Corrigendum: Progression-free survival and time to progression as potential surrogate endpoints for overall survival in chemoradiotherapy trials in limited-stage small-cell lung cancer: A systematic review and meta-analysis
- 1Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
Purpose: To investigate whether progression-free survival (PFS) or time to progression (TTP) could be a valid surrogate endpoint for overall survival (OS) in patients with limited-stage small-cell lung cancer (LS-SCLC) receiving combined chemoradiotherapy.
Methods: Literature searching was performed in PubMed, Embase, and The Cochrane Library up to 2021. Prediction models were firstly established using data from phase III randomized controlled trials (RCTs) and then externally validated in phase II and retrospective studies. Correlation analysis was evaluated by a weighted linear regression model at both trial and arm levels. Cross-validation was performed to assess the consistency and robustness of the established models.
Results: 37 studies, including 15 phase III RCTs, 12 phase II studies, and 10 retrospective studies, were selected in the final analysis. In trial-level surrogacy, a very good correlation was observed between hazard ratios (HRs) of PFS/TTP and OS (R2 = 0.783, 95% CI 0.771–0.794). In arm-level surrogacy, very good correlations were also observed between 2-year (R2 = 0.823, 95% CI 0.814–0.832), 3-year (R2 = 0.843, 95% CI 0.833–0.850), 5-year (R2 = 0.852, 95% CI 0.843–0.859) PFS/TTP, and 5-year OS. An excellent correlation was observed between 4-year PFS/TTP and 5-year OS (R2 = 0.906, 95% CI 0.901–0.910). Cross-validation demonstrated reasonable overall consistency. External validation in phase II and retrospective studies showed good agreement (R2, 0.728–0.824).
Conclusions: PFS/TTP was a valid surrogate endpoint for OS in patients with LS-SCLC receiving combined chemoradiotherapy. The finding provides high-level evidence to support PFS/TTP as the primary endpoint in clinical trials so as to speed up introducing novel agents to the treatment of LS-SCLC.
Introduction
Small-cell lung cancer (SCLC) is the most aggressive subtype of lung cancer, with an estimated incidence of 4% and 250,000 cancer deaths worldwide (1, 2). Limited disease accounts for one third of the total cases. Besides the patients with T1–2N0M0 disease (AJCC 8th) who may be surgical candidates, chemoradiotherapy is the standard of care for most of limited-stage small-cell lung cancer (LS-SCLC) (95%) (3) and results in a 5-year overall survival (OS) of 20%–30% (4, 5).
OS is the gold-standard endpoint in randomized controlled trials (RCTs) as it is simple and unbiased. Especially, the 5-year OS rate is commonly used to assess the long-term benefits and toxicities of the treatment. However, using OS as the primary endpoint requires a large number of patients and long-term follow-up, leading to higher costs and delays in introducing novel drugs. Given these disadvantages, using an early surrogate endpoint in RCTs would shorten the time duration and save the research resources. Until now, The Food and Drug Administration has granted accelerated approval of many drugs based on surrogate endpoints of progression-free survival (PFS) or time to progression (TTP). For example, crizotinib was approved for anaplastic lymphoma kinase-positive non-small-cell lung cancer on the basis of PFS (6) and sunitinib for gastrointestinal stromal tumor and renal cell carcinoma on the basis of TTP (7). PFS and TTP have also been demonstrated to be valid surrogate endpoints for OS in some malignancies (8–12). However, an early valid surrogate endpoint has never been reported in LS-SCLC patients.
Reviewing various endpoints in clinical trials of LS-SCLC, PFS and TTP were potential surrogate endpoint for OS (4, 13, 14). Hereby, we investigated whether PFS/TTP could be used as an early efficient surrogate endpoint in LS-SCLC through literature-based analysis at trial and arm-level.
Literature Search and Study Selection
Search Strategy
Articles published before December 25, 2021 were identified via a systematic literature search of PubMed, Embase, and The Cochrane Library. The keywords were “Limited” and “Small Cell Lung Cancer” and “Chemoradiotherapy”. The search strategy is shown in Supplementary Table 1. The database searches were carried out independently by two authors (YY and JY, W).
Study Selection
The inclusion criteria of studies were as following: (1) LS-SCLC; (2) all patients received chemoradiotherapy but not surgery; (3) phase III RCTs, phase II trials, and retrospective studies; (4) the outcomes of studies include the following endpoints: hazard ratios (HRs) for OS and PFS/TTP, or absolute PFS/TTP rates (1, 2, 3, 4, 5-year) and 5-year OS. (5) English language; (6) at least 30 patients per arm. (7) published after 1990.
We excluded literatures without original data, phase I studies, inadequate survival data, systematic reviews, case reports, and other irrelevant publications.
Data Extraction
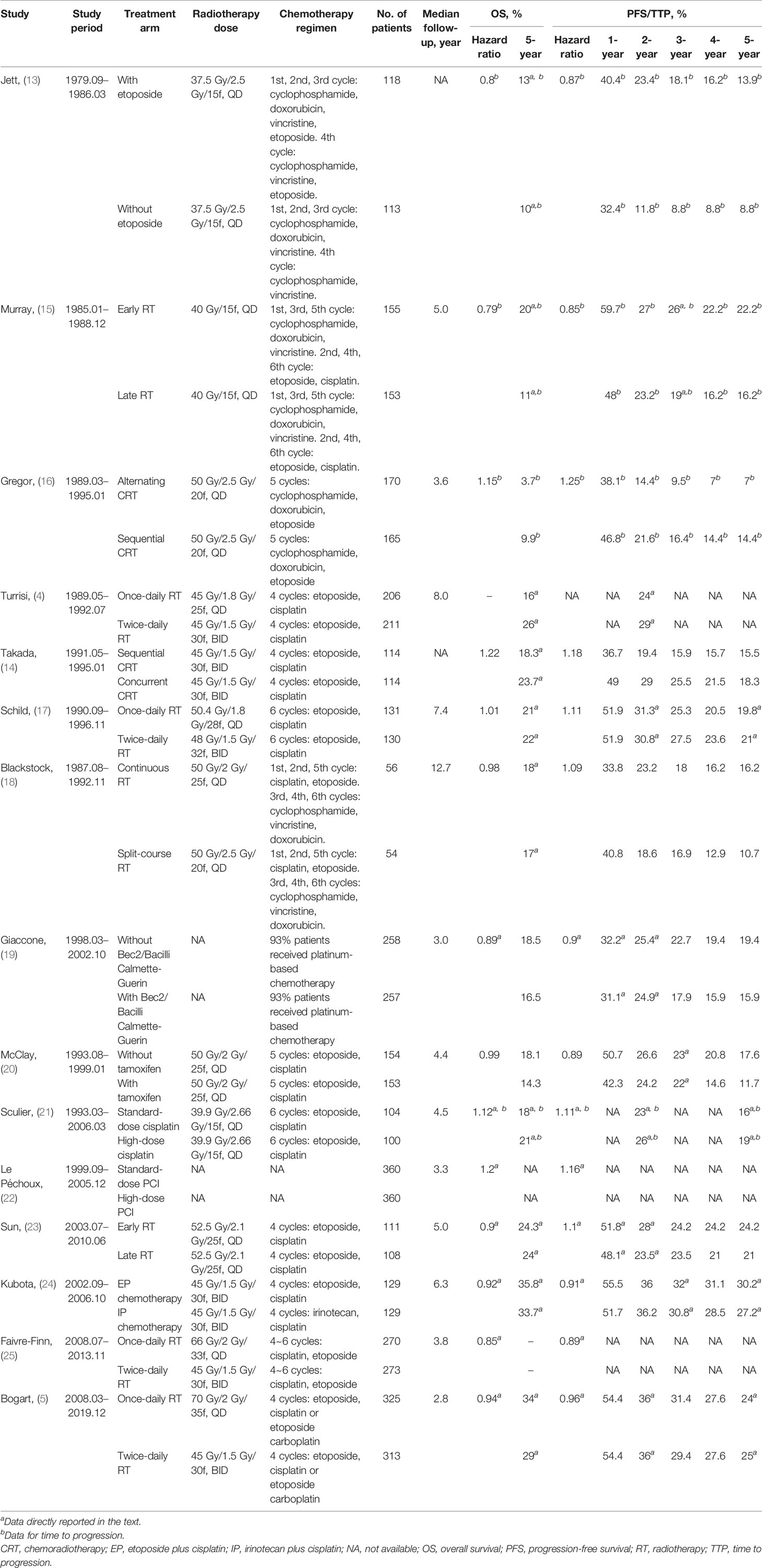
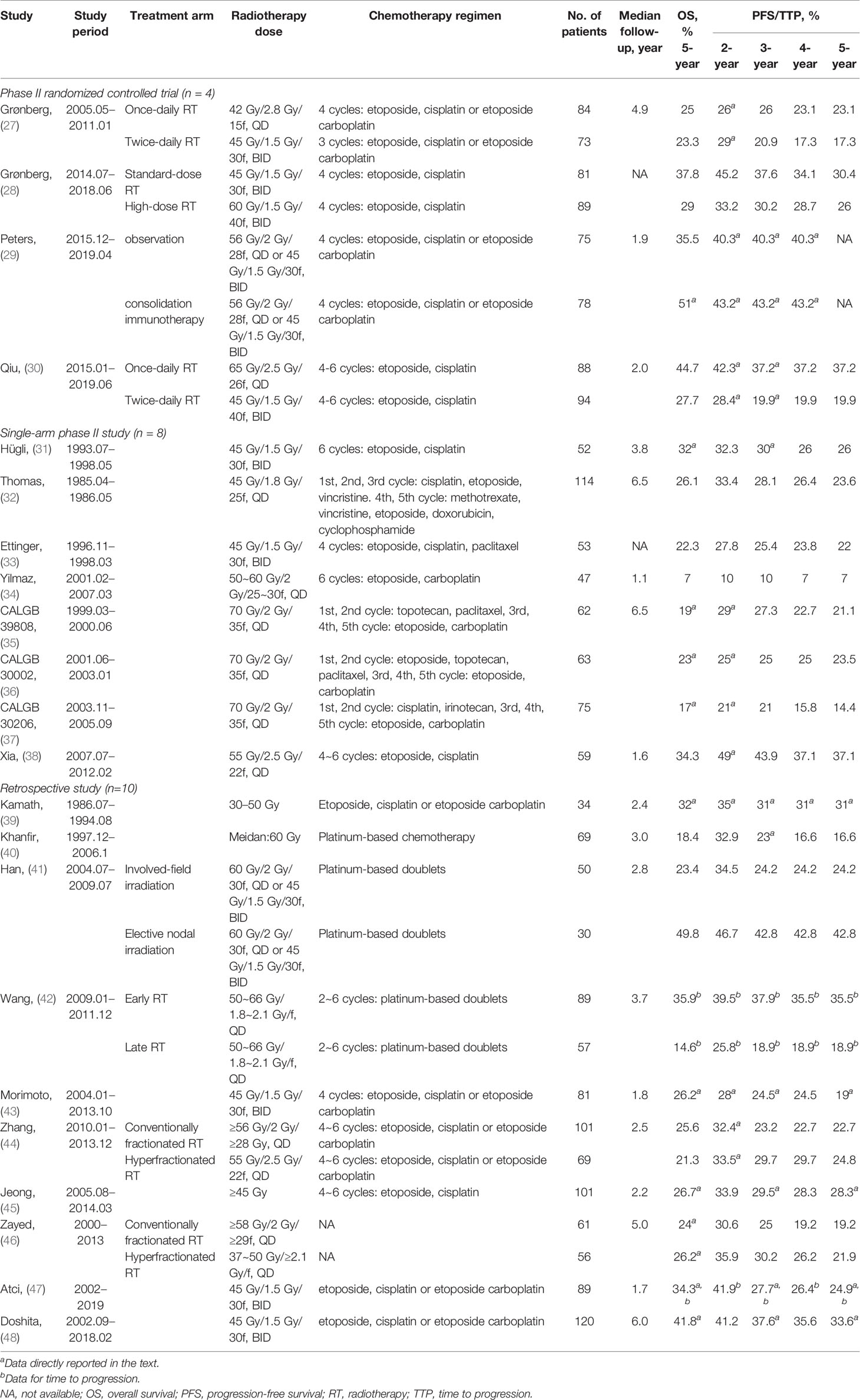
The following information from included studies were extracted: publication year, design, treatments of groups, number of patients, median follow-up time, and endpoints. For phase III RCTs, the endpoints were HRs for OS and PFS/TTP, absolute PFS/TTP rates (1, 2, 3, 4, 5-year) and 5-year OS (Table 1). The HRs or survival rates at different time point were obtained from the text or Kaplan–Meier curves, according to methods by Tierney et al. (26). For phase II trials and retrospective studies, the endpoints were absolute PFS/TTP rates (year 1, 2, 3, 4, 5) and 5-year OS (Table 2).
Endpoint Definition
OS was defined as the time from randomization, registration, diagnosis or the first day of treatment to death. PFS was defined as the time from randomization, registration, diagnosis or the first day of treatment to disease progression or death. TTP was defined as the time from randomization, registration, diagnosis or the first day of treatment to disease progression (Supplementary Tables 2, 3). As surrogate endpoints were defined differently between the trials, two investigators (YY and JY,W) labelled an endpoint of a trial as PFS or TTP according to our established definitions. For the literature without detailed definition of PFS/TTP, we tried to contact authors of original research, otherwise the definition from the text was adopted.
Quality Assessment
The quality of the candidate Phase II, III RCTs was evaluated on 7 domains according to the Cochrane Collaboration tool. The trials were excluded if high risk of bias in any domain was detected (Supplementary Table 4).
The quality of the candidate single-arm phase II, and retrospective studies was assessed in 3 domains with 9 items according to Newcastle-Ottawa Scale for cohort study. The studies were excluded if their scores were less than 6 points (Supplementary Table 5).
Statistical Analysis
Correlation Evaluation
The correlations between surrogate endpoints and OS in phase III RCTs were performed at both trial- and arm-level. At trial level, the correlation of HRs for PFS/TTP and HRs for OS was qualified through a linear regression model, weighted by trial size. At arm-level, the linear correlation between the 1-, 2-, 3-, 4-, and 5-year PFS/TTP rates and 5- year OS rate was also evaluated by the linear regression model, with weight equal to each treatment-arm sample size. The coefficient of determination R2 was calculated to assess the strength of correlation. R2 values of 0–0.25, 0.25–0.5, 0.5–0.75, 0.75–0.9, 0.9–1 indicated poor, moderate, good, very good and excellent correlation. If the R2 value was greater than 0.75, the following sensitivity analysis, leave-one-out cross-validation, and external validation were performed. If R2 values showed great discrepancy between two adjacent time points, further subdivision of the time period and corresponding PFS/TTP rate extraction was performed to find a cut-off value.
Sensitivity Analysis
Phase III RCTs were classified into four subgroups depending on study designs (Supplementary Table 6). To assess the consistency and robustness of prediction models across different settings, sensitivity analyses were performed by leaving each subgroup of trials out at a time. The coefficient of determination R2 value and its 95% CI were calculated by the weighted linear regression method mentioned above.
Leave-One-Out Cross-Validation
To assess the accuracy of prediction models, a leave-one-out cross-validation approach was performed. Each trial or treatment arm was left out once, and at each leave-one-out step a linear regression model was rebuilt on the other trials or arms (n-1). This model was then applied to the left-out trial or arm and the corresponding 95% prediction interval was calculated to compare the predicted and actually observed treatment effect on OS.
External Validation of Phase III RCT Prediction Model
The arm-level predictive linear regression models established by phase III RCTs were applied to the phase II and retrospective studies for external validation. The predicted 5-year OS rate was calculated from the actual 1–5-year PFS/TTP rates in the phase II or retrospective studies using the established linear regression model from the phase III RCTs. More specifically, the equation “5-year OS = α × 1-, 2-, 3-, 4-, or 5-year PFS/TTP + β” was derived from the phase III RCTs. The reported 1~5-year PFS/TTP rates derived from the phase II and retrospective studies were put into the equation, then the predicted 5-year OS rate was generated. The actual and predicted 5-year OS rates were plotted in scatter plots.
Statistical analysis was performed with SPSS (version 26.0), data visualization was performed using the ggplot2 package in R software (version 4.0.4) and GraphPad Prism (version 8.4.0).
Results
Study Characteristics
A total of 4,212 records were searched, and 40 records were screened to quality assessment. Among the 40 records, 3 records (40, 49, 50) were excluded for high risk of bias and 37 records, consisting of 15 phase III RCTs (4, 5, 13–25), 12 phase II (27–38), and 10 retrospective studies (39, 41–48, 51), were finally included for analysis (Supplementary Figure 1). Long-term survival data of three single-arm phase II studies (35–37) were updated in another report (52). The HRs for PFS and OS of a phase III trial (23) were corrected later (53). Thus, we conducted meta-analysis with these updated data.
Trial-Level Correlation Between PFS/TTP on OS in Phase III RCTs
14 RCTs reported pairs of HRs for PFS/TTP and OS. A very good correlation was observed between 14 pairs of HRs for PFS/TTP and OS (R2 = 0.783, 95% CI 0.771–0.794) (Figure 1). Sensitivity analysis showed very good correlations and robust consistency in most subgroups, except when leaving out 6 trials of different radiotherapy model (R2 = 0.645, 95% CI 0.587–0.674) (Supplementary Figure 2A). This result was expected as the subgroup of different radiotherapy model close to half of the number of trials. Exclusion of these trials probably results in a lower correlation and wider confidence interval. The cross-validation showed good consistency, as the observed HRs for OS were all in the 95% prediction intervals in 13 of 14 trials, and the HRs were very close to 95% prediction intervals in the remaining one trial (23) (Figure 2).
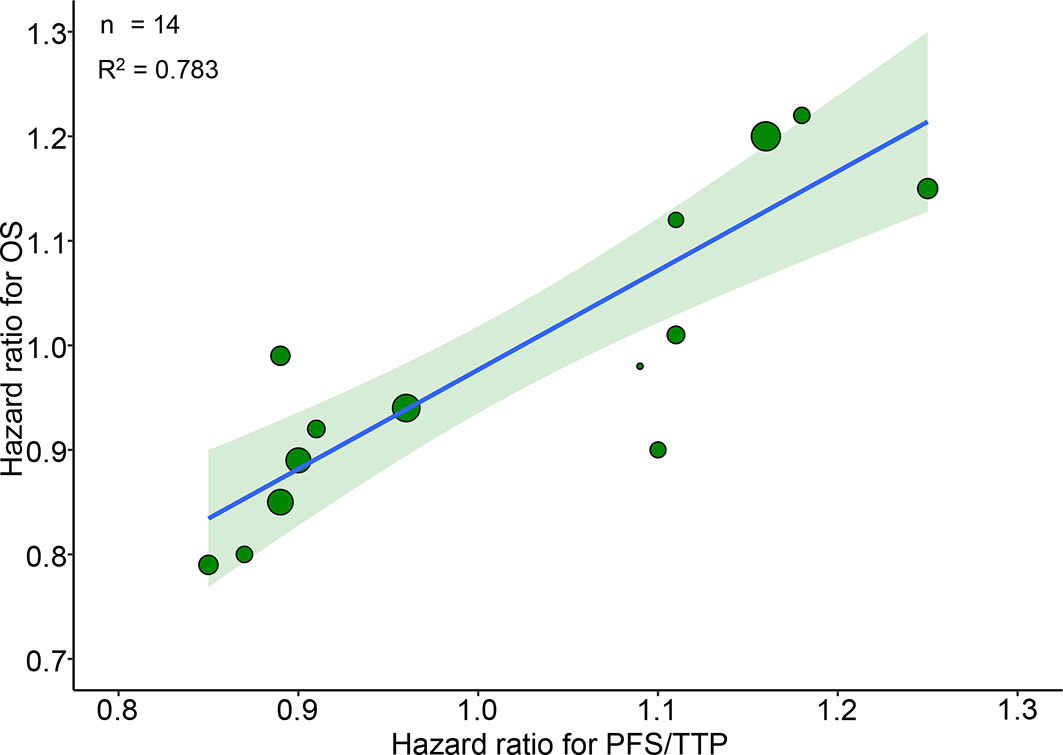
Figure 1 Trial-level correlation between hazard ratios for OS and PFS/TTP in phase III RCTs. Green circles represent trials with a size proportional to the number of patients, blue line for the estimated regression line and the light green zone for 95% confidence intervals. OS, overall survival; PFS/TTP, progression free survival/time to progression; RCTs, randomized controlled trials.
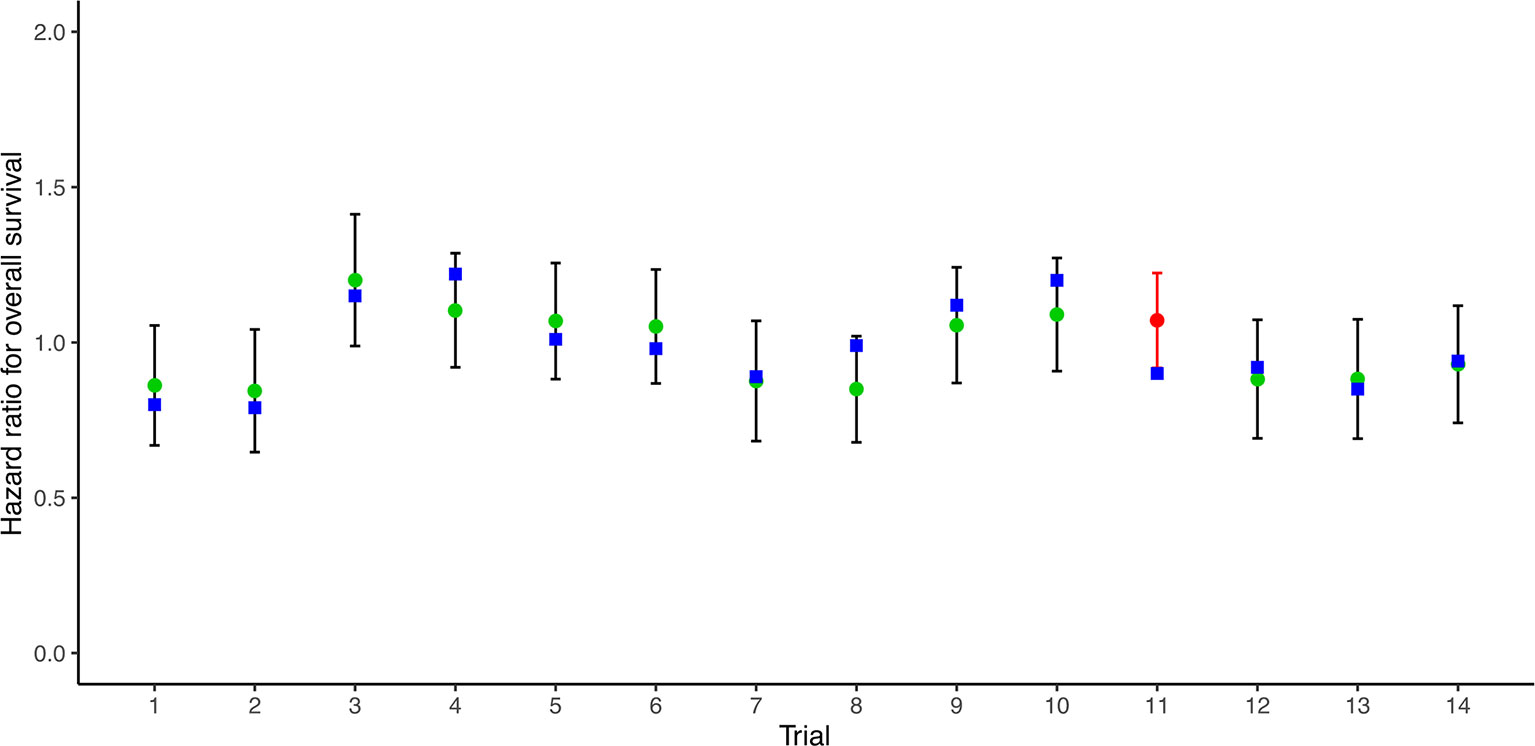
Figure 2 Leave-one-out cross-validation analysis of the prediction of HR for OS based on HR for PFS/TTP. Green circles represent predicted hazard ratio for OS, vertical lines for 95% prediction intervals, and blue squares for observed hazard ratios for OS. Red circles and lines indicate that the observed HR is beyond the 95% prediction intervals. HR, hazard ratio; OS, overall survival; PFS/TTP, progression-free survival/time to progression.
Treatment Arm-Level Correlation Between PFS/TTP and OS in Phase III RCTs
26 arms from 13 phase III RCTs reported 5-year OS, among which 22 arms from 11 trials, 26 arms from 13 trials, 22 arms from 11 trials, 22 arms from 11 trials, and 24 arms from 12 trials reported 1-, 2-, 3-, 4-, and 5-year PFS/TTP, respectively. The correlation between 1-year PFS/TTP and 5-year OS was moderate (R2 = 0.379, 95% CI 0.358–0.394). However, very good correlations were observed analyzing 26 pairs of 2-year PFS/TTP and 5-year OS (R2 = 0.823, 95% CI 0.814–0.832), 22 pairs of 3-year PFS/TTP and 5-year OS (R2 = 0.852, 95% CI 0.843–0.859), and 22 pairs of 5-year PFS/TTP and 5-year OS (R2 = 0.845, 95% CI 0.834–0.852). Moreover, an excellent correlation was observed analyzing 26 pairs of 4-year PFS/TTP and 5-year OS (R2 = 0.906, 95% CI 0.901–0.910) (Figure 3).
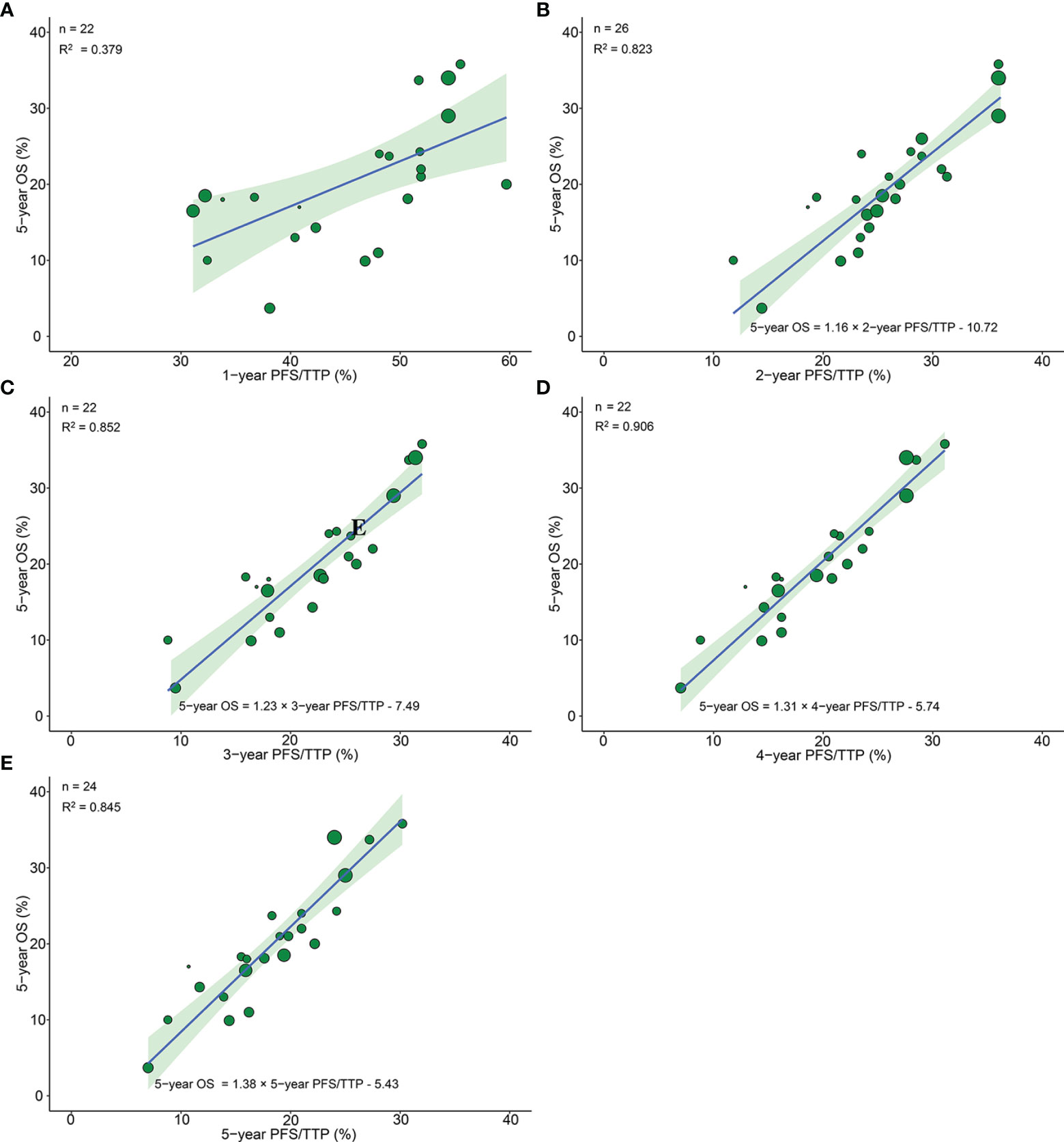
Figure 3 Treatment arm-level correlation between 5-year OS and 1-year PFS/TTP (A), 2-year PFS/TTP (B), 3-year PFS/TTP (C), 4-year PFS/TTP (D), 5-year PFS/TTP (E) in phase III RCTs. Green circles represent treatment arms with a size proportional to the number of patients, blue lines for the estimated regression lines and the light green zones for 95% confidence intervals. OS, overall survival; PFS/TTP, progression free survival/time to progression; RCTs, randomized controlled trials.
Because R2 showed great discrepancy between 1-year PFS/TTP and 2-year PFS/TTP, we further divided the time duration from 1 to 2 years into 5 parts with 4 time points (1.2, 1.4. 1.6, and 1.8 years); corresponding PFS/TTP rates were extracted to calculate R2 with a 5-year OS rate. The plot of R2 and PFS/TTP time showed that the best cutoff time point was 2 years, which indicated that the ≥2-year PFS/TTP rate was the valid surrogate endpoint (Supplementary Figure 3).
Sensitivity analysis showed very good correlations and robust consistency in most subgroups, except when leaving out subgroups of different radiotherapy model due to fewer trials (Supplementary Figures 2B–E).
The prediction results of cross-validation analyses showed that the observed 5-year OS rate fell within the 95% prediction intervals in all arms based on 2-, 3-, and 4-year PFS/TTP. With respect to the 5-year PFS/TTP, the observed 5-year OS rates were all in the 95% prediction intervals in 22 of 24 arms, and the 5-year OS rates of the remaining two trials (5, 18) are very close to the 95% prediction intervals (Figure 4).
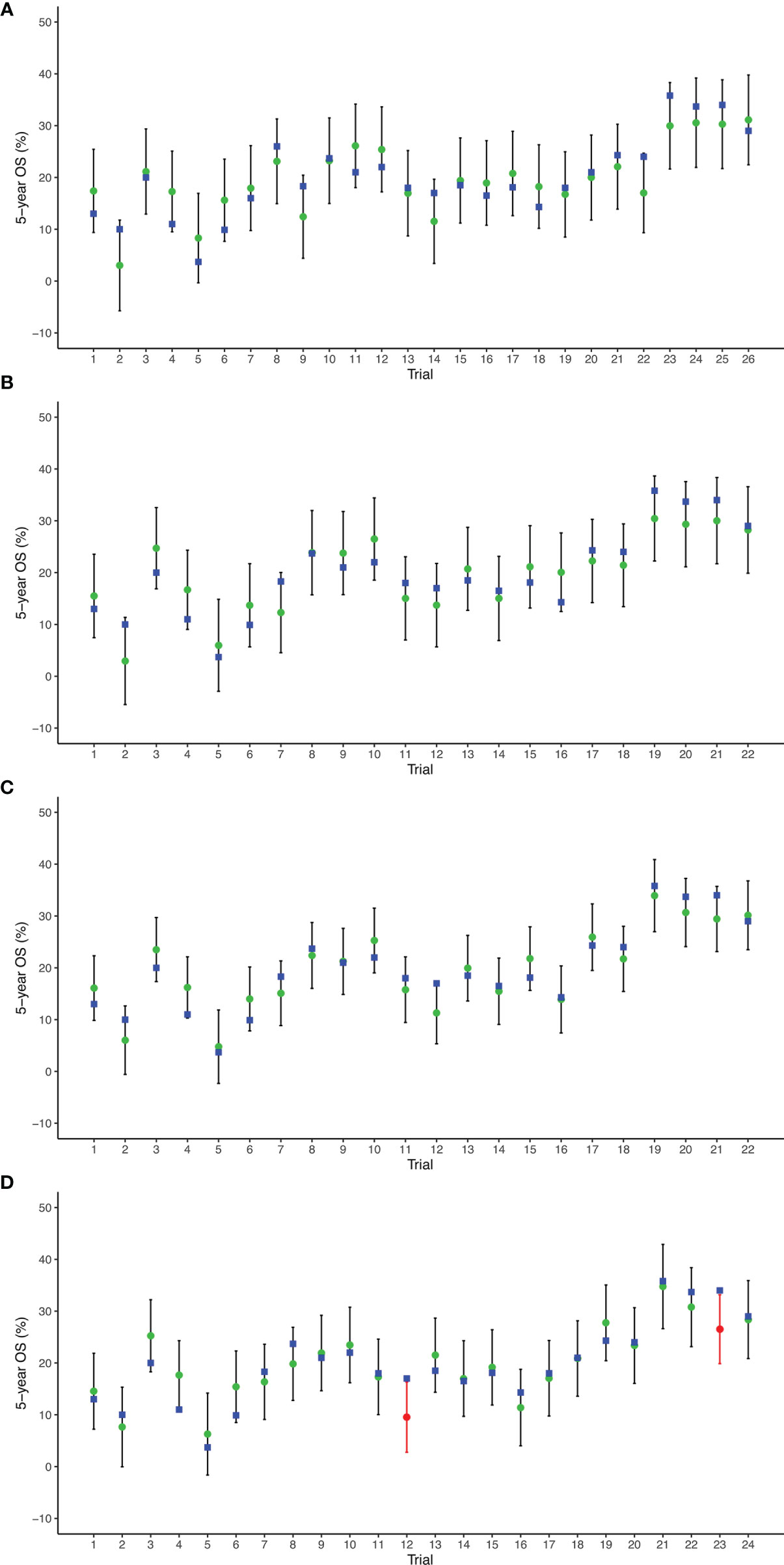
Figure 4 Leave-one-out cross-validation analysis of the prediction of 5-year OS based on 2-year PFS/TTP (A), 3-year PFS/TTP (B), 4-year PFS/TTP (C), and 5-year PFS/TTP (D). Green circles represent predicted 5-year OS, vertical lines for 95% prediction intervals, and blue squares for observed 5-year OS. Red circles and lines indicate that observed 5-year OS is beyond the 95% prediction intervals. OS, overall survival; PFS/TTP, progression-free survival/time to progression.
These findings indicated that improvements in 2–5-year PFS/TTP are strongly associated with a higher 5-year OS.
External Validation of the Correlation Between PFS/TTP and OS
30 treatment arms from 12 phase II and 10 retrospective studies were used for external validation. Using the arm-level prediction models from the phase III RCTs, we calculated the predicted 5-year OS rate for each phase II and retrospective studies using the actual 2-, 3-, 4-, or 5-year PFS/TTP rate. The actual and predicted 5-year OS rates were plotted in scatter plots, which indicated that the predicted 5-year OS was approximated to the actual 5-year OS. The predicted 5-year OS rate greatly correlated with the actual 5-year OS rate, with the R2 ranging from 0.728 to 0.824 (Figure 5). These results validated the hypothesis that PFS/TTP is the efficient surrogate endpoint of OS.
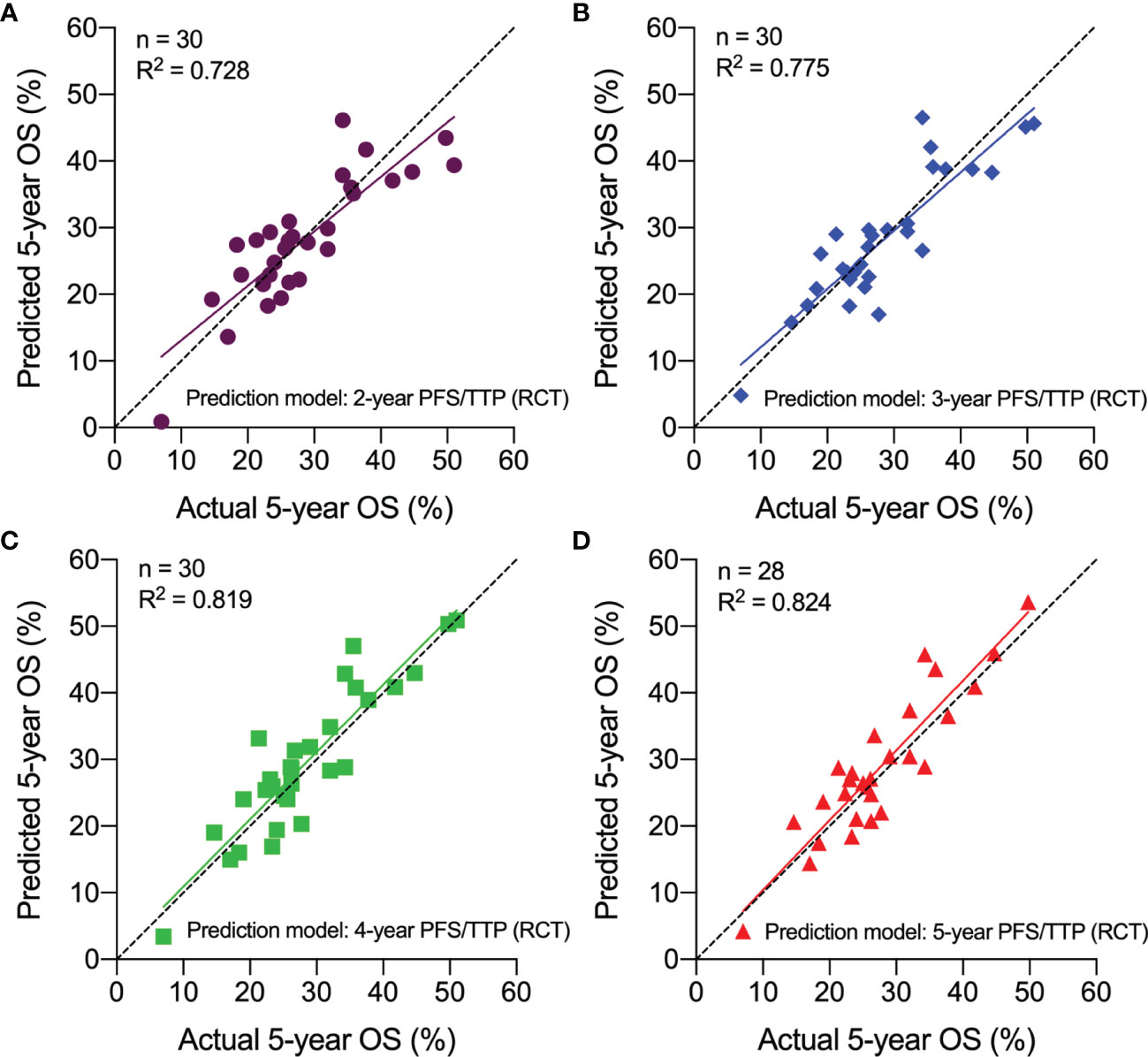
Figure 5 External validation of the correlation between PFS/TTP and OS in Phase II and retrospective studies. The predicted 5-year OS based on actual 2-year PFS/TTP (A), 3-year PFS/TTP (B), 4-year PFS/TTP (C), and 5-year PFS/TTP (D) is plotted against the actual 5-year OS. OS, overall survival; PFS/TTP, progression-free survival/time to progression; RCT, randomized controlled trial.
Discussion
This is the first study combining data from high-quality Phase III RCTs, Phase II studies, and retrospective studies to explore the efficacy of PFS or TTP as a surrogate endpoint of OS in patients with limited-stage SCLC who underwent chemoradiotherapy. Previous meta-analyses have assessed surrogate endpoints in other types or stages of lung cancer. Mauguen et al. (11) indicated that PFS and DFS are valid surrogate endpoints in locally advanced non-small-cell lung cancer. As for extensive-stage SCLC, Foster et al. (12) firstly reported that PFS was a potential surrogate endpoint using individual data from 6 single-arm and 3 RCTs in 2011 and then validated the results by seven new phase II/III trials in 2015 (54). However, analysis of surrogate endpoints in limited-stage SCLC has never been tested.
Our study showed that there were strong correlations between PFS/TTP and OS at the trial level, and 2–5-year PFS/TTP and 5-year OS at the treatment arm level. The coefficient R2 ranged from 0.783 to 0.907, which indicated that nearly 78.3%–90.7% of the variation on OS can be indicated by PFS or TTP. The sensitivity analysis showed good consistency across different settings, and the cross-validation also showed good accuracy of the prediction models. The external validation with phase II and retrospective studies showed excellent agreement between the actual and predicted 5-year OS rates derived from the established linear regression models. These findings confirmed the feasibility of taking PFS/TTP as the primary endpoint for clinical trials of LS-SCLC.
However, the predictive value of 1-year PFS/TTP for 5-year OS was quite lower compared with that of the 2-year PFS/TTP (correlation R2 0.379 vs. 0.823), which indicated that 1-year PFS/TTP was not an appropriate surrogate endpoint. PFS/TTP data of phase III RCT (Table 1) showed that around 50% of patients without progression relapsed during the second year after upfront treatment. From the third year, the PFS/TTP did not reduce significantly. Table 2 also showed that the PFS/TTP was relatively stable after 2 years of chemoradiotherapy in phase II and retrospective studies. This decreasing trend of PFS/TTP in LS-SCLC was consistent with our clinical experience. Thus, longer follow-up time such as 2–5-year PFS/TTP was necessary.
There has been debate on how a surrogate endpoint should be considered as valid. We employed the correlation approach which has been used to assess the possibility of PFS or TTP as a surrogate endpoint for OS in locally advanced NSCLC (11), nasopharyngeal carcinoma (8), and diffuse large B-cell lymphoma (10). Candidate surrogate endpoints could be valid only if the correlation coefficient was greater than 0.75 (55).
Exploring effective treatment or drugs for SCLC is urgent as its prognosis is still poor compared with other malignancy. There was no improvement of outcome for extensive-stage SCLC in the past more than three decades until atezolizumab was added in the classic regimen of etoposide and cisplatin as first-line chemotherapy (56). However, the immunotherapy plus chemotherapy only prolonged the median OS by 2 months compared with chemotherapy alone after more than a 2-year study period. For locally advanced lung cancer, new treatments have significantly increased the OS in NSCLC based on results of the PACIFIC trial (57), and the mature OS was achieved after 6 years from the beginning of the first patients enrolled (58). For limited-stage SCLC under standard concurrent chemoradiotherapy followed by prophylactic cranial irradiation, 5-year OS was still low ranging from 26% to 34% and did not change in the past two decades (4, 25). There are several ongoing phase II and III trials investigating the PD-1/PD-L1 consolidation immunotherapy (59–62). High-level evidence for immunotherapy as concurrent of consolidation treatment has not been reported until now. However, parts of these ongoing trials (59, 60) have already defined PFS as the primary endpoint, OS as the second endpoint. Given that no study has reported valid surrogate endpoints for limited-stage SCLC, our analysis is of great importance to provide a rationale to define PFS or TTP as the primary endpoint in clinical trials, so as to speed up introducing novel effective agents to improve outcome of LS-SCLC.
Another advantage of this study is comprehensively enrolled published literatures with high quality and proper sample size. In addition to the strong correlations demonstrated by phase III RCTs, the positive relationships between 2–5-year PFS/TTP and 5-year OS rates were externally validated by independent data from phase II and retrospective studies. The validation method was unique and firstly used in diffuse large B-cell lymphoma (10) by our department, which showed good efficacy to find early surrogate endpoints. This time, the validation method was used again in limited-stage SCLC to improve the reliability of the conclusions. Moreover, our study found multiple time points between 1-year and 2-year PFS/TTP which were not suitable, indicating that the 2-year PFS/TTP rate or more was really valid when used as a primary endpoint.
One major statistical challenge is inconsistencies and absences of the definition of endpoints across the trials in the current study. The starting time for endpoints was defined from randomization, registration, diagnosis, or the first day of treatment, differently. As SCLC is one of the most aggressive cancers, a difference of 1 or 2 months caused by definition of starting time may result in bias from different arms. Second, this is a literature-based systematic review and meta-analysis without individual patient data; therefore, a potential publication bias cannot be excluded. Third, the prediction models were based on data of patients who received first-line combined chemoradiotherapy, so the extrapolation to other treatments was cautious, especially when more effective second or more line treatments were developed in the future. Nevertheless, LS-SCLC patients usually died after disease progression because there are still no effective second-line treatments nowadays (25).
In conclusion, the current study provides first literature-based evidence to evaluate the correlation of PFS/TTP with OS in patients with limited-stage SCLC. The finding supports PFS/TTP as a valid surrogate endpoint for OS in LS-SCLC patients who underwent combined upfront chemoradiotherapy.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Concept and design: YL, LW, NB. Acquisition, analysis, or interpretation of data: YY, JW, LW, NB. Drafting of the manuscript: YY, JW. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: YY, TZ, YL. Obtained funding: LW, NB. All authors contributed to the article and approved the submitted version.
Funding
This study is supported by the National Natural Science Foundation of China (81572971) and Beijing Hope Run Special Fund of Cancer Foundation of China (LC2018A04).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.810580/full#supplementary-material
References
1. Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med (2020) 383(7):640–9. doi: 10.1056/NEJMoa1916623
2. Gazdar AF, Bunn PA, Minna JD. Small-Cell Lung Cancer: What We Know, What We Need to Know and the Path Forward. Nat Rev Cancer (2017) 17(12):725–37. doi: 10.1038/nrc.2017.87
3. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol (2016) 11(1):39–51. doi: 10.1016/j.jtho.2015.09.009
4. Turrisi Iii AT, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, et al. Twice-Daily Compared With Once-Daily Thoracic Radiotherapy in Limited Small-Cell Lung Cancer Treated Concurrently With Cisplatin and Etoposide. New Engl J Med (1999) 340(4):265–71. doi: 10.1056/NEJM199901283400403
5. Bogart JA, Wang XF, Masters GA, Gao J, Komaki R, Kuzma CS, et al. Phase 3 Comparison of High-Dose Once-Daily (QD) Thoracic Radiotherapy (TRT) With Standard Twice-Daily (BID) TRT in Limited Stage Small Cell Lung Cancer (LSCLC): CALGB 30610 (Alliance)/RTOG 0538. J Clin Oncol (2021) 39(15_suppl):8505. doi: 10.1200/JCO.2021.39.15_suppl.8505
6. Kazandjian D, Blumenthal GM, Chen HY, He K, Patel M, Justice R, et al. FDA Approval Summary: Crizotinib for the Treatment of Metastatic Non-Small Cell Lung Cancer With Anaplastic Lymphoma Kinase Rearrangements. Oncologist (2014) 19(10):e5–11. doi: 10.1634/theoncologist.2014-0241
7. Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, et al. Food and Drug Administration Drug Approval Summary: Sunitinib Malate for the Treatment of Gastrointestinal Stromal Tumor and Advanced Renal Cell Carcinoma. Oncologist (2007) 12(1):107–13. doi: 10.1634/theoncologist.12-1-107
8. Chen YP, Sun Y, Chen L, Mao YP, Tang LL, Li WF, et al. Surrogate Endpoints for Overall Survival in Combined Chemotherapy and Radiotherapy Trials in Nasopharyngeal Carcinoma: Meta-Analysis of Randomised Controlled Trials. Radiother Oncol (2015) 116(2):157–66. doi: 10.1016/j.radonc.2015.07.030
9. Hirai T, Nemoto A, Ito Y, Matsuura M. Meta-Analyses on Progression-Free Survival as a Surrogate Endpoint for Overall Survival in Triple-Negative Breast Cancer. Breast Cancer Res Treat (2020) 181(1):189–98. doi: 10.1007/s10549-020-05615-4
10. Zhu J, Yang Y, Tao J, Wang SL, Chen B, Dai JR, et al. Association of Progression-Free or Event-Free Survival With Overall Survival in Diffuse Large B-Cell Lymphoma After Immunochemotherapy: A Systematic Review. Leukemia (2020) 34(10):2576–91. doi: 10.1038/s41375-020-0963-1
11. Mauguen A, Pignon J-P, Burdett S, Domerg C, Fisher D, Paulus R, et al. Surrogate Endpoints for Overall Survival in Chemotherapy and Radiotherapy Trials in Operable and Locally Advanced Lung Cancer: A Re-Analysis of Meta-Analyses of Individual Patients’ Data. Lancet Oncol (2013) 14(7):619–26. doi: 10.1016/s1470-2045(13)70158-x
12. Foster NR, Qi Y, Shi Q, Krook JE, Kugler JW, Jett JR, et al. Tumor Response and Progression-Free Survival as Potential Surrogate Endpoints for Overall Survival in Extensive Stage Small-Cell Lung Cancer: Findings on the Basis of North Central Cancer Treatment Group Trials. Cancer (2011) 117(6):1262–71. doi: 10.1002/cncr.25526
13. Jett JR, Everson L, Therneau TM, Krook JE, Dalton RJ, Marschke RF Jr., et al. Treatment of Limited-Stage Small-Cell Lung Cancer With Cyclophosphamide, Doxorubicin, and Vincristine With or Without Etoposide: A Randomized Trial of the North Central Cancer Treatment Group. J Clin Oncol (1990) 8(1):33–8. doi: 10.1200/jco.1990.8.1.33
14. Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, et al. Phase III Study of Concurrent Versus Sequential Thoracic Radiotherapy in Combination With Cisplatin and Etoposide for Limited-Stage Small-Cell Lung Cancer: Results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol (2002) 20(14):3054–60. doi: 10.1200/jco.2002.12.071
15. Murray N, Coy P, Pater JL, Hodson I, Arnold A, Zee BC, et al. Importance of Timing for Thoracic Irradiation in the Combined Modality Treatment of Limited-Stage Small-Cell Lung Cancer. J Clin Oncol (1993) 11(2):336–44. doi: 10.1200/JCO.1993.11.2.336
16. Gregor A, Drings P, Burghouts J, Postmus PE, Morgan D, Sahmoud T, et al. Randomized Trial of Alternating Versus Sequential Radiotherapy/Chemotherapy in Limited-Disease Patients With Small-Cell Lung Cancer: A European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group Study. J Clin Oncol (1997) 15(8):2840–9. doi: 10.1200/jco.1997.15.8.2840
17. Schild SE, Bonner JA, Shanahan TG, Brooks BJ, Marks RS, Geyer SM, et al. Long-Term Results of a Phase III Trial Comparing Once-Daily Radiotherapy With Twice-Daily Radiotherapy in Limited-Stage Small-Cell Lung Cancer. Int J Radiat Oncol Biol Phys (2004) 59(4):943–51. doi: 10.1016/j.ijrobp.2004.01.055
18. Blackstock AW, Bogart JA, Matthews C, Lovato JF, McCoy T, Livengood K, et al. Split-Course Versus Continuous Thoracic Radiation Therapy for Limited-Stage Small-Cell Lung Cancer: Final Report of a Randomized Phase III Trial. Clin Lung Cancer (2005) 6(5):287–92. doi: 10.3816/CLC.2005.n.007
19. Giaccone G, Debruyne C, Felip E, Chapman PB, Grant SC, Millward M, et al. Phase III Study of Adjuvant Vaccination With Bec2/Bacille Calmette-Guerin in Responding Patients With Limited-Disease Small-Cell Lung Cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study). J Clin Oncol (2005) 23(28):6854–64. doi: 10.1200/JCO.2005.17.186
20. McClay EF, Bogart J, Herndon JE 2nd, Watson D, Evans L, Seagren SL, et al. A Phase III Trial Evaluating the Combination of Cisplatin, Etoposide, and Radiation Therapy With or Without Tamoxifen in Patients With Limited-Stage Small Cell Lung Cancer: Cancer and Leukemia Group B Study (9235). Am J Clin Oncol (2005) 28(1):81–90. doi: 10.1097/01.coc.0000139940.52625.d0
21. Sculier JP, Lafitte JJ, Efremidis A, Florin MC, Lecomte J, Berchier MC, et al. A Phase III Randomised Study of Concomitant Induction Radiochemotherapy Testing Two Modalities of Radiosensitisation by Cisplatin (Standard Versus Daily) for Limited Small-Cell Lung Cancer. Ann Oncol (2008) 19(10):1691–7. doi: 10.1093/annonc/mdn354
22. Le Pechoux C, Dunant A, Senan S, Wolfson A, Quoix E, Faivre-Finn C, et al. Standard-Dose Versus Higher-Dose Prophylactic Cranial Irradiation (PCI) in Patients With Limited-Stage Small-Cell Lung Cancer in Complete Remission After Chemotherapy and Thoracic Radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): A Randomised Clinical Trial. Lancet Oncol (2009) 10(5):467–74. doi: 10.1016/s1470-2045(09)70101-9
23. Sun JM, Ahn YC, Choi EK, Ahn MJ, Ahn JS, Lee SH, et al. Phase III Trial of Concurrent Thoracic Radiotherapy With Either First- or Third-Cycle Chemotherapy for Limited-Disease Small-Cell Lung Cancer. Ann Oncol (2013) 24(8):2088–92. doi: 10.1093/annonc/mdt140
24. Kubota K, Hida T, Ishikura S, Mizusawa J, Nishio M, Kawahara M, et al. Etoposide and Cisplatin Versus Irinotecan and Cisplatin in Patients With Limited-Stage Small-Cell Lung Cancer Treated With Etoposide and Cisplatin Plus Concurrent Accelerated Hyperfractionated Thoracic Radiotherapy (JCOG0202): A Randomised Phase 3 Study. Lancet Oncol (2014) 15(1):106–13. doi: 10.1016/s1470-2045(13)70511-4
25. Faivre-Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, et al. Concurrent Once-Daily Versus Twice-Daily Chemoradiotherapy in Patients With Limited-Stage Small-Cell Lung Cancer (CONVERT): An Open-Label, Phase 3, Randomised, Superiority Trial. Lancet Oncol (2017) 18(8):1116–25. doi: 10.1016/s1470-2045(17)30318-2
26. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical Methods for Incorporating Summary Time-to-Event Data Into Meta-Analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
27. Grønberg BH, Halvorsen TO, Fløtten Ø, Brustugun OT, Brunsvig PF, Aasebø U, et al. Randomized Phase II Trial Comparing Twice Daily Hyperfractionated With Once Daily Hypofractionated Thoracic Radiotherapy in Limited Disease Small Cell Lung Cancer. Acta Oncol (2016) 55(5):591–7. doi: 10.3109/0284186x.2015.1092584
28. Grønberg BH, Killingberg KT, Fløtten Ø, Brustugun OT, Hornslien K, Madebo T, et al. High-Dose Versus Standard-Dose Twice-Daily Thoracic Radiotherapy for Patients With Limited Stage Small-Cell Lung Cancer: An Open-Label, Randomised, Phase 2 Trial. Lancet Oncol (2021) 22(3):321–31. doi: 10.1016/s1470-2045(20)30742-7
29. Peters S, Pujol JL, Dafni U, Domine M, Popat S, Reck M, et al. Consolidation Nivolumab and Ipilimumab Versus Observation in Limited-Disease Small-Cell Lung Cancer After Chemo-Radiotherapy - Results From the Randomised Phase II ETOP/IFCT 4-12 STIMULI Trial. Ann Oncol (2022) 33(1):67–79. doi: 10.1016/j.annonc.2021.09.011
30. Qiu B, Li Q, Liu J, Huang Y, Pang Q, Zhu Z, et al. Moderately Hypofractionated Once-Daily Compared With Twice-Daily Thoracic Radiation Therapy Concurrently With Etoposide and Cisplatin in Limited-Stage Small Cell Lung Cancer: A Multicenter, Phase II, Randomized Trial. Int J Radiat Oncol Biol Phys (2021) 111(2):424–35. doi: 10.1016/j.ijrobp.2021.05.003
31. Hügli A, Moro D, Mermillod B, Bolla M, Alberto P, Bonnefoi H, et al. Phase II Trial of Up-Front Accelerated Thoracic Radiotherapy Combined With Chemotherapy and Optional Up-Front Prophylactic Cranial Irradiation in Limited Small-Cell Lung Cancer. Groupe d’Oncologie Thoracique Des Régions Alpines. J Clin Oncol (2000) 18(8):1662–7. doi: 10.1200/jco.2000.18.8.1662
32. Thomas CR Jr., Giroux DJ, Janaki LM, Turrisi Iii AT, Crowley JJ, Taylor SA, et al. Ten-Year Follow-Up of Southwest Oncology Group 8269: A Phase II Trial of Concomitant Cisplatin-Etoposide and Daily Thoracic Radiotherapy in Limited Small-Cell Lung Cancer. Lung Cancer (2001) 33(2-3):213–9. doi: 10.1016/S0169-5002(01)00181-7
33. Ettinger DS, Berkey BA, Abrams RA, Fontanesi J, Machtay M, Duncan PJ, et al. Study of Paclitaxel, Etoposide, and Cisplatin Chemotherapy Combined With Twice-Daily Thoracic Radiotherapy for Patients With Limited-Stage Small-Cell Lung Cancer: A Radiation Therapy Oncology Group 9609 Phase II Study. J Clin Oncol (2005) 23(22):4991–8. doi: 10.1200/jco.2005.00.414
34. Yilmaz U, Anar C, Korkmaz E, Yapicioglu S, Karadogan I, Ozkök S. Carboplatin and Etoposide Followed by Once-Daily Thoracic Radiotherapy in Limited Disease Small-Cell Lung Cancer: Unsatisfactory Results. Tumori (2010) 96(2):234–40. doi: 10.1177/030089161009600208
35. Bogart JA, Herndon Ii JE, Lyss AP, Watson D, Miller AA, Lee ME, et al. 70 Gy Thoracic Radiotherapy is Feasible Concurrent With Chemotherapy for Limited-Stage Small-Cell Lung Cancer: Analysis of Cancer and Leukemia Group B Study 39808. Int J Radiat Oncol Biol Phys (2004) 59(2):460–8. doi: 10.1016/j.ijrobp.2003.10.021
36. Miller AA, Wang XF, Bogart JA, Hodgson LD, Rocha Lima CM, Radford JE, et al. Phase II Trial of Paclitaxel-Topotecan-Etoposide Followed by Consolidation Chemoradiotherapy for Limited-Stage Small Cell Lung Cancer: CALGB 30002. J Thorac Oncol (2007) 2(7):645–51. doi: 10.1097/JTO.0b013e318074bbf5
37. Kelley MJ, Bogart JA, Hodgson LD, Ansari RH, Atkins JN, Pang H, et al. Phase II Study of Induction Cisplatin and Irinotecan Followed by Concurrent Carboplatin, Etoposide, and Thoracic Radiotherapy for Limited-Stage Small-Cell Lung Cancer, CALGB 30206. J Thorac Oncol (2013) 8(1):102–8. doi: 10.1097/JTO.0b013e31827628e1
38. Xia B, Hong LZ, Cai XW, Zhu ZF, Liu Q, Zhao KL, et al. Phase 2 Study of Accelerated Hypofractionated Thoracic Radiation Therapy and Concurrent Chemotherapy in Patients With Limited-Stage Small-Cell Lung Cancer. Int J Radiat Oncol Biol Phys (2015) 91(3):517–23. doi: 10.1016/j.ijrobp.2014.09.042
39. Kamath SS, McCarley DL, Zlotecki RA. Decreased Metastasis and Improved Survival With Early Thoracic Radiotherapy and Prophylactic Cranial Irradiation in Combined-Modality Treatment of Limited-Stage Small Cell Lung Cancer. Radiat Oncol Investig (1998) 6(5):226–32. doi: 10.1002/(SICI)1520-6823(1998)6:5<226::AID-ROI4>3.0.CO;2-H
40. Maurer LH, Herndon JE 2nd, Hollis DR, Aisner J, Carey RW, Skarin AT, et al. Randomized Trial of Chemotherapy and Radiation Therapy With or Without Warfarin for Limited-Stage Small-Cell Lung Cancer: A Cancer and Leukemia Group B Study. J Clin Oncol: Off J Am Soc Clin Oncol (1997) 15(11):3378–87. doi: 10.1200/jco.1997.15.11.3378
41. Han TJ, Kim HJ, Wu HG, Heo DS, Kim YW, Lee SH. Comparison of Treatment Outcomes Between Involved-Field and Elective Nodal Irradiation in Limited-Stage Small Cell Lung Cancer. Jpn J Clin Oncol (2012) 42(10):948–54. doi: 10.1093/jjco/hys114
42. Wang P, Liu W, Zhao L, Wang P. Does the Response to Induction Chemotherapy Impact the Timing of Thoracic Radiotherapy for Limited-Stage Small-Cell Lung Cancer? Thorac Cancer (2015) 6(5):605–12. doi: 10.1111/1759-7714.12229
43. Morimoto M, Okishio K, Akira M, Omachi N, Tamiya A, Asami K, et al. Duration of Twice-Daily Thoracic Radiotherapy and Time From the Start of Any Treatment to the End of Chest Irradiation as Significant Predictors of Outcomes in Limited-Disease Small-Cell Lung Cancer. Clin Lung Cancer (2017) 18(2):e117–e27. doi: 10.1016/j.cllc.2016.09.004
44. Zhang J, Fan M, Liu D, Zhao KL, Wu KL, Zhao WX, et al. Hypo- or Conventionally Fractionated Radiotherapy Combined With Chemotherapy in Patients With Limited Stage Small Cell Lung Cancer. Radiat Oncol (2017) 12(1):51. doi: 10.1186/s13014-017-0788-x
45. Jeong JU, Jeon W, Ahn SJ, Kim YC, Oh IJ, Park CK, et al. Treatment Time to the End of Thoracic Radiotherapy has More Predictive Power for Survival Than Radiation Dose Intensity in Patients With Limited-Stage Small-Cell Lung Cancer Receiving Concurrent Chemoradiation of More Than 45 Gy. Oncol Lett (2020) 19(1):239–46. doi: 10.3892/ol.2019.11107
46. Zayed S, Chen H, Ali E, Rodrigues GB, Warner A, Palma DA, et al. Is There a Role for Hypofractionated Thoracic Radiation Therapy in Limited-Stage Small Cell Lung Cancer? A Propensity Score Matched Analysis. Int J Radiat Oncol Biol Phys (2020) 108(3):575–86. doi: 10.1016/j.ijrobp.2020.06.008
47. Atci MM, Sakin A, Uysal E, Aksaray F, Selvi O, Can O. Survival and Prognostic Factors in Limited-Stage Small-Cell Lung Cancer. J Coll Phys Surg Pak (2021) 31(12):1433–7. doi: 10.29271/jcpsp.2021.12.1433
48. Doshita K, Kenmotsu H, Omori S, Tabuchi Y, Kawabata T, Kodama H, et al. Long-Term Survival Data of Patients With Limited Disease Small Cell Lung Cancer: A Retrospective Analysis. Invest N Drugs (2021). doi: 10.1007/s10637-021-01183-6
49. Hu X, Bao Y, Zhang L, Guo Y, Chen YY, Li KX, et al. Omitting Elective Nodal Irradiation and Irradiating Postinduction Versus Preinduction Chemotherapy Tumor Extent for Limited-Stage Small Cell Lung Cancer: Interim Analysis of a Prospective Randomized Noninferiority Trial. Cancer (2012) 118(1):278–87. doi: 10.1002/cncr.26119
50. Han D, Hao S, Tao C, Zhao Q, Wei Y, Song Z, et al. Comparison of Once Daily Radiotherapy to 60Gy and Twice Daily Radiotherapy to 45Gy for Limited Stage Small-Cell Lung Cancer. Thorac Cancer (2015) 6(5):643–8. doi: 10.1111/1759-7714.12262
51. Khanfir K, Elhfidh M, Anchisi S, Matzinger O, Bieri S, Mirimanoff RO, et al. Sequential or Concomitant Chemotherapy in Limited Stage Small-Cell Lung Cancer. Swiss Med Wkly (2011) 141:w13205. doi: 10.4414/smw.2011.13205
52. Salama JK, Hodgson L, Pang H, Urbanic JJ, Blackstock AW, Schild SE, et al. A Pooled Analysis of Limited-Stage Small-Cell Lung Cancer Patients Treated With Induction Chemotherapy Followed by Concurrent Platinum-Based Chemotherapy and 70 Gy Daily Radiotherapy: CALGB 30904. J Thorac Oncol (2013) 8(8):1043–9. doi: 10.1097/JTO.0b013e318293d8a4
53. Park K, Sun JM. Letter to the Editor on ‘Phase III Trial of Concurrent Thoracic Radiotherapy With Either First- or Third-Cycle Chemotherapy for Limited-Disease Small Cell Lung Cancer’. Ann Oncol (2014) 25(9):1866. doi: 10.1093/annonc/mdu244
54. Foster NR, Renfro LA, Schild SE, Redman MW, Wang XF, Dahlberg SE, et al. Multitrial Evaluation of Progression-Free Survival as a Surrogate End Point for Overall Survival in First-Line Extensive-Stage Small-Cell Lung Cancer. J Thorac Oncol (2015) 10(7):1099–106. doi: 10.1097/jto.0000000000000548
55. Michiels S, Le Maître A, Buyse M, Burzykowski T, Maillard E, Bogaerts J, et al. Surrogate Endpoints for Overall Survival in Locally Advanced Head and Neck Cancer: Meta-Analyses of Individual Patient Data. Lancet Oncol (2009) 10(4):341–50. doi: 10.1016/s1470-2045(09)70023-3
56. Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab Plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med (2018) 379(23):2220–9. doi: 10.1056/NEJMoa1809064
57. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab After Chemoradiotherapy in Stage III non-Small-Cell Lung Cancer. N Engl J Med (2017) 377(20):1919–29. doi: 10.1056/NEJMoa1709937
58. Faivre-Finn C, Vicente D, Kurata T, Planchard D, Paz-Ares L, Vansteenkiste JF, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J Thorac Oncol (2021) 16(5):860–7. doi: 10.1016/j.jtho.2020.12.015
59. Sun J-M. Chemoradiation With Durvalumab Followed by Durvalumab Maintenance for Limited Disease Small Cell Lung Cancer (2018). Available at: https://clinicaltrialsgov/show/NCT03585998.
60. Roche H-L. A Study of Atezolizumab With or Without Tiragolumab Consolidation in Limited Stage Small Cell Lung Caner (2020). Available at: https://clinicaltrialsgov/show/NCT04308785.
61. Higgins K, Hu C, Ross H, Jabbour S, Kozono D, Owonikoko T, et al. P48.02 NRG Oncology/Alliance LU005: Chemoradiation vs. Chemoradiation Plus Atezolizumab in Limited Stage Small Cell Lung Cancer. J Thorac Oncol (2021) 16(3):S499–500. doi: 10.1016/j.jtho.2021.01.872
62. Senan S, Okamoto I, Lee GW, Chen Y, Niho S, Mak G, et al. Design and Rationale for a Phase III, Randomized, Placebo-Controlled Trial of Durvalumab With or Without Tremelimumab After Concurrent Chemoradiotherapy for Patients With Limited-Stage Small-Cell Lung Cancer: The ADRIATIC Study. Clin Lung Cancer (2020) 21(2):e84–e8. doi: 10.1016/j.cllc.2019.12.006
Keywords: limited-stage small-cell lung cancer, surrogate endpoint, overall survival, progression-free survival, time to progression, chemoradiotherapy
Citation: Yang Y, Wang J, Wang W, Zhang T, Zhao J, Wang Y, Li Y, Wang L and Bi N (2022) Progression-Free Survival and Time to Progression as Potential Surrogate Endpoints for Overall Survival in Chemoradiotherapy Trials in Limited-Stage Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 12:810580. doi: 10.3389/fonc.2022.810580
Received: 07 November 2021; Accepted: 03 January 2022;
Published: 28 January 2022.
Edited by:
Kristin Higgins, Emory University, United StatesReviewed by:
Lothar Bergmann, University Hospital Frankfurt, GermanyChukwuka Eze, Ludwig Maximilian University of Munich, Germany
Copyright © 2022 Yang, Wang, Wang, Zhang, Zhao, Wang, Li, Wang and Bi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Bi, binan_email@163.com
†These authors have contributed equally to this work
 Yin Yang
Yin Yang Jianyang Wang1†
Jianyang Wang1† Tao Zhang
Tao Zhang Jingjing Zhao
Jingjing Zhao Yu Wang
Yu Wang Yexiong Li
Yexiong Li Nan Bi
Nan Bi