- 1Department of Medical Ultrasound, West China Hospital, Sichuan University, Chengdu, China
- 2West China School of Medicine, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Pathology, West China Hospital, Sichuan University, Chengdu, China
Prostatic malakoplakia (PMP) is a rare inflammatory disease, and misdiagnosis on imaging is a major reason for unnecessary punctures; however, information on imaging is even rarer. Five patients with PMP between May 2022 and February 2023 were enrolled in this study to summarize the imaging manifestations. All patients underwent ultrasound (US)-guided prostate biopsy and were confirmed by pathology, and the presence of prostate cancer was also excluded by pathology. The five patients, with a median age of 71 years (range = 58–74 years), had a median total prostate-specific antigen (T-PSA) of 10.40 ng/mL (range = 1.74–63.42 ng/mL). In two patients, chest computed tomography showed pulmonary infections. All patients underwent magnetic resonance imaging (MRI). Of these patients, four had a Prostate Imaging–Reporting and Data System (PIRADS) score of 5, while one had a score of 4. The lesions were mostly distributed in the peripheral zone of the prostate and appeared as a high signal on T1-weighted imaging (T1WI) and a low signal on T2-weighted imaging (T2WI). In the US examination, four patients had abnormal prostate morphology, with an unsmooth envelope and non-uniform parenchymal echogenicity. Four patients had increased prostate volume. US showed a hypoechoic nodule with non-uniform internal echogenicity, and an abundant internal blood flow signal was detected by color Doppler US. PSA, MRI, and US were not specific for PMP in our study, but we found that a history of co-infection may be helpful in an accurate diagnosis and to avoid unnecessary biopsy.
1 Introduction
Malakoplakia is a rare chronic inflammatory disorder that was first observed by Michaelis and Gutmann, who named it in 1902 (1). It is commonly found in immunocompromised patients and frequently invades the urinary system, particularly the bladder (2). Prostatic malakoplakia (PMP) is an extremely rare condition (3–5). It usually occurs in men over the age of 60 and may be overtly symptomatic or clinically silent. A puncture biopsy remains the gold standard for diagnosis (5, 6). Imaging information on PMP is extremely limited, which may cause misdiagnosis and unnecessary punctures. In this study, we retrospectively included patients who had confirmed PMP and excluded prostate cancer by pathology after prostate biopsy at our institution between May 2022 and February 2023 to further describe the clinical manifestations, pathology, laboratory tests, and imaging performance of this rare disease.
2 Case presentation
2.1 Clinical manifestations and laboratory test findings
This series included five patients with a median age of 71 years (range = 58–74 years), a median total prostate-specific antigen (T-PSA) of 10.40 ng/mL (range = 1.74–63.42 ng/mL, reference value <4ng/mL), a median free prostate-specific antigen (F-PSA) of 0.805 ng/mL (range = 0.135–2.92ng/mL, reference value <0.75 ng/mL), and a median free/total (F/T) ratio of 7.74% (range = 4.6%–16.57%, reference value 25%–100%). The results of the general physical examinations of the patients were normal. Four had elevated T-PSA levels, three had elevated F-PSA levels, and all had low F/T. Only one patient came to clinic due to frequent, urgent, and painful urination; the other patients were clinically silent (Table 1). The chest computed tomography of two of the patients indicated coexisting pulmonary infections. Quantitative urine sediment analysis was available for two patients, with one showing qualitative urine protein of 0.3 g/L (1+), nitrite (++), occult blood of 167 cells/μL (2+), white blood cell (WBC) count of 500 cells/μL (3+), urinary sediment bacteria of 12,271/μL (reference value < 230/μL), and electrical conductivity of 8 mS/cm (reference value = 19.8–42.5 mS/cm). Another patient had a WBC count of 15/μL in the urine sediment (reference value = 0–11/μL) and an electrical conductivity of 13 mS/cm. The urine culture was available for one patient, with the isolated bacteria including Escherichia coli. No other malignant tumors were found in any of the patients.
2.2 Multi-parameter magnetic resonance imaging findings
All MRI examinations were performed on a 3.0-T MRI system (uMR 780, United Imaging Healthcare, Shanghai, China) with a phased-array body surface coil. A multi-parameter prostate magnetic resonance imaging (MP-MRI) protocol, which included T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), micro-view diffusion-weighted imaging (DWI), and three-phase dynamic contrast-enhanced (DCE) imaging using a three-dimensional (3D) fat-suppressed spoiled gradient-echo T1W sequence, was acquired. DWI with three different b-values (50, 200, and 1,400 s/mm2) was obtained, and apparent diffusion coefficient (ADC) maps were calculated and constructed based on two b-values (1,400 and 50 s/mm2).
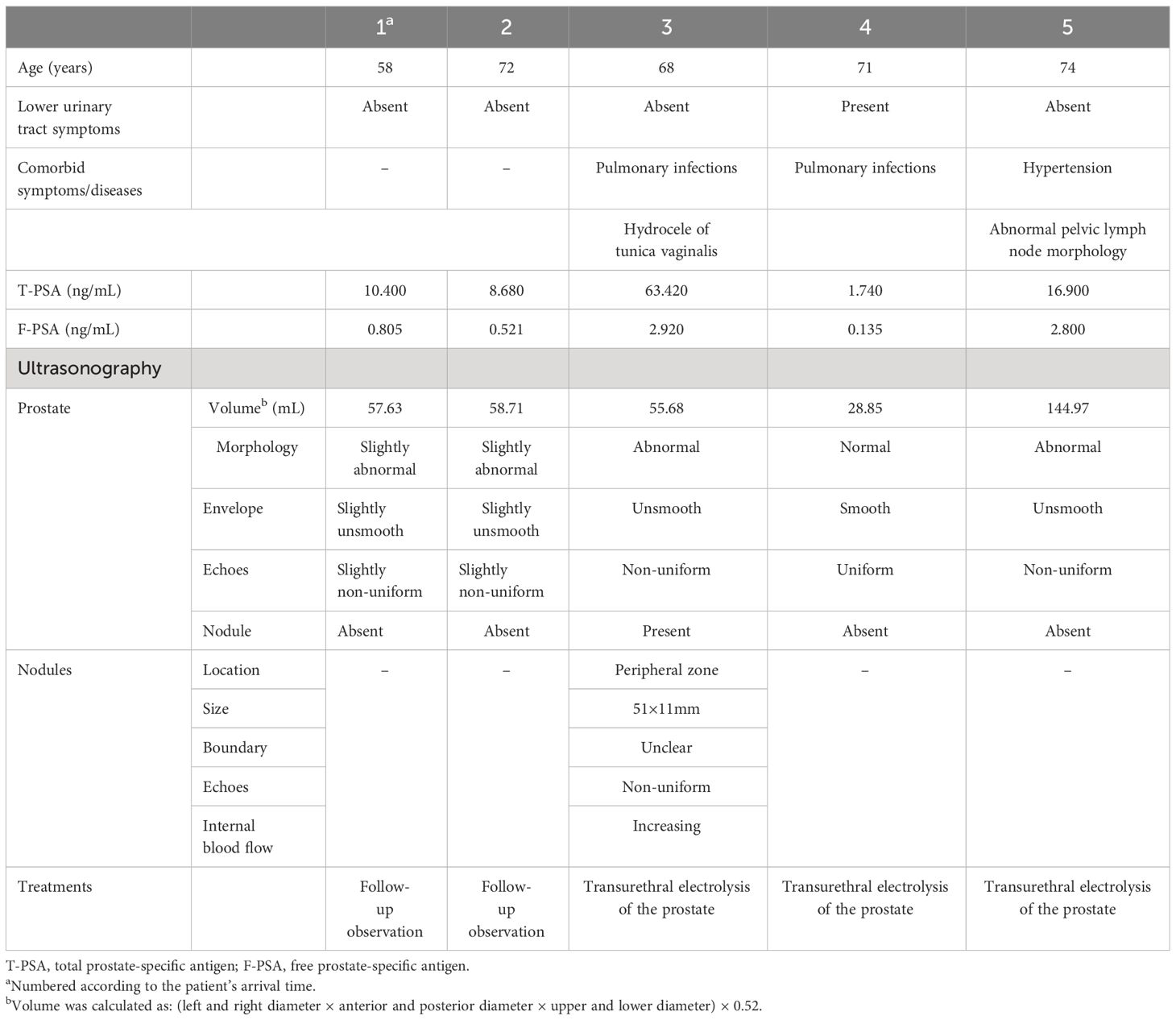
MP-MRI was available for all patients. Four of the patients had a Prostate Imaging–Reporting and Data System (PIRADS) score of 5, while another had a score of 4. The results showed bilateral involvement of the prostate, and the lesions could be located in the peripheral zone, central zone, and migratory zone. Peripheral zone involvement was the most common. The lesions mostly showed a high signal on T1WI, a low signal on T2WI (Figure 1), a high signal on DWI, and a low signal on ADC. A more uniform and obvious strengthening after enhancement was also seen. One patient showed abnormal lymph node morphology in the pelvis and suspected tumor metastasis or infection.
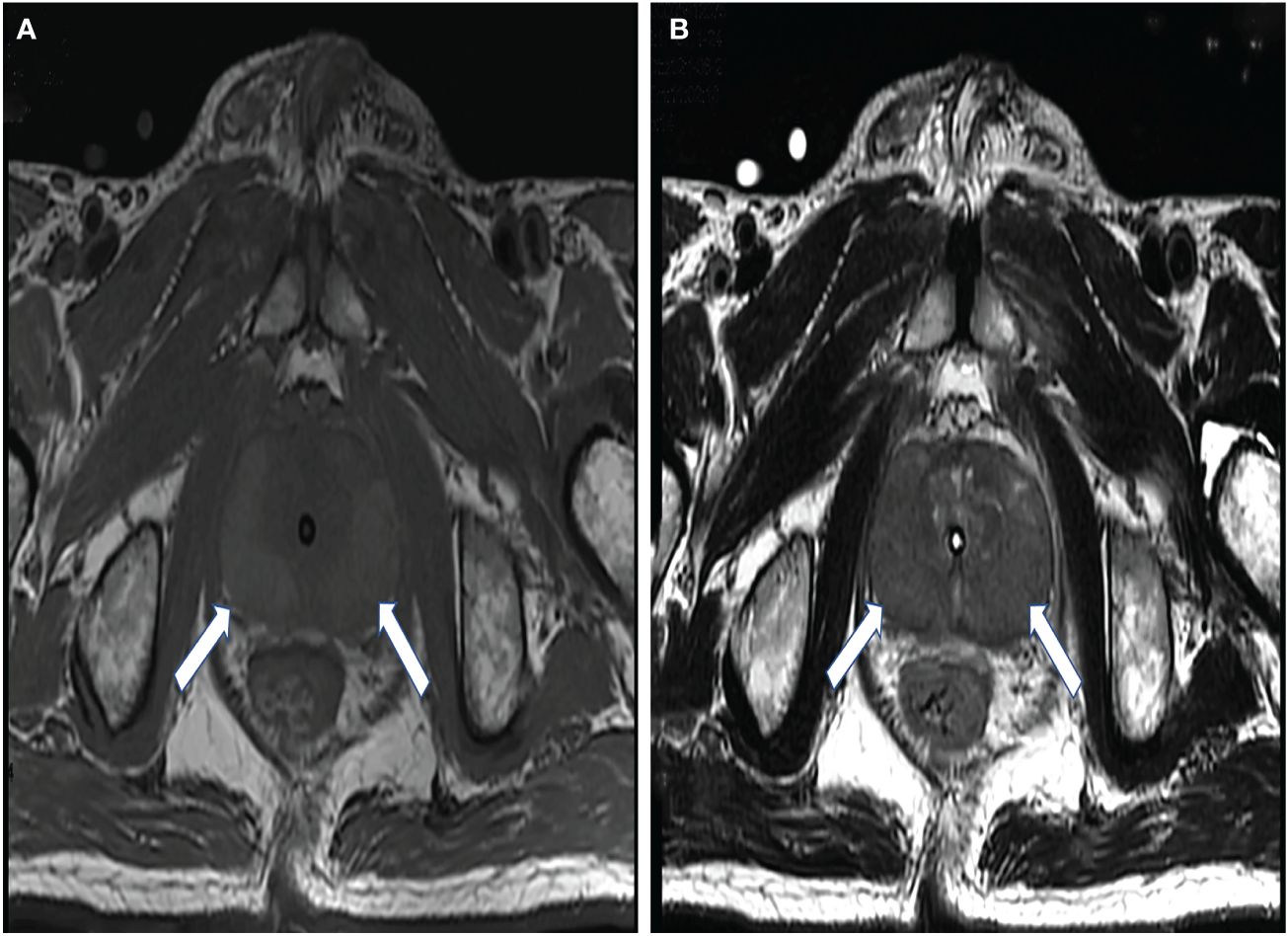
Figure 1 A 68-year-old man with elevated prostate-specific antigen (PSA). The lesions (arrows) showed a high signal on T1-weighted images (A) and a low signal on T2-weighted images (B).
2.3 Ultrasound examinations
All of the patients underwent ultrasound (US) examinations and a prostate biopsy with US guidance. The transperineal approach was chosen. The US images showed four of the patients having abnormal prostate morphology, with an unsmooth envelope and non-uniform parenchymal echogenicity. Four patients had increased prostate volume (Table 1). Only one nodule was detected by US, the size of which was approximately 51 × 11 mm, at the 3–8 o’clock direction in the peripheral zone. The boundary of the lesion was not clear, and the internal echogenicity was not uniform. The internal blood flow signal increased significantly. In addition, a strongly echogenic calcified focus in the prostate parenchyma was detected by grayscale (Figure 2).
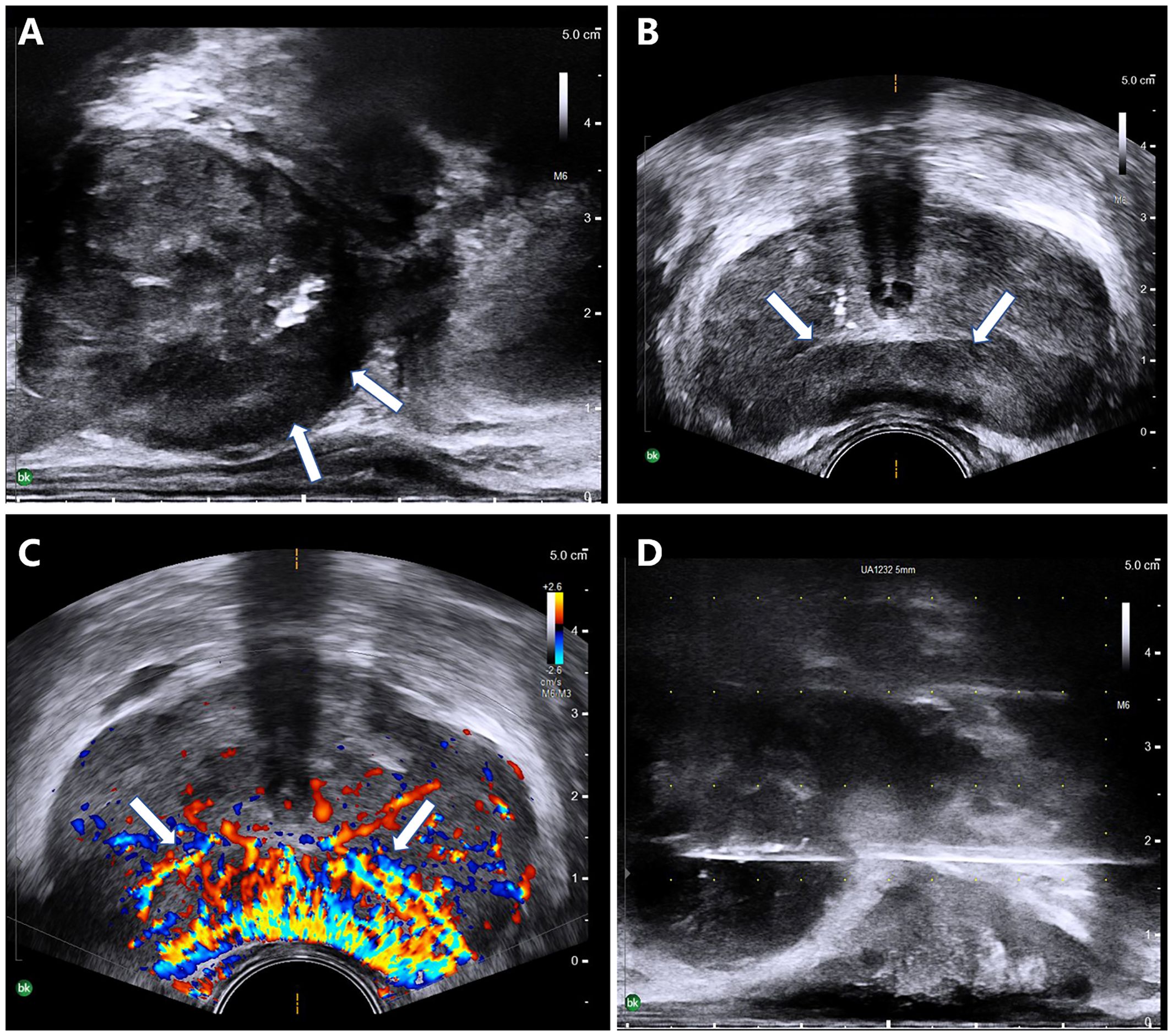
Figure 2 A 68-year-old man with elevated prostate-specific antigen (PSA). (A–D) Ultrasound revealed a morphologically abnormal prostate (A) and a hypoechoic nodule (arrows) at 3–8 o’clock in the peripheral zone (B), with an abundant blood flow signal in it (C). (D) A transperineal prostate biopsy was performed under ultrasound guidance.
2.4 Pathological findings
All patients underwent pathological examinations, with the results indicating PMP. All of the patients showed a large number of histiocytic infiltrates in the interstitium, with lymphocytic infiltration seen in two patients, eosinophilic infiltration seen in one patient, and focal granulomas seen in one patient. Histochemical stains showed calcium staining of Michealis–Gutmann (MG) bodies (+), PAS (+), iron staining (±), and antacid (−) (Figure 3). In the patients for whom prostate adenocarcinoma could not be directly excluded, further immunohistochemistry was performed. Three patients showed CD68 (histiocyte +), and two patients showed P63 (+) and high-molecular-weight cytokeratin (HCK) (+) in immunohistochemistry. PCR for Mycobacterium tuberculosis (TB-PCR) was performed in four patients, but no M. tuberculosis DNA fragments were detected.
Figure 3 Prostatic malakoplakia misdiagnosed as prostate cancer. A large number of Michaelis–Gutmann bodies (arrows) were seen in the interstitium. (A) Histochemical staining showed large intracellular deposits of calcium (B) and iron (C).
3 Discussion
The pathogenesis of malakoplakia is still not clear. It frequently occurs in immunocompromised patients (7), and the impairment in the bactericidal activity of the macrophages could be important in its pathogenesis (8). Malakoplakia has a different prognosis depending on where it occurs. Studies have shown that conservative medical intervention through the use of antibiotics may be effective, but surgical intervention may sometimes be necessary (6, 9–12). There are only isolated reports on malakoplakia imaging in the literature, which recognize that the imaging findings of malakoplakia often mimic different diseases and cancers. PMP is extremely rare, but imaging information on it is even rarer (13, 14).
PMP can be easily misdiagnosed as prostate cancer due to similarities in the clinical symptoms and laboratory findings (15). Both have a similar peak incidence period over 60 years of age and similar clinical symptoms, including lower urinary tract infection or urinary tract obstruction. Moreover, they have elevated PSA on serological testing (4, 16–18). On MP-MRI, both show similar presentations, with the lesions being commonly found in the peripheral zone of the prostate and showing a low signal on T2WI, a high signal on DWI, a low signal on ADC, and an early enhancement with retention of the contrast on DCE (19, 20). In this study, most patients (4/5, 80%) did not present with significant clinical symptoms, and the PSA level of one patient (1/5, 20%) was normal. All of the patients had a PIRADS score of 4 or 5, and one patient showed abnormal pelvic lymph node morphology, which caused high suspicion of prostate cancer. These demonstrate that the clinical features of PMP are not unique and that it is difficult to correctly distinguish PMP from prostate cancer. Pathological findings provide strong evidence for the diagnosis of PMP. The histological features of PMP are macrophages containing calcified lysosomes, called MG bodies, which is the main factor that could differentiate PMP from prostate cancer (21, 22). However, finding a noninvasive and effective imaging tool for the diagnosis of PMP can avoid unnecessary punctures.
US is widely used in the evaluation of prostate disease because of its convenience and inexpensive features (23). The transrectal approach to US of the prostate is the method of choice; however, transabdominal approaches are also available when the patient does not meet the conditions for the transrectal approach (24). The literature indicated that the US of PMP could depict localized hypoechoic areas with indistinct borders and uneven internal echogenicity in the prostate (15, 22, 25). In this study, four patients had an elevated PSA level, and their US showed the prostate to have an abnormal morphology, an unsmooth envelope, and non-uniform internal echogenicity. A hypoechoic nodule with irregular morphology and non-uniform internal echogenicity was detected by the gray-scale mode, while color Doppler US showed an abundant blood flow signal within it. These results were similar to those reported in a previous study (18). These presentations of gray-scale US and color Doppler US are similar to those of prostate cancer. In this study, US could not differentiate PMP or prostate cancer, including MP-MRI (26).
Some studies are of the opinion that defective bacterial phagocytosis and lysosome function are the cause of the disease, as most cases also present with bacterial infections, 80% of which were E. coli (2, 9). Previous studies have also reported two patients with malakoplakia with bronchial asthma and another three with pulmonary cancer (10, 27). In this study, two patients (2/5, 40%) had pulmonary infections, which implies that the occurrence of PMP may be related to pulmonary disease. Combining the patient’s history of infection, pulmonary disease, and imaging findings might provide a correct diagnosis of PMP.
In recent years, some studies have shown that shear wave elastography (SWE) can be effective in the identification of prostate disease (28, 29). PMP is an inflammatory lesion in nature, and the prostate cancer tissue is generally harder than normal tissues. SWE can measure tissue stiffness and therefore could provide more significant information on PMP and be beneficial to avoiding unnecessary biopsies (30). However, further studies are needed.
This is a retrospective study. In addition, the number of cases collected was small due to the rarity of PMP. Insufficient comprehensive clinical data also resulted in an inability to discuss more details.
4 Conclusion
In conclusion, PMP is an extremely rare condition, and imaging reports on it are limited. The similarities between PMP and prostate cancer make diagnosis difficult. Pathological findings are still the most useful evidence to diagnose PMP. The occurrence of an infection in the body could be a signal, such as a pulmonary infection. For this disease, the combination of clinical manifestations and comorbidities could reduce the misdiagnosis rate.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study involving humans was approved by West China Hospital, Sichuan University. The studies were conducted in accordance with local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YR: Writing – original draft, Data curation, Formal analysis, Investigation. WC: Data curation, Investigation, Writing – original draft. MZ: Data curation, Formal analysis, Investigation, Writing – review & editing. XZ: Data curation, Investigation, Software, Writing – original draft. JZ: Data curation, Investigation, Writing – review & editing. YL: Investigation, Writing – review & editing. DC: Data curation, Investigation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lusco MA, Fogo AB, Najafian B, Alpers CE. AJKD atlas of renal pathology: malakoplakia. Am J Kidney Dis. (2016) 68:e27–8. doi: 10.1053/j.ajkd.2016.08.006
2. Hyun KH, Shin HD, Kim DH. Malakoplakia in a healthy young female patient. Korean J Internal Med. (2013) 28:475–80. doi: 10.3904/kjim.2013.28.4.475
3. Medlicott S, Magi-Galluzzi C, Jimenez RE, Trpkov K. Malakoplakia associated with prostatic adenocarcinoma: Report of 4 cases and literature review. Ann Diagn pathology. (2016) 22:33–7. doi: 10.1016/j.anndiagpath.2016.03.004
4. Alpern HD. Malakoplakia of the prostate. Int Urol nephrology. (1991) 23:257–9. doi: 10.1007/BF02550421
5. Acosta AM, Sangoi AR, Maclean F, Trpkov K, Osunkoya AO, Collins K, et al. Prostatic malakoplakia: clinicopathological assessment of a multi-institutional series of 49 patients. Histopathology. (2022) 81:520–8. doi: 10.1111/his.14729
6. Parkin CJ, Acland G, Sulaiman B, Johnsun ML, Latif E. Malakoplakia, a Malignant mimic. Bladder (San Francisco Calif). (2020) 7:e44. doi: 10.14440/bladder.2020.818
7. Purnell SD, Davis B, Burch-Smith R, Coleman P. Renal malakoplakia mimicking a Malignant renal carcinoma: a patient case with literature review. BMJ Case Rep. (2015) 2015. doi: 10.1136/bcr-2014-208652
8. Graves AL, Texler M, Manning L, Kulkarni H. Successful treatment of renal allograft and bladder malakoplakia with minimization of immunosuppression and prolonged antibiotic therapy. Nephrol (Carlton Vic). (2014) 19 Suppl 1:18–21. doi: 10.1111/nep.12194
9. Cięszczyk K, Puderecki M, Wronecki L, Burdan F, Szumiło J. Malakoplakia of the urinary system. Folia Med Cracoviensia. (2019) 59:67–74. doi: 10.24425/fmc.2019.128455
10. Dong H, Dawes S, Philip J, Chaudhri S, Subramonian K. Malakoplakia of the urogenital tract. Urol Case Rep. (2015) 3:6–8. doi: 10.1016/j.eucr.2014.10.002
11. Chaudhry N, Vazzano J, Parwani A. Case study: Malakoplakia of the bladder. Pathology Res practice. (2022) 237:153852. doi: 10.1016/j.prp.2022.153852
12. Leão CA, Duarte MI, Gamba C, Ramos JF, Rossi F, Galvão MM, et al. Malakoplakia after renal transplantation in the current era of immunosuppressive therapy: case report and literature review. Transplant Infect Dis. (2012) 14:E137–141. doi: 10.1111/tid.12012
13. Sujka SK, Malin BT, Asirwatham JE. Prostatic malakoplakia associated with prostatic adenocarcinoma and multiple prostatic abscesses. Urology. (1989) 34:159–61. doi: 10.1016/0090-4295(89)90254-9
14. Répássy DL, Iványi A, Csata S, Tamás G. Combined occurrence of prostate carcinoma and malacoplakia. Pathol Oncol research: POR. (2002) 8:202–3. doi: 10.1007/BF03032396
15. Agostinho AD, Corrêa LA, Amaro JL, Bacchi CE, Viana de Camargo JL. Malacoplakia or prostate cancer? Similarities and differences. Urologia internationalis. (1998) 61:47–9. doi: 10.1159/000030284
16. Cazzaniga MG, Tommasini-Degna A, Negri R, Pioselli F. Cytologic diagnosis of prostatic malakoplakia. Rep three cases. Acta cytologica. (1987) 31:48–52.
17. Sarma HN, Ramesh K, Fituri O, Saeed SO, Majeed SA. Malakoplakia of the prostate gland–report of two cases and review of the literature. Scandinavian J Urol nephrology. (1996) 30:155–7. doi: 10.3109/00365599609180909
18. Görgel SN, Balcı U, Sarı AA, Ermete M, Girgin C, Dinçel C. Malakoplakia of the prostate diagnosed by elevated PSA level and transrectal prostate biopsy. Kaohsiung J Med Sci. (2011) 27:163–5. doi: 10.1016/j.kjms.2010.12.012
19. Velasquez MC, Taylor Smith PJ, Prakash NS, Kava B, Kryvenko ON, Castillo-Acosta R, et al. Malakoplakia of the prostate diagnosed on multiparametric-MRI ultrasound fusion guided biopsy: A case report and review of the literature. Urol Case Rep. (2018) 18:94–6. doi: 10.1016/j.eucr.2017.11.009
20. Ueno Y, Tamada T, Sofue K, Murakami T. Diffusion and quantification of diffusion of prostate cancer. Br J Radiol. (2022) 95:20210653. doi: 10.1259/bjr.20210653
21. Stamatiou K, Chelioti E, Tsavari A, Koulia K, Papalexandrou A, Efthymiou E, et al. Renal failure caused by malakoplakia lesions of the urinary bladder. Nephro-urology monthly. (2014) 6:e18522. doi: 10.5812/numonthly
22. Guner G, Akdogan B, Baydar DE. Malakoplakia of prostate as a complication of transrectal needle biopsy. Can J urology. (2012) 19:6124–7.
23. Mitterberger M, Horninger W, Aigner F, Pinggera GM, Steppan I, Rehder P, et al. Ultrasound of the prostate. Cancer Imaging. (2010) 10:40–8. doi: 10.1102/1470-7330.2010.0004
24. AIUM practice guideline for the performance of an ultrasound evaluation of the prostate (and surrounding structures). J ultrasound Med. (2015) 34:1–6. doi: 10.7863/ultra.34.8.15.13.0004
25. Morgan TA, Rabban JT, Filly RA. Testicular malakoplakia: a rare sonographic mimic of Malignancy. J Clin ultrasound: JCU. (2015) 43:199–202. doi: 10.1002/jcu.v43.3
26. Lotti F, Frizza F, Balercia G, Barbonetti A, Behre HM, Calogero AE, et al. The European Academy of Andrology (EAA) ultrasound study on healthy, fertile men: Prostate-vesicular transrectal ultrasound reference ranges and associations with clinical, seminal and biochemical characteristics. Andrology. (2022) 10:1150–71. doi: 10.1111/andr.13217
27. Koga S, Arakaki Y, Matsuoka M, Ohyama C. Malakoplakia of prostate. Urology. (1986) 27:160–1. doi: 10.1016/0090-4295(86)90374-2
28. Kapoor A, Kapoor A, Mahajan G, Sidhu BS. Real-time elastography in the detection of prostate cancer in patients with raised PSA level. Ultrasound Med Biol. (2011) 37:1374–81. doi: 10.1016/j.ultrasmedbio.2011.05.014
29. Boehm K, Salomon G, Beyer B, Schiffmann J, Simonis K, Graefen M, et al. Shear wave elastography for localization of prostate cancer lesions and assessment of elasticity thresholds: implications for targeted biopsies and active surveillance protocols. J urology. (2015) 193:794–800. doi: 10.1016/j.juro.2014.09.100
Keywords: prostatic malakoplakia, prostate-specific antigen, ultrasound, magnetic resonance imaging, pulmonary disease
Citation: Ren Y, Chen W, Zhang M, Zhang X, Zhou J, Li Y and Cai D (2024) Case report: Prostatic malakoplakia: a rare disease that has a profile mimicking prostate cancer. Front. Oncol. 14:1348797. doi: 10.3389/fonc.2024.1348797
Received: 03 December 2023; Accepted: 25 March 2024;
Published: 11 April 2024.
Edited by:
Cosmin Caraiani, University of Medicine and Pharmacy, Iuliu Hatieganu, RomaniaReviewed by:
Iulia Andras, Iuliu Haţieganu University of Medicine and Pharmacy, RomaniaBing Ren, Dartmouth Hitchcock Medical Center, United States
Copyright © 2024 Ren, Chen, Zhang, Zhang, Zhou, Li and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diming Cai, doccai@163.com
 Yelei Ren
Yelei Ren Weihao Chen
Weihao Chen Mengni Zhang3
Mengni Zhang3 Xuhui Zhang
Xuhui Zhang Jiaojiao Zhou
Jiaojiao Zhou Yongzhong Li
Yongzhong Li Diming Cai
Diming Cai