- 1Department of Science and Education, The Third Hospital of Changsha, Changsha, China
- 2Department of Preventive Medicine, School of Medicine, Hunan Normal University, Changsha, China
- 3Key Laboratory of Molecular Epidemiology of Hunan Province, School of Medicine, Hunan Normal University, Changsha, China
- 4Department of Geriatrics, The Third Hospital of Changsha, Changsha, China
Objectives: The aim of the current study is to explore the association between extended adjuvant endocrine treatment and prognosis of women with hormone receptor-positive (HR+) early breast cancer.
Methods: Databases including PubMed, Web of Science, Embase and the Cochrane Library databases were electronically searched to identify randomized controlled trials (RCTs) that reported extended endocrine therapy for women with HR+ early breast cancer. The retrieval time was limited from inception to September 2022. Two reviewers independently screened literature, extracted data, and assessed risk bias of included studies. Meta-analysis was performed by using R software Version 4.1.2 and STATA Version 12.0.
Results: A total of 15 RCTs involving 29497 cases were included. The overall analysis showed that compared with the control, extended adjuvant endocrine therapy increased disease-free survival (DFS) (HR=0.814, 95% CI: 0.720-0.922, 95% PI: 0.556-1.194), overall survival (OS) (HR=0.885, 95% CI: 0.822-0.953, 95% PI: 0.771-1.035), relapse-free survival (RFS) (HR=0.833, 95% CI: 0.747-0.927, 95% PI: 0.575-1.159), distant metastatic-free survival (DMFS) (HR=0.824, 95% CI: 0.694-0.979, 95% PI: 0.300-2.089) and reduced new breast cancer cumulative incidence (NBCCI) (HR=0.484, 95% CI: 0.403-0.583, 95% PI: 0.359-0.654). For adverse events, extended adjuvant endocrine treatment was associated with a significantly higher risk of bone fracture (RR=1.446, 95% CI: 1.208-1.730, 95% PI: 1.154-1.854) and osteoporosis (RR=1.377, 95% CI: 1.018-1.862, 95% PI: 0.347-5.456).
Conclusion: Our study showed that extended adjuvant endocrine therapy increased DFS, OS, RFS, DMFS, the incidence of bone fracture and osteoporosis, and reduced NBCCI.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero, identifier (CRD42022351295)
Introduction
In recent decades, the treatment of breast cancer has changed substantially with an improved survival over time (1). Over the past 40 years, the risk of dying from breast cancer has fallen by more than 1/3 in the US and Europe, which was attributed to the early detection and improved therapy (2). Adjuvant endocrine therapy (AET) is the foundation of systemic therapy for hormone receptor-positive (HR+) breast cancer patients (3, 4). At present, the standard AET for early breast cancer includes 5-10 years of tamoxifen (TAM), 5-10 years of sequential tamoxifen followed by an aromatase inhibitor (AI), or 5 years of an AI (5–7). Several trials have shown that the risk of late recurrence was reduced after more than 5 years of extended endocrine therapy and prolonged endocrine therapy in patients with early breast cancer has clearly improved patient outcomes (5, 8–10). However, three trials presented at San Antonio Breast Cancer Symposium in 2016 did not show similar benefits for extended adjuvant endocrine therapy beyond 5 years (11–13). Therefore, the research conclusions for extended endocrine therapy are controversial.
Two recently published meta-analyses have reported the effect of extended endocrine therapy on patients with early breast cancer (14, 15). The first meta-analysis revealed that extended endocrine therapy was associated with improvement in breast cancer-specific survival, disease-free survival (DFS), disease recurrence and contralateral breast recurrence. However, the pooled effect of the first meta-analysis was reported as odds ratio (OR) (14). The second meta-analysis showed that prolonged 10-year endocrine treatment improved DFS in patients with early breast cancer (15). Nevertheless, the second study merely reported the outcomes for DFS and overall survival (OS).
Therefore, the objectives of the present study were to assess the the clinical outcomes of extended adjuvant endocrine therapy in women with HR+ early breast cancer by estimating a pooled effect of hazard ratio (HR) extracted from randomized controlled trials (RCTs). In addition, we aimed to supplement the clinical outcomes of relapse-free survival (RFS), distant metastatic-free survival (DMFS), new breast cancer cumulative incidence (NBCCI) and adverse events (AEs), and update DFS and OS of extended endocrine treatment for HR+ early breast cancer.
Materials and methods
This study does not require ethical approval and informed consent because it is a systematic review and meta-analysis of previously published literature and does not address ethics or patient privacy. Our study was analyzed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (16). This systematic review protocol has been registered in PROSPERO’s database (registration number: CRD42022351295).
Search strategy
Two reviewers independently performed a comprehensive literature search in four electronic databases, including PubMed, Web of Science, Embase and the Cochrane Library. All databases were searched through September 2022. The following MeSH terms and keywords were searched: ((breast neoplasm) OR (breast tumor) OR (breast cancer) OR (mammary cancer) OR (breast carcinoma)) AND (hormone OR endocrine OR anti-hormone OR adjuvant OR tamoxifen OR letrozole OR exemestane OR anastrozole OR (aromatase inhibitor)) AND (therapy OR treatment) AND (extend OR extended OR extension OR prolonged OR prolongation) AND ((controlled clinical trial) OR (randomized controlled trial)). The details of the search strategy were provided in Supplemental Files 1.
Inclusion and exclusion criteria
The following criteria were predefined for inclusion of a study: (i) randomized controlled trials (RCTs) comparing the prolonged AET to a control group (placebo, observation or extended treatment for 2-5 years); (ii) the participants were women with HR+ early breast cancer; (iii) hazard ratio (HR) and their 95% confidence intervals (CIs) were reported in the original article or could be extracted from Kaplan-Meier curves. The exclusion criteria were as follows: (i) observational studies; (ii) different endocrine therapy drugs were used in experimental and control arm during extended treatment; (iii) literature reviews, conference abstract, and study protocols.
Assessment of study quality
We used the modified Jadad scale to assess the quality of RCTs (17). The evaluation criteria of the modified Jadad scale included four items: randomization, randomization concealment, double blind, and withdrawals and dropouts. The score 0-3 out of 7 is considered a low-quality study and a score of 4-7 is a high-quality study. When inconsistency exists, a third reviewer will make the final decision after verification and discussion.
Data extraction
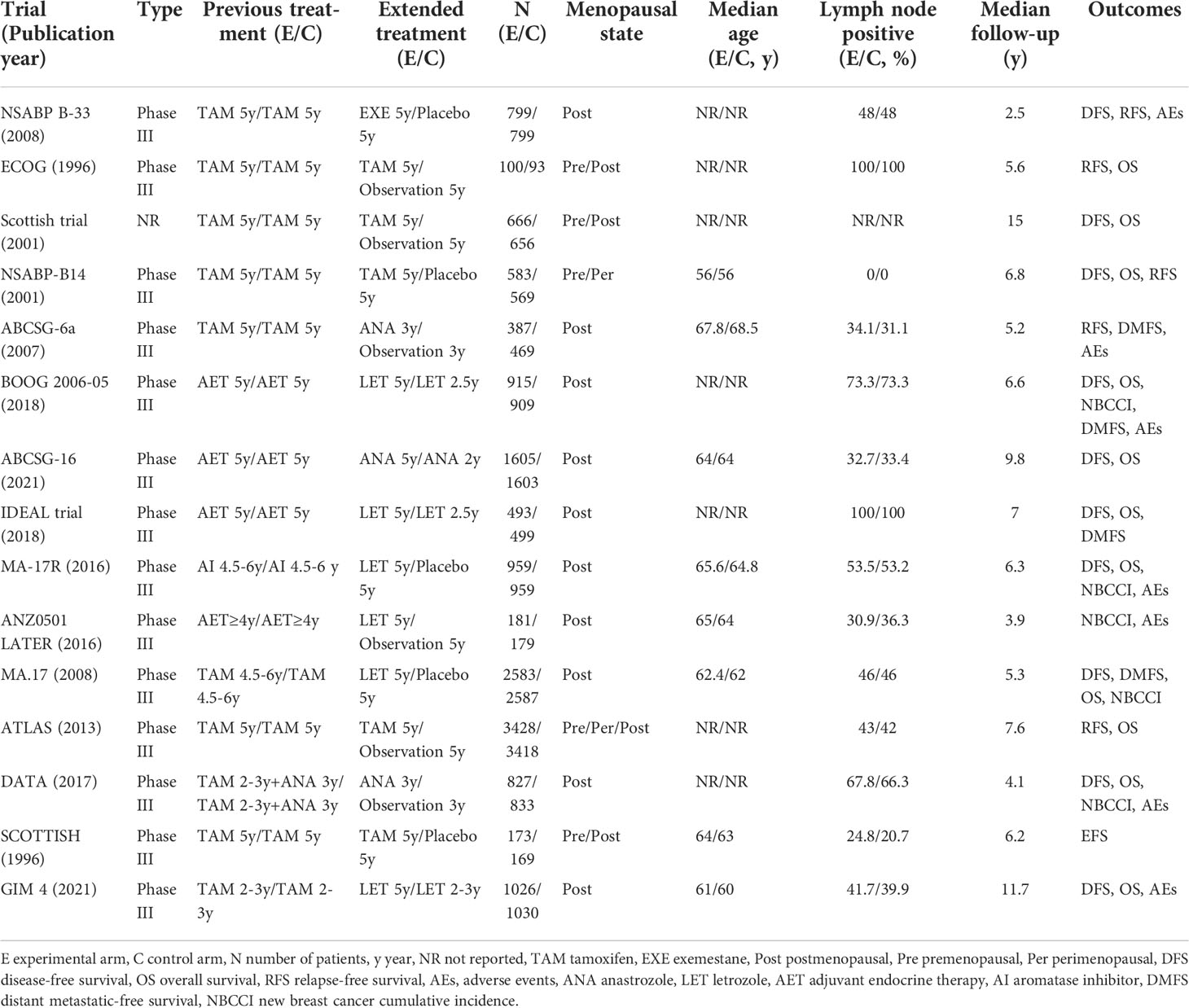
Screening of studies, selection, exclusion, and data extraction were performed by two reviewers independently. Any disagreements were discussed and reached a consensus. We extracted the following information from RCTs: trial and publication year, RCT type, previous and extended treatment, menopausal state, number and median age of participants, lymph node positive rate, median follow-up, and outcomes (the primary outcomes were DFS and OS, the secondary outcomes were RFS, DMFS, NBCCI and AEs).
Statistical analysis
Statistical analyses were performed with R software Version 4.1.2 and STATA Version 12.0 (StataCorp, College Station, TX, USA). HR and 95% CI were extracted from Kaplan-Meier curves using Engauge Digitizer version 10.8 (http://markummitchell.github.io/engauge-digitizer/) and methodology by Tierney et al. (18). Data of dichotomous outcomes were pooled using the risk ratio (RR) and presented as the 95% CI. Heterogeneity was assessed statistically by using the Cochran’s Q test, I2 and Tau2 statistic and 95% prediction interval (PI) (19, 20). When I2 < 50%, the results of the associated studies were considered to have acceptable heterogeneity, and a fixed-effects model (using inverse variance method) was utilized. When I2 ≥ 50%, it was considered that there was heterogeneity in the results of the included studies, and a random-effects model (using DerSimonian and Laird method) was selected (21). The trim-and-fill method was used to test and adjust for publication bias (22). All the P-values are two-sided.
Trial sequential analysis
We performed a trial sequential analysis (TSA) to assess if the available evidence is up to the required information size (RIS) for robust conclusion (23). For dichotomous outcomes, the TSA was performed using TSA v0.9.5.10 Beta software (www.ctu.dk/tsa). STATA Version 12.0 (metacumbounds and rsource function) and R software Version 4.1.2 (foreign and ldbounds packages) were used to perform TSA for outcomes of DFS, OS, RFS, DMFS and NBCCI with an a priori information size (APIS) method. In the present TSA, we estimated the RIS and built O’Brien-Fleming α-spending boundaries by using type I error of 5% and type II error of 20%, which were two-side values. If the cumulative Z-curve crossed the trial sequential monitoring boundary or RIS boundary, no further trials were considered to be needed and firm evidence was obtained.
Results
Procedure of literature selection
The initial search identified 2836 relevant studies (Pubmed: 642, Web of science: 976, Embase: 389, the Cochrane Library: 829). After 1016 duplicate studies were excluded, 1820 articles remained. Then 1774 articles were excluded after screening the titles and abstracts according to the eligibility criteria, eventually, 46 potential articles were reviewed for full-text. After reading the full text, 31 articles did not meet the inclusion criteria: 8 articles did not report Kaplan-Meier curves, HR and their 95%CI; 4 articles were not RCTs of extended endocrine therapy; 19 were conference abstract. Finally, 15 eligible studies (5, 9–11, 24–33) were included in the present meta-analysis (Figure 1).
Characteristics and quality assessment of the included studies
15 RCTs involving 29497 cases were included in our meta-analysis, of which 14 were phase III trials, and one article did not report the trial type. The characteristics of the included trials are shown in Table 1. All the studies included scored 4-7, which were considered as high quality, because the study design had been described in detail (Supplemental Files 2).
DFS and OS of extended endocrine treatment versus the control
As shown in Table 2, 10 trials with a total of 20900 subjects reported DFS. There was medium heterogeneity among the studies concerning DFS, a random-effect model was used to analyze the pooled DFS (I2 = 65.0%, Tau2 = 0.0236, P=0.002). The overall analysis showed that extended endocrine therapy significantly increased DFS compared with the control (HR=0.814, 95% CI: 0.720-0.922, 95% PI: 0.556-1.194) (Figure 2A). The subgroup analysis was undertaken based on the extended treatment method in experimental and control arm (Subgroup 1), the duration of adjuvant endocrine therapy (Subgroup 2), menopausal state of patients (Subgroup 3), lymph node positive/negative (Subgroup 4) and the type of prior endocrine treatment (Subgroup 5). We found that prolonged treatment with drugs increased DFS compared with observation in the control (HR=0.752, 95% CI: 0.665-0.851) and compared with AET for 5 years, AET for 10 years (HR=0.790, 95% CI: 0.632-0.988, 95% PI: 0.371-1.685) or for 7-8 years (HR=0.783, 95% CI: 0.677-0.906) increased DFS. The significant benefit of extended endocrine therapy for DFS was obtained respectively in postmenopausal women (HR=0.793, 95% CI: 0.702-0.895, 95% PI: 0.568-1.107) or lymph node positive/negative patients (HR=0.804, 95% CI: 0.707-0.914, 95% PI: 0.561-1.152). Additionally, we found that extended endocrine treatment increased DFS only in the group that the prior endocrine treatment was TAM (HR=0.811, 95% CI: 0.675-0.974, 95% PI: 0.438-1.500) (Figure 3).
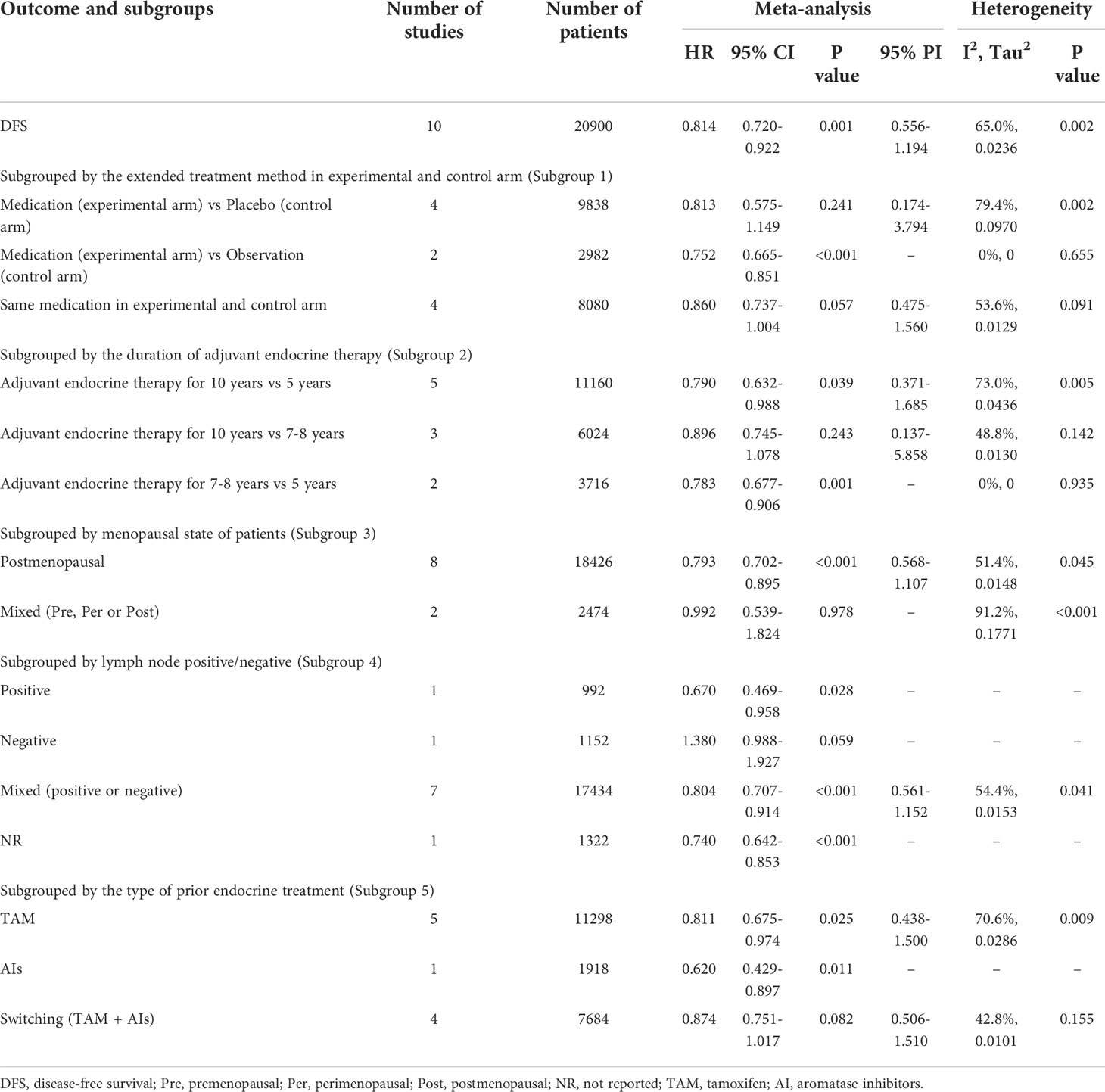
Table 2 Meta-analysis of DFS for extended versus routinely endocrine treatment in HR+ early breast cancer.
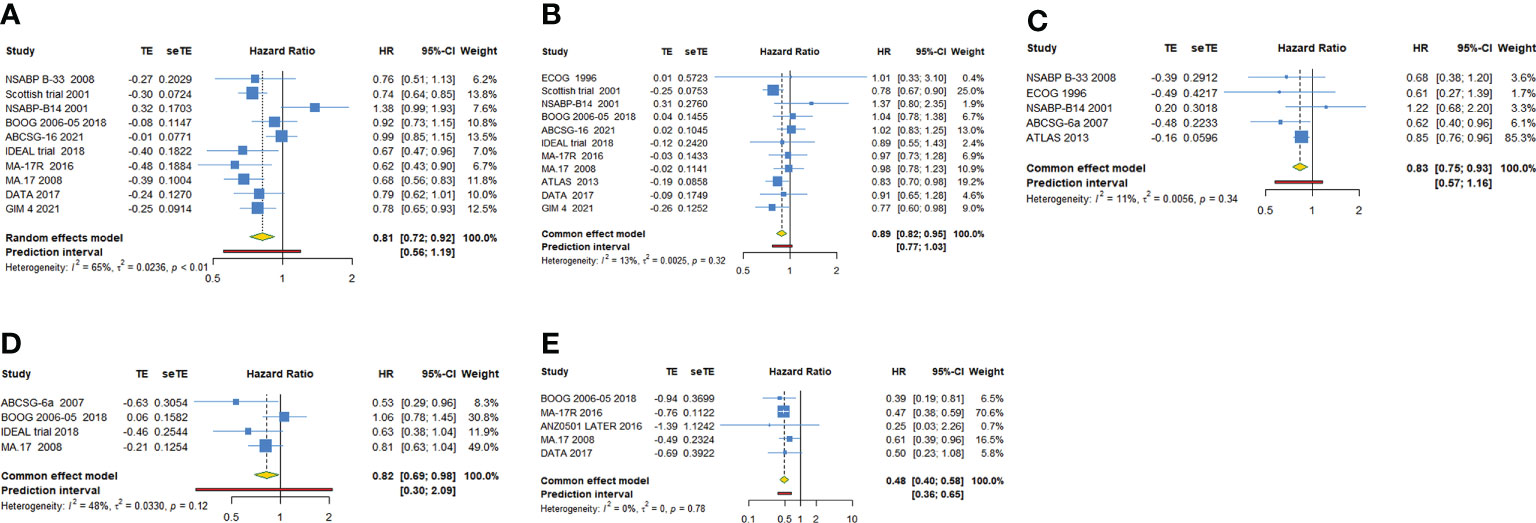
Figure 2 Forest plot of meta-analysis of extended adjuvant endocrine therapy for (A) disease-free survival, (B) overall survival, (C) relapse-free survival, (D) distant metastatic-free survival, and (E) new breast cancer cumulative incidence.
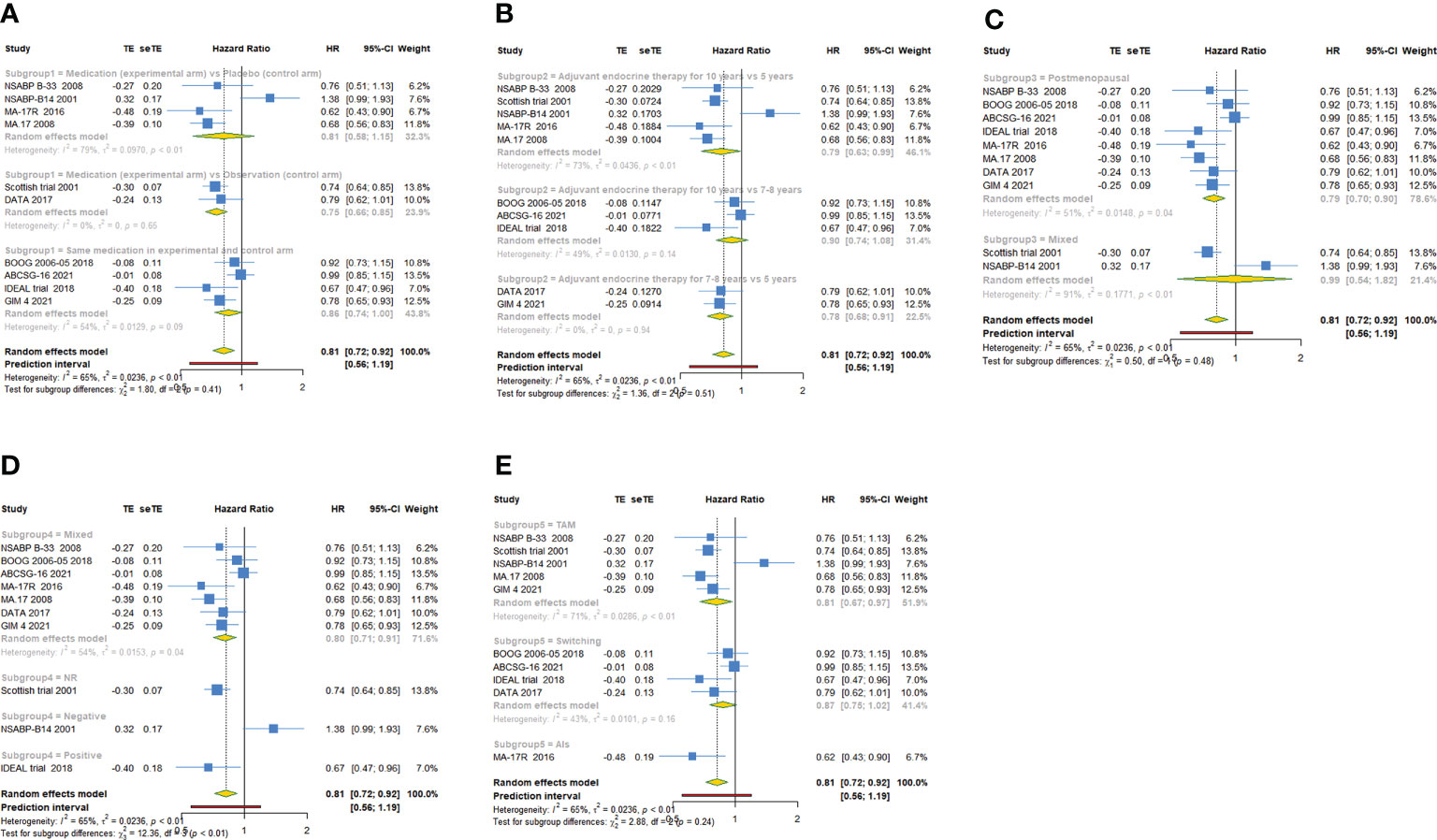
Figure 3 Forest plot of subgroup analysis of extended adjuvant endocrine therapy for disease-free survival (DFS). (A) Subgrouped by the extended treatment method in experimental and control arm; (B) subgrouped by the duration of adjuvant endocrine therapy; (C) subgrouped by menopausal state of patients; (D) subgrouped by lymph node positive/negative; (E) subgrouped by the type of prior endocrine treatment.
11 trials with a total of 26341 subjects reported OS. There was no significant heterogeneity among the studies concerning OS, a fixed-effect model was used to analyze the pooled OS (I2 = 12.9%, Tau2= 0.0025, P=0.321). The overall analysis showed that extended endocrine therapy significantly increased OS compared with the control (HR=0.885, 95% CI: 0.822-0.953, 95% PI: 0.771-1.035) (Figure 2B). As shown in Table 3, compared with observation in the control, prolonged treatment with drugs increased OS (HR=0.813, 95% CI: 0.732-0.903, 95% PI: 0.645-1.024). In addition, compared with AET for 5 years, AET for 10 years (HR=0.861, 95% CI: 0.785-0.944, 95% PI: 0.675-1.148) or for 7-8 years (HR=0.814, 95% CI: 0.668-0.994) increased OS. The significant benefit of extended endocrine treatment for OS was obtained in the group that the prior endocrine treatment was TAM (HR=0.837, 95% CI: 0.765-0.917, 95% PI: 0.664-1.083). No significant benefit of extended endocrine therapy for OS was observed in postmenopausal women (HR=0.946, 95% CI: 0.855-1.046, 95% PI: 0.829-1.079) or lymph node positive patients (HR=0.907, 95% CI: 0.586-1.404) (Figure 4).
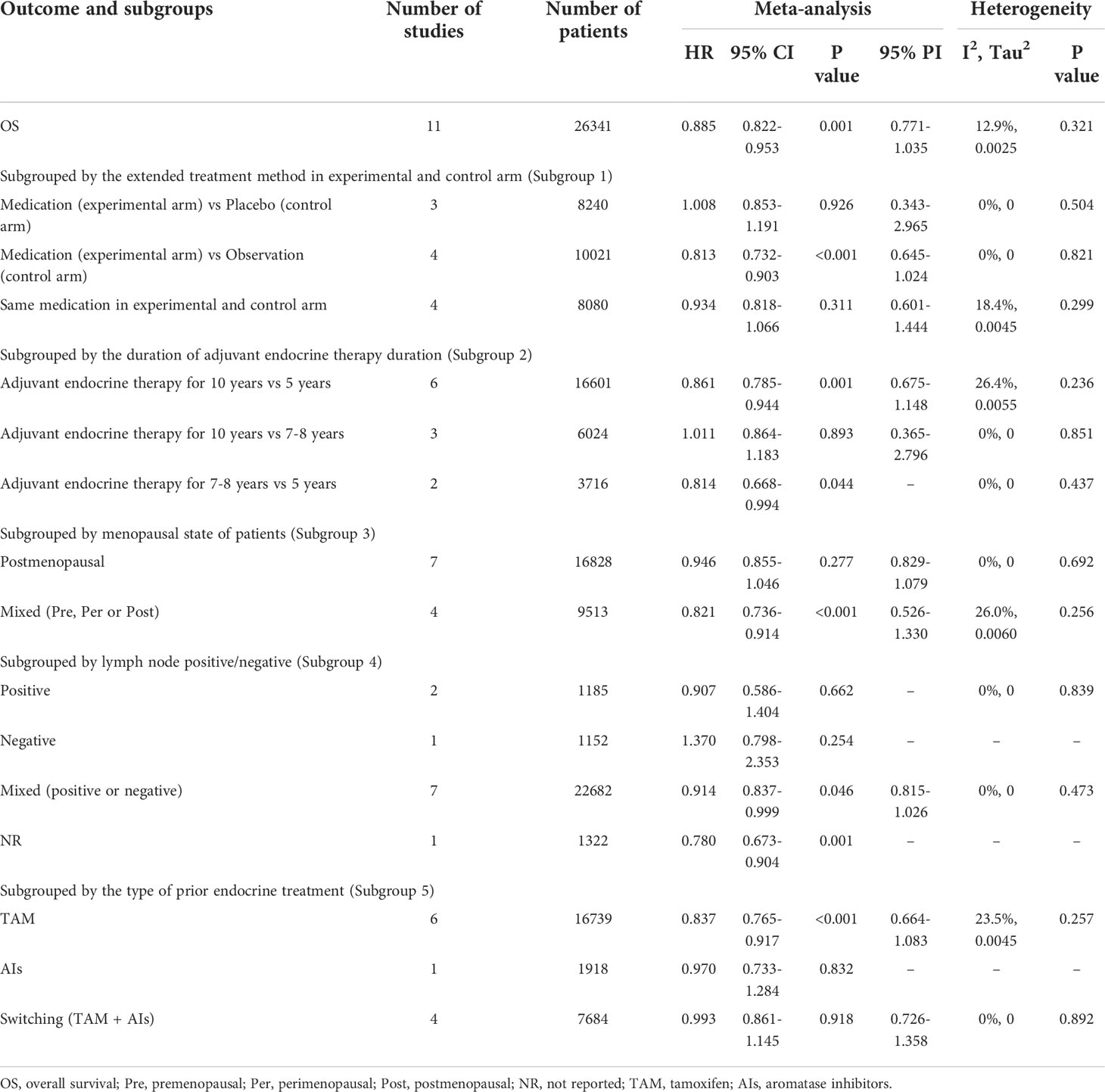
Table 3 Meta-analysis of OS for extended versus routinely endocrine treatment in HR+ early breast cancer.
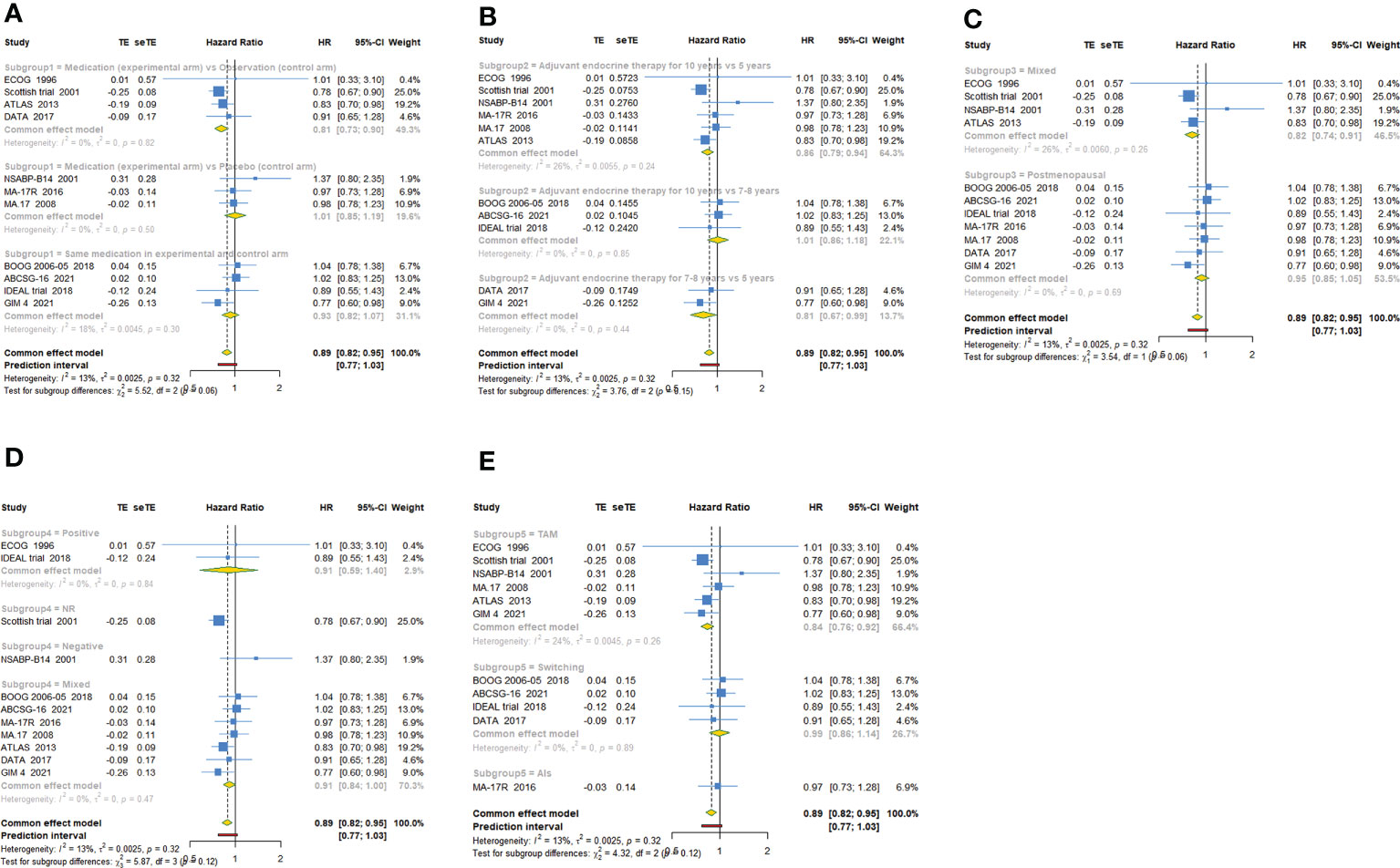
Figure 4 Forest plot of subgroup analysis of extended adjuvant endocrine therapy for overall survival (OS). (A) Subgrouped by the extended treatment method in experimental and control arm; (B) subgrouped by the duration of adjuvant endocrine therapy; (C) subgrouped by menopausal state of patients; (D) subgrouped by lymph node positive/negative; (E) subgrouped by the type of prior endocrine treatment.
RFS, DMFS and NBCCI of extended endocrine treatment versus the control
For RFS, five trials with a total of 10645 subjects reported the HR and 95%CI. Our analysis with a fixed-effect model showed that extended adjuvant endocrine therapy was associated with an increased RFS (HR=0.833, 95% CI: 0.747-0.927, 95% PI: 0.575-1.159, I2= 11.0%, Tau2= 0.0056) (Table 4, Figure 2C). Four trials involving 8842 participants reported DMFS. Our analysis with a fixed-effect model observed that extended endocrine treatment significantly increased DMFS (HR=0.824, 95% CI: 0.694-0.979, 95% PI: 0.300-2.089, I2 = 47.9%, Tau2 = 0.0330) (Table 4; Figure 2D). For NBCCI, five trials with a total of 10932 subjects were included in our analysis. The result with a fixed-effect model showed that extended adjuvant endocrine therapy reduced NBCCI (HR=0.484, 95% CI: 0.403-0.583, 95% PI: 0.359-0.654, I2 = 0, Tau2 = 0) (Table 4; Figure 2E).
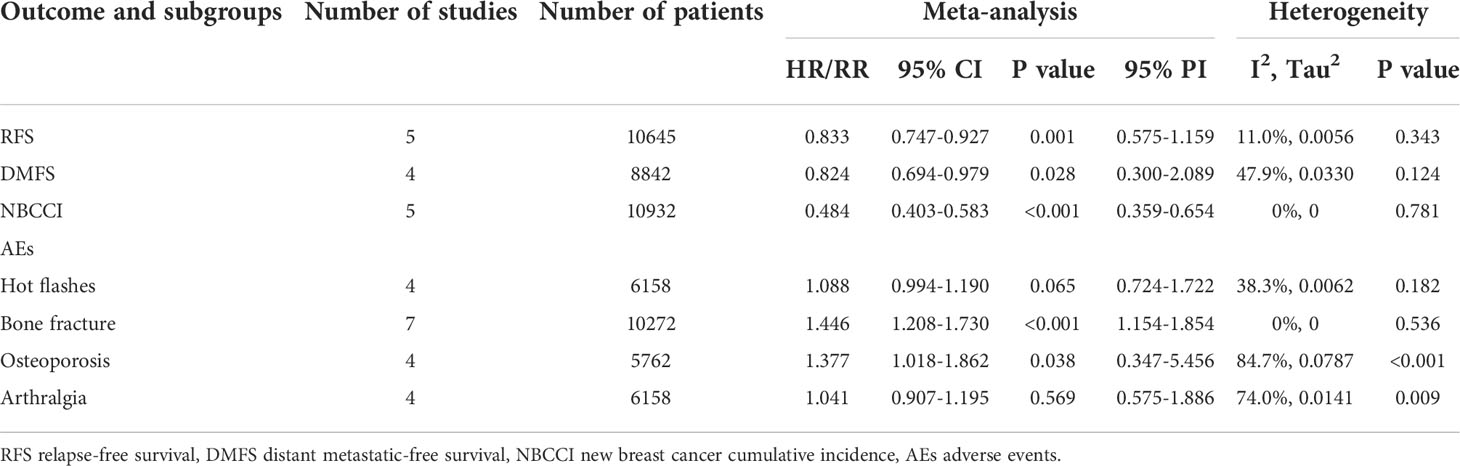
Table 4 Meta-analysis of RFS, DMFS, NBCCI and AEs for extended versus routinely endocrine treatment in HR+ early breast cancer.
EFS of extended endocrine treatment versus the control
For EFS, the trial SCOTTISH reported that extended TAM therapy for 5 years had no effect on EFS (HR=1.270, 95%CI: 0.871-1.852). Since only 1 article reported the effect of extended adjuvant endocrine therapy on prognosis of EFS, it cannot be performed with a meta-analysis in our study.
Adverse events of extended endocrine treatment versus the control
Four studies reported hot flashes, with no statistical heterogeneity between the included studies (I2 = 38.3%, Tau2 = 0.0062, P=0.182). A fixed-effect model was used for statistical analysis. Our meta-analysis revealed that extended adjuvant endocrine therapy group was without a significant risk of hot flashes (RR=1.088, 95% CI: 0.994-1.190, 95% PI: 0.724-1.722) compared with routine group (Table 4; Figure 5A).
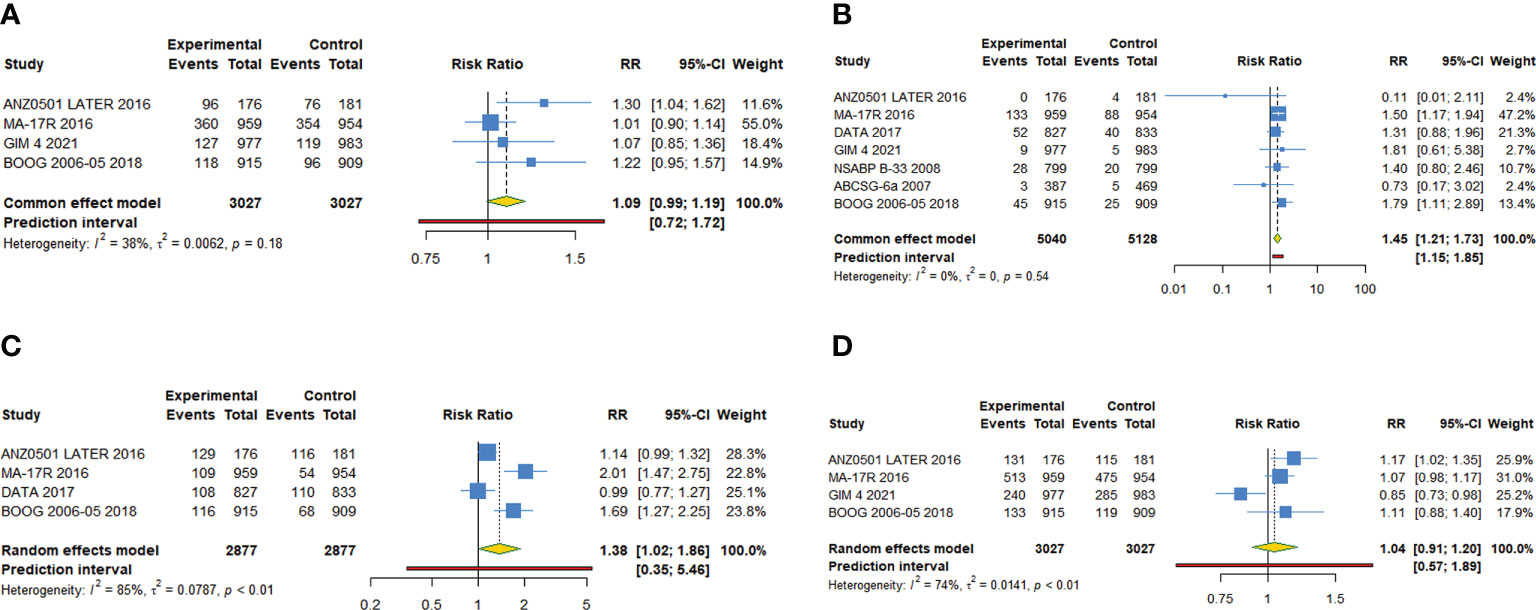
Figure 5 Forest plot of meta-analysis of extended adjuvant endocrine therapy for adverse events. (A) Hot flashes, (B) bone fracture, (C) osteoporosis, (D) arthralgia.
Seven studies reported bone fracture, revealing that extended treatment group was associated with a significantly higher risk of bone fracture (RR=1.446, 95% CI: 1.208-1.730, 95% PI: 1.154-1.854) compared with control group (Table 4; Figure 5B). There was no statistical heterogeneity between the included studies (I2 = 0%, Tau2 = 0, P=0.536).
Four studies reported osteoporosis, revealing that extended adjuvant endocrine treatment was associated with a significantly higher risk of osteoporosis (RR=1.377, 95% CI: 1.018-1.862, 95% PI: 0.347-5.456) compared with the control (Table 4; Figure 5C). There was significant heterogeneity between the included studies (I2 = 84.7%, Tau2 = 0.0787, P<0.001).
Four studies reported arthralgia, revealing that no risk difference (RR=1.041, 95% CI: 0.907-1.195, 95% PI: 0.575-1.886) between extended and routinely endocrine treatment (Table 4; Figure 5D). There was significant heterogeneity between the included studies (I2 = 74.0%, Tau2 = 0.0141, P=0.009).
Trial sequential analysis results
In trial sequential analysis of DFS, OS, RFS, DMFS and NBCCI, we estimated a required sample size of 1990 (APIS=1990), and the cumulative Z-curve significantly crossed the conventional monitoring boundary and RIS boundary, further suggesting that no additional studies were needed for a stable conclusion (Supplemental Files 3; Figure S1). For adverse events, trial sequential analysis estimated a required information size of 28854, 10890 and 13025 respectively for hot flashes, osteoporosis and arthralgia, which greatly exceeded the accumulated sample size in our study. Furthermore, although accumulative Z-curve crossed conventional monitoring boundary, it did not cross the trial sequential monitoring boundary. Therefore, we cannot draw a definitive conclusion about hot flashes, osteoporosis and arthralgia due to the presence of false positive. However, a relatively definite conclusion of bone fracture can be obtained from this meta-analysis, as the the cumulative Z-curve crossed both the conventional monitoring boundary and RIS boundary (Supplemental Files 3; Figure S2).
Sensitivity analysis and publication bias
We performed a sensitivity analysis for DFS and OS by calculating the pooled HRs and the corresponding 95% CIs after individual studies were omitted to assess whether the pooled results were affected by a single study. The removal of any single study had no significant effect on the quantitative results, suggesting that the pooled results were robust and reliable. The results were shown in Figure S3 (Supplementary Files 3). Trim-and-fill analysis was conducted for DFS and OS, and a funnel plot with imputed studies was obtained in OS, suggesting the existence of publication bias. After the trim-and-fill analysis, the correction for potential publication bias did not alter the result of OS. The funnel plots of trim-and-fill method were shown in Figure S4 (Supplementary Files 3).
Discussion
Due to the role of estrogen receptor (ER) in the biology of breast cancer, modulation of estrogen signal through endocrine therapy has long been an important component of the treatment for all stages of HR+ breast cancer (34). HR+ breast cancer accounts for an estimated 75% of breast cancer patients, and therefor early breast cancer can benefit from endocrine treatment (35). Standard endocrine therapy consists of daily oral anti-estrogens for five consecutive years, with different treatment options according to menopausal status. Tamoxifen is a selective ER modulator, which competitively inhibits the binding of estrogen and ER and is effective for premenopausal and postmenopausal women (36). AIs (anastrozole, exemestane, and letrozole) reduce circulating estrogen levels by inhibiting androgen to estrogen conversion and are effective only in postmenopausal women (including those in postmenopausal women due to medical ovarian suppression or oophorectomy) (37). The current 5- or 10-year AET for early breast cancer is based on the early results of tamoxifen adjuvant therapy (5, 38). However, it was reported that about 50% of breast cancer recurrences happened after the initial 5-years adjuvant treatment (39). These results initiated a debate about the extended adjuvant endocrine therapy, and numerous studies was carried out to elucidate on this matter.
The present meta-analysis compared the extended adjuvant endocrine therapy and non-extended standard endocrine therapy for HR+ early breast cancer. Our results demonstrated the benefits of extended endocrine therapy for DFS, OS, RFS, DMFS and NBCCI in patients with HR+ early breast cancer and showed that extended treatment group was associated with a significantly higher risk of bone fracture and osteoporosis compared with control group. Subgroup analysis revealed that AET for 10 years or 7-8 years increased DFS and OS compared with AET for 5 years, the significant benefit of extended endocrine therapy for DFS was obtained in postmenopausal women, and extended adjuvant endocrine treatment increased DFS and OS only in the group that the prior endocrine treatment was TAM.
Whether prolonged AET can further improve the clinical benefit has been a controversial topic. Several large clinical trials have shown that AET for 5 years significantly decreases the risks of breast cancer death, local and distant recurrence, contralateral breast cancer and death from any cause (7, 38, 40). The clinical application of extended endocrine treatment should be carefully weighed against the differences in study population and background in different trials (15). Our results showed that extended adjuvant endocrine therapy increased DFS and OS in patients with hormone receptor-positive early breast cancer. A recent meta-analysis demonstrated that compared with routinely 5-year therapy, extended adjuvant endocrine therapy for 10 years had no effect on OS, which was inconsistent with our result. The causes for the inconsistence may be the differences in studies included in the two meta-analysis and difference in the follow-up duration between included studies (15). For the trial GIM 4 with a median follow-up of 11.7 years included in our study, 15y-DFS was reported with a significant difference (HR=0.78, 95% CI: 0.65-0.93), while 5y-DFS extracted from Kaplan-Meier curves showed no significant difference (HR=1.05, 95%CI: 0.67-1.65). Indeed, the endocrine therapy has a carryover effect with an increase in absolute survival benefit over time, which becomes very pronounced in the second decade after diagnosis compared with the first 5 years of follow-up (7, 8). It becomes therefore obvious that an adequate follow-up duration is crucial in the attempt to assess the benefit of extended adjuvant endocrine treatment (41). Moreover, since OS is the result of breast cancer-related and non-related events, it could be considered that statistically significant OS differences are difficult to be obtained in the presence of a small number of events. Events unrelated to disease recurrence may overtake the ones related to early breast cancer morbidity and mortality, thus masking the actual experimental therapeutic benefits (42). We speculated that the benefit of extended adjuvant endocrine therapy for DFS and OS in early breast cancer patients was more pronounced over time. More RCTs with longer follow-up duration were required to verify our hypothesis.
Additionally, our meta-analysis showed a NBCCI reduction of extending endocrine treatment. The result was consistent with the MA.17R trial, in which the majority of the effect of 5-year LET was explained as prevention of contralateral breast cancer (10). It could be argued that prolonged AI adjuvant therapy for 5-10 years has a significant effect on preventing the recurrence of breast cancer (43, 44). The benefits of extended endocrine therapy for RFS and DFMS were also obtained in our analysis. Evidence from the ATLAS trial existed that 10-year tamoxifen in estrogen receptor-positive breast cancer significantly reduced the recurrence rate and mortality rate of breast cancer not only during the first decade of continuous treatment but also during the second decade after the end of treatment (5). The mechanism of extended adjuvant endocrine therapy to reduce DMFS has not been clear. It may be that mutations in the gene encoding for estrogen receptor are associated with resistance against aromatase inhibitors (34, 45). Dormant tumor cells may become less resistant to AIs, causing the extended therapy to have significant benefit. Nevertheless, our study was still unable to determine the optimal duration of prolonged treatment. For the IDEAL trial and ABCSG-16, patients receiving sequential TAM and AIs or TAM alone or AIs alone for an initial 5 years were randomly assigned to receive 5-year extended AIs treatment (the total duration of AET was 10 years) and 2-2.5 years of prolonged AIs treatment (the total duration of AET was 7-7.5 years). Both trials showed that AET for 10 years was not superior to 7-7.5 years. Subgroup analysis showed that AET for 10 years or 7-8 years improved DFS and OS compared with AET for 5 years. We cannot conclude that the 10-year benefit of adjuvant endocrine treatment is superior to that of 7-8 years by comparing the size of HR values (DFS: 10 years: HR=0.790, 7-8 years: HR=0.783; OS: 10 years: HR=0.861, 7-8 years: HR=0.814).
Our analysis revealed that extended adjuvant endocrine treatment was associated with a significantly higher risk of bone fracture and osteoporosis. The adverse events and clinical efficacy are two decisive factors for extended adjuvant endocrine treatment. The persistence of endocrine therapy can be interrupted by serious adverse events (46, 47). Thus, accurate assessment of adverse events caused by extended treatment is critical to smooth implementation of the trial. There was sufficient evidence that the use of AIs increased the risk of bone related adverse events, such as bone loss and fracture rate (48). The administration of AI can inhibit the conversion of androgens to estrogens, leading to osteopenia and osteoporosis (49, 50). This may cause an increase in the incidence of fragility fractures among patients taking AI (51, 52). A mouse model study of breast cancer demonstrated that AIs, used as blockers of estrogen biosynthesis for standard endocrine therapy, can cause muscle weakness and bone loss (53).
There were some important limitations in our work. First, some of the clinical outcomes (DFS, OS or RFS, etc) were extracted from the Kaplan-Meier curves, which may cause accidental error for HR value and their 95% CI and mask some statistically significant results. Second, different trials have different follow-up times, and this limitation may not capture some late deaths or recurrences that are commonly observed in this disease. Third, due to the differences of outcome definition between included trials (Supplemental Files 4), uncorrectable heterogeneity existed in present analysis (e.g., RFS in MA.17R included only recurrences of original breast cancer or a new breast cancer while NSABP-B33 included also new non-breast primary cancer and death from any cause). Fourth, the TSA results of hot flashes, osteoporosis and arthralgia showed that the cumulative Z-curve crossed neither the the trial sequential monitoring boundary nor RIS boundary, suggesting that more high-quality RCTs with large sample size were needed in future research.
Conclusion
In summary, our meta-analysis demonstrated the benefits of extended adjuvant endocrine therapy for DFS, OS, RFS, DMFS and NBCCI in women with HR+ early breast cancer and showed that extended treatment group was associated with a significantly higher risk of bone fracture and osteoporosis compared with control group.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
MX and YN conceived and participated in the design of this review. YZ and MX performed the literature searches, study selection, data extraction and assessed the risk of bias. MX and YZ drafted the manuscript. YY and FS helped in performing the analysis with constructive discussions. YY and YN revised the final version. All authors have made substantial contributions to this work and have approved the final version of the manuscript.
Funding
This work was supported by the Scientific Research Project of Health Commission of Changsha (2017) and Construction Project of Clinical Teaching Base of Central South University (2020, 2021).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1039320/full#supplementary-material
References
1. De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE–5-a population-based study. Lancet Oncol (2014) 15(1):23–34. doi: 10.1016/s1470-2045(13)70546-1
2. DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA: Cancer J Clin (2019) 69(6):438–51. doi: 10.3322/caac.21583
3. Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KA, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol (2019) 37(5):423–38. doi: 10.1200/jco.18.01160
4. Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol (2019) 30(8):1194–220. doi: 10.1093/annonc/mdz173
5. Davies C, Pan HC, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet (2013) 381(9869):805–16. doi: 10.1016/s0140-6736(12)61963-1
6. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. New Engl J Med (2003) 349(19):1793–802. doi: 10.1056/NEJMoa032312
7. Group EBCTC. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet (2015) 386(10001):1341–52. doi: 10.1016/s0140-6736(15)61074-1
8. Gray RG, Rea D, Handley K, Bowden SJ, Perry P, Earl HM, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol (2013) 31(18):2631–2.
9. Jakesz R, Greil R, Gnant M, Schmid M, Kwasny W, Kubista E, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian breast and colorectal cancer study group trial 6a. J Natl Cancer Institute (2007) 99(24):1845–53. doi: 10.1093/jnci/djm246
10. Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. New Engl J Med (2016) 375(3):209–19. doi: 10.1056/NEJMoa1604700
11. Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E, Duijm-de Carpentier M, Putter H, van den Bosch J, et al. Optimal duration of extended adjuvant endocrine therapy for early breast cancer; results of the IDEAL trial (BOOG 2006-05). J Natl Cancer Institute (2018) 110(1). doi: 10.1093/jnci/djx134
12. Tjan-Heijnen VC, Van Hellemond IE, Peer PG, Swinkels AC, Smorenburg CH, van der Sangen M, et al. First results from the multicenter phase III DATA study comparing 3 versus 6 years of anastrozole after 2-3 years of tamoxifen in postmenopausal women with hormone receptor-positive early breast cancer. Cancer Res (2017) 77(SUPPL 4):S1–3. doi: 10.1158/1538-7445.Sabcs16-s1-03
13. Mamounas EP, Bandos H, Lembersky BC, Geyer CE, Fehrenbacher L, Graham ML, et al. A randomized, double-blinded, placebo-controlled clinical trial of extended adjuvant endocrine therapy (tx) with letrozole (L) in postmenopausal women with hormone-receptor (+) breast cancer (BC) who have completed previous adjuvant tx with an aromatase inhibitor (AI): Results from NRG Oncology/NSABP b-42. Cancer Res (2017) 77(SUPPL 4):S1–5. doi: 10.1158/1538-7445.Sabcs16-s1-05
14. Ibrahim EM, Al-Hajeili MR, Bayer AM, Abulkhair OA, Refae AA. Extended adjuvant endocrine therapy in early breast cancer: a meta-analysis of published randomized trials. Med Oncol (2017) 34(7):131. doi: 10.1007/s12032-017-0986-2
15. Li L, Chang B, Jiang X, Fan X, Li Y, Li T, et al. Clinical outcomes comparison of 10 years versus 5 years of adjuvant endocrine therapy in patients with early breast cancer. BMC Cancer (2018) 18(1):977. doi: 10.1186/s12885-018-4878-4
16. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (2009) 339:b2535. doi: 10.1136/bmj.b2535
17. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clin trials (1996) 17(1):1–12. doi: 10.1016/0197-2456(95)00134-4
18. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
19. Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised q statistics. BMC Med Res Method (2011) 11:41. doi: 10.1186/1471-2288-11-41
20. IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open (2016) 6(7):e010247. doi: 10.1136/bmjopen-2015-010247
21. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
22. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics (2000) 56(2):455–63. doi: 10.1111/j.0006-341x.2000.00455.x
23. Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Method (2017) 17(1):39. doi: 10.1186/s12874-017-0315-7
24. Mamounas EP, Jeong JH, Wickerham DL, Smith RE, Ganz PA, Land SR, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: Intention-to-treat analysis of the national surgical adjuvant breast and bowel project b-33 trial. J Clin Oncol (2008) 26(12):1965–71. doi: 10.1200/jco.2007.14.0228
25. Tormey DC, Gray R, Falkson HC. Postchemotherapy adjuvant tamoxifen therapy beyond five years in patients with lymph node-positive breast cancer. Eastern cooperative oncology group. J Natl Cancer Institute (1996) 88(24):1828–33. doi: 10.1093/jnci/88.24.1828
26. Stewart HJ, Prescott RJ, Forrest APM. Scottish Adjuvant tamoxifen trial: a randomized study updated to 15 years. Jnci-Journal Natl Cancer Institute (2001) 93(6):456–62. doi: 10.1093/jnci/93.6.456
27. Fisher B, Dignam J, Bryant J, Wolmark N. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: Updated findings from the national surgical adjuvant breast and bowel project b-14 randomized trial. Jnci-Journal Natl Cancer Institute (2001) 93(9):684–90. doi: 10.1093/jnci/93.9.684
28. Gnant M, Fitzal F, Rinnerthaler G, Steger GG, Greil-Ressler S, Balic M, et al. Duration of adjuvant aromatase-inhibitor therapy in postmenopausal breast cancer. New Engl J Med (2021) 385(5):395–405. doi: 10.1056/NEJMoa2104162
29. Zdenkowski N, Forbes JF, Boyle FM, Kannourakis G, Gill PG, Bayliss E, et al. Observation versus late reintroduction of letrozole as adjuvant endocrine therapy for hormone receptor-positive breast cancer (ANZ0501 LATER): an open-label randomised, controlled trial(aEuro). Ann Oncol (2016) 27(5):806–12. doi: 10.1093/annonc/mdw055
30. Ingle JN, Tu D, Pater JL, Muss HB, Martino S, Robert NJ, et al. Intent-to-treat analysis of the placebo-controlled trial of letrozole for extended adjuvant therapy in early breast cancer: NCICCTG MA. 17 Ann Oncol (2008) 19(5):877–82. doi: 10.1093/annonc/mdm566
31. Tjan-Heijnen VCG, van Hellemond IEG, Peer PGM, Swinkels ACP, Smorenburg CH, van der Sangen MJC, et al. Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol (2017) 18(11):1502–11. doi: 10.1016/s1470-2045(17)30600-9
32. Stewart HJ, Forrest AP, Everington D, McDonald CC, Dewar JA, Hawkins RA, et al. Randomised comparison of 5 years of adjuvant tamoxifen with continuous therapy for operable breast cancer. Scottish Cancer Trials Breast Group Br J Cancer (1996) 74(2):297–9. doi: 10.1038/bjc.1996.356
33. Del Mastro L, Mansutti M, Bisagni G, Ponzone R, Durando A, Amaducci L, et al. Extended therapy with letrozole as adjuvant treatment of postmenopausal patients with early-stage breast cancer: a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2021) 22(10):1458–67. doi: 10.1016/s1470-2045(21)00352-1
34. Jeselsohn R, Buchwalter G, De Angelis C, Brown M, Schiff R. ESR1 mutations–a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol (2015) 12(10):573–83. doi: 10.1038/nrclinonc.2015.117
35. Orlando L, Fedele P, Cinefra M, Sponziello F, Calvani N, Chetrì MC, et al. Adjuvant endocrine treatment in premenopausal early breast cancer. Oncology (2009) 77(Suppl 1):9–13. doi: 10.1159/000258490
36. Waks AG, Winer EP. Breast cancer treatment: A review. JAMA (2019) 321(3):288–300. doi: 10.1001/jama.2018.19323
37. Osborne C, Tripathy D. Aromatase inhibitors: rationale and use in breast cancer. Annu Rev Med (2005) 56:103–16. doi: 10.1146/annurev.med.56.062804.103324
38. Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet (2011) 378(9793):771–84. doi: 10.1016/s0140-6736(11)60993-8
39. Brewster AM, Hortobagyi GN, Broglio KR, Kau SW, Santa-Maria CA, Arun B, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Institute (2008) 100(16):1179–83. doi: 10.1093/jnci/djn233
40. Group EBCTC. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet (2005) 365(9472):1687–717. doi: 10.1016/s0140-6736(05)66544-0
41. Qian X, Li Z, Ruan G, Tu C, Ding W. Efficacy and toxicity of extended aromatase inhibitors after adjuvant aromatase inhibitors-containing therapy for hormone-receptor-positive breast cancer: a literature-based meta-analysis of randomized trials. Breast Cancer Res Treat (2020) 179(2):275–85. doi: 10.1007/s10549-019-05464-w
42. Corona SP, Roviello G, Strina C, Milani M, Madaro S, Zanoni D, et al. Efficacy of extended aromatase inhibitors for hormone-receptor-positive breast cancer: A literature-based meta-analysis of randomized trials. Breast (2019) 46:19–24. doi: 10.1016/j.breast.2019.04.004
43. Ruhstaller T, Giobbie-Hurder A, Colleoni M, Jensen MB, Ejlertsen B, de Azambuja E, et al. Adjuvant letrozole and tamoxifen alone or sequentially for postmenopausal women with hormone receptor-positive breast cancer: Long-term follow-up of the BIG 1-98 trial. J Clin Oncol (2019) 37(2):105–14. doi: 10.1200/jco.18.00440
44. Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol (2008) 9(1):45–53. doi: 10.1016/s1470-2045(07)70385-6
45. Jeselsohn R, Yelensky R, Buchwalter G, Frampton G, Meric-Bernstam F, Gonzalez-Angulo AM, et al. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res (2014) 20(7):1757–67. doi: 10.1158/1078-0432.Ccr-13-2332
46. Barron TI, Connolly R, Bennett K, Feely J, Kennedy MJ. Early discontinuation of tamoxifen: a lesson for oncologists. Cancer (2007) 109(5):832–9. doi: 10.1002/cncr.22485
47. Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology (2006) 71(1-2):1–9. doi: 10.1159/000100444
48. Group EBCTC. Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: a patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol (2022) 23(3):382–92. doi: 10.1016/s1470-2045(21)00758-0
49. Riggs BL, Khosla S, Melton LJ 3rd. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone mineral Res (1998) 13(5):763–73. doi: 10.1359/jbmr.1998.13.5.763
50. Rachner TD, Coleman R, Hadji P, Hofbauer LC. Bone health during endocrine therapy for cancer. Lancet Diabetes Endocrinol (2018) 6(11):901–10. doi: 10.1016/s2213-8587(18)30047-0
51. Yao S, Laurent CA, Roh JM, Lo J, Tang L, Hahn T, et al. Serum bone markers and risk of osteoporosis and fragility fractures in women who received endocrine therapy for breast cancer: a prospective study. Breast Cancer Res Treat (2020) 180(1):187–95. doi: 10.1007/s10549-019-05518-z
52. Ramchand SK, Cheung YM, Grossmann M. Bone health in women with breast cancer. Climacteric (2019) 22(6):589–95. doi: 10.1080/13697137.2019.1580257
Keywords: aromatase inhibitor, extended adjuvant endocrine therapy, prognosis, disease-free survival, overall survival
Citation: Xie M, Zhong Y, Yang Y, Shen F and Nie Y (2022) Extended adjuvant endocrine therapy for women with hormone receptor-positive early breast cancer: A meta-analysis with trial sequential analysis of randomized controlled trials. Front. Oncol. 12:1039320. doi: 10.3389/fonc.2022.1039320
Received: 08 September 2022; Accepted: 12 October 2022;
Published: 27 October 2022.
Edited by:
Fabio Puglisi, University of Udine, ItalyReviewed by:
Mattia Garutti, Aviano Oncology Reference Center (IRCCS), ItalyGiovanna Garufi, Agostino Gemelli University Polyclinic (IRCCS), Italy
Lucia Del Mastro, University of Genoa, Italy
Copyright © 2022 Xie, Zhong, Yang, Shen and Nie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Nie, yue-nie@cssdsyy.com
 Ming Xie
Ming Xie Yan Zhong2
Yan Zhong2 Yide Yang
Yide Yang