- 1Department of Orthopedic Surgery, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Department of Orthopaedics, Jiujiang First People’s Hospital, Jiujiang, Jiangxi, China
Background: Due to the low incidence of adult fibrosarcoma (AFS), it is difficult for clinicians to assess cancer-specific survival (CSS) in elderly patients based on this study. The study aimed to develop nomograms capable of accurately predicting 3-, 5-, and 8-year CSS in patients over 40 years of age with AFS.
Methods: Data were collected from The Surveillance, Epidemiology, and End Results (SEER) registry. 586 patients were included in this study. Univariate as well as multivariate Cox regression analyses were applied to identify independent risk factors. A nomogram was constructed and validated to predict the 3-, 5-, and 8-year CSS of patients.
Results: Five variables including age, sex, stage, grade, and chemotherapy status were considered independent risk factors and were used to construct the nomogram. The nomogram was well validated. The C-indexes of the training cohort and the validation cohort are 0.766 and 0.780, respectively. In addition, the area under the curves for 3-, 5- and 8-year CSS are 0.824, 0.846 and 0.840 in the training cohort, 0.835, 0.806 and 0.829 in the validation cohort. Calibration curves were also plotted to show that predicted endings have a well fit for the true endings. Finally, decision curve analysis demonstrates that the nomogram can bring a high benefit to patients.
Conclusion: We successfully constructed a highly accurate nomogram to predict the CSS of AFS patients at 3-, 5-, and 8 years. The nomogram can greatly help clinicians and patients with AFS.
Introduction
Adult fibrosarcoma (AFS) is a rare tumor consisting of malignant spindle-shaped fibroblasts. (1) It is classified by the World Health Organization as a fibroblastic and myofibroblastic tumor and is defined as a diagnosis of exclusion (2–4). What needs to be distinguished from infantile fibrosarcoma (IFS) is that IFS occurs primarily in children under 24 months of age (4–6). AFS occurs mostly in the median age of 50 years and is extremely rare in the adolescent population (3).
AFS was once incorrectly considered to be the most common soft tissue sarcoma. 66% of all sarcoma patients diagnosed at the Mayo Clinic between 1910 and 1930 were considered to have AFS (3). However, with the development of experimental diagnostic techniques, the incidence of AFS has declined rapidly. According to current reports, AFS accounts for less than 3.6% of all soft tissue sarcomas (3, 7, 8). Due to the extremely low incidence of AFS, it is easily overlooked by clinicians.
Relying only on the TNM staging system and AJCC staging system can’t meet the needs of clinicians to accurately predict patient prognosis. The nomogram is a visual model that integrates risk factors and assesses individual survival (9). It has been used in the prognosis prediction of a variety of cancers (10–12). Therefore, the study aimed to screen for risk factors that can influence cancer-specific survival (CSS) in elderly people over 40 years old with AFS and to develop a nomogram that can accurately predict the incidence of CSS at 3-, 5-, and 8-year.
Methods
Collection and selection of patients
The Surveillance, Epidemiology, and End Results (SEER) registry program collects patients’ information from 18 cancer registries, covering about 28% of the US population. All of the patient’s information in this study was obtained in the SEER database via SEER∗Stat Software Version 8.3.6.
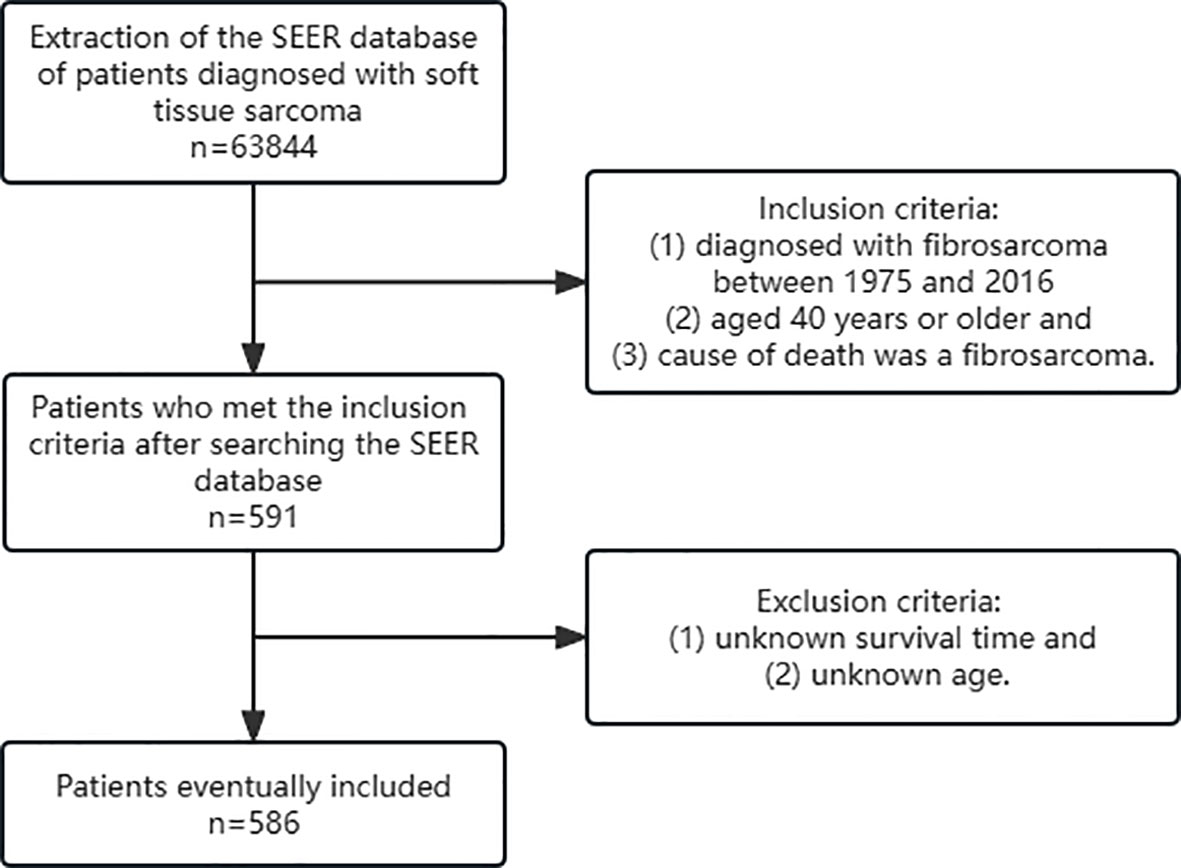
Inclusion criteria were as follows: (1) diagnosed with fibrosarcoma between 1975 and 2016 (2) aged 40 years or older and (3) cause of death was a fibrosarcoma. Exclusion criteria were as follows: (1) unknown survival time and (2) unknown age. At last, 586 patients were included in this retrospective study (Figure 1).
Characteristics of patients
We collected clinicopathological information on patients including patients’ id, survival time, survival status, sex, age, race, tumor primary site, stage, grade, radiotherapy status, and chemotherapy status. The optimal cutoff point for age grouping is selected by the X-tile software (40-57, 58-69,>69). The race was categorized as black, white, and other. The pathological grade into three categories including grades I&II, grades III& IV, and unknown. According to “SEER historic stage A (1973-2015)”, the tumor stage was divided into localized, regional, distant and unknown.
Statistical analysis
Patients were divided into a training cohort and a validation cohort in a ratio of 7:3 by R software (version 4.2.1). IBM SPSS Statistics 26.0 software was used to screen risk factors by univariate analysis cox proportional-hazards model (Cox). The endpoint was defined as CSS. Then these risk factors were included in a multivariate Cox analysis to exclude confounding effects between variables. The obtained independent risk factors were used to construct the nomogram. The C-index was calculated to estimate the prediction accuracy of the nomogram. The receiver operating characteristic (ROC) curve and the area under the curve (AUC) was used to evaluate the predictive ability of the nomogram. AUC is a valid method to summarize the overall diagnostic accuracy of a test. It has a range of values from 0 to 1, where a value of 0 indicates a completely inaccurate test and a value of 1 indicates a completely accurate test. A value of AUC between 0.7 and 0.8 is considered acceptable in the accepted statistical significance assessment, and the closer to 1 the better (1). Calibration curves were used to demonstrate the degree of fitness of predicted endings to true endings. In addition, decision curve analysis (DCA) was also applied to evaluate the actual benefit of this nomogram bringing to patients. Finally, we calculated the nomogram score of the patients. The X-tile software was used to calculate the best cutoff point for the nomogram score. We classified the patients into three risk subgroups: high, middle, and low, based on the cut-off points obtained. Kaplan-Meier curves were plotted to observe the prognosis of the three risk subgroups. The significant prognostic gap between different subgroups proves that this nomogram has a well prognostic prediction. All plots above were plotted using R software (version 4.2.1) and were considered statistically significant when the p-value was less than 0.05.
Results
Patients’ demographic and pathological characteristics
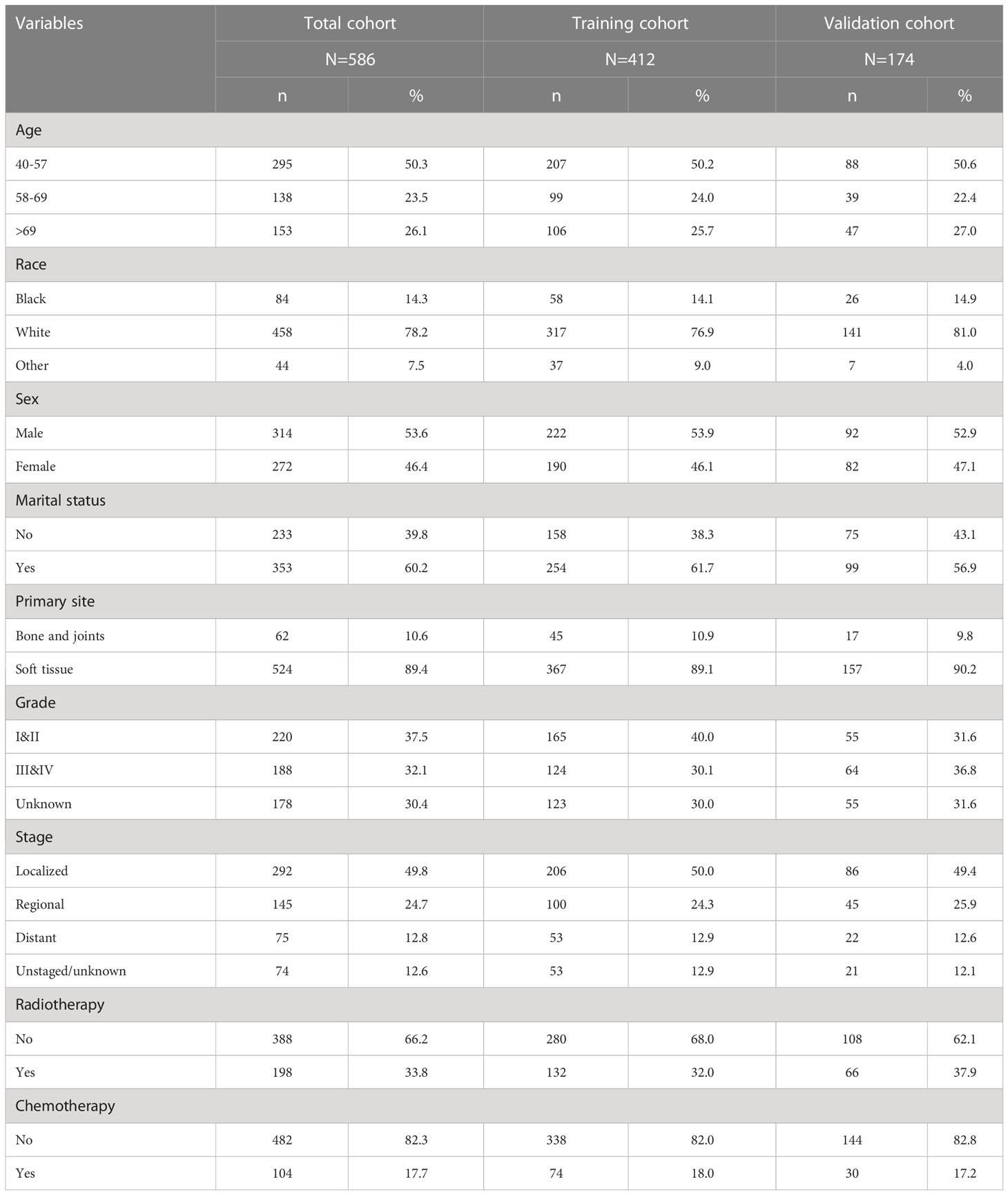
A total of 586 patients were included in this study according to the inclusion and exclusion criteria. Patients were randomized in a 7:3 ratio into a training cohort (n=412) and a validation cohort (n=174). Of these patients, 50.3% were between 40-57 years of age, 23.5% were between 58-69 years of age, and 26.1% were over 69 years of age. More details are presented in Table 1.
Prognostic risk factors for CSS
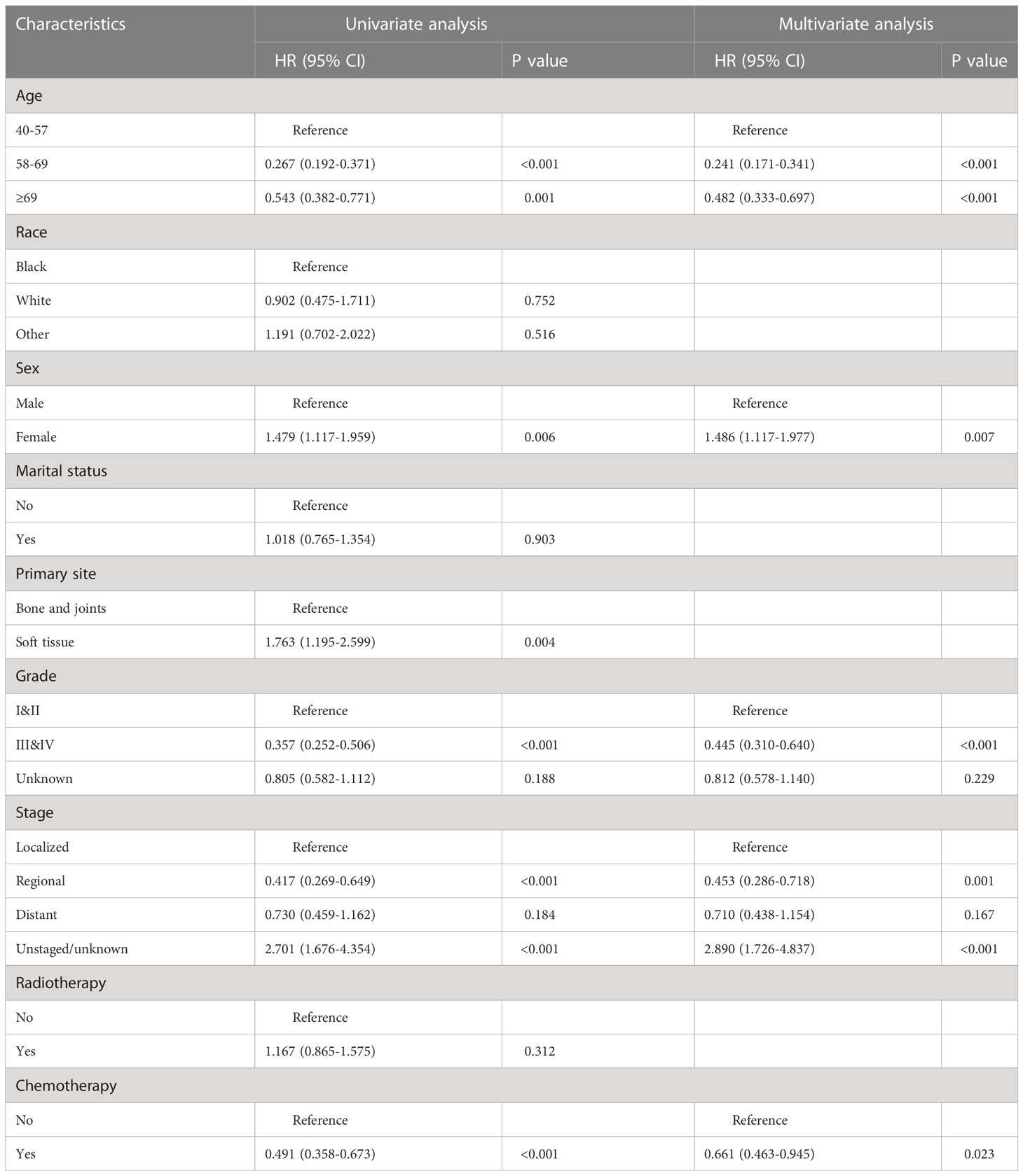
After univariate Cox regression analysis, age, sex, tumor primary site, stage, grade, and chemotherapy status were shown to be prognostic risk factors for CSS. The above risk factors were included in the multivariate Cox regression analysis. After excluding the effect of confounders among variables, age (58-69, HR= 0.241, 95%CI= 0.171-0.341, P< 0.001; ≥69, HR= 0.482, 95%CI= 0.333-0.697, P< 0.001), sex (female, HR= 1.486, 95%CI= 1.117-1.977, P= 0.007), stage (regional, HR= 0.453, 95%CI= 0.286-0.718, P=0.001;unstaged/unknown, HR= 2.890, 95%CI= 1.726-4.837, P< 0.001), grade (III&IV, HR= 0.445, 95%CI= 0.310-0.640, P< 0.001), and chemotherapy status (yes, HR= 0.661, 95%CI= 0.463-0.945, P= 0.023)were screened out as independent CSS prognostic influences (Table 2), but tumor primary site is no longer significant.
Construction and validation of a nomogram
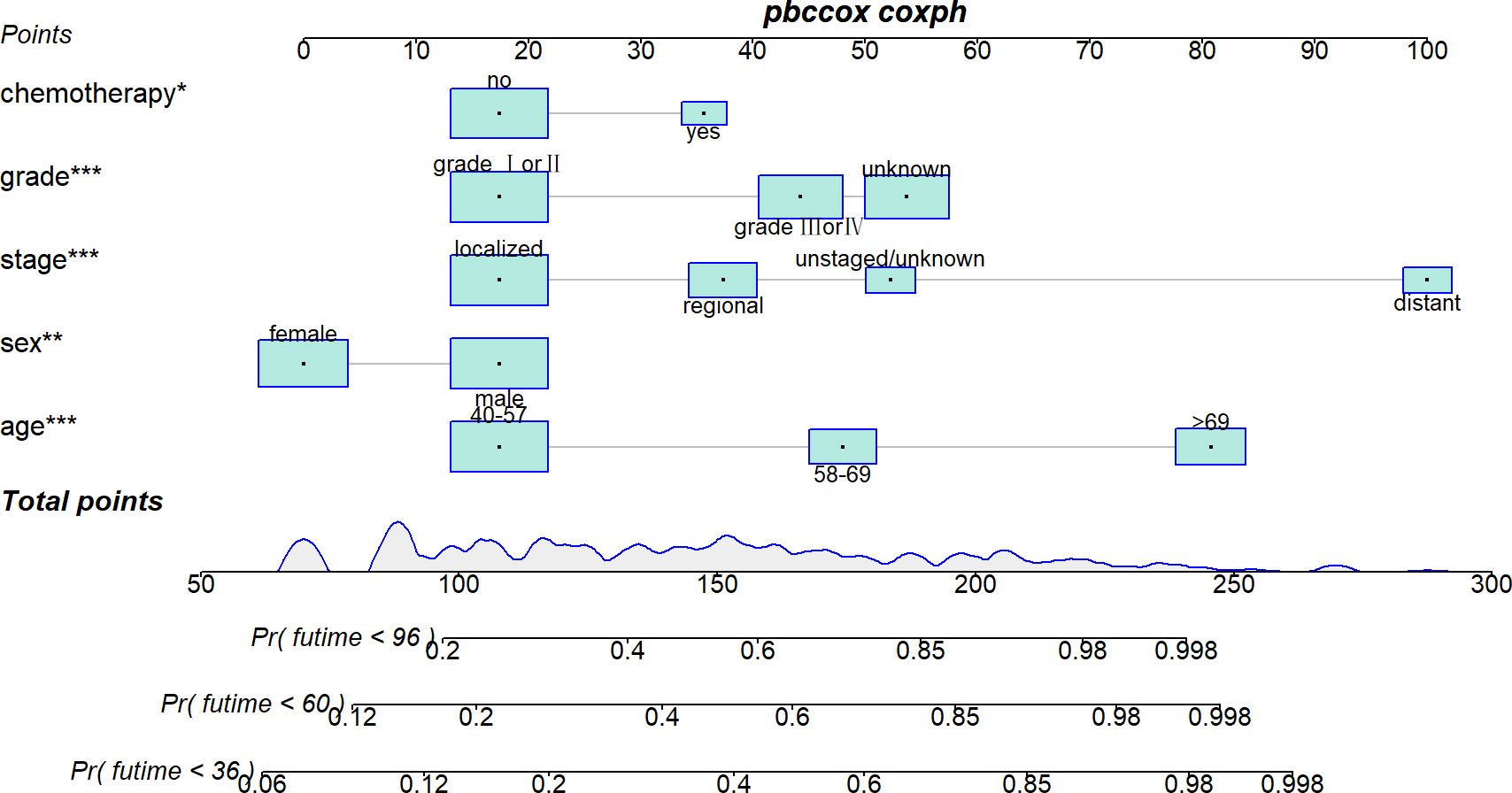
Five variables were used to construct the nomogram, including age, sex, stage, grade, and chemotherapy status (Figure 2). After calculation, the C-index is 0.766 in the training cohort and 0.780 in the validation cohort. This indicates that the prediction results of the nomogram have high accuracy. The AUCs for 3-, 5- and 8-year CSS are 0.824, 0.846 and 0.840 in the training cohort, 0.835, 0.806 and 0.829 in the validation cohort (Figure 3). In addition, calibration curves show that predicted endings have a well fit for the true endings (Figure 4). Finally, DCA demonstrates that the nomogram can bring a benefit to the patient (Figure 5). It dedicates that the nomogram has a high clinical application value.
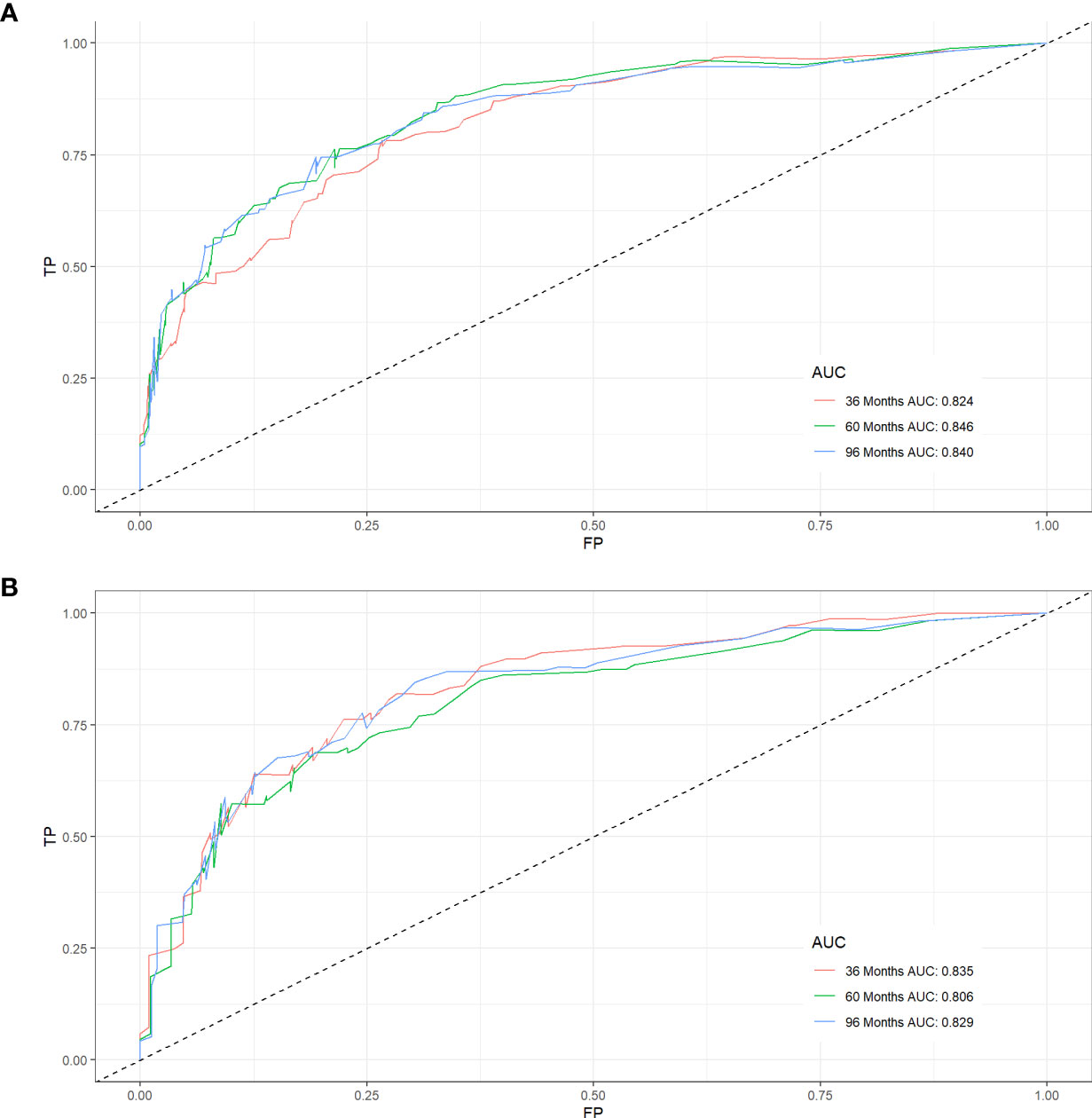
Figure 3 ROC curves for CSS prediction of elderly patients with AFS. (A) ROC curves of 3-, 5-, and 8-years in the training cohort, (B) ROC curves of 3-, 5-, and 8-years in the validation cohort.
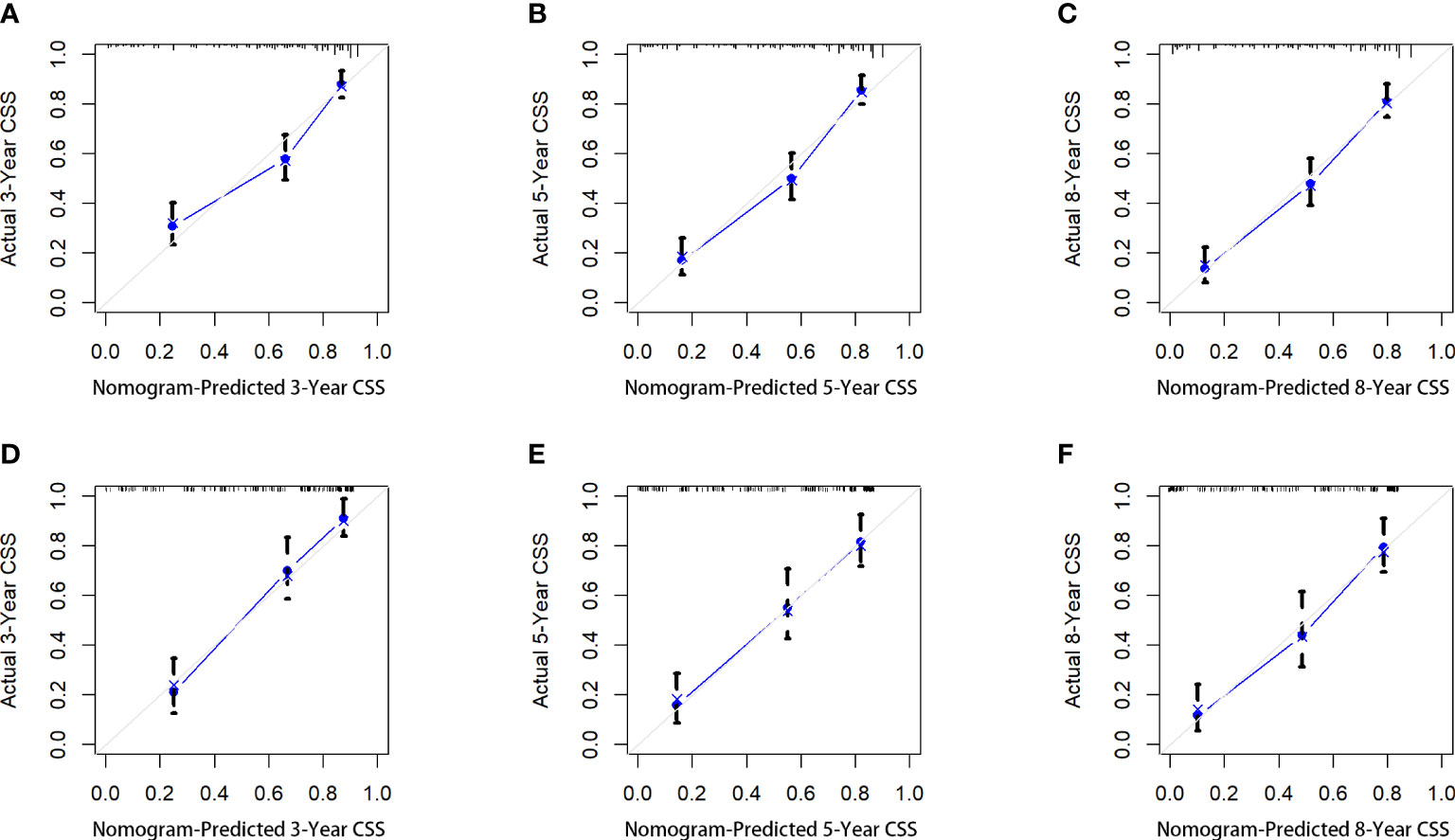
Figure 4 The calibration curves of the nomogram for predicting the 3-year, 5-years, and 8-years the CSS of the training cohort (A–C) and the validation cohort (D–F).
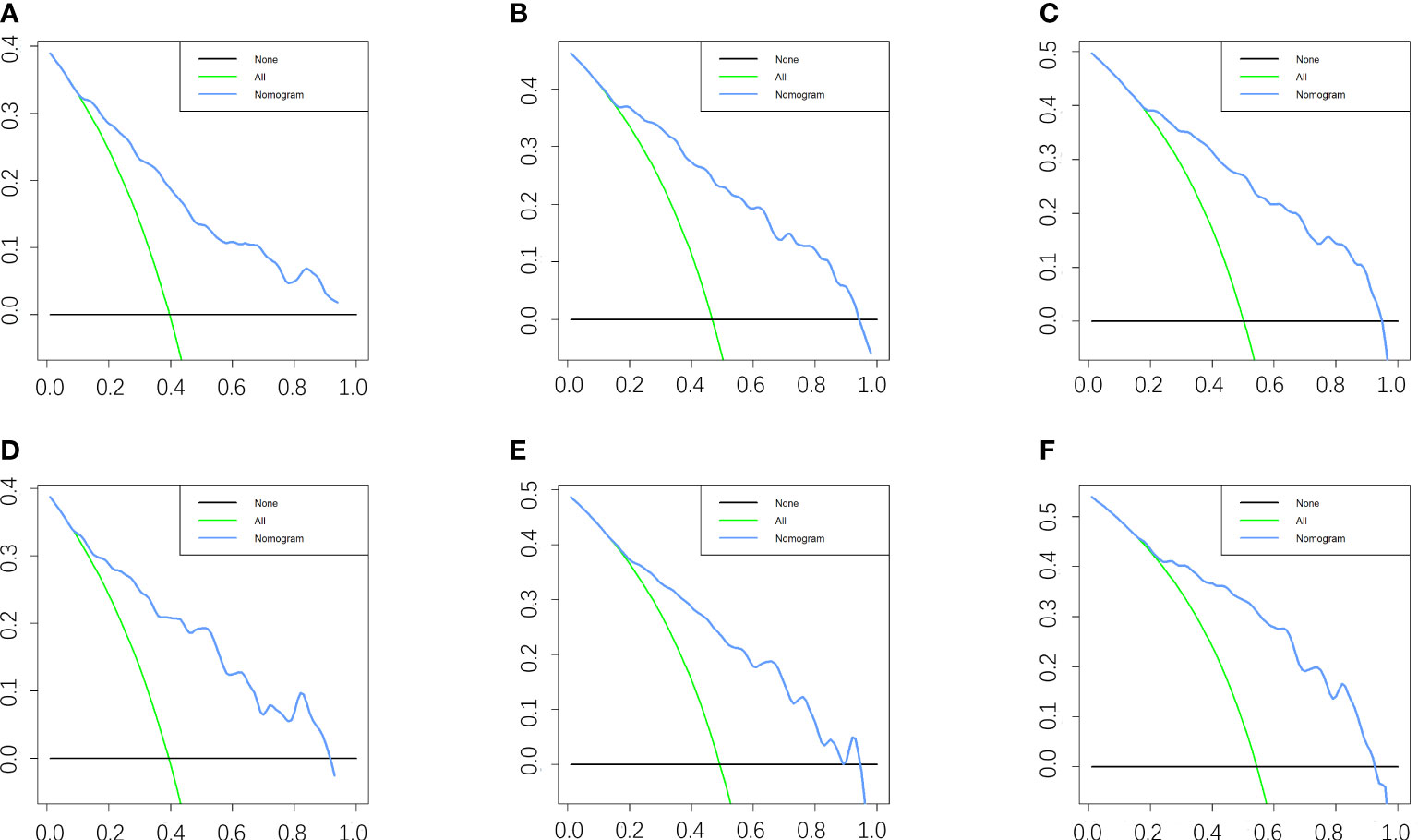
Figure 5 DCA of the nomogram for predicting the 3-, 5- and 8- years CSS in the training cohort (A–C) and the 3-, 5- and 8- years CSS in the validation cohort (D–F).
Risk stratification
By X-tile software, the best cutoff points were confirmed. Patients with nomogram scores below 144 were defined as low-risk patients, with nomogram scores above 207 were defined as high-risk patients, and the remaining patients were defined as mid-risk patients. As shown in Figure 6, the prognosis of CSS varies significantly among patients in different risk groups. This can help clinicians quickly obtain prognosis information of different patients.
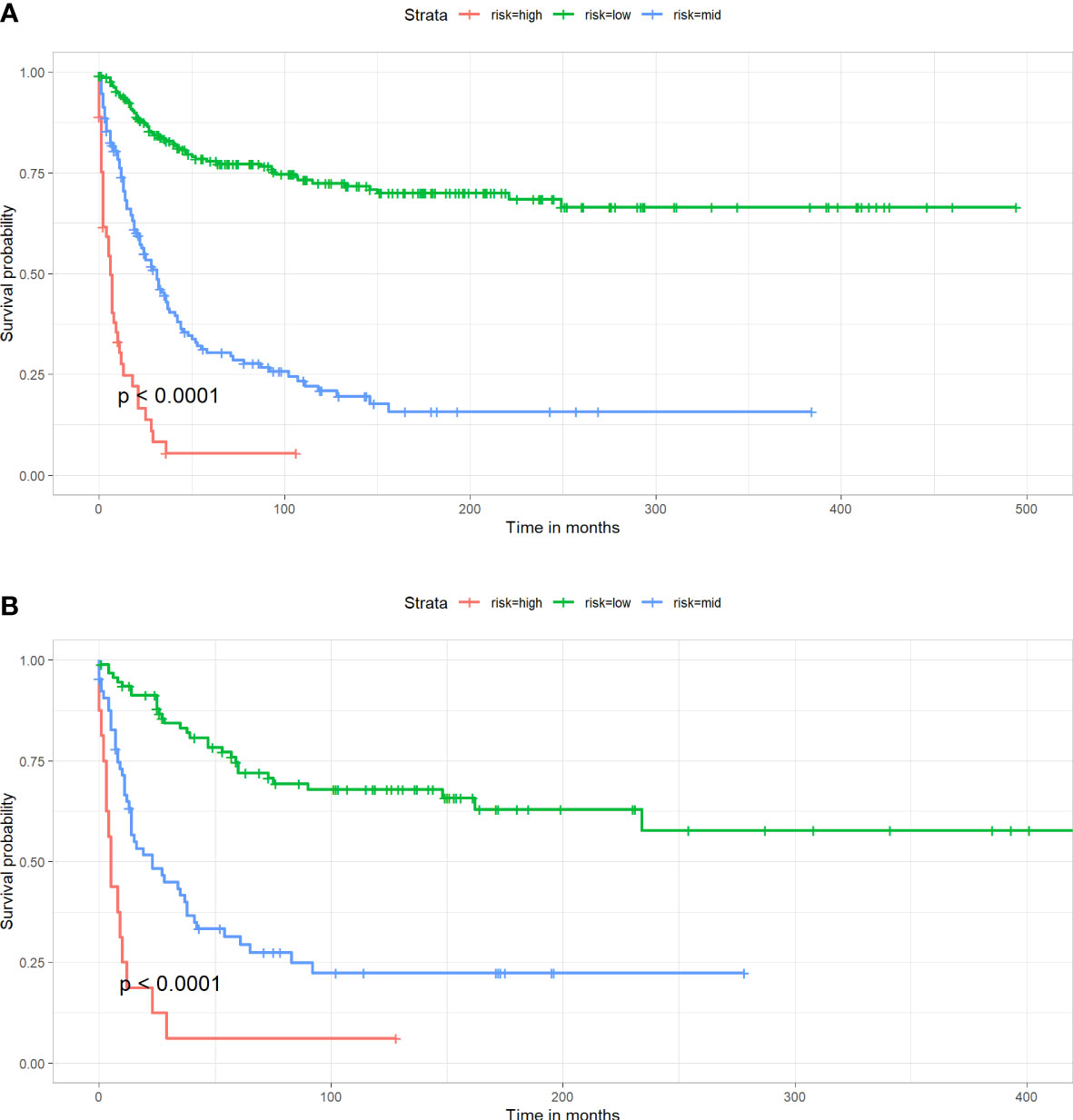
Figure 6 Kaplan-Meier survival curve for patients in different risk groups in both cohorts. (A) Training cohort, (B) Validation cohort.
Discussion
Our study focused on cancer-specific survival, which means that the results will be more representative in the treatment and evaluation of AFS. We found that age, sex, stage, grade, and chemotherapy status were independent risk factors affecting CSS of elderly people over 40 years old with AFS. Age is thought to be associated with poor prognosis in many cancers (13–15).
In previous studies, age has been reported to be an onset predisposing factor and an important prognostic risk factor for AFS in related studies (16–18). This may be related to the accumulation of harmful substances and long-term exposure to risk factors. As seen in our nomogram (Figure 2), except for distant tumor metastasis, high age was the factor that allowed patients to obtain the highest nomogram score. This indicates that higher-age patients deserve more attention, which is the purpose of our study.
Many reports suggest that AFS has a higher incidence in the male population (19). However, this is not enough to prove that men are a risk factor for the prognosis of AFS patients. In a retrospective study by Xiang et al., they obtained the same conclusion as ours (7). Interestingly, in the report by Criscito et al. on dermatofibrosarcoma, men were found to be an important prognostic risk factor and it was noted that men were more likely to develop head and neck tumors (20). Since the data for the relevant retrospective studies were obtained from the SEER database, this may have led to bias. But it is also possible that new clinical findings, which need to be confirmed by more studies.
Similar to other sarcomas, multivariate Cox regression analysis showed that tumor stage and tumor grade were also independent risk factors in AFS (21–23). Distant metastases and high-grade of tumors indicate the widespread spread of tumor cells and multi-organ involvement. Bahrami et al. suggested that more than 80% of AFS patients had high-grade tumors (19). It indicates that without early diagnosis the patient’s survival expectancy will be significantly reduced. In addition, our study also found that not receiving chemotherapy was also a risk factor for CSS in patients over forty years of age with AFS. Although AFS has a low sensitivity to chemotherapy, undergoing surgery and chemotherapy is also the best treatment option for patients with AFS (4). Doxorubicin in combination with other chemotherapeutic agents is regarded as the most common drug applied to patients. While AFS has a low sensitivity to radiotherapy, we didn’t find an effect of radiotherapy on patients’ CSS, which is consistent with the findings of other scholars (20, 24). The use of radiotherapy for patients with AFS is still controversial, so it is not a standard treatment (25, 26).
We still need to acknowledge the limitations of our study. The low prevalence of AFS resulted in an insufficiently rich sample size, which may have led to some bias in our study. But we still have a better validation of the nomogram. This demonstrates that this nomogram has good clinical predictive power and can provide assistance to clinicians.
Conclusion
Age, sex, stage, grade and chemotherapy status were shown to be risk factors affecting 3-, 5- and 8-year CSS in patients with AFS. The above five variables were used to construct the nomogram and were well validated. The development of the nomogram can provide a powerful aid to clinicians.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZH and QK conceived and designed the study. ZH and ZZ performed the literature search. ZH and YL generated the figures and tables. ZH, ZGZ, and WZ analyzed the data. ZH wrote the manuscript and QK critically reviewed the manuscript. ZH and QK supervised the research. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by, China, Sichuan Science and Technology Program (2020YFS0080, 2020YFQ0007, 2021JDRC0159), Science and Technology Project of Tibet Autonomous Region (XZ201901-GB-08), The National Natural Science Foundation of China (81171731), and the 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21026, ZYJC21077).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AFS, Adult fibrosarcoma; IFS, Infantile fibrosarcoma; SEER, Surveillance, Epidemiology, and End Results; CSS, Cancer-specific survival; ROC, Receiver operating characteristic; AUC, Area under the curve; DCA, Decision curve analysis.
References
1. Wang H, Nie P, Dong C, Li J, Huang Y, Hao D, et al. CT and MRI findings of soft tissue adult fibrosarcoma in extremities. BioMed Res Int (2018) 2018:6075705. doi: 10.1155/2018/6075705
2. Choi JH, Ro JY. The 2020 WHO classification of tumors of soft tissue: selected changes and new entities. Adv Anat Pathol (2021) 28(1):44–58. doi: 10.1097/PAP.0000000000000284
3. Folpe AL. Fibrosarcoma: a review and update. Histopathology. (2014) 64(1):12–25. doi: 10.1111/his.12282
4. Augsburger D, Nelson PJ, Kalinski T, Udelnow A, Knösel T, Hofstetter M, et al. Current diagnostics and treatment of fibrosarcoma -perspectives for future therapeutic targets and strategies. Oncotarget. (2017) 8(61):104638–53. doi: 10.18632/oncotarget.20136
5. Sparber-Sauer M, Vokuhl C, Seitz G, Stegmaier S, Hallmen E, Kalle T, et al. The impact of local control in the treatment of children with advanced infantile and adult-type fibrosarcoma: experience of the cooperative weichteilsarkom studiengruppe (CWS). J Pediatr Surg (2020) 55(9):1740–7. doi: 10.1016/j.jpedsurg.2019.10.051
6. Iwasaki H, Enjoji M. Infantile and adult fibrosarcomas of the soft tissues. Acta Pathol Jpn (1979) 29(3):377–88. doi: 10.1111/j.1440-1827.1979.tb00195.x
7. Xiang GH, Zhu JJ, Ke CR, Weng Y, Fang M, Zhu S, et al. Nomograms predict overall survival and cancer-specific survival in patients with fibrosarcoma: a SEER-based study. J Oncol (2020) 2020:8284931. doi: 10.1155/2020/8284931
8. Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CDM, Devesa SS, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer (2006) 119(12):2922–30. doi: 10.1002/ijc.22239
9. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol (2015) 16(4):e173–180. doi: 10.1016/S1470-2045(14)71116-7
10. Zhou Q, Li AB, Lin ZQ, Zhang H. A nomogram and a risk classification system predicting the cancer-specific survival of patients with initially-diagnosed osseous spinal and pelvic tumors. Spine (Phila Pa 1976) (2020) 45(12):E713–e720. doi: 10.1097/BRS.0000000000003404
11. Wu J, Zhang H, Li L, Hu M, Chen L, Xu B, et al. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: a population-based analysis. Cancer Commun (Lond) (2020) 40(7):301–12. doi: 10.1002/cac2.12067
12. Jin C, Cao J, Cai Y, Wang L, Liu K, Shen W, et al. A nomogram for predicting the risk of invasive pulmonary adenocarcinoma for patients with solitary peripheral subsolid nodules. J Thorac Cardiovasc Surg (2017) 153(2):462–469.e461. doi: 10.1016/j.jtcvs.2016.10.019
13. Ferguson JL, Turner SP. Bone cancer: diagnosis and treatment principles. Am Fam Physician (2018) 98(4):205–13.
14. Mizuno K, Takeuchi M, Kikuchi M, Omori K, Kawakami K. Outcomes in patients diagnosed with tongue cancer before and after the age of 45 years. Oral Oncol (2020) 110:105010. doi: 10.1016/j.oraloncology.2020.105010
15. Aljabery F, Liedberg F, Häggström C, Ströck V, Hosseini A, Gårdmark T, et al. Treatment and prognosis of patients with urinary bladder cancer with other primary cancers: a nationwide population-based study in the bladder cancer data base Sweden (BladderBaSe). BJU Int (2020) 126(5):625–32. doi: 10.1111/bju.15198
16. Yang F, Xie H, Wang Y. Prognostic nomogram and a risk classification system for predicting overall survival of elderly patients with fibrosarcoma: a population-based study. J Oncol (2021) 2021:9984217. doi: 10.1155/2021/9984217
17. Cozzolino I, Caleo A, Di Crescenzo V, Cinelli M, Carlomagno C, Garzi A, et al. Cytological diagnosis of adult-type fibrosarcoma of the neck in an elderly patient. report of one case and review of the literature. BMC Surg (2013) 13 Suppl 2(Suppl 2):S42. doi: 10.1186/1471-2482-13-S2-S42
18. Dahl M, Aurit SJ, Silberstein PT, Gootee J. Primary site and other prognostic factors for fibrosarcoma: an analysis of the national cancer database. Cureus. (2021) 13(10):e19163. doi: 10.7759/cureus.19163
19. Bahrami A, Folpe AL. Adult-type fibrosarcoma: a reevaluation of 163 putative cases diagnosed at a single institution over a 48-year period. Am J Surg Pathol (2010) 34(10):1504–13. doi: 10.1097/PAS.0b013e3181ef70b6
20. Criscito MC, Martires KJ, Stein JA. Prognostic factors, treatment, and survival in dermatofibrosarcoma protuberans. JAMA Dermatol (2016) 152(12):1365–71. doi: 10.1001/jamadermatol.2016.1886
21. Milgrom SA, Million L, Mandeville H, Safwat A, Ermoian RP, Terezakis S. Non-rhabdomyosarcoma soft-tissue sarcoma. Pediatr Blood Cancer (2021) 68(Suppl 2):e28279. doi: 10.1002/pbc.28279
22. Zhan H, Mo F, Zhu M, Xu X, Zhang B, Liu H, et al. A SEER-based nomogram accurately predicts prognosis in ewing's sarcoma. Sci Rep (2021) 11(1):22723. doi: 10.1038/s41598-021-02134-0
23. Sampo MM, Klintrup K, Tukiainen EJ, Böhling TO, Blomqvist CP. Improved prognosis in soft-tissue sarcoma of extremity and trunk wall. Acta Orthop (2017) 88(1):116–20. doi: 10.1080/17453674.2016.1196429
24. Slovak ML, Hoeltge GA, Dalton WS, Trent JM. Pharmacological and biological evidence for differing mechanisms of doxorubicin resistance in two human tumor cell lines. Cancer Res (1988) 48(10):2793–7.
25. Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2014) 25 Suppl 3:iii102–112. doi: 10.1093/annonc/mdu254
Keywords: adult fibrosarcoma, seniors, nomogram, retrospective study, prediction model
Citation: Huang Z, Zhao Z, Liu Y, Zhou Z, Zhang W and Kong Q (2023) Identification and validation of a nomogram predicting cancer-specific survival for elderly patients with adult fibrosarcoma: a multicenter retrospective study. Front. Oncol. 13:1187942. doi: 10.3389/fonc.2023.1187942
Received: 16 March 2023; Accepted: 13 June 2023;
Published: 12 July 2023.
Edited by:
Sanjit Mukherjee, National Institutes of Health (NIH), United StatesReviewed by:
Dipranjan Laha, National Cancer Institute (NIH), United StatesAsit Kumar Manna, The University of Utah, United States
Copyright © 2023 Huang, Zhao, Liu, Zhou, Zhang and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingquan Kong, kqqspine@126.com
†These authors have contributed equally to this work
 Zhangheng Huang
Zhangheng Huang Zhen Zhao
Zhen Zhao Yuheng Liu1
Yuheng Liu1 Qingquan Kong
Qingquan Kong