Pain Management in a Model of Interstitial Cystitis/Bladder Pain Syndrome by a Vaccinal Strategy
- 1Urosphere, Department of Pain and Inflammation, Toulouse, France
- 2IRSD, Université de Toulouse, INSERM, INRA, ENVT, UPS, Toulouse, France
- 3Department of Physiology and Pharmacology, Faculty of Medicine, University of Calgary, Calgary, AB, Canada
- 4Urology Department, Rangueil University Hospital, Toulouse, France
- 5INSERM, I2MC-U1048, CHU Rangueil, Toulouse, France
Current analgesic treatments for Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) are limited. Here, we propose a novel antinociceptive strategy exploiting the opioid-mediated analgesic properties of T lymphocytes to relieve from bladder pain. In a chronic model of IC/BPS in rats, we show that a secondary T cell response against intravesically administered ovalbumin prevents from visceral pain in OVA-primed animals. The analgesic effect is associated with the recruitment of T lymphocytes within the inflamed mucosa and is reversed by naloxone-methiodide, a peripheral opioid receptor antagonist. Similarly, intravesical instillation of BCG or tetanus toxoid antigens in vaccinated rats protects from pain in the same model. We show opioid-dependent analgesic properties of local vaccine antigen recall in a preclinical rat model of chronic cystitis. Since BCG bladder instillation is regularly used in humans (as anticancer therapy), our results open it as a new therapeutic positioning for a pain management indication for IC/BPS patients.
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic disorder characterized by pelvic pain, pressure or discomfort perceived to be related to the urinary bladder accompanied by at least one other urinary symptom such as persistent urge to void or frequency of voiding (1). Although the etiology and pathogenesis of IC/BPS have not yet been elucidated, numerous hypotheses including defects of the urothelial barrier, autoimmunity and neurogenic disorder have been proposed (2).
A number of therapies have been proposed to treat IC/BPS but, to date, only pentosan polysulfate (Elmiron®) medication is approved by United States Food and Drug Administration (FDA). Pentosan polysulfate is an oral heparinoid that would induce the regeneration of the urothelial glycosaminoglycan layer. A meta-analysis concluded, however, to a significant pain improvement only in 20–37% of the patients (3). Intravesical treatment with dimethyl sulfoxide (DMSO) display anti-inflammatory and muscle relaxant effects but seems to be efficient only in patients with Hunner's ulcers who represent <7% of IC/PBS patients (4). Other treatments dedicated to alleviate IC/BPS-related vesical pain, including non-steroidal anti-inflammatory drugs (NSAIDs), the anticonvulsant gabapentin (5) or opioid drugs (6), are frequently used but their efficacy remains limited and cause undesirable side effects (7).
It is now widely accepted that T lymphocytes display analgesic activity dependent on their ability to produce and locally release endogenous opioids (8–16). We have previously demonstrated that this property requires their activation by antigens (12, 17). Because peripheral delivery of opioids is preferred to achieve analgesia, mainly because it avoids central side effects (18–22), we hypothesize that, as previously shown in intestinal inflammatory disorders (23), recruitment of opioid-producing T cells may represent an interesting strategy to alleviate pain in IC/BPS (24, 25). Therefore, a vaccine strategy that triggers both T cell recruitment and local opioid release appears as a promising therapeutic option in the pain management in IC/BPS.
In this study, we presented a new anti-nociceptive potency of vaccine strategy exploiting the analgesic properties of T lymphocytes.
Materials and Methods
Animals
Considering that IC/BPS in men is relatively unusual, experiments were performed on female animals. Six to seven weeks-old Sprague-Dawley female rats (Janvier Labs, Saint-Berthevin, France) were acclimatized to the laboratory conditions for at least 3 days before starting experiments. Two or three rats were housed in polysulfone type Sealsafe plus GR900 cages (Tecniplast, Lyon, France) on a bed of wood chips (1200, Souralit, Girona, Spain) with free access to food (Rodent Maintenance Diet A04/10 from Safe) and water (0.2 μm filtered water) ad libitum. Species-appropriate environmental enrichment (Aspen brick, Plexx, Uden, Netherlands) was added in the cages. Animals were maintained under artificial lighting between 7:00 am to 7:00 pm (12 h) in a controlled ambient temperature of 22 ± 2°C, and relative humidity at 55 ± 10%. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the European Council Directive (2010/63/UE) and were approved by French Animal Ethical Committee (application number CEEA-122-2014-28 and APAFIS#16506-2018082411278474).
Vaccination and Induction of Cystitis
Animals were primed by subcutaneous injection of BCG vaccine (0.5 to 2 × 105 UFC; 25 μL) (Statens Serum Institut, Copenhagen, Denmark), tetanus toxoid vaccine (40 IU/0.5 ml; 100 μl) (Sanofi Pasteur MSD SNC, Lyon, France) or OVA (200 μg) emulsified in complete Freund's adjuvant (CFA). Antigen recall was performed 2 weeks later with the same amounts of BCG vaccine, tetanus toxoid vaccine or OVA in incomplete Freund's adjuvant (IFA). One month after immunization, chronic cystitis was induced by 3 i.p. injections of CYP (Fisher-Scientific, Illkirch, France) 3 days apart (on days 0, 3 and 6) at 40 mg/kg as previously described (26). For antigen challenge, animals received intravesical instillation (0.8 ml) of either BCG (3 × 105 to 5 × 106 UFC) (Medac, Hamburg, Germany), a mixture of two tetanus immunodominant toxoid-derived peptides (IDKISDVSTIVPYIGPALNI and NNFTVSFWLRVPKVSASHLE) (50 μg/ml) (27, 28), OVA peptide 323–339 (ISQAVHAAHAEINEAGR) (5 mg/ml) (29, 30), OVA (1 mg/ml) or BSA (1 mg/ml) on days 3, 6, and 7. When indicated, rats were intraperitoneally injected with 200 μl naloxone-methiodide (10 mg/ml) at day 10 (11, 31). The experimental design is shown in Figure 1.
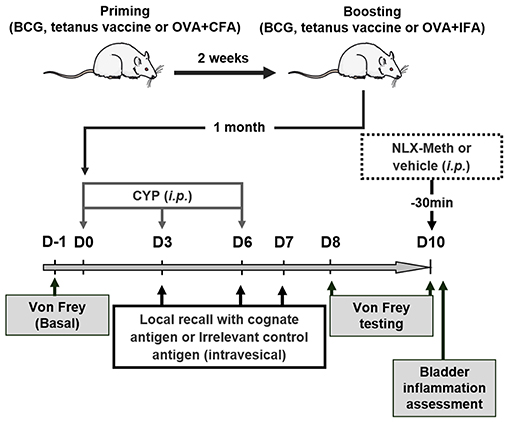
Figure 1. Experimental design. Rats were first vaccinated (immunized) 2 weeks apart. One month later, chronic cystitis was induced by three injections of CYP (40 mg/kg, i.p.) at days 0, 3, and 6. On day 3, 6, and 7 following the first injection of CYP, antigens previously used for vaccination (immunization) or irrelevant control antigens were intravesically instilled. Nociceptive response to mechanical stimuli (von Frey filaments) applied to vicinity of the bladder was performed 1 day before injecting CYP (D-1, basal bladder sensitivity) and at days 8 and 10 following the first injection of CYP to evaluate the effects of the vaccine strategy on CYP-induced chronic visceral pain. The role of peripheral opioid receptors in anti-nociceptive effects was investigated at day 10 using a general opioid receptor antagonist; the naloxone-methiodide (NLX-Meth, 10 mg/ml, i.p.), injected 30 min prior von Frey testing.
Pain and Bladder Inflammation Assessment
Before the experiment starts, the animals were randomly assigned to treatment groups using the block method, which consists of distributing at least one animal per treatment or control in the same block. In addition, a different position in the von Frey chamber was assigned for animals of the same treatment group. Nociceptive response was assessed using von Frey filaments (26, 32). Scoring of nociceptive response was as follows: 0 = no response; 1 = reaction of the animal (e.g., retraction of the abdomen); 2 = reaction of the animal and change of position; 3 = reaction of the animal, change of position, licking of the area stimulated and/or vocalization. Macroscopic evaluation of bladder inflammation was performed as previously described (26). Bladder wall thickness was measured with an electronic caliper. Edema scoring was based on Gray et al. criteria (33) as follows: absent (0), mild (1), moderate (2) and severe (3).
Immunochemistry
Bladders were fixed in 10% formalin and embedded in paraffin. For immunofluorescence staining of T lymphocytes five-micrometer tissue sections were incubated with1:50 diluted rabbit anti-CD3 monoclonal antibody (Clone SP7, Diagnostic-BioSystems, Pleasanton, CA) followed by Alexa Fluor 555-labeled goat anti-rabbit IgG antibody (Invitrogen, Carlsbad, CA); both for 1 h at room temperature. Nuclei were counterstained with 4′,6-Diamidino-2-Phenylindole (DAPI) fluorescent mounting medium (Vector laboratories Inc., Burlingame, CA). Images were acquired using a TCS SP8 confocal laser-scanning microscope with 20× objective (LEICA microsystems, Nanterre, France).
Statistics
Data were analyzed with Prism 6 software (Graphpad, San-Diego, CA, USA). Before performing statistical tests, we determined whether the data were normally distributed and evaluated their variance. When normality condition was not met, non-parametric test was used (i.e., Mann-Whitney test). When variances were unequal, Welch's correction was applied. For nociceptive scores, a two-way analysis of variance (ANOVA) was used to determine whether there was a significant main difference among groups. When performing same measurement on a given animal at multiple time points (i.e., Basal, D8, D10), repeated measures (RM) were used. The accepted level of significance was p < 0.05. Data were expressed as mean ± SEM. The number of animal used is indicated in the legends.
Results
Evaluation of the Vaccine Strategy as Anti-nociceptive Treatment in an Interstitial Cystitis/Bladder Pain Syndrome Model
The anti-nociceptive effect of antigen secondary challenge was first assessed by the use of the classical ovalbumin (OVA) antigen model. Rats were immunized with OVA and 1 month after the last immunization with OVA, cystitis was induced by CYP repeated injections (26). Animals were then intravesically instilled with the cognate antigen OVA or irrelevant control antigen BSA (Figure 1). As shown in Figure 2, as compared to basal values, CYP injection induced an increase in nociceptive responses in BSA-challenged rats whereas in OVA-challenged rat the pain response stay in basal level (Figures 2A,B). Furthermore, OVA-immunized rats locally injected with OVA displayed a significant reduction of vesical pain as compared to those injected with the irrelevant control antigen BSA (Figures 2C–F). No significant difference was observed for bladder inflammation between OVA- and BSA-challenged rats (Figure 2G). Immunohistochemistry revealed a widespread T lymphocyte mucosal tissue infiltration associated with OVA local intravesical antigen recall (Figures 2H,I). In contrast, very rare T lymphocyte staining was present into bladder mucosa of BSA-challenged rats (Figures 2H,I). The analgesia accompanying the recruitment of T lymphocytes induced by intravesical OVA was reversed by naloxone-methiodide (a general opioid receptor antagonist unable to cross blood-brain barrier) (Figures 3A–C). This indicates that vesical pain inhibition was dependent on the local release of endogenous opioids. The administration of naloxone methiodide 30 min before pain assessment had no effect on urinary bladder inflammation (Figure 3D).
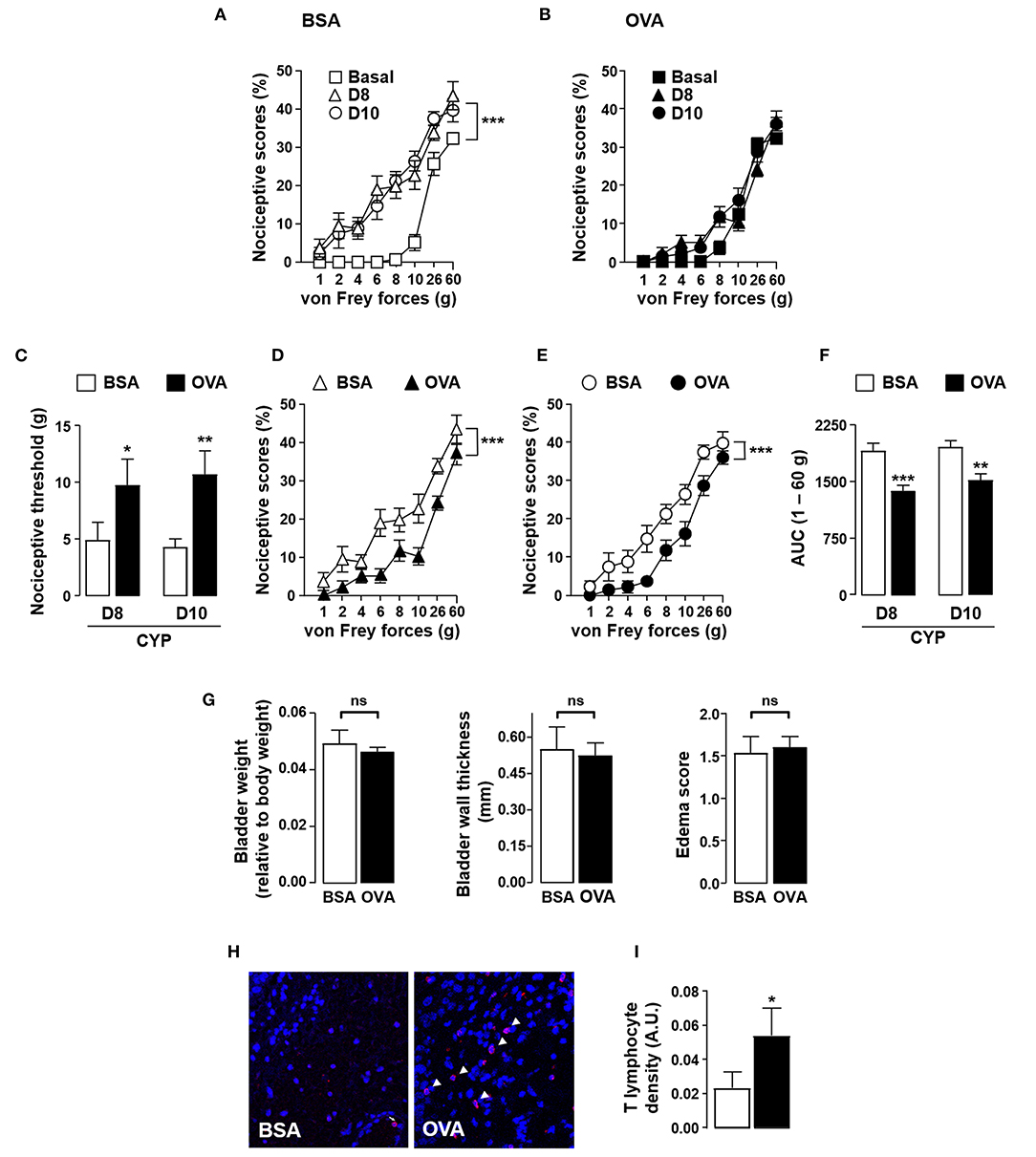
Figure 2. Instillation of OVA alleviates CYP-induced bladder pain in OVA-immunized rats. Rats were primed by subcutaneous injection of OVA emulsified in CFA and then boosted 2 weeks later with the same amount of OVA emulsified in IFA. One month after the last immunization, chronic cystitis was induced as described above. OVA-immunized rats were intravesically administered with either OVA or irrelevant control BSA antigen on days 3, 6 and 7. Von Frey testing was performed at days 8 and 10. (A,B) Nociceptive scores against von Frey forces from 1 to 60 g (hyperalgesia) before (basal, square) and at days 8 (triangles) and 10 (circles) after CYP treatment in OVA-primed rats intravesically instilled with BSA (A) or OVA (B). (C) Nociceptive threshold defined as the von Frey force in grams at which a first score of at least one was obtained (allodynia) (n = 15 / group). (D,E) Nociceptive scores against von Frey forces at days 8 (D) and 10 (E) after CYP treatment in OVA-primed rats intravesically instilled with BSA or OVA. (F) Area under the curve (AUC) calculated by plotting individual nociceptive score against von Frey forces from 1 to 60 g (n = 15 / group). (G) Urinary bladder inflammation assessed at day 10 by bladder weight (left panel), wall thickness (middle panel) and edema scores (right panel). (H) Representative images of CD3 (T cells) immunostaining (red; arrowhead) in bladder from CYP-treated OVA-immunized rats intravesically instilled with either control antigen BSA (left panel) or OVA (right panel). Nuclei are counterstained with DAPI (blue). (I) Density of CD3+ T lymphocytes within bladder determined by quantifying anti-CD3 fluorescence relative to tissue surface delimited manually with DAPI staining (n = 11). Data represent mean values ± SEM. ns, no significant; *p < 0.05; **p < 0.01 and ***p < 0.001.
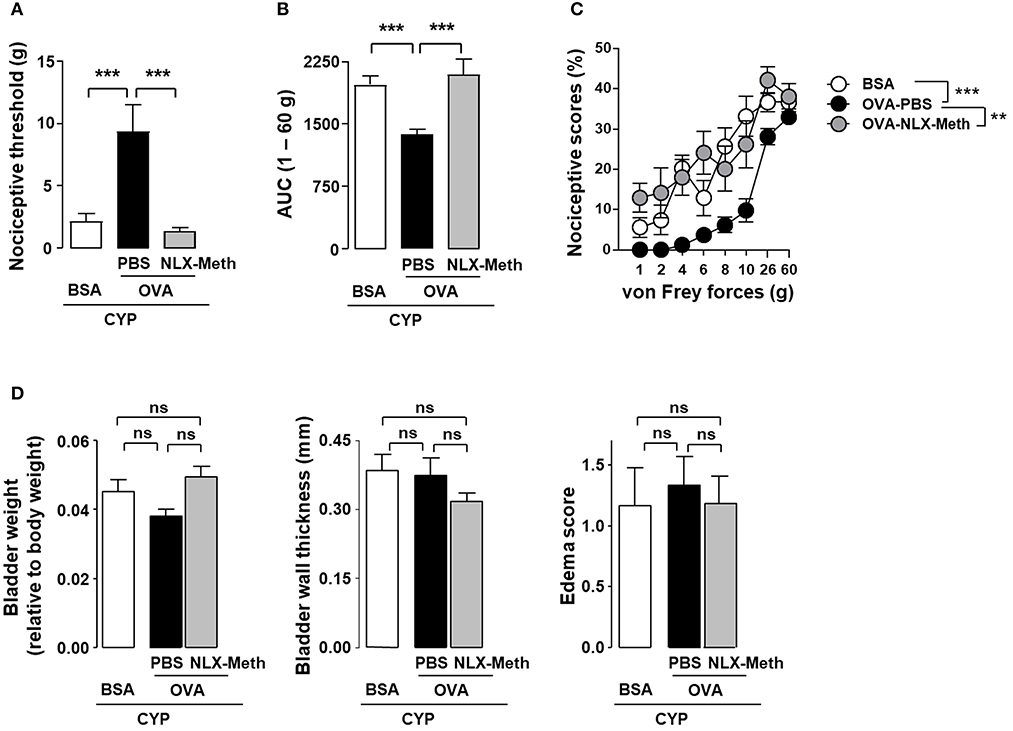
Figure 3. Peripheral opioid receptor blockade inhibits the analgesic effect of OVA instillation in OVA-immunized rats with cystitis. Rats were primed by subcutaneous injection of OVA emulsified in CFA and then boosted 2 weeks later with the same amount of OVA emulsified in IFA. One month after the last immunization, chronic cystitis was induced as described above. OVA-immunized rats were intravesically instilled with either OVA or irrelevant control BSA antigen on days 3, 6, and 7. CYP-induced allodynia (A) and hyperalgesia (B) was assessed at day 10, in OVA-immunized rats intravesically instilled with either irrelevant control BSA antigen (n = 6) or cognate OVA antigen. In addition, in OVA-instilled rats, PBS (n = 9) or naloxone-methiodide (NLX-Meth, n = 11) was intraperitoneally injected. Nociceptive scores against von Frey forces from 1 to 60 g used to calculate AUC are shown in (C). Urinary bladder inflammation (D) was assessed at day 10 by measuring bladder weight (left panel), wall thickness (middle panel) and edema scores (right panel). Data are means values ± SEM. ns, no significant; **p < 0.01 and ***p < 0.001.
Analgesic Effects of BCG and Tetanus Toxoid Vaccines in Interstitial Cystitis/Bladder Pain Syndrome Model
In order to establish that the results observed with OVA could be extended to a general vaccine strategy, we investigated whether BCG or tetanus toxoid vaccine could alleviate vesical pain in our model. In rats vaccinated against BCG, local challenge with BCG resulted in a decrease in CYP-induced allodynia and hyperalgesia as compared to irrelevant control antigen OVA (Figures 4A–D). BCG instillation had no effect on vesical sensitivity of vaccinated non-CYP-treated rats (data not shown). It is noteworthy that BCG had no effect on inflammatory parameters in the bladder of CYP-induced cystitic rats (Figure 4E). In agreement with the results obtained with BCG, intravesical instillation of universal immunodominant tetanus-derived peptides in rats previously vaccinated with tetanus toxoid significantly reduced CYP-induced bladder pain as compared to irrelevant control OVA peptide (Figures 4F–H).
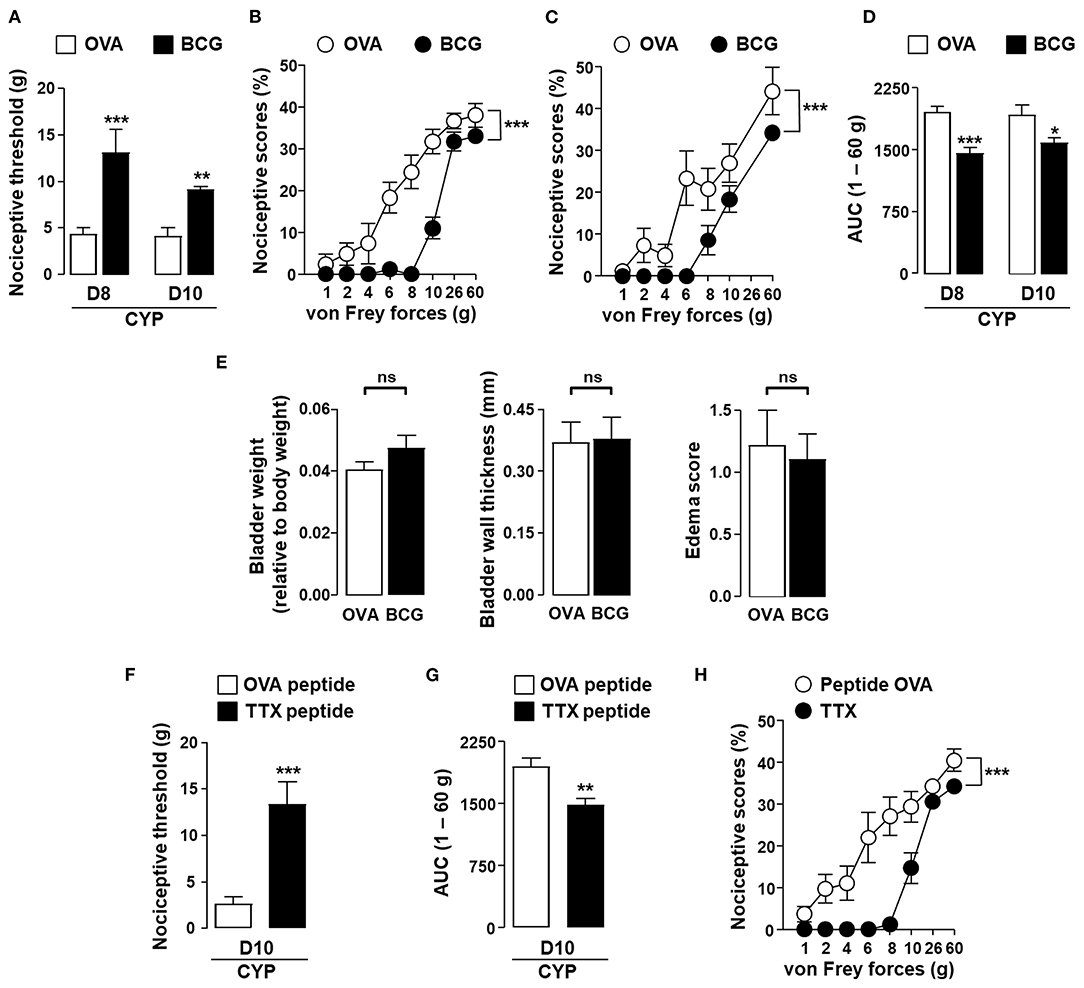
Figure 4. In situ instillation of vaccine antigens alleviates bladder pain associated with chronic cystitis in vaccinated rats. Rats were vaccinated by subcutaneous injection of BCG or tetanus toxoid (TTX) as indicated and boosted 2 weeks later with the same amount of vaccine antigens. One month after the last immunization, chronic cystitis was induced as described above. Rats vaccinated against BCG were intravesically instilled with either irrelevant control antigen OVA (white symbols) or BCG (black symbols) on days 3, 6, and 7. (A) Nociceptive threshold defined as the von Frey force in grams at which a first score of at least one was obtained (allodynia). (B,C) Nociceptive scores against von Frey forces at days 8 (B) and 10 (C) after CYP treatment. (D) Area under the curve (AUC) calculated by plotting individual nociceptive score against von Frey forces from 1 to 60 g. (E) Urinary bladder inflammation assessed at day 10 in rats vaccinated with BCG. Rats vaccinated against tetanus toxoid were intravesically instilled with an equimolar mixture of the two immune-dominant TTX-derived peptides (black symbols) or irrelevant control OVA (323–339) peptide (white symbols) on days 3, 6, and 7. Nociceptive threshold (F) and AUC 1–60 g (G) calculated from nociceptive scores against von Frey forces from 1 to 60 g (H) were assessed at day 10 after CYP treatment. Data represent mean values ± SEM (n = 9/group). ns, no significant; *p < 0.05, **p < 0.01, and ***p < 0.001.
Discussion
Current treatments of IC/BPS have controversial efficacy, particularly on pain symptoms. Therefore, development of new therapies is needed. In the present study, we demonstrated that intravesical antigens application on previously vaccinated rats, significantly relieved from CYP-induced bladder pain. These results point out the potential use of vaccine therapy for pain management in IC/BPS patients on the basis of any commonly used vaccine.
As previously reported for intestinal inflammation (23), in vaccinated animals, the induction of a secondary immune response by instillation of vaccine antigens results in the recruitment of T lymphocytes into the mucosa and organ pain relief. In the bladder, we report that this analgesic effect occurs in a naloxone methiodide-dependent fashion indicating that vesical pain inhibition could be due to the local release of endogenous opioids by T lymphocytes accumulating into the mucosa, thereby increasing the constitutive tonic activation of peripheral opioid receptors on sensory neurons (34). Because primed T lymphocytes release opioids upon antigen stimulation (17), the anti-nociceptive effect of antigen recall in vaccinated individuals probably would need regular antigen injections to be maintained.
In humans, the use of intravesical BCG as a potent immune-stimulator for IC/BPS was first reported by Zeidman et al. (35). Several other non-randomized clinical trials including highly heterogeneous patients with IC/BPS resulted in disparate clinical outcomes (36–38). Most importantly, the vaccination status of the patients was never mentioned or investigated. In order to be used in patients, a vaccine recall that would insure a functional vaccination status would be necessary before applying intravesical BCG. Possible correlations between vaccination status and symptomatic responses to BCG treatments could then be investigated. Although BCG-antigen recall therapy has been used for years in the treatment of bladder cancer, its exact mechanism of action is largely unknown. It has been suggested that intravesical instillation of BCG leads to stimulation of the type 1 helper T-cell response (37) and sometimes to type 1 and type 2 helper T-lymphocytes (39), all of these T cell subsets producing endogenous opioids (11, 17, 40).
Our results also demonstrated that the strategy of antigen instillation into cystitis bladder did not modify inflammatory parameters. This is in agreement with the results obtained in patients IC/BPS receiving intravesical BCG that report no worsening of interstitial cystitis symptoms (37).
Taking together, our results suggest that local induction of a secondary T cell response to vaccine antigens, against which the immune system has been primed, prevents from vesical pain by the release of endogenous opioids and further activation of peripheral opioid receptors. These results tend to support that vaccinal approaches represent a promising strategy to manage visceral pain occurring in IC/BPS. To confirm our findings in humans, clinical trial comparing BCG therapy with or without previous BCG vaccination should be set up. Considering the universality of BCG or tetanus vaccination programs worldwide, our results open new therapeutic avenues in the management of IC/BPS.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by French Animal Ethical Committee application number CEEA-122-2014-28 and APAFIS#16506-2018082411278474.
Author Contributions
NV, PL, SC, and GD elaborated the study concept. CA, LB, and CB performed and planned the experiments. CA, LB, SC, and GD designed and analyzed the data. GD, CA, SC, and NV wrote the manuscript. GD and PL supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM) and the Université Paul Sabatier, Toulouse III. This work was part of the MAGenTA Program overseen by La Banque Publique d'Investissement (BPI) given to Urosphere for implementation of experimental model for urogenital tract pathologies.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank ANEXPLO platform (UMS 006) histopathology core facility (F. Capilla and C. Salon), the cellular imaging core facility (S. Allart and S. Lachambre) and the Integrated Screening Plateform of Toulouse (PICT) for the peptide synthesis.
References
1. Hanno PM, Erickson D, Moldwin R, and Faraday MM. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. (2015) 193:1545–53. doi: 10.1016/j.juro.2015.01.086
2. Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology. (2007) 69:9–16. doi: 10.1016/j.urology.2006.03.084
3. Hwang P, Auclair B, Beechinor D, Diment M, and Einarson TR. Efficacy of pentosan polysulfate in the treatment of interstitial cystitis: a meta-analysis. Urology. (1997) 50:39–43. doi: 10.1016/S0090-4295(97)00110-6
4. Tomoe H. In what type of interstitial cystitis/bladder pain syndrome is DMSO intravesical instillation therapy effective? Transl Androl Urol. (2015) 4:600–4. doi: 10.3978/j.issn.2223-4683.2015.09.01
5. Kwon WA, Ahn SH, Oh TH, Lee JW, Han DY, and Jeong HJ. Effect of low-dose triple therapy using gabapentin, amitriptyline, and a nonsteroidal anti-inflammatory drug for overactive bladder symptoms in patients with bladder pain syndrome. Int Neurourol J. (2013) 17:78–82. doi: 10.5213/inj.2013.17.2.78
6. Wesselmann U, Burnett AL, and Heinberg LJ. The urogenital and rectal pain syndromes. Pain. (1997) 73:269–94. doi: 10.1016/S0304-3959(97)00076-6
7. Hanno PM. Analysis of long-term Elmiron therapy for interstitial cystitis. Urology. (1997) 49:93–9. doi: 10.1016/S0090-4295(97)00179-9
8. Baddack-Werncke U, Busch-Dienstfertig M, Gonzalez-Rodriguez S, Maddila SC, Grobe J, Lipp M, et al. Cytotoxic T cells modulate inflammation and endogenous opioid analgesia in chronic arthritis. J Neuroinflammation. (2017) 14:30. doi: 10.1186/s12974-017-0804-y
9. Basso L, Boue J, Mahiddine K, Blanpied C, Robiou-du-Pont S, Vergnolle N, et al. Endogenous analgesia mediated by CD4(+) T lymphocytes is dependent on enkephalins in mice. J. Neuroinflammation. (2016) 13:132. doi: 10.1186/s12974-016-0591-x
10. Basso L, Benamar M, Mas-Orea X, Deraison C, Blanpied C, Cenac N, et al. Endogenous control of inflammatory visceral pain by T cell-derived opioids in IL-10-deficient mice. Neurogastroenterol Motil. (2020) 32:e13743. doi: 10.1111/nmo.13743
11. Boue J, Basso L, Cenac N, Blanpied C, Rolli-Derkinderen M, Neunlist M, et al. Endogenous regulation of visceral pain via production of opioids by colitogenic CD4(+) T cells in mice. Gastroenterology. (2014) 146:166–75. doi: 10.1053/j.gastro.2013.09.020
12. Boue J, Blanpied C, Brousset P, Vergnolle N, and Dietrich G. Endogenous opioid-mediated analgesia is dependent on adaptive T cell response in mice. J Immunol. (2011) 186:5078–84. doi: 10.4049/jimmunol.1003335
13. Cabot PJ, Carter L, Gaiddon C, Zhang Q, Schäfer M, Loeffler J, et al. Immune cell-derived ß-endorphin. Production, release, and control of inflammatory pain in rats. J Clin Invest. (1997) 100:142–8. doi: 10.1172/JCI119506
14. Labuz D, Schreiter A, Schmidt Y, Brack A, and Machelska H. T lymphocytes containing beta-endorphin ameliorate mechanical hypersensitivity following nerve injury. Brain Behav Immun. (2010) 24:1045–53. doi: 10.1016/j.bbi.2010.04.001
15. Valdez-Morales E, Guerrero-Alba R, Ochoa-Cortes F, Benson J, Spreadbury I, Hurlbut D, et al. Release of endogenous opioids during a chronic IBD model suppresses the excitability of colonic DRG neurons. Neurogastroenterol Motil. (2013) 25:39–46. doi: 10.1111/nmo.12008
16. Verma-Gandhu M, Bercik P, Motomura Y, Verdu EF, Khan WI, Blennerhassett PA, et al. CD4+ T-cell modulation of visceral nociception in mice. Gastroenterology. (2006) 130:1721–8. doi: 10.1053/j.gastro.2006.01.045
17. Boue J, Blanpied C, Djata-Cabral M, Pelletier L, Vergnolle N, and Dietrich G. Immune conditions associated with CD4+ T effector-induced opioid release and analgesia. Pain. (2012) 153:485–93. doi: 10.1016/j.pain.2011.11.013
18. Basso L, Bourreille A, and Dietrich G. Intestinal inflammation and pain management. Curr Opin Pharmacol. (2015) 25:50–5. doi: 10.1016/j.coph.2015.11.004
19. Carbone SE, and Poole DP. Inflammation without pain: immune-derived opioids hold the key. Neurogastroenterol Motil. (2020) 32:e13787. doi: 10.1111/nmo.13787
20. Feng J, Lepetre-Mouelhi S, Gautier A, Mura S, Cailleau C, Coudore F, et al. A new painkiller nanomedicine to bypass the blood-brain barrier and the use of morphine. Sci Adv. (2019) 5:eaau5148. doi: 10.1126/sciadv.aau5148
21. Laumet G, Ma J, Robison AJ, Kumari S, Heijnen CJ, and Kavelaars A. T cells as an emerging target for chronic pain therapy. Front Mol Neurosci. (2019) 12:216. doi: 10.3389/fnmol.2019.00216
22. Machelska H, and Celik MO. Advances in achieving opioid analgesia without side effects. Front Pharmacol. (2018) 9:1388. doi: 10.3389/fphar.2018.01388
23. Basso L, Boue J, Auge C, Deraison C, Blanpied C, Cenac N, et al. Mobilization of CD4+ T lymphocytes in inflamed mucosa reduces pain in colitis mice: toward a vaccinal strategy to alleviate inflammatory visceral pain. Pain. (2018) 159:331–41. doi: 10.1097/j.pain.0000000000001103
24. Machelska H, and Celik MO. Immune cell-mediated opioid analgesia. Immunol Lett. (2020) 227:48–59. doi: 10.1016/j.imlet.2020.08.005
25. Stein C. New concepts in opioid analgesia. Expert Opin Investig Drugs. (2018) 27:765–75. doi: 10.1080/13543784.2018.1516204
26. Augé C, Gamé X, Vergnolle N, Lluel P, and Chabot S. Characterization and validation of a chronic model of cyclophosphamide-induced interstitial cystitis/bladder pain syndrome in rats. Front Pharmacol. (2020) 11:1305. doi: 10.3389/fphar.2020.01305
27. Diethelm-Okita BM, Okita DK, Banaszak L, and Conti-Fine BM. Universal epitopes for human CD4+ cells on tetanus and diphtheria toxins. J Infect Dis. (2000) 181:1001–9. doi: 10.1086/315324
28. Diethelm-Okita BM, Raju R, Okita DK, and Conti-Fine BM. Epitope repertoire of human CD4+ T cells on tetanus toxin: identification of immunodominant sequence segments. J Infect Dis. (1997) 175:382–91. doi: 10.1093/infdis/175.2.382
29. Jaume M, Laffont S, Chapey E, Blanpied C, and Dietrich G. Opioid receptor blockade increases the number of lymphocytes without altering T cell response in draining lymph nodes in vivo. J Neuroimmunol. (2007) 188:95–102. doi: 10.1016/j.jneuroim.2007.06.013
30. Robertson JM, Jensen PE, and Evavold BD. DO11.10 and OT-II T cells recognize a C-terminal ovalbumin 323-339 epitope. J Immunol. (2000) 164:4706–12. doi: 10.4049/jimmunol.164.9.4706
31. Jaume M, Jacquet S, Cavailles P, Mace G, Stephan L, Blanpied C, et al. Opioid receptor blockade reduces Fas-induced hepatitis in mice. Hepatology. (2004) 40:1136–43. doi: 10.1002/hep.20428
32. Auge C, Chene G, Dubourdeau M, Desoubzdanne D, Corman B, Palea S, et al. Relevance of the cyclophosphamide-induced cystitis model for pharmacological studies targeting inflammation and pain of the bladder. Eur J Pharmacol. (2013) 707:32–40. doi: 10.1016/j.ejphar.2013.03.008
33. Gray KJ, Engelmann UH, Johnson EH, and Fishman IJ. Evaluation of misoprostol cytoprotection of the bladder with cyclophosphamide (Cytoxan) therapy. J Urol. (1986) 136:497–500. doi: 10.1016/S0022-5347(17)44929-9
34. Quallo T, Alkhatib O, Gentry C, Andersson DA, and Bevan S. G protein βγ subunits inhibit TRPM3 ion channels in sensory neurons. eLife. (2017) 6:e26138. doi: 10.7554/eLife.26138
35. Zeidman EJ, Helfrick B, Pollard C, and Thompson IM. Bacillus Calmette-Guerin immunotherapy for refractory interstitial cystitis. Urology. (1994) 43:121–4. doi: 10.1016/S0090-4295(94)80284-X
36. Aghamir SM, Mohseni MG, and Arasteh S. Intravesical Bacillus Calmette-Guerin for treatment of refractory interstitial cystitis. Urol J. (2007) 4:18–23.
37. Irani D, Heidari M, and Khezri AA. The efficacy and safety of intravesical Bacillus-Calmette-Guerin in the treatment of female patients with interstitial cystitis: a double-blinded prospective placebo controlled study. Urol J. (2004) 1:90–3.
38. Propert KJ, Mayer R, Nickel JC, Payne CK, Peters KM, Teal V, et al. Followup of patients with interstitial cystitis responsive to treatment with intravesical bacillus Calmette-Guerin or placebo. J Urol. (2008) 179:552–5. doi: 10.1016/j.juro.2007.09.035
39. Peters KM, Diokno AC, and Steinert BW. Preliminary study on urinary cytokine levels in interstitial cystitis: does intravesical bacille Calmette-Guerin treat interstitial cystitis by altering the immune profile in the bladder? Urology. (1999) 54:450–3. doi: 10.1016/S0090-4295(99)00162-4
Keywords: vaccination, T lymphocytes, opioid, bladder pain syndrome, cyclophosphamide, cystitis
Citation: Augé C, Basso L, Blanpied C, Vergnolle N, Gamé X, Chabot S, Lluel P and Dietrich G (2021) Pain Management in a Model of Interstitial Cystitis/Bladder Pain Syndrome by a Vaccinal Strategy. Front. Pain Res. 2:642706. doi: 10.3389/fpain.2021.642706
Received: 16 December 2020; Accepted: 09 February 2021;
Published: 08 March 2021.
Edited by:
Thomas J. Martin, Wake Forest School of Medicine, United StatesReviewed by:
Thomas Voets, VIB-KU Leuven Center for Brain Disease Research, BelgiumJun-Ming Zhang, University of Cincinnati, United States
Copyright © 2021 Augé, Basso, Blanpied, Vergnolle, Gamé, Chabot, Lluel and Dietrich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philippe Lluel, philippe.lluel@urosphere.com
†Present address: Lilian Basso, Infinity, Université de Toulouse, INSERM, CNRS, UPS, Toulouse, France
 Céline Augé
Céline Augé Lilian Basso
Lilian Basso Catherine Blanpied2
Catherine Blanpied2  Nathalie Vergnolle
Nathalie Vergnolle Philippe Lluel
Philippe Lluel Gilles Dietrich
Gilles Dietrich