High levels of cerebrospinal fluid soluble triggering receptor expressed on myeloid cells 2 might be a biomarker of activity in pediatric patients with MOG-AD
- 1Department of Neurology, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, China
- 2China National Clinical Research Center for Neurological Disease, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
Myelin oligodendrocyte glycoprotein antibody disease (MOG-AD) is characterized by its monophasic or relapsing course and inflammatory demyelinating condition which is unable to be classified in typical multiple sclerosis (MS) or other known neuroinflammatory conditions. In the condition of neuroinflammatory, activated microglia are essential for demyelination. The secreted ectodomain of soluble triggering receptor expressed on myeloid cells 2 (sTREM2), expressed by microglial cells, is associated with abnormal biological pathways. It is known that the cerebrospinal fluid (CSF) sTREM2 concentration is much higher in neuroinflammatory and neurodegeneration diseases. However, the role of activated microglia has not been reported in MOG-AD pediatric patients. For the first time, the increased CSF and serum sTREM2 concentration in pediatric patients with MOG-AD is investigated in this work, showing evidence of microglia activation in MOG-AD. CSF sTREM2 levels significantly correlated with clinical inflammatory indexes and adapted modified Rankin Scale score, indicating the potential value of sTREM2 as a severity biomarker.
Introduction
It is well known that myelin oligodendrocyte glycoprotein antibody-associated disease (MOG-AD), an antibody-mediated autoimmune demyelinating disorder in the central nervous system, is still a challenging nosologically entity. The frequency of anti-MOG antibody seropositivity is high in children with acute demyelinating syndrome presentations (1). In MOG-antibody-positive children, the median age at presentation is 6 years with same-gender bias as adults (2, 3). The clinical manifestation includes various phenotypes: from (mostly recurrent) optic neuritis (ON), via transverse myelitis (TM), and longitudinally extensive transverse myelitis (LETM) to acute disseminated encephalomyelitis (ADEM) and cortical encephalitis (4). This mono- or multiphasic course of neurological deficits makes MOG-AD difficult to diagnose with other known neuroinflammatory illnesses. With the new-generation cell-based assays, MOG-IgG demonstrated a robust association with the immunopathogenesis in MOG-AD (5). Even though testing serum MOG antibody IgG made the distinguishment of MOG-AD from typical multiple sclerosis (MS) or other common forms of neuroinflammation more specific and faster, the role of MOG antibody IgG in MOG-AD remains unclear (6).
On the other hand, in neuroinflammation, demyelination is associated with activated microglia (7). Triggering receptor expressed on myeloid cell-2 (TREM-2), a membrane-bound immune receptor, expressed on the differentiated and activated macrophages. Specifically, it is only expressed in peripheral macrophages and central microglia (8). The expression of TREM-2 is upregulated on activated microglia, modulating the biological function of phagocytosis (8, 9), survival (10), chemotaxis, and response to neuronal injury (11). The soluble triggering receptor expressed on myeloid cells 2 (sTREM2) is measurable by cleavage of the ectodomain, as the TREM-2 releases sTREM2 in cerebrospinal fluid (CSF) and blood (8). By this means, it is possible for the sTREM2 to become a marker to describe neuroinflammatory conditions (12).
The objective of this work is to explore the relationship between the pediatric MOG-AD and the sTREM2 levels in serum and CSF. In the meantime, the correlation between the CSF sTREM2 levels and the clinical inflammatory indexes is investigated by analyzing the sTREM2 in CSF samples from the pediatric MOG-AD group and control group.
Method
Patient selection and biofluid collection
The study included 19 control patients and 19 pediatric MOG-AD patients. Nineteen patients were selected from non-neuroinflammatory diseases as control groups, including two cases of mitochondrial disorders, one case of dizziness, one case of febrile convulsion, one case of migraine, three cases of somatization disorder, one case of acute concomitant strabismus, one case of intracranial hypertension, and nine cases of functional headache. Clinical data were retrospectively collected from hospitalized patients diagnosed with MOG-AD at the neurology department of Beijing Children’s Hospital between June 2018 and August 2021. The data was acquired from The FUTang Updating medical REcords (FUTURE) database. More details and the process of data cleaning have been presented in a previous publication (13). We recorded the medical history, neurological symptoms and signs, and biofluid laboratory testing results. According to the guidelines of MOG-AD in the USA, Europe, and related literature (4, 14, 15), the criteria for the diagnosis of MOG-AD in our study include (1) ON; (2) myelitis; (3) ADEM; (4) fundoscopy; 5) the onset of disease from 4 days to 4 weeks after vaccination, co-existing teratoma or NMDAR encephalitis; and (6) anti-MOG antibody testing.
All patients with MOG-AD were in the acute phase, and the samples were collected prior to treatment. CSF and serum samples were immediately centrifuged and the supernatant was collected and stored at −80°C until the time of the ELISA assays.
This study was approved by the Ethics Committee of Beijing Children’s Hospital Affiliated to Capital Medical University, Beijing, People’s Republic of China, and written informed consent was obtained from all selected participants and their parents.
ELISA for detection of soluble triggering receptor expressed on myeloid cells 2
The CSF and serum analyses on sTREM2 were performed using a commercially available ELISA assay from Abcam (Abcam, USA, ab224881) according to the manufacturer. Neat CSF samples and serum samples were analyzed on the same day using assays from the same lot to avoid inter-lot variations. CSF samples and serum samples from the control group were evenly distributed on the plates.
Statistical analysis
Data were analyzed by SPSS software (IBM SPSS 22.0 version) and Prism (GraphPad software 8.0 version). As a small sample quantity, the distribution of CSF and serum level of sTREM2 obey normal (Shapiro–Wilk test, p > 0.05), both in pediatric MOG-AD and control groups. The distribution of CSF WBC count and CSF protein concentration were not normal (Shapiro–Wilk test, p < 0.05) in the pediatric MOG-AD group, but were normal (Shapiro–Wilk test, p > 0.05) in the control groups. Differences between pediatric MOG-AD and control groups were assessed with an independent two-sample t-test when the data were parametric. Correlation coefficients were calculated using Pearson’s two-tailed correlation test when all variables satisfy normal distribution. Spearman’s two-tailed correlation test was employed for correlation coefficients when any variables did not satisfy normal distribution. Receiver operating characteristic (ROC) curves were performed to assess the diagnostic value.
Results
Patients’ characteristics
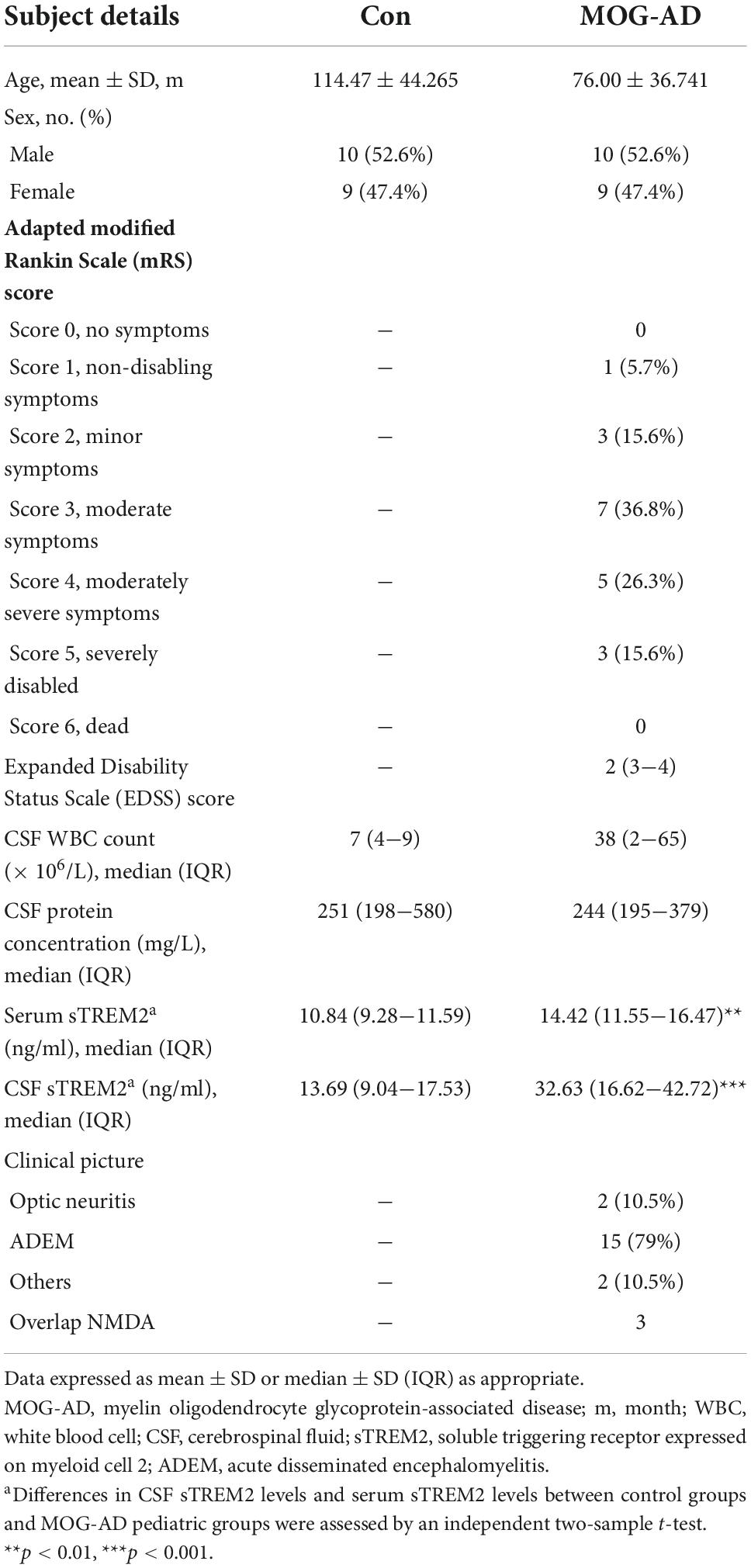
The clinical characteristics and concentrations of biomarkers for the enrolled patients are shown in Table 1. As described in Table 1, a total of 19 MOG-AD pediatric patients (10 male and 9 female) and 19 control patients (10 male and 9 female) were included. All patients from the control group were selected from non-neuroinflammatory diseases. Informed consent was obtained from all participants and their parents. The study was approved by the local ethical board of Beijing Children’s Hospital.
Cerebrospinal fluid and serum soluble triggering receptor expressed on myeloid cells 2 levels are higher in MOG-AD pediatric patients
The CSF and serum sTREM2 levels are presented in Table 1. The median levels of CSF sTREM2 were 32.63 (9.76–74.61) ng/ml in the MOG-AD pediatric group and 13.69 (4.53–27.55) ng/ml in the control group. The median levels of serum sTREM2 were 14.42 (6.08–21.91) ng/ml in the MOG-AD pediatric group and 10.84 (8.49–14.5) ng/ml in the control group. The sTREM2 levels were significantly higher in the MOG-AD pediatric group than in the control group, both in CSF and serum (p < 0.001, Figure 1A, and p = 0.0012, Figure 1B, respectively).
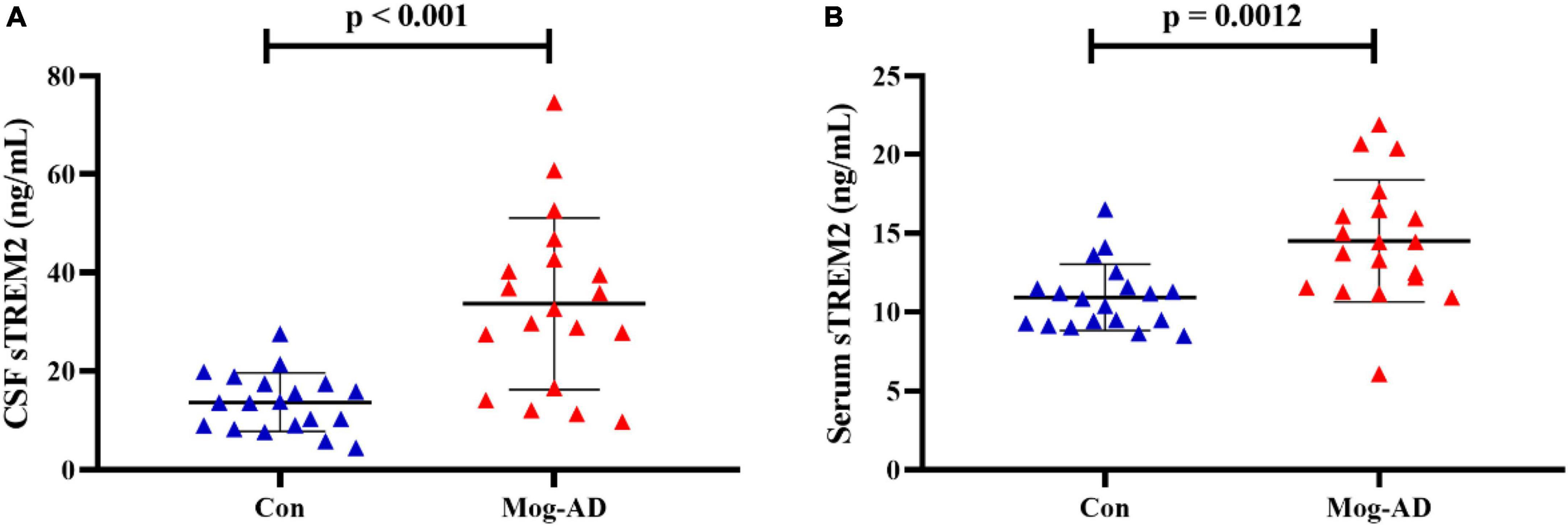
Figure 1. High level of CSF and serum sTREM2 in MOG-AD pediatric patients. (A) sTREM2 levels in cerebrospinal fluid samples from the pediatric MOG-AD group (N = 19) were significantly higher than the control groups (N = 19) (p < 0.001). (B) sTREM2 levels in serum samples from the pediatric MOD-AD group (N = 19) were significantly higher, compared with the control group (N = 19) (p = 0.0012). Independent two-sample t-test were used for differential analysis.
Cerebrospinal fluid soluble triggering receptor expressed on myeloid cells 2 levels concerning clinical inflammatory indexes and adapted modified Rankin Scale score
Since there are no randomized controlled trials or consensus guidelines for the treatment of MOG-AD, we used an adapted modified Rankin Scale (mRS) to assess the disease severity (16). The CSF sTREM2 levels were mildly correlated with adapted mRS score (r = 0.620, p = 0.005; Figure 2B) and Expanded Disability Status Scale (EDSS) score (r = 0.476, p = 0.039; Figure 2E) in MOG-AD pediatric groups. Correlations between CSF sTREM2 levels and clinical inflammatory indexes were shown in Figures 2C,D. The CSF sTREM2 level was correlated with CSF biomarkers of inflammatory (CSF WBC count and CSF protein concentration). We discovered that, in MOG-AD pediatric group, CSF sTREM2 concentration were significantly associated with both CSF WBC count (r = 0.657, p = 0.002; Figure 2C) and CSF protein concentration (r = 0.507, p = 0.027; Figure 2D), while found no association described above in the control group (r = −0.199, p = 0.415, Figure 2F; r = 0.272, p = 0.259, Figure 2G; respectively). In this work, there is no correlation between patients’ age and CSF sTREM2 levels in MOG-AD group (data not shown).
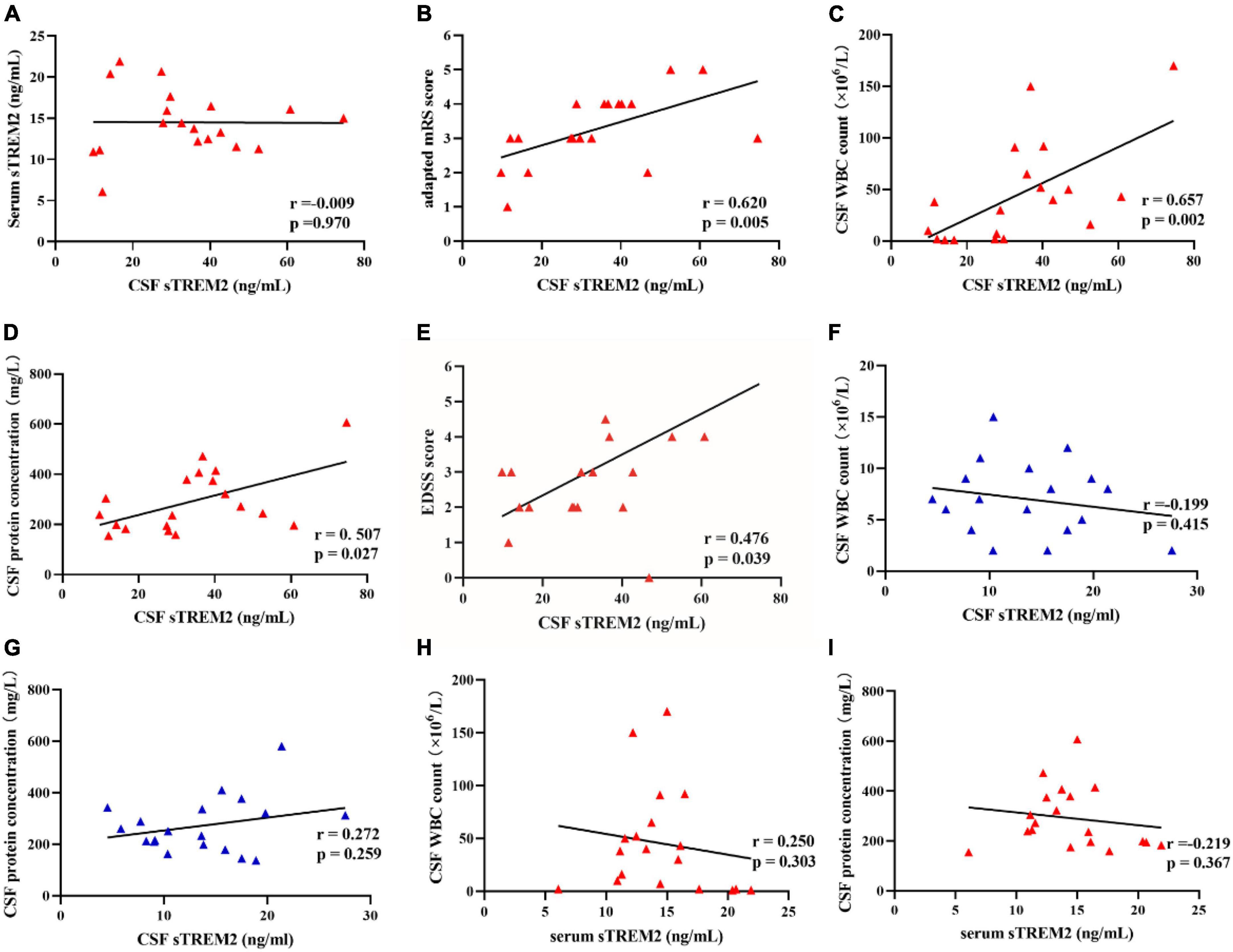
Figure 2. The CSF sTREM2 level was related to the adapted mRS score, EDSS score, and clinical inflammatory indexes. (A) There was no correlation between CSF sTREM2 levels and serum sTREM2 levels (Pearson correlation, r = –0.009, p = 0.970). (B) There was a mildly positive correlation between the adapted modified Rankin Scale (mRS) score and CSF sTREM2 levels (Spearman correlation, r = 0.620, p = 0.005). (C,D) There were significant correlations between CSF sTREM2 levels and central clinical inflammatory indexes. CSF sTREM2 levels were significantly positive correlated with the CSF WBC count (Spearman correlation, r = 0.657, p = 0.002). CSF sTREM2 levels were significantly positive correlated with CSF protein concentration (Spearman correlation, r = 0.507, p = 0.027). (E) The CSF sTREM2 levels were positively correlated to the Expanded Disability Status Scale (EDSS) score (Spearman correlation, r = 0.476, p = 0.039; E). (F,G) CSF sTREM2 levels had no correlation with the CSF WBC counts (Pearson correlation, r = –0.199, p = 0.415) and the CSF protein concentration (Pearson correlation, r = 0.272, p = 0.259) in control groups, respectively. (H,I) Serum sTREM2 levels had no correlation with the CSF WBC counts (Spearman correlation, r = –0.250, p = 0.303) and the CSF protein concentration (Spearman correlation, r = –0.219, p = 0.367) in pediatric MOG-AD groups, respectively.
However, the serum sTREM2 level showed no correlation with CSF WBC count (r = −0.25, p = −0.303, Figure 2H) in the pediatric MOG-AD group. There was no correlation between serum sTREM2 level with CSF protein concentration (r = −0.219, p = 0.367, Figure 2I) in the pediatric MOG-AD group, either. Besides, there was no association between the serum sTREM2 level with the CSF sTREM2 levels in the pediatric MOG-AD group (r = −0.009, p = 0.970, Figure 2A). These results indicated that sTREM2 had different sources in serum and CSF.
The cerebrospinal fluid soluble triggering receptor expressed on myeloid cells 2 and serum soluble triggering receptor expressed on myeloid cells 2 levels can be a biomarker for MOG-AD diagnosis
To further investigate how well sTREM2 levels are capable of discriminating between pediatric MOG-AD patients and control groups, the ROC curve analysis was established. The CSF sTREM2 levels could differentiate pediatric MOG-AD patients (N = 19) well from the control group (N = 19), with the area under the curve (AUC) of CSF sTREM2 levels of 0.861 (p < 0.001; Figure 3A). The serum sTREM2 levels showed a slightly weaker result than the CSF sTREM2 levels, with an AUC of 0.812 (p = 0.001; Figure 3B).
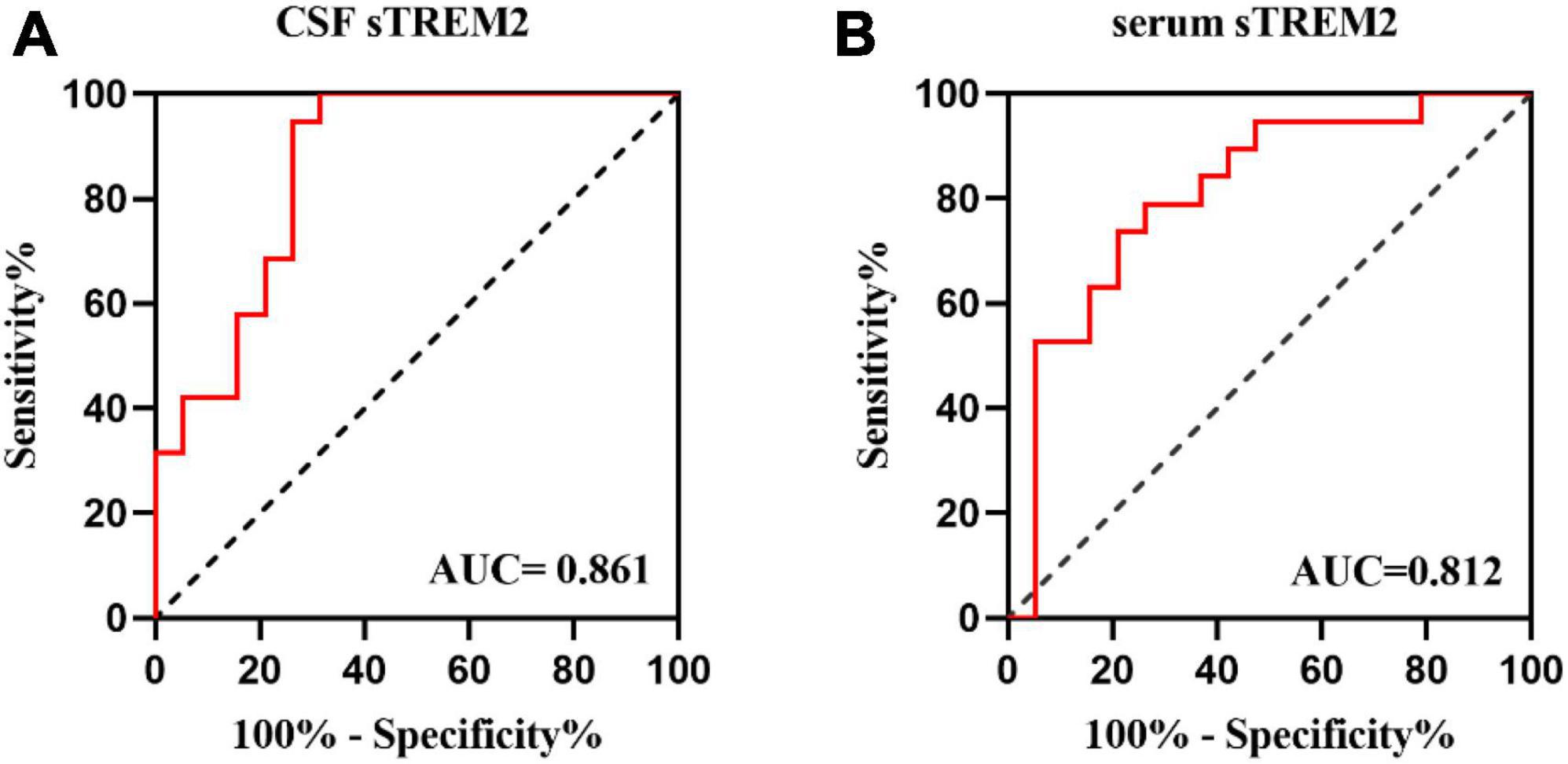
Figure 3. The CSF and serums TREM-2 levels can be a specific MOG-AD diagnosis biomarker. (A,B) Receiver operating characteristic (ROC) curve analysis of sTREM2 in CSF and serum for MOG-AD diagnosis (N = 38). (A) The area under the curve (AUC) of CSF sTREM2 levels was 0.861 (p < 0.001). (B) The area under the curve (AUC) of serum sTREM2 levels was 0.812 (p = 0.001).
Discussion
The pathogenic mechanism of MOG-AD is still unclear. In the CNS, MOG locates on the external lamellae of myelin sheaths and the surface of the oligodendrocyte, which is associated with the myelination and the maturation of oligodendrocytes (17). There are a few types of research on the biological function of MOG. Experimental studies have revealed that MOG plays an essential role in maintaining the adhesion mechanisms of myelin (18) and interacting with the immune system. As the lesion of ADEM demonstrated (19), MOG-laden macrophages are found in the perivascular areas and demyelinating lesions. In this work, the higher level of CSF sTREM2 in the pediatric MOG-AD group further demonstrates that the activated microglia and macrophage may take part in the response to the MOG antibodies mediated neuroinflammation.
In this work, we revealed that: (1) the CSF and serum sTREM2 levels are higher in MOG-AD pediatric patients; (2) the CSF sTREM2 levels are correlated with CSF WBC count and CSF protein concentration; (3) the CSF sTREM2 levels are correlated with mRS score; and (4) the CSG sTREM2 levels could be a biomarker for MOG-AD diagnosis.
Besides, we also noted that there are no relationships between the serum sTREM2 levels with both CSF WBC count and CSF protein concentration. These negative results demonstrated that, compared with serum sTREM2 level, the CSF sTREM2 level has more reference value in measuring the degree of inflammation and may indicate the time phase of the inflammatory process.
As a soluble variant of TREM-2 detected in human biofluids (20), the differences in the sTREM2 levels in these biofluids might be a potential clue attributed to the microglial dysfunction and innate immunity. Although CSF sTREM2 levels have been studied extensively in neuroinflammatory diseases and neurodegeneration diseases, few studies have investigated the serum sTREM2 level. Several experimental studies have investigated that CSF sTREM2 levels were significantly elevated in Alzheimer’s disease (AD). It is strongly correlated with biomarkers of peripheral inflammation and the blood-brain barrier integrity biomarker (i.e., CSF Albumin/Serum Albumin ratio) (21). In MS, both CSF and serum sTREM2 level was higher in the MS group than in healthy control groups (22). By comparing the effects of natalizumab and mitoxantrone, only CSF sTRME2 has shown the potential utility as a biomarker for MS treatment effects (12). In addition, the level of CSF sTREM2 was increased in patients with neurosyphilis (NS), and peaked at the late stage, compared with the control groups (23).
To date, the clinical characteristics and prognosis of pediatric patients need more exploration. Pediatric patients had different clinical features: earlier age of onset, better recovery of visual acuity, lower annual rates of relapse, and more intracranial optic nerve involvement than middle-aged patients (3). The discovery of advanced antibodies refined our hypothesis about the immunopathogenesis of MOG-AD. MOG-AD is now recognized as an autonomous, antibody-mediated inflammatory demyelinating disorder. Thus, the approvement of MOG-AD diagnosis criteria requires more development in the pathophysiological mechanisms and the identification of markers. Our research is the first one investigating on the sTREM2 level in the pediatric MOG-AD patients’ biofluid and revealing the relationship between the CSF sTREM2 level with the clinical inflammatory indexes. TREM-2 is expressed on tissue macrophages in the peripheral and on microglia in the central (24). Research proved sTREM2 promotes microglial survival via PI3K/Akt pathway and activates the microglial depending on NF-κB (25).
We acknowledged a few limitations in this study, that is, lack of other neuroinflammatory disorders data, cross-sectional study design, single-center scope, and small sample size of Asians. In addition, as MOG-AD is rare, the sample of pediatric patients is limited. Since all the samples were collected before treatments, the treatment effects were not discussed in this study. It was difficult for pediatric MOG-AD patients to be accurately diagnosed and large treatment trials have not been performed (14).
Conclusion
In conclusion, the higher level of CSF sTRME2 in pediatric MOG-AD patients supported the evidence that sTREM2 could be a marker of microglia activation in pediatric MOG-AD. These significant results indicated that sTREM2 could be useful in monitoring the degree of central inflammation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Beijing Children’s Hospital Affiliated to Capital Medical University, Beijing, People’s Republic of China (Approved No. of the ethic committee: 2019-k-272). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
AZ prepared the reagents, performed the experiments, and analyzed the data. CR and HC selected and clinically characterized the patients. JZ carried out statistical analyses. XR obtained the financial support. WZ designed the study. AZ wrote the manuscript with revisions from all authors.
Funding
This work was supported by the Beijing Hospitals Authority Youth Program (QMS20201204).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Marignier R, Hacohen Y, Cobo-Calvo A, Pröbstel AK, Aktas O, Alexopoulos H, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. (2021) 20:762–72.
2. Hacohen Y, Mankad K, Chong WK, Barkhof F, Vincent A, Lim M, et al. Diagnostic algorithm for relapsing acquired demyelinating syndromes in children. Neurology. (2017) 89:269–78.
3. Song H, Zhou H, Yang M, Xu Q, Sun M, Wei S. Clinical characteristics and outcomes of myelin oligodendrocyte glycoprotein antibody-seropositive optic neuritis in varying age groups: a cohort study in China. J Neurol Sci. (2019) 400:83–9. doi: 10.1016/j.jns.2019.03.014
4. Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflamm. (2018) 15:134.
5. Waters P, Woodhall M, O’Connor KC, Reindl M, Lang B, Sato DK, et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm. (2015) 2:e89.
6. Hoftberger R, Guo Y, Flanagan EP, Lopez-Chiriboga AS, Endmayr V, Hochmeister S, et al. The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol. (2020) 139:875–92.
7. Benveniste EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med (Berlin, Germany). (1997) 75:165–73.
8. Kleinberger G, Yamanishi Y, Suárez-Calvet M, Czirr E, Lohmann E, Cuyvers E, et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med. (2014) 6:243ra86. doi: 10.1126/scitranslmed.3009093
9. N’Diaye EN, Branda CS, Branda SS, Nevarez L, Colonna M, Lowell C, et al. TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria. J Cell Biol. (2009) 184:215–23.
10. Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. (2015) 160:1061–71. doi: 10.1016/j.cell.2015.01.049
11. Mazaheri F, Snaidero N, Kleinberger G, Madore C, Daria A, Werner G, et al. TREM2 deficiency impairs chemotaxis and microglial responses to neuronal injury. EMBO Rep. (2017) 18:1186–98. doi: 10.15252/embr.201743922
12. Öhrfelt A, Axelsson M, Malmeström C, Novakova L, Heslegrave A, Blennow K, et al. Soluble TREM-2 in cerebrospinal fluid from patients with multiple sclerosis treated with natalizumab or mitoxantrone. Mult Scler (Houndmills, Basingstoke, England). (2016) 22:1587–95.
13. Wang X, Zeng Y, Tian J, Xu H, Song F, Guo Y, et al. A brief introduction to the FUTang updating medical REcords (FUTURE) database. Pediatric Investigat. (2021) 5:247–8. doi: 10.1002/ped4.12297
14. Ambrosius W, Michalak S, Kozubski W, Kalinowska A. Myelin oligodendrocyte glycoprotein antibody-associated disease: current insights into the disease pathophysiology, diagnosis and management. Int J Mol Sci. (2020) 22:100. doi: 10.3390/ijms22010100
15. Wynford-Thomas R, Jacob A, Tomassini V. Neurological update: MOG antibody disease. J Neurol. (2019) 266:1280–6.
16. Nosadini M, Thomas T, Eyre M, Anlar B, Armangue T, Benseler SM, et al. International consensus recommendations for the treatment of pediatric NMDAR antibody encephalitis. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e1052. doi: 10.1212/NXI.0000000000001052
17. Barkovich AJ. Concepts of myelin and myelination in neuroradiology. AJNR Am J Neuroradiol. (2000) 21:1099–109.
18. Morise J, Takematsu H, Oka S. The role of human natural killer-1 (HNK-1) carbohydrate in neuronal plasticity and disease. Biochim Biophys Acta Gen Subj. (2017) 1861:2455–61. doi: 10.1016/j.bbagen.2017.06.025
19. Takai Y, Misu T, Kaneko K, Chihara N, Narikawa K, Tsuchida S, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease: an immunopathological study. Brain J Neurol. (2020) 143:1431–46. doi: 10.1093/brain/awaa102
20. Thornton P, Sevalle J, Deery MJ, Fraser G, Zhou Y, Ståhl S, et al. TREM2 shedding by cleavage at the H157-S158 bond is accelerated for the Alzheimer’s disease-associated H157Y variant. EMBO Mol Med. (2017) 9:1366–78. doi: 10.15252/emmm.201707673
21. Bekris LM, Khrestian M, Dyne E, Shao Y, Pillai JA, Rao SM, et al. Soluble TREM2 and biomarkers of central and peripheral inflammation in neurodegenerative disease. J Neuroimmunol. (2018) 319:19–27.
22. Ioannides ZA, Csurhes PA, Swayne A, Foubert P, Aftab BT, Pender MP. Correlations between macrophage/microglial activation marker sTREM-2 and measures of T-cell activation, neuroaxonal damage and disease severity in multiple sclerosis. Mult Scler J Exp Transl Clin. (2021) 7:20552173211019772. doi: 10.1177/20552173211019772
23. Li W, Chang H, Wu W, Xu D, Jiang M, Gao J, et al. Increased CSF soluble TREM2 concentration in patients with neurosyphilis. Front Neurol. (2020) 11:62. doi: 10.3389/fneur.2020.00062
24. Hickman SE, El Khoury J. TREM2 and the neuroimmunology of Alzheimer’s disease. Biochem Pharmacol. (2014) 88:495–8.
Keywords: MOG-AD, CSF, sTREM2, microglia, autoimmune diseases
Citation: Zhou A, Zhang W, Ren C, Zhou J, Chang H and Ren X (2022) High levels of cerebrospinal fluid soluble triggering receptor expressed on myeloid cells 2 might be a biomarker of activity in pediatric patients with MOG-AD. Front. Pediatr. 10:908527. doi: 10.3389/fped.2022.908527
Received: 30 March 2022; Accepted: 09 September 2022;
Published: 13 October 2022.
Edited by:
Trevor Owens, University of Southern Denmark, DenmarkReviewed by:
Matthew Adams, Wayne State University, United StatesPhilippe Horellou, INSERM U1184 Centre de Recherche en Immunologie des Infections Virales et des Maladies Auto-Immunes, France
Susanna Felsenstein, University of Liverpool, United Kingdom
Copyright © 2022 Zhou, Zhang, Ren, Zhou, Chang and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haoxiao Chang, changhaoxiao@sina.com; Xiaotun Ren, renxiaotun@bch.com.cn
 Anna Zhou
Anna Zhou Weihua Zhang
Weihua Zhang Changhong Ren
Changhong Ren Ji Zhou
Ji Zhou Haoxiao Chang
Haoxiao Chang Xiaotun Ren
Xiaotun Ren