- Fujian Medical University Union Hospital, Fujian Institute of Hematology, Fujian Provincial Key Laboratory on Hematology, Fuzhou, Fujian, China
The aim of this study was to examine the characteristics and prognosis of patients with myelodysplastic syndrome (MDS) accompanied by TP53 abnormalities and explore potential prognostic factors and treatment responses. This retrospective analysis included 95 patients with MDS and TP53 abnormalities and 173 patients with MDS without TP53 abnormalities at the Fujian Medical University Union Hospital between January 2016 and June 2023. Among patients with TP53 abnormalities, 26 (27.4%) developed AML during the disease course, with a median transformation time of 5.7 months. Complex karyotypes were observed in 73.1% of patients, and the proportions of -5 or del(5q), -7 or del(7q), +8, and -20 or del(20q) were 81.8%, 54.5%, 30.7%, and 25.0%, respectively. These patients exhibited poor survival, with a median overall survival (OS) of 7.3 months, and had 1- and 2-year OS rates of 42.2% and 21.5%, respectively. The complete response rates for azacitidine monotherapy, venetoclax combined with azacitidine, decitabine monotherapy, and decitabine combined with low-dose chemotherapy were 9.1%, 41.7%, 37.5%, and 33.3%, respectively. Long-term survival was similar among the four treatment groups. Patients who underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) had a median OS of 21.3 months, which trended to be longer than that of patients who did not undergo allo-HSCT (5.6 months; P = 0.1449). Patients with pulmonary infection at diagnosis experienced worse OS than those without pulmonary infection (2.3 months vs. 15.4 months; P < 0.0001). Moreover, 61.9% of patients with pulmonary infection had immune dysfunction, with a ratio of CD4+ to CD8+ T lymphocytes below two. Pulmonary infections and complex karyotypes were independent adverse prognostic factors for OS. In conclusion, TP53 abnormalities in patients with MDS were frequently accompanied by complex karyotypes, and treatments based on hypomethylating agents or venetoclax have limited efficacy. Pulmonary infections associated with immune dysfunction is associated with poor prognosis.
1 Introduction
Since its identification in 1979 and the revelation of its role as a tumor suppressor gene in 1989, tumor protein 53 (TP53) has been a hot topic in the field of cancer research (1). TP53 is the most frequently mutated gene in cancer (2, 3). The frequency of TP53 mutations is highly variable among the different types and stages of cancers (4). Myelodysplastic syndrome (MDS) is a group of acquired clonal stem cell disorders that is very heterogeneous in its morphology, clinical features, and survival (5). TP53 mutations are detected in approximately 10%-20% of patients with de novo MDS and 30%-40% of patients with therapy-related MDS (6, 7), and TP53 abnormalities occur in 70–80% of patients with complex karyotypes or a loss of chromosome 17/17p, 5/5q, or 7/7q (8, 9). TP53 abnormalities in MDS are associated with high-risk disease, rapid transformation to acute myeloid leukemia (AML), resistance to conventional therapies, and poor outcomes (10–13). However, the clinical value of TP53 abnormalities in patients with MDS has not been fully investigated.
Despite being one of the most studied genes since its discovery, TP53 is considered “undruggable.” Therefore, TP53-mutated MDS remains a long-standing therapeutic challenge, with a median survival of only 5–10 months, irrespective of the therapies administered (14). Hypomethylating agents (HMAs) are the current standard treatment for newly diagnosed high-risk MDS and offer an overall response rate (ORR) of 17%–77% in patients with TP53-mutated MDS, with International Working Group complete response (CR) in 10–25% of patients and a median overall survival (OS) of 8.2–12.4 months (15, 16). Venetoclax, a selective small-molecule B-cell lymphoma 2 inhibitor, is a promising agent for the treatment of myeloid malignancies. A retrospective study showed an ORR of 57.2% and a median OS of 14 months in patients with refractory/relapsed (R/R) MDS treated with venetoclax combined with HMAs (17). Recent clinical trials have shown that venetoclax with azacitidine is effective in patients with high-risk R/R MDS, provides clinically meaningful benefits, and improves OS (18, 19). Presently, treatments based on HMAs or venetoclax are the main choices for high-risk MDS; however, the efficacy of these regimens in the treatment of MDS with TP53 abnormalities needs to be further clarified.
Additionally, immune dysfunction associated with TP53 mutation has recently been observed in patients with MDS. Sallman et al. concluded that the microenvironment of TP53-mutated MDS has an immune-privileged, evasive phenotype that may be a primary driver of poor outcomes and suggested that immunomodulatory therapeutic strategies may improve survival in this molecularly defined subpopulation (20). Recently, novel immunotherapeutic approaches have been developed for TP53-mutated MDS and AML and have demonstrated promising results (21, 22). Currently, few studies have focused on the relationship between immune dysfunction and infection in MDS with TP53 abnormalities.
The aim of this study was to examine the characteristics and prognosis of MDS patients with TP53 abnormalities and explore potential prognostic factors for OS in patients receiving HMA- or venetoclax-based treatments. We also evaluated the prognostic value of pulmonary infections associated with immune dysfunction in this cohort.
2 Methods
2.1 Patients
This single-center retrospective study included 95 consecutive patients diagnosed with MDS and TP53 abnormalities and 173 patients diagnosed with MDS without TP53 abnormalities between January 2016 and June 2023. This study was approved by the Ethics Committee of Fujian Medical University Union Hospital (2023KY154), and all patients provided written informed consent for treatment.
2.2 Definition and classification of MDS
MDS was diagnosed and classified according to the 5th edition of the World Health Organization Classification of Haematolymphoid Tumours (23).
2.3 Definition of complex karyotype and assessment of TP53 mutations
Complex karyotype was defined as having three or more chromosomal abnormalities. TP53 gene mutation assessment was performed by Kangsheng Global Medical Technology Co., Ltd. gDNA was extracted from the patient’s bone marrow (BM) sample, then amplified by multiplex polymerase chain reaction (PCR). The detection of a whole exon of the TP53 gene was performed by an illumina-based NEXTSeq550 sequencer.
2.4 Definition of pulmonary infection and ratio of CD4+ to CD8+ lymphocyte
The diagnosis of pulmonary infection was based on the patient’s clinical symptoms and signs, in combination with a computed tomography (CT) scan of the lung and pathogenic indicators, such as sputum culture and nucleic acid testing of pathogens. Pleural effusion was diagnosed according to a CT scan of the lung.
The samples for analysis of T cell populations were acquired from patients’ peripheral blood and examined by flow cytometry using the BD FACS Canto II. CD45/SSC, CD3/SSC, and CD19/SSC were used for gating. B lymphocytes were labeled with anti-human CD10/FITC, CD20/PE, CD19/PE-Cy7, CD20/APC-Cy7, and CD45/PerCP; T and natural killer (NK) lymphocytes were labeled with anti-human CD3/FITC, CD16/PE, CD56/PE, CD45/PerCP, CD8/PE-Cy7, and CD4/APC-Cy7. All the antibodies used were acquired from BD Biosciences. The ratio of CD4+ to CD8+ lymphocytes below two was classified as immune dysfunction.
2.5 Treatment protocols
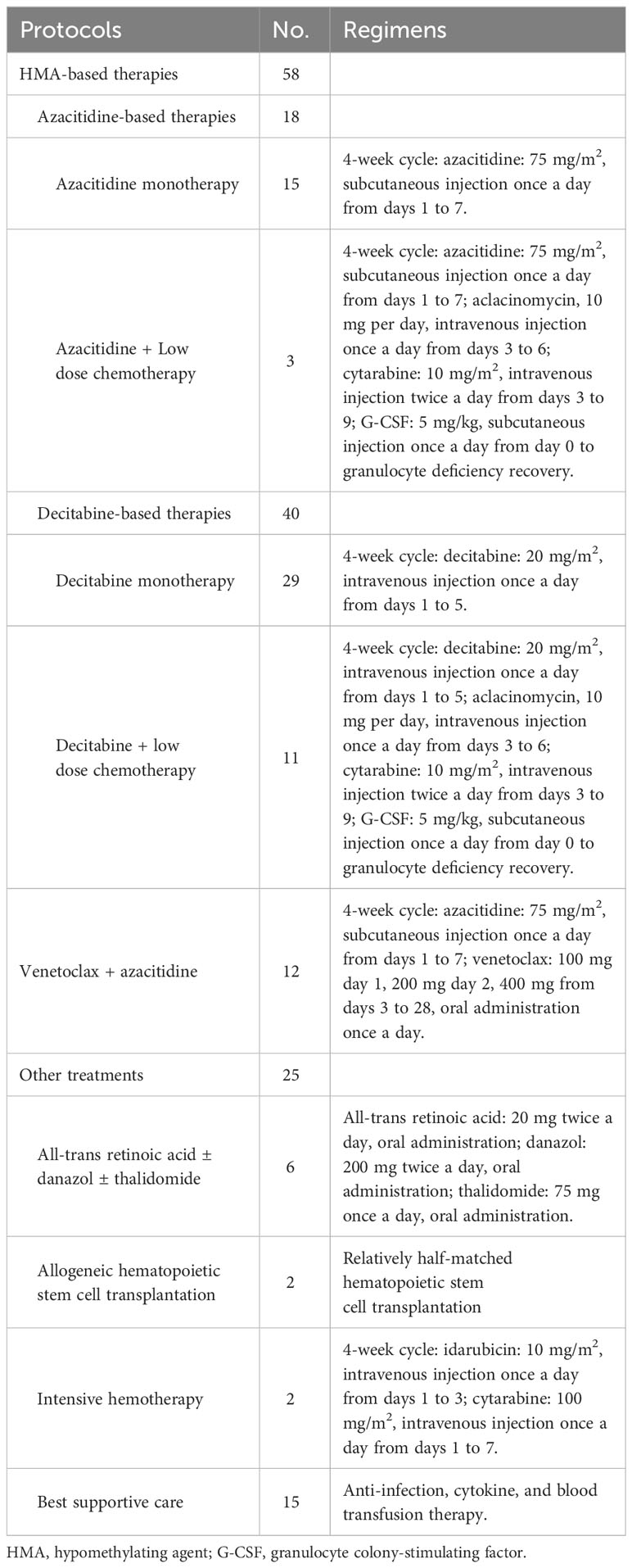
Among the patients with MDS and TP53 abnormalities, fifty-eight patients received HMA-based therapies, including 18 who received azacitidine and 40 who received decitabine. Twelve patients were administered a combination of venetoclax and azacitidine. Ten patients underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT). Details of the initial treatment protocols for these patients are summarized in Table 1.
2.6 Definition of response and outcomes
The therapeutic effects were evaluated according to the revised International Working Group 2023 response criteria for high-risk MDS (24). Peripheral blood counts were obtained for each cycle before treatment. BM aspiration and biopsy were performed every one or two cycles for response assessment. The ORR included CR and partial remission (PR) rates. Duration of response (DOR) was calculated as the time from CR or PR to progression or relapse, and OS was calculated from diagnosis to death or the last follow-up visit.
2.7 Statistical analysis
Differences between patient subgroups were analyzed using the chi-square test, t-test, or nonparametric test, as appropriate. Survival analysis was performed using the Kaplan–Meier method, and survival curves were compared using the log-rank test. The impact of prognostic factors such as sex, age, cytogenetic abnormalities, and other parameters on OS was analyzed using the Cox regression model. Parameters that had significant impact on OS in the univariate analysis were further included in the multivariate analysis, which was performed with the Cox regression model. All data were analyzed using GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA, USA), the Statistical Package for the Social Sciences software (SPSS version 21.0; IBM Corp., Armonk, NY, USA), and R software (Version 4.03, R Project for Statistical Computing, Vienna, Austria). Statistical significance was set at P < 0.05.
3 Results
3.1 Clinical characteristics
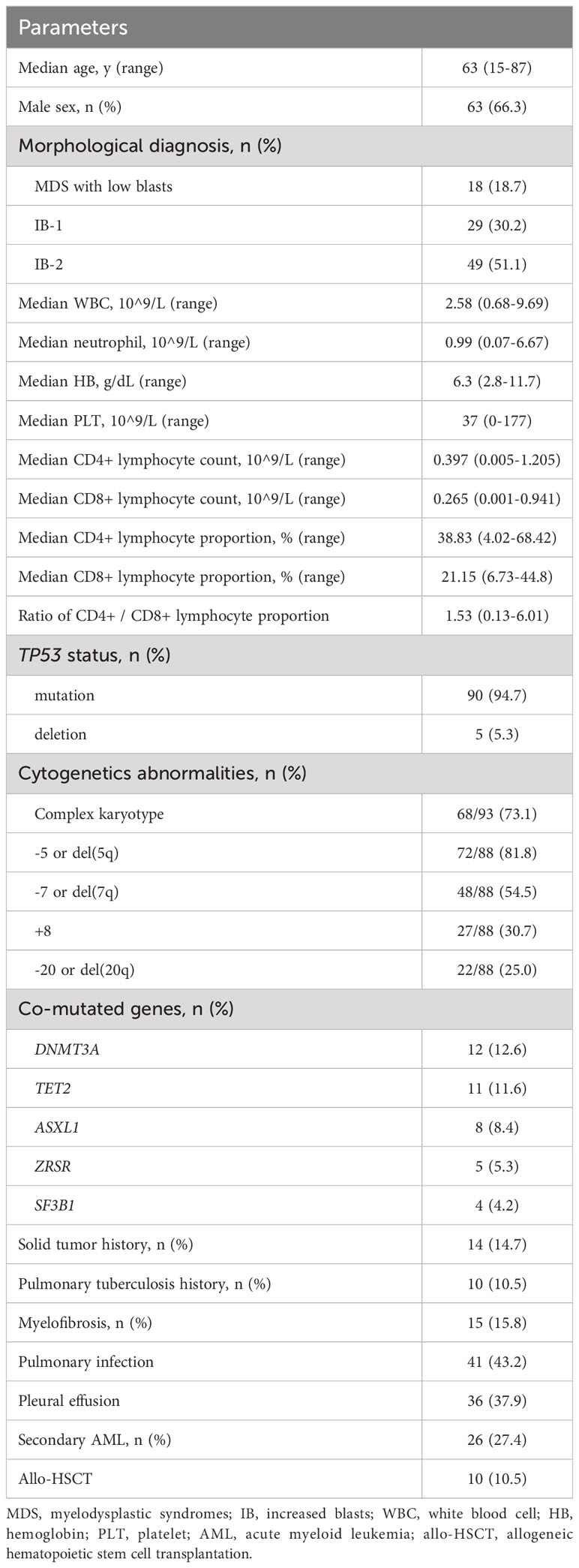
Among the 95 patients with TP53 abnormalities, 90 (94.7%) had TP53 mutations and 5 (5.3%) had TP53 deletions. The group consisted of 63 male (66.3%) and 32 female (33.7%) patients, with a male-to-female ratio of 1.97:1. Patients’ ages ranged from 15 to 87 years, with a median age of 63 years. Based on morphology, 19 patients (20.0%) had MDS with low blasts, 27 (28.4%) had increased blasts-1 (IB-1), and 49 (51.6%) had increased blasts-2 (IB-2). The median white blood cell count, neutrophil count, hemoglobin concentration, and platelet count were 2.58 × 10 (9)/L, 0.99 × 10 (9)/L, 63 g/L, and 37 × 10 (9)/L, respectively. Fifteen patients (15.8%) had myelofibrosis at the time of diagnosis, as detected by BM biopsy. Chromosomal karyotypes were available for 93 patients, and complex karyotypes were observed in 68 patients (73.1%). Among the 88 patients with fluorescence in situ hybridization results, 81.8%, 54.5%, 30.7%, and 25.0% had -5 or del(5q), -7 or del(7q), +8, and -20 or del(20), respectively. DNMT3A, TET2, ASXL1, SF3B1, and ZRSR mutations were observed in 12 (12.6%), 11 (11.6%), 8 (8.4%), 5 (5.3%), and 4 (4.2%) patients, respectively. Fourteen patients (14.7%) had a history of solid tumors, and 10 (10.5%) had a history of pulmonary tuberculosis. Twenty-six patients (27.4%) developed AML during the disease course, with a median transformation time of 5.7 months. Pulmonary infection was diagnosed in 41 patients (43.2%), and pleural effusion was observed in 36 of the 41 (87.8%) patients with pulmonary infection at the time of diagnosis. The most common microorganisms identified were Escherichia coli, Klebsiella pneumoniae, and Candida albicans. The median proportion of CD4+ lymphocyte and CD8+ lymphocyte were 38.83% (4.02% - 68.42%) and 21.15% (6.73% - 44.8%), respectively. The median CD4+ lymphocyte count in patients with pulmonary infection was 0.312 × 10 (9)/L, which was lower than that of 0.482 × 10 (9)/L in patients without pulmonary infection (P = 0.0941); CD8+ lymphocyte counts were similar between the two groups. Details about the patient characteristics are summarized in Table 2.
3.2 Treatment outcomes
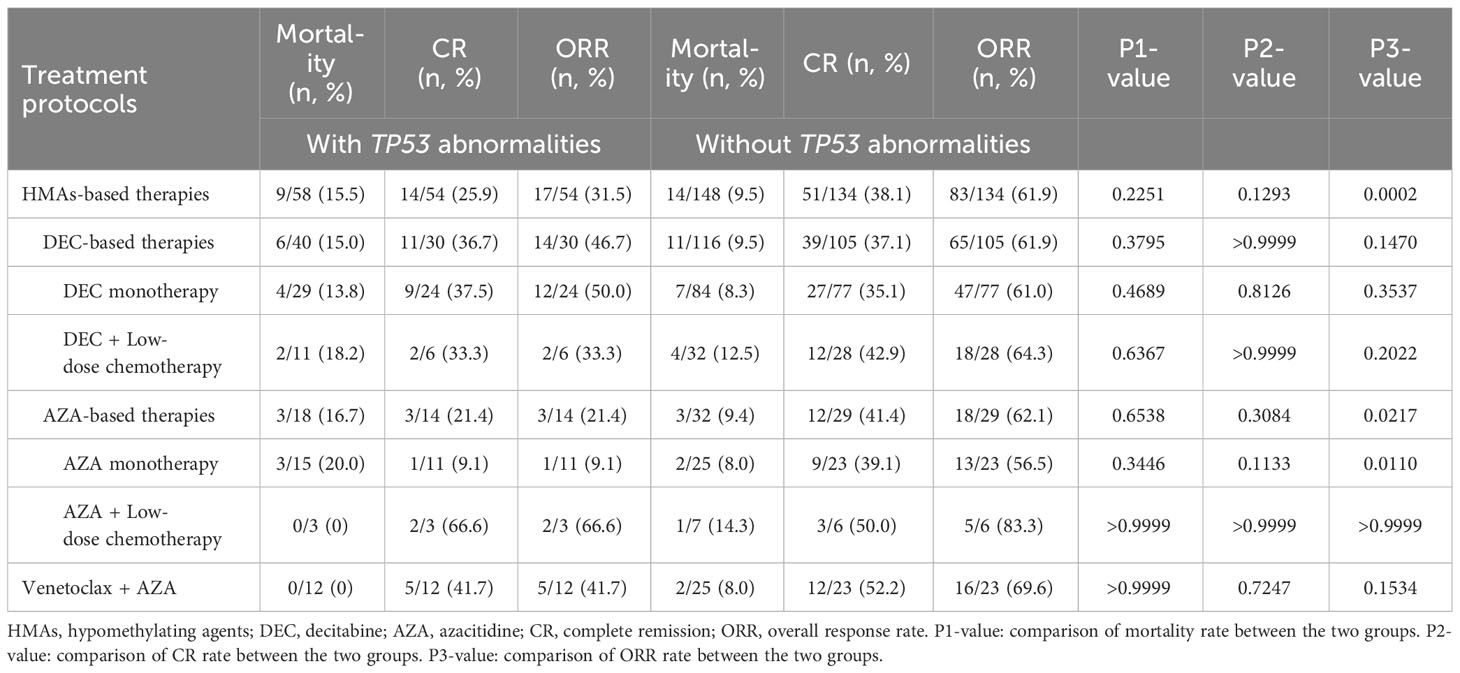
Seventy patients (73.9%) who received HMA- or venetoclax-based treatments were included in the efficacy analysis. Among the patients treated with HMA-based therapies, 25.9% achieved CR, with an ORR of 31.5%. The treatment-related mortality rate was 15.5%, and no significant difference was observed between the azacitidine and decitabine groups (P > 0.9999). The CR rate of azacitidine monotherapy was only 9.1%, which was lower than those observed for decitabine monotherapy (37.5%; P = 0.1197) and decitabine combined with low-dose chemotherapy (33.3%; P = 0.5147). Patients receiving venetoclax combined with azacitidine achieved a CR rate of 41.7%, which was superior to that of azacitidine monotherapy (P = 0.1550) and similar to that of decitabine-based treatment (P > 0.9999). Importantly, the median DOR was only 4.4 months in the venetoclax and azacitidine treatment group, which was significantly inferior to that in the decitabine monotherapy treatment group (11.2 months; P = 0.0093). The response rate of patients with TP53 abnormalities was lower than those without TP53 abnormalities when treating with HMA- or venetoclax-based therapies (Table 3).
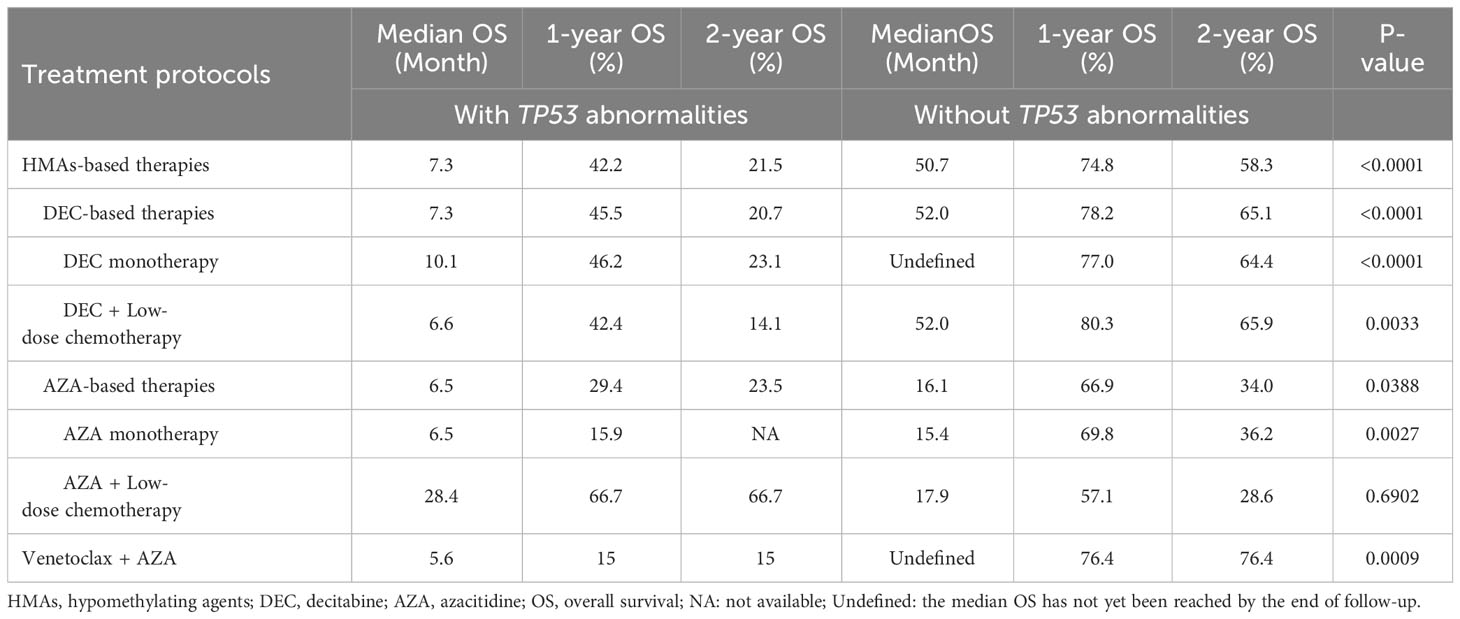
Patients with MDS accompanied by TP53 abnormalities had poor long-term survival when treated with HMA-based therapies, with a median OS of 7.3 months, 1-year OS rate of 42.2%, and 2-year OS rate of 21.5% (Figure 1). Patients who received azacitidine- or decitabine-based treatment had similar outcomes, with a median OS of 7.3 months and 9.0 months, respectively (P = 0.9167; Figure 1). The median OS of patients treated with venetoclax and azacitidine was 5.6 months, which was not superior to that of patients treated with HMA-based regimens (Figure 1). Patients who underwent allo-HSCT trended to have favorable survival, with a median OS of 21.3 months, whereas patients who did not undergo allo-HSCT had a median OS of 5.6 months (P = 0.1449; Figure 1). Patients who developed AML had an extremely poor prognosis, with a median OS of only 1.8 months, 1-year OS rate of 14.7%, and 2-year OS rate of 7.4%. The long-term survival of patients with TP53 abnormalities was significantly inferior to those without TP53 abnormalities when treating with HMA- or venetoclax-based therapies (Table 4).
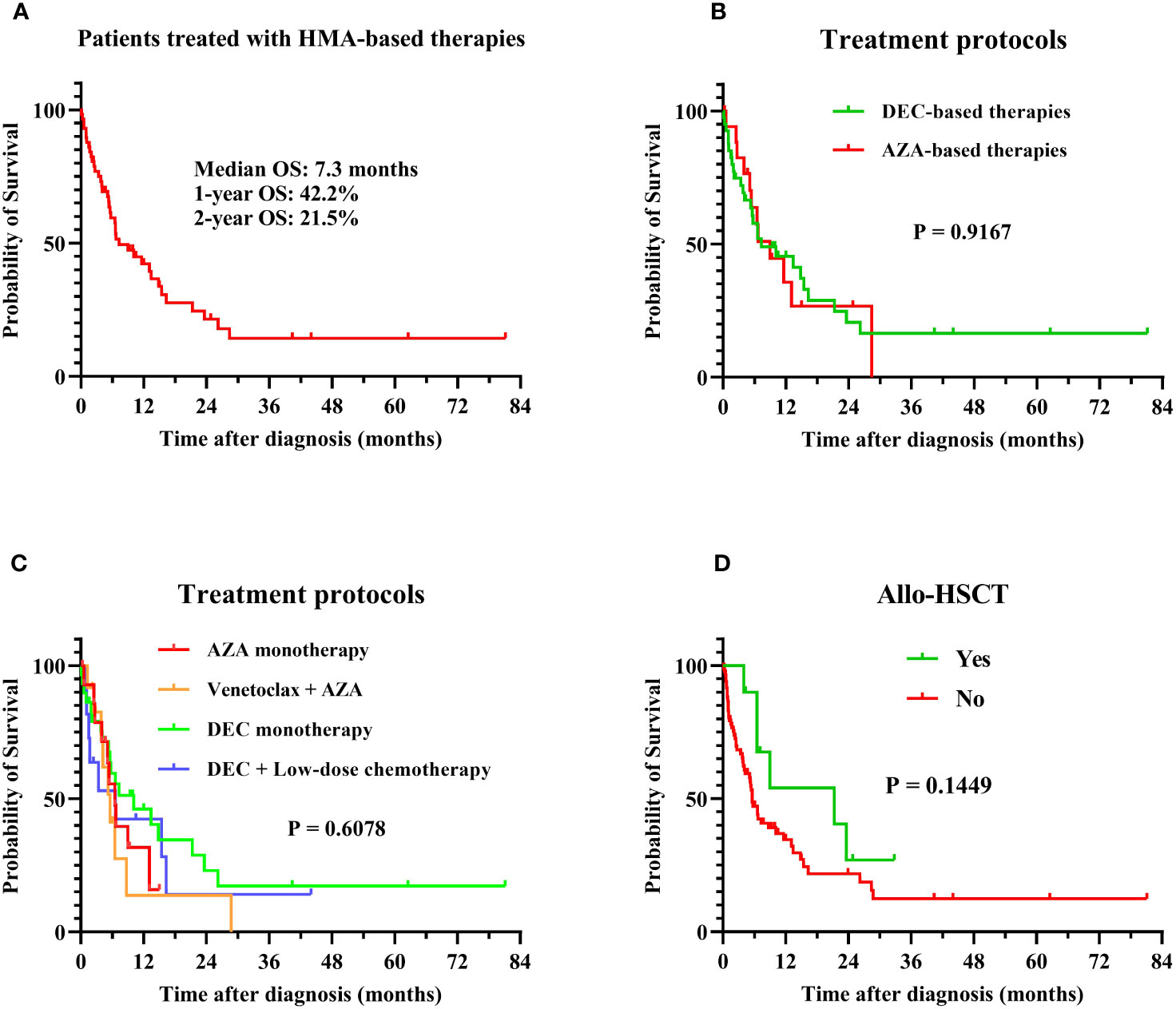
Figure 1 Overall survival in MDS patients with TP53 abnormalities. Survival analysis was performed using the Kaplan–Meier method, and survival curves were compared using the log-rank test. (A) OS of all patients in this study: the median OS was 7.3 months, with 1-year and 2-year OS rates of 42.2% and 21.5%; (B) OS of patients treated with HMA-based therapies: the median OS was 7.3 and 9.0 months for AZA-based (N = 18) and DEC-based therapies (N = 40), P = 0.9167; (C) OS of patients treated with different regimens: the median OS was 6.5, 5.6, 10.1, and 6.6 months in azacitidine monotherapy (N = 15), venetoclax combined with azacitidine (N = 12), decitabine monotherapy (N = 29), and decitabine with low-dose chemotherapy (N = 11) treatment groups, respectively (P = 0.6078); (D) OS of patients treated with or without allo-HSCT: The median OS was 21.3 months and 5.6 months in patients treated with (N = 12) or without (N = 83) allo-HSCT, P = 0.1449. MDS, myelodysplastic syndrome; TP53, tumor protein 53; HMA, hypomethylating agent; allo-HSCT, allogenic hematopoietic stem cell transplantation; DEC, decitabine; AZA, azacitidine; OS, overall survival.
3.3 Prognostic factors
To identify potential prognostic factors for patients with MDS accompanied by TP53 abnormalities, we performed univariate and multivariate analyses for OS in patients treated with HMA- or venetoclax-based therapies (Figure 2). Univariate analysis identified that IB-2 (hazard ratio [HR] = 1.025, 95% CI: 1.005–1.045, P = 0.012), complex karyotype (HR = 2.870, 95% CI: 1.361–6.053, P = 0.006), pulmonary infection (HR = 6.185, 95% CI: 3.125–12.241, P < 0.001), and pleural effusion (HR = 5.344, 95% CI: 2.728–10.468, P < 0.001) at the time of diagnosis were significant adverse prognostic factors. Additionally, male sex, -7 or del(7q), and +8 aberrations had a trend toward adverse effects on OS, whereas decitabine-based therapies and allo-HSCT had a trend toward favorable effects on OS. We included the IB-2, complex karyotype, pulmonary infection, and pleural effusion in the multivariate analysis and found that complex chromosomal karyotypes (HR = 2.493, 95% CI: 1.164–5.338, P = 0.019) and pulmonary infections (HR = 7.666, 95% CI: 1.605–36.608, P = 0.011) were significant independent adverse prognostic factors for OS.
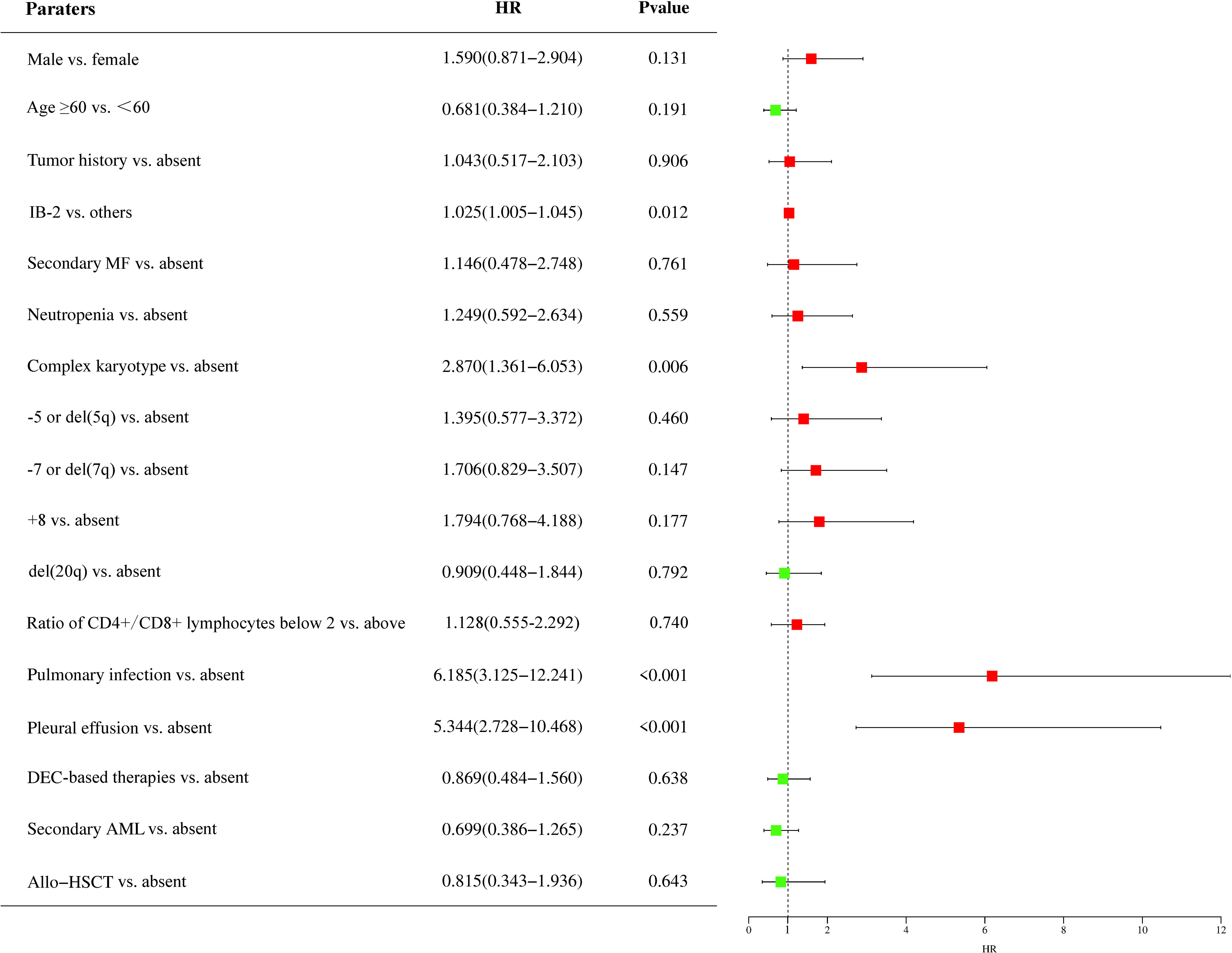
Figure 2 Prognostic factors of patients with MDS accompanied by TP53 abnormalities. The impact of prognostic factors was analyzed using the Cox regression model, and the results are presented as hazard ratios. HR, hazard ratio; IB-2, increase blast-2; MF, myelofibrosis; DEC, decitabine; AML, acute myeloid leukemia; allo-HSCT, allogeneic hematopoietic stem cell transplantation; MDS, myelodysplastic syndrome; TP53, tumor protein 53.
Forty-one patients (43.2%) had pulmonary infection at the time of diagnosis, and these patients had a worse prognosis than those without pulmonary infection (median OS, 2.3 months vs. 15.4 months; P < 0.0001). The median neutrophil counts were 1.24 (0.13-6.67) × 10 (9)/L and 0.99 (0.07-5.23) × 10 (9)/L in patients with and without pulmonary infection, respectively (P = 0.6377). Pleural effusion was observed in 36 of 41 (87.8%) patients with pulmonary infection. Additionally, myeloid blasts were detected by flow cytometry in some patients with massive pleural effusion, indicating that MDS with TP53 abnormalities may present with extramedullary invasion. A T cell subpopulation analysis was performed in 21 patients with pulmonary infection, and 13 (61.9%) had a ratio of CD4+ to CD8+ T lymphocytes below two, which revealed immune dysfunction in these patients.
4 Discussion
In our study, 25.9% of patients treated with HMA-based regimens achieved CR, with an ORR of 31.5%. Treatment-related mortality rates were similar between the azacitidine and decitabine groups. The CR rate of azacitidine monotherapy was lower than those of venetoclax combined with azacitidine, decitabine monotherapy, and decitabine combined with low-dose chemotherapy. Our data showed that patients with TP53 abnormalities had poor long-term survival when treated with HMA-based therapies, with median OS, 1-year survival, and 2-year survival that were significantly inferior to those of patients without TP53 abnormalities in our studies (25). A previous study revealed that venetoclax plus azacitidine improved remission rates, but not DOR or OS, compared with azacitidine alone in patients with TP53-mutated MDS with high-risk cytogenetics (26). Our results also confirmed that despite a higher remission rate in the venetoclax with azacitidine and decitabine-based treatment groups compared to the azacitidine monotherapy group, no significant difference in long-term survival was observed. Hence, a higher CR rate did not directly lead to better long-term survival in patients with MDS and TP53 abnormalities, and subsequent therapies are needed after patients respond to these initial treatment protocols.
MDS is characterized by a high risk for transformation to AML, and approximately 30% of patients with MDS eventually progress to AML as reported in previous studies. In our study, 27.4% of patients developed AML during the disease course with a median transformation time of 5.7 months, which was significantly shorter than that in patients without TP53 abnormalities in previous studies (27). These patients had an extremely poor prognosis, with a median survival period of only 1.8 months. Therefore, improving the outcomes in this population remains challenging.
Although several drugs have been found to improve disease control in MDS, allo-HSCT remains the only curative treatment (28). However, multiple analyses have shown that patients with TP53-mutated MDS and AML harbor an 80–90% higher risk of relapse and death after allo-HSCT than patients with TP53 wild-type MDS (13, 29–32). The majority of these relapses and deaths following allo-HSCT occur in patients with concomitant chromosome 17 abnormalities or complex karyotypes, leading to multi-hit disease (33). Our results demonstrated that patients with MDS accompanied by TP53 abnormalities who underwent allo-HSCT trended to show better survival than those who only received HMAs or venetoclax-based treatments, but this advantage was limited and not as good as that in patients without TP53 abnormalities, as reported previously (34).
Most MDS prognostic scoring systems are based on the BM blast percentage, depth of cytopenia, and cytogenetics, and these three major features have been shown to have a significant impact on the prognosis and risk of AML transformation (35). Recently, TP53 and other genes mutations were incorporated into the Molecular International Prognostic Scoring System (36). It is well established that a complex chromosomal karyotype is an adverse prognostic factor for MDS, and the absence of TP53 mutations in patients with complex karyotypes is associated with much better survival than that in patients with TP53 mutations (37). Our data showed that 73.1% of patients with MDS accompanied by TP53 abnormalities had complex chromosomal karyotypes, and complex karyotypes were identified as an independent adverse prognostic factor of OS in patients treated with HMA- or venetoclax-based therapies. Thus, TP53 abnormalities were frequently associated with complex chromosomal karyotypes and dismal prognosis in patients with MDS.
TP53-mutated MDS is associated with a risk of severe infection. Neutropenia was previously believed to be the main predisposing factor for this increased risk, but recent studies have discovered immune abnormalities in these patients (38, 39). Previous studies have demonstrated that patients with TP53-mutated MDS with infection have worse survival than those without infection (40). In our study, patients with pulmonary infection had extremely poor survival, and pulmonary infection was identified as an independent adverse prognostic factor. Importantly, patients with pulmonary infection had neutrophil counts similar to those in patients without pulmonary infection, and the T cell subpopulation analysis revealed that the majority of these patients had immune dysfunction. Therefore, immune dysfunction might be the main reason for uncontrollable infection and contribute to poor prognosis. However, the specific mechanisms responsible for this phenomenon need to be studied further. Pleural effusion was observed in 87.8% of patients with pulmonary infection, and myeloid blasts were detected by flow cytometry in some patients with massive pleural effusion. Whether patients with pleural infiltration and a percentage of BM blasts less than 20% should be diagnosed with MDS/AML, as proposed by the International Consensus Classification 2022 classification, requires further examination (41).
This study has some limitations that must be considered. Due to the single-center retrospective design and long observation period, some information bias was unavoidable. Additionally, our patients were heterogeneous with several therapeutic regimens used; therefore, the stratification of this population into small groups undermines the statistical power of the study. Because of the limited number of cases and the difficulty in detecting some microorganisms, detecting all pathogens and classifying them according to bacteria, fungi, and virus was impossible, which may have resulted in an additional source of bias. Therefore, prospective studies are still needed to confirm these findings.
In conclusion, TP53 abnormalities in MDS patients are frequently accompanied by complex karyotypes. Decitabine-based therapies are associated with a higher CR rate but similar long-term survival rates compared to azacitidine-based therapies. Venetoclax plus azacitidine improved the CR rate but did not improve survival due to the short DOR in this population. Pulmonary infection and complex karyotypes are independent adverse prognostic factors for OS. Further studies are required to overcome the poor survival of these patients.
Data availability statement
The raw data that support the conclusions of this article are available from the corresponding author upon reasonable request.
Ethics statement
The studies involving humans were approved by Ethical Commission of the Fujian Medical University Union Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
YC: Conceptualization, Data curation, Writing – original draft. JZ: Data curation, Investigation, Methodology, Writing – original draft. YQ: Formal Analysis, Resources, Validation, Writing – original draft. ZW: Resources, Validation, Writing – original draft. XL: Resources, Software, Validation, Writing – original draft. LZ: Project administration, Resources, Writing – original draft. YW: Conceptualization, Funding acquisition, Writing – review & editing. YL: Conceptualization, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Science and Technology Innovation Joint Fund Project of Fujian Province (2018Y9062), Construction Project of Fujian Medical Center of Hematology (Min 2017-04), and National and Fujian Provincial Key Clinical Specialty Discipline Construction Program of China.
Acknowledgments
We greatly appreciate every patient with MDS who supported this study. We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hu J, Cao J, Topatana W, Juengpanich S, Li S, Zhang B, et al. Targeting mutant p53 for cancer therapy: direct and indirect strategies. J Hematol Oncol (2021) 14(1):157. doi: 10.1186/s13045-021-01169-0
2. Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med (2017) 23(6):703–13. doi: 10.1038/nm.4333
3. Giacomelli AO, Yang X, Lintner RE, McFarland JM, Duby M, Kim J, et al. Mutational processes shape the landscape of TP53 mutations in human cancer. Nat Genet (2018) 50(10):1381–7. doi: 10.1038/s41588-018-0204-y
4. Donehower LA, Soussi T, Korkut A, Liu Y, Schultz A, Cardenas M, et al. Integrated analysis of TP53 gene and pathway alterations in the cancer genome atlas. Cell Rep (2019) 28(5):1370–1384.e5. doi: 10.1016/j.celrep.2019.07.001
5. Garcia-Manero G. Myelodysplastic syndromes: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol (2023) 98(8):1307–25. doi: 10.1002/ajh.26984
6. Hunter AM, Sallman DA. Current status and new treatment approaches in TP53 mutated AML. Best Pract Res Clin Haematol (2019) 32:134–44. doi: 10.1016/j.beha.2019.05.004
7. Hunter AM, Sallman DA. Targeting TP53 mutations in myelodysplastic syndromes. Hematol Oncol Clin North Am (2020) 34:421–40. doi: 10.1016/j.hoc.2019.11.004
8. Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia (2014) 28(2):241–7. doi: 10.1038/leu.2013.336
9. Weinberg OK, Siddon A, Madanat YF, Gagan J, Arber DA, Dal Cin P, et al. TP53 mutation defines a unique subgroup within complex karyotype de novo and therapy-related MDS/AML. Blood Adv (2022) 6(9):2847–53. doi: 10.1182/bloodadvances.2021006239
10. Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med (2011) 364(26):2496–506. doi: 10.1056/NEJMoa1013343
11. Kitagawa M, Yoshida S, Kuwata T, Tanizawa T, Kamiyama R. p53 expression in myeloid cells of myelodysplastic syndromes. Assoc Evol overt leukemia. Am J Pathol (1994) 145(2):338–44.
12. Jädersten M, Saft L, Smith A, Kulasekararaj A, Pomplun S, Göhring G, et al. TP53 mutations in low-risk myelodysplastic syndromes with del(5q) predict disease progression. J Clin Oncol (2011) 29(15):1971–9. doi: 10.1200/JCO.2010.31.8576
13. Yoshizato T, Nannya Y, Atsuta Y, Shiozawa Y, Iijima-Yamashita Y, Yoshida K, et al. Genetic abnormalities in myelodysplasia and secondary acute myeloid leukemia: impact on outcome of stem cell transplantation. Blood (2017) 129(17):2347–58. doi: 10.1182/blood-2016-12-754796
14. Daver NG, Maiti A, Kadia TM, Vyas P, Majeti R, Wei AH, et al. TP53-mutated myelodysplastic syndrome and acute myeloid leukemia: biology, current therapy, and future directions. Cancer Discovery (2022) 12(11):2516–29. doi: 10.1158/2159-8290.CD-22-0332
15. Cai L, Zhao X, Ai L, Wang H. Role Of TP53 mutations in predicting the clinical efficacy of hypomethylating therapy in patients with myelodysplastic syndrome and related neoplasms: a systematic review and meta-analysis. Clin Exp Med (2020) 20(3):361–71. doi: 10.1007/s10238-020-00641-4
16. Bernard E, Nannya Y, Hasserjian RP, Devlin SM, Tuechler H, Medina-Martinez JS, et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat Med (2020) 26:1549–56. doi: 10.1038/s41591-020-1008-z
17. Chen M, Li Ye, Yanling R, Zhou X, Ma L, Xu G, et al. 15-days duration of venetoclax combined with azacitidine in the treatment of relapsed/refractory high-risk myelodysplastic syndromes: A retrospective single-center study. Hematol Oncol (2023) 41(3):546–54. doi: 10.1002/hon.3112
18. Zeidan AM, Borate U, Pollyea DA, Brunner AM, Roncolato F, Garcia JS, et al. A phase 1b study of venetoclax and azacitidine combination in patients with relapsed or refractory myelodysplastic syndromes. Am J Hematol (2023) 98(2):272–81. doi: 10.1002/ajh.26771
19. Bazinet A, Darbaniyan F, Jabbour E, Montalban-Bravo G, Ohanian M, Chien K, et al. Azacitidine plus venetoclax in patients with high-risk myelodysplastic syndromes or chronic myelomonocytic leukaemia: phase 1 results of a single-centre, dose-escalation, dose-expansion, phase 1-2 study. Lancet Haematol (2022) 9(10):e756–65. doi: 10.1016/S2352-3026(22)00216-2
20. Sallman DA, McLemore AF, Aldrich AL, Komrokji RS, McGraw KL, Dhawan A, et al. TP53 mutations in myelodysplastic syndromes and secondary AML confer an immunosuppressive phenotype. Blood (2020) 136(24):2812–23. doi: 10.1182/blood.2020006158
21. Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med (2016) 375:143–53. doi: 10.1056/NEJMoa1601202
22. Ravandi F, Assi R, Daver N, Benton CB, Kadia T, Thompson PA, et al. Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a single-arm, phase 2 study. Lancet Haematol (2019) 6:e480–8. doi: 10.1016/S2352-3026(19)30114-0
23. Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia (2022) 36(7):1703–19. doi: 10.1038/s41375-022-01613-1
24. Zeidan AM, Platzbecker U, Bewersdorf JP, Stahl M, Adès L, Borate U, et al. Consensus proposal for revised International Working Group 2023 response criteria for higher-risk myelodysplastic syndromes. Blood (2023) 141(17):2047–61. doi: 10.1182/blood.2022018604
25. Sekeres MA, Taylor J. Diagnosis and treatment of myelodysplastic syndromes: A review. JAMA (2022) 328(9):872–80. doi: 10.1001/jama.2022.14578
26. Pollyea DA, Pratz KW, Wei AH, Pullarkat VA, Jonas BA, Recher C, et al. Outcomes in patients with poor-risk cytogenetics with or without TP53 mutations treated with venetoclax combined with hypomethylating agents. Blood (2021) 138:224. doi: 10.1182/blood-2021-145639
27. Adès L, Itzykson R, Fenaux P. Myelodysplastic syndromes. Lancet (2014) 383(9936):2239–52. doi: 10.1016/S0140-6736(13)61901-7
28. Vittayawacharin P, Kongtim P, Ciurea SO. Allogeneic stem cell transplantation for patients with myelodysplastic syndromes. Am J Hematol (2023) 98(2):322–37. doi: 10.1002/ajh.26763
29. Lindsley RC, Saber W, Mar BG, Redd R, Wang T, Haagenson MD, et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med (2017) 376:536–47. doi: 10.1056/NEJMoa1611604
30. Della Porta MG, Gallì A, Bacigalupo A, Zibellini S, Bernardi M, Rizzo E, et al. Clinical effects of driver somatic mutations on the outcomes of patients with myelodysplastic syndromes treated with allogeneic hematopoietic stem-cell transplantation. J Clin Oncol (2016) 34:3627–37. doi: 10.1200/JCO.2016.67.3616
31. Kim M, Yahng S-A, Kwon A, Park J, Jeon Y-W, Yoon J-H, et al. Mutation in TET2 or TP53 predicts poor survival in patients with myelodysplastic syndrome receiving hypomethylating treatment or stem cell transplantation. Bone Marrow Transplant. (2015) 50(8):1132–4. doi: 10.1038/bmt.2015.110
32. Hamilton BK, Rybicki L, Hirsch C, Przychodzen B, Nazha A, Gerds AT, et al. Mutation clonal burden and allogeneic hematopoietic cell transplantation outcomes in acute myeloid leukemia and myelodysplastic syndromes. Bone Marrow Transplant. (2019) 54(8):1281–6. doi: 10.1038/s41409-019-0444-1
33. Loke J, Labopin M, Craddock C, Cornelissen JJ, Labussière-Wallet H, Wagner-Drouet EM, et al. Additional cytogenetic features determines outcome in patients allografted for TP53 mutant acute myeloid leukemia. Cancer (2022) 128:2922–31. doi: 10.1002/cncr.34268
34. Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol (2017) 35(11):1154–61. doi: 10.1200/JCO.2016.70.7091
35. Bersanelli M, Travaglino E, Meggendorfer M, Matteuzzi T, Sala C, Mosca E, et al. Classification and personalized prognostic assessment on the basis of clinical and genomic features in myelodysplastic syndromes. J Clin Oncol (2021) 39(11):1223–33. doi: 10.1200/JCO.20.01659
36. Sauta E, Robin M, Bersanelli M, Travaglino E, Meggendorfer M, Zhao L-P, et al. Real-world validation of molecular international prognostic scoring system for myelodysplastic syndromes. J Clin Oncol (2023) 41(15):2827–42. doi: 10.1200/JCO.22.01784
37. Haase D, Stevenson KE, Neuberg D, Maciejewski JP, Nazha A, Sekeres MA, et al. TP53 mutation status divides myelodysplastic syndromes with complex karyotypes into distinct prognostic subgroups. Leukemia (2019) 33(7):1747–58. doi: 10.1038/s41375-018-0351-2
38. Toma Andréa, Fenaux P, Dreyfus François, Cordonnier C. Infections in myelodysplastic syndromes. Haematologica (2012) 97(10):1459–70. doi: 10.3324/haematol.2012.063420
39. Caira M, Latagliata R, Girmenia C. The risk of infections in patients with myelodysplastic syndromes in 2016. Expert Rev Hematol (2016) 9(6):607–14. doi: 10.1080/17474086.2016.1181540
40. Marvin-Peek J, Mason EF, Kishtagari A, Jayani RV, Dholaria B, Kim TK, et al. TP53 mutations are associated with increased infections and reduced hematopoietic cell transplantation rates in myelodysplastic syndrome and acute myeloid leukemia. Transplant Cell Ther (2023) 29(6):390.e1–390.e10. doi: 10.1016/j.jtct.2023.03.008
Keywords: myelodysplastic syndrome, tumor protein 53, pulmonary infection, immune dysfunction, prognosis
Citation: Chen Y, Zheng J, Qiu Y, Wu Z, Luo X, Zhu L, Wu Y and Lin Y (2023) Pulmonary infection associated with immune dysfunction is associated with poor prognosis in patients with myelodysplastic syndrome accompanied by TP53 abnormalities. Front. Oncol. 13:1294037. doi: 10.3389/fonc.2023.1294037
Received: 14 September 2023; Accepted: 10 November 2023;
Published: 30 November 2023.
Edited by:
J. Luis Espinoza, Kanazawa University, JapanReviewed by:
Shohei Mizuno, Aichi Medical University, JapanMai Ha Thi thao, Can Tho University of Medicine and Pharmacy, Vietnam
Copyright © 2023 Chen, Zheng, Qiu, Wu, Luo, Zhu, Wu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanjuan Lin, yjlin.88@163.com; Yong Wu, wuyong9195@126.com
†These authors have contributed equally to this work
 Yi Chen
Yi Chen Jing Zheng†
Jing Zheng† Yanyan Qiu
Yanyan Qiu Zhengjun Wu
Zhengjun Wu Xiaofeng Luo
Xiaofeng Luo