- 1College of Medicine and Public Health, Flinders University, Adelaide, SA, Australia
- 2Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States
- 3Department of Medical Oncology, Flinders Centre for Innovation in Cancer, Flinders Medical Centre, Adelaide, SA, Australia
Background: Immune checkpoint inhibitors (ICIs) is the main treatment option for patients with metastatic renal cell carcinoma (mRCC); however, significant heterogeneity in response is commonly observed. This study aimed to evaluate the ability of C-reactive protein (CRP) to predict overall survival (OS) and progression-free survival (PFS) in patients with mRCC treated with immunotherapy.
Patients and Methods: Data from patients with mRCC treated with atezolizumab plus bevacizumab in the IMmotion150 and IMmotion151 trials were pooled. Cox proportional regression was used to model prognostic associations. The relative importance of CRP against International Metastatic RCC Database Consortium (IMDC) factors was confirmed using machine learning.
Results: CRPs were available from 527 patients (mean[range] CRP, 6.3[0.21–340]mg/L). Elevated CRP was significantly associated with worse OS (HR[95%CI], 1.71[1.54–1.90], p<0.001) and PFS (1.27[1.18–1.35], p<0.001). CRP was the most prognostic factor for survival within the available clinicopathological data. The prognostic performance of CRP was superior to IMDC model for OS (CRP c=0.76, IMDC c=0.67, p<0.001) and PFS (CRP OS c=0.62, IMDC c=0.59, p=0.03). Predicted 2-year OS probabilities for patients with CRP values of 0.5, 5, 40, and 150 mg/L were 96%, 73%, 42%, and 23%, respectively.
Conclusions: CRP is a powerful prognostic marker for survival, and its prognostic value was superior to the IMDC risk model. This study highlights that CRP could be implemented as stratification factor for mRCC immunotherapy trials and potentially as an easy-to-use prognostic tool in the clinic.
Introduction
Renal cell carcinoma (RCC) is the most common kidney malignancy, with approximately 20% of patients presenting with metastatic disease at diagnosis (1). Immune checkpoint inhibitors (ICIs) are an established treatment option for metastatic RCC (mRCC). ICIs target the programmed death 1 (PD-1) or the PD-ligand 1 (PD-L1) pathway to remove cytotoxic T-cell inhibitions weakening antitumor immune responses (2). However, at present, there are no markers that accurately predict/prognosticate clinical outcomes of ICI treatment, and significant heterogeneity in response to ICIs remains between patients (2, 3). Therefore, prognostication of clinical outcomes for patients treated with immunotherapy remains of significant clinical interest.
Tumor-associated systemic inflammatory response, consisting of overexpression of proinflammatory cytokines, plays a critical role in cancer cell proliferation, angiogenesis, and metastasis (4, 5). C-reactive protein (CRP) is a clinicopathological marker of systemic inflammation and immune activation and can be readily measured in peripheral blood samples. Elevated CRP has been demonstrated to be a poor prognostic marker in many cancers including mRCC (6–9). While the underlying mechanisms are not yet fully understood, studies suggest that high CRP is correlated with an immunosuppressive tumor microenvironment via the infiltration of immune suppressor cells (including regulatory T cells and tumor-associated microphages) (10, 11). Coincidingly, it is hypothesized that elevated CRP may be associated with a downregulation of the antitumor immune responses of ICIs due to its correlation with immunophenotypes of ICI resistance, tumor growth, and poor prognosis (10, 11). These hypotheses are supported by recent studies highlighting CRP as the most prognostic clinicopathological marker for survival in non-small cell lung cancer (n=751) (12) and urothelial cancer (n=896) (13) cohorts treated with the ICI atezolizumab. As data on the prognostic significance of CRP in patients with mRCC initiating ICI are limited, we sought to investigate the prognostic significance of CRP in patients with mRCC treated with immunotherapy. The prognostic significance of CRP was studied in a combined two clinical trial cohorts of patients receiving atezolizumab plus bevacizumab.
Materials and methods
Study population
Individual participant data from randomized phase 2 IMmotion150 (ClinicalTrials.gov identifier: NCT01984242) and phase 3 IMmotion151 (ClinicalTrials.gov identifier: NCT02420821) clinical trials were utilized for this post-hoc analysis. IMmotion150 involved patients with untreated mRCC randomized 1:1:1 to receive atezolizumab (1,200 mg IV every 3 weeks) with or without bevacizumab (15 mg/kg IV every 3 weeks) versus sunitinib (50 mg orally once daily for 28 days of each 6 weeks cycle) (14). IMmotion151 randomized patients with untreated mRCC to receive atezolizumab (1,200 mg IV every 3 weeks) plus bevacizumab (15 mg/kg IV every 3 weeks) versus sunitinib (50 mg orally once daily for 28 days of each 6 weeks cycle) (15).
Data were accessed according to the Hoffmann–La Roche policy and has been made available through Vivli, Inc. (www.vivli.org). Secondary analysis of de-identified data was confirmed exempt from review by the Southern Adelaide Local Health Network, Office for Research and Ethics, as it was classified as negligible-risk research.
Predictors and outcomes
The primary evaluated outcome was overall survival (OS). Progression-free survival (PFS) was a secondary outcome. OS was defined as the time from randomization to the last follow-up or death from any cause. PFS was assessed by the investigator per the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 (14, 15).
The primary assessed covariate was baseline CRP. The International Metastatic RCC Database Consortium (IMDC) risk tool and pre-treatment levels of hemoglobin, neutrophils, platelets, corrected calcium, and Eastern Cooperative Oncology Group Performance Status (ECOG PS) were available.
Statistical analysis
The atezolizumab plus bevacizumab arms within IMmotion150 and IMmotion151 were used in this post-hoc analysis. Cox proportional hazard regression was used to assess the prognostic associations with OS and PFS. Results were reported as hazard ratios (HRs) with 95% confidence intervals (95% CIs). Continuous variables were explored for potential non-linear associations using restricted cubic splines, and skewed data were log-transformed. Prognostic performance was evaluated using the c-statistic (c) by Harrell (16).
The IMDC risk tool was developed and validated for patients receiving antivascular endothelial growth factor therapy (anti-VEGF) (17). The prognostic performance of CRP was compared to IMDC risk tool and its individual factors (i.e., hemoglobin, neutrophils, platelets, corrected calcium, and ECOG PS). The relative importance of CRP against IMDC factors was confirmed using a machine learning random forest approach (18). The relative importance of variables in the random forest model was determined using a permutation variable importance measures (19), where, on a scale of 0–100, the prognostic strength of a variable is represented. All analyses were stratified, and statistical significance was set to p < 0.05.
Survival probability curves were predicted using flexible parametric survival analysis (20). Exploratory analysis on the sunitinib arms was conducted. A new interactive web-based application incorporating the CRP-prognostic model was developed using the Shiny R package (21). A sensitivity analysis for assessing the prediction performance of CRP using optimal cut points was conducted. Optimal cut points were selected based on maintaining the best discrimination performance compared to using the continuous predictor. All analyses were conducted using the R statistical environment (version 3.6.2).
Results
Study population
A total of 552 patients were randomized to atezolizumab plus bevacizumab within IMmotion150 and IMmotion151 trials, of which 527 (95%) had available pre-treatment CRP (median CRP [range], 6.3 [0.21–340] mg/L). Pre-treatment patient characteristics are presented in Supplementary Table S1. Pre-treatment patient characteristics according to CRP optimal cut groups is presented in Supplementary Table S2. Median (95% CI) follow-up was 19 (18–19) months within the atezolizumab plus bevacizumab cohorts.
Prognostic significance of CRP with survival
The continuous associations of CRP, neutrophils, hemoglobin, platelets, and corrected calcium with OS and PFS within the cohort of patients initiated on atezolizumab plus bevacizumab was best described via a log-linear relationship. Elevated CRP was significantly associated with worse OS (log-CRP HR [95% CI], 1.71 [1.54–1.90], p < 0.001) and PFS (1.27 [1.18–1.35], p < 0.001) (Table 1). According to the c-statistic, elevated CRP was identified as more prognostic than neutrophils, hemoglobin, platelets, corrected calcium levels, and ECOG PS for OS and PFS (Table 1). This finding was validated using a random forest approach, which similarly ranked CRP as the most prognostic variable for OS and PFS (Figure 1).
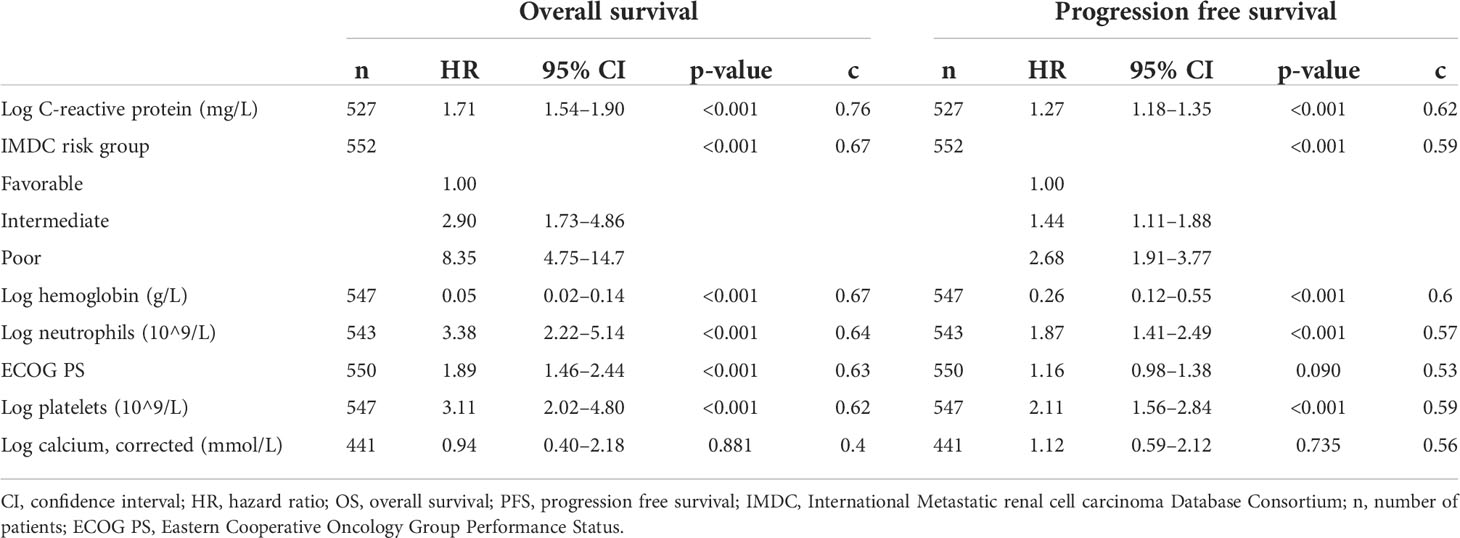
Table 1 Prediction performance and effect size of the association of C-reactive protein and IMDC risk tool with overall survival and progression-free survival for patients treated with atezolizumab plus bevacizumab.
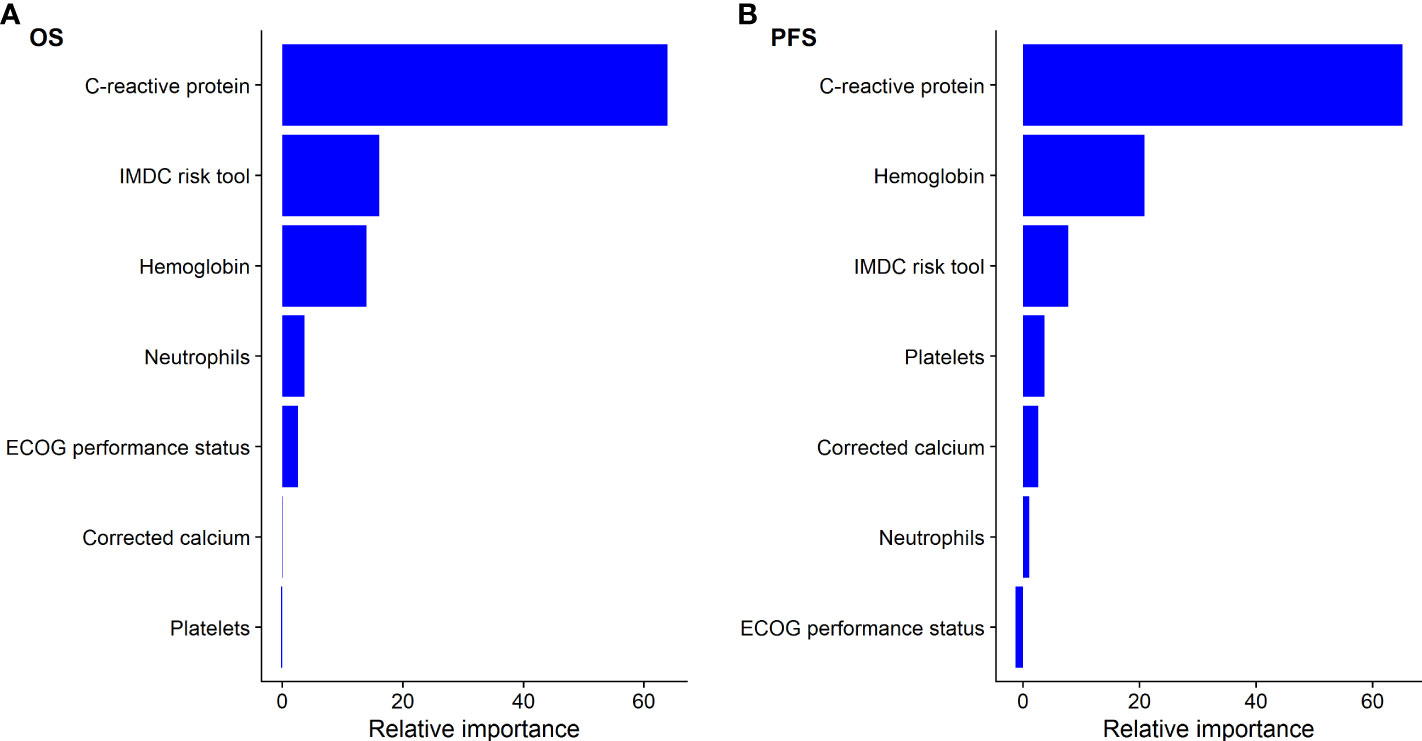
Figure 1 Relative importance of C-creative protein to IMDC factors for predicting (A) overall survival (OS) and (B) progression-free survival (PFS) using random forest for patients treated with atezolizumab plus bevacizumab.
Furthermore, the prognostic performance (mean c, 95% CI) for CRP was superior to IMDC risk tool for OS (CRP c=0.76 [0.73–0.80], IMDC tool c = 0.67 [0.63–0.71], p < 001) and PFS (CRP OS, 0.62 [0.59–0.67], IMDC tool c = 0.59 [0.56–0.62], p = 0.03). Demonstrating the higher discrimination of CRP to IMDC risk tool, the 2-year OS probability for the range of reported CRP data (min CRP, 0.2 and max, 340 mg/ml) ranged from 97% to 15%. Comparatively, the 2-year OS probability for the “favorable” versus “poor” IMDC risk groups ranged from 85%% to 33% (Supplementary Figure S1). Similar findings were identified within the sunitinib-treated cohort (Supplementary Table S3; Supplementary Figures 2, 3).
Figure 2 presents predicted survival curves of OS and PFS according to pre-treatment CRP levels within the analysis cohort of patients initiated on atezolizumab plus bevacizumab treatment. Figure 2 demonstrates that pre-treatment CRP levels of 0.5, 5, 40, and 150 mg/L were associated with 2-year OS probabilities of 96%, 73%, 42%, and 23%, respectively, and 2-year PFS probabilities of 44%, 26%, 14%, and 9%. Further predictions of OS and PFS prognosis according to pre-treatment CRP levels can be estimated using the interactive web-based application at https://pmg-flinders.shinyapps.io/crpprognostic/.
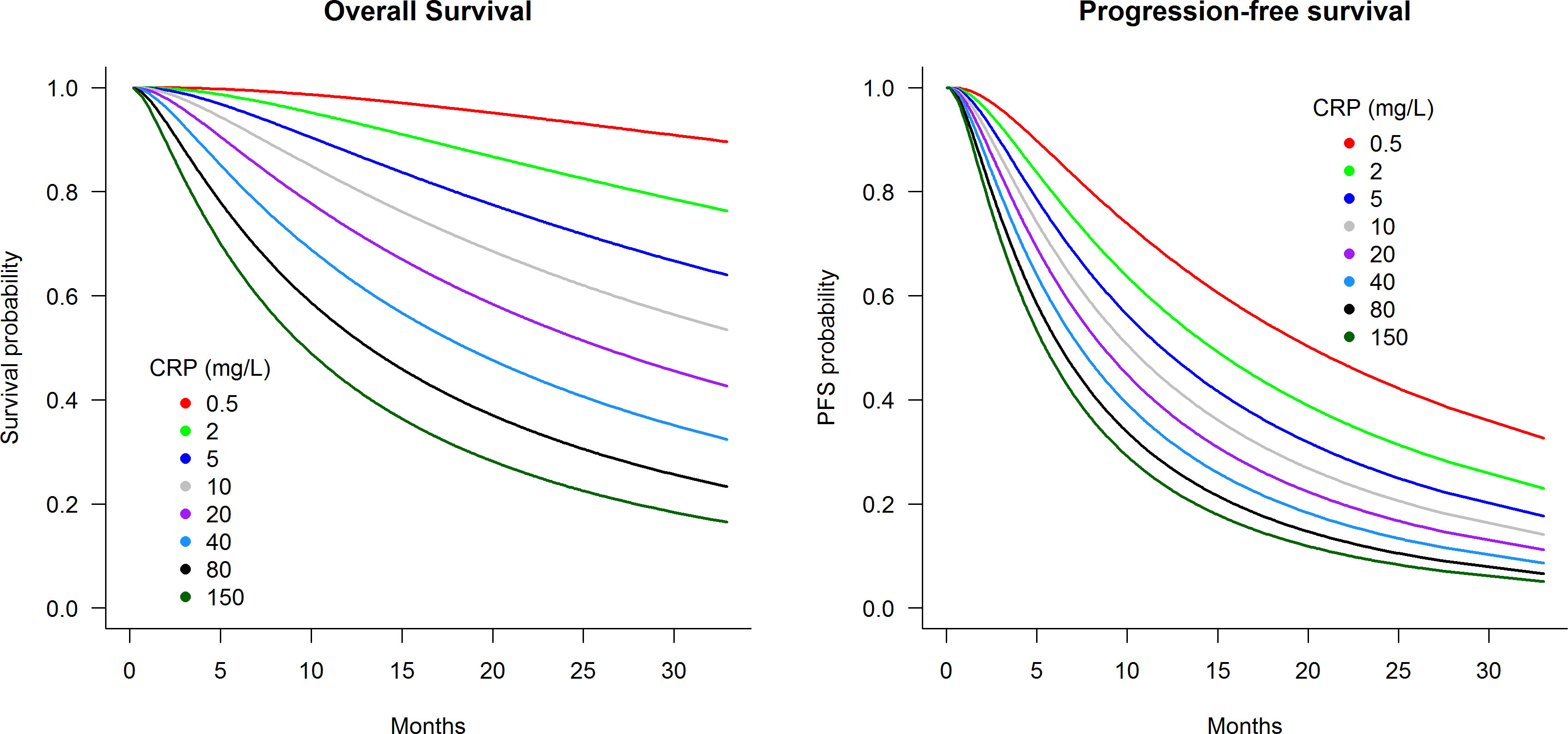
Figure 2 Predicted survival curves according to pre-treatment C-reactive protein level for patients treated with atezolizumab plus bevacizumab.
As a sensitivity analysis, CRP was optimally cut into three groups (<5, ≥5 <30, and ≥30 mg/L). The HR and prognostic performance of CRP groups are presented in Supplementary Table S3 and Kaplan–Meir plots in Supplementary Figure S4. The OS prognostic performance of CRP groups (c=0.75) was substantially higher than the IMDC risk tool (c=0.67). Furthermore, CRP was associated with significantly reduced objective response rate both when used as a linear predictor (OR [95%CI]: 0.86[0.77–0.96]) and when using CRP optimal cut groups (Supplementary Table S4).
Discussion
Using pooled data from two clinical trials, this study demonstrated that CRP is a strong prognostic marker for survival in patients with mRCC initiating atezolizumab ICI treatment. Furthermore, the performance of CRP as a single factor was demonstrated to be superior to the IMDC risk tool comprised of six factors.
Cancer-related inflammation plays an important role in the progression of tumors and survival of patients with cancer (22, 23). The tumor microenvironment can trigger the release of key proinflammatory mediators including interleukin-6 (IL-6), IL-1, and IL-1β, which stimulate hepatocyte CRP production, leading to a marked increase in plasma CRP (24–26). Furthermore, various studies have demonstrated the ability of RCC cells to locally express CRP, which may also contribute to plasma CRP elevation (27, 28). Elevated CRP has therefore been used as a marker of systemic inflammation with substantial evidence, suggesting that elevated CRP is predictive of poor prognosis in patients with multiple malignancies including mRCC (8, 9, 24). Although the underlying mechanism of CRP correlation with poor prognosis is not fully elucidated, studies suggest that elevated CRP is associated with infiltration of immune suppressor cells including regulatory T cells and tumor-associated macrophages (10, 11). Previous subgroup meta-analysis studies of patients with RCC have identified CRP as a prognostic marker for OS and PFS (6–8). However, the RCC studies included in the meta-analysis were often limited by a small sample size and were based on data from patients who have undergone nephrectomy or were treated with targeted therapies. To our knowledge, the presented study is the first study to compare CRP to the IMDC risk tool and the first to demonstrate CRP as the most prognostic marker for survival in patients with mRCC initiating ICI treatment. This finding is similar to that of prior research for non-small-cell lung cancer and urothelial cancer where CRP was demonstrated as the most prognostic variable in patients initiating ICI treatment (13, 29).
Although including CRP as an additional factor to the IMDC risk tool improved the prognostic performance (c=0.77) compared to the IMDC risk tool (c=0.67), the prognostic performance of CRP as a single factor (c=0.76) was comparable to the combined CRP-IMDC risk model. Therefore, the focus of this paper was on using CRP as a single factor rather than updating the existing IMDC risk model to a seven-factor model.
Currently, there is no recommendation to check CRP prior to initiating treatment for ICI in metastatic RCC. The CRP-prognostic tool presented herein is intended for its prognostic value for immunotherapy rather than being predictive to different types of treatments. This study identified strong capacity of CRP in predicting OS and PFS, which outperformed the IMDC risk tool comprised of six factors. Patients with CRP levels of 0.5, 5, 40, and 150 mg/L had predicted median 2-year OS probabilities of 96%, 73%, 42%, and 23%, respectively, and 2-year PFS probabilities of 44%, 26%, 14%, and 9%. Such prognostic power highlights that CRP should be considered as a stratification factor for the design of ICI trials and as a marker to provide realistic expectations to patients initiating ICI treatment. Similar findings were identified in the sunitinib-treated cohort consistent with prior studies (30, 31).
A potential study limitation is that the analysis was only focused on patients treated with atezolizumab plus bevacizumab. Confirming the prognostic association of CRP with survival for other ICIs and combination of immunotherapy/kinase inhibitors is a future direction of research. Furthermore, clinical trial inclusion criteria may limit the generalizability of findings to real-world patient populations. For example, IMmotion150 and IMmotion151 were restricted to patients with Karnofsky performance score ≥70, no history of autoimmune diseases, and no active hepatitis B/C infection or significant cardiovascular disease (14, 15). Future research should validate the prognostic association of CRP in real-world populations to allow implementation of CRP prognostic model for mRCC in the clinic.
Conclusion
CRP was identified as the most prognostic marker of OS and PFS outcomes for patients with mRCC treated with atezolizumab plus bevacizumab. The study highlights that CRP could be considered as a stratification factor for immunotherapy trials and explored as a prognostic tool for mRCC in the clinic.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Data was accessed according to Hoffmann-La Roche policy and has been made available through Vivli, Inc (www.vivli.org). Requests to access these datasets should be directed to Vivli, Inc (www.vivli.org).
Ethics statement
The studies involving human participants were reviewed and approved by Data was accessed according to Hoffmann-La Roche policy and has been made available through Vivli, Inc (www.vivli.org). Secondary analysis of de-identified data was confirmed exempt from review by the Southern Adelaide Local Health Network, Office for Research and Ethics as it was classified as negligible risk research. The patients/participants provided their written informed consent to participate in this study.
Authors contributions
AA, JB, AH, MS, RM, GK, and AR contributed to conception, design, assembly of data, data analysis, and interpretation. AA, RM, AH, MS, and AR wrote the main manuscript text. All authors reviewed and approved the manuscript. All authors contributed to the article and approved the submitted version.
Funding
RM, AR, and MS are supported by the Beat Cancer Research Fellowships from Cancer Council South Australia. AH is supported by a Postdoctoral Fellowship from the National Breast Cancer Foundation, Australia (PF-17-007).
Acknowledgments
This publication is based on research using de-identified individual participant data from data contributor Hoffmann-La Roche that have been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication.
Conflict of interest
RM, AR, and MS report investigator-initiated project grants from Pfizer outside the submitted work. AA, JB, GK, and AH have no conflicts of interest to disclose. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript or in the decision to publish the results.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.918993/full#supplementary-material
References
1. Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, et al. Epidemiology of renal cell carcinoma. Eur Urol (2019) 75:74–84. doi: 10.1016/j.eururo.2018.08.036
2. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annu Rev Pathol (2021) 16:223–49. doi: 10.1146/annurev-pathol-042020-042741
3. Perrier A, Didelot A, Laurent-Puig P, Blons H, Garinet S. Epigenetic Mechanisms of Resistance to Immune Checkpoint Inhibitors. Biomolecules. (2020) 10:1–30. doi: 10.3390/biom10071061
5. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol (2014) 15:e493–503. doi: 10.1016/S1470-2045(14)70263-3
6. Dai J, Tang K, Xiao W, Yu G, Zeng J, Li W, et al. Prognostic significance of c-reactive protein in urological cancers: A systematic review and meta-analysis. Asian Pac J Cancer Prev (2014) 15:3369–75. doi: 10.7314/APJCP.2014.15.8.3369
7. Zhou L, Cai X, Liu Q, Jian ZY, Li H, Wang KJ, et al. Prognostic role of c-reactive protein in urological cancers: A meta-analysis. Sci Rep (2015) 5:12733. doi: 10.1038/srep12733
8. Wang Z, Peng S, Wang A, Xie H, Guo L, Jiang N, et al. C-reactive protein is a predictor of prognosis in renal cell carcinoma patients receiving tyrosine kinase inhibitors: A meta-analysis. Clin Chim Acta (2017) 475:178–87. doi: 10.1016/j.cca.2017.10.021
9. Saito K, Kihara K. Role of c-reactive protein in urological cancers: a useful biomarker for predicting outcomes. Int J Urol (2013) 20:161–71. doi: 10.1111/j.1442-2042.2012.03121.x
10. Riedl JM, Barth DA, Brueckl WM, Zeitler G, Foris V, Mollnar S, et al. C-reactive protein (CRP) levels in immune checkpoint inhibitor response and progression in advanced non-small cell lung cancer: A bi-center study. Cancers (Basel) (2020) 12:1–21. doi: 10.3390/cancers12082319
11. Nakayama T, Saito K, Kumagai J, Nakajima Y, Kijima T, Yoshida S, et al. Higher serum c-reactive protein level represents the immunosuppressive tumor microenvironment in patients with clear cell renal cell carcinoma. Clin Genitourin Cancer (2018) 16:e1151–8. doi: 10.1016/j.clgc.2018.07.027
12. Hopkins AM, Kichenadasse G, Garrett-Mayer E, Karapetis CS, Rowland A, Sorich MJ. Development and validation of a prognostic model for patients with advanced lung cancer treated with the ICI atezolizumab. Clin Cancer Res (2020) 26(13):3280–86. doi: 10.1158/1078-0432.CCR-19-2968
13. Abuhelwa AY, Kichenadasse G, McKinnon RA, Rowland A, Hopkins AM, Sorich MJ. Machine learning for prediction of survival outcomes with immune-checkpoint inhibitors in urothelial cancer. Cancers (Basel) (2021) 13:1–9. doi: 10.3390/cancers13092001
14. McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med (2018) 24:749–57. doi: 10.1038/s41591-018-0053-3
15. Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet (2019) 393:2404–15. doi: 10.1016/S0140-6736(19)30723-8
16. Harrell FE Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med (1996) 15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4
17. Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol (2009) 27:5794–9. doi: 10.1200/JCO.2008.21.4809
19. Ishwaran H. Variable importance in binary regression trees and forests. Electronic J Stat (2007) 1:519–37. doi: 10.1214/07-EJS039
20. Jackson C. Flexsurv: A platform for parametric survival modeling in r. J Stat software (2016) 70(8):1–33. doi: 10.18637/jss.v070.i08
21. Chang W, Cheng J, Allaire J, Xie Y, McPherson JJ. Shiny: Web application framework for r, Vol. 1. (2017). p. 2017.
22. Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest (2015) 125:3347–55. doi: 10.1172/JCI80007
23. Kim SH, Kwon WA, Kim S, Joung JY, Seo HK, Lee KH, et al. The neutrophil-to-lymphocyte ratio makes the heng risk model improve better the prediction of overall survival in metastatic renal cell cancer patients. Jpn J Clin Oncol (2018) 48:835–40. doi: 10.1093/jjco/hyy098
24. Saito K, Kihara K. C-reactive protein as a biomarker for urological cancers. Nat Rev Urol (2011) 8:659–66. doi: 10.1038/nrurol.2011.145
25. Kushner I, Jiang SL, Zhang D, Lozanski G, Samols D. Do post-transcriptional mechanisms participate in induction of c-reactive protein and serum amyloid a by IL-6 and IL-1? Ann N Y Acad Sci (1995) 762:102–7. doi: 10.1111/j.1749-6632.1995.tb32318.x
26. Li N, Tian GW, Wang Y, Zhang H, Wang ZH, Li G, et al. Prognostic role of the pretreatment c-reactive Protein/Albumin ratio in solid cancers: A meta-analysis. Sci Rep (2017) 7:41298. doi: 10.1038/srep41298
27. Jabs WJ, Busse M, Krüger S, Jocham D, Steinhoff J, Doehn C, et al. Expression of c-reactive protein by renal cell carcinomas and unaffected surrounding renal tissue. Kidney Int (2005) 68:2103–10. doi: 10.1111/j.1523-1755.2005.00666.x
28. Johnson TV, Ali S, Abbasi A, Kucuk O, Harris WB, Ogan K, et al. Intratumor c-reactive protein as a biomarker of prognosis in localized renal cell carcinoma. J Urol (2011) 186:1213–7. doi: 10.1016/j.juro.2011.06.014
29. Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol (2020) 6:512–8. doi: 10.1001/jamaoncol.2019.5241
30. Pilskog M, Beisland C, Akslen LA, Bostad L, Haug Å, Heinrich D, et al. Predictive value of c-reactive protein in patients treated with sunitinib for metastatic clear cell renal cell carcinoma. BMC Urol (2017) 17:74. doi: 10.1186/s12894-017-0267-6
Keywords: C-reactive protein, renal cell carcinoma, survival prognosis, immunotherapy, IMDC model
Citation: Abuhelwa AY, Bellmunt J, Kichenadasse G, McKinnon RA, Rowland A, Sorich MJ and Hopkins AM (2022) C-reactive protein provides superior prognostic accuracy than the IMDC risk model in renal cell carcinoma treated with Atezolizumab/Bevacizumab. Front. Oncol. 12:918993. doi: 10.3389/fonc.2022.918993
Received: 13 April 2022; Accepted: 30 June 2022;
Published: 01 August 2022.
Edited by:
Saby George, University at Buffalo, United StatesReviewed by:
Viktor Grünwald, Universitätsklinikum Essen, GermanyBassel Nazha, Department of Hematology and Medical Oncology, School of Medicine (EU), United States
Copyright © 2022 Abuhelwa, Bellmunt, Kichenadasse, McKinnon, Rowland, Sorich and Hopkins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashley M. Hopkins, Ashley.hopkins@flinders.edu.au; Joaquim Bellmunt, jbellmun@bidmc.harvard.edu
†These authors have contributed equally to this work
‡ORCID: Ahmad Y. Abuhelwa, orcid.org/0000-0002-4182-065X
Ashley M. Hopkins, orcid.org/0000-0001-7652-4378
 Ahmad Y. Abuhelwa
Ahmad Y. Abuhelwa Joaquim Bellmunt2*
Joaquim Bellmunt2* Ganessan Kichenadasse
Ganessan Kichenadasse Ross A. McKinnon
Ross A. McKinnon Andrew Rowland
Andrew Rowland Michael J. Sorich
Michael J. Sorich Ashley M. Hopkins
Ashley M. Hopkins