- 1Department of Hematology, Xiangya Hospital, Central South University, Changsha, China
- 2National Clinical Research Center for Geriatric Diseases, (Xiangya Hospital), Changsha, China
- 3Hunan Hematologic Neoplasms Clinical Medical Research Center, Xiangya Hospital, Central South University, Changsha, China
- 4National Clinical Research Center for Hematologic Diseases The First Affiliated Hospital of Soochow University, Suzhou, China
Recent studies have shown that haploidentical hematopoietic stem cell transplantation supported by third-party cord blood (haplo-cord-HSCT) results in rapid hematopoietic recovery, low incidences of graft-versus-host disease (GVHD), and relapse of hematologic malignancies. However, few reports on haploidentical peripheral blood stem cell transplantation supported by third-party cord blood (haplo-cord-PBSCT) have been published. To evaluate the outcomes of patients who underwent haplo-cord-PBSCT or human leukocyte antigen (HLA)-matched sibling donor peripheral blood stem cell transplantation (MSD-PBSCT), we retrospectively reviewed the clinical data of patients with hematologic malignancies who underwent haplo-cord-PBSCT (n = 93) or MSD-PBSCT (n = 72) in our hospital from March 2017 to December 2020. In the haplo-cord-PBSCT and MSD-PBSCT groups, the median time for neutrophil and platelet engraftment was 13 vs. 12 days (p = 0.07) and 16 vs. 13 days (p = 0.06), respectively. The 30-day cumulative incidences of neutrophil engraftment were 100.0% and 98.6% (p = 0.12). The 100-day cumulative incidences of platelet engraftment were 96.8% and 98.6% (p = 0.01). The 100-day cumulative incidences of grade II–IV and grade III–IV acute GVHD were 29.1% vs. 23.6% (p = 0.42) and 9.7% vs. 4.2% (p = 0.18). The cumulative incidences of total and moderate/severe chronic GVHD at 1 year were 26.5% vs. 17.4% and 8.1% vs. 4.5%, respectively, and at 3 years were 34.7% vs. 34.3% (p = 0.60) and 13.6% vs. 10.6% (p = 0.49), respectively. The cumulative incidences of relapse at 1 year were 9.3% and 7.2% and at 3 years were 17.0% and 17.0% (p = 0.98). Non-relapse mortality (NRM) at 1 year was 14.6% and 8.6% and at 3 years was 17.4% and 8.6% (p = 0.13) in two groups. The probabilities of overall survival (OS), disease-free survival (DFS), and GVHD-free/relapse-free survival (GRFS) at 1 year were 81.7% vs. 88.6%, 76.1% vs. 84.2%, and 71.7% vs. 79.7%, respectively, and at 3 years were 78.7% vs. 79.0%, 65.6% vs. 74.4%, and 55.5% vs. 63.6%, respectively, in the corresponding group, p > 0.05. In conclusion, for patients with acute myeloid leukemia/myelodysplastic syndrome (AML/MDS) and acute lymphoid leukemia (ALL), haplo-cord-PBSCT results in similar outcomes compared with MSD-PBSCT, and it may be a valid alternative transplantation method.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an important choice for treating hematologic malignancies. The development of haploidentical hematopoietic stem cell transplantation (haplo-HSCT) has not only contributed to solving the issues with the shortage of donors but also brought hope for patients urgently needing hematopoietic stem cell transplantation but lacking human leukocyte antigen (HLA)-matched donors. The data from the worldwide Network for Blood and Marrow Transplantation Group show that haplo-HSCT procedures have increased rapidly in recent years (1, 2). According to the data of Chinese Bone Marrow Transplantation Registration (CBMTR) for 2019, haploidentical transplantation accounted for 60% of all allo-HSCT, showing an increasing yearly trend (3). In China, haploidentical donors have become the main allo-HSCT donor sources. Three major haplo-HSCT modalities have been defined: T-cell depletion (TCD) transplantation with posttransplant high-dose cyclophosphamide (PTCY), granulocyte colony-stimulating factor (G-CSF)-primed bone marrow (BM) plus peripheral blood (PB) graft, and anti-thymocyte globulin (ATG)-based regimens, called “Beijing Protocol,” and in vitro TCD protocol (3). The application of the aforementioned schemes has reduced the incidence of graft-versus-host disease (GVHD) (4, 5). In China, the “Beijing Protocol” was implemented in approximately 94% of the haplo-HSCT, based on previous data (3). Earlier studies confirmed that haplo-HSCT using the “Beijing Protocol” had similar overall survival (OS) and disease-free survival (DFS) to those of HSCT from HLA-matched sibling donors (MSDs) or HLA-matched unrelated donors (MUDs) (6, 7). Furthermore, haplo-HSCT showed a stronger graft-versus-leukemia (GVL) effect than MSD-HSCT, especially in high-risk, refractory, and pretransplantation measurable residual-positive acute leukemia (AL) (6–9). The stem cell sources of haplo-HSCT are various, including BM, PB, or a combination of them (BM + PB). PB is the main stem cell source in both the United States and Europe (1). However, in China, BM + PB constitutes 59% of the haplo-HSCT cases, while simple PB or BM transplantation accounts for less than 30% (3). Little data exist about PB stem cell transplantation (PBSCT).
Haplo-HSCT has achieved promising results in the treatment of hematologic malignancies. However, the delayed and unstable immune reconstitution and the incidence of GVHD increase the rate of non-relapse mortality (NRM) in haplo-HSCT (10, 11). Umbilical cord blood (UCB) transplantation (UCBT) is another option for HSCT due to its rapid graft acquisition and low incidence of GVHD and relapse (12, 13), while UCB stem cells are less prone to delay and failure of engraftment (14, 15). Recent studies reported that haplo-HSCT combined with third-party cord blood accelerated engraftment reduced the incidence of GVHD and the recurrence of hematologic malignancies, which was superior to either haploidentical or UCB transplantation alone (16–18). To investigate the effects of haplo-cord-HSCT, some studies have compared this transplantation scheme with HLA-matched transplantation and obtained similar OS, DFS, NRM, and GVHD incidence rates (19–21). However, the stem cell sources examined in previous studies have predominantly been BM + PB, whereas very few studies have investigated haploidentical PBSCT supported by third-party cord blood (haplo-cord-PBSCT). To further explore the effect of haplo-cord-PBSCT, we retrospectively compared this transplantation scheme with MSD-PBSCT.
Materials and Methods
Patients
From March 2017 to December 2020, patients who received allo-HSCT at Xiangya Hospital, Central South University (Changsha, Hunan, China), were included in this study if they met the following criteria: 1) they were older than 14 years and were diagnosed with AL or myelodysplastic syndrome (MDS), 2) received haplo-cord-PBSCT or MSD-PBSCT 3) received modified Bu/Cy myeloablative preparative regimen. This research was approved by the Ethics Committee of Xiangya Hospital. Written informed consent was obtained from all patients or their legal guardians.
Preparative Regimen
Modified Bu/Cy regimen was used in all patients: cytarabine (Ara-C) (haplo-cord-PBSCT group, 4 g/m2/day, from days −8 to −7; MSD-PBSCT group, 2 g/m2/day, from days −8 to −7), busulfan (Bu) (3.2 mg/kg/day, from days −6 to −4), cyclophosphamide (CTX) (1.8 g/m2/day, from days −3 to −2), and semustine (250 mg/m2, day −2). r-ATG (rabbit anti-human thymocyte immunoglobulin) was administered at 2.5 mg/kg/day (days −3 to −1) in the haplo-cord-PBSCT group.
Human Leukocyte Antigen Typing and Donor Selection
High-resolution techniques were used for HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 typing to select haploidentical donors. All patients receiving haplo-cord-PBSCT were tested for the presence of donor-specific anti-HLA antibodies (DSAs), including class I and class II HLA antibodies. The median fluorescence intensity (MFI) of DSA in all patients was lower than 4,000, and they did not receive treatment before transplantation. Haploidentical donors were selected on the basis of HLA typing, DSA testing, age, gender, health status, and willingness to donate. The UCB unit was selected based on the high resolution of HLA-A, HLA-B, HLA-DRB1, and HLA-DQB1. The following criteria for cord blood unit selection were applied: 1) at least 5/10 matched-HLA loci and 2) total nucleated cells not less than 1 × 107/kg of the recipient’s body weight after thawing. Blood type-matched cord blood was preferred at an equal level of HLA-type matching.
Graft Collection and Infusion
G-CSF (7.5–10 μg/kg/day) was used to mobilize PB stem cells 4 days before HSCT. In the haplo-cord-PBSCT group, the haploidentical cells were infused on the first day, followed by cord blood mononuclear cell (MNC) infusion (1 × 107/kg) on the second day. The interval between the cord blood transfusion and the end of PBSC transfusion was at least 12 h.
Graft-Versus-Host Disease Prophylaxis and Treatment
All transplantation recipients received cyclosporine A (CsA), mycophenolate mofetil (MMF), and short-term methotrexate (MTX) as GVHD prophylaxis.
CsA was given to the haplo-cord-PBSCT recipients by continuous infusion at 2.5 mg/kg/day from day −9 until the patients could switch to oral intake after the recovery of their gastrointestinal function, with a target blood concentration ranging from 200 to 250 μg/L. MTX at 15 mg/m2 was administered on day +1, and MTX at 10 mg/m2 was given on days +3, +6, and +11. MMF was given at 0.5 g PO twice per day from day −9; the dose was halved from day +30 until day +45 to day +60 if no GVHD occurred.
CsA was given to MSD-PBSCT recipients by continuous infusion at 2.5 mg/kg/day from day −1 until patients could switch to oral intake, with a target blood concentration ranging from 200 to 250 μg/L. MTX at 15 mg/m2 was administered on day +1, and MTX at 10 mg/m2 was given on days +3 and +6. MMF was given at 0.5 g PO twice per day from day −1 until engraftment.
The first-line treatment for grade II–IV acute GVHD (aGVHD) was methylprednisolone infusion at 2 mg/kg/day. Second-line therapy such as basiliximab was used in patients with methylprednisolone intolerance or poor response.
Supportive Care and Posttransplantation Evaluation
Patients started gut decontamination with gentamicin and nystatin prior to conditioning 3–5 days before HSCT. Prophylactic antibiotics and antifungal and antiviral drugs were used during the preparative regimen and immunosuppression period; sulfamethoxazole was administrated to prevent Pneumocystis carinii infection. Bone marrow aspirations were performed at least once a month in the first 6 months after transplantation and then every 1–1.5 months in the second 6 months, every 1.5–2 months in the second year, and every 2–3 months thereafter to evaluate the remission status and chimerism.
Endpoints and Definitions
The diagnosis of acute lymphoid leukemia (ALL), acute myeloid leukemia (AML), and MDS was based on the WHO criteria (22). Risk stratifications were performed with reference to the National Comprehensive Cancer Network (NCCN) guidelines (23, 24) and the International Prognostic Scoring System (IPSS) (25). Patients with refractory/relapsed disease were included in the high-risk group. Granulocyte recovery was defined as the first day of three consecutive days when absolute neutrophil count (ANC) >0.5 × 109/L. Platelet recovery was defined as the first day of platelet counts >20 × 109/L without transfusion support for seven consecutive days. The diagnosis was made, and GVHD grading criteria were implemented with reference to earlier publications (26–28). Relapse was defined on the basis of BM histology analysis with more than 5% blasts or extramedullary relapse. NRM was defined as death due to any cause without previous disease progression or relapse. OS was calculated from the date of transplantation to the date of death due to any cause, and surviving patients were censored at the last follow-up examination. DFS was defined as survival in continuous complete remission without relapse. GVHD-free/relapse-free survival (GRFS) was calculated from the date of HSCT to the date of events that included grade III–IV aGVHD, chronic GVHD (cGVHD) requiring systemic therapy, relapse, or death.
Statistical Analysis
The χ2 or Fisher’s exact test was used to compare categorical variables. A non-parametric test (Mann–Whitney U‐test) was employed to compare continuous variables. One- and three-year OS or DFS was calculated using the Kaplan–Meier outcome curve and compared by log-rank test. Considering the competing risks, the cumulative incidence rate (CIR) of engraftment, aGVHD, cGVHD, NRM, and relapse were calculated by the Gray test. Competing events were defined as follows: death due to any cause without engraftment as the competing event for engraftment; relapse, engraftment failure, or death as the competing event for GVHD; relapse as the competing event for NRM; and death as the competing event for relapse. Multivariate analyses of transplantation-related covariates affecting survival were determined using the Cox proportional hazard model. All variables presented in Table 1 were included in a univariate analysis (Supplementary Table). The forced factor (haplo-cord-PBSCT vs. MSD-PBSCT) and variables with p < 0.10 were included in further multivariate analysis. The SPSS26.0 software package (SPSS, Chicago, IL, USA) was used for data analysis. R software (version 4.1.1; https://www.r-project.org/) was utilized for competing risk analysis. All tests were two-sided, and p < 0.05 was considered to indicate statistically significant differences.
Results
Patients’ Characteristics
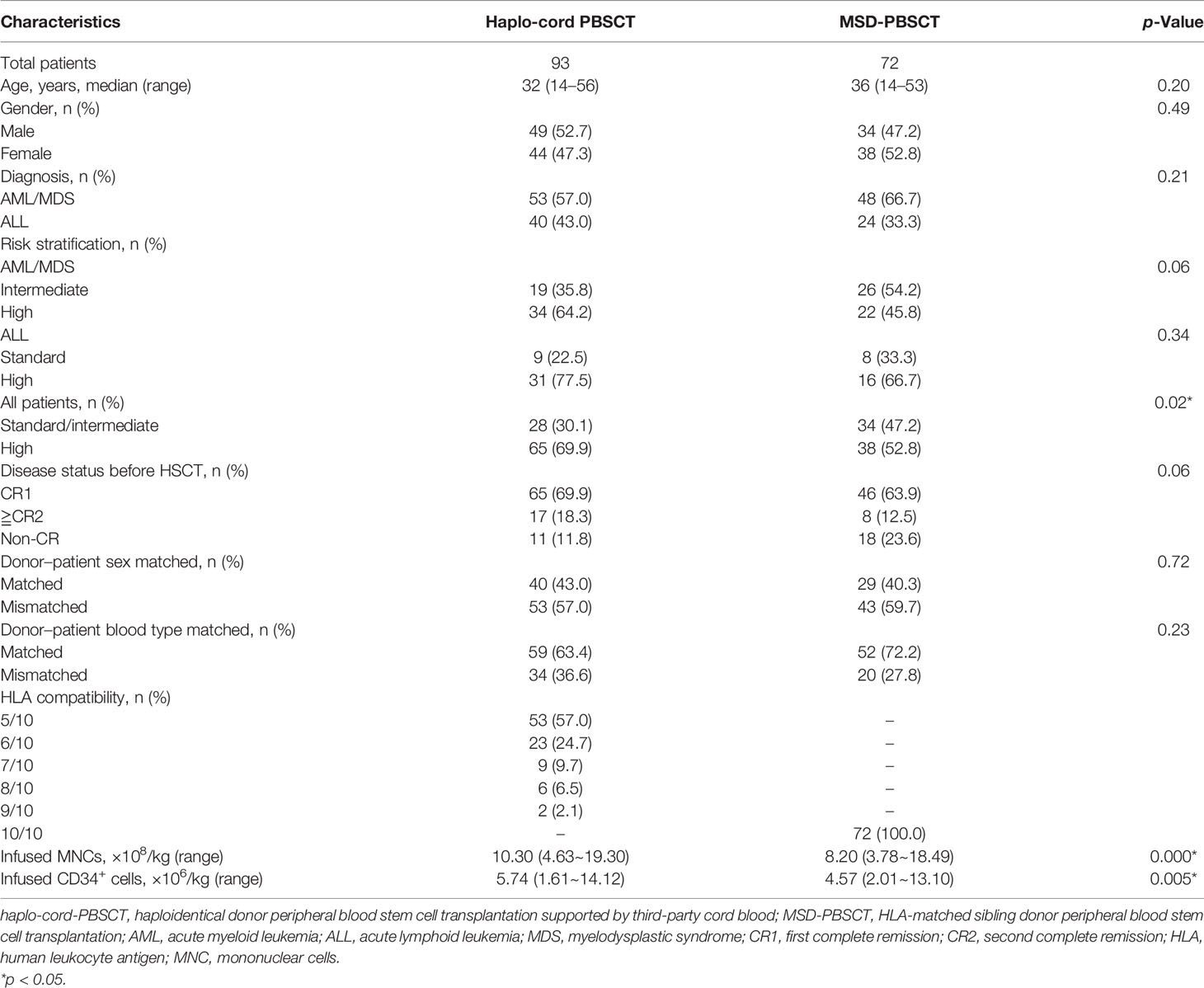
A number of 165 patients who underwent haplo-cord-PBSCT (n = 93, 56.4%) or MSD-PBSCT (n = 72, 43.6%) from March 2017 to December 2020 were enrolled in this study. The characteristics of the patients are displayed in Table 1. There were 53 (57.0%) patients with AML/MDS and 40 (43.0%) patients with ALL in the haplo-cord-PBSCT group, and 48 (66.7%) patients with AML/MDS and 24 (33.3%) patients with ALL in the MSD-PBSCT group. The patients with high-risk features in the haplo-cord-PBSCT group were higher in number than those in the MSD-PBSCT group (69.9% vs. 52.8%, p = 0.02).
The last follow-up date was January 31, 2022. The median follow-up time in the haplo-cord- and MSD-PBSCT groups was 26 (3–59) and 28 (2–60) months, respectively.
Donor and Graft Characteristics
Table 1 shows the characteristics of donor and graft. In the haplo-cord-PBSCT group, 31 (33.3%) haploidentical related donors were patients’ parents, 24 (25.8%) were patients’ children, and 38 (40.9%) were patients’ siblings. The median doses of infused MNCs and CD34+ cells in the haplo-cord-PBSCT group were 10.30 × 108/kg (range, 4.63–19.30) and 5.74 × 106/kg (range, 1.61–14.12), respectively. The number of UCB MNCs infused on the second day was 1 × 107/kg.
For the MSD-PBSCT group, the median MNC content was 8.20 × 108/kg (range, 3.78–18.49), and the median CD34+ cell content was 4.57 × 106/kg (range, 2.01–13.10), which were lower than those in the haplo-cord-HSCT group (MNC, p = 0.000; CD34+ cell, p = 0.005).
Hematopoietic Recovery and Engraftment
Full donor chimerism was achieved in all patients by day 30 posttransplantation, except for two patients in the haplo-cord-PBSCT group who died of infection before platelet engraftment on day +76 and day +81, as well as with the exception of one primary graft failure (PGF) patient who died of sepsis on day +63 after MSD-PBSCT. No cord blood chimerism or mixed chimerism was established in the haplo-cord-PBST group.
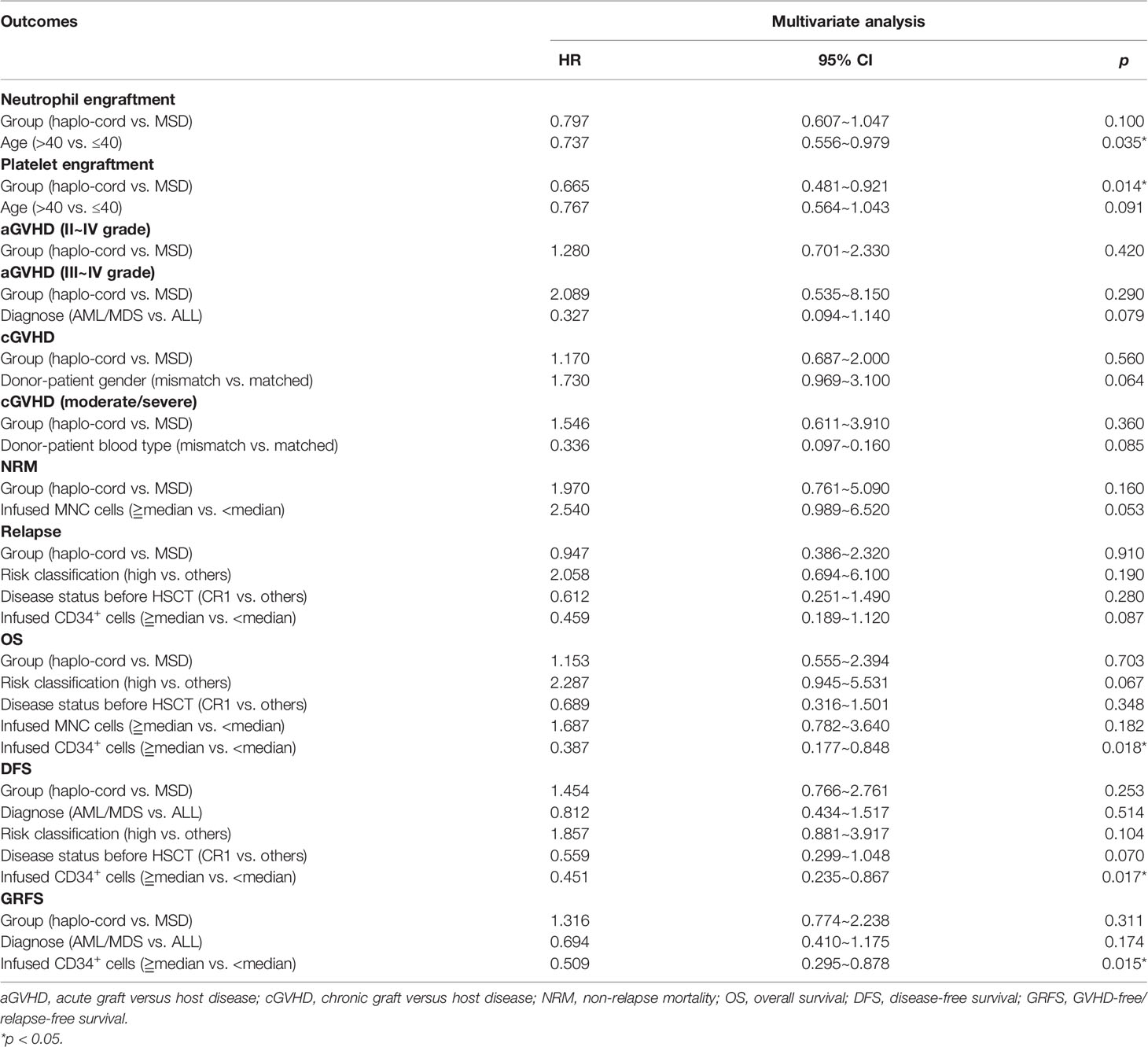
In the haplo-cord and MSD-PBSCT groups, the median time to neutrophil engraftment was 13 days (range, 9–22) and 12 days (range, 10–21), respectively (p = 0.07). The cumulative incidence of neutrophil engraftment at day 30 was 100.0% and 98.6% (95% CI: 84.1–99.9) in the two groups (p = 0.12, Figure 1A). Multivariate analysis revealed no significant difference in the neutrophil engraftment between the two groups (hazard ratio (HR) = 0.797, 95% CI: 0.607–1.047, p = 0.100), but recipient age >40 years was confirmed as an independent risk factor for neutrophil engraftment (HR = 0.737, 95% CI: 0.556–0.979, p = 0.035, Table 2).
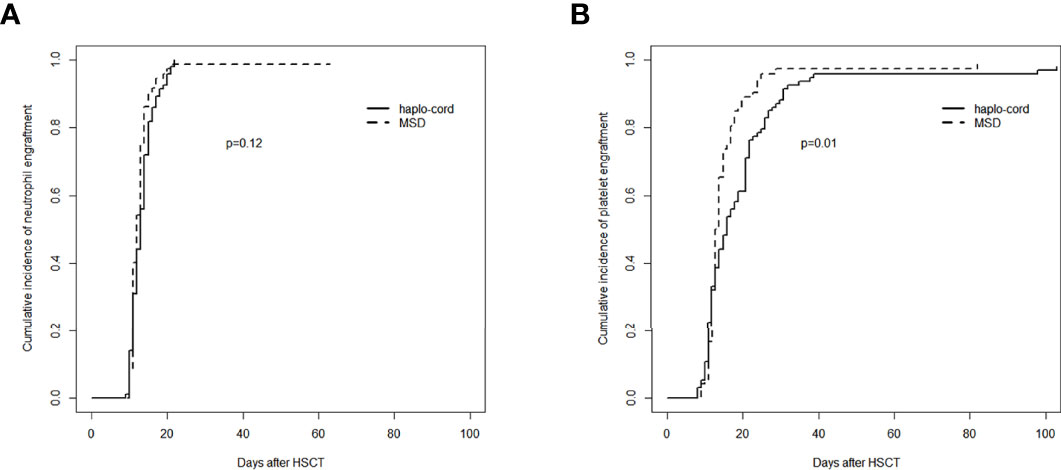
Figure 1 Assessment of hematopoietic recovery after transplantation in haplo-cord group and MSD group: (A) cumulative incidence of neutrophil engraftment and (B) cumulative incidence of platelet engraftment. MSD, matched sibling donor.
The median time to platelet engraftment was 16 days (range, 8–103) and 13 days (range, 9–82) in the haplo-cord and MSD-PBSCT groups, respectively (p = 0.06). The cumulative incidence of platelet engraftment at day 100 in the haplo-cord-PBSCT group (96.8% [95% CI: 88.7%–99.1%]) was significantly lower than that in the MSD-PBSCT group (98.6% [95% CI: 71.0%–99.9%]) (p = 0.01, Figure 1B). On multivariate analysis, inferior platelet engraftment was associated with haplo-cord-PBSCT (HR = 0.665, 95% CI: 0.481–0.921, p = 0.014, Table 2).
Graft-Versus-Host Disease
The cumulative incidence of aGVHD (grade II–IV) at day 100 after haplo-cord-PBSCT was 29.1% (95% CI: 20.2%–38.5%), comparable to that of the MSD-PBSCT group (23.6% [95% CI: 14.5%–34.0%], p = 0.42, Figure 2A). The cumulative incidence of aGVHD (grade III–IV) at day 100 was 9.7% (95% CI: 4.7%–16.7%) in the haplo-cord-PBSCT group versus 4.2% (95% CI: 1.1%–10.7%) in the MSD-HSCT group (p = 0.18, Figure 2B). The cumulative incidences of cGVHD at 1 year were 26.5% (95% CI: 17.7–36.1%) and 17.4% (95% CI: 9.5%–27.2%) (p = 0.60) and at 3 years were 34.7% (95% CI: 24.5%–45.1%) and 34.3% (95% CI: 22.2%–46.8%) (p = 0.60, Figure 3A). The cumulative incidences of moderate/severe cGVHD at 1 year were 8.1% (95% CI: 3.5%–15.0%) and 4.5% (95% CI: 1.2%–11.4%) (p = 0.49) and at 3 years were 13.6% (95% CI: 7.1%–22.3%) and 10.6% (95% CI: 4.0%–20.8%) (p = 0.49, Figure 3B) for the haplo-cord and MSD-PBSCT groups, respectively. Our multivariate analysis (Table 2) showed also no significant differences in the risk of grade II–IV aGVHD (HR = 1.280, 95% CI: 0.701–2.330, p = 0.420), grade III–IV aGVHD (HR = 2.089, 95% CI: 0.535–8.150, p = 0.290), cGVHD (HR = 1.170, 95% CI: 0.687–2.000, p = 0.560), and moderate/severe cGVHD (HR = 1.546, 95% CI: 0.611–3.910, p = 0.360) in the haplo-cord-PBSCT group versus the MSD-PBSCT group.
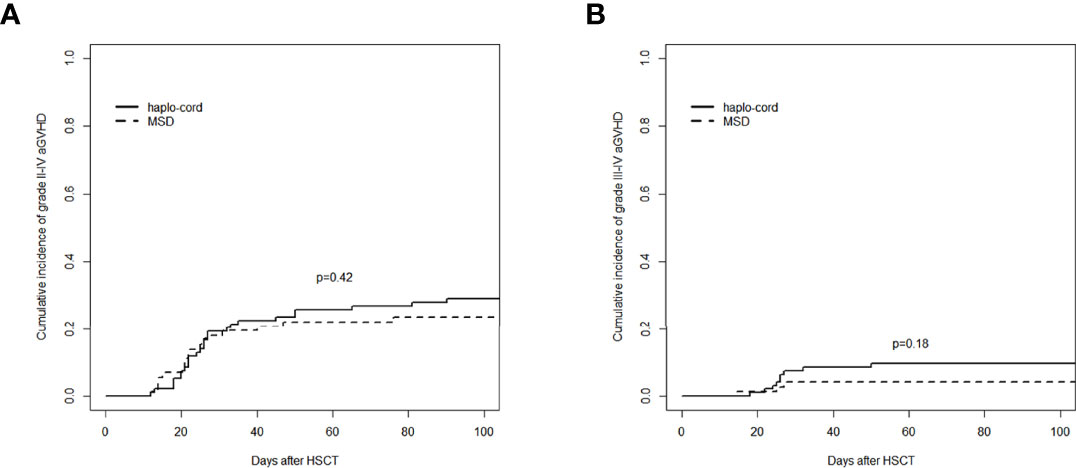
Figure 2 Assessment of cumulative incidence of aGVHD in haplo-cord group and MSD group: (A) grade II–IV aGVHD and (B) grade III–IV aGVHD. aGVHD, acute graft-versus-host disease; MSD, matched sibling donor.
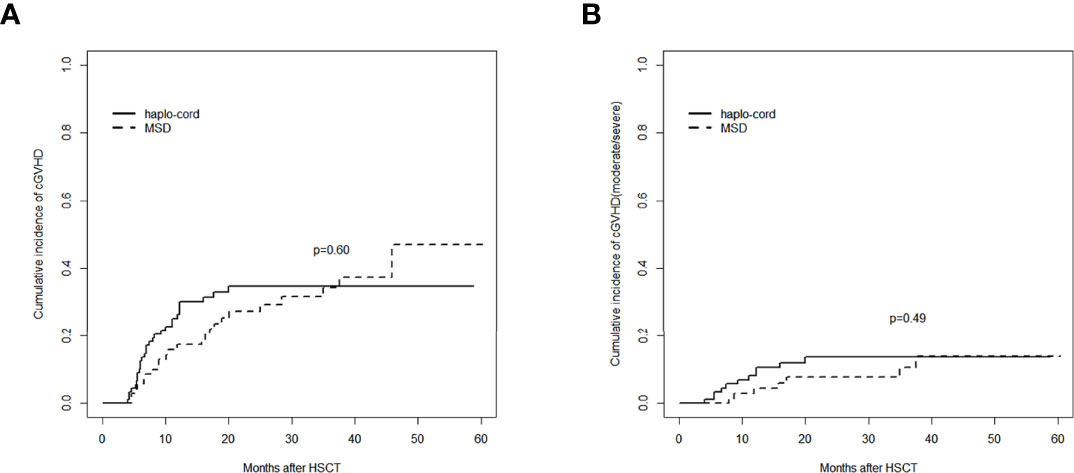
Figure 3 Assessment of cumulative incidence of cGVHD in haplo-cord group and MSD group: (A) all grades of cGVHD and (B) cGVHD (moderate/severe). cGVHD, chronic graft-versus-host disease; MSD, matched sibling donor.
Non-Relapse Mortality
The 1-year cumulative incidence of NRM in the haplo-cord-PBSCT group was 14.6% (95% CI: 8.2%–22.7%), whereas it was 8.6% (95% CI: 3.5%–16.6%) in the MSD-PBSCT group (p = 0.13). The 3-year cumulative incidences of NRM in these two groups were 17.4% (95% CI: 10.2%–26.2%) and 8.6% (95% CI: 3.5%–16.6%) (p = 0.13, Figure 4A).
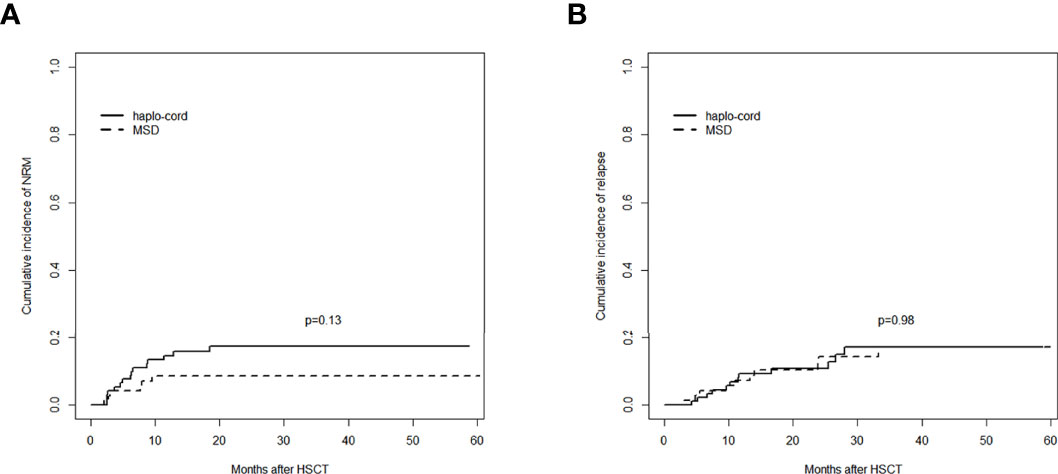
Figure 4 Comparisons of outcomes between the haplo-cord group and the MSD group: (A) cumulative incidence of NRM and (B) cumulative incidence of relapse. MSD, matched sibling donor; NRM, non-relapse mortality.
The causes of NRM included severe GVHD and severe infections such as pneumonia or sepsis. Fifteen NRM cases occurred in the haplo-cord-PBSCT group, whereas six cases occurred in the MSD-PBSCT group (p = 0.14). The multivariate analysis results revealed (Table 2) no difference in the risk of NRM (HR = 1.970, 95% CI: 0.761–5.090, p = 0.160) between the haplo-cord-PBSCT and the MSD-PBSCT groups.
Relapse
The 1- and 3-year cumulative incidences of relapse in the haplo-cord-PBSCT group were 9.3% (95% CI: 4.3%–16.6%) and 17.0% (95% CI: 9.0%–27.3%), respectively, whereas they were 7.2% (95% CI: 2.6%–14.9%) and 17.0% (95% CI: 8.4%–28.2%), respectively, in the MSD-PBSCT group (p = 0.98, Figure 4B). The multivariate analysis (Table 2) showed no significant difference in the risk of relapse (HR = 0.947, 95% CI: 0.386–2.320, p = 0.910) between the haplo-cord and MSD-PBSCT groups.
Survival
The 1- and 3-year OS in the haplo-cord-PBSCT group was 81.7% (95% CI: 73.9%–90.2%) and 78.7% (95% CI: 70.3%–88.1%), respectively, compared with 88.6% (95% CI: 81.4–96.4%) and 79.0% (95% CI: 69.3%–90.2%), in the MSD-PBSCT group (p = 0.73, Figure 5A). The multivariate analysis results showed no significant difference in OS (HR = 1.153, 95% CI: 0.555–2.394, p = 0.703) between the two groups. Inferior OS was associated with a low dose of CD34+ cells (HR = 2.584, 95% CI: 1.179–5.650, p = 0.018).
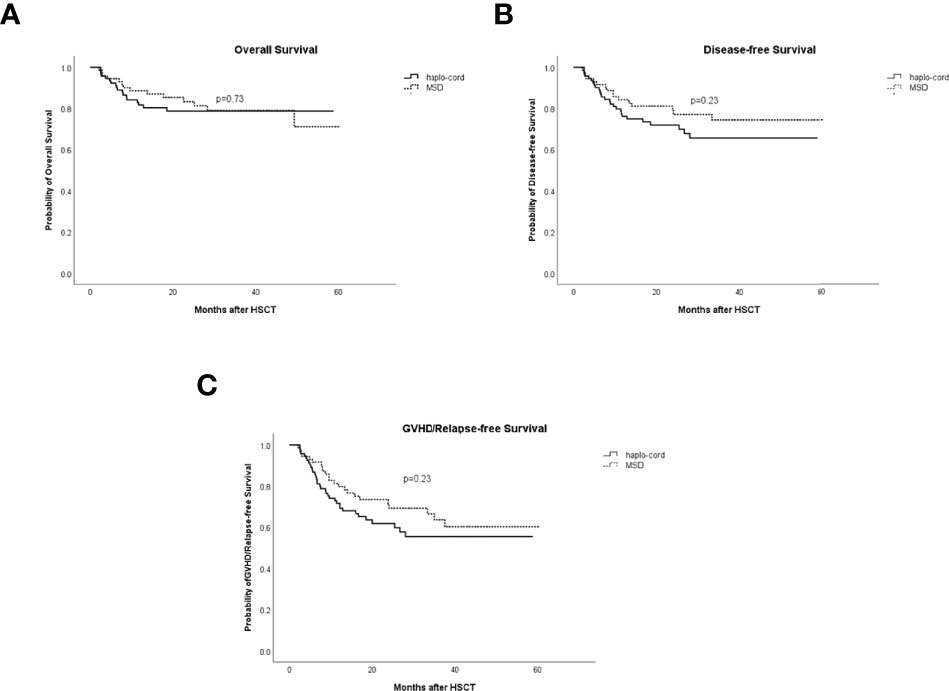
Figure 5 Comparisons of survival rates in haplo-cord group and MSD group. (A) Overall survival (OS). (B) Disease-free survival (DFS). (C) GVHD/relapse-free survival (GRFS). MSD, matched sibling donor; GVHD, graft-versus-host disease.
The 1- and 3-year DFS in the haplo-cord- and MSD-PBSCT was similar at 76.1% (95% CI: 67.7%–85.6%) versus 84.2% (95% CI: 76.0%–93.2%) (p = 0.23) and 65.6% (95% CI: 55.4%–77.8%) versus 74.4% (95% CI: 64.0%–86.5%) (p = 0.23, Figure 5B), respectively. Our multivariate analysis data revealed no significant difference in DFS (HR = 1.454, 95% CI: 0.766–2.761, p = 0.253) between the two groups. A lower dose of infused CD34+ cells was the only risk factor for DFS identified in our multivariate analysis (HR = 2.217, 95% CI: 1.195–4.255, p = 0.017, Table 2).
The 1- and 3-year GRFS was 71.7% (95% CI: 62.8%–81.8%) versus 79.7% (95% CI: 70.8%–89.8%) (p = 0.23) and 55.5% (95% CI: 44.9%–68.5%) versus 63.6% (95% CI: 51.9%–77.9%), respectively, in the haplo-cord-PBSCT and MSD-PBSCT groups (p = 0.23, Figure 5C). Multivariate analysis showed no significant difference in GRFS (HR = 1.316, 95% CI: 0.774–2.238, p = 0.311) between the haplo-cord and MSD-PBSCT groups; the infusion with a low amount of CD34+ cells was the risk factor affecting GRFS (HR = 1.965, 95% CI: 1.139–3.390, p = 0.015, Table 2).
Discussion
The application of the “Beijing Protocol” and PTCY has contributed to an improvement in the effect of haploidentical transplantation applied in the treatment of patients with hematologic malignancies. However, high NRM incidence still occurs in haplo-HSCT due to delayed, unstable immune reconstitution and high GVHD incidence (10, 11). PBSCT has the advantages of rapid engraftment, low incidence of relapse, and greater convenience and ease of collection as compared with BM; however, it is often accompanied by a high GVHD incidence (29, 30). UCBT has a low GVHD incidence but a high incidence of delayed engraftment, and its failure is caused by the limited stem cell number of UCB, which increases the risk of early death (14, 15). Here, we designed haploidentical PBSCT supported by third-party cord blood by combining the characteristics of these two types of grafts. We found that this method could achieve outcomes similar to those of HLA-MSD PBSCT.
In the present analysis, we observed similar median time and cumulative incidence of neutrophil engraftment at day 30 in the haplo-cord and MSD-PBSCT groups. The median time of platelet engraftment was 16 days in the haplo-cord-PBSCT group, which was similar to that in the MSD-PBSCT group. The cumulative incidence of platelet engraftment at 100 days after haplo-cord-PBSCT was significantly lower than that after MSD-PBSCT but similar to that achieved in previous studies of haplo-cord-HSCT (21, 31). Bashey et al. (32) established that the median time of platelet engraftment was 31 days, and the cumulative engraftment rate at day 100 was 84.0% after haploidentical PBSCT alone with PTCY. In another investigation, Zhao et al. (33) reported that the median time of platelet engraftment in haplo-PBSCT with the use of ATG/G-CSF was 16 days, and the cumulative engraftment rate at day 100 was 90.5%. We observed higher cumulative incidence of platelet engraftment in the haplo-cord-PBSCT group than the ones obtained in the aforementioned previous research work, indicating that haploidentical transplantation supported by cord blood may promote hematopoietic recovery. In our study, full haploidentical chimerism without evidence of UCB or mixed chimerism was achieved in all survival patients who underwent haplo-cord-PBSCT, which was different from the findings of other studies (31, 34). This outcome may be attributed to the low number of UCB MNCs (1 × 107/kg) that were infused at least 12 h after the end of the haploid graft infusion.
Our data showed similar cumulative incidences of grade II–IV aGVHD, grade III–IV aGVHD, cGVHD, and moderate/severe cGVHD between the haplo-cord and MSD-PBSCT groups. These outcomes may be due to the addition of ATG and UCB in the haplo-cord-PBSCT group, which prevented GVHD. Previous studies indicated that UCB-Treg exhibits predominantly naïve (CD45RAhi) and almost no activated or memory subsets, which allowed UCB-Treg to demonstrate a superior proliferative capability to PB-Treg (35). The adoptive transfer of UCB-derived Tregs reduced the risk of acute GVHD (36). In concordance, we did not observe a higher incidence rate of GVHD in the haplo-cord-PBSCT group compared with the MSD-PBSCT group. Ma et al. (30) analyzed the outcomes of PBSCT alone with ATG/G-CSF and found that the cumulative incidences of grade II–IV aGVHD at day 100, grade III–IV aGVHD at day 100, cGVHD at one year, and moderate/severe cGVHD at 1 year were 29.9%, 7.5%, 54.9%, and 17.4%, respectively, which were higher than those in our haplo-cord-PBSCT group, especially in cGVHD incidence. A possible explanation of these results could be that UCB may regulate the hematopoietic microenvironment and reduce immune rejection (37). However, compared with haplo-cord-PBSCT in our study, other studies showed lower cumulative incidences of 100-day aGVHD (20.0%–27.0%) and 1-year cGVHD (20.0%–27.0%) in haplo-cord-HSCT (17, 19, 20). One reason may be that our ATG dosage (7.5 mg/kg) was lower than that of other studies (10 mg/kg) (17, 20). From another perspective, we only used PB as haploid graft, whereas other researchers utilized mainly PB + BM. Notably, G-CSF administration significantly decreased the expression of adhesion molecules involved in GVHD on naïve CD4+ and CD8+ T cells in BM grafts and led to polarization of BM naïve CD4+ and CD8+ T cells from Th1 to Th2 phenotype, which may lead to a lower incidence of GVHD (38, 39). However, the incidence rates of grade III–IV aGVHD and moderate/severe cGVHD in our haplo-cord-PBSCT group were lower than those of other studies of haplo-cord-HSCT (20).
Here, we found no significant difference in the cumulative incidence of NRM between the haplo-cord-PBSCT and MSD-PBSCT groups. Salvatore et al. (10) retrospectively analyzed data for the period 2011–2015 obtained from the European Society of Blood and Marrow Transplantation. These scientists established that the NRM in haploidentical transplantation was higher than that of HLA-matched sibling transplantation. Haplo-HSCT supported by UCB reduced the incidence of GVHD and promoted hematopoietic recovery; it lowered the risk of infection associated with early neutropenia and the bleeding associated with platelet deficiency. Moreover, we presumed that the low ATG dosage in our investigation also decreased the risk of cytomegalovirus/Epstein–Barr virus (CMV/EBV) infection (40). Therefore, no high NRM incidence was observed in the haplo-cord-PBSCT group. However, the NRM of haplo-cord-PBSCT in the present study was similar to that established in another study on haplo-PBSCT alone (41), which may have been due to the high proportion of patients with high-risk features in our study.
We observed a similar 3-year cumulative incidence of relapse between the haplo-cord and MSD-PBSCT groups (17.0% vs. 17.0%). In MDS patients, Ke et al. (20) reported a similar 2-year cumulative incidence of relapse between haplo-cord-HSCT and MSD-HSCT (12.0% vs. 14.0%). Furthermore, Lu et al. (31) also found no significant difference in the 2-year cumulative relapse incidence between haplo-cord-HSCT and MSD-HSCT (14.2% vs. 12.0%), which was consistent with our conclusion. However, the results of another study suggested that haplo-cord-HSCT had a stronger GVL effect and lower 2-year cumulative incidence of relapse than MUD-HSCT (7.5% vs. 21.9%) (21). Wang et al. (42) also revealed that the additional infusion of UCB with a haplo graft reduced the relapse rate of refractory AL due to the graft-versus-graft (GVG) effect, similar to that of double UCB. However, a similar effect was not observed in our study, which may be related to the fact that more high-risk patients were included in the haplo-cord-PBSCT group than in the MSD-PBSCT group (p = 0.02).
Similar OS, DFS, and GRFS in the haplo-cord and MSD-PBSCT groups were obtained in the present study, which is consistent with the results of previous studies (19, 20, 31). In addition, we confirmed that OS, DFS, and GRFS were related to the dosage of CD34+ cells. Törlén et al. also found that the CD34+ dose was associated with survival after allogeneic transplantation for AML/MDS (43).
Our study has some limitations. First, it was a retrospective cohort study, and some imbalances might have existed, although multivariate analysis has adjusted the imbalanced factors. Second, because of the small sample size, we did not compare patients with different diseases and different risk stratification categories. Thus, the sample size needs to be increased to analyze subgroups and design a prospective randomized study for further research. Furthermore, the mechanism of action of UCB in haploidentical transplantation needs to be additionally explored.
In summary, the findings of this study indicate that haploidentical PBSCT supported by third-party cord blood results in OS and cumulative incidences of GVHD, NRM, and relapse, similar to those of MSD PBSCT, although the patients with high-risk features in the haplo-cord-PBSCT group were more than those in the MSD-PBSCT group. Our study also confirmed the safety and efficacy of haplo-cord-PBSCT. In terms of stem cell collection, we were able to avoid the risks of anesthesia, cross-infection, and local hemorrhage during BM harvest. Furthermore, the rapid acquisition of UCB and the simplicity and convenience of PBSC collection were more easily accepted by donors. Therefore, in patients with hematologic malignancies, especially those with high-risk features, haplo-cord-PBSCT provides a viable and promising therapeutic option.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
YX and TC conceived and designed the study and helped to draft the manuscript. YC, YL, XM, and CZ performed the data collection. TC, SW, and XC performed the statistical analysis. All authors read and critically revised the manuscript for intellectual content and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81974002) and Translational Research Grant of NCRCH (No. 2021WWC02).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all of the physicians and nurses for their unevaluated contribution to this study and the patients for participating in this research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.922120/full#supplementary-material
References
1. Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, et al. Use of Haploidentical Stem Cell Transplantation Continues to Increase: The 2015 European Society for Blood and Marrow Transplant Activity Survey Report. Bone Marrow Transplant (2017) 52(6):811–17. doi: 10.1038/bmt.2017.34
2. Niederwieser D, Baldomero H, Szer J, Gratwohl M, Aljurf M, Atsuta Y, et al. Hematopoietic Stem Cell Transplantation Activity Worldwide in 2012 and a Swot Analysis of the Worldwide Network for Blood and Marrow Transplantation Group Including the Global Survey. Bone Marrow Transplant (2016) 51(6):778–85. doi: 10.1038/bmt.2016.18
3. Xu LP, Lu PH, Wu DP, Sun ZM, Liu QF, Han MZ, et al. Hematopoietic Stem Cell Transplantation Activity in China 2019: A Report From the Chinese Blood and Marrow Transplantation Registry Group. Bone Marrow Transplant (2021) 56(12):2940–47. doi: 10.1038/s41409-021-01431-6
4. Wang Y, Wu DP, Liu QF, Xu LP, Liu KY, Zhang XH, et al. Low-Dose Post-Transplant Cyclophosphamide and Anti-Thymocyte Globulin as an Effective Strategy for Gvhd Prevention in Haploidentical Patients. J Hematol Oncol (2019) 12(1):88. doi: 10.1186/s13045-019-0781-y
5. Nagler A, Kanate AS, Labopin M, Ciceri F, Angelucci E, Koc Y, et al. Post-Transplant Cyclophosphamide Versus Anti-Thymocyte Globulin for Graft-Versus-Host Disease Prevention in Haploidentical Transplantation for Adult Acute Lymphoblastic Leukemia. Haematologica (2021) 106(6):1591–98. doi: 10.3324/haematol.2020.247296
6. Chang YJ, Wang Y, Liu YR, Xu LP, Zhang XH, Chen H, et al. Haploidentical Allograft Is Superior to Matched Sibling Donor Allograft in Eradicating Pre-Transplantation Minimal Residual Disease of Aml Patients as Determined by Multiparameter Flow Cytometry: A Retrospective and Prospective Analysis. J Hematol Oncol (2017) 10(1):134. doi: 10.1186/s13045-017-0502-3
7. Yu S, Huang F, Wang Y, Xu Y, Yang T, Fan Z, et al. Haploidentical Transplantation Might Have Superior Graft-Versus-Leukemia Effect Than Hla-Matched Sibling Transplantation for High-Risk Acute Myeloid Leukemia in First Complete Remission: A Prospective Multicentre Cohort Study. Leukemia (2020) 34(5):1433–43. doi: 10.1038/s41375-019-0686-3
8. Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Chen YH, et al. Superior Graft-Versus-Leukemia Effect Associated With Transplantation of Haploidentical Compared With Hla-Identical Sibling Donor Grafts for High-Risk Acute Leukemia: An Historic Comparison. Biol Blood Marrow Transplant (2011) 17(6):821–30. doi: 10.1016/j.bbmt.2010.08.023
9. Yu S, Huang F, Fan Z, Xuan L, Nie D, Xu Y, et al. Haploidentical Versus Hla-Matched Sibling Transplantation for Refractory Acute Leukemia Undergoing Sequential Intensified Conditioning Followed by Dli: An Analysis From Two Prospective Data. J Hematol Oncol (2020) 13(1):18. doi: 10.1186/s13045-020-00859-5
10. Salvatore D, Labopin M, Ruggeri A, Battipaglia G, Ghavamzadeh A, Ciceri F, et al. Outcomes of Hematopoietic Stem Cell Transplantation From Unmanipulated Haploidentical Versus Matched Sibling Donor in Patients With Acute Myeloid Leukemia in First Complete Remission With Intermediate or High-Risk Cytogenetics: A Study From the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica (2018) 103(8):1317–28. doi: 10.3324/haematol.2018.189258
11. Ringdén O, Labopin M, Ciceri F, Velardi A, Bacigalupo A, Arcese W, et al. Is There a Stronger Graft-Versus-Leukemia Effect Using Hla-Haploidentical Donors Compared With Hla-Identical Siblings? Leukemia (2016) 30(2):447–55. doi: 10.1038/leu.2015.232
12. Mayani H, Wagner JE, Broxmeyer HE. Cord Blood Research, Banking, and Transplantation: Achievements, Challenges, and Perspectives. Bone Marrow Transplant (2020) 55(1):48–61. doi: 10.1038/s41409-019-0546-9
13. Shimomura Y, Sobue T, Hirabayashi S, Kondo T, Mizuno S, Kanda J, et al. Comparing Cord Blood Transplantation and Matched Related Donor Transplantation in Non-Remission Acute Myeloid Leukemia. Leukemia (2021) 36(4):1132–38. doi: 10.1038/s41375-021-01474-0
14. Gómez-Santos C, González-Vicent M, Molina B, Deltoro N, Herrero B, Ruiz J, et al. Comparison of Clinical Outcomes Between Unrelated Single Umbilical Cord Blood and “Ex-Vivo” T-Cell Depleted Haploidentical Transplantation in Children With Hematological Malignancies. World J Pediatr (2021) 17(6):609–18. doi: 10.1007/s12519-021-00461-w
15. Fuchs EJ, O’Donnell PV, Eapen M, Logan B, Antin JH, Dawson P, et al. Double Unrelated Umbilical Cord Blood Vs Hla-Haploidentical Bone Marrow Transplantation: The Bmt Ctn 1101 Trial. Blood (2021) 137(3):420–28. doi: 10.1182/blood.2020007535
16. van Besien K, Artz A, Champlin RE, Guarneri D, Bishop MR, Chen J, et al. Haploidentical Vs Haplo-Cord Transplant in Adults Under 60 Years Receiving Fludarabine and Melphalan Conditioning. Blood Adv (2019) 3(12):1858–67. doi: 10.1182/bloodadvances.2019000200
17. Chen J, Wang RX, Chen F, Sun AN, Qiu HY, Jin ZM, et al. Combination of a Haploidentical Sct With an Unrelated Cord Blood Unit: A Single-Arm Prospective Study. Bone Marrow Transplant (2014) 49(2):206–11. doi: 10.1038/bmt.2013.154
18. Li H, Li X, Chen Y, Li D, Chen X, Zhu Z, et al. Sequential Transplantation of Haploidentical Stem Cell and Unrelated Cord Blood With Using Atg/Ptcy Increases Survival of Relapsed/Refractory Hematologic Malignancies. Front Immunol (2021) 12:733326. doi: 10.3389/fimmu.2021.733326
19. Xu J, Zhao R, Yang L, Gong H, Ma S, Chen J, et al. Haploidentical Stem Cells Combined With a Small Dose of Umbilical Cord Blood Transplantation Exert Similar Survival Outcome of Hla-Matched Stem Cells Transplantation in T-Cell Acute Lymphoblastic Leukemia. Bone Marrow Transplant (2020) 55(6):1197–99. doi: 10.1038/s41409-019-0666-2
20. Ke P, Bao XB, Hu XH, Zhuang J, Wu XJ, Liu YJ, et al. Myeloablative Conditioning Regimens With Combined of Haploidentical and Cord Blood Transplantation for Myelodysplastic Syndrome Patients. Bone Marrow Transplant (2018) 53(2):162–68. doi: 10.1038/bmt.2017.229
21. Lyu H, Lu W, Yao J, Xiao X, Li Q, Wang J, et al. Comparison of Outcomes of Haploidentical Donor Hematopoietic Stem Cell Transplantation Supported by Third-Party Cord Blood With Hla-Matched Unrelated Donor Transplantation. Leuk Lymphoma (2020) 61(4):840–47. doi: 10.1080/10428194.2019.1695053
22. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood (2016) 127(20):2391–405. doi: 10.1182/blood-2016-03-643544
23. Brown PA, Shah B, Advani A, Aoun P, Boyer MW, Burke PW, et al. Acute Lymphoblastic Leukemia, Version 2.2021, Nccn Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2021) 19(9):1079–109. doi: 10.6004/jnccn.2021.0042
24. Pollyea DA, Bixby D, Perl A, Bhatt VR, Altman JK, Appelbaum FR, et al. Nccn Guidelines Insights: Acute Myeloid Leukemia, Version 2.2021. J Natl Compr Canc Netw (2021) 19(1):16–27. doi: 10.6004/jnccn.2021.0002
25. Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International Scoring System for Evaluating Prognosis in Myelodysplastic Syndromes. Blood (1997) 89(6):2079–88.
26. Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR, et al. Ebmt-Nih-Cibmtr Task Force Position Statement on Standardized Terminology & Guidance for Graft-Versus-Host Disease Assessment. Bone Marrow Transplant (2018) 53(11):1401–15. doi: 10.1038/s41409-018-0204-7
27. Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring Therapeutic Response in Chronic Graft-Versus-Host Disease. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-Versus-Host Disease: Iv. The 2014 Response Criteria Working Group Report. Biol Blood Marrow Transplant (2015) 21(6):984–99. doi: 10.1016/j.bbmt.2015.02.025
28. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-Versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant (2015) 21(3):389–401.e1. doi: 10.1016/j.bbmt.2014.12.001
29. Ruggeri A, Labopin M, Bacigalupo A, Gülbas Z, Koc Y, Blaise D, et al. Bone Marrow Versus Mobilized Peripheral Blood Stem Cells in Haploidentical Transplants Using Posttransplantation Cyclophosphamide. Cancer (2018) 124(7):1428–37. doi: 10.1002/cncr.31228
30. Ma YR, Zhang X, Xu L, Wang Y, Yan C, Chen H, et al. G-Csf-Primed Peripheral Blood Stem Cell Haploidentical Transplantation Could Achieve Satisfactory Clinical Outcomes for Acute Leukemia Patients in the First Complete Remission: A Registered Study. Front Oncol (2021) 11:631625. doi: 10.3389/fonc.2021.631625
31. Lu W, Jin X, Lyu H, Bai X, Zhu H, Li X, et al. A Prospective Trial Comparing Haploidentical Donor Transplantation With Cord Blood Versus Hla-Matched Sibling Donor Transplantation for Hematologic Malignancy Patients. Cell Transplant (2022) 31:9636897221076050. doi: 10.1177/09636897221076050
32. Bashey A, Zhang MJ, McCurdy SR, St Martin A, Argall T, Anasetti C, et al. Mobilized Peripheral Blood Stem Cells Versus Unstimulated Bone Marrow as a Graft Source for T-Cell-Replete Haploidentical Donor Transplantation Using Post-Transplant Cyclophosphamide. J Clin Oncol (2017) 35(26):3002–09. doi: 10.1200/jco.2017.72.8428
33. Zhao X, Gao F, Zhang X, Wang Y, Xu L, Liu K, et al. Improved Clinical Outcomes of Rhg-Csf-Mobilized Blood and Marrow Haploidentical Transplantation Compared to Propensity Score-Matched Rhg-Csf-Primed Peripheral Blood Stem Cell Haploidentical Transplantation: A Multicenter Study. Sci China Life Sci (2016) 59(11):1139–48. doi: 10.1007/s11427-016-0014-8
34. Xu X, Yang J, Cai Y, Li S, Niu J, Zhou K, et al. Low Dose Anti-Thymocyte Globulin With Low Dose Posttransplant Cyclophosphamide (Low Dose Atg/Ptcy) Can Reduce the Risk of Graft-Versus-Host Disease as Compared With Standard-Dose Anti-Thymocyte Globulin in Haploidentical Peripheral Hematopoietic Stem Cell Transplantation Combined With Unrelated Cord Blood. Bone Marrow Transplant (2021) 56(3):705–08. doi: 10.1038/s41409-020-01047-2
35. Vanichapol T, Pongsakul N, Srisala S, Apiwattanakul N, Chutipongtanate S, Hongeng S. Suppressive Characteristics of Umbilical Cord Blood-Derived Regulatory T Cells After Ex Vivo Expansion on Autologous and Allogeneic T Effectors and Various Lymphoblastic Cells. J Immunother (2019) 42(4):110–18. doi: 10.1097/cji.0000000000000262
36. Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, et al. Umbilical Cord Blood-Derived T Regulatory Cells to Prevent Gvhd: Kinetics, Toxicity Profile, and Clinical Effect. Blood (2016) 127(8):1044–51. doi: 10.1182/blood-2015-06-653667
37. Harada K, Fuji S, Seo S, Kanda J, Ueki T, Kimura F, et al. Comparison of the Outcomes After Haploidentical and Cord Blood Salvage Transplantations for Graft Failure Following Allogeneic Hematopoietic Stem Cell Transplantation. Bone Marrow Transplant (2020) 55(9):1784–95. doi: 10.1038/s41409-020-0821-9
38. Zhang C, Zhang X, Chen XH. Cellular Mechanism for Granulocyte-Colony Stimulating Factor in the Prevention of Graft-Versus-Host Disease in Combined Bone Marrow and Peripheral Blood Transplantation for Hematological Malignancies: The Composition in Collection. Transfus Apher Sci (2013) 48(1):3–9. doi: 10.1016/j.transci.2012.08.004
39. Chang YJ, Zhao XY, Huo MR, Huang XJ. Expression Profiles of Adhesion Molecules on Naïve T Cells in Bone Marrow Grafts of Healthy Donors Treated With Granulocyte Colony-Stimulating Factor. Transpl Immunol (2009) 21(4):228–33. doi: 10.1016/j.trim.2009.05.005
40. Lin R, Wang Y, Huang F, Fan Z, Zhang S, Yang T, et al. Two Dose Levels of Rabbit Antithymocyte Globulin as Graft-Versus-Host Disease Prophylaxis in Haploidentical Stem Cell Transplantation: A Multicenter Randomized Study. BMC Med (2019) 17(1):156. doi: 10.1186/s12916-019-1393-7
41. Massoud R, Gagelmann N, Fritzsche-Friedland U, Zeck G, Heidenreich S, Wolschke C, et al. Comparison of Immune Reconstitution Between Anti-T-Lymphocyte Globulin and Posttransplant Cyclophosphamide as Acute Graft-Versus-Host Disease Prophylaxis in Allogeneic Myeloablative Peripheral Blood Stem Cell Transplantation. Haematologica (2022) 107(4):857–67. doi: 10.3324/haematol.2020.271445
42. Wang J, Wang Z, Wei W, Zhang W, Zhang T, Cheng H, et al. Cord Haploidentical Non-In Vitro T Cell Depletion Allogeneic Hematopoietic Stem Cell Transplantation Reduces Relapse of Refractory Acute Leukemia. Biol Blood Marrow Transplant (2019) 25(1):121–28. doi: 10.1016/j.bbmt.2018.09.002
43. Törlén J, Ringdén O, Le Rademacher J, Batiwalla M, Chen J, Erkers T, et al. Low Cd34 Dose Is Associated With Poor Survival After Reduced-Intensity Conditioning Allogeneic Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transplant (2014) 20(9):1418–25. doi: 10.1016/j.bbmt.2014.05.021
Keywords: haploidentical donor, peripheral blood, cord blood, stem cell transplantation, HLA-matched sibling donor, hematologic malignancy
Citation: Cheng T, Chen Y, Liu Y, Ma X, Zeng C, Chen X, Wang S and Xu Y (2022) Comparison of Outcomes of Haploidentical Peripheral Blood Stem Cell Transplantation Supported by Third-Party Cord Blood Versus Human Leukocyte Antigen-Matched Sibling Peripheral Blood Stem Cell Transplantation in Hematologic Malignancy Patients. Front. Oncol. 12:922120. doi: 10.3389/fonc.2022.922120
Received: 17 April 2022; Accepted: 13 June 2022;
Published: 14 July 2022.
Edited by:
Weili Zhao, Shanghai Jiao Tong University, ChinaReviewed by:
Lucien A. Noens, Ghent University Hospital, BelgiumLinghui Xia, Huazhong University of Science and Technology, China
Copyright © 2022 Cheng, Chen, Liu, Ma, Zeng, Chen, Wang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yajing Xu, xyyajingxu@csu.edu.cn
 Tingting Cheng
Tingting Cheng Yan Chen
Yan Chen Yi Liu1,2,3,4
Yi Liu1,2,3,4 Xia Ma
Xia Ma Cong Zeng
Cong Zeng Xu Chen
Xu Chen Shiyu Wang
Shiyu Wang Yajing Xu
Yajing Xu