- 1Prostate Cancer Biomarker Laboratory, Faculty of Clinical Research, Sri Ramachandra Institute of Higher Education and Research, Chennai, India
- 2Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Southern Medical University, Guangzhou, Guangdong, China
- 3Department of Urology, H.H. Sheikh Khalifa General Hospital, Al Salama, Opp. Ministry of Community Development, Umm Al Quwain, United Arab Emirates
- 4Department of Pharmacology and Therapeutics, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, United Arab Emirates
- 5Translational Research & Sustainable Healthcare Management, Institute of Advanced Materials, IAAM, Ulrika, Sweden
- 6NanoBioTech Laboratory, Department of Environmental Engineering, Florida Polytechnic University, Lakeland, FL, United States
- 7School of Engineering, University of Petroleum and Energy Studies (UPES), Dehradun, India
- 8Department of Biochemistry, Chonnam National University Medical School, Gwangju, Republic of Korea
- 9Thumbay Research Institute for Precision Medicine, Gulf Medical University, Ajman, United Arab Emirates
- 10INSERM UMR1186, Integrative Tumor Immunology and Genetic Oncology, Gustave Roussy, Equipe Labellisée par la Ligue Contre le Cancer, EPHE, Faculté de Médecine, Université Paris-Sud, Université Paris-Saclay, Villejuif, France
The development of new therapeutic strategies is on the increase for prostate cancer stem cells, owing to current standardized therapies for prostate cancer, including chemotherapy, androgen deprivation therapy (ADT), radiotherapy, and surgery, often failing because of tumor relapse ability. Ultimately, tumor relapse develops into advanced castration-resistant prostate cancer (CRPC), which becomes an irreversible and systemic disease. Hence, early identification of the intracellular components and molecular networks that promote prostate cancer is crucial for disease management and therapeutic intervention. One of the potential therapeutic methods for aggressive prostate cancer is to target prostate cancer stem cells (PCSCs), which appear to be a primary focal point of cancer metastasis and recurrence and are resistant to standardized therapies. PCSCs have also been documented to play a major role in regulating tumorigenesis, sphere formation, and the metastasis ability of prostate cancer with their stemness features. Therefore, the current review highlights the origin and identification of PCSCs and their role in anti-androgen resistance, as well as stemness-related signaling pathways. In addition, the review focuses on the current advanced therapeutic strategies for targeting PCSCs that are helping to prevent prostate cancer initiation and progression, such as microRNAs (miRNAs), nanotechnology, chemotherapy, immunotherapy, the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) gene-editing system, and photothermal ablation (PTA) therapy.
1 Introduction
Prostate cancer is a complex disease that affects millions of men globally, predominantly in regions that rank highly on the Human Development Index (1). The identification and molecular characterization of genes associated with inherited susceptibility to prostate cancer and of genes in prostate cancer cells that tend to have somatic alterations suggest that infection or inflammation of the prostate contributes to the development of prostate cancer (2). In spite of various therapies, such as radiopharmaceutical therapy, hormonal therapy, chemotherapy, and immunotherapy, Lang et al. proposed that there is evidence that solid tumors originate from undifferentiated stem cell-like cells, which coexist within a heterogeneous tumor mass, that drive tumor formation, maintain tumor homeostasis, and initiate metastases (3). In addition, cancer cells display features of normal tissue organization, whereas cancer stem cells (CSCs) can drive tumor growth, evolution of the cancer stem cells model, and genetics proposed by Kreso et al. to describe the role of genetic diversity and non-genetic influences in contributing to tumor heterogeneity (4). The exocrine gland of prostatic ducts is lined with secretory luminal cells, rare neuroendocrine cells, and basal cells (5). Interestingly, basal cells in the prostate gland are intrinsically enriched in gene sets that are usually associated with stem cells, neurogenesis, and ribosomal RNA biogenesis (6). With regard to theragnosis, prostate cancer stem cells (PCSCs) are the driving force in the tumorigenesis, relapse, metastasis, and therapeutic resistance of prostate cancer (7). Clarke et al. recently proposed that DNA methylation and chromatin modification dictate the difference between stem cells and other types of cells. One part of the polycomb repressor complex 1 (PRC1), a chromatin modifier known as BMI1, usually mediates gene silencing by regulating chromatin structure required for self-renewal of hematopoietic stem cells, as well as for self-renewal of prostate gland stem cells. Collectively, epigenetic regulators, transcription factors, and miRNAs play very important roles in the self-renewal of stem cells (8, 9).
Survival of PCSCs could promote tumor initiation, migration, and relapse, and induce therapy resistance (10, 11). In addition, PCSC plasticity is anticipated to play a pivotal role in bone metastases of prostate cancer (12). PCSCs can be identified with cell surface markers expressed, and studies suggest that CD44 harbors them to exhibit tumor invasive properties (13, 14). Extensive endogenous signaling pathways and wide-ranging epigenetic modifications are involved in the maintenance of the stemness features of PCSCs, such as the wingless-related integration site (Wnt)/β-catenin, phosphoinositide 3-kinase (PI3K)/AKT, sonic hedgehog (SHH), Notch, and androgen receptor (AR) signaling pathways, and histone modifications (15). Accordingly, targeting these pathways to eradicate PCSCs is believed to have potential therapeutic value in prostate cancer therapy. Recently, Han et al. suggested that AR blockade induces reprogramming to a stem-like state characterized by hybrid epithelial and mesenchymal (E/M) traits and clinical aggressivity. Moreover, drug-persistent mesenchymal and stem-like prostate cancer cells are rescued by exogenous recombinant human neuregulin-b1 (NRG1) generating metastases (16). In this review, we briefly describe the origin and the identification of PCSCs as well as their specific stemness properties and processes associated with signaling pathways. Furthermore, we review the current emerging therapeutic strategies for targeting PCSCs, such as miRNAs, nanotechnology, chemotherapy, immunotherapy, the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) system, and photothermal ablation (PTA). These distinctive strategies could impede prostate cancer initiation and development via targeting PCSCs’ stemness-associated signaling pathways.
2 Origin and identification of PCSCs
The origin of PCSCs is still subject to debate. Prostate stem cells (PSCs) are found in the basal and luminal layers, and gain mutations in tumor oncogene and suppressor gene led to become PCSCs during carcinogenesis (17). An adult PSC resembles the same transcriptional landscape of aggressive prostate cancer phenotype (18). Many research studies have demonstrated that PCSCs can originate from differentiated epithelial cells via epithelial–mesenchymal transition (EMT), in which epithelial cells gain the invasion and migration properties of stem cells through various molecular mechanisms and epigenetic modification. In vitro study has implied that the transition of the EMT phenotype to prostate cancer cells exhibits a high level of clone- and sphere-forming capability (19). Lee et al.’s experimental data showed that the reduction of DNA methyltransferase 1 (DNMT1) expression with 5-azacytidine treatment, a global demethylation agent, could induce EMT, and the cancer stem cell phenotype in prostate cancer cells and in turn promote cancer metastasis (20). Kong et al. documented that inducing a high level of expression of platelet-derived growth factor D in prostate cancer cells promotes the EMT phenotype via activating nuclear factor kappa B (NF-κB) signaling and the mammalian target of rapamycin kinase (mTOR) (21). Talati et al.’s study revealed that Janus kinase 2 (JAK2) and the signal transducers and activators of transcription 5 (STAT5) A/B (JAK2-STAT5A/B) signaling pathway is involved in promoting the EMT phenotype and expression of the cancer stem cell maker BMI1 in prostate cancer cells. Knockdown of Twist1, an EMT marker, in prostate cancer cells, resulted in the suppression of the STAT5A/B signaling that can induce EMT phenotype and sphere formation ability in prostate cancer (22).
Furthermore, PTEN (phosphatase and tensin homolog deleted on chromosome 10) is a tumor suppressor gene, and the deletion of PTEN in prostate cancer murine models leads to expansion of the PSC subpopulation and then tumor initiation (23). In addition, loss of the PTEN gene has been involved in promoting the transformation of prostate epithelial cells to the EMT phenotype (24). Estrogen receptors α and β (i.e., ERα and ERβ) play a differential role in prostate cancer development: overexpression of ERα cuases the tumor oncogene effect and, in contrast, overexpression of ERβ could induce the tumor suppressor effect (25, 26). Recent research has clarified the underlying molecular mechanism between ERβ and prostate tumorigenesis. PTEN deletion causes BMI1 activation, which is a critical regulator of PSCs’ self-renewal and malignant transformation, which in turn suppresses ERβ expression. Consequently, the suppression of ERβ has been implicated in prostate tumorigenesis initiation (27). In vitro study revealed that ERα promotes the EMT process of PCSCs via activating the NOTCH1 signaling pathway, which was mediated by the enhancer of Zeste homolog 2 (EZH2) (28).
In cancer therapy, cell surface markers could be used as paradigms for the specific identification and targeting of cancer cells as a means to eradicate them. The study by Collins et al. revealed that a population of CD44+/α2β1high/CD133+ cells can be isolated from prostate cancer patients. This population of cells has a high capacity to be self-renewable and to be able to proliferate (29). Furthermore, CD44+ PCSCs exhibited higher levels of cell proliferation, tumorigenesis, and metastasis potential than CD44– PCSCs (13). Hurt et al. reported that CD44+- and CD24–enriched cells expressed stem cell-related transcription factors, such as BMI1 and octamer-binding transcription factor 3/4 (OCT-3/4), and exhibited in vitro prostasphere cell formation (30). The activity of the enzyme aldehyde dehydrogenase (ALDH) was increased in prostate cancer cells. The enzyme was also involved in intracellular retinoic acid synthesis, which is associated with prostate cancer development, and metastasis formation (31). Li et al. found that ALDH1A1 is a potential marker for malignant PSCs (32). Therefore, ALDH acted as a potential marker for identifying PCSC populations. Trerotola et al. revealed that cancer cell populations with CD133, trophoblast cell surface antigen 2 (TROP-2), and α2β1 integrin surface receptors displayed a stem cell phenotype and served as the ideal markers to characterize PCSCs (33). Sui et al. reported that a PC-3 and a DU145 cell line with a CD51 population exhibited high levels of sphere formation and metastatic initiation, and enhanced transcription factors Nanog and SRY-box transcription factor 2 (SOX2), compared with the CD51– population. Therefore, CD51 acts as a novel surface marker to identify PCSCs (34). An in vitro and in vivo study demonstrated that CD54 contributed to the self-renewal and tumorigenesis of a PC-3 cell line. Knockdown of CD54 by short hairpin RNAs (shRNAs) attenuated tumorigenesis in a PC-3 cell line and demonstrated an increase in the survival period for mouse prostate xenograft models (35).
3 Stemness-related signaling pathways
Stem-like traits of PCSCs have been implicated in prostate cancer progression and metastasis formation. Deciphering the molecular network underlying the stem-like signature will provide novel insights for the development of potential therapeutic interventions for prostate cancer (Figure 1). Several signal transduction cascades are thought to contribute to the regulation of stemness properties of PCSCs, such as Wnt/β-catenin, PI3K/AKT, SHH, Notch, and AR signaling pathways. The Wnt pathway plays a critical role in the development process during the embryonic period, and it regulates adult stem cell self-renewal and differentiation (36). An in vitro study highlighted that the inclusion of WNT3A in prostate cancer cells augments Wnt signaling, and significantly increases self-renewal and sphere formation, which is correlated with β-catenin, CD44, and CD133 expression (37). Glycogen synthase kinase 3 beta (GSK3β) directly inhibits Wnt signaling via inducing β-catenin degradation. As a result, the Wnt pathway could be activated by the addition of AR79, a GSK3β inhibitor, which could lead to an increase in levels of ALDH and CD133 enrichment in a population of PCSCs via increasing nuclear β-catenin accumulation (38).
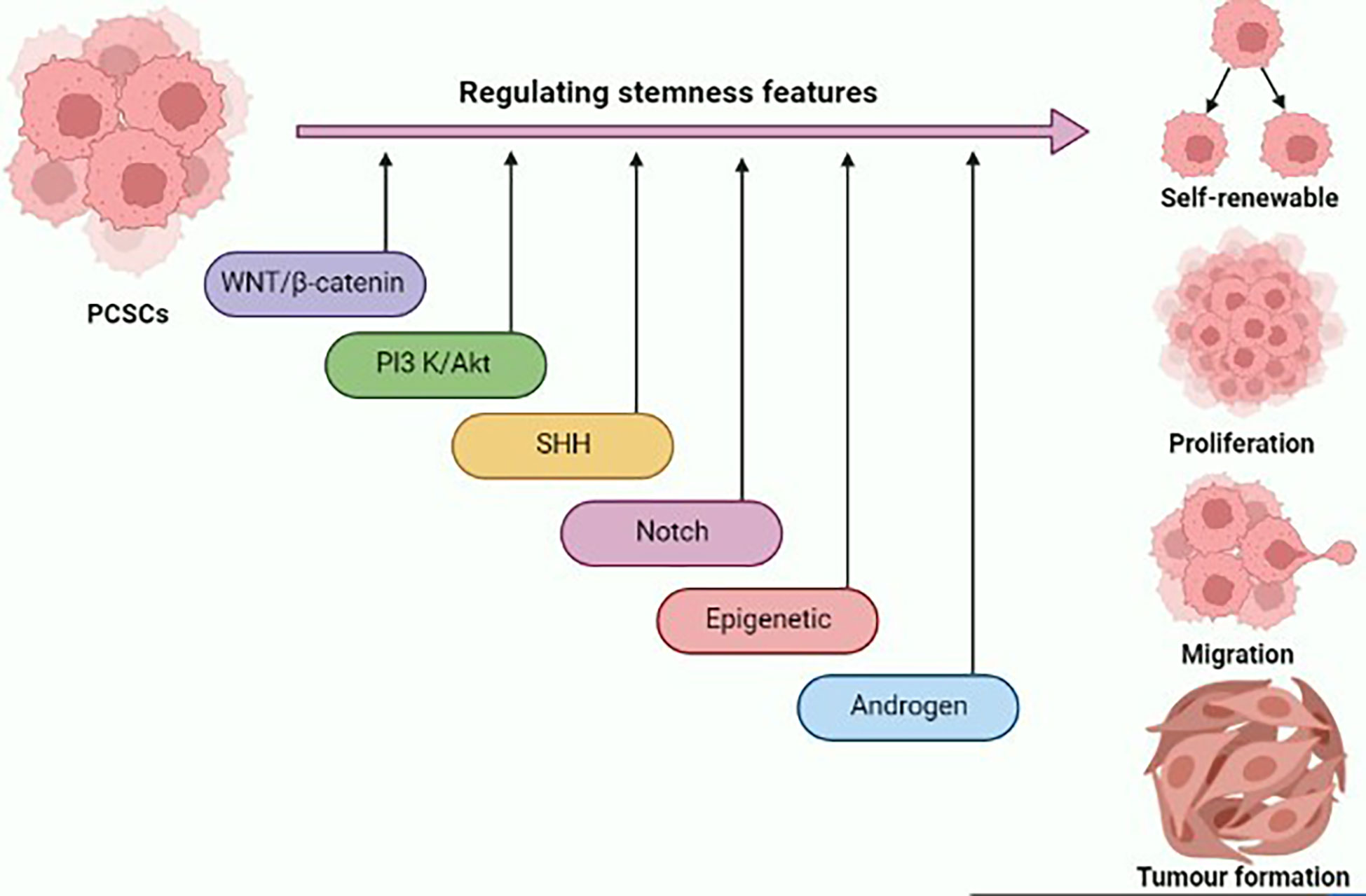
Figure 1 Graphical illustration representing the various signaling pathways and epigenetic landscape contributing to the regulation of stemness features of prostate cancer stem cells (PCSCs) such as self-renewal, proliferation, migration, and tumor formation. This figure was created with biorender.com.
A recent study found that ASPM (an abnormal spindle-like microcephaly-associated protein) is a novel Wnt co-activator, which maintains cancer stem cells, such as the phenotype in prostate cancer by exerting Wnt signaling. ASPM binds to disheveled segment polarity protein 3 (DVL-3), a key upstream regulator of canonical Wnt signaling, and prevents it from being degraded by the proteasome, thereby strengthening protein stability and enhancing Wnt-induced β-catenin transcriptional activity in prostate cancer cells (39). Li et al. found that the expression of the period circadian regulator 3 (PER3) gene was negatively correlated with PCSC stemness via the targeting of the Wnt/βcatenin pathway. Therefore, low levels of PER3 promote brain and muscle ARNT-like 1 (BMAL1) expression, which in turn prompts enhanced β-catenin phosphorylation and activates the WNT/β-catenin pathway, thereby expanding the PCSCs (40). In addition, a CCL5 chemokine derived from tumor associated macrophages such as CCL5, not only support the rejuvenation and tumorigenicity of PCSCs, but also promote PCSC metastasis via activating the β-catenin/STAT3 signaling pathways (41). Knockdown of PTEN by shRNAs in prostate cancer cells leads to the activation of the PI3K/AKT pathway, which in turn increases the viability of cancer stem-like cell populations in prostate cancer (42). Hou et al.’s experimental data showed that up-regulation of thrombospondins 4 (THBS4) could promote the revitalization, proliferation, and tumorigenicity of prostate cells via activating the PI3K/AKT pathway (43). In addition, AKT can directly activate the Wnt pathway, not only promoting β-catenin transcriptional activity via phosphorylation of β-catenin at Ser552, but also inhibiting GSK3β function (44, 45). These findings suggest that Wnt/β-catenin and PI3K/AKT signaling work together to regulate PCSC stemness properties and targeting the pathways demonstrated potential therapeutic ways to eradicate prostate cancer.
Furthermore, the SHH pathway is associated with PCSC stemness regulation. Components of the SHH pathway, including GLI1 (GLI family zinc finger 1), PTCH1 (protein patched homolog 1), and SHH, are highly up-regulated in human prostate cancer tissues, compared with prostatic epithelium. SHH signaling is stimulated by androgen deprivation (46). The SHH signaling pathway promotes androgen-independent prostate cancer and causes therapy resistance via increasing ABC transporter levels (47). Inhibiting the SHH signaling pathways with anti-SHH antibodies diminishes prostate cancer growth (48). Chang et al.’s in vivo study demonstrated that overexpression of hedgehog signaling leads to the promotion of PCSC stemness properties (49). In contrast, the Notch signaling pathway also contributed to sustaining the stemness of PCSCs. CD54+ prostate cancer cells exhibited greater tumorigenicity, orb-forming capability, and resistance to the chemotherapeutic agent than CD54– prostate cancer cells through activating the p38-Notch1 signaling pathway (35). Cheng et al. reported that cell–cell interaction governs PCSC stemness properties; mechanistically, the direct contact of prostate cancer cells with mesenchymal stem cells promotes sphere formation and tumorigenesis via activation of the Jagged1/Notch1 pathway both in vitro and in vivo (50).
Various epigenetic mechanisms, such as ubiquitination and modification of histone domains by methylation and acetylation, help to ameliorate the stem-like state in prostate cancer cells. For instance, the coregulation of EZH2 and breast cancer 1 (BRCA1) pathways is involved in the regulation of the stemness phenotype of PCSCs. Loss of EZH2 can induce down-regulation of BRAC1 that in turn triggers reprogramming capability, resulting in significantly increased tumor sphere formation, Levels of ALDH, and express stem cells phenotype in prostate cancer cells (51). Wu et al. demonstrated that when lymph node carcinoma of the prostate (LNCaP) cells are exposed to phenethyl isothiocyanate, the epigenetic modulator can alter the epigenome by decreasing the levels of H3K9ac by activating the PI3K/AKT pathway. These epigenetic and signaling mechanisms endow a cancer stem-like phenotype to LNCaP cells (52). Recent research has found that the bromodomain adjacent to zinc finger domain 2A (BAZ2A) is required for prostate cancer cells to acquire features similar to stem cells. The BAZ2A genomic population in prostate cancer cells correlates with the H3K14ac chromatin region; this BAZ2A/H3K14ac association is facilitated by BAZ2A-bromodomain (BAZ2A-BRD) via binding with H3K14ac. BAZ2A-BRD inhibits treatment, resulting in the prevention of the BAZ2A-BRD/H3K14ac interaction, which in turn hampers PCSC progression (53).
Cullin 4B (CUL4B), a member of the ubiquitin E3 ligase family, acts as tumor oncogene via inhibiting the tumor suppressor. However, the role of CUL4B in prostate cancer remains unclear. Jiao et al. found that activation of CUL4B in prostate cancer cells stimulates the stem-like state by up-regulating BMI1 via suppressing the miR200b/c expression. In vitro study revealed that activation of CUL4B in PC-3 and DU145 cells results in increased sphere formation and colony formation, and promotes pluripotent marker expression (54). In addition, in vitro study revealed that overexpression of basic transcription factor 3 (BTF3) could up-regulate BMI1 expression, which is a crucial mechanism for the acquisition of a stem cells-like phenotype in prostate cancer (55). Moreover, ubiquitin C-terminal hydrolase L1 (UCH-L1) and ubiquitin C-terminal hydrolase L3 (UCH-L3) differentially govern the stem-like traits in prostate cancer cells through regulating the PI3K/AKT pathway. Mechanistically, UCH-L1 and UCH-L3 inhibit the phosphorylation of downstream targets of the PI3K/AKT signaling pathway in DU145 cells, and up-regulate UCH-L1 or down-regulate UCH-L3 expression, which can in turn promote sphere formation ability, chemoresistance, and stem cell-related gene expression (56).
Androgen receptor (AR) signaling plays a vital role in prostate epithelium differentiation and development. Although androgen deprivation therapy (ADT) is the most often used treatment for prostate cancer, the majority of individuals eventually develop castration-resistant prostate cancer (CRPC), as the tumor is refractory to ADT. The primary CRPC is made up of both AR+ and AR– cell populations; however, AR+ cell populations are abundantly present in CRPC (57). In addition, ARs are highly expressed in differentiated prostate cancer (58). PCSCs mostly contain AR– cell populations, as they are in an undifferentiated state (59). Sánchez et al. reported that androgen deprivation could induce the reprogramming of prostate cancer cells into stem-like cells. In vitro study demonstrated that androgen depletion in LNCaP cells could decrease the expression of AMP-activated protein kinase (AMPK) and increase stem cell marker expression including CD133, ABCB1A (ATP-binding cassette subfamily B member 1), and ALDH1A1, as well as activating stem cell-related transcription factors such as Nanog and OCT4. Therefore low levels of expression of ARs could activate stemness-related transcription factors, thereby increasing stemness features of PCSCs through an AMPK-dependent pathway (60). When prostate cancer is subjected to ADT, AR– cell populations and PCSCs are not completely eradicated, and instead undergo some genetic alterations to possess stemness properties of CRPC (61). Yang et al. studied the relationship between AR and annexin A1 in prostate cancer cell lines. In vitro study results revealed that hampering the AR signaling ameliorates the migration of prostate cancer cells via the up-regulation of annexin A1 expression (62). Consequently, targeting AR alone might not provide therapeutic efficacy; hence, co-targeting the PCSCs with AR could improve the therapeutic efficacy against the prostate cancer.
4 Role of PCSCs in the development of anti-androgen resistance
The prostate gland consists of two layers of epithelial cells: luminal and basal cells. These two cell types exhibit different properties; the bulk of luminal epithelial cells highly express ARs and enable androgen signaling for cell survival, whereas basal cells are AR negative (63). Many studies have highlighted that overexpression of AR signaling relies on prostate cancer initiation and development (64, 65). Therefore, initially targeting the AR with an anti-androgen drug is efficacious; however, after ADT, most patients develop CRPC (66). Enzalutamide, a second-generation AR antagonist, has been shown in phase III clinical studies to prolong the survival of CRPC patients (67). However, even those who respond to enzalutamide at first eventually acquire chemoresistance and their disease advances (68). Therefore, identifying the underlying chemoresistance mechanism is imperative. Kregel et al. found that on AR-mediated repression, the pluripotent transcription factor SOX2 was significantly up-regulated. In this case, most of the tumors become resistant to the anti-androgen drug by evading targeted therapies through switching their lineage plasticity-like stemness state (69). In addition, Verma et al. also confirmed that androgen deprivation could induce the reprogramming of prostate cancer cells into stem-like cells. Treating the LNCaP cells with enzalutamide over the long term, exerting floating sphere formation, not only promotes the expression of stem cell markers, such as CD133 and ALDH1A1, but also modulates the transcriptional signature in prostate cancer cells for the acquisition of the stem cell phenotype by up-regulating Nanog and OCT4 (70). Mu et al. have reported that prostate CRPC cells develop resistance to enzalutamide by switching their phenotype lineage from AR-dependent luminal cells to AR-independent basal cells. This lineage plasticity switch is driven by the loss of tumor suppressor gene function, such as RB1 and TP53, is prompted by an increase in the expression of SOX2. It can be reversed by inhibiting SOX2 expression, thereby restoring the RB1 (RB transcriptional corepressor 1) and TP53 (tumor protein P53) function and inducing sensitivity lineage for anti-androgen drugs (71). The aforementioned Wnt/β-catenin pathway is involved in the regulation of stemness characteristics in PCSCs (72). Zhang et al. found that β-catenin is highly up-regulated in enzalutamide-resistant prostate cancer cells, and on activation of the Wnt/β-catenin pathway, stem cells markers are significantly increased. Furthermore, combination treatment with β-catenin inhibitor ICG001 and enzalutamide in a patient-derived LuCaP35CR xenograft model significantly reduced cancer cell proliferation, stem cell markers, and tumor growth compared to enzalutamide alone (73). Therefore, inhibiting PCSC stemness-associated signaling pathways and transcription factors circumvents anti-androgen drug resistance in prostate cancer. Subsequently, in the following sections we describe advanced therapeutic strategies for targeting PCSCs to ameliorate anti-androgen drug therapeutic function.
5 miRNAs
MicroRNAs (miRNAs) are a small group of non-coding RNAs of 19–24 nucleotides, which are capable of regulating the tumorigenesis-related genes via binding the 3’ untranslated regions (UTRs) of the target gene mRNAs (74). Several studies suggest that miRNAs have the ability to manipulate the stemness properties of PCSCs via targeting surface markers, stem cell-related transcription factors, and signaling pathways (Figure 2).
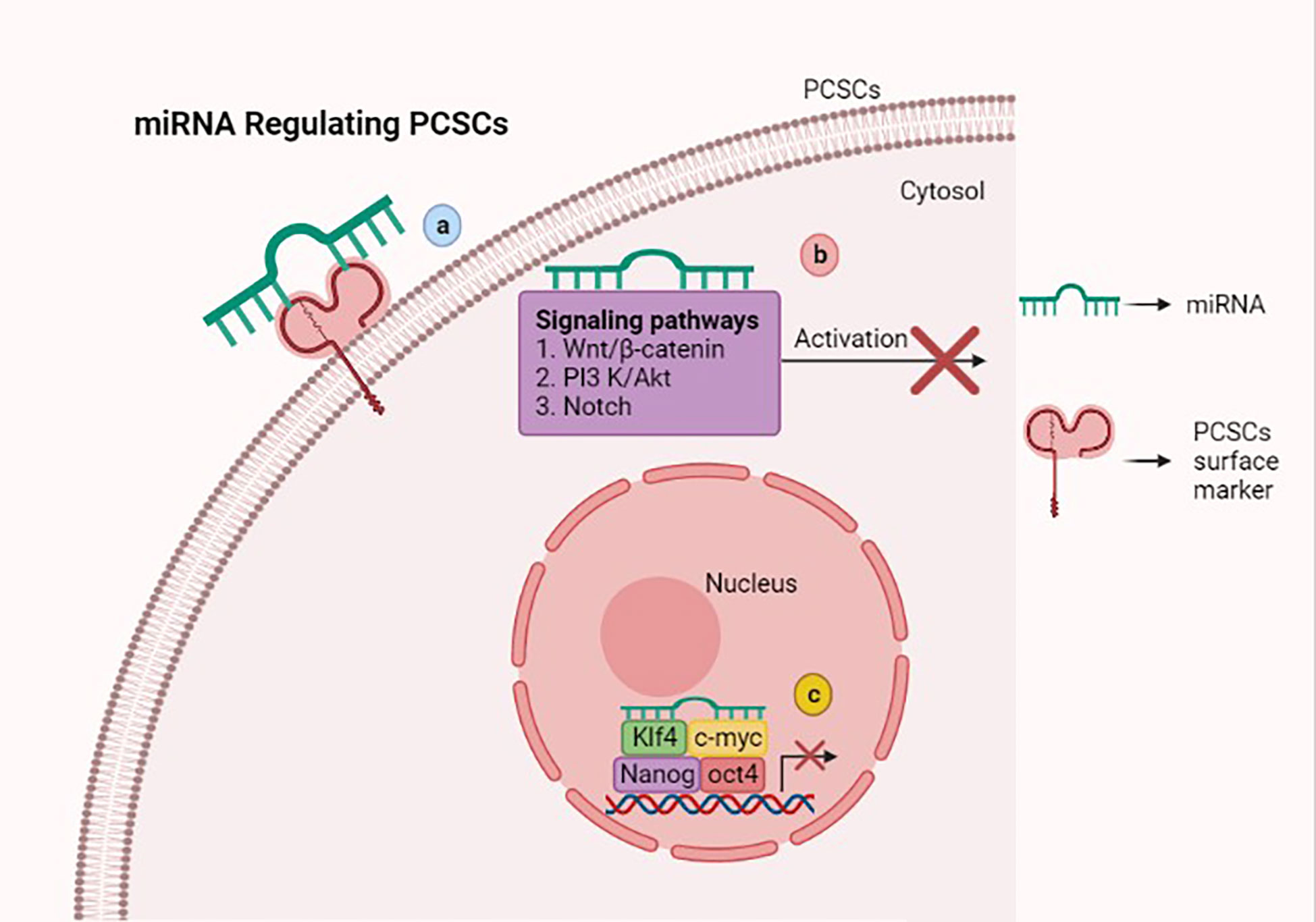
Figure 2 Scheme showing that microRNAs (miRNAs) regulate stemness features of prostate cancer stem cells (PCSCs) via various mechanisms. (A) miRNAs inhibiting stem-like traits of PCSCs via targeting the surface marker. (B) Attenuating Wnt/β-catenin, PI3K/AKT, and Notch signaling pathways with miRNAs suppresses PCSC growth and induces cell death. (C) miRNAs suppress the stem cell-related transcription factors such as OCT4, KLF4, Nanog, and c-myc, which hamper PCSC stemness properties. This figure was created with biorender.com.
CD44, a cell adhesion molecule, has been identified as a major cell surface marker in PCSs that expresses cancer stem cell-like properties, including tumorigenic, clonogenic, and metastatic properties (13). Liu et al. reported that miR-34a is a key negative regulator of CD44 in PCSs, and acts as a promising therapeutic agent against prostate cancer by repressing CD44, and, thus, inhibiting the metastasis of PCSs (75). Similarly, miR-708 negatively correlates with the CD44 subpopulation in PCSs and inhibits in vitro tumorigenicity by down-regulating CD44 expression (76). Further in vitro research reported that miR-143 and miR-145 hamper the tumor sphere formation by inhibiting stemness factors such as CD133 and CD44 in PC-3 PCSs (77).
The miRNA let-7c is down-regulated in prostate cancer and restoration of let-7c induces robust suppression of prostate cancer growth in vitro and in vivo (78). In a comprehensive study, Kong et al. demonstrated that overexpression of let-7c inhibits stemness of PCSs by suppressing EZH2, which plays a major role in cancer cell acquisition of stem cells’ signature. Indeed, after BR-DIM (metabolite 3,3′–diindolylmethane) treatment of prostate cancer cells, let-7c is up-regulated and EZH2 is down-regulated, which hampers the self-renewal and clonogenic properties of prostate cancer cells (79, 80). Jin et al. showed that the overexpression of miR-128 has negative effects on prostate cancer cells by hindering their proliferation, invasion, and sphere- and clonogenic-forming capacities. This can be achieved by targeting the cohort of stem cell regulatory factors including BMI1, Nanog, epidermal growth factor receptor (EGFR), and transforming growth factor beta receptor 1 (TGFBR1), which are all involved in maintaining the stemness of PCSs (81). Similarly, miR-100 has a negative impact on prostate cancer cells by down-regulating the oncogene argonaute 2 (AGO2), which can mediate stemness factors of cancer cells such as OCT4, Krüppel-like factor 4 (KLF4), and c-myc. The suppression of argonaute 2 with miR-100 expression inhibits the migration, invasion, and stemness of cancer cells (82). As previously mentioned, miR-143 and miR-145 attenuate tumorigenesis of PC-3 PCSs not only by targeting surface makers, but also by down-regulating stemness factors such as OCT4, c-myc, and KLF4 (77). In addition, miR-34a attenuates prostate cancer aggressiveness via down-regulating AR and Notch-1, which are highly expressed in prostate cancer (83). Guan et al. conducted an in vitro study based on miR-218 with prostate cancer cells, which revealed that miR-218 serves as a potential therapeutic target for prostate cancer by suppressing the expression of the GLI1 protein. This in vitro study elucidates the role of the GLI1 protein in the mechanism of tumor suppression of miR-218 in prostate cancer (84).
Several scientific reports have documented the role of miRNA in regulating prostate cancer stem cell-like characteristics by targeting various signaling pathways. Chang et al. reported that miR-7 acts as a critical inhibitor for prostate cancer. Mechanistically, a lower level of miR-7 expression existed in prostate cancer cells than in non-tumorigenic prostate epithelial cells, resulting in an over-expression of miR-7, which suppressed the stemness and tumorigenesis properties of PCSCs by targeting the KLF4/PI3K/AKT/p21 pathways (85). Similarly, miR-199a-3p exhibited tumor-suppressive functions in PCSs through the targeting of mitogen signaling-related biomolecules, including EGFR, c-myc, and cyclin D (86). Another in vitro study showed that miR-141 exerts the ability to inhibit tumor growth and metastasis properties of PCSs via targeting the Rho GTPase signaling-associated components, including CDC42, CDC42EP3, RAC1 (ras-related C3 botulinum toxin substrate 1), and ARPC5 (actin-related protein 2/3 complex subunit) (87). Furthermore, the expression of miR-320 in prostate cancer cells significantly inhibits stem cell-like characteristics by down-regulating the Wnt/β-catenin signaling pathways in vitro and in vivo (88).
Numerous studies reported that miR-200 families could regulate the EMT phenotype in PCSCs. Emerging evidence suggests that overexpression of platelet-derived growth factor-D (PDGFD) in PSCSs could mediate acquisition of the EMT phenotype. In PC-3 cells, miR-200 was significantly down-regulated and the expression of ZEB1, ZEB2, and SNAIL2 was up-regulated when exposed to PDGFD. As a result, reconstruction of miR-200 in PC-3 + PDGFD cells inhibits the EMT phenotype by suppressing expression of ZEB1, ZEB2, and snail2 (89). Simultaneously, SLUG is a key regulator of EMT initiation and mesenchymal differentiation, inducing the expression of miR-1 and miR-200 in PC-3 cells, thus suppressing the EMT via SLUG-dependent mechanisms and inhibiting tumorigenesis via SLUG-independent mechanisms (90). Furthermore, cancer-associated fibroblasts (CAFs) are a strong determinant for eliciting a redox-dependent EMT phenotype in prostate cancer cells. Ectopic expression of miR-205 prevents CAF-mediated EMT, thus hampering cell invasion, stemness characteristics, and tumorigenicity in vitro and in vivo (91).
Alternatively, miRNAs expression is directly proportional to PCS growth. Fan et al. reported that up-regulation of miR-143 expression in PCSs ameliorates the stemness properties of prostate cancer cells, such as differentiation and metastasis, by suppressing FNDC3B expression, which regulates cell motility (92). At the same time, ectopic expression of miR-1301–3p enhances expansion of PCSs by targeting the GSK3β and SFRP1 pathways and increasing the expression of stemness-associated factors, such as OCT4, KLF4, Nanog, SOX2, and c-myc (93). Further in vitro studies confirmed that down-regulation of miR-601 in PCSCs counteracts proliferation, migration, and invasion via promoting KRT5 and subsequently blocking the Wnt pathway (94).
NUMB is an evolutionarily conserved protein, widely present in mammalian tissues, and it plays a crucial role in cell fate determination, proliferation, differentiation, and migration (95). In addition, NUMB exerts a tumor suppressor function and inhibits the stemness of PCSCs (96). MiR-543 and miR-9–5P potentially target the NUMB and attenuate NUMB expression in PC-3 cells, promoting stemness and metastasis of PCSCs (97, 98). The role miR-424 plays depends on the cancer. In ovarian cancer, miR-424 can act as a tumor suppressor by inhibiting cell migration, invasion, and EMT via down-regulating the expression of doublecortin-like kinase 1 (DCLK1) and MYB (99, 100). Similarly, in breast cancer, miR-424 suppresses the cell invasion and stem cell traits under hyperglycemic conditions through the attenuated inhibitory function of miR-424 on CDC42, leading to the activation of PR/SET domain 14 (PRDM14), which is a repressor of pluripotent transcription factors (101, 102). However, miR-424 exhibits oncogenic effects in prostate cancer. Dallavalle et al. reported that miR-424 expression was up-regulated in prostate tumors, promoting EMT and stem-like features by silencing COP1, which mediates the STAT3 accumulation to activate stemness factors (103). In addition, circulating extracellular vesicles release higher levels of miR-424 in metastatic prostate cancer that drive the acquisition of stemness features and tumorigenesis properties in prostate cancer (104). A recent study found that miR-375 is significantly elevated in CRPC patients compared with normal prostate cancer patients. Overexpression of miR-375 in DU145 and PC-3 cells promotes cell proliferation, migration, invasion, and enzalutamide drug resistance, and inhibits cell apoptosis via targeting the phosphatase non-receptor type 4 (PTPN4), resulting in the activation of STAT3. Targeting miR-375 with e-375i could inhibit prostate cancer cell proliferation, migration, and invasion, and promote cell apoptosis and enzalutamide drug sensitivity in prostate cancer via down-regulating STAT3 expression by up-regulating PTPN4 expression (105). As a result, down-regulating miR-143, miR-1301-3p, miR-601, miR-543, miR-9-5P, miR-424, and miR-375 in PCSCs could be a potential therapeutic method for treating prostate cancer.
6 Support of nanotechnology
Nanotechnology has become a rapidly growing field in recent years, with potential applications in academic and industrial contexts for the purpose of developing novel materials at the nanoscale (106). The unique size, shape, and desirable surface modification of nanomaterials are considered to have potential for therapeutic nanomedicine and could be extensively used as a nano drug carrier for prostate cancer therapy (107). The clinical utility of biochemical inhibitors is hampered by their hydrophobicity and poor pharmacokinetics; in order to increase their bioavailability, these inhibitors are conjugated with nanoparticles and they attenuate stemness of PCSCs (108) (Figure 3). Cis-dichlorodiamminoplatinum (CDDP) is a potential anti-tumor agent for various cancers. Malek et al. fabricated CDDP-loaded hyaluronic acid (HA) nanoparticles, as a substrate for CD44, by using a drug-induced ionic gelation technique. In vitro study results revealed that HA-CDDP could act as a targeted drug delivery system and significantly prevent the sphere- and colony-forming capacity in PC-3 and DU145 cell lines (109).
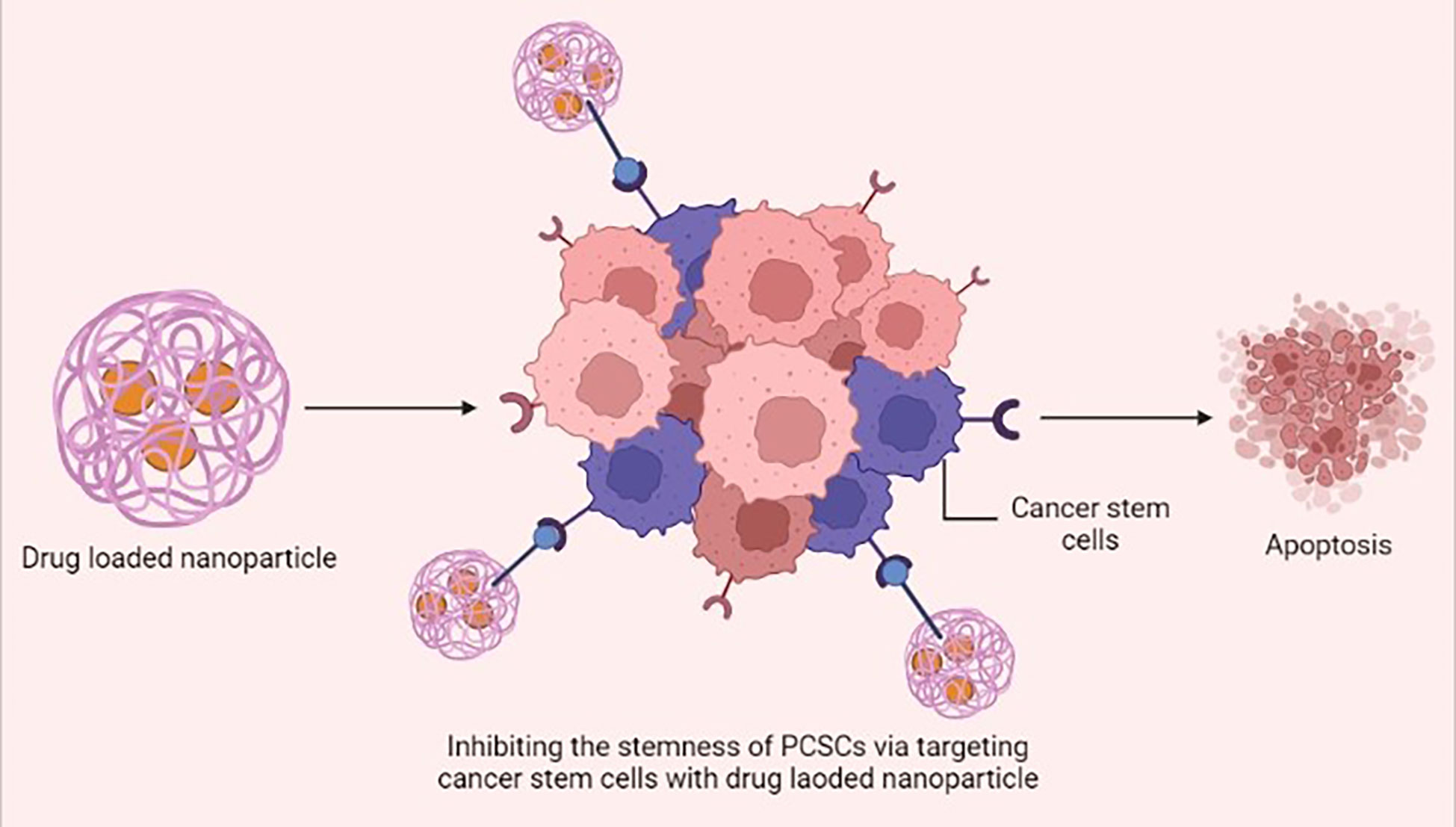
Figure 3 Drug-loaded nanoparticles inhibiting the stemness of prostate cancer stem cells (PCSCs) via specifically targeting the cancer stem cells and inducing apoptosis. This figure was created with biorender.com.
Zhou et al. evaluated N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer-based combination therapy with the PI3K/mTOR inhibitor GDC-0980 and the traditional chemotherapy agent docetaxel, both in vitro and in vivo. In vitro study indicated that HPMA + GDC-0980 could inhibit sphere-forming capacity and decrease the CD133 PCSC-rich population in the PC-3 cell line. In the PC-3 tumor xenograft mice model, combination treatment of HPMA + GDC-0980 with docetaxel significantly prolonged mice survival compared with treatment without docetaxel administration (110). As such, HPMA + cyclopamine treatment could also reduce sphere-forming capacity and the percentage of CD133 PCSCs in PC-3 and RC-92a/hTERT cells. In vivo study highlighted that combination administration of HPMA + cyclopamine with docetaxel led to significantly decreased tumor volume compared with the single drug in PC-3 tumor xenograft mice models (111). Yang et al. combined the paclitaxel and cyclopamine with poly(ethylene glycol)-block-poly(2-methyl-2-carboxyl-propylene carbonate) (mPEG-b-PCC) nanoparticles. Both the drug-loaded nanoparticles exhibited sustained drug release and suppressed the colony-forming ability in PC-3 cells by up-regulating tumor suppressor miRNAs. The combination of paclitaxel and cyclopamine-loaded nanoparticles induced significant tumor inhibition in PC-3 tumor xenograft mice models compared with monotherapy (112). All these findings suggest that combination therapy targeting PCSCs is a promising approach to enhancing therapeutic effect against prostate cancer.
Interestingly, similar to drug-loaded nanomaterials, some nanomaterials themselves can have an antitumor effect. Recent research found that graphene oxide (GO) potentially inhibits the sphere-like capacity of not only PCSCs, but also ovarian cancer cells, glioblastoma cells, lung cancer cells, and pancreatic cancer cells. Notably, GO is non-toxic for normal fibroblasts (113). Cuprous oxide (CO) nanoparticles naturally exhibit a potential antitumor function as a chemotherapeutic agent (114). Wang et al. reported that CO nanoparticles could selectively induce cell death and inhibit prostate cancer cell proliferation both in vitro and in vivo without damaging normal prostate epithelial cells. Correspondingly, CO nanoparticles can also prevent stemness of PCSCs via inhibiting the Wnt signaling pathway (115).
7 Chemotherapy
Chemotherapy is a conventional treatment in cancer therapy involving administration of chemotherapeutic agents to eradicate tumor cells. Several scientific reports have demonstrated that these agents can act as a potential inhibitor for various endogenous signaling pathways that are involved in regulating PCSCs stemness phenotype, thereby possessing potential therapeutic value for prostate cancer treatment. A recent study found that the JAK/STAT signaling pathway was up-regulated in CRPC patients and conferred resistance to AR-targeted therapies. Inhibiting the JAK/STAT pathway with a pharmacological agent resensitizes the AR-resistant prostate tumor to anti-androgen drugs (116). In addition, ectopic activation of the JAK/STAT pathway in LNCaP cells enables transition to a stem-like state and thereby induces the resistance to enzalutamide drugs. Inhibiting the JAK/STAT pathway restores luminal lineage and sensitizes the prostate tumor to enzalutamide drugs (117). Therefore, the JAK/STAT pathway plays an important role in regulating PCSC phenotype. Canesin et al. showed that direct inhibition of the STAT3 transcription factor with galiellalactone in a DU145 cell line could reduce the expression of stem cell markers and sphere and colony formation, and also decrease the viability of the docetaxel-resistant cell line (118). An in vitro study demonstrated that administration of capsaicin to PC-3 and DU145 cells could down-regulate PCSCs markers, such as CD44, CD133, ALDH1A1, SOX2, OCT4, and Nanog, and suppress cell growth via inhibiting the Wnt/β-catenin pathways (119). In addition, saikosaponin D could act as a potential chemotherapeutic agent for CRPC through the suppression of stemness abilities, such as self-renewal, sphere formation, and EMT, by blocking the Wnt/β-catenin signaling pathways by down-regulating GSK3b phosphorylation (120). The combination therapy with apigenin and cisplatin stimulates apoptosis and anti-migration of CD44 PCSCs. This combination therapy promotes apoptosis by up-regulation of apoptotic factors p53, p21, and apoptotic protease activating factor 1 (APAF1); down-regulation of anti-apoptotic factor BCL-2; and suppression of the PI3K/AKT pathway (121). Wang et al.’s in vitro study demonstrated that exposure of shikonin to PC-3 and DU145 cells could inhibit proliferation, migration, and invasion, and induce cell death through a mitochondrial-mediated apoptosis pathway. Furthermore, shikonin could reverse the resistant state of cabazitaxel by inhibiting ABCG2 and ALDH3A1 expression, which are linked with drug resistance. Therefore, the combination of shikonin and cabazitaxel causes a synergistic effect and can enable the use of lower doses of cabazitaxel for CRPC therapy (122). Comparably, Liu et al. found that inhibition of the Notch signaling pathway could improve chemosensitivity in PCSCs via decreasing the ABCC1 expression (123). According to this study, Wang et al. examined the effect of docetaxel on a PC-3 cell line with γ-secretase inhibitor (GSI), a potent inhibitor of the Notch pathway. In vitro study results revealed that the combination of docetaxel and GSI significantly inhibited cell growth and sphere formation, and induced apoptosis and cell cycle arrest in PCSCs when compare with docetaxel alone (124). Furthermore, PCSC growth could be attenuated by rottlerin treatment, which can activate the autophagy mechanism via up-regulation of the AMPK pathway and induction of apoptosis via inhibiting the PI3K/AKT/mTOR pathway (125). In vitro research showed that neferine inhibits the cell viability and migration of CD44 PCSCs via mediating JNK and p38 MAPK phosphorylation, and blocking PI3K and NF-ĸβ signaling (126). Bahmad et al. developed and examined the antitumor mechanism of synthetic retinoid ST1926 on PCSCs. Results indicated that ST1926 reduced cell proliferation, migration, and invasion and also mediated p53‐independent apoptosis via early DNA damage in PCSCs (127). Details of current clinical trials utilizing monotherapy and combinational therapy have been highlighted in Tables 1, 2.
8 Immunotherapy
Immunotherapy is an alternative potential therapeutic option, associated with immunological agents such as vaccines, antibodies, and immune cells, and chimeric antigen receptor T (CAR-T) cell therapy for the management of advanced cancer (144). The prostate stem cell antigen (PSCA) is a notable antigen; owing to its overexpression in prostate cancer, and exclusively in metastatic tissues; thus, it is a promising candidate for prostate cancer immunotherapy (145, 146). In vivo research showed that PSCA vaccination could mediate the expression of CD4 and CD8 T cells and induce a long-term protective immune responses against advanced prostate cancer (147). The stemness of PCSCs is regulated by angiogenin and plexin B2: the bioactive 3′-end fragments of 5S ribosomal RNA are synthesized in order to inhibit the function of angiogenin and plexin B2 with monoclonal antibodies, resulting in reduced stemness of PCSCs and PCSCs sensitized to chemotherapy (148). Wang et al. investigated an innovative immunotherapeutic platform based on non-peptide-stimulated dendritic cells-activated cytokine-induced killer (NP-DC-CIK) cells and immunogenic peptide-stimulated P-DC-CIK cells. These immunogenic peptides were derived from PCSC-related antigens such as CD44 and the epithelial cell adhesion molecule (EpCAM). In vitro and in vivo study results showed that peptide-stimulated dendritic cells-activated cytokine-induced killer (P-DC-CIK) cells exhibited significant cytotoxicity against PCSCs. Cytokine-induced killer cells are specifically activated on dendritic cell sensitization by immunogenic peptides with PCSCs, which significantly prompts in vitro cytotoxicity against PCSCs and induces an in vivo anti-tumor effect (149). Alternatively, Seki et al. reported that co-cultured KHYG-1 human NK cells can preferentially target PCSCs via the TRAIL/DR5 signaling pathway. In vitro data showed that KHYG-1 cells could induce more cytotoxicity against LNCaP stem-like cells than androgen-dependent LNCaP cells (normal prostate cancer cells) (150).
Recently, CAR-T cell therapy has garnered significant research interest as a cancer therapy, owing to its profound target specificity and low major histocompatibility complex restriction in immunotherapy and its ability to exert cellular adoptive immunotherapy. With the advancement of genetic engineering technology, chimeric antigen receptors (CARs) can be designed according to the antigen specific to the target cell and introduced into patient T lymphocytes by in vitro transduction (151). To illustrate, Dang et al. designed a novel immunotherapy strategy for targeting the EpCAM, a PCSC antigen. It was designed in EpCAM-specific CARs and introduced into human peripheral blood lymphocytes (PBLs); results suggested that EpCAM-specific PBLs significantly inhibit PC-3 tumor cell growth in vitro and in vivo by targeting PCSCs (Figure 4) (152). PCSCs are resistant to radiation therapy, specifically fractionated irradiation, which significantly up-regulates the immunoregulatory protein B7-H3 on PCSCs compared with prostate cancer cells. In order to, Zhang et al. developed B7-H3 CAR-T cells that can potentially target radiation-resistant PCSCs. Hence, the combination treatment of B7-H3 CAR-T cells with radiation therapy could induce robust cytotoxicity against PCSCs in vitro and in vivo (153). Another study demonstrated that PSCA-specific engineered CAR-T cells in combination with interleukin-2 (IL2) eradicate PSCA-expressing cancer cells (154).
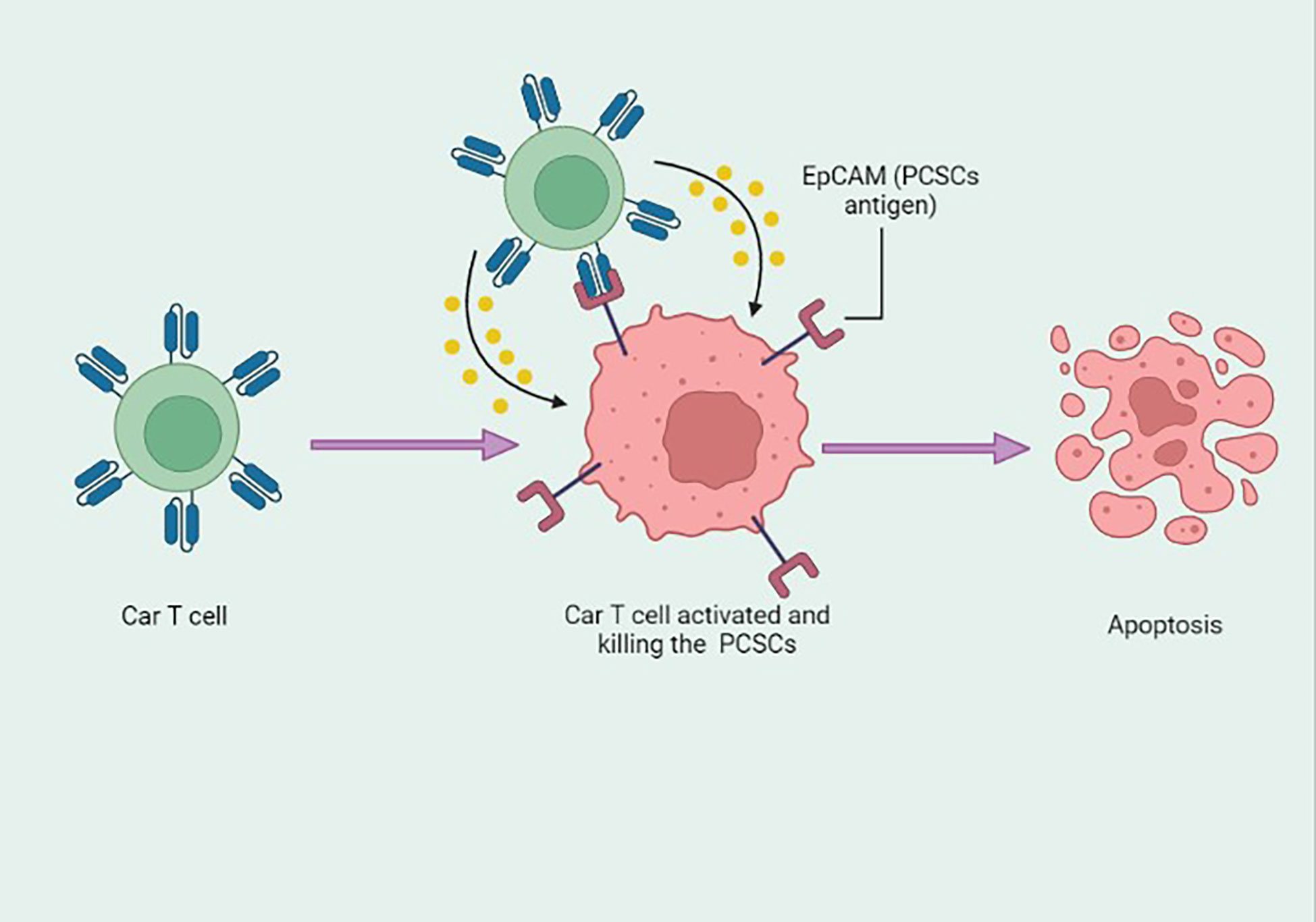
Figure 4 Epithelial cell adhesion molecule (EpCAM) antigen-based engineered CAR-T cells specifically targeting the prostate cancer stem cells (PCSCs) and inducing apoptosis. This figure was created with biorender.com.
9 Concomitant immunotherapy strategy
PCSCs are responsible for tumor initiation, growth, and relapse in prostate cancer; consequently, TLR3 activation-based immunotherapy using polyinosinic:polycytidylic acid could be a good option for treatment, as it generates apoptosis and an inflammatory response in tumor cells (155).
The plasticity of cancer stem cells is responsible for tumor phenotype progression and response to therapy; therefore, an understanding of biomarkers is important. Understanding the molecular basis has led to the development of patient therapies (156). PCSCs play a central role in the development of treatment resistance and subsequent disease progression. PCSCs exhibit surface markers, such as ALDH, CD133, and CD44, possessing states of self-renewal, colonization and recurrence (157). Various other cellular incidents promote PCSCs. It is likely that signaling pathways, such as Wnt/β-catenin, hedgehog, NF-κB, and Notch, ABC transporters, and the tumor microenvironment could be the putative target(s) for PCSCs (158). Figure 5 demonstrates the signaling routes controlling PCSCs and the work of inhibitors in suppressing these pathways (157). Therefore, targeting PCSCs could be an improved approach for the treatment of CRPC, and several inhibitors, antibodies, and other combinative therapy could be evaluated with clinical trials.
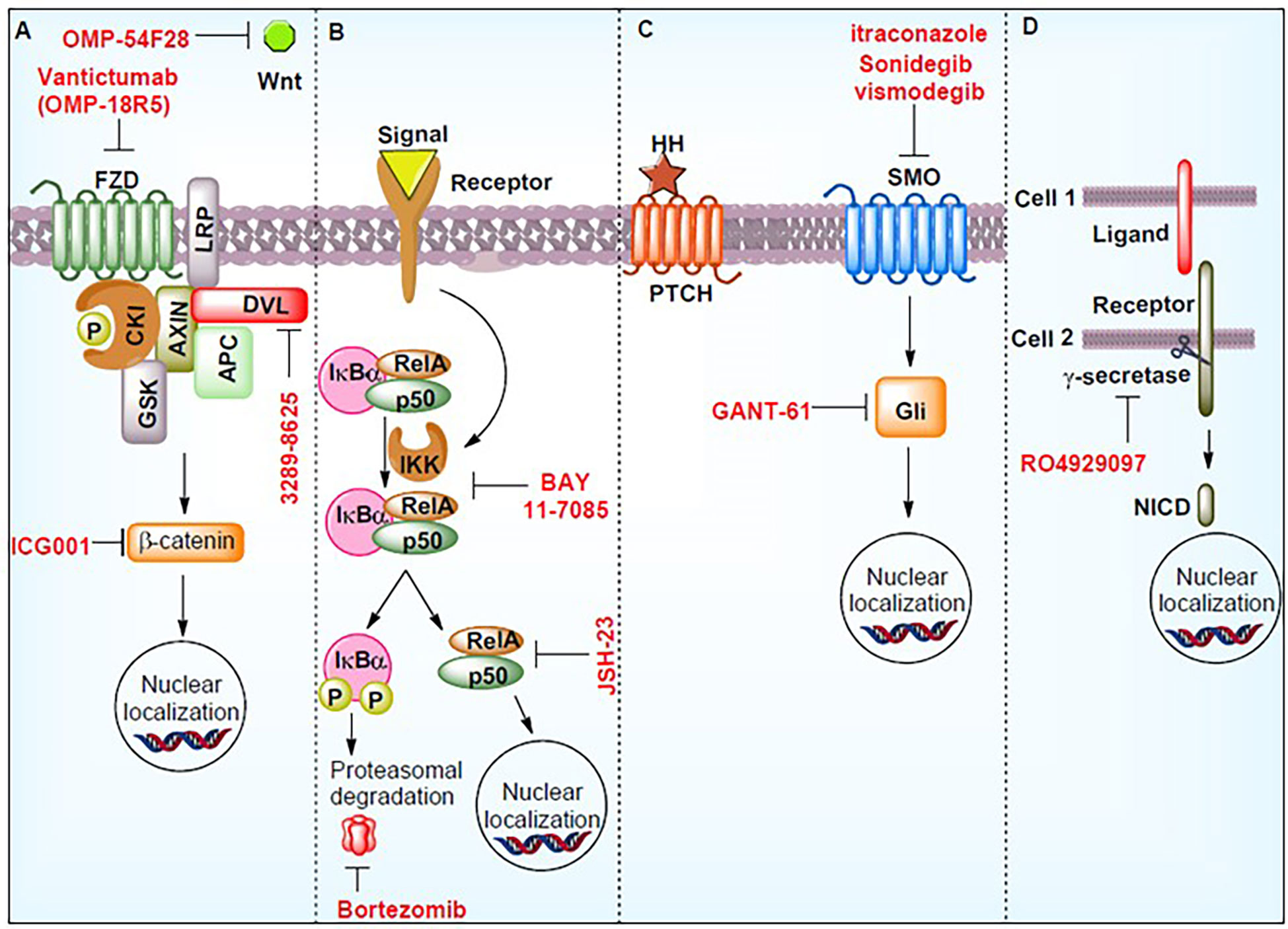
Figure 5 Signaling pathways controlling prostate cancer stem cells (PCSCs) and the work of inhibitors in suppressing these pathways. These molecules may act as potential therapeutics. The figure demonstrates the hedgehog signaling pathway (HH), notch intracellular domain (NICD), phosphorylation (P), smoothened (SMO), Wnt signaling pathways (Wnt), nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IkBα), RELA Proto-Oncogene (RelA), Frizzled (FZD), casein kinase I (CKI), axin (AXIN), APC regulator of the WNT signaling Pathway (APC), disheveled (DVL), glycogen synthase kinase (GSK), and inhibitor of nuclear factor kappa B kinase (IKK). Reproduced with permission from (157).
Studies have found these therapies to be effective in improving survival outcomes for prostate cancer patients, although full treatment is still challenging for clinicians owing to the development of drug-resistant features over time. However, understanding PCSCs’ differentiation, identification, and characterization, and their isolation through pathway-dependent expansion, is of great importance for therapeutic opportunities.
10 CRISPR/Cas9 system
With its profound limited off-targets and high accuracy, the CRISPR/Cas9 system represents an unbiased genome editing system. The system has been widely exploited to elucidate molecular mechanisms of cancer progression via gene knockout or knockdown (159). Single guide RNA (sgRNA), and Cas9 are essential components of the CRISPR system, which have been extensively delivered by viral and non-viral approaches (160). In prostate cancer therapy, the viral- and non-viral-mediated CRISPR/Cas9 delivery system is a pioneering tool that performs systematic genome-wide genetic screening to identify the function of novel genes and drug resistance mechanisms, which in turn enables new drug development for feasible therapeutic approaches (Figure 6) (161). Fei et al. used a lentiviral vector to deliver CRISPR components to prostate cancer cells. The use of genome-wide CRISPR-knockout screens found that the heterogeneous nuclear ribonucleoprotein L (HNRNPL) gene is essential and is required for prostate cancer cell growth. The gene can directly regulate alternate splicing of its RNA targets, including the AR-encoding region (162). Das et al. performed genome-wide CRISPR-interference screens in metastatic prostate cancer models and found two prostate cancer-specific driver genes, KIF4A, and WDR62. Both genes induced the phenotypically aggressive prostate cancer in both in vitro and in vivo models (163). Lei et al. found that CDK12 is conservatively required for prostate cancer cell growth by performing a kinome-scale CRISPR/Cas9 screen. Repression of CDK12 with covalent inhibitor THZ531 exerts an anti-prostate cancer effect; therefore, CDK12 represents a druggable target of prostate cancer (164).
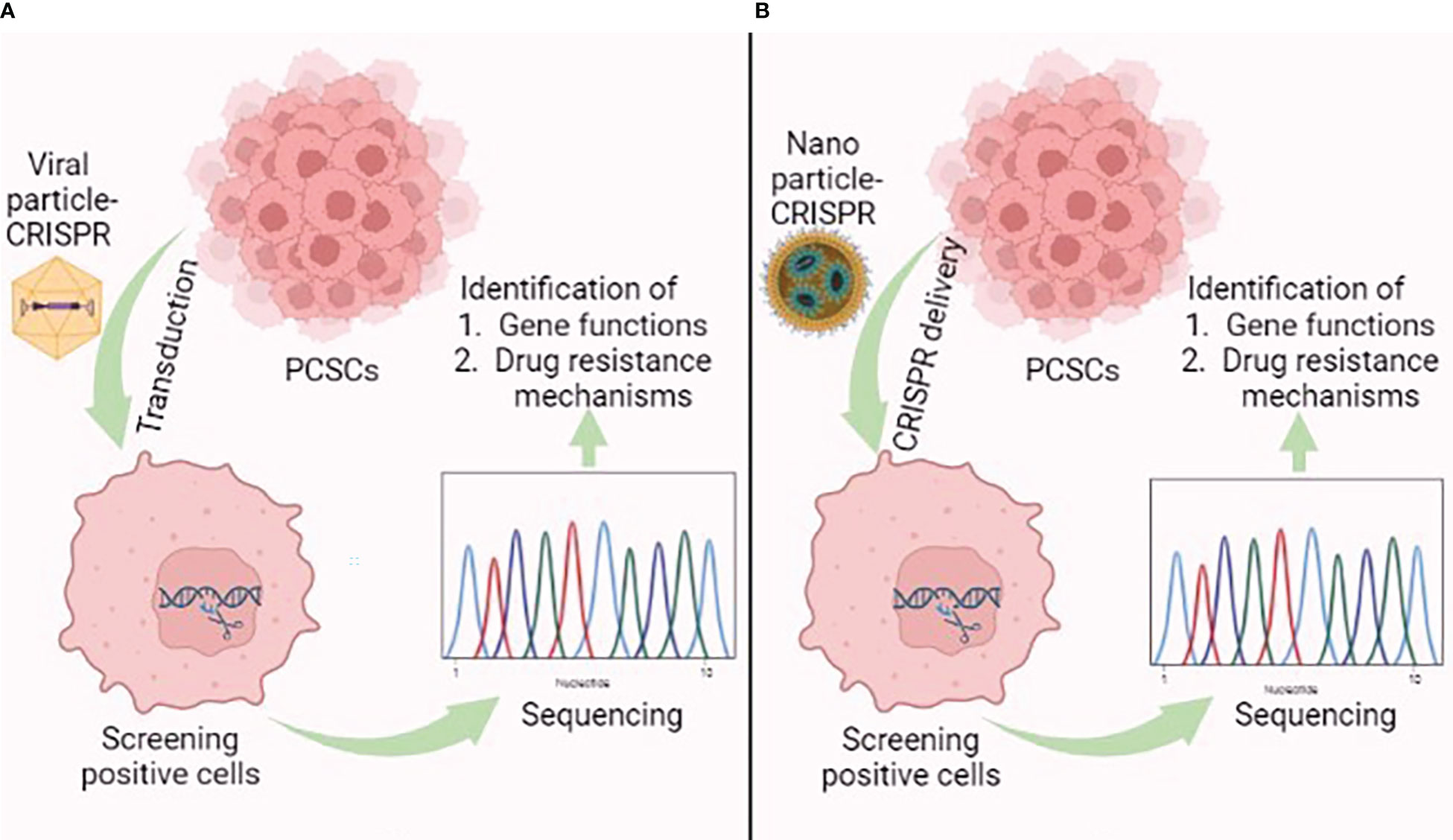
Figure 6 Graphical illustration showing the application of the (A) viral-mediated and (B) nanoparticle-mediated CRISPR/Cas9 delivery system in prostate cancer stem cells (PCSCs). This figure was created with biorender.com.
Alternatively, an in vitro study demonstrated that CRISPR/Cas9 system-mediated lipocalin 2 gene knockout decreased PC-3 cell proliferation and migration, as well as increasing the sensitivity of cisplatin drug-induced apoptosis (165). Rushworth et al. conducted an in vivo study based on a prostate cancer orthograft model sensitized with the genome-wide CRISPR/Cas9 knockout system under docetaxel treatment. Screening results highlighted that the combination of docetaxel with loss of TCEAL1 exhibited a higher therapeutic value than docetaxel alone. Therefore, silencing the TCEAL1 expression improves docetaxel efficacy in prostate cancer treatment (166). AR inhibitors such as apalutamide and enzalutamide are a mainstay of prostate cancer therapy. Palit et al. showed through the genome-wide CRISPR/Cas9 screen that on the loss of TLE3, prostate cancer cells are resistant to AR inhibitors. Notably, consistent with the interaction of TLE3 and AR at the glucocorticoid receptor (GR) locus, loss of TLE3 results in the up-regulation of GR expression, which in turn leads to resistant AR inhibitors. Thus, this study provides novel insights on GR-mediated AR inhibitor resistance, as regulated by TLE3 (167). Correspondingly, it was found through performing a kinome-scale CRISPR/Cas9 screen in CWR-R1 prostate cancer cells that an activated BRAF signaling pathway is resistant to enzalutamide. Inhibition of BRAF or downstream components of MAPK, such as MEK and ERK, ameliorate the enzalutamide sensitivity in prostate cancer cells. As a result, the combined inhibition of MAPK and AR pathways provides a potential therapeutic effect in BRAF-mutated prostate cancer patients (168).
In prostate cancer, NANOG is highly associated with cancer stem cell traits and resistance to androgen deprivation therapy. Kawamura et al. generated a Nanog and Nanogp8 knockout prostate cell line DUI45 using the CRISPR/Cas9 system. Nanog and Nanogp8 knockout significantly impaired the malignant potential, sphere formation, and drug resistance of cancer stem cells compared with the control DU145 cell line (169). However, all experiments mentioned above were conducted by the viral-mediated CRISPR/Cas9 delivery system, which comes with a risk of immunogenicity. As a result, non-viral particles are a promising vehicle for delivering CRISPR/Cas9 components. For example, Zhen et al. designed an aptamer–liposome–CRISPR/Cas9 chimera for specifically targeting prostate cancer. The chimera-inserted RNA aptamer acts as an antigen for prostate cancer, and the CRISPR/Cas9-loaded cationic liposomes are attached to the aptamer. The aptamer–liposome–CRISPR/Cas9 chimera significantly binds to LNCaP cells and attenuates cancer cell growth via silencing the polo-like kinase 1, which is a cell survival gene (170). Hence, targeting stemness-related transcription factors or signaling pathways with a non-viral-based CRISPR/Cas9 system could be a potential therapeutic strategy for prostate cancer treatment.
11 Photothermal ablation therapy
In recent years, photothermal ablation (PTA) therapy has become a promising treatment in the management of various types of cancer with minimal invasiveness. In this approach, light energy is converted into heat energy by exploiting photothermal agents with a high-power near-infrared (NIR) laser to place cancer cells in remission (hyperthermia) (171). Cancer cells are more sensitive than normal cells to heat stress owing to the different pattern expressions of heat shock proteins that are involved in the cellular defense mechanism. As a result of elevating the tumor microenvironment temperature, cancer cells are irretrievably damaged because of the denaturation of proteins (172).
In 2019, Rastinehad et al. conducted the first clinical pilot study of PTA in 16 prostate cancer patients, using laser-excited gold–silica nanoshells. In this experiment, NIR (810 ± 10 nm) light was used to excite gold–silica nanoshells for producing the hyperthermia condition for focal ablation of prostate cancer (173). Kim et al. demonstrated that clustering photothermal agents exhibited higher therapeutic efficiency against prostate cancer than with the single photothermal molecule. They conducted in vitro and in vivo studies with gold (Au) nanoparticles, gold nanoparticle clusters (AuNCs), and silica-coated AuNCs@SiO2 under the NIR laser at 808 nm wavelength against a prostate cancer model. Results showed that AuNCs@SiO2 possess a greater photothermal effect than AuNCs and Au nanoparticles against prostate cancer, owing to its unique nanostructure and silica layer coating, which provides stability for a long time under NIR laser irradiation (Figure 7) (174). Similarly, Yang et al. designed novel enzyme-triggered self-assembled gold nanoparticles with a CREKA-YP FFK(Nph) peptide sequence. Under alkaline phosphatase enzyme treatment, the CREKA-YP FFK(Nph) peptide can be self-assembled; as a result gold nanoparticles self-aggregate inside the tumor cells, and, therefore, enhance retention and photothermal effects against in vitro and in vivo prostate cancer models (175).
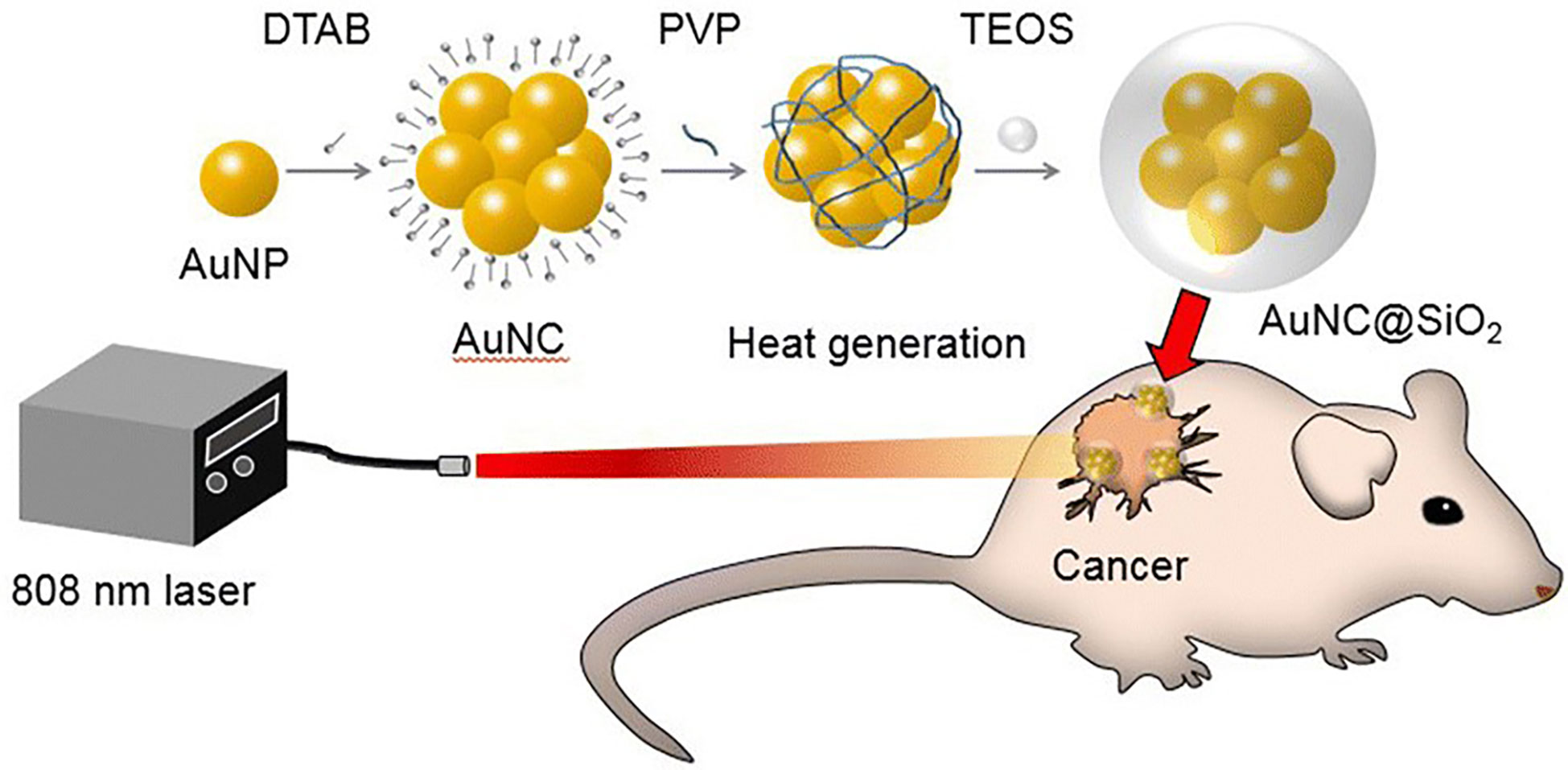
Figure 7 Graphical illustration showing the photothermal effect of gold nanoparticle clusters (AuNCs)@SiO2 in a prostate cancer model under near-infrared (NIR) laser irradiation (174).
Target specification of tumor cells is a major concern in the modern oncology era. To overcome this, the surface of the photothermal agent can be optimized with nanopeptides for specifically targeting the cancer cells. For example, Avvakumova et al. developed neuropeptide Y (NPY)-conjugated gold nanocages and this has been exploited for localized PTA therapy against in vitro prostate cancer models under NIR laser treatment at a wavelength of 800 nm (176). This is because of NPY being highly expressed in prostate cancer cells and being involved in aggressive tumor growth and progression (177). In addition, Gobin et al. demonstrated that ephrinAl-conjugated gold-coated silica nanoshells have shown a potential PTA effect against a PC-3 cell line under NIR laser irradiation at a wavelength of 800 nm. This is because ephrinAl ligands easily bind to the ephrinA2 receptors, which are highly expressed on the PC-3 cell line, and thus can mediate target specificity and enhance PTA therapy (178). Therefore, targeting PCSCs with surface-functionalized photothermal agents could be a potential therapeutic treatment for prostate cancer.
12 Conclusions and future perspectives
Over the decades, a significant amount of research has been conducted into PCSCs and has suggested that they play a critical role in prostate cancer initiation and progression. Current conventional therapies for prostate cancer are ineffective, owing to the fact that cancer stem cells, which are not specifically targeted in most therapies, induce tumor relapse, metastasis, and therapy resistance. Therefore, the development of therapeutic strategies for targeting the PCSCs is imperative to increase treatment efficiency. miRNA-based BR-DIM and NUMB targets (Section 5), nanomedicine-based combinatorial drugs, and nanomaterials such as graphene oxide and cuprous oxide have shown promise in improving therapeutic efficacy and halting stemness features in prostate cancer stem cells. A nanotechnology platform can be adapted to target prostate cancer epithelial cells or prostatecancer stem cells by optimizing the size and shape of the nanomaterials and antibodies or nanobodies for (section 6) (72, 179), biosensor approaches (180), several enhanced chemotherapeutics agents approaches in the clinical trials and are summarized in Tables 1, 2 under the monotherapy and combinational therapy could be a potential strategy to employ them in the prostate cancer stem cells populations (Section 7), Immunotherapy strategies such as immune checkpoint inhibitors in the combination of core-shell nanoparticles and biomimetic nanoparticles (Section 8), CAR-T cell-based nano immunotherapeutic approaches could be more effective strategies for targeting prostate cancer stem cells for their differentiation, identification, characterization for the concomitant immunotherapeutic regime (Section 9), CRISPR-Cas nanoparticle-based strategy for understanding Yamanaka factors (section 10), different nanosystem-based hyperthermia targeting the PCSCs with photothermal ablation therapy could be potential therapeutic treatment for PCSCs for relapse by fulfilling specific markers identification, deciphering stemness-related signaling pathways and their interaction with tumor microenvironment.
Author contributions
SR and V-KL contributed to the conception and design of the study. SR wrote the first draft of the manuscript. PS, MA, SL, AA, SO, AM, AT, AK, YJ, SC, and V-KL finalized the second and third draft of manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by the Department of Science and Technology (DST), SERB (Research Grant number CRG/2021/001369) to V-KL and holding adjunct position Professor Nicole Steinmetz Fellow and group leader at IAAM. AM acknowledged the International Association of Advanced Materials (IAAM) for its Professor Herbert Gleiter Fellowship.
Acknowledgments
The authors acknowledge their respective institutions for providing support and facilities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang L, Lu B, He M, Wang Y, Wang Z, Du L. Prostate cancer incidence and mortality: Global status and temporal trends in 89 countries from 2000 to 2019. Front Public Health (2022) 10:811044. doi: 10.3389/fpubh.2022.811044
2. Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. New Engl J Med (2003) 349:366–81. doi: 10.1056/NEJMra021562
3. Lang SH, Frame FM, Collins AT. Prostate cancer stem cells. J Pathol (2009) 217:299–306. doi: 10.1002/path.2478
4. Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell (2014) 14:275–91. doi: 10.1016/j.stem.2014.02.006
5. Zhang D, Park D, Zhong Y, Lu Y, Rycaj K, Gong S, et al. Stem cell and neurogenic gene-expression profiles link prostate basal cells to aggressive prostate cancer. Nat Commun (2016) 7:10798. doi: 10.1038/ncomms10798
6. Kelsey R. “Stem-like” prostate basal cells. Nat Rev Urol (2016) 13:238. doi: 10.1038/nrurol.2016.55
7. Wolf I, Gratzke C, Wolf P. Prostate cancer stem cells: Clinical aspects and targeted therapies. Front Oncol (2022) 12:935715. doi: 10.3389/fonc.2022.935715
8. Bhattacharya R, Banerjee Mustafi S, Street M, Dey A, Dwivedi SKD. Bmi-1: At the crossroads of physiological and pathological biology. Genes Dis (2015) 2:225–39. doi: 10.1016/j.gendis.2015.04.001
9. Clarke MF. Clinical and therapeutic implications of cancer stem cells. New Engl J Med (2019) 380:2237–45. doi: 10.1056/NEJMra1804280
10. Rybak AP, Bristow RG, Kapoor A. Prostate cancer stem cells: deciphering the origins and pathways involved in prostate tumorigenesis and aggression. Oncotarget (2015) 6:1900–19. doi: 10.18632/oncotarget.2953
11. Zhou H-M, Zhang J-G, Zhang X, Li Q. Targeting cancer stem cells for reversing therapy resistance: mechanism, signaling, and prospective agents. Signal Transduct Targeted Ther (2021) 6:62. doi: 10.1038/s41392-020-00430-1
12. Kim D, Ko Y, Park M, Kim B, Sohn H, Lim W. Regulation of osteosclerosis by inoculated Cd133+ PC3 cells in bone-marrow microenvironmental niches. JBMR Plus (2019) 3:e10189. doi: 10.1002/jbm4.10189
13. Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene (2006) 25:1696–708. doi: 10.1038/sj.onc.1209327
14. Yun E-J, Lo U-G, Hsieh J-T. The evolving landscape of prostate cancer stem cell: Therapeutic implications and future challenges. Asian J Urol (2016) 3:203–10. doi: 10.1016/j.ajur.2016.09.006
15. Giridharan M, Rupani V, Banerjee S. Signaling pathways and targeted therapies for stem cells in prostate cancer. ACS Pharmacol Trans Sci (2022) 5:193–206. doi: 10.1021/acsptsci.2c00019
16. Han H, Wang Y, Curto J, Gurrapu S, Laudato S, Rumandla A, et al. Mesenchymal and stem-like prostate cancer linked to therapy-induced lineage plasticity and metastasis. Cell Rep (2022) 39:110595. doi: 10.1016/j.celrep.2022.110595
17. Mei W, Lin X, Kapoor A, Gu Y, Zhao K, Tang D. The contributions of prostate cancer stem cells in prostate cancer initiation and metastasis. Cancers (2019) 11. doi: 10.3390/cancers11040434
18. Smith AB, Artem S, Vladislav U, Robert B, Yulia N, Kiley G, et al. A basal stem cell signature identifies aggressive prostate cancer phenotypes. Proc Natl Acad Sci (2015) 112:E6544–52. doi: 10.1073/pnas.1518007112
19. Kong D, Banerjee S, Ahmad A, Li Y, Wang Z, Sethi S, et al. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PloS One (2010) 5:e12445. doi: 10.1371/journal.pone.0012445
20. Lee E, Wang J, Yumoto K, Jung Y, Cackowski FC, Decker AM, et al. DNMT1 regulates epithelial-mesenchymal transition and cancer stem cells, which promotes prostate cancer metastasis. Neoplasia (2016) 18:553–66. doi: 10.1016/j.neo.2016.07.007
21. Kong D, Wang Z, Sarkar SH, Li Y, Banerjee S, Saliganan A, et al. Platelet-derived growth factor-d overexpression contributes to epithelial-mesenchymal transition of PC3 prostate cancer cells. Stem Cells (Dayton Ohio) (2008) 26:1425–35. doi: 10.1634/stemcells.2007-1076
22. Talati PG, Gu L, Ellsworth EM, Girondo MA, Trerotola M, Hoang DT, et al. Jak2-Stat5a/b signaling induces epithelial-to-Mesenchymal transition and stem-like cell properties in prostate cancer. Am J Pathol (2015) 185:2505–22. doi: 10.1016/j.ajpath.2015.04.026
23. Wang S, Garcia AJ, Wu M, Lawson DA, Witte ON, Wu H. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc Natl Acad Sci United States America (2006) 103:1480–5. doi: 10.1073/pnas.0510652103
24. Martin P, Liu Y-N, Pierce R, Abou-Kheir W, Casey O, Seng V, et al. Prostate epithelial Pten/TP53 loss leads to transformation of multipotential progenitors and epithelial to mesenchymal transition. Am J Pathol (2011) 179:422–35. doi: 10.1016/j.ajpath.2011.03.035
25. Chaurasiya S, Widmann S, Botero C, Lin C-Y, Gustafsson J-Å, Strom AM. Estrogen receptor β exerts tumor suppressive effects in prostate cancer through repression of androgen receptor activity. PloS One (2020) 15:e0226057–e0226057. doi: 10.1371/journal.pone.0226057
26. Yeh C-R, Da J, Song W, Fazili A, Yeh S. Estrogen receptors in prostate development and cancer. Am J Clin Exp Urol (2014) 2:161–8.
27. Mak P, Li J, Samanta S, Chang C, Jerry DJ, Davis RJ, et al. Prostate tumorigenesis induced by PTEN deletion involves estrogen receptor β repression. Cell Rep (2015) 10:1982–91. doi: 10.1016/j.celrep.2015.02.063
28. Shen Y, Cao J, Liang Z, Lin Q, Wang J, Yang X, et al. Estrogen receptor α-NOTCH1 axis enhances basal stem-like cells and epithelial-mesenchymal transition phenotypes in prostate cancer. Cell Commun Signaling (2019) 17:50. doi: 10.1186/s12964-019-0367-x
29. Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res (2005) 65:10946–51. doi: 10.1158/0008-5472.CAN-05-2018
30. Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+CD24– prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer (2008) 98:756–65. doi: 10.1038/sj.bjc.6604242
31. van den Hoogen C, van der Horst G, Cheung H, Buijs JT, Lippitt JM, Guzmán-Ramírez N, et al. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res (2010) 70:5163–73. doi: 10.1158/0008-5472.CAN-09-3806
32. Li T, Su Y, Mei Y, Leng Q, Leng B, Liu Z, et al. ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients’ outcome. Lab Invest (2010) 90:234–44. doi: 10.1038/labinvest.2009.127
33. Trerotola M, Rathore S, Goel HL, Li J, Alberti S, Piantelli M, et al. CD133, trop-2 and alpha2beta1 integrin surface receptors as markers of putative human prostate cancer stem cells. Am J Trans Res (2010) 2:135–44.
34. Sui X, Cai J, Li H, He C, Zhou C, Dong Y, et al. p53-dependent CD51 expression contributes to characteristics of cancer stem cells in prostate cancer. Cell Death Dis (2018) 9:523. doi: 10.1038/s41419-018-0541-x
35. Li C, Liu S, Yan R, Han N, Wong K-K, Li L. CD54-NOTCH1 axis controls tumor initiation and cancer stem cell functions in human prostate cancer. Theranostics (2017) 7:67–80. doi: 10.7150/thno.16752
36. Van Camp JK, Beckers S, Zegers D, Van Hul W. Wnt signaling and the control of human stem cell fate. Stem Cell Rev Rep (2014) 10:207–29. doi: 10.1007/s12015-013-9486-8
37. Bisson I, Prowse DM. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res (2009) 19:683–97. doi: 10.1038/cr.2009.43
38. Jiang Y, Dai J, Zhang H, Sottnik JL, Keller JM, Escott KJ, et al. Activation of the wnt pathway through AR79, a GSK3β inhibitor, promotes prostate cancer growth in soft tissue and bone. Mol Cancer Res (2013) 11:1597–610. doi: 10.1158/1541-7786.MCR-13-0332-T
39. Pai VC, Hsu C-C, Chan T-S, Liao W-Y, Chuu C-P, Chen W-Y, et al. ASPM promotes prostate cancer stemness and progression by augmenting wnt–Dvl-3–β-catenin signaling. Oncogene (2019) 38:1340–53. doi: 10.1038/s41388-018-0497-4
40. Li Q, Xia D, Wang Z, Liu B, Zhang J, Peng P, et al. Circadian rhythm gene PER3 negatively regulates stemness of prostate cancer stem cells via WNT/β-catenin signaling in tumor microenvironment. Front Cell Dev Biol (2021) 9:656981. doi: 10.3389/fcell.2021.656981
41. Huang R, Wang S, Wang N, Zheng Y, Zhou J, Yang B, et al. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating β-catenin/STAT3 signaling. Cell Death Dis (2020) 11:234. doi: 10.1038/s41419-020-2435-y
42. Anna D, Sungeun K, Salamone JR, Walker RJ, Sauveur-Michel M, Carlos G-E GSP, et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci (2009) 106:268–73. doi: 10.1073/pnas.0810956106
43. Hou Y, Li H, Huo W. THBS4 silencing regulates the cancer stem cell-like properties in prostate cancer via blocking the PI3K/Akt pathway. Prostate (2020) 80:753–63. doi: 10.1002/pros.23989
44. He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet (2007) 39:189–98. doi: 10.1038/ng1928
45. Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, et al. Phosphorylation of β-catenin by AKT promotes β-catenin transcriptional activity *. J Biol Chem (2007) 282:11221–9. doi: 10.1074/jbc.M611871200
46. Chen M, Tanner M, Levine AC, Levina E, Ohouo PY, Buttyan R. Androgenic regulation of hedgehog signaling pathway components in prostate cancer cells. Cell Cycle (2009) 8:149–57. doi: 10.4161/cc.8.1.7532
47. Chen M, Feuerstein MA, Levina E, Baghel PS, Carkner RD, Tanner MJ, et al. Hedgehog/Gli supports androgen signaling in androgen deprived and androgen independent prostate cancer cells. Mol Cancer (2010) 9:89. doi: 10.1186/1476-4598-9-89
48. Pilar S, Maria HA, Barbara S, Kahler JA, DeGueme MA, Andrea B, et al. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci (2004) 101:12561–6. doi: 10.1073/pnas.0404956101
49. Chang H-H, Chen B-Y, Wu C-Y, Tsao Z-J, Chen Y-Y, Chang C-P, et al. Hedgehog overexpression leads to the formation of prostate cancer stem cells with metastatic property irrespective of androgen receptor expression in the mouse model. J Biomed Sci (2011) 18:6. doi: 10.1186/1423-0127-18-6
50. Cheng J-W, Duan L-X, Yu Y, Wang P, Feng J, Feng G-Z, et al. Bone marrow mesenchymal stem cells promote prostate cancer cell stemness via cell-cell contact to activate the Jagged1/Notch1 pathway. Cell biosci (2021) 11:87. doi: 10.1186/s13578-021-00599-0
51. Gorodetska I, Lukiyanchuk V, Peitzsch C, Kozeretska I, Dubrovska A. BRCA1 and EZH2 cooperate in regulation of prostate cancer stem cell phenotype. Int J Cancer (2019) 145:2974–85. doi: 10.1002/ijc.32323
52. Wu J, Cang S, Liu C, Ochiai W, Chiao JW. Development of human prostate cancer stem cells involves epigenomic alteration and PI3K/AKT pathway activation. Exp Hematol Oncol (2020) 9:12. doi: 10.1186/s40164-020-00168-0
53. Peña-Hernández R, Aprigliano R, Carina Frommel S, Pietrzak K, Steiger S, Roganowicz M, et al. BAZ2A-mediated repression via H3K14ac-marked enhancers promotes prostate cancer stem cells. EMBO Rep (2021) 22:e53014. doi: 10.15252/embr.202153014
54. Jiao M, Qi M, Zhang F, Hu J, Feng T, Zhao M, et al. CUL4B regulates cancer stem-like traits of prostate cancer cells by targeting BMI1 via miR200b/c. Prostate (2019) 79:1294–303. doi: 10.1002/pros.23835
55. Hu J, Sun F, Chen W, Zhang J, Zhang T, Qi M, et al. BTF3 sustains cancer stem-like phenotype of prostate cancer via stabilization of BMI1. J Exp Clin Cancer Res (2019) 38:227. doi: 10.1186/s13046-019-1222-z
56. Lee JE, Lim YH, Kim JH. UCH-L1 and UCH-L3 regulate the cancer stem cell-like properties through PI3 K/Akt signaling pathway in prostate cancer cells. Anim Cells Syst (2021) 25:312–22. doi: 10.1080/19768354.2021.1987320
57. Deng Q, Tang DG. Androgen receptor and prostate cancer stem cells: biological mechanisms and clinical implications. Endocrine-Related Cancer (2015) 22:T209–20. doi: 10.1530/ERC-15-0217
58. Jamroze A, Chatta G, Tang DG. Androgen receptor (AR) heterogeneity in prostate cancer and therapy resistance. Cancer Lett (2021) 518:1–9. doi: 10.1016/j.canlet.2021.06.006
59. Liu X, Li WJ, Puzanov I, Goodrich DW, Chatta G, Tang DG. Prostate cancer as a dedifferentiated organ: androgen receptor, cancer stem cells, and cancer stemness. Essays Biochem (2022), EBC20220003. doi: 10.1042/EBC20220003
60. Sánchez BG, Bort A, Vara-Ciruelos D, Díaz-Laviada I. Androgen deprivation induces reprogramming of prostate cancer cells to stem-like cells. Cells (2020) 9. doi: 10.3390/cells9061441
61. Ojo D, Lin X, Wong N, Gu Y, Tang D. Prostate cancer stem-like cells contribute to the development of castration-resistant prostate cancer. Cancers (2015) 7:2290–308. doi: 10.3390/cancers7040890
62. Yang W, Wang K, Ma J, Hui K, Lv W, Ma Z, et al. Inhibition of androgen receptor signaling promotes prostate cancer cell migration via upregulation of annexin A1 expression. Arch Med Res (2021) 52:174–81. doi: 10.1016/j.arcmed.2020.10.005
63. Xie Q, Liu Y, Cai T, Horton C, Stefanson J, Wang ZA. Dissecting cell-type-specific roles of androgen receptor in prostate homeostasis and regeneration through lineage tracing. Nat Commun (2017) 8:14284. doi: 10.1038/ncomms14284
64. Fujita K, Nonomura N. Role of androgen receptor in prostate cancer: A review. World J Men’s Health (2019) 37:288–95. doi: 10.5534/wjmh.180040
65. Tan MHE, Li J, Xu HE, Melcher K, Yong E. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacol Sin (2015) 36:3–23. doi: 10.1038/aps.2014.18
66. Obinata D, Lawrence MG, Takayama K, Choo N, Risbridger GP, Takahashi S, et al. Recent discoveries in the androgen receptor pathway in castration-resistant prostate cancer. Front Oncol (2020) 10:581515. doi: 10.3389/fonc.2020.581515
67. Fizazi K, Scher HI, Miller K, Basch E, Sternberg CN, Cella D, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol (2014) 15:1147–56. doi: 10.1016/S1470-2045(14)70303-1
68. Attard G, Borre M, Gurney H, Loriot Y, Andresen-Daniil C, Kalleda R, et al. Abiraterone alone or in combination with enzalutamide in metastatic castration-resistant prostate cancer with rising prostate-specific antigen during enzalutamide treatment. J Clin Oncol (2018) 36:2639–46. doi: 10.1200/JCO.2018.77.9827
69. Kregel S, Kiriluk KJ, Rosen AM, Cai Y, Reyes EE, Otto KB, et al. Sox2 is an androgen receptor-repressed gene that promotes castration-resistant prostate cancer. PloS One (2013) 8:e53701. doi: 10.1371/journal.pone.0053701
70. Verma S, Shankar E, Kalayci FN, Mukunda A, Alassfar M, Singh V, et al. Androgen deprivation induces transcriptional reprogramming in prostate cancer cells to develop stem cell-like characteristics. Int J Mol Sci (2020) 21. doi: 10.3390/ijms21249568
71. Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen C-C, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science (2017) 355:84–8. doi: 10.1126/science.aah4307
72. Qin W, Zheng Y, Qian B-Z, Zhao M. Prostate cancer stem cells and nanotechnology: A focus on wnt signaling. Front Pharmacol (2017) 8:153. doi: 10.3389/fphar.2017.00153
73. Zhang Z, Cheng L, Li J, Farah E, Atallah NM, Pascuzzi PE, et al. Inhibition of the wnt/β-catenin pathway overcomes resistance to enzalutamide in castration-resistant prostate cancer. Cancer Res (2018) 78:3147–62. doi: 10.1158/0008-5472.CAN-17-3006
74. Yoshida K, Yamamoto Y, Ochiya T. miRNA signaling networks in cancer stem cells. Regenerative Ther (2021) 17:1–7. doi: 10.1016/j.reth.2021.01.004
75. Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med (2011) 17:211–5. doi: 10.1038/nm.2284
76. Saini S, Majid S, Shahryari V, Arora S, Yamamura S, Chang I, et al. miRNA-708 control of CD44+ prostate cancer–initiating cells. Cancer Res (2012) 72:3618–30. doi: 10.1158/0008-5472.CAN-12-0540
77. Huang S, Guo W, Tang Y, Ren D, Zou X, Peng X. miR-143 and miR-145 inhibit stem cell characteristics of PC-3 prostate cancer cells. Oncol Rep (2012) 28:1831–7. doi: 10.3892/or.2012.2015
78. Nadiminty N, Tummala R, Lou W, Zhu Y, Shi X-B, Zou JX, et al. MicroRNA let-7c is downregulated in prostate cancer and suppresses prostate cancer growth. PloS One (2012) 7:e32832–2. doi: 10.1371/journal.pone.0032832
79. Kong D, Heath E, Chen W, Cher ML, Powell I, Heilbrun L, et al. Loss of let-7 up-regulates EZH2 in prostate cancer consistent with the acquisition of cancer stem cell signatures that are attenuated by BR-DIM. PloS One (2012) 7:e33729. doi: 10.1371/journal.pone.0033729
80. Liu C, Kelnar K, Vlassov AV, Brown D, Wang J, Tang DG. Distinct microRNA expression profiles in prostate cancer Stem/Progenitor cells and tumor-suppressive functions of let-7. Cancer Res (2012) 72:3393–404. doi: 10.1158/0008-5472.CAN-11-3864
81. Jin M, Zhang T, Liu C, Badeaux MA, Liu B, Liu R, et al. miRNA-128 suppresses prostate cancer by inhibiting BMI-1 to inhibit tumor-initiating cells. Cancer Res (2014) 74:4183–95. doi: 10.1158/0008-5472.CAN-14-0404
82. Wang M, Ren D, Guo W, Wang Z, Huang S, Du H, et al. Loss of miR-100 enhances migration, invasion, epithelial-mesenchymal transition and stemness properties in prostate cancer cells through targeting argonaute 2. Int J Oncol (2014) 45:362–72. doi: 10.3892/ijo.2014.2413
83. Kashat M, Azzouz L, Sarkar SH, Kong D, Li Y, Sarkar FH. Inactivation of AR and notch-1 signaling by miR-34a attenuates prostate cancer aggressiveness. Am J Trans Res (2012) 4:432–42.
84. Guan B, Mu L, Zhang L, Wang K, Tian J, Xu S, et al. MicroRNA−218 inhibits the migration, epithelial−mesenchymal transition and cancer stem cell properties of prostate cancer cells. Oncol Lett (2018) 16:1821–6. doi: 10.3892/ol.2018.8877
85. Chang Y-L, Zhou P-J, Wei L, Li W, Ji Z, Fang Y-X, et al. MicroRNA-7 inhibits the stemness of prostate cancer stem-like cells and tumorigenesis by repressing KLF4/PI3K/Akt/p21 pathway. Oncotarget (2015) 6:24017–31. doi: 10.18632/oncotarget.4447
86. Liu R, Liu C, Zhang D, Liu B, Chen X, Rycaj K, et al. miR-199a-3p targets stemness-related and mitogenic signaling pathways to suppress the expansion and tumorigenic capabilities of prostate cancer stem cells. Oncotarget (2016) 7:56628–42. doi: 10.18632/oncotarget.10652
87. Liu C, Liu R, Zhang D, Deng Q, Liu B, Chao H-P, et al. MicroRNA-141 suppresses prostate cancer stem cells and metastasis by targeting a cohort of pro-metastasis genes. Nat Commun (2017) 8:14270. doi: 10.1038/ncomms14270
88. Hsieh I-S, Chang K-C, Tsai Y-T, Ke J-Y, Lu P-J, Lee K-H, et al. MicroRNA-320 suppresses the stem cell-like characteristics of prostate cancer cells by downregulating the wnt/beta-catenin signaling pathway. Carcinogenesis (2013) 34:530–8. doi: 10.1093/carcin/bgs371
89. Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim H-RC, et al. miR-200 regulates PDGF-D-Mediated epithelial–mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells (2009) 27:1712–21. doi: 10.1002/stem.101
90. Liu Y-N, Yin JJ, Abou-Kheir W, Hynes PG, Casey OM, Fang L, et al. MiR-1 and miR-200 inhibit EMT via slug-dependent and tumorigenesis via slug-independent mechanisms. Oncogene (2013) 32:296–306. doi: 10.1038/onc.2012.58
91. Gandellini P, Giannoni E, Casamichele A, Taddei ML, Callari M, Piovan C, et al. miR-205 hinders the malignant interplay between prostate cancer cells and associated fibroblasts. Antioxid Redox Signaling (2013) 20:1045–59. doi: 10.1089/ars.2013.5292
92. Fan X, Chen X, Deng W, Zhong G, Cai Q, Lin T. Up-regulated microRNA-143 in cancer stem cells differentiation promotes prostate cancer cells metastasis by modulating FNDC3B expression. BMC Cancer (2013) 13:61. doi: 10.1186/1471-2407-13-61
93. Song X-L, Huang B, Zhou B-W, Wang C, Liao Z-W, Yu Y, et al. miR-1301-3p promotes prostate cancer stem cell expansion by targeting SFRP1 and GSK3β. Biomed Pharmacother (2018) 99:369–74. doi: 10.1016/j.biopha.2018.01.086
94. Du H, Wang X, Dong R, Hu D, Xiong Y. miR-601 inhibits proliferation, migration and invasion of prostate cancer stem cells by targeting KRT5 to inactivate the wnt signaling pathway. Int J Clin Exp Pathol (2019) 12:4361–79.
95. Guo Y, Zhang K, Cheng C, Ji Z, Wang X, Wang M, et al. Numb–/low enriches a castration-resistant prostate cancer cell subpopulation associated with enhanced notch and hedgehog signaling. Clin Cancer Res (2017) 23:6744–56. doi: 10.1158/1078-0432.CCR-17-0913
96. un J, Wang K, Teng J, Yu Y, Hua R, Zhou H, et al. Numb had anti-tumor effects in prostatic cancer. Biomed Pharmacother (2017) 92:108–15. doi: 10.1016/j.biopha.2017.04.134
97. Wang X, Yang J-Y, Cai J, Zhang D-J, Zhao L, Luo L-H, et al. MiR-543/Numb promotes proliferation, metastasis, and stem-like cell traits of prostate cancer cells. Am J Trans Res (2021) 13:617–31.
98. Wang X, Cai J, Zhao L, Zhang D, Xu G, Hu J, et al. NUMB suppression by miR-9-5P enhances CD44+ prostate cancer stem cell growth and metastasis. Sci Rep (2021) 11:11210. doi: 10.1038/s41598-021-90700-x
99. Li P, Xin H, Lu L. Extracellular vesicle-encapsulated microRNA-424 exerts inhibitory function in ovarian cancer by targeting MYB. J Trans Med (2021) 19:4. doi: 10.1186/s12967-020-02652-x
100. Wu X, Ruan Y, Jiang H, Xu C. MicroRNA-424 inhibits cell migration, invasion, and epithelial mesenchymal transition by downregulating doublecortin-like kinase 1 in ovarian clear cell carcinoma. Int J Biochem Cell Biol (2017) 85:66–74. doi: 10.1016/j.biocel.2017.01.020
101. Nandy SB, Orozco A, Lopez-Valdez R, Roberts R, Subramani R, Arumugam A, et al. Glucose insult elicits hyperactivation of cancer stem cells through miR-424–cdc42–prdm14 signalling axis. Br J Cancer (2017) 117:1665–75. doi: 10.1038/bjc.2017.335
102. Yamaji M, Ueda J, Hayashi K, Ohta H, Yabuta Y, Kurimoto K, et al. PRDM14 ensures naive pluripotency through dual regulation of signaling and epigenetic pathways in mouse embryonic stem cells. Cell Stem Cell (2013) 12:368–82. doi: 10.1016/j.stem.2012.12.012
103. Dallavalle C, Albino D, Civenni G, Merulla J, Ostano P, Mello-Grand M, et al. MicroRNA-424 impairs ubiquitination to activate STAT3 and promote prostate tumor progression. J Clin Invest (2016) 126:4585–602. doi: 10.1172/JCI86505
104. Albino D, Falcione M, Uboldi V, Temilola DO, Sandrini G, Merulla J, et al. Circulating extracellular vesicles release oncogenic miR-424 in experimental models and patients with aggressive prostate cancer. Commun Biol (2021) 4:119. doi: 10.1038/s42003-020-01642-5
105. Gan J, Liu S, Zhang Y, He L, Bai L, Liao R, et al. MicroRNA-375 is a therapeutic target for castration-resistant prostate cancer through the PTPN4/STAT3 axis. Exp Mol Med (2022) 54:1290–305. doi: 10.1038/s12276-022-00837-6
106. Baig N, Kammakakam I, Falath W. Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Mater Adv (2021) 2:1821–71. doi: 10.1039/D0MA00807A
107. Cifuentes-Rius A, Butler LM, Voelcker NH. Precision nanomedicines for prostate cancer. Nanomedicine (2018) 13:803–7. doi: 10.2217/nnm-2018-0034
108. Jana B, Kim D, Choi H, Kim M, Kim K, Kim S, et al. Drug resistance-free cytotoxic nanodrugs in composites for cancer therapy. J Mater Chem B (2021) 9:3143–52. doi: 10.1039/D0TB02850A
109. Jafari Malek S, Khoshchehreh R, Goodarzi N, Khoshayand MR, Amini M, Atyabi F, et al. Cis-dichlorodiamminoplatinum (II) glyconanoparticles by drug-induced ionic gelation technique targeted to prostate cancer: Preparation, optimization and in vitro characterization. Colloids Surfaces B: Biointerfaces (2014) 122:350–8. doi: 10.1016/j.colsurfb.2014.06.065
110. Zhou Y, Yang J, Zhang R, Kopeček J. Combination therapy of prostate cancer with HPMA copolymer conjugates containing PI3K/mTOR inhibitor and docetaxel. Eur J Pharmaceut Biopharmaceut (2015) 89:107–15. doi: 10.1016/j.ejpb.2014.11.025
111. Zhou Y, Yang J, Rhim JS, Kopeček J. HPMA copolymer-based combination therapy toxic to both prostate cancer stem/progenitor cells and differentiated cells induces durable anti-tumor effects. J Controlled Release (2013) 172:946–53. doi: 10.1016/j.jconrel.2013.09.005
112. Yang R, Mondal G, Wen D, Mahato RI. Combination therapy of paclitaxel and cyclopamine polymer-drug conjugates to treat advanced prostate cancer. Nanomed: Nanotechnol Biol Med (2017) 13:391–401. doi: 10.1016/j.nano.2016.07.017
113. Fiorillo M, Verre AF, Iliut M, Peiris-Pagés M, Ozsvari B, Gandara R, et al. Graphene oxide selectively targets cancer stem cells, across multiple tumor types: implications for non-toxic cancer treatment, via “differentiation-based nano-therapy”. Oncotarget (2015) 6:3553–62. doi: 10.18632/oncotarget.3348
114. Wang Y, Yang F, Zhang H-X, Zi X-Y, Pan X-H, Chen F, et al. Cuprous oxide nanoparticles inhibit the growth and metastasis of melanoma by targeting mitochondria. Cell Death Dis (2013) 4:e783–3. doi: 10.1038/cddis.2013.314
115. Wang Y, Yang Q-W, Yang Q, Zhou T, Shi M-F, Sun C-X, et al. Cuprous oxide nanoparticles inhibit prostate cancer by attenuating the stemness of cancer cells via inhibition of the wnt signaling pathway. Int J Nanomed (2017) 12:2569–79. doi: 10.2147/IJN.S130537
116. Chan JM, Zaidi S, Love JR, Zhao JL, Setty M, Wadosky KM, et al. Lineage plasticity in prostate cancer depends on JAK/STAT inflammatory signaling. Science (2022) 377:1180–91. doi: 10.1126/science.abn0478
117. Deng S, Wang C, Wang Y, Xu Y, Li X, Johnson NA, et al. Ectopic JAK–STAT activation enables the transition to a stem-like and multilineage state conferring AR-targeted therapy resistance. Nat Cancer (2022) 3:1071–87. doi: 10.1038/s43018-022-00431-9
118. Canesin G, Maggio V, Palominos M, Stiehm A, Contreras HR, Castellón EA, et al. STAT3 inhibition with galiellalactone effectively targets the prostate cancer stem-like cell population. Sci Rep (2020) 10:13958. doi: 10.1038/s41598-020-70948-5
119. Zhu M, Yu X, Zheng Z, Huang J, Yang X, Shi H. Capsaicin suppressed activity of prostate cancer stem cells by inhibition of wnt/β-catenin pathway. Phytother Res (2020) 34:817–24. doi: 10.1002/ptr.6563
120. Zhong D, Zhang H, Jiang Y, Wu P, Qi H, Cai C, et al. Saikosaponin-d: A potential chemotherapeutics in castration resistant prostate cancer by suppressing cancer metastases and cancer stem cell phenotypes. Biochem Biophys Res Commun (2016) 474:722–9. doi: 10.1016/j.bbrc.2016.05.017
121. Erdogan S, Turkekul K, Serttas R, Erdogan Z. The natural flavonoid apigenin sensitizes human CD44+ prostate cancer stem cells to cisplatin therapy. Biomed Pharmacother (2017) 88:210–7. doi: 10.1016/j.biopha.2017.01.056
122. Wang L, Stadlbauer B, Lyu C, Buchner A, Pohla H. Shikonin enhances the antitumor effect of cabazitaxel in prostate cancer stem cells and reverses cabazitaxel resistance by inhibiting ABCG2 and ALDH3A1. Am J Cancer Res (2020) 10:3784–800. doi: 10.21203/rs.3.rs-58823/v1
123. Liu C, Li Z, Bi L, Li K, Zhou B, Xu C, et al. NOTCH1 signaling promotes chemoresistance via regulating ABCC1 expression in prostate cancer stem cells. Mol Cell Biochem (2014) 393:265–70. doi: 10.1007/s11010-014-2069-4
124. Wang L, Zi H, Luo Y, Liu T, Zheng H, Xie C, et al. Inhibition of notch pathway enhances the anti-tumor effect of docetaxel in prostate cancer stem-like cells. Stem Cell Res Ther (2020) 11:258. doi: 10.1186/s13287-020-01773-w
125. Kumar D, Shankar S, Srivastava RK. Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett (2014) 343:179–89. doi: 10.1016/j.canlet.2013.10.003
126. Erdogan S, Turkekul K. Neferine inhibits proliferation and migration of human prostate cancer stem cells through p38 MAPK/JNK activation. J Food Biochem (2020) 44:e13253. doi: 10.1111/jfbc.13253
127. Bahmad HF, Samman H, Monzer A, Hadadeh O, Cheaito K, Abdel-Samad R, et al. The synthetic retinoid ST1926 attenuates prostate cancer growth and potentially targets prostate cancer stem-like cells. Mol Carcinogene (2019) 58:1208–20. doi: 10.1002/mc.23004
128. Aggarwal R, Starodub AN, Koh BD, Xing G, Armstrong AJ, Carducci MA. Phase ib study of the BET inhibitor GS-5829 as monotherapy and combined with enzalutamide in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res (2022) 28:3979–89. doi: 10.1158/1078-0432.CCR-22-0175
129. de Bono JS, Fleming MT, Wang JS, Cathomas R, Miralles MS, Bothos J, et al. Phase I study of MEDI3726: A prostate-specific membrane antigen-targeted antibody–drug conjugate, in patients with mCRPC after failure of abiraterone or enzalutamide. Clin Cancer Res (2021) 27:3602–9. doi: 10.1158/1078-0432.CCR-20-4528
130. Shore ND, Renzulli J, Fleshner NE, Hollowell CMP, Vourganti S, Silberstein J, et al. Enzalutamide monotherapy vs active surveillance in patients with low-risk or intermediate-risk localized prostate cancer: The ENACT randomized clinical trial. JAMA Oncol (2022) 8:1128–36. doi: 10.1001/jamaoncol.2022.1641
131. Freedland SJ, De Giorgi U, Gleave M, Rosbrook B, Shen Q, Sugg J, et al. A phase 3 randomised study of enzalutamide plus leuprolide and enzalutamide monotherapy in high-risk non-metastatic hormone-sensitive prostate cancer with rising PSA after local therapy: EMBARK study design. BMJ Open (2021) 11:e046588. doi: 10.1136/bmjopen-2020-046588
132. Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: Multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol (2020) 38:395–405. doi: 10.1200/JCO.19.01638
133. Cappuccini F, Bryant R, Pollock E, Carter L, Verrill C, Hollidge J, et al. Safety and immunogenicity of novel 5T4 viral vectored vaccination regimens in early stage prostate cancer: a phase I clinical trial. J Immunother Cancer (2020) 8. doi: 10.1136/jitc-2020-000928
134. de Bono JS, Mehra N, Scagliotti GV, Castro E, Dorff T, Stirling A, et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial. Lancet Oncol (2021) 22:1250–64. doi: 10.1016/S1470-2045(21)00376-4
135. Lim EA, Bendell JC, Falchook GS, Bauer TM, Drake CG, Choe JH, et al. Phase ia/b, open-label, multicenter study of AZD4635 (an adenosine A2A receptor antagonist) as monotherapy or combined with durvalumab, in patients with solid tumors. Clin Cancer Res (2022) 28:4871–84. doi: 10.1158/1078-0432.CCR-22-0612
136. Boevé LMS, Hulshof MCCM, Vis AN, Zwinderman AH, Twisk JWR, Witjes WPJ, et al. Effect on survival of androgen deprivation therapy alone compared to androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomised clinical tria. Eur Urol (2019) 75:410–8. doi: 10.1016/j.eururo.2018.09.008
137. Kyriakopoulos CE, Chen Y-H, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol (2018) 36:1080–7. doi: 10.1200/JCO.2017.75.3657
138. Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. New Engl J Med (2018) 378:1408–18. doi: 10.1056/NEJMoa1715546
139. Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. New Engl J Med (2022) 386:1132–42. doi: 10.1056/NEJMoa2119115
140. Attard G, Murphy L, Clarke NW, Cross W, Jones RJ, Parker CC, et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet (2022) 399:447–60. doi: 10.1016/S0140-6736(21)02437-5
141. Vaishampayan UN, Heilbrun LK, Monk IIIP, Tejwani S, Sonpavde G, Hwang C, et al. Clinical efficacy of enzalutamide vs bicalutamide combined with androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: A randomized clinical trial. JAMA Network Open (2021) 4:e2034633–e2034633. doi: 10.1001/jamanetworkopen.2020.34633
142. Schöffski P, Tan DSW, Martín M, Ochoa-de-Olza M, Sarantopoulos J, Carvajal RD, et al. Phase I/II study of the LAG-3 inhibitor ieramilimab (LAG525) ± anti-PD-1 spartalizumab (PDR001) in patients with advanced malignancies. J Immunother Cancer (2022) 10. doi: 10.1136/jitc-2021-003776
143. Graff JN, Beer TM, Alumkal JJ, Slottke RE, Redmond WL, Thomas GV, et al. A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone. J Immunother Cancer (2020) 8. doi: 10.1136/jitc-2020-000642
144. Handa S, Hans B, Goel S, Bashorun HO, Dovey Z, Tewari A. Immunotherapy in prostate cancer: current state and future perspectives. Ther Adv Urol (2020) 12:1756287220951404. doi: 10.1177/1756287220951404
145. Dannull J, Diener P-A, Prikler L, Fürstenberger G, Cerny T, Schmid U, et al. Prostate stem cell antigen is a promising candidate for immunotherapy of advanced prostate Cancer1. Cancer Res (2000) 60:5522–8.
146. Lam JS, Yamashiro J, Shintaku IP, Vessella RL, Jenkins RB, Horvath S, et al. Prostate stem cell antigen is overexpressed in prostate cancer metastases. Clin Cancer Res (2005) 11:2591–6. doi: 10.1158/1078-0432.CCR-04-1842
147. de la Luz Garcia-Hernandez M, Gray A, Hubby B, Klinger OJ, Kast WM. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res (2008) 68:861–9. doi: 10.1158/0008-5472.CAN-07-0445
148. Li S, Goncalves KA, Lyu B, Yuan L, Hu G. Chemosensitization of prostate cancer stem cells in mice by angiogenin and plexin-B2 inhibitors. Commun Biol (2020) 3:26. doi: 10.1038/s42003-020-0750-6
149. Wang Z, Li Y, Wang Y, Wu D, Lau AHY, Zhao P, et al. Targeting prostate cancer stem-like cells by an immunotherapeutic platform based on immunogenic peptide-sensitized dendritic cells-cytokine-induced killer cells. Stem Cell Res Ther (2020) 11:123. doi: 10.1186/s13287-020-01634-6
150. Seki T, Shimizu Y, Ishii K, Takahama Y, Kato K, Yano T. NK cells can preferentially target prostate cancer stem-like cells via the TRAIL/DR5 signaling pathway. Biomolecules (2021) 11. doi: 10.3390/biom11111702
151. Yu H, Pan J, Guo Z, Yang C, Mao L. CART cell therapy for prostate cancer: status and promise. OncoTargets Ther (2019) 12:391–5. doi: 10.2147/OTT.S185556
152. Deng Z, Wu Y, Ma W, Zhang S, Zhang Y-Q. Adoptive T-cell therapy of prostate cancer targeting the cancer stem cell antigen EpCAM. BMC Immunol (2015) 16:1. doi: 10.1186/s12865-014-0064-x
153. Zhang Y, He L, Sadagopan A, Ma T, Dotti G, Wang Y, et al. Targeting radiation-resistant prostate cancer stem cells by B7-H3 CAR T cells. Mol Cancer Ther (2021) 20:577–88. doi: 10.1158/1535-7163.MCT-20-0446
154. Hillerdal V, Ramachandran M, Leja J, Essand M. Systemic treatment with CAR-engineered T cells against PSCA delays subcutaneous tumor growth and prolongs survival of mice. BMC Cancer (2014) 14:30. doi: 10.1186/1471-2407-14-30
155. Escudero-Lourdes C, Alvarado-Morales I, Tokar EJ. Stem cells as target for prostate cancer therapy: Opportunities and challenges. Stem Cell Rev Rep (2022). doi: 10.1007/s12015-022-10437-6
156. Li JJ, Shen MM. Prostate stem cells and cancer stem cells. Cold Spring Harbor Perspect Med (2019) 9. doi: 10.1101/cshperspect.a030395
157. Kushwaha PP, Verma S, Kumar S, Gupta S. Role of prostate cancer stem-like cells in the development of antiandrogen resistance. Cancer Drug Resistance (Alhambra Calif) (2022) 5:459–71. doi: 10.20517/cdr.2022.07
158. Han L, Shi S, Gong T, Zhang Z, Sun X. Cancer stem cells: therapeutic implications and perspectives in cancer therapy. Acta Pharm Sin B (2013) 3:65–75. doi: 10.1016/j.apsb.2013.02.006
159. Balon K, Sheriff A, Jacków J, Łaczmański Ł. Targeting cancer with CRISPR/Cas9-based therapy. Int J Mol Sci (2022) 23. doi: 10.3390/ijms23010573
160. Yip BH. Recent advances in CRISPR/Cas9 delivery strategies. Biomolecules (2020) 10. doi: 10.3390/biom10060839
161. Tsujino T, Komura K, Inamoto T, Azuma H. CRISPR screen contributes to novel target discovery in prostate cancer. Int J Mol Sci (2021) 22. doi: 10.3390/ijms222312777
162. Fei T, Chen Y, Xiao T, Li W, Cato L, Zhang P, et al. Genome-wide CRISPR screen identifies HNRNPL as a prostate cancer dependency regulating RNA splicing. Proc Natl Acad Sci (2017) 114:E5207–15. doi: 10.1073/pnas.1617467114
163. ?>Das R, Sjöström M, Shrestha R, Yogodzinski C, Egusa EA, Chesner LN, et al. An integrated functional and clinical genomics approach reveals genes driving aggressive metastatic prostate cancer. Nat Commun (2021) 12:4601. doi: 10.1038/s41467-021-24919-7
164. Lei H, Wang Z, Jiang D, Liu F, Liu M, Lei X, et al. CRISPR screening identifies CDK12 as a conservative vulnerability of prostate cancer. Cell Death Dis (2021) 12:740. doi: 10.1038/s41419-021-04027-6
165. Rahimi S, Roushandeh AM, Ebrahimi A, Samadani AA, Kuwahara Y, Roudkenar MH. CRISPR/Cas9-mediated knockout of Lcn2 effectively enhanced CDDP-induced apoptosis and reduced cell migration capacity of PC3 cells. Life Sci (2019) 231:116586. doi: 10.1016/j.lfs.2019.116586
166. Rushworth LK, Harle V, Repiscak P, Clark W, Shaw R, Hall H, et al. In vivo CRISPR/Cas9 knockout screen: TCEAL1 silencing enhances docetaxel efficacy in prostate cancer. Life Sci Alliance (2020) 3:e202000770. doi: 10.26508/lsa.202000770
167. Palit SA, Vis D, Stelloo S, Lieftink C, Prekovic S, Bekers E, et al. TLE3 loss confers AR inhibitor resistance by facilitating GR-mediated human prostate cancer cell growth. eLife (2019) 8. doi: 10.7554/eLife.47430
168. Palit SAL, van Dorp J, Vis D, Lieftink C, Linder S, Beijersbergen R, et al. A kinome-centered CRISPR-Cas9 screen identifies activated BRAF to modulate enzalutamide resistance with potential therapeutic implications in BRAF-mutated prostate cancer. Sci Rep (2021) 11:13683. doi: 10.1038/s41598-021-93107-w
169. Kawamura N, Nimura K, Nagano H, Yamaguchi S, Nonomura N, Kaneda Y. CRISPR/Cas9-mediated gene knockout of NANOG and NANOGP8 decreases the malignant potential of prostate cancer cells. Oncotarget (2015) 6:22361–74. doi: 10.18632/oncotarget.4293
170. Zhen S, Takahashi Y, Narita S, Yang Y-C, Li X. Targeted delivery of CRISPR/Cas9 to prostate cancer by modified gRNA using a flexible aptamer-cationic liposome. Oncotarget (2017) 8:9375–87. doi: 10.18632/oncotarget.14072
171. Salimi M, Mosca S, Gardner B, Palombo F, Matousek P, Stone N. Nanoparticle-mediated photothermal therapy limitation in clinical applications regarding pain management. Nanomaterials (2022) 12. doi: 10.3390/nano12060922
172. Lin T-C, Lin F-H, Lin J-C. In vitro feasibility study of the use of a magnetic electrospun chitosan nanofiber composite for hyperthermia treatment of tumor cells. Acta Biomater (2012) 8:2704–11. doi: 10.1016/j.actbio.2012.03.045
173. Rastinehad AR, Anastos H, Wajswol E, Winoker JS, Sfakianos JP, Doppalapudi SK, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Natl Acad Sci (2019) 116:18590–6. doi: 10.1073/pnas.1906929116
174. Kim J, Chun SH, Amornkitbamrung L, Song C, Yuk JS, Ahn SY, et al. Gold nanoparticle clusters for the investigation of therapeutic efficiency against prostate cancer under near-infrared irradiation. Nano Convergence (2020) 7:5. doi: 10.1186/s40580-019-0216-z
175. Yang S, Yao D, Wang Y, Yang W, Zhang B, Wang D. Enzyme-triggered self-assembly of gold nanoparticles for enhanced retention effects and photothermal therapy of prostate cancer. Chem Commun (2018) 54:9841–4. doi: 10.1039/C8CC05136D
176. Avvakumova S, Galbiati E, Sironi L, Locarno SA, Gambini L, Macchi C, et al. Theranostic nanocages for imaging and photothermal therapy of prostate cancer cells by active targeting of neuropeptide-y receptor. Bioconjugate Chem (2016) 27:2911–22. doi: 10.1021/acs.bioconjchem.6b00568
177. Ruscica M, Dozio E, Boghossian S, Bovo G, Martos Rianão V, Motta M, et al. Activation of the Y1 receptor by neuropeptide y regulates the growth of prostate cancer cells. Endocrinology (2006) 147:1466–73. doi: 10.1210/en.2005-0925
178. Gobin AM, Moon JJ, West JL. EphrinA I-targeted nanoshells for photothermal ablation of prostate cancer cells. Int J Nanomed (2008) 3:351–8.
179. Lakshmanan V-K, Jindal S, Packirisamy G, Ojha S, Lian S, Kaushik A, et al. Nanomedicine-based cancer immunotherapy: recent trends and future perspectives. Cancer Gene Ther (2021) 28:911–23. doi: 10.1038/s41417-021-00299-4
Keywords: prostate cancer, cancer stem cells, immunotherapy, chemotherapy, CRISPR, nanotechnology, photothermal ablation therapy
Citation: Ramesh S, Selvakumar P, Ameer MY, Lian S, Abdullah Alzarooni AIM, Ojha S, Mishra A, Tiwari A, Kaushik A, Jung YD, Chouaib S and Lakshmanan V-K (2023) State-of-the-art therapeutic strategies for targeting cancer stem cells in prostate cancer. Front. Oncol. 13:1059441. doi: 10.3389/fonc.2023.1059441
Received: 01 October 2022; Accepted: 30 January 2023;
Published: 09 March 2023.
Edited by:
Saji Uthaman, Iowa State University, United StatesReviewed by:
Eswar Shankar, The Ohio State University, United StatesSanthosh Kalash Rajendrakumar, University of Warwick, United Kingdom
Copyright © 2023 Ramesh, Selvakumar, Ameer, Lian, Abdullah Alzarooni, Ojha, Mishra, Tiwari, Kaushik, Jung, Chouaib and Lakshmanan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vinoth-Kumar Lakshmanan, vinoth.lakshmanan@sriramachandra.edu.in
†ORCID: Vinoth-Kumar Lakshmanan, https://orcid.org/0000-0002-7420-8459
Anshuman Mishra, https://orcid.org/0000-0002-9390-9971
Ashutosh Tiwari, orcid.org/0000-0001-5634-7749
 Saravanan Ramesh1
Saravanan Ramesh1 Anshuman Mishra
Anshuman Mishra Ashutosh Tiwari
Ashutosh Tiwari Vinoth-Kumar Lakshmanan
Vinoth-Kumar Lakshmanan