Risk factors for necrotizing enterocolitis in neonates: A meta-analysis
- Department of Pediatrics, The First Hospital of Hebei Medical University, Shijiazhuang, China
Objective: The objective is to identify the risk factors for necrotizing enterocolitis (NEC) in neonates by a meta-analysis, and to provide a reference for the prevention of NEC.
Methods: The databases, including Chinese Biomedical Literature Datebase, China National Knowledge Infrastructure, Wanfang database, and Weipu Periodical database, PubMed, Web of Science, Embase, Cochrane Library, were searched for studies on the risk factors for NEC in neonates. The meta-analysis was carried out with the aid of Stata software.
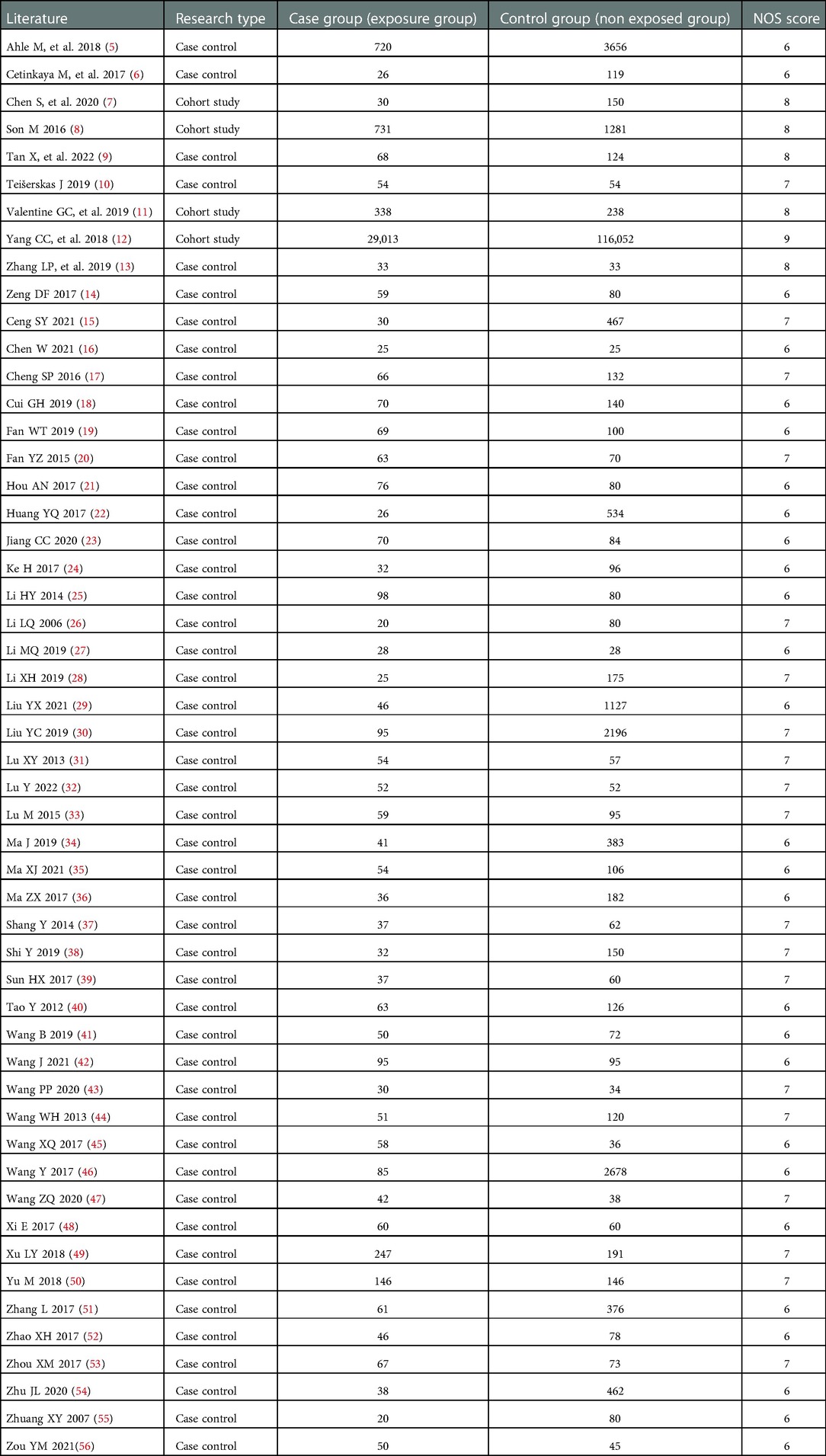
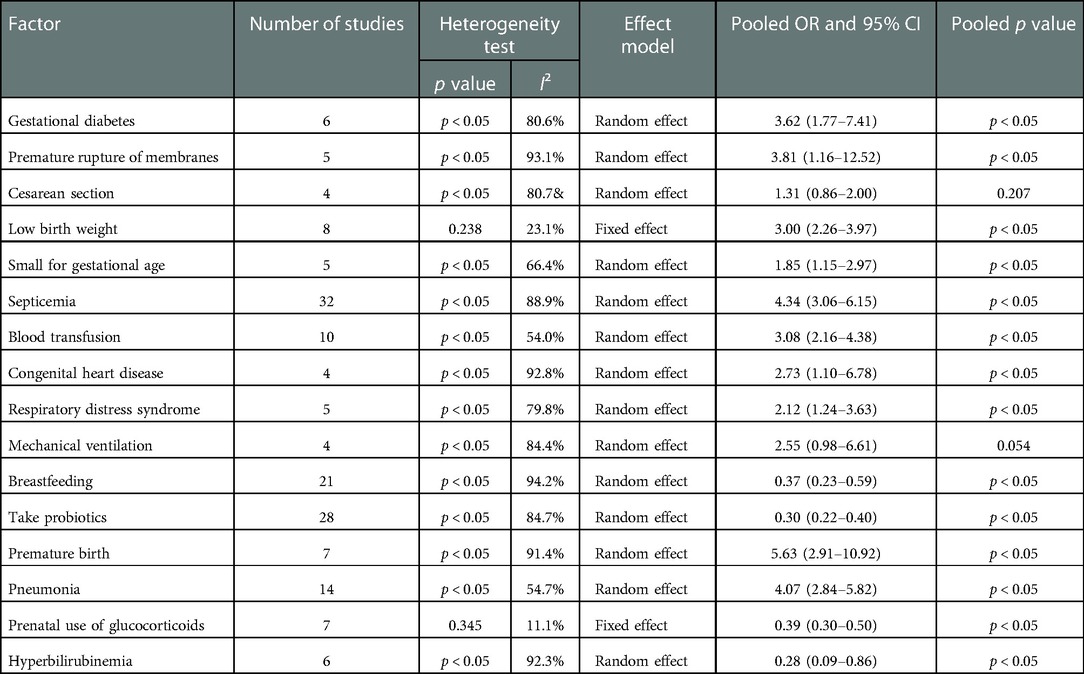
Results: A total of 52 studies were included, with 48 case-control studies and 4 cohort studies. There were 166,580 neonates in total, with 33,522 neonates in the case group and 133,058 neonates in the control group. The meta-analysis showed that gestational diabetes (OR = 3.62, 95% CI:1.77–7.41), premature rupture of membranes (OR = 3.81, 95% CI:1.16–12.52), low birth weight (OR = 3.00, 95% CI:2.26–3.97), small for gestational age (OR = 1.85, 95% CI:1.15–2.97), septicemia (OR = 4.34, 95% CI:3.06–6.15), blood transfusion (OR = 3.08, 95% CI:2.16–4.38), congenital heart disease (OR = 2.73, 95% CI:1.10–6.78), respiratory distress syndrome (OR = 2.12, 95% CI:1.24–3.63), premature birth (OR = 5.63, 95% CI:2.91–10.92), pneumonia (OR = 4.07, 95% CI:2.84–5.82) were risk factors for NEC in neonates. Breastfeeding (OR = 0.37, 95% CI:0.23–0.59), take probiotics (OR = 0.30, 95% CI:0.22–0.40), prenatal use of glucocorticoids (OR = 0.39, 95% CI:0.30–0.50), Hyperbilirubinemia (OR = 0.28, 95% CI:0.09–0.86) were protective factors for NEC in neonates.
Conclusions: Gestational diabetes, premature rupture of membranes, low birth weight, small for gestational age, septicemia, blood transfusion, congenital heart disease, respiratory distress syndrome, premature birth, and pneumonia may increase the risk of NEC in neonates. Breastfeeding, taking probiotics, prenatal use of glucocorticoids, and Hyperbilirubinemia may reduce the risk of NEC in neonates.
Introduction
Necrotizing enterocolitis (NEC) in neonates is a severe muti-factorial disease characterized by intestinal necrosis in the ileum, jejunum, and colon. It is one of the leading causes of morbidity and mortality in preterm infants (1), with which clinical manifestations involve abdominal distension, vomiting, bloody stool, septic shock, and DIC in severe cases. Therefore, early diagnosis and treatment to avoid its devastating consequences are essential. However, due to the poor insight into its pathogensis, reliable tools and effective strategies are short to prevent and treat NEC in neonates (2). Indeed, there are many pathogenic factors of NEC in neonates, some of which still need to be clearly defined. Since the identification and intervention of neonates at risk for NEC can reduce the incidence and improve the prognosis (3), this study comprehensively searched domestic and foreign literature on risk factors for NEC in neonates by a meta-analysis, which aims to provide a reference for the prevention of NEC in neonates.
Materials and methods
Document retrieval
China National Knowledge Infrastructure (CNKI), Wanfang Database, VIP Chinese Journal Database, Chinese Biomedical Literature Database, PubMed, Web of Science, Embase and Cochrane Library were searched to systematically collect published studies on risk factors of NEC in neonates. The search strategy of combining keywords and subject terms was adopted. The Chinese search terms were “newborn”, “necrotizing enterocolitis”, “risk factors”, “case-control study”, “cohort study”, etc. English search words “Enterocolitis, Necrotizing”, “Infant, Newborn”, “relative risk”, “cohort”, etc., supplemented by manual search and literature tracing.
Inclusion and exclusion criteria
Inclusion criteria: 1. The diagnosis of NEC is clear; 2. The study type was a case-control study or cohort study; 3. The subjects were neonates (<28 days); 4. The original data is available. The OR (odds ratio) value and 95% confidence interval (CI) are provided, or the OR value and 95% CI can be calculated from the data.
Exclusion criteria: 1. Conference summary, comments and review articles; 2. Unable to extract effective outcome indicators from the literature; 3. The experimental design is not rigorous (non case-control or cohort studies, as well as grouping non case-control/exposure group and control group/unexposed group); 4. Unable to get a full text; 5. The sample size is too small.
Data extraction and quality assessment
Two researchers strictly followed the inclusion and exclusion criteria to independently conduct literature screening, quality evaluation, data extraction, and discuss possible differences to reach an agreement. The final results were confirmed by more senior researchers. The Newcastle-Ottawa Scale (NOS) (4) was used for the quantitative assessment of case-control studies and cohort studies, including research object evaluation (4 points), inter group comparability evaluation (2 points), and result evaluation (3 points). The NOS score ≥ 6 is a high-quality study.
Method of statistics
The Q test and I2 statistic were used to evaluate the heterogeneity, and the test level was set as 0.1. If the heterogeneity test results were P > 0.1 and I2 < 50%, the pooled effect size OR and 95% CI were calculated using the fixed effects model. Otherwise, the random effect model is used to calculate; Sensitivity analysis uses different models to analyze the same data. Egger's or Begg's test was used to evaluate publication bias, and Stata12.0 was used for statistical analysis.
Result
Literature screening results
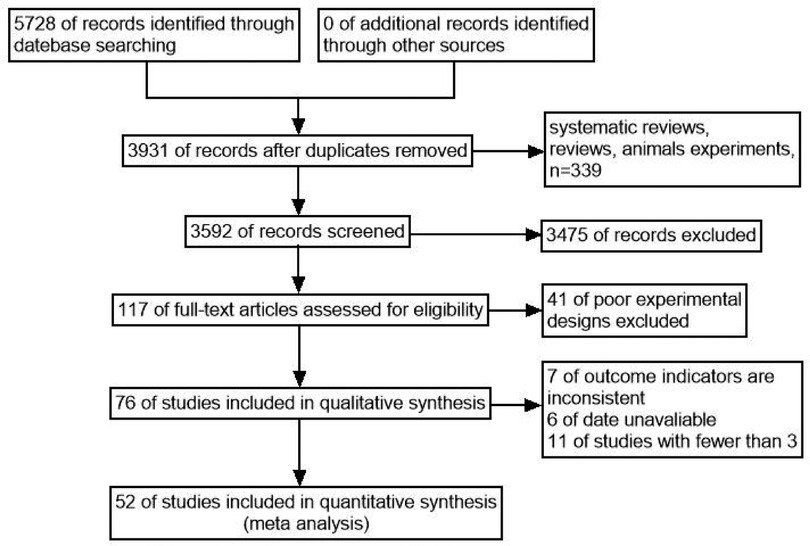
A total of 5,728 relevant articles were obtained through preliminary screening, and 3,931 articles remained after being re-selected by Endnote software. Ulteriorly, 3,475 articles were excluded after reading the title and abstract, and 117 articles remained for further evaluation. Finally, after reading the full text, 52 articles were included according to the inclusion and exclusion criteria, as shown in Figure 1. There were 166,580 subjects, including 33,522 cases in the case group and 133,058 cases in the control group. 48 case-control studies and 4 cohort studies are shown in Table 1. The NOS score of 52 included studies was no less than 6 points.
Results of meta-analysis
According to the risk factors involved in the included literature, gestational diabetes, premature rupture of membranes, cesarean section, low birth weight, small for gestational age, sepsis, blood transfusion, congenital heart disease, respiratory distress syndrome, mechanical ventilation, breast feeding, probiotics, preterm delivery, pneumonia, prenatal use of glucocorticoids, hyperbilirubinemia, ect., were selected for analysis. Heterogeneity test results showed that there was heterogeneity among the studies of diabetes in pregnancy, premature rupture of membranes, cesarean section, small for gestational age, sepsis, blood transfusion, congenital heart disease, respiratory distress syndrome, mechanical ventilation, breast-feeding, probiotics, preterm delivery, pneumonia and hyperbilirubinemia, and the random effect model was used to combine the effect amount. In contrast, there is no heterogeneity in other related factors, and the fixed effect model is used to combine the effects. The meta-analysis results demonstrates that: Cesarean section and mechanical ventilation were not statistically significant with NEC in neonates. Gestational diabetes mellitus, premature rupture of membranes, low birth weight, small for gestational age, sepsis, blood transfusion, congenital heart disease, respiratory distress syndrome, premature birth and pneumonia were risk factors for NEC in neonates. Breastfeeding, probiotics, prenatal glucocorticoid use, and hyperbilirubinemia were protective factors for NEC in neonates, as shown in Table 2.
Sensitivity analysis and bias test
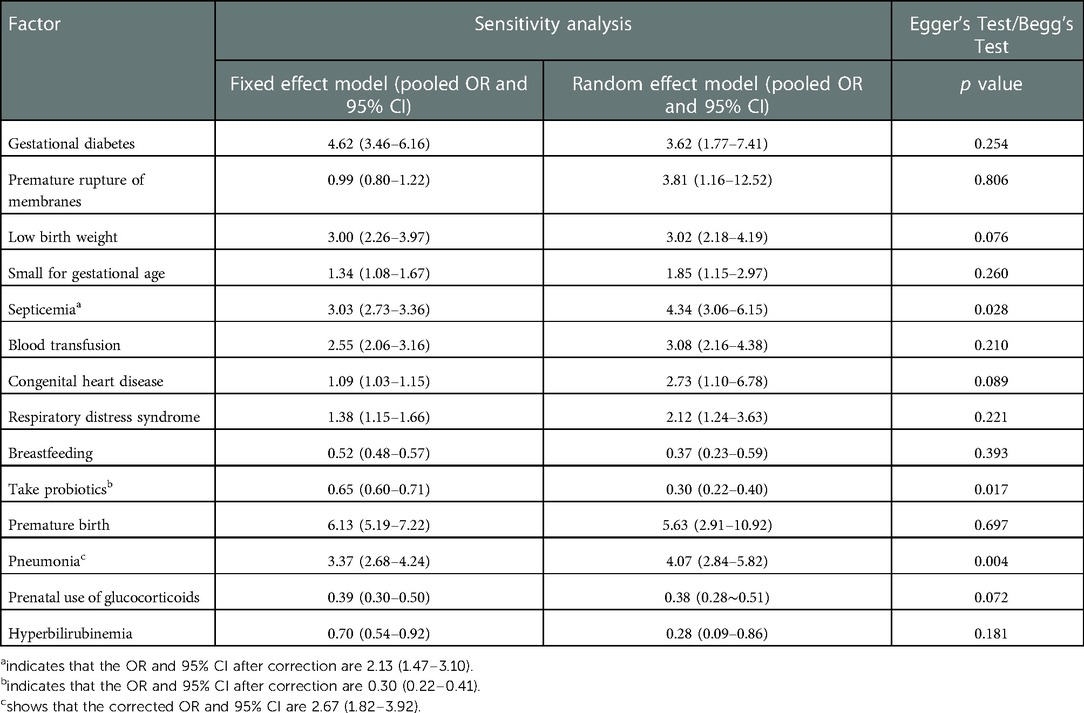
For the screened risk factors, the fixed effect and random effect models were used to recalculate the combined effect size. The calculation results of these two models were basically consistent, indicating that the results of this study were basically reliable. However, the results of premature rupture of membranes are not robust enough and should be treated with caution. The results of Egger's or Begg's test suggest that the results of sepsis, probiotics, and pneumonia are biased (P < 0.05), as shown in Table 3. The pruning method evaluated the publication bias of the results of sepsis, probiotics and pneumonia. It was found that the results of sepsis [OR and 95% CI: 2.13 (1.47–3.10)], probiotics [OR and 95% CI: 0.30 (0.22–0.41)], and pneumonia [OR and 95% CI: 2.67 (1.82–3.92)] were basically consistent before and after pruning, suggesting that the meta-analysis conclusion of risk factors was stable and reliable.
Discussion
This study conducted a meta-analysis of the domestic and foreign studies on the risk factors of NEC in neonates, and conducted a quantitative combined analysis and comprehensive evaluation of the results of multiple studies with the same research factors, to make the research conclusions more comprehensive and reliable.
This meta-analysis shows that gestational diabetes is a risk factor for NEC in neonates. As the nutrition needed for the development of the fetus in the abdomen comes from the mother, the blood sugar of the mother after diabetes during pregnancy is higher than the normal level, and high blood sugar will inhibit the blood circulation of the fetus' intestinal tract, causing ischemic necrosis of the intestinal mucosa. After birth, pathogenic microorganisms easily invade the gastrointestinal tract and colonize the damaged intestinal mucosa, causing inflammation and morbidity (57).
This meta-analysis shows that premature rupture of membranes may increase the risk of NEC. Research shows that PROM, as one of the main causes of premature delivery, can increase the incidence of NEC, ROP, BPD and other complications. Timely treatment of PROM can reduce the occurrence of NEC in neonates (58).
This meta-analysis showed that low birth weight was a risk factor for NEC. The reasons may be as follows: (1) Due to the immature intestinal function and slow intestinal peristalsis of low birth weight infants, food residues are easy to be detained and fermented, providing a good environment for bacterial growth, leading to a large number of bacterial proliferation; (2) The intestinal microbiota of low birth weight infants is very immature, and direct contact with pathogenic microorganisms will cause inflammation related to mucosal damage, leading to NEC (59).
This meta-analysis showed that small for gestational age (SGA) was a risk factor for NEC. SGA infants have a high probability of NEC, neonatal asphyxia, brain injury and respiratory distress syndrome (60). One study found that the risk of NEC in SGA infants was twice that of appropriate for gestational age infants (61).
This meta-analysis showed that septicemia, congenital heart disease, respiratory distress syndrome, and pneumonia were risk factors for NEC. In severe infection, the body is in a state of inflammation activation, producing a variety of inflammatory transmitters. These substances directly or indirectly cause damage to the intestinal mucosa, and then participate in the occurrence and development of NEC (62, 63). Meanwhile, the combined effects of pathogenic bacteria, intestinal flora imbalance, intestinal wall barrier dysfunction, toxic intestinal paralysis, etc., during sepsis can also lead to NEC (64). In addition, in the case of sepsis and other severe infections, in addition to the direct destruction of intestinal epithelial cells by bacteria, endotoxin and other products produced by bacteria can also cause intestinal necrosis (62). In the previous report, the proportion of NEC in children with CHD was 6.8%∼13% (65), significantly higher than that in normal premature infants and neonates. Baxi et al. (66) found that children with CHD were prone to abnormal blood distribution, decreased mesenteric blood supply, and a large number of free radicals, which mediated reperfusion injury. It has been previously reported that asphyxia at birth is closely related to the occurrence of necrotizing enterocolitis. The severity of necrotizing enterocolitis increases with that of respiratory distress (67). When respiratory distress occurs or pneumonia occurs, the body is in an anoxic state. At this time, in order to ensure the oxygen supply of the vital organs of the child, the whole body's blood flow is redistributed, mainly because the intestinal vessels contract and the blood flow is reduced, leading to intestinal hypoperfusion, resulting in intestinal mucosa hypoxia and damage, leading to necrotizing enterocolitis (68, 69).
This meta-analysis showed that blood transfusion was a risk factor for NEC. The possible pathogenesis of NEC is as follows: the inflammatory mediators such as TNF-α, IL-6, and PAF produced during the processing of whole blood and the storage of red blood cells, and the residual white blood cells, free hemoglobin, red cell membrane fragments, etc. promote the occurrence of NEC. The pathological changes of red blood cells occurred during storage, including decreased erythrocyte deformability, increased oxygen affinity ability and decreased nitric oxide resulting in the loss of vasodilator activity, etc., resulting in the failure to improve intestinal microcirculation perfusion flow after blood transfusion; NEC may be caused by anemia (70).
This meta-analysis showed that preterm birth was a risk factor for NEC. Due to the unsound development of the enteric nervous system and poor regularity of small intestinal peristalsis, premature infants are prone to excessive bacterial growth and gas after food fermentation, and are prone to NEC (71).
This meta-analysis showed that breastfeeding, probiotics, prenatal glucocorticoid use, and hyperbilirubinemia were protective factors for NEC. Breast milk is known as the most natural and safe natural food for infants and young children, containing nutrients and antibodies necessary for the development of organized organs, especially beneficial antibodies, which can help maintain the immune function of newborns, inhibit the inflammatory reaction, and speed up the repair of the damaged intestinal mucosa (72). Compared with formula, breast milk has a lower osmotic pressure, which can minimize the osmotic load of food and reduce the impact on intestinal function, thereby reducing the incidence of NEC (73). The supplementation of probiotics may improve gastrointestinal tolerance (74). The raw materials of probiotics come from microorganisms that are beneficial to the body. In the intestinal tract of newborns, probiotics can play a role in improving the microecological balance and promoting intestinal peristalsis, which is of great significance in preventing the occurrence and development of neonatal necrotizing enterocolitis (75). Prenatal hormones can promote the generation of alveolar surfactants, contribute to the development of alveoli, and reduce the incidence of neonatal respiratory distress syndrome and mortality of preterm infants (76). Bilirubin is considered to have antioxidant activity, can scavenge free radicals in the body, and is one of the plasma free radical scavenger to defend against the damage of various oxides (77, 78).
Limitations
This study has some limitations: First, sensitivity analysis found that the results of premature rupture of membranes were not robust enough. Therefore, the relationship between premature rupture of membranes and NEC in neonates needs further study. Secondly, there exist differences in sample size, case selection, and definition of exposure factors among the studies, which may lead to heterogeneity among the studies and have a certain impact on the results. Finally, only Chinese and English literature ware included in the included study, and literature published in other languages could not be analyzed, which may result in language bias.
Conclusion
We conducted a meta-analysis to evaluate the risk factors of NEC. This meta-analysis showed that gestational diabetes mellitus, premature rupture of membranes, low birth weight, small for gestational age, sepsis, blood transfusion, congenital heart disease, respiratory distress syndrome, premature birth, and pneumonia maght increase the risk of NEC in neonates. Therefore, perinatal health care should be strengthened to reduce the incidence of neonatal complications, so as to prevent the occurrence of NEC in neonates.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
YS, R-HX, L-YH and C-JR contributed to conception and design of the study. L-YG organized the database. XC performed the statistical analysis. W-XH wrote the first draft of the manuscript. YS, J-JM and J-JL wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1079894/full#supplementary-material.
References
1. Marseglia L, D'Angelo G, Manti S, Aversa S, Reiter RJ, Antonuccio P, et al. Oxidative stress-mediated damage in newborns with necrotizing enterocolitis: a possible role of melatonin. Am J Perinatol. (2015) 32:905–9. doi: 10.1055/s-0035-1547328
2. Lim JC, Golden JM, Ford HR. Pathogenesis of neonatal necrotizing enterocolitis. Pediatr Surg Int. (2015) 31:509–18. doi: 10.1007/s00383-015-3697-9
3. Yang QY, Lin ZL. Research progress on the risk factors of neonatal necrotizing enterocolitis. Chin J Birth Health Hered. (2022) 30:905–9. doi: 10.13404/j.cnki.cjbhh.2022.05.028
4. Lichtenstein MJ, Mulrow CD, Elwood PC. Guidelines for Reading case-control studies. J Chronic Dis. (1987) 40:893–903. doi: 10.1016/0021-9681(87)90190-1
5. Ahle M, Drott P, Elfvin A, Andersson RE. Maternal, fetal and perinatal factors associated with necrotizing enterocolitis in Sweden. A national case-control study. PLoS One. (2018) 13:e0194352. doi: 10.1371/journal.pone.0194352
6. Cetinkaya M, Erener-Ercan T, Kalayci-Oral T, Babayiğit A, Cebeci B, Semerci SY, et al. Maternal/neonatal vitamin D deficiency: a new risk factor for necrotizing enterocolitis in preterm infants? J Perinatol. (2017) 37:673–8. doi: 10.1038/jp.2017.18
7. Chen S, Wang XQ, Hu XY, Guo L, He Y, Wang ZL, et al. Meconium-stained amniotic fluid as a risk factor for necrotizing enterocolitis in very low-birth weight preterm infants: a retrospective cohort study. J Matern Fetal Neonatal Med. (2020) 33:4102–7. doi: 10.1080/14767058.2019.1597045
8. Son M, Grobman WA, Miller ES. Is mode of delivery associated with the risk of necrotizing enterocolitis? Am J Obstet Gynecol. (2016) 215:389. doi: 10.1016/j.ajog.2016.04.058
9. Tan X, Zhou Y, Xu L, Zhang L, Wang J, Yang W. The predictors of necrotizing enterocolitis in newborns with low birth weight: a retrospective analysis. Med (Baltimore). (2022) 101:e28789. doi: 10.1097/md.0000000000028789
10. Teišerskas J, Bartašienė R, Tamelienė R. Associations between red blood cell transfusions and necrotizing enterocolitis in very low birth weight infants: ten-year data of a tertiary neonatal unit. Med (Kaunas). (2019) 55:16. doi: 10.3390/medicina55010016
11. Valentine GC, Burgess A, Hagan J, Gandhi M, Hurst N, Aagaard K, et al. 937: decreased rates of necrotizing enterocolitis associated with exclusive human milk-based diet. Am J Obstet Gynecol. (2019) 220:S603–S4. doi: 10.1016/j.ajog.2018.11.961
12. Yang CC, Tang PL, Liu PY, Huang WC, Chen YY, Wang HP, et al. Maternal pregnancy-induced hypertension increases subsequent neonatal necrotizing enterocolitis risk: a nationwide population-based retrospective cohort study in Taiwan. Med (Baltimore). (2018) 97:e11739. doi: 10.1097/md.0000000000011739
13. Zhang LP, Lei XP, Luo LJ, Dong WB. Risk factors for necrotizing enterocolitis in very preterm infants: a case-control study in southwest China. J Matern Fetal Neonatal Med. (2019) 32:896–901. doi: 10.1080/14767058.2017.1395011
14. Zeng DF, Tan ZY. Risk factors of neonatal necrotizing enterocolitis and prognostic factors of surgical treatment. J Chongqing Med Univ. (2017) 42:361–4. doi: 10.13406/j.cnki.cyxb.001189
15. Ceng SY, Deng C. Analysis of risk factors of very low birth weight infants with necrotizing enterocolitis. J Chongqing Med Univ. (2021) 46:335–40. doi: 10.13406/j.cnki.cyxb.002644
16. Chen W. Factors related to the occurrence of neonatal necrotizing enterocolitis and intervention countermeasures. Mod Diagn Treat. (2021) 32:2982–3.,3012.
17. Cheng SP, Lu Q, Zhou M, Yu JL. Risk factors for necrotizing enterocolitis in gestational age < 34 weeks preterm neonates: a case-control study. Chin J Evid-Based Pediatr. (2016) 11:122–5. doi: 10.3969/j.issn.1673-5501.2016.02.008
18. Cui GH, Lian TF, Fan L, Du YY, Zhou HJ, Chen FS, et al. Risk factors and surgical timing of necrotizing enterocolitis in neonates. Lingnan Mod Clin Surg. (2019) 19:264–7. doi: 10.3969/j.issn.1009-976X.2019.03.004
19. Fan WT, Liao W. Retrospective analysis of the main risk factors for neonatal necrotizing enterocolitis and the prognosis influence of surgical timing. J North Sichuan Med Coll. (2019) 34:679–82. doi: 10.3969/j.issn.1005-3697.2019.06.06
20. Fan YZ, Xiao ZX, Liu L, Xie CE, Wang Q. Clinical analysis of influence factors of neonatal necrotizing enterocolitis. China Mod Doct. (2015) 53:36–9.
21. Hou AN, Li X, Fu JH. Risk factors for neonatal necrotizing enterocolitis: an analysis of 76 cases. Chin J Pract Pediatr. (2017) 32:611–4. doi: 10.19538/j.ek20170806013
22. Huang YQ. Analysis on prevalence and risk factors of neonatal necrotizing enterocolitis. Matern Child Health Care China. (2017) 32:3222–4. doi: 10.7620/zgfybj.j.issn.1001-4411.2017.14.46
23. Jiang CC, Li W, Liu MY. Analysis of influencing factors of neonatal necrotizing enterocolitis. Chin Gen Practi Nurs. (2020) 18:2033–6. doi: 10.12104/j.issn.1674-4748.2020.16.036
24. Ke H. Risk factors for necrotizing enterocolitis in preterm infants. J Bethune Med Sci. (2017) 15:491–3. doi: 10.16485/j.issn.2095-7858.2017.04.040
25. Li HY, Tian SP, Li N. Risk factor analysis for neonatal necrotizing enterocolitis. J Ningxia Med Univ. (2014) 36:1389–91.
26. Li LQ, Wu B, Gao XX, Wang SX, Zheng ZS, Xu JL. Role of probiotics in the prevention of neonatal necrotizing enterocolitis: a case-control study. Chin J Contemp Pediatr. (2006) 8:464–6. doi: 10.3969/j.issn.1008-8830.2006.06.007
27. Li MQ. Risk factors for necrotizing enterocolitis in very low birth weight infants of prematurity. World Latest Med Inf. (2019) 19:64–5. doi: 10.19613/j.cnki.1671-3141.2019.91.038
28. Li XH. Clinical features and influencing factors of necrotizing enterocolitis in preterm infants. Matern Child Health Care China. (2019) 34:5673–5. doi: 10.7620/zgfybj.j.issn.1001-4411.2019.24.37
29. Liu YX, Lin ZB, He B, Zheng ZL. Construction of nomogram model for personalized prediction of neonatal necrotizing enterocolitis. Chin J Child Health Care. (2021) 29:838–42. doi: 10.11852/zgetbjzz2020-1901
30. Liu YC. An analysis on risk factors suffered from necrotizing enterocolitis in low and very low birth weight infants from author’s hospital (2012-2018yr). Pract J Med Pharm. (2019) 36:985–8. doi: 10.14172/j.issn1671-4008.2019.11.008
31. Lu XY, Tan W. Clinical analysis of influencing factors in 54 cases of necrotizing enterocolitis in preterm infants. Jiangxi Med Journal. (2013) 48:610–2. doi: 10.3969/j.issn.1006-2238.2013.07.023
32. Lu Y, Pan SS. Analysis of risk factors for necrotizing enterocolitis in preterm infants. China Mod Doct. (2022) 60:60–3.
33. Lu M, Liu DL, Lu YD, Li YB. A retrospective analysis of the influencing factors of necrotizing enterocolitis in preterm infants. Matern Child Health Care China. (2015) 30:2397–400. doi: 10.7620/zgfybj.j.issn.1001-4411.2015.15.39
34. Ma J, Qiao YX, Cao QY, Liu WN, Zhang M, Bai XY. Analysis of factors related to neonatal necrotizing enterocolitis in 41 cases. Chin J Neonatol. (2019) 34:291–4. doi: 10.3760/cma.j.issn.2096-2932.2019.04.010
35. Ma XJ, Zheng TT. Relationship between maternal risk factors and occurrence of necrotizing enterocolitis in premature infants before and during the first trimester. World Chin J Digestol. (2021) 29:557–62. doi: 10.11569/wcjd.v29.i10.557
36. Ma ZX, Chen YJ. Risk factors affecting necrotizing enterocolitis in newborns and preventive countermeasures. J Shanxi Med Coll Contin Educ. (2017) 27:83–5.
37. Shang Y, Yang J. Preterm infants necrotizing enterocolitis risk factors for clinical analysis. Jilin Med J. (2014) 35:6920–2. doi: 10.3969/j.issn.1004-0412.2014.31.018
38. Shi Y. Analysis of influencing factors of necrotizing enterocolitis in preterm infants. Mod Diagn Treat. (2019) 30:3416–8.
39. Sun HX, Gao RR, Zheng JF, Sun LS, Liu W, Wang XJ, et al. Clinical analysis of risk factors for premature infant necrotizing enterocolitis in premature infants. Acta Acad Med Weifang. (2017) 39:60–2. doi: 10.16846/j.issn.1004-3101.2017.01.021
40. Tao Y, Jiang Y, Chao J. Clinical analysis of risk factors for the pathogenesis of necrotizing enterocolitis in neonates. Healthmust-Readmagazine. (2012) 11:296–7.
41. Wang B. Risk factors for neonatal necrotizing enterocolitis are discussed. Cap Food Med. (2019) 26:29–30. doi: 10.3969/j.issn.1005-8257.2019.17.023
42. Wang J, Tang LF, Gu MQ, Xu XY, Yu JH, He S, et al. Risk factors and early clinical characteristics of neonatal necrotizing enterocolitis. J Kunming Med Univ. (2021) 42:99–104. doi: 10.12259/j.issn.2095-610X.S20211118
43. Wang PP, Huang NN, Wang GZ, Yu FQ. High risk factors and coping analysis of premature infants with necrotizing enterocolitis. Clin Res. (2020) 28:23–4.
44. Wang WH, Wang QH, Miao JJ. Risk factors for the development of neonatal necrotizing enterocolitis. Chin J Postgrad Med. (2013) 36:59–61. doi: 10.3760/cma.j.issn.1673-4904.2013.20.021
45. Wang XQ, Li ZB, Lian J. Case-control study on risk factors of necrotizing enterocolitis in premature infants. Med J Chin People Health. (2017) 29:65–7.
46. Wang Y, You CM, Liang J, Liang ZY. Analysis on the etiology of 85 cases of neonatal necrotizing enterocolitis. Clin Med Eng. (2017) 24:579–80. doi: 10.3969/j.issn.1674-4659.2017.04.0579
47. Wang ZQ. Analysis of risk factors associated with necrotizing enterocolitis in very low birth weight infants. Mod Med Health Res. (2020) 4:104–5.
48. Xi E, Zhu XF. Risk factors for the pathogenesis and mortality of neonatal necrotizing enterocolitis. Mod Dig Interv. (2017) 22:819–21. doi: 10.3969/j.issn.1672-2159.2017.06.021
49. Xu LY, Lin ZL. Risk factors for necrotizing enterocolitis in premature infants and observation on prophylactic effect of probiotics. Matern Child Health Care China. (2018) 33:5150–3. doi: 10.7620/zgfybj.j.issn.1001-4411.2018.22.33
50. Yu M, Xu H, Lu YJ, Zhu ZH, Shi BZ. Determinants and surgery effects of low birth weight neonates with necrotizing enterocolitis. Anhui Med Pharm J. (2018) 22:1949–52. doi: 10.3969/j.issn.1009-6469.2018.10.027
51. Zhang L, Xu YL, Li MX. Risk factors for necrotizing enterocolitis in very low birth weight infants born preterm. Matern Child Health Care China. (2017) 32:3515–7. doi: 10.7620/zgfybj.j.issn.1001-4411.2017.15.41
52. Zhao XH, Hua Z, Yang J. Risk factors affecting the occurrence of neonatal necrotizing enterocolitis and analysis of intervention countermeasures. J Shanxi Med Coll Contin Educ. (2017) 27:53–4.
53. Zhou XM. Risk factors for the occurrence of necrotizing enterocolitis in neonates. Contemp Med Symp. (2017) 15:51–2. doi: 10.3969/j.issn.2095-7629.2017.06.037
54. Zhu JL, Zhu XG, Ma LY, Zhou LX, Duan CS. Analysis on factors associated with neonatal necrotizing enterocolitis. Chin Prev Med. (2020) 21:990–4. doi: 10.16506/j.1009-6639.2020.09.008
55. Zhuang XY, Li LQ, Gao XX, Su LD. Relative factors of neonatal necrotizing enterocolitis and preventive effect of microeco-preparation. J Appl Clin Pediatr. (2007) 22:1392–3. doi: 10.3969/j.issn.1003-515X.2007.18.015
56. Zou YM, Ruan X, Deng XM, Su JY. Analysis of risk factors for the occurrence of necrotizing enterocolitis in neonates. Mod Med Health Res Electron J. (2021) 5:110–2.
57. Rose AT, Patel RM. A critical analysis of risk factors for necrotizing enterocolitis. Semin Fetal Neonatal Med. (2018) 23:374–9. doi: 10.1016/j.siny.2018.07.005
58. Duan SY, Kong XY, Xu FD, Lv HY, Ju R, Li ZK, et al. Impact of premature rupture of membranes on neonatal complications in preterm infants with gestational age< 37 weeks. J Southern Med University. (2016) 36:887–91.
59. Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. (2005) 147:192–6. doi: 10.1016/j.jpeds.2005.03.054
60. You JY, Su Z, Pan LL. Research progress in small for gestational age. Chin J Pract Pediatr. (2021) 36:602–7. doi: 10.19538/j.ek2021080609
61. Ree IM, Smits-Wintjens VE, Rijntjes-Jacobs EG, Pelsma IC, Steggerda SJ, Walther FJ, et al. Necrotizing enterocolitis in small-for-gestational-age neonates: a matched case-control study. Neonatol. (2014) 105:74–8. doi: 10.1159/000356033
62. Neu J, Pammi M. Necrotizing enterocolitis: the intestinal microbiome, metabolome and inflammatory mediators. Semin Fetal Neonatal Med. (2018) 23:400–5. doi: 10.1016/j.siny.2018.08.001
63. Tirone C, Pezza L, Paladini A, Tana M, Aurilia C, Lio A, et al. Gut and lung microbiota in preterm infants: immunological modulation and implication in neonatal outcomes. Front Immunol. (2019) 10:2910. doi: 10.3389/fimmu.2019.02910
64. Yi XL, Zhang BH, Yan CX, Meng L. Research progress on the pathogenesis of neonatal necrotizing enterocolitis. Chin J Neonatol. (2011) 26:130–2. doi: 10.3969/j.issn.1673-6710.2011.02.20
65. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. (1986) 33:179–201. doi: 10.1016/s0031-3955(16)34975-6
66. Baxi AC, Josephson CD, Iannucci GJ, Mahle WT. Necrotizing enterocolitis in infants with congenital heart disease: the role of red blood cell transfusions. Pediatr Cardiol. (2014) 35:1024–9. doi: 10.1007/s00246-014-0891-9
67. Ozcan B, Aydemir O, Isik DU, Bas AY, Demirel N. Severe anemia is associated with intestinal injury in preterm neonates. Am J Perinatol. (2020) 37:603–6. doi: 10.1055/s-0039-1683982
68. Knee D, Knoop S, Davis AT, Rawson B, DiCarlo A, Olivero R. Outcomes after implementing restrictive blood transfusion criteria in extremely premature infants. J Perinatol. (2019) 39:1089–97. doi: 10.1038/s41372-019-0408-8
69. Lu M, Zhu XY, Liu DL, Lu YD, Ben XM. Clinical analysis of risk factors of neonatal necrotizing enterocolitis. Chin J Neonatol. (2012) 27:382–5. doi: 10.3969/j.issn.1673-6710.2012.06.006
70. Tao HK, Tang Q, Hei MY, Yu B. Meta-analysis of post-transfusion necrotizing enterocolitis in neonates. Chin J Pediatr. (2013) 51:336–9.
71. Neu J. Gastrointestinal development and meeting the nutritional needs of premature infants. Am J Clin Nutr. (2007) 85:629s–34s. doi: 10.1093/ajcn/85.2.629S
72. Xiao NR, Liu XH, Chen HM, Sun J, Wu JH, Xiao MH. Effect of donor feeding in breast milk banks on common complications in preterm infants with low body mass. J Pract Med. (2018) 34:1734–6. doi: 10.3969/j.issn.1006-5725.2018.10.039
73. D'Angelo G, Impellizzeri P, Marseglia L, Montalto AS, Russo T, Salamone I, et al. Current status of laboratory and imaging diagnosis of neonatal necrotizing enterocolitis. Ital J Pediatr. (2018) 44:84. doi: 10.1186/s13052-018-0528-3
74. Al-Hosni M, Duenas M, Hawk M, Stewart LA, Borghese RA, Cahoon M, et al. Probiotics-supplemented feeding in extremely low-birth-weight infants. J Perinatol. (2012) 32:253–9. doi: 10.1038/jp.2011.51
75. Cai YJ, Qu LH, Li W, Feng X, Ma LY, Yang BY, et al. A multicenter study on the clinical features and risk factors of poor prognosis in neonatal necrotizing enterocolitis. J Appl Clin Pediatr. (2019) 34:24–9. doi: 10.3760/cma.j.issn.2095-428X.2019.01.006
76. Li TH, Lin XZ. Long-term effects of antenatal corticosteroid therapy on newborns. Chin J Neonatol. (2015) 30:464–7. doi: 10.3969/j.issn.1673-6710.2015.06.019
77. Chen J, Li CF, Song GW. Determination of elimination activity in vitro of bilirubin for the hydroxyl radical by UV-vis spectrophotometry. J Hubei Univ (Nat Sci). (2008) 30:199–201. doi: 10.3969/j.issn.1000-2375.2008.02.024
Keywords: necrotizing enterocolitis, risk factor, meta analysis, neonate, newborn
Citation: Su Y, Xu R, Guo L, Chen X, Han W, Ma J, Liang J, Hao L and Ren C (2023) Risk factors for necrotizing enterocolitis in neonates: A meta-analysis. Front. Pediatr. 10:1079894. doi: 10.3389/fped.2022.1079894
Received: 25 October 2022; Accepted: 13 December 2022;
Published: 6 January 2023.
Edited by:
Shi Yuan, Children's Hospital of Chongqing Medical University, ChinaReviewed by:
Francesco Cresi, University of Turin, ItalyMayank Priyadarshi, All India Institute of Medical Sciences, India
© 2023 Su, Xu, Guo, Chen, Han, Ma, Liang, Hao and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Hao 2475759137@qq.com Chang-Jun Ren 137544907@qq.com
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 Yan Su
Yan Su Rui-Hong Xu
Rui-Hong Xu Li-Yan Guo
Li-Yan Guo Xin-Qing Chen
Xin-Qing Chen  Wen-Xiao Han
Wen-Xiao Han