Hereditary pulmonary arterial hypertension burden in pediatrics: A single referral center experience
- 1Department of Pediatrics, Critical Care, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, United States
- 2Department of Pathology & Immunology, Baylor College of Medicine, Houston, TX, United States
- 3John Welsh Cardiovascular Diagnostic Laboratory, Division of Genomic Medicine, Department of Pathology, Texas Children’s Hospital, Houston, TX, United States
- 4Department of Pediatrics, Pulmonology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, United States
- 5Division of Genomic Medicine, Department of Pathology, Texas Children’s Hospital, Houston, TX, United States
- 6Department of Pediatrics, Cardiology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, United States
- 7Departments of Pathology & Immunology and Pediatrics-Cardiology, Baylor College of Medicine, Houston, TX, United States
Introduction: Hereditary pulmonary arterial hypertension (HPAH) is a rare yet serious type of pulmonary arterial hypertension (PAH). The burden in the pediatric population remains high yet underreported. The objective of this study is to describe the distribution of mutations found on targeted PAH panel testing at a large pediatric referral center.
Methods: Children with PAH panel administered by the John Welsh Cardiovascular Diagnostic Laboratory at Texas Children's Hospital and Baylor College of Medicine in Houston, Texas between October 2012 to August 2021 were included into this study. Medical records were retrospectively reviewed for clinical correlation.
Results: Sixty-six children with PAH underwent PAH genetic testing. Among those, 9 (14%) children were found to have pathogenic mutations, 16 (24%) children with variant of unknown significance and 41 (62%) children with polymorphism (classified as likely benign and benign). BMPR2 mutation was the most common pathogenic mutation, seen in 6 of the 9 children with detected mutations. Hemodynamic studies showed higher pulmonary vascular resistance among those with pathogenic mutations than those without (17.4 vs. 4.6 Wood units). All children with pathogenic mutations had severe PAH requiring triple therapy. There were tendencies for higher lung transplantation rate but lower mortality among those with pathogenic mutations.
Conclusions: Abnormalities on genetic testing are not uncommon among children with PAH, although majority are of unclear significance. However, children with pathogenic mutations tended to present with more severe PAH requiring aggressive medical and surgical therapies. Genetic testing should be routinely considered due to consequences for treatment and prognostic implications. Larger scale population studies and registries are warranted to characterize the burden of HPAH in the pediatric population specifically.
Introduction
Heritable pulmonary arterial hypertension (HPAH) is a rare yet serious type of pulmonary arterial hypertension (PAH). Although adult epidemiologic studies report the median age of diagnosis for PAH is currently in the 3rd–4th decade of life (1, 2), age of diagnosis for HPAH is extremely variable. Emerging clinical and genetic data indicate that there are fundamental differences between pediatric and adult-onset disease (3, 4), reflecting the influence of epigenetic and possibly environmental factors. There is a greater genetic burden in children with genetic factors contributing to 42% of pediatric onset PAH compared to 13% of adult-onset PAH (3). At present, mutations in 16 genes have been linked to development of HPAH, many of them involved in or affecting the BMPR2 (Bone morphogenetic protein receptor 2) signaling pathway (3, 5). BMPR2 is highly expressed on pulmonary vascular endothelium and mutations in BMPR2 comprise the 70%–80% of HPAH cases (6). The co-factors of BMPR2 endoglin (ENG) and activin receptor like kinase 1 (ACVRL1) are predominantly altered in hereditary hemorrhagic telangiectasia-associated PAH (7, 8). Mutations in the BMP receptor type IA and type 1B [BMPR1A and BMPR1B, also called activin receptor-like kinase 6 (ALK6)], caveolin-1 (CAV1), eukaryotic initiation translation factor 2 alpha kinase 4 (EIF2AK4), potassium two-pore-domain channel subfamily K member 3 (KCNK3), SMAD family members 4 and 9 (SMAD4 and SMAD9), and T-box 4 (TBX4) have all been identified as less frequent or rare causes of PAH (9).
Pediatric PAH differs from adult-onset PAH in many important aspects, including clinical presentation, etiology, genetic burden, and specific genes involved. In pediatric-onset PAH, transcription factors TBX4 and SOX17 are seen with high frequency following BMPR2 (3). TBX4 and SOX 17 are not expressed in pulmonary arterial endothelial cells or smooth muscles, but in embryonic tissues and have prominent roles in lung and vasculature development (10, 11). De novo variants are a frequent characteristic of pediatric-onset PAH, contributing to 15% of patient population (3). These discoveries have prompted considerable development of targeted gene mutation panels for evaluation of pediatric PAH patients. Here we describe the distribution of mutations in a pediatric PAH population of a large referral center.
Materials and methods
Patient population
This was a single center study. Our institution is the largest children's hospital in North America and is also home to the most active pediatric lung transplant program in the country, rendering a diverse and large sample population. Therefore, we retrospectively reviewed the data of children with PAH who had genetic testing at Texas Children's Hospital (TCH) from October 2012 to August 2021. Diagnosis of PAH was made based on echocardiography and right heart catheterization when patients’ clinical status allowed.
Genetic testing
The applied PAH gene panel included ACVRL1, BMPR2, CAV1, EIF2AK4, ENG, FLNA, GDF2, KCNA5, KCNK3, NOTCH1, NOTCH3, SMAD4, SMAD8, SOX17 and TOPBP1 genes. This panel was developed and administered by the John Welsh Cardiovascular Diagnostic Laboratory. Specifically, genomic DNA isolated from patient's blood sample was analyzed by sequencing for exons, splice junctions, and flanking regions of all genes tested in this panel. Sequencing analysis was performed by oligonucleotide-based in-solution hybridization target capture (SeqCap EZ, NimbleGen or KAPA HyperChoice, Roche) followed by next generation sequencing (MiSeq, Illumina). Sanger sequencing (3730XL DNA Analyzer, ABI) was used to fill in for gaps/bases that were not sufficiently covered. All clinically significant and novel variants were confirmed by independent Sanger sequencing. DNA sequence was assembled to and analyzed in comparison with the genomic reference sequences (GRCh37/hg19 or GRCh38/hg38) published in the NCBI database to generate variant calls. Variants were annotated through Esembl VEP program, curated via AlaMut and open source databases including gnomAD, ClinVar, VarSome and PubMed, and classified according to the ACMG guideline (12). Genetic variations were classified as pathogenic, variant with unknown significance (VUS) and polymorphisms (likely benign and benign). Further/correlating genetic testing was offered to parents as indicated.
Clinical information
Clinical data correlates were obtained by reviewing medical records. Demographic data of the study patients, including age, gender, race, ethnicity, comorbidity and family history of PAH was collected. Clinical information including echocardiography findings, cardiac catheterization results, treatment combinations and outcome were also collected and analyzed. Presence of right ventricular failure was defined qualitatively based on the echo reports. Medical therapy was categorized as monotherapy, dual therapy and triple therapy.
Statistical analysis
Data were analyzed using STATA 13.1 (StataCorp, College Station, TX). Summary statistics were described with mean and standard deviation for parametric variables and median with interquartile range (IQR) for nonparametric variables. Comparison of continuous variables in subgroups was performed using independent t-test or Wilcoxon rank-sum test, one-way analysis of variance, or Kruskal-Wallis depending on normality of distribution. Comparisons of categorical variables between subgroups were analyzed using the Chi-square test. The threshold for statistical significance was p-value <0.05.
Results
There were 66 children with diagnosis of PAH who had genetic testing through John Welsh Laboratory during the study period (Table 1). Median (IQR) age of study patients was 4.4 (0.94, 11.4) years old. There was female predominance (n = 38, 58%). The majority of patients were Caucasian (n = 47, 71%) and 19 (40.4%) identified as Hispanic or Latino. The greater part of the study population (n = 40, 61%) had comorbidities either in the cardiac or respiratory system. Approximately 10% of children who underwent genetic testing had known family history of PAH.
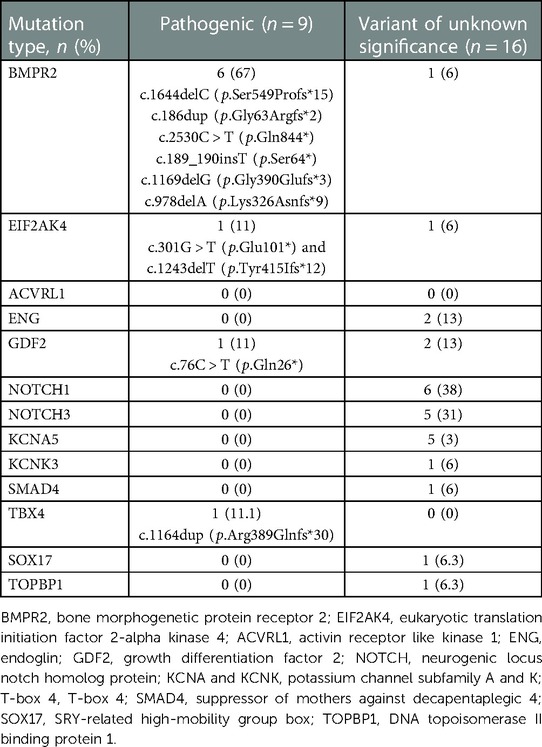
Nine children (14%) were found to have pathogenic mutations, 16 (24%) with variant of unknown significance and 41 (62%) with –polymorphisms (likely benign or benign) noted. Children with pathogenic mutations older than those without pathogenic mutations. BMPR2 mutation was the most common pathogenic mutation, seen in 6 children (Table 2). Other recorded mutations included EIF2AK4 pathogenic mutation, GDF2 pathogenic mutation in one child each respectively. And there was a child who was found to have TBX4 pathogenic mutation later on through a genetic testing out of our lab. The majority of the variants of unknown significance were located in the gene NOTCH1 (38%), followed by NOTCH3 (31%) and KCNA5 (31%).
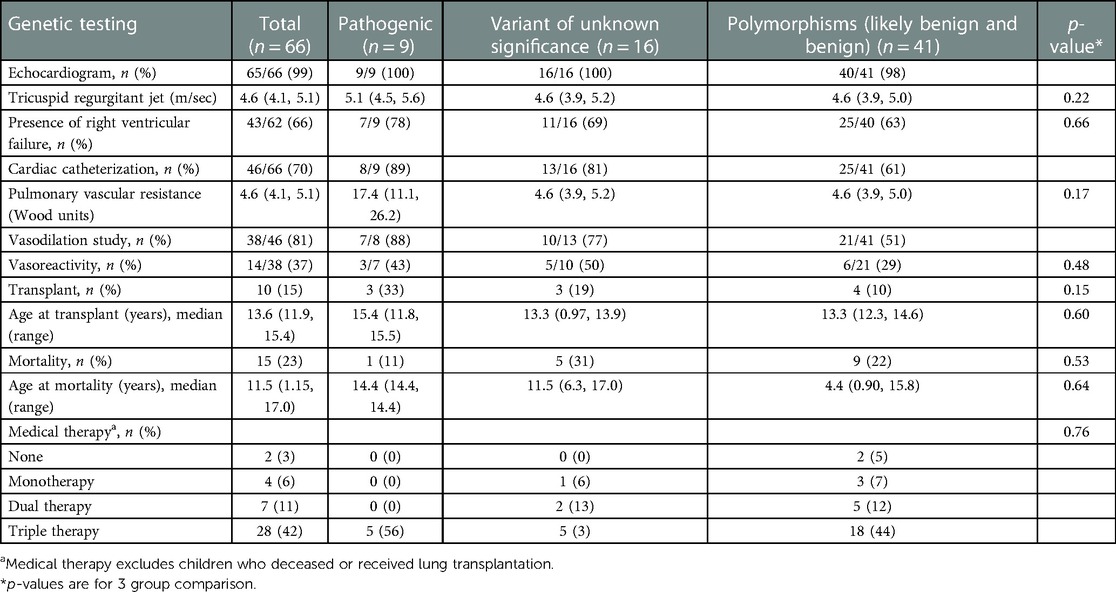
Eighty-three percent of the study population was classified as World Symposium of Pulmonary Hypertension (WSPH) Group 1, WSPH Group 3 was 21% of patients. Children with features of Group 1 and Group 3 disease were classified in the WSPH group that corresponded to the driver of disease pathogenesis. There were 10 (15%) children who were classified as multifactorial disease (i.e., fit more than 1 WSPH classification), however none of them had pathologic mutations. All children with pathogenic mutations were classified as WHO group 1 (Group 1.2 for HPAH, 1.6 for PVOD). All children with pathogenic mutations had severe PAH requiring triple therapy (Table 3).
On echocardiogram, tricuspid regurgitant (TR) doppler signal was measured in 43 children. Median TR jet was elevated to 5.1 (4.5, 5.6) vs. 4.6 (3.9, 5.2) vs. 4.6 (3.9, 5.0) among children with pathogenic, VUS and polymorphism mutations respectively. The rate of RV failure was the highest in pathogenic mutation group [78% vs. 69% vs. 63%]. Cardiac catheterization revealed the highest pulmonary vascular resistance (PVR) with pathogenic mutations [17.4 (11.1, 26.2) vs. 4.6 (3.9, 5.2) vs. 4.6 (3.9, 5.0)]. About half of the children with pathogenic and VUS mutations had positive vasoreactivity during cardiac catheterization (43%, 50%). Vasoreactivity was least observed among those with polymorphism mutations (29%).
Outcomes for the cohort with identified pathogenic mutation trended to higher rates of transplantation, 33% (n = 3) compared to those without pathogenic mutations (n = 7, 12%). The age of lung transplantation was similar at 13–15 years old among all genetic groups. The median (IQR) time to the lung transplant was 9.8 (1.2, 72.0) months ranging from 0.78 months to 12.5 years.
Mortality was higher in the non-pathogenic group (VUS and polymorphism composite) compared to the group of patients with pathogenic mutations (n = 14, 53% vs. n = 1, 11%, p = 0.53). The one death in the pathogenic mutation group was a 14 year-old child who died during diagnostic admission; later discovered to have BMPR2 mutation.
Genetic testing was offered to four families with known pathogenic mutation and two families with detected variant of unknown significance. Inherited pathogenic mutations were identified in two families of patients with pathogenic mutation. The first family had a mutation in EIF2AK4. The parents had c.1243delT (p.Tyr415Ifs*12) and c.301G > T (p.Glu101*) and the child inherited both of these mutations. The second family had a mutation in GDF2: both of the parents had c.76C > T (p.Gln26*), which the child inherited. In families with children who resulted with variant of unknown significance, no no pathogenic mutations were identified.
Discussion
We describe the genetic burden of disease within a large pediatric referral center for PH. In our cohort, we identified PAH-associated pathogenic genetic mutations in 13.6% of the patients who otherwise had no obvious etiology for their disease. This is lower than previous studies reporting 20%–50% prevalence of genetic mutations in pediatric onset PAH (3, 13, 14). One of the possible explanations is limitations on our genetic panel not including newer PAH genes such as BMP10, aquaporin 1 (AQP1), ATPase 13A3 (ATP13A3) or kinase insert domain receptor (KDR). BMPR2 mutation was the most common mutation seen among 66.7% of mutations in our population, consistent with the literature. BMPR2 mutation has been reported as the most common mutation in both pediatrics and adults, comprising 6.5%–12.5% of all unexplained pediatric PAH and 36%–65% of pediatric PAH with pathogenic genetic variants (3, 5, 13, 15).
In our patient population, higher TR jet and more RV failure was noted on initial echocardiograms among children with pathogenic mutation, suggesting more clinical burden of disease (16). This trend was confirmed by cardiac catheterization, showing higher PVR among children with pathogenic mutations compared to those without them. PVR among children with pathogenic mutation in our population was similar to the previously reported values of 19.9 WU among children with idiopathic and heritable PAH by Zhang et al. (14). All children with pathologic mutation had severe PAH at presentation and all were started on upfront combination therapy. Outcomes for HPAH in our population were commensurate with reports of severe disease in the literature, including high rate of transplantation. It is interesting to note that the mortality in the HPAH cohort was overall less than the non-HPAH cohort. This may reflect the benefit of upfront aggressive medical treatment that was offered to the group with pathogenic mutation and the expectant referral provided for transplantation in the face of poor prognosis. Although the majority of our population did not have an identified pathogenic mutation on targeted screening, this non-genetic disease group did demonstrate progressive and aggressive disease, consistent with idiopathic PAH, WSPH Group 1 (86%). This percentage is higher than literature reports of 58% idiopathic in the pediatric population (3). This discrepancy may reflect a limitation in our institutional genetic panel or reflect the diversity of our patient population. Advancement in genetic studies including identification of new genetic mutations is required for these patient population.
A previous population-based study among insured United States (US) patients reported that 36% of pediatric PAH patients had a history of prematurity, 75% with congenital heart defect and 13% with trisomy 21 (17). In this selected population that underwent genetic testing, only 8 (12%) children had documented history of prematurity, 18 (27%) had cardiac defect and none of the referred patients had other known PAH associated genetic syndromes such as trisomy 21. This difference is likely reflective of some sample bias as genetic studies were only offered for unexplained or disproportionate PH symptoms.
In addition to the limitations already stated, there was insufficient data on familial testing to report on the benefit of that modality in this study. Although pathogenic mutations were discovered and group classification was changed to WSPH Group 1.2, further familial studies were necessary to confirm hereditary nature of the discovered mutations. Secondly, the overall number of children with pathogenic mutation was small. This is likely a limitation of targeted or known familial mutation testing. Application of broader panels will likely find additional genetic abnormalities. Finally, due to the retrospective nature of the study, our data was limited for data prior to presentation at our center and to testing available during a specific era. Genetic panels are evolving quickly and negative testing in 2012 may not be negative on repeat, expanded testing. The panel that was used for genetic testing in this population was updated over time by adding new genes. Although BMPR2 has been on the panel since testing was started, and this diagnostic yield was not significantly affected over study period, it is possible that mutations in other genes of interest were missed in early testing.
We recognize that the findings in this study may be unique and not be generalizable for the greater pediatric PAH population. However, we hope that this descriptive study informs on the genetic burden of disease in pediatric PH patients and the consideration for genetic screening to affect morbidity and mortality. Certainly, larger population studies and registries for pediatric PAH are warranted to better characterize the greater burden of HPAH and phenotype-genotype correlations.
Conclusion
Genetic mutations are not uncommon among children with PAH. Children affected with pathogenic mutations presented with more severe PAH, higher pulmonary vascular resistance and higher rate of RV failure requiring upfront triple therapy. These children had higher rates of transplantation but improved survival, perhaps because of more aggressive treatment in the setting of known HPAH. Genetic testing should be routinely considered due to consequences for treatment and prognostic implications. Further studies in larger population and registries are warranted to better characterize pediatric HPAH.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, SCV002575026 - SCV002575059.
Ethics statement
The studies involving human participants were reviewed and approved by Baylor College of Medicine, Institutional Review Board. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
MI: Generated research idea, collected and analyzed data, developed manuscript. WZ: Performed genetic testing. EW: Collected data and edited manuscript. EE: Collected data. RDC: Collected data. DHL-T: Supervised genetic testing. DJP: Supervised genetic testing. YF: Supervised genetic testing. NPV: Supervised research idea development, collected data and supervised manuscript development. All authors contributed to the article and approved the submitted version.
Conflict of interest
NPV receives research support from the PePH registry.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa-Hahnle K, et al. A global view of pulmonary hypertension. Lancet Respir Med. (2016) 4:306–22.26975810
2. Taichman DB, Mandel J. Epidemiology of pulmonary arterial hypertension. Clin Chest Med. (2013) 34:619–37. doi: 10.1016/j.ccm.2013.08.010
3. Welch CL, Chung WK. Genetics and genomics of pediatric pulmonary arterial hypertension. Genes. (2020) 11:1–16. doi: 10.3390/genes11101213
4. Ivy DD, Abman SH, Barst RJ, Berger RMF, Bonnet D, Fleming TR, et al. Pediatric pulmonary hypertension. J Am Coll Cardiol. (2013) 62:D117–26. doi: 10.1016/j.jacc.2013.10.028
5. Eichstaedt CA, Saßmannshausen Z, Shaukat M, Cao D, Xanthouli P, Gall H, et al. Gene panel diagnostics reveals new pathogenic variants in pulmonary arterial hypertension. Respir Res. (2022) 23(1):74. doi: 10.1186/s12931-022-01987-x
6. Evans JDW, Girerd B, Montani D, Wang XJ, Galiè N, Austin ED, et al. BMPR2 Mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med. (2016) 4(2):129–37. doi: 10.1016/S2213-2600(15)00544-5
7. Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, Loyd JE, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. (2000) 26(1):81–4. doi: 10.1038/79226
8. Harrison RE, Berger R, Haworth SG, Tulloh R, Mache CJ, Morrell NW, et al. Transforming growth factor-beta receptor mutations and pulmonary arterial hypertension in childhood. Circulation. (2005) 111(4):435–41. doi: 10.1161/01.CIR.0000153798.78540.87
9. Zhu N, Gonzaga-Jauregui C, Welch CL, Ma L, Qi H, King AK, et al. Exome sequencing in children with pulmonary arterial hypertension demonstrates differences compared with adults. Circ Genom Precis Med. (2018) 11(4):e001887. doi: 10.1161/CIRCGEN.117.001887
10. Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. (2002) 129(10):2367–79. doi: 10.1242/dev.129.10.2367
11. Arora R, Metzger RJ, Papaioannou VE. Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet. (2012) 8(8):e1002866. doi: 10.1371/journal.pgen.1002866
12. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. (2015) 17(5):405–24. doi: 10.1038/gim.2015.30
13. Levy M, Eyries M, Szezepanski I, Ladouceur M, Nadaud S, Bonnet D, et al. Genetic analyses in a cohort of children with pulmonary hypertension. Eur Respir J. (2016) 48(4):1118–26. doi: 10.1183/13993003.00211-2016
14. Zhang HS, Liu Q, Piao CM, Zhu Y, Li QQ, Du J, et al. Genotypes and phenotypes of Chinese pediatric patients with idiopathic and heritable pulmonary arterial hypertension-A single-center study. Can J Cardiol. (2019) 35(12):1851–6. doi: 10.1016/j.cjca.2019.07.628
15. Haarman MG, Kerstjens-Frederikse WS, Vissia-Kazemier TR, Breeman KTN, Timens W, Vos YJ, et al. The genetic epidemiology of pediatric pulmonary arterial hypertension. J Pediatr. (2020) 225:65–73.e5. doi: 10.1016/j.jpeds.2020.05.051
16. Augustine DX, Coates-Bradshaw LD, Willis J, Harkness A, Ring L, Grapsa J, et al. Echocardiographic assessment of pulmonary hypertension: a guideline protocol from the British society of echocardiography. Echo Res Pract. (2018) 5(3):G11–G24. doi: 10.1530/ERP-17-0071
Keywords: pediatrcis, pulmonary arterial hypertension, hereditary, tertiary referral center, genetic
Citation: Ishizuka M, Zou W, Whalen E, Ely E, Coleman RD, Lopez-Terrada DH, Penny DJ, Fan Y and Varghese NP (2023) Hereditary pulmonary arterial hypertension burden in pediatrics: A single referral center experience. Front. Pediatr. 11:1050706. doi: 10.3389/fped.2023.1050706
Received: 22 September 2022; Accepted: 24 February 2023;
Published: 29 March 2023.
Edited by:
Marie-Christine Seghaye, University Hospital of Liège, BelgiumReviewed by:
Hao Zhang, Shanghai Children's Medical Center, ChinaPaul Critser, Cincinnati Children's Hospital Medical Center, United States
© 2023 Ishizuka, Zou, Whalen, Ely, Coleman, Lopez-Terrada, Penny, Fan and Varghese. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuxin Fan Yuxin.fan@bcm.edu
†These authors share last authorship
Specialty Section: This article was submitted to Pediatric Cardiology, a section of the journal Frontiers in Pediatrics
 Maki Ishizuka
Maki Ishizuka Wenxin Zou
Wenxin Zou Elise Whalen
Elise Whalen Erin Ely4
Erin Ely4  Yuxin Fan
Yuxin Fan Nidhy P. Varghese
Nidhy P. Varghese