Clinical and Laboratory Characteristics of Kikuchi-Fujimoto Disease According to Age
- 1Department of Pediatrics, Medical Research Institute, Pusan National University School of Medicine, Pusan National University Hospital, Busan, South Korea
- 2Department of Pediatrics, Pusan National University Hospital, Busan, South Korea
- 3Department of Pediatrics, Seoul National University Children's Hospital & College of Medicine, Seoul, South Korea
Background: Little information exists regarding the differences in the clinical and laboratory characteristics of Kikuchi-Fujimoto disease (KFD) according to age.
Objective: To evaluate the clinical and laboratory characteristics of KFD according to age.
Methods: The relevance of sex, age, clinical features, laboratory findings, courses, and follow-up results were retrospectively evaluated in patients diagnosed with KFD at Pusan National University Hospital between 2010 and 2020.
Results: Eighty patients (46 children and 34 adults) with a mean age of 21.5 ± 11.8 years (range, 3–49 years) were included in the study. Those aged 10–19 years accounted for the largest number of patients (42.5%). Among children, the male sex ratio was higher, especially for patients aged ≤ 9 years. In adults, the female sex ratio was higher, especially for patients aged 20–29 years. Fever, tenderness in the lymph node, and skin rashes were more common in children, while myalgia and weight loss were more common in adults. In children, the recurrence rate was significantly higher among boys than among girls (15.8 vs. 0.0%, P = 0.001); lower platelet count and higher CRP levels were observed among boys than among girls. EBV and ANA positivity rates were higher in boys than in girls. In adults, the recurrence rate was significantly higher in women than in men (18.2 vs. 0.0%, P = 0.005). ANA positivity rates were higher in women than in men.
Conclusion: The clinical features, laboratory findings, and recurrence of KFD may differ depending on age and sex. Clinicians should be aware of this.
Introduction
Kikuchi-Fujimoto disease (KFD), also called Kikuchi disease or histiocytic necrotizing lymphadenitis, is a rare, generally self-limiting condition of unknown cause, usually characterized by cervical lymphadenopathy, fever, and leukopenia (1). As the symptoms are non-specific, differential diagnoses, including viral infections, malignancies, and autoimmune conditions such as systemic lupus erythematosus (SLE), are often considered. The diagnosis of KFD is usually based on lymph node histology, which features variably confluent paracortical necrosis surrounded by a prominent collar of histiocytes with crescentic nuclei, immunoblasts, and plasmacytoid monocytes.
While the pathogenesis of Kikuchi disease is unknown, the clinical presentation, course, and histologic changes suggest an immune response of T cells and histiocytes to an infectious agent. Infectious agents, including Yersinia, Toxoplasma, Epstein–Barr virus, human herpesvirus 6 and 8, human T-lymphotropic virus type 1, and parvovirus B19 have been reported to play a causative role, but this has not been confirmed (2, 3).
Although KFD affects all age and sex groups, the clinical features of KFD may differ according to age and sex. It was primarily thought to be a disease affecting women under the age of 30 years. However, in a Korean report of 20 individuals younger than 18 years of age with Kikuchi disease, the sex distribution is equal (4). Others have suggested that, among children, boys are slightly more frequently affected than girls, in contrast to older patients (5, 6). In addition, KFD has a reported recurrence rate of 3–4% (7), but pediatric studies have shown a higher recurrence rate of up to 42.4% (8–10).
The age- and sex-related differences in KFD characteristics are not yet fully understood. Many KFD studies have focused on adults; thus, there is little information on the differences in the presentation of KFD according to age. Therefore, it is necessary to understand the differences in the clinical and laboratory features of KFD according to age and sex. We analyzed the clinical and laboratory characteristics of patients with KFD according to age and sex.
Materials and Methods
We reviewed the medical records of KFD patients who were diagnosed between January 2010 and September 2020 at Pusan National University Hospital. Our university hospital is a reference center for six million inhabitants in Busan and Gyeongnam, Korea. The study protocol was approved by the institutional review board of Pusan National University Hospital (PNUHIRB 2012-032-098). The diagnosis of KFD was made on the basis of histopathologic findings of affected lymph nodes obtained by fine-needle aspiration (FNA) or excisional biopsy after exclusion of other diseases, such as multifocal necrosis in the paracortical area with karyorrhectic debris and various histiocytes with crescentic nuclei in the absence of neutrophils (11–13). Most of our pathology results were as follows: (1) Karryorhectic debris, necrosis, and histiocytes, suspicious for histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto disease) (2) Histiocytic necrotizing lymphadenitis, consistent with Kikuchi disease. Immunohistochemistry of CD3, CD20, and Ki67 for the lymphoid tumor was done and revealed mostly reactive patterns. Unfortunately, we did not perform the Epstein-Barr virus (EBV)-encoded RNA in-situ hybridization.
Initially, FNA was performed in 68 patients, and an excisional biopsy was performed in 12 patients. FNA cytology was used as a diagnostic test in 61 of 68 patients, and the remaining seven patients required an additional excisional biopsy. The decision on the performance and timing of the histopathologic confirmation was made depending on the patient's condition and the severity and duration of their symptom manifestations. Clinical features, laboratory findings, courses, and follow-up results were collected and analyzed. Subjects under 19 years of age were defined as children, and those above or equal to 19 years of age were defined as adults. EBV positivity was defined when the EBV IgM viral capsid antigen (VCA) or EBV polymerase chain reaction (PCR) blood assays were positive.
Statistical analysis was performed using SPSS for Windows (version 21.0; SPSS, Chicago, IL, USA). The data are expressed as means ± standard deviations or as percentages where appropriate. The clinical and demographic data were compared between groups using the chi-square analysis and the Mann-Whitney U-test. P < 0.05 were considered statistically significant.
Results
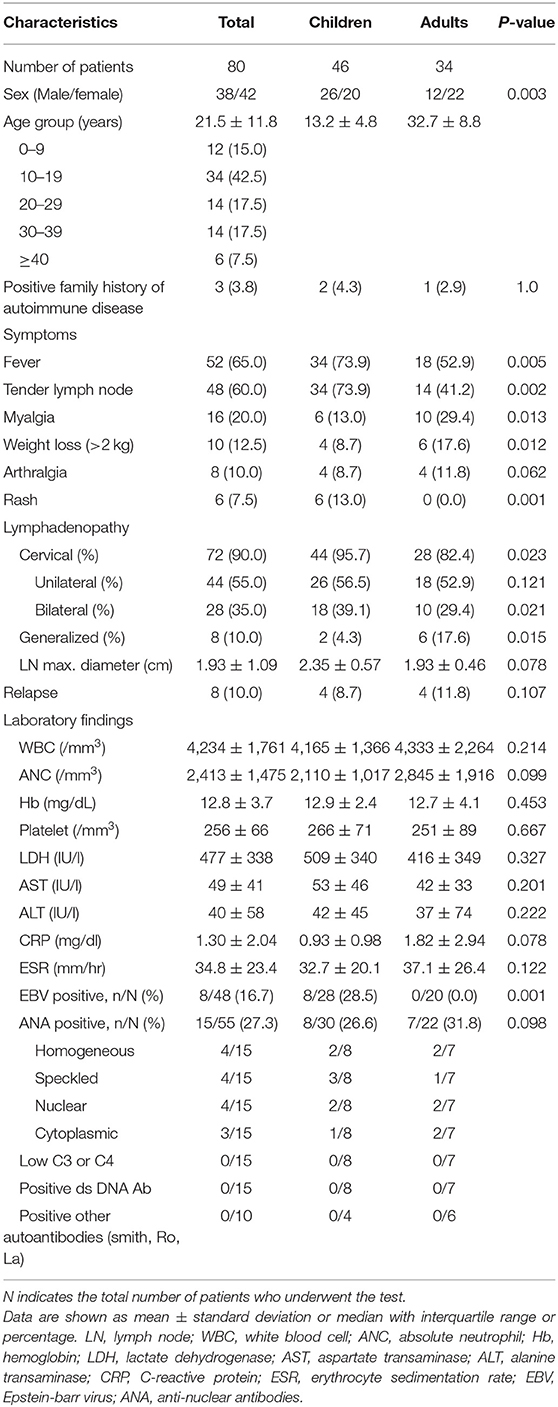
A total of 80 patients (46 children and 34 adults) were included in the study. Twenty (43.5%) children and 22 (64.7%) adults were female, and the proportion of female patients was significantly higher among adults than among children (P = 0.003) (Table 1). The mean age of the study subjects was 21.5 ± 11.8 years (range, 3–49 years). The mean age was 13.2 ± 4.8 years for children and 32.7 ± 8.8 years for adults. Among the 80 patients, the age distribution was as follows: 12 were (15.0%) aged ≤ 9, 34 (42.5%) were aged 10–19, 14 (17.5%) were aged 20–29, 14 (17.5%) were aged 30–39, and 6 (7.5%) were aged ≥ 40 years. Those aged between 10 and 19 years accounted for the largest number of patients (42.5%). Two children and one adult had a positive family history of autoimmune diseases, all of which were SLE.
Fifty-two patients (65.0%) had a fever, and tender lymph nodes were observed in 60.0% of patients. Fever and tender lymph nodes were far more prevalent in children than in adults (73.9 vs. 52.9%, P = 0.005; 73.9 vs. 41.2%, P = 0.002, respectively). Myalgia and weight loss (>2 kg) occurred in 13.0 and 8.7% of the children and in 29.4 and 17.6% of the adults, respectively. These symptoms were significantly higher in adults than in children (P = 0.013 and P = 0.012, respectively). Although rash was not common, it was more common in children (13.0 vs. 0%, P = 0.001). Most skin lesions in children were like non-specific erythematous maculopapular rashes, mainly on the face trunk and upper extremities accompanied by mild itching.
All patients presented with lymphadenopathy, with cervical nodes involved in 72 (90.0%) patients, axillary nodes involved in 7 (8.8%) patients, inguinal nodes involved in 5 (6.3%), mesenteric nodes involved in 2 (2.5%), and generalized lymphadenopathy involving two or more anatomic sites in eight (10.0%) patients. While cervical lymphadenopathy, especially bilateral lymphadenopathy, was more common in children than in adults, generalized lymphadenopathy was more common in adults than in children (Table 1). There was no difference in lymph node size between the children and adults. There were four recurrent cases in children and adults, respectively. The rate of EBV positivity was higher in children than in adults (28.5 vs. 0.0%, P = 0.001). The other laboratory findings, including WBC, Hb, platelet, AST ALT, C-reactive protein (CRP), ESR, and antinuclear antibody (ANA) positivity rates, were not different between the two groups (Table 1).
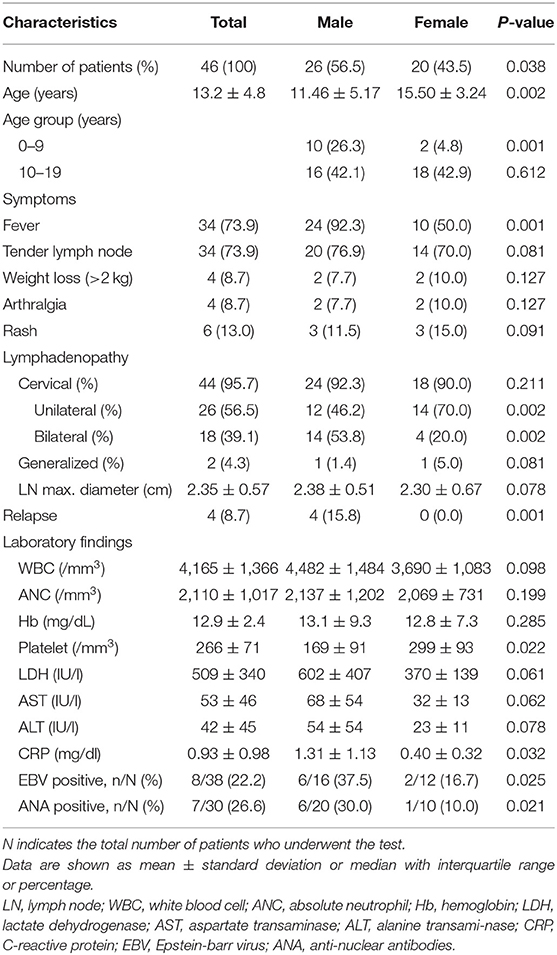
In children, the male sex ratio was higher, especially for patients aged ≤ 9 years (Table 2). Fever was more common in boys than in girls (92.3 vs. 50.0%, P = 0.002). Bilateral cervical lymphadenopathy was observed more frequently in boys than in girls (53.8 vs. 20.0%, P = 0.001). The recurrence rate was also significantly higher in boys than in girls (15.8 vs. 0.0%, P = 0.001). In boys, we found lower platelet counts and higher CRP levels than in girls. Furthermore, EBV and ANA positivity rates were higher in boys than in girls (Table 2).
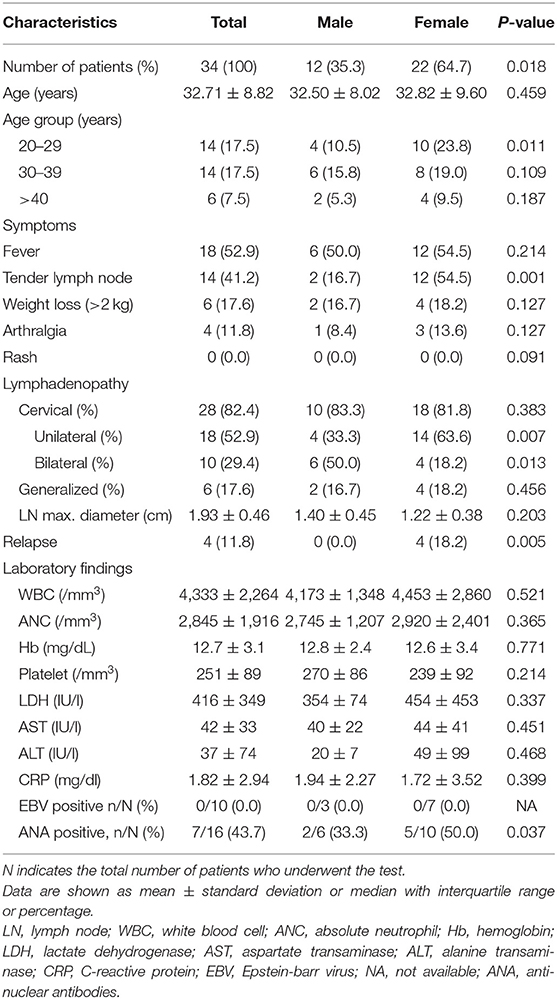
In adults, the female sex ratio was higher, especially for patients aged 20–29 years (Table 3). Tender lymph nodes were more common in women than in men (54.5 vs. 16.7%, P = 0.001). Unilateral cervical lymphadenopathy was observed more frequently in women than in men (63.6 vs. 33.3%, P = 0.007). The recurrence rate was also significantly higher in women than in men (18.2 vs. 0.0%, P = 0.005). ANA positivity rates were higher in women than in men (Table 3).
Most patients received non-steroidal anti-inflammatory drugs (NSAIDs) as a first-line treatment for KFD. Ibuprofen (30–40 mg/kg in three divided doses) was given in most young children and naproxen (10–20 mg/kg, two divided doses, max 1,000 mg/day) in older children and adults. Corticosteroid treatment was added if the fever did not improve even 2–5 days after starting the NSAIDs. The usual dose of oral corticosteroid (prednisolone) was 1 mg/kg (max 60 mg) in three divided and used for 3–10 days in 15 adults and 18 children. There were no patients requiring different therapies other than oral steroids and NSAIDs. In some patients (11 adults and 8 children), the symptoms were improved without any specific treatment. In the case of relapse, treatment was performed at each time of relapse in a similar way.
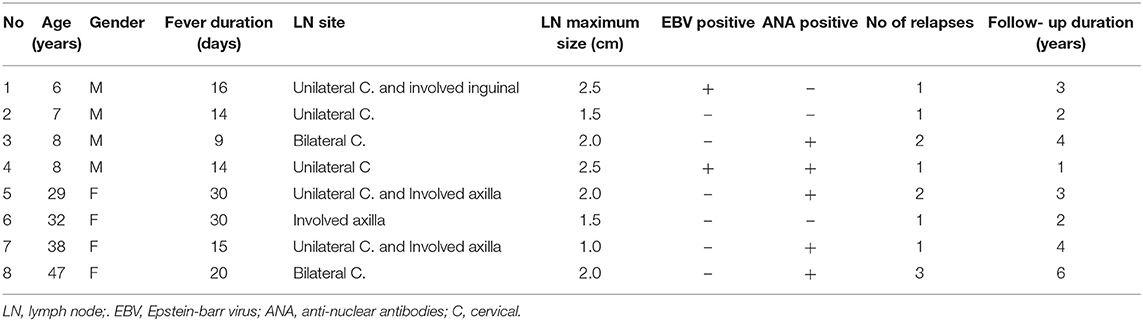
The clinical characteristics of the patients with Kikuchi disease recurrence are described in Table 4. There were four children and four adults. All children were male, while all adults were female. The range of fever duration was 9–30 days. Two children were EBV-positive. ANA positive results were observed in two children and three adults.
Discussion
KFD has a broad clinical spectrum, including fever and cervical lymphadenopathy, the most common symptoms. Typically, patients with KFD present clinically as fever and posterior cervical lymphadenopathy. These patients can have leukopenia as a clue. In our study, we investigated the clinical and laboratory characteristics and KFD according to age and sex. We found that there were some differences in clinical patterns with age. The prevalence of KFD is known to be higher in Asian and Eastern European populations (1, 3, 14). KFD usually occurs in the 30s and 40s and has a female predominance with a ratio of ~3–4:1 in young adults (15); however, in children, there are inconsistent results in terms of sex differences, although it seems that female predominance, as seen in young adults, is not evident (2, 6, 16–18). Moreover, some studies reported male predominance in children (2, 17, 18). Kim et al. showed a male predominance under the age of 14 (1:1.6) and a female predominance over the age of 15 (3:1) (2). This difference may be related to race and the small number of pediatric patients in the studies. To the best of our knowledge, there have been few papers comparing adults and children in one institution, and our study has the advantage of being performed under controlling the variables of race and region. We found male predominance among children and female predominance among adults in one institution, and these differences were statistically significant. Although the exact pathophysiology of KFD is still unknown, it may be assumed that there are different pathological mechanisms in children compared to those in adults. The youngest child was 3 years old, with a mean age of 13.2 years, similar to previous studies (6, 15, 17).
We identified differences in symptoms and laboratory findings between children and adults. Fever, tenderness in the lymph node, and skin rashes were more common in children, while myalgia and weight loss were more common in adults, with more systemic inflammatory symptoms in children than in adults. Lymphadenopathy was less common in children. These results are almost consistent with those of previous studies by Kim et al. (2). Although it is not clear why there were more systemic symptoms in children, we suspect that differences in immune responses between children and adults, roles of sex hormones, and infection frequencies may play a role; however, further research is needed. In a study comparing clinical-cytological features in adults and children, children were significantly less likely to have high cellularity and Kikuchi histiocyte counts >5% than adults (19).
Our study also showed that the positive rate of EBV, one of the trigger factors of KFD, was higher in children, particularly among boys compared to adults. Although ANA positivity was higher in women than in men, ANA positivity was higher in boys than in girls. ANA-positive findings in boys are suspected to be related to EBV infection rather than persistent autoimmunity. EBV during the acute infection or reactivation phase could lead to the formation of the ANA and extractable nuclear antigen autoantibodies (ENA) (20). Although there were no patients with overt systemic lupus erythematosus (SLE) during the study period, careful observation and follow-ups are required due to the fact that autoimmunity due to EBV infection could be related to the development of SLE (21, 22).
SLE is a close differential diagnosis in patients with KFD. We should be careful if SLE occurs during follow-up, especially in the case of positive ANA, because some patients with KFD have associated lupus or develop lupus on follow-up (1.3~25%) (23, 24). Monogenic lupus is a kind of SLE that generally presents early in life, usually at <5 years of age. Recently, a significant number of genes have been implicated in monogenic lupus, such as several complement deficiencies, ACP5, DNASE1, DNASE1L3, PRKCD, RAG2 genes, etc. (25). An interesting association between C1q deficiency lupus with Kikuchi-Fujimoto disease and macrophage activation syndrome was reported (26). C1q deficiency is a rare cause of early-onset SLE. As in typical SLE, KFD can also occur in early-onset SLE due to complement deficiency such as C1q deficiency.
KFD is generally known to be self-limiting local lymphadenopathy; however, pediatricians sometimes experience recurrent or refractory KFD in children. Although the recurrence rate of KFD is usually known to be around 3–4%, it has a higher recurrence rate in children (3, 27, 28). Yoo et al., in a multivariate analysis, reported a 42% recurrence rate in 33 children with KFD; they suggested that a past history of systemic illnesses and a higher absolute lymphocyte count were risk factors associated with recurrent KFD (9). This study may have shown a slightly higher rate of recurrence because it included patients who had relapsed before the KFD was confirmed. In our study, the total relapse rate was 10%; interestingly, all relapsed patients were male in children, while all were female in the adult group. In a recent study of 98 children with KFD, there was a higher proportion of boys who had recurrent KFD, although this was not statistically significant (6). We ruled out relapsed patients before the KFD was confirmed because we were not sure whether it was a definite symptom of KFD.
Corticosteroids are commonly used for treatment. Patients with frequent recurrences are likely to suffer from side effects of corticosteroids; thus, they usually require the administration of steroid-sparing drugs instead of long-term use corticosteroids, as in other autoimmune diseases. In KFD, hydroxychloroquine is known to be effective and can be an alternative to corticosteroids because of its favorable effects and safety (29–31). Hydroxychloroquine, developed initially as an antimalarial drug, is commonly used in the treatment of rheumatic diseases such as systemic lupus erythematosus and dermatomyositis due to its immunomodulatory effects (32–34). Hydroxychloroquine suppresses the production of proinflammatory cytokines produced by peripheral mononuclear cells in the blood, such as IFNγ, TNFα interleukin (IL)-1, and IL-6 (27, 35, 36). Impaired apoptosis of self-reactive effector T cells is an essential mechanism for autoimmunity. Hydroxychloroquine can suppress autoimmunity by promoting apoptosis in effector T cells and inhibiting T cell antigen receptor signaling (37, 38). In KFD, hydroxychloroquine can be effective for the resolution of fever and systemic symptoms by impairing the production of IFN-γ (30, 39). In our study, there were no patients who took hydroxychloroquine because there were no cases of steroid dependence or refractory, and patients with multiple recurrences were rare (only one patient with three relapses, The interval between each relapse was about 1–2 years).
This study has some limitations. First, this was a retrospective study with a relatively small number of patients in a single hospital. Second, this was not a cohort study, so we could not fully understand the patients' current conditions. Third, we described the pathologic findings (description only). However, finer pathologic details of individual cases were not included since the focus of the manuscript was on clinical characteristics. In conclusion, our findings that suggest evident differences in the clinical and laboratory features of KFD according to age are encouraging. Ideally, our results aid in improving our understanding of KFD according to age and sex and are helpful for clinicians.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Pusan National University Hospital, Busan, Korea (11 January 2021; protocol code 2012-032-098). Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
H-YK, HJ, and SK contributed to conception and design of the study and wrote sections of the manuscript. HJ organized the database. SK performed the statistical analysis. H-YK and SK wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kucukardali Y, Solmazgul E, Kunter E, Oncul O, Yildirim S, Kaplan M. Kikuchi-Fujimoto disease: analysis of 244 cas-es. Clin Rheumatol. (2007) 26:50–4. doi: 10.1007/s10067-006-0230-5
2. Kim TY, Ha KS, Kim Y, Lee J, Lee K, Lee J. Characteristics of Kikuchi-Fujimoto disease in children compared with adults. Eur J Pediatr. (2014) 173:111–16. doi: 10.1007/s00431-013-2131-3
3. Bosch X, Guilabert A, Miquel R, Campo E. Enigmatic Kikuchi-Fujimoto disease: a comprehensive review. Am J Clin Pathol. (2004) 122:141–52. doi: 10.1309/YF08-1L4T-KYWV-YVPQ
4. Seo JH, Shim HS, Park JJ, Jeon SY, Kim JP, Ahn SK, et al. A clinical study of histi-ocytic necrotizing lymphadenitis (Kikuchi's disease) in children. Int J Pediatr Otorhinolaryngol. (2008) 72:1637–42. doi: 10.1016/j.ijporl.2008.07.019
5. Batton E, Alali M, Hageman JR, Parilla M, Yu KOA. Kikuchi-Fujimoto disease in children: an important diagnostic consideration for cervical lymphadenitis. Pediatr Ann. (2019) 48:e406–11. doi: 10.3928/19382359-20190920-01
6. Selvanathan SN, Suhumaran S, Sahu VK, Chong CY, Tan NWH, Thoon KC. Kikuchi-Fujimoto disease in children. J Paediatr Child Health. (2020) 56:389–93. doi: 10.1111/jpc.14628
7. Hutchinson CB, Wang E. Kikuchi-Fujimoto disease. Arch Pathol Lab Med. (2010) 134:289–93. doi: 10.5858/134.2.289
8. Kang HM, Kim JY, Choi EH, Lee HJ, Yun KW, Lee H. Clinical characteristics of severe histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto disease) in children. J Pediatr. (2016) 171:208–12.e1. doi: 10.1016/j.jpeds.2015.12.064
9. Yoo IH, Na H, Bae EY, Han SB, Lee SY, Jeong DC, et al. Recurrent lymphadenopathy in children with Kikuchi-Fujimoto disease. Eur J Pediatr. (2014) 173:1193–9. doi: 10.1007/s00431-014-2306-6
10. Park HS, Sung MJ, Park SE, Lim YT. Kikuchi-Fujimoto disease of 16 children in a single center of Korea. Pediatr Al-lergy Immunol. (2007) 18:174–8. doi: 10.1111/j.1399-3038.2006.00505.x
11. Dorfman RF, Berry GJ. Kikuchi's histiocytic necrotizing lymphadenitis: an analysis of 108 cases with emphasis on differential diagnosis. Semin Diagn Pathol. (1988) 5:329–45.
12. Kuo TT. Kikuchi's disease (histiocytic necrotizing lymphadenitis). A clinicopathologic study of 79 cases with an analysis of histologic subtypes, immunohistology, and DNA ploidy. Am J Surg Pathol. (1995) 19:798–809. doi: 10.1097/00000478-199507000-00008
13. Pileri S, Kikuchi M, Helbron D, Lennert K. Histiocytic necrotizing lymphadenitis without granulocytic infiltration Vir-chows. Arch A Pathol Anat Histol. (1982) 395:257–71. doi: 10.1007/BF00429352
14. Bosch X, Guilabert A. Kikuchi-Fujimoto disease. Orphanet J Rare Dis. (2006) 1:18. doi: 10.1186/1750-1172-1-18
15. Lin HC, Su CY, Huang SC. Kikuchi's disease in Asian children. Pediatrics. (2005) 115:e92–6. doi: 10.1542/peds.2004-0924
16. Adhikari RC, Sayami G, Lee MC, Basnet RB, Shrestha PK, Shrestha HG. Kikuchi-Fujimoto disease in Nepal: a study of 6 cases. Arch Pathol Lab Med. (2003) 127:1345–8. doi: 10.5858/2003-127-1345-KDINAS
17. Lee KY, Yeon YH, Lee BC. Kikuchi-Fujimoto disease with prolonged fever in children. Pediatrics. (2004) 114:e752–6. doi: 10.1542/peds.2004-0485
18. Zou CC, Zhao ZY, Liang L. Childhood Kikuchi-Fujimoto disease. Indian J Pediatr. (2009) 76:959–62. doi: 10.1007/s12098-009-0194-y
19. Das DK, Haji BI, Al-Boijan RA, Sheikh ZA, Pathan SK, Mannan AA. Kikuchi-Fujimoto disease in fine needle aspiration smears: a clinicocytologic study of 18 pediatric cases and correlation with 68 adult patients. Indian J Pathol Micro-biol. (2012) 55:333–8. doi: 10.4103/0377-4929.101739
20. Cuomo L, Cirone M, Di Gregorio AO, Vitillo M, Cattivelli M, Magliocca V, et al. Elevated antinuclear antibodies and altered anti-Epstein-Barr virus immune responses. Virus Res. (2015) 195:95–9. doi: 10.1016/j.virusres.2014.09.014
21. Harley JB, James JA. Epstein-Barr virus infection induces lupus autoimmunity. Bull NYU Hosp Jt Dis. (2006) 64:45–50.
22. James JA, Robertson JM. Lupus and Epstein-Barr. Curr Opin Rheumatol. (2012) 24:383–8. doi: 10.1097/BOR.0b013e3283535801
23. Guleria S, Gupta A, Pilania RK, Pandiarajan V, Rawat A, Saikia UN, et al. Kikuchi-Fujimoto disease: an under recognized cause of fever with lymphadenopathy. Indian J Pediatr. (2020) 87:85. doi: 10.1007/s12098-019-03070-8
24. Dumas G, Prendki V, Haroche J, Amoura Z, Cacoub P, Galicier L, et al. Kikuchi-Fujimoto disease: retrospective study of 91 cases and review of the literature. Medicine. (2014) 93:372–82. doi: 10.1097/MD.0000000000000220
25. Alperin JM, Ortiz-Fernández L, Sawalha AH. Monogenic lupus: a developing paradigm of disease. Front Immunol. (2018) 9:2496. doi: 10.3389/fimmu.2018.02496
26. Chaudhary H, Daniel R, Pilania RK, Anjani G, Sharma M, Pandiarajan V, et al. Catastrophes due to missing complements: C1q deficiency lupus with Kikuchi-Fujimoto disease and macrophage activation syndrome. Rheumatology. (2020) 59:1778–80. doi: 10.1093/rheumatology/kez625
27. Han HJ, Lim GY, Yeo DM, Chung NG. Kikuchi's disease in children: clinical manifestations and imaging features. J Korean Med Sci. (2009) 24:1105–9. doi: 10.3346/jkms.2009.24.6.1105
28. Wang TJ, Yang YH, Lin YT, Chiang BL. Kikuchi-Fujimoto disease in children: clinical features and disease course. J Microbiol Immunol Infect. (2004) 37:219–24.
29. Chen PH, Huang YF, Tang CW, Wann SR, Chang HT. Kikuchi-Fujimoto disease: an amazing response to hydroxychloroquine. Eur J Pediatr. (2010) 169:1557–9. doi: 10.1007/s00431-010-1256-x
30. Lin YC, Huang HH, Nong BR, Liu PY, Chen YY, Huang YF, et al. Pediatric Kikuchi-Fujimoto disease: a clinicopathologic study and the therapeutic effects of hydroxychloroquine. J Microbiol Immunol Infect. (2019) 52:395–401. doi: 10.1016/j.jmii.2017.08.023
31. Hyun M, So IT, Kim HA, Jung H, Ryu SY. Recurrent Kikuchi's Disease treated by hydroxychloroquine. Infect Chemother. (2016) 48:127–31. doi: 10.3947/ic.2016.48.2.127
32. Canadian Hydroxychloroquine Study Group. A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med. (1991) 324:150–4. doi: 10.1056/NEJM199101173240303
33. Dos Reis Neto ET, Kakehasi AM, de Medeiros Pinheiro M, Ferreira GA, Marques CDL, da Mota LMH, et al. Revisiting hydroxychloroquine and chloroquine for patients with chronic immunity-mediated inflammatory rheumatic diseases. Adv Rheumatol. (2020) 60:32. doi: 10.1186/s42358-020-00134-8
34. Danza Á, Graña D, Goñi M, Vargas A, Ruiz-Irastorza G. Hydroxychloroquine for autoimmune diseases. Rev Med Chil. (2016) 144:232–40. doi: 10.4067/S0034-98872016000200012
35. Barrera P, Boerbooms AM, van de Putte LB, van der Meer JW. Effects of antirheumatic agents on cytokines. Semin Arthritis Rheum. (1996) 25:234–53. doi: 10.1016/s0049-0172(96)80035-7
36. Nirk EL, Reggiori F, Mauthe M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol Med. (2020) 12:e12476. doi: 10.15252/emmm.202012476
37. Goldman FD, Gilman AL, Hollenback C, Kato RM, Premack BA, Rawlings DJ. Hydroxychloroquine inhibits calcium signals in T cells: a new mechanism to explain its immunomodulatory properties. Blood. (2000) 95:3460–6. doi: 10.1182/blood.V95.11.3460
38. van Loosdregt J, Spreafico R, Rossetti M, Prakken BJ, Lotz M, Albani S. Hydroxychloroquine preferentially induces apoptosis of CD45RO+ effector T cells by inhibiting autophagy: a possible mechanism for therapeutic modulation of T cells. J Allergy Clin Immunol. (2013) 131:1443–6.e1. doi: 10.1016/j.jaci.2013.02.026
Keywords: Kikuchi-Fujimoto disease, histiocytic necrotizing lymphadenitis, prognosis, children, adults, age
Citation: Kim H-Y, Jo HY and Kim SH (2021) Clinical and Laboratory Characteristics of Kikuchi-Fujimoto Disease According to Age. Front. Pediatr. 9:745506. doi: 10.3389/fped.2021.745506
Received: 22 July 2021; Accepted: 12 October 2021;
Published: 02 November 2021.
Edited by:
Amit Rawat, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaReviewed by:
Rakesh Kumar Pilania, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaHimanshi Chaudhary, Post Graduate Institute of Medical Education and Research (PGIMER), India
Copyright © 2021 Kim, Jo and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seong Heon Kim, pedksh@gmail.com
 Hye-Young Kim1,2
Hye-Young Kim1,2  Ha Young Jo
Ha Young Jo Seong Heon Kim
Seong Heon Kim