- 1Laboratory of Molecular Biology, Henan Luoyang Orthopedic Hospital (Henan Provincial Orthopedic Hospital), Zhengzhou, China
- 2Henan University of Chinese Medicine, Zhengzhou, China
- 3Hunan University of Chinese Medicine, Changsha, China
- 4Department of Orthopaedics, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
The clinical manifestations of bone metastases are diversified while many sites remain asymptomatic at early stage. As the early diagnosis method is not perfect and the early symptoms of tumor bone metastasis are not typical, bone metastasis is not easy to be detected. Therefore, the search for bone metastasis-related markers is effective for timely detection of tumor bone metastases and the development of drugs to inhibit bone metastases. As a result, bone metastases can only be diagnosed when symptoms are found, increasing the risk of developing skeletal-related event (SREs), which significantly impairs the patient’s quality of life. Therefore, the early diagnosis of bone metastases is of great importance for the treatment and prognosis of cancer patients. Changes of bone metabolism indexes appear earlier in bone metastases, but the traditional biochemical indexes of bone metabolism lack of specificity and could be interfered by many factors, which limits their application in the study of bone metastases. Some new biomarkers of bone metastases have good diagnostic value, such as proteins, ncRNAs, circulating tumor cells (CTCs). Therefore, this study mainly reviewed the initial diagnostic biomarkers of bone metastases which were expected to provide references for the early detection of bone metastases.
1 Introduction
Bone metastasis occurs when tumor cells spread to the bones. When people suffering from cancer, with the progession of the disease, the cancer cells invade the blood vessels. As the blood flows, the cancer cells may travel to the bone marrow and continue to rise, forming bone metastases (1). Distant metastases are a typical characteristic of malignant tumor, as well as one of the main reasons leading to treatment failure of tumor patients (2). On average, 1 out of every 5 patients will suffer from bone metastases. Theoretically, almost all types of cancers may metastasize to bone, among which lung cancer, breast cancer and prostate cancer are the most frequent (3). Digestive tract tumors such as stomach cancer, bowel cancer, pancreatic cancer, etc., can also appear, relatively low risk. There are three types of bone metastases: osteolytic, osteoblastic and mixed (4, 5). Only clear diagnosis and symptomatic treatment will have beneficial clinical effect (6). Osteogenic bone metastases are widespread in prostate cancer, accounting for about 10% of bone metastases. Lytic bone metastases account for 70%, which are atypical lung and breast cancer (4).
The early diagnosis of malignant tumors is very critical to the recovery. In clinical practice, some of cancer patients showed symptoms such as waist and leg pain or anemia (especially those who had a history of this, such as rheumatic inflammation, lumbar disc herniation, etc.), but they did not pay enough attention (7). In fact, it is highly likely that this is a precursor of tumor bone metastases. If the bone lesions and complications of bone metastases cannot be treated reasonably, it will do great harm, such as pathological fractures, which often paralyze patients in bed, as well as the severe pain will seriously affect the quality of life of patients (8, 9).
Early diagnosis of bone metastases is of major importance. The main symptom of bone metastases is persistent pain with continuously aggravated, which may also cause mobility impairment. The commonly used imaging methods for the diagnosis of bone metastases have different characteristics. As for X-ray, specificity is high but sensitivity is low. The positive rate of bone ECT imaging is high, but there exist false positive and false negative problems (5, 10). CT and MRI have high specificity and accuracy, but are not appropriate for general examination. positron emission computed tomography PET has a high positive rate, but it doesn’t applicable to simple bone lesions, and the price is relatively high, which limited its application in clinic (11, 12). Theoretically, the changes of biochemical indexes of bone metabolism during bone metastases are earlier than those in imaging (13, 14). However, traditional biochemical indexes of bone metabolism with low specificity limits their application in the study of bone metastases (15, 16).
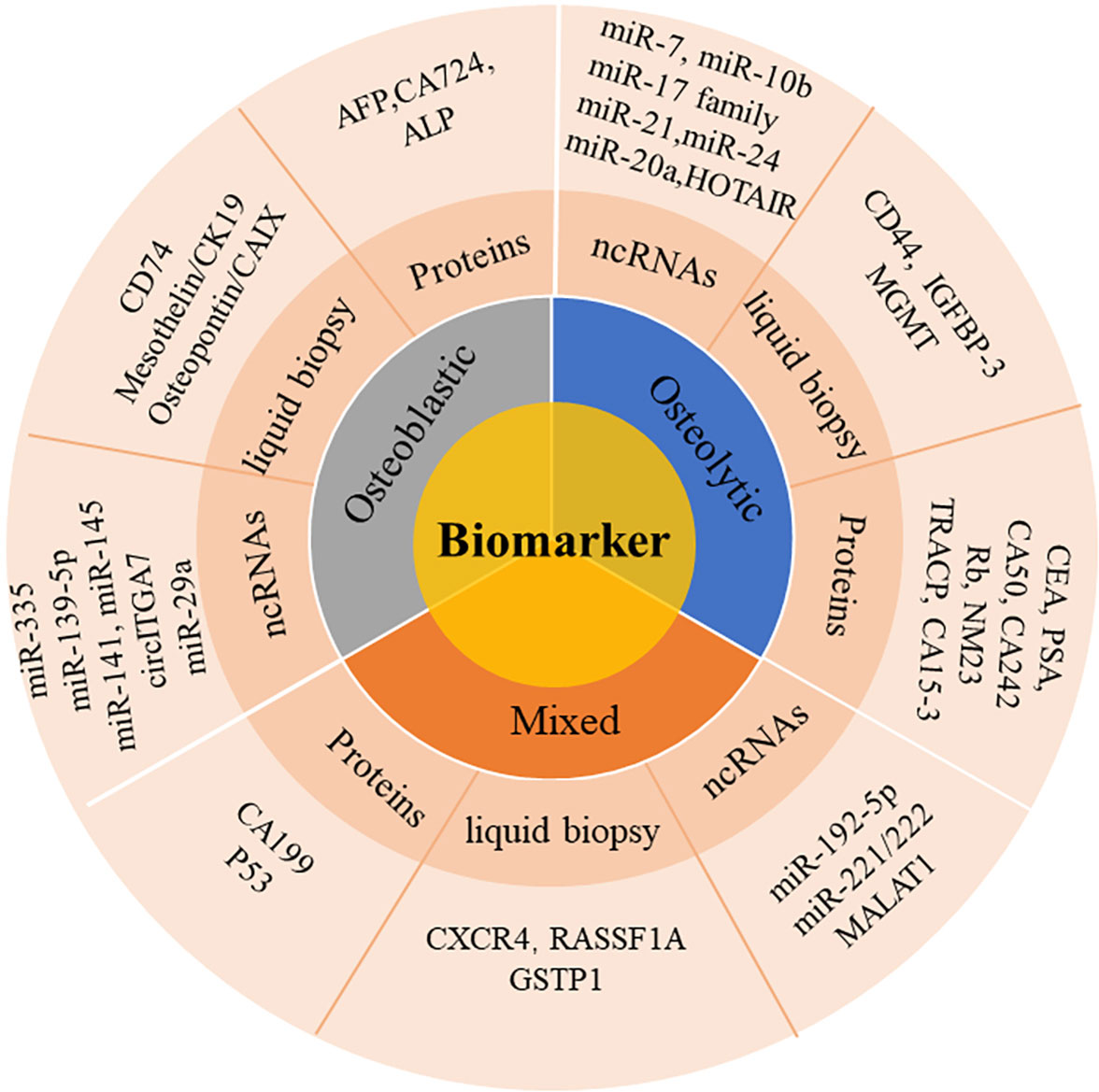
Some new biomarkers of bone metastases have good diagnostic value, such as proteins, ncRNAs, biomarkers in liquid biopsy and other biochemical indicators. These new types of biomarkers have demonstrated great potential in the initial diagnosis of bone metastases. In the study we searched relevant researches for bone metastases biomarkers, which mainly provides reference for early diagnosis of bone metastases, as shown in Figure 1.
2 Application of commonly used protein biomarkers in bone metastases
Protein biomarkers are most commonly used in the clinical diagnosis and prognosis of bone metastases. It indicates proteins in the blood whose presence or abnormal expression is often associated with certain types of tumors. These proteins can be detected in tumor cells, surrounding tissues, and blood, these biomarkers can be employed to monitor patient responsiveness and effectiveness during treatment. However, it is important to emphasize that a single blood biomarker is not enough to detect the tumor. It is usually used in conjunction with other tests, imaging and clinical symptoms to determine the status of the tumor. The presence of digestive system tumors and the occurrence of bone metastases may lead to increasing carbohydrate resistance, such as the indexes of alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), prostate specific antigen (PSA), CA199, CA724, CA50, and CA242.
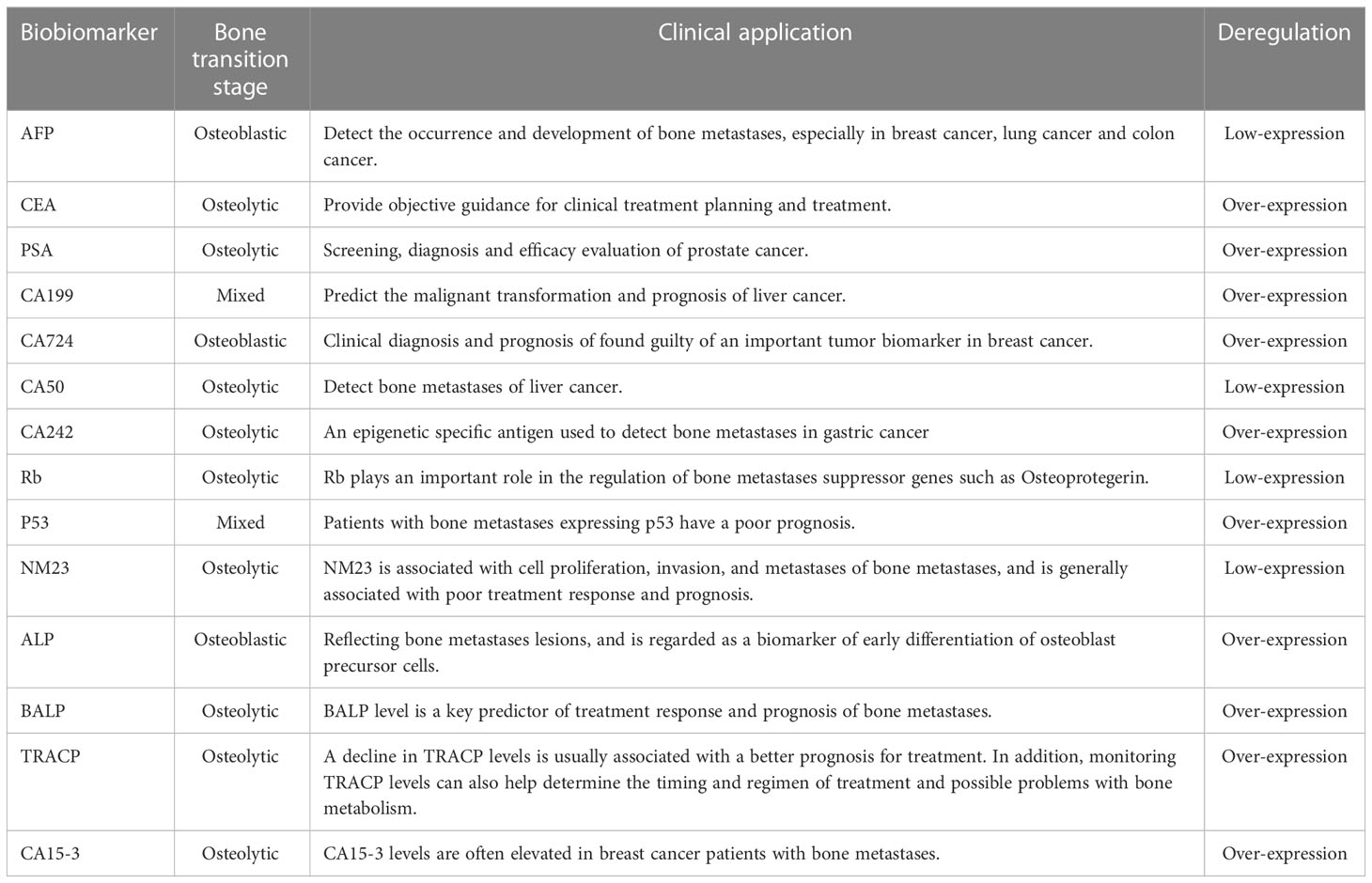
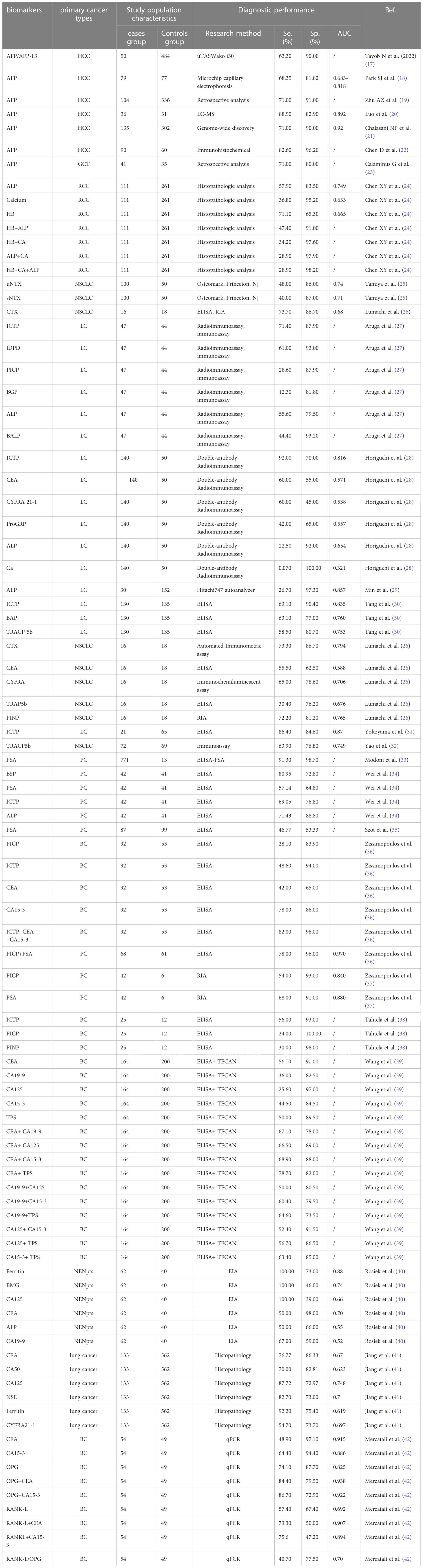
Except that most commonly used for bone metastases tumor biomarkers include bone specific alkaline phosphatase (BALP), tartrate-resistant acid phosphatase (TRACP), tumor necrosis factor (TNF), carbohydrate antigen 15-3 (CA15-3). The exact contents were shown in Table 1. The diagnostic performance of each biomarker was presented shown in Table 2.
2.1 AFP
AFP, known as hepatoembryonic antigen, is a biomarker for the identification of bone metastases (43). It plays a major role in embryonic and early embryonic development, but the adult owned the low level of AFP. AFP is commonly used as the diagnostic biomarker for liver, testicular, and ovarian carcinoma. Moreover, AFP can be used to predict bone metastases, which is a manifestation of antigen movement in a specific direction (43, 44). Studies showed that the serum level of AFP in patients with non-small cell lung cancer can be utilized to predict location-based tumor susceptibility and duration of location-based tumor treatment (45, 46). Another study showed that higher serum AFP level in the patients of cancer indicated the risk of bone metastases and thus to infer more effective cancer treatment options (44, 47). High level of serum AFP has been shown to help to diagnose patients with bone metastases with diagnostic accuracy of 75% as well as to predict tumor size, location, risk of metastases, and duration of treatment (40, 48). Recent studies have found that it can be utilized to assess location-based tumor susceptibility, as well as tumor size, location, and duration of treatment. To sum up, AFP is a significant biomarker for the detection of bone metastases.
2.2 CEA
CEA is a common antigenic factor that plays an important role in a variety of cancers, such as Colon cancer, stomach cancer, pancreatic cancer, small intestinal adenocarcinoma, lung cancer, liver cancer, breast cancer (49). CEA is a biomarker widely used in colorectal cancer screening and monitoring treatment response. However, its low sensitivity and specificity in bone tumors limit its application in bone metastasis. CEA is of particular importance in bone metastases. At present, CEA is used primarily to detect the occurrence and development of bone metastases, especially in breast cancer, lung cancer and gastrointestinal tumors (50, 51). CEA has excellent sensitivity and specificity, which can be used to assess the existence of bone metastases. The sensitivity and specificity of serum CEA were 19.0%-56.1% and 50%-92%, in the gastrointestinal tumors (39). At present, more and more studies have pointed out that CEA can help accurately diagnose bone metastases and improve the curative effect. Clinical trials have shown that increased CEA levels were linked to reduced efficacy in patients with breast cancer bone metastases (52, 53). In addition, CEA also has significant application value for clarifying tumor manifestations, namely the range of bone metastases and bone changes, so as to provide objective guidance for clinical treatment planning.
2.3 ALP and PSA
ALP and PSA are widely used to predict bone metastases of prostate cancer, but their accuracy and reliability in the diagnosis of bone metastases are inconsistent (54). Serum ALP is derived from osteoblasts with isoenzyme activities, which can hydrolyze phosphate esters. Moreover, serum ALP, can be used to indicate the specificity of reflecting bone metastases lesions, regarded as a biomarker of early differentiation of osteoblast precursor cells. ALP is specific biomarkers of bone tissue and widely utilized in bone tumors. The expression level of ALP can be used to estimate the balance between bone reconstruction and destruction. (55). Salter et al. found that ALP was oleophilic, which was an important biomarker reflecting osteoblast activity and tumor progression (56). Rao et al. suggested that ALP was a serum biomarker in predicting bone metastases of prostate cancer (57). Serum PSA, a serine protease, is commonly used in screening, diagnosis and efficacy evaluation of prostate cancer (58). In patients of prostate cancer with bone metastases, due to the proliferation of prostate cancer cells, a large amount of PSA was produced and secreted into the blood, resulting in elevated serum PSA (59, 60). PSA is a good indicator of bone metastases of prostate cancer. The higher the PSA, the greater the risk of bone metastases. When PSA < 20ng/ml, the risk of bone metastases was relatively small, while when PSA > 100ng/ml, the risk of bone metastases was higher than 80%. Therefore, further testing and prophylaxis were recommended when PSA > 20ng/ml (61). Although bone metastases are common sites of prostate cancer, the use of PSA in the diagnosis of bone metastases is limited.
2.4 CA and Rb
CA is used more frequently for the detection of breast and bowel cancer. CA199 is an important biomarker and apparent specific antigen for the detection of bone metastases of liver cancer. Studies have shown that the expression level of CA199 was related to the metastases of liver cancer, with the excellent ability to predict the malignant transformation and prognosis of liver cancer (39, 62). CA724 used for clinical diagnosis and prognosis of found guilty of an important tumor biomarker in breast cancer. Studies have shown that increased level of CA724 may represent increased bone metastases potential of breast cancer, which was more accurate for symptomatic radiotherapy (63, 64). CA50 is an apparent exclusive cancer biomarker used to detect bone metastases of liver cancer. The experimental results indicated that the level of CA50 can serve as a biomarker to predict the potential of bone metastases of liver cancer (65, 66). CA242 is an epigenetic specific antigen used to detect bone metastases in gastric cancer. Studies have indicated that increased level of CA242 can be used to predict bone metastases in gastric cancer, and can effectively help to improve the treatment efficiency and anti-cancer therapeutic effect of tumors (67, 68).
Rb is widely used in the diagnosis of bone-derived tumors, whose reduced expression indicates an increased risk of bone metastasis. (69). P53 is a tumor suppressor gene protein that is abnormally expressed in a variety of tumors. NM23 is an RNA-binding protein that is abnormally expressed in non-small cell lung cancer and some other cancers, whose application in bone tumors is restricted.
In conclusion, the current researches on protein biomarkers of bone metastases are still in the primary stage. Despite the fact that some biomarkers have been proved to have certain application value, more biomarkers need to be explored and applied in the accurate diagnosis of bone metastases and the formulation of treatment plans.
3 Application of ncRNA as biomarkers in bone metastases
With the development of high-throughput sequencing technology and bioinformatics, a large number of ncRNA, such as miRNA, lncRNA and circRNA, have been found to be involved in gene expression regulation, cell differentiation, etc (70, 71). In addition, they are closely related to the occurrence and development of tumors.
3.1 miRNA
miRNA in mammalian serum and plasma have high stability and can be stable under repeated freeze-thaw and different pH conditions (70, 72–74).
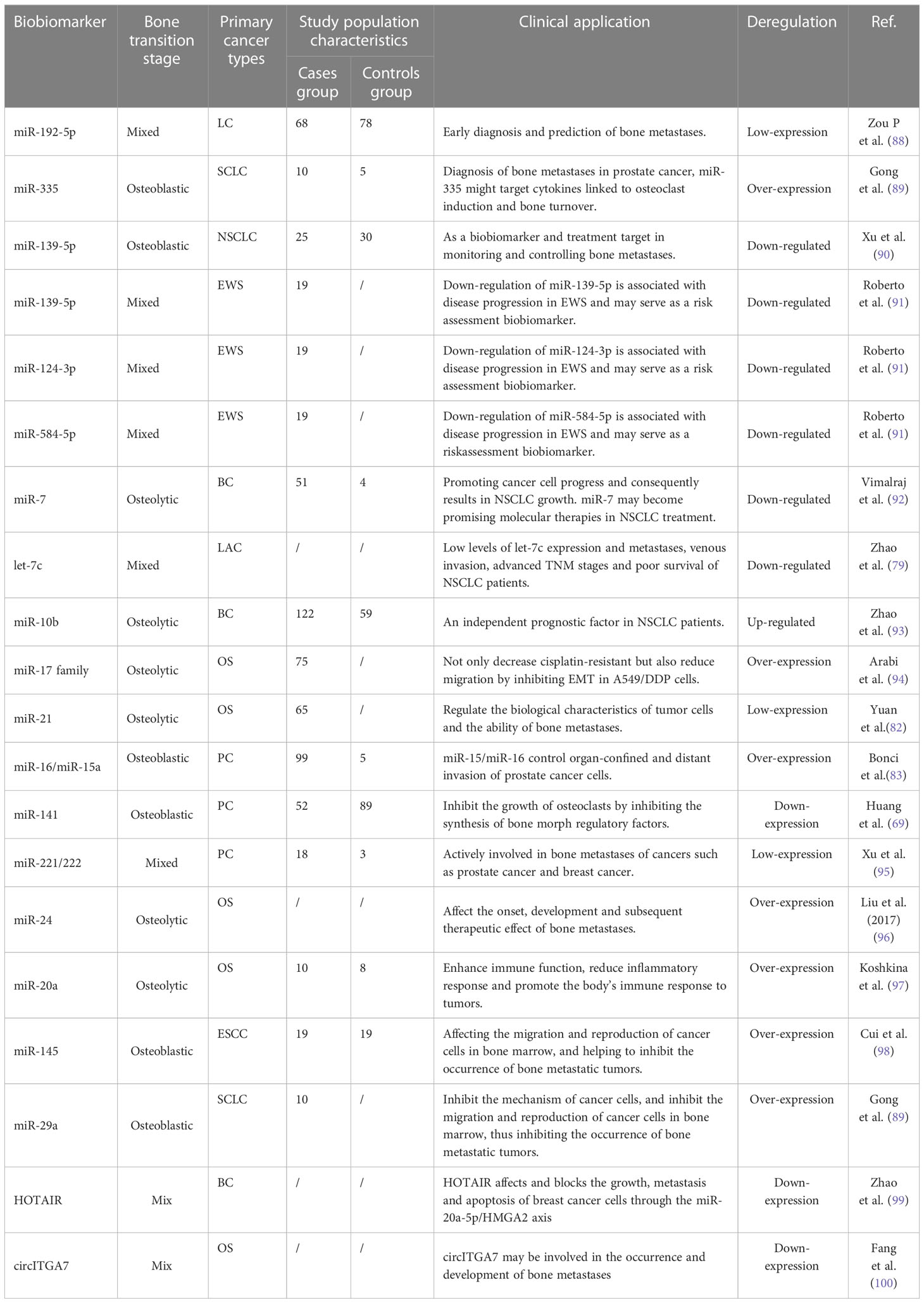
miRNA plays an important role in the diagnosis of bone metastases, which can help doctors to identify cancer metastases to bone in order to provide timely treatment (70, 71). Currently, many studies have shown that the expression level of miRNA from samples can be used to identify the presence of bone partially implanted cancer cells (70, 72, 75–77). Some miRNA such as let-7 (78, 79), miR-125b (80, 81), and miR-21 were significantly expressed in experimental tumor migration into the mouse bone, contributing to the identification and diagnosis of bone metastatic cancer (82–84). miRNA plays an important role in tumor therapy, and it has attracted more and more attention as new therapeutic biomarkers (85, 86). Targeting miRNA therapy can reduce drug toxicity and achieve higher efficacy by accurately identifying and treating bone metastases. Contemporary studies have shown that miRNAs-based therapy has a significant promoting effect on inhibiting the growth, invasion and immune resistance of bone metastases (7, 87). Currently, miRNAs that have been considered as biomarkers of bone metastases include miR-21, miR-141, miR-221/222, miR-24, miR-20a, miR-145, miR-29a, miR-26a, miR-22, miR-125b, miR-15b, miR-193b, miR-196a, and miR-101 et al., which were shown in Table 3.
3.1.1 miR-21
miR-21 has been extensively studied as a key biomarker for various types of cancer, including breast, lung, prostate, ovarian, and colorectal cancers (82–84). One study found that miR-21 was significantly up regulated in bone metastases tissue samples, compared to primary tumor tissue samples from patients with breast cancer (9). Furthermore, they observed that serum levels of miR-21 were significantly higher in breast cancer patients with bone metastases. They suggested that miR-21 could be used as a non-invasive biomarker to detect bone metastases in breast cancer patients. Similarly, another study found that miR-21 was over expressed in bone metastases tissue samples from patients with prostate cancer. They observed that miR-21 expression was positively correlated with bone metastases, suggesting that miR-21 could be used as a prognostic biomarker to predict the progression of bone metastases in prostate cancer patients (101). One study analyzed miR-21 expression in serum samples from patients with breast cancer and bone metastases, as well as healthy controls, drawing a conclusion that serum levels of miR-21 were significantly higher in breast cancer patients with bone metastases, compared to healthy controls. (102). Overall, the above studies suggested that miR-21 was a promising biomarker in the detecting and monitoring of bone metastases in various types of cancer. Its potential use as a therapeutic target warrants further investigation in preclinical and clinical studies.
3.1.2 miR-141
miR-141 has been a top priority in the study of bone metastases in recent years (103, 104). miR-141 can inhibit adenovirus transcription factors, immune response and apoptosis-mediated response, and exert a huge effect on inhibiting tumor growth to promote factor expression and inhibit gene expression regulation (105, 106). Studies have shown that miR-141 is paramount in preventing the development of bone metastases (106). In previous studies, miR-141 can prohibit the growth of osteoclasts by inhibiting the synthesis of bone morph regulatory factors, thus delaying the metastases process (107, 108). Meanwhile, miR-141 interdicted the migration and invasion of bone metastases. In addition, miR-141 can also induce tumor cell apoptosis, thus playing a momentous role in the process of bone metastases (109, 110). In conclusion, miR-141 is instrumental in inhibiting the development of bone metastatic tumors and may be essential in clinical diagnosis and treatment of bone metastatic tumors in the future.
3.2 lncRNA and circRNA
lncRNA and circRNA are a class of emerging ncRNA, playing important roles in the occurrence and development of human diseases. In recent years, more and more studies have shown that lncRNA and circRNA may also be strong candidates for tumor biomarkers of bone metastasis. There are some studies have found that lncRNA is crucical in bone metastasis. For example, one research has shown that metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) can promote tumor cell invasion and migration, whose expression level was elevated in patients with bone metastasis (111). Other lncRNAs such as HOX antigens intergenic RNA (HOTAIR) and taurine unregulated gene 1 (TUG1) have also been found to be closely associated with the occurrence and development of bone metastases. HOTAIR affected and blocked the growth, metastasis, and apoptosis of breast cancer cells through the miR-20a-5p/HMGA2 axis. In the past few years, studies have found that lncRNA-SOX2OT may have clinical diagnostic value and can be employed as an in vitro diagnostic biomarker for bone metastases (110). It was found that the level of lncRNA-SOX2OT in serum in patients with bone metastases were significantly higher than those in the control group (112). Besides, studies had found that lncRNA-SOX2OT might regulate the phenotype of bone metastatic tumor cells. It was also found that lncRNA-SOX2OT inhibited the expression of MMP-13, which explained why lncRNA -Sox2OT may be associated with the regulation of bone metastases (113). Moreover, by combining multiple gene factors, we found that HIF-1, Hypoxia, and LCC-Sox2OT gene regulatory networks may present in bone metastases. What’ more, the researchers suggested that the expression of LCC-Sox2OT may be related to cell status, which can be used to identify biomarkers in vitro, and to identify and forecast the incidence of bone metastatic tumors in vivo (54, 112, 114, 115).
In contrast, circRNA has been relatively poorly studied in bone metastasis (99). What’s more, some studies have shown that circRNA may also be a biomarker of bone metastases. For instance, there reported a study showing that circITGA7 (circular RNA-integrin subunit alpha 7) may be involved in the occurrence and development of bone metastases. This circular transcription can inhibit apoptosis of a variety of cells, whose expression level was significantly increased in patients with bone metastasis (100). Of course, studies on tumor biomarkers for bone metastases in lncRNA and circRNA are still in the preliminary stage, and their potential mechanisms and clinical application value need to be further verified and explored.
4 Bone metastasis biomarkers in liquid biopsy
Compared with traditional tissue sample biopsies, liquid biopsy-based markers have the following advantages:1. Non-invasive: Liquid sample collection is relatively simple, such as blood, urine, etc., without tissue excision or puncture, which can reduce patients’ pain and risk. 2. Systemic: Liquid samples can reflect the situation of the whole body, avoiding local errors in the collection of tissue samples, making them more representative and comprehensive. 3. High sensitivity: the concentration of markers in liquid samples is relatively stable and is not affected by tissue heterogeneity, making the detection results more accurate and reliable. 4. Good repeatability: liquid sample collection is relatively simple and non-invasive, which can be collected multiple times to monitor tumor growth and metastasis. 5. Forward-looking: in the detection and monitoring of early tumors, liquid biopsy can provide a more flexible and sensitive detection method, and improve the rate of early diagnosis and treatment of tumors. For tumor biomarkers of bone metastasis in liquid biopsy, molecular indicators related to bone metastasis, such as ctDNA, exosomes and circulating tumor cells (CTCs), were mainly screened from biological fluids such as blood or urine. These indicators have the advantages of high sensitivity, non-trauma and dynamic monitoring, which can be utilized to achieve early detection, monitor and prediction of bone metastasis. Corresponding contents were shown in Table 4.
4.1 ctDNA
ctDNA is a piece of DNA which was released into the blood by cancer cells with certain specificity and sensitivity. ctDNA is a piece of DNA that is released into the bloodstream when cancer cells die or die. Unlike normal plasma DNA, ctDNA contains specific variations from tumor cells. Therefore, ctDNA can be used as a non-invasive “liquid biopsy” method, which can be widely used in the early diagnosis, treatment monitoring and prognosis assessment of tumors. ctDNA has the following advantages: 1. Non-invasive: ctDNA sampling is simple and non-invasive, requiring no painful tissue removal or cancer cell culture. 2. High sensitivity: The proportion of ctDNA in the blood is very low, so it can be detected even in the mild disease, especially in the primary tumor detection has a better application prospect. 3. High specificity: ctDNA contains specific variations from tumor cells, which can distinguish different subtypes and tumors at different stages of synchronization. 4. Real-time dynamic monitoring can be realized: ctDNA can reflect real-time treatment progress, drug resistance and relapse, which can provide doctors with better treatment strategies. To sum up, ctDNA as a tumor marker has great advantages and has gradually become a hot spot in cancer research.
In the detection of bone metastases, studies on ctDNA as a kind of biomarker in bone metastases mainly focus on the following aspects. ctDNA tests based on gene mutations. Firstly, some mutations associated with bone metastases, such as the fatty acid acylase gene (ACSL5) and the fusion gene TMPRSS2-ERG, had been shown to have high sensitivity and specificity when ctDNA was detected in the blood. These mutations were valuable for the detection of bone metastases (120). For example, one study found that ctDNA, which detected a deletion of the PTEN and mutation of the TP53, had high sensitivity and specificity in the plasma of prostate cancer patients. Secondly, the detection of ctDNA is based on epigenetic changes. Bone metastasis is also closely associated with epigenetic changes in DNA methylation and histone modification. Studies had shown that some epigenetic biomarkers such as RASSF1A (121), IGFBP-3 (74), MGMT and ctDNA of GSTP1 can be detected in patients with bone metastases. These biomarkers provided an accurate value for the early detection and evaluation of bone metastases. Finally, the detection of ctDNA based on microsatellite instability (MSI), which is usually caused by the depletion of mismatch repair systems in vivo and is a hallmark of many familial non-multiple systemic tumors. It has been noted that the appearance of MSI in cancer cells is closely related to the occurrence and development of bone metastasis. There was a study showed that the detection of MSI in ctDNA could be used to evaluate the prognosis of bone metastases in intestinal cancer, providing a reference for the selection of treatment (122). In conclusion, the research and application of ctDNA as tumor biomarkers in bone metastases are developing and improving all the time. Although it still faces some technical and methodological bottlenecks, future studies will continuously improve its application prospect and clinical value. It is expected to become an important indicator in the timely detection, prognosis assessment and treatment monitoring of bone metastases.
4.2 CTCs
CTCs are cells shed from tumors and enter the peripheral blood of the body, which are the highest manifestation of the spread of malignant tumors. The genetic characteristics or antigens of CTCs are identical to those of primary tumor cells, but the method of obtaining CTCS is less invasive and highly reproducible (123). Systematic monitoring of CTCS through liquid biopsies enables monitoring of disease processes, detecting emerging resistance genes, and identifying new molecular targets (124). Relevant studies had shown that CTCs were highly invasive and malignant, and could evade immune surveillance of the body. CTCs can reflect the characteristics of tumor metastases and disease changes in patients with malignant tumors, playing crucial part in the curative effect and recurrence prediction of malignant tumors, so as to provide a reference for the early diagnosis and treatment of diseases (125). Detection of CTCs is a prerequisite for distant metastases of solid tumors (126). The specific contents were shown in Table 4.
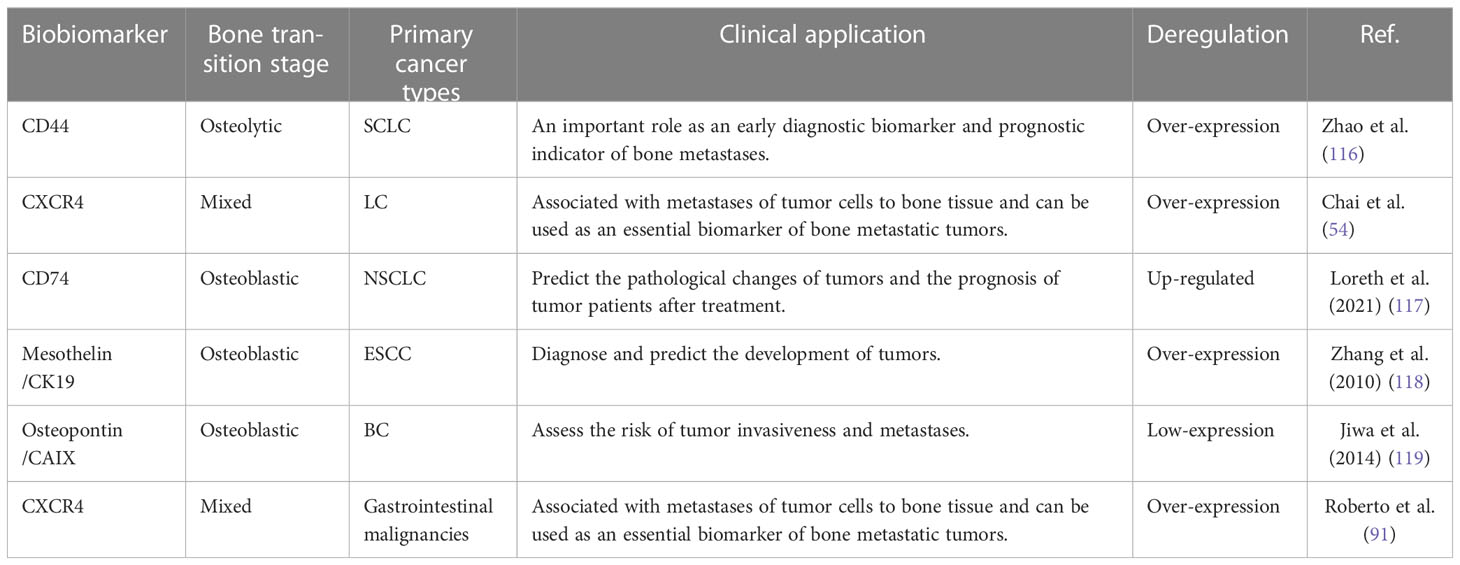
Taking CD44 for example. CD44 is a protein, which is deemed to be a pathological indicator. It is generically known as CD44 receptor, also known as adhesion molecule, which is a variety of tumor cell adhesion molecule genes, associated with signal activation and cell cross-coupling of cell molecules (127, 128). Clinical studies had shown that CD44 was a diagnostic biomarker and prognostic indicator in a variety of tumors, including liver cancer, stomach cancer, esophageal cancer, ovarian cancer, prostate cancer, etc. It can be found in blood, cellular mediators, tissue biopsy specimens, tumor cells, and normal cells (129, 130). Studies had shown that the expression of CD44 was related to the expression of late genes such as PD-L1. Its expression may also matter in the early detection of tumors and later forms of metastases. Laboratory studies have demonstrated that CD44 can form binding with chemical factors of mitogen and cell surface, improve cell binding to other cell surface molecules and thus increase the risk of bone metastases (127, 131).
4.3 Exosomes
Extracellular vehicles (EVs) include apoptotic bodies (ABs), microvesicles (MVs), and exosomes, encapsulate tumor-specific content, and transmit them into environmental cells and circulation. Exosomes as molecular biomarkers, play major roles in diagnostic decisions and treatment selection in the detection of cancer bone metastases (132). Exosomes have relatively stable components that confer biological effects on adjacent or distal cells. Exosomes are also nanoparticles secreted by all cell types (133, 134). Due to their nature as nanovesicles, exosomes can be transferred proximal and distal across different biological barriers. Exosomes have been used as transport carriers for a variety of molecules including proteins and different RNA (135).
Therefore, exosomes can be used not only as reaction markers of different diseases and physiological states, but also as tools of in vitro genetic engineering for the treatment of different diseases and organs. This shows that exosomes, as communication mediators between cells, have infinite potential as biomarkers. From the perspective of exosome functioned as molecular biomarkers, exosomes function importantly in the molecular linkage of bone metastases tumor, accurate detection and quantification of bone metastases tumor biomarkers, which are extremely important (136). On the one hand, the studies of exosome molecular biomarker will provide useful information that can help clinicians more accurately in diagnosing bone metastases. Exosomes can be detected diagnostic cancer biomarkers in body fluids, such as prostate specific nucleic acid expression (PNA), gastrointestinal specific protein expression (GIP), and respiratory specific nucleic acid expression (RNA) (137, 138), which can identify cancer cells faster and more accurately, providing more detailed and reliable molecular information of cancer cells, so as to better predict the trend of cancer cell metastases and provide more accurate treatment guidance.
miR-375 and miR-141, which from exosomes, are the main biomarkers of bone metastases, which are mainly involved in regulating the respiration and proliferation of cancer cells (7, 57). The increased expression of miR-375 can promote the malignant proliferation of cancer cells. On the contrary, miR-141 will promote and inhibit the proliferation of cancer cells, reduce the damage to sensitive cancer cells, and decrease the resistance to drug-resistant cancer cells (139). In addition, TM256, LAMTOR1 and VATL were tumor biomarkers associated with miR-141 and miR-375. TM256 can recognize the increased expression of miR-141 and promote the proliferation and growth of cancer cells (103). LAMTOR1 can recognize the increased expression of miR-141 and miR-375 and inhibit the proliferation and growth of cancer cells (69). VATL can recognize the increased expression of miR-375 and promote malignant proliferation of cancer cells. ADIRF was a specific tumor biomarker that can detect and recognize increased expression of miR-375 and miR-141, thereby contributing to the growth and proliferation of cancer cells (104, 140).
5 Application of other kinds of biomarkers in bone metastases
DNA methylation is a joint biological modification that affects gene expression by introducing methyl groups into DNA molecules through methylase. In tumor cells, the change of DNA methylation degree is closely linked to tumor growth, cell proliferation and development. Currently, there are many biomarkers of bone metastases based on DNA methylation, which include many different types. Glutathione S transferase P1 (GSTP1) is an antioxidant enzyme whose DNA methylation leaded to decreased expression levels, which had been demonstrated in many tumor cases, including bone metastases (141). SEPT9 was often considered a biomarker of DNA methylation. Recent studies had shown that exon 8 methylation of SEPT9 was a valid biomarker for blood samples (both venous and serum) from lung cancer patients (141, 142). The HOXB gene family is a member of the HOX gene superfamily, and HOXB7 may acted as a proto-oncogene in a variety of malignancies (143). DNA methylation of HOXB7 gene played an essential role in bone metastasis of prostate cancer cells (144). That is to say, DNA methylation of bone metastases tumor biomarkers provides a novel idea and means for the diagnosis, monitor and treatment of bone metastases. However, more studies are required to confirm their clinical application prospects as well as their sensitivity, specificity and stability.
Histone methylation is a key epigenetic modification, which plays a balancing and regulating role in gene transcription and expression. Tumor markers of bone metastases targeted at histone methylation mainly include the following aspects. H3K9me3 is the triumphalist form of the 9th lysine of histone H3 and is a silencing marker for many genes. The loss or reduction of H3K9me3 in bone metastases may be related to its enhanced ability to metastasize and the difference in prognosis (145). H3K27me3 is the triumphalist form of the 27th lysine of histone H3, which plays an important role in cell growth and differentiation. Reduction of H3K27me3 in bone metastasis may lead to inhibition of apoptosis and the growth and metastasis of cancer cells (146). H3K4me3 is the triumphalist form of lysine at the fourth position of histone H3, which is a marker of enrichment in genes with high transcriptional activity. During the treatment of patients with bone metastases, prominent expression of H3K4me3 was associated with the prognosis and progression of bone metastases (147). In conclusion, the study of histone methylation tumor markers of bone metastasis provides a novel idea and means for the early detection and treatment of bone metastasis. Although there are still some challenges in the application, they are expected to be one of the principal markers of bone metastasis in the future.
6 Perspectives and future opportunities
This paper mainly introduces the commonly used clinical protein biomarkers, ncRNA, and liquid biopsy biomarkers. Each type has its specific advantages, limitations in the clinical application. Protein-based tumor biomarkers have been extensively studied and have a wide range of applications, including diagnosis, disease surveillance and therapeutic strategies. Numerous protein measurement techniques and automated methods have been rapidly developed, making high-throughput identification and measurement easy and fast. Proteins can be interfered with by external factors (such as diet and preparations), and in some cases of proteins may be non-specific, which can lead to false positives. So, the interpretation of the results does not necessarily reflect accurate. Compared with proteins, the structure and function of ncRNAs are still being studied, so understanding the role of ncRNAs and their detection techniques are limited. Some ncRNAs may be raised at similar levels in multiple tumor types and non-tumor diseases, so there may be some limitations in the differential diagnosis process. To sum up, these types of biomarkers have their peculiar advantages and disadvantages, and the future development will be different depending on the specific application. Among them, miRNA, as an emerging method, may be the future direction while further understanding its biological role and mechanism. Due to the wide variety of biomarkers, this study mainly elaborated protein, ncRNAs, liquid biopsy biomarkers and other studied biomarkers, which were mainly derived from serum plasma and tissue. Our team will conduct a more comprehensive and detailed description of such biomarkers in subsequent studies, so as to provide reference for the clinical application of biomarkers of bone metastases and the early diagnosis of diseases.
Future research on how to find new methods of screening and detecting biomarkers, and the set of cut-off value, etc., not only for detection but also for prognosis is needed. Firstly, large-scale prospective clinical studies are required. More large-scale prospective clinical studies are needed to confirm the sensitivity, specificity, and stability of different markers, as well as their feasibility for early detection, classification, and treatment of bone metastases. Secondly, combinations of multiple biomarkers can be studied. Combined with biomarkers of different types of bone metastases, a more accurate diagnosis and prediction model was established. In the process of integration, it is necessary to investigate the interaction, influence and cooperation among different biomarkers, and establish the corresponding bioinformatics model and algorithm combined with bioinformatics. Finally, multidisciplinary cooperation and communication is important. There is necessary to have closer collaboration among clinicians, basic scientists, bioinformatics specialists and engineers to leverage their expertise and skills to better support the research and application of markers for bone metastases.
In conclusion, in the future, the study of bone metastases tumor markers will gradually develop from a single biomarker study to a systematic and integrated research model, so as to more accurately and comprehensively understand the biological characteristics and clinical manifestations of bone metastases, promoting more significant progress in the diagnosis and treatment of bone metastases.
Author contributions
JL and HL conceived the research. YH and FZ conducted the study and drafted the manuscript, and they contributed equally to this work. YM, YL, YZ, NY, ML contributed to the acquisition, or interpretation of data and critically reviewed and revised the article for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (82004397), the Innovation Fund of National Clinical Research Center for Orthopedics, Sports Medicine & Rehabilitation (2021-NCRC-CXJJ-PY-13), Young Elite Scientists Sponsorship Program by CAST (2021-QNRC2-A06), and the Major Project of TCM research in Henan Province (2023ZY2136).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yin JJ, Pollock CB, Kelly K. Mechanisms of cancer metastases to the bone. Cell Res (2005) 15:57–62. doi: 10.1038/sj.cr.7290266
2. Jimenez-Andrade JM, Mantyh WG, Bloom AP, Ferng AS, Geffre CP, Mantyh PW. Bone cancer pain. Ann N Y Acad Sci (2010) 1198:173–81. doi: 10.1111/j.1749-6632.2009.05429.x
3. Coleman RE, Croucher PI, Padhani AR, Clézardin P, Chow E, Fallon M, et al. Bone metastases. Nat Rev Dis Primers (2020) 6:83. doi: 10.1038/s41572-020-00216-3
4. Alfranca A, Martinez-Cruzado L, Tornin J, Abarrategi A, Amaral T, de Alava E, et al. Bone microenvironment signals in osteosarcoma development. Cell Mol Life Sci (2015) 72:3097–113. doi: 10.1007/s00018-015-1918-y
5. Yip RKH, Rimes JS, Capaldo BD, Vaillant F, Mouchemore KA, Pal B, et al. Mammary tumour cells remodel the bone marrow vascular microenvironment to support metastases. Nat Commun (2021) 12:6920. doi: 10.1038/s41467-021-26556-6
6. Kruger TE, Miller AH, Godwin AK, Wang J. Bone sialoprotein and osteopontin in bone metastases of osteotropic cancers. Crit Rev Oncol Hematol (2014) 89:330–41. doi: 10.1016/j.critrevonc.2013.08.013
7. Li Z, Li LX, Diao YJ, Wang J, Ye Y, Hao XK. Identification of urinary exosomal miRNAs for the non-invasive diagnosis of prostate cancer. Cancer Manag Res (2021) 13:25–35. doi: 10.2147/cmar.S272140
8. Hirai T, Shinoda Y, Tateishi R, Asaoka Y, Uchino K, Wake T, et al. Early detection of bone metastases of hepatocellular carcinoma reduces bone fracture and paralysis. Jpn J Clin Oncol (2019) 49:529–36. doi: 10.1093/jjco/hyz028
9. Wang Y, Ding Y, Guo N, Wang S. MDSCs: key criminals of tumor pre-metastatic niche formation. Front Immunol (2019) 10:172. doi: 10.3389/fimmu.2019.00172
10. Shackleton M, Yuen K, Little AF, Schlicht S, McLachlan SA. Reliability of X-rays and bone scans for the assessment of changes in skeletal metastases from breast cancer. Intern Med J (2004) 34:615–20. doi: 10.1111/j.1445-5994.2004.00637.x
11. Wei Y, Xiao J, Zou L. Masticator space: CT and MRI of secondary tumor spread. AJR Am J Roentgenol (2007) 189:488–97. doi: 10.2214/ajr.07.2212
12. Rong J, Wang S, Ding Q, Yun M, Zheng Z, Ye S. Comparison of 18 FDG PET-CT and bone scintigraphy for detection of bone metastases in breast cancer patients. a meta-analysis. Surg Oncol (2013) 22:86–91. doi: 10.1016/j.suronc.2013.01.002
13. Dyrberg E, Hendel HW, Huynh THV, Klausen TW, Løgager VB, Madsen C, et al. (68) Ga-PSMA-PET/CT in comparison with (18)F-fluoride-PET/CT and whole-body MRI for the detection of bone metastases in patients with prostate cancer: a prospective diagnostic accuracy study. Eur Radiol (2019) 29:1221–30. doi: 10.1007/s00330-018-5682-x
14. Donners R, Figueiredo I, Tunariu N, Blackledge M, Koh DM, de la Maza M, et al. Multiparametric bone MRI can improve CT-guided bone biopsy target selection in cancer patients and increase diagnostic yield and feasibility of next-generation tumour sequencing. Eur Radiol (2022) 32:4647–56. doi: 10.1007/s00330-022-08536-6
15. Aryal A, Kumar VS, Shamim SA, Gamanagatti S, Khan SA. What is the comparative ability of 18F-FDG PET/CT, 99mTc-MDP skeletal scintigraphy, and whole-body MRI as a staging investigation to detect skeletal metastases in patients with osteosarcoma and Ewing sarcoma? Clin Orthop Relat Res (2021) 479:1768–79. doi: 10.1097/corr.0000000000001681
16. Ottosson F, Baco E, Lauritzen PM, Rud E. The prevalence and locations of bone metastases using whole-body MRI in treatment-naïve intermediate- and high-risk prostate cancer. Eur Radiol (2021) 31:2747–53. doi: 10.1007/s00330-020-07363-x
17. Tayob N, Kanwal F, Alsarraj A, Hernaez R, El-Serag HB. The performance of AFP, AFP-3, DCP as biomarkers for detection of hepatocellular carcinoma (HCC): a phase 3 biobiomarker study in the united states. Clin Gastroenterol Hepatol (2023) 21(2):415–23. doi: 10.1016/j.cgh.2022.01.047
18. Park SJ, Jang JY, Jeong SW, Cho YK, Lee SH, Kim SG, et al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Med (Baltimore) (2017) 96(11):e5811. doi: 10.1097/MD.0000000000005811
19. Zhu AX, Dayyani F, Yen CJ, Ren Z, Bai Y, Meng Z, et al. Alpha-fetoprotein as a potential surrogate biobiomarker for atezolizumab + bevacizumab treatment of hepatocellular carcinoma. Clin Cancer Res (2022) 28(16):3537–45. doi: 10.1158/1078-0432.CCR-21-3275
20. Luo P, Yin P, Hua R, Tan Y, Li Z, Qiu G, et al. Multicenter serum metabolite biobiomarker identification study for the early detection of hepatocellular carcinoma. Hepatology (2018) 67(2):662–75. doi: 10.1002/hep.29561
21. Chalasani NP, Ramasubramanian TS, Bhattacharya A, Olson MC, Edwards V DK, Roberts LR, et al. A novel blood-based panel of methylated DNA and protein biomarkers for detection of early-stage hepatocellular carcinoma. Clin Gastroenterol Hepatol (2021) 19(12):2597–2605.e4. doi: 10.1016/j.cgh.2020.08.065
22. Chen D, Li Z, Song Q, Qian L, Xie B, Zhu J. Clinicopathological features and differential diagnosis of hepatocellular carcinoma in extrahepatic metastases. Med (Baltimore). (2018) 97(50):e13356. doi: 10.1097/MD.0000000000013356
23. Calaminus G, Schneider DT, Bökkerink JP, Gadner H, Harms D, Willers R, et al. Prognostic value of tumor size, metastases, extension into bone, and increased tumor biomarker in children with malignant sacrococcygeal germ cell tumors: a prospective evaluation of 71 patients treated in the German cooperative protocols maligne keimzelltumoren (MAKEI) 83/86 and MAKEI 89. J Clin Oncol (2003) 21(5):781–6. doi: 10.1200/JCO.2003.03.125
24. Chen XY, Lan M, Zhou Y, Chen WZ, Hu D, Liu JM, et al. Risk factors for bone metastasis from renal cell cancer. J Bone Oncol (2017) 9:29–33. doi: 10.1016/j.jbo.2017.10.004
25. Tamiya M, Tokunaga S, Okada H, Suzuki H, Kobayashi M, Sasada S, et al. Prospective study of urinary and serum cross-linked n-telopeptide of type I collagen (NTx) for diagnosis of bone metastasis in patients with lung cancer. Clin Lung Cancer. (2013) 14(4):364–9. doi: 10.1016/j.cllc.2012.11.006
26. Lumachi F, Santeufemia DA, Del Conte A, Mazza F, Tozzoli R, Chiara GB, et al. Carboxy-terminal telopeptide (CTX) and amino-terminal propeptide (PINP) of type I collagen as biomarkers of bone metastases in patients with non-small cell lung cancer. Anticancer Res (2013) 33(6):2593–6.
27. Aruga A, Koizumi M, Hotta R, Takahashi S, Ogata E. Usefulness of bone metabolic biomarkers in the diagnosis and follow-up of bone metastasis from lung cancer. Br J Cancer (1997) 76(6):760–4. doi: 10.1038/bjc.(1997).458
28. Horiguchi T, Tachikawa S, Kondo R, Hirose M, Teruya S, Ishibashi A, et al. Usefulness of serum carboxy-terminal telopeptide of type I collagen (ICTP) as a biomarker of bone metastasis from lung cancer. Jpn J Clin Oncol (2000) 30(4):174–9. doi: 10.1093/jjco/hyd043
29. Min JW, Um SW, Yim JJ, Yoo CG, Han SK, Shim YS, et al. The role of whole-body FDG PET/CT, Tc 99m MDP bone scintigraphy, and serum alkaline phosphatase in detecting bone metastasis in patients with newly diagnosed lung cancer. J Korean Med Sci (2009) 24(2):275–80. doi: 10.3346/jkms.2009.24.2.275
30. Tang C, Liu Y, Qin H, Li X, Guo W, Li J, et al. Clinical significance of serum BAP, TRACP 5b and ICTP as bone metabolic biomarkers for bone metastasis screening in lung cancer patients. Clin Chim Acta (2013) 426:102–7. doi: 10.1016/j.cca.2013.09.011
31. Yokoyama T, Yamamoto M, Shima K, Suzuki K, Sako C, Ito G, et al. Clinical usefulness of serum pyridinoline cross-linked carboxyterminal telopeptide of type I collagen for diagnosis of bone metastases in patients with primary lung cancer. Respirology (2005) 10:300–304. doi: 10.1111/j.1440-1843.2005.00713.x
32. Yao NS, Wu YY, Janckila AJ, Ku CH, Hsieh AT, Ho CL, et al. Serum tartrate-resistant acid phosphatase 5b (TRACP5b) activity as a biobiomarker for bone metastasis in non-small cell lung cancer patients. Clin Chim Acta (2011) 412(1-2):181–5. doi: 10.1016/j.cca.2010.09.038
33. Modoni S, Calò E, Nardella G, Ritrovato G, Frusciante V. PSA and bone scintigraphy. Int J Biol biomarkers. (1997) 12(4):158–61. doi: 10.1177/172460089701200404
34. Wei RJ, Li TY, Yang XC, Jia N, Yang XL, Song HB. Serum levels of PSA, ALP, ICTP, and BSP in prostate cancer patients and the significance of ROC curve in the diagnosis of prostate cancer bone metastases. Genet Mol Res (2016) 15(2):gmr7707. doi: 10.4238/gmr.15027707
35. Szot W, Kostkiewicz M, Zając J, Owoc A, Bojar I. Prostate cancer in patients from rural and suburban areas–PSA value, Gleason score and presence of metastases in bone scan. Ann Agric Environ Med (2014) 21(4):888–92. doi: 10.5604/12321966.1129953
36. Zissimopoulos A, Stellos K, Matthaios D, Petrakis G, Parmenopoulou V, Babatsikou F, et al. Type I collagen biomarkers in the diagnosis of bone metastases in breast cancer, lung cancer, urinary bladder cancer and prostate cancer. comparison to CEA, CA 15-3, PSA and bone scintigraphy. J BUON. (2009) 14(3):463–72.
37. Zissimopoulos A, Stellos C, Petrakis G, Baziotis N. In process citation correlation of procollagen (I) with prostate specific antigen and bone scan for the diagnosis of bone metastases in patients with prostate carcinoma. Hell J Nucl Med (2004) 7(3):162–7.
38. Tähtelä R, Thölix E. Serum concentrations of type I collagen carboxyterminal telopeptide (ICTP) and type I procollagen carboxy-and aminoterminal propeptides (PICP, PINP) as biomarkers of metastatic bone disease in breast cancer. Anticancer Res (1996) 16(4B):2289–93.
39. Wang W, Xu X, Tian B, Wang Y, Du L, Sun T, et al. The diagnostic value of serum tumor biomarkers CEA, CA19-9, CA125, CA15-3, and TPS in metastatic breast cancer. Clin Chim Acta (2017) 470:51–5. doi: 10.1016/j.cca.2017.04.023
40. Rosiek V, Wójcik-Giertuga M, Kos-Kudła B. Serum tumor biomarkers for detection of bone metastases in patients with lung neuroendocrine neoplasms". Cancer Treat Res Commun (2022) 31:100533. doi: 10.1016/j.ctarc.2022.100533
41. Jiang M, Chen P, Zhang X, Guo X, Gao Q, Ma L, et al. Metabolic phenotypes, serum tumor biomarkers, and histopathological subtypes in predicting bone metastasis: analysis of 695 patients with lung cancer in China. Quant Imaging Med Surg (2023) 13(3):1642–54. doi: 10.21037/qims-22-741
42. Mercatali L, Ibrahim T, Sacanna E, Flamini E, Scarpi E, Calistri D, et al. Bone metastases detection by circulating biomarkers: OPG and RANK-l. Int J Oncol (2011) 39(1):255–61. doi: 10.3892/ijo.2011.1001
43. Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int (2019) 39:2214–29. doi: 10.1111/liv.14223
44. Evdokimova VN, Butterfield LH. Alpha-fetoprotein and other tumour-associated antigens for immunotherapy of hepatocellular cancer. Expert Opin Biol Ther (2008) 8:325–36. doi: 10.1517/14712598.8.3.325
45. Okunaka T, Kato H, Konaka C, Yamamoto H, Furukawa K. Primary lung cancer producing alpha-fetoprotein. Ann Thorac Surg (1992) 53:151–2. doi: 10.1016/0003-4975(92)90778-3
46. Xiong S, Tang K, Luo F. An extensive surgical resection in stage T4 small cell lung cancer with cardiac invasion: a case report and literature review. Ann Med Surg (Lond) (2022) 81:104448. doi: 10.1016/j.amsu.2022.104448
47. Tonyali O, Gonullu O, Ozturk MA, Kosif A, Civi OG. Hepatoid adenocarcinoma of the lung and the review of the literature. J Oncol Pharm Pract (2020) 26:1505–10. doi: 10.1177/1078155220903360
48. Aass N, Klepp O, Cavallin-Stahl E, Dahl O, Wicklund H, Unsgaard B, et al. Prognostic factors in unselected patients with nonseminomatous metastatic testicular cancer: a multicenter experience. J Clin Oncol (1991) 9:818–26. doi: 10.1200/jco.1991.9.5.818
49. Beauchemin N, Arabzadeh A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastases. Cancer Metastases Rev (2013) 32:643–71. doi: 10.1007/s10555-013-9444-6
50. Shibata C, Nakano T, Yasumoto A, Mitamura A, Sawada K, Ogawa H, et al. Comparison of CEA and CA19-9 as a predictive factor for recurrence after curative gastrectomy in gastric cancer. BMC Surg (2022) 22:213. doi: 10.1186/s12893-022-01667-z
51. Teijeira A, Migueliz I, Garasa S, Karanikas V, Luri C, Cirella A, et al. Three-dimensional colon cancer organoids model the response to CEA-CD3 T-cell engagers. Theranostics (2022) 12:1373–87. doi: 10.7150/thno.63359
52. Ayan AK, Erdemci B, Orsal E, Bayraktutan Z, Akpinar E, Topcu A, et al. Is there any correlation between levels of serum ostepontin, CEA, and FDG uptake in lung cancer patients with bone metastases? Rev Esp Med Nucl Imagen Mol (2016) 35:102–6. doi: 10.1016/j.remn.2015.09.002
53. Numata T, Endo T, Yanai H, Ota K, Yamamoto Y, Shimizu K, et al. Serum CEA and CYFRA levels in ALK-rearranged NSCLC patients: correlation with distant metastases. In Vivo (2020) 34:2095–100. doi: 10.21873/invivo.12013
54. Chai X, Yinwang E, Wang Z, Wang Z, Xue Y, Li B, et al. Predictive and prognostic biomarkers for lung cancer bone metastases and their therapeutic value. Front Oncol (2021) 11:692788. doi: 10.3389/fonc.2021.692788
55. Ge YW, Liu XL, Yu DG, Zhu ZA, Ke QF, Mao YQ, et al. Graphene-modified CePO4 nanorods effectively treat breast cancer-induced bone metastases and regulate macrophage polarization to improve osteo-inductive ability. J Nanobiotechnology (2021) 19:11. doi: 10.1186/s12951-020-00753-9
56. Salter RS, Fitchen J. Evaluation of a chemiluminescence method for measuring alkaline phosphatase activity in whole milk of multiple species and bovine dairy drinks: interlaboratory study. J AOAC Int (2006) 89:1061–70. doi: 10.1093/jaoac/89.4.1061
57. Liu Z, Dong N, Hui H, Wang Y, Liu F, Xu L, et al. Endothelial cell-derived tetrahydrobiopterin prevents aortic valve calcification. Eur Heart J (2022) 43:1652–64. doi: 10.1093/eurheartj/ehac037
58. Oketch-Rabah HA, Roe AL, Rider CV, Bonkovsky HL, Giancaspro GI, Navarro V, et al. United states pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol Rep (2020) 7:386–402. doi: 10.1016/j.toxrep.2020.02.008
59. Barry MJ, Simmons LH. Prevention of prostate cancer morbidity and mortality: primary prevention and early detection. Med Clin North Am (2017) 101:787–806. doi: 10.1016/j.mcna.2017.03.009
60. Maestroni U, Cavalieri DM, Campobasso D, Guarino G, Ziglioli F. PSA-IgM and iXip in the diagnosis and management of prostate cancer: clinical relevance and future potential. a review. Acta BioMed (2022) 92:e2021344. doi: 10.23750/abm.v92i6.12058
61. Conteduca V, Oromendia C, Eng KW, Bareja R, Sigouros M, Molina A, et al. Clinical features of neuroendocrine prostate cancer. Eur J Cancer. (2019) 121:7–18. doi: 10.1016/j.ejca.2019.08.011
62. Ren W, Hou J, Yang C, Wang H, Wu S, Wu Y, et al. Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J Exp Clin Cancer Res (2019) 38:62. doi: 10.1186/s13046-019-1027-0
63. Xu Y, Zhang P, Zhang K, Huang C. The application of CA72-4 in the diagnosis, prognosis, and treatment of gastric cancer. Biochim Biophys Acta Rev Cancer (2021) 1876:188634. doi: 10.1016/j.bbcan.2021.188634
64. Healthcare Engineering JO. Retracted: effect of apatinib combined with seggio on the expression of serum AFP and CA724 and long-term survival rate in patients with advanced gastric cancer undergoing comfortable nursing intervention. J Healthc Eng (2022) 2022:9756408. doi: 10.1155/2022/9756408
65. Pan Q, Law COK, Yung MMH, Han KC, Pon YL, Lau TCK. Novel RNA aptamers targeting gastrointestinal cancer biomarkers CEA, CA50 and CA72-4 with superior affinity and specificity. PloS One (2018) 13:e0198980. doi: 10.1371/journal.pone.0198980
66. Huang H, Yu X, Han X, Hao J, Zhao J, Bebek G, et al. Piwil1 regulates glioma stem cell maintenance and glioblastoma progression. Cell Rep (2021) 34:108522. doi: 10.1016/j.celrep.2020.108522
67. Zhang Y, Yang J, Li H, Wu Y, Zhang H, Chen W. Tumor biomarkers CA19-9, CA242 and CEA in the diagnosis of pancreatic cancer: a meta-analysis. Int J Clin Exp Med (2015) 8:11683–91. doi: 10.1136/bmjopen-2017-018175
68. Dou H, Sun G, Zhang L. CA242 as a biobiomarker for pancreatic cancer and other diseases. Prog Mol Biol Transl Sci (2019) 162:229–39. doi: 10.1016/bs.pmbts.2018.12.007
69. Huang P, Chen A, He W, Li Z, Zhang G, Liu Z, et al. BMP-2 induces EMT and breast cancer stemness through Rb and CD44. Cell Death Discovery (2017) 3:17039. doi: 10.1038/cddiscovery.2017.39
70. Iaquinta MR, Lanzillotti C, Mazziotta C, Bononi I, Frontini F, Mazzoni E, et al. The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies. Theranostics (2021) 11:6573–91. doi: 10.7150/thno.55664
71. Puppo M, Taipaleenmäki H, Hesse E, Clézardin P. Non-coding RNAs in bone remodelling and bone metastases: mechanisms of action and translational relevance. Br J Pharmacol (2021) 178:1936–54. doi: 10.1111/bph.14836
72. Croset M, Santini D, Iuliani M, Fioramonti M, Zoccoli A, Vincenzi B, et al. MicroRNAs and bone metastases: a new challenge. Molecules (2014) 19:10115–28. doi: 10.3390/molecules190710115
73. Nugent M. MicroRNA function and dysregulation in bone tumors: the evidence to date. Cancer Manag Res (2014) 6:15–25. doi: 10.2147/cmar.S53928
74. Lang J, Zhao Q, He Y, Yu X. Bone turnover biomarkers and novel biomarkers in lung cancer bone metastases. biomarkers (2018) 23:518–26. doi: 10.1080/1354750x.2018.1463566
75. Zhao Q, Li P, Ma J, Yu X. MicroRNAs in lung cancer and lung cancer bone metastases: biomarkers for early diagnosis and targets for treatment. Recent Pat Anticancer Drug Discovery (2015) 10:182–200. doi: 10.2174/1574892810666150120163617
76. Croset M, Pantano F, Kan CWS, Bonnelye E, Descotes F, Alix-Panabières C, et al. miRNA-30 family members inhibit breast cancer invasion, osteomimicry, and bone destruction by directly targeting multiple bone metastases-associated genes. Cancer Res (2018) 78:5259–73. doi: 10.1158/0008-5472.Can-17-3058
77. Puppo M, Valluru MK, Clézardin P. MicroRNAs and their roles in breast cancer bone metastases. Curr Osteoporos Rep (2021) 19:256–63. doi: 10.1007/s11914-021-00677-9
78. Zhang YK, Zhu WY, He JY, Chen DD, Huang YY, Le HB, et al. miRNAs expression profiling to distinguish lung squamous-cell carcinoma from adenocarcinoma subtypes. J Cancer Res Clin Oncol (2012) 138:1641–50. doi: 10.1007/s00432-012-1240-0
79. Zhao B, Han H, Chen J, Zhang Z, Li S, Fang F, et al. MicroRNA let-7c inhibits migration and invasion of human non-small cell lung cancer by targeting ITGB3 and MAP4K3. Cancer Lett (2014) 342:43–51. doi: 10.1016/j.canlet.2013.08.030
80. Bao X, Ren T, Huang Y, Wang S, Zhang F, Liu K, et al. Induction of the mesenchymal to epithelial transition by demethylation-activated microRNA-125b is involved in the anti-migration/invasion effects of arsenic trioxide on human chondrosarcoma. J Exp Clin Cancer Res (2016) 35:129. doi: 10.1186/s13046-016-0407-y
81. Maroni P, Bendinelli P, Matteucci E, Desiderio MA. The therapeutic effect of miR-125b is enhanced by the prostaglandin endoperoxide synthase 2/cyclooxygenase 2 blockade and hampers ETS1 in the context of the microenvironment of bone metastases. Cell Death Dis (2018) 9:472. doi: 10.1038/s41419-018-0499-8
82. Yuan J, Chen L, Chen X, Sun W, Zhou X. Identification of serum microRNA-21 as a biobiomarker for chemosensitivity and prognosis in human osteosarcoma. J Int Med Res (2012) 40:2090–7. doi: 10.1177/030006051204000606
83. Bonci D, Coppola V, Patrizii M, Addario A, Cannistraci A, Francescangeli F, et al. A microRNA code for prostate cancer metastases. Oncogene (2016) 35:1180–92. doi: 10.1038/onc.2015.176
84. Ren X, Shen Y, Zheng S, Liu J, Jiang X. miR-21 predicts poor prognosis in patients with osteosarcoma. Br J BioMed Sci (2016) 73:158–62. doi: 10.1080/09674845.2016.1220710
85. He B, Zhao Z, Cai Q, Zhang Y, Zhang P, Shi S, et al. miRNA-based biomarkers, therapies, and resistance in cancer. Int J Biol Sci (2020) 16:2628–47. doi: 10.7150/ijbs.47203
86. Kara G, Calin GA, Ozpolat B. RNAi-based therapeutics and tumor targeted delivery in cancer. Adv Drug Delivery Rev (2022) 182:114113. doi: 10.1016/j.addr.2022.114113
87. Mishra S, Yadav T, Rani V. Exploring miRNA-based approaches in cancer diagnostics and therapeutics. Crit Rev Oncol Hematol (2016) 98:12–23. doi: 10.1016/j.critrevonc.2015.10.003
88. Zou P, Zhu M, Lian C, Wang J, Chen Z, Zhang X, et al. miR-192-5p suppresses the progression of lung cancer bone metastasis by targeting TRIM44. Sci Rep (2019) 9(1):19619. doi: 10.1038/s41598-019-56018-5
89. Gong M, Ma J, Guillemette R, Zhou M, Yang Y, Yang Y, et al. miR-335 inhibits small cell lung cancer bone metastases via IGF-IR and RANKL pathways. Mol Cancer Res (2014) 12(1):101–10. doi: 10.1158/1541-7786.MCR-13-0136
90. Xu S, Yang F, Liu R, Li X, Fan H, Liu J, et al. Serum microRNA-139-5p is downregulated in lung cancer patients with lytic bone metastasis. Oncol Rep (2018) 39(5):2376–84. doi: 10.3892/or.2018.6316
91. Roberto GM, Delsin LEA, Vieira GM, Silva MO, Hakime RG, Gava NF, et al. ROCK1-PredictedmicroRNAs dysregulation contributes to tumor progression in Ewing sarcoma. Pathol Oncol Res (2020) 26(1):133–9. doi: 10.1007/s12253-017-0374-4
92. Vimalraj S, Miranda PJ, Ramyakrishna B, Selvamurugan N. Regulation of breast cancer and bone metastasis by microRNAs. Dis biomarkers. (2013) 35(5):369–87. doi: 10.1155/2013/451248
93. Zhao FL, Hu GD, Wang XF, Zhang XH, Zhang YK, Yu ZS. Serum overexpression of microRNA-10b in patients with bone metastatic primary breast cancer. J Int Med Res (2012) 40(3):859–66. doi: 10.1177/147323001204000304
94. Arabi L, Gsponer JR, Smida J, Nathrath M, Perrina V, Jundt G, et al. Upregulation of the miR-17-92 cluster and its two paraloga in osteosarcoma - reasons and consequences. Genes Cancer. (2014) 5(1-2):56–63. doi: 10.18632/genesandcancer.6
95. Xu Q, Li P, Chen X, Zong L, Jiang Z, Nan L, et al. miR-221/222 induces pancreatic cancer progression through the regulation of matrix metalloproteinases. Oncotarget (2015) 6:14153–64. doi: 10.18632/oncotarget.3686
96. Liu Z., Liu Z., Zhang Y., Li Y., Liu B., Zhang K. miR-24 represses metastasis of human osteosarcoma cells by targeting Ack1 via AKT/MMPs pathway. Biochem Biophys Res Commun (2017) 486(2):211–7. doi: 10.1016/j.bbrc.2017.02.045
97. Koshkina N, Yang Y, Kleinerman ES. The Fas/FasL signaling pathway: its role in the metastatic process and as a target for treating osteosarcoma lung metastases. Adv Exp Med Biol (2020) 1258:177–87. doi: 10.1007/978-3-030-43085-6_12
98. Cui XB, Li S, Li TT, Peng H, Jin TT, Zhang SM, et al. Targeting oncogenic PLCE1 by miR-145 impairs tumor proliferation and metastases of esophageal squamous cell carcinoma. Oncotarget (2016) 7:1777–95. doi: 10.18632/oncotarget.6499
99. Zhao W, Geng D, Li S, Chen Z, Sun M. LncRNA HOTAIR influences cell growth, migration, invasion, and apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer. Cancer Med (2018) 7(3):842–55. doi: 10.1002/cam4.1353
100. Fang C, Wang X, Guo D, Fang R, Zhu T. Circular RNA CircITGA7 promotes tumorigenesis of osteosarcoma via miR-370/PIM1 axis. Comput Math Methods Med (2020) 2020:1367576. doi: 10.1155/2020/1367576
101. Li F, Li H, Hou Y. Identification and analysis of survival-associated ceRNA triplets in prostate adenocarcinoma. Oncol Lett (2019) 18(4):4040–7. doi: 10.3892/ol.2019.10752
102. Liu M., Mo F., Song X., He Y., Yuan Y., Yan J., et al. Exosomal hsa-miR-21-5p is a biomarker for breast cancer diagnosis. PeerJ (2021) 9:e12147. doi: 10.7717/peerj.12147
103. Zhang HL, Qin XJ, Cao DL, Zhu Y, Yao XD, Zhang SL, et al. An elevated serum miR-141 level in patients with bone-metastatic prostate cancer is correlated with more bone lesions. Asian J Androl (2013) 15:231–5. doi: 10.1038/aja.2012.116
104. Ye Y, Li SL, Ma YY, Diao YJ, Yang L, Su MQ, et al. Exosomal miR-141-3p regulates osteoblast activity to promote the osteoblastic metastases of prostate cancer. Oncotarget (2017) 8:94834–49. doi: 10.18632/oncotarget.22014
105. Guo X, Han T, Hu P, Guo X, Zhu C, Wang Y, et al. Five microRNAs in serum as potential biomarkers for prostate cancer risk assessment and therapeutic intervention. Int Urol Nephrol (2018) 50:2193–200. doi: 10.1007/s11255-018-2009-4
106. Yang G, Lu Z, Meng F, Wan Y, Zhang L, Xu Q, et al. Circulating miR-141 as a potential biobiomarker for diagnosis, prognosis and therapeutic targets in gallbladder cancer. Sci Rep (2022) 12:10072. doi: 10.1038/s41598-022-13430-8
107. Yang S, Zhang W, Cai M, Zhang Y, Jin F, Yan S, et al. Suppression of bone resorption by miR-141 in aged rhesus monkeys. J Bone Miner Res (2018) 33:1799–812. doi: 10.1002/jbmr.3479
108. Tian L, Sun S, Li W, Yuan L, Wang X. Down-regulated microRNA-141 facilitates osteoblast activity and inhibits osteoclast activity to ameliorate osteonecrosis of the femoral head via up-regulating TGF-β2. Cell Cycle (2020) 19:772–86. doi: 10.1080/15384101.2020.1731053
109. Wang CY, Li SY, Xiao YX, Zhen L, Wei XG, Tang XB, et al. miR-141-3p affects β-catenin signaling and apoptosis by targeting Ubtd2 in rats with anorectal malformations. Ann N Y Acad Sci (2022) 1518:315–27. doi: 10.1111/nyas.14924
110. Ni Z, Shen Y, Wang W, Cheng X, Fu Y. miR-141-5p affects the cell proliferation and apoptosis by targeting BTG1 in cervical cancer. Cancer Biother Radiopharm (2021). doi: 10.1089/cbr.2021.0227
111. Liu M, Sun W, Liu Y, Dong X. The role of lncRNA MALAT1 in bone metastasis in patients with non-small cell lung cancer. Oncol Rep (2016) 36(3):1679–85. doi: 10.3892/or.2016.4909
112. Chang X, Zhang H, Yang Q, Pang L. LncRNA SOX2OT affects cervical cancer cell growth, migration and invasion by regulating SOX2. Cell Cycle (2020) 19:1391–403. doi: 10.1080/15384101.2020.1750812
113. Chen K, Yu B, Liao J. LncRNA SOX2OT alleviates mesangial cell proliferation and fibrosis in diabetic nephropathy via Akt/mTOR-mediated autophagy. Mol Med (2021) 27:71. doi: 10.1186/s10020-021-00310-6
114. Stewart CL, Warner S, Ito K, Raoof M, Wu GX, Kessler J, et al. Cytoreduction for colorectal metastases: liver, lung, peritoneum, lymph nodes, bone, brain. when does it palliate, prolong survival, and potentially cure? Curr Probl Surg (2018) 55:330–79. doi: 10.1067/j.cpsurg.2018.08.004
115. Wang N, Liu F, Xi W, Jiang J, Xu Y, Guan B, et al. Development and validation of risk and prognostic nomograms for bone metastases in Chinese advanced colorectal cancer patients. Ann Transl Med (2021) 9:875. doi: 10.21037/atm-21-2550
116. Zhao C, Zhang Z, Hu X, Zhang L, Liu Y, Wang Y, et al. Hyaluronic acid correlates with bone metastasis and predicts poor prognosis in small-cell lung cancer patients. Front Endocrinol (Lausanne). (2022) 12:785192. doi: 10.3389/fendo.2021.785192
117. Loreth D., Schuette M., Zinke J., Mohme M., Piffko A., Schneegans S., et al. CD74 and CD44 Expression on CTCs in Cancer Patients with Brain Metastasis. Int. J. Mol. Sci (2021) 22(13):6993. doi: 10.3390/ijms22136993
118. Zhang X., Chen S.B., Chen J.X., Wen J., Yang H., Xie M.R., et al. CK19 mRNA expression in the bone marrow of patients with esophageal squamous cell carcinoma and its clinical significance. Dis Esophagus (2010) 22(13):6993. doi: 10.3390/ijms22136993.x
119. Jiwa L. S., van Diest P. J., Hoefnagel L. D., Wesseling J., Wesseling P., Moelans C. B. Upregulation of Claudin-4, CAIX and GLUT-1 in distant breast cancer metastases. BMC Cancer (2014) 14:864. doi: 10.1186/1471-2407-14-864
120. Ma W, Li T, Wu S, Li J, Wang X, Li H. LOX and ACSL5 as potential relapse biomarkers for pancreatic cancer patients. Cancer Biol Ther (2019) 20(6):787–98. doi: 10.1080/15384047.2018.1564565
121. Mehrotra J, Vali M, McVeigh M, Kominsky SL, Fackler MJ, Lahti-Domenici J, et al. Very high frequency of hypermethylated genes in breast cancer metastasis to the bone, brain, and lung. Clin Cancer Res (2004) 10(9):3104–9. doi: 10.1158/1078-0432.ccr-03-0118
122. Barata P, Agarwal N, Nussenzveig R, Gerendash B, Jaeger E, Hatton W, et al. Clinical activity of pembrolizumab in metastatic prostate cancer with microsatellite instability high (MSI-h) detected by circulating tumor DNA. J Immunother Cancer. (2020) 8(2):e001065. doi: 10.1136/jitc-2020-001065
123. Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells - mechanisms of immune surveillance and escape. Nat Rev Clin Oncol (2017) 14:155–67. doi: 10.1038/nrclinonc.2016.144
124. Garcés JJ, Cedena MT, Puig N, Burgos L, Perez JJ, Cordon L, et al. Circulating tumor cells for the staging of patients with newly diagnosed transplant-eligible multiple myeloma. J Clin Oncol (2022) 40:3151–61. doi: 10.1200/jco.21.01365
125. Iuliani M, Simonetti S, Ribelli G, Napolitano A, Pantano F, Vincenzi B, et al. Current and emerging biomarkers predicting bone metastases development. Front Oncol (2020) 10:789. doi: 10.3389/fonc.2020.00789
126. Leblanc R, Peyruchaud O. Metastases: new functional implications of platelets and megakaryocytes. Blood (2016) 128:24–31. doi: 10.1182/blood-2016-01-636399
127. Feng S, Wu ZX, Zhao Z, Liu J, Sun K, Guo C, et al. Engineering of bone- and CD44-Dual-Targeting redox-sensitive liposomes for the treatment of orthotopic osteosarcoma. ACS Appl Mater Interfaces (2019) 11:7357–68. doi: 10.1021/acsami.8b18820
128. Sun X, Li K, Hase M, Zha R, Feng Y, Li BY, et al. Suppression of breast cancer-associated bone loss with osteoblast proteomes via Hsp90ab1/moesin-mediated inhibition of TGFβ/FN1/CD44 signaling. Theranostics (2022) 12:929–43. doi: 10.7150/thno.66148
129. Pang X, Gong K, Zhang X, Wu S, Cui Y, Qian BZ. Osteopontin as a multifaceted driver of bone metastases and drug resistance. Pharmacol Res (2019) 144:235–44. doi: 10.1016/j.phrs.2019.04.030
130. Niu Y, Yang H, Yu Z, Gao C, Ji S, Yan J, et al. Intervention with the bone-associated tumor vicious cycle through dual-protein therapeutics for treatment of skeletal-related events and bone metastases. ACS Nano (2022) 16:2209–23. doi: 10.1021/acsnano.1c08269
131. Liu L, Zhang C, Wang J, Liu X, Qu H, Zhang G, et al. A high level of lncFGD5-AS1 inhibits epithelial-to-Mesenchymal transition by regulating the miR-196a-5p/SMAD6/BMP axis in gastric cancer. BMC Cancer (2021) 21:453. doi: 10.1186/s12885-021-08192-x
132. Yu L, Sui B, Fan W, Lei L, Zhou L, Yang L, et al. Exosomes derived from osteogenic tumor activate osteoclast differentiation and concurrently inhibit osteogenesis by transferring COL1A1-targeting miRNA-92a-1-5p. J Extracell Vesicles (2021) 10:e12056. doi: 10.1002/jev2.12056
133. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells (2019) 8(7):727. doi: 10.3390/cells8070727
134. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science (2020) 367(6478):eaau6977. doi: 10.1126/science.aau6977
135. O'Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA Delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol (2020) 21(10):585–606. doi: 10.1038/s41580-020-0251-y
136. Wu K, Feng J, Lyu F, Xing F, Sharma S, Liu Y, et al. Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastases of estrogen receptor-positive breast cancer. Nat Commun (2021) 12:5196. doi: 10.1038/s41467-021-25473-y
137. Medeiros B, Allan AL. Molecular mechanisms of breast cancer metastases to the lung: clinical and experimental perspectives. Int J Mol Sci (2019) 20(9):2272. doi: 10.3390/ijms20092272
138. Akoto T, Saini S. Role of exosomes in prostate cancer metastases. Int J Mol Sci (2021) 22(7):3528. doi: 10.3390/ijms22073528
139. Colletti M, Tomao L, Galardi A, Paolini A, Di Paolo V, De Stefanis C, et al. Neuroblastoma-secreted exosomes carrying miR-375 promote osteogenic differentiation of bone-marrow mesenchymal stromal cells. J Extracell Vesicles (2020) 9:1774144. doi: 10.1080/20013078.2020.1774144
140. Ge J, Liu M, Zhang Y, Xie L, Shi Z, Wang G. SNHG10/miR-141-3p/WTAP axis promotes osteosarcoma proliferation and migration. J Biochem Mol Toxicol (2022) 36:e23031. doi: 10.1002/jbt.23031
141. Warton K, Samimi G. Methylation of cell-free circulating DNA in the diagnosis of cancer. Front Mol Biosci (2015) 2:13. doi: 10.3389/fmolb.2015.00013
142. Palanca-Ballester C, Rodriguez-Casanova A, Torres S, Calabuig-Fariñas S, Exposito F, Serrano D, et al. Cancer epigenetic biomarkers in liquid biopsy for high incidence malignancies. Cancers (Basel). (2021) 13(12):3016. doi: 10.3390/cancers13123016
143. Jiang W, Kai J, Li D, Wei Z, Wang Y, Wang W. lncRNA HOXB-AS3 exacerbates proliferation, migration, and invasion of lung cancer via activating the PI3K-AKT pathway. J Cell Physiol (2020) 235(10):7194–203. doi: 10.1002/jcp.29618
144. Shi Z, Zhang H, Jie S, Yang X, Huang Q, Mao Y, et al. Long non-coding RNA SNHG8 promotes prostate cancer progression through repressing miR-384 and up-regulating HOXB7. J Gene Med (2021) 23(3):e3309. doi: 10.1002/jgm.3309
145. Chen M, Jiang Y, Sun Y. KDM4A-mediated histone demethylation of SLC7A11 inhibits cell ferroptosis in osteosarcoma. Biochem Biophys Res Commun (2021) 550:77–83. doi: 10.1016/j.bbrc.2021.02.137
146. Deligezer U, Yaman F, Darendeliler E, Dizdar Y, Holdenrieder S, Kovancilar M, et al. Post-treatment circulating plasma BMP6 mRNA and H3K27 methylation levels discriminate metastatic prostate cancer from localized disease. Clin Chim Acta (2010) 411(19-20):1452–6. doi: 10.1016/j.cca.2010.05.040
Keywords: bone metastases, biomarkers, ncRNAs, circulating tumor cells, exosome
Citation: Hao Y, Zhang F, Ma Y, Luo Y, Zhang Y, Yang N, Liu M, Liu H and Li J (2023) Potential biomarkers for the early detection of bone metastases. Front. Oncol. 13:1188357. doi: 10.3389/fonc.2023.1188357
Received: 17 March 2023; Accepted: 01 June 2023;
Published: 19 June 2023.
Edited by:
Feifei Pu, Huazhong University of Science and Technology, ChinaReviewed by:
Junfei Guo, Third Hospital of Hebei Medical University, ChinaYanxia Chen, Second Affiliated Hospital of Nanchang University, China
Jing Chen, Nanjing University of Chinese Medicine, China
Copyright © 2023 Hao, Zhang, Ma, Luo, Zhang, Yang, Liu, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jitian Li, jitianlee@hotmail.com; Hongjian Liu, hongjianmd@126.com
†These authors have contributed equally to this work and share first authorship
 Yang Hao
Yang Hao Feifan Zhang
Feifan Zhang Yan Ma
Yan Ma Yage Luo
Yage Luo Yongyong Zhang
Yongyong Zhang Ning Yang
Ning Yang Man Liu
Man Liu Hongjian Liu4*
Hongjian Liu4* Jitian Li
Jitian Li