Inotropic score and vasoactive inotropic score as predictors of outcomes in congenital diaphragmatic hernia: A single center retrospective study
- 1Section of Neonatology, Department of Pediatrics, Baylor College of Medicine/Texas Children's Hospital, Houston, TX, United States
- 2Department of Pharmacy, Texas Children's hospital, Houston, TX, United States
- 3Section of Pediatric Cardiology, Department of Pediatrics, Baylor College of Medicine/Texas Children's Hospital, Houston, TX, United States
- 4Department of Obstetrics and Gynecology, Maternal Fetal Medicine/Fetal Intervention Baylor College of Medicine, Houston, TX, United States
- 5Department of Pediatric Surgery, Baylor College of Medicine/Texas Children's Hospital, Houston, TX, United States
Background: Neonates with congenital diaphragmatic hernia (CDH) have varying degrees of pulmonary hypoplasia, pulmonary hypertension (PH) and cardiac dysfunction. These neonates frequently require vasoactive support and are at high risk for mortality and morbidity, including prolonged ventilator support, need for extracorporeal membrane oxygenation (ECMO), prolonged length of stay, and need for tracheostomy. However, identifying which infants are at increased risk can be challenging. In this study, we sought to investigate the utility of the inotropic score (IS) and vasoactive inotropic score (VIS) as tools to predict significant clinical outcomes and overall survival in patients with CDH. Additionally, we evaluated the correlation between IS/VIS and postnatal echocardiographic variables.
Methods: This was a retrospective chart review of 57 patients with CDH whose postnatal care was based on a standardized institutional protocol. We calculated the IS/VIS at 6-, 12-, 24-, 48 hours of life (HOL), on the day of CDH repair and 24- and 48 hours after surgical repair. The association of these scores with postnatal echocardiographic markers was analyzed using Pearson's correlation and linear regression, while logistic regression was used for binary outcomes, and Cox proportional hazards regression was used to assess associations with survival.
Results: We found that every one-unit increase in IS/VIS at 6 HOL was associated with 13% increase in the odds of ECMO (p = 0.034) and 10.1% increase in risk of death (p = 0.021). An increase in IS/VIS at 12-, 24- and 48-HOL was associated with posterior septal bowing in the first postnatal echocardiogram (p < 0.05 for all). Additionally, we noted an inverse relationship between IS (r = −0.281, p = 0.036) and VIS (r = −0.288, p = 0.031) on the day of repair and left ventricle (LV) systolic function in first postnatal echocardiogram. Increase in IS (r = −0.307, p = 0.024) and VIS (r = −0.285, p = 0.037) on the day of repair was associated with decreased LV function on the post-repair echocardiogram.
Conclusion: This retrospective study showed a significant association between IS/VIS obtained at various time points with clinical outcomes and echocardiographic findings in CDH, which could be used to guide prognosis and management in this patient population.
Introduction
Congenital diaphragmatic hernia (CDH) is a congenital defect that presents with varying degrees of pulmonary hypoplasia, pulmonary hypertension and ventricular dysfunction, which play significant roles in the overall morbidity and mortality (1, 2). Despite advances in clinical and surgical management of CDH, the overall morbidity and mortality remain high (3). The trifecta of pulmonary hypertension, pulmonary hypoplasia and myocardial dysfunction in CDH leads to hypoxic respiratory failure and circulatory insufficiency, which, when left untreated, can progress to end organ hypoperfusion and shock. This multiorgan dysfunction contributes to need for prolonged ventilation and need for circulatory support in the form of inotropes and ECMO (4, 5).
In critically ill patients, utilizing scoring indices that accurately reflect severity of illness have been shown to be valuable by providing guidance to bedside clinical management and patient care research (6, 7). Congenital heart surgeons and cardiac critical care physicians use the inotrope score (IS), vasoactive-inotrope score (VIS) and total inotrope exposure score (TIES) to correlate postoperative hemodynamics following surgical repairs (8–11). The VIS obtained at 24 h after admission to a cardiac ICU has been shown to be strongly associated with morbidity and mortality in infants undergoing cardiac surgery (11).
With the interplay of complex physiology that exists in the CDH population, we hypothesized that the inotropic score and vasoactive inotropic score would be useful and effective bedside tools to predict clinical outcomes in CDH. To the best of our knowledge, no such studies have been conducted in the CDH population.
Our primary objective with this study was to determine the correlation of the inotropic and vasoactive inotropic scores at 6-, 12-, 24-, 48-HOL, on the day of CDH repair and 24- and 48 hours following repair with various clinical outcomes and postnatal echocardiographic parameters. Inotropic score (IS) and vasoactive inotropic scores (VIS) are calculated as shown below (8):
Materials and methods
Setting and infrastructure
This single center study was conducted in a 187 bed NICU of a quaternary care children's hospital, which is a CDH/ECMO referral center. All patients were treated on a standardized protocol. Following approval from the Baylor College of Medicine Institutional Review Board (IRB # H-49059), data was extracted from the medical records, and stored in encrypted folders on secure institutional servers.
Study design
This retrospective cohort study included neonates ≤28 days of age admitted to the level IV NICU between January 2017 and December 2021 with a diagnosis of CDH, and who received one or more inotropic medications (including dopamine, dobutamine, epinephrine, vasopressin, milrinone, norepinephrine and hydrocortisone) during their hospital stay. Our exclusion criteria consisted of patients with critical congenital heart disease (defined as ductal dependent systemic and pulmonary circulation requiring prostaglandin E1 (PGE) or cardiac intervention within first few days of life), multiple congenital anomalies, and lethal genetic abnormalities who underwent redirection of care within 48 h of life.
Data collection
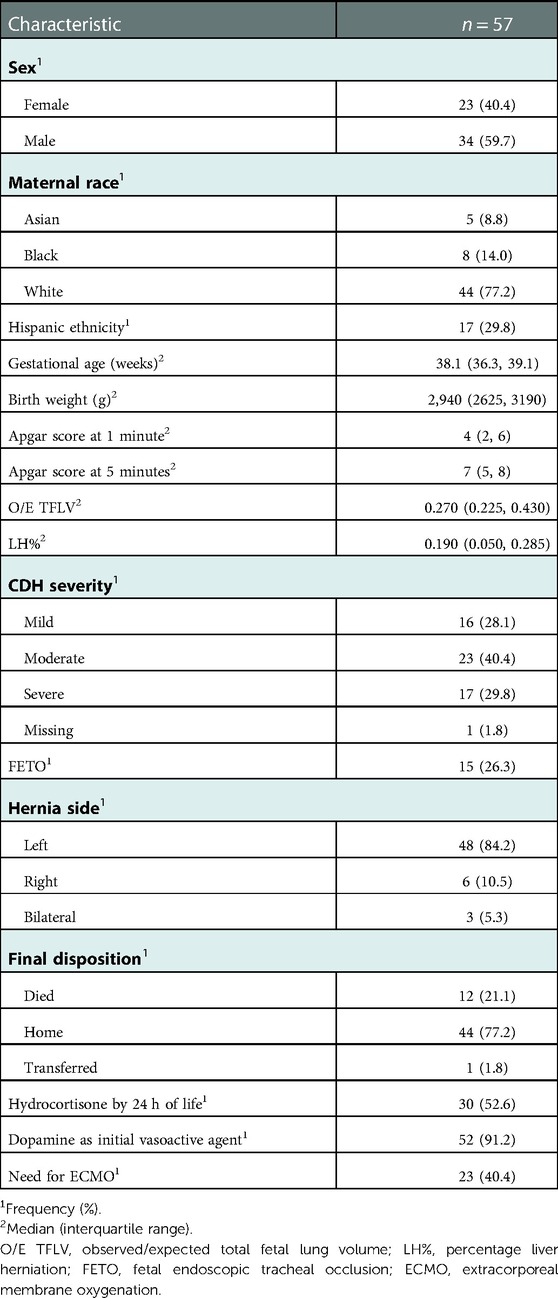
Baseline demographics, clinical, and outcomes data were obtained. Demographic data included gestational age, birthweight, sex, and APGAR scores at 1- and 5 min. Clinical data included fetal CDH measurements {Observed/Expected Total Fetal Lung Volume (O/E TFLV), Lung-Head Ratio (LHR) and percent liver herniation (%LH)}, severity of hernia (based on prenatal CDH measurements described by Ruano et al.) (12), side of hernia, fetal intervention such as Fetal Tracheal Balloon Occlusion (FETO), and inotropic medications used. Postnatal use of vasoactive medications was obtained for different time points: 6-, 12-, 24-, 48-HOL, on the day of CDH repair and 24- and 48 h after surgical repair. This data was extracted and screened by 2 study personnel (SHaG and CT) to ensure accuracy. We obtained echocardiographic data from the first postnatal study, following CDH surgical repair, and at 28 days of life. Echocardiographic measures included data on septal defects (presence of atrial septal defect(s) (ASD), ventricular septal defect(s) (VSD) and/or patent foramen ovale (PFO)), ASD/VSD shunt direction, tricuspid regurgitation (TR) velocity, ventricular septal position, patent ductus arteriosus (PDA) size (small, moderate and large), PDA shunt direction (left to right, right to left or bidirectional), qualitative right and left ventricular function (mild, moderate or severely depressed) and ejection fraction of LV based on American Society for Echocardiography (ASE) guidelines (13). Primary clinical outcomes were use of ECMO and mortality, and secondary clinical outcomes were duration of ECMO, length of hospital stay and postnatal echocardiographic markers.
IS/VIS calculations
The inotropic score and vasoactive inotropic scores were calculated (using the formulas above) for each of the following time points: 6-, 12-, 24-, 48-HOL, on the day of CDH repair and 24- and 48 hours.
Clinical endpoints and outcomes
Following data extraction, we analyzed IS/VIS at different time points with echocardiographic data and clinical outcomes. We performed the following analyses:
1. Associated IS/VIS at 6-, 12-, 24- and 48-HOL, on the day of repair and 24- and 48 hours following repair with primary and secondary outcomes measures.
2. Associated 6-, 12-, 24- and 48-HOL IS/VIS with echocardiographic measures obtained in first postnatal echocardiogram.
3. Associated IS/VIS on the day of repair and 24- and 48 hours following repair with first postnatal, post repair and 28-day echocardiographic measurements.
Statistical analysis
Logistic regression and receiver operating characteristic (ROC) curve analyses were used to investigate associations of IS/VIS with binary outcomes, while Pearson's correlation and linear regression analysis were used for continuous outcomes and Cox proportional hazards regression was used to assess associations with survival time. Multiple logistic regression analysis was used to examine associations of IS/VIS with ECMO, after controlling for CDH severity. SAS version 9.4 (SAS Institute Inc., Cary, North Carolina) was used for data analysis. A two-sided 5% significance level was used for hypothesis tests.
Results
Cohort characteristics
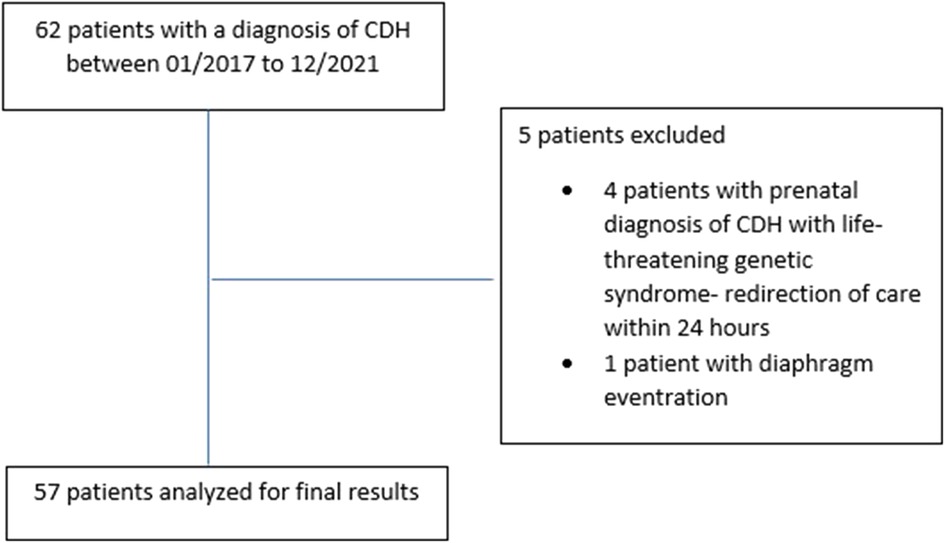
After excluding 4 patients with lethal genetic syndrome who had redirection of care within the 24 h of birth and 1 patient with diaphragmatic eventration, 57 patients were included in the final study cohort (Figure 1).
Patient characteristics are shown in Table 1. Based on prenatal imaging, 28%, 40%, and 30% of the cohort had mild, moderate and severe disease, respectively (12). Prenatal imaging was not available for one out-born patient who had a postnatal diagnosis of CDH. Twenty-six percent of patients in the cohort had prenatal CDH intervention in the form of Fetal Endoscopic Tracheal Occlusion (FETO). The primary inotropic therapy was dopamine in 91% of the patients. Fifty-three percent of the patients were treated with hydrocortisone by 24 h of life for the management of hypotension, after escalation of dopamine therapy per institutional guidelines. Forty percent required ECMO. Overall, 77% of all patients survived to discharge. Out of the twelve patients who died, ten required ECMO. Four died on ECMO-Two of which were due to bleeding on ECMO. Four Babies died following decannulation due to cardiorespiratory decompensation. Two babies died a few weeks after being on ECMO due to sepsis.
VIS/is for predicting primary outcomes
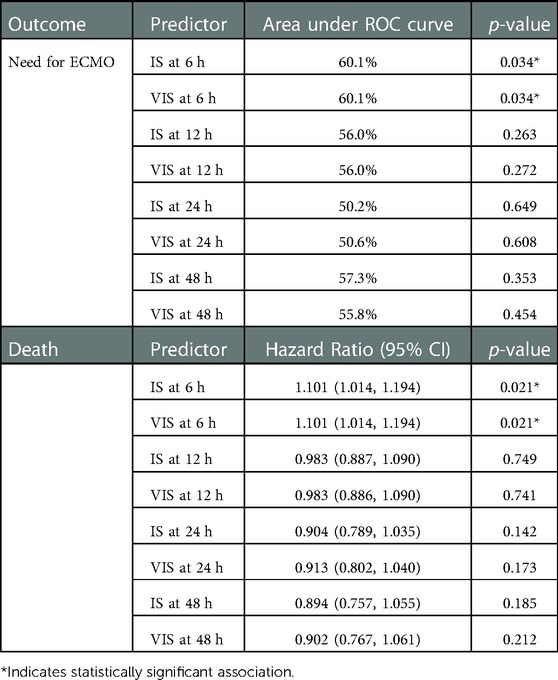
IS and VIS at 6 HOL were significantly (p = 0.034) associated with ECMO (Table 2). Every one-unit increase in IS and VIS at 6-HOL was associated with a 13% increase in the odds of ECMO (OR: 1.130, 95% CI: 1.010–1.264). Using an IS and VIS at 6-HOL cut-off of ≥7.5 to predict ECMO yielded the highest sensitivity and specificity average (sensitivity = 39.1%, specificity = 88.2%). After controlling for CDH severity, IS and VIS at 6-HOL were still significantly (p = 0.045) associated with ECMO (adjusted OR: 1.138, 95% CI: 1.003, 1.291, 66.9% area under ROC curve). Additionally, we found that every one-unit increase in IS and VIS at 6 HOL was associated with a 10.1% increase in risk of death (hazard ratio = 1.101, 95% CI: 1.014–1.194, p = 0.021) (Table 2).
IS/VIS and its correlation with echocardiographic measures
We correlated the IS/VIS obtained at various time points in the first 48 h of life (6-, 12-, 24- and 48-HOL) with echocardiographic markers of pulmonary hypertension and ventricular function seen in the first postnatal echocardiogram. We found that early increased VIS/IS was associated with worsening pulmonary hypertension.
We found that IS/VIS obtained at 6-HOL was associated with increased odds of bidirectional atrial level shunting, with every one-unit increase in IS/VIS at 6-HOL associated with 13.9% increase in the odds of bidirectional atrial level shunting (odds ratio (OR) = 1.139, 95% CI: 1.007–1.289, p = 0.038). We also noted that higher VIS at 12-HOL was significantly associated with bowing of interventricular septum (IVS) (OR: 1.135, 95% CI: 1.019–1.265, p = 0.021), higher IS at 12-HOL was significantly associated with IVS bowing (OR: 1.136, 95% CI: 1.019–1.265, p = 0.021) and higher IS and VIS at 24 hours were associated with bowing IVS (OR: 1.167, 95% CI: 1.038–1.313, p = 0.010 for both). Higher VIS at 48 hours were significantly associated with bowing IVS (OR: 1.140, 95% CI: 1.002, 1.298, p = 0.047). There was an inverse relationship between in IS/VIS at 6 HOL (OR: 0.896, 95% CI: 0.804–0.999, p = 0.048) 12 HOL (IS OR: 0.894, 95% CI: 0.810–0.986, p = 0.026, VIS OR: 0.895, 95% CI: 0.811–0.987, p = 0.027) and 24-HOL (IS OR: 0.897, 95% CI: 0.811–0.991, p = 0.033, VIS OR: 0.898, 95% CI: 0.813–0.993, p = 0.036) with septal flattening, with higher scores associated with lower odds of septal flattening. The associations of IS/VIS obtained in first 48-HOL with first postnatal echocardiogram is shown in Table 3.
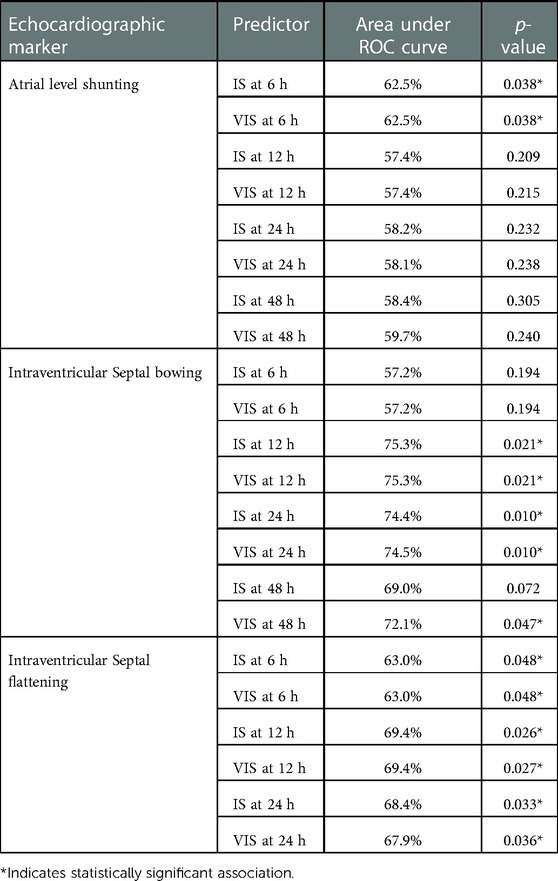
Table 3. Associations of iS/VIS obtained in first 48-HOL with echocardiographic markers in 1st postnatal echocardiogram.
When examining associations of IS/VIS obtained at various time points following the CDH repair (day of repair, 24- and 48 hours after repair) with the first post-natal, post-repair and 28-day echocardiogram (Table 4), we noted that for every one-unit increase in the IS at day of repair the predicted LV function at first post-natal Echocardiogram decreased by 0.045 (r = −0.281, p = 0.036, Figure 2A). Similarly, for every one-unit increase in the VIS at day of repair the predicted LV function at first post-natal ECHO decreased by 0.047 (r = −0.288, p = 0.031, Table 4, Figure 2B). Additionally, we found that VIS on day of repair explained 8.1% of the variation in LV function, with the predicted LV function on the post-repair echocardiogram decreasing by 0.062 for every one-unit increase in VIS on day of repair (r = −0.285, p = 0.037). Similarly, IS at day of repair explained 9.4% of the variation in LV function with every one-unit increase in IS predicting a 0.065-unit decrease in LV function in the post-repair echocardiogram (r = −0.307, p = 0.024) (Table 4 and Figures 2C,D). Finally, we found direct relationships between IS/VIS on the day of repair, 24 h following repair and 48 h following repair with LV function in the 28-day echocardiogram. IS and VIS at 24 h post repair explained the largest proportion of the variation in LV function (29.4% and 29.5%, respectively). For every one-unit increase in IS and VIS at 24 h post repair, the predicted LV function increased by 0.073 (p < 0.001) and 0.074 (p < 0.001), respectively (Table 4 and Figures 2E,F). We found no statistically significant correlation between IS/VIS at different time points with markers of RV function, PDA flow direction and TR velocity.
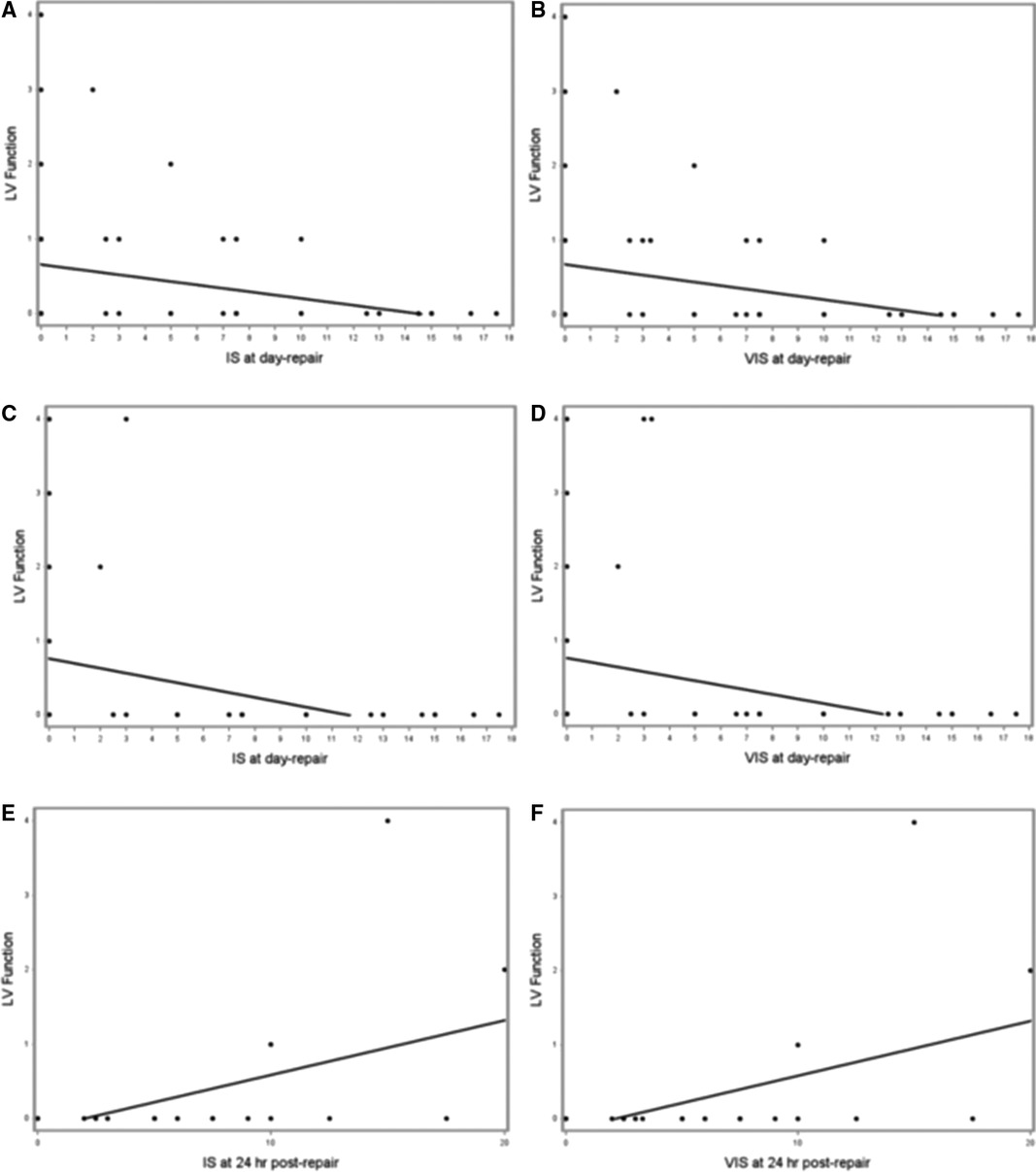
Figure 2. (A–F). Scatterplots of IS and VIS on the day of repair vs. LV function in first postnatal echocardiogram (A and B, respectively), IS and VIS on the day of repair vs. LV function in post-repair echocardiogram (C and D, respectively), and IS and VIS at 24 h following repair vs. LV function in 28-day echocardiogram (E and F, respectively).
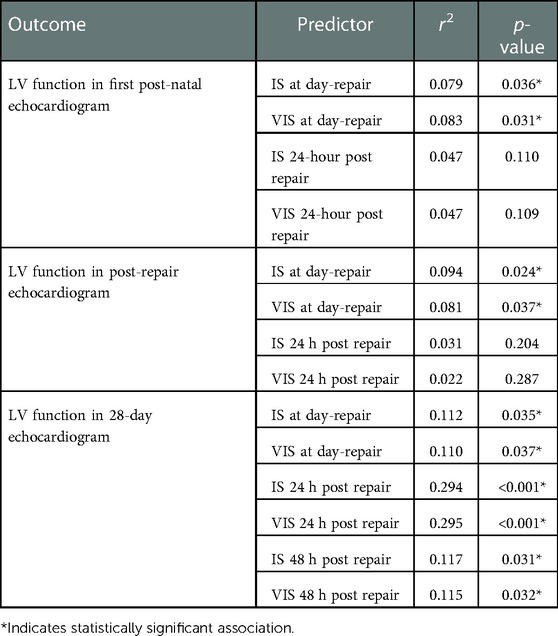
Table 4. Associations of iS/VIS obtained following CDH repair (on the day of repair, 24- and 48 hours after repair) with left ventricular function in 1st postnatal, post-repair and 28-day echocardiogram.
Discussion
We found IS and VIS to be a useful tool for assessing patients with CDH. We observed increased IS/VIS obtained at 6- HOL was associated with ECMO and mortality. IS/VIS obtained at different time points correlated well with various echocardiographic markers of PH and ventricular function, thus highlighting their potential utility as bedside tools for clinical assessment and guiding management.
A thorough understanding of the pathophysiology of systemic hypotension in CDH is still evolving. Elevated pulmonary vascular resistance (PVR) and right ventricular (RV) afterload decreases pulmonary blood flow leading to decreased pulmonary venous return and net cardiac output. Recently, ventricular dysfunction has emerged as an important area of clinical significance (14). LV dysfunction seen in this patient population contributes to low cardiac output, which has been reported as an independent predictor for death and ECMO use (14). Our study validated this by demonstrating an increase in IS/VIS at 6-HOL, which was associated with mortality and increased need for ECMO.
Etiology of LV dysfunction in CDH is incompletely understood. The fetal and post-natal cardiac physiology contributing to LV dysfunction has been summarized in Figures 3, 4.
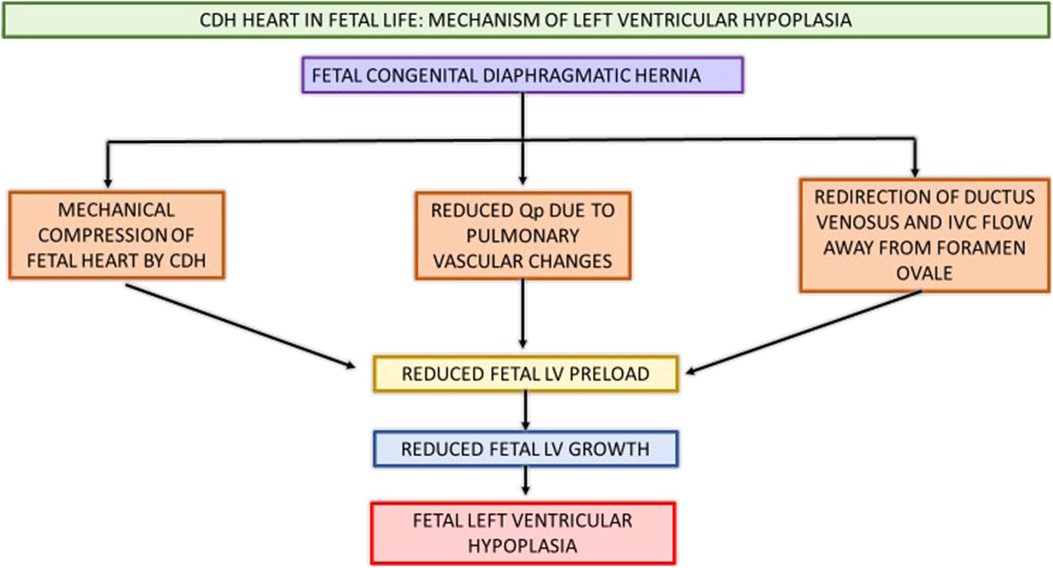
Figure 3. Schematic showing CDH heart in fetal life and pathophysiology contributing to postnatal LV hypoplasia and systemic hypotension. CDH, congenital diaphragmatic hernia; Qp, pulmonary blood flow; IVC, inferior vena cava; LV, left ventricle.
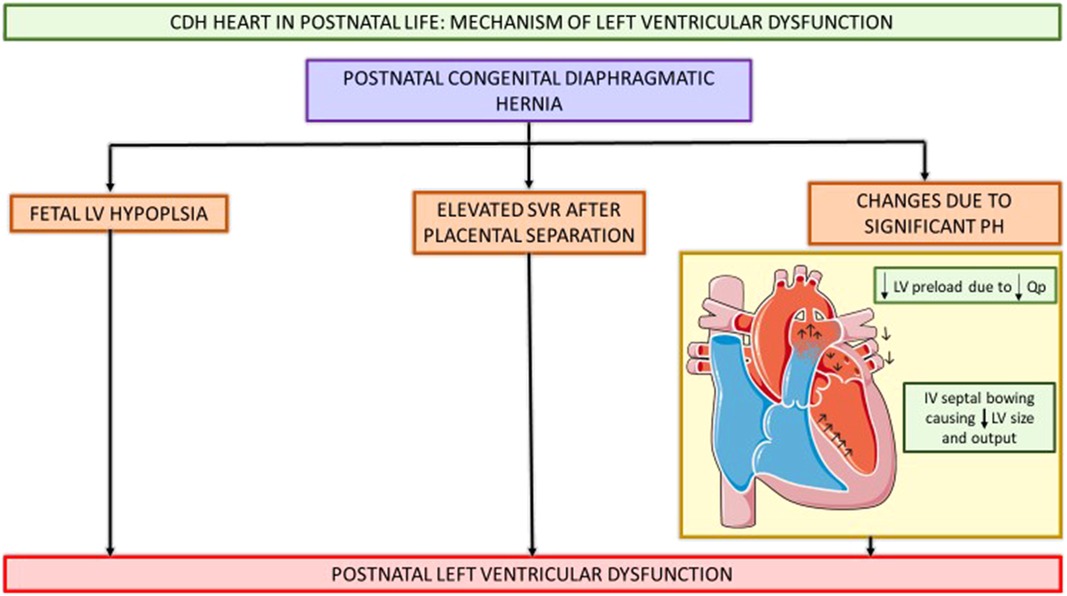
Figure 4. Schematic showing CDH heart in postnatal life and pathophysiology contributing to postnatal LV hypoplasia and systemic hypotension. CDH, congenital diaphragmatic hernia; Qp, pulmonary blood flow; LV, left ventricle; SVR, systemic vascular resistance; IV, intraventricular septum. (This Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.).
Various fetal and postnatal mechanisms for LV dysfunction in the CDH population have been hypothesized, such as underdeveloped left sided cardiac structures, increased SVR and ventricular interdependence (15–17). Early fetal echocardiograms show evidence of smaller left heart structures (16, 18). Left ventricular hypoplasia is thought to be a product of mechanical compression of the fetal heart by the herniated contents in the thoracic cavity (19). Re-direction of ductus venosus and inferior vena cava (IVC) flow away from the foramen ovale leads to decreased LV preload and inadequate fetal myocardial growth (15, 20). Additionally, postnatal stress with elevated systemic vascular resistance (SVR) after placental separation further worsens ventricular dysfunction and is associated with adverse postnatal outcomes (14, 16, 21, 22). Thirdly, fetal lung growth is impeded by intrathoracic herniation of abdominal contents causing reduction in total fetal lung volume and pulmonary hypoplasia. This pulmonary vasculature is maladaptive and maldeveloped as a result of this prenatal insult, thus leading to pulmonary hypertension. The resultant reduced pulmonary blood flow in the setting of this pulmonary hypertension causes decreased pulmonary venous return to the left atrium, and ultimately can lead to postnatal systemic hypotension (15, 17). The RV dilation and hypertrophy alter the myocardial contractility as ventricular inter-dependence is compromised due to septal displacement. With inadequate RV contractility and increased RV afterload, a vicious cycle of decreased pulmonary blood flow, with further reduction in pulmonary venous return, and thus lower LV preload with resultant systemic and coronary hypoperfusion and worsening myocardial contractility ensues (15, 17, 23).
In our study, we observed a significant correlation between IS/VIS at 12-, 24- and 48-HOL with septal bowing seen in the first postnatal echocardiogram-with higher scores associated with septal bowing. We also found an inverse relationship between IS/VIS at 6-,12- and 24-HOL with septal flattening, which can be explained by preserved interventricular interaction with septal flattening, as opposed to septal bowing where the LV preload is decreased, and myocardial contractility is altered with geometric distortion of the left ventricle. This results ultimately in decreased stroke volume and cardiac output thus needing additional inotropic support and higher IS/VIS scores.
Our results showed that an increase in IS/VIS on the day of repair was associated with decreased LV function in the first postnatal and post-repair echocardiogram which may be secondary to persistence of fetal cardiac maladaptation and delays in postnatal transition, resulting in increased need for vasoactive support during the repair and increased metabolic demands during the procedure. In addition, our results showed that an increase in IS/VIS correlated with increase in LV function in the 28-day echocardiogram. This can be explained by the fact that after CDH repair, the mediastinum and cardiac structures shift into anatomically appropriate positions, improving venous return and optimizing preload, permitting recovery of LV function and remodeling of myocardium, all of which contribute to the improved function noted on the echocardiogram obtained after 28 days of life.
The IS/VIS have been validated as a clinical measure of cardioactive medication use in both the adult and pediatric population (8, 10, 24, 25). They have been specifically studied in the cardiac ICU as a predictor for adverse outcomes such as prolonged length of stay, duration of mechanical ventilation, mechanical circulatory support, renal replacement therapy and death (8). Studies in the neonatal population have been emerging and have shown promising results on the clinical use of these scoring tools (26, 27). These studies specifically looked at the preterm population and calculated the VISmax (calculated as the maximum score during birth hospitalization). Aziz et al. reported VISmax to have significant utility to predict mortality in the preterm population, with VISmax >30 associated with universal mortality (26). Kharrat et al. reported the VISmax during the first 12-, 24- and 48 hours of treatment in preterm infants and concluded that a VISmax score ≥20 within 48 hours of treatment initiation was associated with adverse outcomes (27).
Strengths
We report that IS and VIS are useful prognostic variables in the CDH patient population, where there is an increased need for inotropic support and associated adverse outcomes, such as mortality and ECMO. We add a unique perspective by correlating these scoring modalities with specific echocardiographic markers in neonates with CDH. Additionally, we have taken both pre- and post-repair CDH physiologies into consideration by obtaining serial IS/VIS measurements up to 48 hours of life and 48 h following surgical repair. This provides a temporal understanding of changing CDH physiology from birth until after surgical repair, thus validating the contribution of ventricular remodeling and improvement in cardio-pulmonary physiology following repair in CDH.
Limitations
One of the major limitations of the study is the retrospective nature of the study due to which the specific timing of echocardiogram, timing, rate of escalation, dosing and type of vasopressor support was not standardized. Additionally, since the study focused specifically on inotropic use, we could not account for other management strategies for systemic hypotension such as use of fluid boluses, blood transfusions and/or use of hydrocortisone, which may have impacted the overall clinical picture of this patient population. We could also not account for contributors of systemic hypotension such as pulmonary vasodilators and/or sedation medications. VIS and IS ≥ 7.5 at 6-HOL had high specificity but low sensitivity in predicting ECMO. Thus, scores <7.5 at 6-HOL could be useful for identifying patients at low risk for ECMO, but other prognostic variables should be investigating to discriminate those who will versus will not receive ECMO among those with VIS and IS ≥ 7.5 at 6-HOL. Predictive accuracy for ECMO decreases after 6-HOL but it is fortunate that the highest predictive accuracy occurs early when it is most useful. Examining predictive accuracy at numerous time points increases the probability of Type I Error (i.e., concluding there is an association with IS/VIS where there really is not). Therefore, findings from this study should be considered exploratory and future prospective studies should focus on IS/VIS at 6-HOL and within the first day of repair. Despite these limitations, this is one of the first studies validating the use of these scoring tools in predicting outcomes and correlating with echocardiographic markers in the CDH population.
Future research
Our goal with this study is to stimulate further research into the use of IS/VIS following its validation in the CDH population. This data has now laid the foundation for a potential pilot prospective model to further investigate these scoring tools in a standardized protocol-based setting and help predict various short-term and long-term clinical outcomes. This would further add to the evidence and encourage the use of these scoring modalities in the CDH population. Additionally, it would be beneficial to investigate the role of variables such as sex and ethnicity, to supplement these scoring tools, to further understand the impact of these factors on the overall clinical outcomes in CDH. In our study, IS and VIS ≥ 7.5 at 6 HOL was found to have high specificity but low sensitivity in predicting ECMO. These results suggest that IS and VIS < 7.5 at 6 HOL indicate low risk for needing ECMO, but future research should attempt to identify other prognostic variables to discriminate those who will versus will not need ECMO among CDH patients with IS and VIS ≥ 7.5 at 6 HOL. Finally, with the advent of fetal interventions, the impact of FETO on IS/VIS would be an indirect assessment of the impact of this fetal intervention on ventricular function in CDH, which is an area of ongoing research.
Conclusion
The IS/VIS are objective markers of ventricular function and, with this study, we have correlated these scores at different time points with short term outcomes, and echocardiographic markers of PH/myocardial dysfunction in CDH. This study encourages the use of these scoring modalities in the CDH population and has opened avenues for further research.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Baylor College of Medicine-Institutional Review Board- IRB # H-49059. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
SHaG: Conceptualization/design, methodology, data collection, investigation, writing initial manuscript and editing of manuscript. CLT Methodology, data collection, investigation, review and editing manuscript. MH: Data collection, investigation, review and editing manuscript. BYF: Conceptualization/design, methodology, review and editing manuscript. JLH: Formal analysis, review and editing manuscript. AAN: Conceptualization/design, review and editing manuscript. CJF: Conceptualization/design, review and editing manuscript. SK: Conceptualization/design, review and editing manuscript. SHG: Conceptualization/design, methodology, investigation, supervision/oversight, review and editing manuscript. All authors contributed to the article and approved the submitted version.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Avitabile CM, Wang Y, Zhang X, Griffis H, Saavedra S, Adams S, et al. Right ventricular strain, brain natriuretic peptide, and mortality in congenital diaphragmatic hernia. Ann Am Thorac Soc. (2020) 17(11):1431–9. Available at: www.atsjournals.org doi: 10.1513/AnnalsATS.201910-767OC
2. Karpuz D, Giray D, Celik Y, Hallioglu O. Prognostic markers in congenital diaphragmatic hernia: left ventricular diameter and pulmonary hypertension. Pediatr Int. (2018) 60(2):122–6. doi: 10.1111/ped.13464
3. Putnam LR, Harting MT, Tsao K, Morini F, Yoder BA, Luco M, et al. Congenital diaphragmatic hernia study group. Congenital diaphragmatic hernia defect size and infant morbidity at discharge. Pediatrics. (2016) 138(5). doi: 10.1542/peds.2016-2043
4. Altit G, Bhombal S, van Meurs K, Tacy TA. Diminished cardiac performance and left ventricular dimensions in neonates with congenital diaphragmatic hernia. Pediatr Cardiol. (2018) 39(5):993–1000. doi: 10.1007/s00246-018-1850-7
5. Murthy K, Pallotto EK, Gien J, Brozanski BS, Porta NF, Zaniletti I, et al. Predicting death or extended length of stay in infants with congenital diaphragmatic hernia. J Perinatol. (2016) 36(8):654–9. doi: 10.1038/jp.2016.26
6. Strand K, Flaatten H. Severity scoring in the ICU: a review. Acta Anaesthesiol Scand. (2008) 52:467–78. doi: 10.1111/j.1399-6576.2008.01586.x
7. Wynn JL, Mayampurath A, Carey K, Slattery S, Andrews B, Sanchez-Pinto LN. Multicenter validation of the neonatal sequential organ failure assessment score for prognosis in the neonatal intensive care unit. J Pediatr. (2021) 236:297–300. doi: 10.1016/j.jpeds.2021.05.037
8. Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. (2010) 11(2):234–8. doi: 10.1097/PCC.0b013e3181b806fc
9. Bangalore H, Checchia P, Ocampo E, Heinle J, Guffey D, Minard C, et al. Total inotrope exposure score: an extension of the vasoactive inotrope score. Intensive Care Med Exp. (2015) 3(1):1–2 doi: 10.1186/2197-425X-3-S1-A211
10. Bangalore H, Gaies M, Ocampo EC, Heinle JS, Guffey D, Minard CG, et al. The total inotrope exposure score: an extension of the vasoactive inotrope score as a predictor of adverse outcomes after paediatric cardiac surgery. Cardiol Young. (2017) 27(6):1146–52. doi: 10.1017/S1047951116002602
11. Gaies MG, Jeffries HE, Niebler RA, Pasquali SK, Donohue JE, Yu S, et al. Vasoactive-Inotropic score (VIS) is associated with outcome after infant cardiac surgery: an analysis from the pediatric cardiac critical care consortium (PC 4) and virtual PICU system registries. Pediatr Crit Care Med. (2014) 15(6):529–37. doi: 10.1097/PCC.0000000000000153
12. Ruano R, Lazar DA, Cass DL, Zamora IJ, Lee TC, Cassady CI, et al. Fetal lung volume and quantification of liver herniation by magnetic resonance imaging in isolated congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. (2014) 43:662–9. doi: 10.1002/uog.13223
13. Mertens L, Seri I, Marek J, Arlettaz R, Barker P, McNamara P, et al. Targeted neonatal echocardiography in the neonatal intensive care unit: practice guidelines and recommendations for training. J Am Soc Echocardiogr. (2011) 24(10):1057–78. doi: 10.1016/j.echo.2011.07.014
14. Patel N, Lally PA, Kipfmueller F, Massolo AC, Luco M, Van Meurs KP, et al. Ventricular dysfunction is a critical determinant of mortality in congenital diaphragmatic hernia. Am J Respir Crit Care Med. (2019) 200(12):1522–30. doi: 10.1164/rccm.201904-0731OC
15. Patel N, Massolo AC, Kraemer US, Kipfmueller F. The heart in congenital diaphragmatic hernia: knowns, unknowns, and future priorities. Front Pediatr. (2022) 10:890422. doi: 10.3389/fped.2022.890422.
16. Kinsella JP, Steinhorn RH, Mullen MP, Hopper RK, Keller RL, Ivy DD, et al. The left ventricle in congenital diaphragmatic hernia: implications for the management of pulmonary hypertension. J Pediatr. (2018) 197:17–22. doi: 10.1016/j.jpeds.2018.02.040
17. Patel N, Massolo AC, Kipfmueller F. Congenital diaphragmatic hernia-associated cardiac dysfunction. Semin Perinatol. (2020) 44(1):151168. WB Saunders. doi: 10.1053/j.semperi.2019.07.007
18. Siebert JR, Haas JE, Beckwith JB. Left ventricular hypoplasia in congenital diaphragmatic hernia. J Pediatr Surg. (1984) 19(5):567–71. doi: 10.1016/S0022-3468(84)80105-0
19. Byrne FA, Keller RL, Meadows J, Miniati D, Brook MM, Silverman NH, et al. Severe left diaphragmatic hernia limits size of fetal left heart more than does right diaphragmatic hernia. Ultrasound Obstet Gynecol. (2015) 46:688–94. doi: 10.1002/uog.14790
20. Stressig R, Fimmers R, Eising K, Gembruch U, Kohl T. Preferential streaming of the ductus venosus and inferior caval vein towards the right heart is associated with left heart underdevelopment in human fetuses with left-sided diaphragmatic hernia. Heart. (2010) 96(19):1564–8. doi: 10.1136/hrt.2010.196550
21. Massolo AC, Romiti A, Viggiano M, Vassallo C, Ledingham MA, Lanzone A, et al. Fetal cardiac dimensions in congenital diaphragmatic hernia: relationship with gestational age and postnatal outcomes. J Perinatol. (2021) 41(7):1651–9. doi: 10.1038/s41372-021-00986-y
22. Patel N, Massolo AC, Paria A, Stenhouse EJ, Hunter L, Finlay E, et al. Early postnatal ventricular dysfunction is associated with disease severity in patients with congenital diaphragmatic hernia. J Pediatr. (2018) 203:400–7. doi: 10.1016/j.jpeds.2018.07.062
23. Gan C T-J, Lankhaar JW, Marcus JT, Westerhof N, Marques KM, Bronzwaer JG, et al. Impaired left ventricular filling due to right-to-left ventricular interaction in patients with pulmonary arterial hypertension. Am J Physiol-Heart and Circ Physiol. (2006) 290(4):H1528–33. doi: 10.1152/ajpheart.01031.2005
24. Yamazaki Y, Oba K, Matsui Y, Morimoto Y. Vasoactive-inotropic score as a predictor of morbidity and mortality in adults after cardiac surgery with cardiopulmonary bypass. J Anesth. (2018) 32(2):167–73. doi: 10.1007/s00540-018-2447-2
25. Belletti A, Lerose CC, Zangrillo A, Landoni G. Vasoactive-inotropic score: evolution, clinical utility, and pitfalls. J Cardiothorac Vasc Anesth. (2021) 35(10):3067–77. doi: 10.1053/j.jvca.2020.09.117
26. Aziz KB, Lavilla OC, Wynn JL, Lure AC, Gipson D, de la Cruz D. Maximum vasoactive-inotropic score and mortality in extremely premature, extremely low birth weight infants. J Perinatol. (2021) 41(9):2337–44. doi: 10.1038/s41372-021-01030-9
Keywords: congenital diaphragmatic hernia, pulmonary hypertension, ventricular dysfunction, inotropic score, vasoactive inotropic score, extracorporeal membrane oxygenation
Citation: Hari Gopal S, Toy CL, Hanna M, Furtun BY, Hagan JL, Nassr AA, Fernandes CJ, Keswani S and Gowda SH (2023) Inotropic score and vasoactive inotropic score as predictors of outcomes in congenital diaphragmatic hernia: A single center retrospective study. Front. Pediatr. 11:1101546. doi: 10.3389/fped.2023.1101546
Received: 18 November 2022; Accepted: 10 January 2023;
Published: 1 February 2023.
Edited by:
Jason Gien, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Upender Munshi, Albany Medical College, United StatesAdrian Holloway, University of Maryland Medical Center, United States
© 2023 Hari Gopal, Toy, Hanna, Furtun, Hagan, Nassr, Fernandes, Keswani and Gowda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sharada H. Gowda sharada.gowda@bcm.edu
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 Srirupa Hari Gopal
Srirupa Hari Gopal Cynthia L. Toy2
Cynthia L. Toy2  Ahmed A. Nassr
Ahmed A. Nassr Caraciolo J. Fernandes
Caraciolo J. Fernandes Sharada H. Gowda
Sharada H. Gowda