Off-label use of cinacalcet in pediatric primary hyperparathyroidism: A French multicenter experience
- 1Centre de Référence des Maladies Rares du Calcium et du Phosphore, Centre de Référence des Maladies Rénales Rares, Filières de Santé Maladies Rares OSCAR, ORKID et ERKNet, Service de Néphrologie Rhumatologie et Dermatologie Pédiatriques, Hôpital Femme Mère Enfant, Bron, France
- 2INSERM UMR S1033 Research Unit, Lyon, France
- 3Service de Néphrologie Pédiatrique, CHU de Nice, Hôpital Archet, Nice, France
- 4Faculté de Médecine, Université Côte d'Azur, Nice, France
- 5Centre de Référence des Maladies Rares du Calcium et du Phosphore, Unité d'Endocrinologie, Génétique et Pathologies Osseuses, Filières Santé Maladies Rares OSCAR et BOND, Hôpital des Enfants, Toulouse, France
- 6Service d'Endrocrinologie et Néphrologie Pédiatrique, Filière de Santé Maladies Rares OSCAR, Hôpital Arnaud de Villeneuve - CHU Montpellier, Université de Montpellier, Montpellier, France
- 7Centre de Référence des Maladies Rares du Calcium et du Phosphore, Département de Pédiatrie, Filière Santé Maladies Rares OSCAR, CHU Rouen, Rouen, France
- 8Service de Pédiatrie, CHU de Limoges, Limoges, France
- 9Service d'Endocrinologie Pédiatrique, Centre de Référence des Maladies Endocriniennes Rares de la Croissance et du Développement (CRMERCD), Hôpital Robert Debré, Assistance Publique - Hôpitaux de Paris, Paris, France
- 10Center for Pediatric and Adolescent Medicine, University of Heidelberg, Heidelberg, Germany
- 11Institute of Medical Biometry and Informatics, University of Heidelberg, Heidelberg, Germany
- 12AP-HP, Centre de référence des maladies rares du métabolisme du calcium et du phosphate, Plateforme d'expertise maladies rares Paris Saclay, filière OSCAR, EndoRare and BOND ERN, Hôpital de Bicêtre Paris Saclay, Le Kremlin-Bicêtre, France
- 13Université Paris-Saclay, AP-HP, Service d'endocrinologie et diabète de l'enfant, Service de médecine des adolescents, Hôpital de Bicêtre Paris Saclay, INSERM U1185, Le Kremlin-Bicêtre, France
- 14Faculté de Médecine Lyon Est, Université de Lyon, Lyon, France
Background: Cinacalcet is a calcimimetic approved in adults with primary hyperparathyroidism (PHPT). Few cases reports described its use in pediatric HPT, with challenges related to the risk of hypocalcemia, increased QT interval and drug interactions. In this study, we report the French experience in this setting.
Methods: We retrospectively analyzed data from 18 pediatric patients from 7 tertiary centers who received cinacalcet for PHPT. The results are presented as median (interquartile range).
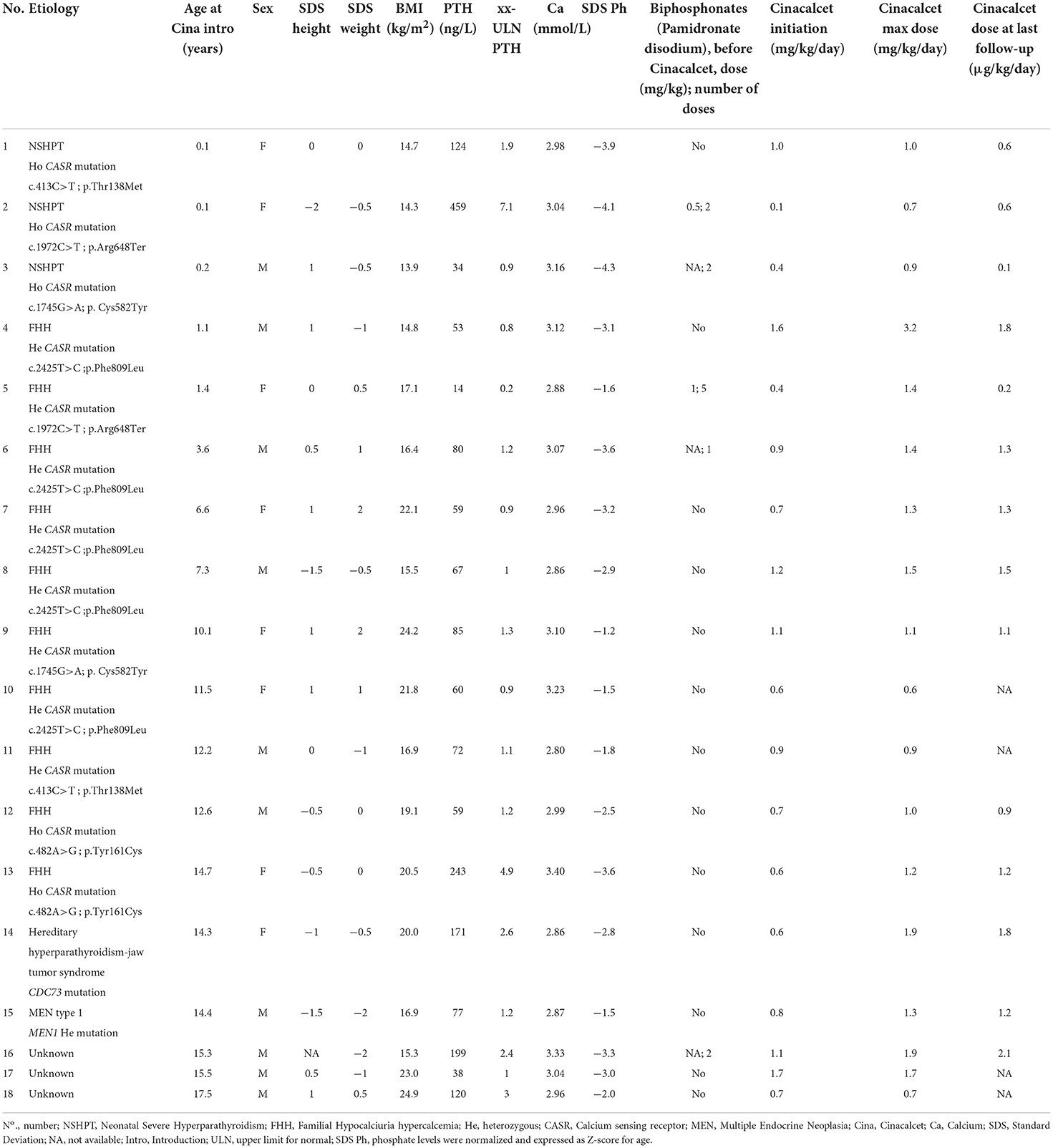
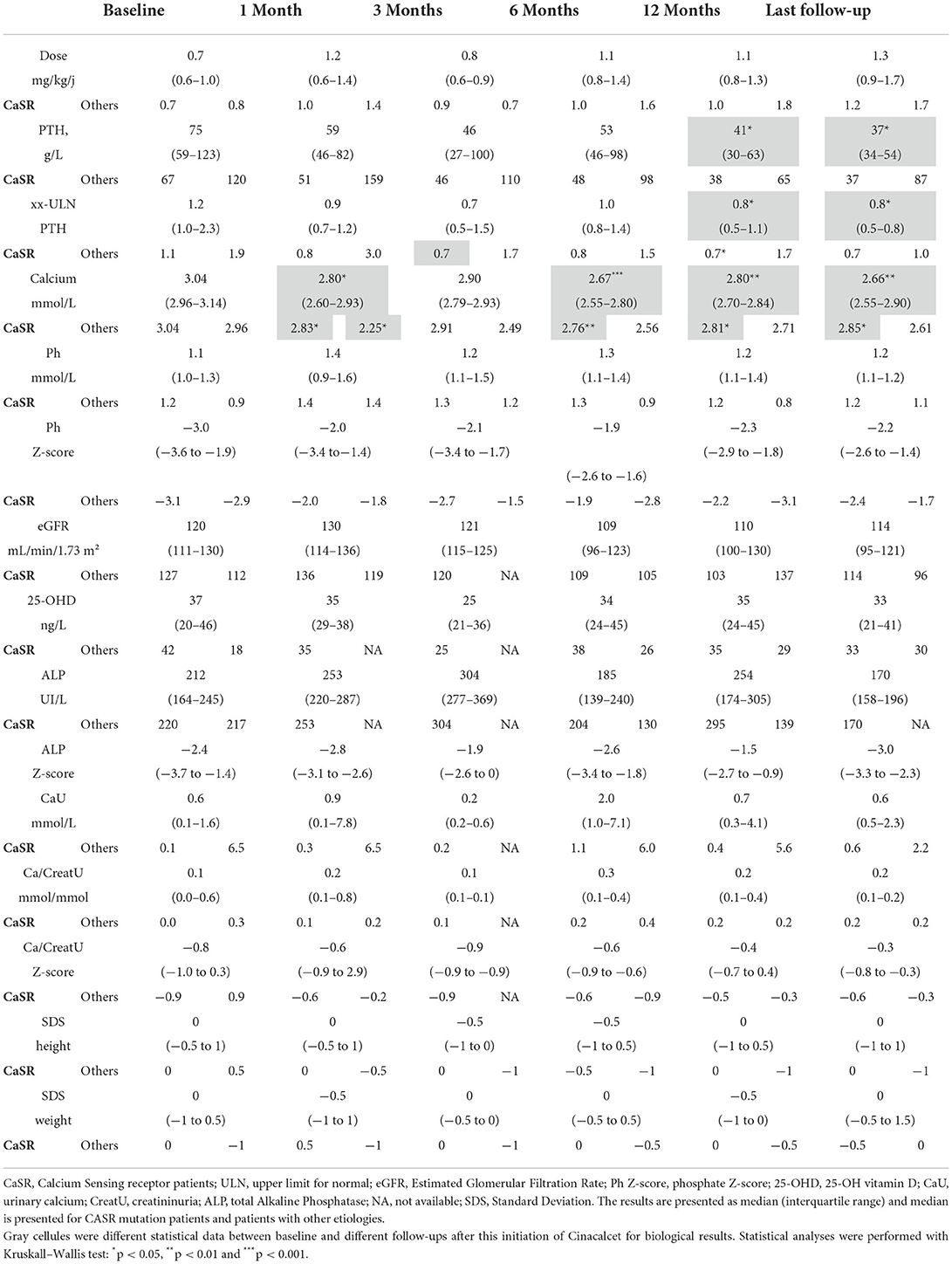
Results: At a median age of 10.8 (2.0–14.4) years, 18 patients received cinacalcet for primary HPT (N = 13 inactive CASR mutation, N = 1 CDC73 mutation, N = 1 multiple endocrine neoplasia type 1, N=3 unknown etiology). Cinacalcet was introduced at an estimated glomerular filtration rate (eGFR) of 120 (111–130) mL/min/1.73 m2, plasma calcium of 3.04 (2.96–3.14) mmol/L, plasma phosphate of 1.1 (1.0–1.3) mmol/L, age-standardized (z score) phosphate of −3.0 (−3.5;−1.9), total ALP of 212 (164–245) UI/L, 25-OHD of 37 (20–46) ng/L, age-standardized (z score) ALP of −2.4 (−3.7;−1.4), PTH of 75 (59–123) ng/L corresponding to 1.2 (1.0–2.3)-time the upper limit for normal (ULN). The starting daily dose of cinacalcet was 0.7 (0.6–1.0) mg/kg, with a maximum dose of 1.0 (0.9–1.4) mg/kg per day. With a follow-up of 2.2 (1.3–4.3) years on cinacalcet therapy, PTH and calcium significantly decreased to 37 (34–54) ng/L, corresponding to 0.8 (0.5–0.8) ULN (p = 0.01), and 2.66 (2.55–2.90) mmol/L (p = 0.002), respectively. In contrast, eGFR, 25-OHD, ALP and phosphate and urinary calcium levels remained stable. Nephrocalcinosis was not reported but one patient displayed nephrolithiasis. Cinacalcet was progressively withdrawn in three patients; no side effects were reported.
Conclusions: Cinacalcet in pediatric HPT can control hypercalcemia and PTH without significant side effects.
Introduction
Pediatric primary hyperparathyroidism (PHPT) is an uncommon endocrine disorder secondary to an increased secretion of parathyroid hormone (PTH), with an incidence of 2–5 per 100 000 children, responsible for hypercalcemia and hypophosphatemia (1). Heterogenous clinical features such as polyuria, nausea, constipation, abdominal pain and failure to thrive, may be the presenting symptoms of chronic hypercalcemia (2). The diagnosis is usually made in children with hypercalcemia with elevated or non-adapted “normal” PTH levels; genetic screening is recommended, especially because of the risk of adenocarcinoma of the parathyroid with specific mutations as in the CDC73 gene.
In children, single benign parathyroid adenoma may cause PHPT occurring during adolescence and requires parathyroid resection for definitive treatment (1, 3). PHPT may also be part of genetic endocrine syndromes including 1/ Multiple endocrine neoplasia (MEN) syndromes, type I (PHPT, duodenopancreatic neuroendocrine tumors and/or pituitary adenomas, MEN1 gene mutation) (4), or type II (PHPT, medullary thyroid carcinoma and/or pheochromocytoma, RET proto-oncogene mutation) (5); 2/ Hereditary hyperparathyroidism-jaw tumor (HPT-JT) syndrome secondary to CDC73 mutation (also known as HRPT2) (3); and 3/ Inactive Calcium-sensing Receptor (CaSR) secondary to genetic disorders (AP2S1, GNA11, CASR mutations) or anti-CaSR antibodies (6, 7). Patients with CASR mutations can display parathyroid hyperplasia or adenoma (3).
In 2014, the Fourth international workshop proposed indications for surgery in adults with asymptomatic PHPT more frequently than the last set of guidelines (8). This workshop published surgical management of asymptomatic primary hyperparathyroidism and stated that age below 50 years should be considered as one of indications for surgery (9). Children with sporadic PHPT, symptomatic MEN1 and HPT-JT, should be treated surgically if there are no contraindications (9, 10). In other cases or before surgery, medical management is proposed, aiming at decreasing calcium levels, obtaining normal phosphate levels, repleting native vitamin D, and avoiding hypercalciuria that may further induce nephrolithiasis and/or nephrocalcinosis (8). In adults, the conventional management uses bisphosphonates, with a preference to alendronate, to improve Bone Mass Density (BMD), since it does not alter serum calcium and PTH concentration; and in an acute setting in case of symptomatic severe hypercalcemia, calcimimetics are combinate (8). Restriction of calcium intake below established guidelines for all individuals is not recommended to avoid bone defects and impaired peak bone mass (11).
The calcimimetic cinacalcet inhibits PTH secretion through the sensitization of the parathyroid CaSR by enhancing signal transduction (12). In adults, the recent international guidelines recommend to use calcimimetics in PHPT patients unable to undergo surgical parathyroidectomy (8). A recent meta-analysis of 28 studies, including four randomized clinical trials (RCTs), has reported that serum levels of calcium and PTH levels were significantly reduced, and phosphate levels significantly increased with cinacalcet use in PHPT (13).
Cinacalcet was recently licensed in Europe in dialysis children above 3 years with secondary hyperparathyroidism due to end-stage kidney disease; European guidelines have delineated its use in this peculiar setting (14). Alternatively, the use of cinacalcet, as an off-label therapy in PHPT, has been reported only in few pediatric case reports and is considered to be a challenge in daily practice, because of the putative risk of hypocalcemia, increased QT interval and drug interactions (15, 16).
Thus, we aimed to retrospectively report the French experience of cinacalcet in pediatric PHPT. We hypothesized that cinacalcet improves calcium levels with a good safety profile.
Patients and methods
Patients
This is a retrospective multicenter series of 18 pediatric patients (below the age of 18 years), followed in seven tertiary centers of rare diseases of calcium and phosphate metabolism (Toulouse N = 6; Montpellier N = 4; Lyon N = 2; Bicêtre Paris Saclay N = 2; Rouen N = 2; Paris-Robert Debré N = 1; Limoges N = 1), in France, for PHPT. We included all children who started cinacalcet between November 2013 and January 2020, as an off-label therapy, with at least 1 year of follow-up for 17 patients.
Biochemicals and assessment of growth parameters
As part of the routine follow-up of these patients with PHPT, total calcium, phosphate, magnesium, alkaline phosphatase (ALP), PTH, creatinine, 25-OH vitamin D (25-OHD) and urinary calcium were regularly assessed. Calcium (Ca), phosphate (Ph), creatinine, and 25-OHD levels were locally measured by standard methods. Variables with age-dependent reference values (plasma phosphate concentration, ALP, urinary calcium/creatinine ratio) are expressed as z score, calculated as follows: z score = (measured value – mean normal value)/SD. Reference values for across corresponding age groups and/or gender were used from prior literature (17, 18) whereby a square root transformation and corresponding reference values for ALP (19). Estimated Glomerular Filtration Rate (eGFR) was estimated using the 2009 Schwartz formula (20). Laboratory parameters were collected at six different time points: initiation of cinacalcet therapy (baseline), and after 1, 3, 6, and 12 months; data at last follow-up were also recorded. In the youngest children in whom 24-h urinary calcium assessment was not possible, spot mictions were obtained to measure urinary calcium/creatinine ratio.
Because the local assays used for routine assessment of PTH were different, as illustrated in Table 1, we decided to present PTH data as xx-time the upper limit for normal (xx-ULN).
Main outcome parameters [height, weight and Body Mass Index (BMI)] were referenced to sex and age according to new French growth curves (www.afpa.org) and are reported as standard deviation scores (SDS).
Ethics
The study was approved by the local ethical committee (Comité d'Ethique des Hospices Civils de Lyon, session 18/02/2019, approval 19-26), and declared to the Information Technology and Liberty Commission (CNIL n°19-168). As per French law, parents and patients do not need to give a written consent for retrospective studies.
Statistical analysis
Non-parametric Kruskal-Wallis tests followed by Dunn's tests compared to baseline were used. In all cases, p-values below 0.05 were considered statistically significant using GraphPad Prism software 8.0 (GraphPad, La Jolla, CA, USA). Results are presented as median (inter-quartile range, i.e., 25th−75th percentile) for biochemical routine parameters.
Results
Cinacalcet introduction
Relevant demographic, clinical and biochemical features of the 18 patients (8 girls) at the time of cinacalcet initiation are presented in Table 2. At a median age of 10.8 (2.0–14.4) years, cinacalcet was introduced at an estimated glomerular filtration rate (eGFR) of 120 (111–130) mL/min/1.73 m2, plasma calcium of 3.04 (2.96–3.14) mmol/L, plasma phosphate of 1.1 (1.0–1.3) mmol/L, age-standardized (z score) phosphate of −3.0 (−3.5;−1.9), total ALP of 212 (164–245) UI/L age-standardized (z score) ALP of −2.4 (−3.7;−1.4), 25-OHD of 37 (20-46) ng/L, and PTH of 75 (59–123) ng/L corresponding to 1.2 (1.0–2.3)-time the ULN. The starting daily dose of cinacalcet was 0.7 (0.6–1.0) mg/kg, with a maximum dose of 1.0 (0.9–1.4) mg/kg per day (median in CaSR group: 1.1 (0.9–1.3) mg/kg/day; other etiologies: 1.7 (1.3–1.9) mg/kg/day). Six patients (32%) had received bisphosphonates (Pamidronate disodium) and no parathyroidectomy was performed before cinacalcet introduction. Seventeen patients had at least a 1-year follow-up. The median time of follow-up was 2.2 (1.3–4.3) years. No patients have received phosphate supplementation and active vitamin D analogs during the follow-up. Main outcome parameters (height, weight and BMI) remained stable during the follow-up (Table 3). Nephrocalcinosis was improved for one patient and another one displayed nephrolithiasis secondary to hypercalciuria (this patient had nephrolithiasis history before the Cinacalcet introduction).
Evolution of PTH and calcium levels in response to cinacalcet
The evolution of the main biochemical parameters at different time points under cinacalcet therapy is reported in Table 3. We observed a nearly 50% significant decrease in PTH levels between cinacalcet initiation [75 (59–123) ng/L] and the last follow-up [37 (34-54) ng/L]. The ULN PTH levels significantly decreased between cinacalcet initiation and after 12 months (p = 0.02), and at the last follow-up (p = 0.01), as shown in Figure 1A. We observed a significant decrease in calcium levels at 1, 6, 12 months and at the last follow-up as compared to baseline, with a similar pattern in the CaSR sub-group (Figure 1B). At the time of cinacalcet initiation, all patients were hypercalcemic ≥2.80 mmol/L; calcium levels remained above 2.8 mmol/L in 6 patients after initiation of cinacalcet therapy.
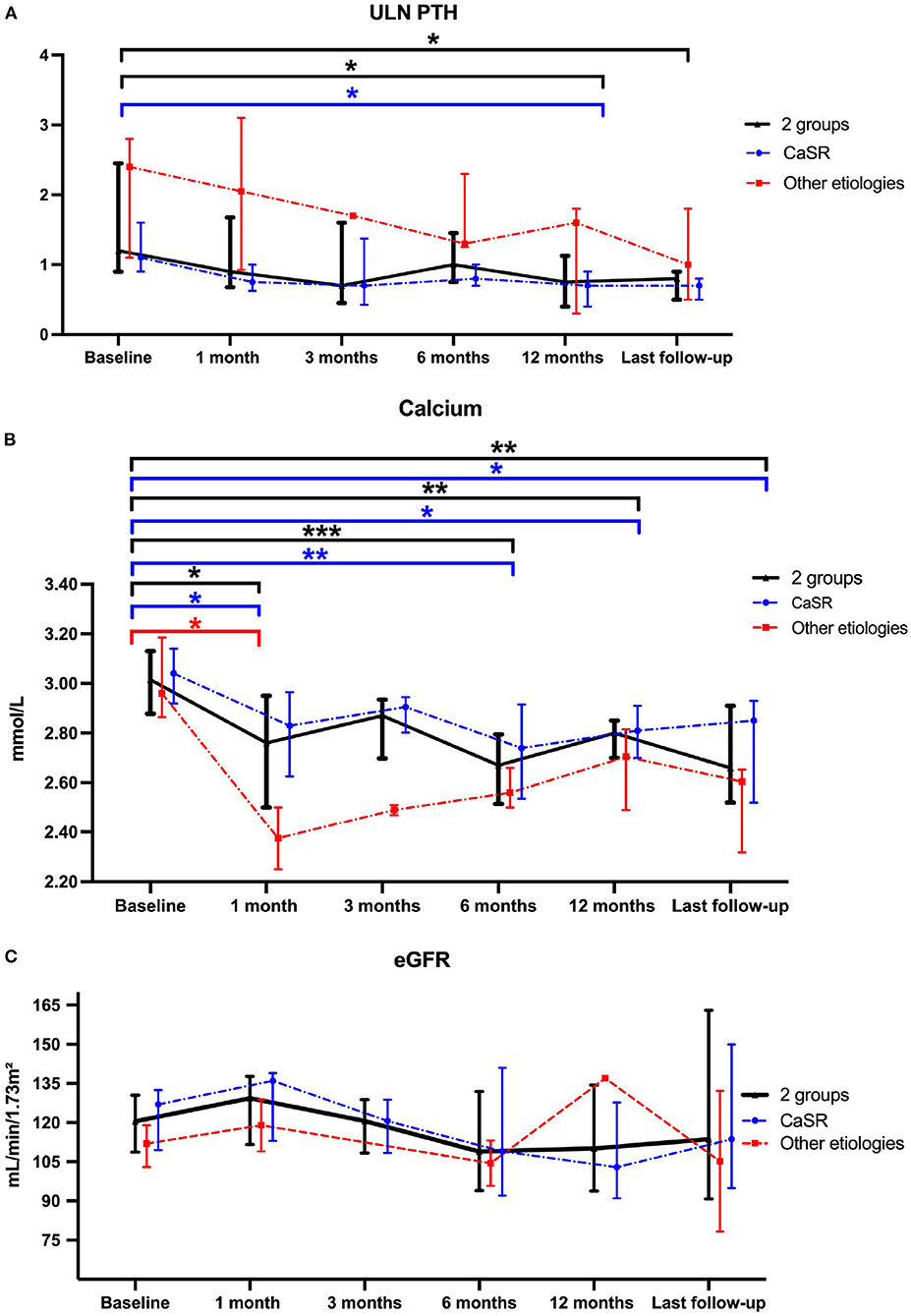
Figure 1. Comparison of ULN-PTH (A), total calcium (B) and eGFR (C) data at Cinacalcet cinacalcet initiation and during follow-up. Each dot on the graph represents median with interquartile range of different biological data levels at the different time-points. Statistical analyses were performed with Kruskall–Wallis test: *p < 0.05, **p < 0.01 and ***p < 0.001. The blue line represents the “CASR mutation patients” sub-group, the red line represents the sub-group of patients without CASR mutation and the black line represents these 2 sub-groups.
Evolution of eGFR and urinary calcium in response to cinacalcet
Renal function assessed by eGFR remained stable during follow-up (Figure 1C). Results were similar in patients with CASR mutations as compared to patients with other etiologies (Figure 1C). Urinary absolute calcium excretion and urinary calcium/creatinine ratio remained stable, whatever the underlying cause; as expected patients with CASR mutations displayed lower urinary calcium levels (Figure 2). Interestingly, one may discuss a trend toward decreased absolute urinary calcium levels in patients without CASR mutations at the end of the follow-up (Figure 2A); this could be relevant in practice since median urinary calcium at baseline in this sub-group is far higher (i.e., 6.5 mmol.L) than the crystallization threshold (i.e., 3.8 mmol/L).
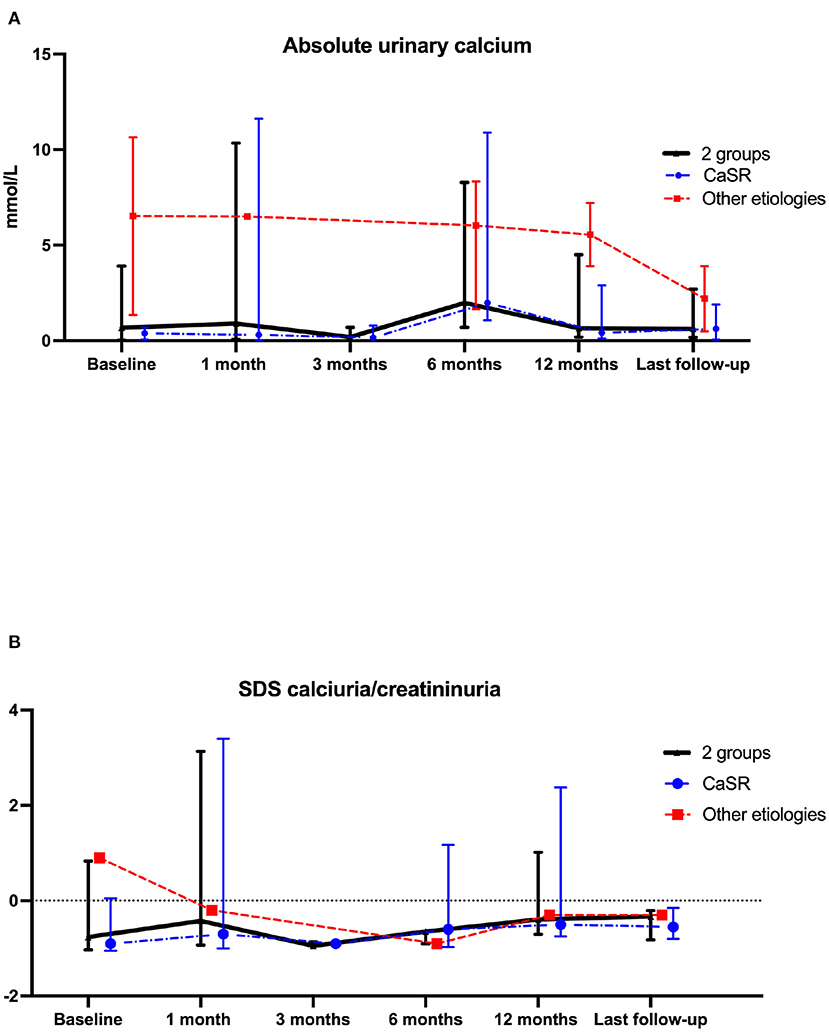
Figure 2. Comparison of calciuria (A) and z-score for age calciuria/creatininuria on one urine sample (B) data at cinacalcet initiation and during follow-up. Each dot on the graph represents median with interquartile range of biological data at the different time-points. Statistical analyses were performed with Kruskall–Wallis test: p = not statistically significant (NS). The blue line represents the “CASR mutation patients” sub-group, the red line represents the sub-group of patients without CASR mutation and the black line represents these 2 sub-groups.
Evolution of phosphate, ALP, and 25-OHD in response to cinacalcet
As shown in Figure 3A, there has been no significant change of phosphate SDS levels before and after cinacalcet therapy. As illustrated in Figure 3B, age-standardized (z score) ALP remained stable. Of note, following European recommendations guidelines (8), 12 children received native vitamin D supplementation to obtain normal 25-OHD levels between 20 and 40 ng/ and 25-OHD levels remained stable during the observational period (Figure 3C).
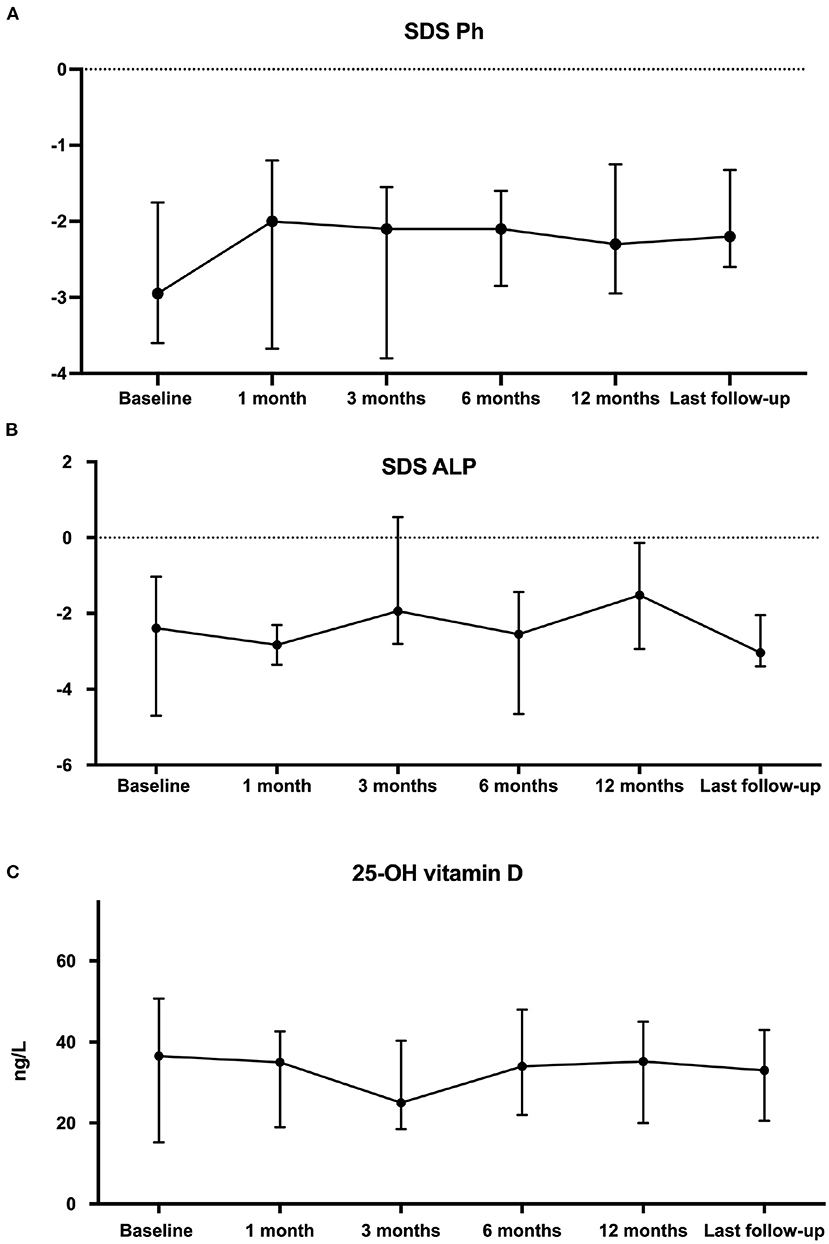
Figure 3. Comparison of phosphate levels as z-score for age (A), z-score for age alkaline phosphatases (B) and 25-OH vitamin D (C) data at cinacalcet initiation and during follow-up. Each dot on the graph represents median with interquartile range of biological data at the different time-points. Statistical analyses were performed with Kruskall–Wallis test: p = not statistically significant (NS).
Side effects
No severe side effects were reported in 18 patients, and notably no iatrogenic hypocalcemia and no increased QT interval. Renal ultrasounds (when available, N = 6 at baseline; N = 5 at 1 year and N = 6 at the last follow-up) were performed during the follow-up. We observed a disappearance in the last ultrasounds for one patient who presented nephrocalcinosis at baseline. Cinacalcet was withdrawn in three patients: in one patient because of complete calcium normalization after 1 year of follow-up, in one patient because of secondary surgery of an adenoma, and for the last patient because of bad therapeutic compliance.
Discussion
This retrospective multicenter observational study evaluated the off-label use of cinacalcet to treat pediatric PHPT. The main results are the following: 1/ cinacalcet allows better control of calcium and PTH levels, without any substantial changes in urinary calcium excretion, and 2/ cinacalcet is safe and effective without notable side effects.
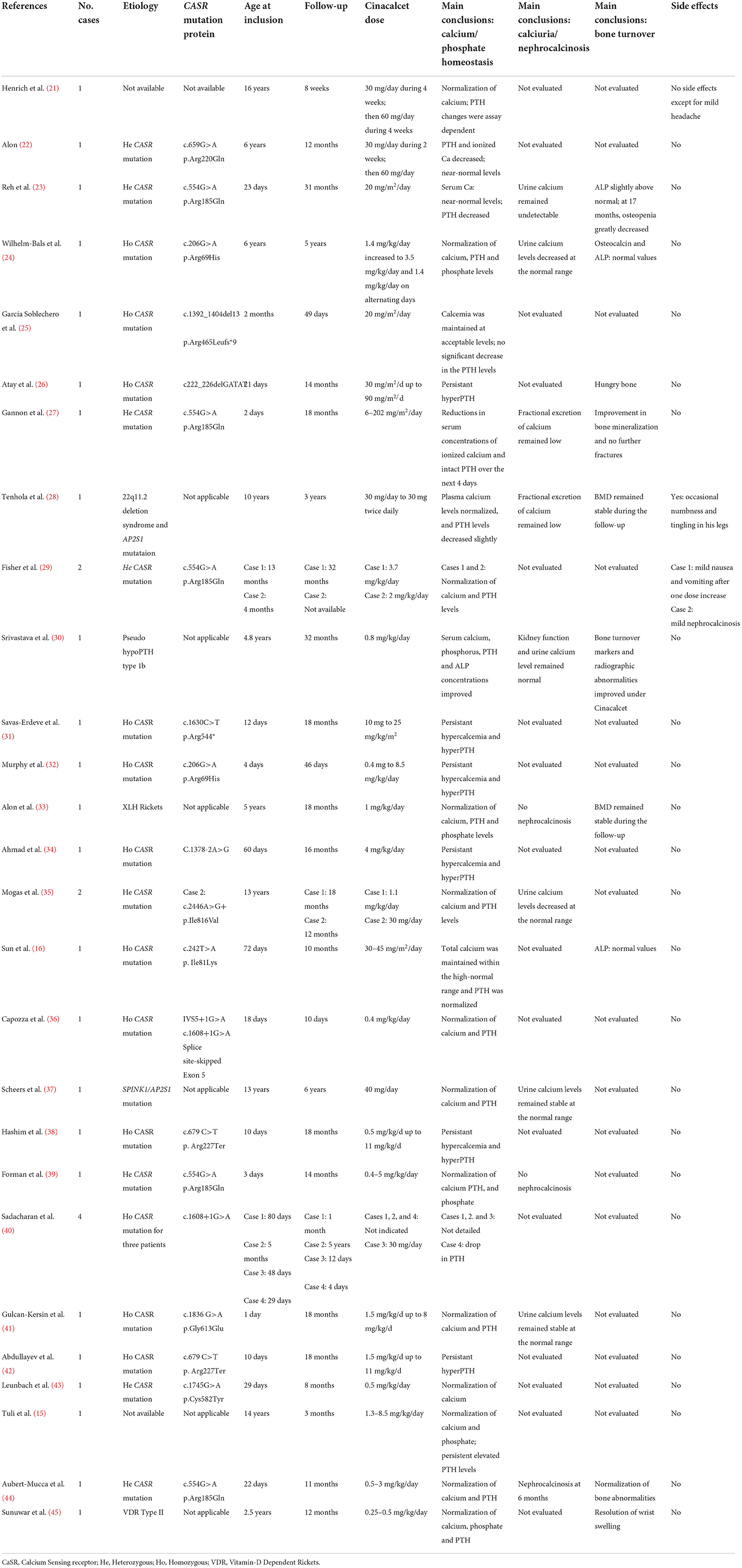
In pediatrics, data are scarce, and to our knowledge, only 50 pediatric patients (including the 18 patients presented here) out of a total of almost 1,200 pediatric patients reported in the literature with PHPT, have been treated with cinacalcet, as summarized in Table 4 (15, 16, 21–25, 27–30, 32, 33, 35–37, 40, 43–45). The present study almost doubles the number of reported pediatric patients having received cinacalcet as an off-label drug for PHPT.
A recent meta-analysis has evaluated 28 studies including 722 adults receiving cinacalcet for PHPT (because of contra-indication to surgical procedure or parathyroidectomy failure) (13). It reported a normalization of calcium levels in 90% of cases; PTH levels were significantly reduced but only 10% of cases normalized PTH levels (13). The effect of cinacalcet was greater when baseline calcium values were above 3 mmol/L. In our study, we observed a significant decrease of calcium levels after 1 month of therapy that was sustained until the last follow-up; no hypocalcemia was reported. All patients displayed calcium levels above 2.80 mmol/L at the time of cinacalcet initiation; twelve patients normalized their calcium levels after cinacalcet therapy with doses that remained stable.
In the present study, different PTH assays were used in the different centers, with different normal ranges, explaining why we presented PTH data as ULN-PTH. The ULN-PTH significantly decreased after 12 months under cinacalcet treatment, and we observed a nearly 50% decrease of PTH levels between cinacalcet initiation and the last follow-up. The patients nevertheless remained within the “normal” range during follow-up, even though the normal range in case of hypercalcemia remains quite challenging to define. The main objective for physicians was to obtain acceptable calcium levels allowing to obtain optimal growth with adequate nutritional calcium intake for age.
Hypercalcemia due to neonatal severe HPT (NSHPT) secondary to homozygous or heterozygous mutations of CASR, has been treated since 2011 with cinacalcet, in association with biphosphonates, in order to avoid post-surgical hypoparathyroidism (2, 16, 23, 25, 36, 40). Clinical presentation or response to treatment, such as Cinacalcet, may differ depending to CASR mutation (homozygous or heterozygous) and interindividual differences (46, 47). Some mutations only partially affect the CaSR function secondary to an alteration of the configuration of the calcium binding domain or a partial loss of the transduction domain. As suggested by Gulcan-Kersin et al. a therapeutic test with Cinacalcet could be started before genetic result to treat NSHPT or severe acute hypercalcemia and suspended if we do not observe hypercalcemia correction (41).
Maximum doses of cinacalcet were similar between the group with “CASR mutation” and “other etiologies” (1.0 mg/kg/day). The European consensus in pediatric dialysis recommends ≤ 0.2 mg/kg per day for the starting dose and 2 mg/kg per day for the maximal dose of cinacalcet (14). However, this consensus was established to stay on the safe side because of the risk of hypocalcemia in dialysis; here, in patients with hypercalcemia due to PHPT and normal renal function at baseline, the risk of hypocalcemia is lower.
In a meta-analysis of studies in adults, 23% of 722 patients suffered from nausea or vomiting and 3% had hypocalcemia (most of them are mild or asymptomatic); the majority of these adverse reactions were not severe enough to stop the treatment (13). In pediatrics (Table 4), one paper described gastrointestinal symptoms (29). Other reported adverse effects included mild headache and occasional numbness and tingling in legs but were not observed in our cohort (21, 28). Despite these favorable outcomes, we believe that it is of utmost importance to provide a global information to children and their caregivers on the risk and symptoms of hypocalcemia, on the risk of drug interactions and further QTc interval prolongation and not only through hypocalcemia with cinacalcet treatment. Indeed, a special caution should be given in children to the association between cinacalcet and macrolides or ondansetron, that is contra-indicated (48).
In the kidney, CaSR is expressed in all nephron segments; it plays a crucial role in calcium excretion (49, 50). Activating CASR mutations lead to hypercalcemia that reduces calcium and sodium transport in the Henle loop (Na-K-2Cl and paracellular pathways) through claudins, in association with a decreased urinary concentrating ability (51, 52). Our results are similar to previous reports, demonstrating stable renal function and no direct effects of cinacalcet on urinary calcium (22–24, 27, 28, 30, 35, 37). Only one paper has reported the onset of nephrocalcinosis after 6 months of cinacalcet therapy in a patient with heterozygous CASR mutation (44). Multiple CASR mutations have been identified (53) which reduce receptor function or produce truncated inactive CaSR and heterogenous response to cinacalcet have been observed and influence urinary calcium excretion (22–24, 27, 28, 30, 35, 37). In vitro studies showed that calcimimetics were able to restore sensitivity on intra- and extracellular CASR mutations and deactivations mutations (54, 55). This mechanism in the kidney is still under investigation and requires further studies.
This study has some limitations, and notably the (relatively) small number of patients. However, we are in the field of orphan diseases in pediatrics, with an off-label use of novel therapies; here, we almost double the number of reported pediatric patients having received cinacalcet as an off-label drug for PHPT. Data on bone metabolism (such as CTX, bone density) and calcium intake are unfortunately not available after cinacalcet treatment in the present study; however in most studies, growth improved or remained stable at the end of the follow-up, similarly to what is observed here (16, 23, 24, 27).
In conclusion, our observational data suggest that cinacalcet could be a safe alternative therapy in pediatric PHPT to treat hypercalcemia without major side effects. Even though European guidelines already propose this management as a second-line off-label therapy in adults with PHPT, larger international studies may evaluate its effect with the main objective to treat hypercalcemia and obtain optimal growth with normal calcium intake and vitamin D supplementation. Its long-term consequences especially for the potential risk of hypercalciuria and nephrocalcinosis later in life of these children should be further assessed.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Comité d'Ethique des Hospices Civils de Lyon, session 18/02/2019, approval 19-26), and declared to the Information Technology and Liberty Commission (CNIL n°19-168). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
JBe and JBa: data collection and writing the manuscript. SF: data collection. J-PS, CA, MC, ALie, LM, and ALin: physicians of patients from different center and proofreading of the manuscript. ID: statistical analysis required for the review. All authors contributed to the article and approved the submitted version.
Conflict of interest
JBa is a clinical investigator for industry-sponsored clinical trials on the use of calcimimetics (cinacalcet and etelcalcetide) in pediatric dialysis (Amgen). JBa has received research grants from Amgen (RENOCLASTE study: ID-RCB 2017-A03241-52).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kollars J, Zarroug AE, Heerden J., van, Lteif A, Stavlo P, Suarez L, Moir C, Ishitani M, Rodeberg D. Primary hyperparathyroidism in pediatric patients. Pediatrics. (2005) 115:974–80. doi: 10.1542/peds.2004-0804
2. Roizen J, Levine MA. Primary hyperparathyroidism in children and adolescents. J Chin Med Assoc. (2012) 75:425–34. doi: 10.1016/j.jcma.2012.06.012
3. Bilezikian JP, Cusano NE, Khan AA, Liu J-M, Marcocci C, Bandeira F. Primary hyperparathyroidism. Nat Rev Dis Primers. (2016) 2:16033. doi: 10.1038/nrdp.2016.33
4. Al-Salameh A, Cadiot G, Calender A, Goudet P, Chanson P. Clinical aspects of multiple endocrine neoplasia type 1. Nat Rev Endocrinol. (2021) 17:207–24. doi: 10.1038/s41574-021-00468-3
5. Conte-Devolx B, Niccoli-Sire P. Groupe d'etude des tumeurs à calcitonine. [Polyadenomatoses: type 2 multiple endocrine neoplasms]. Presse Med. (2002) 31:1224–30.
6. Belcher R, Metrailer AM, Bodenner DL, Stack BC Jr. Characterization of hyperparathyroidism in youth and adolescents: a literature review. Int J Pediatr Otorhinolaryngol. (2013) 77:318–22. doi: 10.1016/j.ijporl.2012.12.008
7. Dandurand K, Ali DS, Khan AA. Primary hyperparathyroidism: a narrative review of diagnosis and medical management. J Clin Med. (2021) 10:1604. doi: 10.3390/jcm10081604
8. Bilezikian JP, Brandi ML, Eastell R, Silverberg SJ, Udelsman R, Marcocci C, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. (2014) 99:3561–9. doi: 10.1210/jc.2014-1413
9. Udelsman R, Åkerström G, Biagini C, Duh Q-Y, Miccoli P, Niederle B, et al. The surgical management of asymptomatic primary hyperparathyroidism: proceedings of the fourth international workshop. J Clin Endocrinol Metab. (2014) 99:3595–606. doi: 10.1210/jc.2014-2000
10. Alagaratnam S, Kurzawinski TR. Aetiology, diagnosis and surgical treatment of primary hyperparathyroidism in children: new trends. Horm Res Paediatr. (2015) 83:365–375. doi: 10.1159/000381622
11. Soyka LA, Fairfield WP, Klibanski A. Hormonal determinants and disorders of peak bone mass in children1. J Clin Endocrinol Metab. (2000) 85:3951–63. doi: 10.1210/jcem.85.11.6994
12. Joy MS, Kshirsagar AV, Franceschini N. Calcimimetics and the treatment of primary and secondary hyperparathyroidism. Ann Pharmacother. (2004) 38:1871–80. doi: 10.1345/aph.1D108
13. Ng CH, Chin YH, Tan MHQ, Ng JX, Yang SP, Kiew JJ, et al. Cinacalcet and primary hyperparathyroidism: systematic review and meta regression. Endocr Connect. (2020) 9:724–35. doi: 10.1530/EC-20-0221
14. Bacchetta J, Schmitt CP, Ariceta G, Bakkaloglu S, Wan M, Vervloet MG, et al. Cinacalcet in pediatric dialysis: a position statement from the ESPN and the CKD-MBD working groupe of the ERA-EDTA. Nephrol Dial Transplant. (2020) 35:47–64. doi: 10.1093/ndt/gfz159
15. Tuli G, Munarin J, Tessaris D, Buganza R, Matarazzo P, De Sanctis L. Primary Hyperparathyroidism (PHPT) in children: two case reports and review of the literature. Case Rep Endocrinol. (2021) 2021:5539349. doi: 10.1155/2021/5539349
16. Sun X, Huang L, Wu J, Tao Y, Yang F. Novel homozygous inactivating mutation of the calcium-sensing receptor gene in neonatal severe hyperparathyroidism responding to cinacalcet therapy. Medicine. (2018) 97:e13128. doi: 10.1097/MD.0000000000013128
17. Ardeshirpour L, Cole DEC, Carpenter TO. Evaluation of bone and mineral disorders. Pediatr Endocrinol Rev. (2007) 5:584–598.
18. Matos V, van Melle G, Boulat O, Markert M, Bachmann C, Guignard J-P. Urinary phosphate/creatinine, calcium/creatinine, and magnesium/creatinine ratios in a healthy pediatric population. J Pediatr. (1997) 131:252–7. doi: 10.1016/S0022-3476(97)70162-8
19. Turan S, Topcu B, Gökçe I, Güran T, Atay Z, Omar A, et al. Serum alkaline phosphatase levels in healthy children and evaluation of alkaline phosphatase z-scores in different types of rickets. J Clin Res Pediatr Endocrinol. (2011) 3:7–11. doi: 10.4274/jcrpe.v3i1.02
20. Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. (2009) 4:1832–43. doi: 10.2215/CJN.01640309
21. Henrich LM, Rogol AD, D'Amour P, Levine MA, Hanks JB, Bruns DE. Persistent hypercalcemia after parathyroidectomy in an adolescent and effect of treatment with cinacalcet HCl. Clin Chem. (2006) 52:2286–93. doi: 10.1373/clinchem.2006.070219
22. Alon US, VandeVoorde RG. Beneficial effect of cinacalcet in a child with familial hypocalciuric hypercalcemia. Pediatr Nephrol. (2010) 25:1747–50. doi: 10.1007/s00467-010-1547-5
23. Reh CMS, Hendy GN, Cole DEC, Jeandron DD. Neonatal hyperparathyroidism with a heterozygous calcium-sensing receptor (CASR) R185Q mutation: clinical benefit from cinacalcet. J Clin Endocrinol Metab. (2011) 96:E707o12. doi: 10.1210/jc.2010-1306
24. Wilhelm-Bals A, Parvex P, Magdelaine C, Girardin E. Successful use of bisphosphonate and calcimimetic in neonatal severe primary hyperparathyroidism. Pediatrics. (2012) 129:e812–6. doi: 10.1542/peds.2011-0128
25. García Soblechero E, Ferrer Castillo MT, Jiménez Crespo B, Domínguez Quintero ML, González Fuentes C. Neonatal hypercalcemia due to a homozygous mutation in the calcium-sensing receptor: failure of cinacalcet. Neonatology. (2013) 104:104–8. doi: 10.1159/000350540
26. Atay Z, Bereket A, Haliloglu B, Abali S, Ozdogan T, Altuncu E, et al. Novel homozygous inactivating mutation of the calcium-sensing receptor gene (CASR) in neonatal severe hyperparathyroidism-lack of effect of cinacalcet. Bone. (2014) 64:102t A, Haliloglu B, Abali S, Ozdogan
27. Gannon AW, Monk HM, Levine MA. Cinacalcet monotherapy in neonatal severe hyperparathyroidism: a case study and review. J Clin Endocrinol Metab. (2014) 99:7–11. doi: 10.1210/jc.2013-2834
28. Tenhola S, Hendy GN, Valta H, Canaff L, Lee BSP, Wong BYL, et al. Mäkitie O. Cinacalcet treatment in an adolescent with concurrent 22q112 deletion syndrome and familial hypocalciuric hypercalcemia type 3 caused by AP2S1 mutation. J Clin Endocrinol Metab. (2015) 100:2515–8. doi: 10.1210/jc.2015-1518
29. Fisher MM, Cabrera SM, Imel EA. Successful treatment of neonatal severe hyperparathyroidism with cinacalcet in two patients. Endocrinol Diabetes Metab Case Rep. (2015) 2015:150040. doi: 10.1530/EDM-15-0040
30. Srivastava T, Krudys J, Mardis NJ, Sebestyen-VanSickle J, Alon US. Cinacalcet as adjunctive therapy in pseudohypoparathyroidism type 1b. Pediatr Nephrol. (2016) 31:795–800. doi: 10.1007/s00467-015-3271-7
31. Savas-Erdeve S, Sagsak E, Keskin M, Magdelaine C, Lienhardt-Roussie A, Kurnaz E, et al. Treatment experience and long-term follow-up data in two severe neonatal hyperparathyroidism cases. J Pediatr Endocrinol Metab. (2016) 29:1103–10. doi: 10.1515/jpem-2015-0261
32. Murphy H, Patrick J, Báez-Irizarry E, Lacassie Y, Gómez R, Vargas A, et al. Neonatal severe hyperparathyroidism caused by homozygous mutation in CASR: a rare cause of life-threatening hypercalcemia. Eur J Med Genet. (2016) 59:227–31. doi: 10.1016/j.ejmg.2016.02.001
33. Alon US, Jarka D, Monachino PJ, Sebestyen VanSickle J, Srivastava T. Cinacalcet as an alternative to phosphate therapy in X-linked hypophosphataemic rickets. Clin Endocrinol. (2017) 114-16. doi: 10.1111/cen.13346
34. Ahmad N, Bahasan M, Al-Ghamdi BAA, Al-Enizi HF, Al-Zahrani AS. Neonatal severe hyperparathyroidism secondary to a novel homozygous CASR gene mutation. Clin Cases Miner Bone Metab. (2017) 14:354–8. doi: 10.11138/ccmbm/2017.14.3.354
35. Mogas E, Campos-Martorell A, Clemente M, Castaño L, Moreno-Galdó A, Yeste D, Carrascosa A. Successful use of cinacalcet to treat parathyroid-related hypercalcemia in two pediatric patients. Endocrinol Diabetes Metab Case Rep. (2018) 2018:EDM180009. doi: 10.1530/EDM-18-0009
36. Capozza M, Chinellato I, Guarnieri V, Di Lorgi N, Accadia M, Traggiai C, et al. Case report: acute clinical presentation and neonatal management of primary hyperparathyroidism due to a novel CaSR mutation. BMC Pediatr. (2018) 18:340. doi: 10.1186/s12887-018-1319-0
37. Scheers I, Sokal E, Limaye N, Denoncin C, Stephenne X, Pirson Y, et al. Cinacalcet sustainedly prevents pancreatitis in a child with a compound heterozygous SPINK1/AP2S1 mutation. Pancreatology. (2019) 19:801–4. doi: 10.1016/j.pan.2019.07.045
38. Hashim R, Levine MA, Somasundarum K, Lamahewage A, Atapattu N. Neonatal severe hyperparathyroidism due to a homozygous mutation of calcium-sensing receptor; a challenging case. Ceylon Med J. (2019) 64:155–7. doi: 10.4038/cmj.v64i4.8988
39. Forman TE, Niemi A-K, Prahalad P, Shi RZ, Nally LM. Cinacalcet therapy in an infant with an R185Q calcium-sensing receptor mutation causing hyperparathyroidism: a case report and review of the literature. J Pediatr Endocrinol Metabol. (2019) 32:305–10. doi: 10.1515/jpem-2018-0307
40. Sadacharan D, Mahadevan S, Rao SS, Kumar AP, Swathi S, Kumar S, et al. Neonatal severe primary hyperparathyroidism: a series of four cases and their long-term management in India. Indian J Endocrinol Metab. (2020) 24:196–201. doi: 10.4103/ijem.IJEM_53_20
41. Gulcan-Kersin S, Kirkgoz T, Eltan M, Rzayev T, Ata P, Bilgen H, et al. Cinacalcet as a first-line treatment in neonatal severe hyperparathyroidism secondary to calcium sensing receptor (CaSR) mutation. Horm Res Paediatr. (2020) 93:313atr.irkgoz T, Eltan M, Rzay
42. Abdullayev T, Korkmaz M, Kul M, Koray N. A rare cause of neonatal hypercalcemia: Neonatal severe primary hyperparathyroidism: a case report and review of the literature. Int J Surg Case Rep. (2020) 66:365–9. doi: 10.1016/j.ijscr.2019.12.024
43. Leunbach TL, Hansen AT, Madsen M, Cipliene R, Christensen PS, Schou AJ, et al. novel case of neonatal severe hyperparathyroidism successfully treated with a type II calcimimetic drug. Bone Rep. (2021) 14:100761. doi: 10.1016/j.bonr.2021.100761
44. Aubert-Mucca M, Dubucs C, Groussolles M, Vial J, Le Guillou E, Porquet-Bordes V, et al. Prenatal features and neonatal management of severe hyperparathyroidism caused by the heterozygous inactivating calcium-sensing receptor variant, Arg185Gln: a case report and review of the literature. Bone Rep. (2021) 15:101097. doi: 10.1016/j.bonr.2021.101097
45. Sunuwar N, Gautam S, Twayana A, Adhikari Yadav S, Anjum F, Kandel K. Hereditary vitamin-D dependent rickets type II: a case report. JNMA J Nepal Med Assoc. (2021) 59:597–600. doi: 10.31729/jnma.6411
46. Jack MM, Stone ML, Clifton-Bligh R. Neonatal hypercalcemia due to polymorphisms of the calcium sensing receptor. J Pediatr Endocrinol Metab. (2009) 22:561–3. doi: 10.1515/JPEM.2009.22.6.561
47. Gerbino A, Colella M. The different facets of extracellular calcium sensors: old and new concepts in calcium-sensing receptor signalling and pharmacology. Int J Mol Sci. (2018) 19:999. doi: 10.3390/ijms19040999
48. Temiz G, Yalçin AU, Mutluay R, Bozaci I, Bal C. Effects of cinacalcet treatment on QT interval in hemodialysis patients. Anatol J Cardiol. (2016) 16:520–3. doi: 10.5152/AnatolJCardiol.2015.6284
49. Rainone F, Arcidiacono T, Terranegra A, Aloia A, Dogliotti E, Mingione A, et al. Calcium sensing receptor and renal mineral ion transport. J Endocrinol Invest. (2011) 34:8–12.
50. Ba J, Brown D, Friedman PA. Calcium-sensing receptor regulation of PTH-inhibitable proximal tubule phosphate transport. Am J Physiol Renal Physiol. (2003) 285:F1233–43. doi: 10.1152/ajprenal.00249.2003
51. Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, et al. Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J. (2012) 31:1999–2012. doi: 10.1038/emboj.2012.49
52. Sands JM, Naruse M, Baum M, Jo I, Hebert SC, Brown EM, et al. Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J Clin Invest. (1997) 99:1399–405. doi: 10.1172/JCI119299
53. Leach K, Hannan FM, Josephs TM, Keller AN, Møller TC, Ward DT, et al. International union of basic and clinical pharmacology. CVIII. Calcium-sensing receptor nomenclature, pharmacology, and function. Pharmacol Rev. (2020) 72:558–604. doi: 10.1124/pr.119.018531
54. Rus R, Haag C, Bumke-Vogt C, Bähr V, Mayr B, Möhlig M, et al. Novel inactivating mutations of the calcium-sensing receptor: the calcimimetic NPS R-568 improves signal transduction of mutant receptors. J Clin Endocrinol Metab. (2008) 93:4797–803. doi: 10.1210/jc.2008-1076
55. Lu JYL, Yang Y, Gnacadja G, Christopoulos A, Reagan JD. Effect of the calcimimetic R-568 [3-(2-chlorophenyl)-N-((1R)-1-(3-methoxyphenyl)ethyl)-1-propanamine] on correcting inactivating mutations in the human calcium-sensing receptor. J Pharmacol Exp Ther. (2009) 331:775 Ther.dja G, Christopoulos A, Rea
Keywords: children, primary hyperparathyroidism, cinacalcet, hypercalcemia, Calcium-sensing Receptor (CaSR)
Citation: Bernardor J, Flammier S, Salles J-P, Amouroux C, Castanet M, Lienhardt A, Martinerie L, Damgov I, Linglart A and Bacchetta J (2022) Off-label use of cinacalcet in pediatric primary hyperparathyroidism: A French multicenter experience. Front. Pediatr. 10:926986. doi: 10.3389/fped.2022.926986
Received: 23 April 2022; Accepted: 11 July 2022;
Published: 24 August 2022.
Edited by:
Hala M. Tfayli, American University of Beirut, LebanonReviewed by:
Elizaveta Mamedova, Endocrinology Research Center, RussiaSerap Demircioglu Turan, Marmara University, Turkey
Copyright © 2022 Bernardor, Flammier, Salles, Amouroux, Castanet, Lienhardt, Martinerie, Damgov, Linglart and Bacchetta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julie Bernardor, bernardor.j@chu-nice.fr
†ORCID: Julie Bernardor orcid.org/0000-0002-1356-1507
 Julie Bernardor
Julie Bernardor Sacha Flammier
Sacha Flammier Jean-Pierre Salles5
Jean-Pierre Salles5  Laetitia Martinerie
Laetitia Martinerie Agnès Linglart
Agnès Linglart