- Department of Urology, The Forth Hospital of China Medical University, Shenyang, China
Bladder cancer is one of the most common malignant tumors in urinary system. Intravesical chemotherapy is a common adjuvant therapy after transurethral resection of bladder tumors. However, it has several disadvantages such as low drug penetration rate, short residence time, unsustainable action and inability to release slowly, thus new drug delivery and new modalities in delivery carriers need to be continuously explored. Nano-drug delivery system is a novel way in treatment for bladder cancer that can increase the absorption rate and prolong the duration of drug, as well as sustain the action by controlling drug release. Currently, nano-drug delivery carriers mainly included liposomes, polymers, and inorganic materials. In this paper, we reveal current researches in nano-drug delivery system in bladder cancer intravesical chemotherapy by describing the applications and defects of liposomes, polymers and inorganic material nanocarriers, and provide a basis for the improvement of intravesical chemotherapy drugs in bladder cancer.
Introduction
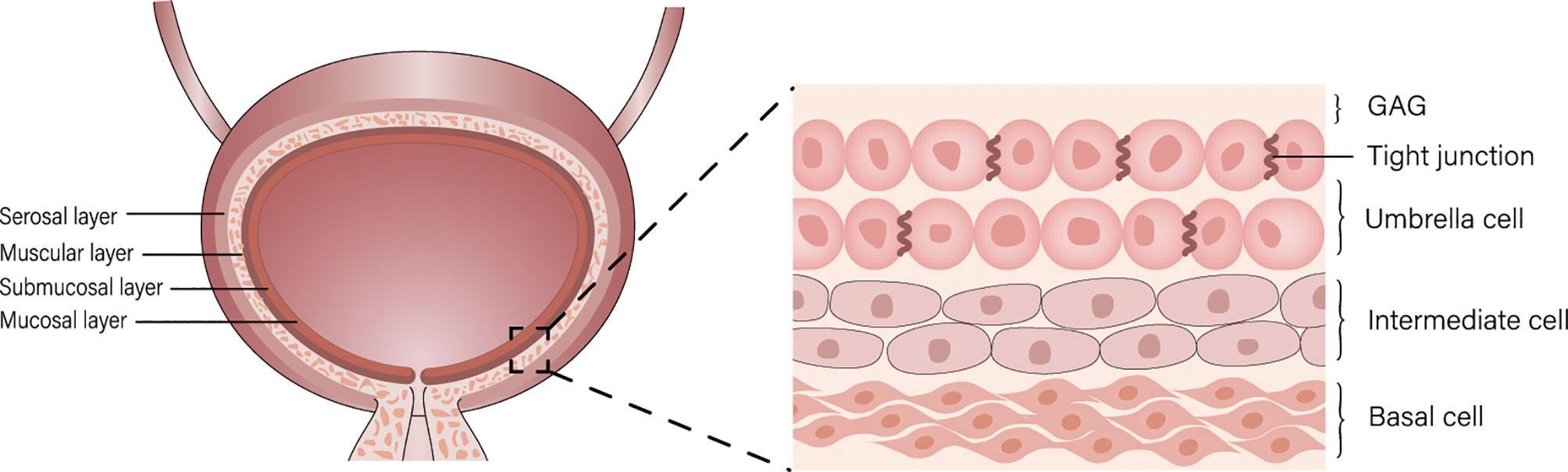
Bladder cancer (BC) is a common disease of the urinary tract, and its incidence ranks tenth in the world among oncological diseases (1). Approximately 75% of patients with BC present with disease confined to the mucosa (Ta or CIS) or submucosa (T1) (2), transurethral resection of bladder tumor (TURBt) is a common treatment for bladder cancer and often following intravesical chemotherapy (3). However, the bladder permeability barrier (BPB) including umbrella cells and glycosaminoglycans (GAG) on its surface affect drug penetration, and the regular emptying of the bladder dilutes or excretes the drug, resulting in a short drug residence time, which makes intravesical chemotherapy less efficient (Figure 1) (4). Therefore, new drug delivery systems are urgently needed to be developed.
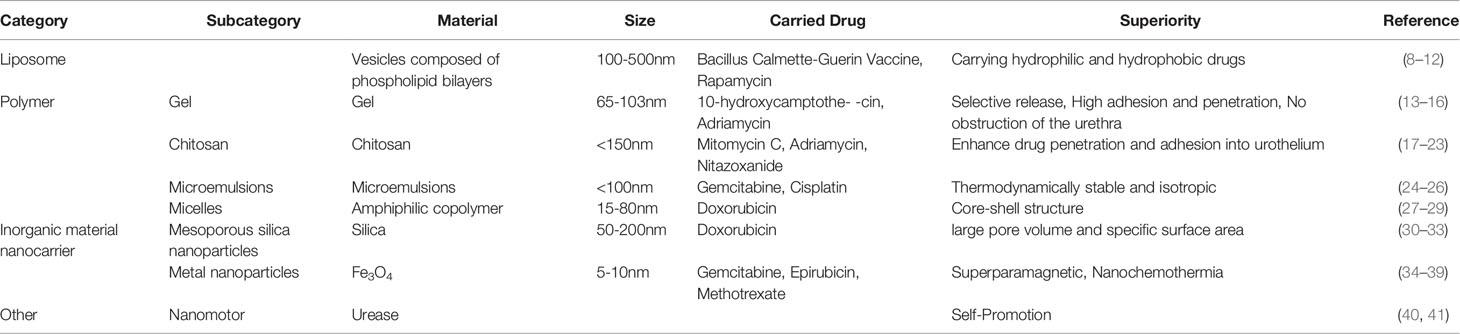
In the field of medicine and pharmacology, nanoparticles are substances with diameters of 1-200 nm and 1-1000 nm separately. Nanomaterials are currently used to construct drug delivery carriers as a novel drug delivery system. Liposomes, polymers, and inorganic nanomaterials are the most dominant drug delivery carriers currently. These carriers exert longer duration of action and reduce side effects by controlling drug release, and can encapsulate multiple drugs to achieve combination therapy, which helps to improve the efficacy of intravesical chemotherapy (5–7). To provide a basis for the improvement of bladder cancer intravesical chemotherapy, we elaborate current researches on the application of nano-drug delivery systems. (Table 1)
Liposomes
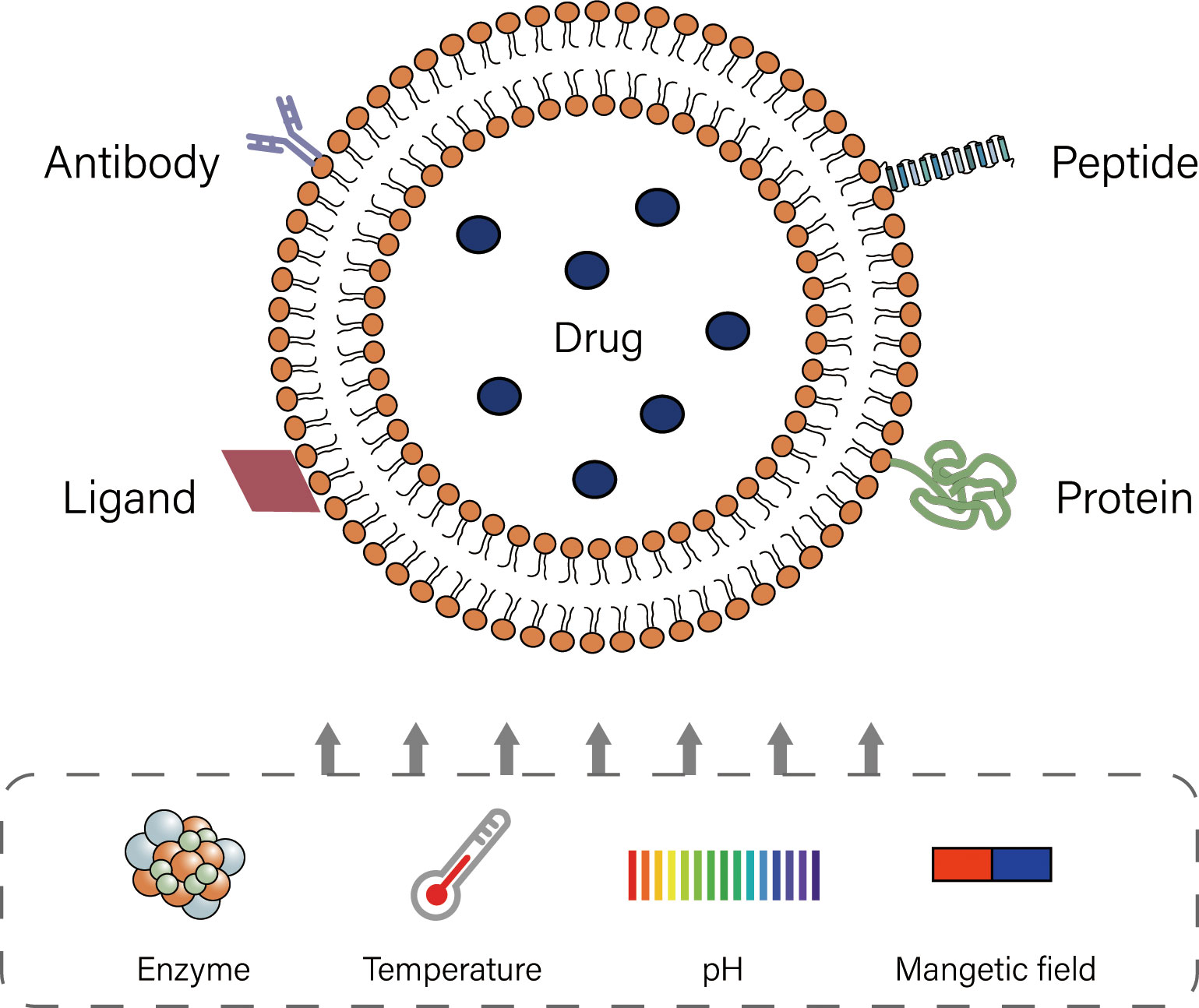
Liposomes are lipid based on spherical shaped vesicular systems, in which a lipophilic bilayer is sandwiched between two hydrophilic layers (8, 9). The biodegradability, biocompatibility and high encapsulation rate of liposomes, as well as their relatively simple production and stable properties, are of great significance in the clinical application of drug delivery (42, 43). During drug delivery, dynamic changes in the in vivo microenvironment of liposomes can promote the release of drug at specific locations or control the release in targeted tissues, which is known as “triggered release” (44). Liposomes mainly include thermosensitive, pH-sensitive, ultrasound-sensitive, enzyme-triggered, magnetic field-sensitive and ligand-targeted liposomes (45). Most liposomes currently used in infusion chemotherapy for bladder cancer are ligand-targeted (Figure 2). Though modification, liposomes show excellent effect in intravesical chemotherapy.
Modified liposomes can effectively overcome the poor water solubility of some drugs, which affects the cellular uptake rate and perfusion effect (46). Bacillus Calmette-Guérin (BCG) is an important agent for intravesical chemotherapy in non-muscle-invasive bladder cancer, but it has greater local or systemic adverse effects (47). BCG cell wall skeleton (BCG-CWS) has relatively few adverse effects and can replace live BCG for bladder perfusion, but it is poor in water solubility and low in cancer cells uptake (10). Nakamura et al. (10) obtained homogeneous and water-soluble nanoparticles (CWS-NP/LEEL) by the liposome evaporated via emulsified lipid (LEEL) method. CWS-NP/LEEL has a high uptake rate and significant tumor suppression ability in rats. Although the drug-loaded liposome preparation was enhanced by the addition of stearylated octaarginine (STR-R8) to enhance internalization, the problem of targeted drug uptake by cancer cells was not addressed.
Taking advantage of the high metabolism of tumors, modification of liposomes by specific metabolites can improve the targeted uptake of drugs by tumors. Folic acid (FA) is essential for tumor cell growth, and it has the advantages of high receptor affinity, small size, economy, and stability. In addition, folate receptor (FR) is deficiently expressed in normal cells and abundantly expressed on a variety of tumor cancer cells (48–52). Consequently, Yoon et al. (11) used folic acid (FA) and the cell-penetrating peptide (Pep1) to improve the encapsulation of LEEL and changed the solvent from pentane to dichloromethane to form a novel vector CWS-FPL (FA- and Pep1-modified liposomes). FA contributes to targeted drug delivery to cancer cells, and positively charged Pep1 facilitates drug delivery into the cells. The mean fluorescence intensity (MFI) of CWS-FPL uptake rates in both 5637 and MBT2 cell lines was significantly higher (138.26 and 132.59) than that of ordinary liposomes (34.95 and 35.2). CWS-FPL also significantly inhibited tumor growth. Rapamycin (Rap) encapsulated in FA-modified liposomes (R-FL) can effectively remedy its disadvantage of poor water solubility and improve the tumor killing ability. Co-cultured with low doses of Rap, plain encapsulated liposomes (R-CL) or R-FL for 48 h, the bladder cancer cell viability varied, Rap and R-CL decreased the viability less than 10%, while R-FL decreased the viability by 40%, demonstrating that R-FL performed a significant cytotoxic effect. This tumor inhibition ability resulted from the increased cell adhesion by FA modification (12).
Functionalized liposome also improves the poor retention and poor permeation of the drug in regular urination of the bladder (53). Kaldybekov et al. (54) explored maleimide-functionalized PEGylated liposomes (PEG-Mal) with efficient mucosal adhesion and penetration ability. PEG-Mal WO50 (wash out50, volume of artificial urine required to wash out 50% of liquid formulation) (55) was significantly higher than that of conventional liposomes (48 ml versus 15 ml), reflecting the excellence in vitro retention, fluorescence microscopy of PEG-Mal and conventional liposomes in the porcine bladder mucosa also showed good mucosal penetration, the release time of PEG-Mal was up to 8 h, which is significantly higher than the 2h saturation release time of conventional liposomes. This method effectively solves the problem of drug dilution and rapid loss due to urination.
Polymers
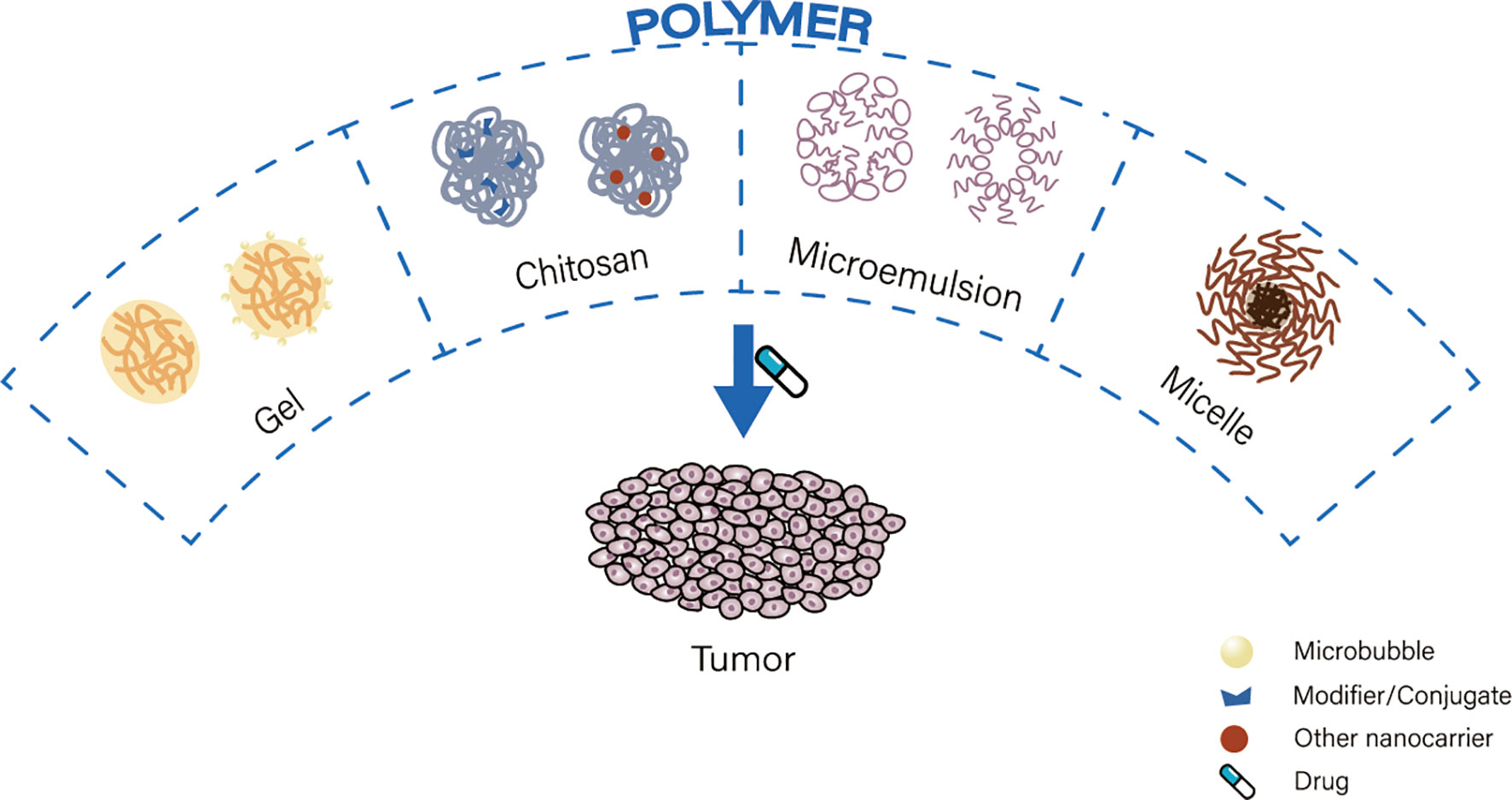
Polymers are macromolecules composed of repeating subunits (56). Drug delivery carriers composed of polymers are characterized by controlled release times, biocompatibility, and hydrophilic and hydrophobic selective release (57). Polymeric carriers commonly used for nano-drug delivery are gels, chitosan, microemulsions and micelles (Figure 3).
Gels
Gels are a hydrophilic three-dimensional polymer network (58) and are classified as macrogels, microgels and nanogels according to their size (59). Gels have sufficient adhesion to the uroepithelial mucosa and remain attached to the bladder wall after urination, which can avoid repeated drug instillation (60, 61). Nanogel delivery system can achieve floating or smart release, slow drug release and reduce lumen obstruction, this delivery system is appliable in intravesical chemotherapy (62–66).
The modified gel nanomaterials provide intelligent drug release and enhanced drug adhesion and penetration. Guo et al. synthesized a positively charged disulfide-core-crosslinked polypeptide nanogel of poly(L-lysine)–poly(L-phenylalanine-co-L-cystine) (PLL-P(LP-co-LC)) to form a drug-loaded nanogel (NG/HCPT) based on 10-Hydroxycamptothecin (HCPT). The release of NG/HCPT was slow in normal tissues and accelerated in tumor sites. Confocal laser scanning microscopy (CLSM) of whole bladder wall sections showed that the optical density of NG/HCPT at 0.5h, 2h and 6h was 1.7 times, 2.5 times and 5.3 times higher than that of free HCPT, reflecting good permeability. This carrier maintained a high concentration in bladder tissue and could penetrate the bladder wall, and the anti-tumor effect was obvious in animal model (13). In response to reduced efficacy in some patients after repeated treatments, Guo et al. also synthesized a smart disulfide-crosslinked polypeptide nanogel of poly-(l-lysine)-poly(l-phenylalanine-co-l-cystine), which triggered the breakage of the disulfide bond by high intracellular concentration of glutathione, and led to the selective release of hydroxycamptothecin from NG/HCPT (14). Then Guo et al. synthesized a new optimized oligoarginine-poly(ethylene glycol)-poly(L-phenylalanine-L-cysteine) nanogels (R9-PEG-P(LP-co-LC)). The PEG significantly improved the dispersion of the particles in water, and the non-specific interaction of PEG chains with bladder mucosa and the electrostatic interaction between cationic R9 and negatively charged bladder mucosa further enhanced the adhesion of the gels. Besides, as a cell-penetrating peptide, R9 effectively penetrated cell membranes and delivered drugs with more optimized antitumor effects than previous nanogels (67).
A floating gel modified by chemical reaction can compensate for ordinary gels defects that dislodged gel has direct contact irritation to bladder mucosa and can obstruct the urethra (68). A floating gel consisting of adriamycin (ADR), poloxamer 407 (P407) and NaHCO3 was developed, in which NaHCO3 produces microbubbles to float the gel in an acidic environment. This nanogel can avoid the possible direct contact and obstruction of the urinary tract (15). In addition, NH4HCO3 instead of NaHCO3 in modified floating gel can achieve temperature-controlled release. The microbubbles can be produced spontaneously at body temperature (37°C) and the gel float. This process was confirmed by ultrasound in rabbit bladder (16).
Chitosan
Chitosan (CS) nanoparticles are applicable in drug delivery, transport, targeted drug uptake due to their excellent bioadhesion and permeability, unique polycationic, non-toxic and bioresorbable properties (69, 70). Similar to gel systems, chitosan plays an important role in bladder cancer perfusion therapy by modification through different chemical reactions or in combination with specific bioactive nanomaterials (71–76).
By coupling different substances, chitosan shows significant advantages in increasing the adhesion rate of perfused drugs. Some researchers have pioneered the reaction of chitosan with methacrylic anhydride to prepare methacrylate chitosan, and detected the adhesion and cytotoxicity on isolated porcine bladder. This methacrylate chitosan had a best adhesion effect compared with dextran and chitosan, and the adhesion ability depended on the methylation degree (17). Kolawole et al. (18) prepared high molecular weight chitosan (HCHI) with β-glycerophosphate to form an new nanocarrier, which was transparent and non-coagulable at room temperature. This nanocarrier binds to mitomycin C and forms a mucoadhesive gel layer with a large bladder area at elevated temperatures, allowing prolonged drug diffusion. However, this study was performed in isolated bladders filled with artificial urine, the specific adhesion, release efficiency and other potential defects need to be further verified and explored. In addition, the synthetic borate-coupled chitosan derivatives (FS/LBCHI) exhibited significantly elevated mucoadhesive properties by applying coupling agents to interact chitosan with 4-carboxyphenylboronic acid. It can be seen that coupling with modified chitosan significantly improves the adhesion of perfused drugs (19).Moustafa et al. used tripolyphosphate (TPP)-treated chitosan and loaded with nanodiamond-bound doxorubicin (DOX), which showed superior cytotoxic effects on human bladder cancer cells than DOX or NDs alone and increased drug retention in the isolated bovine bladder wall, suggesting that this carrier may enhance the drug effect of bladder perfusion (20, 21).
Besides, specific chitosan nanocarriers can increase chemotherapeutic drug bioavailability and enhance tumor suppression. Tumor-selective photosensitizer dyes in photodynamic therapy are retained and accumulated by aberrant or overproliferating cells (e.g., tumor cells) and combined with tissue oxygen and targeted illumination, intracellularly produce cytotoxic reactive oxygen species (ROS) to kill tumor cells and selectively destroy tissue in the diseased area (77, 78). Specific chitosan nanocarriers based on photodynamic therapy can increase the effect of chemotherapeutic agents. Nitazoxanide (NTZ) and chlorine e6 (Ce6)-conjugated human serum albumin (HSA-Ce6) formed self-assembled human serum albumin-Ce6/NTZ nanoparticles (NPs), which were further compounded across the mucosal carrier fluorinated chitosan (FCS) to form HSA- Ce6/NTZ/FCS nanoparticle. The highest AMPK α phosphorylation levels were detected in cells treated with this nanoparticle, which effectively improved tumor tissue hypoxia and inhibited tumor cell overproliferation. The 5-week survival rate of mice with in situ bladder cancer perfused with this nanoparticle reached 83%, which was significantly prolonged compared to 17%-33% in other treatment groups, and could effectively alleviate the effect of tumor hypoxia on drug resistance (22). Manan et al. developed a novel Mn : ZnS quantum dot-bound chitosan nanocarrier (CS-Mn : ZnS), which can load drug such as Mitomycin C (MMC) and promote engulfment of drug into target tumor cells efficiently and continuously. This chitosan nanocarrier improves the bioavailability of drugs, and it is possible to be a practical tool for intravesical perfusion chemotherapy (23).
Microemulsions
Microemulsions are spontaneously formed single-phase dispersion systems, which are thermodynamically stable and isotropic. The small and uniform particle size of microemulsion can improve the dispersion of the drug sealed in it and promote the transdermal absorption of the drug (24, 25). Chen et al. (26) used a microemulsion carrier to carry gemcitabine and cisplatin. This carrier was chemically and physically stable, with significantly better permeability than the corresponding aqueous solution and significantly less bladder irritation than the chemotherapeutic drug alone.
Micelles
Micelles are colloidal systems formed spontaneously by amphiphilic copolymers in aqueous media (27). In aqueous environment, hydrophobic chain segments gather each other to form a hydrophobic core due to the exclusion of water molecules, while hydrophilic chains surround them to form a hydrophilic layer, eventually forming micelles with a core-shell structure that remains stable in water. Unlike liposomes, micelles have only one lipid layer, ranging in size from 15 nm to 80 nm (28). DOX and IR780 dyes (a near infrared dye) were used to form self-assembled micelles (DOX&IR780@PEG-PCL-SS NPs) with PEG-PCL-SS (An amphiphilic copolymer containing disulfide bonds) and cross-linked them internally to form nanoparticles under disulfide bonding (DTT) catalytic conditions. The DOX release rate was faster in bladder cancer cells with high GSH concentration. In addition, the photothermal effect of the nanoparticles by the photosensitizer IR780 could also greatly promote the release of the drug. The good photothermal properties and tumor targeting of this micelle gives it a greater advantage in bladder intravesical chemotherapy (29).
Inorganic Material Nanocarriers
Common inorganic drug nanocarriers can be broadly classified into non-metallic nanoparticles and metallic nanoparticles, which show promising applications in targeted drug delivery, controlled release and sustained release of drugs (79).
Non-metallic Nanoparticles
Mesoporous silica nanoparticles (MSNPs) are the more widely used carriers among the non-metallic nanoparticle types. Their excellent biocompatibility, high stability, rigid backbone, good pore structure, tunable surface chemistry and controlled release for drugs, determine they to be excellent drug carriers (30, 31). Wei et al. (32) prepared novel targeting adriamycin mesoporous silica nanoparticles and peptide CSNRDARRC couples (DOX-loaded MSNs@PDA-PEP), which could load DOX more efficiently compared to MSNs alone. It had higher internalization rate and targeting efficiency in HT-1376 cells, which could improve the therapeutic effect on bladder cancer and show non-toxicity to mice model. Another highly mucoadhesive nano-drug delivery system was prepared using poly-amidoamine (PAMAM) modified MSNPs (MSNPs-G0~MSNPs-G3) and loaded with DOX. With the dilution of urine, the release of DOX increased significantly with the decrease of pH, in addition MSNPs-G2 had the best mucoadhesive property and the mucoadhesive ability of MSNPs-G2 remained unchanged after loading DOX (33).
Metal Nanoparticles
Common Metal Nanoparticles
Currently, metal nanoparticles are often prepared using biological systems, which are nontoxic, economical and highly efficient compared to traditional physical or chemically mediated methods, and their applications are gradually expanding to bladder cancer treatment (80). Ferreira et al. synthesized Fusarium biogenic silver nanoparticle for the treatment of NMIBC patients who are highly malignant, ineffective with BCG treatment or relapsed, this nanoparticle can directly induce DNA damage and have significant antitumor effects in vivo (81). Cuprous oxide nanoparticles (CONPs) could activate the ROS/ERK signaling pathway to induce apoptosis in bladder cancer cells, and they were more metabolizable, less toxic, and more suitable as drug carriers for intravesical chemotherapy. Combining CONPs with gemcitabine chemotherapy reduced the recurrence rate by synergistically exerting a more optimal effect than single agents (82). In addition, studies have also confirmed the killing effect of gold nanoparticles on different bladder cancer cell lines with the same effect (83, 84).
Magnetic Nanomaterials
Magnetic nanoparticles (MNPs) are dominated by oxides of iron. MNPs are appropriately sized, easily prepared and surface modified, with good adsorption capacity and they can combine with magnetic guidance (Figure 4). These characteristics make MNPs as important tools in drug delivery, imaging and clinical diagnosis (34, 35). With appropriately size, MNPs can increase endocytosis by cancer cells. Jasna Lojk et al. (85) synthesized polyacrylic acid (PAA)-coated MNPs and studied the differences in their endocytosis in normal primary urothelial (NPU) cells, RT4 cell line and T24 cell line. They confirmed that cancer cells can selectively take up this vector. The surface modification of MNPs can facilitate the binding of antitumor drugs and the targeted uptake by tumor cells. Suo et al. prepared a novel magnetic carboxylated multiwalled carbon nanotubes (mMWCNTs) for loading epirubicin (EPI). Application of such magnetic multi-walled carbon nanotubes not only alleviated the toxicity of the drug to normal cells, but also increased the dispersion and efficiency of the loaded drug, resulting in good stability of the solution and anti-tumor activity in vitro and in vivo (36). In addition, magnetic fields acting on MNPs can lead to an increase in local temperature and thus kill tumor cells (37, 38). Studies have shown that methotrexate coupled with MNPs (MTX/MNPs) in combination with magnetic heat achieves better cancer suppression than drug therapy alone and adjuvant heat therapy alone. The advantage of MTX/MNPs combined with magnetic heat therapy was the low CEM43T90 value (the cumulative equivalent minutes representing 43°C in 90% of the tumor area) was sufficient for rapid tumor destruction and no recurrence (39). Further the superparamagnetic nature of magnetic nanomicrospheres allows for tumor localization and vascular imaging for early diagnosis of disease (11, 32, 86, 87). These advantages of MNPs can reduce the toxicity of perfused drugs, induce slow and sustained drug release, increase targeted binding, uptake and targeted killing of tumor cells, and will enhance the efficacy of perfusion chemotherapy for bladder cancer.
Others
In addition to the above mentioned nanocarriers, there are some special carriers that can be applied for chemotherapy of bladder cancer. With self-propulsive properties, nanomotors show great potential in overcoming drug delivery barriers (88). One investigator prepared urease powered nanomotors of MSNPs containing both polyethylene glycol and anti-FGFR3 antibodies on their outer surface. The urease driven nanomotor converts urea to carbon dioxide and ammonia thereby triggering the propulsion of the nanomotor. The targeting functions of substrate-dependent enzyme nanomotors was demonstrated in spheroid culture (3D culture) of human bladder cancer cells (40). Choi et al. prepared a biocompatible and bioavailable nanomotor using dopamine (PDA) hollow nanoparticles. This nanomotor was fluorescently labeled and showed strong fluorescence in the bladder wall even after 12 hours of perfusion and penetrated to a greater depth than the control group (41). Active motion increased the penetration ability of the nanomotor, and active antibody-modified nanomotors were more efficient than these without antibody modification.
Application of Nanocarriers in the Diagnosis and Monitoring of Bladder Cancer
The diagnosis and follow-up of BC are mainly based on cystoscopy and urine cytology. Cystoscopy is invasive and costly, while urinary exfoliative cytology has low sensitivity (89). The efficient binding and easy detection characteristics of nanocarriers may become an important imaging tool for the diagnosis and monitoring of bladder cancer. One investigator used chitosan and ferromagnetic iron oxide nanocubes to design peptide-conjugated chitosan nanoparticles (pMCNP). The nanoparticles were administered to mice through the tail vein, and then good MRI and optical dual-modality imaging were detected. The pMCNP loaded with vincristine accumulated at the tumor site and showed controlled release for up to 50 hours (90). Besides the multi-binding site modification of MNPs has the potential for drug perfusion with simultaneous imaging detection (91). MNPs can also be used for urine protein capture and detection. Researchers synthesized a novel bladder cancer biosensor based on polycrystalline silicon nanowire field effect transistor (Poly-SiNW-FET) for the quantification of apolipoprotein A II protein (APOA2) in urine. The biosensor can clearly differentiate urine from non-bladder cancer patients, and the results are consistent with those of suspension chip analysis system (Bio-Plex). The detection is non-invasive, simple and fast. In addition, the biosensor accelerates purification during immobilization of anti-APOA2, effectively preventing denaturation of anti-APOA2 and increasing the accuracy of the assay (92). MNPs can selectively capture urinary glycoproteins from BC patients, contributing to the study of glycoproteome and having the potential to uncover glycoprotein biomarkers (93). Magnetic nanocarriers can also be used for the detection of exfoliated tumor cells. Xu et al. combined Fe3O4 and SiO2 to form new positively charged multifunctional nanoprobes that can specifically capture and enrich tumor cells in urine in a magnetic field, increasing the sensitivity of the detection (94).
Discussion
The recurrence rate after TURBT for NMIBC is 50% to 80% (95), and prevention of bladder cancer recurrence after surgery is a key aspect to improve the prognosis of bladder cancer patients. Bladder perfusion chemotherapy is an effective mean of preventing tumor recurrence, but the tumor suppressive effect of the drugs reduces due to the barrier effect of bladder epithelium and regular urination behavior (61, 96, 97). Through the application of nanocarriers, both lipid-soluble drugs and water-soluble drugs can better act on tumor location. Meanwhile, drug adhesion, targeted uptake and killing to cancer cell increases, toxicity, release rate and adverse effects reduce. In addition, nanocarriers provide a low-invasive and efficient way to monitor bladder cancer through specific imaging and targeted binding detection of urine proteins or tumor cells.
However, there are still some drawbacks in the application of nanocarrier technology in bladder cancer intravesical chemotherapy. Firstly, liposome-based carriers have low water solubility, large molecular weight, non-uniform particle size, aggregate formation, and even affect the drug effect. Liposomes still have a certain amount of binding between carriers and healthy cells (28), which also proves that there may be toxic side effects on normal cells. In contrast, most chemically cross-linked hydrogel injections require high pressure and long delivery time when using small-diameter catheters for bladder perfusion (68), resulting a high attrition. Some polymer-based carriers are cumbersome to prepare, with complicated preparation processes and high development costs, limiting their translation to the clinic (36). Inorganic nanomaterial carriers are also in the early stage of research, lacking exact animal experiments and clinical experiments for effect verification. The targeting, drug resistance, encapsulation rate and release rate of the carriers themselves still need to be optimized and improved.
The high recurrence rate of bladder cancer, the limitations of perfusion drugs and their organ specificity require continuous improvement of perfusion drugs and drug delivery system, and the innovation and optimization of nano-delivery system is expected to provide a guiding idea for perfusion chemotherapy of bladder cancer and become a powerful tool for the treatment of bladder cancer.
Author Contributions
DX contributed to design of the review. YLu and SW collected the data and information. LY wrote the first draft of the manuscript. YW wrote parts of the manuscript. ML and YLi embellished the manuscript. All authors participated in the revision of the manuscript and read and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Babjuk M, Burger M, Comperat EM, Gontero P, Mostafid AH, Palou J, et al. European Association of Urology Guidelines on Non-Muscle-Invasive Bladder Cancer (Tat1 and Carcinoma in Situ) - 2019 Update. Eur Urol (2019) 76(5):639–57. doi: 10.1016/j.eururo.2019.08.016
3. Sylvester RJ, Oosterlinck W, Holmang S, Sydes MR, Birtle A, Gudjonsson S, et al. Systematic Review and Individual Patient Data Meta-Analysis of Randomized Trials Comparing a Single Immediate Instillation of Chemotherapy After Transurethral Resection With Transurethral Resection Alone in Patients With Stage Pta-Pt1 Urothelial Carcinoma of the Bladder: Which Patients Benefit From the Instillation? Eur Urol (2016) 69(2):231–44. doi: 10.1016/j.eururo.2015.05.050
4. Tyagi P, Wu PC, Chancellor M, Yoshimura N, Huang L. Recent Advances in Intravesical Drug/Gene Delivery. Mol Pharm (2006) 3(4):369–79. doi: 10.1021/mp060001j
5. Buss JH, Begnini KR, Bender CB, Pohlmann AR, Guterres SS, Collares T, et al. Nano-BCG: A Promising Delivery System for Treatment of Human Bladder Cancer. Front Pharmacol (2017) 8:977. doi: 10.3389/fphar.2017.00977
6. Xu X, Liu K, Jiao B, Luo K, Ren J, Zhang G, et al. Mucoadhesive Nanoparticles Based on Ros Activated Gambogic Acid Prodrug for Safe and Efficient Intravesical Instillation Chemotherapy of Bladder Cancer. J Control Release (2020) 324:493–504. doi: 10.1016/j.jconrel.2020.03.028
7. Edgar JYC, Wang H. Introduction for Design of Nanoparticle Based Drug Delivery Systems. Curr Pharm Des (2017) 23(14):2108–12. doi: 10.2174/1381612822666161025154003
8. Yan W, Leung SS, To KK. Updates on the Use of Liposomes for Active Tumor Targeting in Cancer Therapy. Nanomedicine (Lond) (2020) 15(3):303–18. doi: 10.2217/nnm-2019-0308
9. Almeida B, Nag OK, Rogers KE, Delehanty JB. Recent Progress in Bioconjugation Strategies for Liposome-Mediated Drug Delivery. Molecules (2020) 25(23):5672. doi: 10.3390/molecules25235672
10. Nakamura T, Fukiage M, Higuchi M, Nakaya A, Yano I, Miyazaki J, et al. Nanoparticulation of BCG-CWS for Application to Bladder Cancer Therapy. J Control Release (2014) 176:44–53. doi: 10.1016/j.jconrel.2013.12.027
11. Yoon HY, Yang HM, Kim CH, Goo YT, Hwang GY, Chang IH, et al. Enhanced Intracellular Delivery of Bcg Cell Wall Skeleton Into Bladder Cancer Cells Using Liposomes Functionalized With Folic Acid and Pep-1 Peptide. Pharmaceutics (2019) 11(12):652. doi: 10.3390/pharmaceutics11120652
12. Yoon HY, Chang IH, Goo YT, Kim CH, Kang TH, Kim SY, et al. Intravesical Delivery of Rapamycin Via Folate-Modified Liposomes Dispersed in Thermo-Reversible Hydrogel. Int J Nanomedicine (2019) 14:6249–68. doi: 10.2147/IJN.S216432
13. Guo H, Xu W, Chen J, Yan L, Ding J, Hou Y, et al. Positively Charged Polypeptide Nanogel Enhances Mucoadhesion and Penetrability of 10-Hydroxycamptothecin in Orthotopic Bladder Carcinoma. J Control Release (2017) 259:136–48. doi: 10.1016/j.jconrel.2016.12.041
14. Guo H, Li F, Xu W, Chen J, Hou Y, Wang C, et al. Mucoadhesive Cationic Polypeptide Nanogel With Enhanced Penetration for Efficient Intravesical Chemotherapy of Bladder Cancer. Adv Sci (Weinh) (2018) 5(6):1800004. doi: 10.1002/advs.201800004
15. Lin T, Wu J, Zhao X, Lian H, Yuan A, Tang X, et al. In Situ Floating Hydrogel for Intravesical Delivery of Adriamycin Without Blocking Urinary Tract. J Pharm Sci (2014) 103(3):927–36. doi: 10.1002/jps.23854
16. Lin T, Zhang Y, Wu J, Zhao X, Lian H, Wang W, et al. A Floating Hydrogel System Capable of Generating CO2 Bubbles to Diminish Urinary Obstruction After Intravesical Instillation. Pharm Res (2014) 31(10):2655–63. doi: 10.1007/s11095-014-1362-y
17. Kolawole OM, Lau WM, Khutoryanskiy VV. Methacrylated Chitosan as a Polymer With Enhanced Mucoadhesive Properties for Transmucosal Drug Delivery. Int J Pharm (2018) 550(1-2):123–9. doi: 10.1016/j.ijpharm.2018.08.034
18. Kolawole OM, Lau WM, Khutoryanskiy VV. Chitosan/beta-Glycerophosphate in Situ Gelling Mucoadhesive Systems for Intravesical Delivery of Mitomycin-C. Int J Pharm X (2019) 1:100007. doi: 10.1016/j.ijpx.2019.100007
19. Kolawole OM, Lau WM, Khutoryanskiy VV. Synthesis and Evaluation of Boronated Chitosan as a Mucoadhesive Polymer for Intravesical Drug Delivery. J Pharm Sci (2019) 108(9):3046–53. doi: 10.1016/j.xphs.2019.05.006
20. Lien ZY, Hsu TC, Liu KK, Liao WS, Hwang KC, Chao JI. Cancer Cell Labeling and Tracking Using Fluorescent and Magnetic Nanodiamond. Biomaterials (2012) 33(26):6172–85. doi: 10.1016/j.biomaterials.2012.05.009
21. Ali MS, Metwally AA, Fahmy RH, Osman R. Chitosan-Coated Nanodiamonds: Mucoadhesive Platform for Intravesical Delivery of Doxorubicin. Carbohyd Polym (2020) 245:116528. doi: 10.1016/j.carbpol.2020.116528
22. Wang S, Jin S, Li G, Xu M, Deng D, Xiao Z, et al. Transmucosal Delivery of Self-Assembling Photosensitizer-Nitazoxanide Nanocomplexes With Fluorinated Chitosan for Instillation-Based Photodynamic Therapy of Orthotopic Bladder Tumors. ACS Biomater Sci Eng (2021) 7(4):1485–95. doi: 10.1021/acsbiomaterials.0c01786
23. Manan FAA, Yusof NA, Abdullah J, Mohammad F, Nurdin A, Yasan LS, et al. Drug Release Profiles of Mitomycin C Encapsulated Quantum Dots-Chitosan Nanocarrier System for the Possible Treatment of Non-Muscle Invasive Bladder Cancer. Pharmaceutics (2021) 13(9):1379. doi: 10.3390/pharmaceutics13091379
24. Gupta S, Moulik SP. Biocompatible Microemulsions and Their Prospective Uses in Drug Delivery. J Pharm Sci (2008) 97(1):22–45. doi: 10.1002/jps.21177
25. Jalali-Jivan M, Garavand F, Jafari SM. Microemulsions as Nano-Reactors for the Solubilization, Separation, Purification and Encapsulation of Bioactive Compounds. Adv Colloid Interfac (2020) 283:102227. doi: 10.1016/j.cis.2020.102227
26. Chen TY, Tsai MJ, Chang LC, Wu PC. Co-Delivery of Cisplatin and GemcitabineVia Viscous Nanoemulsion for Potential Synergistic Intravesical Chemotherapy. Pharmaceutics (2020) 12(10):949. doi: 10.3390/pharmaceutics12100949
27. Alai MS, Lin WJ, Pingale SS. Application of Polymeric Nanoparticles and Micelles in Insulin Oral Delivery. J Food Drug Anal (2015) 23(3):351–8. doi: 10.1016/j.jfda.2015.01.007
28. Oliveira MB, Villa Nova M, Bruschi ML. A Review of Recent Development sonMicro/Nanostructured Pharmaceutical Systems for Intravesical Therapy of the Bladder Cancer. Pharm Dev Technol (2018) 23(1):1–12. doi: 10.1080/10837450.2017.1312441
29. Zhu G, Wang K, Qin H, Zhao X, Chen W, Xu L, et al. Internal Cross-Linked Polymeric Nanoparticles With Dual Sensitivity for Combination Therapy of Muscle-Invasive Bladder Cancer. J Nanobiotech (2020) 18(1):124. doi: 10.1186/s12951-020-00686-3
30. Vallet-Regi M, Colilla M, Izquierdo-Barba I, Manzano M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules (2017) 23(1):47. doi: 10.3390/molecules23010047
31. Xu C, Lei C, Yu C. Mesoporous Silica Nanoparticles for Protein Protection and Delivery. Front Chem (2019) 7:290. doi: 10.3389/fchem.2019.00290
32. Wei Y, Gao L, Wang L, Shi L, Wei E, Zhou B, et al. Polydopamine and Peptide Decorated Doxorubicin-Loaded Mesoporous Silica Nanoparticles as a Targeted Drug Delivery System for Bladder Cancer Therapy. Drug Deliv (2017) 24(1):681–91. doi: 10.1080/10717544.2017.1309475
33. Wang B, Zhang K, Wang J, Zhao R, Zhang Q, Kong X. Poly(amidoamine)-Modified Mesoporous Silica Nanoparticles as a Mucoadhesive Drug Delivery System for Potential Bladder Cancer Therapy. Colloids Surf B Biointerfaces (2020) 189:110832. doi: 10.1016/j.colsurfb.2020.110832
34. Thevenot J, Oliveira H, Sandre O, Lecommandoux S. Magnetic Responsive Polymer Composite Materials. Chem Soc Rev (2013) 42(17):7099–116. doi: 10.1039/c3cs60058k
35. Huang RY, Liu Z-H, Weng W-H, Chang C-W, et al. Magnetic Nanocomplexes for Gene Delivery Applications. J Mater Chem B (2021) 9(21):4267–86. doi: 10.1039/D0TB02713H
36. Suo N, Wang M, Jin Y, Ding J, Gao X, Sun X, et al. Magnetic Multiwalled Carbon Nanotubes With Controlled Release of Epirubicin: An Intravesical Instillation System for Bladder Cancer. Int J Nanomedicine (2019) 14:1241–54. doi: 10.2147/IJN.S189688
37. Gavilan H, Avugadda SK, Fernandez-Cabada T, Soni N, Cassani M, Mai BT, et al. Magnetic Nanoparticles and Clusters for Magnetic Hyperthermia: Optimizing Their Heat Performance and Developing Combinatorial Therapies to Tackle Cancer. Chem Soc Rev (2021) 50(20):11614–67. doi: 10.1039/D1CS00427A
38. Singh A, Jain S, Sahoo SK. Magnetic Nanoparticles for Amalgamation of Magnetic Hyperthermia and Chemotherapy: An Approach Towards Enhanced Attenuation of Tumor. Mater Sci Eng C Mater Biol Appl (2020) 110:110695. doi: 10.1016/j.msec.2020.110695
39. Stapf M, Teichgraber U, Hilger I. Methotrexate-Coupled Nanoparticles and Magnetic Nanochemothermia for the Relapse-Free Treatment of T24 Bladder Tumors. Int J Nanomedicine (2017) 12:2793–811. doi: 10.2147/IJN.S120969
40. Hortelao AC, Carrascosa R, Murillo-Cremaes N, Patino T, Sanchez S. Targeting 3d Bladder Cancer Spheroids With Urease-Powered Nanomotors. ACS Nano (2019) 13(1):429–39. doi: 10.1021/acsnano.8b06610
41. Choi H, Cho SH, Hahn SK. Urease-Powered Polydopamine Nanomotors for Intravesical Therapy of Bladder Diseases. ACS Nano (2020) 14(6):6683–92. doi: 10.1021/acsnano.9b09726
42. Wang G, Wu B, Li Q, Chen S, Jin X, Liu Y, et al. Active Transportation of Liposome Enhances Tumor Accumulation, Penetration, and Therapeutic Efficacy. Small (2020) 16(44):e2004172. doi: 10.1002/smll.202004172
43. Sturm L, Poklar Ulrih N. Basic Methods for Preparation of Liposomes and Studying Their Interactions With Different Compounds, With the Emphasis on Polyphenols. Int J Mol Sci (2021) 22(12):6547. doi: 10.3390/ijms22126547
44. Li M, Du C, Guo N, Teng Y, Meng X, Sun H, et al. Composition Design and Medical Application of Liposomes. Eur J Med Chem (2019) 164:640–53. doi: 10.1016/j.ejmech.2019.01.007
45. Moosavian SA, Bianconi V, Pirro M, Sahebkar A. Challenges and Pitfalls in the Development of Liposomal Delivery Systems for Cancer Therapy. Semin Cancer Biol (2021) 69:337–48. doi: 10.1016/j.semcancer.2019.09.025
46. Yingchoncharoen P, Kalinowski DS, Richardson DR. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol Rev (2016) 68(3):701–87. doi: 10.1124/pr.115.012070
47. Larsen ES, Joensen UN, Poulsen AM, Goletti D, Johansen IS. Bacillus Calmette-Guerin Immunotherapy for Bladder Cancer: A Review of Immunological Aspects, Clinical Effects and Bcg Infections. APMIS (2020) 128(2):92–103. doi: 10.1111/apm.13011
48. Zhao X, Li H, Lee RJ. Targeted Drug Delivery via Folate Receptors. Expert Opin Drug Deliv (2008) 5(3):309–19. doi: 10.1517/17425247.5.3.309
49. Lu Y, Low PS. Folate-Mediated Delivery of Macromolecular Anticancer Therapeutic Agents. Adv Drug Deliv Rev (2002) 54(5):675–93. doi: 10.1016/s0169-409x(02)00042-x
50. Serini S, Cassano R, Bruni M, Servidio C, Calviello G, Trombino S. Characterization of a Hyaluronic Acid and Folic Acid-Based Hydrogel for Cisplatin Delivery: Antineoplastic Effect in Human Ovarian Cancer Cells In Vitro. Int J Pharm (2021) 606:120899. doi: 10.1016/j.ijpharm.2021.120899
51. Law S, Leung AW, Xu C. Folic Acid-Modified Celastrol Nanoparticles: Synthesis, Characterization, Anticancer Activity in 2D and 3D Breast Cancer Models. Artif Cells Nanomed Biotechnol (2020) 48(1):542–59. doi: 10.1080/21691401.2020.1725025
52. Stallivieri A, Baros F, Jetpisbayeva G, Myrzakhmetov B, Frochot C. The Interest of Folic Acid in Targeted Photodynamic Therapy. Curr Med Chem (2015) 22(27):3185–207. doi: 10.2174/0929867322666150729113912
53. Tyagi P, Kashyap M, Majima T, Kawamorita N, Yoshizawa T, Yoshimura N. Intravesical Liposome Therapy for Interstitial Cystitis. Int J Urol (2017) 24(4):262–71. doi: 10.1111/iju.13317
54. Kaldybekov DB, Tonglairoum P, Opanasopit P, Khutoryanskiy VV. Mucoadhesive Maleimide-Functionalised Liposomes for Drug Delivery to Urinary Bladder. Eur J Pharm Sci (2018) 111:83–90. doi: 10.1016/j.ejps.2017.09.039
55. Mun EA, Williams AC, Khutoryanskiy VV. Adhesion of Thiolated Silica Nanoparticles to Urinary Bladder Mucosa: Effects of PEGylation, Thiol Content and Particle Size. Int J Pharm (2016) 512(1):32–8. doi: 10.1016/j.ijpharm.2016.08.026
56. Ilyas RA, Sapuan SM. The Preparation Methods and Processing of Natural Fibre Bio-Polymer Composites. Curr Org Synth (2019) 16(8):1068–70. doi: 10.2174/157017941608200120105616
57. Wang B, Wang S, Zhang Q, Deng Y, Li X, Peng L, et al. Recent Advances in Polymer-Based Drug Delivery Systems for Local Anesthetics. Acta Biomater (2019) 96:55–67. doi: 10.1016/j.actbio.2019.05.044
58. Cao H, Duan L, Zhang Y, Cao J, Zhang K. Current Hydrogel Advances in Physicochemical and Biological Response-Driven Biomedical Application Diversity. Signal Transduct Target Ther (2021) 6(1):426. doi: 10.1038/s41392-021-00830-x
59. Yoon HY, Yang HM, Kim CH, Goo YT, Kang MJ, Lee S, et al. Current Status of the Development of Intravesical Drug Delivery Systems for the Treatment of Bladder Cancer. Expert Opin Drug Deliv (2020) 17(11):1555–72. doi: 10.1080/17425247.2020.1810016
60. Zacche MM, Srikrishna S, Cardozo L. Novel Targeted Bladder Drug-Delivery Systems: A Review. Res Rep Urol (2015) 7:169–78. doi: 10.2147/RRU.S56168
61. GuhaSarkar S, Banerjee R. Intravesical Drug Delivery: Challenges, Current Status, Opportunities and Novel Strategies. J Control Release (2010) 148(2):147–59. doi: 10.1016/j.jconrel.2010.08.031
62. Vashist A, Kaushik A, Vashist A, Sagar V, Ghosal A, Gupta YK, et al. Advances in Carbon Nanotubes-Hydrogel Hybrids in Nanomedicine for Therapeutics. Adv Healthc Mater (2018) 7(9):e1701213. doi: 10.1002/adhm.201701213
63. Farjadian F, Rezaeifard S, Naeimi M, Ghasemi S, Mohammadi-Samani S, Welland ME, et al. Temperature and pH-Responsive Nano-Hydrogel Drug Delivery System Based on Lysine-Modified Poly (Vinylcaprolactam). Int J Nanomedicine (2019) 14:6901–15. doi: 10.2147/IJN.S214467
64. Mei E, Chen C, Li C, Ding X, Chen J, Xi Q, et al. Injectable and Biodegradable Chitosan Hydrogel-Based Drug Depot Contributes to Synergistic Treatment of Tumors. Biomacromolecules (2021) 22(12):5339–48. doi: 10.1021/acs.biomac.1c01279
65. Ding L, Cui X, Jiang R, Zhou K, Wen Y, Wang C, et al. Design, Synthesis and Characterization of a Novel Type of Thermo-Responsible Phospholipid Microcapsule-Alginate Composite Hydrogel for Drug Delivery. Molecules (2020) 25(3):694. doi: 10.3390/molecules25030694
66. Tan B, Huang L, Wu Y, Liao J. Advances and Trends of Hydrogel Therapy Platform in Localized Tumor Treatment: A Review. J BioMed Mater Res A (2021) 109(4):404–25. doi: 10.1002/jbm.a.37062
67. Guo H, Li F, Qiu H, Xu W, Li P, Hou Y, et al. Synergistically Enhanced Mucoadhesive and Penetrable Polypeptide Nanogel for Efficient Drug Delivery to Orthotopic Bladder Cancer. Res (Wash D C) (2020) 2020:8970135. doi: 10.34133/2020/8970135
68. Qiu HP, Guo H, Li D, Hou YC, Kuang TR, Ding JX. Intravesical Hydrogels as DrugReservoirs. Trends Biotechnol (2020) 38(6):579–83. doi: 10.1016/j.tibtech.2019.12.012
69. Wang W, Meng Q, Li Q, Liu J, Zhou M, Jin Z, et al. Chitosan Derivatives and Their Application in Biomedicine. Int J Mol Sci (2020) 21(2):487. doi: 10.3390/ijms21020487
70. Muxika A, Etxabide A, Uranga J, Guerrero P, de la Caba K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int J Biol Macromol (2017) 105:1358–68. doi: 10.1016/j.ijbiomac.2017.07.087
71. Assa F, Jafarizadeh-Malmiri H, Ajamein H, Vaghari H, Anarjan N, Ahmadi O, et al. Chitosan Magnetic Nanoparticles for Drug Delivery Systems. Crit Rev Biotechnol (2017) 37(4):492–509. doi: 10.1080/07388551.2016.1185389
72. Shariatinia Z, Jalali AM. Chitosan-Based Hydrogels: Preparation, Properties and Applications. Int J Biol Macromol (2018) 115:194–220. doi: 10.1016/j.ijbiomac.2018.04.034
73. Kumar S, Dutta J, Dutta PK, Koh J. A Systematic Study on Chitosan-Liposome Based Systems for Biomedical Applications. Int J Biol Macromol (2020) 160:470–81. doi: 10.1016/j.ijbiomac.2020.05.192
74. Shariatinia Z. Carboxymethyl Chitosan: Properties and Biomedical Applications. Int J Biol Macromol (2018) 120(Pt B):1406–19. doi: 10.1016/j.ijbiomac.2018.09.131
75. Kurakula M NRN. Prospection of Recent Chitosan Biomedical Trends: Evidence From Patent Analysis (2009-2020). Int J Biol Macromol (2020) 165(Pt B):1924–38. doi: 10.1016/j.ijbiomac.2020.10.043
76. Sacco P, Cok M, Scognamiglio F, Pizzolitto C, Vecchies F, Marfoglia A, et al. Glycosylated-Chitosan Derivatives: A Systematic Review. Molecules (2020) 25(7):1534. doi: 10.3390/molecules25071534
77. Dobson J, de Queiroz GF, Golding JP. Photodynamic Therapy and Diagnosis: Principles and Comparative Aspects. Veterinary J (2018) 233:8–18. doi: 10.1016/j.tvjl.2017.11.012
79. Xu JS, Liao KL, Jiang HX, Zhou WM. Research Progress of Novel Inorganic Nanometre Materials Carriers in Nanomedicine for Cancer Diagnosis and Treatment. Artif Cells Nanomedicine Biotechnol (2018) 46:S492–502. doi: 10.1080/21691401.2018.1499665
80. Chugh H, Sood D, Chandra I, Tomar V, Dhawan G, Chandra R. Role of Gold and Silver Nanoparticles in Cancer Nano-Medicine. Artif Cells Nanomed Biotechnol (2018) 46(sup1):1210–20. doi: 10.1080/21691401.2018.1449118
81. Ferreira LAB, Garcia-Fossa F, Radaic A, Duran N, Favaro WJ, de Jesus MB. Biogenic Silver Nanoparticles: In Vitro and In Vivo Antitumor Activity in Bladder Cancer. Eur J Pharm Biopharm (2020) 151:162–70. doi: 10.1016/j.ejpb.2020.04.012
82. Xiong Q, Liu A, Ren Q, Xue Y, Yu X, Ying Y, et al. Cuprous Oxide Nanoparticles Trigger Reactive Oxygen Species-Induced Apoptosis Through Activation of Erk-Dependent Autophagy in Bladder Cancer. Cell Death Dis (2020) 11(5):366. doi: 10.1038/s41419-020-2554-5
83. Wu T, Duan X, Hu C, Wu C, Chen X, Huang J, et al. Synthesis and Characterization of Gold Nanoparticles From Abies Spectabilis Extract and its Anticancer Activity on Bladder Cancer T24 Cells. Artif Cells Nanomed Biotechnol (2019) 47(1):512–23. doi: 10.1080/21691401.2018.1560305
84. Daei S, Ziamajidi N, Abbasalipourkabir R, Khanaki K, Bahreini F. Anticancer Effects of Gold Nanoparticles by Inducing Apoptosis in Bladder Cancer 5637 Cells. Biol Trace Elem Res (2021), 10.1007/s12011–021-02895-9. doi: 10.1007/s12011-021-02895-9
85. Lojk J, Bregar VB, Strojan K, Hudoklin S, Veranic P, Pavlin M, et al. Increased Endocytosis of Magnetic Nanoparticles Into Cancerous Urothelial Cells Versus Normal Urothelial Cells. Histochem Cell Biol (2018) 149(1):45–59. doi: 10.1007/s00418-017-1605-1
86. Xu C, Lu X, Dai H. The Synthesis of Size-Adjustable Superparamagnetism Fe3O4 Hollow Microspheres. Nanoscale Res Lett (2017) 12(1):234. doi: 10.1186/s11671-017-1986-z
87. Abarca-Cabrera L, Fraga-Garcia P, Berensmeier S. Bio-Nano Interactions: Binding Proteins, Polysaccharides, Lipids and Nucleic Acids Onto Magnetic Nanoparticles. Biomater Res (2021) 25(1):12. doi: 10.1186/s40824-021-00212-y
88. Zhang JT, Chen ZJ, Kankala RK, Wang SB, Chen AZ. Self-Propelling Micro-/Nano-Motors: Mechanisms, Applications, and Challenges in Drug Delivery. Int J Pharmaceutics (2021) 596:120275. doi: 10.1016/j.ijpharm.2021.120275
89. Ferro M, La Civita E, Liotti A, Cennamo M, Tortora F, Buonerba C, et al. Liquid Biopsy Biomarkers in Urine: A Route Towards Molecular Diagnosis and Personalized Medicine of Bladder Cancer. J Pers Med (2021) 11(3):237. doi: 10.3390/jpm11030237
90. Key J, Dhawan D, Cooper CL, Knapp DW, Kim K, Kwon IC, et al. Multicomponent, Peptide-Targeted Glycol Chitosan Nanoparticles Containing Ferrimagnetic Iron Oxide Nanocubes for Bladder Cancer Multimodal Imaging. Int J Nanomedicine (2016) 11:4141–55. doi: 10.2147/IJN.S109494
91. Wu K, Su D, Liu J, Saha R, Wang JP. Magnetic Nanoparticles in Nanomedicine: A Review of Recent Advances. Nanotechnology (2019) 30(50):502003. doi: 10.1088/1361-6528/ab4241
92. Chen HC, Chen YT, Tsai RY, Chen MC, Chen SL, Xiao MC, et al. A Sensitive and Selective Magnetic Graphene Composite-Modified Polycrystalline-Silicon Nanowire Field-Effect Transistor for Bladder Cancer Diagnosis. Biosens Bioelectron (2015) 66:198–207. doi: 10.1016/j.bios.2014.11.019
93. Azevedo R, Soares J, Gaiteiro C, Peixoto A, Lima L, Ferreira D, et al. Glycan Affinity Magnetic Nanoplatforms for Urinary Glycobiomarkers Discovery in Bladder Cancer. Talanta (2018) 184:347–55. doi: 10.1016/j.talanta.2018.03.028
94. Xu J, Zeng S, Li J, Gao L, Le W, Huang X, et al. Novel Non-Invasive Diagnosis of Bladder Cancer in Urine Based on Multifunctional Nanoparticles. Front Cell Dev Biol (2021) 9:813420. doi: 10.3389/fcell.2021.813420
95. Wang Z, Gao W, Li J, Wang T, Zhu M, Duan Y. Development and Validation of a Novel Recurrence Risk Stratification for Initial Non-Muscle Invasive Bladder Cancer in the Han Chinese Population. J Cancer (2020) 11(7):1668–78. doi: 10.7150/jca.38649
96. Giannantoni A, Di Stasi SM, Chancellor MB, Costantini E, Porena M. New Frontiers in Intravesical Therapies and Drug Delivery. Eur Urol (2006) 50(6):1183–93. doi: 10.1016/j.eururo.2006.08.025
Keywords: bladder cancer, intravesical chemotherapy, nano-drug delivery system, liposomes, polymers, inorganic material
Citation: Lu Y, Wang S, Wang Y, Li M, Liu Y and Xue D (2022) Current Researches on Nanodrug Delivery Systems in Bladder Cancer Intravesical Chemotherapy. Front. Oncol. 12:879828. doi: 10.3389/fonc.2022.879828
Received: 20 February 2022; Accepted: 21 April 2022;
Published: 24 May 2022.
Edited by:
Francesco Del Giudice, Sapienza University of Rome, ItalyReviewed by:
M.Carmen Martinez-Bisbal, University of Valencia, SpainFrancesca Della Sala, Italian National Research Council, Italy
Ettore De Berardinis, Sapienza University of Rome, Italy
Copyright © 2022 Lu, Wang, Wang, Li, Liu and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongwei Xue, cmu4h-xdw@126.com
†These authors have contributed equally to this work
 Yilei Lu
Yilei Lu Siqi Wang
Siqi Wang Yuhang Wang
Yuhang Wang Mingshan Li
Mingshan Li Yili Liu
Yili Liu Dongwei Xue
Dongwei Xue