- Department of Radiation Oncology and Molecular Radiation Sciences, School of Medicine, Johns Hopkins University, Baltimore, MD, United States
Radioligand therapy (RLT) agents are demonstrating a crucial role in the clinical approach to aggressive malignancies such as metastatic castrate-resistant prostate cancer (m-CRPC). With the recent FDA approval of prostate-specific membrane antigen (PSMA)-targeted RLT for m-CRPC, the field has broadened its gaze to explore other cancers that express PSMA in the tumor parenchyma or tumor neovasculature. In this review article, we discuss current progress in the clinical use of PSMA RLTs in non-prostate cancers such salivary gland cancers, renal cell carcinoma, high grade glioma, and soft tissue sarcoma. We highlight early reports in small case series and clinical trials indicating promise for PSMA-targeted RLT and highlighting the importance of identifying patient cohorts who may most benefit from these interventions. Further study is indicated in non-prostate cancers investigating PSMA RLT dosimetry, PSMA PET/CT imaging as a biomarker, and assessing PSMA RLT safety and efficacy in these cancers.
Introduction
In the era of personalized medicine, theranostic approaches are continuing to evolve and shape the clinical landscape for many cancer types. In particular, radioligand therapies (RLT) comprised of radioactive isotopes conjugated to peptide ligands are allowing for targeted approaches for aggressive neoplasms, such as metastatic castrate-resistant prostate cancer (m-CRPC). These hybrid therapies offer a unique modality to deliver localized radiation to endogenous targets, and are not only safe but also efficacious (1, 2). The recent phase III VISION trial of patients with m-CRPC who failed hormone and taxane therapies demonstrated a significant increase in median progression-free survival (8.7 vs. 3.4 months) and overall survival (15.3 vs. 11.3 months) when 177Lu-PSMA-617 (lutetium-177 conjugated to prostate-specific membrane antigen-617 targeting ligand) was added to standard of care2. The efficacy of 177Lu-PSMA-617 has generated interest in applying PSMA RLTs earlier in prostate cancer management, as well as in other cancer types in which PSMA is expressed.
In this review article, we discuss current progress in the clinical use of PSMA RLTs in non-prostate cancers. We highlight early reports in small case series and relevant ongoing early clinical trials, in particular recent progress in salivary gland cancers, renal cell carcinoma, high grade glioma, and soft tissue sarcoma. We also discuss the limitations and future directions for this growing field.
PSMA expression in non-prostate malignancies
Prostate-specific membrane antigen (PSMA), a type II transmembrane glycoprotein, was first described as highly expressed in benign and malignant prostate epithelium (3, 4), with substantially higher PSMA expression in prostate adenocarcinoma compared to normal tissue (5, 6). However, contrary to its name, PSMA also exhibits expression in several non-prostatic tissues including salivary glands, kidneys, and gastrointestinal mucosa (7, 8). Expression of PSMA has also been reported in the tissues of many solid tumors and their neovascular endothelium (9). PSMA has a functional role in promoting angiogenesis within tumors, a process mediated by laminin substrates to promote endothelial cell activation (10). In contrast, this function is absent in normal tissue endothelium. In most solid tumors, the percentages of tumor neovascular endothelium with positive PSMA typically exceed that of the tumor cells themselves, as is the case for primary brain tumors, lung cancer, breast cancer, gastrointestinal tumors, and renal cell carcinoma (RCC) (9, 11).
Certain tumors like pancreatic ductal adenocarcinoma (PDAC) and salivary gland tumors also express PSMA in a significant proportion of the tumor parenchyma. PDACs express PSMA in 67% of cases, with higher expression possibly associated with worse survival outcomes (9, 12). However, clinical applications of PSMA RLTs in PDAC have yet to be investigated. In contrast, PSMA RLTs for salivary gland cancers (adenoid cystic carcinoma and salivary ductal carcinoma) have garnered significant interest. PSMA levels are detectable in the majority (as high as 90%) of certain salivary gland tumors by PSMA PET/CT, which has been demonstrated in small cohort clinical trials (13, 14).
Types of radionuclide emitters
Currently, the small molecules PSMA-I&T and PSMA-617 are the most developed PSMA-targeted small molecule therapies. Both are conjugated to lutetium-177 (177Lu). While the two ligands are fairly dissimilar apart from the urea-binding motif, it appears that in m-CRPC, the toxicity and efficacy profiles are generally similar (15). Both conjugates likely kill tumor cells with the same mechanism. After localization to the tumor, they rely on the beta-emitter properties of 177Lu to induce DNA damage. Radioisotopes emitting beta particles (β-), high speed electrons ejected from nuclei during radioactive decay, have certain physical, biological, and dosimetric properties that are particularly suitable for RLTs (16, 17). 177Lu emits photons detectable on imaging (SPECT or planar) and has a desirable half-life of 6.6 days, enabling practical shelf life and biologic effect. Beta particles can penetrate tissue in the millimeter range, in contrast to alpha (40-100 µm range) and Auger (<1 µm range) emitters. For non-prostate tumors with heterogeneous PSMA expression or in which the majority of PSMA expression occurs in the neovasculature, this may be preferable in order to maximize impact on tumor tissue and bystander-mediated cell killing (18). However, the higher linear energy transfer of alpha emitters may have benefit for tumor types with lower but more homogeneous PSMA expression. The relative strengths and limitations of different types of emitters for different applications is an area of ongoing discussion and investigation in the field.
PSMA imaging
The use of PSMA RLTs is typically guided by the ability to detect PSMA-positive tumors via PET/CT using gallium-68 PSMA radioligands (68Ga-PSMA) or piflufolastat F 18 (18F-DCFPyL). Numerous studies have established PSMA PET/CT as a potential screening modality for patient selection in early stage RLT trials in these non-prostatic cancers (9, 19). Overall, the SUVmax values for non-prostate malignancies appear lower than for prostate cancer. However, data on the sensitivity of specific SUV cutoffs are generally lacking. As a result, current trials often require detectable disease on PSMA PET/CT with tumor/liver SUV ratio greater than 1.
Materials and methods
The materials for this review were obtained through a systematic search of existing literature. Studies describing therapeutic administration of PSMA-based therapy were queried using the following terms in PubMed: (Lutetium-PSMA OR Lu-PSMA OR Lu-EB-PSMA OR Lu-prostate) AND (therapy OR radioligand OR radiopharmaceutical OR theranostic), and existing reviews of RLT in non-prostate cancers were cross-referenced (20, 21). Studies describing RLT use (Table 1) were found for salivary gland tumors (14, 22–26), high grade glioma (27–30, 37), sarcoma (31, 32), thyroid carcinoma (33, 34), breast cancer (35), and hepatocellular carcinoma (36). Ongoing clinical trials (Table 2) were collated by searching ClinicalTrials.gov for “PSMA 177Lu”. Search results were current as of June 30th, 2023. The PRISMA flow chart outlining the search process is depicted in Supplemental Figure 1.
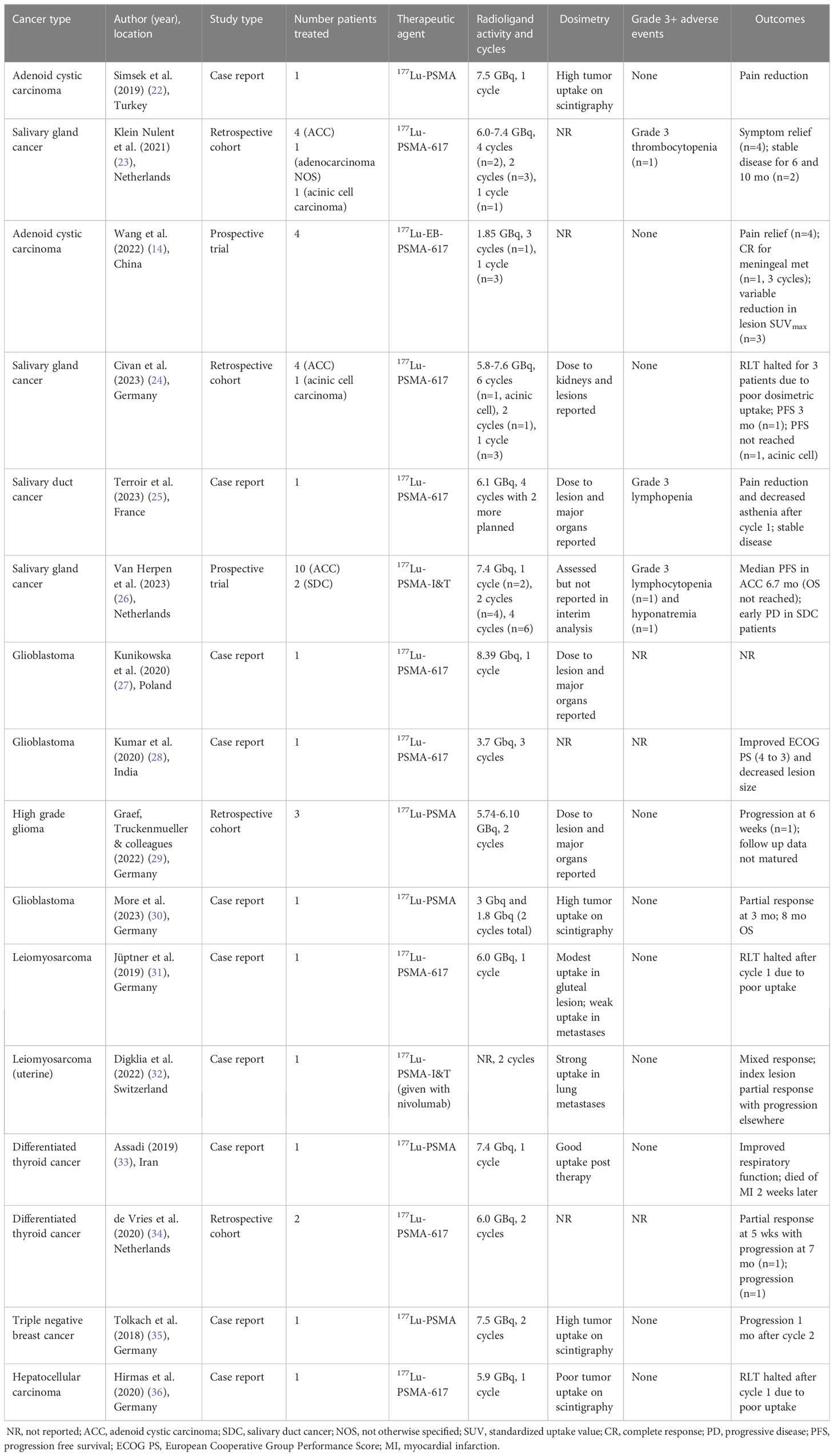
Table 1 Reports on patients treated with PSMA radioligand therapy (RLT) for non-prostate malignancies.
Salivary gland malignancies
Investigation of PSMA RLTs for non-prostate cancers has continued to grow in recent years, with early phase clinical trials ongoing for salivary gland malignancies (Table 2). Salivary gland carcinomas (SGCs) represent up to 6% of all head and neck malignancies (38), and are categorized into more than 20 histologic subtypes. The majority of RLT development to date has focused on adenoid cystic carcinoma (ACC) and to a lesser extent salivary duct carcinoma (SDC).
ACC arises from the major and minor salivary glands, with a classic cribriform histology, though tubular and solid components can also be present (39). The 10-year overall survival (OS) for ACC has been reported at 37-65% (40). The standard treatment for resectable disease consists of surgery, often followed by radiation to improve local control. However, ACC has a predilection for perineural invasion and recurrence. Distant metastatic progression (commonly to lung, bone, and liver) is the leading cause of mortality, and occurs in nearly half of patients (40). The majority of ACC patients who develop distant metastases do so within the first 5 years (41), after which the median OS is 20-32 months (42). Unfortunately, response rates to chemotherapy and targeted inhibitors (e.g. anti-VEGF) are poor in the progressive/metastatic setting (38, 43). Hence, alternative targeted approaches including PSMA RLTs are being pursued. More than 90% of recurrent or metastatic ACC tumors express PSMA detectable on PET/CT (SUVmax range of 1.1-30.2) (13, 44). Additionally, histology shows >50% of cells in primary tumors and up to 92% of cells in metastatic lesions express PSMA (44).
Salivary ductal carcinoma (SDC) is rarer than ACC and typically diagnosed at a more advanced stage. Histologically, SDC resembles ductal carcinoma of the breast, with the majority expressing androgen receptor (AR) and/or Her-2 receptors (9). SDC is an aggressive malignancy, with 3-year OS of around 36% (45, 46). For local disease, treatment typically consists of surgical resection followed by adjuvant therapy with radiation or chemoradiation. For recurrent or metastatic disease, recent retrospective and early stage clinical studies support a role for targeted therapies (47), including androgen deprivation and trastuzumab (46). Recurrent or metastatic SDC also express PSMA detectable on PET/CT, which was reported in a prospective phase II study for 10 patients (SUVmax range of 0.3-25.9) (13), with 40% of patients showing a tumor/liver ratio >1 in all tumor sites.
Data on the usage of PSMA RLTs in salivary gland tumors come from case reports and small cohort studies (Table 1) (14, 22–26). An early report from Simsek et al. described a patient with adenoid cystic carcinoma that received 177Lu-PSMA (7.5 GBq) for palliation of painful bone metastases (22). The patient reported pain relief without notable side effects after one cycle but ended up dying 6 weeks later. Subsequently, Klein Nulent et al. reported retrospective data from patients treated with up to 4 cycles of 177Lu-PSMA-617 (6.0-7.4 GBq) on a compassionate use protocol (23). Among the patients treated four had ACC, one had salivary gland adenocarcinoma (not otherwise specified), and one had acinic cell carcinoma. Two patients – both with ACC – completed 4 cycles of therapy. One showed stable lung metastases and minimal progression of liver disease. The other had stable disease with decreased SUVmax (from 6.5 to 4.5) and was alive 10 months after treatment start, at which point progression was noted on PSMA PET/CT. Of the 4 patients that progressed on therapy, 2 stopped after cycle 2, one stopped after cycle 2 due to grade 3 thrombocytopenia (the only grade 3 event), and one stopped due to demotivation from side effects after cycle 1. Notably, 4 patients reported reduction in tumor-related symptoms, particularly pain, after the first cycle.
More recent studies have also begun to report dosimetry details. Terroir et al. described treatment of one patient with SDC with 177Lu-PSMA-617 on a compassionate use protocol (25). The patient received 4 cycles for a cumulative dose of 24.3 GBq, which was well tolerated with grade 3 lymphopenia being the worst adverse effect. The patient reported improvement in bone pain after 1 cycle. The index femoral head lesion (PSMA PET/CT SUVmax of 10 pre-treatment) received 0.04 Gy/GBq, and was stable on follow up PSMA PET/CT. A separate study by Civan et al. also reported 5 patients (4 with ACC and 1 with acinic cell carcinoma) treated with 177Lu-PSMA-617 (5.8-7.6 GBq) (24). The absorbed dose to index lesions ranged from 0.06-0.68 Gy/GBq on SPECT/CT. Due to poor lesion PSMA uptake on 24-hour post-treatment scintigraphy, RLT was discontinued for 3 patients with ACC. One patient with ACC had a progression free survival of 3 months after 2 cycles. The patient with acinic cell carcinoma had continued response after 6 cycles, with accumulated dose of 0.41 and 0.49 Gy/GBq for two index lesions after cycle 1.
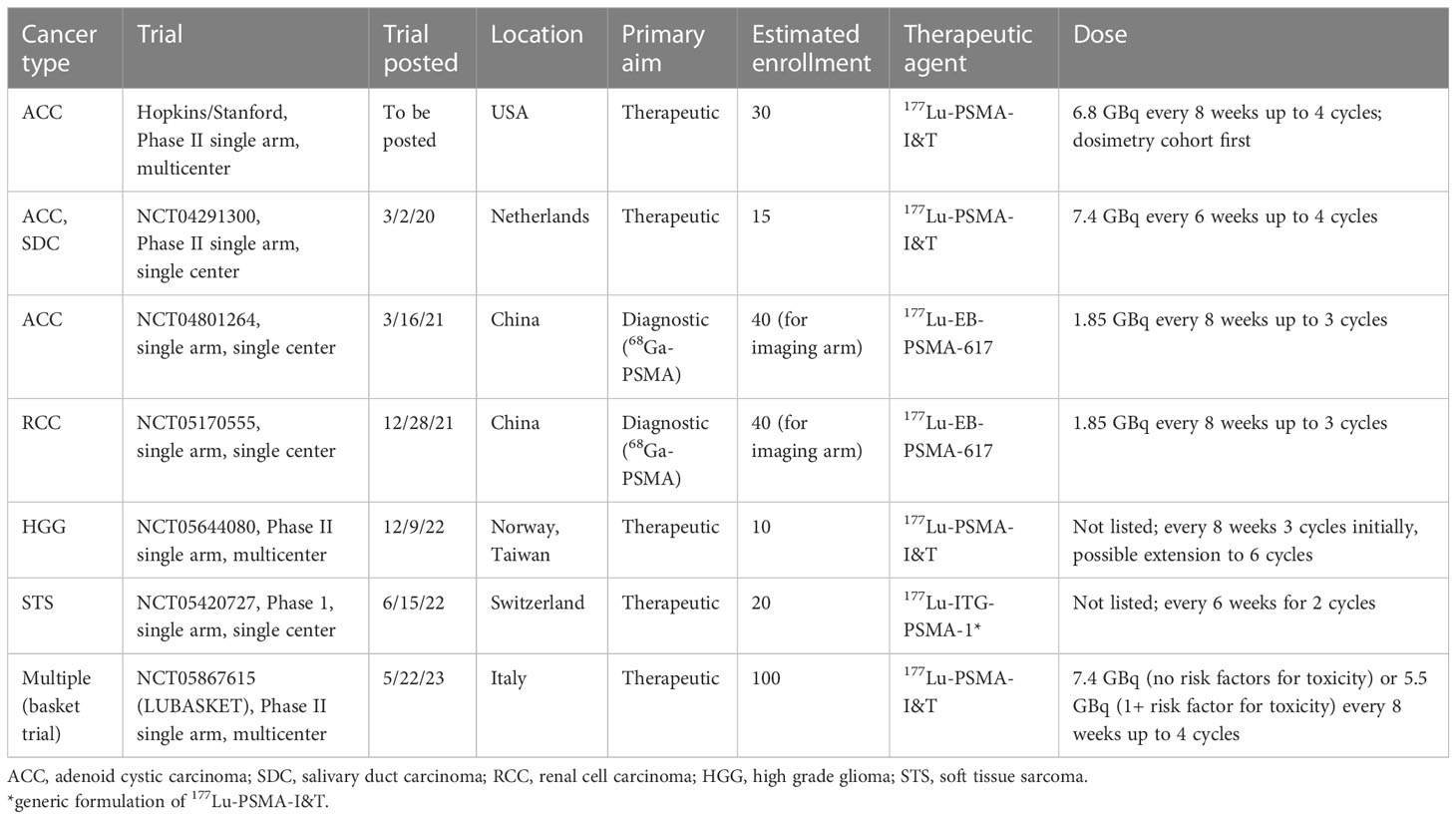
There are two early-stage clinical trials for recurrent/metastatic ACC that are enrolling and one that will begin enrolling soon (Table 2). The forthcoming Hopkins/Stanford trial and NCT04291300 (Dutch) are phase II single arm studies and NCT04801264 is an early phase study. The primary endpoint of the NCT04801264 study is diagnostic evaluation of 68Ga-PSMA-617. The trial also incorporates a pilot study of 177Lu-EB-PSMA-617 therapy (1.85 GBq), a derivative of 177Lu-PSMA-617 in which the Evans blue (EB) motif helps to bind albumin and slow plasma clearance, which may promote tumor accumulation (14). This therapy was ultimately received by 4 patients, with one patient receiving 3 cycles and three patients receiving only 1 cycle (due to restrictions during the COVID pandemic). Subjective pain symptoms were improved for all 4 patients and no grade 3 side effects were reported. The patient who received 3 cycles had a significant reduction in SUVmax (from 7.0 to 1.1) in a solitary meningeal metastasis. Metabolic responses after cycle 1 were variable, however. One notable finding was that diagnostically, 68Ga-PSMA-617 outperformed traditional 18F-FDG PET except in lung metastases, which may have implications for patient selection given that ACC most commonly metastasizes to lung.
The phase II Dutch trial is a single-center study using 177Lu-PSMA-I&T dosed at 7.4 GBq every 6 weeks up to 4 cycles and includes both ACC and salivary ductal carcinoma. The Hopkins/Stanford trial will be a multi-center study using 177Lu-PSMA-I&T dosed at 6.8 GBq every 8 weeks up to 4 cycles for ACC. Enrollment in both require detectable disease on PSMA PET/CT (with tumor/liver ratio greater than 1). The Dutch trial recently reported results for the first 12 patients treated (10 ACC and 2 SDC) (26). RLT was discontinued for two patients after 1 cycle and 4 patients after 2 cycles due to early progression. Six patients with ACC received 4 cycles, with median PFS of 6.7 months (3 patients with stable disease and 3 with progression on 3-month imaging). The treatment was well tolerated, with two grade 3 toxicities: lymphopenia and hyponatremia. Dosimetry results were not yet reported.
Renal cell carcinoma
The clinical role of PSMA RLT in renal cell carcinoma (RCC) is being investigated in an early phase trial NCT05170555 (Table 2). RCC originates from the renal epithelium and accounts for >90% of kidney cancers. Within RCC, most have clear cell histology. RCC is relatively resistant to conventional chemotherapy and radiation, with current median OS of 13 months for metastatic disease. While targeted therapies against mTOR and VEGFR and immunotherapies are approved in metastatic RCC, they have limited efficacy (48). RCC is a highly vascular tumor, and PSMA is over-expressed in the neovasculature in the majority of tumors. Expression occurs primarily in clear cell (84% on average) and chromophobe subtypes (61% on average), whereas papillary and transitional RCC have <30% endothelial expression by IHC (9, 11, 49, 50). Increased PSMA expression may be associated with adverse pathologic features and worse overall survival (51, 52). 68Ga-PSMA PET/CT has been shown to have greater sensitivity than conventional modalities for detecting metastatic disease, particularly for clear cell (ccRCC) (50, 53). For metastatic ccRCC lesions, SUVmax values have been reported in the range of 1.2-48, with lung metastases appearing to have lower SUVmax values than other sites (54–56).
Given these promising data, several early stage trials are in progress to determine the role of PSMA PET/CT in diagnosis and restaging of advanced RCC (57). To date, no case reports for RCC patients treated with PSMA RLTs exist. The NCT05170555 trial has a primary goal of assessing the diagnostic value of 68Ga-PSMA PET/CT. Additionally, it will evaluate the safety and efficacy of 177Lu-EB-PSMA-617, which will be dosed at 1.85 GBq every 8 weeks up to 3 cycles in select patients based on PSMA avidity. Renal dosimetry and toxicity may be important for these studies.
High grade glioma
The role of PSMA RLTs in the setting of recurrent high-grade glioma is under evaluation in the phase II trial NCT05644080 (Table 2). PSMA is highly expressed in the neovasculature of high-grade gliomas, due to the increased vascularity compared to low-grade glioma. For glioblastoma multiforme (GBM), tumor vascular epithelia demonstrate PSMA expression in all cases compared to only 5% of tumor cells (9). With standard treatment consisting of surgery followed by chemoradiation, the median OS for GBM is 15 months. After recurrence or progression, effective options are limited. Six patients to date have been treated with 177Lu-PSMA-617 in the setting of refusing or exhausting standard therapeutic options (Table 1) (27–30, 37). The first case reported by Kunikowska et al. was for recurrent GBM previously treated with surgery and chemoradiation, with SUVmax of 10.3 on 68Ga-PSMA-11 PET/CT (27). One dose (8.39 GBq) was given with estimated 14 Gy delivered to the tumor and limited dose to other organs. No outcomes were reported. Kumar et al. described a second patient with recurrent GBM who received 3 cycles of 3.7 GBq at 2 month intervals (28). No adverse effects were reported, and the patient had improvement in performance score and reduction in lesion size. Recently, More et al. reported a case of recurrent GBM treated with 2 cycles of 177Lu-PSMA, 3.0 GBq followed by 1.8 GBq (30). The tumor PSMA PET/CT SUVmax was 10.2 (compared to 2.9 for liver) and post-treatment scintigraphy showed good uptake. The treatment was well tolerated. The tumor showed partial response at 3 months, and the patient survived for 8 months after initiation of RLT.
The aforementioned cases suggest good CNS penetrance for PSMA RLT, though recent dosimetry studies from a retrospective cohort by Graef, Truckenmueller and colleagues raise concerns that delivered dose and efficacy may be lower than previously described (29, 37). The authors screened for eligible patients with high grade glioma using 68Ga-PSMA-11 and found that for a tumor-to-background (liver) cutoff ratio > 1, only 3 of 20 patients (15%) met criteria. The tumor SUVmax values for these patients were 8.65, 7.97, and 6.39. Patients were treated with 177Lu-PSMA (5.74-6.10 GBq) up to 2 cycles with median dose of 0.56 Gy. At time of reporting, only one patient underwent follow up imaging at 6 weeks, which showed tumor growth. Overall, the treatments were well tolerated.
Soft-tissue sarcoma
The phase I trial NCT05420727 is currently aimed at testing the feasibility of PSMA RLT in soft tissue sarcomas (Table 2). Soft tissue sarcomas arise from connective tissues, such as muscle, fat, nerves, lymphatics, and blood vessels. Due to the heterogeneity of presentation, sarcoma management varies depending on histology, though typically consists of gross total resection, when possible, along with chemotherapy and/or radiation. Recurrences and metastatic disease are notoriously difficult to treat. PSMA expression in soft tissue sarcoma occurs primarily in the neovasculature and varies based on subtype. From IHC studies, reported values range from 23% of dedifferentiated liposarcoma to >50% of pleomorphic rhabdomyosarcoma and liposarcoma (58). Uptake of 68Ga-PSMA-11 on PET/CT also varies based on histology (59), with reports of liposarcomas showing PSMA uptake up to SUVmax of 13 (60) and metastatic leiomyosarcoma SUVmax of 16.5 (31). There have also been two case reports of sarcoma patients treated with PSMA RLT. In one case, a patient with progressive metastatic leiomyosarcoma was considered for 177Lu-PSMA-617 as part of a compassionate use protocol after refusing chemotherapy. She was treated with one dose of 6.0 Gbq, which was reportedly well tolerated (31). However, whole body scan revealed only weak uptake of the radiotracer, and hence the therapy was discontinued. Follow up PSMA PET/CT three months later showed disease progression. For the second case, a patient with metastatic uterine leiomyosarcoma was treated with a combination of 177Lu-PSMA-I&T in 8 week intervals and nivolumab given 1 week after each RLT treatment for 2 cycles (32). PSMA PET/CT 4 months after treatment initiation showed reduction of primary tumor growth rate of initially reported lesions, with reduction in size of the lung nodule with highest uptake (SUVmax 8.9). However, due to progression of a neighboring lesion with low uptake, the patient was switched to next line chemotherapy.
Other non-prostate malignancies
While not being studied in clinical trials yet, the use of PSMA RLTs have also been reported in thyroid cancer, hepatocellular carcinoma, and breast cancer (21).
Thyroid malignancies also express PSMA in the neovasculature (but not tumor cells) of all histologic subtypes (21). Three patients have been treated with PSMA RLTs to date (33, 34). One patient had differentiated thyroid cancer (DTC) that progressed after multiple lines of therapy, including radioactive iodine, sorafenib, and 177Lu-DOTATATE (33). The patient had severe dyspnea due to progression in the neck and lungs and was reported to have some improvement in respiratory function following 7.4 GBq of 177Lu-PSMA. However, he died unexpectedly from cardiac arrest 2 weeks following treatment. In a separate study, two patients underwent 177Lu-PSMA-617 for treatment-refractory DTC (34). Patients were selected based on predominance of PSMA-positivity on 68Ga-PSMA PET/CT, though no pre-defined SUV cutoff value was used. Of three eligible patients, two underwent 2 cycles of 6 GBq. One patient demonstrated partial response 5 weeks after the second treatment, with progression 7 months after. The other patient showed disease progression on 18F-FDG PET/CT 1 month after second treatment with stable disease at 6 months.
For hepatocellular carcinoma, two patients received 177Lu-PSMA-617 as part of a larger study evaluating the diagnostic capabilities of 68Ga-PSMA PET/CT (36). Unfortunately, dosimetric analysis showed accumulated doses 10-fold lower than typically achieved by 1 cycle of external beam radiation, and hence the therapy was stopped prior to a second cycle.
For breast cancer, one patient with triple-negative breast cancer refractory to other systemic agents received 2 cycles (7.5 GBq) of 177Lu-PSMA based on PSMA imaging (35). However, the patient progressed 4 weeks after cycle 2 and the treatment was stopped. Overall, these treatments were reported as well tolerated.
To address the broad range of cancers that express PSMA, there is new phase II basket trial NCT05867615 (LUBASKET) that recently began enrolling (Table 2). The trial does allow for inclusion of metastatic prostate cancer, so the overall non-prostate cancer enrollment potential is unclear.
Discussion
With the recent FDA approval of 177Lu-PSMA-617 based on significant survival benefit in patients with mCRPC, there is significant interest in expanding PSMA-targeted RLT for other indications including non-prostate malignancies that express PSMA. Many of these cancers have much more limited treatment options than prostate cancer. For example, advanced salivary adenoid cystic carcinoma has no FDA-approved treatment options and has been shown to express PSMA in over 90% of patients in early studies (13). While most solid tumor express PSMA in neovasculature, there is also tumor cell expression of PSMA in ACC and other salivary malignancies, as well as some pancreatic cancers.
There are several potential concerns and limitations regarding RLT use for non-prostate cancers. A major issue continues to be heterogeneity of expression of PSMA, and the fact that the PSMA PET/CT SUV for non-prostate cancers tend to be markedly lower than for prostate adenocarcinoma (23). In prostate cancer, lower and heterogeneous PSMA expression on PSMA PET/CT imaging has been shown to correlate with lower response rate (61). A complicating factor here is the fact that unlike for prostate cancer, in non-prostate cancer there is not consistent correlation between expression of PSMA on IHC and signal on PSMA PET/CT (13). This suggests a lack of appreciation for all the factors that contribute to PSMA-ligand binding and accumulation in tumor tissue and neovasculature. Furthermore, response to PSMA RLTs in the limited non-prostatic cases discussed does not consistently associate with lesion SUVmax (14, 23, 25, 37, 59). There may be also different kinetics and distribution of binding for diagnostic 68Ga-PSMA ligands versus RLT ligands, which may be influenced by factors including tissue location, an important consideration for tumors like soft tissue sarcomas. Also problematic are dosimetric studies showing variable lesion uptake within and across studies (24, 25, 37), the underlying cause of which is unclear. It is promising that across tumor types there do appear to be cases in which PSMA RLT show good uptake on scintigraphy and partial metabolic responses (14, 23–25, 30). Dedicated dosimetry studies will continue to be essential for PSMA RLT in non-prostate cancers to determine absorbed dose to tumors and normal organs at different administered activities.
Another concern is optimal patient selection. As noted above, PSMA PET/CT imaging has potential for patient selection but needs to be further validated as a response biomarker for non-prostate malignancies. The European Association of Urology (EAU) and European Association of Nuclear Medicine (EANM) recently collaboratively released consensus guidelines regarding usage of PSMA PET/CT imaging for RLT applications (62). There was unanimous recommendation to use PSMA PET/CT imaging to demonstrate expression for patient selection, though no specific cutoffs were postulated. 68Ga-PSMA-11 and 18F-DCFPyL were the preferred PET tracers. The utility of 18F-FDG PET for patient selection has been demonstrated for prostate cancer but is an unanswered question for non-prostate cancers. A recent retrospective analysis by Seifert et al. found that in metastatic CRPC, mismatches in detection favoring 18F-FDG were rare (3%) compared to those favoring PSMA PET/CT (18%). However, studies specific to non-prostate cancers may be needed, as supported by the data from Wang et al. in ACC showing that detection mismatches favored 18F-FDG for lung metastases (52).
Specific PET criteria should be investigated for balancing likelihood of response while not excluding patients who may benefit. Data from prostate cancer RLT trials suggest that baseline PET uptake parameters such as mean and max SUV are associated with outcomes (63, 64). Currently for non-prostate cancers, patient selection tends to include patients with more advanced stage and comorbidities. This may limit completion of enough cycles to achieve therapeutic benefit, as seen in the pilot studies in non-prostate cancers to date. Therefore, prospective phase II-III trials with further inclusion and exclusion criteria in addition to dosimetric analysis are needed and may be more likely to show treatment response and yield information regarding PET screening parameters. There is interest in developing trials with criteria for target expression rather than specific disease indications (65, 66), and there is precedent for FDA approval of therapy based on biomarkers (e.g. pembrolizumab for microsatellite instability-high solid tumors) (67).
A third concern is limited data on dose-limiting toxicities. Thus far, 177Lu-PSMA-617 and 177Lu-PSMA-I&T have demonstrated favorable safety profiles for metastatic CRPC patients in large retrospective cohorts (68). Renal doses for both compounds were <1 Gy/GBq, with 1-1.5x higher accumulation for 177Lu-PSMA-I&T across studies, with no reports of grade 3 nephrotoxicity (68–70). While there has been concern about renal toxicity, a recent study of metastatic CRPC patients with at least CKD stage 3 showed that RLT (at least 2 cycles of 177Lu-PSMA-617; median 5 cycles) led to stable or improved GFR in most patients (71). However, RLT should still be administered with caution given reports of patients developing renal thrombotic microangiopathy following extensive treatment (72). Analysis of the VISION trial demonstrated that 177Lu-PSMA-617 treatments were associated with additional risk of grade 3 and 4 hematologic side effects, including anemia, lymphopenia, and thrombocytopenia (73). For 177Lu-PSMA-I&T, incidence of grade 3 or 4 toxicity is less than 5% (70). Different conjugates like 177Lu-EB-PSMA-617 may have better target accumulation, but also have increased uptake in normal organs like the normal salivary glands, kidneys, and bone marrow (74). Xerostomia occurs in approximately 30% of patients after 177Lu-EB-PSMA-617 (75), which is concordant with numbers from 177Lu-PSMA-617 use in the VISION trial (73). Effects could be more severe in patients who have had prior salivary gland resection or external beam radiation. Receipt of prior chemotherapy such as taxanes can also predispose patients to more significant hematopoietic toxicities (76).
In general, salivary gland cancer (SGC) is likely to be the first non-prostate cancer investigated in a prospective randomized trial as a potential indication for PSMA RLT, and pilot studies in SGC have shown some of the challenges noted above. Early results of PSMA RLTs in SGC have shown a less profound impact of PSMA RLTs compared to prostate cancer. The potential reasons for this are multifactorial including potential differences in PSMA expression, radiosensitivity, and patient selection in the pilot studies. The mean 68Ga-PSMA SUVmax for SGCs noted above tend to be lower in comparison to mean SUVmax reported in prostate cancer (median of 13.3) (77). In a combined analysis of PSMA PET/CT studies in SGC, Tan et al. reported overall local recurrence and metastatic mean SUVmax of 6.33 [2.41-13.8] and 6.82 [2.04-14.9] respectively for ACC; those respective mean SUVmax values for other SGCs (largely SDC) were 10.57 [4.0-16.8] and 6.06 [1.43-14.27] respectively (20). Of the 29 patients with SGC who have received PSMA RLT in pilot studies, 11 received 3 or more cycles on protocol. This contrasts with recently reported prospective studies in prostate cancer, as patients received a median of 5 cycles (37.5 GBq) in both the TheraP and VISION trials. This may be related to the selection criteria and late stage of patients with SGC treated in the pilot studies, and prospective phase II studies may show more favorable response. Interestingly, two ACC patients with partial metabolic response were among those that completed 3 or more cycles, and both had intracranial metastases (14, 23).
Overall, there is significant promise for PSMA-targeted therapy in non-prostate malignancies. Even if there is lower absorbed tumor dose due to lower and more heterogeneous PSMA expression, there may be significant treatment response in comparison with currently available treatment options for these malignancies. Further study is indicated investigating PSMA RLT dosimetry, PSMA PET/CT imaging as a biomarker in non-prostate cancers, and prospective phase II and III trials will be required for assessment of PSMA RLT treatment safety and efficacy.
Author contributions
Conceptualization, writing, editing, and final approval of manuscript: JW and AK.
Conflict of interest
AK has institutional clinical research funding from Bayer, Novartis/AAA, and Merck and has participated in unpaid consulting and advisory board for Novartis.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1220586/full#supplementary-material
References
1. Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet (2021) 397(10276):797–804. doi: 10.1016/S0140-6736(21)00237-3
2. Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med (2021) 385(12):1091–103. doi: 10.1056/NEJMoa2107322
3. Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res (1987) 7(5B):927–35.
4. Pinto JT, Suffoletto BP, Berzin TM, Qiao CH, Lin S, Tong WP, et al. Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin Cancer Res Off J Am Assoc Cancer Res (1996) 2(9):1445–51.
5. Minner S, Wittmer C, Graefen M, Salomon G, Steuber T, Haese A, et al. High level PSMA expression is associated with early psa recurrence in surgically treated prostate cancer. Prostate (2011) 71(3):281–8. doi: 10.1002/pros.21241
6. Satapathy S, Singh H, Kumar R, Mittal BR. Diagnostic accuracy of 68 ga-PSMA PET/CT for initial detection in patients with suspected prostate cancer: A systematic review and meta-analysis. Am J Roentgenol (2021) 216(3):599–607. doi: 10.2214/AJR.20.23912
7. Mhawech-Fauceglia P, Zhang S, Terracciano L, Sauter G, Chadhuri A, Herrmann FR, et al. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology (2007) 50(4):472–83. doi: 10.1111/j.1365-2559.2007.02635.x
8. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res Off J Am Assoc Cancer Res (1997) 3(1):81–5.
9. Salas Fragomeni RA, Amir T, Sheikhbahaei S, Harvey SC, Javadi MS, Solnes LB, et al. Imaging of nonprostate cancers using PSMA-targeted radiotracers: Rationale, current state of the field, and a call to arms. J Nucl Med (2018) 59(6):871–7. doi: 10.2967/jnumed.117.203570
10. Conway RE, Petrovic N, Li Z, Heston W, Wu D, Shapiro LH. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Mol Cell Biol (2006) 26(14):5310–24. doi: 10.1128/MCB.00084-06
11. Al-Ahmadie HA, Olgac S, Gregor PD, Tickoo SK, Fine SW, Kondagunta GV, et al. Expression of prostate-specific membrane antigen in renal cortical tumors. Mod Pathol (2008) 21(6):727–32. doi: 10.1038/modpathol.2008.42
12. Ren H, Zhang H, Wang X, Liu J, Yuan Z, Hao J. Prostate-specific membrane antigen as a marker of pancreatic cancer cells. Med Oncol Northwood Lond Engl (2014) 31(3):857. doi: 10.1007/s12032-014-0857-z
13. van Boxtel W, Lütje S, van Engen-van Grunsven ICH, Verhaegh GW, Schalken JA, Jonker MA, et al. 68 ga-PSMA-HBED-CC PET/CT imaging for adenoid cystic carcinoma and salivary duct carcinoma: a phase 2 imaging study. Theranostics (2020) 10(5):2273–83. doi: 10.7150/thno.38501
14. Wang G, Zhou M, Zang J, Jiang Y, Chen X, Zhu Z, et al. A pilot study of 68 ga-PSMA-617 PET/CT imaging and 177Lu-EB-PSMA-617 radioligand therapy in patients with adenoid cystic carcinoma. EJNMMI Res (2022) 12(1):52. doi: 10.1186/s13550-022-00922-x
15. Hartrampf PE, Weinzierl FX, Buck AK, Rowe SP, Higuchi T, Seitz AK, et al. Matched-pair analysis of [177Lu]Lu-PSMA I&T and [177Lu]Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging (2022) 49(9):3269–76. doi: 10.1007/s00259-022-05744-6
16. Sgouros G, Bodei L, McDevitt MR, Nedrow JR. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat Rev Drug Discov (2020) 19(9):589–608. doi: 10.1038/s41573-020-0073-9
17. English KK, Knox S, Graves SA, Kiess AP. Basics of physics and radiobiology for radiopharmaceutical therapies. Pract Radiat Oncol (2022) 12(4):289–93. doi: 10.1016/j.prro.2022.04.004
18. Boyd M, Ross SC, Dorrens J, Fullerton NE, Tan KW, Zalutsky MR, et al. Radiation-induced biologic bystander effect elicited In vitro by targeted radiopharmaceuticals labeled with α-, β-, and auger electron–emitting radionuclides. J Nucl Med (2006) 47(6):1007–15.
19. de Galiza Barbosa F, Queiroz MA, Nunes RF, Costa LB, Zaniboni EC, Marin JFG, et al. Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings. Cancer Imaging (2020) 20(1):23. doi: 10.1186/s40644-020-00300-7
20. Tan BF, Tan WCC, Wang FQ, Lechner M, Schartinger VH, Tan DSW, et al. PSMA PET imaging and therapy in adenoid cystic carcinoma and other salivary gland cancers: A systematic review. Cancers (2022) 14(15):3585. doi: 10.3390/cancers14153585
21. Uijen MJM, Derks YHW, Merkx RIJ, Schilham MGM, Roosen J, Privé BM, et al. PSMA radioligand therapy for solid tumors other than prostate cancer: background, opportunities, challenges, and first clinical reports. Eur J Nucl Med Mol Imaging (2021) 48(13):4350–68. doi: 10.1007/s00259-021-05433-w
22. Has Simsek D, Kuyumcu S, Agaoglu FY, Unal SN. Radionuclide therapy with 177Lu-PSMA in a case of metastatic adenoid cystic carcinoma of the parotid. Clin Nucl Med (2019) 44(9):764–6. doi: 10.1097/RLU.0000000000002645
23. Klein Nulent TJW, van Es RJJ, Willems SM, ArthurJAT B, LA D, de Bree R, et al. First experiences with 177Lu-PSMA-617 therapy for recurrent or metastatic salivary gland cancer. EJNMMI Res (2021) 11(1):126. doi: 10.1186/s13550-021-00866-8
24. Civan C, Kasper S, Berliner C, Fragoso-Costa P, Grünwald V, Pogorzelski M, et al. PSMA-directed imaging and therapy of salivary gland tumors: A single-center retrospective study. J Nucl Med Off Publ Soc Nucl Med (2023) 64(3):372–8. doi: 10.2967/jnumed.122.264342
25. Terroir M, Lamesa C, Krim M, Vija L, Texier JS, Cassou-Mounat T, et al. RadioLigand therapy with [177Lu]Lu-PSMA-617 for salivary gland cancers: Literature review and first compassionate use in france. Pharm Basel Switz (2023) 16(5):754. doi: 10.3390/ph16050754
26. Van Herpen CML, Uijen M, van Ruitenbeek N, Driessen CML, Gotthardt M, Nagarajah J. 177Lu-PSMA radioligand therapy for patients with recurrent/metastatic (R/M) salivary gland cancer (SGC): A phase II pilot study. J Clin Oncol (2023) 41(16_suppl):6099–9. doi: 10.1200/JCO.2023.41.16_suppl.6099
27. Kunikowska J, Charzyńska I, Kuliński R, Pawlak D, Maurin M, Królicki L. Tumor uptake in glioblastoma multiforme after IV injection of [177Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging (2020) 47(6):1605–6. doi: 10.1007/s00259-020-04715-z
28. Kumar A, Ballal S, Yadav MP, ArunRaj ST, Haresh KP, Gupta S, et al. 177Lu-/68Ga-PSMA theranostics in recurrent glioblastoma multiforme: Proof of concept. Clin Nucl Med (2020) 45(12):e512. doi: 10.1097/RLU.0000000000003142
29. Truckenmueller P, Graef J, Scheel M, Vajkoczy P, Capper D, Kaul D, et al. [68Ga]Ga-PSMA PET/MRI, histological PSMA expression and preliminary experience with [177Lu]Lu-PSMA therapy in relapsing high-grade glioma. Front Oncol (2022) 12. doi: 10.3389/fonc.2022.980058
30. More S, Naiker T, Jacobs N, Oompie F, Prasad V. Short-interval, low-dose [177Lu]Lu–Prostate-Specific membrane antigen in the treatment of refractory glioblastoma. Clin Nucl Med (2023) 48(5):e217. doi: 10.1097/RLU.0000000000004612
31. Jüptner M, Marx M, Zuhayra M, Lützen U. Experimental 177Lu-PSMA-617 radioligand therapy in a patient with extended metastasized leiomyosarcoma. Nukl - Nucl (2019) 58(04):328–30. doi: 10.4103/wjnm.WJNM_112_18
32. Digklia A, Boughdad S, Homicsko K, Dromain C, Trimech M, Dolcan A, et al. First communication on the efficacy of combined 177Lutetium-PSMA with immunotherapy outside prostate cancer. J Immunother Cancer (2022) 10(10):e005383. doi: 10.1136/jitc-2022-005383
33. Assadi M, Ahmadzadehfar H. 177Lu-DOTATATE and 177Lu-prostate-specific membrane antigen therapy in a patient with advanced metastatic radioiodine-refractory differentiated thyroid cancer after failure of tyrosine kinase inhibitors treatment. World J Nucl Med (2019) 18(4):406–8. doi: 10.4103/wjnm.WJNM_112_18
34. de Vries LH, Lodewijk L, Braat AJAT, Krijger GC, Valk GD, Lam MGEH, et al. 68Ga-PSMA PET/CT in radioactive iodine-refractory differentiated thyroid cancer and first treatment results with 177Lu-PSMA-617. EJNMMI Res (2020) 10:18. doi: 10.1186/s13550-020-0610-x
35. Tolkach Y, Gevensleben H, Bundschuh R, Koyun A, Huber D, Kehrer C, et al. Prostate-specific membrane antigen in breast cancer: a comprehensive evaluation of expression and a case report of radionuclide therapy. Breast Cancer Res Treat (2018) 169(3):447–55. doi: 10.1007/s10549-018-4717-y
36. Hirmas N, Leyh C, Sraieb M, Barbato F, Schaarschmidt BM, Umutlu L, et al. 68Ga-PSMA-11 PET/CT improves tumor detection and impacts management in patients with hepatocellular carcinoma. J Nucl Med (2021) 62(9):1235–41. doi: 10.2967/jnumed.120.257915
37. Graef J, Bluemel S, Brenner W, Amthauer H, Truckenmueller P, Kaul D, et al. [177Lu]Lu-PSMA therapy as an individual treatment approach for patients with high-grade glioma: Dosimetry results and critical statement. J Nucl Med (2023) 64(6):892–5. doi: 10.2967/jnumed.122.264850
38. Laurie SA, Licitra L. Systemic therapy in the palliative management of advanced salivary gland cancers. J Clin Oncol (2006) 24(17):2673–8. doi: 10.1200/JCO.2005.05.3025
39. Moskaluk CA. Adenoid cystic carcinoma: Clinical and molecular features. Head Neck Pathol (2013) 7(1):17–22. doi: 10.1007/s12105-013-0426-3
40. Jang S, Patel PN, Kimple RJ, Mcculloch TM. Clinical outcomes and prognostic factors of adenoid cystic carcinoma of the head and neck. Anticancer Res (2017) 37(6):3045–52. doi: 10.21873/anticanres.11659
41. Sung MW, Kim KH, Kim JW, Min YG, Seong WJ, Roh JL, et al. Clinicopathologic predictors and impact of distant metastasis from adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Neck Surg (2003) 129(11):1193–7. doi: 10.1001/archotol.129.11.1193
42. van der Wal JE, Becking AG, Snow GB, van der Waal I. Distant metastases of adenoid cystic carcinoma of the salivary glands and the value of diagnostic examinations during follow-up. Head Neck (2002) 24(8):779–83. doi: 10.1002/hed.10126
43. Di Villeneuve L, Souza IL, Tolentino FDS, Ferrarotto R, Schvartsman G. Salivary gland carcinoma: Novel targets to overcome treatment resistance in advanced disease. Front Oncol (2020) 10. doi: 10.3389/fonc.2020.580141
44. Klein Nulent TJW, Valstar MH, Smit LA, Smeele LE, Zuithoff NPA, de Keizer B, et al. Prostate-specific membrane antigen (PSMA) expression in adenoid cystic carcinoma of the head and neck. BMC Cancer (2020) 20(1):519. doi: 10.1186/s12885-020-06847-9
45. Mifsud M, Sharma S, Leon M, Padhya T, Otto K, Caudell J. Salivary duct carcinoma of the parotid: Outcomes with a contemporary multidisciplinary treatment approach. Otolaryngol Neck Surg (2016) 154(6):1041–6. doi: 10.1177/0194599816636812
46. Takahashi H, Tada Y, Saotome T, Akazawa K, Ojiri H, Fushimi C, et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2–positive salivary duct carcinoma. J Clin Oncol (2019) 37(2):125–34. doi: 10.1200/JCO.18.00545
47. Uijen MJM, Lassche G, van Engen-van Grunsven ACH, Tada Y, Verhaegh GW, Schalken JA, et al. Systemic therapy in the management of recurrent or metastatic salivary duct carcinoma: A systematic review. Cancer Treat Rev (2020), 89. doi: 10.1016/j.ctrv.2020.102069
48. Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, et al. Renal cell carcinoma. Nat Rev Dis Primer (2017) 3(1):1–19. doi: 10.1038/nrdp.2017.9
49. Zschäbitz S, Erlmeier F, Stöhr C, Herrmann E, Polifka I, Agaimy A, et al. Expression of prostate-specific membrane antigen (PSMA) in papillary renal cell carcinoma - overview and report on a large multicenter cohort. J Cancer (2022) 13(6):1706–12. doi: 10.7150/jca.63509
50. Ahn T, Roberts MJ, Abduljabar A, Joshi A, Perera M, Rhee H, et al. A review of prostate-specific membrane antigen (PSMA) positron emission tomography (PET) in renal cell carcinoma (RCC). Mol Imaging Biol (2019) 21(5):799–807. doi: 10.1007/s11307-018-01307-0
51. Spatz S, Tolkach Y, Jung K, Stephan C, Busch J, Ralla B, et al. Comprehensive evaluation of prostate specific membrane antigen expression in the vasculature of renal tumors: Implications for imaging studies and prognostic role. J Urol (2018) 199(2):370–7. doi: 10.1016/j.juro.2017.08.079
52. Gao J, Xu Q, Fu Y, He K, Zhang C, Zhang Q, et al. Comprehensive evaluation of 68Ga-PSMA-11 PET/CT parameters for discriminating pathological characteristics in primary clear-cell renal cell carcinoma. Eur J Nucl Med Mol Imaging (2021) 48(2):561–9. doi: 10.1007/s00259-020-04916-6
53. Muselaers S, Erdem S, Bertolo R, Ingels A, Kara Ö, Pavan N, et al. PSMA PET/CT in renal cell carcinoma: An overview of current literature. J Clin Med (2022) 11(7):1829. doi: 10.3390/jcm11071829
54. Gühne F, Seifert P, Theis B, Steinert M, Freesmeyer M, Drescher R. PSMA-PET/CT in patients with recurrent clear cell renal cell carcinoma: Histopathological correlations of imaging findings. Diagnostics (2021) 11(7):1142. doi: 10.3390/diagnostics11071142
55. Siva S, Callahan J, Pryor D, Martin J, Lawrentschuk N, Hofman MS. Utility of 68Ga prostate specific membrane antigen – positron emission tomography in diagnosis and response assessment of recurrent renal cell carcinoma. J Med Imaging Radiat Oncol (2017) 61(3):372–8. doi: 10.1111/1754-9485.12590
56. Rhee H, Blazak J, Tham CM, Ng KL, Shepherd B, Lawson M, et al. Pilot study: use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res (2016) 6:76. doi: 10.1186/s13550-016-0231-6
57. Urso L, Castello A, Rocca GC, Lancia F, Panareo S, Cittanti C, et al. Role of PSMA-ligands imaging in renal cell carcinoma management: current status and future perspectives. J Cancer Res Clin Oncol (2022) 148(6):1299–311. doi: 10.1007/s00432-022-03958-7
58. Heitkötter B, Trautmann M, Grünewald I, Bögemann M, Rahbar K, Gevensleben H, et al. Expression of PSMA in tumor neovasculature of high grade sarcomas including synovial sarcoma, rhabdomyosarcoma, undifferentiated sarcoma and MPNST. Oncotarget (2016) 8(3):4268–76. doi: 10.18632/oncotarget.13994
59. Kleiburg F, Heijmen L, Gelderblom H, Kielbasa SM, Bovée JV, De Geus-Oei LF. Prostate-specific membrane antigen (PSMA) as a potential target for molecular imaging and treatment in bone and soft tissue sarcomas. Br J Radiol (2023) 20220886. doi: 10.1259/bjr.20220886
60. Militano V, Afaq A, Bomanji J. 68Ga–Prostate-Specific membrane antigen PET/CT: Incidental finding of a liposarcoma. Clin Nucl Med (2019) 44(2):e90. doi: 10.1097/RLU.0000000000002389
61. Seifert R, Kessel K, Schlack K, Weber M, Herrmann K, Spanke M, et al. PSMA PET total tumor volume predicts outcome of patients with advanced prostate cancer receiving [177Lu]Lu-PSMA-617 radioligand therapy in a bicentric analysis. Eur J Nucl Med Mol Imaging (2021) 48(4):1200–10. doi: 10.1007/s00259-020-05040-1
62. Fanti S, Briganti A, Emmett L, Fizazi K, Gillessen S, Goffin K, et al. EAU-EANM consensus statements on the role of prostate-specific membrane antigen positron emission Tomography/Computed tomography in patients with prostate cancer and with respect to [177Lu]Lu-PSMA radioligand therapy. Eur Urol Oncol (2022) 5(5):530–6. doi: 10.1016/j.euo.2022.05.003
63. Groener D, Schneider S, Baumgarten J, Happel C, Klimek K, Mader N, et al. Baseline [68Ga]Ga-PSMA-11 PET/CT before [177Lu]Lu-PSMA-617 radioligand therapy: Value of PSMA-uptake thresholds in predicting targetable lesions. Cancers (2023) 15(2):473. doi: 10.3390/cancers15020473
64. Buteau JP, Martin AJ, Emmett L, Iravani A, Sandhu S, Joshua AM, et al. PSMA and FDG-PET as predictive and prognostic biomarkers in patients given [177Lu]Lu-PSMA-617 versus cabazitaxel for metastatic castration-resistant prostate cancer (TheraP): a biomarker analysis from a randomised, open-label, phase 2 trial. Lancet Oncol (2022) 23(11):1389–97. doi: 10.1016/S1470-2045(22)00605-2
65. Seifert R, Emmett L, Rowe SP, Herrmann K, Hadaschik B, Calais J, et al. Second version of the prostate cancer molecular imaging standardized evaluation framework including response evaluation for clinical trials (PROMISE V2). Eur Urol (2023) 83(5):405–12. doi: 10.1016/j.eururo.2023.02.002
66. Radiometabolic therapy with 177Lu PSMA in PSMA PET/CT positive Advanced/Metastatic tumours. Available at: https://clinicaltrials.gov/ct2/show/NCT05867615.
67. Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res (2019) 25(13):3753–8. doi: 10.1158/1078-0432.CCR-18-4070
68. Schuchardt C, Zhang J, Kulkarni HR, Chen X, Müller D, Baum RP. Prostate-specific membrane antigen radioligand therapy using 177Lu-PSMA I&T and 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: Comparison of safety, biodistribution, and dosimetry. J Nucl Med (2022) 63(8):1199–207. doi: 10.2967/jnumed.121.262713
69. Uijen MJM, Privé BM, van Herpen CML, Westdorp H, van Gemert WA, de Bakker M, et al. Kidney absorbed radiation doses for [177Lu]Lu-PSMA-617 and [177Lu]Lu-PSMA-I&T determined by 3D clinical dosimetry. Nucl Med Commun (2023) 44(4):270. doi: 10.1097/MNM.0000000000001658
70. Hartrampf PE, Weinzierl FX, Serfling SE, Pomper MG, Rowe SP, Higuchi T, et al. Hematotoxicity and nephrotoxicity in prostate cancer patients undergoing radioligand therapy with [177Lu]Lu-PSMA I&T. Cancers (2022) 14(3):647. doi: 10.3390/cancers14030647
71. Rosar F, Kochems N, Bartholomä M, Maus S, Stemler T, Linxweiler J, et al. Renal safety of [177Lu]Lu-PSMA-617 radioligand therapy in patients with compromised baseline kidney function. Cancers (2021) 13(12):3095. doi: 10.3390/cancers13123095
72. Schäfer H, Mayr S, Büttner-Herold M, Knorr K, Steinhelfer L, Böger CA, et al. Extensive 177Lu-PSMA radioligand therapy can lead to radiation nephropathy with a renal thrombotic microangiopathy–like picture. Eur Urol (2023) 83(5):385–90. doi: 10.1016/j.eururo.2022.05.025
73. Fizazi K, Herrmann K, Krause BJ, Rahbar K, Chi KN, Morris MJ, et al. Health-related quality of life and pain outcomes with [177Lu]Lu-PSMA-617 plus standard of care versus standard of care in patients with metastatic castration-resistant prostate cancer (VISION): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2023) 24(6):597–610. doi: 10.1016/S1470-2045(23)00158-4
74. Zang J, Fan X, Wang H, Liu Q, Wang J, Li H, et al. First-in-human study of 177Lu-EB-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging (2019) 46(1):148–58. doi: 10.1007/s00259-018-4096-y
75. Heynickx N, Herrmann K, Vermeulen K, Baatout S, Aerts A. The salivary glands as a dose limiting organ of PSMA- targeted radionuclide therapy: A review of the lessons learnt so far. Nucl Med Biol (2021) 98–99:30–9. doi: 10.1016/j.nucmedbio.2021.04.003
76. Groener D, Nguyen CT, Baumgarten J, Bockisch B, Davis K, Happel C, et al. Hematologic safety of 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration-resistant prostate cancer. EJNMMI Res (2021) 11(1):61. doi: 10.1186/s13550-021-00805-7
Keywords: PSMA, radioligand therapy (RLT), non-prostate cancers, lutetium-177, salivary gland cancer, glioblastoma, renal cell carcinoma, soft tissue sarcoma
Citation: Wang JH and Kiess AP (2023) PSMA-targeted therapy for non-prostate cancers. Front. Oncol. 13:1220586. doi: 10.3389/fonc.2023.1220586
Received: 10 May 2023; Accepted: 25 July 2023;
Published: 14 August 2023.
Edited by:
Arthur Johannes Anthonius Theodorus Braat, Utrecht University, NetherlandsReviewed by:
Laura Evangelista, University of Padua, ItalyTobias Maurer, Martini Klinik Prostate Cancer Center, Germany
Copyright © 2023 Wang and Kiess. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jarey H. Wang, jwang446@jh.edu; Ana P. Kiess, akiess1@jhmi.edu
 Jarey H. Wang
Jarey H. Wang Ana P. Kiess
Ana P. Kiess