Fluid Balance in the Critically Ill Child Section: “How Bad Is Fluid in Neonates?”
- 1Department of Pediatrics, Medical University of South Carolina, Charleston, SC, United States
- 2Department of Pediatrics (Neonatology), University of Wisconsin, Madison, WI, United States
- 3Stead Family Department of Pediatrics (Nephrology), University of Iowa Health Care, Iowa City, IA, United States
Fluid overload (FO) in neonates is understudied, and its management requires nuanced care and an understanding of the complexity of neonatal fluid dynamics. Recent studies suggest neonates are susceptible to developing FO, and neonatal fluid balance is impacted by multiple factors including functional renal immaturity in the newborn period, physiologic postnatal diuresis and weight loss, and pathologies that require fluid administration. FO also has a deleterious impact on other organ systems, particularly the lung, and appears to impact survival. However, assessing fluid balance in the postnatal period can be challenging, particularly in extremely low birth weight infants (ELBWs), given the confounding role of maternal serum creatinine (Scr), physiologic weight changes, insensible losses that can be difficult to quantify, and difficulty in obtaining accurate intake and output measurements given mixed diaper output. Although significant FO may be an indication for kidney replacement therapy (KRT) in older children and adults, KRT may not be technically feasible in the smallest infants and much remains to be learned about optimal KRT utilization in neonates. This article, though not a meta-analysis or systematic review, presents a comprehensive review of the current evidence describing the effects of FO on outcomes in neonates and highlights areas where additional research is needed.
Introduction
In adults and pediatric patients, FO is associated with adverse outcomes including respiratory failure, cardiovascular events, prolonged hospitalization, and mortality (1–4). Recent studies suggest FO is similarly deleterious in neonates, but limited data are available. Neonates have unique physiologic renal adaptations, and neonatal fluid dynamics are complex. An understanding of the factors that impact fluid balance is required to prevent and/or treat neonatal FO as is a working knowledge of the available literature regarding associated morbidities and clinical outcomes.
Postnatal Renal Adaptation and Fluid Dynamics
At birth the kidney is functionally immature; function slowly improves as renal blood flow and glomerular filtration increase during the neonatal period. In pre-term newborns, nephrogenesis is also not yet complete. Due to this functional renal immaturity, neonatal fluid dynamics are different from that of older patients, and quantifying fluid balance and detecting FO can be difficult. For example, total body water (TBW), which encompasses extracellular water (ECW) and intracellular water, is high in the fetus accounting for ~95% of body weight. Throughout gestation, the proportion of body weight represented by water decreases, but even newborns at term have TBW accounting for nearly 75% of their birth weight (BW) (5). After birth, isotonic contraction of the extracellular fluid compartment occurs with loss of ECW and accompanying weight loss. This physiologic diuresis is likely mediated via atrial natriuretic peptide. Regardless of gestational age (GA), newborns lose 10–15% of their BW in the first days of life and are then expected to regain their BW over the next 2 weeks (5, 6). Excessive fluid administration can confound this process and is associated with increased incidence of bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), and patent ductus arteriosus (PDA) (7–10). In addition to measurable losses, insensible losses via the skin or respiratory tract can be considerable, especially in pre-mature neonates (6, 11). While fluid-based therapies are necessary for a variety of neonatal conditions, appropriate fluid regimens are highly debated. Fluid requirements are based on GA with differing hydration needs and nutritional goals for growth. With the expected volume contraction followed by restoration and varying insensible losses, establishing the ideal weight for use in fluid calculations can be challenging and requires ongoing careful evaluation utilizing weight changes, intake and output measurements, and serum and/or urine biochemistries.
Fluid Balance Management Strategies
Fluid balance management strategies often include modifying environmental factors to minimize insensible losses rather than replacing estimated fluid losses given the risk of FO if estimates are incorrect. As described, excessive fluid administration can be harmful. Fluid restriction is another strategy to prevent complications associated with FO; however, presently available studies are inconclusive. A Cochrane review evaluating fluid restriction demonstrated a decreased risk of PDA and NEC in pre-term infants, but the five randomized controlled trials (RCTs) included are outdated and likely do not reflect current practices (12). In a more recent RCT, fluid restriction reduced the duration of respiratory support for severe transient tachypnea of the newborn (13). Conversely, Nicholson et al. found no difference in outcomes with post-operative fluid restriction in a cohort of neonates after cardiac surgery, a finding that suggests fluid restriction may not be helpful in all populations (14). Moreover, fluid restriction is not without consequences, risking adverse effects including dehydration, hypotension, and decreased end-organ perfusion. Optimal fluid therapy thus should allow for adequate postnatal diuresis with adjustment for increasing post-menstrual age as the kidneys mature and the degree of insensible losses diminish.
There is no consensus on how best to define FO (4), especially in neonates. FO is typically assessed by one of two methods: (1) weight-based methods, which quantify percent change in weight from baseline, or (2) cumulative fluid balance methods, which utilize daily fluid intake and output measurements from time of intensive care unit (ICU) admission (or other start point). Selewski et al. confirmed both methods correlate well in a cohort of pediatric patients receiving KRT (15). However, both approaches have disadvantages in neonates. First, the degree of postnatal diuresis and weight loss varies based on GA and confounds weight-based methods. Second, accurate recording of fluid intake and output is challenging. Van Asperen et al. found that fluid balance charted in the medical record correlated poorly with daily weight changes, and therefore providers may be unable to rely on recorded fluid balances as a sole measure in assessing FO in neonates (16). Third, diaper outputs are often recorded as “mixed” (consisting of both urine and stool), leading to uncertainty about how much of recorded volumes represent urine.
Further complicating fluid management is the co-occurrence of AKI with FO. Altered renal function secondary to AKI hinders diuresis after significant fluid accumulation, pre-disposing to the development of FO. Just as other biomarkers have been studied to better evaluate renal function (17), FO can be a marker of AKI (18). FO can also dilute Scr, hindering the ability to detect AKI. Once the existence of both disease processes is determined, it is difficult to differentiate and isolate the effects of AKI from FO; both can have profound, independent impacts on response to fluids and kidney function (19).
When FO is detected, it is unclear at what threshold treatment should be initiated in neonates. Specific FO thresholds ranging from 10 to 20% have been identified in older pediatric populations and adults as (1) requiring interventions and (2) associated with adverse outcomes (2, 36, 37). Unfortunately, similar thresholds have not yet been identified for neonates; depending on the clinical context, differing thresholds may exist.
Fluid Overload Treatment Options
Treatment options for FO include diuretics, peritoneal dialysis (PD), and continuous KRT (CKRT). Diuretics are frequenty utilized in the ICU and are the mainstay of therapy for the prevention or treatment of pulmonary edema or congestive heart failure in infants with congenital heart defects (38). However, more research is needed to guide optimal diuretic dosing, timing, and type (loop vs. thiazide) in order to achieve desired outcomes without causing AKI or other organ injury. Belik et al. found diuretics improved pulmonary function in ventilated patients (39), though RCTs have failed to demonstrate improvement in outcomes in pre-term infants with respiratory distress syndrome, a precursor of BPD (40). Similarly, diuretics do not prevent the development or worsening of AKI in patients with oliguria, and the optimal use of diuretics to treat oliguria and FO in patients with or at risk for AKI is not yet clearly established (41).
Dialytic modalities including PD and CKRT are also options for fluid removal, but are not used as frequently for this indication in neonates, likely because of the lack of equipment designed specifically for small patients as well as lack of high quality data supporting optimal use. In the Assessment of Worldwide Acute Kidney Epidemiology in Neonates (AWAKEN) study, some form of dialysis was used in only 4.1% of those with AKI (42). Unlike diuretics, dialytic modalities offer the benefit of managing electrolyte imbalances while allowing for adequate nutrition provision that otherwise may be restricted in patients with oliguria and AKI. PD is often the preferred modality in neonates because of the avoidance of large fluid shifts and the need for large vascular catheters. A recent systematic review and meta-analysis by Flores et al. highlighted the challenges associated with using available data to guide clinical decision making around the use of PD (43). Their meta-analysis demonstrated an increased risk of mortality in patients who received PD post-operatively compared with those who were supported with diuretics, but a larger proportion of infants in this group came from centers that implemented PD following failed diuretic response and thus may represent a group at higher risk for poor outcomes. Outcomes by which efficacy of PD was assessed varied across studies, making cross-study comparisions difficult.
CKRT is another dialytic modality used to support neonates with FO and/or AKI. As mentioned previously, currently available machines have, until recently, been designed for adults and adapted for use in neonates. Studies assessing the outcomes of patients supported with CKRT consistently show higher mortality rates in patients <10 kg than in older and larger patients (44). The combination of technical challenges and published rates of high mortality likely contribute to hesitancy around the use of this therapy in neonates. However, newer devices with lower extracorporeal volumes are now becoming available. Menon et al. published multi-center data on the adapted use of the Aquadex FlexFlow system (CHS solutions Inc., Eden Prairie, MN) (45). In their study, FO was the indication for this therapy in 46% of their sample and FO with AKI in another 15%. Even with this smaller circuit, survival rates were lower in patients <10 kg than in the other patient groups, with 60% of patients <10 kg surviving to treatment discontinuation compared with 97–100% in older/larger patients (overall survival: 32% in patients <10 kg vs. 68–85% in older/larger patients). The Cardio Renal Pediatric Dialysis Emergency Machine (Carpediem™) is the only machine specifically designed for neonates and recently received FDA approval for treatment of AKI and FO in patients 2.5–10 kg. Published survival outcomes for neonates with AKI and FO are higher using this machine, with 97% of the sample surviving to treatment discontinuation, and 50% overall survival of this group (46). These newer devices have significant potential to expand our therapeutic options and improve our ability to manage FO (and AKI) in neonates.
Subsections: Specific Populations at Risk for FO
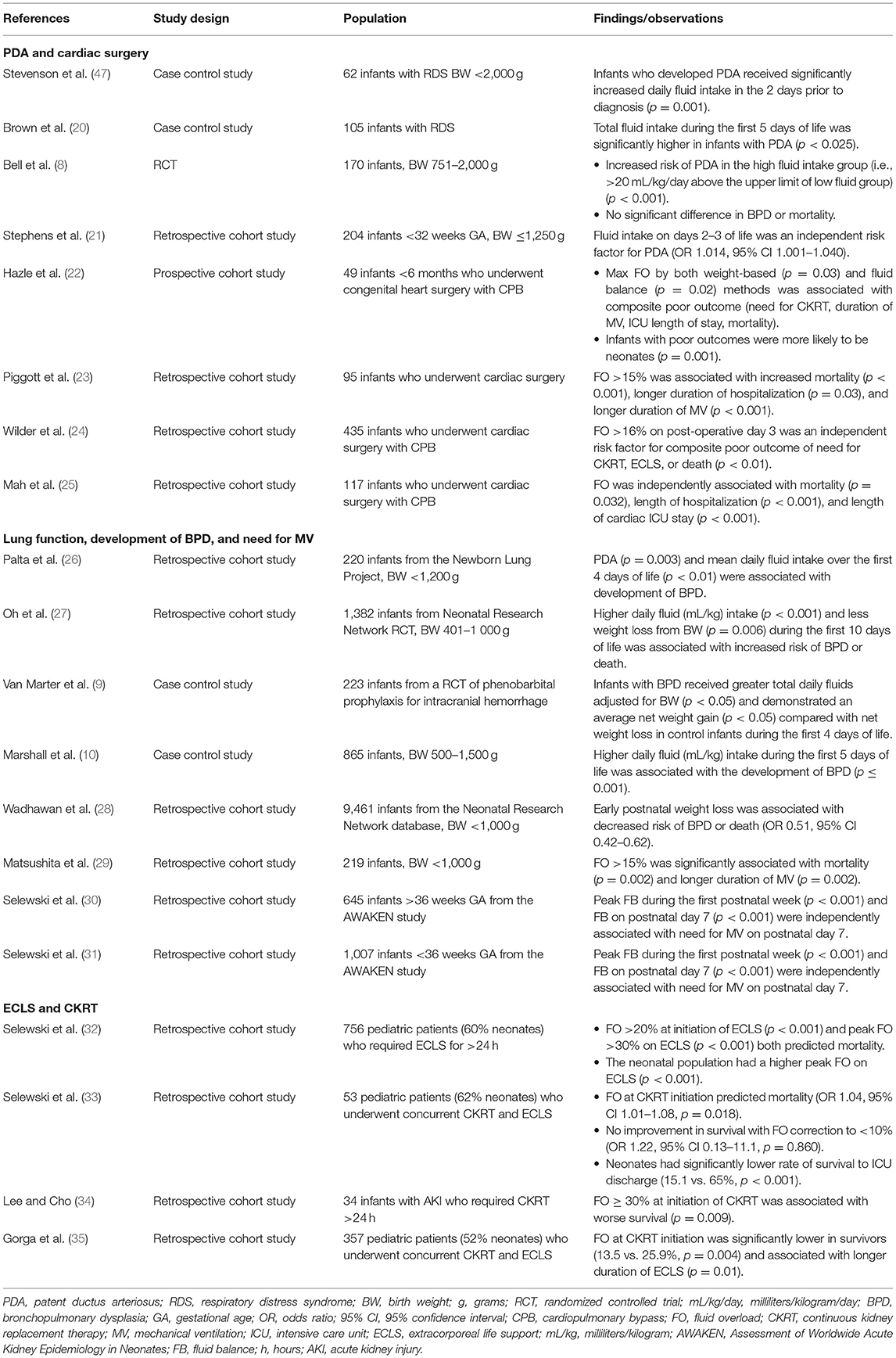
Below is a brief review of key studies (Table 1) of FO in several specific neonatal populations: those with cardiac disease or requiring cardiac surgery, those with lung disease, and those requiring extracorporeal life support (ECLS) and CKRT Table 1.
PDA and Cardiac Surgery
Persistence of the ductus arteriosus (i.e., PDA), the most common cardiac problem among pre-term infants, is associated with excessive fluid intake (8, 20, 21, 47). Fluid intakes on days 2 and 3 of life are independently associated with increased risk of PDA indicating that early fluid administration can be problematic even before FO develops (21). In this same study, the odds of PDA increased 22% for every 10 mL/kg of fluid received on day 3, and those who received total fluid intakes >170 mL/kg/day were 4.5 times more likely to have a PDA.
Cardiac surgery is a risk factor for FO as well as AKI. Both complications often occur with cardiopulmonary bypass (CPB) support and are associated with significant morbidity and mortality (22–25). Peri-operative fluid management is complicated by the need for blood products and diuretics, impaired renal function, and low cardiac output necessitating volume resuscitation balanced with inotropic/vasopressor support. In a retrospective cohort of neonates undergoing CPB, FO was an independent risk factor for the composite outcome of death and need for CKRT or ECLS (24). Notably, those with poor outcomes were more likely to be <3 days old at the time of the operation signifying acuity of illness but also possibly reflecting inadequate diuresis pre-operatively. The authors determined that >16% FO on post-operative day (POD) 3 held the highest predictive value for poor outcomes, suggesting POD 3 and a FO >16% could represent important therapeutic thresholds. FO can also impact recovery by prolonging the duration of mechanical ventilation, time to sternal closure, and overall length of stay in neonates after cardiac surgery (23, 24). Mah et al. have also demonstrated the independent association of FO on length of stay as well as mortality (25).
Lung Function, Development of BPD, and Need for Mechanical Ventilation
Most research evaluating the relationship between fluid balance and the lung in neonates has focused on ELBW infants and BPD-related outcomes. Multiple factors are implicated in the pathogenesis of BPD including barotrauma, oxygen toxicity, and PDA; positive fluid balance and subsequent pulmonary edema are proposed to contribute as well (20, 26). Researchers hypothesize excessive fluid administration leads to increased pulmonary blood flow (especially if PDA is present), and this excess fluid subsequently shifts from the vessels into the pulmonary interstitium. Resultant pulmonary edema negatively affects lung compliance, requiring increased respiratory support and risking potential lung injury (27, 48).
Multiple studies have found increased risk of BPD in infants who received higher total fluid intakes and less postnatal weight loss through the first 10 days of life (9, 10, 27, 28). More recent investigations explored the link between neonatal fluid balance and mechanical ventilation and found FO in the first 72 h of life was associated with higher ventilator settings and longer duration of mechanical ventilation (29). In a secondary analysis of the AWAKEN cohort (42), Selewski et al. demonstrated multiple measurements of positive fluid balance as risk factors for the need for mechanical ventilation at the end of the first week of life (30, 31); every 1% increase in peak fluid balance led to a 12–14% increased risk of requiring mechanical ventilation on postnatal day 7, suggesting even incremental fluid changes can adversely affect lung function.
ECLS and CKRT
Neonates requiring ECLS are the most critically ill patients in the neonatal ICU. Severe respiratory pathology is a common neonatal indication for ECLS, and FO with pulmonary edema can be detrimental to the recovery of lung function (49). FO is common in pediatric patients requiring ECLS with the majority developing >30% FO in one multi-center observational study (32). Although this study included all pediatric patients, the median patient age was 10 days. Positive fluid balance alone during ECLS was an independent risk factor for mortality, and neonates had significantly higher peak FO (35.3 vs. 26.3%; p < 0.001) than pediatric patients.
CKRT is often used in patients receiving ECLS to prevent or treat FO. In another retrospective study examining pediatric ECLS patients comprised of 62% neonates, Selewski et al. demonstrated increased risk of mortality with a higher degree of FO at CKRT initiation (33). Although CKRT may be able to improve fluid balance, the authors also showed that once FO was established, fluid removal had no impact on survival (33). This associated risk of mortality was later reiterated in an exclusively neonatal observational study using a FO threshold of 30% (34). Gorga et al. conducted a multi-center cohort study in which neonates represented 52% of the sample and also found an independent association between the degree of FO at CKRT initiation and mortality with graded increases in both ECLS and hospital mortality for every 10% increase in FO (35). However, the median change in FO from CKRT initiation to discontinuation did not seem to impact either ECLS or hospital mortality. The mortality risk with positive fluid balance on ECLS and the lack of survival benefit with fluid removal suggests earlier recognition and intervention with CKRT may be indicated prior to the development of FO. However, the threshold at which this should occur remains unknown.
Discussion and Conclusions
Neonatal fluid dynamics are complex, evolve throughout the neonatal period, and present clinical challenges. Neonatal fluid balance is impacted by a variety of factors, and FO can be difficult to quantify accurately and reliably. Moreover, FO occurs frequently in high-risk neonates including those with cardiac disease, lung disease, and those requiring ECLS, and is associated with worse outcomes. In addition, neonates with AKI and FO requiring dialytic support consistently have higher mortality rates across studies than do older and larger patients. Whether it is the FO itself, the combination of AKI and FO, or an overall increased severity of illness for which FO may simply be a marker is not yet known.
It is also unclear whether FO is a modifiable risk factor. That is, can we improve outcomes by preventing fluid accumulation? If so, what are the thresholds at which to intervene? As we recognize the harmful effects of FO in neonates, additional research is warranted to evaluate this relationship. Research efforts will be enhanced by the standardization of definitions of FO, methods by which to quantify fluid balance (recognizing that these may vary by GA and day of life), and ideal outcomes by which to assess interventions (e.g., time to extubation, ICU and hospital length of stay, AKI occurrence and duration, and mortality). Furthermore, multi-center RCTs are needed to guide clinical management by identifying therapeutic thresholds at which to intervene, and the indications for and appropriate use of FO treatment strategies (both diuretics and dialytic modalities). Finally, the impact of these treatment strategies on long-term outcomes will need to be explored. Greater awareness of this important clinical issue at the bedside, enhanced research focus, and the availability of dialysis equipment designed specifically for neonates have the potential to expand treatment options and improve outcomes for these vulnerable patients.
Author Contributions
AR is responsible for the conception, design, drafting, and completion of this review. HM, MH, and JJ have assisted with analysis and interpretation of his work and critically revised this manuscript. All authors are fully accountable for ensuring the integrity and accuracy of this work and have approved this final version.
Funding
The Division of Neonatology at the Medical University of South Carolina will fund the publication of this review.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. (2009) 76:422–7. doi: 10.1038/ki.2009.159
2. Arikan AA, Zappitelli M, Goldstein SL, Naipaul A, Jefferson LS, Loftis LL. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med. (2012) 13:253–8. doi: 10.1097/PCC.0b013e31822882a3
3. Hung SC, Lai YS, Kuo KL, Tarng DC. Volume overload and adverse outcomes in chronic kidney disease: clinical observational and animal studies. J Am Heart Assoc. (2015) 4:e001918. doi: 10.1161/jaha.115.001918
4. Alobaidi R, Morgan C, Basu RK, Stenson E, Featherstone R, Majumdar SR, et al. Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr. (2018) 172:257–68. doi: 10.1001/jamapediatrics.2017.4540
5. Dell K. Fluid, electrolytes, and acid-base homeostasis. In: Martin RJ, Fanaroff AA, Walsh MC, editors. Fanaroff and Martin's Neonatal-Perinatal Medicine. 11th ed. Cleveland, Ohio: Elsevier (2020). p. 1854–70.
6. Chawla D, Agarwal R, Deorari AK, Paul VK. Fluid and electrolyte management in term and preterm neonates. Indian J Pediatr. (2008) 75:255–9. doi: 10.1007/s12098-008-0055-0
7. Bell EF, Warburton D, Stonestreet BS, Oh W. High-volume fluid intake predisposes premature infants to necrotising enterocolitis. Lancet. (1979) 2:90. doi: 10.1016/s0140-6736(79)90135-1
8. Bell EF, Warburton D, Stonestreet BS, Oh W. Effect of fluid administration on the development of symptomatic patent ductus arteriosus and congestive heart failure in premature infants. N Engl J Med. (1980) 302:598–604. doi: 10.1056/nejm198003133021103
9. Van Marter LJ, Leviton A, Allred EN, Pagano M, Kuban KC. Hydration during the first days of life and the risk of bronchopulmonary dysplasia in low birth weight infants. J Pediatr. (1990) 116:942–9. doi: 10.1016/s0022-3476(05)80658-4
10. Marshall DD, Kotelchuck M, Young TE, Bose CL, Kruyer L, O'Shea TM. Risk factors for chronic lung disease in the surfactant era: a North Carolina population-based study of very low birth weight infants. N C Neonatol Assoc Pediatr. (1999) 104:1345–50. doi: 10.1542/peds.104.6.1345
11. Segar JL. A physiological approach to fluid and electrolyte management of the preterm infant: review. J Neonatal Perinatal Med. (2020) 13:11–9. doi: 10.3233/npm-190309
12. Bell EF, Acarregui MJ. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. (2014) 2014:Cd000503. doi: 10.1002/14651858.CD000503.pub3
13. Stroustrup A, Trasande L, Holzman IR. Randomized controlled trial of restrictive fluid management in transient tachypnea of the newborn. J Pediatr. (2012) 160:38–43.e31. doi: 10.1016/j.jpeds.2011.06.027
14. Nicholson GT, Clabby ML, Mahle WT. Is there a benefit to postoperative fluid restriction following infant surgery? Congenit Heart Dis. (2014) 9:529–35. doi: 10.1111/chd.12165
15. Selewski DT, Cornell TT, Lombel RM, Blatt NB, Han YY, Mottes T, et al. Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med. (2011) 37:1166–73. doi: 10.1007/s00134-011-2231-3
16. van Asperen Y, Brand PL, Bekhof J. Reliability of the fluid balance in neonates. Acta Paediatr. (2012) 101:479–83. doi: 10.1111/j.1651-2227.2012.02591.x
17. Gubhaju L, Sutherland MR, Horne RS, Medhurst A, Kent AL, Ramsden A, et al. Assessment of renal functional maturation and injury in preterm neonates during the first month of life. Am J Physiol Renal Physiol. (2014) 307:F149–58. doi: 10.1152/ajprenal.00439.2013
18. Selewski DT, Goldstein SL. The role of fluid overload in the prediction of outcome in acute kidney injury. Pediatr Nephrol. (2018) 33:13–24. doi: 10.1007/s00467-016-3539-6
19. Askenazi DJ, Koralkar R, Hundley HE, Montesanti A, Patil N, Ambalavanan N. Fluid overload and mortality are associated with acute kidney injury in sick near-term/term neonate. Pediatr Nephrol. (2013) 28:661–6. doi: 10.1007/s00467-012-2369-4
20. Brown ER, Stark A, Sosenko I, Lawson EE, Avery ME. Bronchopulmonary dysplasia: possible relationship to pulmonary edema. J Pediatr. (1978) 92:982–4. doi: 10.1016/s0022-3476(78)80382-5
21. Stephens BE, Gargus RA, Walden RV, Mance M, Nye J, McKinley L, et al. Fluid regimens in the first week of life may increase risk of patent ductus arteriosus in extremely low birth weight infants. J Perinatol. (2008) 28:123–8. doi: 10.1038/sj.jp.7211895
22. Hazle MA, Gajarski RJ, Yu S, Donohue J, Blatt NB. Fluid overload in infants following congenital heart surgery. Pediatr Crit Care Med. (2013) 14:44–9. doi: 10.1097/PCC.0b013e3182712799
23. Piggott KD, Soni M, Decampli WM, Ramirez JA, Holbein D, Fakioglu H, et al. Acute kidney injury and fluid overload in neonates following surgery for congenital heart disease. World J Pediatr Congenit Heart Surg. (2015) 6:401–6. doi: 10.1177/2150135115586814
24. Wilder NS, Yu S, Donohue JE, Goldberg CS, Blatt NB. Fluid overload is associated with late poor outcomes in neonates following cardiac surgery. Pediatr Crit Care Med. (2016) 17:420–7. doi: 10.1097/pcc.0000000000000715
25. Mah KE, Hao S, Sutherland SM, Kwiatkowski DM, Axelrod DM, Almond CS, et al. Fluid overload independent of acute kidney injury predicts poor outcomes in neonates following congenital heart surgery. Pediatr Nephrol. (2018) 33:511–20. doi: 10.1007/s00467-017-3818-x
26. Palta M, Gabbert D, Weinstein MR, Peters ME. Multivariate assessment of traditional risk factors for chronic lung disease in very low birth weight neonates. Newborn Lung Project J Pediatr. (1991) 119:285–92. doi: 10.1016/s0022-3476(05)80746-2
27. Oh W, Poindexter BB, Perritt R, Lemons JA, Bauer CR, Ehrenkranz RA, et al. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J Pediatr. (2005) 147:786–90. doi: 10.1016/j.jpeds.2005.06.039
28. Wadhawan R, Oh W, Perritt R, Laptook AR, Poole K, Wright LL, et al. Association between early postnatal weight loss and death or BPD in small and appropriate for gestational age extremely low-birth-weight infants. J Perinatol. (2007) 27:359–64. doi: 10.1038/sj.jp.7211751
29. Matsushita FY, Krebs VLJ, Ferraro AA, de Carvalho WB. Early fluid overload is associated with mortality and prolonged mechanical ventilation in extremely low birth weight infants. Eur J Pediatr. (2020) 179:1665–71. doi: 10.1007/s00431-020-03654-z
30. Selewski DT, Akcan-Arikan A, Bonachea EM, Gist KM, Goldstein SL, Hanna M, et al. The impact of fluid balance on outcomes in critically ill near-term/term neonates: a report from the AWAKEN study group. Pediatr Res. (2019) 85:79–85. doi: 10.1038/s41390-018-0183-9
31. Selewski DT, Gist KM, Nathan AT, Goldstein SL, Boohaker LJ, Akcan-Arikan A, et al. The impact of fluid balance on outcomes in premature neonates: a report from the AWAKEN study group. Pediatr Res. (2020) 87:550–7. doi: 10.1038/s41390-019-0579-1
32. Selewski DT, Askenazi DJ, Bridges BC, Cooper DS, Fleming GM, Paden ML, et al. The impact of fluid overload on outcomes in children treated with extracorporeal membrane oxygenation: a multicenter retrospective cohort study. Pediatr Crit Care Med. (2017) 18:1126–35. doi: 10.1097/pcc.0000000000001349
33. Selewski DT, Cornell TT, Blatt NB, Han YY, Mottes T, Kommareddi M, et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med. (2012) 40:2694–9. doi: 10.1097/CCM.0b013e318258ff01
34. Lee ST, Cho H. Fluid overload and outcomes in neonates receiving continuous renal replacement therapy. Pediatr Nephrol. (2016) 31:2145–52. doi: 10.1007/s00467-016-3363-z
35. Gorga SM, Sahay RD, Askenazi DJ, Bridges BC, Cooper DS, Paden ML, et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy: a multicenter retrospective cohort study. Pediatr Nephrol. (2020) 35:871–82. doi: 10.1007/s00467-019-04468-4
36. Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. (2010) 55:316–25. doi: 10.1053/j.ajkd.2009.10.048
37. Woodward CW, Lambert J, Ortiz-Soriano V, Li Y, Ruiz-Conejo M, Bissell BD, et al. Fluid overload associates with major adverse kidney events in critically ill patients with acute kidney injury requiring continuous renal replacement therapy. Crit Care Med. (2019) 47:e753–60. doi: 10.1097/ccm.0000000000003862
38. Hsu DT, Pearson GD. Heart failure in children: part II: diagnosis, treatment, and future directions. Circ Heart Fail. (2009) 2:490–8. doi: 10.1161/circheartfailure.109.856229
39. Belik J, Spitzer AR, Clark BJ, Gewitz MH, Fox WW. Effect of early furosemide administration in neonates with respiratory distress syndrome. Pediatr Pulmonol. (1987) 3:219–25. doi: 10.1002/ppul.1950030405
40. Stewart A, Brion LP, Soll R. Diuretics for respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev. (2011) 2011:Cd001454. doi: 10.1002/14651858.CD001454.pub3
41. Guignard JP, Iacobelli S. Use of diuretics in the neonatal period. Pediatr Nephrol. (2021). doi: 10.1007/s00467-021-04921-3. [Epub ahead of print].
42. Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. (2017) 1:184–94. doi: 10.1016/s2352-4642(17)30069-x
43. Flores S, Loomba RS, Elhoff JJ, Bronicki RA, Mery CM, Alsaied T, et al. Peritoneal dialysis vs. diuretics in children after congenital heart surgery. Ann Thorac Surg. (2019) 108:806–12. doi: 10.1016/j.athoracsur.2019.03.066
44. Askenazi DJ, Goldstein SL, Koralkar R, Fortenberry J, Baum M, Hackbarth R, et al. Continuous renal replacement therapy for children ≤10 kg: a report from the prospective pediatric continuous renal replacement therapy registry. J Pediatr. (2013) 162:587–92.e583. doi: 10.1016/j.jpeds.2012.08.044
45. Menon S, Broderick J, Munshi R, Dill L, DePaoli B, Fathallah-Shaykh S, et al. Kidney support in children using an ultrafiltration device: a multicenter, retrospective study. Clin J Am Soc Nephrol. (2019) 14:1432–40. doi: 10.2215/cjn.03240319
46. Garzotto F, Vidal E, Ricci Z, Paglialonga F, Giordano M, Laforgia N, et al. Continuous kidney replacement therapy in critically ill neonates and infants: a retrospective analysis of clinical results with a dedicated device. Pediatr Nephrol. (2020) 35:1699–705. doi: 10.1007/s00467-020-04562-y
47. Stevenson JG. Fluid administration in the association of patent ductus arteriosus complicating respiratory distress syndrome. J Pediatr. (1977) 90:257–61. doi: 10.1016/s0022-3476(77)80645-8
48. Bland RD. Edema formation in the lungs and its relationship to neonatal respiratory distress. Acta Paediatr Scand Suppl. (1983) 305:92–9. doi: 10.1111/j.1651-2227.1983.tb09868.x
Keywords: fluid overload, acute kidney injury, fluid balance, kidney replacement therapy, continuous renal replacement therapy, neonate
Citation: Rutledge A, Murphy HJ, Harer MW and Jetton JG (2021) Fluid Balance in the Critically Ill Child Section: “How Bad Is Fluid in Neonates?” Front. Pediatr. 9:651458. doi: 10.3389/fped.2021.651458
Received: 09 January 2021; Accepted: 15 March 2021;
Published: 20 April 2021.
Edited by:
Katja Michelle Gist, Children's Hospital Colorado, United StatesReviewed by:
Zaccaria Ricci, Bambino Gesù Children Hospital (IRCCS), ItalyDick Tibboel, Erasmus Medical Center, Netherlands
Copyright © 2021 Rutledge, Murphy, Harer and Jetton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heidi J. Murphy, murphyh@musc.edu
 Austin Rutledge
Austin Rutledge Heidi J. Murphy
Heidi J. Murphy Matthew W. Harer
Matthew W. Harer Jennifer G. Jetton
Jennifer G. Jetton