- 1Department of Oncology, Fengcheng People’s Hospital, Yichun, China
- 2Department of Oncology, The Affiliated Fengcheng Hospital of Yichun University, Yichun, China
- 3Department of Oncology, Shangrao People’s Hospital, Shangrao, China
- 4Department of Thoracic Surgery, The Second Affiliated Hospital of Nanchang University, Nanchang, China
Background: In recent years, we have observed the pivotal role of immunotherapy in improving survival for patients with non-small cell lung cancer (NSCLC). However, the effectiveness of immunotherapy in the perioperative (neoadjuvant + adjuvant) treatment of resectable NSCLC remains uncertain. We conducted a comprehensive analysis of its antitumor efficacy and adverse effects (AEs) by pooling data from the KEYNOTE-671, NADIM II, and AEGEAN clinical trials.
Methods: For eligible studies, we searched seven databases. The randomized controlled trials (RCTs) pertaining to the comparative analysis of combination neoadjuvant platinum-based chemotherapy plus perioperative immunotherapy (PIO) versus perioperative placebo (PP) were included. Primary endpoints were overall survival (OS) and event-free survival (EFS). Secondary endpoints encompassed drug responses, AEs, and surgical outcomes.
Results: Three RCTs (KEYNOTE-671, NADIM II, and AEGEAN) were included in the final analysis. PIO group (neoadjuvant platinum-based chemotherapy plus perioperative immunotherapy) exhibited superior efficacy in OS (hazard ratio [HR]: 0.63 [0.49-0.81]), EFS (HR: 0.61 [0.52, 0.72]), objective response rate (risk ratio [RR]: 2.21 [1.91, 2.54]), pathological complete response (RR: 4.36 [3.04, 6.25]), major pathological response (RR: 2.79 [2.25, 3.46]), R0 resection rate (RR: 1.13 [1.00, 1.26]) and rate of adjuvant treatment (RR: 1.08 [1.01, 1.15]) compared with PP group (neoadjuvant platinum-based chemotherapy plus perioperative placebo). In the subgroup analysis, EFS tended to favor the PIO group in almost all subgroups. BMI (>25), T stage (IV), N stage (N1-N2) and pathological response (with pathological complete response) were favorable factors in the PIO group. In the safety assessment, the PIO group exhibited higher rates of serious AEs (28.96% vs. 23.51%) and AEs leading to treatment discontinuation (12.84% vs. 5.81%). Meanwhile, although total adverse events, grade 3-5 adverse events, and fatal adverse events tended to favor the PP group, the differences were not statistically significant.
Conclusion: PIO appears to be superior to PP for resectable stage II-III NSCLC, demonstrating enhanced survival and pathological responses. However, its elevated adverse event (AE) rate warrants careful consideration.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/#recordDetails, identifier CRD42023487475.
Introduction
For decades, lung cancer (LC) has been the leading global cause of cancer-related deaths, with over 80% attributed to non-small cell lung cancer (NSCLC) (1, 2). Comprehensive treatment based on surgery is the standard of care for selected resectable stages II-III NSCLC (3). In previous approaches to neoadjuvant and adjuvant treatment for stage II-III NSCLC, chemotherapy played a vital role, but its solitary use yielded unsatisfactory results (4). In recent years, immunotherapy has gained widespread acceptance in solid tumor treatment, demonstrating superior efficacy in both neoadjuvant and adjuvant treatment for resectable NSCLC (5–7). Nevertheless, controversy persists in clinical settings regarding whether perioperative immunotherapy (neoadjuvant+adjuvant) can yield superior results (8).
The use of immunotherapy in the perioperative period of resectable lung cancer has been a hot topic in recent years. In neoadjuvant therapy, the CheckMate 816 study demonstrated that the addition of nivolumab to platinum-based chemotherapy (PBC) could significantly increase event-free survival (EFS) and drug responses (9). Similar results were also validated in the TD-FOREKNOW study (Camrelizumab) (10). In adjuvant therapy, the KEYNOTE-091 study showed that the addition of pembrolizumab to PBC could significantly increase disease-free survival (DFS) (11). The IMpower010 study also confirmed that adding atezolizumab to PBC could improve DFS and overall survival (OS), especially in patients with programmed cell death 1 ligand 1 (PD-L1)-positive NSCLC (12). Regarding the use of immunotherapy in combination of neoadjuvant and adjuvant therapy, both the KEYNOTE-671 study (pembrolizumab) and the AEGEAN study (durvalumab) found that perioperative immunotherapy could significantly improve OS and EFS, and similar results were also validated in the NADIM II study (nivolumab) (13–15).
This study conducted a meta-analysis based on randomized controlled trials (RCTs) to evaluate the impact of perioperative immunotherapy with neoadjuvant PBC on survival, pathological responses, and adverse reactions.
Materials and methods
This study was conducted in accordance with PRISMA guidelines and registered in PROSPERO (ID: CRD42023487475) (Supplementary Table S1).
Search strategy
The search strategy involved the use of keywords: “lung cancer,” “randomized,” and immune checkpoint inhibitors (nivolumab, pembrolizumab, treprinumab, cedilimumab, camrelizumab, tislelizumab, penpulimab, zimberelimab, serplulimab, durvalumab, atezolizumab, envolizumab, sugemalimab, adebrelimab, ipilimumab, and tremelimumab). Seven databases (PubMed, ScienceDirect, Ovid MEDLINE, the Cochrane Library, Scopus, EMBASE and Web of Science) were thoroughly searched for eligible RCTs from the inception of the databases to November 15, 2023 (Supplementary Table S2). Additionally, we reviewed the reference lists of the included RCTs to identify any further eligible studies.
Selection criteria
The studies published in English were selected following PICOS criteria:
(1) Participants (P): patients with stage II-III NSCLC, evaluated per the American Joint Committee on Cancer staging system, 8th edition (16).
(2) Intervention (I): neoadjuvant (PBC+immunotherapy) + adjuvant (immunotherapy), defined as the perioperative immunotherapy (PIO) group.
(3) Control (C): neoadjuvant (PBC+placebo) + adjuvant (placebo), defined as the perioperative placebo (PP) group.
(4) Outcomes (O): survival (OS, EFS), pathological responses, and adverse events (AEs).
(5) Study design (S): RCTs.
Articles lacking initial data, as well as meta-analyses, conference articles, and case reports, were not considered for inclusion. Distinct articles covering the same trial with diverse outcomes were included, but for identical outcomes, only the most recent data were utilized in the analysis.
Data extraction
Two investigators independently extracted data, including study characteristics (publication date, first author, etc.), participant details (sex, age, etc.), cancer specifics (histopathology, stage, etc.), antitumor effectiveness (OS, EFS, pathological responses, etc.), and counts of adverse events (total AEs, serious AEs, etc.). Disagreements were resolved through a process of re-evaluation and discussion.
Outcome assessments
The primary endpoints analyzed were OS and EFS. Simultaneously, the overall survival rate (OSR) and event-free survival rate (EFSR) at 6, 12, 18, 24, 30, 36, 42, and 48 months were compared between the two groups. Additionally, we examined EFS within specific subgroups, including patient characteristics (sex, age, etc.), histologic features, pathological stage, T stage, N stage, PD-L1 tumor cell proportion score (TPS), epidermal growth factor receptor (EGFR) mutation, anaplastic lymphoma kinase (ALK) translocation, pathological response (major pathological response [MPR]), and pathological response (pathological complete response [PCR]).
Quality assessment
We assessed the quality of RCTs using the Jadad scale, a 5-point system reflecting randomization, blinding, and patient inclusion. A score of ≥3 points was considered indicative of high quality (17). Additionally, the Cochrane Risk Assessment Tool was employed, which evaluates bias related to selection, performance, detection, attrition, and reporting and categorizes risk as low, unclear, or high (18). The results are presented in a bias graph.
We assessed the quality of the results using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) method, which primarily encompasses bias, indirectness, inaccuracy, and publication bias. The outcomes are classified into four levels: very low, low, medium, and high (19).
Statistical analysis
The pooled data were assessed using Review Manager 5.3. Hazard ratios (HR) were employed for the analysis of survival data, favoring the PIO group when HR < 1. For dichotomous variables, we used the risk ratio (RR), with results favoring the PP group when RR > 1, particularly in the AE analysis. Conversely, support for the PIO group emerged in the analysis of OSR, EFSR, and drug responses. Heterogeneity was assessed using the I2 statistic and χ2 test. In cases where I2 was less than 50% or p was greater than 0.1, indicating the absence of significant heterogeneity, we employed a fixed-effects model; otherwise, a random-effects model was utilized. Statistical significance was defined by P values less than 0.05, and we assessed publication bias by visually inspecting funnel plots.
Results
Search results
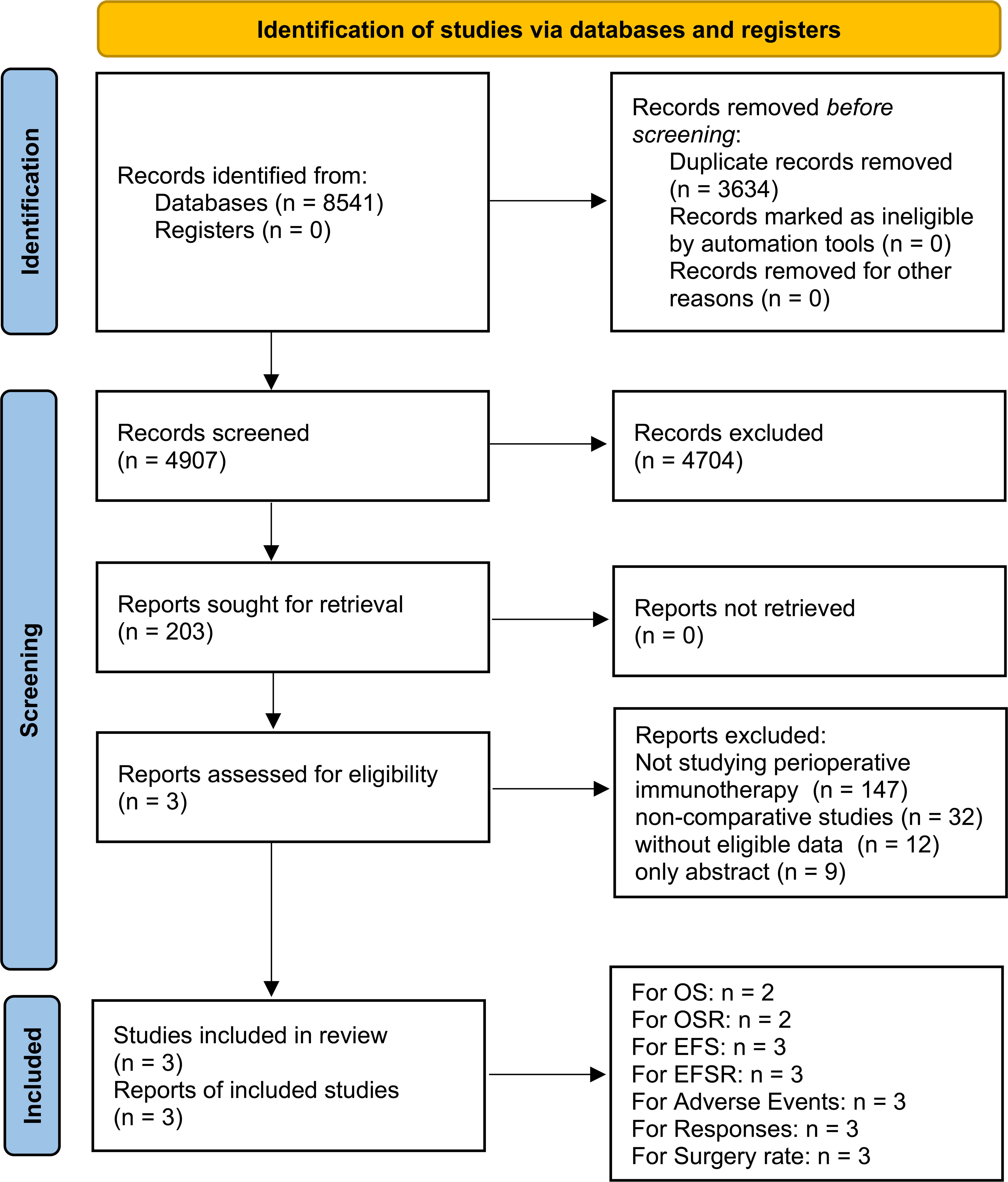
Three high-quality RCTs (KEYNOTE-671, NADIM II, and AEGEAN) were included in the analysis. The PIO group included 820 patients, and the PP group included 803 patients (Figure 1, Supplementary Figure S1, Supplementary Table S3) (13–15). These comprised two global multicenter studies (KEYNOTE-671 and AEGEAN) and one study conducted in Spain (NADIM II) (13–15). As per the GRADE method, the quality of all results was categorized within the medium-high range (Supplementary Table S4). Table 1 provided a summary of the baseline information for the included studies.
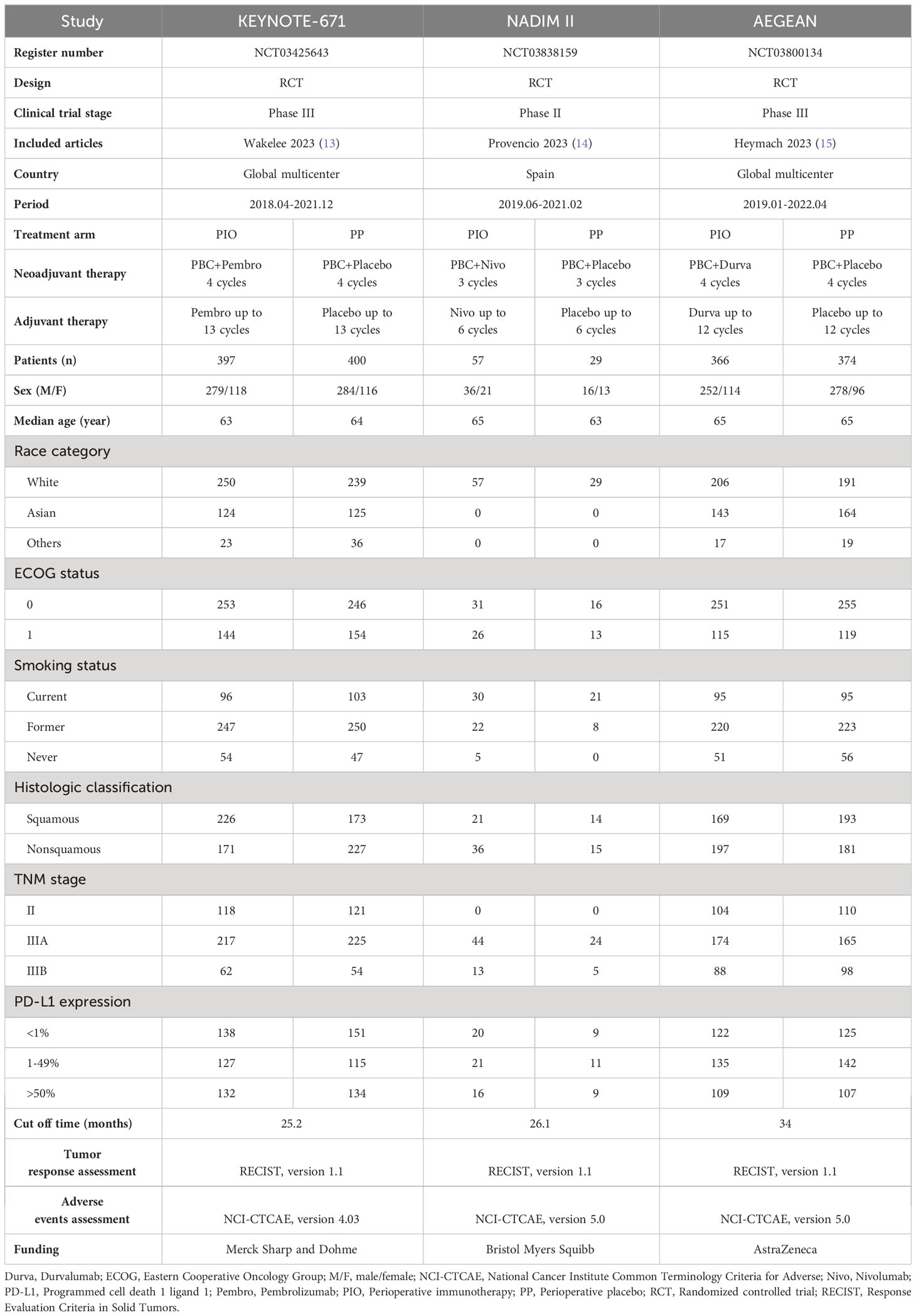
Table 1 Characteristics of the three randomized controlled trials (KEYNOTE-671, NADIM II and AEGEAN).
Antitumor efficacy
The OS in the PIO group surpassed that in the PP group (HR: 0.63 [0.49-0.81], p = 0.0003; Figure 2). At 24-48 months, OSR favored the PIO group (OSR-24 m, RR: 1.07 [1.00, 1.15]; OSR-30 m, RR: 1.16 [1.07, 1.26]; OSR-36 m, RR: 1.23 [1.12, 1.35]; OSR-42 m, RR: 1.23 [1.12, 1.36]; OSR-48 m, RR: 1.49 [1.32, 1.68]) (Supplementary Figure S2). As survival extended, PIO demonstrated an increasing OS advantage compared to PP (Figures 3A, C).

Figure 2 Forest plots of overall survival and event-free survival associated with perioperative immunotherapy versus perioperative placebo.
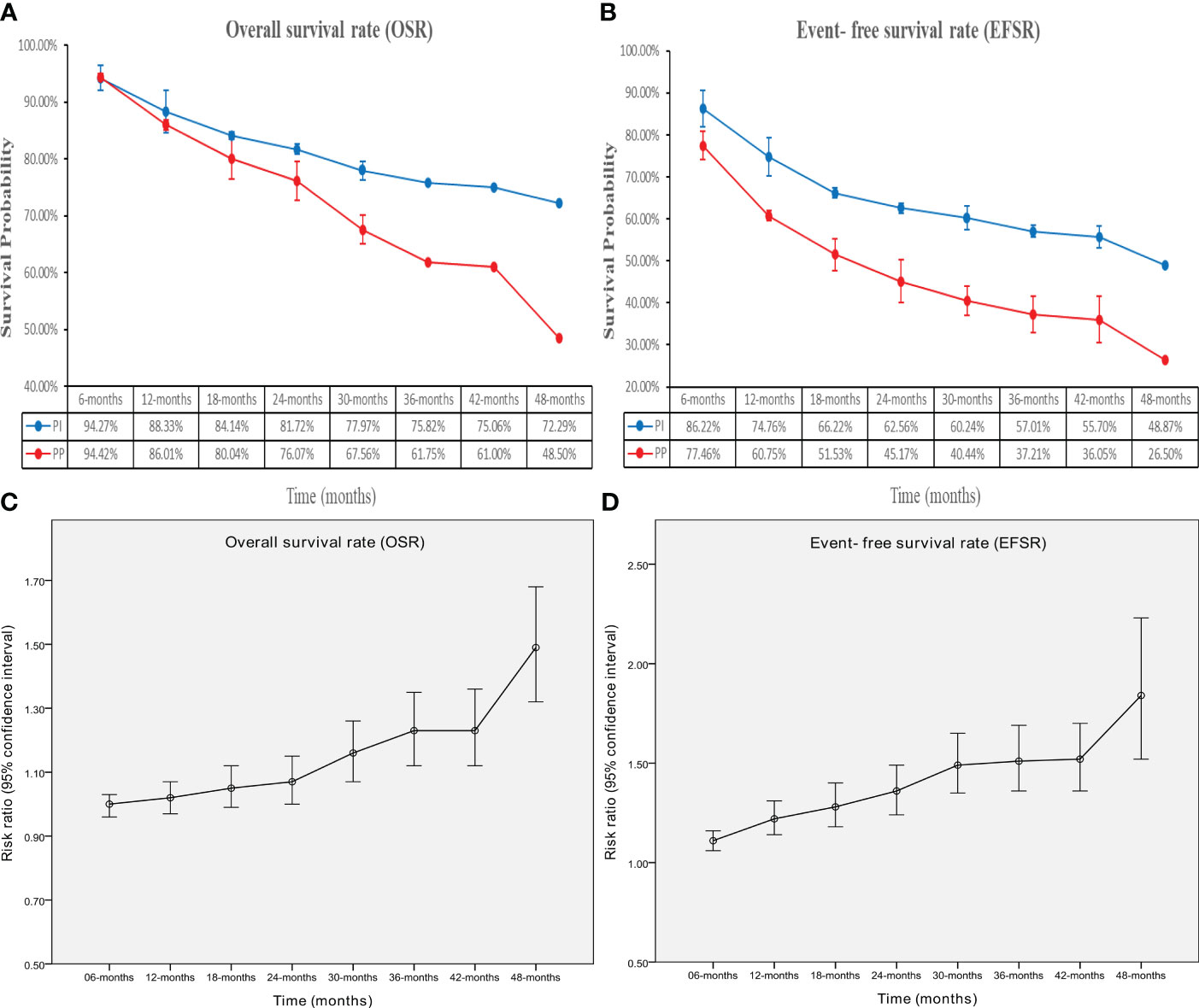
Figure 3 Comparisons of overall survival rate (6-48 months, A: trend of overall survival rate; C: trend of risk ratios) and event-free survival rate (6-48 months, B: trend of event-free survival rate; D: trend of risk ratios) associated with perioperative immunotherapy versus perioperative placebo according to survival time.
The EFS in the PIO group surpassed that in the PP group (HR: 0.61 [0.52, 0.72], p < 0.00001; Figure 2). At 6-48 months, EFSR favored the PIO group (EFSR-6 m, RR: 1.11 [1.06, 1.16]; EFSR-12 m, RR: 1.22 [1.14, 1.31]; EFSR-18 m, RR: 1.28 [1.18, 1.40]; EFSR-24 m, RR: 1.36 [1.24, 1.49]; EFSR-30 m, RR: 1.49 [1.35, 1.65]; EFSR-36 m, RR: 1.51 [1.36, 1.69]; EFSR-42 m, RR: 1.52 [1.36, 1.70]; EFSR-48 m, RR: 1.84 [1.52, 2.23]; Supplementary Figure S3). Regarding extended survival, PIO demonstrated an increasing advantage in EFS compared to PP (Figures 3B, D).
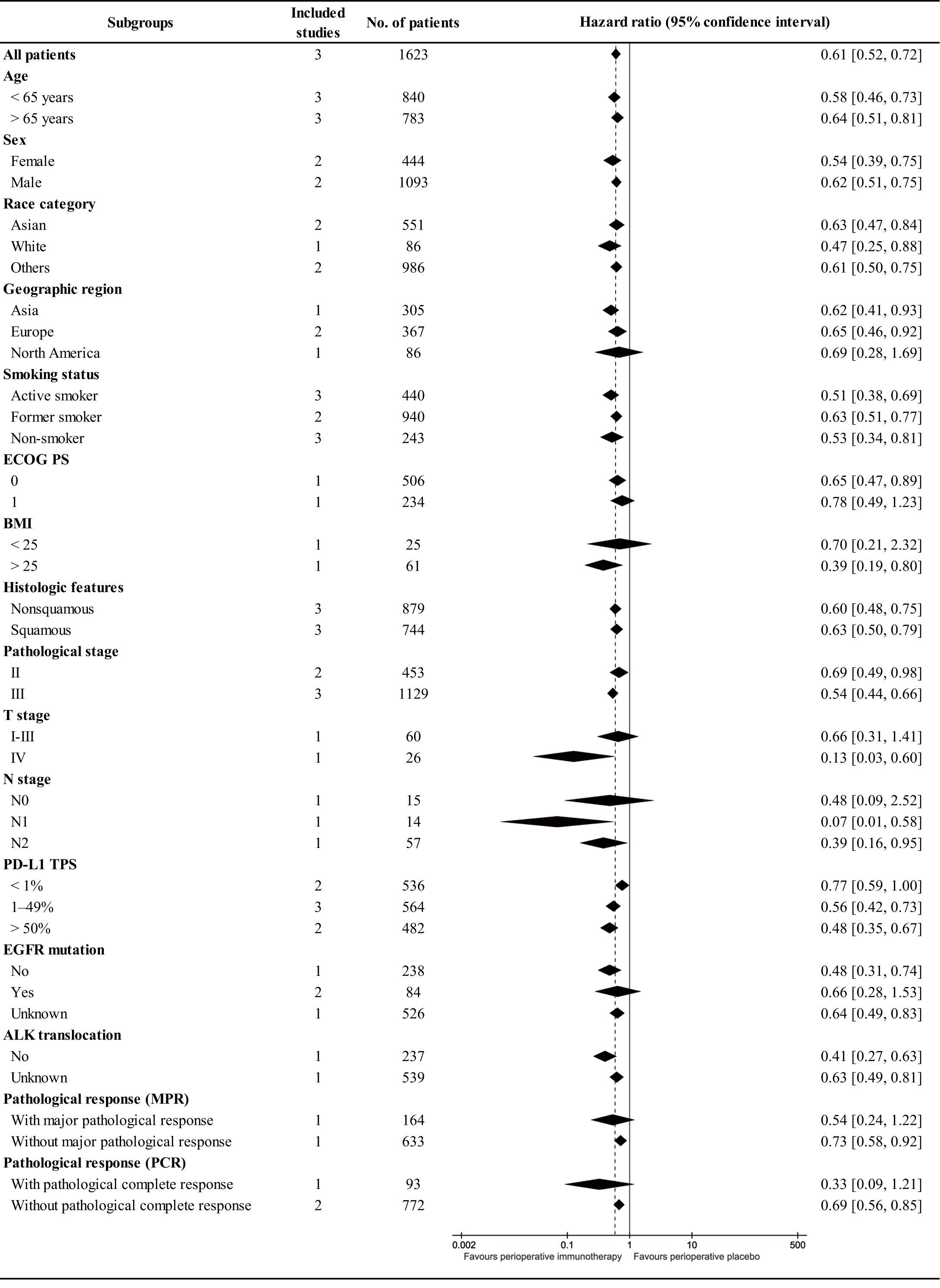
In subgroup analysis, EFS tended to favor the PIO group across most subgroups. High BMI (>25), advanced T stage (IV), involved N stage (N1-N2), and favorable pathological response (with PCR) might benefit PIO treatment. Simultaneously, the EFS advantage of PIO increased with higher PD-L1 expression (PD-L1 TPS, < 1%, RR: 0.77 [0. 59-1.00]; 1-49%, RR: 0.56 [0. 42-0.73]; > 50%, RR: 0.48 [0. 35-0.67]) (Figure 4).
The objective response rate (ORR, RR: 2.21 [1.91, 2.54]), PCR (RR: 4.36 [3.04, 6.25]), and MPR (RR: 2.79 [2.25, 3.46]) surpassed those in the PIO group (Figure 5). The surgery rates were similar between the two groups, and the R0 resection rate (RR: 1.08 [1.01, 1.16]) was higher in the PIO group (Supplementary Figure S4). The started rate (RR: 1.08 [1.01, 1.15]) and completed rate (RR: 1.13 [0.98, 1.30]) of adjuvant therapy tended to favor the PIO group (Supplementary Figure S5).

Figure 5 Forest plots of pathological responses (objective response rate, pathological complete response, and major pathological response) associated with perioperative immunotherapy versus perioperative placebo according to survival time.
Toxicity
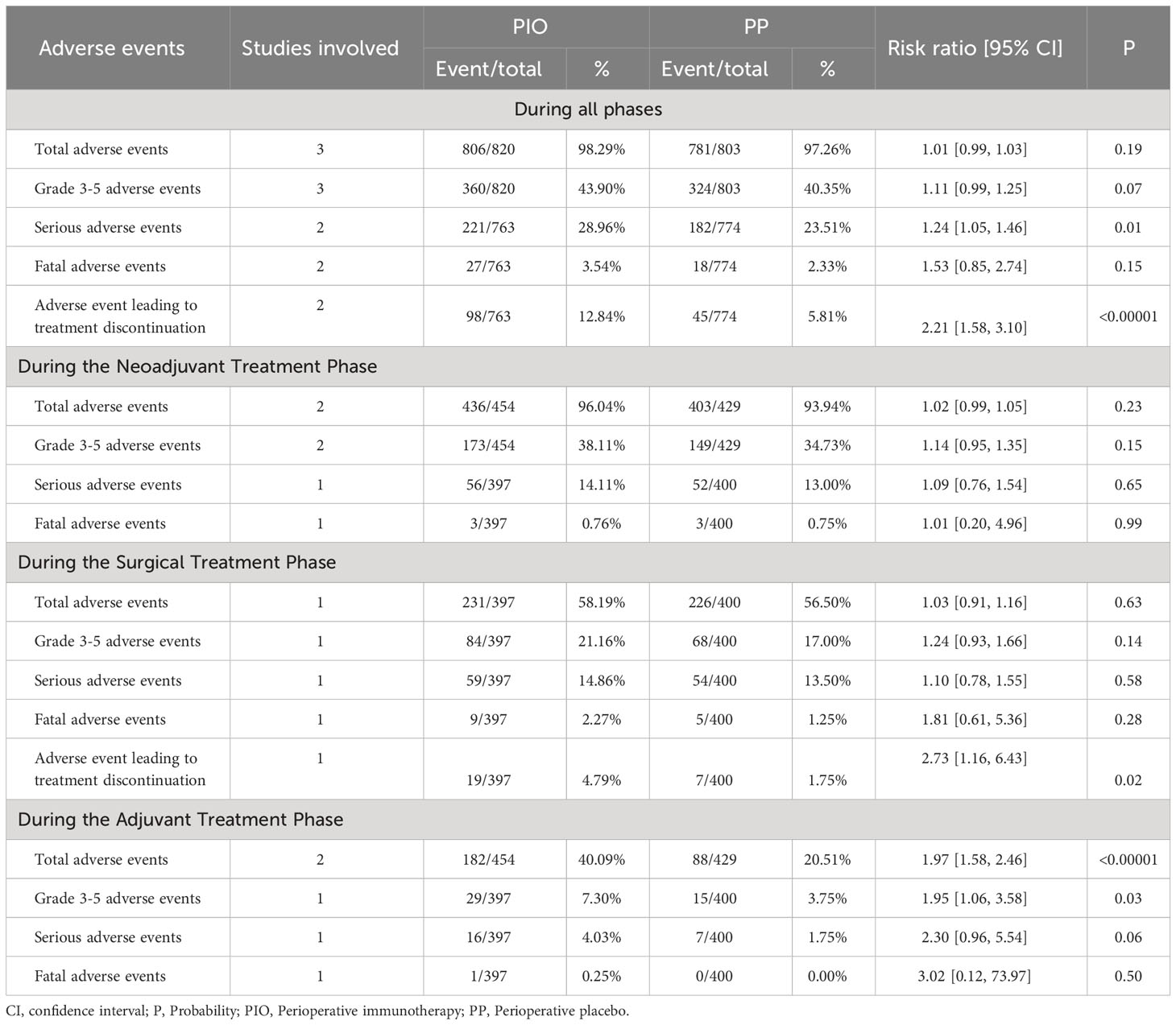
To summarize, PIO treatment resulted in a greater incidence of serious AEs (28.96% vs. 23.51%, RR: 1.24 [1.05, 1.46]) and AEs leading to treatment discontinuation (ALTD, 12.84% vs. 5.81%, RR: 2.21 [1.58, 3.10]). Total AEs, grade 3-5 AEs and fatal AEs tended to favor the PP group without significant differences (Table 2, Supplementary Figure S6).
In the neoadjuvant treatment phase, total AEs, grade 3-5 AEs, serious AEs, and fatal AEs tended to favor the PP group without a significant difference (Table 2, Supplementary Figure S7). More cases of rash, pruritus, increased alanine aminotransferase, hypothyroidism, and pneumonitis were found in the PIO group (Supplementary Table S5). There was no significant difference in the incidence of all grade 3-5 adverse events between the two groups in the neoadjuvant treatment phase (Supplementary Table S6).
In the surgical treatment phase, total AEs, grade 3-5 AEs, serious AEs, and fatal AEs tended to favor the PP group without a significant difference. PIO treatment was associated with more ALTD (4.79% vs. 1.75%, RR: 2.73 [1.16, 6.43]) (Table 2, Supplementary Figure S8). More diarrhea of any grade was found in the PIO group (Supplementary Table S7). There was no significant difference in the incidence of all grade 3-5 adverse events between the two groups in the surgical treatment phase (Supplementary Table S8).
In the adjuvant treatment phase, PIO treatment resulted in a greater incidence of total AEs (40.09% vs. 20.51%, RR: 1.97 [1.58, 2.46]) and grade 3-5 AEs (7.30% vs. 3.75%, RR: 1.95 [1.06, 3.58]). Serious AEs and fatal AEs tended to favor the PP group, but the difference was not significant (Table 2, Supplementary Figure S9). More grade pruritus, rash, and hypothyroidism were found in the PIO group (Supplementary Table S9). There was no significant difference in the incidence of all grade 3-5 adverse events between the two groups in the adjuvant treatment phase (Supplementary Table S10).
Sensitivity analysis
Analysis of ORR, surgery rate, and R0 resection rate revealed significant heterogeneity. Excluding any study did not affect the stability or reliability of the results, as indicated by the sensitivity analysis (Supplementary Figure S10).
Publication bias
Symmetrical funnel plots were observed for survival summary (Figure 6A), pathological responses (Figure 6B), and AEs (Figures 6C-F), indicating acceptable publication bias.

Figure 6 Funnel plots of survival summary (A), pathological responses (B), adverse events’ summary during all treatment phase (C), adverse events’ summary during the neoadjuvant treatment phase (D), adverse events’ summary during the surgical treatment phase (E), adverse events’ summary during the adjuvant treatment phase (F) associated with perioperative immunotherapy versus perioperative placebo according to survival time.
Discussion
Resectable stage II-III NSCLC cases can have improved outcomes if neoadjuvant and/or adjuvant treatment is given in addition to surgery (20–22). However, although traditional PBC can improve patient survival, it is very limited (23, 24). In recent years, the introduction of immunotherapy in neoadjuvant therapy and adjuvant therapy for resectable NSCLC has brought new hope to the long-term survival of these patients (9–15). This study represents the first meta-analysis analyzing the perioperative use (neoadjuvant+adjuvant) of immunotherapy for stage II-III NSCLC based on RCTs. The results suggested that PIO exhibited superior efficacy in OS, EFS, ORR, PCR, MPR, R0 resection rate, and rate of adjuvant treatment compared with PP. In safety assessment, more serious AEs and ALTD were found in the PIO group.
The primary advantage of PIO treatment lies in improved survival, particularly in terms of OS. In this study, the HR for survival was 0.63 [0.49-0.81] for OS and 0.61 [0.52, 0.72] for EFS. EFS is currently the primary endpoint in most RCTs on the perioperative treatment of NSCLC. In neoadjuvant therapy, the HR of EFS was 0.63 [0.43-0.91] in the CheckMate 816 study (9). In adjuvant therapy, the HR of EFS was 0.66 [0.50-0.88] in the Impower 010 study and 0.76 [0.63-0.91] in the KEYNOTE-091 study (11, 12). In addition, the Neotorch study (toripalimab) has reported interim research results with EFS (HR, 0.40 [0. 277-0. 565]) in ASCO 2023 (25). Thus, many scholars believed that the combined use of immunotherapy during the perioperative period might bring more survival benefits to patients than using neoadjuvant therapy and adjuvant therapy alone (8, 26). Meanwhile, this study also confirmed that PIO demonstrated an increasing advantage in survival (OS, EFS) compared to PP, which was consistent with the tail effect of immunotherapy (27). In the subgroup analysis, EFS tended to favor the PIO group in almost all subgroups. BMI (>25), T stage (IV), N stage (N1-N2) and pathological response (with PCR) were favorable factors in the PIO group, as substantiated in several studies (28, 29). Additionally, the EFS advantage of the PIO group increased with increasing PD-L1 expression (PD-L1 TPS, < 1%, RR: 0.77 [0.59-1.00]; 1-49%, RR: 0.56 [0.42-0.73]; > 50%, RR: 0.48 [0.35-0.67]).
Neoadjuvant immunotherapy may have improved survival benefits, although a direct comparative randomized trial would need to be conducted to determine this (30, 31). Therefore, the pathological response and its impact on surgical treatment are crucial indicators for evaluating drug efficacy. In summary, the ORR, PCR and MPR were 51.46%, 19.02% and 32.44% in the PIO group, which was similar to the results of NADIM study and SAKK 16/14 study (32, 33). In this study, patients in the PIO group achieved better ORR (RR: 2.21 [1.91, 2.54]), PCR (RR: 4.36 [3.04, 6.25]) and MPR (RR: 2.79 [2.25, 3.46]) compared to patients in the PP group. Similar results were also confirmed by the CheckMate 816 study and the Neotorch study (9, 25). Better pathological response was also associated with increased surgery rate (82.07% vs. 79.58%) and R0 resection rate (75.24% vs. 67.87%), playing a crucial role in the long-term survival of patients. Furthermore, we confirmed that the EFS advantage in the PIO group was particularly notable in the PCR subgroup. Therefore, it can be indirectly confirmed that a better pathological response could lead to a better prognosis in perioperative immunotherapy.
Safety is another concern in the perioperative and long-term use of immunotherapy after surgery. The IMpower010 trial reported that Atezolizumab-related adverse events leading to hospitalization occurred in 7% of the surgery groups (34). In clinical practice, although the incidence of AEs in immunotherapy is often much lower than that in chemotherapy, immune related AEs (such as pneumonitis, myocarditis, etc.) are often challenging to manage and can substantially impact the quality of life (35). At different periods of this study, it was observed that the incidence of total AEs, grade 3-5 AEs, serious AEs, and fatal AEs was higher in the PIO group than in the PP group in varying degrees, especially during the neoadjuvant treatment phase. In this phase, the top 5 AEs in the PIO group were nausea (41.15%), anemia (36.17%), neutrophil count decreased (30.28%), constipation (26.87%), and fatigue (23.17%), similar to those in the PP group. These common AEs are often associated with chemotherapy (13). The incidences of rash, pruritus, alanine aminotransferase increased, hypothyroidism, and pneumonitis were significant higher in the PIO group. These significantly increased AEs are often associated with immunotherapy (36). Therefore, although PIO can substantially improve survival, the monitoring and treatment of AEs at different phases still requires close attention.
This meta-analysis has limitations. Firstly, the inclusion of only English articles may introduce language bias. Secondly, including only 3 RCTs may reduce the overall clinical value. Thirdly, all the data analyzed were extracted from previously published articles, leading to increased data heterogeneity. Fourthly, the absence of individual patient data prevented a meta-analysis at the patient level, potentially decreasing the clinical value. Fifthly, variations in median follow-up times across studies might contribute to increased data heterogeneity.
Conclusion
PIO appears superior to PP for resectable stage II-III NSCLC, exhibiting better survival (OS and EFS) and improved pathological responses. Survival tended to favor the PIO group across almost all subgroups. Additionally, PIO demonstrated an increased advantage in survival compared to PP with longer follow up and increased PD-L1 expression. However, the higher rate of AEs in the PIO group warrants serious consideration.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
AY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FF: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. XL: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. MW: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. MY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. WZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Natural Science Foundation of China (NSFC), number of grants (81560345). The funding had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
The authors thank professor Wenxiong Zhang, MD (Department of Thoracic Surgery, The second affiliated hospital of Nanchang University) for his data collection and statistical advice.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1351359/full#supplementary-material
Supplementary Figure 1 | Cochrane Risk Assessment.
Supplementary Figure 2 | Comparisons of overall survival rate (6-48 months) associated with perioperative immunotherapy versus perioperative placebo according to survival time.
Supplementary Figure 3 | Comparisons of event-free survival rate (6-48 months) associated with perioperative immunotherapy versus perioperative placebo according to survival time.
Supplementary Figure 4 | Forest plots of surgery rate and R0 resection rate associated with perioperative immunotherapy versus perioperative placebo according to survival time.
Supplementary Figure 5 | Treatment summary of adjuvant phase.
Supplementary Figure 6 | Forest plots of adverse events’ summary during all treatment phase associated with perioperative immunotherapy versus perioperative placebo.
Supplementary Figure 7 | Forest plots of adverse events’ summary during the neoadjuvant treatment phase associated with perioperative immunotherapy versus perioperative placebo.
Supplementary Figure 8 | Forest plots of adverse events’ summary during the surgical treatment phase associated with perioperative immunotherapy versus perioperative placebo.
Supplementary Figure 9 | Forest plots of adverse events’ summary during the adjuvant treatment phase associated with perioperative immunotherapy versus perioperative placebo.
Supplementary Figure 10 | Sensitivity analysis of objective response rate (A), surgery rate (B), and R0 resection rate (C).
Abbreviations
AEs, Adverse effects; ALK, Anaplastic lymphoma kinase; ALTD, AEs leading to treatment discontinuation; BMI, Body mass index; DFS, Disease-free survival; Durva, Durvalumab; ECOG, Eastern Cooperative Oncology Group; EFS, Event-free survival; EFSR, Event-free survival rate; EGFR, Epidermal growth factor receptor; GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; HR, Hazard ratio; LC, Lung cancer; M/F, male/female; MPR, Major pathological response; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse; Nivo, Nivolumab; NSCLC, Non-small cell lung cancer; ORR, Objective response rate; OS, Overall survival; OSR, Overall survival rate; P, Probability; PCR, Pathological complete response; PD-L1, Programmed cell death 1 ligand 1; Pembro, Pembrolizumab; PICOS, Participants, Intervention, Control, Outcome and Study design; PIO, Perioperative immunotherapy; PP, Perioperative placebo; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis; RCT, randomized controlled trial; RECIST, Response Evaluation Criteria in Solid Tumors; RR, Risk ratio; TPS, Tumor cell Proportion Score.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763.
2. Wolf AMD, Oeffinger KC, Shih TY, Walter LC, Church TR, Fontham ETH, et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin. (2023). doi: 10.3322/caac.21811
3. Expert Consensus Panel, Kidane B, Bott M, Spicer J, Backhus L, Chaft J, et al. The American Association for Thoracic Surgery (AATS) 2023 Expert Consensus Document: Staging and multidisciplinary management of patients with early-stage non-small cell lung cancer. J Thorac Cardiovasc Surg. (2023) 166:637–54. doi: 10.1016/j.jtcvs.2023.04.039.
4. Daly ME, Singh N, Ismaila N, Antonoff MB, Arenberg DA, Bradley J, et al. Management of stage III non-small-cell lung cancer: ASCO guideline. J Clin Oncol. (2022) 40:1356–84. doi: 10.1200/JCO.21.02528.
5. Fillon M. Adding immunotherapy to chemotherapy improves survival for endometrial cancer patients. CA Cancer J Clin. (2023) 73:445–7. doi: 10.3322/caac.21809.
6. Yu S, Zhai S, Gong Q, Xiang C, Gong J, Wu L, et al. Neoadjuvant immunotherapy and non-small cell lung cancer: A systematic review and meta-analysis of randomized controlled trials. Am J Clin Oncol. (2023) 46:517–28. doi: 10.1097/COC.0000000000001046.
7. Li Z, Zhang X, Wang Y, Yu Z, Yang C, Zhou Y, et al. Adjuvant therapy in completely resected, EGFR-mutant non-small cell lung cancer: a comparative analysis of treatment efficacy between EGFR-TKI and anti-PD-1/PD-L1 immunotherapy. J Immunother Cancer. (2023) 11:e007327. doi: 10.1136/jitc-2023-007327.
8. Lovly CM. Perioperative immunotherapy-A KEY toward improved outcomes for early-stage lung cancer? N Engl J Med. (2023) 389:560–1. doi: 10.1056/NEJMe2305762.
9. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. (2022) 386:1973–85. doi: 10.1056/NEJMoa2202170.
10. Lei J, Zhao J, Gong L, Ni Y, Zhou Y, Tian F, et al. Neoadjuvant camrelizumab plus platinum-based chemotherapy vs chemotherapy alone for chinese patients with resectable stage IIIA or IIIB (T3N2) non-small cell lung cancer: the TD-FOREKNOW randomized clinical trial. JAMA Oncol. (2023) 9:1348–55. doi: 10.1001/jamaoncol.2023.2751.
11. O’Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. (2022) 23:1274–86. doi: 10.1016/S1470-2045(22)00518-6
12. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. (2021) 398:1344–57. doi: 10.1016/S0140-6736(21)02098-5.
13. Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. (2023) 389:491–503. doi: 10.1056/NEJMoa2302983.
14. Provencio M, Nadal E, González-Larriba JL, Martínez-Martí A, Bernabé R, Bosch-Barrera J, et al. Perioperative nivolumab and chemotherapy in stage III non-small-cell lung cancer. N Engl J Med. (2023) 389:504–13. doi: 10.1056/NEJMoa2215530.
15. Heymach JV, Harpole D, Mitsudomi T, Taube JM, Galffy G, Hochmair M, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. (2023) 389:1672–84. doi: 10.1056/NEJMoa2304875.
16. Edwards JG, Chansky K, Van Schil P, Nicholson AG, Boubia S, Brambilla E, et al. The IASLC lung cancer staging project: analysis of resection margin status and proposals for residual tumor descriptors for non-small cell lung cancer. J Thorac Oncol. (2020) 15:344–59. doi: 10.1016/j.jtho.2019.10.019
17. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4.
18. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928.
19. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. (2011) 64:380–2. doi: 10.1016/j.jclinepi.2010.09.011.
20. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines® Insights: non-small cell lung cancer, version 2.2023. J Natl Compr Canc Netw. (2023) 21:340–50. doi: 10.6004/jnccn.2023.0020
21. Wang C, Chen KN, Chen Q, Wu L, Wang Q, Li X, et al. Neoadjuvant nivolumab plus chemotherapy versus chemotherapy for resectable NSCLC: subpopulation analysis of Chinese patients in CheckMate 816. ESMO Open. (2023) 8:102040. doi: 10.1016/j.esmoop.2023.102040.
22. Felip E, Altorki N, Zhou C, Vallières E, Martínez-Martí A, Rittmeyer A, et al. Overall survival with adjuvant atezolizumab after chemotherapy in resected stage II-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase III trial. Ann Oncol. (2023) 34:907–19. doi: 10.1016/j.annonc.2023.07.001.
23. NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. (2014) 383:1561–71. doi: 10.1016/S0140-6736(13)62159-5.
24. Burdett S, Pignon JP, Tierney J, Tribodet H, Stewart L, Le Pechoux C, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev. (2015) 2015:CD011430. doi: 10.1002/14651858.CD011430.
25. Lu S, Wu L, Zhang W, Zhang P, Wang WX, Fang WT, et al. Perioperative toripalimab+platinum-doublet chemotherapy vs chemotherapy in resectable stage II/III non-small cell lung cancer (NSCLC): interim event-free survival (EFS) analysis of the phase III Neotorch study. J Clin Oncol. (2023) 41:Suppl:425126. doi: 10.1200/JCO.2023.41.36_suppl.425126
26. Ni Y, Lei J, Huang W, Wang J, Guo H, Lv F, et al. Systematic review of the perioperative immunotherapy in patients with non-small cell lung cancer: evidence mapping and synthesis. Front Oncol. (2023) 13:1092663. doi: 10.3389/fonc.2023.1092663.
27. Sim JK, Choi J, Lee SY. Perioperative immunotherapy in stage IB-III non-small cell lung cancer: a critical review of its rationale and considerations. Korean J Intern Med. (2023) 38:787–96. doi: 10.3904/kjim.2023.345.
28. Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. (2019) 25:141–51. doi: 10.1038/s41591-018-0221-5.
29. Zhao J, Hao S, Li Y, Liu X, Liu Z, Zheng C, et al. Comparative efficacy and safety of neoadjuvant immunotherapy with chemotherapy versus chemotherapy alone in non-small cell lung cancer: A propensity score and inverse probability treatment weighting analysis. Immunotargets Ther. (2023) 12:113–33. doi: 10.2147/ITT.S437911.
30. Gaudreau PO, Negrao MV, Mitchell KG, Reuben A, Corsini EM, Li J, et al. Neoadjuvant chemotherapy increases cytotoxic T cell, tissue resident memory T cell, and B cell infiltration in resectable NSCLC. J Thorac Oncol. (2021) 16:127–39. doi: 10.1016/j.jtho.2020.09.027.
31. Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. (2020) 367:eaax0182. doi: 10.1126/science.aax0182.
32. Provencio M, Serna-Blasco R, Nadal E, Insa A, García-Campelo MR, Casal Rubio J, et al. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial). J Clin Oncol. (2022) 40:2924–33. doi: 10.1200/JCO.21.02660.
33. Rothschild SI, Zippelius A, Eboulet EI, Savic Prince S, Betticher D, Bettini A, et al. SAKK 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small-cell lung cancer-A multicenter single-arm phase II trial. J Clin Oncol. (2021) 39:2872–80. doi: 10.1200/JCO.21.00276.
34. Lee JM, Vallières E, Ding B, Johnson A, Bhagwakar J, Rashidi S, et al. Safety of adjuvant atezolizumab after pneumonectomy/bilobectomy in stage II-IIIA non-small cell lung cancer in the randomized phase III IMpower010 trial. J Thorac Cardiovasc Surg. (2023) 166:655–666.e7. doi: 10.1016/j.jtcvs.2023.01.012.
35. Xu Y, Lyu X, Qin Y, Ma D, Wang M, Shi J, et al. Multi-organs perioperative immune-related adverse events and postoperative bronchial anastomotic fistula in a patient receiving neoadjuvant immunotherapy with NSCLC. Thorac Cancer. (2022) 13:2340–5. doi: 10.1111/1759-7714.14567.
Keywords: immunotherapy, neoadjuvant, adjuvant, surgery, non-small cell lung cancer, meta-analysis
Citation: Yu A, Fu F, Li X, Wu M, Yu M and Zhang W (2024) Perioperative immunotherapy for stage II-III non-small cell lung cancer: a meta-analysis base on randomized controlled trials. Front. Oncol. 14:1351359. doi: 10.3389/fonc.2024.1351359
Received: 06 December 2023; Accepted: 05 February 2024;
Published: 22 February 2024.
Edited by:
Aakash Desai, University of Alabama at Birmingham, United StatesReviewed by:
Pietro Bertoglio, IRCCS Azienda Ospedaliero Universitaria di Bologna, ItalyMitchell Von Itzstein, University of Texas, United States
Copyright © 2024 Yu, Fu, Li, Wu, Yu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meijian Yu, Ymj_yy_happy@163.com
 Anping Yu1,2
Anping Yu1,2 Meijian Yu
Meijian Yu Wenxiong Zhang
Wenxiong Zhang