Stress-induced changes in nociceptive responding post-surgery in preclinical rodent models
- 1Physiology, School of Medicine, University of Galway, Galway, Ireland
- 2Centre for Pain Research, University of Galway, Galway, Ireland
- 3Galway Neuroscience Centre, University of Galway, Galway, Ireland
- 4Pharmacology and Therapeutics, School of Medicine, University of Galway, Galway, Ireland
Chronic post-surgical pain affects up to 85% of individuals depending on the type of surgery, the extent of inflammation, tissue and/or nerve damage. Pre-surgical stress is associated with greater pain intensity, prolonged recovery and is one of the main risk factors for the development of chronic post-surgical pain. Clinically valid animal models provide an important means of examining the mechanisms underlying the effects of stress on post-surgical pain and identifying potential novel therapeutic targets. This review discusses the current data from preclinical animal studies examining the effect of stress on post-surgical pain, the potential underlying mechanisms and gaps in the knowledge that require further investigation.
1. Introduction
Chronic post-surgical pain is defined as chronic pain that develops or increases in intensity after a surgical procedure or a tissue injury and persists beyond the healing process, ie, at least 3 months after the surgery or tissue trauma (1). Chronic post-surgical pain affects between 5%–85% of individuals depending on the type of surgery, the extent of inflammation, tissue and/or nerve damage (1). Although the pathophysiological mechanisms underlying the development of chronic post-surgical pain and possible treatment strategies have been widely explored (for recent reviews see (2–4)), chronic post-surgical pain remains poorly treated and current prevention approaches have been proven inadequate. Pre-surgical stress is one of the main risk factors for the development of chronic post-surgical pain [for recent review see (5)]. While the ability to mount an appropriate response to stress is critical to maintain homeostasis, suboptimal or excessive stress responses can be maladaptive leading to short- and long-term changes in physiological functioning. Stress can range from short-term intense acute physical or psychological stress such as pre-surgical fear, to a more long-term persistent stress states such as catastrophizing or stress-related disorders such as depression. Indeed, pre-existing depression and pain catastrophizing are associated with greater pain intensity and/or a prolonged recovery in patients undergoing different surgical procedures (6–10). Furthermore, stress or injury in early life can induce long-term changes that have been shown to alter nociceptive pathways and pain responding in later-life. There has been a wealth of clinical and preclinical research examining the impact of stress on nociceptive responding in acute and chronic inflammatory and neuropathic pain conditions as well as the mechanisms underlying such effects [for reviews see (11, 12)]. The effect of stress on pain depends on the nature, intensity and duration of the stressor and the type of the pain under investigation. In general, brief acute intense stress is most commonly associated with reduced pain responding, termed stress-induced analgesia, although hyperalgesia has also been reported. Chronic persistent low-grade stress tends to be associated with increased pain responding, termed stress-induced hyperalgesia. Despite clinical observations demonstrating that pre-surgical stress enhances and prolongs post-surgical pain, there have been a paucity of preclinical animal studies in this area. Multiple mechanisms have been shown to mediate the effects of stress on pain, including enhanced hypothalamic-pituitary-adrenal axis activity, alterations in neurotransmitter systems, enhanced neuroimmune signaling and reduced descending inhibition (11, 12). However, the role of such mechanisms in stress-induced changes in nociceptive responding in preclinical animal post-surgical pain models has been limited. Such studies play a useful role in advancing our mechanistic understanding of the impact of acute, chronic or early life stress on post-surgical pain, and in the identification and characterisation of novel therapeutic approaches. This review provides an overview of the preclinical animal studies examining the effect of stress on post-surgical nociceptive responding, the potential underlying mechanisms involved and gaps in the knowledge.
2. The effect of stress on post-surgical nociceptive responding
2.1. The effect of acute short-term stress exposure on post-surgical nociceptive responding
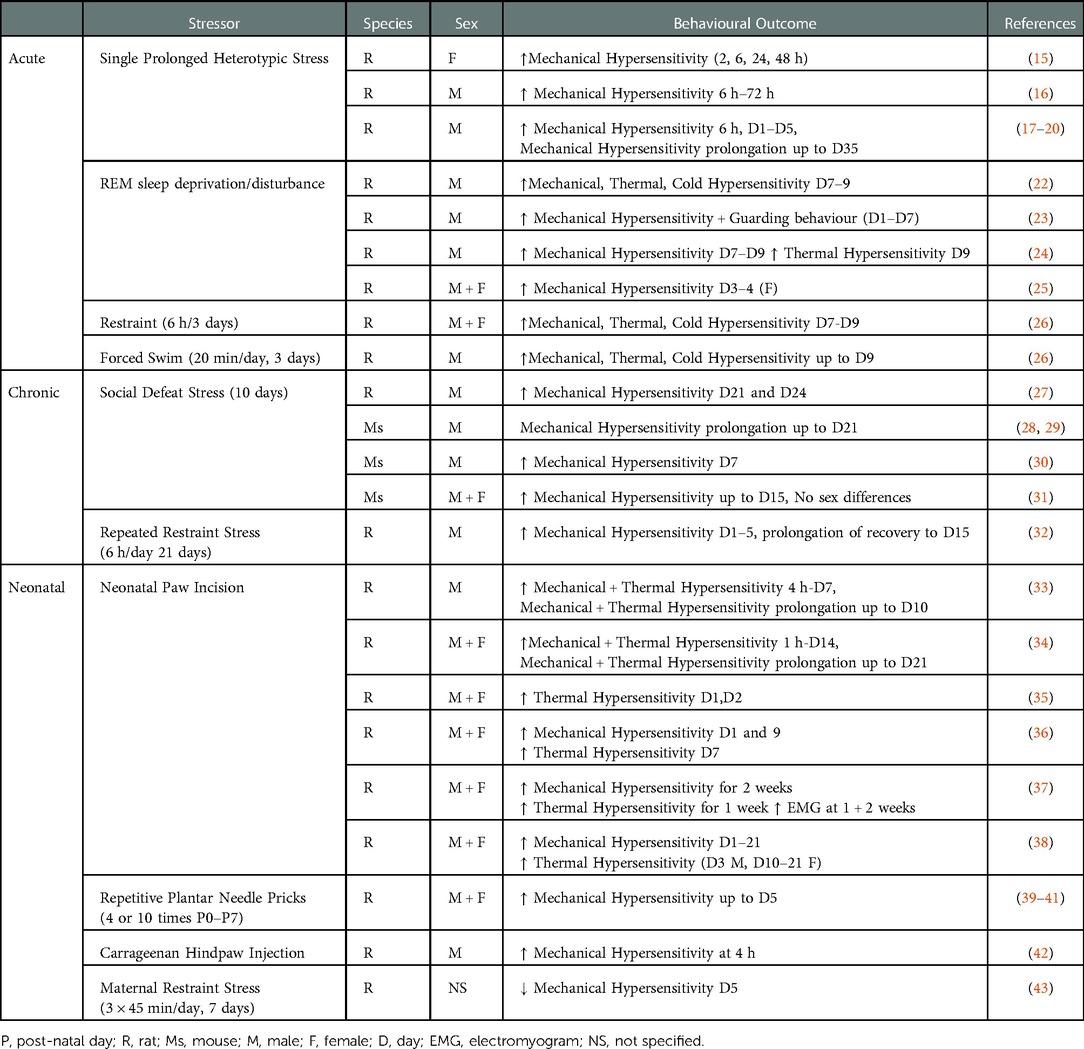
Several preclinical animal studies have investigated the effect of acute short-term stress exposure (up to 3 days) both pre- and post-surgery on nociceptive responding (see Table 1). The majority of these studies have used the paw incision model, originally described in rats by Brennan and colleagues (13) and adapted to mice by Pogatzki–Zahn and Raja (14). The effect of a single prolonged heterotypic stress episode (SPS) - which involves 2 h of restraint, 20 min of forced swim, 15 min of rest and ether inhalation until loss of consciousness - on nociceptive responding following paw incision, has been examined by several groups. SPS has been shown to increase the magnitude of mechanical hypersensitivity 6 h following surgery, an effect which persisted up to 48 h in female (15) and 72 h in male (16) rats. Mechanical withdrawal thresholds typically return to baseline levels 4–5 days post-paw incision surgery. However, male rats pre-exposed to SPS exhibited a decrease in paw withdrawal thresholds for up to 35 days (17–20) post-surgery, demonstrating that SPS prolongs the recovery of post-surgical mechanical hypersensitivity. Whether such effects are also observed in female rats remains to be determined. However, SPS has been shown to be associated with prolonged hysterectomy-induced mechanical hypersensitivity (21). Thus, SPS enhances and prolongs post-surgical mechanical hypersensitivity in both male and female rats, however the effects on other pain-related modalities and affective pain responding remain to be examined.
Perioperative sleep disturbance is a risk factor for the development of persistent pain after surgery (44–46). Pre-clinically, a single pre-operative exposure to REM sleep deprivation/disturbance (RSD) for 6 h has been shown to enhance mechanical hypersensitivity in female rats on day 3–4 post-surgery (25), while pre- or peri-operative RSD for 24 h or 3 days (6 h/day) increases mechanical, thermal and cold hypersensitivity in male rats for up to 9 days post paw-incision surgery (22–24) (Table 1). Immobilisation/restraint stress (6 h/day for 3 days), prior to and post paw-incision surgery has been shown to increase mechanical, thermal and cold hypersensitivity in both male and female rats up to day 9 post-surgery (26). Similarly, post-surgical exposure to repeated forced swim (20 min/day 1 h, 24 h, 48 h post-surgery) resulted in prolonged mechanical, heat and cold hypersensitivity in male rats for up to 9 days (26). Thus, taken together, the data suggest that exposure of rodents to multiple forms of acute short-term stress prior to, or following, surgery results in increased and prolonged mechanical and thermal hypersensitivity post-surgery.
2.2. The effect of chronic stress and anxio-depressive phenotype on post-surgical nociceptive responding
Chronic persistent stress is a risk factor for a host of psychiatric disorders including anxiety and depression. To date the effects of only two forms of chronic stress, social defeat stress (SDS) and repeated immobilization/restraint stress, have been examined on post-surgical pain responding in rodents. Male mice or rats subjected to pre-operative SDS over a period of 10–14 days exhibited an anxio-depressive-like phenotype associated with increased and prolonged mechanical hypersensitivity for up to 24 days post paw-incision surgery (27–30) (Table 1). Although female rodents have been reported to be resistant to the depressive-like phenotype associated with SDS (47), SDS results in an equivalent decrease in paw withdrawal thresholds in both male and female mice post-paw incision surgery (31). In modified versions of the SDS test, both chronic non-discriminatory SDS, in which male and female mice are simultaneously exposed to the aggressor, and vicarious SDS, which involves a mouse solely witnessing a social defeat, increased mechanical hypersensitivity for up to 15 days post-surgery (31). Thus, various forms of SDS can increase mechanical hypersensitivity of male and female rodents post-surgery. Exposure to repeated restraint/immobilisation (6 h/day for 21 days) results in a depressive-like phenotype (48, 49) and recent data has demonstrated that this increases and prolongs mechanical hypersensitivity in male rats up to day 15 post-paw-incision (32). Thus, stress-induced depressive and anxiety-like phenotypes in male rodents is associated with enhanced and prolonged post-surgical nociceptive responding to mechanical noxious stimuli. However, further studies are required to examine the effects of sex, other pain-related modalities and affective pain responding.
2.3. The effect of early-life stress on post-surgical pain responding
In addition to the effects of stress prior to and post-surgery, stress or injury in early life is associated with altered pain responding in later life. For example, maternal restraint stress results in anxiety and depressive-like behaviours (50), reduced thermal noxious thresholds and increased inflammatory pain responding (51) in the offspring. Interestingly, and in contrast with the effects reported above, post-surgical mechanical hypersensitivity was reduced in adult male offspring of dams that underwent maternal restraint stress (3 × 45 min/day, 7 days) (43). Thus, the effect of maternal stress on nociceptive responding is dependent on the noxious stimulus examined (thermal, mechanical post-surgery, inflammatory). While the effects of early-life psychological stress such as maternal separation, have been shown to alter long-term nociceptive responding in inflammatory and neuropathic pain models (52), to our knowledge there have been no studies examining effects on nociceptive responding post-surgery. However, injury in early-life which may represent a form of physical stress, such as hindpaw inflammation (53–55), full thickness skin wound (56), peripheral nerve injury (57, 58) and visceral injury (59–61), can significantly influence developing nociception-related pathways and lead to altered pain processing during adulthood. Several studies have demonstrated that hindpaw plantar incision during the first post-natal week increases mechanical and thermal hypersensitivity following subsequent re-incision during adulthood, lasting from the first post-surgical hours (33–36) up to 1–2 weeks later (33, 34, 36, 37) (Table 1). While much of the research in this area has been conducted in male rodents, Moriarty and colleagues (38) have demonstrated that post-operative mechanical and thermal hyperalgesia were equivalently enhanced in males and females. Similarly, Repetitive Plantar Needle Pricking (39–41) or neonatal hindpaw administration of carrageenan (42), further models of neonatal injury, are also associated with increased mechanical hypersensitivity post re-incision surgery in male adult rats. Thus, while the data indicate that neonatal injury/stress can profoundly alter post-surgical hypersensitivity, it should be noted that it is also not possible to disentangle the effects of early-life injury on stress from the possible direct effects on the nociceptive circuitry.
As described above, exposure to various types of stress prior to, and post, surgery can modulate both the magnitude and the duration of post-surgical somatosensory hypersensitivity. For the most part, prior acute or chronic stress exposure increases post-surgical nociceptive-related behaviour. These preclinical models provide a means of examining neurobiological substrates that may underlie the stress-induced exacerbation of post-surgical pain.
3. Mechanisms that may underlie stress-induced modulation of post-surgical pain
3.1. Hypothalamic-pituitary-adrenal (HPA) axis
Hypothalamic-pituitary-adrenal (HPA) axis dysfunction is well recognised in both chronic pain and affective disorders, and as such it is unsurprising that several studies have focused on alterations in the HPA axis in stress-induced exacerbation of post-surgical pain (Figure 1). Serum corticosterone levels have been shown to be increased post-surgery in rats subjected to RSD (22) or post-operative immobilization stress (26). Furthermore, glucocorticoid receptor inhibition during immobilisation stress or prior bilateral adrenalectomy, significantly decreased the duration of surgery-induced hypersensitivity to mechanical, thermal and cold stimuli (26). Although corticosterone levels were not altered post-surgery in the SPS model compared to non-surgery counterparts, glucocorticoid receptor inhibition prior to SPS exposure attenuates SPS-induced exacerbation of post-surgical mechanical hypersensitivity, potentiation of microglial activation and cytokine expression and decrease in GABAergic expression (16, 18). Thus, acute stress-induced activation of the HPA axis plays a key role in the enhanced post-surgical hypersensitivity. Further studies are required to elucidate the role of the HPA axis in chronic and early-life psychological stress-induced exacerbation of post-surgical pain/hypersensitivity. However, SDS is associated with decreased serum corticosterone levels post-paw incision (27) and adult rats that have been exposed to maternal restraint stress displayed decreased corticosteroid binding globulin (CBG) levels (43), indicating a possible role for the HPA axis in mediating or modulating chronic stress-induced modulation of post-surgical pain.
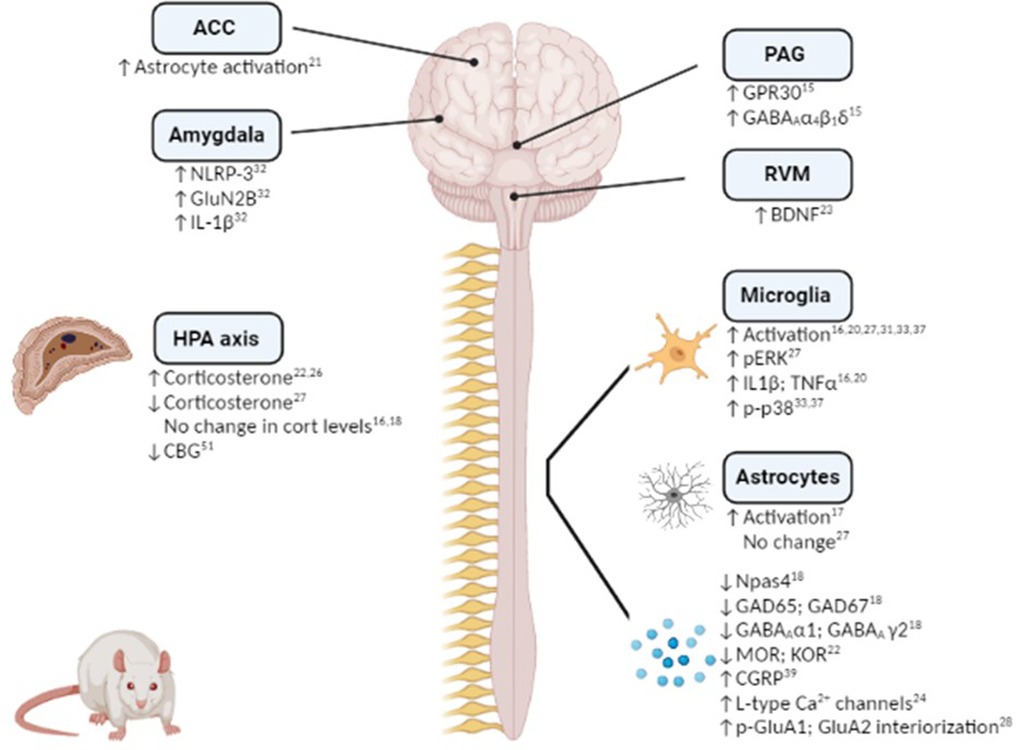
Figure 1. Mechanistic changes associated with stress-induced exacerbation and/or prolongation of post-surgical hypersensitivity in preclinical rodent models. Figure created using Biorender.
3.2. Neuroimmune system
Several studies have demonstrated a role for neuroimmune and glial involvement in stress-induced increase and prolongation of post-surgical hypersensitivity to noxious stimuli (Figure 1). For example, microglial activation and elevated expression of the proinflammatory cytokines IL-1β and TNF-α in the spinal cord have been shown to accompany SPS-induced increase in post-surgical mechanical hypersensitivity on Day 1, 3, 7 and 28 post-injury (16, 20). Similarly, microglial and pERK activation has been observed in the dorsal horn of the spinal cord 8 days following paw-incision surgery in rats pre-exposed to SDS compared to non-stressed counterparts (27). Perioperative intrathecal inhibition of microglial activation decreased IL-1β and TNF-α expression and shortened the duration of SDS-induced post-operative mechanical hypersensitivity by one week (20). Interestingly, spinal microglial activation was only observed in male, but not female, mice exposed to SDS and subsequent paw incision surgery (31), indicating possible sex differences in the mechanisms underlying stress-induced hypersensitivity. However, both male and female rats exposed to neonatal injury exhibited increased spinal microglial reactivity and phosphorylated-p38 up to 14 days following a second paw-incision surgery during adulthood (33, 37). Inhibition of microglial reactivity and p-p38 at the time of the second surgery prevented the increased mechanical hypersensitivity associated with neonatal injury in both sexes (33, 37). However, microglial inhibition at the time of the neonatal incision was associated with the prevention of enhanced mechanical and thermal hypersensitivity following re-incision in adulthood in males, but not females, revealing sex-dependent effects (38). Thus, the data highlight a key role for spinal microglia in stress-induced exacerbation of post-surgical pain in several models, an effect which appears to be sex specific.
In addition to microglial activation, enhanced spinal astrocyte activation has been reported in rats exposed to SPS and paw incision surgery, when compared to non-stressed counterparts (17). SPS-induced post-surgical mechanical hypersensitivity is associated with glucocorticoid-dependent release of ATP from spinal astrocytes (19), thus stress induced alterations in the HPA axis may drive astrocyte activation in the spinal cord and as a consequence mechanical hypersensitivity. Although surgery induces astrocyte activation in the spinal cord, Arora and colleagues reported this was not altered by prior SDS (27). Further research is require to determine if spinal astrocytes play a role in mediating or modulating stress-induced post-surgical hypersensitivity.
In addition to changes in the spinal cord there have been two studies to date that have examined neuroimmune alterations within the brain. Exacerbated mechanical hypersensitivity of female rats exposed to SPS + hysterectomy was associated with increased astrocyte activation in the anterior cingulate cortex (ACC) (21), if this change underlies the change in post-surgical nociceptive responding was not examined. In comparison, repeated restraint stress-induced post-surgical mechanical hypersensitivity was associated with NLRP-3 inflammasome-mediated IL-1β release in the basolateral amygdala (BLA) of male rats (32). Perioperative inhibition of NLRP-3 in the BLA attenuated both the magnitude and the duration of stress-induced post-surgical hypersensitivity (47), indicating a key role for neuroimmune changes in the amygdala in mediating stress-induced enhancement of post-surgical pain. Given the key role of the amygdala and the ACC in the emotional-affective components of post-surgical pain (62–65), future studies could examine if neuroimmune alterations in these regions may underlie stress-induced changes in sensory and affective responding post-surgery.
3.3. Neurotransmitter systems
3.3.1. GABA and glutamate
There is a wealth of evidence demonstrating roles for GABAergic and glutamatergic neurotransmission in the brain and spinal cord in mediating and modulating both stress and pain responding, as well as the interactions between these conditions [see (66, 67)]. A few studies have demonstrated alterations in these systems in stress-induced exacerbation of post-surgical mechanical hypersensitivity (Figure 1). Npas4 is a neuronal PAS domain protein that facilitates the development of glutamatergic and GABAergic synapses and preserves neuronal homeostasis (68). Npas4 was significantly decreased in the spinal cord of male rats exposed to SPS at 7, 10 and 14 days after surgery (18). Furthermore, reduced levels of glutamic acid decarboxylase (GAD)-65, GAD-67 and GABA type-A receptor α1 and γ2 subunits were noted, indicating an overall impairment of the GABAergic system. Glucocorticoid receptor antagonism restored the decrease of Npas4, reversed the impairment of the GABAergic system, and attenuated SPS-induced exacerbation of mechanical hypersensitivity (18). Additionally, the impairment in GABAergic expression was restored by overexpressing Npas4, and exacerbated by underexpression, highlighting a key role for Npas4 in the modulation of GABAergic signaling and the associated changes in post-operative mechanical hypersensitivity (18). Therefore, this study showed that pre-surgical SPS results in impairments in the GABAergic system and subsequent heightened mechanical hypersensitivity, effects which may be mediated by stress-induced HPA axis activation.
It is known that estrogen levels can be modulated by stress (69), and can in turn modulate pain (69, 70). Interestingly, levels of the estrogen receptor G-protein coupled receptor 30 (GPR30) are increased following acute restraint and forced swim stress exposure, an effect associated with an upregulation of GABAA receptor in the amygdala of female mice (71). Similarly, female rats that underwent SPS prior to paw incision displayed enhanced nociceptive hypersensitivity and up-regulated GPR30 and GABAA α4, β1 and δ levels in the periaqueductal gray (PAG) (15). Moreover, microinjection of the GPR30 antagonist G15 into the PAG significantly reduced GPR30, PKA and GABAA α4, β1 and δ levels and prevented SPS-induced post-operative mechanical hypersensitivity (15). Furthermore, intra-PAG injection of the GPR30 agonist G1 resulted in increased post-surgical mechanical hypersensitivity for up to 48 h post-incision (15). Thus, preoperative SPS-induced postoperative mechanical hypersensitivity in female rats is due at least in part to increased GPR30 and GABAA activity in the PAG, and if this is a sex-specific mechanism remains to be determined.
Alterations in glutamate signaling in stress-induced exacerbation of post-operative hypersensitivity to noxious stimuli have also been reported. Intrathecal administration of the spinal NMDA receptor agonists MK-DL-APV and L-NAME, 4 h after paw incision, reversed the incision-induced mechanical hypersensitivity in rats neonatally treated with carrageenan (42). Mice subjected to SDS exhibit enhanced AMPA GluA1 phosphorylation in the spinal cord 48 h post-paw incision surgery and prolonged post-surgical nociceptive responding for up to 40 days (28). GluA1 S831A-phospho-deficient mice showed a significant inhibition of SDS-induced prolongation of mechanical hypersensitivity after paw incision surgery (28), indicating a key role for spinal GluA1 in mediating the transition from acute to chronic post-surgical mechanical hypersensitivity. It was also noted that stress-induced AMPA receptor phosphorylation led to increased GluA2 internalization in the spinal dorsal horn neurons, causing AMPA receptor subunit switch from Ca2+ impermeable to Ca2+ permeable (28), a mechanism known to enhance persistent inflammatory hyperalgesia (72). Whether this mechanism also underlies stress-induced exacerbation of post-surgical pain remains to be determined. A key role for GluN2B receptors in the central amygdala has recently been reported with rats subjected to repeated restraint stress exhibited an up-regulation of GluN2B expression in the central nucleus of the amygdala 24 h following paw incision (32). Inhibition of NLRP3 in the basolateral amygdala resulted in reduced expression of GluN2B in the central nucleus of the amygdala, which in turn reversed the stress-induced exacerbation of post-surgical mechanical hypersensitivity (32). Taken together, the data suggest that alterations in the glutamatergic system in the spinal cord may mediate or modulate stress-induced exacerbation of post-surgical pain.
3.3.2. Endocannabinoids and opioids
Although there is limited data to date directly investigating the endocannabinoid and opioid systems in stress-induced exacerbation of post-surgical pain, given the well described role for these systems in the modulation of both stress and nociception (73–77), it is likely that they are also involved in this pathophysiological state. Furthermore, it has been reported that both of these systems are key players in stress-induced alterations in inflammatory and neuropathic nociceptive responding in several animal models (11). For example, impaired endocannabinoid signaling in the rostral ventromedial medulla (RVM) mediates hyper-responsivity to inflammatory noxious stimuli in Wistar–Kyoto rats, a strain that exhibits a stress-hyper-responsive phenotype (78). Administration of URB597, an inhibitor of the anandamide-degrading enzyme fatty acid amide hydrolase (FAAH), increased anandamide levels and decreased the mechanical hyperalgesia and anxiety-like behavior induced by chronic unpredictable stress in mice (79). Thus, while there is no direct evidence to date for a role for the endocannabinoid system in stress-induced exacerbation of post-surgical pain, data from other pain models suggest that impaired endocannabinoid signaling may be implicated. Similarly, in relation to the opioid system, several studies have demonstrated that alterations in this system play a key role in stress-induced hyperalgesia [for review see (76)]. Nevertheless, to our knowledge, only one study to date has examined whether stress-induced exacerbation of post-surgical hypersensitivity was associated with alterations in the opioidergic system. Wang and colleagues showed decreased mu- and kappa-opioid receptor expression in spinal cord and dorsal root ganglia (DRG) in male and female rats subjected to RSD and subsequent paw incision surgery (22). However, further studies are required to determine if such changes underlie the effects of stress on post-surgical nociceptive responding and/or effects on analgesic efficacy of opioids post-surgery.
3.4. Other mechanisms
The expression and activity of L-type calcium channels in the DRG was increased in RSD rats 9 days after paw incision surgery and blocking these channels significantly shortened the duration of mechanical and thermal hyperalgesia (24). Thus, in addition to changes at a central level, stress may induce alterations at the level of the DRGs that can modulate post-surgical hypersensitivity to noxious stimuli. Adenosine A2A receptors in the median preoptic nucleus are important for sleep regulation (80). Antagonism of adenosine A2A receptor in the median preoptic nucleus attenuates post-surgical mechanical hypersensitivity induced by RSD (25), revealing a key role of adenosine in sleep-post-surgical pain interactions. RSD rats subjected to paw incision exhibit increased BDNF levels in the RVM (23). Given the involvement of descending pain pathways in stress-induced hyperalgesia (11), it is possible the BDNF alterations in the RVM may play a role in stress-induced exacerbation of post-surgical pain.
Calcitonin gene-related peptide expression in the lumbar spinal cord (39) and orexin neurons activation in the lateral hypothalamus (35) has been shown to be increased in rats that were subjected to early-life injury followed by paw incision surgery during adulthood. Early-life repeated touch and needle-prick stimulation results in increased dorsal horn neurons sensitivity after paw-incision during adulthood (81). Thus, early-life events can modulate neural circuits related to pain processing and significantly alter nociceptive responding post-surgery in adulthood.
4. Discussion
This review has demonstrated that both pre- and/or post-surgical acute and chronic stress exposure increases both the magnitude and duration of post-operative somatosensory hypersensitivity. The effects of early-life stress on post-surgical pain in later life may depend on sex, the type and timing of the stress. Alterations in the HPA axis, neuroinflammation, neurotransmitter and neuromodulatory systems are a few of the mechanisms that are likely to mediate this pathophysiological state, although interplay among these several different systems is highly likely. However, important gaps in the knowledge remain to be addressed in future studies. Notably, most studies have to date focused on post-surgical mechanical hypersensitivity, with few studies examining responses to thermal nociceptive stimuli. Assessing the effects across a range of noxious and innocuous stimuli would be important given that clinically patients report different sensitivities to different stimuli post-surgery. It is important to note that withdrawal thresholds as measured in the aforementioned studies are reflexive in nature and as such may not incorporate the full spectrum of the chronic post-surgical pain response. To our knowledge, no study to date has examining effects of stress on spontaneous, affective or cognitive component of chronic post-surgical pain. Understanding the effects of stress on these latter components of the pain response is critical as these are the more widely reported changes associated with chronic post-surgical pain clinically. Furthermore, the studies to date have primarily used male rodents, thus ignoring possible behavioural and mechanistic sex differences. Surprisingly, the effect of a single short-term stress exposure on nociceptive responding post surgery has not been reported to date. Finally, the majority of preclinical animal studies in this field have using the hind paw-incision model, which although a useful as a screening tool, questions have been raised over its clinical relevance and validity. Assessing the impact of stress in more clinically relevant animal models such as the recently developed hernia repair model (82, 83) or laporatomy model of abdominal surgery (84) to name a few, may provide greater mechanistic and therapeutic insight for understanding chronic post-operative pain clinically. Future studies in this field will expand our understanding of the effect of acute and chronic stress on sensory and affective dimensions of chronic post-surgical pain in both males and females, laying the foundation for the development of novel sex-specific and personalised prevention strategies and treatments.
Author contributions
AB and AMD: wrote the article. MR and DPF: revised and reviewed the article. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the European Union’s Horizon 2020 research and innovation program under the Marie Skltodowska–Curie grant agreement grant no. 955684.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACC, anterior cingulate cortex; BDNF, brain-derived neurotrophic factor; CGB, corticosteroid binding globulin; CGRP, calcitonin gene-related peptide; DRG, dorsal root ganglia; GAD65, glutamic acid decarboxylase 65; GAD67, glutamic acid decarboxylase 67; GluN2B, glutamate receptor subunit epsilon-2; GPR30, G protein-coupled receptor 30; HPA, hypothalamic-pituitary-adrenal; IL-1β, interleukin-1 beta; KOR, kappa-opioid receptor; MOR, mu-opioid receptor; NLR family pyrin domain containing 3; Npas4, neuronal PAS domain protein 4; PAG, periaquductal grey; p-p38, phosphorylated p38; pERK, phosphorylated ERK; RSD, REM sleep deprivation/disturbance; RVM, rostral ventromedial medulla; SDS, social defeat stress; SPS, single prolonged heterotypic stress; TNFα, tumour necrosis factor alpha.
References
1. Schug SA, Lavand’homme P, Barke A, Korwisi B, Rief W, Treede RD, et al. The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain. (2019) 160(1):45–52. doi: 10.1097/j.pain.0000000000001413
2. Chapman CR, Vierck CJ. The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J Pain. (2017) 18(4):359.e1–38. doi: 10.1016/j.jpain.2016.11.004
3. Fregoso G, Wang A, Tseng K, Wang J. Transition from acute to chronic pain: evaluating risk for chronic postsurgical pain. Pain Physician. (2019) 22(5):479–88. Retrieved from: https://www.painphysicianjournal.com/linkout?issn=&vol=22&page=47931561647
4. Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain: pathophysiology and preventative pharmacologic considerations. Anesthesiology. (2018) 129(3):590–607. doi: 10.1097/ALN.0000000000002238
5. Rosenberger DC, Pogatzki-Zahn EM. Chronic post-surgical pain - update on incidence, risk factors and preventive treatment options. BJA Educ. (2022) 22(5):190–6. doi: 10.1016/j.bjae.2021.11.008
6. Khan RS, Skapinakis P, Ahmed K, Stefanou DC, Ashrafian H, Darzi A, et al. The association between preoperative pain catastrophizing and postoperative pain intensity in cardiac surgery patients. Pain Med. (2012) 13(6):820–7. doi: 10.1111/j.1526-4637.2012.01386.x
7. Poole L, Ronaldson A, Kidd T, Leigh E, Jahangiri M, Steptoe A. Pre-surgical depression and anxiety and recovery following coronary artery bypass graft surgery. J Behav Med. (2017) 40(2):249–58. doi: 10.1007/s10865-016-9775-1
8. Dunn LK, Durieux ME, Fernandez LG, Tsang S, Smith-Straesser EE, Jhaveri HF, et al. Influence of catastrophizing, anxiety, and depression on in-hospital opioid consumption, pain, and quality of recovery after adult spine surgery. J Neurosurg Spine. (2018) 28(1):119–26. doi: 10.3171/2017.5.SPINE1734
9. AbuRuz ME. Pre-operative depression predicted longer hospital length of stay among patients undergoing coronary artery bypass graft surgery. Risk Manag Healthc Policy. (2019) 12:75–83. doi: 10.2147/RMHP.S190511
10. Gohari J, Grosman-Rimon L, Arazi M, Caspi-Avissar N, Granot D, Gleitman S, et al. Clinical factors and pre-surgical depression scores predict pain intensity in cardiac surgery patients. BMC Anesthesiol. (2022) 22(1):204. doi: 10.1186/s12871-022-01740-3
11. Jennings EM, Okine BN, Roche M, Finn DP. Stress-induced hyperalgesia. Prog Neurobiol. (2014) 121:1–18. doi: 10.1016/j.pneurobio.2014.06.003
12. Olango WM, Finn DP. Neurobiology of stress-induced hyperalgesia. Curr Top Behav Neurosci. (2014) 20:251–80. doi: 10.1007/7854_2014_302
13. Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. (1996) 64(3):493–502. doi: 10.1016/0304-3959(95)01441-1
14. Pogatzki EM, Raja SN. A mouse model of incisional pain. Anesthesiology. (2003) 99(4):1023–7. doi: 10.1097/00000542-200310000-00041
15. Jiang M, Sun Y, Lei Y, Hu F, Xia Z, Liu Y, et al. GPR30 receptor promotes preoperative anxiety-induced postoperative hyperalgesia by up-regulating GABAA-alpha4beta1delta subunits in periaqueductal gray in female rats. BMC Anesthesiol. (2020) 20(1):93. doi: 10.1186/s12871-020-01017-7
16. Sun R, Zhao Z, Feng J, Bo J, Rong H, Lei Y, et al. Glucocorticoid-potentiated spinal microglia activation contributes to preoperative anxiety-induced postoperative hyperalgesia. Mol Neurobiol. (2017) 54(6):4316–28. doi: 10.1007/s12035-016-9976-1
17. Liu Y, Hou B, Zhang W, Sun YE, Li L, Ma Z, et al. The activation of spinal astrocytes contributes to preoperative anxiety-induced persistent post-operative pain in a rat model of incisional pain. Eur J Pain. (2015) 19(5):733–40. doi: 10.1002/ejp.596
18. Wu H, Huang Y, Tian X, Zhang Z, Zhang Y, Mao Y, et al. Preoperative anxiety-induced glucocorticoid signaling reduces GABAergic markers in spinal cord and promotes postoperative hyperalgesia by affecting neuronal PAS domain protein 4. Mol Pain. (2019) 15:1744806919850383. doi: 10.1177/1744806919850383
19. Zhang Z, Wu H, Liu Y, Gu X, Zhang W, Ma Z. The GCs-SGK1-ATP signaling pathway in spinal astrocytes underlied presurgical anxiety-induced postsurgical hyperalgesia. Anesth Analg. (2019) 129(4):1163–9. doi: 10.1213/ANE.0000000000003682
20. Sun R, Liu Y, Hou B, Lei Y, Bo J, Zhang W, et al. Perioperative activation of spinal alpha7 nAChR promotes recovery from preoperative stress-induced prolongation of postsurgical pain. Brain Behav Immun. (2019) 79:294–308. doi: 10.1016/j.bbi.2019.02.017
21. Gu D, Zhou M, Han C, Lei D, Xie S, Yuan Y, et al. Preoperative anxiety induces chronic postoperative pain by activating astrocytes in the anterior cingulate cortex region. Rev Assoc Med Bras. (2019) 65(9):1174–80. doi: 10.1590/1806-9282.65.9.1174
22. Wang PK, Cao J, Wang H, Liang L, Zhang J, Lutz BM, et al. Short-term sleep disturbance-induced stress does not affect basal pain perception, but does delay postsurgical pain recovery. J Pain. (2015) 16(11):1186–99. doi: 10.1016/j.jpain.2015.07.006
23. Xue J, Li H, Xu Z, Ma D, Guo R, Yang K, et al. Paradoxical sleep deprivation aggravates and prolongs incision-induced pain hypersensitivity via BDNF signaling-mediated descending facilitation in rats. Neurochem Res. (2018) 43(12):2353–61. doi: 10.1007/s11064-018-2660-2
24. Li Q, Zhu ZY, Lu J, Chao YC, Zhou XX, Huang Y, et al. Sleep deprivation of rats increases postsurgical expression and activity of L-type calcium channel in the dorsal root ganglion and slows recovery from postsurgical pain. Acta Neuropathol Commun. (2019) 7(1):217. doi: 10.1186/s40478-019-0868-2
25. Hambrecht-Wiedbusch VS, Gabel M, Liu LJ, Imperial JP, Colmenero AV, Vanini G. Preemptive caffeine administration blocks the increase in postoperative pain caused by previous sleep loss in the rat: a potential role for preoptic adenosine A2A receptors in sleep-pain interactions. Sleep. (2017) 40(9):1–15. doi: 10.1093/sleep/zsx116
26. Cao J, Wang PK, Tiwari V, Liang L, Lutz BM, Shieh KR, et al. Short-term pre- and post-operative stress prolongs incision-induced pain hypersensitivity without changing basal pain perception. Mol Pain. (2015) 11:73. doi: 10.1186/s12990-015-0077-3
27. Arora V, Martin TJ, Aschenbrenner CA, Hayashida K, Kim SA, Parker RA, et al. Psychosocial stress delays recovery of postoperative pain following incisional surgery in the rat. Neuroscience. (2018) 382:35–47. doi: 10.1016/j.neuroscience.2018.04.014
28. Li C, Yang Y, Liu S, Fang H, Zhang Y, Furmanski O, et al. Stress induces pain transition by potentiation of AMPA receptor phosphorylation. J Neurosci. (2014) 34(41):13737–46. doi: 10.1523/JNEUROSCI.2130-14.2014
29. Aizawa F, Nakamoto K, Tokuyama S. The involvement of free fatty acid-GPR40/FFAR1 signaling in chronic social defeat stress-induced pain prolongation in C57BL/6J male mice. Psychopharmacology. (2018) 235(8):2335–47. doi: 10.1007/s00213-018-4930-8
30. Aizawa F, Sato S, Yamazaki F, Yao I, Yamashita T, Nakamoto K, et al. N-3 fatty acids modulate repeated stress-evoked pain chronicity. Brain Res. (2019) 1714:218–26. doi: 10.1016/j.brainres.2019.03.001
31. Wang W, Liu WZ, Wang ZL, Duan DX, Wang XY, Liu SJ, et al. Spinal microglial activation promotes perioperative social defeat stress-induced prolonged postoperative pain in a sex-dependent manner. Brain Behav Immun. (2022) 100:88–104. doi: 10.1016/j.bbi.2021.11.010
32. Meng Y, Zhuang L, Xue Q, Zhang J, Yu B. NLRP3-mediated neuroinflammation exacerbates incisional hyperalgesia and prolongs recovery after surgery in chronic stressed rats. Pain Physician. (2021) 24(7):E1099–108. Retrieved from: https://www.painphysicianjournal.com/linkout?issn=&vol=24&page=E109934704719
33. Schwaller F, Beggs S, Walker SM. Targeting p38 mitogen-activated protein kinase to reduce the impact of neonatal microglial priming on incision-induced hyperalgesia in the adult rat. Anesthesiology. (2015) 122(6):1377–90. doi: 10.1097/ALN.0000000000000659
34. Ding X, Liang YJ, Su L, Liao FF, Fang D, Tai J, et al. BDNF Contributes to the neonatal incision-induced facilitation of spinal long-term potentiation and the exacerbation of incisional pain in adult rats. Neuropharmacology. (2018) 137:114–32. doi: 10.1016/j.neuropharm.2018.04.032
35. Low LA, Fitzgerald M. Acute pain and a motivational pathway in adult rats: influence of early life pain experience. PLoS One. (2012) 7(3):e34316. doi: 10.1371/journal.pone.0034316
36. Moriarty O, Harrington L, Beggs S, Walker SM. Opioid analgesia and the somatosensory memory of neonatal surgical injury in the adult rat. Br J Anaesth. (2018) 121(1):314–24. doi: 10.1016/j.bja.2017.11.111
37. Beggs S, Currie G, Salter MW, Fitzgerald M, Walker SM. Priming of adult pain responses by neonatal pain experience: maintenance by central neuroimmune activity. Brain. (2012) 135(Pt 2):404–17. doi: 10.1093/brain/awr288
38. Moriarty O, Tu Y, Sengar AS, Salter MW, Beggs S, Walker SM. Priming of adult incision response by early-life injury: neonatal microglial inhibition has persistent but sexually dimorphic effects in adult rats. J Neurosci. (2019) 39(16):3081–93. doi: 10.1523/JNEUROSCI.1786-18.2019
39. Knaepen L, Patijn J, Tibboel D, Joosten EA. Sex differences in inflammatory mechanical hypersensitivity in later life of rats exposed to repetitive needle pricking as neonates. Neurosci Lett. (2012) 516(2):285–9. doi: 10.1016/j.neulet.2012.04.012
40. van den Hoogen NJ, Tibboel D, Honig WM, Hermes D, Patijn J, Joosten EA. Neonatal paracetamol treatment reduces long-term nociceptive behaviour after neonatal procedural pain in rats. Eur J Pain. (2016) 20(8):1309–18. doi: 10.1002/ejp.855
41. van den Hoogen NJ, Patijn J, Tibboel D, Joosten EA. Repetitive noxious stimuli during early development affect acute and long-term mechanical sensitivity in rats. Pediatr Res. (2020) 87(1):26–31. doi: 10.1038/s41390-019-0420-x
42. Chu YC, Chan KH, Tsou MY, Lin SM, Hsieh YC, Tao YX. Mechanical pain hypersensitivity after incisional surgery is enhanced in rats subjected to neonatal peripheral inflammation: effects of N-methyl-D-aspartate receptor antagonists. Anesthesiology. (2007) 106(6):1204–12. doi: 10.1097/01.anes.0000267604.40258.d1
43. Knaepen L, Rayen I, Charlier TD, Fillet M, Houbart V, van Kleef M, et al. Developmental fluoxetine exposure normalizes the long-term effects of maternal stress on post-operative pain in Sprague–Dawley rat offspring. PLoS One. (2013) 8(2):e57608. doi: 10.1371/journal.pone.0057608
44. Bjurstrom MF, Irwin MR, Bodelsson M, Smith MT, Mattsson-Carlgren N. Preoperative sleep quality and adverse pain outcomes after total hip arthroplasty. Eur J Pain. (2021) 25(7):1482–92. doi: 10.1002/ejp.1761
45. Poole L, Kidd T, Leigh E, Ronaldson A, Jahangiri M, Steptoe A. Preoperative sleep complaints are associated with poor physical recovery in the months following cardiac surgery. Ann Behav Med. (2014) 47(3):347–57. doi: 10.1007/s12160-013-9557-8
46. Wang JP, Lu SF, Guo LN, Ren CG, Zhang ZW. Poor preoperative sleep quality is a risk factor for severe postoperative pain after breast cancer surgery a prospective cohort study. Medicine. (2019) 98(44):e17708. doi: 10.1097/MD.0000000000017708
47. Palanza P, Parmigiani S. How does sex matter? Behavior, stress and animal models of neurobehavioral disorders. Neurosci Biobehav Rev. (2017) 76(Pt A):134–43. doi: 10.1016/j.neubiorev.2017.01.037
48. Zain MA, Pandy V, Majeed ABA, Wong WF, Mohamed Z. Chronic restraint stress impairs sociability but not social recognition and spatial memoryin C57BL/6J mice. Exp Anim. (2019) 68(1):113–24. doi: 10.1538/expanim.18-0078
49. Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo X, Sheridan JF, et al. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS One. (2013) 8(3):e58488. doi: 10.1371/journal.pone.0058488
50. Weinstock M. Prenatal stressors in rodents: effects on behavior. Neurobiol Stress. (2017) 6:3–13. doi: 10.1016/j.ynstr.2016.08.004
51. Knaepen L, Pawluski JL, Patijn J, van Kleef M, Tibboel D, Joosten EA. Perinatal maternal stress and serotonin signaling: effects on pain sensitivity in offspring. Dev Psychobiol. (2014) 56(5):885–96. doi: 10.1002/dev.21184
52. Burke NN, Finn DP, McGuire BE, Roche M. Psychological stress in early life as a predisposing factor for the development of chronic pain: clinical and preclinical evidence and neurobiological mechanisms. J Neurosci Res. (2017) 95(6):1257–70. doi: 10.1002/jnr.23802
53. Walker SM, Meredith-Middleton J, Cooke-Yarborough C, Fitzgerald M. Neonatal inflammation and primary afferent terminal plasticity in the rat dorsal horn. Pain. (2003) 105(1-2):185–95. doi: 10.1016/S0304-3959(03)00201-X
54. Hohmann AG, Neely MH, Pina J, Nackley AG. Neonatal chronic hind paw inflammation alters sensitization to intradermal capsaicin in adult rats: a behavioral and immunocytochemical study. J Pain. (2005) 6(12):798–808. doi: 10.1016/j.jpain.2005.07.009
55. LaPrairie JL, Murphy AZ. Female rats are more vulnerable to the long-term consequences of neonatal inflammatory injury. Pain. (2007) 132(Suppl 1):S124–33. doi: 10.1016/j.pain.2007.08.010
56. Reynolds ML, Fitzgerald M. Long-term sensory hyperinnervation following neonatal skin wounds. J Comp Neurol. (1995) 358(4):487–98. doi: 10.1002/cne.903580403
57. Howard RF, Walker SM, Mota MP, Fitzgerald M. The ontogeny of neuropathic pain: postnatal onset of mechanical allodynia in rat spared nerve injury (SNI) and chronic constriction injury (CCI) models. Pain. (2005) 115(3):382–9. doi: 10.1016/j.pain.2005.03.016
58. Vega-Avelaira D, McKelvey R, Hathway G, Fitzgerald M. The emergence of adolescent onset pain hypersensitivity following neonatal nerve injury. Mol Pain. (2012) 8:30. doi: 10.1186/1744-8069-8-30
59. Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. (2000) 119(5):1276–85. doi: 10.1053/gast.2000.19576
60. Randich A, Uzzell T, DeBerry JJ, Ness TJ. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain. (2006) 7(7):469–79. doi: 10.1016/j.jpain.2006.01.450
61. Wang J, Gu C, Al-Chaer ED. Altered behavior and digestive outcomes in adult male rats primed with minimal colon pain as neonates. Behav Brain Funct. (2008) 4:28. doi: 10.1186/1744-9081-4-28
62. Thompson JM, Neugebauer V. Amygdala plasticity and pain. Pain Res Manag. (2017) 2017:8296501. doi: 10.1155/2017/8296501
63. Seno MDJ, Assis DV, Gouveia F, Antunes GF, Kuroki M, Oliveira CC, et al. The critical role of amygdala subnuclei in nociceptive and depressive-like behaviors in peripheral neuropathy. Sci Rep. (2018) 8(1):13608. doi: 10.1038/s41598-018-31962-w
64. Barthas F, Sellmeijer J, Hugel S, Waltisperger E, Barrot M, Yalcin I. The anterior cingulate cortex is a critical hub for pain-induced depression. Biol Psychiatry. (2015) 77(3):236–45. doi: 10.1016/j.biopsych.2014.08.004
65. Sellmeijer J, Mathis V, Hugel S, Li XH, Song Q, Chen QY, et al. Hyperactivity of anterior cingulate Cortex areas 24a/24b drives chronic pain-induced anxiodepressive-like consequences. J Neurosci. (2018) 38(12):3102–15. doi: 10.1523/JNEUROSCI.3195-17.2018
66. Lau BK, Vaughan CW. Descending modulation of pain: the GABA disinhibition hypothesis of analgesia. Curr Opin Neurobiol. (2014) 29:159–64. doi: 10.1016/j.conb.2014.07.010
67. Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. (2017) 2017:9724371. doi: 10.1155/2017/9724371
68. Sun X, Lin Y. Npas4: linking neuronal activity to memory. Trends Neurosci. (2016) 39(4):264–75. doi: 10.1016/j.tins.2016.02.003
69. Sorge RE, Totsch SK. Sex differences in pain. J Neurosci Res. (2017) 95(6):1271–81. doi: 10.1002/jnr.23841
70. Chen Q, Zhang W, Sadana N, Chen X. Estrogen receptors in pain modulation: cellular signaling. Biol Sex Differ. (2021) 12(1):22. doi: 10.1186/s13293-021-00364-5
71. Tian Z, Wang Y, Zhang N, Guo YY, Feng B, Liu SB, et al. Estrogen receptor GPR30 exerts anxiolytic effects by maintaining the balance between GABAergic and glutamatergic transmission in the basolateral amygdala of ovariectomized mice after stress. Psychoneuroendocrinology. (2013) 38(10):2218–33. doi: 10.1016/j.psyneuen.2013.04.011
72. Kopach O, Krotov V, Goncharenko J, Voitenko N. Inhibition of spinal ca(2+)-permeable AMPA receptors with dicationic compounds alleviates persistent inflammatory pain without adverse effects. Front Cell Neurosci. (2016) 10:50. doi: 10.3389/fncel.2016.00050
73. Corcoran L, Roche M, Finn DP. The role of the brain's endocannabinoid system in pain and its modulation by stress. Int Rev Neurobiol. (2015) 125:203–55. doi: 10.1016/bs.irn.2015.10.003
74. Finn DP. Endocannabinoid-mediated modulation of stress responses: physiological and pathophysiological significance. Immunobiology. (2010) 215(8):629–46. doi: 10.1016/j.imbio.2009.05.011
75. Atwal N, Winters BL, Vaughan CW. Endogenous cannabinoid modulation of restraint stress-induced analgesia in thermal nociception. J Neurochem. (2020) 152(1):92–102. doi: 10.1111/jnc.14884
76. Ferdousi M, Finn DP. Stress-induced modulation of pain: role of the endogenous opioid system. Prog Brain Res. (2018) 239:121–77. doi: 10.1016/bs.pbr.2018.07.002
77. Pol O. The role of carbon monoxide, heme oxygenase 1, and the Nrf2 transcription factor in the modulation of chronic pain and their interactions with opioids and cannabinoids. Med Res Rev. (2021) 41(1):136–55. doi: 10.1002/med.21726
78. Rea K, Olango WM, Okine BN, Madasu MK, McGuire IC, Coyle K, et al. Impaired endocannabinoid signalling in the rostral ventromedial medulla underpins genotype-dependent hyper-responsivity to noxious stimuli. Pain. (2014) 155(1):69–79. doi: 10.1016/j.pain.2013.09.012
79. Lomazzo E, Bindila L, Remmers F, Lerner R, Schwitter C, Hoheisel U, et al. Therapeutic potential of inhibitors of endocannabinoid degradation for the treatment of stress-related hyperalgesia in an animal model of chronic pain. Neuropsychopharmacology. (2015) 40(2):488–501. doi: 10.1038/npp.2014.198
80. Kumar S, Rai S, Hsieh KC, McGinty D, Alam MN, Szymusiak R. Adenosine A(2A) receptors regulate the activity of sleep regulatory GABAergic neurons in the preoptic hypothalamus. Am J Physiol Regul Integr Comp Physiol. (2013) 305(1):R31–41. doi: 10.1152/ajpregu.00402.2012
81. van den Hoogen NJ, Patijn J, Tibboel D, Joosten BA, Fitzgerald M, Kwok CHT. Repeated touch and needle-prick stimulation in the neonatal period increases the baseline mechanical sensitivity and postinjury hypersensitivity of adult spinal sensory neurons. Pain. (2018) 159(6):1166–75. doi: 10.1097/j.pain.0000000000001201
82. Bree D, Moriarty O, O'Mahony CM, Morris B, Bannerton K, Broom DC, et al. Development and characterization of a novel, anatomically relevant rat model of acute postoperative pain. J Pain. (2015) 16(5):421–35.e1-6. doi: 10.1016/j.jpain.2015.01.010
83. Bree D, Moriarty O, Broom DC, Kelly JP, Roche M, Finn DP. Characterization of the affective component of acute postoperative pain associated with a novel rat model of inguinal hernia repair pain. CNS Neurosci Ther. (2016) 22(2):146–53. doi: 10.1111/cns.12483
84. Oliver VL, Thurston SE, Lofgren JL. Using cageside measures to evaluate analgesic efficacy in mice (Mus musculus) after surgery. J Am Assoc Lab Anim Sci. (2018) 57(2):186–201. Retrieved from: https://www.ingentaconnect.com/content/aalas/jaalas/2018/00000057/00000002/art00012#29555008
Keywords: allodynia, surgery, anxiety, depression, stress, von frey, neuroimmune
Citation: Bella A, Diego AM, Finn DP and Roche M (2023) Stress-induced changes in nociceptive responding post-surgery in preclinical rodent models. Front. Pain Res. 3:1106143. doi: 10.3389/fpain.2022.1106143
Received: 23 November 2022; Accepted: 19 December 2022;
Published: 10 January 2023.
Edited by:
Fabien Marchand, INSERM U1107 Douleur et Biophysique Neurosensorielle (Neuro-Dol), FranceReviewed by:
Cyril Rivat, Université de Montpellier, France© 2023 Bella, Diego, Finn and Roche. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michelle Roche michelle.roche@universityofgalway.ie
Specialty Section: This article was submitted to Pain Mechanisms, a section of the journal Frontiers in Pain Research
 Ariadni Bella1,2,3
Ariadni Bella1,2,3  Michelle Roche
Michelle Roche