Inflammatory Myofibroblastic Tumors in the Uterus: Childhood-Case Report and Review of the Literature
- University of Szeged, Szeged, Hungary
Inflammatory myofibroblastic tumor (IMT) is a spindle cell neoplasm with low malignant potential, which may appear in different parts of the body. Uterine localization is rare, especially among children. Etiology is unclear, although some authors suggest underlying trauma or distress. A 3.5-year-old girl was treated at our institute for recurring vaginal bleeding without injury or known pathology. Physical examination and laboratory analysis revealed no specific findings, contrast-enhanced MRI found a 25 × 28 × 30 mm-sized inhomogeneous soft tissue mass in the uterus wall, which was excised in toto. Histological examination identified a spindle cell pattern, and the FISH test revealed ALK gene rearrangement, the lesion was defined as an IMT. Six cases were published to date, and their diagnostic methods are not equivocal, CT, and PET CT were preferred instead of MRI. Aggressive therapy seems to be exaggerated according to low recurrence and metastasis occurrence, and crizotinib is proved as good therapeutic agent in those cases. Biopsy and histology has important role in order to distinguish IMT from malignancies completed with FISH examination because ALK positivity strengthens the diagnosis. No lethal outcome was published among children, as our patient is also symptom-free after 3 years.
Background
In the WHO definition, inflammatory myofibroblastic tumor (IMT) is a rare spindle cell neoplasm with low malignant potential, and it is composed of proliferative myofibroblasts and mixed inflammatory cell infiltrate. The appearance of IMT in childhood is extremely rare. Symptoms and treatment are heterogeneous, mainly depend on the localization of the tumor (1–4). Genetically speaking, approximately half of IMTs harbor clonal rearrangement of the anaplastic lymphoma kinase (ALK) gene, which encodes receptor tyrosine kinase (1–5). Here, the authors present a uterine-located IMT case and review the current literature data.
Case Report
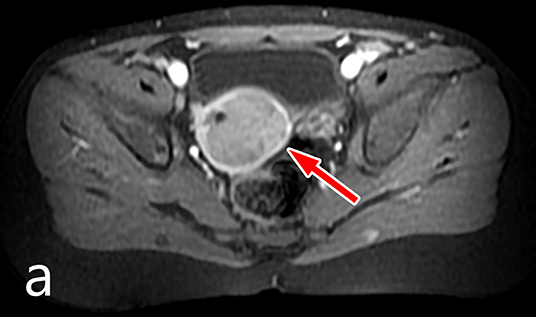
A 3.5-year-old girl was referred to the surgical outpatient clinic complaining of recurring vaginal bleeding. There was no trauma or inflammatory symptoms in her clinical history. Regarding the family history, no malignant, or genetic disease was noted. On physical examination flat and non-tender abdomen was found without any palpable lumps, with negative rectal examination. Abdominal ultrasound scan showed a mildly enlarged uterus, while MRI examination identified a 25 × 28 × 30 mm sized inhomogeneous soft tissue mass in the uterus that compressed the entire endometrium (Figure 1). The lesion enhanced contrast material, and it did not spread through the serosa. AFP and LDH values were in normal range, but NSE level was mildly elevated.
According to the multidisciplinary team's decision, primary open surgery was chosen. Fine needle biopsy was excluded because of pelvic localization, small size, and the chance of inadequate sample size for a certain histologic opinion. Tumor spilling was also considered as risk factor during this procedure. Surgery was performed through suprapubic incision; and the well-circumscribed lesion seemed resectable, so instead of tumor biopsy the entire mass was removed from the posterior wall of the uterus. The myometrium was completely reconstructed. Lymph node metastasis was not found. The postoperative period was uneventful. After a 3-years follow-up period the child was complaint-free, and the abdominal US scans showed no recurrence. Histological examination revealed IMT with irregular borders composed of spindle cells with low cytological atypia. Also, an extensive inflammatory cell infiltrate with eosinophyl granulocytes and lymphocytes was observed in the tumor tissue. Immunohistochemistry was positive for h-caldesmon, smooth muscle actin, CD34, factor XIII and ALK. ALK FISH examination has found ALK translocation in 80% of the tumor cells.
Discussion
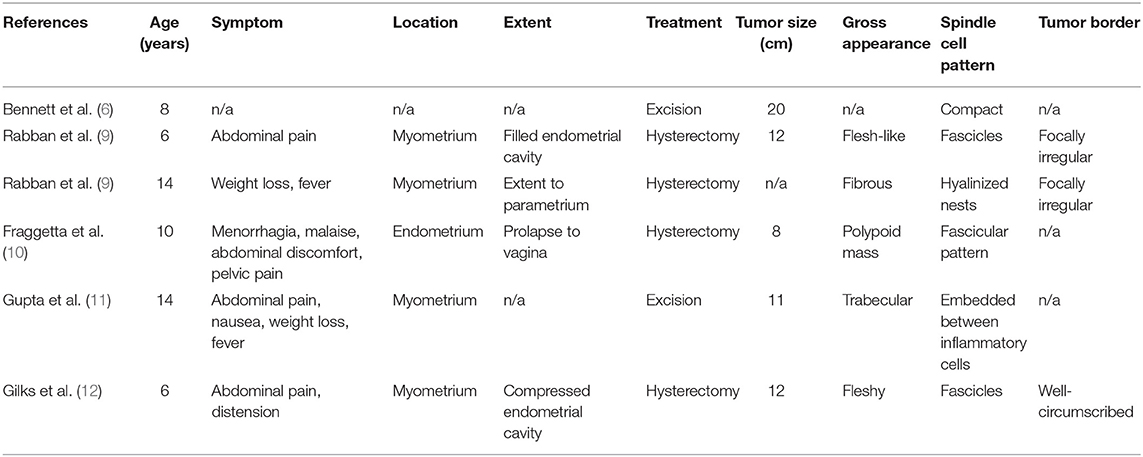
IMTs in children are seldom found, especially in uterine localization (6). It usually appears in the lung or liver, but some reports also mentioned other possible sites such as the stomach (7), mesentery, omentum, retroperitoneum, head and neck region, minor pelvis, urogenital tract, as well as extremities (2, 4). Symptoms vary depending on the localization, e.g., palpable mass, bleeding and vomiting, or bowel obstruction in abdominal located IMTs (8). Etiology is unknown, however reports suggest probability of previous injury, inflammation, or distress (8). In the Pubmed© database only 6 reports concern pediatric uterine IMT cases (6, 9–12). The spectrum of their clinical findings varied from asymptomatic to severe physical signs such as abdominal and pelvic pain (67%), weight loss (40%), fever (40%), and menorrhagia (20%) (see Table 1). Tumor size ranged between 8 and 20 cm, while in our case maximum diameter was only 2.8 cm.
There is no single imaging method that is universally agreed upon; contrast-enhanced CT (11) and PET-CT (10) were also performed in different pediatric cases in order to detect activity and metastases. Our chosen diagnostic modality was MRI for its better soft tissue specificity and to avoid radiation exposure, especially in the ovarian region. Interestingly, this tool has not been used in pediatric uterine IMTs. Diagnostic laparoscopy was performed prior to surgery in one case (11).
All six cases were treated surgically, four by hysterectomy (9, 10, 12) and two by local tumor removal (6, 11). Depending on the size and myometrial infiltration of the tumor, especially in children, organ-sparing local excision was preferred; this was also performed in our case.
Lymph node metastasis was present in one case without progression during follow-up (10). One recurrence was observed (6). She had the first local recurrence 1 month after the surgery, although it could be the result of incomplete resection during local excision (data not available), followed by two (ovarian and unknown) recurrences treated successfully with crizotinib.
The overall recurrence rate for IMT is about 25%, while distant metastases are very uncommon (2%) (2). Biological behavior mostly depends on the size, mitotic activity, tumor cell necrosis and resection line positivity (3). As some authors also reported, recurrence can be the consequence of incomplete resection, however in those cases no further relapse was detected (6, 8).
The histological appearance of each varied from case to case. Immunohistochemistry revealed ALK-positivity in 4 cases as it was also seen in our presented case. The expression of ALK protein and the presence of ALK gene rearrangement are good diagnostic markers; however, they are only present in about 50% of the IMTs, but in 88–100% of uterine IMTs (4, 5, 13, 14). A variable ALK-positivity can be seen in other tumor subtypes, such as anaplastic large cell lymphoma, rhabdomyosarcoma, and neuroblastoma (13), but in the case of female genital tract tumors, it is considered as a specific marker of IMTs thus supporting diagnosis. ALK translocation also offers a therapeutic option, because ALK inhibitors like crizotinib and alectinib can deactivate uncontrolled cell proliferation. ALK inhibitor treatment is recommended in surgically incurable, metastatic and recurrent cases (13, 14).
The “wait-and-see” approach was also described as a safe treatment of IMTs in adults as well as in pediatric cases (8, 15, 16). Data were also published about spontaneous regression of a hepatic IMT (8). However, to distinguish uterine IMTs from highly malignant tumors like leiomyosarcoma, either a biopsy or an excision is recommended. In contrast with pediatric cases, the outcome is worse among adults. Two lethal outcomes and six recurrences in 59 adult patients with uterine IMT were found (6, 17).
In conclusion, the so far published youngest child with uterine IMT was treated successfully by our team. To date, no recurrence or metastasis has been observed. The authors would also like to stress the role of the uterus-sparing surgery to eliminate tumor or gain tissue specimen, and emphasize the importance of the prior MRI imaging instead of other reported methods in order to achieve recovery without harming the normal development of the female genital tract.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
This study was carried out in accordance with the recommendations of the Declaration of Helsinki with written informed consent from the parents. The protocol was approved by the scientific ethic committee of University of Szeged.
Author Contributions
The study conception was performed and the manuscript was written by PE and TK. Data acquisition was by PE, LK, and TK. Revision was by LK and TK.
Funding
This work was supported by University of Szeged Open Access Fund (nr. 4400).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
IMT, Inflammatory myofibroblastic tumor; ALK, Anaplastic lymphoma kinase; MDT, Multidisciplinary team; AFP, Alpha-fetoprotein; LDH, Lactate-dehydrogenase; NSE, Neuron-specific enolase; NPM, Nucleophosmin; FISH, Fluorescence in situ hybridization; TPM4, Trophomyosine 4; CLTC, Clathrin heavy chain.
References
1. Coffin CM, Fletcher JA. Inflammatory myofibroblastic tumor. In: Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F, editors. International Agency for Research on Cancer, Vol. V. World Health Organization Classification of Tumours. Lyon: WHO Press (2013). p. 83–4.
2. Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. (2008) 61:428–37 doi: 10.1136/jcp.2007.049387
3. Parra-Herran C, Howitt BE. Uterine mesenchymal tumors update on classification, staging, and molecular features. Surg Pathol. (2019) 12:363–96. doi: 10.1016/j.path.2019.01.004
4. Shukla PS, Mittal K. Inflammatory myofibroblastic tumor in female genital tract. Arch Pathol Lab Med. (2019) 143:122–9. doi: 10.5858/arpa.2017-0575-RA
5. Mohammad N, Haimes JD, Mishkin S, Kudlow BA, Leong MY, Chew SH. ALK is a specific diagnostic marker for inflammatory myofibroblastic tumor of the uterus. Am J Surg Pathol. (2018) 42:1353–9. doi: 10.1097/PAS.0000000000001120
6. Bennett JA, Nardi V, Rouzbahman M, Morales-Oyarvide V, Nielsen GP, Oliva E. Inflammatory myofibroblastic tumor of the uterus: a clinicopathological, immunohistochemical, and molecular analysis of 13 cases highlighting their broad morphologic spectrum. Mod Pathol. (2017) 30:1489–503. doi: 10.1038/modpathol.2017.69
7. Estevão-Costa J, Correia-Pinto J, Rodrigues FC, Carvalho JL, Campos M, Dias JA, et al. Gastric inflammatory myofibroblastic proliferation in children. Pediatr Surg Int. (1998) 13:95–9. doi: 10.1007/s003830050257
8. Fragoso AC, Eloy C, Estevão-Costa J, Campos M, Farinha N, Lopes JM. Abdominal inflammatory myofibroblastic tumor: a clinicopathologic study with reappraisal of biologic behavior. J Ped Surg. (2011) 46:2076–82. doi: 10.1016/j.jpedsurg.2011.07.009
9. Rabban JT, Zaloudek CJ, Shekitka KM, Tavassoli FA. Inflammatory myofibroblastic tumor of the uterus: a clinicopathologic study of 6 cases emphasizing distinction from aggressive mesenchymal tumors. Am J Surg Pathol. (2005) 29:1348–55. doi: 10.1097/01.pas.0000172189.02424.91
10. Fraggetta F, Doglioni C, Scollo P, Pecciarini L, Massimo Ippolito M, Amico P, et al. Uterine inflammatory myofibroblastic tumor in a 10-year-old girl presenting as polypoid mass. J Clin Oncol. (2015) 33:e7–e10. doi: 10.1200/JCO.2013.48.8304
11. Gupta N, Mittal S, Misra R. Inflammatory pseudotumor of uterus: an unusual pelvic mass. Eur J Obstet Gynecol Reprod Biol. (2011) 156:118–9. doi: 10.1016/j.ejogrb.2011.01.002
12. Gilks CB, Taylor GP, Cldment PB. Inflammatory pseudotumor of the uterus. Int J Gyn Path. (1987) 6:275–86. doi: 10.1097/00004347-198709000-00008
13. Mossé YP. Anaplastic lymphoma kinase as a cancer target in pediatric malignancies. Clin.Cancer Res. (2016) 22:546–52. doi: 10.1158/1078-0432.CCR-14-1100
14. Mossé YP, Voss SD, Lim S, Rolland D, Minard CG, Fox E, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a children's oncology group study. J Clin Onc. (2017) 28:3215–22 doi: 10.1200/JCO.2017.73.4830
15. Jimenez JM, Poustchi-Amin M, Leonidas JC, Pena A. Extraperitoneal abdominopelvic inflammatory pseudotumor: report of four cases. Pediatr Radiol. (1997) 27:170–4. doi: 10.1007/s002470050093
16. Vasiljkovic BM, Plesinac Karapandzic V, Pejcic T, Djuric Stefanovic A, Milosevic Z, Plesinac S. Follow-up imaging of inflammatory myofibroblastic tumor of the uterus and its spontaneous regression. Iran J Radiol. (2016) 13:e12991. doi: 10.5812/iranjradiol.12991
17. Parra-Herran C, Scoolmeester JK, Yuan L, Dal Cin P, Fletcher CDM, Quade BJ, et al. Myxoid leiomyosarcoma of the uterus a clinicopathologic analysis of 30 cases and review of the literature with reappraisal of its distinction from other uterine myxoid mesenchymal neoplasms. Am J Surg Path. (2016) 40:285–301. doi: 10.1097/PAS.0000000000000593
Keywords: inflammatory, myofibroblastic, tumor, uterus, children
Citation: Etlinger P, Kuthi L and Kovács T (2020) Inflammatory Myofibroblastic Tumors in the Uterus: Childhood-Case Report and Review of the Literature. Front. Pediatr. 8:36. doi: 10.3389/fped.2020.00036
Received: 22 October 2019; Accepted: 24 January 2020;
Published: 14 February 2020.
Edited by:
Eugene S. Kim, University of Southern California, United StatesReviewed by:
José Estevão-Costa, São João University Hospital Center, PortugalLuca Pio, Hôpital Robert Debré, France
Ashley Walther, University of Southern California, United States
Copyright © 2020 Etlinger, Kuthi and Kovács. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Péter Etlinger, etlinger.peter@med.u-szeged.hu
 Péter Etlinger
Péter Etlinger Levente Kuthi
Levente Kuthi