 20% compared with 5% in one study of 700 patients (Zipprich et al., 2012). In these patients, the only treatment that alters long-term survival is liver transplantation. Unfortunately, not all patients are transplantation candidates and wait-list mortality remains a concern (Toniutto et al., 2016).
Pathogenesis
Hepatic fibrogenesis involves a dynamic interplay among hepatic stellate cells (HSCs), macrophages, and liver progenitor cells (LPCs). HSCs are pericytes that store vitamin A. During chronic liver injury, they transform to myofibroblasts, acquire a contractile phenotype, and accumulate at sites of injury where they secrete large amounts of ECM including collagen. TGFβ is a major fibrogenic cytokine that triggers HSC activation and ECM production and induces hepatocyte apoptosis (Gressner, 2002). Platelet-derived growth factor (PDGF) is the most potent mitogenic cytokine for HSC (Borkham-Kamphorst et al., 2007). These cytokines are logical targets for drug development. Blocking TGFβ and PDGF signaling has been effective in ameliorating experimental liver fibrosis (Yata et al., 2002; Liu et al., 2011), however, off-target effects hinder clinical development.
Kupffer cells (resident liver macrophages) and recruited circulating monocytes contribute to inflammation, fibrogenesis, and fibrosis resolution. Macrophages are capable of distinct activation states and functions, broadly classified as M1 (classical) or M2 (alternative) (Mantovani et al., 2004). M1 macrophages are classically pro-inflammatory, whereas M2 macrophages are responsible for immunomodulation and wound-healing responses. In addition a fibrolytic macrophage subset (Ly6Clo) that produces high levels of matrix metalloproteinases that contribute to ECM degradation has been described (Ramachandran et al., 2012).
LPCs are rare in healthy tissue but proliferate and differentiate into cholangiocytes or hepatocytes during chronic liver injury. The LPC response corresponds with the degree of liver injury (Lowes et al., 1999; Roskams et al., 2003) because, unlike hepatocytes, LPC resist the anti-proliferative actions of TGFβ (Nguyen et al., 2007). LPC express surface markers representative of their primitive, undifferentiated state such as Thy-1 (CD90), prominin (CD133), and pan-cytokeratin. A close physical relationship exists between HSC and LPC suggesting that the two cell types proliferate in tandem as HSC depletion significantly dampens the LPC response (Roskams, 2008; Ruddell et al., 2009). HSC produce soluble factors that increase LPC proliferation and hepatocyte differentiation (Nagai et al., 2002; Lin et al., 2008) and ECM proteins produced by HSC, such as laminin, may activate the LPC response (Kallis et al., 2011). Conversely, LPC produce lymphotoxin (LT), which recruits HSC through paracrine signaling (Ruddell et al., 2009). LPC also recruit macrophages via CCL2 and CX3CL1. Macrophage-derived TNF and LT, in turn, influence LPC response (Viebahn et al., 2010).
Treatment of Hepatic Fibrosis
The concept that hepatic fibrosis develops from a wound-healing response to chronic injury provides a rational basis for treatment. Diminishing liver injury by inhibiting chronic hepatitis B replication results in significant fibrosis regression in cirrhotic patients (Marcellin et al., 2013). Similar outcomes occur in patients with chronic hepatitis C infection (Hoefs et al., 2011). In diseases without specific therapy, a general anti-fibrotic approach might be useful. However, a recent trial of a monoclonal antibody against lysl-oxidase-like 2, which mediates collagen cross-linkage, was not effective (Meissner et al., 2016). Considering the complex interactions involved in hepatic wound healing, cell-based therapy may provide a strategy to control inflammation, degrade collagen, and promote hepatic parenchymal regeneration. Human clinical trials have utilized MSC with variable cell doses, delivery routes, and administration frequency (Table 2). Trial endpoints commonly include liver tests, ascites volume, or clinical scores (Child–Pugh–Turcotte, model for end-stage liver disease). To date, outcomes have yet to translate into clinical practice. Furthermore, there is experimental evidence that bone marrow-derived MSC can contribute to hepatic fibrosis (Russo et al., 2006). MSCs as an anti-fibrotic therapy has been critically reviewed (Haldar et al., 2016).
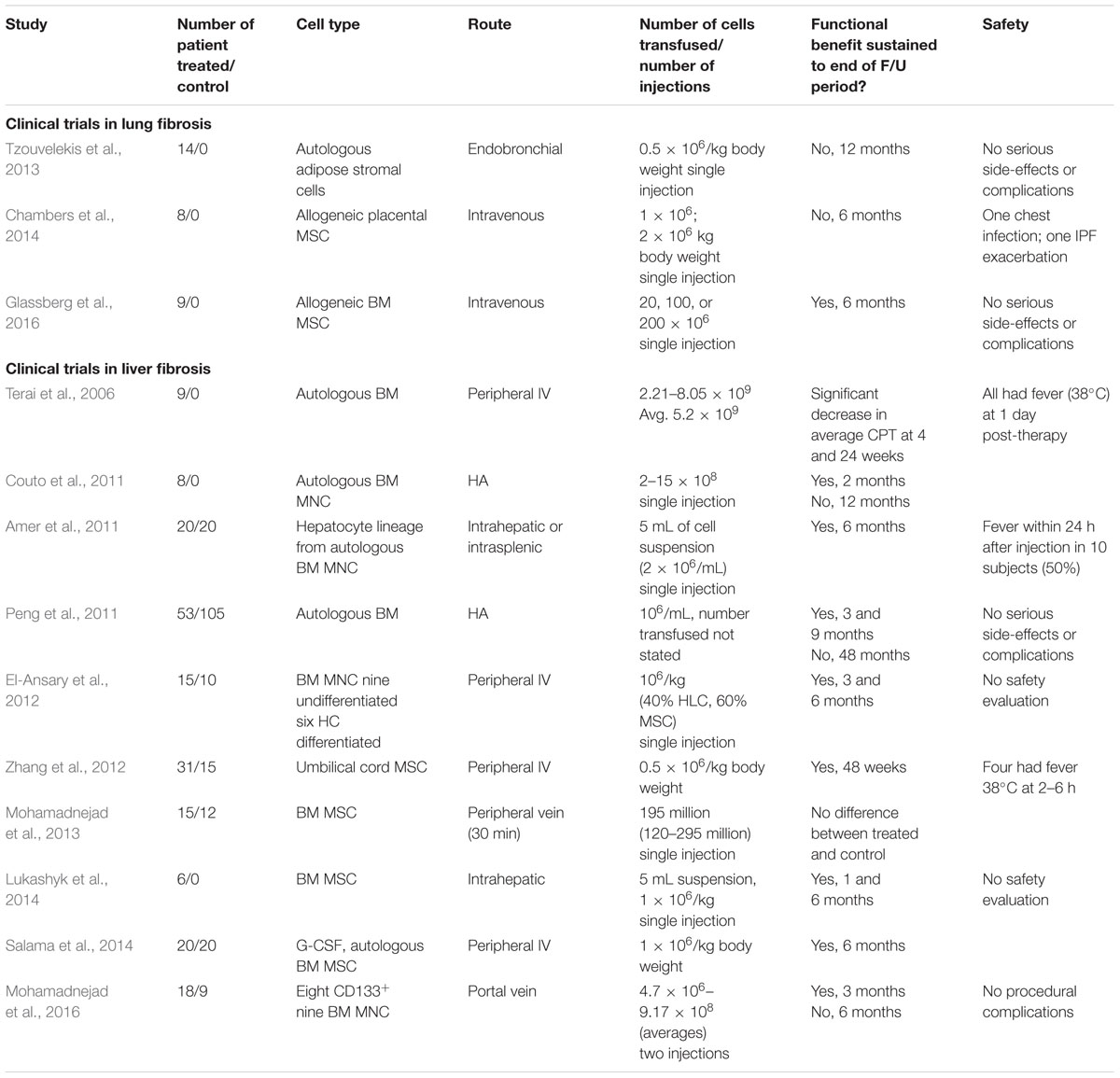
TABLE 2. Summary of reports from clinical trials assessing safety and efficacy of cell therapies for lung and liver fibrosis.
We studied human amnion epithelial cells (hAECs), fetus-derived stem-like cells that arise prior to gastrulation and are easily isolated from the placenta, which is an abundant and ethically undisputed source. hAEC prevent and reverse inflammation and established fibrosis in immunocompetent animal models of liver injury (Manuelpillai et al., 2010), diminish myofibroblast activation, and skew hepatic macrophages toward a reparative phenotype (Manuelpillai et al., 2012). Similar effects are seen with cell-free conditioned media, suggesting that hAEC release factors responsible for the observed outcomes (Hodge et al., 2014). Liver fibrosis reduction also occurs in hAEC-treated mice given a “Western diet” high in lipids and fructose to model fatty liver disease (unpublished). A phase 1 safety trial is planned in patients with compensated cirrhosis.
Summary
The global burden of end-stage fibrotic disease can be seen in the impaired survival of patients with IPF, diabetic CKD, and cirrhosis. Fortunately, the pathogenesis of fibrosis in response to injury is relatively well understood and remarkably similar in different organs, suggesting that an integrated approach may be possible. Control or removal of the injury stimulus should be the primary focus in preventing disease progression, yet for many control is incomplete or unachievable, thus the need for a broadly effective anti-fibrotic therapy that targets multiple fibrogenic pathways remains. Cell-based approaches employing stem cells that are easy to isolate and upscale to sufficient quantities for clinical use have been successfully characterized in animal models of organ fibrosis. While the outcomes of early phase clinical trials indicate that cell-based (primarily MSC) therapies are safe, efficacy data remain scarce. Consequently, cell-based therapies remain largely experimental. The lack of robust efficacy data may be due to the heterogeneity of MSC populations as well as limited agreement regarding differentiation state, doses, and administration regimens. Challenges remain in determining the goals of cell therapy – whether to supply sufficient cells to replace damaged parenchyma, to dampen inflammation with the aim of decreasing fibrosis, or to stimulate endogenous progenitor cells and repair processes. Furthermore, the ability to manufacture, transport, and store stem cells in a cost-effective manner must be considered. Clinical trials will continue to inform us about the most effective stem cell types on which to base therapy as well as the optimal dosages necessary to achieve a clinically meaningful reduction in fibrosis-related organ dysfunction.
Author Contributions
WS contributed the liver fibrosis section; RL contributed the lung fibrosis section; SR contributed the kidney fibrosis section; and all authors reviewed the manuscript and provided critical intellectual input.
Funding
This work was supported by the National Health and Medical Research Council of Australia (1064247 and 1124020) and Diabetes Australia Research Trust.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alder, J. K., Barkauskas, C. E., Limjunyawong, N., Stanley, S. E., Kembou, F., Tuder, R. M., et al. (2015). Telomere dysfunction causes alveolar stem cell failure. Proc. Natl. Acad. Sci. U.S.A. 112, 5099–5104. doi: 10.1073/pnas.1504780112
Amer, M. E., El-Sayed, S. Z., El-Kheir, W. A., Gabr, H., Gomaa, A. A., El-Noomani, N., et al. (2011). Clinical and laboratory evaluation of patients with end-stage liver failure injected with bone marrow-derived hepatocyte-like cells. Eur. J. Gastroenterol. Hepatol. 23, 936–941. doi: 10.1097/MEG.0b013e3283488b00
Balasubramanian, S. (2013). Progression of chronic kidney disease: mechanisms and interventions in retardation. Apollo Med. 10, 19–28. doi: 10.1016/j.apme.2013.01.009
Bjoraker, J. A., Ryu, J. H., Edwin, M. K., Myers, J. L., Tazelaar, H. D., Schroeder, D. R., et al. (1998). Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 157, 199–203. doi: 10.1164/ajrccm.157.1.9704130
Borkham-Kamphorst, E., van Roeyen, C. R. C., Ostendorf, T., Floege, J., Gressner, A. M., and Weiskirchen, R. (2007). Pro-fibrogenic potential of PDGF-D in liver fibrosis. J. Hepatol. 46, 1064–1074. doi: 10.1016/j.jhep.2007.01.029
Chambers, D. C., Enever, D., Ilic, N., Sparks, L., Whitelaw, K., Ayres, J., et al. (2014). A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology 19, 1013–1018. doi: 10.1111/resp.12343
Chambers, D. C., and Hopkins, P. M. A. (2013). Idiopathic pulmonary fibrosis: a degenerative disease requiring a regenerative approach. Am. J. Respir. Crit. Care Med. 188, 252–253. doi: 10.1164/rccm.201301-0192LE
Chevalier, R. L., Thornhill, B. A., Forbes, M. S., and Kiley, S. C. (2010). Mechanisms of renal injury and progression of renal disease in congenital obstructive nephropathy. Pediatr. Nephrol. 25, 687–697. doi: 10.1007/s00467-009-1316-5
Conti, C., Montero-Fernandez, A., Borg, E., Osadolor, T., Viola, P., De Lauretis, A., et al. (2016). Mucins MUC5B and MUC5AC in distal airways and honeycomb spaces: comparison among idiopathic pulmonary fibrosis/usual interstitial pneumonia, fibrotic nonspecific interstitial pneumonitis, and control lungs. Am. J. Respir. Crit. Care Med. 193, 462–464. doi: 10.1164/rccm.201507-1322LE
Couto, B., Goldenberg, R., da Fonseca, L., Thomas, J., Gutfilen, B., Resende, C. M., et al. (2011). Bone marrow mononuclear cell therapy for patients with cirrhosis: a phase 1 study. Liver Int. 31, 391–400. doi: 10.1111/j.1478-3231.2010.02424.x
Delanote, I., Wuyts, W. A., Yserbyt, J., Verbeken, E. K., Verleden, G. M., and Vos, R. (2016). Safety and efficacy of bridging to lung transplantation with antifibrotic drugs in idiopathic pulmonary fibrosis: a case series. BMC Pulm. Med. 16:156. doi: 10.1186/s12890-016-0308-z
El-Ansary, M., Abdel-Aziz, I., Mogawer, S., Abdel-Hamid, S., Hammam, O., Teaema, S., et al. (2012). Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev. 8, 972–981. doi: 10.1007/s12015-011-9322-y
Froese, A. R., Shimbori, C., Bellaye, P.-S., Inman, M., Obex, S., Fatima, S., et al. (2016). Stretch-induced activation of transforming growth factor-β1 in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 194, 84–96. doi: 10.1164/rccm.201508-1638OC
Glassberg, M. K., Minkiewicz, J., Toonkel, R. L., Simonet, E. S., Rubio, G. A., Difede, D., et al. (2016). Allogeneic human mesenchymal stem cells in patients with idiopathic pulmonary fibrosis via intravenous delivery (AETHER): a phase I, safety, clinical trial. Chest 151, 971–981. doi: 10.1016/j.chest.2016.10.061
Gressner, A. M. (2002). Roles of TGF-beta in hepatic fibrosis. Front. Biosci. 7, d793–d807. doi: 10.2741/A812
Haldar, D., Henderson, N. C., Hirschfield, G., and Newsome, P. N. (2016). Mesenchymal stromal cells and liver fibrosis: a complicated relationship. FASEB J. 30, 3905–3928. doi: 10.1096/fj.201600433R
Han, S. H., Mok, Y., Jee, S. H., and Danoff, S. K. (2013). “Incidence and mortality of idiopathic pulmonary fibrosis in South Korea,” in Proceedings of the A42 Interstitial Lung Disease: Epidemiology, Evaluation and Pathogenesis (Washington, DC: American Thoracic Society), doi: 10.1164/ajrccm-conference.2013.187.1_MeetingAbstracts.A1460
Hewitson, T. D. (2009). Renal tubulointerstitial fibrosis: common but never simple. Am. J. Physiol. Renal Physiol. 296, F1239–F1244. doi: 10.1152/ajprenal.90521.2008
Hodge, A., Lourensz, D., Vaghjiani, V., Nguyen, H., Tchongue, J., Wang, B., et al. (2014). Soluble factors derived from human amniotic epithelial cells suppress collagen production in human hepatic stellate cells. Cytotherapy 16, 1132–1144. doi: 10.1016/j.jcyt.2014.01.005
Hoefs, J. C., Shiffman, M. L., Goodman, Z. D., Kleiner, D. E., Dienstag, J. L., Stoddard, A. M., et al. (2011). Rate of progression of hepatic fibrosis in patients with chronic hepatitis C: results from the HALT-C Trial. Gastroenterology 141, 900.e1–2–908.e1–2. doi: 10.1053/j.gastro.2011.06.007
Hutchinson, J., Fogarty, A., Hubbard, R., and McKeever, T. (2015). Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur. Respir. J. 46, 795–806. doi: 10.1183/09031936.00185114
Huuskes, B. M., Wise, A. F., Cox, A. J., Lim, E. X., Payne, N. L., Kelly, D. J., et al. (2015). Combination therapy of mesenchymal stem cells and serelaxin effectively attenuates renal fibrosis in obstructive nephropathy. FASEB J. 29, 540–553. doi: 10.1096/fj.14-254789
Kallis, Y. N., Robson, A. J., Fallowfield, J. A., Thomas, H. C., Alison, M. R., Wright, N. A., et al. (2011). Remodelling of extracellular matrix is a requirement for the hepatic progenitor cell response. Gut 60, 525–533. doi: 10.1136/gut.2010.224436
Lai, C.-C., Wang, C.-Y., Lu, H.-M., Chen, L., Teng, N.-C., Yan, Y.-H., et al. (2012). Idiopathic pulmonary fibrosis in Taiwan - a population-based study. Respir. Med. 106, 1566–1574. doi: 10.1016/j.rmed.2012.07.012
Lin, N., Tang, Z., Deng, M., Zhong, Y., Lin, J., Yang, X., et al. (2008). Hedgehog-mediated paracrine interaction between hepatic stellate cells and marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 372, 260–265. doi: 10.1016/j.bbrc.2008.05.029
Liu, Y., Wang, Z., Kwong, S. Q., Lui, E. L. H., Friedman, S. L., Li, F. R., et al. (2011). Inhibition of PDGF, TGF-β, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J. Hepatol. 55, 612–625. doi: 10.1016/j.jhep.2010.11.035
Lowes, K. N., Brennan, B. A., Yeoh, G. C., and Olynyk, J. K. (1999). Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am. J. Pathol. 154, 537–541. doi: 10.1016/S0002-9440(10)65299-6
Lukashyk, S., Tsyrkunov, V., Isaykina, Y., Romanova, O. N., Shymanskly, A. T., Aleynikova, O. V., et al. (2014). Mesenchymal bone marrow-derived stem cells transplantation in patients with HCV related liver cirrhosis. J. Clin. Transl. Hepatol. 2, 217–221. doi: 10.14218/JCTH.2014.00027
Mantovani, A., Sica, A., Sozzani, S., and Allavena, P. (2004). The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686. doi: 10.1016/j.it.2004.09.015
Manuelpillai, U., Lourensz, D., Vaghjiani, V., Tchongue, J., Lacey, D., Tee, J. Y., et al. (2012). Human amniotic epithelial cell transplantation induces markers of alternative macrophage activation and reduces established hepatic fibrosis. PLOS ONE 7:e38631. doi: 10.1371/journal.pone.0038631
Manuelpillai, U., Tchongue, J., Lourensz, D., Vaghjiani, V., Samuel, C. S., Liu, A., et al. (2010). Transplantation of human amnion epithelial cells reduces hepatic fibrosis in immunocompetent CCl4-treated mice. Cell Transplant. 19, 1157–1168. doi: 10.3727/096368910X504496
Mapel, D. W., Hunt, W. C., Utton, R., Baumgartner, K. B., Samet, J. M., and Coultas, D. B. (1998). Idiopathic pulmonary fibrosis: survival in population based and hospital based cohorts. Thorax 53, 469–476. doi: 10.1136/thx.53.6.469
Marcellin, P., Gane, E., Buti, M., Afdhal, N., Sievert, W., Jacobson, I. M., et al. (2013). Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 381, 468–475. doi: 10.1016/S0140-6736(12)61425-1
McMahon, G. M., Preis, S. R., Hwang, S. J., and Fox, C. S. (2014). Mid-adulthood risk factor profiles for CKD. J. Am. Soc. Nephrol. 25, 2633–2641. doi: 10.1681/ASN.2013070750
Meissner, E., McLaughlin, M., Matthews, L., Gharib, A., Wood, B., Levy, E., et al. (2016). Simtuzumab treatment of advanced liver fibrosis in HIV and HCV-infected adults: results of a 6-month open-label safety trial. Liver Int. 36, 1783–1792. doi: 10.1111/liv.13177
Meran, S., and Steadman, R. (2011). Fibroblasts and myofibroblasts in renal fibrosis. Int. J. Exp. Pathol. 92, 158–167. doi: 10.1111/j.1365-2613.2011.00764.x
Mohamadnejad, M., Alimoghaddam, K., Bagheri, M., Ashrafi, M., Abdollahzadeh, L., Akhlaghpoor, S., et al. (2013). Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 33, 1490–1496. doi: 10.1111/liv.12228
Mohamadnejad, M., Vosough, M., Moossavi, S., Nikfam, S., Mardpour, S., Akhlaghpoor, S., et al. (2016). Intraportal infusion of bone marrow mononuclear or CD133+ cells in patients with decompensated cirrhosis: a double-blind randomized controlled trial. Stem Cells Transl. Med. 5, 87–94. doi: 10.5966/sctm.2015-0004
Munakata, M., Asakawa, M., Hamma, Y., and Kawakami, Y. (1994). Present status of idiopathic interstitial pneumonia–from epidemiology to etiology. Nihon Kyobu Shikkan Gakkai Zasshi 32(Suppl.), 187–192.
Nagai, H., Terada, K., Watanabe, G., Ueno, Y., Aiba, N., Shibuya, T., et al. (2002). Differentiation of liver epithelial (stem-like) cells into hepatocytes induced by coculture with hepatic stellate cells. Biochem. Biophys. Res. Commun. 293, 1420–1425. doi: 10.1016/S0006-291X(02)00406-0
Naikawadi, R. P., Disayabutr, S., Mallavia, B., Donne, M. L., Green, G., La, J. L., et al. (2016). Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight 1:e86704. doi: 10.1172/jci.insight.86704
Nakano, Y., Yang, I. V., Walts, A. D., Watson, A. M., Helling, B. A., Fletcher, A. A., et al. (2016). MUC5B promoter variant rs35705950 affects MUC5B expression in the distal airways in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 193, 464–466. doi: 10.1164/rccm.201509-1872LE
Nguyen, L. N., Furuya, M. H., Wolfraim, L. A., Nguyen, A. P., Holdren, M. S., Campbell, J. S., et al. (2007). Transforming growth factor-beta differentially regulates oval cell and hepatocyte proliferation. Hepatology 45, 31–41. doi: 10.1002/hep.21466
Nita, I., Hostettler, K., Tamo, L., Medová, M., Bombaci, G., Zhong, J., et al. (2017). Hepatocyte growth factor secreted by bone marrow stem cell reduce ER stress and improves repair in alveolar epithelial II cells. Sci. Rep. 7:41901. doi: 10.1038/srep41901
Ohno, S., Nakaya, T., Bando, M., and Sugiyama, Y. (2008). Idiopathic pulmonary fibrosis–results from a Japanese nationwide epidemiological survey using individual clinical records. Respirology 13, 926–928. doi: 10.1111/j.1440-1843.2008.01349.x
Papiris, S. A., Kagouridis, K., Kolilekas, L., Papaioannou, A. I., Roussou, A., Triantafillidou, C., et al. (2015). Survival in Idiopathic pulmonary fibrosis acute exacerbations: the non-steroid approach. BMC Pulm. Med. 15:162. doi: 10.1186/s12890-015-0146-4
Peng, L., Xie, D., Lin, B., Liu, J., Zhu, H. P., Xie, C., et al. (2011). Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology 54, 820–828. doi: 10.1002/hep.24434
Raghu, G., Anstrom, K. J., King, T. E., Lasky, J. A., and Martinez, F. J. (2012). Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 366, 1968–1977. doi: 10.1056/NEJMoa1113354
Raghu, G., Collard, H. R., Egan, J. J., Martinez, F. J., Behr, J., Brown, K. K., et al. (2011). An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 183, 788–824. doi: 10.1164/rccm.2009-040GL
Ramachandran, P., Pellicoro, A., Vernon, M. A., Boulter, L., Aucott, R. L., Ali, A., et al. (2012). Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl. Acad. Sci. U.S.A. 109, E3186–E3195. doi: 10.1073/pnas.1119964109
Rinciog, C., Watkins, M., Chang, S., Maher, T. M., LeReun, C., Esser, D., et al. (2017). A cost-effectiveness analysis of nintedanib in idiopathic pulmonary fibrosis in the UK. Pharmacoeconomics 35, 479–491. doi: 10.1007/s40273-016-0480-2
Roskams, T. (2008). Relationships among stellate cell activation, progenitor cells, and hepatic regeneration. Clin. Liver Dis. 12, 853–860. doi: 10.1016/j.cld.2008.07.014
Roskams, T., Yang, S. Q., Koteish, A., Durnez, A., DeVos, R., Huang, X., et al. (2003). Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am. J. Pathol. 163, 1301–1311. doi: 10.1016/S0002-9440(10)63489-X
Rudd, R. M., Prescott, R. J., Chalmers, J. C., Johnston, I. D. A., and Fibrosing Alveolitis Subcommittee of the Research Committee of the British Thoracic Society (2007). British Thoracic Society Study on cryptogenic fibrosing alveolitis: response to treatment and survival. Thorax 62, 62–66. doi: 10.1136/thx.2005.045591
Ruddell, R. G., Knight, B., Tirnitz-Parker, J. E. E., Akhurst, B., Summerville, L., Subramaniam, V. N., et al. (2009). Lymphotoxin-beta receptor signaling regulates hepatic stellate cell function and wound healing in a murine model of chronic liver injury. Hepatology 49, 227–239. doi: 10.1002/hep.22597
Russo, F. P., Alison, M. R., Bigger, B. W., Amofah, E., Florou, A., Amin, F., et al. (2006). The bone marrow functionally contributes to liver fibrosis. Gastroenterology 130, 1807–1821. doi: 10.1053/j.gastro.2006.01.036
Salama, H., Zekri, A., Medhat, E., Al Alim, S. A., Ahmed, O. S., Bahnassy, A. A., et al. (2014). Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res. Ther. 5, 70. doi: 10.1186/scrt459
Samarakoon, R., Overstreet, J. M., Higgins, S. P., and Higgins, P. J. (2012). TGF-beta1 → SMAD/p53/USF2 → PAI-1 transcriptional axis in ureteral obstruction-induced renal fibrosis. Cell Tissue Res. 347, 117–128. doi: 10.1007/s00441-011-1181-y
Schatteman, G. C., Hanlon, H. D., Jiao, C., Dodds, S. G., and Christy, B. A. (2000). Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J. Clin. Invest. 106, 571–578. doi: 10.1172/JCI9087
Schnaper, H. W., Jandeska, S., Runyan, C. E., Hubchak, S. C., Pasu, R. K., Curley, J. F., et al. (2009). TGF-beta signal transduction in chronic kidney disease. Front. Biosci. 14, 2448–2465. doi: 10.2741/3389
Selman, M., King, T. E., and Pardo, A. (2001). Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann. Intern. Med. 134, 136–151. doi: 10.7326/0003-4819-134-2-200101160-00015
Skyler, J. S., Fonseca, V. A., Segal, K. R., Rosenstock, J., and MSB-DM003 Investigators (2015). Allogeneic mesenchymal precursor cells in Type 2 diabetes: a randomized, placebo-controlled, dose-escalation safety and tolerability pilot study. Diabetes Care 38, 1742–1749. doi: 10.2337/dc14-2830
Stitt, A. W., Lois, N., Medina, R. J., Adamson, P., and Curtis, T. M. (2013). Advances in our understanding of diabetic retinopathy. Clin. Sci. 125, 1–17. doi: 10.1042/CS20120588
Terai, S., Ishikawa, T., Omori, K., Aoyama, K., Marumoto, Y., Urata, Y., et al. (2006). Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells 24, 2292–2298. doi: 10.1634/stemcells.2005-0542
Toniutto, P., Zanetto, A., Ferrarese, A., and Burra, P. (2016). Current challenges and future directions for liver transplantation. Liver Int. 37, 317–327. doi: 10.1111/liv.13255/full
Tzouvelekis, A., Paspaliaris, V., Koliakos, G., Ntolios, P., Bouros, E., Oikonomou, A., et al. (2013). A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J. Transl. Med. 11:171. doi: 10.1186/1479-5876-11-171
Viebahn, C. S., Benseler, V., Holz, L. E., Elsegood, C. L., Vo, M., Bertolino, P., et al. (2010). Invading macrophages play a major role in the liver progenitor cell response to chronic liver injury. J. Hepatol. 53, 500–507. doi: 10.1016/j.jhep.2010.04.010
Wells, A. U., and Maher, T. M. (2017). Update in interstitial lung disease 2016. Am. J. Respir. Crit. Care Med. 196, 132–138. doi: 10.1164/rccm.201702-0351UP
Wise, A. F., Williams, T. M., Klewlet, M. B., Payne, N. L., Slatskas, C., Samuel, C., et al. (2014). Human mesenchymal stem cells alter macrophage phenotype and promote regeneration via homing to the kidney following ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 306, F1222–F1235. doi: 10.1152/ajprenal.00675.2013
Wise, A. F., Williams, T. M., Rudd, S., Wells, C. A., Kerr, P. G., and Ricardo, S. D. (2016). Human mesenchymal stem cells alter the gene profile of monocytes from patients with Type 2 diabetes and end-stage renal disease. Regen. Med. 11, 145–158. doi: 10.2217/rme.15.74
Wynn, T. A. (2004). Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 4, 583–594. doi: 10.1038/nri1412
Yagi, H., Soto-Gutierrez, A., Parekkadan, B., Kitagawa, Y., Tompkins, R. G., Kobayashi, N., et al. (2010). Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 19, 667–679. doi: 10.3727/096368910X508762
Yata, Y., Gotwals, P., Koteliansky, V., and Rockey, D. C. (2002). Dose-dependent inhibition of hepatic fibrosis in mice by a TGF-beta soluble receptor: implications for antifibrotic therapy. Hepatology 35, 1022–1030. doi: 10.1053/jhep.2002.32673
Zhang, Z., Lin, H., Shi, M., Xu, R., Fu, J., Lv, J., et al. (2012). Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J. Gastroenterol. Hepatol. 27(Suppl. 2), 112–120. doi: 10.1111/j.1440-1746.2011.07024.x
Zipprich, A., Garcia-Tsao, G., Rogowski, S., Fleig, W. E., Seufferlein, T., and Dollinger, M. M. (2012). Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int. 32, 1407–1414. doi: 10.1111/j.1478-3231.2012.02830.x
Keywords: fibrosis, stem cells and regenerative medicine, cell therapy, mesenchymal stem cells, progenitor cells
Citation: Lim R, Ricardo SD and Sievert W (2017) Cell-Based Therapies for Tissue Fibrosis. Front. Pharmacol. 8:633. doi: 10.3389/fphar.2017.00633
Received: 03 June 2017; Accepted: 28 August 2017;
Published: 22 September 2017.
Edited by:
Tim David Hewitson, Royal Melbourne Hospital, AustraliaReviewed by:
Andrew J. Boyle, University of Newcastle, AustraliaMustapha Najimi, Catholic University of Louvain, Belgium
Copyright © 2017 Lim, Ricardo and Sievert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: William Sievert, william.sievert@monash.edu
 Rebecca Lim
Rebecca Lim Sharon D. Ricardo
Sharon D. Ricardo William Sievert
William Sievert