Analysis of the association between the acetabular morphology and femoral head in children aged 0–3 years with developmental hip dysplasia
- 1Department of Orthopaedic Surgery, Children’s Hospital of Nanjing Medical University, Nanjing, China
- 2Department of Anesthesiology and Surgery, Children's Hospital of Nanjing Medical University, Nanjing, China
Background: Magnetic resonance imaging (MRI) has been advocated as a routine examination for preoperative and postoperative assessment of Developmental Dysplasia of the Hip (DDH). However, there is limited research regarding the correlation between acetabulum and femoral head morphology using preoperative MRI measurements.
Objective: To explore the correlation between acetabulum and femoral head morphology in children with DDH aged 0–3 years, using MRI measurements as indicators.
Methods: A Retrospective Analysis of MRI Data from 172 Children Diagnosed with Developmental Dysplasia of the Hip (DDH) at Nanjing Medical University Affiliated Children's Hospital, spanning from January 2017 to January 2022. Measurements were taken to assess various parameters reflecting hip socket morphology as well as the development status of the femoral head and ossifying nucleus. The correlation between these factors was explored using Pearson correlation analysis and multiple-factor linear regression. Statistical analysis was conducted using SPSS 18.0 software.
Results: Pearson correlation analysis revealed statistically significant associations between the length of the ossifying nucleus ratio and age(mo.), BAI, BCAD, CTAD, and CTAD. The height of the ossifying nucleus ratio displayed statistically significant correlations with age(mo.) and BTAD. The length of the femoral head ratio exhibited statistically significant correlations with CAI, BCEA, and BCAD. Furthermore, the height of the femoral head ratio demonstrated a statistically significant correlation with BCEA. After adjusting for age(mo.), BMI, BCEA, and CCEA, BPoAcet and CPoAcet was found to be correlated with the length of the ossifying nucleus ratio. Preoperatively, the CAI, BAxAcet, BPoAcet, CPoAcet, and BTAD were correlated with the height of ossifying nucleus ratio after correcting for age, BMI, BCEA, and CCEA.
Conclusion: The measurement parameters of hip socket morphology on MRI are associated with femoral head development, making them potential predictive indicators for femoral head development in DDH patients. These findings offer valuable insights for clinical decisions regarding the timing and approach of surgery in patients with developmental hip dislocation.
Introduction
Developmental Dysplasia of the Hip (DDH) refers to a series of anatomical anomalies in the relationship between the femoral head and the acetabulum during the developmental process, including acetabular dysplasia, subluxation of the hip joint, and dislocation of the hip joint (1). The reported incidence of DDH varies between 0.1% and 5%, depending on the study population, inclusion criteria, and diagnostic methods (2).The surgical treatment of pediatric DDH aims to restore the concentric relationship between the acetabulum and the femoral head, thereby promoting normal acetabular development (6). However, cases where both the acetabulum and the femoral head exhibit developmental dysplasia present greater challenges (35). Previous studies have indicated a strong interdependence between the development of the hip joint and the interaction between the acetabulum and the femoral head (3). Existing research has predominantly focused on primary femoral head abnormalities leading to acetabular dysplasia, with a limited number of studies exploring the influence of acetabular morphology on femoral head development (4, 5).MRI offers the advantage of clear visualization of soft tissues, cartilage, boney structures, and plays a crucial role in the assessment of DDH before and after treatment (22, 23, 24). This study aims to evaluate the correlation between acetabular and femoral head morphology in children aged 0–3 years with DDH using MRI.”
Methods
Study population
A total of 172 pediatric patients diagnosed with Developmental Dysplasia of the Hip (DDH) were collected from Nanjing Medical University Affiliated Children's Hospital between January 2017 and January 2022. Among these patients, there were 15 males and 157 females, with an average age of 18.20 ± 6.86 months.
Inclusion Criteria: Initial diagnosis made before the age of 36 months. Unilateral developmental dysplasia of the hip. Complete pre-treatment radiological data. Exclusion Criteria: Secondary hip dislocation due to conditions such as cerebral palsy, purulent hip joint arthritis, trauma, or multi-joint contractures. Lack of pre-treatment radiological data. Bilateral developmental dysplasia of the hip.
This study has obtained approval from the Ethics Committee of Nanjing Medical University Affiliated Children's Hospital, and informed consent was obtained from the legal guardians of all the patients.
Imaging examination
A superconducting 1.5 T magnetic resonance imaging (MRI) scanner manufactured by GE Healthcare (model: GE SignaHde 1.5 T) was used for the imaging scans. The scanning parameters were as follows: a body coil was used, with the following sequence parameters: coronal and transverse T1-weighted imaging (T1WI) with a repetition time (TR) of 476–623 ms, echo time (TE) of 22–23 ms, a matrix size of 512 × 336, and a field of view (FOV) of 300 mm × 300 mm; fast spin echo (TSE) sequence with coronal T2-weighted imaging (T2WI) with a TR of 3,450–4,000 ms, TE of 88–95 ms, a matrix size of 512 × 336, and a FOV of 250 mm × 250 mm. The scanning range extended from the superior margin of the iliac wing to the middle-upper portion of the femur, with 1–2 excitations, a slice thickness of 2 mm, and an interslice gap of 0.1 mm.
Measurement parameters
The MRI images were analyzed and measured independently by two chief radiologists specialized in pediatric orthopedic radiology. The measurements were taken on the coronal and transverse T1-weighted imaging (T1WI) sequences at the level of the greatest extent of the femoral head epiphysis. The following measurements were performed to evaluate the acetabular morphology: Boney Acetabular Index (BAI), Cartilaginous Acetabular Index (CAI), Boney Center Edge Angle (BCEA), Cartilaginous Center Edge Angle (CCEA), Boney Coronal Acetabular Depth (BCAD), Cartilaginous Coronal Acetabular Depth (CCAD), Boney Axial Acetabular Angle (BAxAcet), Cartilaginous Axial Acetabular Angle (CAxAcet), Boney Posterior Acetabular Angle (BPoAcet), Cartilaginous Posterior Acetabular Angle (CPoAcet), Boney Transverse Acetabular Depth (BTAD), and Cartilaginous Transverse Acetabular Depth (CTAD) (Figure 1).
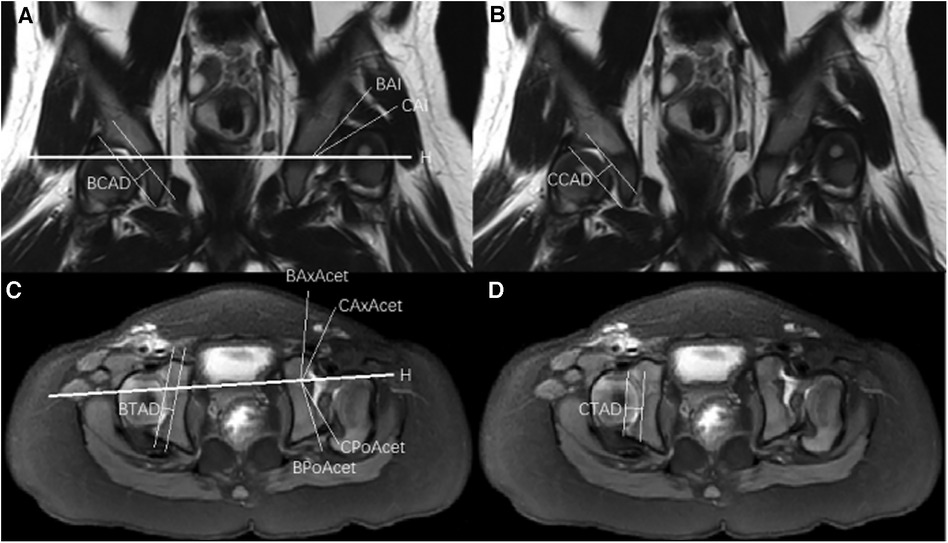
Figure 1. (A) Coronal T2-weighted magnetic resonance imaging demonstrating Hilgenreiner's line (H-H), boney acetabular index (BAI), cartilaginous acetabular index (CAI) and boney coronal acetabular depth (BCAD). (B) Coronal T2-weighted magnetic resonance imaging demonstrating cartilaginous coronal acetabular depth (CCAD). (C) Transverse T1-weighted magnetic resonance imaging demonstrating Boney axial acetabular angle (BAxAcet), cartilaginous axial acetabular angle (CAxAcet), boney posterior acetabular angle (BPoAcet), cartilaginous posterior acetabular angle (CPoAcet) and boney transverse acetabular depth (BTAD). (D) Transverse T1-weighted magnetic resonance imaging demonstrating cartilaginous transverse acetabular depth (CTAD).
The measurements also included the length of the ossifying nucleus, the height of the ossifying nucleus, the length of the femoral head, and the height of the femoral head, all on the affected side and the unaffected side. The ratios between the affected and unaffected sides were used to assess the development of the femoral head.
Statistical analysis
Statistical analysis was conducted using SPSS 18.0 software. Continuous variables were presented as mean ± standard deviation. Pearson linear correlation analysis was employed for the correlation analysis. Multiple linear regression analysis was used to examine the association between hip socket morphology imaging parameters and femoral head development while controlling for confounding factors. A p-value less than 0.05 was considered statistically significant.
Results
Characteristics of the study population
This study included 172 subjects, comprising 15 males and 157 females, with an average age of 18.20 ± 6.86 months and an average BMI of 17.39 ± 2.13. The shortest follow-up time was 1 year. All patients had a BAI of 37.83 ± 5.29°, CAI of 23.62 ± 5.21°, BCEA of −48.40 ± 22.41°, and CCEA of −39.26 ± 19.21°.
Pearson correlation analysis
Pearson correlation analysis revealed statistically significant associations:
The length of ossifying nucleus ratio had significant correlations with age(mo.), BAI, BCAD, CTAD, and CTAD (Figure 2).
The height of ossifying nucleus ratio exhibited statistically significant correlations with age(mo.) and BTAD (Figure 3).
The length of femoral head ratio showed statistically significant correlations with CAI, BCEA, and BCAD (Figure 4).
The height of femoral head ratio displayed a statistically significant correlation with BCEA (Figure 5).
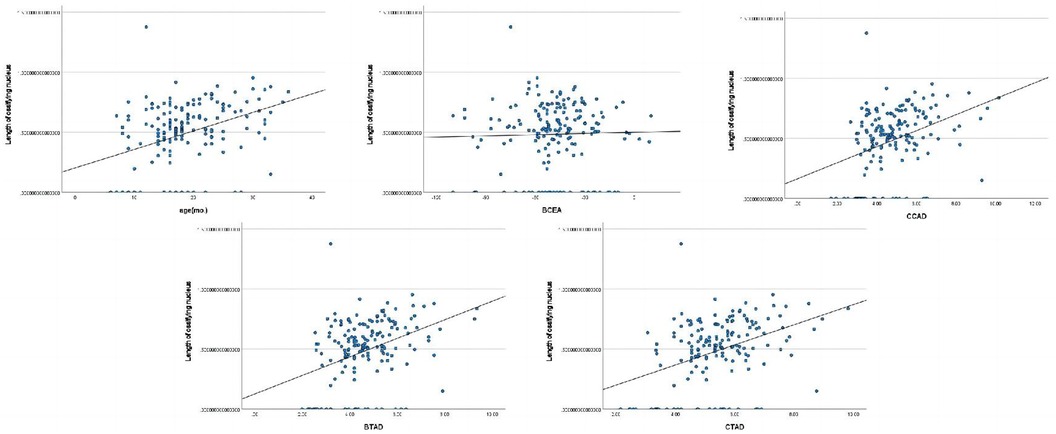
Figure 2. Pearson correlation analysis indicated statistically significant associations between the length of ossifying nucleus ratio and age(mo.), BAI, BCAD, CTAD, and CTAD.
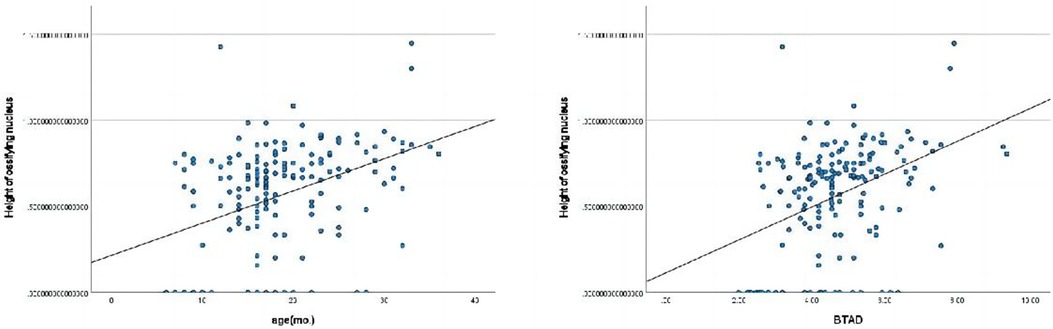
Figure 3. Pearson correlation analysis indicated statistically significant associations between the height of ossifying nucleus ratio and age(mo.), BTAD.
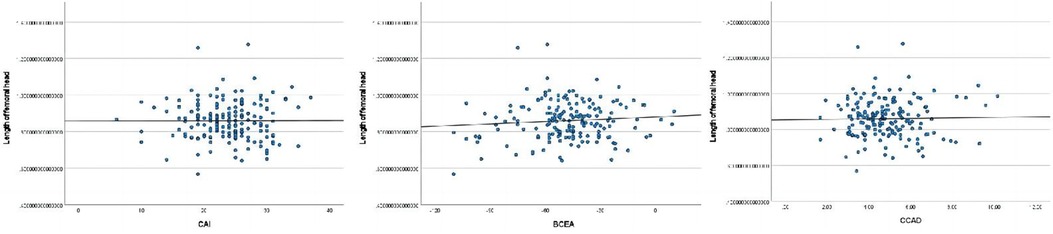
Figure 4. Pearson correlation analysis indicated statistically significant associations between the length of femoral head ratio and CAI, BCEA, BCAD.
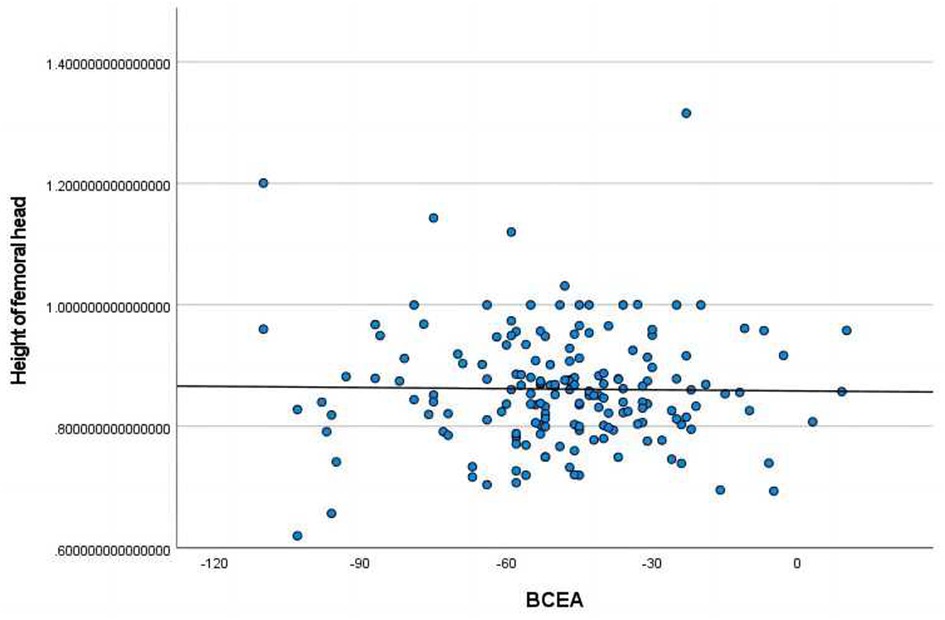
Figure 5. Pearson correlation analysis indicated statistically significant associations between the height of femoral head ratio and BCEA.
Multiple linear regression
Multiple linear regression analysis demonstrated the following associations in models without adjusting for confounding factors: All of the measurements of the acetabular morphology were correlated with the length of ossifying nucleus ratio. After adjusting for age (mo.) and BMI, BAxAcet and CPoAcet were associated with the length of ossifying nucleus ratio. After adjusting for age (mo.), BMI, BCEA, and CCEA, BPoAcet and CPoAcet were related to the length of ossifying nucleus ratio (Table 1).
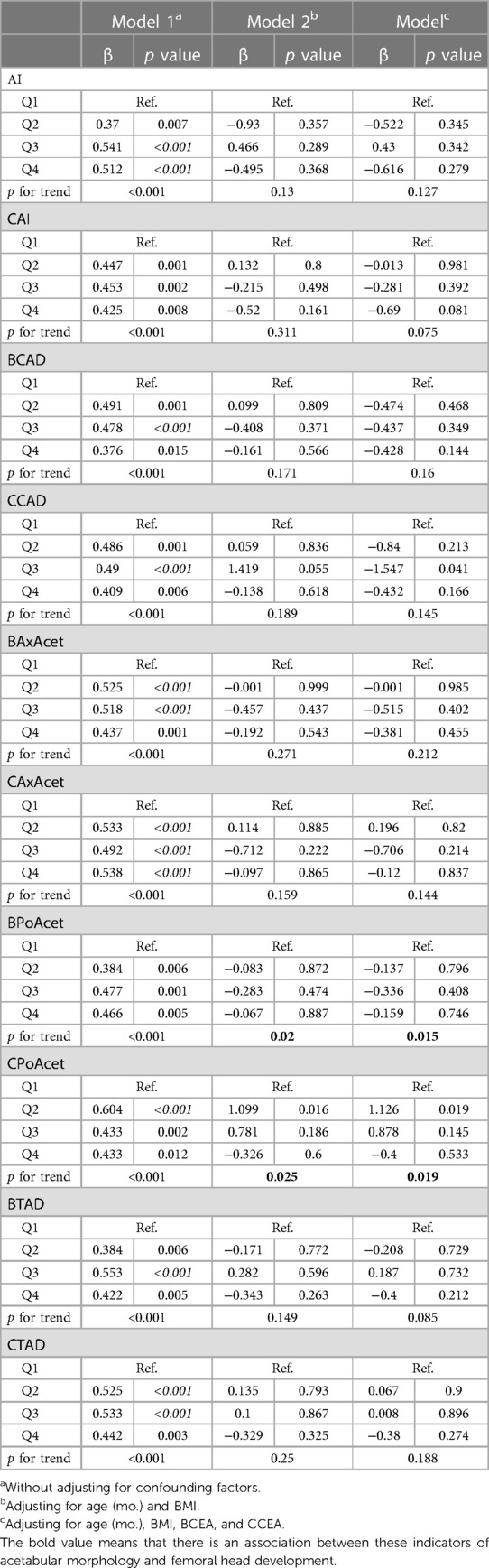
Table 1. Multiple linear regression analysis between the acetabular morphology and the length of ossifying nucleus ratio.
Without adjusting for confounding factors, all of the measurements of the acetabular morphology were correlated with the height of ossifying nucleus ratio. After adjusting for age (mo.) and BMI, CAI, BAxAcet, BPoAcet, and CPoAcet were correlated with the height of ossifying nucleus ratio. After adjusting for age (mo.), BMI, BCEA, and CCEA, CAI, BAxAcet, BPoAcet, CPoAcet, and BTAD were related to the height of ossifying nucleus ratio (Table 2).
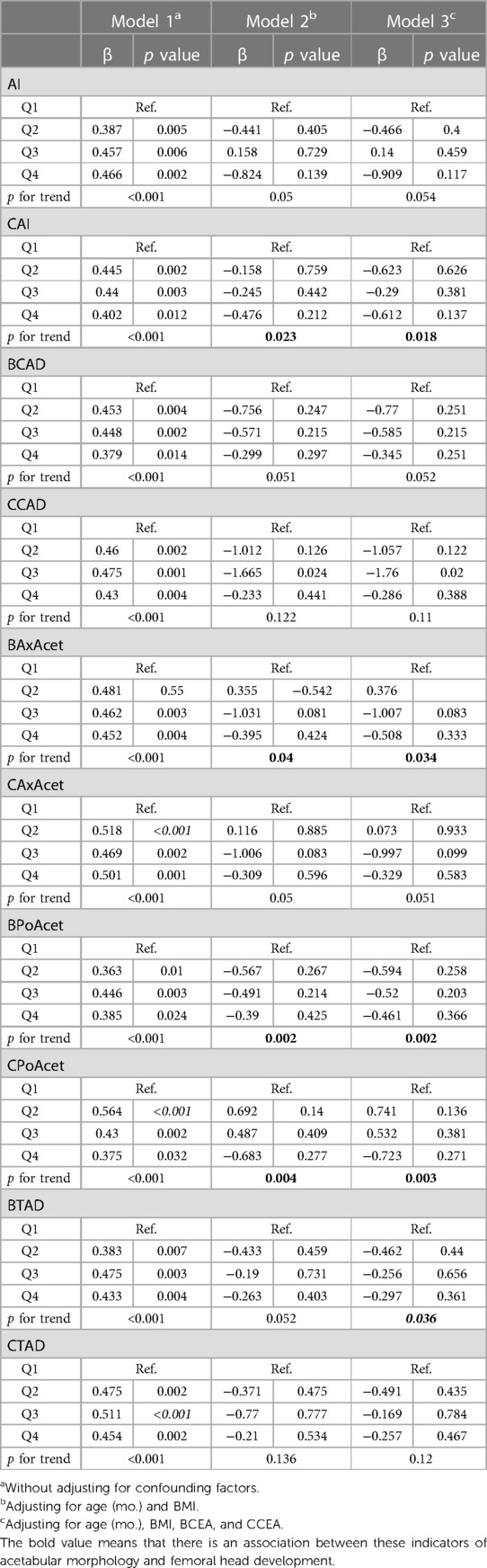
Table 2. Multiple linear regression analysis between the acetabular morphology and the height of ossifying nucleus ratio.
Without adjusting for confounding factors, all of the measurements of the acetabular morphology were not correlated with the length of femoral head ratio.After adjusting for age (mo.) and BMI, all of the measurements of the acetabular morphology were not correlated with the length of femoral head ratio.After adjusting for age (mo.), BMI, BCEA, and CCEA, CAxAcet were related to the length of femoral head ratio (Table 3).
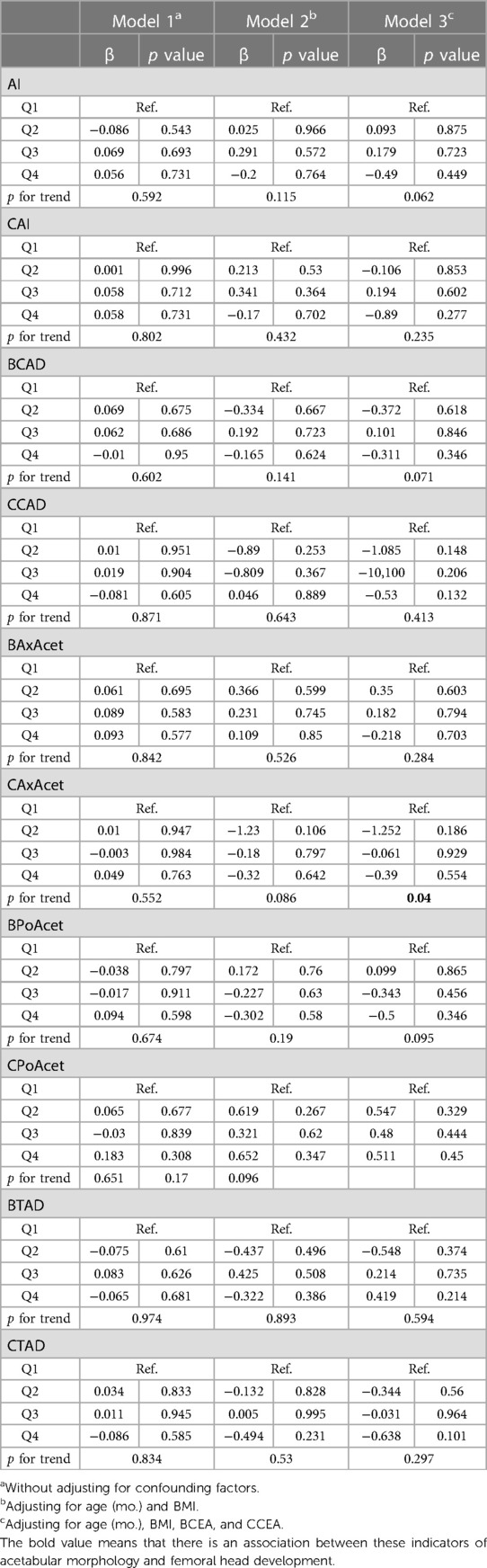
Table 3. Multiple linear regression analysis between the acetabular morphology and the length of femoral head ratio.
Within the three models, all of the measurements of the acetabular morphology were not correlated with the height of femoral head ratio (Table 4).
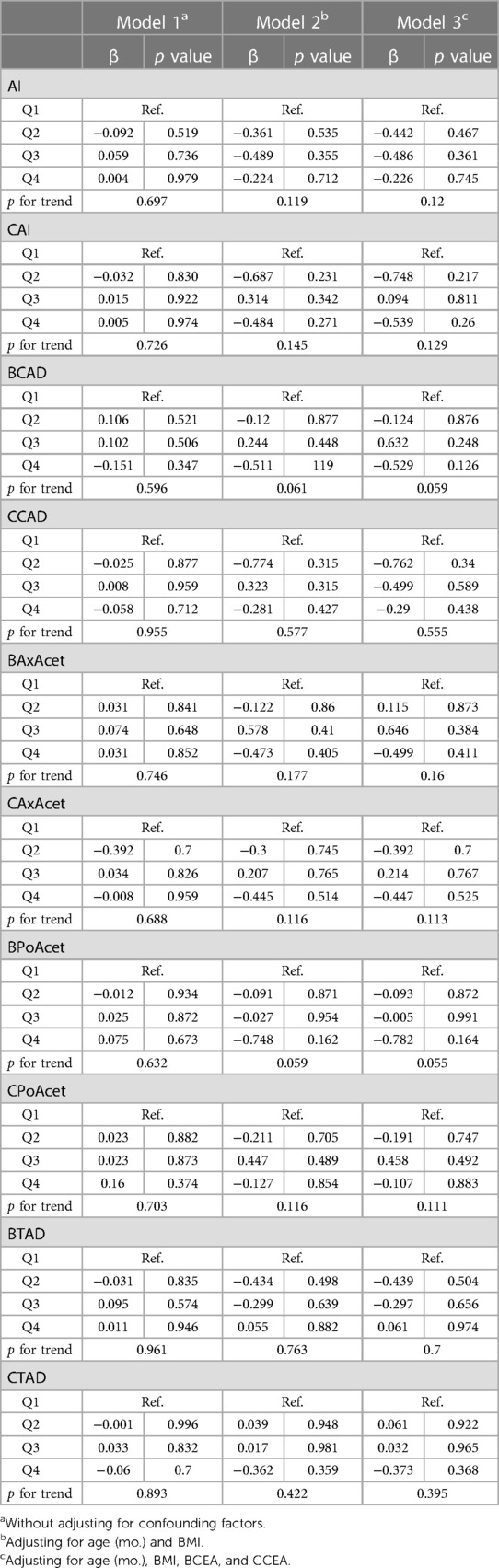
Table 4. Multiple linear regression analysis between the acetabular morphology and the height of femoral head ratio.
Discussion
The hip joint is among the most intricate articulations of the human body, comprising the acetabulum, the proximal femur, and the connecting soft tissues. In children, the hip socket is divided into three parts, formed by the connection of the ischium, pubis, and ilium through the Y-shaped cartilage. The development of the acetabulum is closely intertwined with that of the femoral head, as when they fail to make contact, the acetabulum assumes a flattened shape (6). At birth, the femoral head is entirely composed of cartilaginous tissue, and the appearance of the ossification center of the femoral head occurs around the age of six months. As age progresses, the anteversion angle and neck-shaft angle of the femur decrease. The growth and development of the hip joint are contingent upon the concentric alignment of the femoral head within the acetabulum and the harmonious growth of the Y-shaped cartilage and the acetabular cartilage. Any alteration in either of these factors can potentially lead to the occurrence of developmental dysplasia of the hip (DDH) (7,8).
Previous studies have indicated that the development of the hip joint heavily relies on the interaction between the acetabulum and the femoral head. The acetabulum requires a spherical femoral head as a growth template, while the spherical growth of the femoral head and the symmetrical development of the epiphysis also necessitate the coverage of the acetabulum (3, 9). Existing research findings have primarily focused on the primary deformity of the femoral head leading to acetabular dysplasia (10–16). When hip dislocation occurs and the femoral head fails to make contact with the acetabulum, the acetabulum assumes a flattened shape. An animal study demonstrated that removal of the femoral head in rats results in inadequate development of the acetabulum (12). Complete absence of the proximal femur in humans leads to acetabular deficiency (17). In cases of unstable concentric reduction, hip joint dislocation in children of walking age can result in acetabular “saucerization” (18). Only a few studies have investigated the impact of acetabular morphology on the development of the femoral head. S.D. Steppacher et al. found that in cases of dysplastic hip joints, there is a decreased acetabular depth, along with an elliptical shape of the femoral head, reduced epiphyseal height, and asymmetric extension of the epiphysis on both sides, indicating that different acetabular coverage affects the anatomical morphology of the femoral head (3). Wudbhav N. studied the sphericity of the femoral head in patients with developmental dysplasia of the hip (DDH) and found that the affected hips had lower femoral head sphericity scores compared to the unaffected hips, with no correlation to age (19). Due to anatomical abnormalities and physical factors, the development of the affected hip joint in DDH patients does not correspond to that of the unaffected side. Research suggests that the magnitude of stress load is inversely related to the rate of epiphyseal growth, a pattern applicable to both the acetabulum and the femoral head epiphysis (20).
The objective of this study is to observe the development of the acetabulum and femoral head in children aged 0–3 years with unilateral Developmental Dysplasia of the Hip (DDH) and to explore the relationship between them. To eliminate individual differences, we used the ratio of measurements on the affected side to those on the unaffected side to represent the development of the femoral head. We measured various indicators on MRI that reflect acetabular morphology, which to some extent can reflect the primary acetabular dysplasia in DDH patients.
MRI offers clear visualization of non-ossified femoral heads, acetabular cartilage, acetabular labrum, and fibrous adipose tissue distribution within the acetabular socket (22, 23). It has been widely used for preoperative planning and postoperative evaluation of surgical treatment for developmental hip dysplasia, and some studies have employed it for monitoring joint cartilage growth in children's growth and development (24). Pearson correlation analysis suggests a higher correlation between hip socket morphology and ossifying nucleus development. This aligns with previous research findings, which suggest that the development of the ossifying nucleus is more susceptible to stress-induced ischemic injury (21). Age, BMI, and CCEA may act as confounding factors. Whether in a physiological or pathological context, the ossifying nucleus has developmental potential, and CEA, to some extent, represents the degree of hip joint dislocation. In cases of subluxation, there is abnormal stress between the femoral head and the outer edge of the acetabulum. Fully dislocated femoral heads are exposed to an extremely abnormal developmental environment, and excessive body weight in walking-age children can increase the load on the hip joint. Therefore, we corrected for these three factors in our multiple linear regression analysis. Multiple linear regression analysis reveals an association between hip socket morphology and femoral head development. After adjusting for confounding factors, we found correlations between boney posterior acetabular angle (BPoAcet) and cartilaginous posterior acetabular angle (CPoAcet) with the ossifying nucleus transverse diameter ratio. This may be related to the direction of force in the hip joint, which experiences varying stress directions in different states, with the posterior aspect of the acetabulum bearing more load during walking (25). We observed that factors influencing ossifying nucleus height were more varied. After adjusting for age (mo.) and BMI, CAI, BAxAcet, BPoAcet, CPoAcet, and BTAD were related to the height of ossifying nucleus ratio. As children with dislocated femoral heads experience abnormal stresses, the stress directions from the outer and upper aspects of the acetabulum are most variable. According to Wolff's law (34), bone trabeculae adapt to changes in the mechanical environment, and ossifying nucleus height is more sensitive to changes in stress in the context of DDH. Reduced acetabular depth implies inadequate coverage of the femoral head, resulting in changes in the position and size of the femoral head load, which affects the development of the ossifying nucleus (5). After correcting for all confounding factors, only CAxAcet was related to the femoral head transverse diameter ratio. This might be an expression of continuous stress stimulation leading to thickening of acetabular cartilage. With advancing age, long-term changes in the developmental environment will gradually affect the femoral head's response to stress changes. Long-term changes in the developmental environment disrupt the balance of apoptosis, significantly impacting the normal development of cartilage and ossification (26). In DDH, the acetabulum is smaller than normal, and the peak stresses on the hip joint are significantly higher, leading to joint cartilage damage and directly affecting the development of the femoral head size. As age increases, the remodeling capacity of the acetabulum and femoral head decreases (27, 28). Continuous subluxation increases the load on the outer edge of the acetabulum, inhibiting the development and ossification of the acetabulum's outer edge. Stress concentrates on the complex formed by the labrum and acetabular rim on the outer-upper aspect of the hip joint, resulting in continuous cartilage damage to the femoral head and acetabulum (29–32). Continuous dislocation leads to the absence of a spherical structure of the femoral head as a growth template for the acetabulum, causing the acetabulum to develop into a flatter shape.
This study leans towards the idea that acetabular dysplasia is the initial pathology of DDH, and changes in the femoral head are secondary adaptive phenomena (33). Although there is no direct evidence, we believe that even in the absence of femoral head necrosis, abnormal femoral head morphology can have a detrimental effect on hip joint development, and the morphological abnormalities of the femoral head are often difficult to correct through surgery. Additionally, femoral head development cannot be effectively monitored in the early stages using simple methods such as x-ray. Therefore, identifying reliable acetabular morphology indicators for assessing femoral head development is crucial.
The surgical focus of pediatric DDH remains on acetabular osteotomy and various procedures to improve acetabular coverage, restoring the concentric relationship between the acetabulum and the femoral head and stabilizing it. Some studies (35) have focused on long-term changes in the proximal femoral morphology in DDH, with findings suggesting that the femoral neck length decreases as the severity of the disease increases and tends to incline forward. Notably, there are significant differences in femoral head anterior tilt between male and female DDH patients. Apart from the common complication of femoral head avascular necrosis, femoral head deformity also requires attention during the treatment of DDH.
This study has several limitations. Although we evaluated acetabular morphology from different perspectives on MRI images, the measurements are still two-dimensional, while the complex relationship between the acetabulum and the femoral head should ideally be visualized in three dimensions. Femoral head morphology needs to be comprehensively assessed from a three-dimensional perspective. This study evaluated femoral head morphology in two dimensions, measuring its transverse diameter and width. Additionally, the sample size in this study is relatively small, and it is necessary to conduct multicenter studies to increase the sample size. This cross-sectional study only provides a preliminary exploration of the relationship between acetabular morphology and femoral head development, serving as a foundation for more in-depth mechanistic research. Continuous follow-up is required to observe long-term changes in femoral head morphology.
In conclusion, through a retrospective analysis of imaging data from 172 children with unilateral developmental hip dislocation aged 0–3 years, we found correlations between boney posterior acetabular angle, cartilaginous posterior acetabular angle, preoperative cartilaginous acetabular index, boney axial acetabular angle, boney transverse acetabular depth, and cartilaginous axial acetabular index with femoral head development. These factors may serve as predictive indicators for femoral head development in DDH patients, providing insights for clinical decision-making regarding the timing and method of surgery for developmental hip dislocation patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Children's Hospital Affiliated to Nanjing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
LX: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. BW: Investigation, Supervision, Writing – review & editing. LW: Data curation. ZZ: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Johnson MA, Gohel S, Nguyen JC, Sankar WN. MRI predictors of residual dysplasia in developmental dysplasia of the hip following open and closed reduction. J Pediatr Orthop. (2022) 42(4):179–85. doi: 10.1097/BPO.0000000000002062
2. Hareendranathan AR, Wichuk S, Punithakumar K, Dulai S, Jaremko J. Normal variation of infant hip development: patterns revealed by 3D ultrasound. Bone Joint Open. (2022) 3(11):913–23. doi: 10.1302/2633-1462.311.BJO-2022-0081.R1
3. Steppacher SD, Tannast M, Werlen S, Siebenrock KA. Femoral morphology differs between deficient and excessive acetabular coverage. Clin Orthop Relat Res. (2008) 466(4):782–90. doi: 10.1007/s11999-008-0141-7
4. Grzegorzewski A, Synder M, Kozłowski P, Szymczak W, Bowen RJ. The role of the acetabulum in perthes disease. J Pediatr Orthop. (2006) 26(3):316–21. doi: 10.1097/01.bpo.0000221926.10148.bf
5. Rejholec M, Stryhal F. Behavior of the proximal femur during the treatment of congenital dysplasia of the hip: a clinical long-term study. J Pediatr Orthop. (1991) 11(4):506–13. doi: 10.1097/01241398-199107000-00017
6. Vaquero-Picado A, González-Morán G, Garay EG, Moraleda L. Developmental dysplasia of the hip: update of management. EFORT Open Rev. (2019) 4(9):548–56. doi: 10.1302/2058-5241.4.180019
7. Moraleda L, Bravo C, Forriol F, Albiñana J. Does orientation of the femoral head affect acetabular development? An experimental study in lamb. J Pediatr Orthop. (2019) 39(8):416–21. doi: 10.1097/BPO.0000000000000974
8. Moraleda L, Albiñana J, Forriol F. Selective epiphysiodesis of the triradiate cartilage for treatment of residual experimental acetabular dysplasia. J Pediatr Orthop. (2013) 33(8):821–8. doi: 10.1097/BPO.0b013e31829b2f3f
9. Lee MC, Eberson CP. Growth and development of the child’s hip. Orthop Clin North Am. (2006) 37(2):119–32. doi: 10.1016/j.ocl.2005.12.001
10. Domzalski ME, Glutting J, Bowen JR, Littleton AG. Lateral acetabular growth stimulation following a labral support procedure in legg-calve-perthes disease. J Bone Joint Surg Am Vol. (2006) 88(7):1458–66. doi: 10.2106/JBJS.E.00689
11. Ezoe M, Naito M, Inoue T. The prevalence of acetabular retroversion among various disorders of the hip. J Bone Joint Surg Am Vol. (2006) 88(2):372–9. doi: 10.2106/JBJS.D.02385
12. Harrison TJ. The influence of the femoral head on pelvic growth and acetabular form in the rat. J Anat. (1961) 95(Pt 1):12–24.13711848
13. Hochbergs P, Eckerwall G, Egund N, Jonsson K, Wingstrand H. Femoral head shape in legg-calvé-perthes disease. Correlation between conventional radiography, arthrography and MR imaging. Acta Radiol. (1994) 35(6):545–8. doi: 10.1177/028418519403500607
14. Madan S, Fernandes J, Taylor JF. Radiological remodelling of the acetabulum in Perthes’ disease. Acta Orthop Belg. (2003) 69(5):412–20.14648950
15. Meurer A, Böhm B, Decking J, Heine J. Analysis of acetabular changes in Morbus Perthes disease with radiomorphometry. Z Orthop Ihre Grenzgeb. (2005) 143(1):100–5. doi: 10.1055/s-2004-832408
16. Siffert RS. Patterns of deformity of the developing hip. Clin Orthop Relat Res. (1981) 160:14–29. doi: 10.1097/00003086-198110000-00002
17. Strayer LM Jr. Embryology of he human hip joint. Clin Orthop Relat Res. (1971) 74:221–40. doi: 10.1097/00003086-197101000-00029
18. Shefelbine SJ, Carter DR. Mechanobiological predictions of growth front morphology in developmental hip dysplasia. J Orthop Res. (2004) 22(2):346–52. doi: 10.1016/j.orthres.2003.08.004
19. Sankar WN, Neubuerger CO, Moseley CF. Femoral head sphericity in untreated developmental dislocation of the hip. J Pediatr Orthop. (2010) 30(6):558–61. doi: 10.1097/BPO.0b013e3181e4f53e
21. Salter RB, Kostuik J, Dallas S. Avascular necrosis of the femoral head as a complication of treatment for congenital dislocation of the hip in young children: a clinical and experimental investigation. Can J Surg J Canadien de Chirurgie. (1969) 12(1):44–61.
22. Dibello D, Odoni L, Pederiva F, Di Carlo V. MRI In postreduction evaluation of developmental dysplasia of the hip: our experience. J Pediatr Orthop. (2019) 39(9):449–52. doi: 10.1097/BPO.0000000000001037
23. Satsuma S, Kobayashi D, Kinugasa M, Takeoka Y, Kuroda R, Kurosaka M. A new predictive indicator by arthrography for future acetabular growth following conservative treatment of developmental dysplasia of the hip. J Pediatr Orthop Part B. (2016) 25(3):207–11. doi: 10.1097/BPB.0000000000000265
24. Wang X, Song G, Zhang J. Arthroscopic treatment of labral tears in patients with borderline developmental dysplasia of the hip: a retrospective study with mean 5.8 years follow-up. Orthop Surg. (2021) 13(6):1835–42. doi: 10.1111/os.13042
25. Harris MD, Anderson AE, Henak CR, Ellis BJ, Peters CL, Weiss JA. Finite element prediction of cartilage contact stresses in normal human hips. J Orthop Res. (2012) 30(7):1133–9. doi: 10.1002/jor.22040
26. Hernandez PA, Wells J, Usheva E, Nakonezny PA, Barati Z, Gonzalez R, et al. Early-onset osteoarthritis originates at the chondrocyte level in hip dysplasia. Sci Rep. (2020) 10(1):627. doi: 10.1038/s41598-020-57431-x
27. Tarassoli P, Gargan MF, Atherton WG, Thomas SR. The medial approach for the treatment of children with developmental dysplasia of the hip. Bone Joint J. (2014) 96-b(3):406–13. doi: 10.1302/0301-620X.96B3.32616
28. Sllamniku S, Bytyqi C, Murtezani A, Haxhija EQ. Correlation between avascular necrosis and the presence of the ossific nucleus when treating developmental dysplasia of the hip. J Children’s Orthop. (2013) 7(6):501–5. doi: 10.1007/s11832-013-0538-z
29. Henak CR, Abraham CL, Anderson AE, Maas SA, Ellis BJ, Peters CL, et al. Patient-specific analysis of cartilage and labrum mechanics in human hips with acetabular dysplasia. Osteoarthr Cartil. (2014) 22(2):210–7. doi: 10.1016/j.joca.2013.11.003
30. Henak CR, Ellis BJ, Harris MD, Anderson AE, Peters CL, Weiss JA. Role of the acetabular labrum in load support across the hip joint. J Biomech. (2011) 44(12):2201–6. doi: 10.1016/j.jbiomech.2011.06.011
31. Thomas-Aitken HD, Goetz JE, Dibbern KN, Westermann RW, Willey MC, Brown TS. Patient age and hip morphology alter joint mechanics in computational models of patients with hip dysplasia. Clin Orthop Relat Res. (2019) 477(5):1235–45. doi: 10.1097/CORR.0000000000000621
32. Song K, Anderson AE, Weiss JA, Harris MD. Musculoskeletal models with generic and subject-specific geometry estimate different joint biomechanics in dysplastic hips. Comput Methods Biomech Biomed Engin. (2019) 22(3):259–70. doi: 10.1080/10255842.2018.1550577
33. Elsharkawi KM, Barakat MS, Farahat AAK, Ahmed AAY, Bastawi RA. Role of magnetic resonance imaging in assessment of acetabular and femoral version in developmental dysplasia of the hip. Radiol Bras. (2022) 55(5):299–304. doi: 10.1590/0100-3984.2021.0133
34. Frost HM. Why do marathon runners have less bone than weight lifters? A vital-biomechanical view and explanation. Bone. (1997) 20(3):183–9. doi: 10.1016/S8756-3282(96)00311-0
Keywords: developmental dysplasia of the hip, MRI, acetabulum, femoral head, correlation analysis
Citation: Xu L, Wang B, Wang L and Zhang Z (2023) Analysis of the association between the acetabular morphology and femoral head in children aged 0–3 years with developmental hip dysplasia. Front. Pediatr. 11:1310411. doi: 10.3389/fped.2023.1310411
Received: 9 October 2023; Accepted: 9 November 2023;
Published: 27 November 2023.
Edited by:
Pablo Castañeda, National Autonomous University of Mexico, MexicoReviewed by:
Marco Sapienza, University of Catania, ItalySabit Sllamniku, University Clinical Center of Kosovo, Albania
© 2023 Xu, Wang, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiqun Zhang zhangzqun@njmu.edu.cn
†These authors have contributed equally to this work
 Liukun Xu1,†
Liukun Xu1,†  Zhiqun Zhang
Zhiqun Zhang