- 1Department of Pharmacological Modeling, M&S Decisions LLC, Moscow, Russia
- 2Department of Pharmacology, Institute of Pharmacy, I.M. Sechenov First Moscow State Medical University, Moscow, Russia
- 3MID3 Research Center, I.M. Sechenov First Moscow State Medical University, Moscow, Russia
- 4Artificial Intelligence Research Center, STU Sirius, Sochi, Russia
- 5World-Class Research Center “Digital biodesign and personalized healthcare”, I.M. Sechenov First Moscow State Medical University, Moscow, Russia
- 6Institute of Professional Education, I.M. Sechenov First Moscow State Medical University, Moscow, Russia
- 7Federal State Budgetary Institution “Scientific Centre for Expert Evaluation of Medicinal Products” of the Ministry of Health of the Russian Federation, Moscow, Russia
Immuno-oncology is an emerging field in the treatment of oncological diseases, that is based on recruitment of the host immune system to attack the tumor. Radiation exposure may help to unlock the potential of the immune activating agents by enhancing the antigen release and presentation, attraction of immunocompetent cells to the inflammation site, and eliminating the tumor cells by phagocytosis, thereby leading to an overall enhancement of the immune response. Numerous preclinical studies in mouse models of glioma, murine melanoma, extracranial cancer, or colorectal cancer have contributed to determination of the optimal radiotherapy fractionation, as well as the radio- and immunotherapy sequencing strategies for maximizing the antitumor activity of the treatment regimen. At the same time, efficacy of combined radio- and immunotherapy has been actively investigated in clinical trials of metastatic melanoma, non-small-cell lung cancer and renal cell carcinoma. The present review summarizes the current advancements and challenges related to the aforementioned treatment approach.
Principles of cancer immunotherapy
Recruiting the patient’s immune system for cancer treatment was proposed by the American surgeon William Coley back in 1891 (1). Using bacterial cultures and their metabolic products he developed a vaccine to treat patients with inoperable tumors. Despite the positive results of his research, this treatment strategy did not received an approval and was soon replaced by chemotherapy (CT) and radiation therapy (RT).
The progress in understanding the molecular mechanisms of immunity in the late 20th century, including the role of various cell populations and cytokines in the activation, maintenance and downregulation of the immune response, revitalized the idea of using the host immune system against the tumor cells (2–4). According to the current concept, proposed by D. Chen and I. Mellman (2013) (5), specific antigens released from tumor cells during stress-induced immunogenic cell death (ICD) stimulate clonal expansion of the tumor-specific T-lymphocyte subsets. Next, dendritic cells (DCs) present the antigens on major histocompatibility complex (MHC) class I and MHC class II molecules to T cells. The activated T cells attack the tumor cells and enhance the antitumor immune response. The effects of ICD stimulate the recruitment of T cells and their ability to recognize the tumor cells (6) (Figure 1).
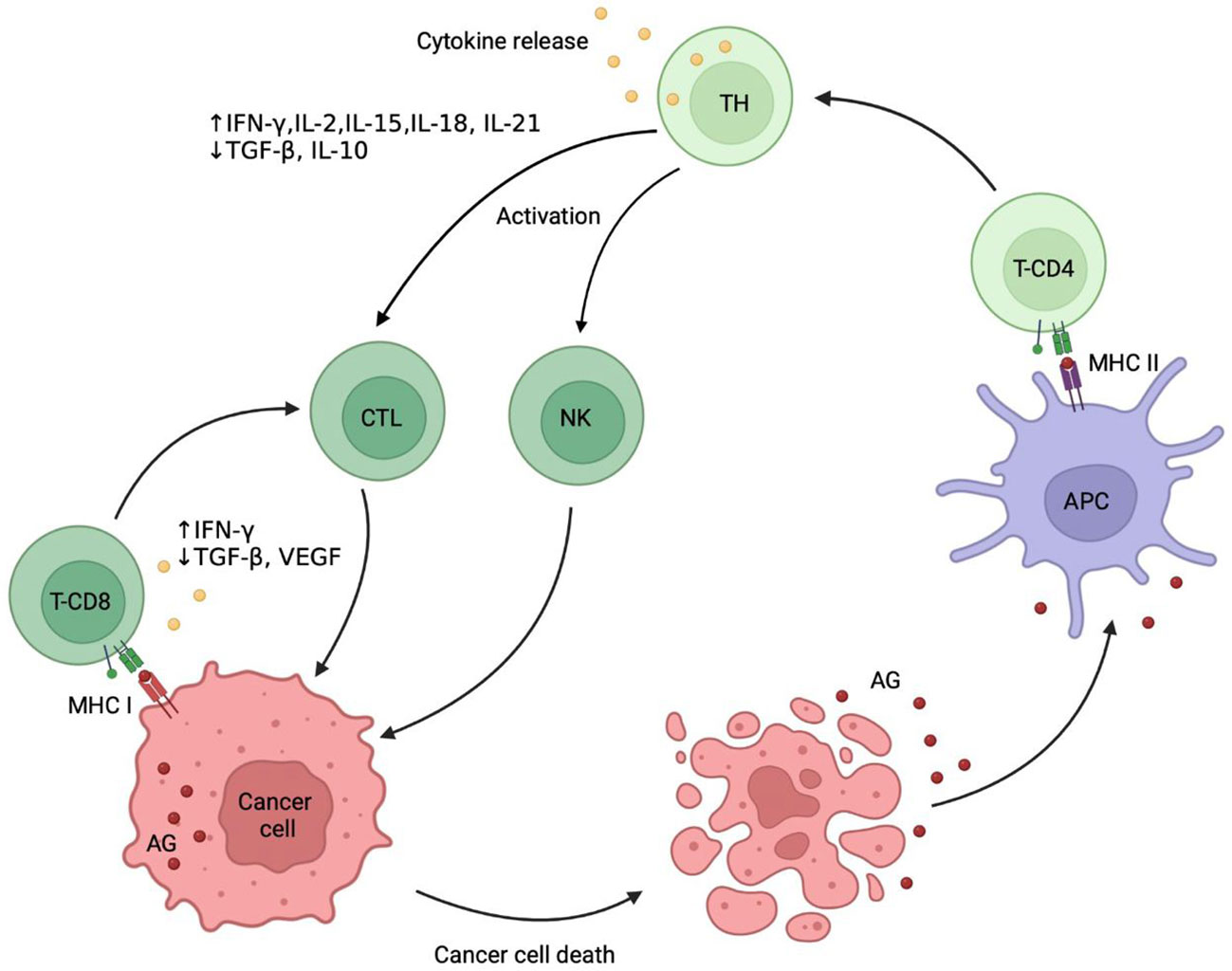
Figure 1 Cancer immunity cycle. Immunogenic death of tumor cells leads to release of tumor-specific antigens (AG) that are captured by antigen presenting cells (APC) and after binding to major histocompatibility complex (MHC) class II are presented on their surface. The receptors CD4 dock to an MHC class II, activate the naive T helper (TH) cells followed by clonal selection of antigen-specific T cells, their proliferation, migration into the tumor site and differentiation into cytotoxic T-lymphocytes (CTL). CTL express receptors CD8, which dock to MHC class I. Finally, CTL recognize tumor-specific antigens and kill the tumor cells, which produces a further release of tumor antigens into the surrounding space and potentiates the immune response. Natural killer (NK) cells recognize tumor cells without the involvement of the MHC class I, making the response mediated very quick. AG, tumor-associated antigens; MHC I, major histocompatibility complex class I; MHC II, major histocompatibility complex class II; TH, T helper cell; NK, natural killer cell.
However, practical observations show that the activation of the immune response is not always sufficient for tumor rejection, which can lead to natural selection of cancer cells, resistant to the immune attack due to multiple immunosuppressive mechanisms. This phenomenon has been termed immunoediting (3, 7). The resistant tumor cell populations recruit various mechanisms to create a tumor microenvironment (TME), which not only reduces the immune response but also stimulates tumor growth. Therefore, one of the main challenges of the current oncotherapy is to shift TME from the immunosuppressive to the immunoreactive state, which requires establishing and implementation of new therapeutic strategies. Immune tolerance was shown to be driven by multiple TME components including immunosuppressive cell populations such as regulatory T (Treg) cells and myeloid derived suppressive cells, various cytokines, soluble factors, enzymes and metabolites (e.g. arginase, adenosine, TGF-β) (8).
Immune checkpoint (IC) proteins were shown to play an important role in the immunosuppression and have been increasingly recognized as an important target for anticancer drug development. In non-pathological conditions IC proteins prevent hyperactivation of the immune system and are crucial for immune tolerance. Cancer cells, however, use these proteins to limit the specific antitumor immune response, thus protecting themselves from the T cell attack. The programmed death 1 (PD-1), its ligand PD-L1, and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) are the best characterized ICs.
PD-1 is a receptor expressed on the surface of T- and B-lymphocytes, macrophages and myeloid cells serving to suppress the autoimmunity via specific modulation of apoptotic signaling mechanisms in distinct populations of immunocompetent cells (9, 10). PD-1 is expressed both in cancer and antigen presenting cells (APC). Binding of PD-L1 to PD-1 activates the Src homology-2-containing protein tyrosine phosphatase 2 (SHP2), which inhibits the phosphatidylinositol-3-kinase (PI3k)/protein kinase B (PKB/AKT) signaling pathway by dephosphorylation of the phosphatase and tensin homolog (PTEN), thereby suppressing the downstream molecular pathways including AKT, mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK) and others (11–15). Deactivation of these cascades leads to a decrease in lymphocytes proliferative activity and effector functions (16, 17).
PD-L1 up-regulation in tumors occurs either by constitutive oncogenic signaling via AKT or signal transducer and activator of transcription 3 (STAT3), a mechanism termed intrinsic immune resistance, or by interferon gamma (IFN-γ) released from activated T cells or natural killer (NK) cells (adaptive resistance) (18). High PD-1 and PD-L1 expression was detected in several tumor cell lines, such as serous ovarian carcinoma (10) and breast cancer (19), although the prognostic value of PD-1 or PD-L1 expression is currently unclear (10, 20). Moreover, conventional cancer therapies such as radio- or chemotherapy have been associated with activation of the PD-1 signaling in tumor cells conferring a certain resistance against these treatments (21–23).
CTLA-4 represents another potential therapeutic target among ICs. CTLA-4 appears on the surface of T cells after their interactions with APC and prevents the T cells activation by competing with CD28 for its ligands, CD80 and CD86. CTLA-4 has a greater affinity for CD28-activating ligands than CD28 itself. The resulting CD28 antagonism suppresses the PI3k/AKT cascade (24–27). In contrast to the PD-1 signaling, CTLA-4 likely acts via the protein phosphatase 2A (PP2A) to decrease the abundance of phosphorylated AKT (15, 28, 29). CTLA-4 is overexpressed under pathophysiological conditions in the tumor environment, which substantially reduces the availability of CD80 and CD86 for the CD28-pathway and limits the proper activation of T-lymphocytes at early maturation stages (14).
CTLA-4 and PD-1 act as negative regulators of T cells function at different stages of the immune response: CTLA-4 regulates the proliferation of T cells in the early stages, primarily in the lymph nodes, whereas PD-1 plays an important role in the regulation of previously activated T cells, predominantly in peripheral tissues (9, 16).
Clinical application of immune checkpoint inhibitors
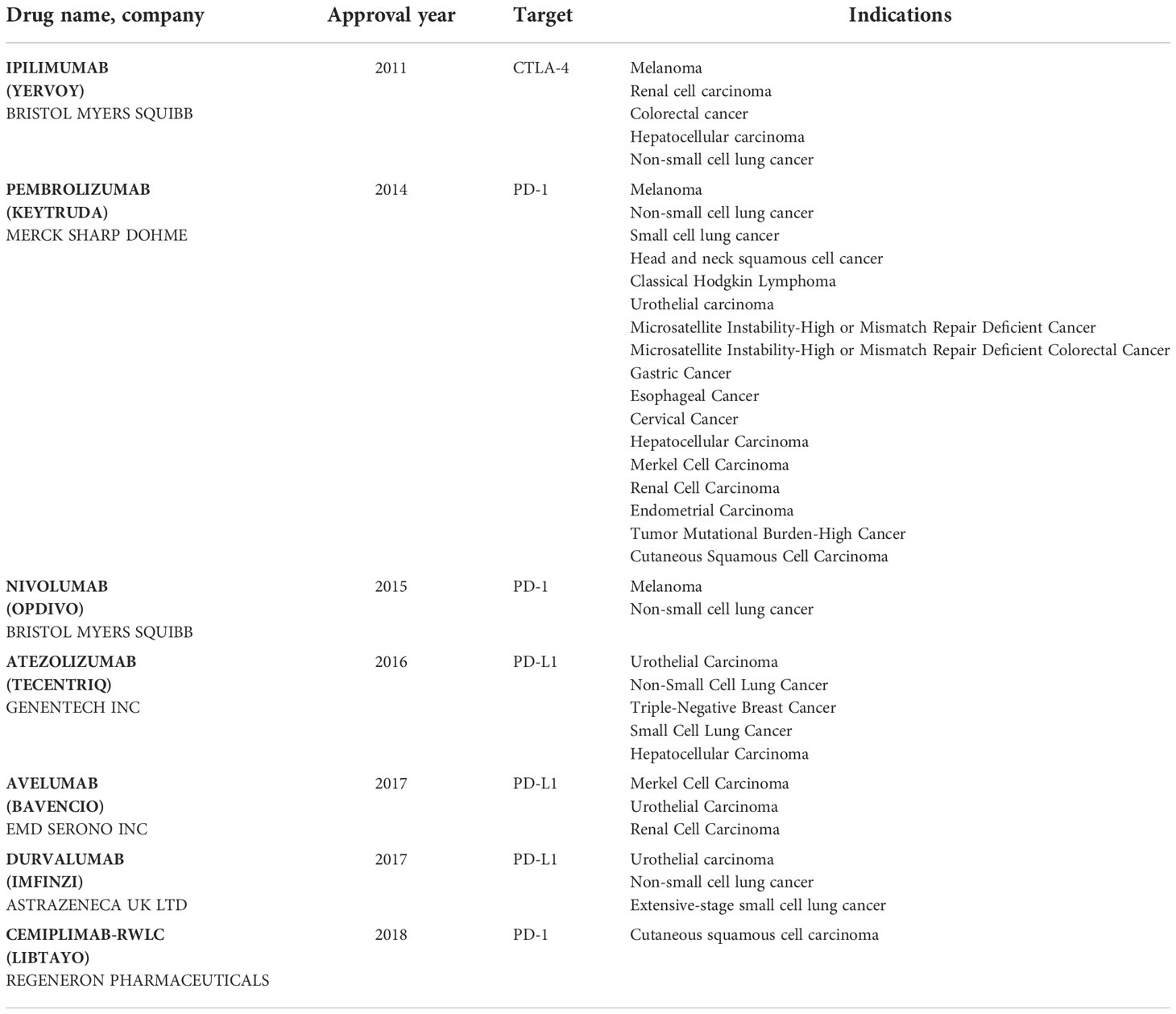
Monoclonal antibodies to PD-1, PD-L1 or CTLA-4 are emerging agents in cancer therapy due to their potential to block the excessive IC activity and restore the antitumor immune response. To date, several preparations of monoclonal antibodies affecting various ICs have been developed and approved for a wide range of cancer treatments (Table 1) and multiple IC inhibitors (ICI) are currently at the stage of preclinical or clinical trials (30).
The first ICI ipilimumab, which is an antibody to CTLA-4, was approved by FDA for treatment of metastatic inoperable melanoma in 2011 (31). The results demonstrated an improved long-term survival over 5 years (more than 18% of patients receiving the treatment), which led to a broad clinical and scientific resonance and stimulated studies of other ICIs.
Nivolumab and pembrolizumab were further medications approved by FDA for treatment of metastatic inoperable melanoma in 2014 and small cell lung carcinoma in 2015. Atezolizumab (PD-L1-specific monoclonal antibody) was approved for the therapy of the urothelial carcinoma in 2016. Further PD-L1-specific antibodies, avelumab and durvalumab, received the FDA support for the treatment of urothelial carcinoma in 2017. In addition, a PD-1-specific monoclonal antibody, cemiplimab, has been approved by FDA for the therapy of squamous cell skin cancer in 2018 (31). Preliminary and early results of these studies indicate that ICIs may bear a broad therapeutic potential for tumors of various histological composition including treatment of advanced metastatic or non-metastatic cancer stages.
Overcoming resistance to immune checkpoint inhibitors
Although, the ICIs have demonstrated clinical efficacy in patients with various types of cancer (see Table 1), the inconsistency of patients’ responses and a relatively high percentage of non-responsive patients remain a major limitation for this therapy. According to various sources, up to 80% of patients with previously treated and advanced non-small-cell lung cancer (NSCLC), recurrent squamous-cell carcinoma of the head and neck, melanoma, as well as relapsed or refractory Hodgkin’s lymphoma showed no adequate response to ICI treatment (32–41).
The response to ICIs has been shown to depend on various factors including the presence of certain T cell and APC subpopulations, immunosuppressive cytokine responses, levels of antigenic molecule inhibition in malignant cells, recruitment of immunoregulatory cells of the myeloid and lymphoid series to the neoplasm area, and dysfunction of DCs (10).
Multiple mechanisms of the tumor resistance to the ICI have been identified. These mechanisms can be classified into the primary mechanisms due to insufficient tumor recognition by the immune system, the adaptive mechanisms related to the immune response downregulation, and the acquired mechanisms driven by the immunoediting of the tumor (42).
Managing the resistance to immune therapy depends on the kind of therapeutic intervention and should be, as much as possible, personalized in each patient. The resistance to ICI can be determined by the absence of tumor antigenic proteins (primary resistance) or development of mechanisms decreasing antigen presentation and enabling immune evasion (secondary resistance). Multiple primary and adaptive tumor-intrinsic mechanisms include signaling through the MAPK pathway and/or loss of PTEN expression, which enhances the PI3K and WNT/β-catenin signaling pathways partially via suppression of the IFNγ (42). In addition, tumors of patients non-responding to the anti-PD-1 therapy showed signs of epithelial-mesenchymal transformation, which may further promote the tumor survival (43). The composition of molecular and cellular tumor microenvironment seems to be critical to the anti-tumor immune response.
The strategies to overcome resistance to the immunotherapies are aimed at selection of patient populations which are likely to respond to the treatment or using combination strategies to target diverse immunosuppressive pathways.
Assessment of PD-L1 and PD-1 as biomarkers of primary and metastatic tumors is often required for making clinical decisions on use of treatment strategies targeting ICs (44). Clinical studies of PD-1/PD-L1 blocking antibodies with patient stratification according to the PD-L1 expression at the tumor site showed a higher overall and relapse free survival, as well as a greater number of responses to therapy in patient groups with a higher biomarker level (45). At the same time, the use of one factor in stratification of patients for the treatment order may not be sufficient considering the complex TME structure. Therefore, various combined biomarker strategies, including tumor genome profiling, assessment of the T-cell repertoire, and studies of TME are currently being tested to develop multivariate predictive models for assessing the likelihood of successful treatment outcomes (46–49).
Combination strategies to combat resistance include coadministration of several immunotherapeutic agents with various mechanisms of action, as well as combinations with chemo-, radio- or targeted therapies (50). Combined administration of CTLA-4 and PD-1/PD-L1 blocking antibodies has been extensively investigated as an option to improve their therapeutic efficacy. To date, the only approved treatment combination consists of ipilimumab and nivolumab. This combination was approbated in 2015 for metastatic melanoma treatment and a 60% response was demonstrated (31, 51, 52). At a minimum follow-up of 60 months, the median overall survival was more than 60.0 months in the nivolumab-plus-ipilimumab group and 36.9 months in the nivolumab group, as compared with 19.9 months in the ipilimumab group (53). Combined nivolumab and ipilimumab therapy has been approved for various indications (54) and demonstrated sustained overall survival benefit in renal cell carcinoma (RCC) (54, 55) and in NSCLC (56). In 2020, the combination of ipilimumab and nivolumab received a further FDA approvement for the treatment of adults with malignant pleural mesothelioma (MPM) that cannot be removed by surgery (57). Other immunotherapy combinations are currently being investigated in clinical settings including coadministration of the registered ICIs with vaccines, other ICIs, adoptive cell transfer or agents, targeting various immunosuppressive TME components (58, 59). Generally, the choice of drug combinations for an optimal treatment is a complex task requiring careful consideration of their individual and synergistic therapeutic vs. side effects. It should be also noted that optimization of combined treatments is challenging and includes not only rationale selection of therapeutic modalities, but also the optimization of dosing regimens for each of them as well as their sequence.
One of the potential effective approaches to treat patients with cancer is a combination of CT and ICI. Some cytotoxic chemotherapeutic drugs such as anthracycline and oxaliplatin could induce ICD and stimulate antitumor immune response (60). Nowadays, CT combined with α-PD-1/PD-L1 has become a standard option for some cancer patient categories. The efficacy and safety of such combinations has been confirmed by a large number of clinical trials. Patients with NSCLC receiving pembrolizumab combined with standard CT (carboplatin and pemetrexed) had a higher response rate and longer progression-free survival, as well as overall survival than patients receiving standard CT only. As a consequence, pembrolizumab plus CT has been approved by the FDA as the first-line treatment for advanced non-squamous NSCLC, regardless of the PD-L1 level. Subsequently, the indication of pembrolizumab plus CT was expanded to the advanced triple-negative breast cancer (TNBC), esophageal cancer, and gastroesophageal junction cancer (GEJC). Apart from α-PD-1 the α-PD-L1-based chemoimmunotherapy attracts an intensive attention too, especially the drug atezolizumab. Based on the results of IMpower150, which is a pioneer clinical trial assessing the efficacy of atezolizumab plus angiogenesis inhibitor and CT in patients with advanced non-squamous NSCLC, the FDA approved atezolizumab plus bevacizumab, paclitaxel, and carboplatin as the first-line treatment for advanced non squamous NSCLC. Later, the FDA approved atezolizumab plus CT for TNBC and SCLC (60).
Moreover Deng et al. (2021) shows that ICIs plus platinum-free, single-agent CT can provide promising progression-free survival and overall response rate benefit, along with a low rate of severe adverse events in patients with epidermal growth factor receptor-tyrosine kinase (EGFR-TKI)-resistant advanced NSCLC (61). The result of another research shows that triple combination therapy with CT or agents eliciting oncolytic virus-like responses may overcome multiple resistance mechanisms (62).
Immunologic aspects of radiation therapy
RT has been widely used in oncology practice as a monotherapy or in combinations with other therapeutic strategies depending on the disease and patient characteristics (63). The first experimental observation pointing to the role of the immune system in antitumor activity of RT was obtained in 1979: the radiation dose required to control the tumor in 50% of mice was twice as large in the group of immunodeficient animals compared to the control group (64). Immune-mediated indirect suppression of tumor metastases by RT was first reported by R. Mole et al. in 1953 and called «abscopal effect» (65). Many years later, in 2004 S. Demaria et al. demonstrated importance of the immune system in abscopal effect of RT implying distant control of unirradiated tumor lesions in mouse (66). In summary, mechanism of RT action is complex and includes both direct cytotoxic effect of RT on cancer cells and an immune-related component (67).
Recent studies showed that the therapeutic effect of RT is mediated by formation of reactive oxygen species (ROS) affecting DNA structure and leading to ICD. This process is accompanied by multiple changes in molecular signaling pathways and intercellular interactions such as release of tumor antigens and damage-associated molecular patterns (DAMPs) into extracellular space followed by activation of signaling pathways involved in the immune response (Figure 2) (23, 68–70).
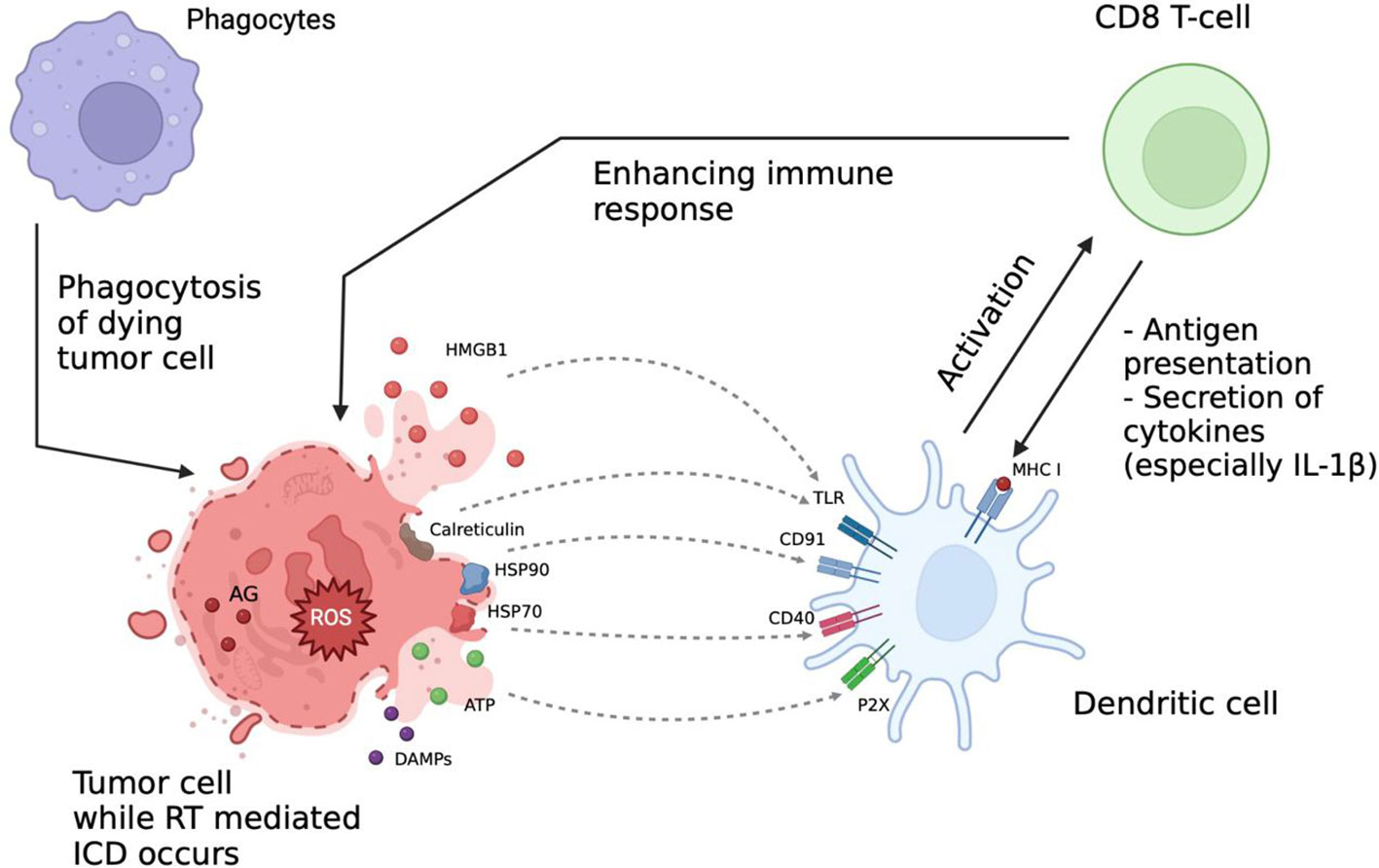
Figure 2 Immunologic aspects of radiation therapy (RT). RT effect is mediated via formation of reactive oxygen species (ROS) affecting the DNA structure and leading to immunogenic cell death (ICD). This process is accompanied by release of tumor antigens (AG) and damage-associated molecular patterns (DAMPs) into extracellular space and activation of signaling pathways involved into the immune response. After RT the interaction between immune cells is increased, ICI can further enhance it, thus increasing the overall therapeutic efficacy. AG, tumor-associated antigens; DAMPs, damage associated molecular patterns; ICD, immunogenic cell death; ROS, reactive oxygen species; HMGB1, high-mobility group protein B1; HSP70, heat shock protein 70; HSP90, heat shock protein 90; TLR, toll-like receptors; MHC I, major histocompatibility complex class I.
The RT-induced immune response is facilitated by release of adenosine triphosphate (ATP), uric acid and other intracellular components serving as chemo-attractants for APC (69). ATP, released in millimolar concentrations, promotes phenotypic maturation of the DCs by binding to their purinergic P2X and P2Y receptors (71–73). Interestingly, upon activation of the P2X7 receptor DCs can synthesize the precursor IL-1β, which is an essential component of the antitumor immune response (74, 75).
Secretion of pro-interleukin is carried out by a cascade activation mechanism including the nod-like receptor family pyrin domain containing 3 (NLRP3) and inflammasome-mediated secretion with following caspase-1-mediated processing (76, 77). It is important to note that the secretion of IL-1β requires concomitant signaling from the P2X7 receptors and toll-like receptors (TLRs), which can be suppressed by heat shock proteins (HSPs) and calreticulin (78, 79). Uric acid can additionally promote the immunological component by stimulating nucleotide-binding oligomerization domain-like receptor NALP3 via urate ion interactions with TLR4 (80, 81).
Alternatively, to the mentioned above ATP-dependent mechanisms of immune response, Y. He et al. (2013) demonstrated stimulation of cytokine secretion without high interstitial concentrations of ATP in a mouse model, suggesting a role of autocrine DCs activation (82).
As a protective reaction to irradiation tumor cells increase surface expression of various plasma membrane proteins, including HSPs and high-mobility group box 1 (HMGB1) (83). HSPs play a critical role in enhancing immunogenicity by signaling to APC and stimulating phagocytosis and cross-presentation of antigens mediated by NK cells. Specifically, HSPs 70 and 90 can bind to CD40 and CD91 receptors of DCs (84). Moreover, HSP70 has been shown to activate the cytotoxic effect of CD8 T cells after interacting with the co-activating molecule CD40 (85), and the interaction of HSP90 with CD91 potentiates the killing of tumor cells via cross-presentation of tumor antigens by DCs (86).
In this context, irradiation causes formation of necrotic tumor areas, characterized by calreticulin overexpression at the cell surface (87), which stimulates phagocytosis of tumor cells and further antigen release promoting the immune response (88, 89). Numerous studies show that it is calreticulin that plays the vital role in potentiating the immune system activity, since the level of its expression on the plasma membrane surface is directly proportional to the degree of immunocompetent cells attraction to the inflammation focus and antigen-specific T cell response (89). In addition, various studies have demonstrated a correlation between calreticulin expression and overall patient survival, which clearly distinguishes calreticulin from other DAMPs suggesting potential use of calreticulin as a prognostic marker for success of the RT in various types of cancer (89–92).
Additional evidence for stimulation of the antitumor immune response caused by radiation comes from the expression of HMGB1 (a nuclear DNA-binding protein synthesized during cell death) on the surface of tumor cells, which promotes its processing by DCs via TLR-mediated pathways and the receptor of advanced glycation end-products (RAGE)-mediated signaling (93, 94).
Moreover, irradiation increases MHC class I expression on the surface of cancer cells, which allows cancer-specific T cells to recognize and destroy the tumor cells. Increased expression of MHC class I is one of the best-characterized major mechanisms for enhancing immune responses to radiation. The activation of mammalian target of rapamycin (mTOR), enhanced translation and antigen presentation are crucial for this process. Enhanced surface expression of Fas induced by radiation promotes the apoptotic tumor cells death and represents another important mechanism mediating effects of RT on the host immune system (95).
The T cell immune response has been shown to be proportionally stimulated by the released of tumor antigen according to the radiation dose (96, 97). The RT destroys the tumor cells leading to the local activation of immune system with later generalization of the anti-tumor immune response. DAMPs may induce migration of neutrophils in the tumor site and enhance antitumor immune response as well (98). Attracted by sterile inflammation to the area of irradiation, neutrophils destroy tumor cells with free oxygen radicals and ensuing phagocytosis (98).
Thus, irradiation contributes to tumor necrosis, inflammation in the tumor site and multiple responses of the immune system to the tumor (99). Among various forms of cell death caused by ionizing radiation, ICD is caused by direct stimulation of tumor-specific immune response. Apart from activating anti-tumor immunity, RT may cause immunosuppressive effects as well. In this context, increased expression of PD-1 and its ligand PD-L1 in tumor in tumor microenvironment may serve as an important predictor for ICI administration (100, 101). Combining RT with ICI may also help to overcome local immunosuppressive effects originating from tumor cells.
The current state of knowledge strongly suggests that RT acts not only on the irradiated tumor site but also elicits a specific immunologic response, which puts forward the combination of radio- and immunotherapy as a promising strategy for improved management of metastatic tumor conditions. The systemic effect of RT on the immune system is further manifested by enhancing the homing effect of NK cells, that is an essential factor of innate antitumor immunity (102). In addition, RT can decrease the suppressive effect of Treg cells which usually down modulate immune responses against cancers (103). At the same time, irradiation has been associated with immunosuppressive mechanisms such as PD-L1 expression (21, 104). Therefore, therapeutic blockade of the PD-1/PD-L1 axis may be considered as a strategy to enhance the antitumor effect of RT (Figure 3). However, these results were obtained in animal models and need further verification.
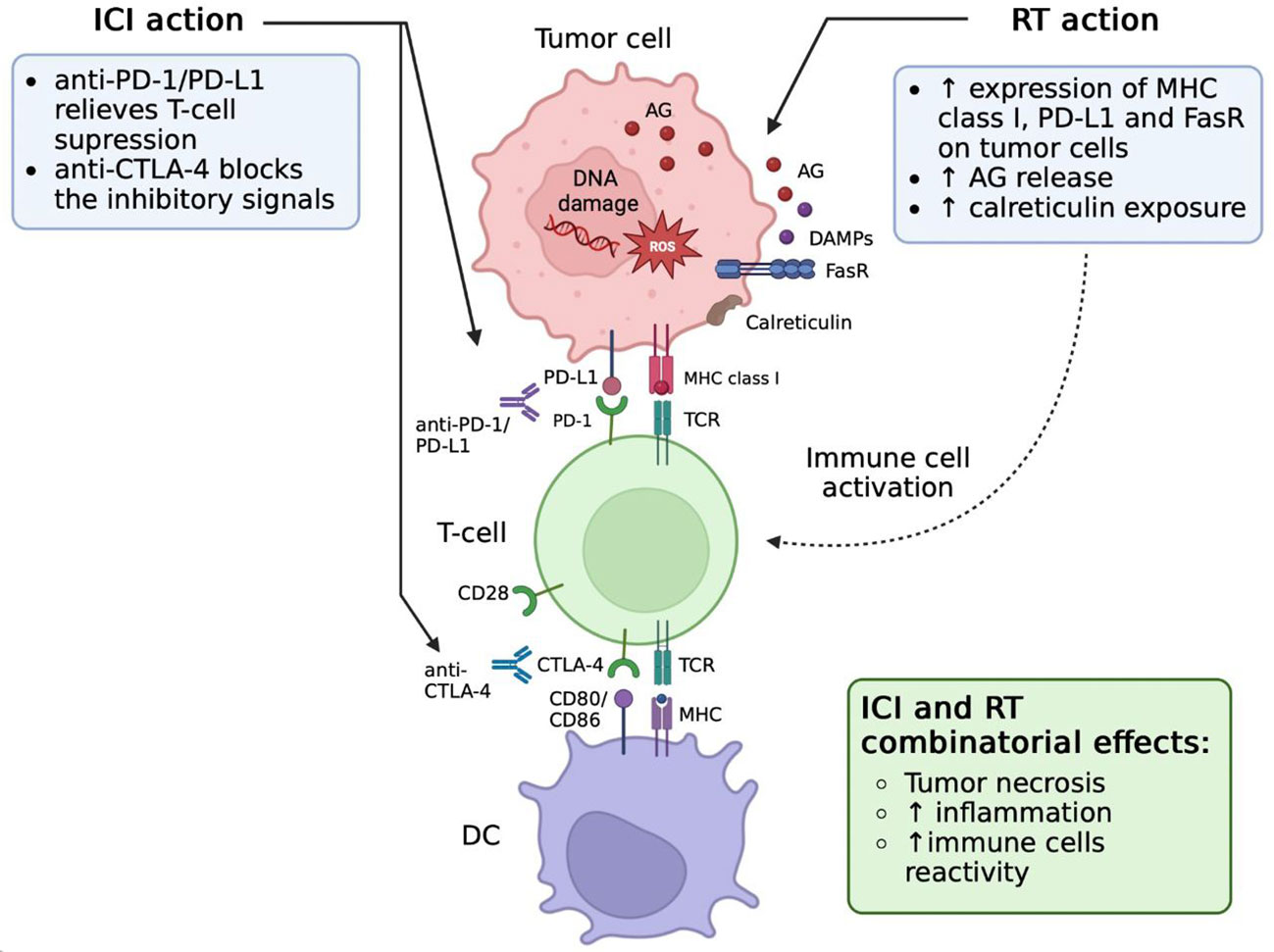
Figure 3 Effects of radiation therapy (RT) and immune checkpoint inhibitors (ICIs) combination. RT induces PD-L1 upregulation on tumor cells. Blockade of PD-L1/PD-1 signaling via antibody therapy repairs the function of CD8 T cells after RT stimulation. These functionally active CD8 T cells are able to effectively attack and kill cancer cells, which leads to tumor cell necrosis and inflammation increase. AG, tumor-associated antigens; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; DAMPs, damage-associated molecular patterns; DC, dendritic cells; ICI, immune checkpoint inhibitors; MHC, major histocompatibility complex; PD-1, programmed cell death protein 1; RT, radiotherapy; TCR, T-cell receptor; FasR, Fas receptor.
The RT exerts not only a direct effect in the area of irradiation but also indirectly stimulates the immune response due to low doses of irradiation beyond the target zone. These low irradiation doses may stimulate an immune response to the general stress, caused by radiation, including the adaptive response and immune system repair (103). The reduction of immune tolerance to tumors caused by RT may amplify the general anti-tumor immune response and mediate the abscopal effect, although mechanisms underlying the abscopal effect require further characterization.
Preclinical efficacy of radio- and immunotherapies
Summarizing the available data, it can be stated that a combination of RT and immunotherapies may represent a biologically rationale approach to improve cancer treatment outcomes (19). Synergy of these treatment modalities has been extensively investigated in several mouse models of cancer (105).
As was mentioned earlier, rational selection of the radiation dose, fractional content and the sequence of prescribing the therapy components are required to achieve the most pronounced treatment benefits. Fractionation (separation of the total dose of radiation into several fraction) allows maximum destruction of malignant cells with minimal damage to healthy tissues.
Currently, there are three optimal modes of dose fractionation used in RT:
● Hypofractionation (3-20 Gy/fraction, one fraction/day, 2-5 fractions/week);
● Conventional fractionation schemes (1.8-2.2 Gy/fraction, one fraction/day, 5 days/week for 3-7 weeks);
● Hyperfractionation (0.5-2.2 Gy/fraction, two fractions/day, 2-5 fractions/week for 2-4 weeks) (106).
Immunological effects of different regimens and the molecular mechanisms underlying the immune response to radiation remain unclear. It’s established that single high-dose (12 Gy) RT did not deplete CD8 T cells but kills tumor cells more effectively when combined with immunotherapy (107).
In experimental mouse models after exposure to hypofractionated RT a strong upregulation of PD-L1 was observed (radiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice), so we can suppose that the combination of the PD-1/PD-L1 blockade and RT may overcome tumor immunosuppression and improve the systemic effect of RT.
In contrast to the conventional and hypofractionated regimen, the single, high-dose irradiation did not increase the surface expression of PD-L1 on B16-F10 melanoma and GL261 glioblastoma cells in vitro (22). The positive effect of a single exposure has been also established in other studies. It was demonstrated that a single high dose of radiation (12 Gy) does not cause the death of immunocompetent cells suggesting that combination of this regimen with ICI may be successful (107). Therapeutic potential of combining PD-1 blockade with a single exposure of 10 Gy has been further demonstrated in a mouse model of glioma (108).
Other studies demonstrated the superiority of fractionated RT over single-session RT. Fractionated RT appears to be more synergistic with ICI therapy, inducing tumor regression and increasing long-term survival rate in various extracranial cancer models (109). It was suggested that the single dose RT has a positive effect on the micrometastases regression but is not active enough against the mature ones. Comparison of two dose regimen of fractionated therapy (3*8 Gy and 5*6 Gy) in combination with CTLA-4 blockade showed that the dose of 3*8 Gy was more efficient (109).
There is evidence for the role of the size of the ablative radiation fraction (minimum 6 Gy), as well as the linear energy transfer level affect the release of immunogenic antigens. A. Lugade et al. (2005) compared 15 Gy in one fraction versus 15 Gy in 5 fractions of 3 Gy in a murine melanoma model. They found that fractions equally lead to tumor infiltration by lymphocytes, but larger fraction sizes produce a better effect (110).
The DNA exonuclease three prime repair exonuclease 1 (TREX 1) may serve as a marker for the optimal dose determination, since its expression proportionally correlates with the radiation dose and reflects the severity of the radiation-induced DNA-damage (111). Cells treated with a single dose of 20 and 30 Gy showed a larger increase in TREX1 exonuclease expression than cells treated with 3*8 Gy. High dose radiation (above 12-18 Gy) in a single fraction promotes the TREX 1-induced DNA degradation and cytosolic accumulation of damaged DNA in irradiated cancer cells, which inhibits the type-I interferon (IFN-I) pathway and decreases the immunogenicity of cancer cells. In contrast, the radiation dose of 3*8 Gy given in a repeated fashion is below the threshold dose for TREX 1 induction. This dose regimen is also optimal in terms of the IFNβ stimulation required for recruitment of Batf3-dependent DCs and CD8 T-cells to the tumor site. Use of ICI, in this context, may promote a sustained regression of the irradiated and non-irradiated tumors via direct and abscopal effects.
Notably, the success of combination therapy is influenced not only by the fractionation scheme but also depends on the type of immunotherapeutic intervention, as well as sequence of the immunotherapeutic and RT modalities. Concurrent PD-1/PD-L1 blockade and RT was shown to be more effective compared to sequential administration of RT followed by ICI (21), whereas anti-CTLA-4 therapy was shown to be the most effective when given before RT in a colorectal cancer model (112). Therefore, disease-specific approaches and personalized medicine should be applied for decisions on the concrete strategy.
Thus, optimal modes of radiation and immunotherapy in pre-clinical studies are various and are determined primarily by the mechanics of action of a specific immunotherapy agent. This should be taken into account when designing clinical trials.
Clinical usage of radio- and immunotherapies
It should be stated that the first clinical trials of combined radio- and immunotherapy were conducted in 80th and included administration of cancer vaccines and RT for treatment of melanoma, breast, colorectal or lung cancer but failed to demonstrate the efficacy of combined therapy (113). Following decades of oblivion, the interest to the radio- and immunotherapies was renewed after ICI discovery.
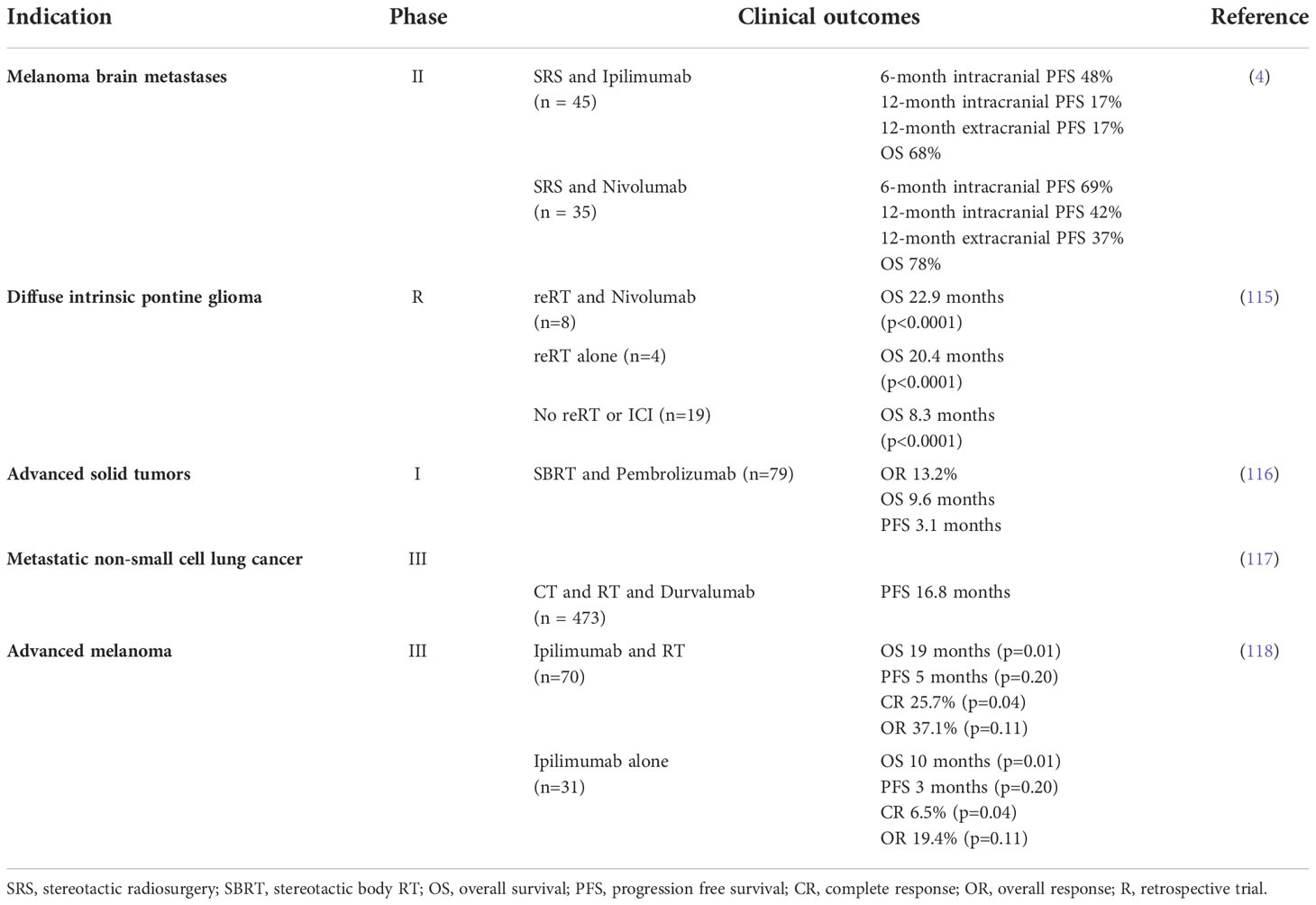
Clinical benefit of combining ICI and RT was initially described by multiple retrospective analyses comparing efficacy of RT alone or RT in combination with ICI in patients with highly immunogenic cancer forms such as metastatic melanoma, NSCLC and RCC (114). The following prospective clinical trials have corroborated the therapeutic potential of the combined ICI and RT approach (Table 2) (119, 120). Identification of the optimal sequencing strategy has been performed retrospectively and comprehensive meta-analysis of the accumulated clinical evidence suggested superiority of concurrent over sequential ICI and RT treatment (121). In contrast to the retrospective observations, the prospective single institution ELEKTRA trial demonstrated superiority of RT given prior to ICI treatment, which was reflected by more pronounced increases in circulating CD4 and CD8 cell populations (122). These discrepancies may be related with confounding factors, affecting conclusions of the retrospective trials (122).
Efficacy of the ICI and RT combination has been also investigated in other tumor types. A retrospective analysis of diffuse intrinsic pontine glioma patients treated with RT alone or RT in combination with PD-1 specific antibodies demonstrated no additional benefit of ICI inclusion into the therapy. Two prospective trials Checkmate-498 and Checkmate-548, evaluating efficacy of the triple combination of nivolumab with RT and temozolomide in patients with primary glioblastoma have failed to meet primary endpoints. The lack of the combination efficacy may be related to low activity of immunotherapies by this indication due to low tumor immunogenicity as well as activation of alternative immunosuppressive pathways (115, 123). In contrast, another prospective study Checkmate-577 demonstrated improvement of the esophageal or gastroesophageal Junction cancer treatment outcomes following the addition of nivolumab to neoadjuvant chemoradiotherapy (124). Efficacy of ICI and RT combination has been also tested in other indications such as metastatic breast (NCT03483012, NCT03807765), pancreatic (NCT0436116), ovarian cancers (NCT03283943), hematological malignancies (NCT03610061) and hepatocellular carcinoma (NCT04913480).
Therefore, the effectiveness of combined radioimmunotherapy depends on the choice of the optimal radiotherapy mode, including both the total dose and the fractionation mode.
The different contribution of TME components of a particular patient to the regulation of the immune system, depending on the stage of immune response development, determines the significance of the RT sequence and the taking of immune drugs.
Prospective predicting of clinical responses to combined radioimmunotherapy is possible by identifying predictive biomarkers. Currently two biomarkers (PD-L1 and MMR/MSI status) have already been implemented in the clinic. Soluble NKG2D ligands and antibodies that neutralize their activity are considered to be easily accessible predictive biomarkers for patients receiving combination of RT and ICIs (125, 126). RT promotes the exposure of NKG2D ligands on the surface of tumor cells, hence rendering them potentially susceptible to NK cell-dependent lysis or improved recognition by CTLs (125). Cancer cells can shed ligands from their surface, resulting in high circulating levels and can have a negative predictive value in melanoma patients treated with various ICIs including nivolumab, pembrolizumab and ipilimumab (127–131).
Markers of cGAS-STING DNA-sensing pathway which is essential for activation of IFN-dependent antitumor immunity are also promising for the identification of patients with positive response to combinatorial regimens involving RT and ICIs (132).
Conclusion
Therapeutic benefits of combined ICI and RT approach has been suggested by numerous studies enrolling patients with melanoma, NSCLC and RCC and is currently being investigated in other indications. The optimal therapeutic regimen in terms of the doses, RT fractionation and sequence of RT and ICI administration has been addressed in preclinical setting but needs a further corroboration in clinical trials. The available studies categorize the combination of ICI and RT as a promising approach for improved treatment of immunogenic cancer forms.
Author contributions
Conceptualization: KP, VV, SL, MS. Literature review: MM, VV, AV. Writing: MM, MSa, AV. Editing: KM, VV, MS. Visualization: MM, NB, SL. Supervision: SL. All authors contributed to the article and approved the submitted version.
Funding
This work was financed by the Ministry of Science and Higher Education of the Russian Federation within the framework of state support for the creation and development of World-Class Research Centers “Digital biodesign and personalized healthcare” №075-15-2022-304
Conflict of interest
Authors VV and KP were employed by company M&S Decisions LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. McCarthy EF. The toxins of William b. coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J (2006) 26:154–8.
2. Burnet FM. Immunological surveillance in neoplasia. Immunol Rev (1971) 7(1):3–25. doi: 10.1111/j.1600-065X.1971.tb00461.x
3. O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol (2019) 16(3):151–67. doi: 10.1038/s41571-018-0142-8
4. Chen DS, Mellman I. Elements of cancer immunity and the cancer–immune set point. Nature (2017) 541(7637):321–30. doi: 10.1038/nature21349
5. Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity (2013) 39(1):1–10. doi: 10.1016/j.immuni.2013.07.012
6. Galluzzi L, Vitale I, Warren S, Adjemian S, Agostinis P, Matinez AB, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer (2020) 8(1):e000337. doi: 10.1136/jitc-2019-000337
7. Teng MWL, Galon J, Fridman WH, Smyth MJ. From mice to humans: developments in cancer immunoediting. J Clin Invest (2015) 125(9):3338–46. doi: 10.1172/JCI80004
8. Sansom DM. CD28, CTLA-4 and their ligands: who does what and to whom? Immunology (2000) 101(2):169–77. doi: 10.1046/j.1365-2567.2000.00121.x
9. Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol (2005) 23(1):515–48. doi: 10.1146/annurev.immunol.23.021704.115611
10. Darb-Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S, Lindner J, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget (2016) 7(2):1486–99. doi: 10.18632/oncotarget.6429
11. Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on akt and ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal (2012) 5(230):ra46. doi: 10.1126/scisignal.2002796
12. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol (2018) 18(3):153–67. doi: 10.1038/nri.2017.108
13. Lu Y, Yu Q, Liu JH, Zhang J, Wang H, Koul D, et al. Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-Kinase/AKT cascades. J Biol Chem (2003) 278(41):40057–66. doi: 10.1074/jbc.M303621200
14. Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, et al. T Cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science (2017) 355(6332):1428–33. doi: 10.1126/science.aaf1292
15. Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol (2005) 25(21):9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005
16. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol (2008) 26(1):677–704. doi: 10.1146/annurev.immunol.26.021607.090331
17. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol (2001) 2(3):261–8. doi: 10.1038/85330
18. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell (2015) 27(4):450–61. doi: 10.1016/j.ccell.2015.03.001
19. Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y, Meeker A, et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol (2016) 47(1):52–63. doi: 10.1016/j.humpath.2015.09.003
20. Evrard D, Hourseau M, Couvelard A, Paradis V, Gauthier H, Raymond E, et al. PD-L1 expression in the microenvironment and the response to checkpoint inhibitors in head and neck squamous cell carcinoma. Oncoimmunology (2020) 9(1):1844403. doi: 10.1080/2162402X.2020.1844403
21. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res (2014) 74(19):5458–68. doi: 10.1158/0008-5472.CAN-14-1258
22. Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol (2008) 45(5):1470–6. doi: 10.1016/j.molimm.2007.08.013
23. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti–PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest (2014) 124(2):687–95. doi: 10.1172/JCI67313
24. Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood (2018) 131(1):58–67. doi: 10.1182/blood-2017-06-741033
25. Stamper CC, Zhang Y, Tobin JF, Erbe DV, Ikemizu S, Davis SJ, et al. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature (2001) 410(6828):608–11. doi: 10.1038/35069118
26. Schwartz JCD, Zhang X, Fedorov AA, Nathenson SG, Almo SC. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature (2001) 410(6828):604–8. doi: 10.1038/35069112
27. Collins AV, Brodie DW, Gilbert RJC, Iaboni A, Manso-Sancho R, Walse B, et al. The interaction properties of costimulatory molecules revisited. Immunity (2002) 17(2):201–10. doi: 10.1016/S1074-7613(02)00362-X
28. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239
29. Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang NAAS, Andrews MC, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell (2017) 170(6):1120–33. doi: 10.1016/j.cell.2017.07.024
30. Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol (2018) 29(1):84–91. doi: 10.1093/annonc/mdx755
31. Rotte A, Jin JY, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol (2018) 29(1):71–83. doi: 10.1093/annonc/mdx686
32. Galluzzi L, Chan TA, Kroemer G, Wolchok JD, López-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med (2018) 10(459):eaat7807. doi: 10.1126/scitranslmed.aat7807
33. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet Lond Engl (2017) 389(10066):255–65. doi: 10.1016/S0140-6736(16)32517-X
34. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
35. Maleki Vareki S, Garrigós C, Duran I. Biomarkers of response to PD-1/PD-L1 inhibition. Crit Rev Oncol Hematol (2017) 116:116–24. doi: 10.1016/j.critrevonc.2017.06.001
36. Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med (2016) 375(19):1856–67. doi: 10.1056/NEJMoa1602252
37. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med (2015) 372(4):320–30. doi: 10.1056/NEJMoa1412082
38. Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, et al. Nivolumab monotherapy for first-line treatment of advanced non–Small-Cell lung cancer. J Clin Oncol (2016) 34(25):2980–7. doi: 10.1200/JCO.2016.66.9929
39. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced non-squamous non-small cell lung cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
40. Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory hodgkin’s lymphoma. N Engl J Med (2015) 372(4):311–9. doi: 10.1056/NEJMoa1411087
41. Kwok G, Yau TCC, Chiu JW, Tse E, Kwong YL. Pembrolizumab (Keytruda). Hum Vaccines Immunother (2016) 12(11):2777–89. doi: 10.1080/21645515.2016.1199310
42. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive and acquired resistance to cancer immunotherapy. Cell (2017) 168(4):707–23. doi: 10.1016/j.cell.2017.01.017
43. Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell (2017) 168(3):542. doi: 10.1016/j.cell.2017.01.010
44. Zou Y, Hu X, Zheng S, Yang A, Li X, Tang H, et al. Discordance of immunotherapy response predictive biomarkers between primary lesions and paired metastases in tumours: A systematic review and meta-analysis. EBioMedicine (2021) 63:103137. doi: 10.1016/j.ebiom.2020.103137
45. Jorgensen JT. PD-L1 expression and efficacy of pembrolizumab as monotherapy in NSCLC. Chin Clin Oncol (2020) 9(4):60–0. doi: 10.21037/cco.2020.01.03
46. Clark DP. Biomarkers for immune checkpoint inhibitors: The importance of tumor topography and the challenges to cytopathology: Biomarkers for checkpoint inhibitors. Cancer Cytopathol (2018) 126(1):11–9. doi: 10.1002/cncy.21951
47. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol (2016) 17(12):e542–51. doi: 10.1016/S1470-2045(16)30406-5
48. Wargo JA, Reddy SM, Reuben A, Sharma P. Monitoring immune responses in the tumor microenvironment. Curr Opin Immunol (2016) 41:23–31. doi: 10.1016/j.coi.2016.05.006
49. Mehnert JM, Monjazeb AM, Beerthuijzen JMT, Collyar D, Rubinstein L, Harris LN. The challenge for development of valuable immuno-oncology biomarkers. Clin Cancer Res Off J Am Assoc Cancer Res (2017) 23(17):4970–9. doi: 10.1158/1078-0432.CCR-16-3063
50. Tang J, Pearce L, O’Donnell-Tormey J, Hubbard-Lucey VM. Trends in the global immuno-oncology landscape. Nat Rev Drug Discovery (2018) 17(11):783–4. doi: 10.1038/nrd.2018.167
51. Zappasodi R, Merghoub T, Wolchok JD. Emerging concepts for immune checkpoint blockade-based combination therapies. Cancer Cell (2018) 33(4):581–98. doi: 10.1016/j.ccell.2018.03.005
52. Hao C, Tian J, Liu H, Li F, Niu H, Zhu B. Efficacy and safety of anti-PD-1 and anti-PD-1 combined with anti-CTLA-4 immunotherapy to advanced melanoma. Med (Baltimore) (2017) 96(26):e7325. doi: 10.1097/MD.0000000000007325
53. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med (2019) 381(16):1535–46. doi: 10.1056/NEJMoa1910836
54. La-Beck NM, Jean GW, Huynh C, Alzghari SK, Lowe DB. Immune checkpoint inhibitors: New insights and current place in cancer therapy. Pharmacother J Hum Pharmacol Drug Ther (2015) 35(10):963–76. doi: 10.1002/phar.1643
55. Motzer RJ, Rini BI, McDermott DF, Frontera OA, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised phase 3 trial. Lancet Oncol (2019) 20(10):1370–85. doi: 10.1016/S1470-2045(19)30413-9
56. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non–Small-Cell lung cancer. N Engl J Med (2019) 381(21):2020–31. doi: 10.1056/NEJMoa1910231
57. Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet (2021) 397(10272):375–86. doi: 10.1016/S0140-6736(20)32714-8
58. Gao H, Xu C, Liang J, Ge S, Zhang F, Tuo Y, et al. Pan-cancer analysis of oncogenic role of programmed cell death 2 like (PDCD2L) and validation in colorectal cancer. Cancer Cell Int (2022) 22:100. doi: 10.1186/s12935-022-02525-x
59. Le Naour J, Galluzzi L, Zitvogel L, Kroemer G, Vacchelli E. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology (2014) 9(1):1777625. doi: 10.1080/2162402X.2020.1777625
60. Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer (2022) 21(1):28. doi: 10.1186/s12943-021-01489-2
61. Deng H, Lin X, Xie X, Yang Y, Wang L, Wu J, et al. Immune checkpoint inhibitors plus single-agent chemotherapy for advanced non-Small-Cell lung cancer after resistance to EGFR-TKI. Front Oncol (2021) 11:700023. doi: 10.3389/fonc.2021.700023
62. Yuan J, Khilnani A, Brody J, Andtbacka RHI, Hu-Lieskovan S, Luke JJ, et al. Current strategies for intratumoural immunotherapy – beyond immune checkpoint inhibition. Eur J Cancer (2021) 157:493–510. doi: 10.1016/j.ejca.2021.08.004
63. Ko EC, Formenti SC. Radiation therapy to enhance tumor immunotherapy: a novel application for an established modality. Int J Radiat Biol (2019) 95(7):936–9. doi: 10.1080/09553002.2019.1623429
64. Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst (1979) 63(5):1229–35.
65. Mole RH. Whole body irradiation; radiobiology or medicine? Br J (1953) 26(305):234–41. doi: 10.1259/0007-1285-26-305-234
66. Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol (2004) 58(3):862–70. doi: 10.1016/j.ijrobp.2003.09.012
67. Johnson CB, Jagsi R. The promise of the abscopal effect and the future of trials combining immunotherapy and radiation therapy. Int J Radiat Oncol (2016) 95(4):1254–6. doi: 10.1016/j.ijrobp.2016.02.067
68. Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol (2015) 1(9):1325. doi: 10.1001/jamaoncol.2015.2756
69. Chajon E, Castelli J, Marsiglia H, De Crevoisier R. The synergistic effect of radiotherapy and immunotherapy: A promising but not simple partnership. Crit Rev Oncol Hematol (2017) 111:124–32. doi: 10.1016/j.critrevonc.2017.01.017
70. Gameiro SR, Malamas AS, Bernstein MB, Tsang KY, Vassantachart A, Sahoo N, et al. Tumor cells surviving exposure to proton or photon radiation share a common immunogenic modulation signature, rendering them more sensitive to T cell-mediated killing. Int J Radiat Oncol Biol Phys (2016) 95(1):120–30. doi: 10.1016/j.ijrobp.2016.02.022
71. Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, et al. Nucleotides released by apoptotic cells act as a find-me signal for phagocytic clearance. Nature (2009) 461(7261):282–6. doi: 10.1038/nature08296
72. Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med (2009) 15(10):1170–8. doi: 10.1038/nm.2028
73. Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X 7 receptor-mediated K + release. Am J Physiol-Cell Physiol (2004) 286(5):C1100–8. doi: 10.1152/ajpcell.00494.2003
74. Franchi L, Kanneganti TD, Dubyak GR, Núñez G. Differential requirement of P2X7 receptor and intracellular k+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem (2007) 282(26):18810–8. doi: 10.1074/jbc.M610762200
75. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol (2009) 27(1):519–50. doi: 10.1146/annurev.immunol.021908.132612
76. Cullen SP, Kearney CJ, Clancy DM, Martin SJ. Diverse activators of the NLRP3 inflammasome promote IL-1β secretion by triggering necrosis. Cell Rep (2015) 11(10):1535–48. doi: 10.1016/j.celrep.2015.05.003
77. Franchi L, Muñoz-Planillo R, Reimer T, Eigenbrod T, Núñez G. Inflammasomes as microbial sensors. Eur J Immunol (2010) 40(3):611–5. doi: 10.1002/eji.200940180
78. Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol (2009) 183(2):787–91. doi: 10.4049/jimmunol.0901363
79. Franchi L, Eigenbrod T, Núñez G. Cutting edge: TNF-α mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol (2009) 183(2):792–6. doi: 10.4049/jimmunol.0900173
80. Xiao J, Fu C, Zhang X, Zhu D, Chen W, Lu Y, et al. Soluble monosodium urate, but not its crystal, induces toll like receptor 4-dependent immune activation in renal mesangial cells. Mol Immunol (2015) 66(2):310–8. doi: 10.1016/j.molimm.2015.03.250
81. Xiao J, Zhang XL, Fu C, Han R, Chen W, Lu Y, et al. Soluble uric acid increases NALP3 inflammasome and interleukin-1β expression in human primary renal proximal tubule epithelial cells through the toll-like receptor 4-mediated pathway. Int J Mol Med (2015) 35(5):1347–54. doi: 10.3892/ijmm.2015.2148
82. He Y, Franchi L, Núñez G. Toll-like receptor agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J Immunol Baltim Md (1950) 190(1):334–9.
83. Rapoport BL, Anderson R. Realizing the clinical potential of immunogenic cell death in cancer chemotherapy and radiotherapy. Int J Mol Sci (2019) 20(4):959. doi: 10.3390/ijms20040959
84. Garg AD, Galluzzi L, Apetoh L, Baert T, Birge RB, Bravo-San Pedro JM, et al. Molecular and translational classifications of DAMPs in immunogenic cell death. Front Immunol (2015) 6:588. doi: 10.3389/fimmu.2015.00588
85. Flechtner JB, Cohane KP, Mehta S, Slusarewicz P, Leonard AK, Barber BH, et al. High-affinity interactions between peptides and heat shock protein 70 augment CD8+ T lymphocyte immune responses. J Immunol (2006) 177(2):1017–27. doi: 10.4049/jimmunol.177.2.1017
86. Salimu J, Spary LK, Al-Taei S, Clayton A, Mason MD, Staffurth J, et al. Cross-presentation of the oncofetal tumor antigen 5T4 from irradiated prostate cancer cells –a key role for heat-shock protein 70 and receptor CD91. Cancer Immunol Res (2015) 3(6):678–88. doi: 10.1158/2326-6066.CIR-14-0079
87. Osman R, Tacnet-Delorme P, Kleman JP, Millet A, Frachet P. Calreticulin release at an early stage of death modulates the clearance by macrophages of apoptotic cells. Front Immunol (2017) 8:1034. doi: 10.3389/fimmu.2017.01034
88. Gameiro SR, Jammed ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget (2013) 5(2):403–16. doi: 10.18632/oncotarget.1719
89. Fucikova J, Truxova I, Hensler M, Becht E, Kasikova L, Moserova I, et al. Calreticulin exposure by malignant blasts correlates with robust anticancer immunity and improved clinical outcome in AML patients. Blood (2016) 128(26):3113–24. doi: 10.1182/blood-2016-08-731737
90. Stoll G, Iribarren K, Michels J, Leary A, Zitvogel L, Cremer I, et al. Calreticulin expression: Interaction with the immune infiltrate and impact on survival in patients with ovarian and non-small cell lung cancer. Oncoimmunology (2016) 5(7):e1177692. doi: 10.1080/2162402X.2016.1177692
91. Harada K, Takenawa T, Ferdous T, Kuramitsu Y, Ueyama Y. Calreticulin is a novel independent prognostic factor for oral squamous cell carcinoma. Oncol Lett (2017) 13(6):4857–62. doi: 10.3892/ol.2017.6062
92. Fucikova J, Kasikova L, Truxova I, Laco J, Skapa P, Ryska A, et al. Relevance of the chaperone-like protein calreticulin for the biological behavior and clinical outcome of cancer. Immunol Lett (2018) 193:25–34. doi: 10.1016/j.imlet.2017.11.006
93. Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol (2010) 28(1):367–88. doi: 10.1146/annurev.immunol.021908.132603
94. Yanai H, Ban T, Taniguchi T. High-mobility group box family of proteins: ligand and sensor for innate immunity. Trends Immunol (2012) 33(12):633–40. doi: 10.1016/j.it.2012.10.005
95. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol (2015) 16(13):e498–509. doi: 10.1016/S1470-2045(15)00007-8
96. Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, K.Wansley E, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med (2006) 203(5):1259–71. doi: 10.1084/jem.20052494
97. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science (2015) 348(6230):69–74. doi: 10.1126/science.aaa4971
98. Takeshima T, Pop LM, Laine A, Iyengar P, Vitetta ES, Hannan R. Key role for neutrophils in radiation-induced antitumor immune responses: Potentiation with G-CSF. Proc Natl Acad Sci U.S.A. (2016) 113(40):11300–5. doi: 10.1073/pnas.1613187113
99. Victor CTS, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activates non-redundant immune mechanisms in cancer. Nature (2015) 520(7547):373–7. doi: 10.1038/nature14292
100. Hecht M, Büttner-Herold M, Erlenbach-Wünsch K, Haderlein M, Croner R, Grützmann R, et al. PD-L1 is upregulated by radiochemotherapy in rectal adenocarcinoma patients and associated with a favourable prognosis. Eur J Cancer (2016), 65:52–60. doi: 10.1016/j.ejca.2016.06.015
101. Rückert M, Deloch L, Fietkau R, Frey B, Hecht M, Gaipl US. Immune modulatory effects of radiotherapy as basis for well-reasoned radioimmunotherapies. Strahlenther Oncol (2018) 194(6):509–19. doi: 10.1007/s00066-018-1287-1
102. Canter RJ, Grossenbacher SK, Foltz JA, Sturgill IR, Park JS, Luna JI, et al. Radiotherapy enhances natural killer cell cytotoxicity and localization in pre-clinical canine sarcomas and first-in-dog clinical trial. J Immunother Cancer (2017) 5:98. doi: 10.1186/s40425-017-0305-7
103. Welsh J, Bevelacqua JJ, Dobrzynski L, Mortazavi SAR, Farjadian Sh, Mortazavi SMJ. Abscopal effect following radiation therapy in cancer patients: A new look from the immunological point of view. J BioMed Phys Eng (2020) 10(4):537–42. doi: 10.31661/jbpe.v0i0.1066
104. Deng L, Liang H, Burnette B, Weicheslbaum RR, Fu YX. Radiation and anti-PD-L1 antibody combinatorial therapy induces T cell-mediated depletion of myeloid-derived suppressor cells and tumor regression. OncoImmunology (2014) 3(4):e28499. doi: 10.4161/onci.28499
105. Mosely SIS, Prime JE, Sainson RCA, Koopmann JO, Wang DYQ, Greenawalt DM, et al. Rational selection of syngeneic preclinical tumor models for immunotherapeutic drug discovery. Cancer Immunol Res (2017) 5(1):29–41. doi: 10.1158/2326-6066.CIR-16-0114
106. Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol OncolJ Hematol Oncol (2018) 11:104. doi: 10.1186/s13045-018-0647-8
107. Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: A clinical review for the radiobiologist. Cancer Lett (2015) 356(1):82–90. doi: 10.1016/j.canlet.2013.09.018
108. Belcaid Z, Phallen JA, Zeng J, See AP, Mathios D, Gottschalk C, et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PloS One (2014) 9(7):e101764. doi: 10.1371/journal.pone.0101764
109. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res Off J Am Assoc Cancer Res (2009) 15(17):5379–88. doi: 10.1158/1078-0432.CCR-09-0265
110. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol (2005) 174:7516–23. doi: 10.4049/jimmunol.174.12.7516
111. Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA Exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun (2017) 8:15618. doi: 10.1038/ncomms15618
112. Young KH, Baird JR, Savage T, Cottam B, Friedman D, Bambina S, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PloS One (2016) 11(6):e0157164. doi: 10.1371/journal.pone.0157164
113. Naghavi AO, Johnstone PAS, Kim S. Clinical trials exploring the benefit of immunotherapy and radiation in cancer treatment: A review of the past and a look into the future. Curr Probl Cancer (2016) 40(1):38–67. doi: 10.1016/j.currproblcancer.2015.10.002
114. Voronova V, Lebedeva S, Sekacheva M, Helmlinger G, Peskov K. Quantification of scheduling impact on safety and efficacy outcomes of brain metastasis radio- and immuno-therapies: A systematic review and meta-analysis. Front Oncol (2020) 10:1609. doi: 10.3389/fonc.2020.01609
115. Kline C, Liu SJ, Duriseti S, Banerjee A, Nicolaides T, Raber S, et al. Reirradiation and PD-1 inhibition with nivolumab for the treatment of recurrent diffuse intrinsic pontine glioma: a single-institution experience. J Neurooncol (2018) 140(3):629–38. doi: 10.1007/s11060-018-2991-5
116. Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol (2018) 36(16):1611–8. doi: 10.1200/JCO.2017.76.2229
117. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-Small-Cell lung cancer. N Engl J Med (2017) 377(20):1919–29. doi: 10.1056/NEJMoa1709937
118. Koller KM, Mackley HB, Liu J, Wagner H, Talamo G, Schell TD, et al. Improved survival and complete response rates in patients with advanced melanoma treated with concurrent ipilimumab and radiotherapy versus ipilimumab alone. Cancer Biol Ther (2016) 18(1):36–42. doi: 10.1080/15384047.2016.1264543
119. Rothschilds AM, Wittrup KD. What, why, where, and when: Bringing timing to immuno-oncology. Trends Immunol (2019) 40(1):12–21. doi: 10.1016/j.it.2018.11.003
120. Reynders K, De Ruysscher D. Radiotherapy and immunotherapy: Improving cancer treatment through synergy. In: Michielin O, Coukos G, editors. Progress in tumor research. S. Karger AG (2015), Basel. p. 67–78. Available at: https://www.karger.com/Article/FullText/437185.
121. Lehrer EJ, Peterson J, Brown PD, Sheehan JP, Quiñones-Hinojosa A, Zaorsky NG, et al. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: An international meta-analysis of individual patient data. Radiother Oncol (2019) 130:104–12. doi: 10.1016/j.radonc.2018.08.025
122. Hassela JC, Schanka TE, Smetakb H, Mühlbauerb J, Salzmanna M, Machirajua D, et al. Evaluation of radio-immunotherapy sequence on immunological responses and clinical outcomes in patients with melanoma brain metastases (ELEKTRA). Oncoimmunol (2022) 11(1). doi: 10.1080/2162402X.2022.2066609
123. Yu MW, Quail DF. Immunotherapy for glioblastoma: Current progress and challenges. Front Immunol (2021) 12:1–12. doi: 10.3389/fimmu.2021.676301
124. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med (2021) 384(13):1191–203. doi: 10.1056/NEJMoa2032125
125. Wu J. Antibody targeting soluble NKG2D ligand sMIC refuels and invigorates the endogenous immune system to fight cancer. Oncoimmunology (2015) 5(3):e1095434. doi: 10.1080/2162402X.2015.1095434
126. Jinushi M, Hodi FS, Dranoff G. Therapy-induced antibodies to MHC class I chain-related protein a antagonize immune suppression and stimulate antitumor cytotoxicity. Proc Natl Acad Sci USA (2006) 103(24):9190–5. doi: 10.1073/pnas.0603503103
127. Ruocco MG, Pilones KA, Kawashima N, Cammer M, Huang J, Babb JS, et al. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Invest (2012) 122(10):3718–30. doi: 10.1172/JCI61931
128. Lopez-Soto A, Gonzalez S, Galluzzi L. Soluble NKG2D ligands limit the efficacy of immune checkpoint blockade. Oncoimmunology (2017) 6(10):e1346766. doi: 10.1080/2162402X.2017.1346766
129. Maccalli C, Giannarelli D, Capocefalo F, Lorenzo P, Fonsatti E, Giacomo AM, et al. Immunological markers and clinical outcome of advanced melanoma patients receiving ipilimumab plus fotemustine in the NIBIT-M1 study. Ocoimmunology (2015) 5(2):e1071007. doi: 10.1080/2162402X.2015.1071007
130. Maccali C, Giannarelli D, Chiarucci C, Cutaia O, Gianluca G, Hendrickx W, et al. Soluble NKG2D ligands are biomarkers associated with the clinical outcome to immune checkpoint blockade therapy of metastatic melanoma patients. Oncoimmunology (2017) 6(7):e1323618. doi: 10.1080/2162402X.2017.1323618
131. Koguchi Y, Hoen HM, Bambina SA, Rynning MD, Fuerstenberg RK, Curti BD, et al. Serum immunoregulatory proteins as predictors of overall survival of metastatic melanoma patients treated with ipilimumab. Cancer Res (2015) 75(23):5084–92. doi: 10.1158/0008-5472.CAN-15-2303
Keywords: clinical trials, combination therapy, immune checkpoint inhibitors, immuno-oncology, radiotherapy
Citation: Voronova V, Vislobokova A, Mutig K, Samsonov M, Peskov K, Sekacheva M, Materenchuk M, Bunyatyan N and Lebedeva S (2022) Combination of immune checkpoint inhibitors with radiation therapy in cancer: A hammer breaking the wall of resistance. Front. Oncol. 12:1035884. doi: 10.3389/fonc.2022.1035884
Received: 03 September 2022; Accepted: 07 November 2022;
Published: 05 December 2022.
Edited by:
Eyad Elkord, University of Salford, United KingdomReviewed by:
Lijie Zhai, Northwestern Medicine, United StatesSachio Fushida, NPO corporation Digestive Disease Support Organization (DDSO), Japan
Elena Muraro, Aviano Oncology Reference Center (IRCCS), Italy
Copyright © 2022 Voronova, Vislobokova, Mutig, Samsonov, Peskov, Sekacheva, Materenchuk, Bunyatyan and Lebedeva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Svetlana Lebedeva, lebedeva502@yandex.ru
 Veronika Voronova
Veronika Voronova Anastasia Vislobokova
Anastasia Vislobokova Kerim Mutig
Kerim Mutig Mikhail Samsonov
Mikhail Samsonov Kirill Peskov
Kirill Peskov Marina Sekacheva
Marina Sekacheva Maria Materenchuk
Maria Materenchuk Natalya Bunyatyan6,7
Natalya Bunyatyan6,7 Svetlana Lebedeva
Svetlana Lebedeva