Severe pneumonia caused by human adenovirus type 55 in children
- 1Department of Respiratory, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China
- 2Center Laboratory, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China
- 3State Key Laboratory of Respiratory Diseases, Guangzhou Medical University, Guangzhou, China
- 4Pediatric Intensive Care Unit, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China
Background: Emerging human adenovirus type 55 (HAdV-55) causes fatal pneumonia in adults. There is a lack of studies on severe pneumonia caused by HAdV-55 in children.
Methods: We conducted a retrospective review of pediatric patients hospitalized at Guangzhou Women and Children’s Medical Center with severe pneumonia from 2013 to 2020 who had human adenovirus (HAdV) detected in throat samples or bronchoalveolar lavage fluid using RT-PCR. The presence of HAdV-55 was determined by PCR amplification of the hypervariable regions of the hexon gene. Demographic, clinical, etiological, and outcome data were collected and analyzed.
Results: Over the eight-year period, HAdV-55 was detected in three severe and six critical pediatric pneumonia patients. None of the patients had any underlying diseases, and had a median age of 18 months (range, 6–108 months). The male to female ratio was 2:1. All patients presented with fever and cough, and three patients presented with wheezing and diarrhea. Six patients had coinfections with other respiratory pathogens, such as bacteria, Mycoplasma pneumoniae and fungi. Three critical patients developed plastic bronchitis (PB). The median lengths of invasive mechanical ventilation and hospital stay of the critical patients were 10 (8, 28.75) days and 25 (13, 32.25) days, respectively. Three critical patients died, although two of them received extracorporeal membrane oxygenation (ECMO) and blood purification. Three surviving patients developed post-infectious bronchiolitis obliterans (PIBO) at the follow-up.
Conclusions: HAdV-55 can cause fatal pneumonia in children, and shows a high rate of co-infection with other respiratory pathogens and a poorer prognosis combined with PB. Thus, HAdV-55 may be an important subtype in patients with HAdV-induced pneumonia who develop PIBO.
Introduction
Human adenovirus (HAdV) is an important pathogen causing community-acquired pneumonia (CAP) in children, accounting for 4%–10% of hospitalized cases (1). With the development of phylogenetic analyses based on complete genomic sequences, novel HAdV types have become increasingly identified and characterized. To date, over 100 HAdV genotypes, classified into seven groups (A–G), have been identified (HAdV Working Group, http://hadvwg.gmu.edu/). Among them, HAdV-3 and HAdV-7 most frequently lead to severe pneumonia in children (2, 3). Inparticular, previous research found that Human adenovirus type 55 (HAdV-55) is emerging as a highly virulent pathogen causing acute fatal adenoviral pneumonia among immunocompetent adults (4, 5). HAdV-55, a variant with a recombination of the hexon gene between HAdV-11 and HAdV-14 strains, has also been reported to be associated with multiple outbreaks of severe respiratory tract infections, mostly occurring in students’ training bases, schools, households, and hospitals (6–9). Different HAdV types have different degrees of infectivity and virulence among children and adults. Although HAdV-55 is not the predominant serotype of adenovirus pneumonia in children in contrast to HAdV-7 and HAdV-3, it can still cause severe or critical pneumonia (3, 10). Indeed, HAdV-55 accounts for 3.1%–6.9% of the hospitalized pediatric patients with acute respiratory disease due to HAdV (3, 10, 11). Besides, current knowledge of HAdV-55-induced severe pneumonia in children is relatively rare. Here we describe the clinical features and outcomes of severe and critical HAdV-55 pneumonia in pediatric patients.
Material and methods
Study population
We retrospectively collected data of pediatric patients hospitalized at Guangzhou Women and Children’s Medical Center with HAdV-55 induced severe pneumonia between 2013 and 2020.
Clinical data collection
Clinical information was collected using a standardized data form, and included demographic characteristics (age and sex), comorbidities, clinical symptoms (fever, cough, sputum, dyspnea, chest pain, rash, nausea, vomiting, abdominal pain, diarrhea, and headache), signs (body temperature, heart rate, respiratory frequency, blood pressure, and crackles in the lungs), laboratory tests (whole-blood cell count and blood chemistry), as well as lung microbiological characterization and imaging (computed tomography). Concomitant medications, respiratory support, complications, and outcomes were registered.
Children with respiratory distress and hypoxemia [sustained saturation of peripheral oxygen (SpO2) <90% at sea level] were considered as having severe pneumonia (12). Children considered as having critical pneumonia had ≥1 of the major or ≥2 of the minor following criteria (12): (1) Major criteria: invasive mechanical ventilation; fluid refractory shock; acute need for non-invasive positive pressure ventilation; hypoxemia requiring fraction of inspired oxygen (FiO2) greater than the inspired concentration or flow feasible in general care area; (2) Minor criteria: respiratory rate greater than the World Health Organization normal classification for age; apnea; increased work of breathing (e.g., retractions, dyspnea, nasal flaring, and grunting), PaO2/FiO2 ratio <250, multilobar infiltrates, pediatric early warning score >6, altered mental status, hypotension, presence of effusion, comorbid conditions (e.g., hemoglobin SS disease, immunosuppression, and immunodeficiency); and unexplained metabolic acidosis.
Sample collection and molecular typing with PCR
Throat swab samples or bronchoalveolar lavage fluid (BAF) were collected from hospitalized pediatric patients with severe or critical pneumonia. The samples were collected and refrigerated at 2–8 °C in a viral transport medium before being transported on ice and analyzed immediately, or stored at −80 °C until analysis. Viral genomic DNA was extracted using a TaKaRa Mini BEST Viral RNA/DNA Extraction Kit Ver.5.0 (TaKaRa, Dalian, China), according to the manufacturer’s instructions and then tested for HAdV using the TaqMan real-time PCR kit (Guangzhou HuYanSuo Medical Technology Co., Ltd., Guangzhou, China) as previously reported (13). HAdV-positive samples were further characterized at the molecular level by PCR amplification of the hypervariable regions of the hexon gene (14, 15). Mycoplasma pneumoniae and respiratory viruses detected by a real-time PCR assay on throat swab sample or BAF. Bacteria and fungi detected by blood, sputum, BAF or bone marrow culture.
Statistical analysis
Non-continuous variables were summarized as median (interquartile range).
Results
Demographics
Over the eight-year period, a total of nine patients with HAdV-55-induced severe pneumonia were admitted to our hospital. All nine patients had a median age of 18 months (range, 6–108 months) and no underlying disease at the time of admission. Five patients were younger than 2 years of age, and four patients were older than 3 years. The male to female ratio was 2:1. Three patients were hospitalized in spring, four in summer, and two in autumn. Five patients were hospitalized in 2019.
Clinical and laboratory characteristics
The clinical characteristics of the patients are summarized in Table 1. All patients had a disease duration >10 days before admission, either because the condition recurred after discharge or the treatment was not satisfactory at the local hospital before they were transferred to our center. All patients presented with cough and mild-to-high fever. Three patients presented with wheezing and diarrhea. Three patients presented with wheezing and moist crackles on lung auscultation.
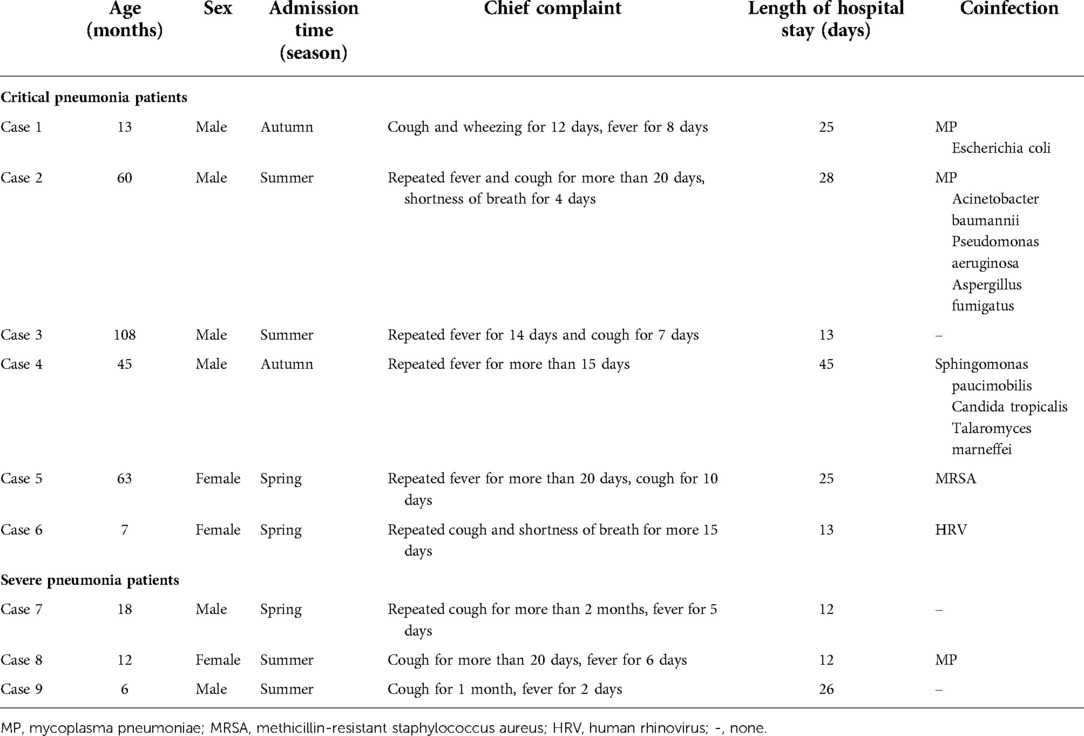
Table 1. The clinical characteristics of human adenovirus type 55 infection in severe and critical pediatric pneumonia patients.
According to the determination criteria, patients 1–6 had critical pneumonia and patients 7–9 had severe pneumonia. Patients with severe pneumonia included two males and one female, with a median age of 12 months (range, 6–18 months). Patients with critical pneumonia included four males and two females, with a median age of 52.5 months (range, 7–108 months). Four critical patients were older than three years of age. The patients’ laboratory characteristics are shown in Table 2.
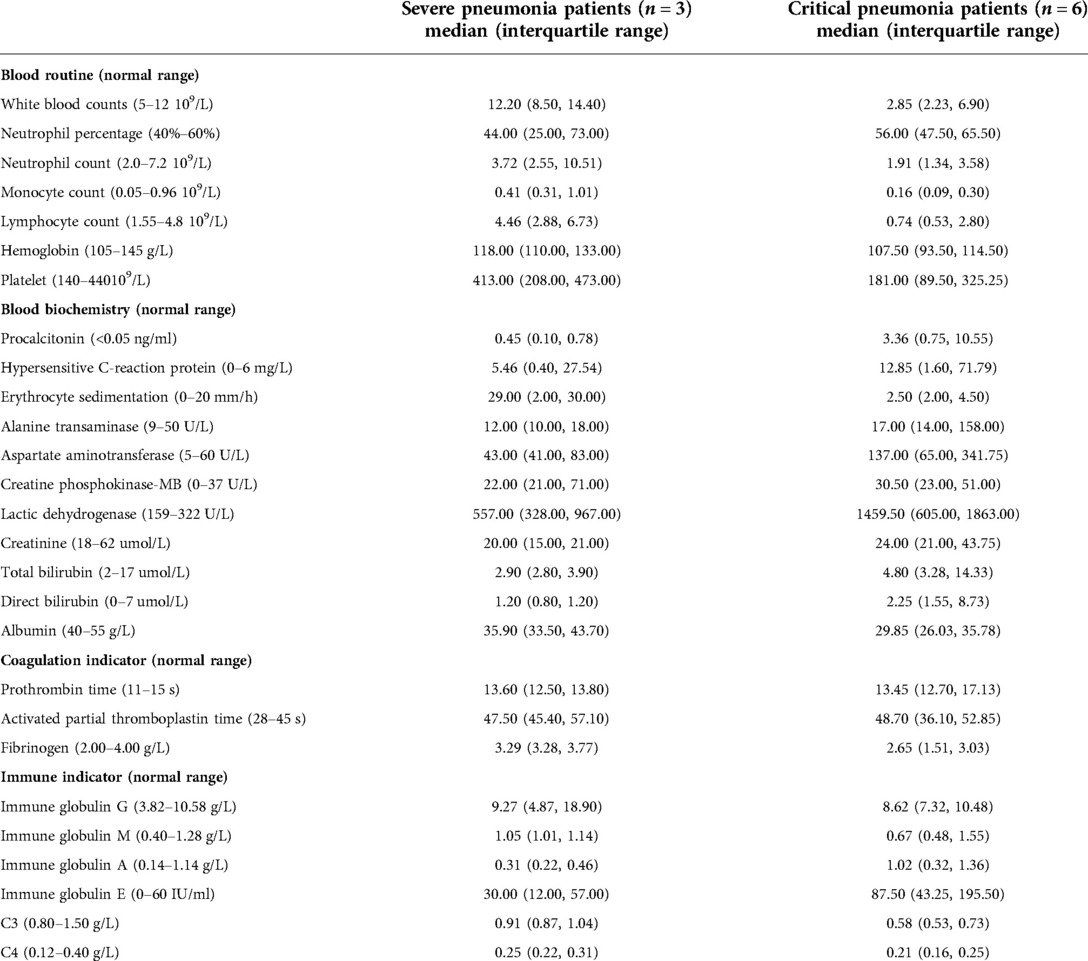
Table 2. The laboratory characteristics of human adenovirus type 55 infection in severe and critical pediatric pneumonia patients on admission.
Microbiological investigation
The co-infections of HAdV-55 with other respiratory pathogens is shown in Table 1. Six patients presented co-infections, of which five were critical. Among the six co-infected patients, three were coinfected with one pathogen, and the other three with more than two pathogen types. The co-infection was of bacterial origin in four patients, Mycoplasma pneumoniae derived in three patients, and induced by fungi with Aspergillus fumigatus and Talaromyces marneffei in two cases respectively.
Chest computed tomography findings
All patients underwent chest computed tomography (CT) scan. The chest CT features were mainly patchy shadows (6/9), lung consolidation (6/9), pleural effusion (5/9), and mediastinal and hilar lymph node enlargement (3/9). Ground-glass opacity was observed in one patient. Among the patients with pulmonary consolidation, four had single lobe consolidation and two had multi-lobe consolidation.
Fiberoptic bronchoscopy findings
Fiberoptic bronchoscopy (FB) and bronchoalveolar lavage were performed in seven patients. Of those, three revealed a whitish rubbery material occluding the right inferior lobar bronchus (patient 2), multi-lobar bronchus (patient 4), and left inferior lobar bronchus (patient 5), which were considered to be associated with plastic bronchitis (PB). One patient was confirmed to have PB, however the plastic cast was removed successfully through lavage and negative pressure suction. In the other two patients, the plastic cast failed to be successfully removed, therefore the patients were clinically considered to have PB, and refractory hypoxemia could not be improved by invasive mechanical ventilation (IMV). None of the patients were able to undergo multiple bronchoscopic alveolar lavages to remove PB because such an operation under IMV would aggravate hypoxia.
Treatment, outcome and sequelae
Among the three patients with severe pneumonia, two required oxygen support through inhalation and received 2 mg/kg/day methylprednisolone during 3–5 days for persistent wheezing. All three patients received intravenous immunoglobulin (1 g/kg/d for 2 days) to alleviate inflammatory storms. The average length of hospital stay of these patients was 16.67 ± 8.08 days.
Treatments and outcomes for the critically ill patients are shown in Table 3. Intravenous methylprednisolone was administered to two patients. All patients received either intravenous immunoglobulin, antibiotics, or antifungal therapy. All critically ill patients with respiratory failure received IMV for respiratory support, with a median IMV length of 10 (8, 28.75) days. The median length of pediatric intensive care unit (PICU) and hospital stay was 17 (8.25, 32.25) and 25 (13, 32.25) days respectively. Ultimately, three critical patients died, although two of them had received extracorporeal membrane oxygenation (ECMO) and blood purification. The detailed causes of death in these three patients are shown in Table 3.
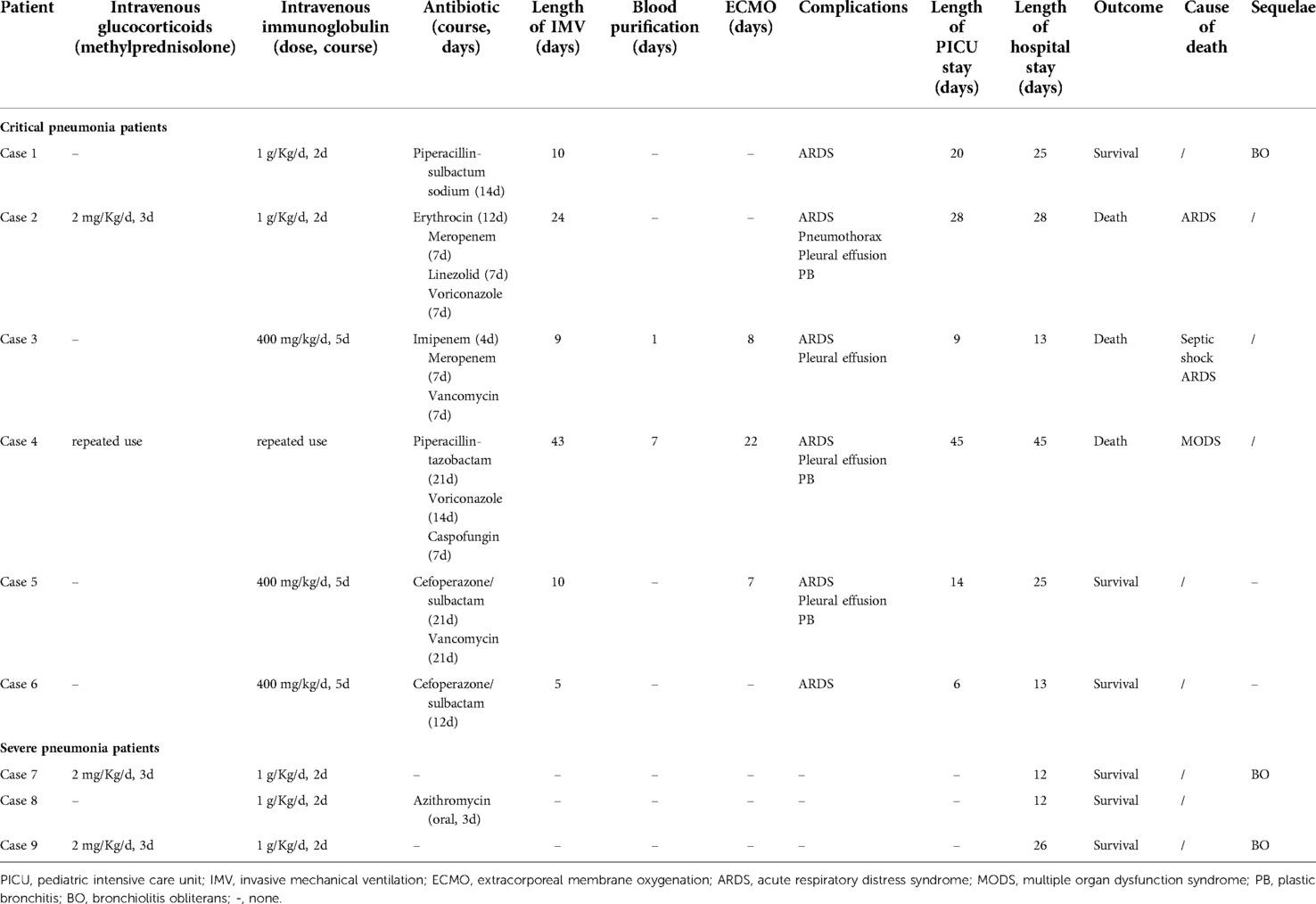
Table 3. Treatment and outcomes for the severe and critical pediatric pneumonia patients with human adenovirus type 55 infection.
Three surviving patients developed post-infectious bronchiolitis obliterans (PIBO) during the follow-up, including two patients with severe pneumonia and one with critical pneumonia. One of them has been symptom-free for 8 years. The other two presented wheezing after physical activity, regularly received inhaled glucocorticoid therapy and have been followed up for 2 years. Chest CT of all patients showed mosaic perfusion and air trapping during follow-up (Figure 1).
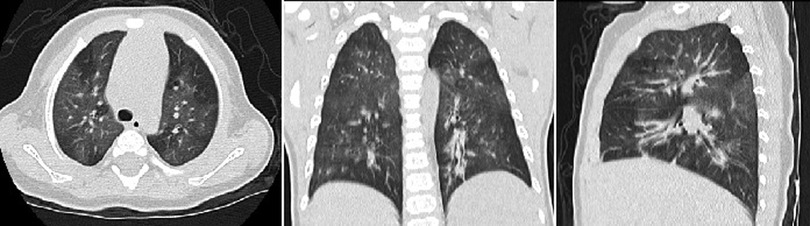
Figure 1. The chest computed tomography of the patient 7 showed mosaic perfusion and air trapping for bronchiolitis obliterans at the follow-up.
Discussion
The number of children with HAdV-induced CAP has increased significantly due to an epidemic of HAdV infection occurred in Southern China in 2019. During the study period, 55.56% of the patients diagnosed with HAdV-55-induced CAP were infected in 2019. Previous studies have found that HAdV-55 can cause fatal pneumonia in immunocompetent adults (4, 5). Although HAdV-55 can cause severe pediatric pneumonia, it is rarely fatal (3, 10). In the present study, 66.67% of the patients were critical and required PICU admission and IMV. In this regard, although HAdV-3 and HAdV-7 are the most frequent agents causing severe HAdV pneumonia in children, HAdV-55, although less frequently, can also cause severe or fatal pneumonia in children. Thus, pediatricians and PICU staff should recognize HAdV-55 as a potential cause of critical illness.
Epidemiological surveys showed that most children with HAdV pneumonia are younger than 5 years of age, and 50% of the pediatric patients with severe HAdV pneumonia are younger than 2 years of age (10, 16). This may occur because the immune systems of young children are not well-developed, which makes them prone to more severe HAdV disease. In our study, the patients with severe pneumonia were all younger than 2 years of age, however four patients with critical pneumonia were older than 3 years of age. The sample size of this study may be too small to truly reflect the age distribution of severe pneumonia caused by HAdV-55 infection. Meanwhile, HAdV-infected children with underlying diseases are prone to develop severe forms (17). None of the patients included in this study had previous serious underlying diseases such as immunodeficiency, bronchopulmonary dysplasia, premature birth, atopy, or congenital heart disease. In particular, patient 4 was co-infected with Talaromyces marneffei which is characteristic of immunocompromised patients, however he was confirmed not to have immunodeficiency by whole gene sequencing. In addition to age, the potential factors leading to critical illness derived from of HAdV-55 are so far undetermined.
The most common clinical manifestations of pediatric HAdV-55-induced pneumonia are fever, cough, and wheezing. However, differences in the relative frequency of symptoms have been reported, such as a higher proportion of patients presenting wheezing and diarrhea in our study than previously published (3). In our study, patients with critical HAdV-55-induced pneumonia manifested shortness of breath, progressive dyspnea, and persistent hypoxia, which were consistent with reported symptoms in adults (18). For patients with severe HAdV-55-induced pneumonia, laboratory tests showed no obvious alterations. However, for critical patients, there were several evident abnormalities in laboratory analyses, such as a decline in white blood cell counts mainly due to a decrease in lymphocytes, increased hypersensitive C-reaction protein and procalcitonin relative to severe cases. Controlled studies showed that the majority of clinical symptoms and signs as well as blood parameters did not significantly differ between HAdV-55 and HAdV-7, with the exception of wheezing, which was higher in patients with HAdV-7-induced pneumonia (3). Concerning laboratory results, in a controlled study of subjects over 7 years of age, the detection rate of procalcitonin (PCT) >1.0 ng/ml, aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) levels were significantly higher in the HAdV-55 group than in the HAdV-7 group, whereas the level of serum albumin was lower. Besides, the HAdV-55 group presented a higher number of severe patients (5). In summary, HAdV-55 is a relevant pathogen causing severe pneumonia in children. A more severe condition is associated with more obvious alterations in laboratory indicators.
Co-infections occurred frequently in our patients. Six patients were co-infected with additional respiratory pathogens. However, when comparing between subgroups, the incidence of co-infection in critical patients was higher than that in severe patients (83.3% vs. 33.3%). Among these patients, three had Mycoplasma pneumoniae infection. M. pneumoniae is not only the most prevalent organism in co-infection with HAdV, but is also known to easily lead to severe illness when co-infected with HAdV in pediatric patients (19). Other combinations of pathogenic co-infections may have influenced the development of a critical condition in our patients. Some studies have reported that HAdV-55 is associated with other respiratory viral infections (3, 10). However, most of our patients were co-infected with bacteria, M. pneumoniae, and fungi. Moreover, two patients who were co-infected with Aspergillus fumigatus and Talaromyces marneffei died. Complications derived from fungal co-infections do worsen the condition and may be lethal. In this sense, the detection of fungi should be performed as soon as possible for critical patients, as timely and standardized antifungal treatment may improve prognosis.
To our knowledge, pediatric patients with PB secondary to HadV-55 have not been reported. Three critical patients had complications with PB, including one case with a definite diagnosis and two cases with a clinical diagnosis. PB is a rare critical disease characterized by the formation of plastic casts of the bronchial tree that obstruct the airways. HAdV-associated PB is mainly observed in case reports and is caused by HAdV-7 (20–22). Removal of plastic casts through FB is the main treatment for children with PB; however, for critical patients, oxygen support, respiratory support (IMV or non-invasive ventilation), and even ECMO are required (20–22). FB in critically ill patients increases the risk of airway obstruction which may lead to aggravating hypoxia. In fact, 70% of patients with HAdV-associated PB require more than two rounds of FB therapy during hospitalization (20). Two of our patients with PB failed to have their casts removed through FB under IMV and were unable to tolerate prolonged FB along with negative pressure suction. In actual clinical practice, the frequency and length of FB examinations need to consider the patient’s tolerance. Even under the current standard treatment, HAdV combined with PB has a high mortality rate (20). Regarding our patients, two among those with PB who had respiratory failure died. These patients, in addition to respiratory failure, also had high ventilator parameters, prolonged IMV, and hypoxia, which may lead to failure of other organs.
It is generally known that HAdV infection predominates in PIBO, which is a chronic obstruction of the airflow associated with inflammatory lesions of the small airways (16, 23). PIBO is the classical long-term sequela of HAdV infection, mainly observed in HAdV-7 and HAdV-3 infections (24). PIBO associated with HAdV-55 infections is relatively rare. Three patients (33.3%) developed PIBO in our study, consistent with that 10.7%–40% of hospitalized patients with HAdV that developed PIBO according to the literature (16, 25, 26). Consistently, these three patients had risk factors for PIBO, such as persistent wheezing or respiratory failure during a severe pneumonia episode. Thus, HAdV-55 is a relevant subtype in patients with HAdV pneumonia who develop PIBO.
This study had several limitations. First, this was a single-center, small sample study. We did not routinely perform virus typing for mild adenovirus pneumonia. In addition, we did not compare clinical features with those of children with HAdV-7 infection, which is the most common cause of severe pneumonia in children. It is difficult to evaluate the severity of the patient’s condition caused by HAdV-55 or other pathogens due to co-infections. The failure to exclude the influence of co-infection on the results is one of the limitations of this study. Future researchers should adjust the variable of co-infection in the experimental design.
Conclusion
Our data provides new insights into the clinical features of severe HAdV-55 pneumonia in pediatric patients. HAdV-55 can cause fatal pneumonia in healthy children. Severe, especially critical, patients showed a high rate of co-infection with other respiratory pathogens. Thus, HAdV-55 may be a relevant subtype in patients with HAdV-induced pneumonia who develop PIBO.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Guangzhou Women and Children’s Medical Centre, Guangzhou Medical University [No. 202063711]. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
DZ, YC and GL designed the study and wrote the manuscript. TS, HF and LH gathered the data. Material preparation and data analysis were performed by XT, RZ and DY. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank all patients and their families involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jobran S, Kattan R, Shamaa J, Marzouqa H, Hindiyeh M. Adenovirus respiratory tract infections in infants: a retrospective chart-review study. Lancet. (2018) 391(Suppl 2):S43. doi: 10.1016/S0140-6736(18)30409-4
2. Xie L, Zhang B, Xiao N, Zhang F, Zhao X, Liu Q, et al. Epidemiology of human adenovirus infection in children hospitalized with lower respiratory tract infections in Hunan, China. J Med Virol. (2019) 91:392–400. doi: 10.1002/jmv.25333
3. Xu L, Liu J, Liu C, Duan Y, Zhu Y, Xu B, et al. Case-control study of the epidemiological and clinical features of human adenovirus 55 and human adenovirus 7 infection in children with acute lower respiratory tract infections in Beijing, China, 2008–2013. BMC Infect Dis. (2018) 18:634. doi: 10.1186/s12879-018-3520-z
4. Cao B, Huang GH, Pu ZH, Qu JX, Yu XM, Zhu Z, et al. Emergence of community-acquired adenovirus type 55 as a cause of community-onset pneumonia. Chest. (2014) 145:79–86. doi: 10.1378/chest.13-1186
5. Zhu Q, Chen S, Gu L, Qu J. Comparative analyses of clinical features reveal the severity of human adenovirus type 55 and type 7 in acute respiratory tract infections. J Med Microbiol. (2021) 70(12). doi: 10.1099/jmm.0.001445
6. Lu G, Peng X, Li R, Liu Y, Wu Z, Wang X, et al. An outbreak of acute respiratory infection at a training base in Beijing, China due to human adenovirus type B55. BMC Infect Dis. (2020) 20:537. doi: 10.1186/s12879-020-05258-2
7. Li P, Wang K, Qiu S, Lin Y, Xie J, Li J, et al. Rapid identification and metagenomics analysis of the adenovirus type 55 outbreak in Hubei using real-time and high-throughput sequencing platforms. Infect Genet Evol. (2021) 93:104939. doi: 10.1016/j.meegid.2021.104939
8. Jing S, Zhang J, Cao M, Liu M, Yan Y, Zhao S, et al. Household transmission of human adenovirus type 55 in case of fatal acute respiratory disease. Emerg Infect Dis. (2019) 25:1756–8. doi: 10.3201/eid2509.181937
9. Yi L, Zou L, Lu J, Kang M, Song Y, Su J, et al. A cluster of adenovirus type B55 infection in a neurosurgical inpatient department of a general hospital in Guangdong, China. Influenza Other Respir Viruses. (2017) 11:328–36. doi: 10.1111/irv.12457
10. Lu QB, Tong YG, Wo Y, Wang HY, Liu EM, Gray GC, et al. Epidemiology of human adenovirus and molecular characterization of human adenovirus 55 in China, 2009–2012. Influenza Other Respir Viruses. (2014) 8:302–8. doi: 10.1111/irv.12232
11. Chen SY, Liu W, Xu Y, Qiu S, Chen Y, Tian X, et al. Epidemiology and genetic variabilities of human adenovirus type 55 reveal relative genome stability across time and geographic space in China. Front Microbiol. (2020) 11:606195. doi: 10.3389/fmicb.2020.606195
12. Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the pediatric infectious diseases society and the infectious diseases society of America. Clin Infect Dis. (2011) 53:e25–76. doi: 10.1093/cid/cir531
13. Liu WK, Chen DH, Liu Q, Liang HX, Yang ZF, Qin S, et al. Detection of human bocavirus from children and adults with acute respiratory tract illness in Guangzhou, Southern China. BMC Infect Dis. (2011) 11:345. doi: 10.1186/1471-2334-11-345
14. Lu X, Erdman DD. Molecular typing of human adenoviruses by PCR and sequencing of a partial region of the hexon gene. Arch Virol. (2006) 151:1587–602. doi: 10.1007/s00705-005-0722-7
15. Han G, Niu H, Zhao S, Zhu B, Wang C, Liu Y, et al. Identification and typing of respiratory adenoviruses in Guangzhou, Southern China using a rapid and simple method. Virol Sin. (2013) 28:103–8. doi: 10.1007/s12250-013-3308-7
16. Yu X, Ma Y, Gao Y, You H. Epidemiology of adenovirus pneumonia and risk factors for bronchiolitis obliterans in children during an outbreak in jilin, China. Front Pediatr. (2021) 9:722885. doi: 10.3389/fped.2021.722885
17. Dotan M, Zion E, Bilavsky E, Nahum E, Ben-Zvi H, Zalcman J, et al. Adenovirus can be a serious, life-threatening disease, even in previously healthy children. Acta Paediatr. (2022) 111:614–9. doi: 10.1111/apa.16207
18. Sun B, He H, Wang Z, Qu J, Li X, Ban C, et al. Emergent severe acute respiratory distress syndrome caused by adenovirus type 55 in immunocompetent adults in 2013: a prospective observational study. Crit Care. (2014) 18:456. doi: 10.1186/s13054-014-0456-6
19. Wei J, Wu S, Jin X, Zhang J, Pan S. Association of Mycoplasma pneumoniae coinfection with adenovirus pneumonia severity in children. Allergol Immunopathol. (2022) 50:31–6. doi: 10.15586/aei.v50i1.476
20. Zeng L, Wei J, Tang Y, Liu E, Li Q, Zang N. Clinical characteristics of human adenovirus plastic bronchitis in 10 pediatric cases: a retrospective study of seven years. Virol Sin. (2021) 36:550–4. doi: 10.1007/s12250-021-00394-8
21. Zhang FZ, Qin L, Yuan JX, Tang LF. Plastic bronchitis due to adenoviral infection: a case report. BMC Pediatr. (2020) 20:61. doi: 10.1186/s12887-020-1954-0
22. Yuan L, Huang JJ, Zhu QG, Li MZ, Zhuo ZQ. Plastic bronchitis associated with adenovirus serotype 7 in children. BMC Pediatr. (2020) 20:268. doi: 10.1186/s12887-020-02119-4
23. Chan KC, Yu MW, Cheung T, Lam D, Leung T, Tsui TK, et al. Childhood bronchiolitis obliterans in Hong Kong-case series over a 20-year period. Pediatr Pulmonol. (2021) 56:153–61. doi: 10.1002/ppul.25166
24. Yu J. Postinfectious bronchiolitis obliterans in children: lessons from bronchiolitis obliterans after lung transplantation and hematopoietic stem cell transplantation. Korean J Pediatr. (2015) 58:459–65. doi: 10.3345/kjp.2015.58.12.459
25. Castro-Rodriguez JA, Daszenies C, Garcia M, Meyer R, Gonzales R. Adenovirus pneumonia in infants and factors for developing bronchiolitis obliterans: a 5-year follow-up. Pediatr Pulmonol. (2006) 41:947–53. doi: 10.1002/ppul.20472
Keywords: human adenovirus type 55, severe pneumonia, critical pneumonia, clinical feature, children
Citation: Zhang D, Chen Y, Shi T, Fan H, Tian X, Zhou R, Huang L, Yang D and Lu G (2022) Severe pneumonia caused by human adenovirus type 55 in children. Front. Pediatr. 10:1002052. doi: 10.3389/fped.2022.1002052
Received: 24 July 2022; Accepted: 27 September 2022;
Published: 13 October 2022.
Edited by:
Bülent Taner Karadağ, Marmara University, TurkeyReviewed by:
Yongdong Yan, Children’s Hospital of Soochow University, ChinaZhengde Xie, Capital Medical University, China
© 2022 Zhang, Chen, Shi, Fan, Tian, Zhou, Huang, Yang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gen Lu lugen5663330@sina.com
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Pediatric Pulmonology, a section of the journal Frontiers in Pediatrics
 Dongwei Zhang
Dongwei Zhang Yi Chen2,†
Yi Chen2,†  Tingting Shi
Tingting Shi Huifeng Fan
Huifeng Fan Rong Zhou
Rong Zhou Li Huang
Li Huang Diyuan Yang
Diyuan Yang Gen Lu
Gen Lu