- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
- 2Department of Health Sciences and Technology, SAIHST, Sungkyunkwan University, Seoul, South Korea
- 3Department of Biopharmaceutical Convergence, Sungkyunkwan University, Seoul, South Korea
Background: Bevacizumab is a humanized monoclonal antibody against vascular endothelial growth factor and is used in combination with first-line chemotherapy in the treatment of metastatic colorectal cancer. One of the side effects of bevacizumab is gastrointestinal perforation. This study was designed to identify the effect of bevacizumab in intestinal anastomosis site healing.
Methods: From January 2010 to December 2020, patients diagnosed with stage IV colorectal cancer treated with palliative chemotherapy or chemoradiotherapy followed by radical surgery were retrospectively reviewed. Clinical signs or symptoms and computed tomography were tools used for diagnosing anastomosis site leakage. The patients were divided into two groups, the bevacizumab group (n = 136) and the non-bevacizumab group (n = 124).
Results: Among the 260 patients 14 (5.4%) patients were diagnosed with anastomosis site leakage. In the bevacizumab group, 13 (9.6%) patients were diagnosed with anastomotic leakage. In the non-bevacizumab group, 1 (0.8%) patient was diagnosed with anastomotic leakage. Anastomosis site leakage was significantly higher in the bevacizumab treatment group (P < 0.001). In the bevacizumab group, period of drug discontinuation before surgery was factor associated with anastomosis site leakage in multivariable analysis (P = 0.031).
Conclusion: Stage IV colorectal patients treated with bevacizumab before radical surgery for primary cancer should be carefully observed of anastomosis site leakage after surgery, and the period of drug discontinuation before surgery should be longer than 5 weeks to avoid anastomosis site leakage.
Introduction
Bevacizumab (Avastin®) is a humanized monoclonal antibody against vascular endothelial growth factor (VEGF) used to inhibit VEGF function and, as a result, inhibit tumor angiogenesis (1). Fluoropyrimidine-based chemotherapy combined with bevacizumab in the first and second-line treatments of metastatic colorectal cancer significantly increased oncologic outcomes in several randomized controlled trials (2, 3). However, the antiangiogenic effect of bevacizumab inhibits the capillary beds of the small bowel villi, contributing to gastrointestinal perforation by provoking the regression of normal blood vessels in the gastrointestinal tract (4). Several studies showed an increased risk of gastrointestinal perforations in patients treated with bevacizumab (5, 6). In an animal model, the administration of bevacizumab inhibited angiogenesis in the intestinal anastomosis site, resulting in a decrease in a-SMA accumulation and collagen deposition in bowel anastomosis site tissue, which might affect the healing of intestinal anastomosis (7). Because of the antiangiogenic effect of bevacizumab, the discontinuation of bevacizumab is recommended at least 6 weeks before surgery (8). Therefore, this study was conducted to evaluate the effect of bevacizumab on intestinal anastomosis site healing in stage IV colorectal cancer patients who underwent preoperative chemotherapy.
Methods
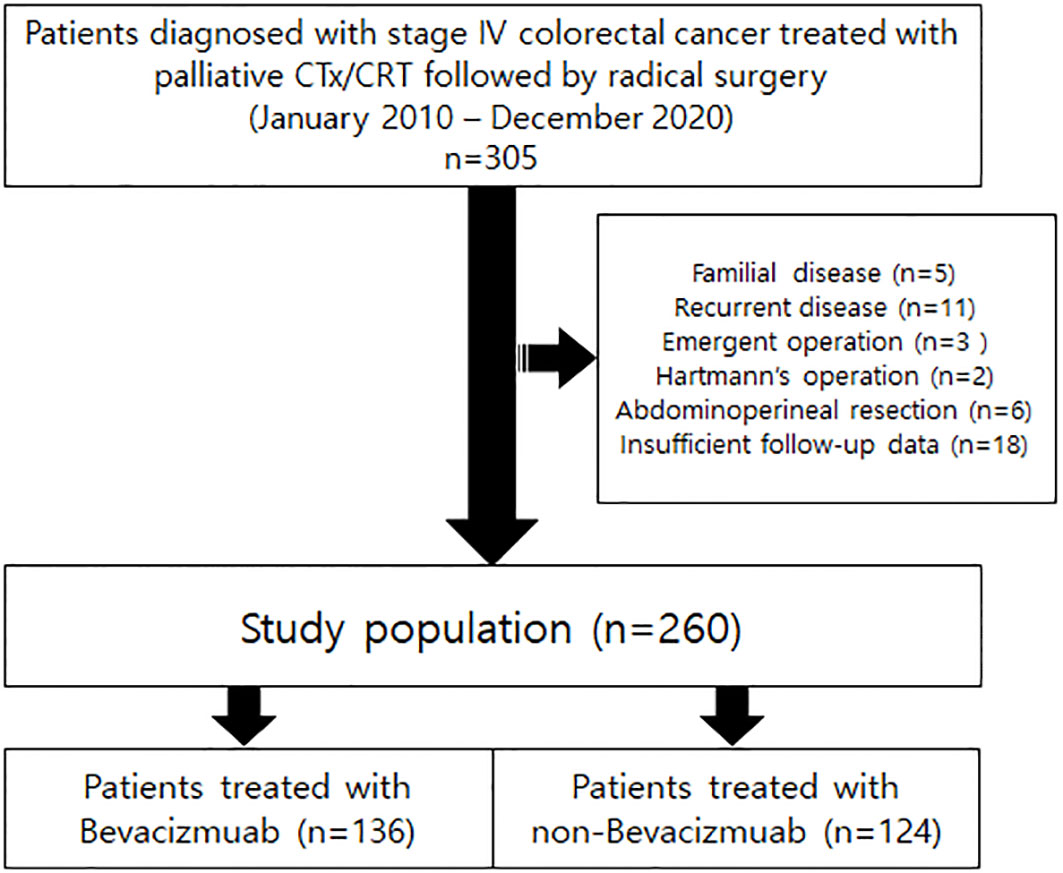
From January 2010 to December 2020, patients diagnosed with stage IV colorectal cancer treated with palliative chemotherapy or chemoradiotherapy followed by radical surgery were retrospectively reviewed. Patients with familial disease, recurrent disease, emergent operations, or who underwent abdominoperineal resections, which has no anastomosis site, or without appropriate follow-up data were excluded from the cohort. A flowchart of patient selection is illustrated in Figure 1. A total of 260 patients were enrolled. This study was reviewed and approved by the Institutional Review Board of Samsung Medical Center (No. 2021-10-041).
Chemotherapy regimens were based on the National Comprehensive Cancer Network (NCCN) guidelines. In all the patients, chemotherapy with FOLFIRI/FOLFOX/XELOX was initiated with or without cetuximab/bevacizumab. All patients underwent tests of tumor gene status for KRAS/NRAS as well as MSI/MMR status. Patients with KRAS or NRAS mutation were not treated with cetuximab or panitumumab and treated with FOLFIRI/FOLFOX/XELOX alone or in combination with bevacizumab. When the disease progressed despite of first-line FOLFOX/XELOX based chemotherapy, chemotherapy regimen was altered. In previous oxaliplatin-based therapy without irinotecan, irinotecan ± aflibercept or pembrolizumab based chemotherapy was continued (8). Also, patients with low to mid-rectal cancer were discussed in the multidisciplinary meeting whether to undergo neoadjuvant radiotherapy before surgery.
Anastomosis site leakage was defined as a defect in the intestinal wall integrity at the colorectal or colo-anal anastomosis site, leading to a communication between the intraluminal and extraluminal compartments. An abscess in the pelvic cavity close to the anastomosis site was considered anastomotic leakage. Clinical symptoms and signs such as fever, tachycardia, abdominal pain or distension, leukocytosis, and elevated C-reactive protein (CRP) levels were indicators suspicious of anastomosis site leakage, so patients with the symptoms or signs listed above underwent computed tomography (CT). Peri-anastomotic loculated fluid containing air or anastomosis wall defects in contrast CT was considered anastomosis site leakage (9).
Statistical analyses were performed using Rex (Version 3.0.3, RexSoft Inc., Seoul, Korea) and SPSS version 27 (SPSS Inc., Chicago, IL, USA). The categorical variables were analyzed using the Chi-squared test, and the continuous variables were analyzed using the Mann-Whitney U-test. The logistic regression model was used to analyze the variables that could independently influence anastomosis site leakage. Variables with a P-value of < 0.1 in univariable analysis were entered into a multivariable analysis. A P-value of < 0.05 in the multivariable analysis was considered statistically significant.
Results
Among the 260 patients, a total of 136 (52.3%) patients were treated with XELOX/FOLFOX/FOLFIRI combined with bevacizumab, 85 (32.7%) patients were treated with FOLFOX/FOLFIRI combined with cetuximab, 25 (9.6%) patients with XELOX only, 8 (3.2%) patients with FOLFOX only, 2 (0.8%) patients with FOLFIRI only, 2 (0.8%) patients with XELOX and XELIRI, 1 (0.3%) patient with FOLFIRI with aflibercept, and 1 (0.3%) patient with pembrolizumab. Three patients received 25Gy to 44Gy of radiation. The patients received a median of 6 courses of bevacizumab (minimum 3, maximum 42), and the median interval days between the last bevacizumab treatment to surgery was 43 days (range 16 – 240 days).
A comparison of the baseline clinicopathologic features between the groups is summarized in Table 1. Age, perineural invasion, and anastomosis site leakage were significantly different between the two groups (P = 0.012 P = 0.040, P < 0.001). In the group treated with bevacizumab, anastomosis site leakage was higher in patients with rectal cancer, and drug discontinuation periods shorter than 35 days (P = 0.020, P = 0.027; Table 2).
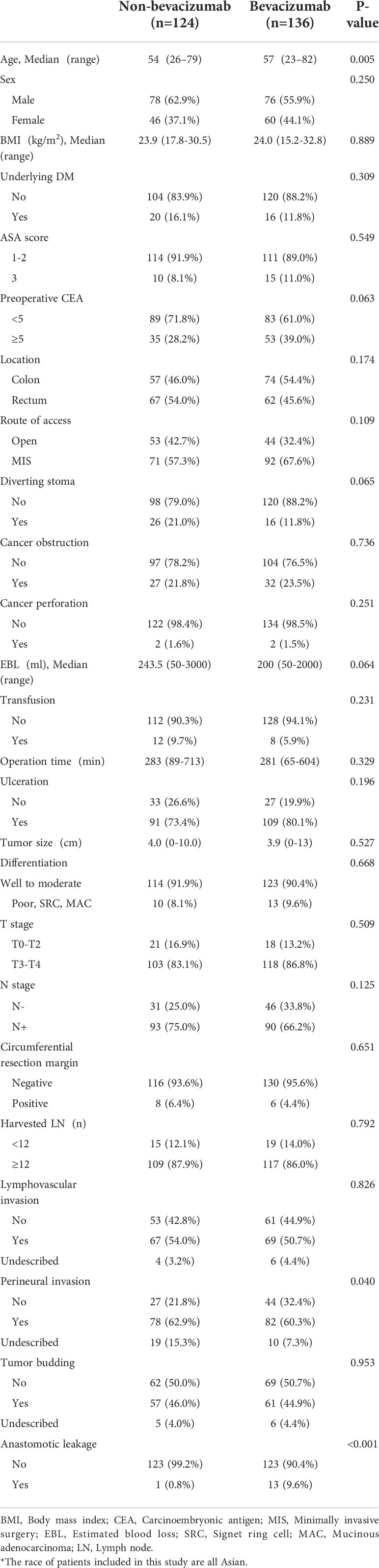
Table 1 Baseline clinicopathologic features of patients in the bevacizumab and non-bevacizumab groups.
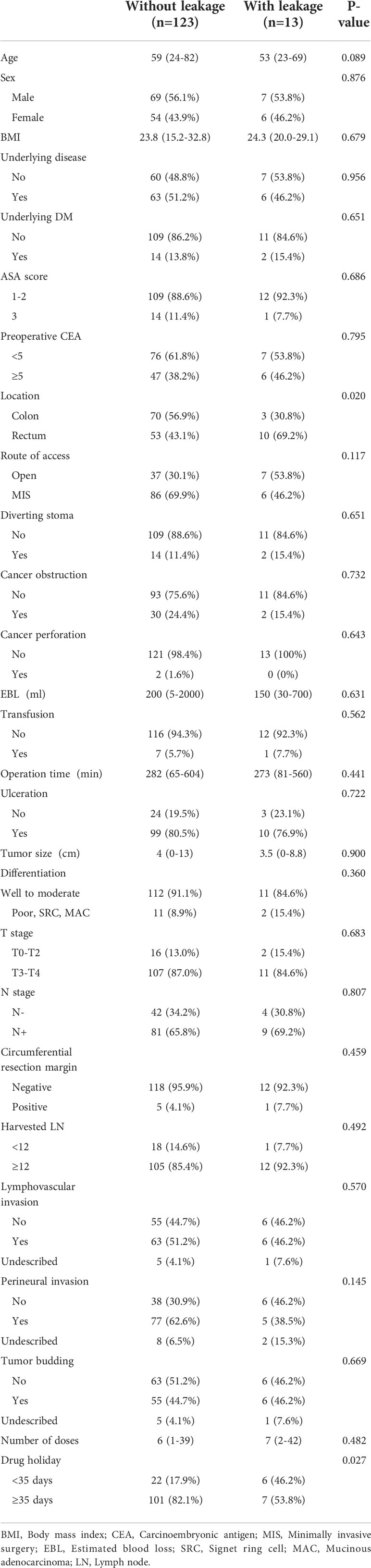
Table 2 Baseline clinicopathologic features of patients with anastomotic leakage and non-leakage treated with bevacizumab.
Among the all patients, 213 patients underwent radical operation of colorectal lesion with metastasectomy. The most common operation site for metastatic organ was liver. One hundred seventy-one (65.8%) patients underwent liver resection or intraoperative radio frequency ablation. Hemihepatectomy, sectionectomy, segmentectomy and wedge resection were conducted for the liver resection. The next common operation for metastasis was distant metastatic lymph node dissection (15.4%). Hysterectomy or oophorectomy were performed in 6.2% patients. Pneumonectomy, small bowel resection, operation for bladder/ureter were performed in 2.3% patients respectively. Splenectomy, pancreatectomy and wedge resection of stomach were performed in each one patient.
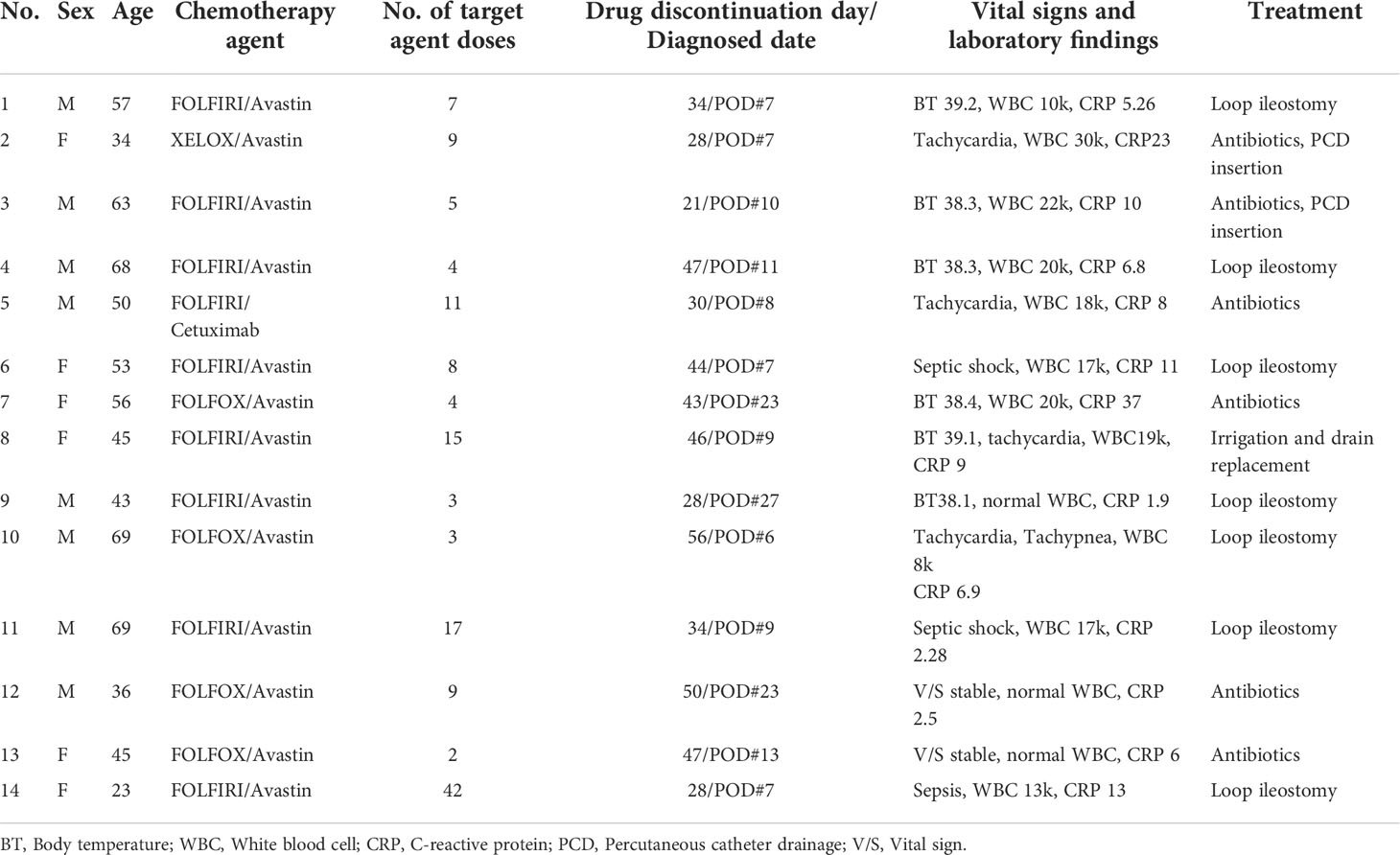
A total of 14 (5.4%) patients were diagnosed with anastomosis site leakage. The anastomosis site leakage events are described in detail in Table 3. Thirteen (92.85%) patients were treated with bevacizumab and 1 (7.15%) patient was treated with cetuximab. Among 14 patients, 1 (7.2%) was diagnosed before 7 postoperative days (PODs), 10 (71.4%) patients between 1 – 2 weeks, 3 (21.4%) patients between 3 – 4 weeks. 10 (71.4%) patients were diagnosed at hospitalization and 4 (28.6%) were diagnosed after discharge. 9 (64.3%) patients had body temperatures higher than 38 °C and 10 (71.4%) patients had elevated white blood cell (WBC) counts with elevated absolute neutrophil counts, and all patients had elevated CRP levels. Two (14.3%) patients had stable vital signs with elevated CRP levels. Among 9 patients with fever, 2 patients had septic shock and 1 patient had sepsis before emergent surgery.
Eight (57.1%) patients underwent emergent operations. Seven underwent intra-abdominal irrigation with loop ileostomy and 1 underwent irrigation and drainage only because this patient already had an ileostomy from the initial operation. Three (18.75%) patients were treated with antibiotics with percutaneous catheter drainage insertion for complicated fluid collection. Three (18.75%) patients were treated with antibiotics only.
Among 14 patients, 3 (21.4%) patients had an ileostomy from the primary operation. One patient underwent irrigation and drainage because she was diagnosed with sepsis with complicated fluid collection with anastomosis site dehiscence in the CT examination. One patient was treated with antibiotics with percutaneous catheter drainage insertion. He had a high fever of over 38 °C with tachycardia and bacteremia. The CT showed air containing fluid collection abutting the anastomosis site with localized peritonitis. One patient was treated with antibiotics only because his vital signs were stable and CT showed air containing fluid collection suspicious of connection with anastomosis site with localized peritonitis. All patients with a stoma underwent ileostomy take down after the end of chemotherapy treatment with the confirmation of anastomosis site healing by colon fluoroscopy using gastrografin. In patients who didn’t need ileostomy for anastomosis site leakage, median days of fistula to close was 18days (range, 12-62). In patient treated with laparotomy with ileostomy, median months of fistula to close was 5.5 months (range, 3-24).
In multivariable analysis, chemotherapy agent was independent factor associated with anastomosis site leakage (P = 0.008, Table 4). In the bevacizumab group, the discontinuation period before surgery was independent factor associated with anastomosis site leakage in multivariable analysis (P = 0.031, Table 5).
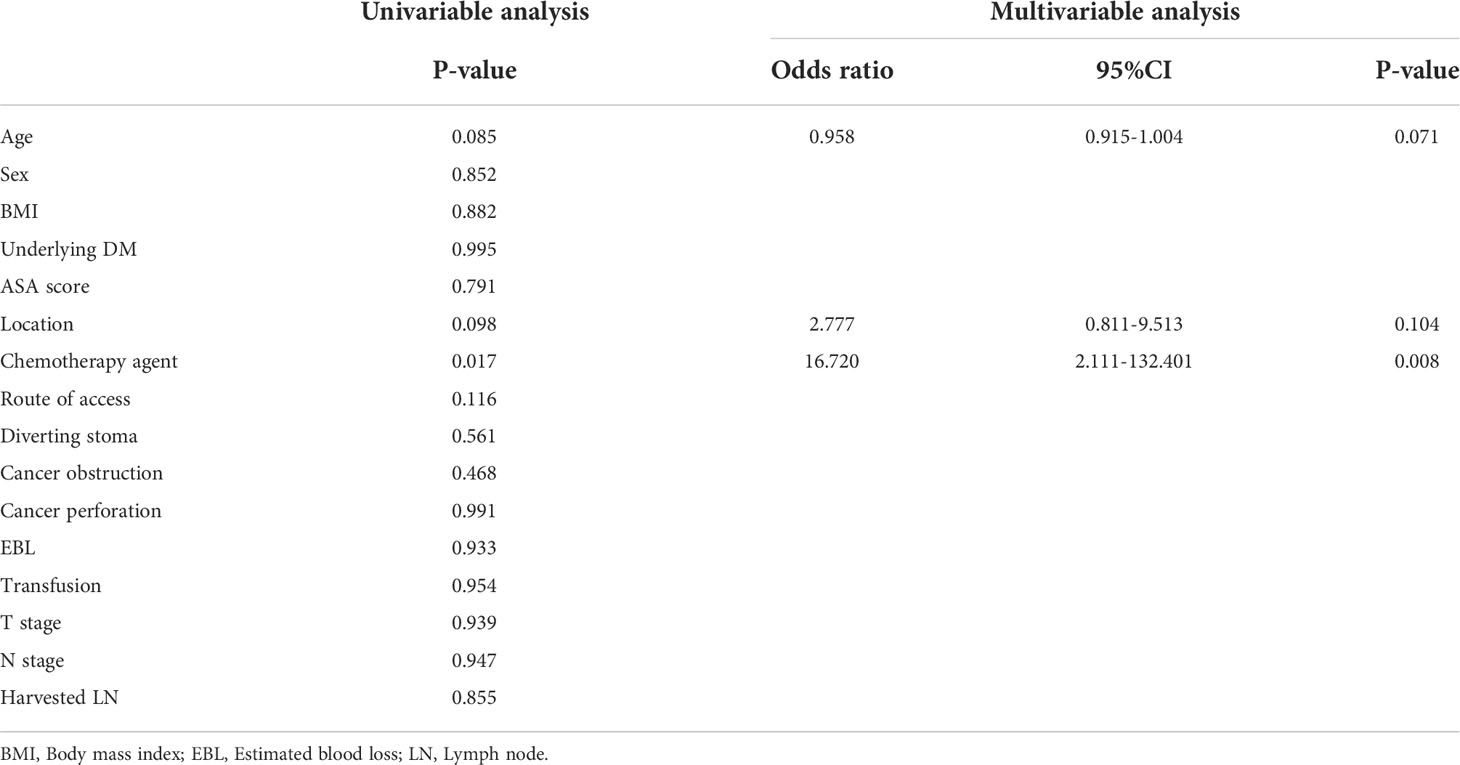
Table 4 Univariable and multivariable analyses of factors associated with anastomosis site leakage in stage IV colorectal cancer patients.
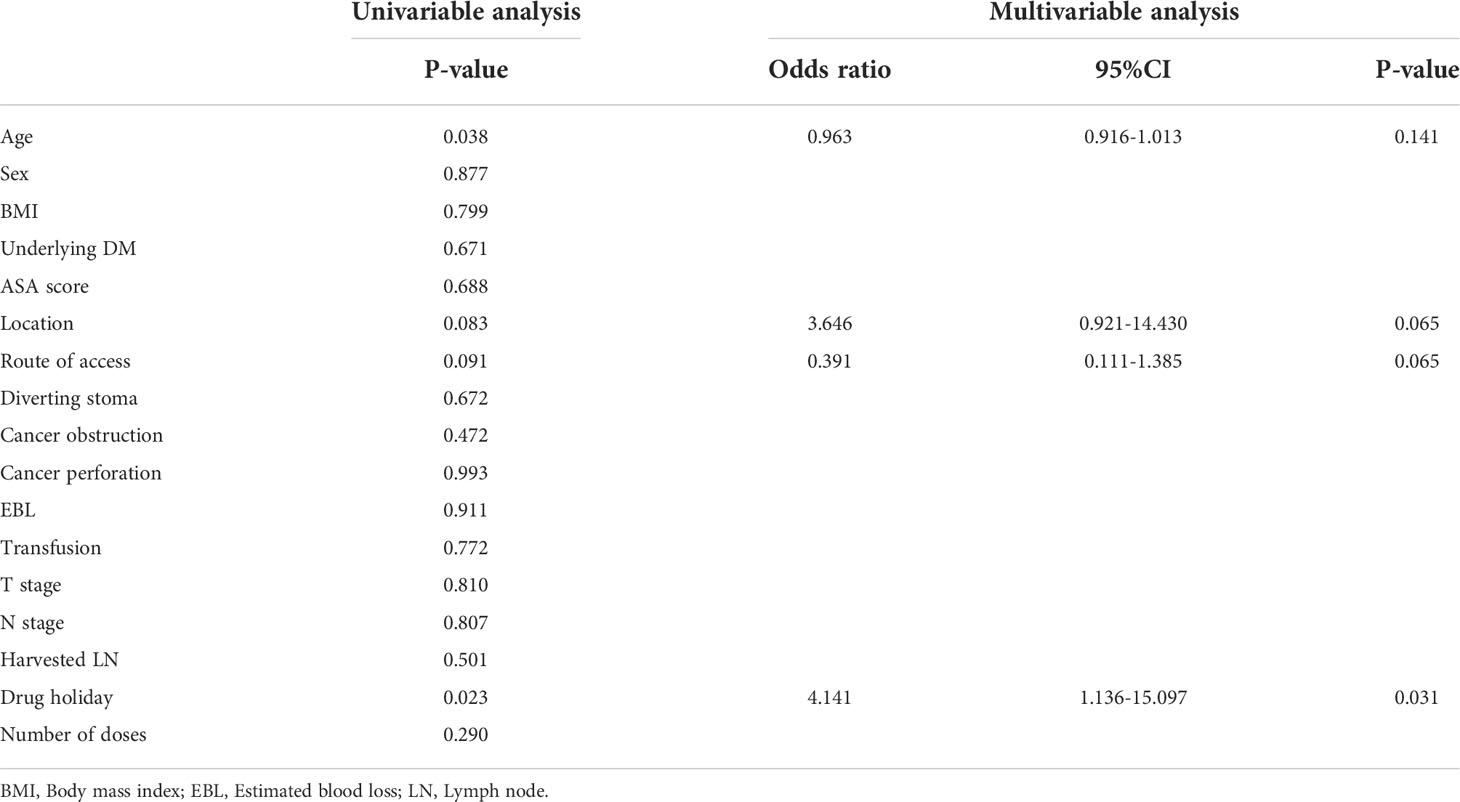
Table 5 Univariable and multivariable analyses of factors associated with anastomosis site leakage in the bevacizumab group.
Discussion
Monoclonal antibodies are chemotherapeutic agents targeting specific receptors on cancer cells (10). Bevacizumab, a monoclonal antibody, acts as an anti-angiogenic agent inhibiting VEGF-A (2). The addition of bevacizumab to fluorouracil-based combination therapy resulted in significant improvement in survival among patients with stage IV colorectal cancer (11, 12). However, because of the anti-angiogenic effects, many studies have reported the complications of surgical wound healing or gastrointestinal perforation in patients treated with bevacizumab (13–16). In previous studies, bevacizumab-associated GI perforation was seen in 1.5% – 1.6% of the patients with metastatic colorectal cancer (4, 11). Also bevacizumab is considered a preoperative risk factor for colorectal anastomotic leakage (17). And some studies reported spontaneous delayed anastomotic complications associated with bevacizumab (18, 19).
In our study, postoperative anastomosis site leakage was observed in 5.4% of the patients with stage IV colorectal cancer treated with preoperative chemotherapy/chemoradiotherapy. Of the anastomosis site leakage patients, 93.75% were treated with bevacizumab combined with FOLFOX/FOLFIRI/XELOX. Patients treated with bevacizumab showed significantly higher anastomosis site leakage compared to the non-bevacizumab group. Bevacizumab was also a factor associated with anastomosis site leakage in stage IV colorectal patients. Koscielny et al. reported that bevacizumab was associated with significantly higher anastomosis site leakage in the non-ileostomy group who underwent debulking surgery for ovarian cancer (20). Also, Uehara et al. reported 27.8% of anastomosis site leakage in rectal cancer patients who underwent neoadjuvant XELOX+Bevacizumab followed by total mesorectal excision (21).
Anastomotic leakage after rectal cancer surgery commonly occurs in the early postoperative period within 7 days (22, 23). Previous studies revealed that treatment with bevacizumab could be associated with delayed anastomosis site perforation, even 15 months after surgery (18, 19). In our study, the median time to leakage was 9 days (range, 6 – 27 days), which was longer than anastomotic leakage after surgery in patients without bevacizumab treatment. Therefore, even if time has passed since the operation, if the patient complains of anal pain or bleeding, anastomotic leakage should be suspected, and further examinations should be performed.
Bevacizumab has a long terminal half-life (20 days) and the bevacizumab prescribing information recommends discontinuing bevacizumab at least 4 weeks before surgery (24).
The NCCN guidelines suggest withholding bevacizumab at least 6 weeks prior to surgery (8). Yoshioka et al. reported that the interval between bevacizumab and surgery was not a risk factor for anastomotic leakage, but study was based on a median interval of 9 weeks so effect of bevacizumab on anastomosis site healing would be small (25). In this study, anastomosis site leakage was significantly higher in patients with discontinuation dates shorter than 5 weeks and there was no significant difference between the two groups when compared based on a 4 or 6-week discontinuation interval. Therefore, the discontinuation of bevacizumab is recommended at least 5 weeks prior to major surgery.
Despite the limitation that this study was a retrospective study from a single-center, to our knowledge, this was the first study to evaluate the effect of bevacizumab on anastomosis site healing in stage IV colorectal patients. Further prospective studies from multi-centers should be conducted to confirm our study results.
In conclusion, bevacizumab affected anastomosis site healing after colorectal cancer, and at least 5 weeks from bevacizumab discontinuation to surgery was associated with lower anastomosis site leakage compared to discontinuation dates shorter than 5 weeks. Thus, stage IV colorectal patients treated with bevacizumab before radical surgery for primary cancer should be carefully observed after the operation, and the period of drug discontinuation before surgery should be longer than 5 weeks to avoid anastomosis site leakage.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Samsung Medical Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Guarantor of integrity of the entire study: WL, YC. Study concepts and design: YC, SY. Literature research: JS, YP. Data analysis: SK, JS. Statistical analysis: SK, JH. Manuscript preparation: SK, YC, HK. Critical revision of manuscript: SK, JH, WL, SY, HK, YC, YP, JS. All authors contributed to the article and approved the submitted version.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR20C0025). This work was supported by the BK21 FOUR Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. McCormack PL, Keam SJ. Bevacizumab: A review of its use in metastatic colorectal cancer. Drugs (2008) 68(4):487–506. doi: 10.2165/00003495-200868040-00009
2. Hurwitz HI, Tebbutt NC, Kabbinavar F, Giantonio BJ, Guan ZZ, Mitchell L, et al. Efficacy and safety of bevacizumab in metastatic colorectal cancer: Pooled analysis from seven randomized controlled trials. Oncologist (2013) 18(9):1004–12. doi: 10.1634/theoncologist.2013-0107
3. Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol (2005) 23(16):3697–705. doi: 10.1200/JCO.2005.05.112
4. Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol (2007) 14(6):1860–9. doi: 10.1245/s10434-006-9337-9
5. Qi WX, Shen Z, Tang LN, Yao Y. Bevacizumab increases the risk of gastrointestinal perforation in cancer patients: a meta-analysis with a focus on different subgroups. Eur J Clin Pharmacol (2014) 70(8):893–906. doi: 10.1007/s00228-014-1687-9
6. Saito S, Hayashi N, Sato N, Iwatsuki M, Baba Y, Sakamoto Y, et al. Chemotherapy with bevacizumab for metastatic colorectal cancer: a retrospective review of 181 Japanese patients. Int J Clin Oncol (2013) 18(4):689–95. doi: 10.1007/s10147-012-0426-4
7. Nakamura H, Yokoyama Y, Uehara K, Kokuryo T, Yamaguchi J, Tsuzuki T, et al. The effects of bevacizumab on intestinal anastomotic healing in rabbits. Surg Today (2016) 46(12):1456–63. doi: 10.1007/s00595-016-1342-4
8. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2021) 19(3):329–59. doi: 10.6004/jnccn.2021.0012
9. Hirst NA, Tiernan JP, Millner PA, Jayne DG. Systematic review of methods to predict and detect anastomotic leakage in colorectal surgery. Colorectal Dis (2014) 16(2):95–109. doi: 10.1111/codi.12411
10. Pasetto LM, Bortolami A, Falci C, Sinigaglia G, Monfardini S. Recent progress in target therapy in colorectal cancer. Anticancer Res (2006) 26(5B):3973–81.
11. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med (2004) 350(23):2335–42. doi: 10.1056/NEJMoa032691
12. Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol (2013) 14(1):29–37. doi: 10.1016/S1470-2045(12)70477-1
13. Scappaticci FA, Fehrenbacher L, Cartwright T, Hainsworth JD, Heim W, Berlin J, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol (2005) 91(3):173–80. doi: 10.1002/jso.20301
14. Gordon CR, Rojavin Y, Patel M, Zins JE, Grana G, Kann B, et al. A review on bevacizumab and surgical wound healing: an important warning to all surgeons. Ann Plast Surg (2009) 62(6):707–9. doi: 10.1097/SAP.0b013e3181828141
15. Erinjeri JP, Fong AJ, Kemeny NE, Brown KT, Getrajdman GI, Solomon SB. Timing of administration of bevacizumab chemotherapy affects wound healing after chest wall port placement. Cancer (2011) 117(6):1296–301. doi: 10.1002/cncr.25573
16. Lordick F, Geinitz H, Theisen J, Sendler A, Sarbia M. Increased risk of ischemic bowel complications during treatment with bevacizumab after pelvic irradiation: report of three cases. Int J Radiat Oncol Biol Phys (2006) 64(5):1295–8. doi: 10.1016/j.ijrobp.2005.12.004
17. McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg (2015) 102(5):462–79. doi: 10.1002/bjs.9697
18. August DA, Serrano D, Poplin E. "Spontaneous," delayed colon and rectal anastomotic complications associated with bevacizumab therapy. J Surg Oncol (2008) 97(2):180–5. doi: 10.1002/jso.20938
19. Jafari M, Tessier W, El Hajbi F, Decanter G, Mirabel X. Delayed anastomotic leakage following bevacizumab administration in colorectal cancer patients. Acta Oncol (2016) 55(9-10):1250–2. doi: 10.3109/0284186X.2016.1171393
20. Koscielny A, Ko A, Egger EK, Kuhn W, Kalff JC, Keyver-Paik MD. Prevention of anastomotic leakage in ovarian cancer debulking surgery and its impact on overall survival. Anticancer Res (2019) 39(9):5209–18. doi: 10.21873/anticanres.13718
21. Uehara K, Hiramatsu K, Maeda A, Sakamoto E, Inoue M, Kobayashi S, et al. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: N-SOG 03 phase II trial. Jpn J Clin Oncol (2013) 43(10):964–71. doi: 10.1093/jjco/hyt115
22. Zhao WT, Hu FL, Li YY, Li HJ, Luo WM, Sun F. Use of a transanal drainage tube for prevention of anastomotic leakage and bleeding after anterior resection for rectal cancer. World J Surg (2013) 37(1):227–32. doi: 10.1007/s00268-012-1812-9
23. Kanellos D, Pramateftakis MG, Vrakas G, Demetriades H, Kanellos I, Mantzoros I, et al. Anastomotic leakage following low anterior resection for rectal cancer. Tech Coloproctol (2010) 14 Suppl 1:S35–7. doi: 10.1007/s10151-010-0620-1
24. Ni M. Update and interpretation of 2021 national comprehensive cancer network (NCCN) "Clinical practice guidelines for bone tumors". Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi (2021) 35(9):1186–91. doi: 10.7507/1002-1892.202103073
Keywords: colorectal (colon) cancer, bevacizumab, stage IV, anastomotic leak in colorectal surgery, chemotherapy
Citation: Kim S, Shin JK, Park Y, Huh JW, Kim HC, Yun SH, Lee WY and Cho YB (2022) Bevacizumab increases the risk of anastomosis site leakage in metastatic colorectal cancer. Front. Oncol. 12:1018458. doi: 10.3389/fonc.2022.1018458
Received: 13 August 2022; Accepted: 10 October 2022;
Published: 21 October 2022.
Edited by:
Tadahiko Masaki, Kyorin University, JapanReviewed by:
Kay Uehara, Nagoya University, JapanGuanyu Yu, Second Military Medical University, China
Copyright © 2022 Kim, Shin, Park, Huh, Kim, Yun, Lee and Cho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Beom Cho, gscyb@skku.edu
 Seijong Kim
Seijong Kim Jung Kyong Shin1
Jung Kyong Shin1 Jung Wook Huh
Jung Wook Huh