- 1Department of Cardiology, Zibo Central Hospital, Zibo, China
- 2Department of Respiratory and Critical Care Medicine, Zibo Central Hospital, Shandong, China
Background: Previous clinical randomized controlled trials (RCTs) have demonstrated that immune checkpoint inhibitors (ICIs) cause various toxicities during cancer treatment, but the effects of different inhibitors in combination with chemotherapy for cardiotoxicity remain controversial. The aim of the present study was to assess cardiotoxicity caused by programmed cell death protein 1 (PD-1), programmed cell death-Ligand 1 (PD-L1), and cytotoxic T lymphocyte associate protein-4 (CTLA-4) in combination with chemotherapy to treat lung cancer.
Methods: The following ICIs were included in the present study: durvalumab, avelumab, ipilimumab, atezolizumab, pembrolizumab, cemiplimab, and nivolumab. The relevant information was extracted using a predefined data extraction table, and the risk of bias was assessed in randomized controlled trials using the Cochrane Bias Risk tool. The main outcomes were hypertension, heart failure, pericardial effusion, and other adverse cardiac events. The random effects model was used to conduct a paired meta-analysis, and a random effects network meta-analysis was then performed within a Bayesian framework.
Results: In total, 17 RCTs were included in the present study. There were 11,063 individuals in the experimental and control groups, with an average age greater than 60 years. Based on the evaluation of all drug classes in RCTs, CTLA-4+chemotherapy (RR, -0.69 [95% CI, 2.91-1.52] and PD-L1 (RR, -0.21 [95% CI, -1.03-0.60]) were less cardiotoxic than the control arm, which indicated they were safer options for adverse cardiac events. PD-L1 alone was less cardiotoxic than PD-1 alone (RR, -0.57 [95% CI, -1.96-0.82]). Further, the dual immunotarget inhibitor, PD-1+CTLA-4, had the lowest SUCRA value and had the highest cardiotoxicity (SUCRA=9).
Conclusion: When classified according to drug type, CTLA-4+chemotherapy is associated with fewer cardiac adverse events compared to other treatments. Dual immunotarget inhibitors are more likely to have adverse cardiac reactions. Therefore, clinicians should consider this evidence when developing an ICI immunotherapy regimen for lung cancer.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier CRD42023360931.
Introduction
In the last five years, immune checkpoint inhibitors (ICIs) have become the first line of treatment for many types of cancer. Tumour cells express neoantigens, and immune cells recognize and destroy mutant proteins (1). Immune checkpoint inhibitors function by blocking inhibitory signals from tumour cells to T cells that recognize them, thus allowing tumour cells to be destroyed by the patient’s own immune system (2). Programmed death 1 (PD-1) is present on the surface of T cells and binds to programmed cell death 1 ligand 1 (PD-L1), which is widely expressed on tumour cells, allowing functional inhibition of the T cell response and tumour immune escape in several malignancies (3). Blocking the PD-1/PD-L1 interaction enhances immune recognition and stimulation of T cells to attack tumour cells. The ICIs that have been used in the clinic include cytotoxic T lymphocyte associate protein-4 (CTLA-4), PD-1, and PD-L1.
ICIs induce tumour responses in a variety of tumour types, including melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), and Hodgkin’s disease. However, ICI treatment is often associated with immune-related adverse events and with multisystemic toxic changes that affect the skin, liver, nerves, and heart (4). ICI-induced cardiotoxicity is rare, with the incidence of specific types of cardiotoxicity as high as 1%, but it is usually serious and may be life-threatening (5). Patients may present with cardiac arrest, angina pectoris, myocarditis, cardiomyopathy, heart failure, and arrhythmia (6). In the present study, we discussed various immunotherapies known to cause cardiotoxicity. Understanding the interaction between lung cancer immunotherapy and the cardiac system will help in the early detection and prevention of cardiotoxicity. In this review, we focus on these differences and conduct qualitative assessments.
Methods
Search strategy
The meta-analysis was designed and conducted in accordance with the Preferred Reporting Project for Systematic Review and Meta-Analysis (PRISMA) reporting guidelines (7).
We conducted rigorous searches of the PubMed, Embase and Cochrane Library from inception to 1 December 2022 to identify all RCTs searched. The Medical Subject Heading (MeSH) terms retrieved and their entry terms were: “Immune Checkpoint Inhibitors”, “ICIs”, “PD-1 inhibitors”, “PD-L1 inhibitors”, “PD-1”, “PD-L1”, “CTLA-4”, “Durvalumab”, “Avelumab”, “Ipilimumab”, ”Atezolizumab”, ”Pembrolizumab”, “Cemiplimab”, “Nivolumab”, “Lung cancer”, “Cardiotoxicity” and “Adverse cardiac events”. The included articles were also limited to those published in English.
Study selection and data extraction
The following medical records of lung cancer patients (>18 years) treated with ICIs were reviewed: all RCTs associated with PD-1/PD-L1 inhibitors or CTLA-4 inhibitors, including randomized controlled trials comparing PD-1, PD-L1 inhibitors, and CTLA-4 inhibitors with placebo or chemotherapy as well as those comparing PD-1, PD-L1 inhibitors, and CTLA-4 inhibitors with chemotherapy versus chemotherapy. Only studies that reported adverse cardiac events as well as those with extractable total number of trials and the number of events were included. Duplicate studies were excluded with literature management software. In addition, studies published as conference abstracts as well as case reports were excluded. Finally, trials that did not report treatment-related cardiotoxicity were also excluded.
Cardiotoxicity data were collected, including defined rates of heart disease and the number of individuals who experienced heart disease. Two researchers independently screened the titles and abstracts of publications, and any publication that the researchers deemed potentially relevant was evaluated in full-text. If disagreements occurred, they were resolved by discussion.
Publication bias
Two researchers independently assessed the risk of bias of each included study according to the Cochrane Risk of Bias tool (8).
Statistical analysis
The gemtc package in R (4.2.1) was used to perform the Bayesian network meta-analysis, and a random effects model was used for the network meta-analysis (9). The odds ratio (OR) and corresponding 95% confidence intervals (CI) were used as measures of comparison. Heterogeneity was assessed by the mtc.anohe command in the gemtc package. Heterogeneity between studies was assessed as high if I2> 50%, whereas heterogeneity between studies was considered to be low if I2< 50%. Trace plots and density plots were used to evaluate the convergence of the model. A P value < 0.05 (bilateral) was considered statistically significant (10). The hierarchy of treatments was determined by calculating ranking probabilities. In addition, for each outcome, the probability of each drug at each possible grade was estimated, and the probability distribution of each treatment for each possible position ranking was presented in the ranking graph. The surface under the cumulative ranking (SUCRA) curve was used to rank the safety of various immunotherapy regiments, in which lower SUCRA rankings indicate greater risks of cardiotoxicity (11). In addition to the network meta-analysis, Stata (15.1) tested publication bias and generated a funnel plot by running Egger’s command.
Results
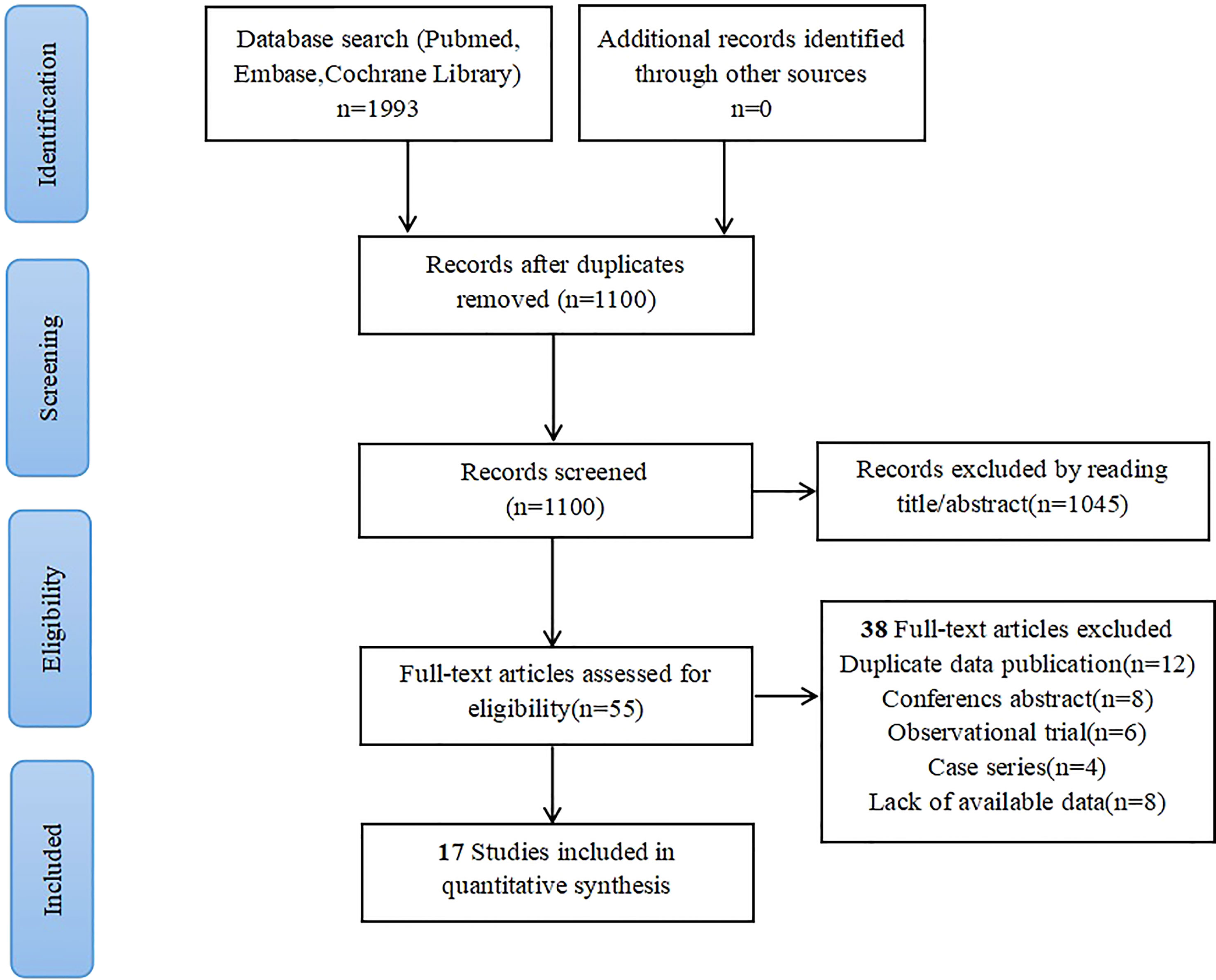
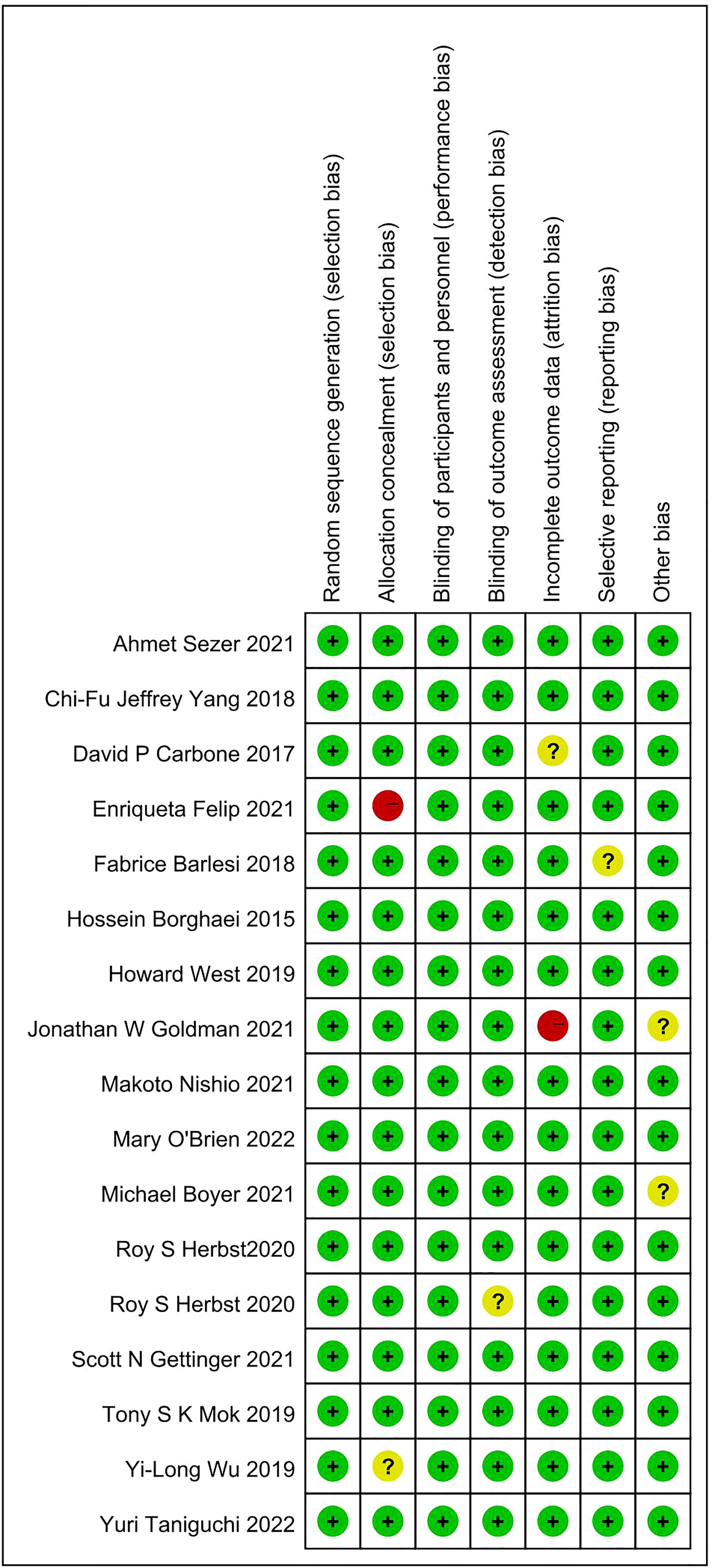
A search of the PubMed, Embase, and Cochrane libraries found 1100 potentially relevant records. After deleting duplicates and filtering titles and abstracts, 55 articles remained. After downloading and reading the full texts, 17 articles met the inclusion criteria and were included in the meta-analysis. One clinical trial had a high risk of bias, mainly due to incomplete report data and other biases (12). The flow chart of the search strategy and research selection is shown in Figure 1. In the meta-analysis, we used the Cochrane bias risk tool to evaluate the data quality. Figure 2 shows a summary of the risk of bias in the included literature.
Characteristics of clinical trials
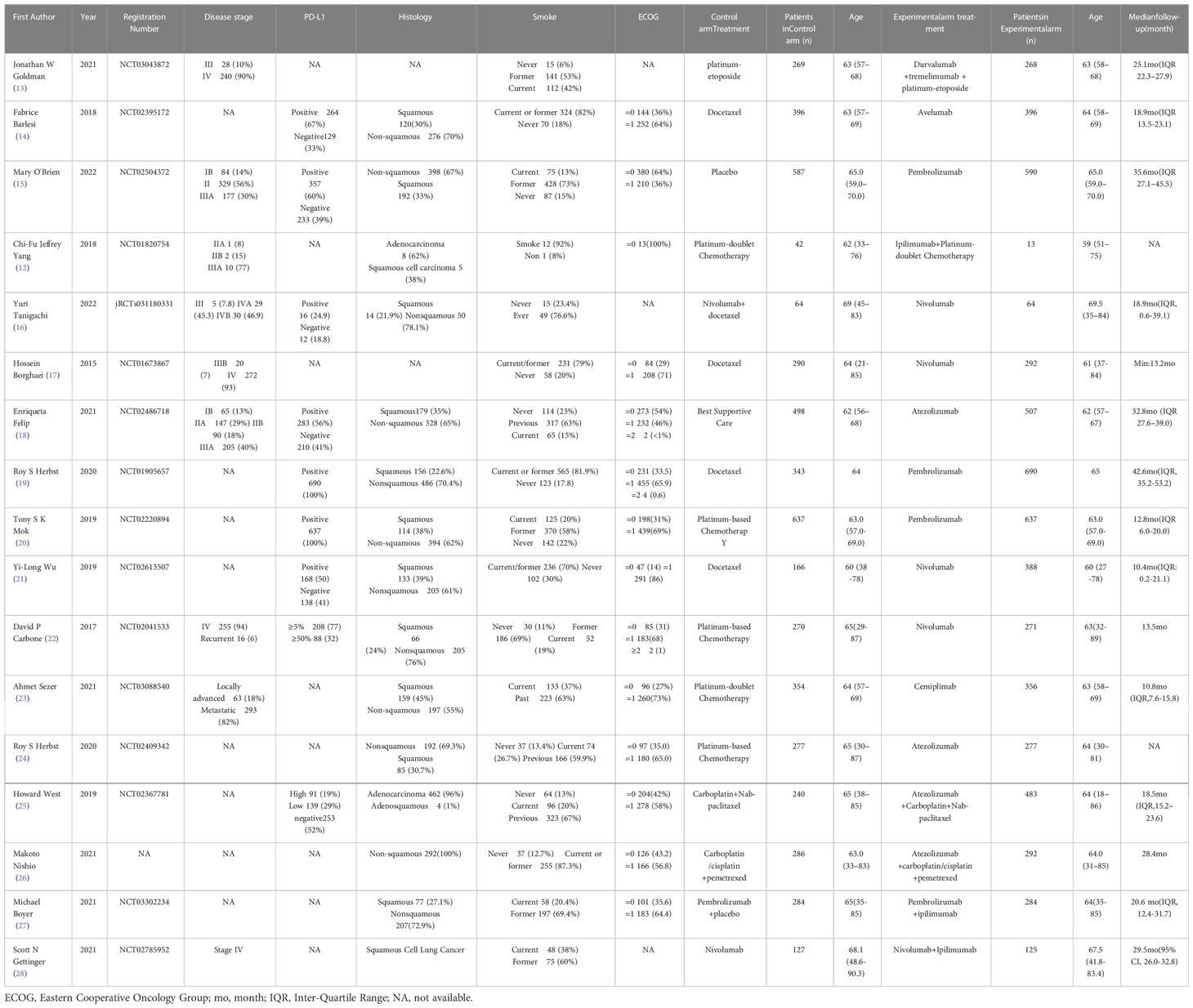
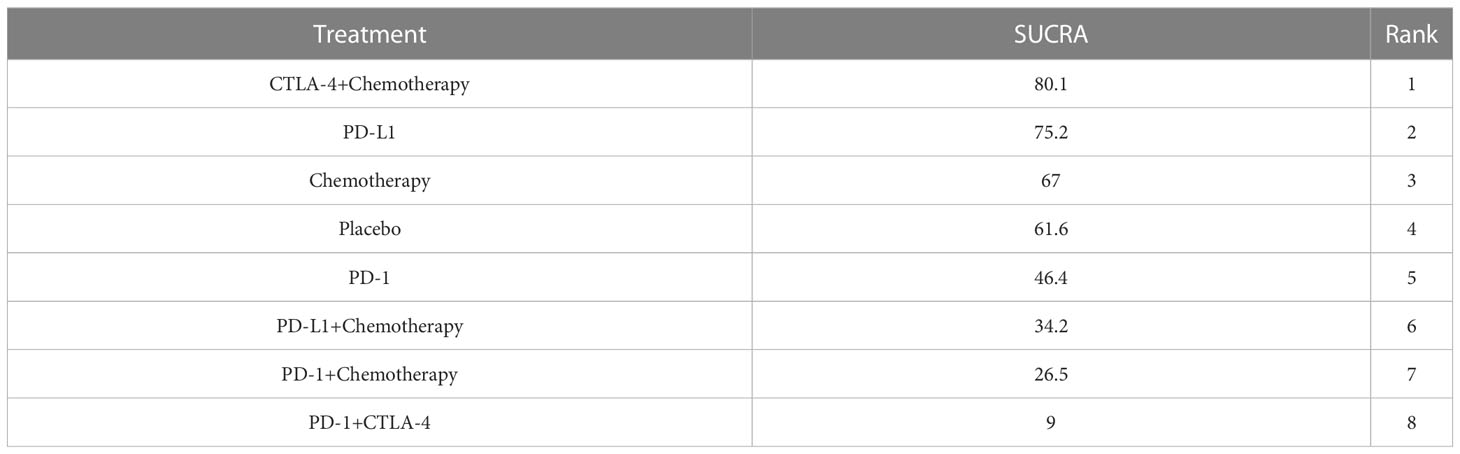
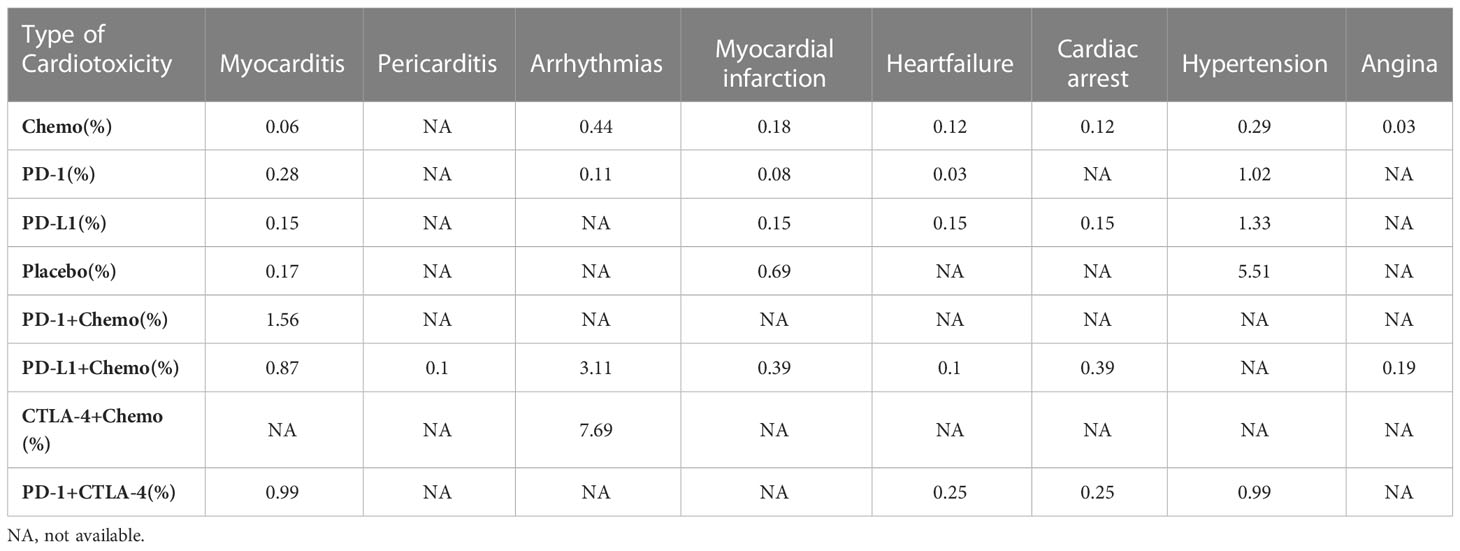
The specific experimental and control group characteristics of the 17 clinical trials are shown in Table 1. Seven ICIs were included, namely, durvalumab, avelumab, ipilimumab, atezolizumab, pembrolizumab, cemiplimab, and nivolumab. The following comparisons were made in the clinical studies: three studies compared PD-L1+chemotherapy to chemotherapy alone (13, 25, 26); two studies compared PD-L1 to chemotherapy (14, 24); two studies compared PD-1+CTLA-4 to PD-1 alone (27, 28); six studies compared PD-1 to chemotherapy (17, 19–23); one study compared PD-1 to placebo (15); one study compared PD-1+chemotherapy to PD-1 alone (16); and one study compared CTLA-4+chemotherapy to chemotherapy (12). Figure 3 shows the network diagram of the comparisons of different immunosuppressant combinations. The average age of the participants in the 17 clinical trials was greater than 60 years old. Table 2 shows the SUCRA ranking of the interventions based on treatment efficacy and cumulative probability plots, indicating the combination of immunological drugs that were least and most toxic to the heart in lung cancer patients. Table 3 shows the specific rates of cardiotoxicity for different treatment combinations.
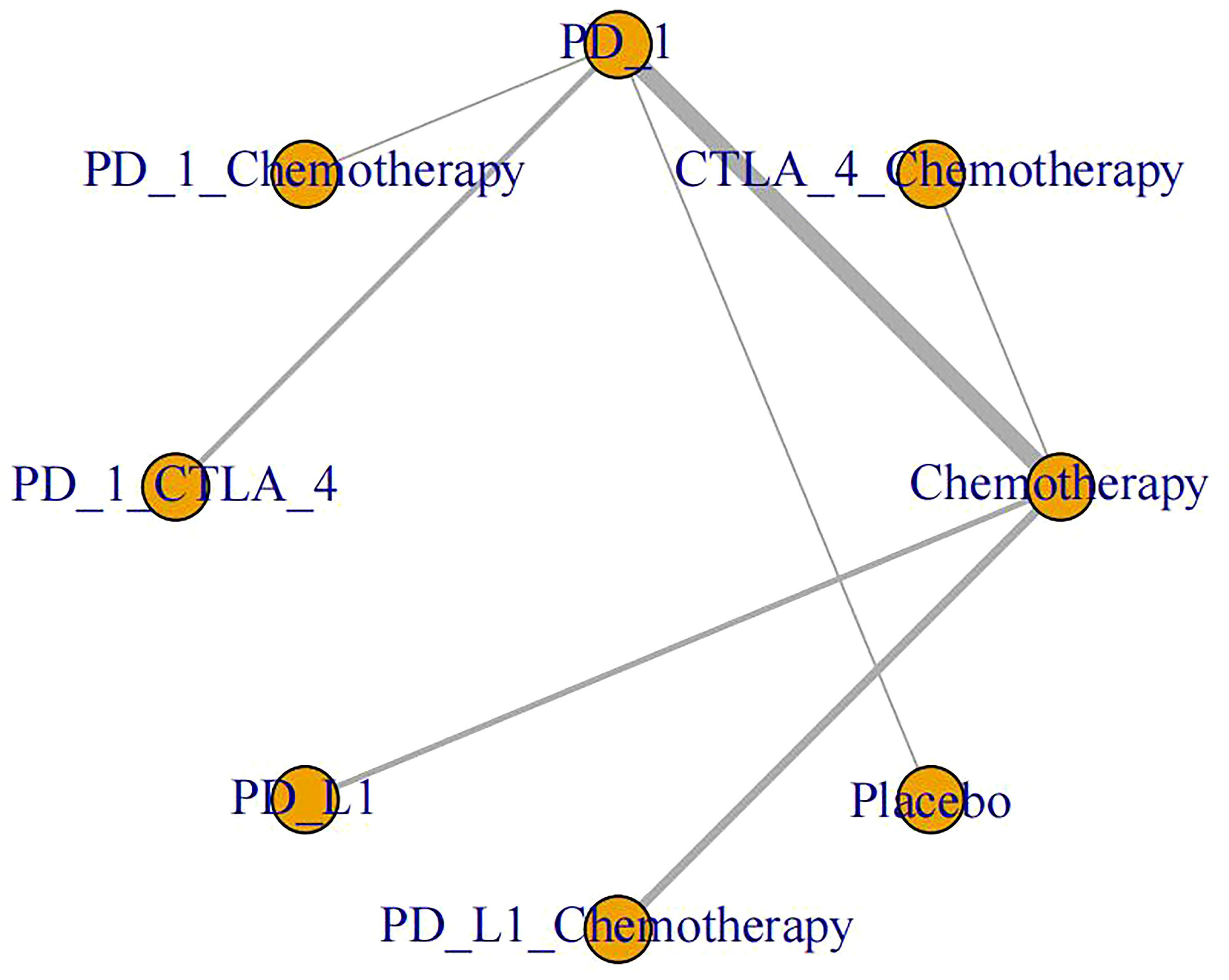
Figure 3 Network plots of different ICI comparisons. Single drug and drug combination comparison network. The size of each node is proportional to the number of participants (sample size). The lines represent the direct comparison available between treatment pairs, and the line width is proportional to the number of trials comparing each pair of treatments.
Risk of cardiotoxicity
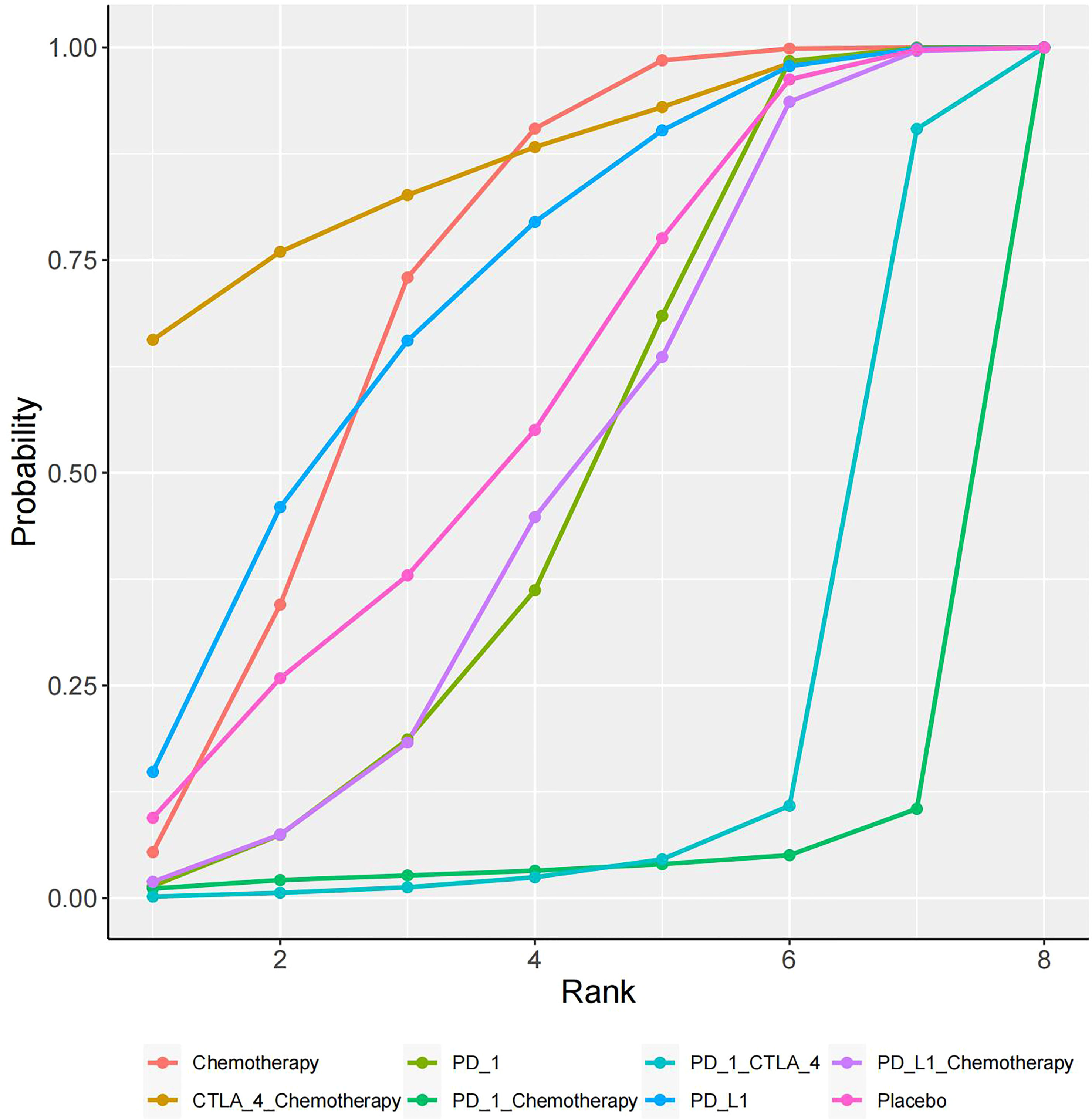
In total, 16 studies were included in a network meta-analysis of the cardiotoxic effects of single inhibitors and combinations in lung cancer. According to the SUCRA rankings, the top three interventions were CTLA-4+chemotherapy, PD-L1, and chemotherapy with SUCRA values of 80.1%, 75.2%, and 67%, respectively. The results of the network meta-analysis of the primary outcomes are shown in Figure 4. CTLA-4+chemotherapy had a lower rate of adverse cardiac events than PD-L1+chemotherapy (OR, -1.28 [95% CI, -3.54-0.99]) or placebo (OR, -0.84 [95% CI, -3.36-1.69]). The effect of PD-L1 alone on cardiac toxicity was significantly less than that of PD-1 alone (OR, -0.57 [95% CI, -1.96-0.82]). PD-1 alone was less cardiotoxic than the PD-1+CTLA-4 dual ICI (OR, -1.58 [95% CI, -3.12-0.18]) and PD-1+chemotherapy (OR, -1.13 [95% CI, -4.35-2.09]). The combination of PD-1 and CTLA-4 (SUCRA=9%), had the greatest cardiotoxicity and the highest probability of adverse cardiac events. Figure 5 shows the cumulative ranking probability of each ICI combination.
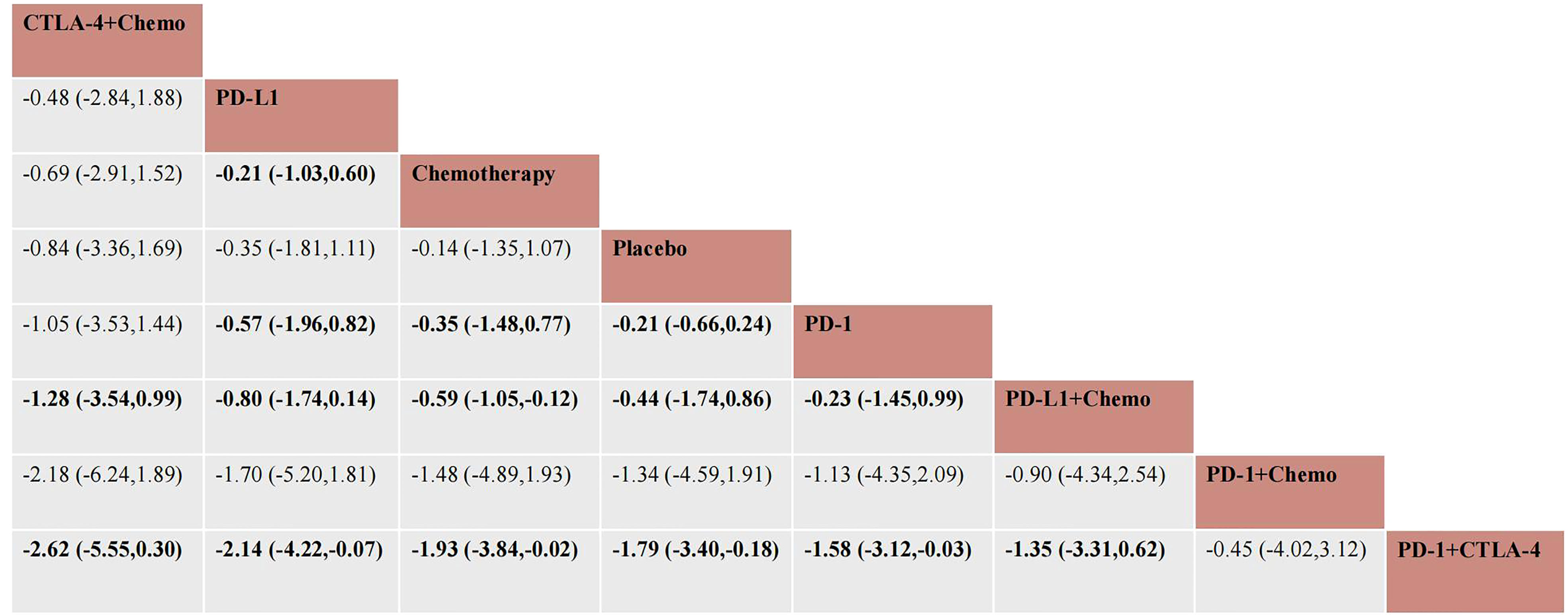
Figure 4 Network meta-analysis estimates for cardiotoxicity of different drug species. Ranking of drugs according to cardiac toxicity using SUCRA. The comparison should be read from left to right. Statistically significant differences are indicated in bold and underlined.
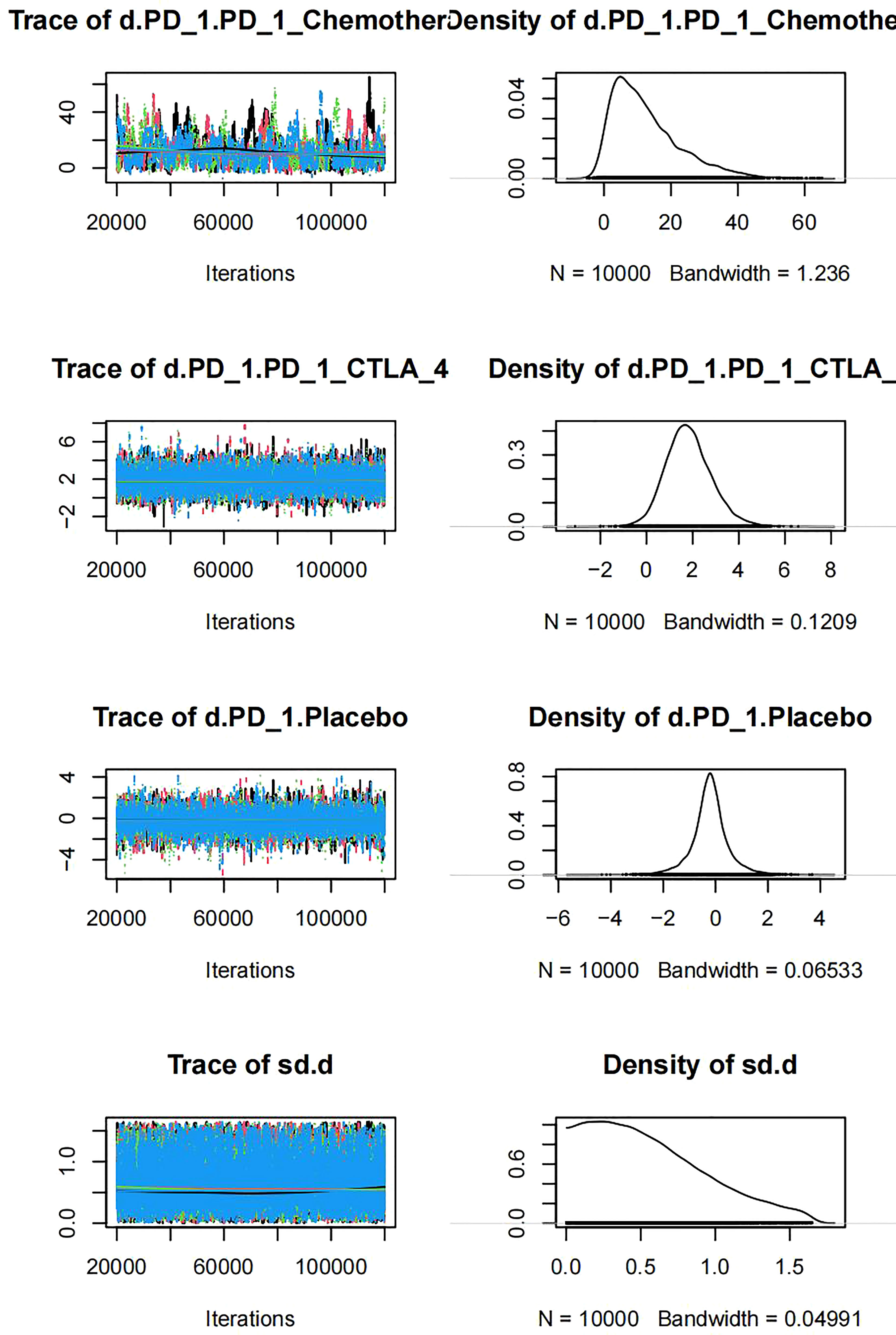
The trajectory indicated that when the number of iterations reached more than 5000, the MCMC chain reached a stable fusion from the initial portion, and the overlapping portion accounted for most of the chain fluctuation range in the subsequent calculation (Figure 6). The density graph indicated that when the number of iterations reached 20000, the bandwidth tended to be zero and reached stability, which indicated that the model converged well.
Publication bias
There were no detectable differences in any of the other comparisons. The network analysis funnel plot was approximately symmetrical (Figure 7). The p value after Egger’s test was 0.949, which indicated that there was no significant publication bias in the data.
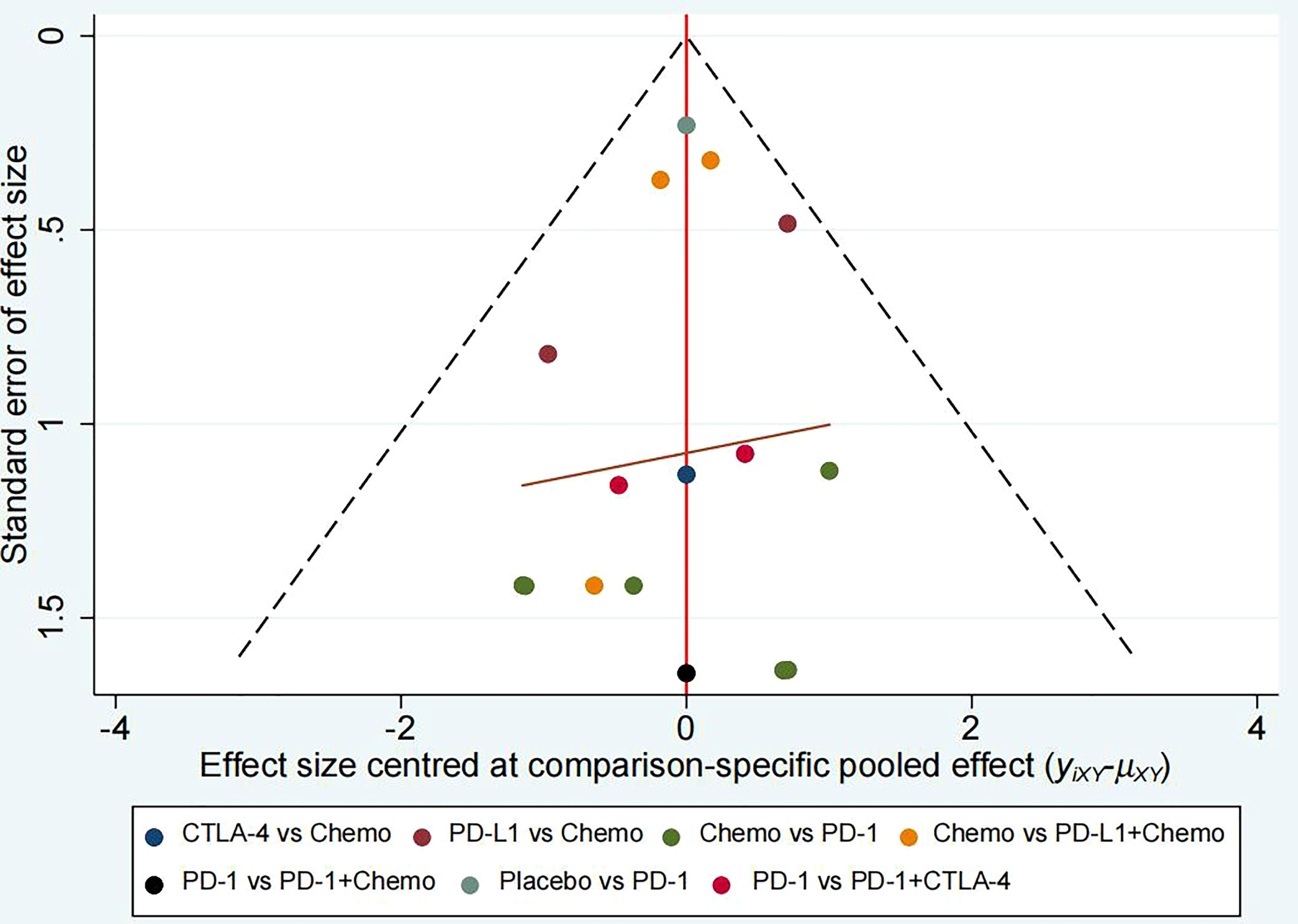
Figure 7 Comparison-adjusted funnel plot for mean overall change in cardiotoxicity in all comparisons.
Discussion
The present network meta-analysis evaluated cardiotoxicity following different combinations of immunosuppressants in lung cancer patients. The cumulative number of patients in the experimental arm of each included clinical trial was 5933.
The magnitude of cardiotoxic effect estimates varied widely across different combinations of immunosuppressants. The CTLA-4+chemotherapy and PD-L1 treatments were less toxic than placebo or control, while PD-L1 ranked higher than PD-1, which indicated less cardiotoxicity (SUCRA, 75.2% vs. 46.4%). The toxicity of PD-L1+chemotherapy (OR, -0.23 [95% CI, -1.45 to 0.99]) and PD-1+CTLA-4 (OR, -1.58 [95% CI, - 3.12–0.03]) and was greater than that of PD-1 treatment alone. Of note, the PD-1+chemotherapy treatment was not statistically significant and may lack clinical significance.
Among cancer survivors, cardiotoxicity is considered a major cause of long-term morbidity and mortality. When cancer patients benefit from anticancer drugs, they should also receive specific interventions to treat cardiotoxic complications (29). The Canadian Cardiovascular Society guidelines have recommended that ACE inhibitors or ARBs and/or β blockers and/or statins should be considered in patients at high risk of left ventricular dysfunction associated with cancer therapy to reduce the risk of cardiotoxicity (29). Clinically, captopril and enalapril have been used in cardioprotective strategies as chemotherapy adjuvants to reduce oxidative stress and minimize the production of free radicals to reduce cardiotoxicity (30).
ICIs have not been introduced for cancer treatment as checkpoint inhibitors until the last 5 years. Tumour cells have the ability to evade or quiesce the host immune system by utilizing immunomodulatory mechanisms (31). ICIs target these escape pathways, allowing the immune system to recognize and target cancer cells (32). ICI is a monoclonal antibody that targets the CTLA-4, PD-1, and PD-L1 inhibitory receptors. These drugs enable the immune system to attack tumour cells (32). Thus, mechanisms to prevent autoimmune responses are inhibited, explaining most of the potential autoimmune-related side effects associated with this class of drugs, such as rash, elevated aminotransferases, hypothyroidism, pneumonia, autoimmune hepatitis, and pituitary inflammation (33). The PD-1/PD-L1 pathway is essential for immune homeostasis within the myocardium and cardiac protection of T cells (34). It has been hypothesized that dysregulated immune cells falsely label surface structures, such as cardiolipin as antigens, resulting in subsequent targeting of normal cardiomyocytes or other cells expressing these antigens (35). The PD-1/PD-L1 pathway provides mechanisms of action for the various cardiotoxicities that occur after ICIs are used.
Myocarditis is inflammation of the heart muscle. ICI therapy can cause myocarditis with signs of dyspnoea, fatigue, and chest pain as well as elevated electrocardiogram (ECG) and myocardial enzyme profiles on examination (36). Similarly, ICIs can cause pericardial disorders, such as pericardial effusion and pericarditis (37). Cardiotoxicity is rare, occurring in only 0.04% to 1.14% of patients receiving immunotherapy (38). The present study indicated that receiving dual ICI combination therapy (e.g., CTLA-4 inhibitor combined with PD-1 inhibitor) was the clearest risk factor for ICI-related cardiotoxicity. Previous studies have reported that the combination of nivolumab and ipilimumab is associated with a 4.74-fold risk of myocarditis compared to nivolumab alone. Compared to myocarditis caused by ICI monotherapy, myocarditis caused by ICI combination therapy is also more likely to be severe (39). A previous trial of patients with advanced renal cell carcinoma who received avelumab and axitinib (antiangiogenic therapy) has indicated that the incidence of fatal myocarditis is 2% (40). Experimental models using transgenic mice have shown that immune checkpoints play a key role in the heart muscle; inflammation is especially harmful in this case because the heart muscle lacks redundancy and cannot regenerate (41). The integrity of PD-1, PD-L1, and CTLA-4 signalling is critical for downregulating excessive immune responses in the myocardia. Dilated cardiomyopathy and premature death in PD-1-deficient mice are due to a high titre of IgG autoantibodies against cardiac troponin I, which increases voltage-dependent L-type calcium currents in normal cardiomyocytes (42).
Drugs may have controllable safety as a single drug treatment, but combined use may induce severe AEs in susceptible patients. Among various types of cancer, tumours with high tumour mutational burden (TMB) caused by dysfunction during the DNA damage response (DDR) may have better clinical outcomes when receiving ICI treatment, such as CTLA-4 and PD-1 (43). The combination of PD-1 and CTLA-4 blockers is associated with an increased incidence of adverse events, accompanied by severe myocarditis in some patients (44). Bintrafusp alfa, a bifunctional fusion protein consisting of the transforming growth factor β (TGF-β) receptor fused to a human immunoglobulin G1 antibody that blocks programmed death ligand 1 (PD-L1), has been evaluated for safety and efficacy in patients with advanced NSCLC, breast cancer, and pancreatic cancer (45–47). Mitra et al. administered TGF-β in combination with PD-L1 antibody to mice for 5 weeks and observed acute bleeding and cardiovascular toxicity (48). In a total of 31 patients with recurrent gastric cancer treated with Bintrafusp alfa, Kang et al. reported that 6 patients (19%) experienced grade 3 treatment-related adverse events and no grade 4 events with a disease control rate of 26% (49). In addition, Kang et al. reported that 59 patients with advanced HPV-related cancer were treated with Bintrafusp alfa and had a clinical response rate of 35.6%. Moreover, treatment-related adverse events occurred in 49 patients (83.1%), and grade 3 or 4 adverse events occurred in 16 patients (27.1%). No treatment-related deaths occurred during follow-up (50). The median overall survival of patients with biliary tract cancer was 12.7 months, and the probability of grade 3 or above TRAE was 37% (51).
In patients treated with ICIs, various types of arrhythmias have been reported, including life-threatening complete atrioventricular block or ventricular tachyarrhythmia. The most common is atrial fibrillation followed by ventricular tachycardia or ventricular fibrillation and atrioventricular conduction disorder. Conduction disorders are associated with increased cardiovascular mortality in patients treated with ICIs (52). The pathological mechanism of arrhythmia induced by immunotherapy remains unclear. Once an ECG shows a prolonged PR interval, QT interval, and bundle branch block, the threshold for introducing cardiac pacing should be lowered because the conduction disorder may rapidly progress to late cardiac block (53). To avoid fatal adverse events, all patients receiving ICI treatment should undergo regular ECG screening.
Management Principles The treatment strategies for ICI-related cardiovascular complications were multifold as follows: discontinuation or reduction of ICI to prevent further toxicity; immunosuppression to alleviate inflammatory changes; and supportive therapy to address cardiac complications. Due to the complexity involved and the limited data available, the management of cardiotoxicity with ICI treatment should be conducted through discussions between oncologists and cardiologists. Patients should be given continuous ECG and haemodynamic monitoring when severe adverse cardiac events occur (54, 55). Immunosuppressive therapy for ICI-associated myocarditis requires large doses of corticosteroids. In cases that do not respond to corticosteroids, both recommendations suggest considering the use of infliximab (54, 55). It should be noted that infliximab may induce exacerbation of severe heart failure. Elevated serum troponin T levels have been used to assess the prognosis and diagnosis of major cardiovascular adverse events. In addition to the use of troponin, natriuretic peptide has also been proposed for screening and surveillance in high-risk patients with immune checkpoint inhibitor-associated myocarditis (38). Patients using ICIs still need regular monitoring of cardiac function in the clinic, such as cardiac troponin, ECG, and cardiac ultrasound. Genetic and tumour-specific factors of the patient also need to be considered when selecting immunotherapy and combination regimens to avoid resistance and adverse effects to these therapies (56). Clinicians should keep a close eye on the clinical manifestations during immunotherapy.
The present study had several limitations. First, because data from clinical trials were analysed, confounding factors, such as the underlying disease and previous treatment of the patient, cannot be ruled out. Second, due to data limitations, no group histological analysis was performed for lung squamous cell carcinoma and adenocarcinoma. Third, the control arm had different chemotherapy drugs and combinations as well as different dose regimens and different study durations included in the trial, which may lead to data bias. As there were few trials, we were unable to divide them in more detail.
Conclusion
The present meta-analysis compared cardiac adverse events following ICI therapy in lung cancer patients. The network meta-analysis showed that CTLA-4+chemotherapy and PD-L1 are least likely to cause cardiotoxicity when classified by inhibitor combination. Moreover, PD-1 alone is more cardiotoxic than PD-L1, and treatment with the PD-1+CTLA-4 dual ICI caused the highest risk of adverse cardiac events. Therefore, evidence of cardiotoxicity should be considered when assessing the benefits and risks of ICI in the treatment of lung cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: CJ, MT. Data curation: CJ, JQ, QW. Formal analysis: CP, CJ. Investigation: MT. Project administration: CJ, MT, JQ. Software: JQ, QW. Supervision: MT. Writing—original draft: JQ, QW, CP. Writing—review, and editing: CJ. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Shandong Medical and Health Science and Technology Development Plan Project (2019WS384), and Key Technology Research and Development Plan of Shandong Province (2018GSF118194).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lenneman CG, Sawyer DB. Cardio-oncology: An update on cardiotoxicity of cancer-related treatment. Circ Res (2016) 118(6):1008–20. doi: 10.1161/CIRCRESAHA.115.303633
2. Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: Pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc (2020) 9(2):e013757. doi: 10.1161/JAHA.119.013757
3. Sun JY, Zhang D, Wu S, Xu M, Zhou X, Lu XJ, et al. Resistance to PD-1/PD-L1 blockade cancer immunotherapy: Mechanisms, predictive factors, and future perspectives. Biomark Res (2020) 8:35. doi: 10.1186/s40364-020-00212-5
4. Qin W, Hu L, Zhang X, Jiang S, Li J, Zhang Z, et al. The diverse function of PD-1/PD-L pathway beyond cancer. Front Immunol (2019) 10:2298. doi: 10.3389/fimmu.2019.02298
5. Canale ML, Camerini A, Casolo G, Lilli A, Bisceglia I, Parrini I, et al. Incidence of pericardial effusion in patients with advanced non-small cell lung cancer receiving immunotherapy. Adv Ther (2020) 37(7):3178–84. doi: 10.1007/s12325-020-01386-y
6. Zhang L, Reynolds KL, Lyon AR, Palaskas N, Neilan TG. The evolving immunotherapy landscape and the epidemiology, diagnosis, and management of cardiotoxicity: JACC: CardioOncology primer. JACC CardioOncology (2021) 3(1):35–47. doi: 10.1016/j.jaccao.2020.11.012
7. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed) (2021) 372:n71. doi: 10.1136/bmj.n71
8. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical Res ed) (2011) 343:d5928. doi: 10.1136/bmj.d5928
9. Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods (2012) 3(2):161–76. doi: 10.1002/jrsm.57
10. Brown S, Hutton B, Clifford T, Coyle D, Grima D, Wells G, et al. A Microsoft-excel-based tool for running and critically appraising network meta-analyses–an overview and application of NetMetaXL. Sys Rev (2014) 3:110. doi: 10.1186/2046-4053-3-110
11. Tonin FS, Rotta I, Mendes AM, Pontarolo R. Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharm Prac (2017) 15(1):943. doi: 10.18549/PharmPract.2017.01.943
12. Yang CJ, McSherry F, Mayne NR, Wang X, Berry MF, Tong B, et al. Surgical outcomes after neoadjuvant chemotherapy and ipilimumab for non-small cell lung cancer. Ann Thorac Surg (2018) 105(3):924–9. doi: 10.1016/j.athoracsur.2017.09.030
13. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol (2021) 22(1):51–65. doi: 10.1016/S1470-2045(20)30539-8
14. Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN lung 200): an open-label, randomised, phase 3 study. Lancet Oncol (2018) 19(11):1468–79. doi: 10.1016/S1470-2045(18)30673-9
15. O'Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol (2022) 23(10):1274–86. doi: 10.1016/S1470-2045(22)00518-6
16. Taniguchi Y, Shimokawa T, Takiguchi Y, Misumi T, Nakamura Y, Kawashima Y, et al. A randomized comparison of nivolumab versus nivolumab + docetaxel for previously treated advanced or recurrent ICI-naïve non-small cell lung cancer: TORG1630. Clin Cancer Res (2022) 28(20):4402–9. doi: 10.1158/1078-0432.CCR-22-1687
17. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-Small-Cell lung cancer. New Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
18. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet (London England) (2021) 398(10308):1344–57. doi: 10.1016/S0140-6736(21)02098-5
19. Herbst RS, Garon EB, Kim DW, Cho BC, Perez-Gracia JL, Han JY, et al. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1−Positive, advanced Non−Small-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol (2020) 38(14):1580–90. doi: 10.1200/JCO.19.02446
20. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (London England) (2019) 393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7
21. Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol (2019) 14(5):867–75. doi: 10.1016/j.jtho.2019.01.006
22. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-Small-Cell lung cancer. New Engl J Med (2017) 376(25):2415–26. doi: 10.1056/NEJMoa1613493
23. Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet (London England) (2021) 397(10274):592–604. doi: 10.1016/S0140-6736(21)00228-2
24. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1-Selected patients with NSCLC. New Engl J Med (2020) 383(14):1328–39. doi: 10.1056/NEJMoa1917346
25. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(7):924–37. doi: 10.1016/S1470-2045(19)30167-6
26. Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: Results from the randomized phase 3 IMpower132 trial. J Thorac Oncol (2021) 16(4):653–64. doi: 10.1016/j.jtho.2020.11.025
27. Boyer M, Şendur MAN, Rodríguez-Abreu D, Park K, Lee DH, Çiçin I, et al. Pembrolizumab plus ipilimumab or placebo for metastatic non-Small-Cell lung cancer with PD-L1 tumor proportion score ≥ 50%: Randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol (2021) 39(21):2327–38. doi: 10.1200/JCO.20.03579
28. Gettinger SN, Redman MW, Bazhenova L, Hirsch FR, Mack PC, Schwartz LH, et al. Nivolumab plus ipilimumab vs nivolumab for previously treated patients with stage IV squamous cell lung cancer: The lung-MAP S1400I phase 3 randomized clinical trial. JAMA Oncol (2021) 7(9):1368–77. doi: 10.1001/jamaoncol.2021.2209
29. Virani SA, Dent S, Brezden-Masley C, Clarke B, Davis MK, Jassal DS, et al. Canadian Cardiovascular society guidelines for evaluation and management of cardiovascular complications of cancer therapy. Can J Cardiol (2016) 32(7):831–41. doi: 10.1016/j.cjca.2016.02.078
30. Janbabai G, Nabati M, Faghihinia M, Azizi S, Borhani S, Yazdani J. Effect of enalapril on preventing anthracycline-induced cardiomyopathy. Cardiovasc Toxicol (2017) 17(2):130–9. doi: 10.1007/s12012-016-9365-z
31. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239
32. Hwu P. Treating cancer by targeting the immune system. New Engl J Med (2010) 363(8):779–81. doi: 10.1056/NEJMe1006416
33. Heinzerling L, Ott PA, Hodi FS, Husain AN, Tajmir-Riahi A, Tawbi H, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer (2016) 4:50. doi: 10.1186/s40425-016-0152-y
34. Grabie N, Lichtman AH, Padera R. T Cell checkpoint regulators in the heart. Cardiovasc Res (2019) 115(5):869–77. doi: 10.1093/cvr/cvz025
35. Lobenwein D, Kocher F, Dobner S, Gollmann-Tepeköylü C, Holfeld J. Cardiotoxic mechanisms of cancer immunotherapy - a systematic review. Int J Cardiol (2021) 323:179–87. doi: 10.1016/j.ijcard.2020.08.033
36. Spallarossa P, Tini G, Sarocchi M, Arboscello E, Grossi F, Queirolo P, et al. Identification and management of immune checkpoint inhibitor-related myocarditis: Use troponin wisely. J Clin Oncol (2019) 37(25):2201–5. doi: 10.1200/JCO.18.02464
37. Imazio M, Gaita F, LeWinter M. Evaluation and treatment of pericarditis: A systematic review. Jama (2015) 314(14):1498–506. doi: 10.1001/jama.2015.12763
38. Kumar M, Thangavel C, Becker RC, Sadayappan S. Monoclonal antibody-based immunotherapy and its role in the development of cardiac toxicity. Cancers (2020) 13(1):86. doi: 10.3390/cancers13010086
39. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. New Engl J Med (2016) 375(18):1749–55. doi: 10.1056/NEJMoa1609214
40. Choueiri TK, Larkin J, Oya M, Thistlethwaite F, Martignoni M, Nathan P, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol (2018) 19(4):451–60. doi: 10.1016/S1470-2045(18)30107-4
41. Ertl G, Frantz S. Healing after myocardial infarction. Cardiovasc Res (2005) 66(1):22–32. doi: 10.1016/j.cardiores.2005.01.011
42. Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Sci (New York NY) (2001) 291(5502):319–22. doi: 10.1126/science.291.5502.319
43. Xu Y, Nowsheen S, Deng M. DNA Repair deficiency regulates immunity response in cancers: Molecular mechanism and approaches for combining immunotherapy. Cancers (2023) 15(5):1619. doi: 10.3390/cancers15051619
44. Lemaire V, Shemesh CS, Rotte A. Pharmacology-based ranking of anti-cancer drugs to guide clinical development of cancer immunotherapy combinations. J Exp Clin Cancer Research: CR (2021) 40(1):311. doi: 10.1186/s13046-021-02111-5
45. Paz-Ares L, Kim TM, Vicente D, Felip E, Lee DH, Lee KH, et al. Bintrafusp Alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in second-line treatment of patients with NSCLC: Results from an expansion cohort of a phase 1 trial. J Thorac Oncol (2020) 15(7):1210–22. doi: 10.1016/j.jtho.2020.03.003
46. Yi M, Wu Y, Niu M, Zhu S, Zhang J, Yan Y, et al. Anti-TGF-β/PD-L1 bispecific antibody promotes T cell infiltration and exhibits enhanced antitumor activity in triple-negative breast cancer. J Immunother Cancer (2022) 10(12):e005543. doi: 10.1136/jitc-2022-005543
47. Melisi D, Oh DY, Hollebecque A, Calvo E, Varghese A, Borazanci E, et al. Safety and activity of the TGFβ receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J Immunother Cancer (2021) 9(3):e002068. doi: 10.1136/jitc-2020-002068
48. Mitra MS, Lancaster K, Adedeji AO, Palanisamy GS, Dave RA, Zhong F, et al. A potent pan-TGFβ neutralizing monoclonal antibody elicits cardiovascular toxicity in mice and cynomolgus monkeys. Toxicol Sci (2020) 175(1):24–34. doi: 10.1093/toxsci/kfaa024
49. Kang YK, Bang YJ, Kondo S, Chung HC, Muro K, Dussault I, et al. Safety and tolerability of bintrafusp Alfa, a bifunctional fusion protein targeting TGFβ and PD-L1, in Asian patients with pretreated recurrent or refractory gastric cancer. Clin Cancer Res (2020) 26(13):3202–10. doi: 10.1158/1078-0432.ccr-19-3806
50. Strauss J, Gatti-Mays ME, Cho BC, Hill A, Salas S, McClay E, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with human papillomavirus-associated malignancies. J Immunother Cancer (2020) 8(2):e001395. doi: 10.1136/jitc-2020-001395
51. Yoo C, Oh DY, Choi HJ, Kudo M, Ueno M, Kondo S, et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J Immunother Cancer (2020) 8(1):e000564. doi: 10.1136/jitc-2020-000564
52. Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation (2017) 136(21):2085–7. doi: 10.1161/CIRCULATIONAHA.117.030571
53. Sławiński G, Wrona A, Dąbrowska-Kugacka A, Raczak G, Lewicka E. Immune checkpoint inhibitors and cardiac toxicity in patients treated for non-small lung cancer: A review. Int J Mol Sci (2020) 21(19):7195. doi: 10.3390/ijms21197195
54. Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol (2018) 19(9):e447–e58. doi: 10.1016/S1470-2045(18)30457-1
55. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol (2018) 36(17):1714–68. doi: 10.1200/JCO.2017.77.6385
Keywords: lung cancer, cardiotoxicity, immunotherapy, CTLA-4, chemotherapy
Citation: Jin C, Qi J, Wang Q, Pu C and Tan M (2023) Cardiotoxicity of lung cancer-related immunotherapy versus chemotherapy: a systematic review and network meta-analysis of randomized controlled trials. Front. Oncol. 13:1158690. doi: 10.3389/fonc.2023.1158690
Received: 04 February 2023; Accepted: 31 March 2023;
Published: 14 April 2023.
Edited by:
Anand Rotte, Arcellx Inc, United StatesCopyright © 2023 Jin, Qi, Wang, Pu and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingming Tan, 413938533@qq.com
 Chengwei Jin
Chengwei Jin Jia Qi1
Jia Qi1