- 1The Third Department of Breast Cancer, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin, China
- 2Key Laboratory of Cancer Prevention and Therapy, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China
- 3Tianjin’s Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China
- 4Key Laboratory of Breast Cancer Prevention and Therapy, Tianjin Medical University, Ministry of Education, Tianjin, China
- 5College of Basic Medical Sciences, Fujian Medical University, Fuzhou, China
- 6Department for Chronic and Noncommunicable Disease Control and Prevention, Fujian Provincial Center for Disease Control and Prevention, Fuzhou, China
- 7First Department of Cadre Clinic, Provincial Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou, China
- 8Department of Oncological Surgery, Provincial Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou, China
- 9Department of Breast Pathology and Lab, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China
Background: Triple-negative breast cancer (TNBC) patients who recur at different times are associated with distinct biological characteristics and prognoses. Research on rapid-relapse TNBC (RR-TNBC) is sparse. In this study, we aimed to describe the characteristics of recurrence, predictors for relapse, and prognosis in rrTNBC patients.
Methods: Clinicopathological data of 1584 TNBC patients from 2014 to 2016 were retrospectively reviewed. The characteristics of recurrence were compared between patients with RR-TNBC and slow relapse TNBC(SR-TNBC). All TNBC patients were randomly divided into a training set and a validation set to find predictors for rapid relapse. The multivariate logistic regression model was used to analyze the data of the training set. C-index and brier score analysis for predicting rapid relapse in the validation set was used to evaluate the discrimination and accuracy of the multivariate logistic model. Prognostic measurements were analyzed in all TNBC patients.
Results: Compared with SR-TNBC patients, RR-TNBC patients tended to have a higher T staging, N staging, TNM staging, and low expression of stromal tumor-infiltrating lymphocytes (sTILs). The recurring characteristics were prone to appear as distant metastasis at the first relapse. The first metastatic site was apt to visceral metastasis and less likely to have chest wall or regional lymph node metastasis. Six predictors (postmenopausal status, metaplastic breast cancer,≥pT3 staging,≥pN1 staging, sTIL intermediate/high expression, and Her2 [1+]) were used to construct the predictive model of rapid relapse in TNBC patients. The C-index and brier score in the validation set was 0.861 and 0.095, respectively. This suggested that the predictive model had high discrimination and accuracy. The prognostic data for all TNBC patients showed that RR-TNBC patients had the worst prognosis, followed by SR-TNBC patients.
Conclusion: RR-TNBC patients were associated with unique biological characteristics and worse outcomes compared to non-RR-TNBC patients.
1 Introduction
Accounting for approximately 15%-20% of instances of breast cancer (BC), triple-negative breast cancer (TNBC) is pathologically defined by a lack of targetable estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) (1, 2). More than 35% of breast cancer-related deaths are caused by TNBC. Compared with other subtypes of BC, TNBC tends to have an increased aggressiveness, higher rate of metastasis, and shorter overall survival (OS) (3–5). The survival time of TNBC patients after the diagnosis of distant metastasis ranges from 17 to 25 months (6–8). Therefore, understanding the recurring characteristics, risk factors, and mechanisms of distant metastasis in TNBC patients are of critical importance to improving the prognosis of these patients.
Up to now, most studies on the recurring characteristics and risk factors for TNBC have focused on the overall recurring and metastatic populations, and have never further subdivided for all recurring and metastatic populations. To more accurately understand the differences in TNBC patients’ outcomes, we divided these patients into three groups: rapid relapse (RR-TNBC; distant relapse or death ≤2 years of diagnosis), slow relapse (SR-TNBC; distant relapse or death > 2 years), and no relapse (NR-TNBC;> 5 years no relapse/death) (9, 10). In several large TNBC cohort studies, the median time to distant metastasis was approximately 2 years, ranging from 19.7 to 31.2 months (6, 11–13). Therefore, we define RR-TNBC as distant relapse or death within 24 months of diagnosis. However, research on RR-TNBC is sparse. In this retrospective, single-institution study, we aimed to nvestigate the characteristics of recurrence, predictors for relapse, and prognosis in RR-TNBC patients.
2 Materials and methods
2.1 Patients
This retrospective analysis included 1584 consecutive triple-negative breast cancer (TNBC) patients pathologically confirmed in the Cancer Institute and Hospital of Tianjin Medical University from January 1, 2014, to December 31, 2016. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Medical Ethics Committee of Tianjin Medical University Cancer Institute and Hospital. Male patients, ductal carcinoma in situ (DCIS), lobular carcinoma in situ (LCIS), hormone receptor-positive (HR+) patients, human epidermal growth factor receptor 2 positives (HER2+) patients, patients with a history of other malignant tumors, bilateral breast cancer, or de-novo metastatic breast cancer patients were excluded. TNBC Patients with <2 years follow-up and no survival event were also excluded.
HER2 status was determined with immunohistochemistry (IHC) and/or fluorescence in situ hybridization (FISH) at the time of the first biopsy or breast surgery and classified according to the American Society of Clinical Oncology and the College of American Pathologists clinical practice guidelines for HER2 testing of 2013, respectively, and the Belgian Guidelines for HER2 testing (14). HER2 expression levels in TNBC included IHC 0, IHC 1+, or IHC 2+/FISH-negative. Hormone receptor and Ki67 status were determined by IHC using the Allred scoring system (15).
P53 expression ≤10% was considered P53-negative, P53 expression >10% was considered P53-positive.The expression levels of cytokeratin 5/6(CK5/6) and epidermal growth factor receptor (EGFR) <1% were considered to be negative, and positive if the expression levels of CK5/6 and EGFR were ≥1%. We evaluated stromal tumor-infiltrating lymphocytes (sTILs) on whole slides according to internationally established guidelines (16). sTILs were evaluated and grouped into three categories: low (≤10%), intermediate (10% to ≤40%), and high (>40%). All IHC readings were independently verified by two blinded and trained pathologists.
2.2 Follow-up strategy
Follow-up data of all TNBC patients in our study were gathered via telephone or our outpatient clinic, and the follow-up results were recorded. The follow-up time began with the first BC diagnosis. The follow-up deadline was June 1, 2022. Our follow-up strategies were as follows: routine breast/liver color ultrasound, ECT, chest and cranium CT plain scan for every initial TNBC patient to exclude the possibility of distant metastasis. In the first 3 years after surgery, we regularly reviewed breast/liver color ultrasound, X-ray, or chest CT plain scan every 3 months. Patients who survived 3-5 years after surgery were regularly reviewed with these items every 6 months. Patients who survived more than 5 years after surgery were regularly reviewed with these items every 1 year. Once distant metastasis was confirmed in follow-up patients, liver, lung, cranium CT, and ECT examinations were routinely performed at the same time.
2.3 Statistical analysis
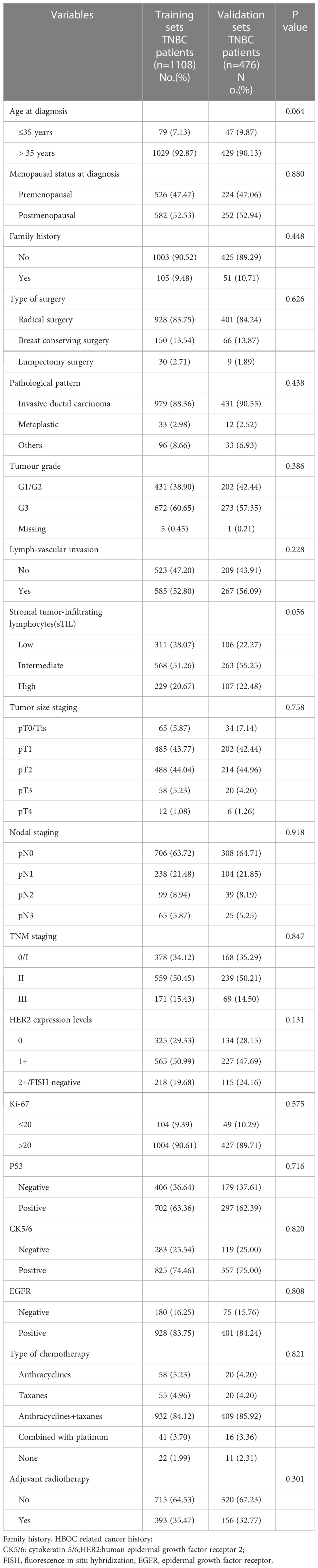
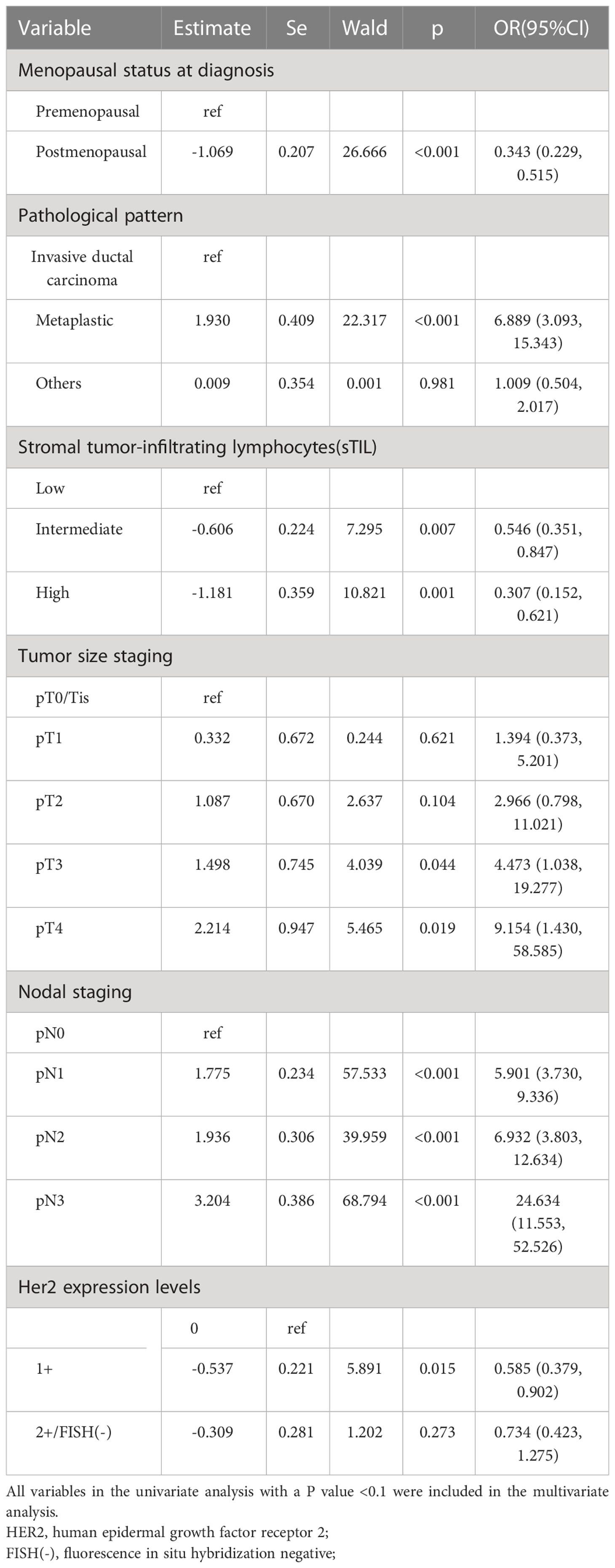
Statistical analysis was performed using R version 4.1.0. The random seed was determined to be 123456. All TNBC patients were randomly divided into the training set and the validation set in a 7:3 ratio. The Chi-square test and Fisher’s exact test were used for comparison between the two groups. The multivariate logistic regression model was used to analyze the data of the training set. All variables in the univariate analysis with a P value <0.1 were included in a stepwise multivariable logistic regression model. The minimum AIC was used to determine the optimal logistic regression model. The C-index and calibration curve was used to evaluate the discrimination and accuracy of the multivariate logistic model. Kaplan-Meier (K-M) method and Log-rank test were used to evaluate the prognosis in all TNBC patients. The statistically significant difference was considered to be a P value<0.05.
3 Results
A total of 12738 breast cancer patients from January 1, 2014, to December 31, 2016, were identified in the Cancer Institute and Hospital of Tianjin Medical University. Among these patients, 1584 TNBC patients (12.43%) fit the criteria to be included in the study. Among these 1584 TNBC patients,1209 patients had no relapse and 375 patients had relapse up until the time of follow-up data. Based on the time first distant metastasis occurred, 375 relapsed patients were divided into 249 patients with rapid-relapse(RR) and 126 patients with slow-relapse (SR) (Figure 1).
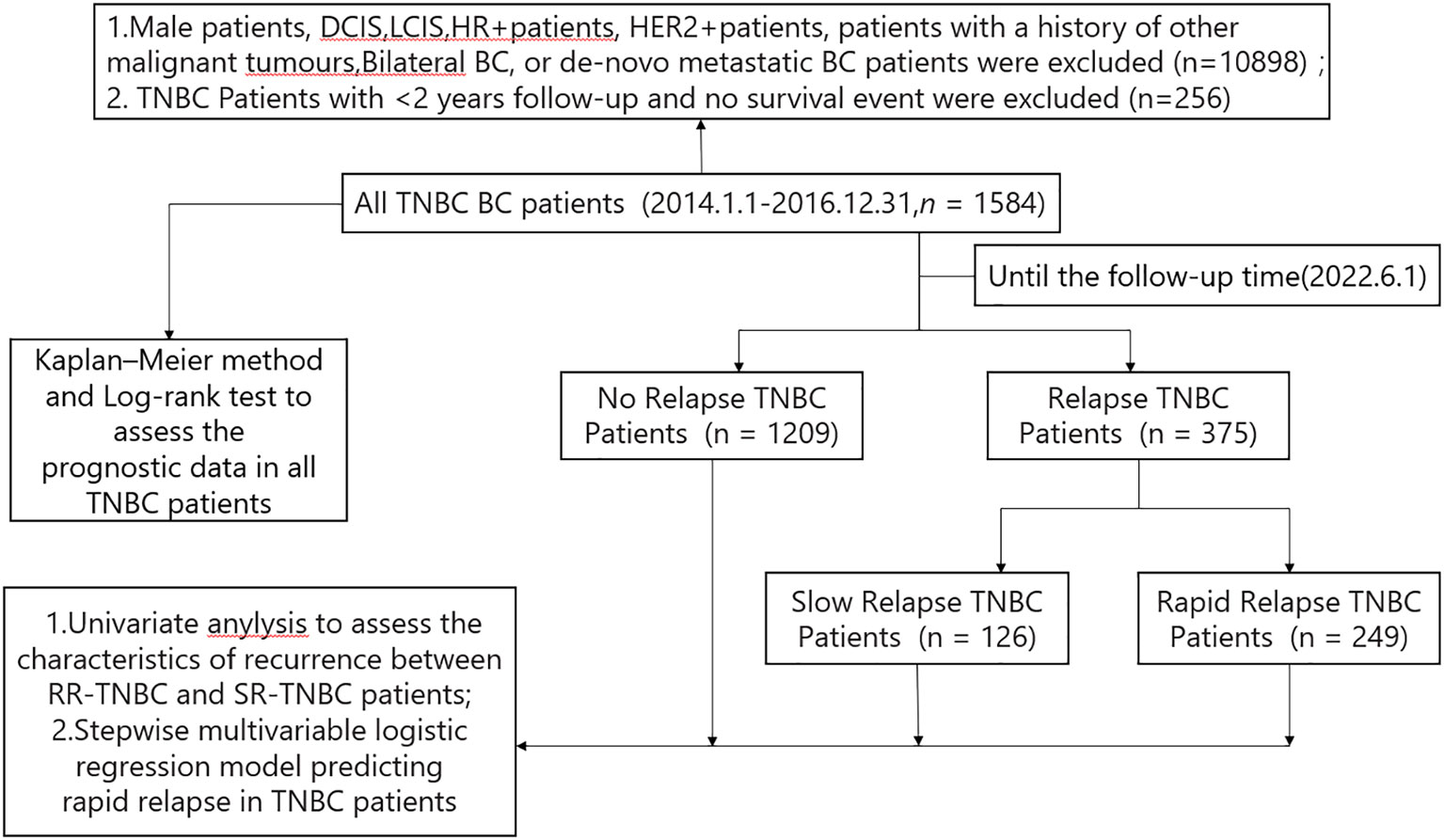
Figure 1 Constitution of the study population.BC, breast cancer; HR+,hormone receptor positive; HER2+, human epidermal growth factor receptor 2 positive; TNBC,triple negative breast cancer; RR-TNBC:rapid relapse-triple negative breast cancer; SR-TNBC:slow relapse-triple negative breast cancer; DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ.
By comparing recurrence characteristics between 249 patients with RR-TNBC and 126 patients with SR-TNBC, RR-TNBC patients tended to have a higher T staging, N staging, TNM staging, and low expression of stromal tumor-infiltrating lymphocytes (sTIL). The recurring characteristics were prone to appear as distant metastasis at the first relapse. The first metastatic site was apt to visceral metastasis and less likely to have chest wall or regional lymph node metastasis (Table 1).
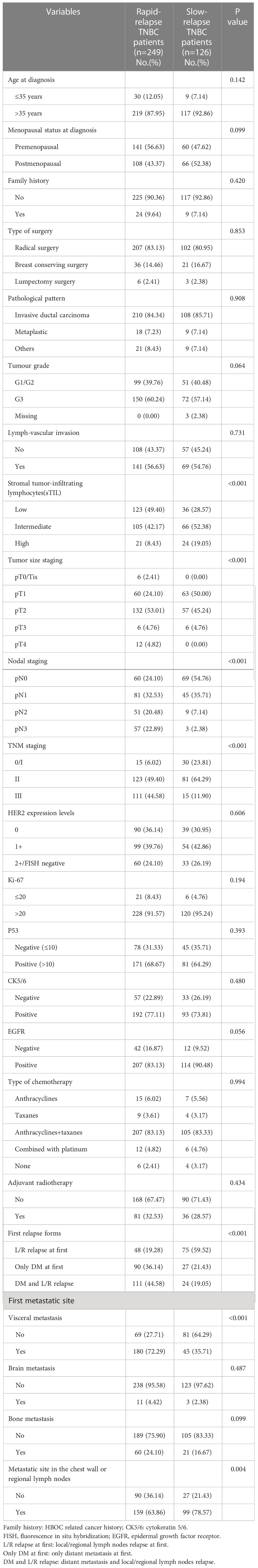
Table 1 The relapse and metastasis characteristics between RR-TNBC and SR-TNBC patients according to stratified variables: univariate analysis.
R version 4.1.0 was used to randomly divide all 1584 TNBC patients into two groups in a 7:3 ratio: the training set (1108 patients) and the validation set (476 patients). The random seed was determined to be 123456. The clinicopathological parameters of these two groups are presented in Table 2. There was no statistical difference between these two groups in terms of clinicopathological parameters (Table 2). Through comparing the clinicopathological parameters between 175 RR-TNBC patients and 933 non-RR-TNBC patients in the training set, the univariate analysis showed that menopausal status, pathological pattern, T staging, N staging, TNM staging, sTIL expression levels, and HER2 expression levels were significantly associated with the risk factors for relapse in RR-TNBC patients (Table 3). Variables with P<0.1 in univariate analysis were included in multivariate analysis and the multivariate analysis showed that metaplastic breast cancer, ≥pT3 staging, and ≥pN1 staging were independent risk factors for relapse in RR-TNBC patients. Postmenopausal status, sTIL intermediate/high expression, and Her2 (1+) were independent protective factors for relapse in RR-TNBC patients (Table 4).
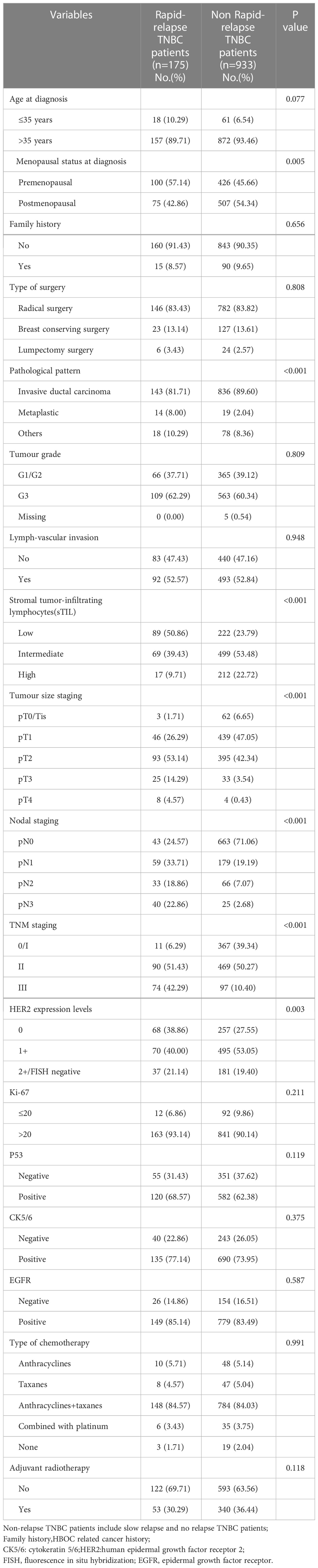
Table 3 Risk factors of rapid relapse in training set according to stratified variables: univariate analysis.
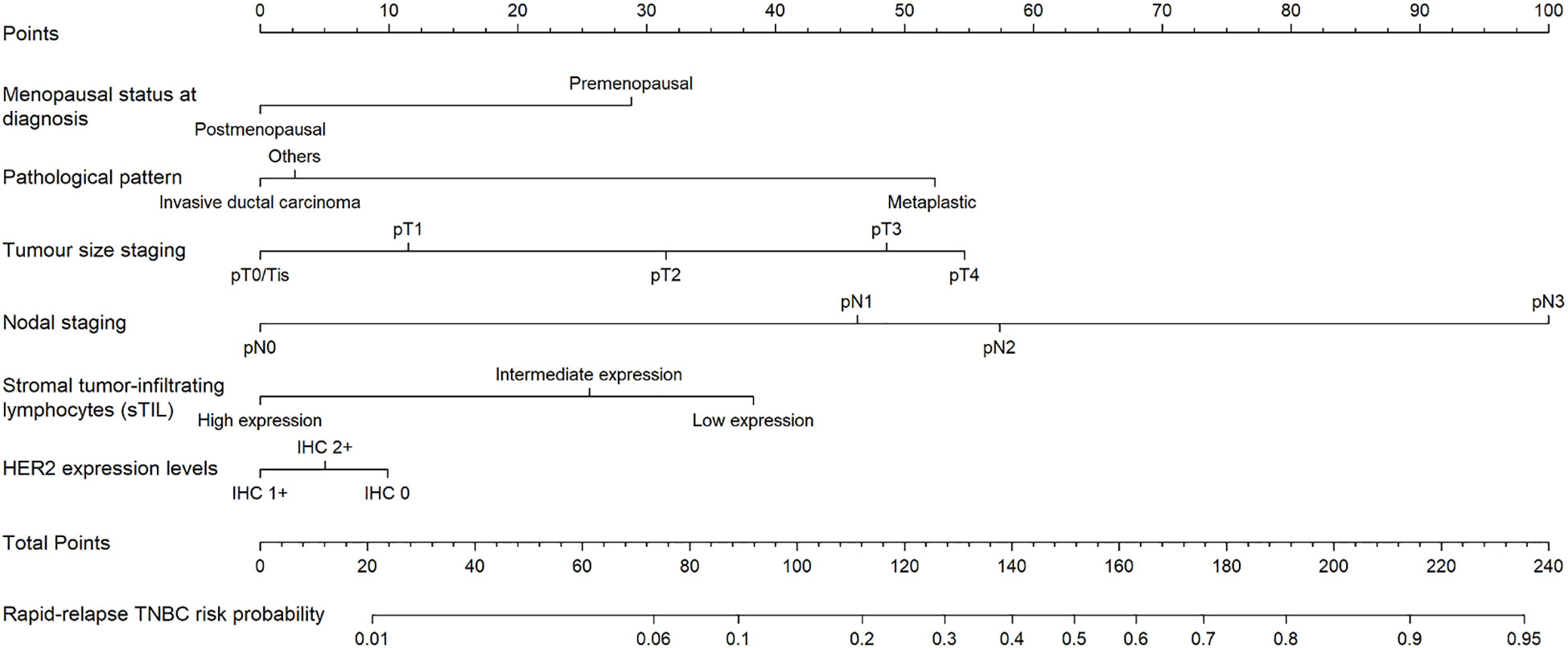
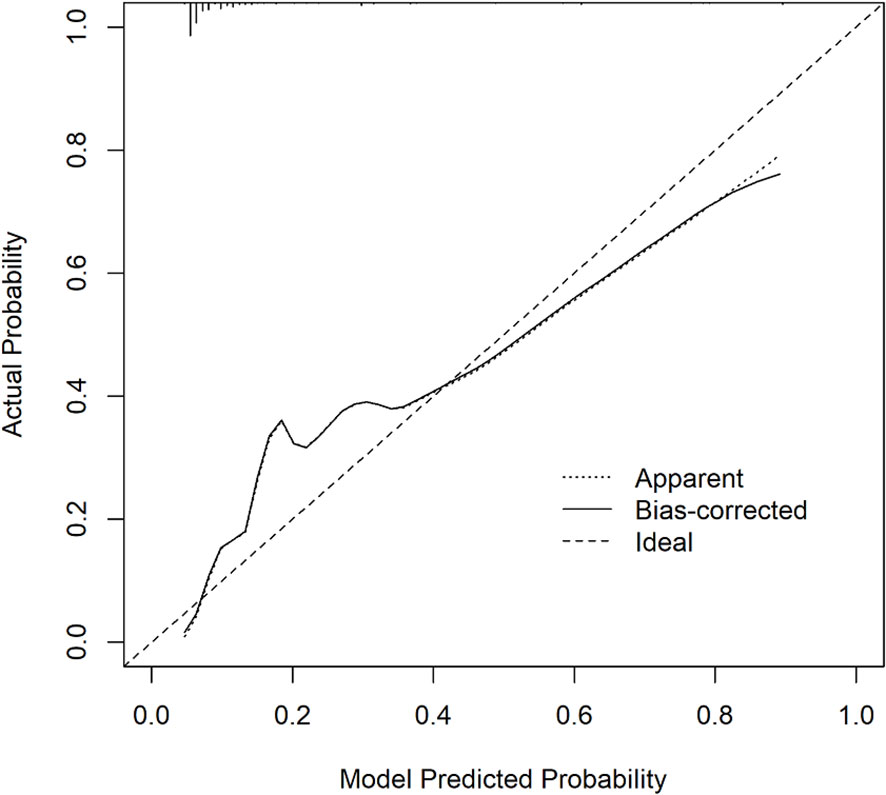
Six predictors (postmenopausal status, metaplastic breast cancer,≥pT3 staging,≥pN1 staging,sTIL intermediate/high expression, and Her2 [1+]) were used to construct the predictive model of rapid relapse in TNBC patients (Figure 2). The C-index and brier score in the validation set was 0.861 and 0.095, respectively. ROC (receiver operating characteristic) analysis for predicting rapid relapse in the validation set showed that AUC (area under the curve) was 0.861(95% confidence interval [CI]: 0.814–0.908). The sensitivity for predicting rapid relapse in the validation set was 0.878 (95% CI: 0.782–0.943). The specificity for predicting rapid relapse in the validation set was 0.731 (95% CI: 0.685–0.774) (Figure 3). The calibration curve for predicting rapid relapse in the validation set is presented in Figure 4. This suggested that the predictive model had high discrimination and accuracy.
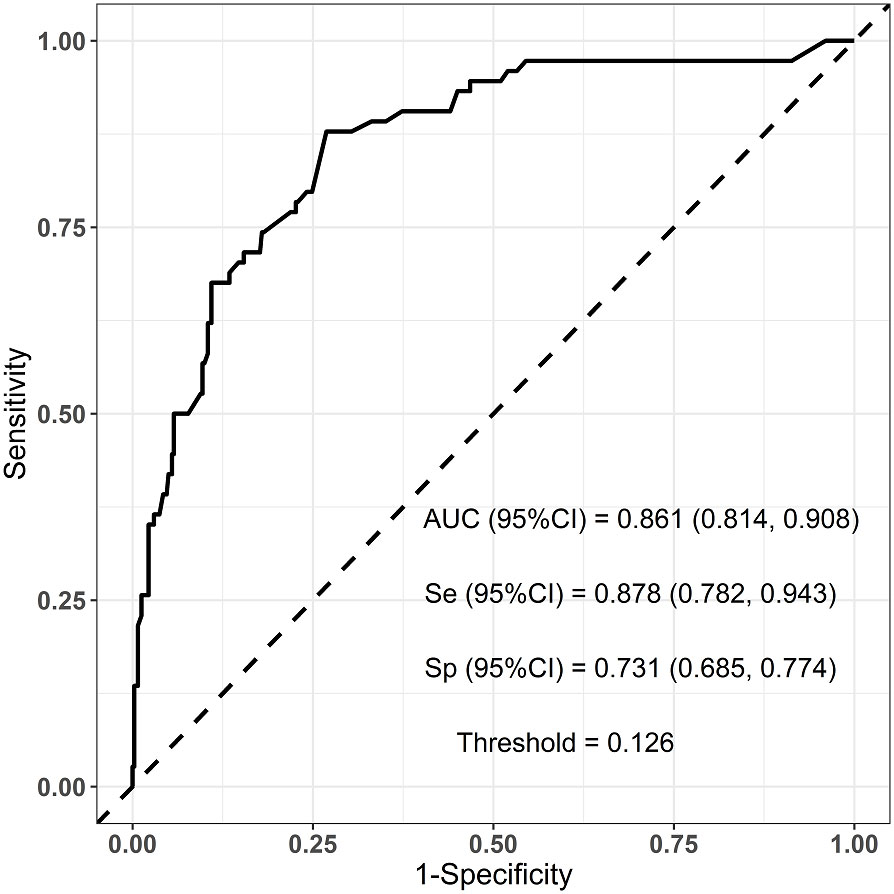
Figure 3 ROC analysis for predicting rapid relapse in the validation set. ROC, receiver operating characteristic; AUC, area under the curve.
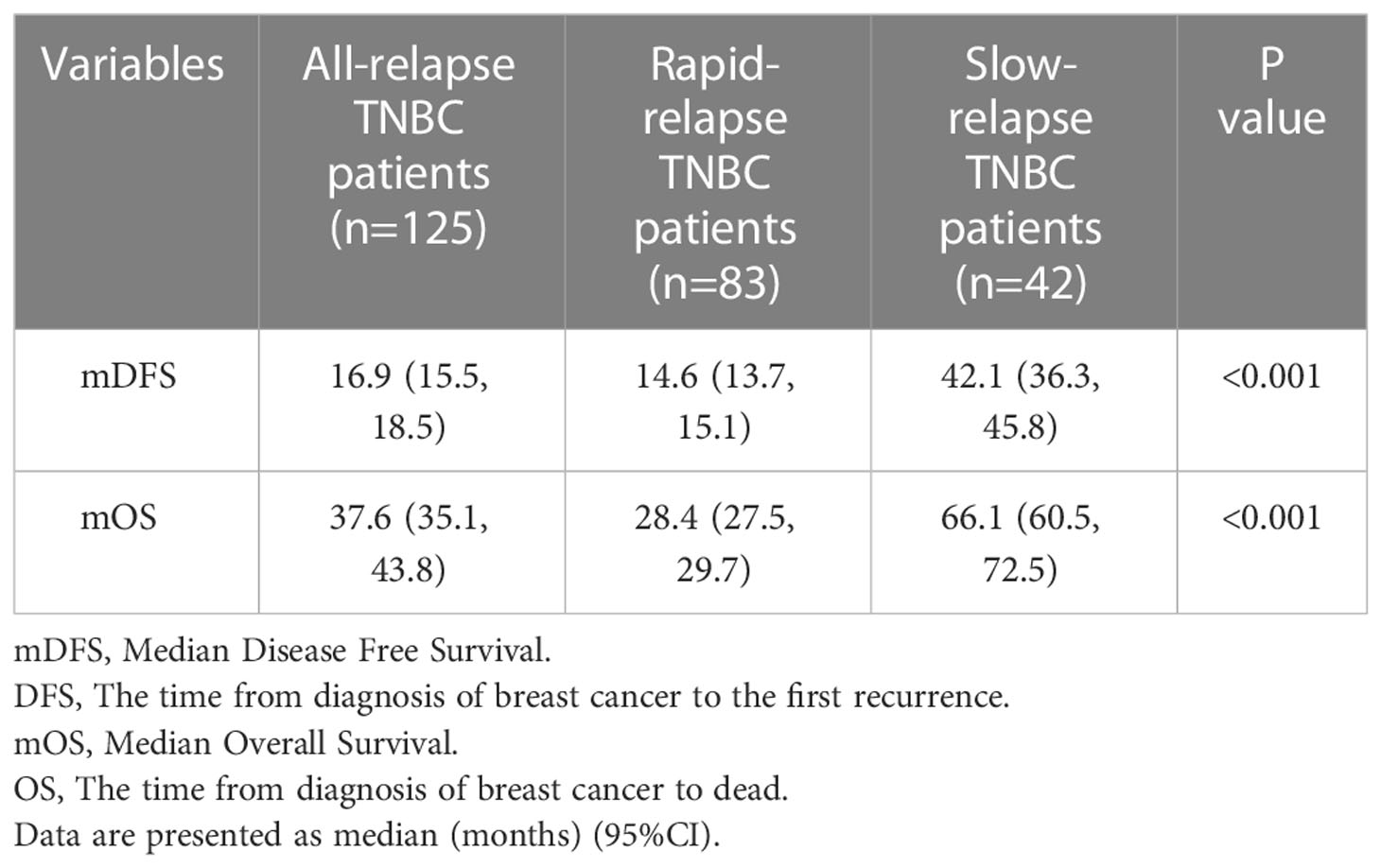
Prognostic data of all TNBC patients showed that RR-TNBC patients had the worst prognosis, followed by SR-TNBC patients (Figure 5). The median disease-free survival and median overall survival of different recurred TNBC patients are presented in Table 5. The overall survival analysis of all TNBC patients in six predictors for rapid relapse are presented in Figure 6. Different expressions of sTILs(Low, intermediate, and high) and different expression levels of Her2 (IHC 0, IHC 1+, or IHC 2+/FISH-negative) were detected in tumors by using hematoxylin and eosin staining (H&E×200, Figure 7).
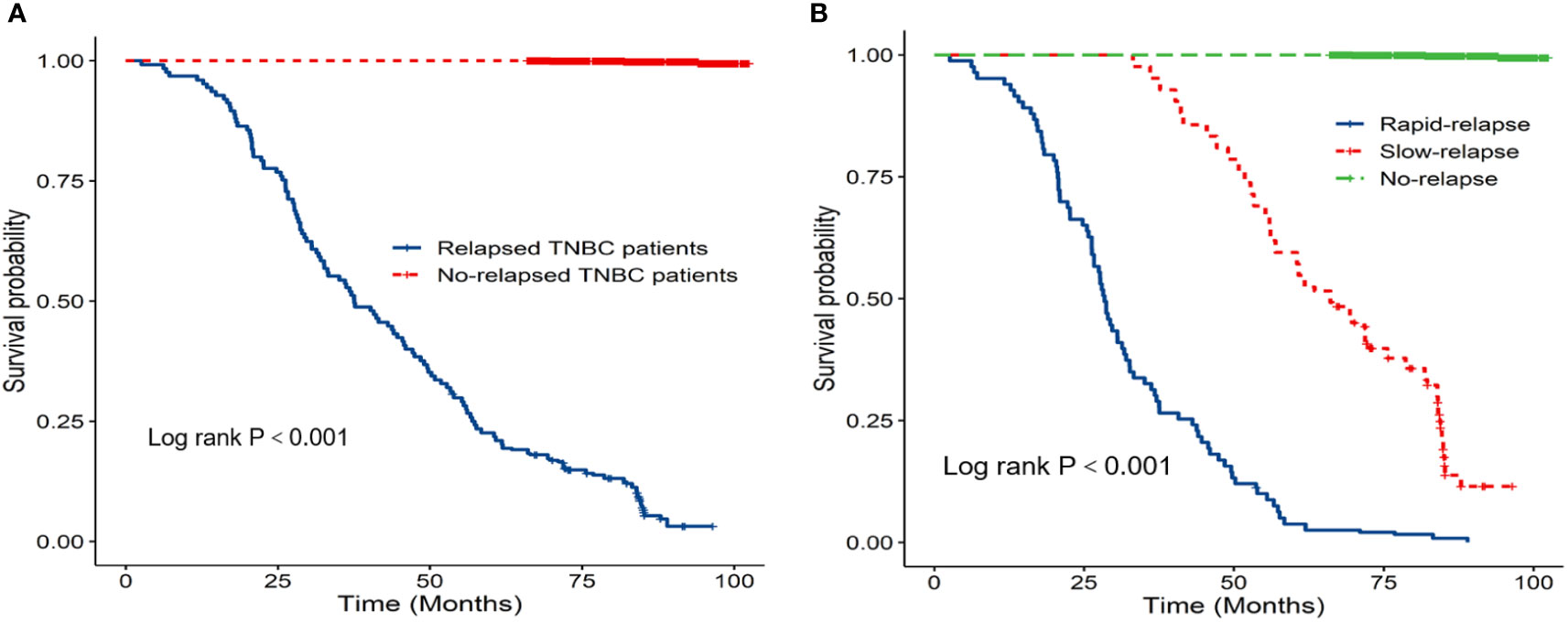
Figure 5 (A) K-M overall survival analysis of relapsed patients and no relapsed patients; (B) K-M overall survival analysis of different relapsed types in 1584 TNBC patients.

Figure 6 K-M overall survival analysis of all TNBC patients in six predictors for rapid relapse (A) Menopausal status at diagnosis; (B) Pathological pattern; (C) Tumour size staging; (D) Nodal staging; (E) Stromal tumor-infiltrating lymphocytes(sTIL); (F) Her2 expression levels).

Figure 7 Different expressions of sTILs [(A) Low, (B) intermediate, (C) high] and different expression levels of Her2 [(D) IHC 0, (E) IHC 1+, (F) IHC 2+/FISH-negative] were detected in tumors by using hematoxylin and eosin staining (H&E×200).
4 Discussion
To our knowledge, relative to other subtypes of breast cancer, TNBC was always associated with a higher aggressiveness and more risk of local recurrence and visceral metastasis (3–5). The highest risk of recurrence in TNBC patients was during the first 3 years after the disease diagnosis. After the first 3 years, the recurrence risk declined rapidly (11). Until recently, most TNBC studies have focused on overall recurred populations, and fewer studies have examined the timing of relapse. However, in our clinical practice, there was an aggressive subset of TNBC patients with marked chemoresistance, rapid metastatic spread, and poor survival. We defined it as the rapid-relapse TNBC (RR-TNBC). Learning more about the biological characteristics of RR-TNBC could help us to better identify these patients, improve patient outcomes, and optimize treatment regimens in the future. In our study, through the prognostic analysis of all 1584 TNBC patients and the characteristics of recurrence analysis in different recurred types of TNBC patients, no matter the median disease-free survival or the median overall survival, RR-TNBC patients have the worst prognosis, followed by SR-TNBC patients. This result was consistent with the observation of TNBC patients from the previous studies (17, 18). Besides that, RR-TNBC patients tended to have a higher T staging, N staging, TNM staging, and low expression of stromal tumor-infiltrating lymphocytes (sTIL). The recurrence characteristics were prone to appear as distant metastasis at the first relapse. The first metastatic site was apt to visceral metastasis and less likely to have chest wall or regional lymph node metastasis. We supposed that the worse prognosis of RR-TNBC patients may be due to: 1) the biological characteristics of TNBC; having no therapeutic target, high heterogeneity, and aggressive behavior: 2) just as we found in our study, through the comparative analysis between RR-TNBC and SR-TNBC groups, the population distribution and relapsed characteristics of RR-TNBC patients may also lead to an earlier recurrence and metastasis, and ultimately affect the prognosis: and 3) a lack of more detailed treatment information may also lead to a different prognosis.
In this study, we also included a series of non-RR-TNBC patients to assess the risk factors for relapse in RR-TNBC patients. We included many clinicopathological factors in predicting risk factors. Among these clinicopathological indicators, primary tumor size, lymph node metastatic status, tumor staging, lymph-vascular invasion or not, tumor grade, and Ki-67 status were important indicators that reflect the characteristics of triple-negative breast tumors. Related research has reported that TNBC patients with larger tumors, more lymph node metastases, later TNM staging,lymph-vascular invasion, higher tumor grade, and Ki-67 were more likely to have metastasis and had a shorter disease-free survival (19, 20). However, our study found that ≥pT3 staging and ≥pN1 staging were the independent risk factors for relapse in RR-TNBC patients. Patients with higher T staging and N staging did have a worse prognosis. The reason for this might be that TNBC patients with a higher T staging or N staging had greater tumor burdens. Therefore, compared with other clinicopathological indicators, ≥pT3 staging and ≥pN1 staging were important factors in rapid recurrence and metastasis.
Most oncologists agreed with that: except for the biological characteristics of the tumor itself, the tumor microenvironment also played an important role in the growth, invasion, and metastasis of tumor cells. Therefore, in our study, we also discussed whether some tumor microenvironment variables were associated with the rapid relapse in TNBC patients or not, such as sTILs, P53, CK5/6, and EGFR. Many studies have reported that TNBC with a high level of sTILs showed better short-term and long-term prognoses (21–23). The main reason for this was that the CD4+ T cells and CD8+ T cells (primary effector sTIL subtypes) had been linked to a better response to anti-tumor treatment in triple-negative breast cancer (24, 25). However, a small number of studies reported that TNBC patients with sTIL enrichment after NAC were at a higher risk of relapse (26). Through univariate and multivariate analysis in the training set, we found that the intermediate/high expression of sTILs was an independent protective factor for recurrence in RR-TNBC patients. Moreover, prognostic analysis of all TNBC patients confirmed that patients with a higher level of sTILs had a better prognosis, which was consistent with many previous reports. Although relevant studies have reported that the positive expression of CK5/6, EGFR, and P53 in TNBC patients had a worse prognosis (4, 27), our study did not find that CK5/6, EGFR, and P53 expression were associated with rapid relapse in TNBC patients. These results requires more studies and larger sample sizes to verify this conclusion.
Sociodemographic factor analysis by Daniel G. Stover et al. found that RR-TNBC patients were associated with Medicaid/indigent insurance, being single, of Black ethnicity, having a lower income, and a younger age at diagnosis (28, 29). Due to a lack of data, we did not focus on the analysis of many sociodemographic factors. Our clinicopathological factors analysis only found that postmenopausal status was an independent protective factor for recurrence in rrTNBC patients. There was no significant difference in overall survival between premenopausal and postmenopausal patients. Female patients of younger age (age ≤ 35 years) and family history were not significantly associated with the rapid relapse of TNBC patients. Moreover, we studied whether some special type of TNBC would increase the risk of rapid relapse or not. Few studies in this area were mainly because the number of these special types of TNBC patients was too small. Most research about the special type of TNBC patients focused on the prognosis. In our study, we also found that metaplastic breast cancer (MBC) patients had the worst prognosis. The five-year overall survival rate of MBC patients was about 60.0% (27/45), which was consistent with previous relevant reports (30–32). Besides that, we found that MBC was an independent risk factor for rapid relapse in TNBC patients. This may be associated with the biological characteristics of MBC. Considerating the low morbidity of MBC, and the small number of MBC patients in our study, more research is required to confirm this.
In recent years, Trastuzumab deruxtecan (T-DXd), a novel HER2-targeting therapy has been developed. Relevant research found that it can not only target HER2-positive tumor cells but also can effectively target tumor cells that express low levels of HER2 through the bystander effect to neighboring tumor cells heterogeneously expressing HER2 (33–35). Therefore, we explored the relationship between HER2 status and RR-TNBC in our study. Although the overexpression of HER2 was associated with shorter overall survival and a higher risk of disease recurrence. Through the univariate and multivariate analysis in our study, only HER2 (1+) status was an independent protective factor for recurrence in RR-TNBC patients. Moreover, survival analysis showed that patients with HER2 (1+) status had a better prognosis. Therefore, the relationship between HER2 status and RR-TNBC remains unclear and needs more research to confirm it. In our study, we also analyzed the relationship between different treatments and RR-TNBC patients. Findings indicated that breast surgery, chemotherapy, and radiotherapy reduce the risk of locoregional failure, recurrence, and breast cancer mortality in TNBC patients, especially patients with intermediate or high-recurring risk factors (36, 37). In our study, we did not find that different treatments were significantly associated with rapid relapse in TNBC patients.
Our study combined the results of univariate and multivariate analysis in the training set and established a predictive model for the rapid relapse in TNBC patients. Through the analysis of receiver operating characteristics (ROC) and the calibration curve in the verification set, we found that our predictive model revealed good discrimination with a C-index of 0.861 and good accuracy with a brier score of 0.095. These findings indicate that our predictive model is suitable for predicting the probability of rapid relapse in TNBC patients.
5 Limitations
Several limitations should be considered in our study: first, it included the heterogeneous TNBC patients and a retrospective study from a single center. Second, it lacked more sociodemographic, genomic data, and detailed treatment information may weaken the accuracy of prediction. For example, the number of chemotherapy regimen cycles, the dose of chemotherapy and radiotherapy, the completion rate of chemoradiotherapy, and whether chemotherapy and radiotherapy were delayed or not. Finally, the generalizability of the predictive model needs to be externally validated with an independent population before it can be applied to clinical practice. Therefore, we still need a larger sample size and longer follow-up time to analyze the biological characteristics of RR-TNBC patients in the future.
6 Conclusion
In this single-center study, by studying the characteristics of recurrence, predictors for relapse, and prognosis in RR-TNBC patients, we verified that TNBC was a highly heterogeneous disease. The different relapse types of TNBC patients had unique biological behaviors and prognoses, especially for RR-TNBC patients. Addressing the limitations of the present study, we will continue to expand our sample sizes and collect more data from different breast cancer treatment centers in the future, to build a better predictive model through internal and external validation methods. In addition, integrating more sociodemographic, clinicopathological, and genomic features in the future will help us excavate more internal and external biological characteristics for RR-TNBC patients. Following on from the progress we have made in immunotherapy and antibody–drug conjugates therapy (35, 38–40), this exploratory and comprehensive research on RR-TNBC patients will help us to find more potential therapeutic targets and optimize treatment regimens, benefitting TNBC patients through individualized treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Tianjin Medical University Cancer Institute and Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
S-LC and JZ contributed to the conception and design of the study. S-LC and SZ developed the methodology. X-QL, H-DC, XF, TM, YL, Y-QL, C-YL, SZ, X-GC, and X-JG took part in the data acquisition, analysis, and interpretation. S-LC, J-JL, Y-XL, S-HY, and XL wrote, reviewed, and/or revised the manuscript. JZ supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by research funding from the General Project of the Scientific Research Program of Tianjin Municipal Education Commission(No.2019KJ186).
Acknowledgments
We thank all patients who participated in our study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
References
1. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumors. Nature (2000) 406(6797):747–52. doi: 10.1038/35021093
2. Harbeck N, Gnant M. Breast cancer. Lancet (2017) 389(10074):1134–50. doi: 10.1016/S0140-6736(16)31891-8
3. Cossetti RJ, Tyldesley SK, Speers CH, Zheng Y, Gelmon KA. Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J Clin Oncol (2015) 33(1):65–73. doi: 10.1200/JCO.2014.57.2461
4. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med (2010) 363(20):1938–48. doi: 10.1056/NEJMra1001389
5. Ge JY, Zuo WJ, Chen YY, Shao ZM, Yu KD. The advance of adjuvant treatment for triple-negative breast cancer. Cancer Biol Med (2021) 19(2):187–201. doi: 10.20892/j.issn.2095-3941.2020.0752
6. Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer (2008) 113(10):2638–45. doi: 10.1002/cncr.23930
7. Kassam F, Enright K, Dent R, Dranitsaris G, Myers J, Flynn C, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: Implications for clinical practice and trial design. Clin Breast Cancer (2009) 9(1):29–33. doi: 10.3816/CBC.2009.n.005
8. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med (2018) 379(22):2108–21. doi: 10.1056/NEJMoa1809615
9. Zhang YQ, Asad S, Weber Z, Tallman D, Nock W, Wyse M, et al. Genomic features of rapid versus late relapse in triple-negative breast cancer. BMC Cancer (2021) 21(1):568. doi: 10.1186/s12885-021-08320-7
10. Wang LY, Zhai QL, Lu QY, Lee KP, Zheng QF, Hong RX, et al. Clinical genomic profiling to identify actionable alterations for very early relapsed triple-negative breast cancer patients in the Chinese population. Ann Med (2021) 53(1):1358–69. doi: 10.1080/07853890.2021.1966086
11. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res (2007) 13(15 Pt 1):4429–34. doi: 10.1158/1078-0432.CCR-06-3045
12. van Roozendaal LM, Smit LHM, Duijsens GHNM, Vries BD, Siesling S, I Lobbes MB, et al. Risk of regional recurrence in triple-negative breast cancer patients: A Dutch cohort study. Breast Cancer Res Treat (2016) 156(3):465–72. doi: 10.1007/s10549-016-3757-4
13. Ghosh J, Agarwal S, Ganguly S, Dabkara D, Biswas B, Nandi S, et al. Patterns of recurrence in triple-negative breast cancer patients (automated IHC): An Indian tertiary care center data [abstract]. J Clin Oncol (2018) 36(Suppl):e13128. doi: 10.1200/JCO.2018.36.15_suppl.e13128
14. Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical Oncology/College of American pathologists clinical practice guideline update. J Clin Oncol (2013) 31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984
15. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol (1999) 17(5):1474–81. doi: 10.1200/JCO.1999.17.5.1474
16. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an international TILs working group 2014. Ann Oncol (2015) 26(2):259–71. doi: 10.1093/annonc/mdu450
17. Pogoda K, Niwińska A, Murawska M, Pieńkowski T. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol (2013) 30(1):388. doi: 10.1007/s12032-012-0388-4
18. Sheng DL, Shen XG, Shi ZT, Chang C, Li JW. Survival outcome assessment for triple-negative breast cancer: A nomogram analysis based on integrated clinicopathological, sonographic, and mammographic characteristics. Eur Radiol (2022) 32(10):6575–87. doi: 10.1007/s00330-022-08910-4
19. Shi H, Wang XH, Gu JW. Guo GL.Development and validation of nomograms for predicting the prognosis of triple-negative breast cancer patients based on 379 Chinese patients. Cancer Manag Res (2019) 30(11):10827–39. doi: 10.2147/CMAR.S234926
20. Yang YP, Wang Y, Deng HR, Tan C, Li Q, He ZH, et al. Development and validation of nomograms predicting survival in Chinese patients with triple-negative breast cancer. BMC Cancer (2019) 19(1):541. doi: 10.1186/s12885-019-5703-4
21. de Jong VM T, Wang YW, Ter Hoeve ND, Opdam M, Stathonikos N, Jóźwiak K, et al. Prognostic value of stromal tumor-infiltrating lymphocytes in young, node-negative, triple-negative breast cancer patients who did not receive (neo)Adjuvant systemic therapy. J Clin Oncol (2022) 40(21):2361–74. doi: 10.1200/JCO.21.01536
22. Gao GX, Wang ZH, Qu X, Zhang ZT. Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: A systematic review and meta-analysis. BMC Cancer (2020) 20(1):179. doi: 10.1186/s12885-020-6668-z
23. Park JH, Jonas SF, Bataillon G, Criscitiello C, Salgado R, Loi S, et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol (2019) 30(12):1941–9. doi: 10.1093/annonc/mdz395
24. Zhang H, Qin GH, Yu H, Han X, Zhu S. Comprehensive genomic and immunophenotypic analysis of CD4 T cell infiltrating human triple-negative breast cancer. Cancer Immunol Immunother (2021) 70(6):1649–65. doi: 10.1007/s00262-020-02807-1
25. Sun XJ, Zhai J, Sun B, Parra ER, Jiang M, Ma W, et al. Effector memory cytotoxic CD3+/CD8+/CD45RO+ T cells are predictive of good survival and a lower risk of recurrence in triple-negative breast cancer. Mod Pathol (2022) 35(5):601–8. doi: 10.1038/s41379-021-00973-w
26. Giacchetti S, Faucheux L, Gardair C, Cuvier C, Roquancourt AD, Campedel L, et al. Negative relationship between post-treatment stromal tumor-infiltrating lymphocyte (TIL) and survival in triple-negative breast cancer patients treated with dose-dense dose-intense NeoAdjuvant chemotherapy. Cancers (Basel) (2022) 14(5):1331. doi: 10.3390/cancers14051331
27. Pan YB, Yuan YF, Liu GS, Wei YC. P53 and ki-67 as prognostic markers in triple-negative breast cancer patients. PloS One (2017) 12(2):e0172324. doi: 10.1371/journal.pone.0172324
28. Asad S, Barcenas CH, Bleicher RJ, Cohen AL, Javid SH, Levine EG, et al. Sociodemographic factors associated with rapid relapse in triple-negative breast cancer: A multi-institution study. J Natl Compr Canc Netw (2021) 19(7):797–804. doi: 10.6004/jnccn.2020.7659
29. Obeng-Gyasi S, Asad S, Fisher JL, Rahurkar S, Stover DG. Socioeconomic and surgical disparities are associated with rapid relapse in patients with triple-negative breast cancer. Ann Surg Oncol (2021) 28(11):6500–9. doi: 10.1245/s10434-021-09688-3
30. Elimimian EB, Samuel TA, Liang H, Elson L, Bilani N, Nahleh ZA. Clinical and demographic factors, treatment patterns, and overall survival associated with rare triple-negative breast carcinomas in the US. JAMA Netw Open (2021) 4(4):e214123. doi: 10.1001/jamanetworkopen.2021.4123
31. Kim JY, Kim JY, Lee HB, Lee YJ, Seong MK, Paik N, et al. Characteristics and prognosis of 17 special histologic subtypes of invasive breast cancers according to world health organization classification: Comparative analysis to invasive carcinoma of no special type. Breast Cancer Res Treat (2020) 184(2), 527–42. doi: 10.1007/s10549-020-05861-6
32. Tadros AB, Sevilimedu V, Giri DD, Zabor EC, Morrow M. Plitas G.Survival outcomes for metaplastic breast cancer differ by histologic subtype. Ann Surg Oncol (2021) 28(8):4245–53. doi: 10.1245/s10434-020-09430-5
33. Tamura K, Tsurutani J, Takahashi S, Iwata H, Krop IE, Redfern C, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: Adose-expansion, phase 1 study. Lancet Oncol (2019) 20(6):816–26. doi: 10.1016/S1470-2045(19)30097-X
34. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients withHER2-low-expressing advanced breast cancer: Results from a phase ib study. J Clin Oncol (2020) 38(17):1887–96. doi: 10.1200/JCO.19.02318
35. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med (2022) 387(1):9–20. doi: 10.1056/NEJMoa2203690
36. Network NCC. NCCN clinical practice guidelines in oncology (NCCN guidelines), breast cancer . Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (Accessed 21 June 2020).
37. Recht A, Comen EA, Fine RE, Fleming GF, Hardenbergh PH, Ho AY, et al. Postmastectomy radiotherapy: An American society of clinical oncology, American society for radiation oncology, and society of surgical oncology focused guideline update. J Clin Oncol (2016) 34(36):4431–42. doi: 10.1200/JCO.2016.69.1188
38. Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med (2022) 386(6):556–67. doi: 10.1056/NEJMoa2112651
39. Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2020) 21(1):44–59. doi: 10.1016/S1470-2045(19)30689-8
Keywords: triple negative breast cancer, rapid relapse, slow relapse, biological characteristics analysis, prognostic analysis
Citation: Cai S-L, Liu J-J, Liu Y-X, Yu S-H, Liu X, Lin X-Q, Chen H-D, Fang X, Ma T, Li Y-Q, Li Y, Li C-Y, Zhang S, Chen X-G, Guo X-J and Zhang J (2023) Characteristics of recurrence, predictors for relapse and prognosis of rapid relapse triple-negative breast cancer. Front. Oncol. 13:1119611. doi: 10.3389/fonc.2023.1119611
Received: 09 December 2022; Accepted: 01 February 2023;
Published: 16 February 2023.
Edited by:
Shengyu Yang, College of Medicine, The Pennsylvania State University, United StatesCopyright © 2023 Cai, Liu, Liu, Yu, Liu, Lin, Chen, Fang, Ma, Li, Li, Li, Zhang, Chen, Guo and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Zhang, zhangjin@tjmuch.com
†These authors have contributed equally to this work and share the first authorship
 Shuang-Long Cai
Shuang-Long Cai Jing-Jing Liu1,2,3,4†
Jing-Jing Liu1,2,3,4† Jin Zhang
Jin Zhang