- 1Flint Animal Cancer Center, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, CO, United States
- 2Department of Microbiology, Immunology, and Pathology, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, CO, United States
- 3Center for Cancer and Blood Disorders, Children’s Hospital Colorado, Aurora, CO, United States
Fueled by support from the National Cancer Institute’s “Cancer Moonshot” program, the past few years have witnessed a renewed interest in the canine spontaneous cancer model as an invaluable resource in translational oncology research. Increasingly, there is awareness that pet dogs with cancer provide an accessible bridge to improving the efficiency of cancer drug discovery and clinical therapeutic development. Canine tumors share many biological, genetic, and histologic features with their human tumor counterparts, and most importantly, retain the complexities of naturally occurring drug resistance, metastasis, and tumor-host immune interactions, all of which are difficult to recapitulate in induced or genetically engineered murine tumor models. The utility of canine models has been particularly apparent in sarcoma research, where the increased incidence of sarcomas in dogs as compared to people has facilitated comparative research resulting in treatment advances benefitting both species. Although there is an increasing awareness of the advantages in using spontaneous canine sarcoma models for research, these models remain underutilized, in part due to a lack of more permanent institutional and cross-institutional infrastructure to support partnerships between veterinary and human clinician-scientists. In this review, we provide an updated overview of historical and current applications of spontaneously occurring canine tumor models in sarcoma research, with particular attention to knowledge gaps, limitations, and growth opportunities within these applications. Furthermore, we propose considerations for working within existing veterinary translational and comparative oncology research infrastructures to maximize the benefit of partnerships between veterinary and human biomedical researchers within and across institutions to improve the utility and application of spontaneous canine sarcomas in translational oncology research.
Introduction
Sarcomas are a heterogeneous group of neoplasms which arise from mesenchymal cells within tissues derived from the embryonic mesoderm. Sarcomas are rare in people and account for only about 1% of all cancers, though pediatric patients are disproportionately burdened by this tumor type as sarcomas represent greater than 20% of pediatric solid tumors (1). Sarcomas are broadly subdivided into categories of bone and soft tissue sarcomas, however there are over 70 histologically and genetically distinct sarcoma subtypes recognized, and the incidence of each of these unique sarcoma subtypes is substantially lower. Thus, patient recruitment for clinical trials in rarer sarcomas remains a significant barrier to the development of new therapies. Despite the rarity of sarcomas, sarcoma research has historically made an outsized contribution to our understanding of the fundamental biology of cancer and laid the groundwork for remarkable advances in cancer treatment including immunotherapy and personalized small-molecule inhibitor therapy (2). Still, treatment advances for sarcoma patients have lagged behind more common cancer types, particularly with regard to the paradigm-shifting cell-based therapies recently developed for hematologic malignancies. Novel therapies for treatment-refractory, relapsed, and metastatic sarcomas are lacking and overall survival remains quite poor for these patients.
Animal models are essential for testing new interventions for these rare cancers at a meaningful and time-efficient scale. Immune competent and immunodeficient murine models are often utilized in pre-clinical sarcoma research and have provided the rationale for novel therapies. While studying experimentally induced tumors in mouse models is cost-effective, accessible, and affords a high degree of reproducibility, the limitations of these models are becoming increasingly apparent. It is estimated that 85% of novel drugs which show promise in preclinical testing will fail in early clinical trials. The failure rate for cancer drugs is even greater, with as few as 8% of novel cancer drugs translated successfully from preclinical animal models to human clinical trials (3–5). The statistics surrounding sarcoma drug development are even more discouraging, with estimates suggesting only 5% of Phase I/II sarcoma trials make it to Phase III studies (6). This unacceptable outcome is in part driven by the fact that many of the drugs tested in sarcoma patients are drugs originally developed, tested, or approved for other cancer types (6). Most of these failures are attributed not to drug safety concerns, but rather to lack of efficacy (7), suggesting that in vivo preclinical testing as currently performed is poorly predictive of clinical efficacy. Given the complex interplay of genetic, biological, and environmental factors influencing disease phenotype and outcomes in cancer patients, it is unsurprising that preclinical rodent models are limited in their ability to predict treatment efficacy in humans and it is apparent that incorporation of additional representative animal models is desperately needed to enhance the efficiency and success of cancer drug development.
Pet dogs with spontaneously occurring cancers have received increasing attention as a promising model to bridge the translational gap between rodent tumor models and human clinical trials. Cancer and associated complications are the leading cause of death in most dog breeds and mixed breed dogs in North America, and their large size, relative outbreeding, and shared environmental exposures with their human caregivers are advantageous for modeling human cancers. In contrast with their rare incidence in people, sarcomas comprise a much larger proprotion of malignant tumors in dogs (8). The increased incidence of sarcomas in dogs provides both an impetus for veterinary sarcoma research and a comparative research opportunity. Canine sarcomas share many genetic, biologic, and histologic features with their human counterparts. Most importantly, canine spontaneous cancer models retain the complexities of naturally occurring therapeutic resistance, spontaneous metastasis, and tumor-host immune interactions seen in human patients. Here, we provide an overview of translationally relevant spontaneously occurring canine sarcomas and offer perspectives on optimal application of these models in current and future translational research (Figure 1), as well as considerations for expanding translational research training and facilitating greater inclusion of veterinary clinician-scientists into cross-disciplinary cancer research teams.
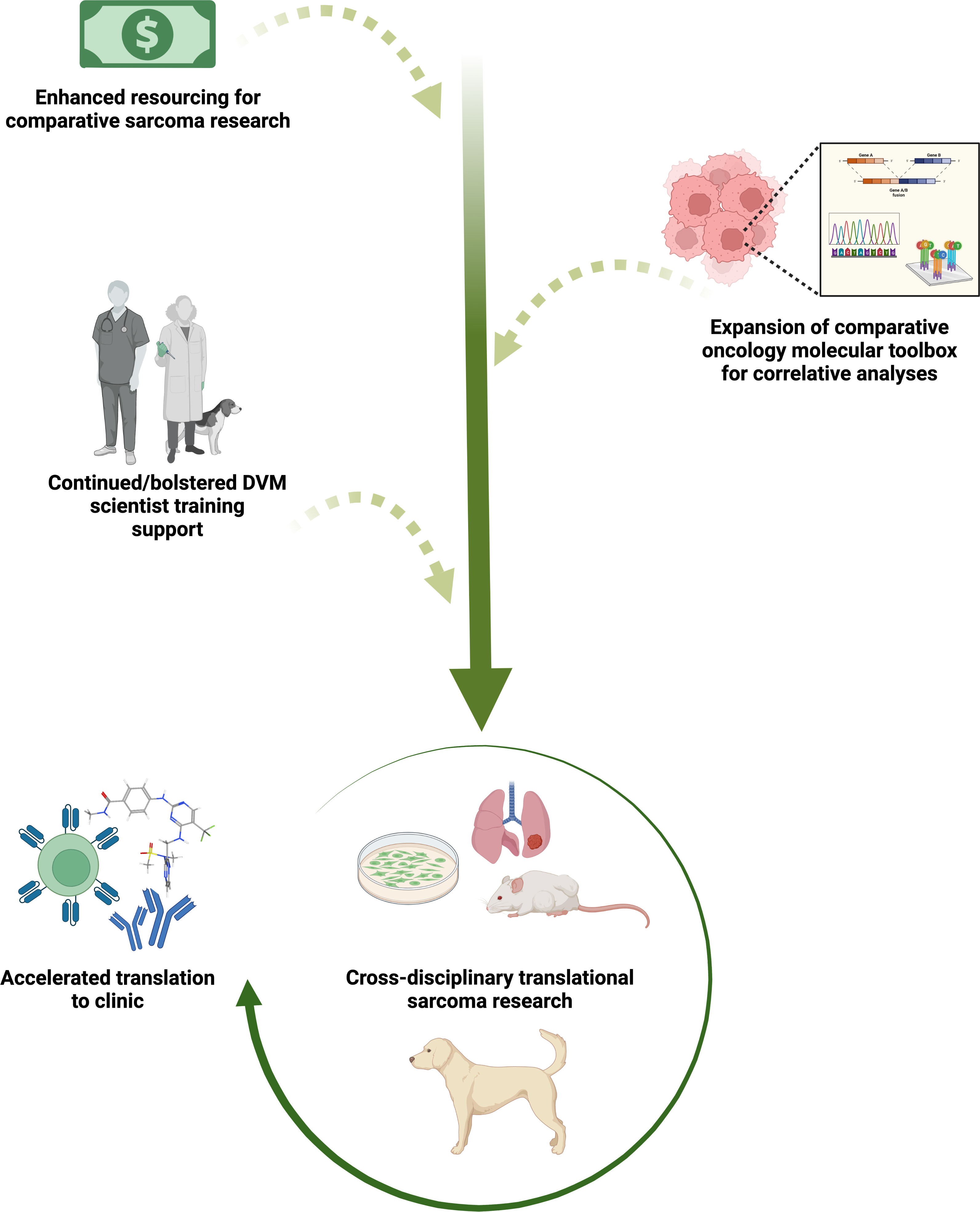
Figure 1 Sustaining and bolstering existing comparative oncology research infrastructure for continued acceleration of sarcoma therapeutic discovery and translation to clinical trial evaluation. Increasing comparative oncology research funding and industry investment in development of species-specific molecular tools, paired with veterinary and medical collaborative sarcoma research, will enhance an already effective pathway for more predictive vetting of novel preclinical agents and movement into human clinical trials. Evaluation of small molecule immunotherapies, novel molecular-targeted agents, monoclonal/bi-specific antibodies, and adoptive cell therapies alone and in combination are likely to be especially informative to the field. Created with BioRender.com.
Naturally occurring canine sarcomas
Osteosarcoma
Osteosarcoma (OS) is the most common primary malignant bone tumor in both dogs and people (1, 8). However, it remains a rare disease in people and accounts for approximately 2% of all childhood cancers, and less than 1% of adult cancers (1). Based on recent OS incidence estimates, less than 1000 new cases of OS are diagnosed yearly in the United States (9), and the rarity of this tumor has greatly limited its study in human patients. In contrast, canine osteosarcoma accounts for about 5% of all canine cancers and the incidence of OS in dogs is estimated to be 10-30 times higher than in people (though incidence rates vary across dog breeds), with at least 10,000 new cases diagnosed in the US each year by conservative estimates (10–12). Thus, the inclusion of dogs with OS can greatly expand the available patient populations for translational OS research.
Appendicular OS arising from the metaphysis of long bones is the most common and best researched form in both people and dogs and has a remarkably similar clinical presentation in both species, including a similar bimodal age distribution (12, 13–15). However, notable differences in affected sites are seen between these species, with a majority of canine OS occurring in the thoracic limbs while OS in people most commonly affects the long bones of the pelvic limb (13, 15). Thoracic limb OS is associated with an increased risk of metastasis and subsequently a more rapid and aggressive disease course, and thus this apparent site predilection makes dogs a beneficial model for studying the biology and treatment of OS within the context of the more aggressive disease course seen with thoracic limb osteosarcoma (13, 15, 16). The histological subtypes of OS observed in dogs also closely parallel those observed in people (17–20), and the most common OS subtypes in people are seen with similar prevalence in the dog (13, 19–21). However, the prognostic importance of OS histological subclassification in dogs is unknown and it is not a feature included in the histological grading of canine OS (22). Additionally, reported cases of juxtacortical (periosteal and parosteal) OS in dogs are rare, and it is not known whether these surface OS subtypes carry an improved prognosis as is documented in people (19, 23, 24). Retrospective analyses indicate that metastatic disease in both human and canine OS results in worse clinical outcomes. Strikingly, over 80% of patients of both species are presumed to have micrometastases at diagnosis, despite a minority of these patients having detectable metastatic disease (25, 26). Thus, prevention and treatment of metastatic disease represents a critical area for utilization of canine OS models to improve survival rates which have been largely stagnant over the past several decades for both species.
The molecular landscape of canine OS has been relatively well characterized compared with other canine sarcomas. Comparative genomic analysis of OS from dogs and people consistently identifies striking similarities between these tumors, with one study finding them indistinguishable by global gene expression signature (27). The genomic structural complexity in canine OS appears to parallel that of human OS, with both patterns of localized somatic hypermutation consistent with kataegis as well as complex chromosomal rearrangements suggestive of chromothripsis identified in canine OS tumors (28). Furthermore, multiple studies corroborate genomic aberrations in both human and canine OS converging on key shared tumorigenic pathways, with both tumors characterized by a high prevalence of TP53 mutations (28–31) and hyperactivated PI3K/MAPK signaling (28, 30–33).
While further exploration is warranted (and ongoing), the genetic similarities between the canine and human tumors suggest rich opportunities for utilization of canine OS models in the development of new prognostication methods and personalized, targeted therapeutics. Indeed, comparison of the genetic signatures of canine and human OS have led to identification of a pair of genes (CXCL8 and SLC1A3) for which high expression in human tumors was linked with poorer clinical outcome (27). The clinical significance of one of these genes, CXCL8, on lung metastatic OS progression and response to macrophage-targeted immunotherapeutic intervention was also subsequently verified in two independent studies in people and dogs, respectively (34, 35). Unlike many rodent tumor models, dogs with OS also offer an opportunity to investigate tumor-immune interactions within an immunocompetnt host, and because of this have proven instrumental in identifying the mechanisms by which OS may modulate local and global anti-tumor immune responses to promote progression and metastasis (36–38). Additionally, though the general principles of treatment protocols are similar across these species (39–42), neoadjuvant or multi-drug adjuvant chemotherapeutic protocols are infrequently pursued in dogs due to quality-of-life concerns in the face of questionable clinical benefit. This makes dogs with OS a unique patient population in which to assess immunologic targets and therapies in the neo-adjuvant setting where they may demonstrate greater prognostic and therapeutic benefit.
Vascular sarcomas
Angiosarcoma (AS) refers to a group of sarcomas which exhibit differentiation toward vascular or lymphatic endothelial cells. These are among the rarest of the sarcoma types in humans, comprising less than 1% of all soft tissue sarcomas (43). The incidence of hemangiosarcoma (HSA), the canine analog of AS, is estimated to be 25 to 100 times higher than in people, and HSA accounts for 5% of all non-cutaneous primary malignant neoplasms in dogs (15, 44). However, it should be noted that while the human angiosarcoma term encompasses multiple vascular sarcomas including lymphangiosarcoma, these tumors are still denoted separately in veterinary literature with hemangiosarcoma commonly described while only 30 cases of lymphangiosarcoma have been reported since this tumor was first documented in the dog in 1981 (45, 46). The extremely rare and heterogeneous nature of AS has made researching this tumor in human patients particularly challenging, and treatment options for people with AS remain limited and patient mortality high. The much higher incidence of a similar tumor in dogs highlights an important opportunity for comparative research with the potential to generate novel treatment strategies.
The clinical presentations of AS and HSA are similar with both tumors diagnosed predominantly in older patients (43, 47). However, the distribution of affected sites differs between dogs and people, likely in part due to differences in environmental exposures (44). UV light exposure and radiation treatment for other cancer types (particularly breast cancer) are well-documented risk factors for AS, and correspondingly this neoplasm most frequently presents in the cutaneous and subcutaneous tissues of the head, neck, and breast (48, 49). In contrast, visceral HSA affecting the spleen or right cardiac atrium is the most common presentation in the dog (47), though UV-light exposure has also been implicated in the development of cutaneous HSA in this species (50, 51). The pathologic features of HSA closely parallel those of AS, with neoplastic cells characterized by variable vascular differentiation and expression of endothelial-associated markers CD34, CD31, and von Willebrand’s factor (52–57). A histologic grading scheme has not been established for canine HSA, but tumor stage and primary site appear to be useful grade-independent prognostic factors for both species (58–62).
AS and HSA exhibit similarly aggressive biologic behavior with highly infiltrative and invasive patterns of local growth and a high risk of metastasis contributing to the poor clinical outcomes seen in both species (44, 48–50). The 1-year survival rate in dogs who underwent surgical resection followed by adjuvant chemotherapy is only about 10% (63), and in humans the five-year survival rate is only about 35% to 40% despite multi-modal standard-of-care treatment with surgery followed by adjuvant radiotherapy and chemotherapy (64–66). Though shorter survival times in dogs with HSA likely also reflect differences in treatment intent and stage at diagnosis, as in OS, the more rapid disease course of HSA in this species allows for the assessment of clinical endpoints within an accelerated timeline.
The cellular origins of both AS and HSA remain incompletely understood, but recent genomic profiling of canine HSA has driven paradigm shifts regarding the histogenesis of this tumor. Though classically thought of as arising from transformed endothelial cells, these data suggest a pluripotent bone marrow progenitor cell of origin for HSA (67, 68). While ontogenic molecular studies have been comparatively limited in human AS, the data also seem to support a similar bone marrow-derived pluripotent progenitor or early endothelial progenitor as a cell of origin in AS (69). A recent whole exome sequencing study of 20 HSA patients, and 30 more in a following study, identified multiple driver mutations shared with AS, including NRAS, PLCG1, PIK3CA and TP53 mutations with net activating effects on both the PI3K and MAPK signaling pathways (70, 71). Another recent study utilizing whole exome and RNA sequencing from a large cohort of Golden Retriever and mixed breed dog HSA showed additional shared molecular drivers including CDKN2A/B deletions and VEGFA, KDR, and KIT gain-of-function mutations (72). The driver mutations of KDR, TP53 and PIK3CA collectively identified across these three canine studies were also the primary recurrently mutated genes observed in an analysis of 47 human AS tumors by Painter et al. (73) Molecular data from both human AS and canine HSA increasingly support the presence of distinct molecular and functional tumor subtypes, which likely reflect differences in both cell-intrinsic mutations and influences from the tumor microenvironment and may have implications for prognosis and personalized treatments (68, 70, 74). Given the commonalities between HSA and AS within these proposed molecular subtypes, molecular subtyping may prove a promising means for overcoming disparate nomenclature schemes and enhancing translation of research between canine HSA and human AS.
Stromal soft tissue sarcomas
In canine patients, multiple histologic subtypes of sarcomas arising from cutaneous and subcutaneous connective tissues are grouped under a shared grading system and are referred to collectively as soft tissue sarcomas (STS) (75, 76). Despite their distinct phenotypes and histogenesis, certain tumor subtypes within this grouping have very similar microscopic features, and immunohistochemical testing is typically required for their definitive diagnosis. Grade and mitotic index have been shown to be prognostic for these tumors, but the clinical significance of subtype in canine STS is less clear (75–78) and thus subtyping is frequently not pursued given the added time and client cost involved. In people, the term soft tissue sarcoma refers more broadly to all sarcomas arising from non-bony tissues and includes multiple sarcoma subtypes which are specifically excluded from the canine STS grouping due to their overall more aggressive biologic behavior in dogs (75, 77, 78). Furthermore, classification of human soft tissue sarcomas is increasingly defined by molecular and immunochemical testing not routinely available in veterinary medicine (18). Thus, facilitating more granular subtyping of canine STS, as well as clarification of the discordant veterinary and medical nomenclature schemes will be necessary prerequisites for fully leveraging this diverse collection of canine tumors in future pre-clinical and clinical research. Utilization of whole exome/genome and RNA sequencing technologies for comprehensive identification of cancer somatic variants, gene fusions, and transcriptomic signatures in canine STS should be a priority of the field to address this gap and better define histogenesis and molecular subcategories of these canine tumors which could inform human research. In this review, we will use the term stromal STS (sSTS) to collectively refer to the subset of human soft tissue sarcomas with histotypes corresponding to those in the canine STS grouping.
While the general histologic features are similar between canine STS and human sSTS the distribution of histologic subtypes varies between these species. In dogs, fibrosarcoma, peripheral nerve sheath tumors, and perivascular wall tumors are most common among STS for which subtype is reported. In contrast, liposarcoma and undifferentiated pleomorphic sarcoma are the most commonly reported sSTS in people (79, 80). As in canine STS, grade also appears to be strongly predictive of clinical outcome in human sSTS (80). Treatment principles for canine STS and human sSTS are similar, with wide surgical resection with complete histologic margins pursued as the primary treatment of choice in patients of both species (79–83). Adjuvant radiation and chemotherapy are also used with variable reported benefit (81–86).
Molecular characterization of canine STS represents an area where increased focus could overcome challenges posed by differences in current classification and nomenclature schemes to facilitate inclusion of these tumors in translational studies. For example, dermatofibrosarcoma protuberans in people is characterized by a pathognomonic COL1A1-PDGFB gene fusion (87), and though this fibrosarcoma subtype is not recognized in canine patients, RNA-seq analysis of a dermatofibrosarcoma protuberans-like tumor from a dog revealed an equivalent COL3A1-PDGFB fusion suggesting the presence of an analogous canine tumor (88).
The genetic landscape of human malignant peripheral nerve sheath tumors (MPNST) reveals recurrent themes of Ras/Raf pathway activation and impairment of DNA repair and P53 regulation resulting from mutations in NF1 (a hallmark of the inherited disorder neurofibromatosis type 1 which confers increased risk of MPNST), BRAF and CDKN2A/B (89–91). Limited genetic characterization of canine PNST indicate that a subset also have a correlate to the activating BRAFV600E mutation described in the human tumor (92), and alterations to the P53 regulatory system in the form of P35 mutations or MDM2 amplification have also been described in PNST in dogs (93). Interestingly, chromosomal rearrangements resulting in loss of heterozygosity in the CDKN2A/B cluster region similar to the alterations in this region in human MPNST have also been documented in two canine STS described as poorly differentiated fibrosarcomas (94). The authors in this study do not state what modalities beyond light microscopy were used in subtyping, and given significant microscopic overlap between canine PNST and fibrosarcoma, it is possible that these tumors may also represent PNST with a similar mutational profile to human MPNST.
Liposarcoma is an uncommonly reported STS subtype in dogs, but one for which histologic classification closely parallels human classification. Several immunohistochemical studies in canine liposarcoma demonstrate some similarities with the human disease, including overexpression of MDM2 in well-differentiated and de-differentiated variants. However these studies fail to provide evidence for other histotype-characteristic genetic alterations used in the diagnosis of myxoid and pleomorphic liposarcoma variants in people (95, 96). More complete genomic characterization of canine liposarcomas and other tumors within the canine STS categorization would be beneficial to better define their potential comparative applications.
Other soft tissue sarcomas
Other rarer sarcoma subtypes occurring in dogs including gastrointestinal sarcomas (gastrointestinal stromal tumors (GIST) and leiomyosarcomas) and rhabdomyosarcoma, require further investigation but may also have potential applications as models for human disease. GIST and leiomyosarcoma are primary gastrointestinal sarcomas which account for only around 1% of GI malignancies in people (97, 98) but are reported to comprise up to 30% of all gastrointestinal neoplasms in dogs (99). These tumors are nearly indistinguishable microscopically, and prior to the routine use of immunohistochemistry for gastrointestinal biopsies, many GIST in both species were misdiagnosed as leiomyosarcomas (100–102). GIST and gastrointestinal leiomyosarcoma exhibit a range of biologic behavior, but distinction between these tumors is clinically important in people as activating mutations in exon 11 of the c-kit gene are implicated in the pathogenesis of many GIST and contribute to remarkable sensitivity of these tumors to tyrosine kinase inhibitors (103). As with many other sarcomas, cytogenetic data for canine gastrointestinal sarcomas are limited, but analogous activating c-kit mutations have been identified in canine GIST (104). Limited patient data has also demonstrated clinical benefit of the KIT-targeted tyrosine kinase inhibitor toceranib in canine GIST suggesting shared oncogenic mechanisms and therapeutic sensitivities that may make canine GIST a useful model for the human tumor.
Rhabdomyosarcoma refers to a group of malignant mesenchymal neoplasms which exhibit varying degrees of skeletal muscle differentiation. These neoplasms are rare in the veterinary literature, though this apparent low prevalence may be partially due to diagnostic challenges posed by the extreme variation in phenotype, age of presentation, and cellular morphology in these tumors (105). In people, rhabdomyosarcoma is the most common soft tissue sarcoma affecting children and teens, and over 50% of cases are diagnosed in children under 10 years of age (106). Similarly, and in contrast with the previously discussed sarcomas, canine rhabdomyosarcoma is also a disease primarily of young and adolescent animals (105). Canine rhabdomyosarcomas are classified using a scheme that is derived from human pathology, but which utilizes only histologic appearance and does not incorporate the immunohistochemical and molecular diagnostics that are now standard in subclassification of rhabdomyosarcomas in people. Based on this histologic classification scheme, the distribution of rhabdomyosarcoma subclasses in the dog appears similar to people (105, 106), suggesting that dogs with rhabdomyosarcomas may represent a yet largely untapped patient population which shares characteristics with the human rhabdomyosarcoma patient population. We recently published a small retrospective study on canine rhabdomyosarcoma which optimized immunohistochemical labeling for the myogenic transcription factors myogenin and MyoD1 in these tumors. This study provides a resource which may increase identification of these tumors in the canine population for future comparative biology investigations (107). To date only one cytogenetic examination has been performed on a single cell line derived from a canine pleomorphic rhabdomyosarcoma, which did not reveal any cytogenetic abnormalities (108). Further genomic characterization of canine rhabdomyosarcoma is needed to determine if the molecular pathogenesis of these tumors is similar to their human counterparts, with particular attention to the presence of PAX3/7-FHKR gene fusions, which are a hallmark of the more aggressive alveolar rhabdomyosarcoma subclass in people and have both diagnostic and prognostic utility (109–111).
Considerations for co-clinical trial approaches in canine sarcoma patients
The conception and implementation of co-clinical trials approaches in translational oncology research has significantly expanded over the last decade. This expansion has been driven by technological advances which continue to provide an ever-increasing understanding of the mutational landscape and key genetic drivers of human cancer types. Our expanded understanding of this genetic complexity has led to an increasingly granular stratification of patients with a common cancer histology into diverse molecular subtypes. Personalized medicine through tailored therapeutic approaches targeting each individual patient’s actionable molecular drivers will no doubt led to additional clinical advances. However, the breadth and depth of these advances, and the time it takes to achieve them, continues to hinge entirely on effective pre-clinical modeling of the in vivo biological complexity of human tumors.
In part, this effective pre-clinical modeling has been achieved through significant advancements in 1) accessibility and feasibility of obtaining, expanding, and utilizing patient-derived xenograft (PDX) mouse models, and 2) insightful development and refinement of compelling genetically engineered mouse models (GEMMs) of human cancers. These pre-clinical in vivo models allow an almost exact recapitulation of treatment protocols for human clinical trial cancer patients in their GEMM or PDX counterpart. This type of mirrored approach can inform strategies and biomarkers for patient stratification, lead to the identification of mechanisms of de novo and acquired drug resistance, and rapidly evaluate drug combinations that overcome this resistance. For certain tumor types, the success of this approach has surmounted increasing cynicism of the basic and clinical oncology research communities around small animal pre-clinical cancer models. Still, these models are not without inherent challenges and disadvantages. PDX models require the use of immunodeficient mice, thus failing to recapitulate tumor-immune interactions and evolution and precluding investigation of anti-metastatic or immunomodulatory therapies. GEMMs alleviate some of these issues via de novo tumor development in the presence of an intact immune system, subsequent establishment of a microenvironment with tumor-host interactions, and in some models, spontaneous metastasis, but GEMMs can be limited in the number of concurrent mutations, limiting their ability to fully recapitulate the molecular heterogeneity of human tumors (112, 113).
Spurred from these challenges is an increasingly renewed interest in employing a similar comparative dog-human co-clinical approach (Figures 1, 2 and Table 1). This approach has already proven to hold translational value and has the potential to fill some of the gaps associated with other pre-clinical models. Historically, the field of comparative oncology has provided the blueprint for the successful utilization of naturally occurring diseases in companion animals to conduct informative translational research. Clinical trials in dogs with spontaneous tumors were being conducted as early as the mid 1970s in pioneering bone marrow transplant studies by Storb and colleagues (114, 115). Other notable past advancements specific to sarcomas include development of human limb-sparing surgical techniques in dogs with osteosarcoma (116), and clinical evaluation of the biologic response modifier and macrophage immune stimulant liposomal muramyl tripeptide phosphatidyl ethanolamine (L-MTP-PE) in dogs with osteosarcoma by MacEwen et al., which provided key data supporting Phase II and Phase III trials of this compound in human patients (117). A more contemporary example of the dog-to-human pipeline includes drug repurposing studies for development of monocyte-targeted immunotherapy in dogs with metastatic osteosarcoma (34, 118), which has led to a Phase I trial in human patients (ClinicalTrials.gov Identifier: NCT03900793). Table 1 provides a non-comprehensive list of active canine trials as a sampling of the intensive ongoing comparative oncology efforts to accelerate sarcoma therapeutic development. Nonetheless, leveraging dogs with naturally occurring tumors also presents its own unique set of barriers and considerations. These include competing trials for highly investigated tumor types like OS, which can result in bottlenecks in patient enrollment and subsequent failure to meet the desired timelines of funding sponsors, both industry and academic investigator alike. A lack of canine species-specific reagents is another gap which limits the ability of the comparative oncology field to provide more valuable bio-correlative data to pharmaceutical sponsors besides just clinical outcome (Figure 1). Moreover, the type of perfectly matched therapeutic mirroring that can be effectively accomplished between mouse and human patient is precluded by several hurdles in our canine patients. Many of the investigational drugs being screened in human patients have not undergone extensive toxicity and pharmacokinetic studies in dogs. If they have, these have often been completed by private pharmaceutical companies, and thus the necessary safety and dosing data required to design a canine-human co-clinical trial is often not publicly available or is entirely unattainable. While dose finding studies of investigational therapeutics are often conducted in dogs, this necessary prerequisite alone can undermine the time and cost efficiency gained from employing the drug in a co-clinical trial type approach. Furthermore, purely from a body size standpoint, obtaining these investigational therapeutics for co-clinical trial studies in dogs can be financially prohibitive when compared to their murine counterpart, if an industry collaboration is not established.

Figure 2 Integrating canine sarcoma trials in human oncology drug development. Considerations for the various types of clinical and biological data that can be generated through trials in dogs with spontaneous sarcoma and where in the human clinical trial pipeline integration of these data may be informative. Created with BioRender.com.
Thus, enhancing engagement and collaboration between academic physician-veterinarian research teams and the pharmaceutical industry is something that must be increasingly addressed in the comparative sarcoma research community. One potential solution to this pressure point is the National Cancer Institute’s (NCI) Cancer Therapy Evaluation Program (CTEP). The long-established NCI Comparative Oncology Trials Consortium (COTC) could work internally with the NCI Division of Cancer Treatment and Diagnosis to develop a secondary function wherein COTC could enter into collaborative agreements with pharmaceutical companies similar to those of CTEP. This could allow COTC, perhaps through CTEP's already established “Non-clinical Use Request” avenue, to supply academic investigators of human-canine veterinary cancer center consortium institutions (e.g. The V Foundation Canine Oncology Research Consortium – CORC) with investigator brochure canine pharmacokinetic and toxicity data and the desired investigational agent or active pharmaceutical ingredient for compounding.
Despite these challenges, co-clinical trial designs in dogs with spontaneous sarcomas can still significantly aid in pharmacokinetic and pharmacodynamic based vetting of pre-clinical therapies (Figure 2). Co-clinical trials may also provide equal or greater efficiency and utility in 1) addressing the biology underlying mechanisms of therapy response and sarcoma progression and metastasis, and 2) in “high-risk high-reward” biomarker evaluation or combination therapy clinical approaches that would otherwise not be attempted in human patients, no matter how positive the pre-clinical supportive data is. These suggestions are not to say that investigations leveraging dogs as a surrogate for human sarcomas should not be supported by strong pre-clinical data from cellular and small animal models. Nor are they meant to undermine the undeniable value of the canine patient for preclinical assessment of safety, efficacy, pharmacokinetics, and pharmacodynamics of novel therapeutic candidates, or in correlative studies on parallel evaluation of biologic responses and predictors to the therapeutic actions of these candidates.
A prime example of the type of “high-risk high-reward” investigations that may best leverage dogs with spontaneous sarcoma is metastasis biology research in osteosarcoma. Metastasis accounts for greater than 90% of all cancer-related mortality (119), and in OS, roughly 30% of patients with localized disease at diagnosis die within 5 years due to metastasis (120). Lung metastasis accounts for up to 90% of OS recurrence, occurring on average 1.6 years after diagnosis and portending an unacceptable 20% 5-year survival (21, 121, 122). Like their human counterparts, dogs with naturally occurring sarcomas develop metastasis spontaneously over a still protracted but more chronologically relevant scale and after successful first-line therapy for their primary tumor. Thus, canine OS patients provide an incredible window of opportunity to conduct high risk clinical studies in the setting of pre-/micro-metastatic disease (Figure 2), an otherwise ethically and technically prohibitive endeavor in their human counterpart (8, 16, 123–126). These studies can inform new biomolecular imaging techniques, screen for early and more robust biomarkers of metastasis development, and test anti-metastatic drug strategies in the adjuvant/minimal residual disease setting. The latter is increasingly important as many anti-cancer drugs screened in early phase clinical trials are often evaluated in the setting of gross metastatic disease, despite their initial pre-clinical efficacy signal first being validated in mouse models of microscopic/minimal residual.
Stephen Paget’s 1889 “seed and soil” hypothesis on metastatic organotropism continues to foresee one of the most intensive areas of cancer research over the last two decades (127–130). Despite this intensive focus on the mechanisms of “pre-metastatic niche” formation in distant organs, mice are essentially the singular model for this area of investigation, and there remain significant obstacles to evaluating the clinical importance of pre-metastatic niche formation in human cancer patients. Nonetheless, experimental data from mouse models has demonstrated the lung’s unique permissiveness to pre-metastatic priming, highlighting it as an archetype of metastatic organotropism for many solid cancers. Retrospective studies estimate pulmonary metastases occur in 20-54% of patients who die from extra-thoracic malignancies, and the lung is a top 3 metastatic site for 9 of the 18 most lethal cancers in the U.S (131). As summarized above, canine OS is an established, well-recognized, and translationally relevant animal model with the potential to rapidly accelerate discovery in human OS (8, 16, 123, 124). Additionally, the significantly high and early lung metastatic tropism of canine OS represents a spontaneous large animal model with significant potential to inform discoveries on basic mechanisms of lung metastasis which are fundamentally applicable across other tumor types. Given the uniquely strong tropism of OS cell dissemination to the lung in both humans and dogs, insights gained into the fundamental biology underlying lung metastasis development in OS from investigations in dogs are likely to provide a translational value equal to those comparative oncology trials solely focused on pre-clinical vetting of novel therapeutic candidates. Moreover, comparative studies addressing these more basic research questions could help to better address potential concerns regarding the predictability of the canine model for human (osteo)sarcoma.
While remarkable clinical and biological similarities of human and canine OS have been previously demonstrated, increasing knowledge of the genomic complexity in human OS obtained through investigations leveraging technological advances is beginning to widen the gap between these parallel patient populations. If not addressed, this widening gap in the molecular understanding of human vs. canine OS could undermine the predictability of the canine model for testing novel preclinical candidates more so than in studies focused on the fundamental biology of sarcoma progression and metastasis. However, work on addressing this knowledge gap has already begun through establishment of the NCI’s Integrated Canine Data Commons (https://caninecommons.cancer.gov/#/home), a centralized, open access repository of clinical and genomic data from spontaneous canine cancers, and by private biotechnology companies such as nanoString® who have made significant investments in canine comparative oncology research (Figure 1), such as development of the nCounter canine IO® panel, and canine cancer atlas GeoMX® spatial transcriptomics. Continued funding and expansion of the comparative oncology molecular toolbox over the next decade will surely close this gap and continue to illuminate key species similarities and differences in sarcoma biology. To this end, another mechanism to incentivize canine species-specific reagent development such as therapeutic and diagnostic antibodies could be continued engagement between private biotechnology companies and the academic investigators and core facilities which are members or supporting laboratories of the NCI Comparative Oncology Trials Consortium or PRECINCT (PRE-medical Cancer Immunotherapy Network Canine Trials; https://www.precinctnetwork.org/). Through an NIH-hosted workshop, these groups of comparative oncology investigators could provide a list of prioritized canine targets for which antibody reagents are needed. Subsequently, working through the channels of the Comparative Oncology Program or Canine Cancer Immunotherapy Network, NCI could produce a Small Business Innovation Research (SBIR) request for proposals for companies to produce these antibody reagents. Once produced, competitive supplemental funding could then be provided to NCI PRECINCT members who submit detailed proposals for validating the specificity and functionality of these antibodies in a variety of immune assays.
The veterinary clinician-scientist pipeline
Continued efforts in diversification of our cancer research workforce training infrastructure is an indispensable prerequisite for enhancing the quantity and quality of comparative sarcoma research. To be successful in this approach, the next generation of cancer researchers must include veterinary biomedical scientists with training backgrounds in medical, surgical, and radiation oncology, pharmacology, pathology, and immunology (Figure 1). In addition to their veterinary training, these individuals need an outlet, through partnerships with medical schools, to participate in comparative training opportunities. In some cases, these opportunities exist in the form of clinical trials design training and MS certification programs, non-MD-restricted molecular genetics fellowships, post DVM residency training with a specific focus on lab animal medicine or pathology, and NIH Clinical and Translational Science Awards (CTSA) sponsored training and partnerships such as TL1 training grants and CTSA One Health Alliance (COHA) training fellowships (https://www.ctsaonehealthalliance.org/). Additional opportunities in areas such as advanced molecular imaging, cross disciplinary training in human molecular pathology and human investigative new drug trial design would allow veterinary clinician scientists to bring these skills back to our patients and better inform comparative oncology trial design and laboratory correlative studies to maximize extraction of data from these trials and increase their impact on clinical sarcoma patient management.
Sustaining and bolstering cross-veterinary and -medical institutional support in the form of expanded financial and faculty training investment into specialized and clinically relevant comparative and translational biomedical research fellowship training programs for veterinary scientists would provide more than just a pipeline of well-trained, diverse clinician scientists to foster co-clinical trial approaches in large animal models. Specifically, these fellowship programs would be separate from the already successfully established, and in some cases National Institutes of Health training grant funded, DVM-PhD and post-DVM residency/PhD programs established at veterinary schools across the USA. Instead, these fellowships could more closely mirror MD post-residency clinical fellowships programs, which often contain a multi-year research intensive component and in many cases lead the trainee to pursue further post-doctoral research following fellowship completion. In veterinary medicine, establishment of these fellowship programs would serve to further develop and fortify a more integrated veterinary and medical oncology research landscape from its current partially siloed state, into one that effectively blends and aligns the comparative oncology trials work of veterinary clinicians and scientists with their human colleague counterparts. Veterinary clinicians and scientists completing these fellowships would likely emerge with strong established collaborations with their human oncology research counterparts, a key benefit that circumvents an otherwise not infrequent barrier for junior veterinary clinician scientist faculty looking to establish independent comparative oncology research programs.
Similar to their medical counterparts, veterinary clinician-scientists are invaluable members of increasingly necessary multi-disciplinary cancer research teams. If and when these types of translational research fellowships are established, recruiting, retaining, and growing the number of clinically trained veterinarians entering into these programs would in part require a paradigm shift in how veterinary medical institutions themselves value these post-DVM trainees. Based on the latest American Veterinary Medical Association (AVMA) compensation report, veterinarians entering academic residency or PhD programs immediately after graduation would likely receive 40-50% of the starting salary of their fellow classmates entering into private clinical practice despite comparably high educational loan burdens. When considering post-residency DVMs entering PhD or the fellowship programs proposed here, this compensation gap exponentially widens. Post-residency academic fellowship programs that provide compensation at the NIH-specified post-doctoral stipend levels, regardless of whether or not the program holds, or the individual successfully competes for, an NIH institutional training grant, would provide the necessary institutional commitment needed to broaden the veterinary clinician-scientist pipeline.
There exists already a strengthening network of key academic, government, and non-profit stakeholders with an expanding intellectual and financial investment in comparative sarcoma research. For example, opportunities are already arising to share ideas and data from members of canine clinical trials and comparative oncology research consortia such as the Comparative Oncology Trials Consortium, the Consortium for Canine Comparative Oncology (c3o) between Duke Cancer Institute and North Carolina State University College of Veterinary Medicine, and NCI PRECINCT (which was created through the aforementioned NCI Cancer Moonshot funding opportunities), with that of their human counterparts such as the Children’s Oncology Group Osteosarcoma Biology and Bone Tumor Steering Committees. These collaborative opportunities occur through 1) focused comparative oncology scientific sessions at national meetings, 2) joint national meetings between canine and human cancer moonshot grant awardees, 3) funding opportunities requiring veterinary and medical scientist co-PI submissions (V foundation for Cancer Research), and 4) inclusion of members of both groups in monthly grant progress and update meetings of these consortia. Additionally, non-profit groups such as Ethos Discovery, the Osteosarcoma Institute, and Make It Better (MIB) Agents are fully invested in engaging academic investigators, leveraging their collaborative networks, and raising funds to support both human and canine sarcoma research.
Conclusion
Although clear gaps in the comparative molecular characterization of the canine sarcoma types reviewed herein remain, these temporary limitations should not preclude the cancer research community’s embracement of these valuable translational models. Leveraging dogs with spontaneous sarcomas in co-clinical trial approaches will undoubtedly provide a valuable and complementary source of high predictive value data to inform clinical practice, and ultimately, improve both human and canine sarcoma patient outcomes. Fully realizing the potential of this comparative approach requires fortifying veterinary and human medical oncology research partnerships alongside continued and enhanced investment in training the next generation of diverse, cross-disciplinary cancer researchers.
Author contributions
MK, LH, LA, AM, ML and DR all drafted and edited the manuscript. MK and DR generated the figures and table. All authors contributed to the article and approved the submitted version.
Funding
DR is supported by the Boettcher Foundation, MIB Agents and National Institutes of Health grants K01 OD022982 and L30TR002126. LH is supported by National Institutes of Health grant 5T32GM136628. ML is supported by the Boettcher Foundation, MIB Agents and Hyundai Hope on Wheels. AM is supported by the Hera Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Burningham Z, Hashibe M, Spector L, Schiffman JD. The epidemiology of sarcoma. Clin Sarcoma Res (2012) 2(1):14–4. doi: 10.1186/2045-3329-2-14
2. Potter JW, Jones KB, Barrott JJ. Sarcoma-the standard-bearer in cancer discovery. Crit Rev oncology/hematology (2018) 126:1–5. doi: 10.1016/j.critrevonc.2018.03.007
3. Ledford H. Translational research: 4 ways to fix the clinical trial. Nature 2011/09 (2011) 477(7366):526–8. doi: 10.1038/477526a
4. Arrowsmith J. Phase III. And submission failures: 2007–2010. Nat Rev Drug Discovery 2011/02 (2011) 10(2):87–7. doi: 10.1038/nrd3375
5. Innovation or stagnation: Challenge and opportunity on the critical path to new medical products (2004) (US Food and Drug Administration Website: US Food and Drug Administration - Critical Path Initiative). Available from: http://wayback.archiveit.org/7993/20180125032208/https://www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative/CriticalPathOpportunitiesReports/ucm077262.htm.
6. Lee DY, Staddon AP, Shabason JE, Sebro R. Phase I and phase II clinical trials in sarcoma: Implications for drug discovery and development. Cancer Med Feb (2019) 8(2):585–92. doi: 10.1002/cam4.1958
7. Kola I. The state of innovation in drug development. Clin Pharmacol Ther 2008/02 (2008) 83(2):227–30. doi: 10.1038/sj.clpt.6100479
8. Gustafson DL, Duval DL, Regan DP, Thamm DH. Canine sarcomas as a surrogate for the human disease. Pharmacol Ther Aug (2018) 188:80–96. doi: 10.1016/j.pharmthera.2018.01.012
9. Cole S, Gianferante DM, Zhu B, Mirabello L. Osteosarcoma: A surveillance, epidemiology, and end results program-based analysis from 1975 to 2017. Cancer Jun 1 (2022) 128(11):2107–18. doi: 10.1002/cncr.34163
10. Anfinsen KP, Grotmol T, Bruland OS, Jonasdottir TJ. Breed-specific incidence rates of canine primary bone tumors–a population based survey of dogs in Norway. Can J Vet Res Jul (2011) 75(3):209–15.
11. Egenvall A, Nødtvedt A, von Euler H. Bone tumors in a population of 400 000 insured Swedish dogs up to 10 y of age: Incidence and survival. Can J Vet Res Oct (2007) 71(4):292–9.
12. Simpson S, Rizvanov AA, Jeyapalan JN, de Brot S, Rutland CS. Canine osteosarcoma in comparative oncology: Molecular mechanisms through to treatment discovery. Front veterinary science (2022) 9:965391. doi: 10.3389/fvets.2022.965391
13. Nie Z, Peng H. Osteosarcoma in patients below 25 years of age: An observational study of incidence, metastasis, treatment and outcomes. Oncol letters (2018) 16(5):6502–14. doi: 10.3892/ol.2018.9453
14. Messerschmitt PJ, Garcia RM, Abdul-Karim FW, Greenfield EM, Getty PJ. Osteosarcoma. J Am Acad Orthopaedic Surgeons 2009/08 (2009) 17(8):515–27. doi: 10.5435/00124635-200908000-00005
15. Vail DM, Thamm DH, Liptak JM. Tumors of the skeletal system. Withrow MacEwen's Small Anim Clin Oncology: Elsevier; (2019) p:773–810. doi: 10.1016/B978-0-323-59496-7.00034-7
16. Khanna C, Fan TM, Gorlick R, Helman LJ, Kleinerman ES, Adamson PC, et al. Toward a drug development path that targets metastatic progression in osteosarcoma. Clin Cancer Res Aug 15 (2014) 20(16):4200–9. doi: 10.1158/1078-0432.Ccr-13-2574
17. Avallone G, Rasotto R, Chambers JK, Miller AD, Behling-Kelly E, Monti P, et al. Review of histological grading systems in veterinary medicine. Veterinary Pathology 2021/03/26 (2021) 58(5):809–28. doi: 10.1177/0300985821999831
18. Board WCoTE. Soft tissue and bone tumors. In: WHO classification of tumors, 5 ed. Lyons, France: International Agency for Research On Cancer (2020).
19. Thompson KG, Dittmer KE. Tumors of bone. Tumors Domest Animals: John Wiley Sons Inc; (2016), 356–424. doi: 10.1002/9781119181200.ch10
20. Beck J, Ren L, Huang S, Berger E, Bardales K, Mannheimer J, et al. Canine and murine models of osteosarcoma. Vet Pathol May (2022) 59(3):399–414. doi: 10.1177/03009858221083038
21. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer. (2009) 115(7):1531–43. doi: 10.1002/cncr.24121
22. Kirpensteijn J, Kik M, Rutteman GR, Teske E. Prognostic significance of a new histologic grading system for canine osteosarcoma. Vet Pathol Mar (2002) 39(2):240–6. doi: 10.1354/vp.39-2-240
23. Cesari M, Alberghini M, Vanel D, Palmerini E, Staals EL, Longhi A, et al. Periosteal osteosarcoma: A single-institution experience. Cancer Apr 15 (2011) 117(8):1731–5. doi: 10.1002/cncr.25718
24. Okada K, Frassica FJ, Sim FH, Beabout JW, Bond JR, Unni KK. Parosteal osteosarcoma. a clinicopathological study. J Bone Joint Surg Am Mar(1994) 76(3):366–78. doi: 10.2106/00004623-199403000-00007
25. Vail DM, Thamm DH, Liptak JM. Miscellaneous tumors. In: Withrow and MacEwen's small animal clinical oncology. St. Louis, MO: Elsevier (2019). p. 773–810.
26. Marcove RC, MikÉ V, Hajek JV, Levin AG, Hutter RVP. Osteogenic sarcoma under the age of twenty-one. J Bone Joint Surgery 1970/04 (1970) 52(3):411–23. doi: 10.2106/00004623-197052030-00001
27. Paoloni M, Davis S, Lana S, Withrow S, Sangiorgi L, Picci P, et al. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genomics (2009) 10:625. doi: 10.1186/1471-2164-10-625
28. Gardner HL, Sivaprakasam K, Briones N, Zismann V, Perdigones N, Drenner K, et al. Canine osteosarcoma genome sequencing identifies recurrent mutations in DMD and the histone methyltransferase gene SETD2. Commun Biol (2019) 2:266–6. doi: 10.1038/s42003-019-0487-2
29. Chu S, Skidmore ZL, Kunisaki J, Walker JR, Griffith M, Griffith OL, et al. Unraveling the chaotic genomic landscape of primary and metastatic canine appendicular osteosarcoma with current sequencing technologies and bioinformatic approaches. PloS One (2021) 16(2):e0246443–e0246443. doi: 10.1371/journal.pone.0246443
30. Perry JA, Kiezun A, Tonzi P, Van Allen EM, Carter SL, Baca SC, et al. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc Natl Acad Sci United States America (2014) 111(51):E5564–73. doi: 10.1073/pnas.1419260111
31. Sakthikumar S, Elvers I, Kim J, Arendt ML, Thomas R, Turner-Maier J, et al. SETD2 is recurrently mutated in whole-exome sequenced canine osteosarcoma. Cancer Res 2018/07/01 (2018) 78(13):3421–31. doi: 10.1158/0008-5472.can-17-3558
32. Zhang J, Yu X-H, Yan Y-G, Wang C, Wang W-J. PI3K/Akt signaling in osteosarcoma. Clinica Chimica Acta 2015/04 (2015) 444:182–92. doi: 10.1016/j.cca.2014.12.041
33. Wu J, Cui L-L, Yuan J, Wang Y, Song S. Clinical significance of the phosphorylation of MAPK and protein expression of cyclin D1 in human osteosarcoma tissues. Mol Med Rep 2017/02/21 (2017) 15(4):2303–7. doi: 10.3892/mmr.2017.6224
34. Regan DP, Chow L, Das S, Haines L, Palmer E, Kurihara JN, et al. Losartan blocks osteosarcoma-elicited monocyte recruitment, and combined with the kinase inhibitor toceranib, exerts significant clinical benefit in canine metastatic osteosarcoma. Clin Cancer Res Feb 15 (2022) 28(4):662–76. doi: 10.1158/1078-0432.ccr-21-2105
35. Gross AC, Cam H, Phelps DA, Saraf AJ, Bid HK, Cam M, et al. IL-6 and CXCL8 mediate osteosarcoma-lung interactions critical to metastasis. JCI Insight (2018) 3(16). doi: 10.1172/jci.insight.99791
36. Wasserman J, Diese L, VanGundy Z, London C, Carson WE, Papenfuss TL. Suppression of canine myeloid cells by soluble factors from cultured canine tumor cells. Veterinary Immunol immunopathology (2012) 145(1-2):420–30. doi: 10.1016/j.vetimm.2011.12.018
37. Hingorani P, Maas ML, Gustafson MP, Dickman P, Adams RH, Watanabe M, et al. Increased CTLA-4(+) T cells and an increased ratio of monocytes with loss of class II (CD14(+) HLA-DR(lo/neg)) found in aggressive pediatric sarcoma patients. J Immunother Cancer (2015) 3:35–5. doi: 10.1186/s40425-015-0082-0
38. Dumars C, Ngyuen J-M, Gaultier A, Lanel R, Corradini N, Gouin F, et al. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget (2016) 7(48):78343–54. doi: 10.18632/oncotarget.13055
39. Morello E, Martano M, Buracco P. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differences with human osteosarcoma. Veterinary J 2011/09 (2011) 189(3):268–77. doi: 10.1016/j.tvjl.2010.08.014
41. Yu D, Zhang S, Feng A, Xu D, Zhu Q, Mao Y, et al. Methotrexate, doxorubicin, and cisplatinum regimen is still the preferred option for osteosarcoma chemotherapy: A meta-analysis and clinical observation. Medicine (2019) 98(19):e15582–2. doi: 10.1097/MD.0000000000015582
42. Ferrari S, Ruggieri P, Cefalo G, Tamburini A, Capanna R, Fagioli F, et al. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: An Italian sarcoma group trial ISG/OS-1. J Clin Oncol 2012/06/10 (2012) 30(17):2112–8. doi: 10.1200/jco.2011.38.4420
43. Gaballah AH, Jensen CT, Palmquist S, Pickhardt PJ, Duran A, Broering G, et al. Angiosarcoma: Clinical and imaging features from head to toe. Br J radiology (2017) 90(1075):20170039–20170039. doi: 10.1259/bjr.20170039
44. Kim J-H, Graef AJ, Dickerson EB, Modiano JF. Pathobiology of hemangiosarcoma in dogs: Research advances and future perspectives. Veterinary Sci (2015) 2(4):388–405. doi: 10.3390/vetsci2040388
45. Kelly WR, Wilkinson GT, Allen PW. Canine angiosarcoma (Lymphangiosarcoma): A case report. Veterinary Pathology 1981/03 (1981) 18(2):224–7. doi: 10.1177/030098588101800210
46. Curran KM, Halsey CHC, Worley DR. Lymphangiosarcoma in 12 dogs: A case series (1998-2013). Veterinary Comp Oncol (2014) 14(2):181–90. doi: 10.1111/vco.12087
47. Schultheiss PC. A retrospective study of visceral and nonvisceral hemangiosarcoma and hemangiomas in domestic animals. J Veterinary Diagn Invest (2004) 16(6):522–6. doi: 10.1177/104063870401600606
48. Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol 2010/10 (2010) 11(10):983–91. doi: 10.1016/s1470-2045(10)70023-1
49. Fury MG, Antonescu CR, Van Zee KJ, Brennan ME, Maki RG. A 14-year retrospective review of angiosarcoma. Cancer J(2005) 11(3):241–7. doi: 10.1097/00130404-200505000-00011
50. Hargis AM, Ihrke PJ, Spangler WL, Stannard AA. A retrospective clinicopathologic study of 212 dogs with cutaneous hemangiomas and hemangiosarcomas. Veterinary Pathol (1992) 29(4):316–28. doi: 10.1177/030098589202900406
51. Hargis AM, Lee AC, Thomassen RW. Tumor and tumor-like lesions of perilimbal conjunctiva in laboratory dogs. J Am Vet Med Assoc Nov 1 (1978) 173(9):1185–90.
52. Ronchi A, Cozzolino I, Zito Marino F, De Chiara A, Argenziano G, Moscarella E, et al. Primary and secondary cutaneous angiosarcoma: Distinctive clinical, pathological and molecular features. Ann Diagn Pathology (2020) 48:151597. doi: 10.1016/j.anndiagpath.2020.151597
53. Yasir S, Torbenson MS. Angiosarcoma of the liver. Am J Surg Pathology (2019) 43(5):581–90. doi: 10.1097/pas.0000000000001228
54. Fosmire SP, Dickerson EB, Scott AM, Bianco SR, Pettengill MJ, Meylemans H, et al. Canine malignant hemangiosarcoma as a model of primitive angiogenic endothelium. Lab Invest (2004) 84(5):562–72. doi: 10.1038/labinvest.3700080
55. Al-Abbadi MA, Almasri NM, Al-Quran S, Wilkinson EJ. Cytokeratin and epithelial membrane antigen expression in angiosarcomas: An immunohistochemical study of 33 cases. Arch Pathol Lab Med (2007) 131(2):288–92. doi: 10.5858/2007-131-288-caemae
56. Warren AL, Summers BA. Epithelioid variant of hemangioma and hemangiosarcoma in the dog, horse, and cow. Veterinary Pathology (2007) 44(1):15–24. doi: 10.1354/vp.44-1-15
57. Ohsawa M, Naka N, Tomita Y, Kawamori D, Kanno H, Aozasa K. Use of immunohistochemical procedures in diagnosing angiosarcoma. Eval 98 cases Cancer (1995) 75(12):2867–74. doi: 10.1002/1097-0142(19950615)75:12<2867::aid-cncr2820751212>3.0.co;2-8
58. Göritz M, Müller K, Krastel D, Staudacher G, Schmidt P, Kühn M, et al. Canine splenic haemangiosarcoma: Influence of metastases, chemotherapy and growth pattern on post-splenectomy survival and expression of angiogenic factors. J Comp Pathol (2013) 149(1):30–9. doi: 10.1016/j.jcpa.2012.11.234
59. Albores-Saavedra J, Schwartz AM, Henson DE, Kostun L, Hart A, Angeles-Albores D, et al. Cutaneous angiosarcoma. analysis of 434 cases from the surveillance, epidemiology, and end results program, 1973-2007. Ann Diagn Pathology 2011/04 (2011) 15(2):93–7. doi: 10.1016/j.anndiagpath.2010.07.012
60. Nascimento AF, Raut CP, Fletcher CDM. Primary angiosarcoma of the breast. Am J Surg Pathol (2008) 32(12):1896–904. doi: 10.1097/pas.0b013e318176dbc7
61. Wang L, Lao IW, Yu L, Wang J. Clinicopathological features and prognostic factors in angiosarcoma: A retrospective analysis of 200 patients from a single Chinese medical institute. Oncol Lett (2017) 14(5):5370–8. doi: 10.3892/ol.2017.6892
62. Fayette J, Martin E, Piperno-Neumann S, Le Cesne A, Robert C, Bonvalot S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: A retrospective study of 161 cases. Ann Oncol 2007/12 (2007) 18(12):2030–6. doi: 10.1093/annonc/mdm381
63. Wendelburg KM, Price LL, Burgess KE, Lyons JA, Lew FH, Berg J. Survival time of dogs with splenic hemangiosarcoma treated by splenectomy with or without adjuvant chemotherapy: 208 cases (2001–2012). J Am Veterinary Med Assoc 2015/08/15 (2015) 247(4):393–403. doi: 10.2460/javma.247.4.393
64. Buehler D, Rice SR, Moody JS, Rush P, Hafez G-R, Attia S, et al. Angiosarcoma outcomes and prognostic factors: A 25-year single institution experience. Am J Clin Oncol (2014) 37(5):473–9. doi: 10.1097/COC.0b013e31827e4e7b
65. Mark RJ, Poen JC, Tran LM, Fu YS, Juillard GF. Angiosarcoma: A report of 67 patients and a review of the literature. Cancer 1996/06/01 (1996) 77(11):2400–6. doi: 10.1002/(sici)1097-0142(19960601)77:11<2400::aid-cncr32>3.0.co;2-z
66. Ye J, Li X-F, Wang Y-D, Yuan Y. Long-term survival of a patient with scalp angiosarcoma and multiple metastases treated using combination therapy: A case report. Oncol letters (2015) 9(4):1725–8. doi: 10.3892/ol.2015.2919
67. Lamerato-Kozicki AR, Helm KM, Jubala CM, Cutter GC, Modiano JF. Canine hemangiosarcoma originates from hematopoietic precursors with potential for endothelial differentiation. Exp Hematology 2006/07 (2006) 34(7):870–8. doi: 10.1016/j.exphem.2006.04.013
68. Gorden BH, Kim J-H, Sarver AL, Frantz AM, Breen M, Lindblad-Toh K, et al. Identification of three molecular and functional subtypes in canine hemangiosarcoma through gene expression profiling and progenitor cell characterization. Am J Pathol (2014) 184(4):985–95. doi: 10.1016/j.ajpath.2013.12.025
69. Liu L, Kakiuchi-Kiyota S, Arnold LL, Johansson SL, Wert D, Cohen SM. Pathogenesis of human hemangiosarcomas and hemangiomas. Hum Pathology 2013/10 (2013) 44(10):2302–11. doi: 10.1016/j.humpath.2013.05.012
70. Wang G, Wu M, Durham AC, Radaelli E, Mason NJ, Xu X, et al. Molecular subtypes in canine hemangiosarcoma reveal similarities with human angiosarcoma. PloS One (2020) 15(3):e0229728–e0229728. doi: 10.1371/journal.pone.0229728
71. Wang G, Wu M, Maloneyhuss MA, Wojcik J, Durham AC, Mason NJ, et al. Actionable mutations in canine hemangiosarcoma. PloS One (2017) 12(11):e0188667–e0188667. doi: 10.1371/journal.pone.0188667
72. Megquier K, Turner-Maier J, Swofford R, Kim J-H, Sarver AL, Wang C, et al. Comparative genomics reveals shared mutational landscape in canine hemangiosarcoma and human angiosarcoma. Mol Cancer Res MCR (2019) 17(12):2410–21. doi: 10.1158/1541-7786.MCR-19-0221
73. Painter CA, Jain E, Tomson BN, Dunphy M, Stoddard RE, Thomas BS, et al. The angiosarcoma project: Enabling genomic and clinical discoveries in a rare cancer through patient-partnered research. Nat Med Feb (2020) 26(2):181–7. doi: 10.1038/s41591-019-0749-z
74. Chan JY, Lim JQ, Yeong J, Ravi V, Guan P, Boot A, et al. Multiomic analysis and immunoprofiling reveal distinct subtypes of human angiosarcoma. J Clin Invest (2020) 130(11):5833–46. doi: 10.1172/JCI139080
75. Dennis MM, McSporran KD, Bacon NJ, Schulman FY, Foster RA, Powers BE. Prognostic factors for cutaneous and subcutaneous soft tissue sarcomas in dogs. Veterinary Pathology (2010) 48(1):73–84. doi: 10.1177/0300985810388820
76. Liptak J. Soft tissue sarcomas. Withrow MacEwen's Small Anim Clin Oncology: Elsevier; (2007) p:425–54.
77. Chase D, Bray J, Ide A, Polton G. Outcome following removal of canine spindle cell tumours in first opinion practice: 104 cases. J Small Anim Practice 2009/10/08 (2009) 50(11):568–74. doi: 10.1111/j.1748-5827.2009.00809.x
78. McSporran KD. Histologic grade predicts recurrence for marginally excised canine subcutaneous soft tissue sarcomas. Veterinary Pathology 2009/05/09 (2009) 46(5):928–33. doi: 10.1354/vp.08-vp-0277-m-fl
79. Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CDM, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int J Cancer (2006) 119(12):2922–30. doi: 10.1002/ijc.22239
80. Gage MM, Nagarajan N, Ruck JM, Canner JK, Khan S, Giuliano K, et al. Sarcomas in the united states: Recent trends and a call for improved staging. Oncotarget. (2019) 10(25):2462–74. doi: 10.18632/oncotarget.26809
81. Hohenhaus AE, Kelsey JL, Haddad J, Barber L, Palmisano M, Farrelly J, et al. Canine cutaneous and subcutaneous soft tissue sarcoma: An evidence-based review of case management. J Am Anim Hosp Assoc 2016/03/01 (2016) 52(2):77–89. doi: 10.5326/jaaha-ms-6305
82. Zagars GK, Ballo MT, Pisters PWT, Pollock RE, Patel SR, Benjamin RS, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy. Cancer 2003/04/30 (2003) 97(10):2530–43. doi: 10.1002/cncr.11365
83. Lintz F, Moreau A, Odri GA, Waast D, Maillard O, Gouin F. Critical study of resection margins in adult soft-tissue sarcoma surgery. Orthopaedics Traumatology: Surg Res (2012) 98(4):S9–S18. doi: 10.1016/j.otsr.2012.04.006
84. Strander H, Turesson I. Cavallin-ståhl e. a systematic overview of radiation therapy effects in soft tissue sarcomas. Acta Oncologica (2003) 42(5-6):516–31. doi: 10.1080/02841860310014732
85. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008/08/01 (2008) 113(3):573–81. doi: 10.1002/cncr.23592
86. Elmslie RE, Glawe P, Dow SW. Metronomic therapy with cyclophosphamide and piroxicam effectively delays tumor recurrence in dogs with incompletely resected soft tissue sarcomas. J Veterinary Internal Med (2008) 22(6):1373–9. doi: 10.1111/j.1939-1676.2008.0179.x
87. Folpe AL. Fibrosarcoma: a review and update. Histopathology. (2013) 64(1):12–25. doi: 10.1111/his.12282
88. Ulvé R, Rault M, Bahin M, Lagoutte L, Abadie J, De Brito C, et al. Discovery of human-similar gene fusions in canine cancers. Cancer Res (2017) 77(21):5721–7. doi: 10.1158/0008-5472.can-16-2691
89. Brohl AS, Kahen E, Yoder SJ, Teer JK, Reed DR. The genomic landscape of malignant peripheral nerve sheath tumors: Diverse drivers of ras pathway activation. Sci Rep (2017) 7(1):14992–2. doi: 10.1038/s41598-017-15183-1
90. Kaplan HG, Rostad S, Ross JS, Ali SM, Millis SZ. Genomic profiling in patients with malignant peripheral nerve sheath tumors reveals multiple pathways with targetable mutations. J Natl Compr Cancer Network (2018) 16(8):967–74. doi: 10.6004/jnccn.2018.7033
91. Lemberg KM, Wang J, Pratilas CA. From genes to -omics: The evolving molecular landscape of malignant peripheral nerve sheath tumor. Genes. (2020) 11(6):691. doi: 10.3390/genes11060691
92. Mochizuki H, Kennedy K, Shapiro SG, Breen M. BRAF mutations in canine cancers. PloS One (2015) 10(6):e0129534–e0129534. doi: 10.1371/journal.pone.0129534
93. Nasir L, Rutteman GR, Reid SWJ, Schulze C, Argyle DJ. Analysis of p53 mutational events and MDM2 amplification in canine soft-tissue sarcomas. Cancer Letters (2001) 174(1):83–9. doi: 10.1016/s0304-3835(01)00637-1
94. Aguirre-Hernández J, Milne BS, Queen C, O'Brien PCM, Hoather T, Haugland S, et al. Disruption of chromosome 11 in canine fibrosarcomas highlights an unusual variability of CDKN2B in dogs. BMC veterinary Res (2009) 5:27–7. doi: 10.1186/1746-6148-5-27
95. Avallone G, Roccabianca P, Crippa L, Lepri E, Brunetti B, Bernardini C, et al. Histological classification and immunohistochemical evaluation of MDM2 and CDK4 expression in canine liposarcoma. Veterinary Pathology (2016) 53(4):773–80. doi: 10.1177/0300985815626573
96. Avallone G, Muscatello LV, Leoni A, Roccabianca P, Lepri E, Crippa L, et al. p53 expression in canine liposarcoma correlates with myxoid variant and higher proliferative activity. Veterinary Pathology (2020) 57(5):620–2. doi: 10.1177/0300985820941501
97. Patel N, Benipal B. Incidence of gastrointestinal stromal tumors in the united states from 2001-2015: A united states cancer statistics analysis of 50 states. Cureus Feb 22 (2019) 11(2):e4120. doi: 10.7759/cureus.4120
98. Senapathi H, Morada A, Perry M, Bertram C, Yeung E, Sultany M, et al. Prognostic factors in gastrointestinal leiomyosarcomas: An analysis using the surveillance, epidemiology, and end results (SEER) database. Cureus Nov (2021) 13(11):e19447. doi: 10.7759/cureus.19447
99. Del Alcazar CM, Mahoney JA, Dittrich K, Stefanovski D, Church ME. Outcome, prognostic factors and histological characterization of canine gastrointestinal sarcomas. Vet Comp Oncol Sep (2021) 19(3):578–86. doi: 10.1111/vco.12696
100. Fletcher CDM, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathology (2002) 33(5):459–65. doi: 10.1053/hupa.2002.123545
101. Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol (1998) 11(8):728–34.
102. Russell KN, Mehler SJ, Skorupski KA, Baez JL, Shofer FS, Goldschmidt MH. Clinical and immunohistochemical differentiation of gastrointestinal stromal tumors from leiomyosarcomas in dogs: 42 cases (1990–2003). J Am Veterinary Med Assoc (2007) 230(9):1329–33. doi: 10.2460/javma.230.9.1329
103. de Silva MC, Reid R. Gastrointestinal stromal tumors (GIST): C-kit mutations, CD117 expression, differential diagnosis and targeted cancer therapy with imatinib. Pathol Oncol Res (2003) 9(1):13–9. doi: 10.1007/bf03033708
104. Gregory-Bryson E, Bartlett E, Kiupel M, Hayes S, Yuzbasiyan-Gurkan V. Canine and human gastrointestinal stromal tumors display similar mutations in c-KIT exon 11. BMC cancer (2010) 10:559–9. doi: 10.1186/1471-2407-10-559
105. Caserto BG. A comparative review of canine and human rhabdomyosarcoma with emphasis on classification and pathogenesis. Veterinary Pathol (2013) 50(5):806–26. doi: 10.1177/0300985813476069
106. Amer KM, Thomson JE, Congiusta D, Dobitsch A, Chaudhry A, Li M, et al. Epidemiology, incidence, and survival of rhabdomyosarcoma subtypes: SEER and ICES database analysis. J Orthopaedic Res (2019) 37(10):2226–30. doi: 10.1002/jor.24387
107. Tuohy JL, Byer BJ, Royer S, Keller C, Nagai-Singer MA, Regan DP, et al. Evaluation of myogenin and MyoD1 as immunohistochemical markers of canine rhabdomyosarcoma. Vet Pathol May (2021) 58(3):516–26. doi: 10.1177/0300985820988146
108. Azakami D, Shibutani H, Dohi M, Takasaki M, Ishioka K, Mori A, et al. Establishment and characterization of canine rhabdomyosarcoma cell line CMS-c. J Veterinary Med Science (2011) 73(8):1105–8. doi: 10.1292/jvms.10-0436
109. Ren Y-X, Finckenstein FG, Abdueva DA, Shahbazian V, Chung B, Weinberg KI, et al. Mouse mesenchymal stem cells expressing PAX-FKHR form alveolar rhabdomyosarcomas by cooperating with secondary mutations. Cancer Res (2008) 68(16):6587–97. doi: 10.1158/0008-5472.can-08-0859
110. Missiaglia E, Williamson D, Chisholm J, Wirapati P, Pierron G, Petel F, et al. PAX3/FOXO1 fusion gene status is the key prognostic molecular marker in rhabdomyosarcoma and significantly improves current risk stratification. J Clin Oncol (2012) 30(14):1670–7. doi: 10.1200/jco.2011.38.5591
111. Williamson D, Missiaglia E, de Reyniès A, Pierron G, Thuille B, Palenzuela G, et al. Fusion gene–negative alveolar rhabdomyosarcoma is clinically and molecularly indistinguishable from embryonal rhabdomyosarcoma. J Clin Oncol (2010) 28(13):2151–8. doi: 10.1200/jco.2009.26.3814
112. Hill W, Caswell DR, Swanton C. Capturing cancer evolution using genetically engineered mouse models (GEMMs). Trends Cell Biol (2021) 31(12):1007–18. doi: 10.1016/j.tcb.2021.07.003
113. Kersten K, de Visser KE, van Miltenburg MH, Jonkers J. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol Med (2017) 9(2):137–53. doi: 10.15252/emmm.201606857
114. Tsoi MS, Weiden PL, Storb R. Lymphocyte reactivity to autochthonous tumor cells in dogs with spontaneous malignancies. Cell Immunol (1974) 13(3):431–9. doi: 10.1016/0008-8749(74)90262-7
115. Weiden PL, Storb R, Lerner KG, Kao GF, Graham TC, Thomas ED. Treatment of canine malignancies by 1200 r total body irradiation and autologous marrow grafts. Exp Hematol (1975) 3(2):124–34.
116. LaRue SM, Withrow SJ, Powers BE, Wrigley RH, Gillette EL, Schwarz PD, et al. Limb-sparing treatment for osteosarcoma in dogs. J Am Veterinary Med Assoc (1989) 195(12):1734–44.
117. Kurzman ID, MacEwen EG, Rosenthal RC, Fox LE, Keller ET, Helfand SC, et al. Adjuvant therapy for osteosarcoma in dogs: Results of randomized clinical trials using combined liposome-encapsulated muramyl tripeptide and cisplatin. Clin Cancer Res an Off J Am Assoc Cancer Res (1995) 1(12):1595–601.
118. Regan DP, Coy JW, Chahal KK, Chow L, Kurihara JN, Guth AM, et al. The angiotensin receptor blocker losartan suppresses growth of pulmonary metastases via AT1R-independent inhibition of CCR2 signaling and monocyte recruitment. J Immunol (2019) 202(10):3087–102. doi: 10.4049/jimmunol.1800619
119. Mehlen P, Puisieux A. Metastasis: A question of life or death. Nat Rev Cancer (2006) 6(6):449–58. doi: 10.1038/nrc1886
120. Meltzer PS, Helman LJ. New horizons in the treatment of osteosarcoma. N Engl J Med (2021) 385(22):2066–76. doi: 10.1056/NEJMra2103423
121. Hawkins DS, Arndt CAS. Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer. (2003) 98(11):2447–56. doi: 10.1002/cncr.11799
122. Kempf-Bielack B, Bielack SS, Jürgens H, Branscheid D, Berdel WE, Exner GU, et al. Osteosarcoma relapse after combined modality therapy: An analysis of unselected patients in the cooperative osteosarcoma study group (COSS). J Clin Oncol (2005) 23(3):559–68. doi: 10.1200/jco.2005.04.063
123. Regan D, Garcia K, Thamm D. Clinical, pathological, and ethical considerations for the conduct of clinical trials in dogs with naturally occurring cancer: A comparative approach to accelerate translational drug development. Ilar J (2018) 59(1):99–110. doi: 10.1093/ilar/ily019
124. LeBlanc AK, Mazcko CN. Improving human cancer therapy through the evaluation of pet dogs. Nat Rev Cancer (2020) 20(12):727–42. doi: 10.1038/s41568-020-0297-3
125. LeBlanc AK, Mazcko CN, Cherukuri A, Berger EP, Kisseberth WC, Brown ME, et al. Adjuvant sirolimus does not improve outcome in pet dogs receiving standard-of-Care therapy for appendicular osteosarcoma: A prospective, randomized trial of 324 dogs. Clin Cancer Res (2021) 27(11):3005–16. doi: 10.1158/1078-0432.ccr-21-0315
126. Selmic LE, Burton JH, Thamm DH, Withrow SJ, Lana SE. Comparison of carboplatin and doxorubicin-based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J Vet Intern Med (2014) 28(2):554–63. doi: 10.1111/jvim.12313
127. Paget S. THE DISTRIBUTION OF SECONDARY GROWTHS IN CANCER OF THE BREAST. Lancet 1889/03 (1889) 133(3421):571–3. doi: 10.1016/s0140-6736(00)49915-0
128. Giles AJ, Reid CM, Evans JD, Murgai M, Vicioso Y, Highfill SL, et al. Activation of hematopoietic Stem/Progenitor cells promotes immunosuppression within the pre-metastatic niche. Cancer Res (2016) 76(6):1335–47. doi: 10.1158/0008-5472.CAN-15-0204
129. Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. (2005) 438(7069):820–7. doi: 10.1038/nature04186
130. Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol (2015) 17(6):816–26. doi: 10.1038/ncb3169
Keywords: canine (dog), sarcoma, osteosarcoma, comparative oncology, immunotherapy
Citation: Klosowski M, Haines L, Alfino L, McMellen A, Leibowitz M and Regan D (2023) Naturally occurring canine sarcomas: Bridging the gap from mouse models to human patients through cross-disciplinary research partnerships. Front. Oncol. 13:1130215. doi: 10.3389/fonc.2023.1130215
Received: 23 December 2022; Accepted: 20 February 2023;
Published: 23 March 2023.
Edited by:
Todd M. Pitts, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Amy LeBlanc, National Cancer Institute (NIH), United StatesMichael Wagner, University of Washington, United States
Copyright © 2023 Klosowski, Haines, Alfino, McMellen, Leibowitz and Regan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Regan, Daniel.regan@colostate.edu
 Marika Klosowski
Marika Klosowski Laurel Haines
Laurel Haines Lauren Alfino1,2
Lauren Alfino1,2 Daniel Regan
Daniel Regan