- 1Department of Oncology, Affiliated Hospital of Guizhou Medical University, Guiyang, China
- 2Department of Clinical Medicine, Guizhou Medical University, Guiyang, China
- 3Department of Abdominal Oncology, Affiliated Cancer Hospital of Guizhou Medical University, Guiyang, China
Background: Fluoropyrimidine is an important component of systemic chemotherapy for colorectal cancer (CRC). Fluoropyrimidine-induced cardiotoxicity (FIC) may result in delay and discontinuation of chemotherapy and, in severe cases, can even be life-threatening. To date, risk factors for FIC have not been well identified. This cohort study aimed to identify the predictors of FIC in CRC patients and develop a risk prediction nomogram model.
Methods: Between January 1, 2018 and December 31, 2020, colorectal cancer patients who received 5-fluoropyrimidine(5-Fu)/capecitabine-based chemotherapy in Affiliated Cancer Hospital of Guizhou Medical University were included. FIC was defined as an adverse cardiovascular event related to fluoropyrimidine that occurred during or within four weeks of completing chemotherapy. Risk factors were determined by LASSO algorithm and multivariate logistic regression analysis. Nomogram for predicting 5-Fu-induced cardiotoxicity was established and internally validated. The concordance index (C-index) and calibration curve were used to evaluate the nomogram’s discrimination and accuracy.
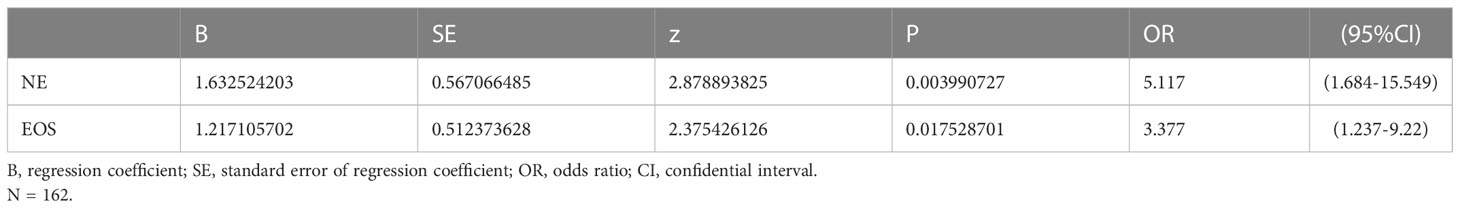
Results: A total of 916 patients were included for analysis, and 200 [21.8%,95% confidence interval (CI):19.12%-24.47%] experienced FIC. LASSO algorithm and multivariate logistic regression analysis determined that chemotherapy ≤3 cycles (OR=4.694, 95%CI=3.184-6.92), age≥ 60 (OR=1.678, 95%CI=1.143-2.464), BMI>22.97 (OR=1.77, 95%CI=1.202-2.606), and simultaneous use of bevacizumab (OR=2.922, 95%CI=1.835-4.653) were significant risk factors, and were included in the prediction model for 5-Fu induced cardiotoxicity. The C-index (95%CI) was 0.751 (0.706-0.795) by internal validation. For patients treated with capecitabine-based regimen, the incidence of FIC increased with the absolute value of neutrophils (OR=5.177, 95%CI=1.684-15.549) and eosinophils (OR=3.377,95% CI=1.237-9.22).
Conclusions: Our study identified risk factors for FIC and established a prediction nomogram model based on chemotherapy cycle, age, BMI and use of target therapy for 5-FU induced Cardiotoxicity. The discriminative prediction model can be used for patient counselling and risk-stratification before undergoing chemotherapy in colorectal cancer.
Background
Globally, colorectal cancer (CRC) is the third most common cancer with the second highest mortality rate (1). Chemotherapy is a critical part of colorectal cancer treatment, significantly reducing the risk of disease recurrence and mortality, and ultimately improving the quality of life (2). Nevertheless, chemotherapeutic agents can cause both reversible and irreversible cardiotoxicity, ultimately increasing the incidence of cardiovascular diseases (2–4). The mortality of cardiovascular diseases currently ranks second in tumor patients, exceeded only by tumor recurrence (5). Fluoropyrimidine remains a cornerstone of chemotherapy for colorectal cancer, which is principally administered as either 5-fluorouracil (5-FU) or its oral prodrug, capecitabine (6). Fluoropyrimidine-induced cardiotoxicity (FIC), the incidence of which is only exceeded by anthracyclines, can affect coronary blood flow or directly damage the myocardium, leading to myocardial infarction, cardiomyopathy, cardiogenic shock or even death (7–10). FIC not only can be life-threatening, but also leads directly to the withdrawal of patients from the optimal chemotherapeutic regimen. Therefore, risk evaluation of FIC is essential for clinical treatment and management. Here, we aimed to identify the incidence and predictors of FIC in a large sample of patients with colorectal cancer and to develop and validate a prediction nomogram model intended to assist clinicians in assessing the risk of FIC in this population.
Patients and methods
Selection of patients
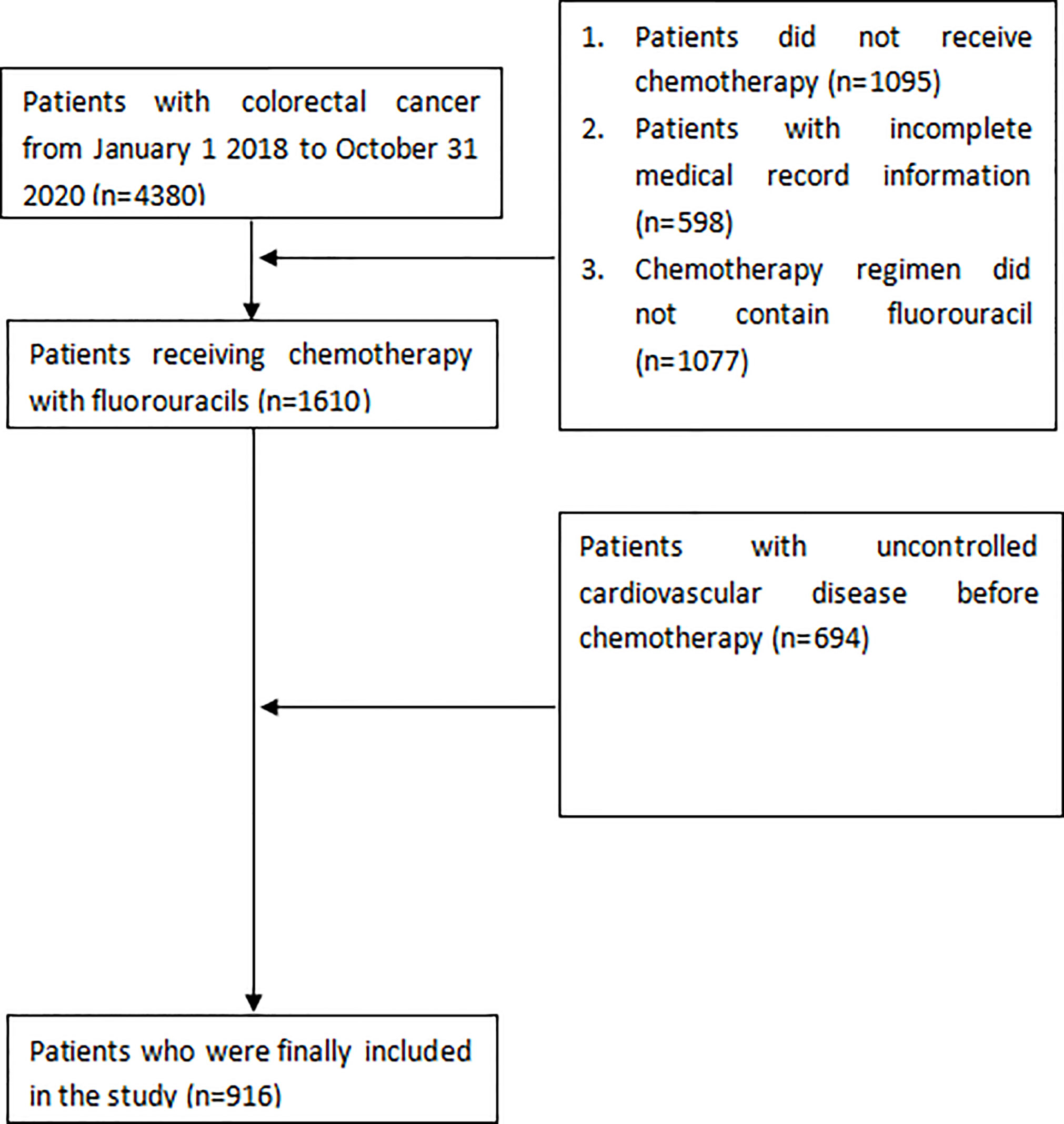
The study was approved by the institutional ethics committee of the Affiliated Cancer Hospital of Guizhou Medical University (FZ 2021-11-298) and the institutional ethics committee waived the requirement for informed consent given the deidentified data. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. Patients who were diagnosed with colorectal adenocarcinoma and received 5-Fu/capecitabine-based chemotherapy at Affiliated Cancer Hospital of Guizhou Medical University from January 1, 2018 to December 31, 2020 were included. Similar to the approach used in prior studies, the inclusion criteria were as follows: (1) pathologically confirmed colon or rectal adenocarcinoma; (2) no severe medical complication prior to treatment (uncontrolled serious high blood pressure, acute stage of myocardial infarction, unstable angina pectoris, uncorrected severe cardiac insufficiency, or serious arrhythmia); (3) normal blood count, hepatorenal function, and electrocardiogram, and left ventricular ejection fraction (LVEF)≥50%; and (4) received fluoropyrimidine-based chemotherapy (11, 12).
Variables
Collected data included demographic factors, medical complications prior to treatment, laboratory tests within 3 days before treatment, smoking and alcohol history, treatment regimen and cycle, and Cancer staging based on the 8th edition of the American Joint Committee on Cancer (AJCC)/TNM system (13). The medical complications we focused on were recorded in electronic medical record system, mainly including: history of cardiovascular disease (acute myocardial infarction, ischemic heart disease, high blood pressure, cardiac failure or stroke), history of endocrinopathy (diabetes, hyperlipidemia, hyperthyroidism and hypothyroidism); in our study, it is represented by yes or no; Results of laboratory tests recorded in electronic medical record system, containing blood count, hepatorenal function, and electrocardiogram, and left ventricular ejection were obtained.
FIC was defined as significant symptoms of likely cardiac origin (chest pain, palpitations, dyspnoea, or cardiogenic shock), electrocardiographic abnormalities (ST-T changes or arrhythmia), abnormal echocardiogram, or obvious elevation of myocardial enzymes that developed during treatment (14–18). These changes occurred during 5-FU/capecitabine treatment or within 4 weeks following completion of treatment but were not present before treatment. Cases of suspected FIC were further reviewed by cardiologists who rendered the final decision of whether FIC was diagnosed. FIC was graded according to Common Terminology Criteria for Adverse Events (CTCAE) (19) for both 5-FU and capecitabine groups.
Statistical analysis
Categorical data were presented as numbers and percentages. For continuous variables, the value of normal distribution was expressed as mean± SD, and the value of non-normal distribution as medians and interquartile ranges (IQRs). The χ2 test or Fisher’s exact tests were used for univariate analysis. The least absolute shrinkage and selection operator (LASSO) regression method was employed for predictor selection. Significant variables in the LASSO analysis were subjected to multivariate logistic regression analysis by backward stepwise selection under the Akaike information criterion (AIC) (20). Receiver operating characteristic (ROC) curve was used to determine the cut-off value of continuous variables. Continuous variables were divided into high or low groups according to the optimal cut-off value. Variables that were statistically significant in the multivariate logistic regression analysis were determined as independent risk factors of the FIC. Nomogram was constructed according to the logistic regression analysis. The concordance index (C-index) applied to evaluate the discriminatory ability of the nomogram, was used to evaluate discriminative ability. C-index values greater than 0.7 suggested a reasonable estimation (19). The calibration curve was used to evaluate the nomogram’s accuracy. Statistical analyses were performed using R software (version 3.6.3; https://www.r-project.org). A value of P <0.05 was considered statistically significant.
Results
Patient characteristics
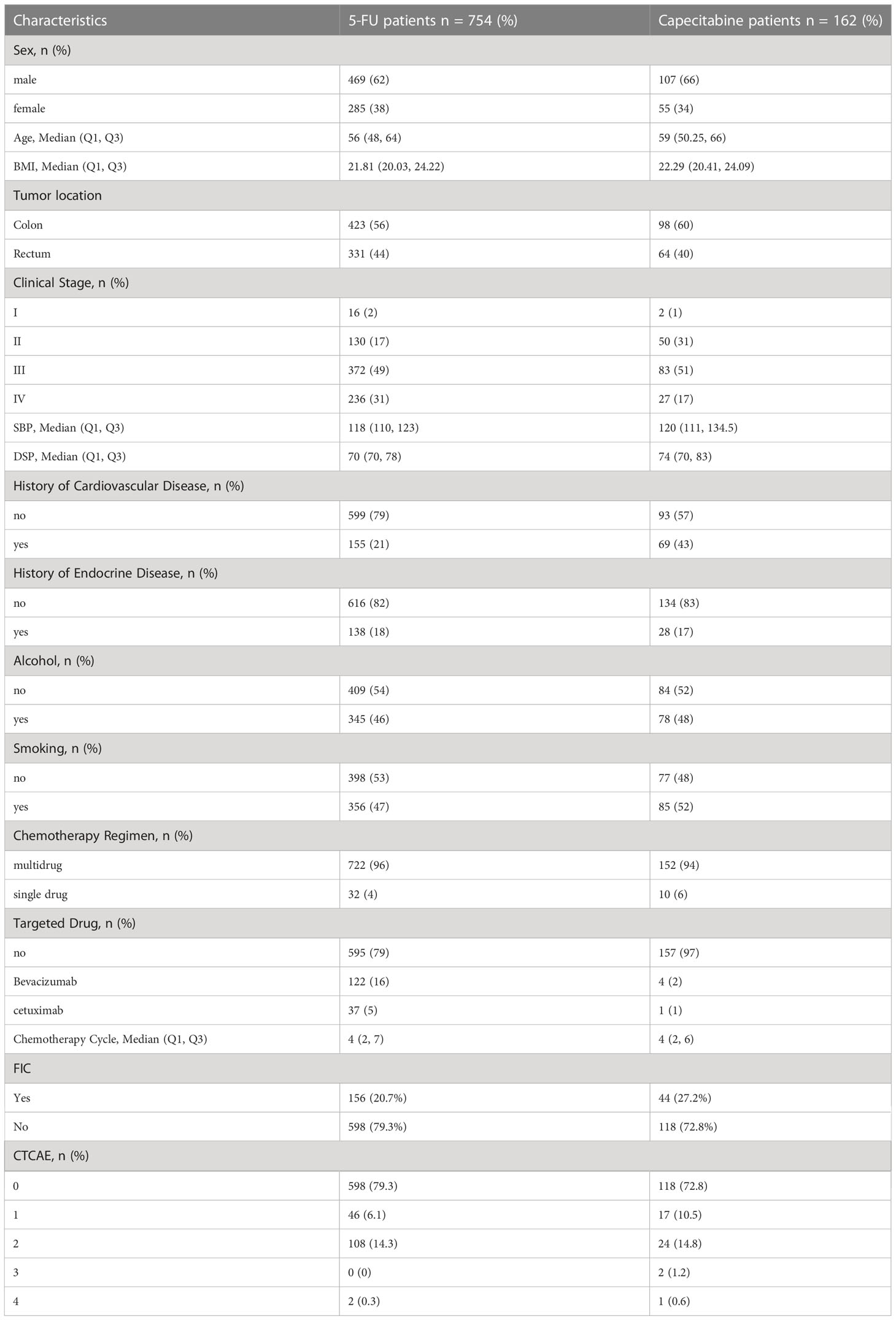
We reviewed 4380 patients diagnosed as colorectal cancer in Affiliated Cancer Hospital of Guizhou Medical University between January 1, 2018, and December 31, 2020. Figure 1 shows the flow chart for enrolment. The study finally included 916 patients, of whom 754 (82.3%) received the 5-FU-based chemotherapy regimen, and 162 (17.7%) received capecitabine-based chemotherapy. Patients’ characteristics prior to treatment are shown in Table 1.
Incidence and presentation of FIC
FIC was diagnosed in 200 patients (21.8%), of whom 156 (78%) occurred in the 5-FU group and 44 (22%) in the capecitabine group. In the 5-FU group, 66/156 (42.3%) experienced arrhythmia (nodal tachycardia, bradycardia, atrioventricular block, premature beat, atrial fibrillation or ventricular fibrillation); 56/156 (35.9%) suffered chest pain and breathing difficulties; 32/156 (20.5%) manifested only ST-T changes (ST-T elevation or depression); and 2/156 (1.3%) suffered myocardial infarction. While in the capecitabine group, 22/44 (50%) had arrhythmia, 10/44 (22.7%) experienced ST-T changes, 9/44 (20.5%) had chest pain and breathing difficulties, 1/44 (2.3%) suffered shock, and 2/44 (4.5%) had myocardial infarction (Figure 2).
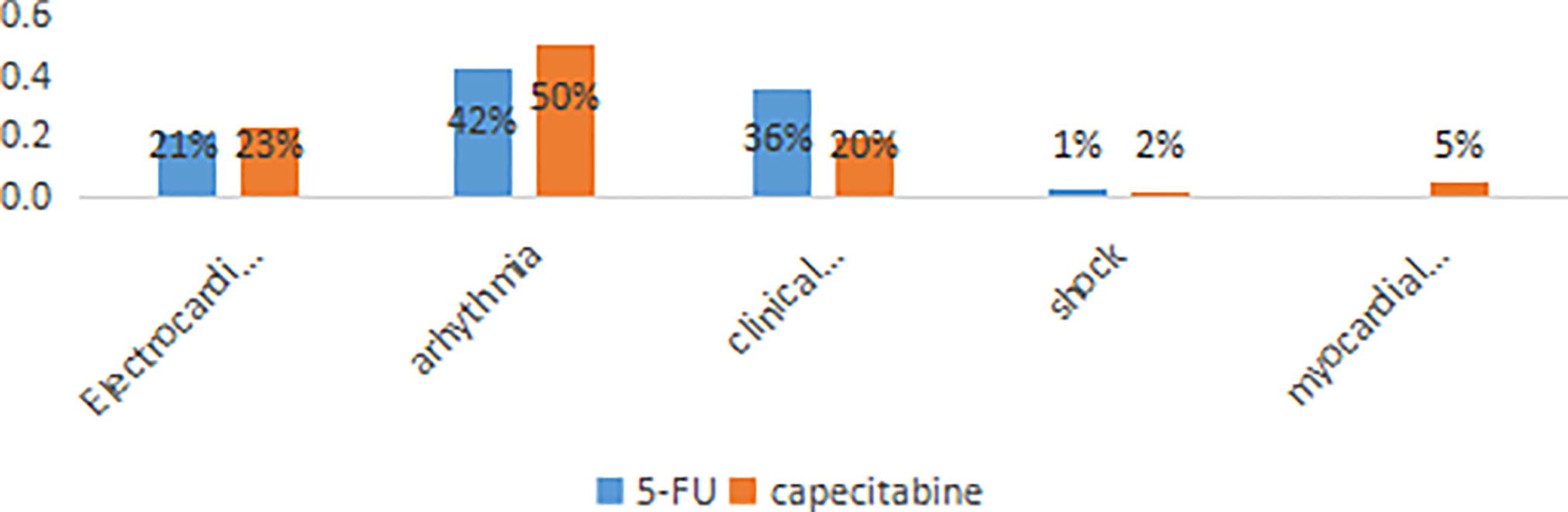
Figure 2 The distribution of the different manifestations of cardiotoxicity. The percentages shown on each bar reflect the distribution of symptoms among the 200 patients with cardiotoxicity.
Univariate analysis
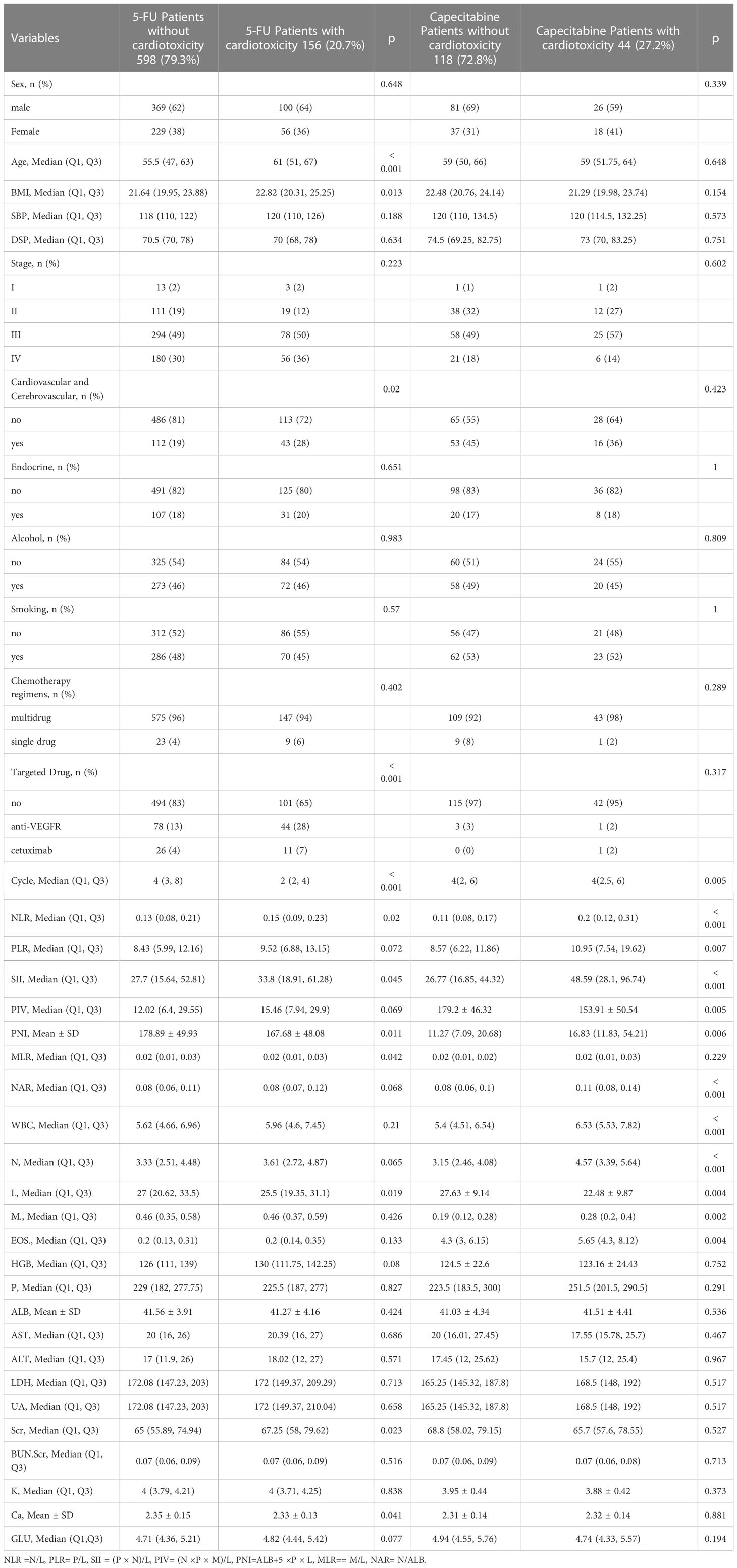
The results of the univariate logistics analyses are listed in Table 2. The variables significantly associated with FIC (p<0.05) in 5-FU group were age, BMI, past history of cardiovascular disease, number of chemotherapy cycles, treatment with targeted drugs, neutrophils(NE), systemic immune-inflammation index(SII), neutrophil to lymphocyte ratio(NLR), creatinine and calcium levels. In the capecitabine group, chemotherapy cycle, white blood cells(WBC), neutrophils(NE), eosinophils(Eos), monocytes(M), NLR, prognostic nutritional index (PNI), SII, Pan-Immune-Inflammation Value(PIV), and neutrophil-to-albumin ratio(NAR) were correlated with FIC (p<0.05).
Multivariate analysis
The significant continuous variables were converted to categorical variables according to the ROC curve. The optimal cut-off points are shown in Appendix 1. The LASSO analysis suggested that chemotherapy cycle, WBC, NLR, PIV, NE, and Eos were associated with 5-Fu induced cardiotoxicity. While in the capecitabine group, age, BMI, chemotherapy cycle, and targeted drugs were correlated with capecitabine induced cardiotoxicity (Figure 3). Subsequently, all VIFs (Variance Inflation Factor, VIF) were found to be less than ten, which means there existed no collinearity among variables (Figure 3).
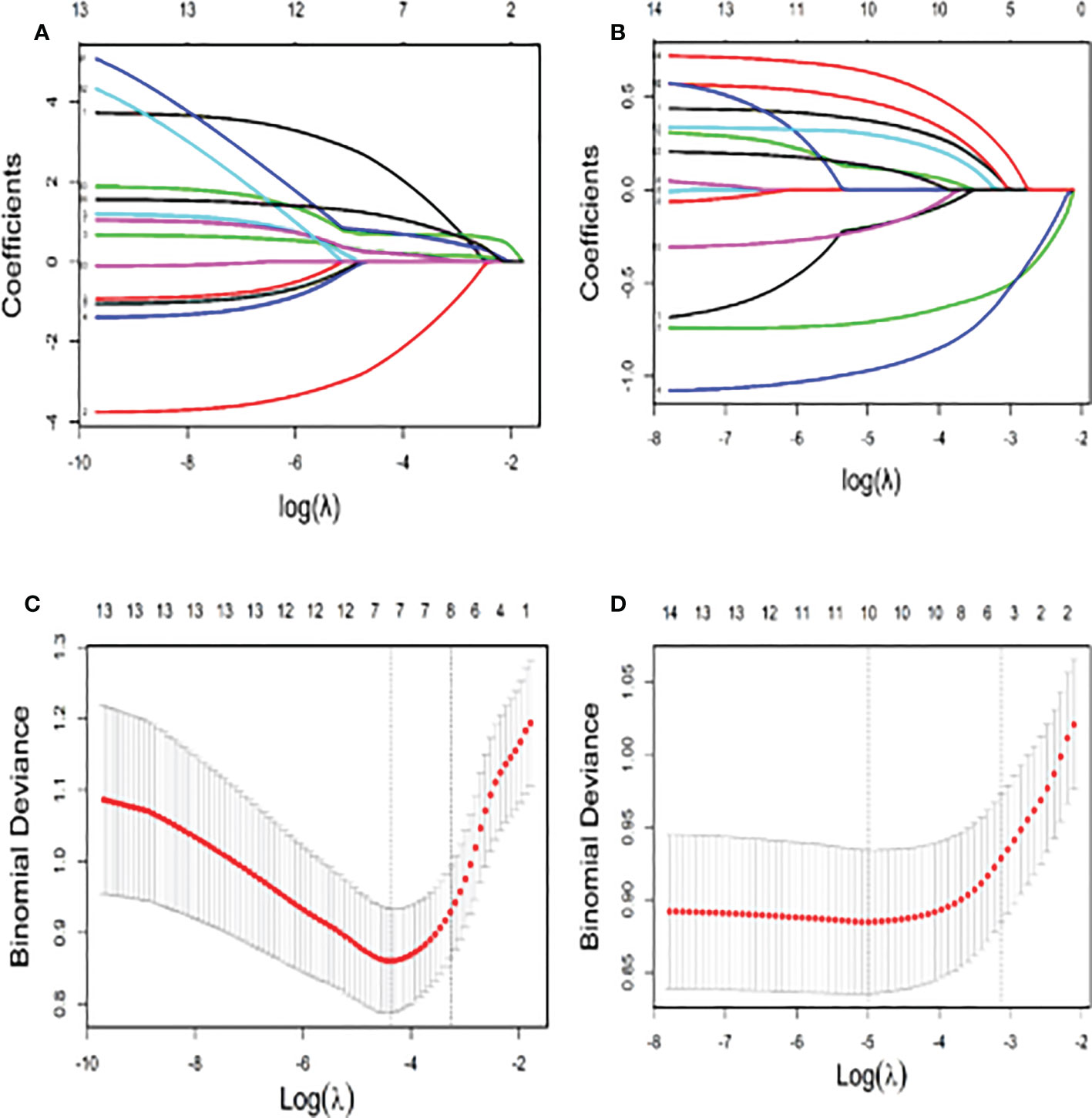
Figure 3 The Lasso Model for Screening of Factors Related to Cardiotoxicity (A) The number of variables screened by the 10-fold cross validation in the capecitabin chemotherapy group; (B) The number of variables screened by the 10-fold cross validation in the 5-fluorouracil chemotherapy group; (C) The coefficient shrink path in the capecitabin chemotherapy group; (D) The coefficient shrink path in the capecitabin chemotherapy group).
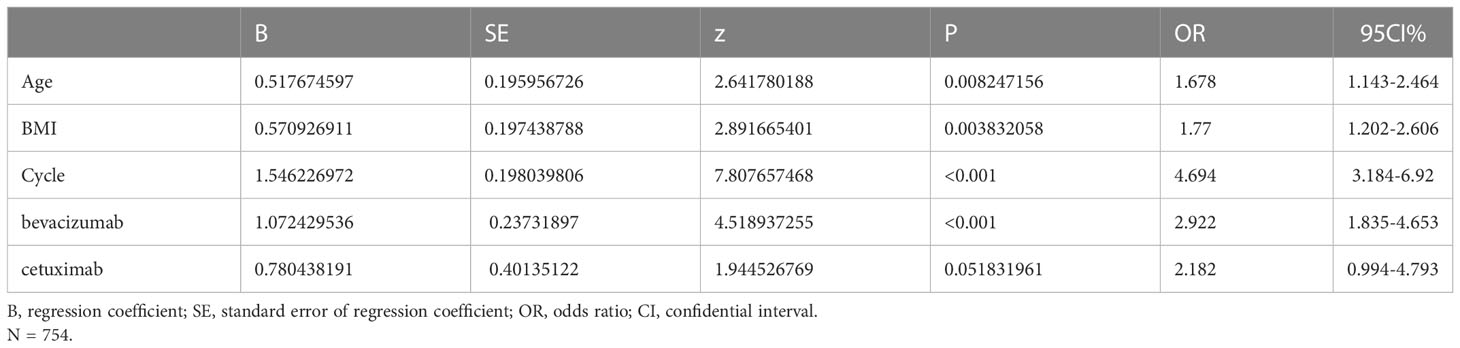
As shown in Table 3, the independent risk factors for FIC identified by multivariate logistic regression analysis in the 5-FU group were: number of chemotherapy cycle ≤ 3 (OR=4.694,95% CI=3.184-6.92, p<0.001), age>60 years old (OR=1.678, 95%CI=1.143-2.464, p=0.008), BMI≥22.97 (OR=1.77, 95%CI=1.202-2.606, p=0.004), and use of bevacizumab (OR=2.922, 95%CI=1.835-4.653, p<0.001. The risk of FIC tended to be higher in patients receiving cetuximab (OR=2.18, 95% CI=0.994-4.793, p=0.051). While in the capecitabine group, the risk of FIC increased by 3.377-fold (OR=3.377, 95%CI=1.237-9.22, p=0.018) and 5.177-fold (OR=5.177,95%CI=1.684-15.549, p=0.004) per unit increase in eosinophils and neutrophils, respectively (Table 4).
Construction and internal validation of nomograms
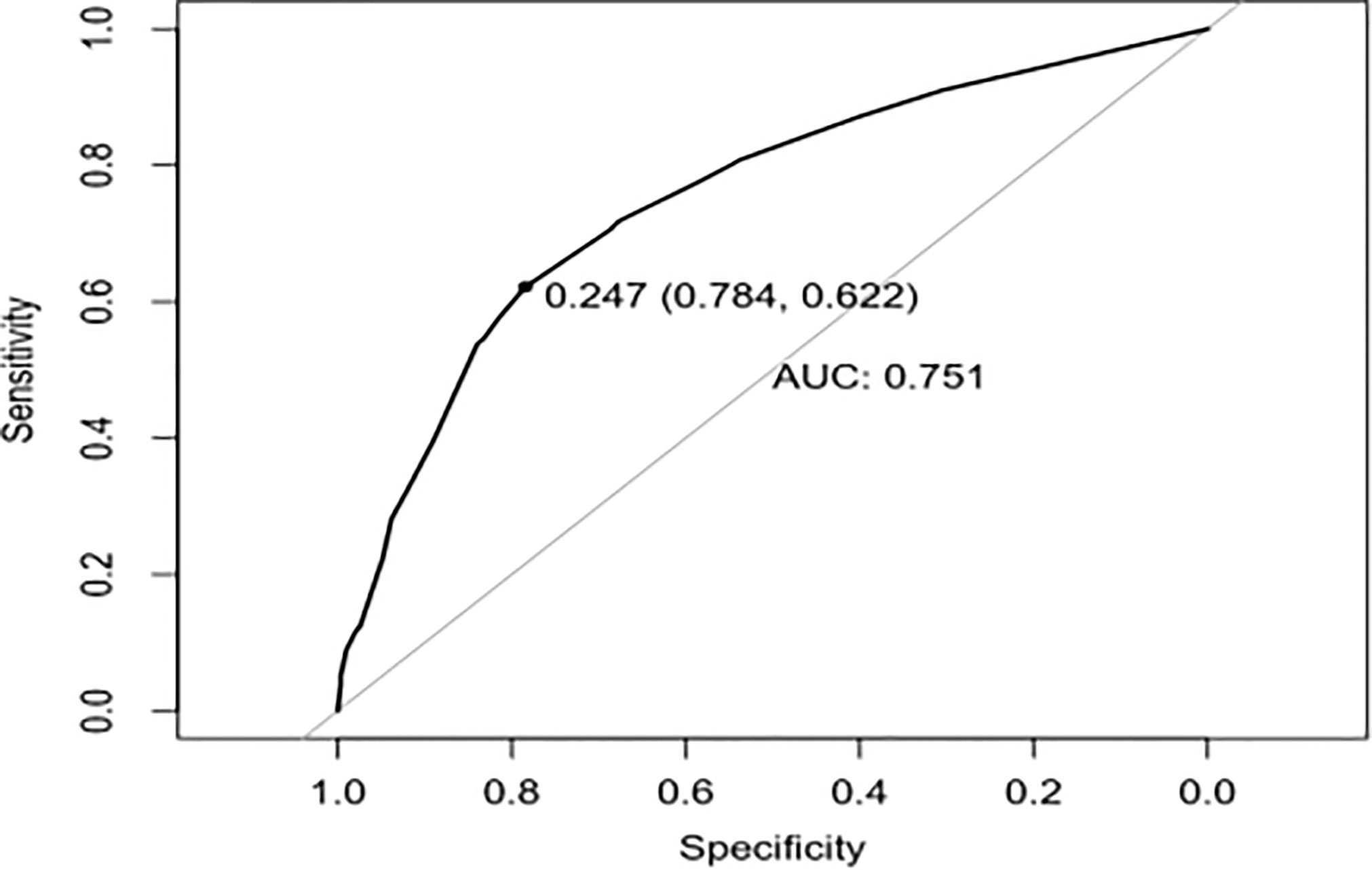
To provide physicians with a quantitative tool for individualized prediction of 5-fluorouracil induced cardiotoxicity, a nomogram was constructed according to results of multivariable logistic regression (Figure 4). The point score for each parameter was obtained through correlation of each variable to the value of the small ruler (top line of the Nomogram). Each predictive variable had its corresponding score on the points scale. The individual scores corresponding to the independent predictive variables were added to get the total score. The higher the total score, the higher the risk of FIC. Then a perpendicular line was drawn from the total points scale to the probability of FIC on the lowest rule to obtain the probability of cardiotoxicity occurring with an individual patient. The predictive accuracy of this model was then assessed, and the AUC of the nomogram was 0.751. A bootstrap resampling was then used to evaluate the efficacy of the constructed nomogram model, the C-index (95%CI) of the model was 0.751 (0.706-0.795) through the internal validation, which demonstrated the discriminatory ability of this model (Figure 5). The calibration curve were used to evaluate the nomogram’s accuracy (Figure 6). A Nomogram was not constructed for the capecitabine group as the sample size was deemed insufficient.
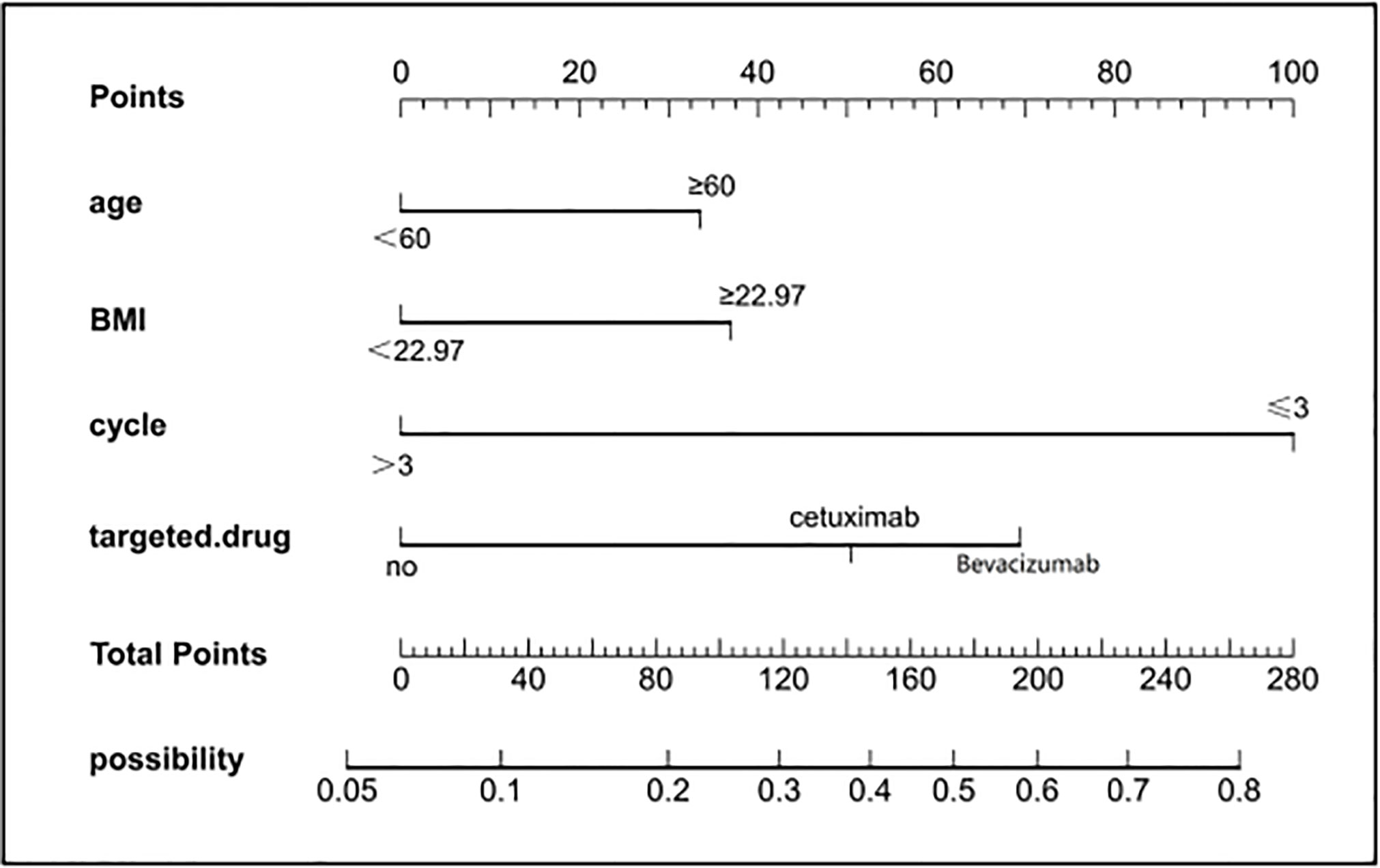
Figure 4 Nomogram for prediction of FIC risk and its predictive performance. First, find the points for each predictor (variable) of a patient on the uppermost rule; then, add all points to calculate the “total points” finally find the corresponding predicted probability of FIC on the lowest rule.
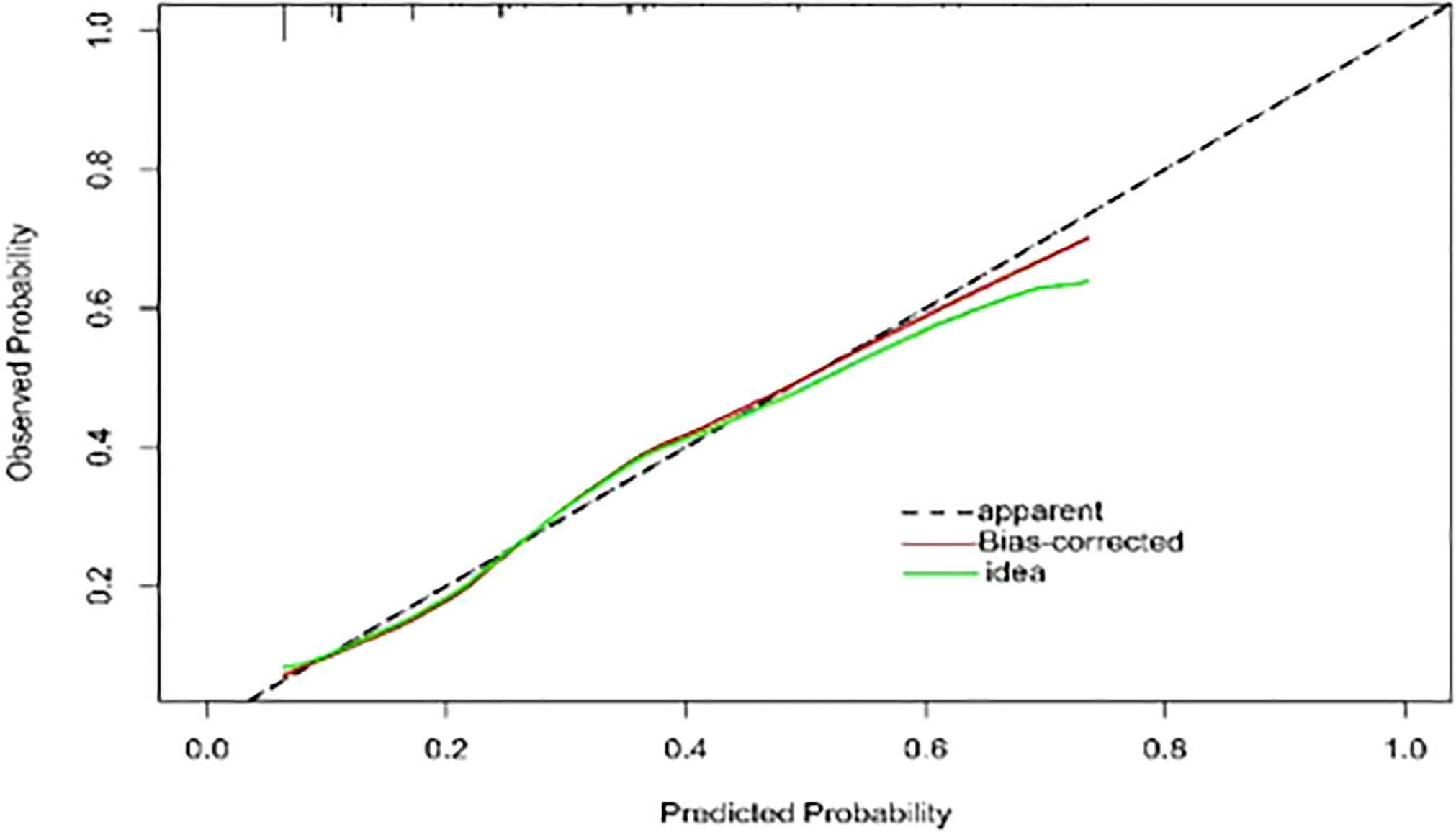
Figure 6 Calibration curve of the model. The calibration of the model in line with the agreement between predicted and observed outcomes of FIC. The Y-axis represents the actual FIC rate. The X-axis represents the predicted risk of FIC. The dotted line represents a perfect prediction by an ideal model. The red line represents the performance of the model, with a closer fit to the shadow line representing a better prediction. FIC, The fluoropyrimidine-induced cardiotoxicity.
Discussion
In the present retrospective cohort study, we evaluated the high-risk factors for FIC among colorectal cancer patients. We found that in the patients using 5-FU-based chemotherapy, FIC was more likely to occur in the early duration of chemotherapy (chemotherapy cycle ≤3), in patients with age ≥60, BMI≥22.97, or use of targeted drugs. While in the capecitabine group, as the neutrophil and eosinophilic granulocyte counts increased, so did the incidence rate of FIC. Furthermore, we developed a nomogram for predicting FIC in patients using 5-FU-based chemotherapy, which had good discriminative power.
Cardiotoxicity has been one of the common toxic side effects of fluoropyrimidine. In 1969, Gaveau et al. first reported FIC (21), and subsequently there have been an increasing number of similar reports. The reported incidence of FIC related specifically to 5-FU among the European and American varied from 7 to 19.9%, with the oral prodrug capecitabine having an even higher incidence of 34.6% (11, 22–24). According to a retrospective cohort study of 129 Chinese patients with gastric cancer, 38(29.5%) patients developed FIC (25). In our study, the incidence rate of cardiotoxicity was 21.8% (200/916), with 156 (20.6%) in the 5-FU group and 44 (27.1%) in the capecitabine group, suggesting that the incidence of cardiotoxicity in the Chinese population might be higher than that in the European and American populations. The incidence rate of FIC in the capecitabine group in our study was 27.1%, higher than that in the 5-FU group, which is consistent with previous studies (14).
In contrast to studies included European and American patients, in which angina has been the most common evidence of FIC (23), the most common manifestation in our study was arrhythmia. The incidence rate of arrhythmia was as high as 42% in the 5-FU group, and 50% in the capecitabine group. A previous Chinese study also found the incidence rate of arrhythmia was as high as 20.9% (14). We also found that ST-T changes accounted for a relatively high proportion of FIC. Such electrocardiographic changes could be a potential harbinger for asymptomatic myocardial ischemia or arrhythmia (26). However, because most related heart ischemia resolved in a short time, the incidence rate of FIC might have been higher if continuous electrocardiographic monitoring had been carried out on patients who were treated with fluoropyrimidine.
The incidence of cardiotoxicity had been reported to increase with increasing cycles of chemotherapy (14). But our study found that cardiotoxicity in 5-FU regimen occurred most frequently in the early duration of chemotherapy, consistent with results that FIC was found to occur mostly during the first cycle of chemotherapy (11, 16) suggesting 5-Fu induced FIC may not be the dose-limiting adverse event. In addition, the incidence rate of FIC was higher in patients who were concurrently treated with targeted drugs (bevacizumab, cetuximab). Previous studies also reported that the regimens containing bevacizumab and panitumumab appeared to have a higher cardiotoxicity risk (27). Bevacizumab significantly reduces cardiomyocyte viability and increases cell apoptosis, which in turn increase the incidence rate of the relevant cardiotoxicity (28).
Whether age is associated with FIC was controversial in published studies (14, 24, 29, 30). Our study found the incidence rate of 5-FU induced FIC rose in patients with advanced age (≥60 years). Furthermore, high BMI (>22.97) was correlated with the occurrence of 5-FU induced FIC, which has not been previously reported. Our results might suggest that when using 5-FU-based chemotherapy in patients with high BMI, we should increase the frequency of cardiovascular-related tests.
Previous studies reported that elevation of inflammatory cell such as neutrophils and eosinophilia, as well as inflammation-related indices may contribute to varied cardiovascular disease (31–35). Therefore, we also collected inflammation-related biomarkers (leukocytes, neutrophils, eosinophils, monocytes and Systemic immune immune-inflammation index(SII)) and included them into analysis. We did not find significant correlation between inflammation-related biomarkers and 5-Fu induced FIC. While in patients using the capecitabine-based chemotherapy, elevated neutrophils and eosinophils were found to be independent risk factors for capecitabine induced FIC, which may indicate the occurrence of capecitabine induced FIC is related to pre-existing inflammation.
Nomograms have been used extensively in oncology and medicine to predict the probability of clinical events. They can assist clinicians to make data-based and easily interpreted decisions because of improved accuracy and readily comprehensible predictions (36). The nomogram predicting 5-Fu induced FIC was established based on the four risk factors identified through Lasso regression and the multivariate logistic regression analysis. The area under the ROC curve is 0.751, showing good predictive accuracy and the calibration curve for predicting the occurrence of the FIC in the CRC patients was identical with the actual curve. According to the result of the Hosmer-Lemeshow goodness of fit, The C-index of 0.751 through the internal validation means the nomogram had a good imitative effect and a good degree of discrimination and accuracy. To the best of our knowledge, this is the first study for construct a nomogram for predicting the risk of 5-Fu related FIC in CRC patients.
However, we did not construct nomogram model for predicting capecitabine induced FIC, because of the relatively small number of patients in the capecitabine group, and only two independent high-risk factors identified.
Nonetheless, our study has some limitations. First, although we collected past history on patients with FIC, duration of comorbidities, was not captured and is, therefore, not included in the assessment. Second, this model was developed using retrospective data at a single center, which inevitably suffered from confounding bias. Third, external validation was not undertaken but would certainly be valuable to confirm the performance of the nomogram. However, because the data are collected as a part of routine clinical records, other hospitals that collect similar data could likely apply this model.
Conclusions
In conclusion, our study demonstrated increased risk of 5-Fu related in early duration of chemotherapy, and in patients with age≥60, high BMI, or treatment with targeted drugs. For patients treated with capecitabine-based regimen, FIC appeared to be related to inflammatory indices. The constructed nomogram showed a high degree of accuracy for prediction of FIC in CRC patients and may eventually help clinicians for risk evaluation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Affiliated Cancer Hospital of Guizhou Medical University. The institutional ethics committee waived the requirement for informed consent given the deidentified data.
Author contributions
YW and WWC designed the study. YW, WWC, WLW, HMD, and WHC contributed to the collection and analysis of the study data. YW wrote the article. HMD, GW, JC and WLW revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Science and Technology Planning Project of Science and Technology Department of Guizhou Province ([2022]340), the Science and Technology Fund Project of the Guizhou Provincial Health Commission (gzwkj2021-052), and the Cultivation Project of Guizhou Medical University (20NSP040).
Acknowledgments
We thank Huan Chai, Guochun Gou, and Guodong Li for providing editorial assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1017237/full#supplementary-material
Abbreviations
CRC, colorectal cancer; 5FU, 5-fluorouracil; FIC, fluoropyrimidine induced cardiotoxicity; LVEF, left ventricular ejection fraction; AJCC, American Joint Committee on Cancer; CTCAE, Common Terminology Criteria for Adverse Events; LASSO, least absolute shrinkage and selection operator; AIC, akaike information criterion; ROC, receiver operating characteristic; C-index, concordance index; NE, neutrophils; SII, systemic immune-inflammation index; NLR, neutrophil to lymphocyte ratio; WBC, white blood cells; NE, neutrophils; Eos, eosinophils; M, monocytes; PNI, prognostic nutritional index; PIV, Pan-Immune-Inflammation value; NAR, neutrophil-to-albumin ratio.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Akazawa H. [Cardiotoxicity of cancer chemotherapy - mechanisms and therapeutic approach]. Gan to kagaku ryoho Cancer chemother (2017) 44(13):2058–63. doi: 10.1093/annonc/mdx575.003
4. Jain D, Aronow W. Cardiotoxicity of cancer chemotherapy in clinical practice. Hosp Pract (1995) (2019) 47(1):6–15. doi: 10.1080/21548331.2018.1530831
5. Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, et al. Causes of death among cancer patients. Ann Oncol Off J Eur Soc Med Oncol (2017) 28(2):400–7. doi: 10.1093/annonc/mdw604
6. Marek J, Linhart A, Rucklová Z, Haber J, Korínek J. [Cardiotoxicity of cancer therapy]. Vnitrni lekarstvi. (2011) 57(5):472–84.
7. Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P, Vymetalkova V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol Ther (2020) 206:107447. doi: 10.1016/j.pharmthera.2019.107447
8. Depetris I, Marino D, Bonzano A, Cagnazzo C, Filippi R, Aglietta M, et al. Fluoropyrimidine-induced cardiotoxicity. Crit Rev oncology/hematol (2018) 124:1–10. doi: 10.1016/j.critrevonc.2018.02.002
9. Raber I, Warack S, Kanduri J, Pribish A, Godishala A, Abovich A, et al. Fluoropyrimidine-associated cardiotoxicity: A retrospective case-control study. oncologist (2020) 25(3):e606–9. doi: 10.1634/theoncologist.2019-0762
10. Layoun ME, Wickramasinghe CD, Peralta MV, Yang EH. Fluoropyrimidine-induced cardiotoxicity: Manifestations, mechanisms, and management. Curr Oncol Rep (2016) 18(6):35. doi: 10.1007/s11912-016-0521-1
11. Meydan N, Kundak I, Yavuzsen T, Oztop I, Barutca S, Yilmaz U, et al. Cardiotoxicity of de gramont's regimen: incidence, clinical characteristics and long-term follow-up. Japanese J Clin Oncol (2005) 35(5):265–70. doi: 10.1093/jjco/hyi071
12. Kwakman JJ, Simkens LH, Mol L, Kok WE, Koopman M, Punt CJ. Incidence of capecitabine-related cardiotoxicity in different treatment schedules of metastatic colorectal cancer: A retrospective analysis of the CAIRO studies of the Dutch colorectal cancer group. Eur J Cancer (Oxford Engl 1990) (2017) 76:93–9. doi: 10.1016/j.ejca.2017.02.009
13. Weiser MR. AJCC 8th edition: Colorectal cancer. Ann Surg Oncol (2018) 25(6):1454–5. doi: 10.1245/s10434-018-6462-1
14. Peng J, Dong C, Wang C, Li W, Yu H, Zhang M, et al. Cardiotoxicity of 5-fluorouracil and capecitabine in Chinese patients: a prospective study. Cancer Commun (London England) (2018) 38(1):22. doi: 10.1186/s40880-018-0292-1
15. Dyhl-Polk A, Vaage-Nilsen M, Schou M, Vistisen KK, Lund CM, Kümler T, et al. Incidence and risk markers of 5-fluorouracil and capecitabine cardiotoxicity in patients with colorectal cancer. Acta Oncol (Stockholm Sweden) (2020) 59(4):475–83. doi: 10.1080/0284186X.2019.1711164
16. de Forni M, Malet-Martino MC, Jaillais P, Shubinski RE, Bachaud JM, Lemaire L, et al. Cardiotoxicity of high-dose continuous infusion fluorouracil: a prospective clinical study. J Clin Oncol (1992) 10(11):1795–801. doi: 10.1200/JCO.1992.10.11.1795
17. Jensen SA, Sørensen JB. 5-fluorouracil-based therapy induces endovascular injury having potential significance to development of clinically overt cardiotoxicity. Cancer chemother Pharmacol (2012) 69(1):57–64. doi: 10.1007/s00280-011-1669-x
18. Kosmas C, Kallistratos MS, Kopterides P, Syrios J, Skopelitis H, Mylonakis N, et al. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol (2008) 134(1):75–82. doi: 10.1007/s00432-007-0250-9
19. Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the common terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas dermo-sifiliograficas (2021) 112(1):90–2. doi: 10.1016/j.ad.2019.05.009
20. Vrieze SI. Model selection and psychological theory: a discussion of the differences between the akaike information criterion (AIC) and the Bayesian information criterion (BIC). psychol Methods (2012) 17(2):228–43. doi: 10.1037/a0027127
21. Gaveau T, Banzet P, Marneffe H, Viars P. [Cardiovascular disorders in the course of antimitotic infusions at high doses. 30 clinical cases]. Anesthesie analgesie reanimation (1969) 26(3):311–27.
22. Khan MA, Masood N, Husain N, Ahmad B, Aziz T, Naeem A. A retrospective study of cardiotoxicities induced by 5-fluouracil (5-FU) and 5-FU based chemotherapy regimens in Pakistani adult cancer patients at shaukat khanum memorial cancer hospital & research center. JPMA J Pakistan Med Assoc (2012) 62(5):430–4.
23. Polk A, Vaage-Nilsen M, Vistisen K, Nielsen DL. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: A systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev (2013) 39(8):974–84. doi: 10.1016/j.ctrv.2013.03.005
24. Labianca R, Beretta G, Clerici M, Fraschini P, Luporini G. Cardiac toxicity of 5-fluorouracil: a study on 1083 patients. Tumori (1982) 68(6):505–10. doi: 10.1177/030089168206800609
25. Jin X, Bai Y, Gao L, Wu S. Incidence of and risk factors for cardiotoxicity after fluorouracil-based chemotherapy in locally advanced or metastatic gastric cancer patients. Cancer chemother Pharmacol (2019) 84(3):599–607. doi: 10.1007/s00280-019-03888-1
26. Deedwania PC, Nelson JR. Pathophysiology of silent myocardial ischemia during daily life. hemodynamic evaluation by simultaneous electrocardiographic and blood pressure monitoring. Circulation (1990) 82(4):1296–304. doi: 10.1161/01.cir.82.4.1296
27. Abdel-Rahman O. 5-fluorouracil-related cardiotoxicity; findings from five randomized studies of 5-fluorouracil-based regimens in metastatic colorectal cancer. Clin colorectal cancer (2019) 18(1):58–63. doi: 10.1016/j.clcc.2018.10.006
28. Li Y, Tian W, Yue D, Chen C, Li C, Zhang Z, et al. Bevacizumab-induced mitochondrial dysfunction, endoplasmic reticulum stress, and ERK inactivation contribute to cardiotoxicity. Oxid Med Cell longevity (2021) 2021:5548130. doi: 10.1155/2021/5548130
29. Jensen SA, Sørensen JB. Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer chemother Pharmacol (2006) 58(4):487–93. doi: 10.1007/s00280-005-0178-1
30. Meyer CC, Calis KA, Burke LB, Walawander CA, Grasela TH. Symptomatic cardiotoxicity associated with 5-fluorouracil. Pharmacotherapy (1997) 17(4):729–36.
31. Nishida K, Otsu K. Sterile inflammation and degradation systems in heart failure. Circ J (2017) 81(5):622–8. doi: 10.1253/circj.CJ-17-0261
32. Strassheim D, Dempsey EC, Gerasimovskaya E, Stenmark K, Karoor V. Role of inflammatory cell subtypes in heart failure. J Immunol Res (2019) 2019:2164017. doi: 10.1155/2019/2164017
33. Cucu F, Chiriloiu C, Georgescu M, Cucu C, Suciu A. The effect of exhaust gases on the serum lipids and on the arterial wall. Exp study Med interne (1975) 13(3):235–40.
34. Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol (2014) 11(5):255–65. doi: 10.1038/nrcardio.2014.28
35. Masaki N, Issiki A, Kirimura M, Kamiyama T, Sasaki O, Ito H, et al. Echocardiographic changes in eosinophilic endocarditis induced by churg-Strauss syndrome. Internal Med (Tokyo Japan) (2016) 55(19):2819–23. doi: 10.2169/internalmedicine.55.7150
Keywords: fluoropyrimidine-related cardiotoxicity, risk factor, colorectal cancer, nomogram, prediction model
Citation: Wang Y, Wang W, Dong H, Wang G, Chen W, Chen J and Chen W (2023) Risk factors for fluoropyrimidine-induced cardiotoxicity in colorectal cancer: A retrospective cohort study and establishment of a prediction nomogram for 5-FU induced cardiotoxicity. Front. Oncol. 13:1017237. doi: 10.3389/fonc.2023.1017237
Received: 11 August 2022; Accepted: 09 January 2023;
Published: 01 March 2023.
Edited by:
Peter Christensen, Aarhus University Hospital, DenmarkReviewed by:
Paola Gargiulo, University of Naples Federico II, ItalySohrab Kazemi, Babol University of Medical Sciences, Iran
Copyright © 2023 Wang, Wang, Dong, Wang, Chen, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiwei Chen, weiweichen@gmc.edu.cn
 Yan Wang1,2,3
Yan Wang1,2,3 Weiwei Chen
Weiwei Chen