- 1Department of Neurosurgery, The First Hospital of Jilin University, Jilin, China
- 2Department of Gynecology Oncology, The First Hospital of Jilin University, Jilin, China
Gliomas are one of the most common primary central nervous system tumors, and surgical treatment remains the principal role in the management of any grade of gliomas. In this study, based on the introduction of gliomas, we review the novel surgical techniques and technologies in support of the extent of resection to achieve long-term disease control and summarize the findings on how to keep the balance between cytoreduction and neurological morbidity from a list of literature searched. With modern neurosurgical techniques, gliomas resection can be safely performed with low morbidity and extraordinary long-term functional outcomes.
Introduction
Gliomas stem from glial cells of the central nervous system (CNS), and they are the most common primary CNS tumors. According to the 2021 World Health Organization (WHO) classification of CNS tumors, gliomas are classified as low-grade gliomas (LGGs; WHO grades I or II) and high-grade gliomas (HGGs; WHO grades III or IV). Supratentorial gliomas account for approximately 30% of all adult primary intracranial tumors, and more than 50% of these are high-grade gliomas (HGGs) (1). Several clinical features (both pathological and non-pathological) determine the grade, with pathological features including nuclear atypia, mitotic activity, vascular proliferation, necrosis, and so on, and non-pathological ones including clinical course and treatment outcome (2). Glioma is more common in whites and blacks, and the incidence of it in men is 1.5 times that in women (3).
China is one of the countries with the largest prevalence and death rates of CNS tumors (4). Overall incidence rates with adjusted age for all gliomas range from 4.67 to 5.73 per 100,000 persons (5, 6). The median overall survival (OS) times were 78.1, 37.6, and 14.4 months for low-grade gliomas, anaplastic gliomas, and glioblastomas, respectively (7). Most cases of gliomas are of sporadic onset, although some are related to Mendelian disorders such as tuberous sclerosis, neurofibromatosis type 1 or type 2, and so on (8). There are several factors that influence prognosis, including the Karnofsky Performance Status Scale at diagnosis, histology, and molecular markers. Age and tumor histology have been identified as primary predictors of patient prognosis (9, 10). Irradiated brain or scalp in the past may increase the risk of developing gliomas (11, 12).
Gliomas have variable presenting symptoms depending on tumor size and location. A systemic review summarizes the symptom prevalence and concludes several symptoms, of which the most prevalent ones are seizures, cognitive deficits, drowsiness, and dysphagia during different phases (13). In these tumors, seizures are the most common presenting symptom since they tend to be highly epileptogenic. It is common in low- and high-grade gliomas. The risk of seizures varies between 60% and 100% among low-grade gliomas and between 40% and 60% among glioblastomas (14). Seizure control and decreasing neurocognitive deficits are the two main purposes for some of the patients diagnosed with gliomas, which they always pursue.
The principle of surgical treatment of gliomas is necessary to reduce the mass effect caused by the tumors, maximize the extent of resection, and in the meantime reduce the damage to the surrounding tissue structure, especially the gliomas located in the eloquent area, to protect neurological function. The treatment procedures are different depending on the grade of the gliomas. We will introduce LGGs, HGGs, and recurrent gliomas separately.
Low-grade gliomas
LGGs account for up to 15% of all brain tumors in adults (15). Some volumetric studies support the idea of “extent of resection” (EOR) to improve survival in patients with LGGs. These ones illustrated mean survival time variation (61.1 to 90.5 months) with maximal resection (10, 16, 17). Low-grade gliomas are the most common and uniformly fatal disease in young adults (mean age 41 years), with survival averaging approximately 7 years (18). EOR is a statistically significant predictor of overall survival. The data from these studies emphasize the importance of achieving a complete resection, cannot be overstated. LGGs have a diverse anatomical, histopathological, and molecular profile, which reflect the clinical outcome (19). In addition to affecting overall survival in LGG patients, the EOR also influences the malignant transformation rate and seizure-free status (20). A retrospective study including 153 glioma patients followed by “watch and wait” and early surgical resection, respectively, demonstrated that patients with LGGs who underwent early surgery had a higher chance of survival, which suggested that the timing of resection was crucial (21).
High-grade gliomas
For patients with primary HGGs such as glioblastoma, retrospective analysis from a randomized trial has concluded that survival and progression-free survival are highly influenced by the extent of tumor resection; the fact that incomplete resections result in more rapid neurological deterioration also attests to the importance of complete resections on progression-free survival (22–24).
One retrospective study on the comparison of “gross total” and “subtotal” resection data demonstrated that the gross total section of HGGs had a greater survival rate for 1 year follow-up, which decreased to 19% at 2 years, than subtotal resection (25). Some MRI scans of these patients diagnosed as HGGs always show a noncontrast-enhancing surrounding abnormality, so the study of the resection rate on the abnormality’s surroundings remains unclear. Li reported the results that the group that underwent gross total resection of ≥53.21% of the surrounding FLAIR abnormality beyond the 100% contrast-enhancing resection was associated with a significant prolongation of survival compared with that following less extensive resection (p <0.001) (26).
In the discussion of the extent and rate of resection, Sanai reported that for glioblastoma multiforme (GBM), aggressive EOR equated to improvement in overall survival, even at the highest levels of resection. A significant survival advantage was seen with as little as 78% EOR, and stepwise improvement in survival was evident even in the 95%–100% EOR range (27).
Recurrent gliomas
No matter which classification the recurrent gliomas (LGGs or HGGs) are, many patients accept repeat resection as a common treatment option to pursue a good quality of life (28). A significant benefit had been seen in 52 patients with reoperated LGGs; the principle of undergoing reoperation demonstrated an overall 10-year survival rate of 57%; variable prognostic factors such as the use of upfront radiation and pathology at recurrence influenced the overall survival rate (29). A large study on recurrent HGGs reported to date that the median overall survival duration was 19 months with a median progression-free survival following re-resection of 5.2 months, suggesting that the survival benefit of microsurgical resection did not diminish despite biological progression (30). At present, the clinical benefit of reoperation for both recurrent LGGs and HGGs demonstrates that the extent of resection can be the strongest predictor of overall survival in each individual patient.
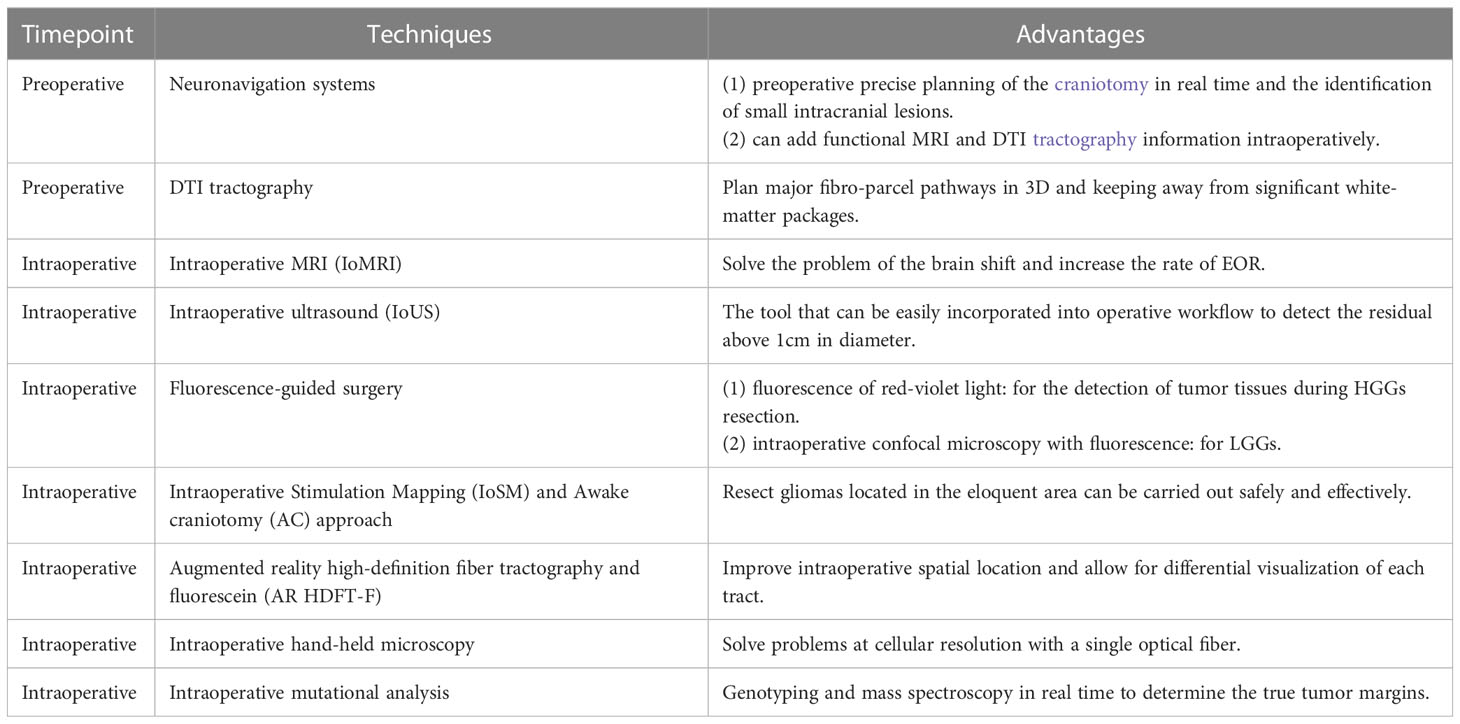
Advances in surgical techniques and neurosurgical tools make neuro-oncology practice easier, meanwhile improving favorable outcomes and maximizing cytoreduction for patients with gliomas. Advanced neurosurgical imaging technologies, including intraoperative neuronavigation (31, 32), DTI tractography (33), intraoperative MRI (IoMRI) (34, 35), intraoperative ultrasonography (IoUS) (36, 37), fluorescence-guided surgery (38, 39), intraoperative stimulation mapping (IoSM), the awake craniotomy (AC) approach (40, 41), augmented reality high-definition fiber tractography and fluorescein (AR HDFT-F) (42, 43), intraoperative hand-held microscopy, and intraoperative mutational analysis have improved the complete radiographic resection rate of gliomas.
Neuronavigation systems
Neuronavigation systems have been widely used in the operative management of gliomas and offer lots of advantages to surgeons. Preoperatively precise planning of the craniotomy in real time and the identification of small intracranial lesions are some of the principal benefits. In addition to the basic settings, it is also now possible to add functional MRI (fMRI) and DTI tractography information as an overlay available to the surgeon intraoperatively. fMRI does not rely on the use of radiation; it can produce images with high spatial resolution by the millimeter and poor temporal resolution because of a 5-second lag between initial neural activity and image; it can capture a clear picture of brain activity picture only if the patient stays still but not moment-to-moment brain activity. Neuronavigation can generate relationships between mass lesions and functional areas. A randomized controlled trial on the study of the effectiveness of neuronavigation demonstrated that the mean amount of residual tumor tissue was 13.8% for surgery involving neuronavigation compared with 28.9% for standard surgery, although there was no rationale for the use of neuronavigation to improve the extent of tumor resection because of the size or location of the lesion (33).
DTI tractography
DTI is utilized to plan major fibro-parcel pathways in 3D and keep away from significant white-matter packages. The imagined white-matter groups are integrated into a 3D model to limit the level of careful dreariness brought about by disturbance of significant white-matter packs.
Nevertheless, some perioperative trials about the effect of DTI neuronavigation on reducing morbidity did not show clear evidence because of tumor infiltration and edema (31, 44, 45). Given the insufficient approximation of functional sites, DTI itself cannot be used as a tool for surgical decision-making. Diffusion-weighted MRI as a novel technique is being used to overcome the previous issue of accurately mapping peritumoral edema tissue (46).
Intraoperative MRI
Intraoperative MRI (IoMRI) changes the way we deal with gliomas. IoMRI not only helps us to solve the problem of brain shift but also assists the neurosurgeon to highlight the tumor remnants and reach a higher EOR. In one prospective study including 100 adult patients operated on for gliomas using IoMRI with neuronavigation, Leroy reported that the median EOR was 100% whatever the type of glioma and location. It was only in the insula area that residue levels were higher. There was no difference between LGGs and HGGs in the median KPS at different follow-up times after surgery. “Staged volume” surgery was also introduced by him to ensure a high level of security for the surgeon and low morbidity for the patients (47). Several other nonrandomized studies also indicate that IoMRI can increase the resection rate of LGGs and HGGs, preserve neurofunction, and prolong patient survival (48–50).
Intraoperative ultrasound
Intraoperative ultrasound (IoUS) is an affordable tool that can be easily incorporated into existing infrastructure and operative workflows. IoUS has dramatically evolved with well-integrated navigation tools and improvements in image quality compared with the previous artifact-prone image quality of ultrasound (51). However, it is difficult to detect residuals below 1 cm in diameter when using IoUS (52), and the cone-shaped field of view can sometimes make it hard to see the lesion.
Fluorescence-guided surgery
Recently, intraoperative fluorescence surgery using 5-aminolevulinic acid (5-ALA) has been widely adopted (22, 53). 5-ALA as a prodrug to heme can be converted to protoporphyrin IX (PpIX), and PpIX can be accumulated preferentially in tumor cells and epithelial tissues when 5-ALA is administered through the intact blood–brain barrier (BBB) (53, 54). The properties of PpIX, which emits fluorescence of red-violet light, have been used for the detection of tumor tissues during glioma resection (38, 39).
The sensitivity of 5-ALA to gliomas has been demonstrated up to 95% in one study as a growing technology (55). Specificity for 5-ALA in predicting malignant tissues has a wide degree of variance, and in most studies it can be above 70% (56–58). The visible fluorescence varies depending on the grade of glioma, which is 95.4% in glioblastomas compared with 24.1%–26.3% in grade I and II gliomas (59). Alessandro reported the resection outcome guided by 5-ALA fluorescence: overall gross total resection of >98% was achieved in 93% of HGG patients, and the boundaries of fluorescent tissue exceeded those of tumoral tissue by neuronavigation in 43% of the patients (60).
Intraoperative 5-ALA fluorescence is ineffective at guiding LGGs because they do not produce a level of fluorescence that is visible to the naked eye (38). Hence, the approaches of intraoperative confocal microscopy have been developed and used to visualize 5-ALA-based tumor fluorescence in LGGs when exposed to resection procedures. With this microscopy, PpIX fluorescence has been detected in cellular infiltration identified at the tumor margins of WHO grade I and II gliomas (61).
Intraoperative stimulation mapping and awake craniotomy approach
Because it allows for a more precise identification of functional areas (especially in the dominant hemisphere), intraoperative stimulation (IS) mapping has emerged as the standard treatment of choice for eloquent tumors. This allows surgeons to achieve higher extents of resection (EOR) and reduce postoperative morbidity.
This technique involves electrical stimulation to depolarize a focal area of the functional cortex. An electrode precisely stimulates the focal neurons to depolarize, passes the signal within the area of interest, and causes local excitation, inhibition, or perhaps diffusion to distant areas (40). The whole procedure can be monitored by an electrophysiologist if the patient is performing a motor or language task. With the help of a bipolar probe, the neurosurgeon can perform more precise mapping.
Sometimes the nidus of gliomas is located within functional cortical areas of the brain. Neurosurgeons cannot use the classic anatomy of the central nervous system (CNS) to predict functional areas (such as language or motor sites) because of individual cortical heterogeneity (62, 63). The mass effects of gliomas can distort the topography of the brain, and the brain’s plasticity can cause functional networks to be rearranged (64).
A meta-analysis of IoSM enrolled patients diagnosed with supratentorial gliomas; the result showed that the gross total resections were 75% of the patients whose resection was guided by IoSM, compared with 58% of the patients without intraoperative mapping; the IoSM did not influence the extent of the resection (65). Another meta-analysis shared similar results, especially the outcome of delayed severe deficits, which demonstrated a lower incidence (3.4%) in patients with IoSM significantly (63), and the deficits were always transient because of brain structures adjacent to the resection cavity. The use of IoSM during resection surgery reduces late, severe neurological deficits (65). The recommendation for awake craniotomy (AC) guided surgery is for gliomas affecting the dominant mid-to-posterior frontal, temporal, and mid-to-anterior parietal lobes. AC techniques can monitor more complex cognitive functions such as spatial and emotional recognition more effectively (41).
Based on some experience, the maximum duration that patients can stay conscious and complete mapping tasks is about 1 h. During this period there are several task series for IoSM, such as language tasks (the major tasks are number counting and picture naming) and non-language tasks (vision and other higher cognitive functions include calculation, working memory, and music) (66, 67). When tumors are near language or motor networks, it is recommended that direct cortical stimulation be used to help preserve function in appropriately selected patients. This often involves proceeding with an awake craniotomy, for which technical nuances and anesthesia considerations have been previously reported (68). If the gliomas are located within the area associated with severe complications, the rate of permanent complications will be about 10% with the use of AC mapping techniques (69).
A random-effects meta-analysis showed that AC (90.1%) had a higher mean EOR than general anesthesia (GA) (81.7%), which was found to be the case (p = 0.06). For analysis, neurological deficits were divided according to their severity and timing. Early neurological deficits, late neurological deficits, and non-severe and severe morbidity were not significantly different between patients who underwent AC and GA, respectively. The results suggested that IoSM when resecting gliomas located in the eloquent area can be carried out safely and effectively with AC (70).
Other adjunct IoSM methods
There are some other adjunct IoSM methods. Functional MRI (fMRI) mainly provides vital information regarding the location of sensory and motor pathways, but it is unable to map language sites (71). Somatosensory-evoked potential (SSEP) phase-reversal techniques can be used to identify the location of the primary sensory cortex. However, the level of SSEP is only useful for locating the primary somatosensory cortex (72).
Augmented reality high-definition fiber tractography and fluorescein
As with simulating three-dimensional virtual objects with real objects, the application of augmented reality (AR) in neurosurgery has the potential to change the way neurosurgeons plan and perform surgical procedures. The AR neuronavigation system has been used in surgical planning (73), integrating MR or CT images into the surgical field (74). López et al., in a review, illustrated that as the second most frequent use in brain tumors, AR enabled the neurosurgeon to locate the fiber tracts and guide resection (42). It can improve intraoperative safety. It facilitates and simplifies the selective anatomy of the lesion and adjacent structures, although there is no established method for precise measurement of 3D error and bias in deep injuries.
DTI high-definition fiber tractography (HDFT) has been tested as a useful tool for glioma resection planning and assessment of postoperative connectivity of the fiber tracts (43). Sodium fluorescein (F) has been used as a fluorescent dye in HGGs to increase the EOR (75). Although there is still a wide range of limitations, there are several studies about the combined utility of AR and HDFT-F. AR HDFT-F improves the neurosurgeon’s intraoperative spatial location and allows for differential visualization of each tract as needed. Luzzi (1) enrolled 117 patients newly diagnosed as supratentorial HGGs, of whom 54 underwent surgery with the AR HDFT-F technique and 63 with conventional neuronavigation surgery. The results suggested that the AR HDFT-F group had a higher extent of resection and longer progression-free survival, although there was no significant difference in complication rates between the two groups. Surgery with AR HDFT-F is regarded as a safe and effective procedure for patients’ neurofunction recovery. However, the present procedures involve only GA patients, and the whole process is still limited by the DTI’s only supply of anatomical information (76).
There are some other intraoperative tools that have been described to achieve maximal tumor resection, and most of them are still in debate.
(1) Intraoperative hand-held microscopy
This is a technique in which a single optical fiber combined with miniaturized scanning and optical systems supplies high-resolution images (77). It can solve problems at the cellular level. The histological characteristics of gliomas, meningiomas, and central neurocytomas have been distinguished by intraoperative confocal microscopy to visualize fluorescein (78). Further study is needed to determine whether this technique can be used to complement resection procedures and conventional neuropathological diagnostic techniques.
(2) Intraoperative mutational analysis
The analysis can be used in real time to theoretically determine the true tumor margins. All these methods include genotyping for known tumor mutations using PCR-based approaches, such as isocitrate dehydrogenase 1 or 2 (IDH1/2) aberrations (79); mass spectroscopy based on defined tumor spectral profiles, which is similar to magnetic resonance spectroscopy techniques (80); All these methods need to be further tested for specificity and sensitivity in resectioning gliomas.
Conclusions
Preoperative (such as neuronavigation systems and DTI tractography) and intraoperative (including IoMRI, IoUS, fluorescence-guided surgery, IoSM and AC approaches, AR HDFT-F, intraoperative hand-held microscopy, and mutational analysis) surgical options for gliomas therapy supply a lot of choices for neurosurgeons to improve the greater extent of resections of any grade of gliomas (Table 1). The neuronavigation system is always used for preoperatively precise planning. DTI tractography can plan surgical fibro-parcel pathways, keeping away from significant white-matter packages. IoMRI can solve the intraoperative brain shift. IoUS can be easily incorporated into operations to detect residuals above 1 cm in diameter. Fluorescence-guided surgery can detect the tumors in HGGs and LGGs with confocal microscopy and fluorescence. IoSM and AC approaches can be used for gliomas located in the eloquent area. AR HDFT-F can improve intraoperative spatial location and allow for differential visualization of each tract. As the methods need to be further tested, intraoperative hand-held microscopy and mutational analysis also provide the methods for glioma resection. The development of surgical techniques has changed the principles of gliomas and characterized them as accurate, effective, and real-time to maximize tumor resection, preserve neurological function of the eloquent area near the lesion, decrease morbidity, and improve outcomes.
Author contributions
XC is the corresponding author, and he designed the content. ZY works on the analysis selected data and wrote the manuscript. CZ, SZ, JP, and YZ are responsible for collecting the papers focused on the surgical treatment of the gliomas from hundreds of papers. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Jiapeng Medical Nutrition Technology (Jilin) Co., Ltd (grant no. 3R2220793428).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Luzzi S, Giotta Lucifero A, Martinelli A, Maestro MD, Savioli G, Simoncelli A, et al. Supratentorial high-grade gliomas: Maximal safe anatomical resection guided by augmented reality high-definition fiber tractography and fluorescein. Neurosurg Focus (2021) 51(2):E5. doi: 10.3171/2021.5.FOCUS21185
2. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol (Berl) (2007) 114:97–109. doi: 10.1007/s00401-007-0243-4
3. Ostrom QT, Gittleman H, Stetson L, Virk SM, Barnholtz-Sloan JS. Epidemiology of gliomas. Cancer Treat Res (2015) 163:1–14. doi: 10.1007/978-3-319-12048-5_1
4. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66:115–32. doi: 10.3322/caac.21338
5. Larjavaara S, Mantyla R, Salminen T, Haapasalo H, Raitanen J, Jääskeläinen J, et al. Incidence of gliomas by anatomic location. Neuro Oncol (2007) 9(3):319–25. doi: 10.1215/15228517-2007-016
6. Gousias K, Markou M, Voulgaris S, Goussia A, Voulgari P, Bai M, et al. Descriptive epidemiology of cerebral gliomas in northwest Greece and study of potential predisposing factors, 2005-2007. Neuroepidemiology (2009) 33(2):89–95. doi: 10.1159/000222090
7. Yang P, Wang Y, Peng X, You G, Zhang W, Yan W, et al. Management and survival rates in patients with glioma in China (2004-2010): A retrospective study from a single-institution. J Neuro-Oncol (2013) 113(2):259–66. doi: 10.1007/s11060-013-1103-9
8. Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro-oncol (2014) 16:896–913. doi: 10.1093/neuonc/nou087
9. Sanai N, Chang S, Berger MS. Low-grade gliomas in adults. J Neurosurg (2011) 115:948–65. doi: 10.3171/2011.7.JNS101238
10. Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery (2008) 62:753–64. doi: 10.1227/01.neu.0000318159.21731.cf
11. Preston DL, Ron E, Yonehara S, Kobuke T, Fujii H, Kishikawa M, et al. Tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure. J Natl Cancer Inst (2002) 94:1555–63. doi: 10.1093/jnci/94.20.1555
12. Sadetzki S, Chetrit A, Freedman L, Stovall M, Modan B, Novikov I. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res (2005) 163:424–32. doi: 10.1667/RR3329
13. IJzerman-Korevaar M, Snijders TJ, de Graeff A, Teunissen SCCM, de Vos FYF. Prevalence of symptoms in glioma patients throughout the disease trajectory: A systematic review. J Neurooncol (2018) 140(3):485–96. doi: 10.1007/s11060-018-03015-9
14. Vecht CJ, Kerkhof M, Duran-Pena A. Seizure prognosis in brain tumors: new insights and evidence-based management. Oncologist (2014) 19(7):751–9. doi: 10.1634/theoncologist.2014-0060
15. Guthrie BL, Laws ER Jr. Supratentorial low-grade gliomas. Neurosurg Clin N Am (1990) 1:37–48. doi: 10.1016/S1042-3680(18)30822-2
16. Hollon T, Nguyen V, Smith BW, Lewis S, Junck L, Orringer DA. Supratentorial hemispheric ependymomas: An analysis of 109 adults for survival and prognostic factors. J Neurosurg (2016) 8:1–9. doi: 10.3171/2015.7.JNS151187
17. Incekara F, Olubiyi O, Ozdemir A, Lee T, Rigolo L, Golby A. The value of pre- and intraoperative adjuncts on the extent of resection of hemispheric low-grade gliomas: A retrospective analysis. J Neurol Surg A Cent Eur Neurosurg (2016) 77(2):79–87. doi: 10.1055/s-0035-1551830
18. Claus EB, Walsh KM, Wiencke JK, Molinaro AM, Wiemels JL, Schildkraut JM, et al. Survival and low-grade glioma: The emergence of genetic information. Neurosurg Focus (2015) 38(1):E6. doi: 10.3171/2014.10.FOCUS12367
19. Reifenberger G, Wirsching HG, Knobbe Thomsen CB, Weller M. Advances in the molecular genetics of gliomas — implications for classification and therapy. Nat Rev Clin Oncol (2017) 14:434–52. doi: 10.1038/nrclinonc.2016.204
20. Xu DS, Awad AW, Mehalechko C, Wilson JR, Ashby LS, Coons SW, et al. An extent of resection threshold for seizure freedom in patients with low-grade gliomas. J Neurosurg (2018) 128(4):1084–90. doi: 10.3171/2016.12.JNS161682
21. Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgård G, et al. Comparison of a strategy favoring early surgical resection versus a strategy favoring watchful waiting in low-grade gliomas. JAMA (2012) 308:1881–8. doi: 10.1001/jama.2012.12807
22. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen H-J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol (2006) 7:392–401. doi: 10.1016/S1470-2045(06)70665-9
23. Stummer W, Tonn JC, Mehdorn HM, Nestler U, Franz K, Goetz C, et al. Counterbalancing risks and gains from extended resections in malignant glioma surgery: A supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. Clin Article J Neurosurg (2011) 114:613–23. doi: 10.3171/2010.3.JNS097
24. Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, Tonn JC, et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery (2008) 62:564–76. doi: 10.1227/01.neu.0000317304.31579.17
25. Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, et al. Association of the extent of resection with survival in glioblastoma: A systematic review and meta-analysis. JAMA Oncol (2016) 2:1460–9. doi: 10.1001/jamaoncol.2016.1373
26. Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J Neurosurg (2016) 124:977–88. doi: 10.3171/2015.5.JNS142087
27. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg (2011) 115:3–8. doi: 10.3171/2011.2.JNS10998
28. Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, Raza SM, Pascual-Gallego M, Ibrahim A, et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol (2014) 16:113–22. doi: 10.1093/neuonc/not137
29. Ramakrishna R, Hebb A, Barber J, Rostomily R, Silbergeld D. Outcomes in reoperated low-grade gliomas. Neurosurgery (2015) 77:175–84. doi: 10.1227/NEU.0000000000000753
30. Oppenlander ME, Wolf AB, Snyder LA, Bina R, Wilson JR, Coons SW, et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg (2014) 120:846–53. doi: 10.3171/2013.12.JNS13184
31. Wu JS, et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: A prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery (2007) 61:935–48. doi: 10.1227/01.neu.0000303189.80049.ab
32. Fountain DM, Bryant A, Barone DG, Waqar M, Hart MG, Bulbeck H, et al. Intraoperative imaging technology to maximise extent of resection for glioma: a network meta-analysis. Cochrane Database Syst Rev (2021) 1(1):CD013630. doi: 10.1002/14651858.CD013630.pub2
33. Willems PW, Taphoorn MJ, Burger H, Berkelbach van der Sprenkel JW, Tulleken CA. Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: A randomized controlled trial. J Neurosurg (2006) 104(3):360–8. doi: 10.3171/jns.2006.104.3.360
34. Pamir MN, Ozduman K, Dinçer A, Yildiz E, Peker S, Ozek MM. First intraoperative, shared-resource, ultrahigh-field 3-Tesla magnetic resonance imaging system and its application in low-grade glioma resection. J Neurosurg (2010) 112:57–69. doi: 10.3171/2009.3.JNS081139
35. Matsumae M, Nishiyama J, Kuroda K. Intraoperative MR imaging during glioma resection. Magn Reson Med Sci (2022) 21(1):148–67. doi: 10.2463/mrms.rev.2021-0116
36. Coenen VA, Krings T, Weidemann J, Hans FJ, Reinacher P, Gilsbach JM, et al. Sequential visualization of brain and fiber tract deformation during intracranial surgery with three-dimensional ultrasound: An approach to evaluate the effect of brain shift. Neurosurgery (2005) 56:133–41. doi: 10.1227/01.neu.0000144315.35094.5f
37. Shi J, Zhang Y, Yao B, Sun P, Hao Y, Piao H, et al. Application of multiparametric intraoperative ultrasound in glioma surgery. BioMed Res Int (2021) 2021:6651726. doi: 10.1155/2021/6651726
38. Ishihara R, Katayama Y, Watanabe T, Yoshino A, Fukushima T, Sakatani K. Quantitative spectroscopic analysis of 5-aminolevulinic acid-induced protoporphyrin IX fluorescence intensity in diffusely infiltrating astrocytomas. Neurol Med Chir (2007) 47:53–7. doi: 10.2176/nmc.47.53
39. Zhang RR, Schroeder AB, Grudzinski JJ, Rosenthal EL, Warram JM, Pinchuk AN, et al. Beyond the margins: Real-time detection of cancer using targeted fluorophores. Nat Rev Clin Oncol (2017) 14:347–64. doi: 10.1038/nrclinonc.2016.212
40. Kunieda T, Yamao Y, Kikuchi T, Matsumoto R. New approach for exploring cerebral functional connectivity: Review of cortico-cortical evoked potential. Neurol Med Chir (2015) 55:374–82. doi: 10.2176/nmc.ra.2014-0388
41. Duffau H. Stimulation mapping of white matter tracts to study brain functional connectivity. Nat Rev Neurol (2015) 11:255–65. doi: 10.1038/nrneurol.2015.51
42. Contreras López WO, Navarro PA, Crispin S. Intraoperative clinical application of augmented reality in neurosurgery: A systematic review. Clin Neurol Neurosurg (2019) 177:6–11. doi: 10.1016/j.clineuro.2018.11.018
43. Henderson F, Abdullah KG, Verma R, Brem S. Tractography and the connectome in neurosurgical treatment of gliomas: the premise, the progress, and the potential. Neurosurg Focus (2020) 48(2):E6. doi: 10.3171/2019.11.FOCUS19785
44. Mickevicius NJ, Carle AB, Bluemel T, Santarriaga S, Schloemer F, Shumate D, et al. Location of brain tumor intersecting white matter tracts predicts patient prognosis. J Neurooncol (2015) 125:393–400. doi: 10.1007/s11060-015-1928-5
45. Nimsky C, Bauer M, Carl B. Merits and limits of tractography techniques for the uninitiated. Adv Tech Stand Neurosurg (2016) 43:37–60. doi: 10.1007/978-3-319-21359-0_2
46. McDonald CR, White NS, Farid N, Lai G, Kuperman JM, Bartsch H, et al. Recovery of white matter tracts in regions of peritumoral FLAIR hyperintensity with use of restriction spectrum imaging. AJNR Am J Neuroradiol (2013) 34:1157–63. doi: 10.3174/ajnr.A3372
47. Leroy HA, Delmaire C, Le Rhun E, Drumez E, Lejeune JP, Reyns N. High-field intraoperative MRI and glioma surgery: Results after the first 100 consecutive patients. Acta Neurochir (2019) 161:1467–74. doi: 10.1007/s00701-019-03920-6
48. Kuhnt D, Ganslandt O, Schlaffer SM, Buchfelder M, Nimsky C. Quantification of glioma removal by intraoperative highfield magnetic resonance imaging: An update. Neurosurgery (2011) 69:852–62. doi: 10.1227/NEU.0b013e318225ea6b
49. Mohammadi AM, Sullivan TB, Barnett GH, Recinos V, Angelov L, Kamian K, et al. Use of high-field intraoperative magnetic resonance imaging to enhance the extent of resection of enhancing and nonenhancing gliomas. Neurosurgery (2014) 74:339. doi: 10.1227/NEU.0000000000000278
50. Reins N, Leroy HA, Delmaire C, Derre B, Le-Rhun E, Lejeune JP. Intraoperative MRI for the management of brain lesions adjacent to eloquent areas. Neurochirurgie (2017) 63:181–8. doi: 10.1016/j.neuchi.2016.12.006
51. Dixon L, Lim A, Grech-Sollars M, Nandi D, Camp S. Intraoperative ultrasound in brain tumor surgery: A review and implementation guide. Neurosurg Rev (2022) 45:2503–15. doi: 10.1007/s10143-022-01778-4
52. Gerganov VM, Samii A, Akbarian A, Stieglitz L, Samii M, Fahlbusch R. Reliability of intraoperative high-resolution 2D ultrasound as an alternative to high-field strength MR imaging for tumor resection control: A prospective comparative study. J Neurosurg (2009) 111:512–9. doi: 10.3171/2009.2.JNS08535
53. Stummer W, Stocker S, Simon W, Herbert S, Clemens F, Claudia G, et al. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery (1998) 42:518–26. doi: 10.1097/00006123-199803000-00017
54. Duffner F, Ritz R, Freudenstein D, Weller M, Dietz K, Wessels J. Specific intensity imaging for glioblastoma and neural cell cultures with 5-aminolevulinic acid-derived protoporphyrin IX. J Neurooncol (2005) 71:107–11. doi: 10.1007/s11060-004-9603-2
55. Yamada S, Muragaki Y, Maruyama T, Komori T, Okada Y. Role of neurochemical navigation with 5-aminolevulinic acid during intraoperative MRI-guided resection of intracranial malignant gliomas. Clin Neurol Neurosurg (2015) 130:134–9. doi: 10.1016/j.clineuro.2015.01.005
56. Coburger J, Engelke J, Scheuerle A, Thal D, Hlavac M, Wirtz CR, et al. Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA–enhanced intraoperative MRI at the border of contrast-enhancing lesions: A prospective study based on histopathological assessment. Neurosurg Focus (2014) 36:E3. doi: 10.3171/2013.11.FOCUS13463
57. Panciani PP, Fontanella M, Garbossa D, Agnoletti A, Ducati A, Lanotte M. 5-aminolevulinic acid and neuronavigation in high-grade glioma surgery: Results of a combined approach. Neurocirugía (2012) 23:23–8. doi: 10.1016/j.neucir.2012.04.003
58. Roberts DW, Valdés PA, Harris BT, Fontaine KM, Hartov A, Fan X, et al. Coregistered flfluorescence-enhanced tumor resection of malignant glioma: Relationships between δ-aminolevulinic acid–induced protoporphyrin IX flfluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. J Neurosurg (2011) 114:595–603. doi: 10.3171/2010.2.JNS091322
59. Ji SY, Kim JW, Park C-K. Experience profifiling of fluorescence-guided surgery I: Gliomas. Brain Tumor Res Treat (2019) 7:98–104. doi: 10.14791/btrt.2019.7.e38
60. Della Puppa A, Ciccarino P, Lombardi G, Rolma G, Cecchin D, Rossetto M. 5-aminolevulinic acid fluorescence in high grade glioma surgery: Surgical outcome, intraoperative findings, and fluorescence patterns. BioMed Res Int (2014) 2014:232561. doi: 10.1155/2014/232561
61. Sanai N, Snyder LA, Honea NJ, Coons SW, Eschbacher JM, Smith KA, et al. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in lowgrade gliomas. J Neurosurg (2011) 115:740–8. doi: 10.3171/2011.6.JNS11252
62. Herholz K, Thiel A, Wienhard K, Pietrzyk U, von Stockhausen HM, Karbe H, et al. Individual functional anatomy of verb generation. Neuroimage (1996) 3:185–94. doi: 10.1006/nimg.1996.0020
63. Skirboll SS, Ojemann GA, Berger MS, Lettich E, Winn HR. Functional cortex and subcortical white matter located within gliomas. Neurosurgery (1996) 38:678–84. doi: 10.1227/00006123-199604000-00008
64. Duffau H. Brain plasticity: From pathophysiological mechanisms to therapeutic applications. J Clin Neurosci (2006) 13:885–97. doi: 10.1016/j.jocn.2005.11.045
65. De Witt Hamer PC, Robles GS, Zwinderman A, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: A meta-analysis. J Clin Oncol (2012) 30:1–7. doi: 10.1200/JCO.2011.38.4818
66. Mandonnet E, Herbet G, Duffffau H. A letter: Introducing new tasks for intraoperative mapping in awake glioma surgery: Clearing the line between patient care and scientific research. Neurosurgery (2019) 86:E256–7. doi: 10.1093/neuros/nyz447
67. Bu L, Lu J, Zhang J, Wu J. Intraoperative cognitive mapping tasks for direct electrical stimulation in clinical and neuroscientific contexts. Front Hum Neurosci (2021) 15:612891. doi: 10.3389/fnhum.2021.612891
68. Hervey-Jumper SL, Li J, Lau D, Molinaro AM, Perry DW, Meng L, et al. Awake craniotomy to maximize glioma resection: methods and technical nuances over a 27-year period. J Neurosurg (2015) 123:325–39. doi: 10.3171/2014.10.JNS141520
69. Rolston JD, Englot DJ, Benet A, Li J, Cha S, Berger MS. Frontal operculum gliomas: Language outcome following resection. J Neurosurg (2015) 122:725–34. doi: 10.3171/2014.11.JNS132172
70. Suarez-Meade P, Marenco-Hillembrand L, Prevatt C, Murguia-Fuentes R, Mohamed A, Alsaeed T, et al. Awake vs. asleep motor mapping for glioma resection: A systematic review and meta-analysis. Acta Neurochir (Wien) (2020) 162(7):1709–20. doi: 10.1007/s00701-020-04357-y
71. Cochereau J, Deverdun J, Herbet G, Charroud C, Boyer A, Moritz-Gasser S, et al. Comparison between resting state fMRI networks and responsive cortical stimulations in glioma patients. Hum Brain Mapp (2016) 37:3721–32. doi: 10.1002/hbm.23270
72. Romstock J, Fahlbusch R, Ganslandt O, Nimsky C, Strauss C. Localisation of the sensorimotor cortex during surgery for brain tumours: Feasibility and waveform patterns of somatosensory evoked potentials. J Neurol Neurosurg Psychiatry (2002) 72:221–9. doi: 10.1136/jnnp.72.2.221
73. Deng W, Li F, Song Z. Easy-to-use augmented reality neuronavigation using wireless tablet PC. Stereotact Funct Neurosurg (2014) 92:17–24. doi: 10.1159/000354816
74. Mahvash M, Tabrizi LB. A novel augmented reality system of image projection for image-guided neurosurgery. Acta Neurochir (2013) 155:943–7. doi: 10.1007/s00701-013-1668-2
75. Acerbi F, Broggi M, Eoli M, Anghileri E, Cavallo C, Boffano C, et al. Is fluorescein-guided technique able to help in resection of high-grade gliomas? Neurosurg Focus (2014) 36(2):E5. doi: 10.3171/2013.11.FOCUS13487
76. Duffau H. The dangers of magnetic resonance imaging diffusion tensor tractography in brain surgery. World Neurosurg (2014) 81(1):56–8. doi: 10.1016/j.wneu.2013.01.116
77. Sanai N, Eschbacher J, Hattendorf G, Coons SW, Preul MC, Smith KA, et al. Intraoperative confocal microscopy for brain tumors: A feasibility analysis in humans. Neurosurgery (2011) 68:282–90. doi: 10.1227/NEU.0b013e318212464e
78. Kubben PL, ter Meulen KJ, Schijns OE, ter Laak-Poort MP, van Overbeeke JJ, van Santbrink H. Intraoperative MRI-guided resection of glioblastoma multiforme: A systematic review. Lancet Oncol (2011) 12:1062–70. doi: 10.1016/S1470-2045(11)70130-9
79. Kanamori M, Kikuchi A, Watanabe M, Shibahara I, Saito R, Yamashita Y, et al. Rapid and sensitive intraoperative detection of mutations in the isocitrate dehydrogenase 1 and 2 genes during surgery for glioma. J Neurosurg (2014) 120:1288–97. doi: 10.3171/2014.3.JNS131505
Keywords: review, intraoperative techniques, extent of resection (EOR), gliomas, imaging technologies, intraoperative stimulation mapping, awake craniotomy, augmented reality high-definition fiber tractography and fluorescein
Citation: Yang Z, Zhao C, Zong S, Piao J, Zhao Y and Chen X (2023) A review on surgical treatment options in gliomas. Front. Oncol. 13:1088484. doi: 10.3389/fonc.2023.1088484
Received: 03 November 2022; Accepted: 24 February 2023;
Published: 16 March 2023.
Edited by:
Zhifeng Shi, Fudan University, ChinaCopyright © 2023 Yang, Zhao, Zong, Piao, Zhao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuan Chen, chen_xuan@jlu.edu.cn
 Zhongxi Yang
Zhongxi Yang Chen Zhao1
Chen Zhao1 Yuhao Zhao
Yuhao Zhao