- 1Centro Unico Regionale Trapianti Cellule Staminali e Terapie Cellulari (CTMO), Grande Ospedale Metropolitano “Bianchi-Melacrino-Morelli”, Reggio Calabria, Italy
- 2Istituto di Fisiologia Clinica del Consiglio Nazionale delle Ricerche (CNR), Roma, Italy
- 3Dipartimento di Oncologia, SSD Trapianto Allogenico di Cellule Staminali, AOU Città della Salute e della Scienza di Torino, Torino, Italy
- 4Dipartimento di Biotecnologie Molecolari e Scienze per la Salute, Divisione di Ematologia, Università di Torino, Torino, Italy
- 5Unità Operativa di Oncoematologia e TMO, Istituto “La Maddalena”, Palermo, Italy
- 6Ematologia e Trapianto di Cellule Staminali, Polo Ospedaliero “Vito Fazzi”, Lecce, Italy
- 7UOC Ematologia con Trapianto CSE, AORN “Antonio Cardarelli”, Napoli, Italy
- 8Dipartimento di Biomedicina e Prevenzione, Università di Roma Tor Vergata, Roma, Italy
- 9UOSD UTMO, AOR Villa “Sofia Cervello”, Palermo, Italy
- 10Ematologia e Trapianto di Midollo Osseo, Ospedale “San Giuseppe Moscati”, Taranto, Italy
- 11UOC di Ematologia con Trapianto, Dipartimento di Emergenza e Trapianti d’Organo, Università degli Studi “Aldo Moro” e AOUC Policlinico di Bari, Bari, Italy
- 12Divisione di Ematologia, Ospedale “Antonio Perrino”, Brindisi, Italy
- 13Unità di Ematologia, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori”, Meldola, Italy
- 14UO Ematologia - CTMO, Polo Ospedaliero “Armando Businco”, Cagliari, Italy
- 15Dipartimento di Medicina, Chirurgia e Odontoiatria, Università di Salerno, Salerno, Italy
- 16Unità di Ematologia, Azienda Ospedaliera “San Giuseppe Moscati”, Avellino, Italy
- 17SC Ematologia, Azienda Ospedaliera “S. Croce e Carle”, Cuneo, Italy
- 18Programma di Trapianto Emopoietico, Azienda Policlinico “Vittorio Emanuele”, Catania, Italy
- 19Divisione Universitaria di Ematologia, Ospedale “San Carlo”, Potenza, Italy
- 20Ematologia e Centro Trapianti Midollo Osseo (CTMO), Dipartimento ad Attività Integrata Medicina Generale e Specialistica, Azienda Ospedaliero-Universitaria di Parma, Parma, Italy
- 21Ospedale I.R.C.C.S. Casa Sollievo della Sofferenza - Centro Trapianti di Cellule Staminali, San Giovanni Rotondo, Italy
- 22Istituto di Fisiologia Clinica del Consiglio Nazionale delle Ricerche (CNR), Reggio Calabria, Italy
Despite effective treatments, cytomegalovirus (CMV) continues to have a significant impact on morbidity and mortality in allogeneic stem cell transplant (allo-SCT) recipients. This multicenter, retrospective, cohort study aimed to evaluate the reproducibility of the safety and efficacy of commercially available letermovir for CMV prophylaxis in a real-world setting. Endpoints were rates of clinically significant CMV infection (CSCI), defined as CMV disease or CMV viremia reactivation within day +100-+168. 204 adult CMV-seropositive allo-SCT recipients from 17 Italian centres (median age 52 years) were treated with LET 240 mg/day between day 0 and day +28. Overall, 28.9% of patients underwent a haploidentical, 32.4% a matched related, and 27.5% a matched unrelated donor (MUD) transplant. 65.7% were considered at high risk of CSCI and 65.2% had a CMV seropositive donor. Low to mild severe adverse events were observed in 40.7% of patients during treatment [gastrointestinal toxicity (36.3%) and skin rash (10.3%)]. Cumulative incidence of CSCI at day +100 and day +168 was 5.4% and 18.1%, respectively, whereas the Kaplan-Meier event rate was 5.8% (95% CI: 2.4-9.1) and 23.3% (95% CI: 16.3-29.7), respectively. Overall mortality was 6.4% at day +100 and 7.3% at day +168. This real-world experience confirms the efficacy and safety of CMV.
Introduction
Clinically significant cytomegalovirus (CMV) infection (CSCI), defined as CMV disease or CMV viremia reactivation after allogeneic stem-cell transplantation (allo-SCT), is often a serious complication given the delayed immune recovery of the host (1–3). Post-transplant CSCI varies from 30% to 70% and has been associated with higher non-relapse mortality (NRM) (4–10). During the past few decades, both clinical trials and real-world experiences have evaluated the role of CMV prophylaxis, reporting conflicting results (11, 12).
Letermovir (LET) is an antiviral agent with a novel mechanism of action characterized by inhibition of the CMV DNA terminase complex (13, 14). In a pivotal registration Phase 3 clinical trial, prophylaxis with LET significantly reduced the incidence of CSCI after allo-SCT (15). The drug was granted fast-track status by the US Food and Drug Administration (FDA), and orphan drug status by the European Medicines Agency. In the US and Europe, LET was approved for prophylaxis of CSCI in adult CMV-seropositive recipients of allo-SCT (16). FDA considers it a first-in-class medication (17).
The majority of Italian transplant programs adopted prophylaxis with LET as standard policy as soon as the drug became commercially available. The aims of the present multicenter, retrospective, cohort study were to investigate whether the results reported in the aforementioned phase 3 trial could be reproduced in a real-world experience and to assess whether prophylaxis could affect pre-emptive CMV therapy.
Methods
Seventeen Italian Transplant Centers took part in the study. The observation period began in January 2019 when the Italian Medicines Agency (AIFA) authorized LET for commercial purposes. Prophylaxis was indicated by AIFA for patients aged 18 years or older who had positive CMV serologic status with an undetectable level of CMV DNAemia in whole blood before transplant. Patients received LET 480 mg tablet once daily between day 0 and day +28 after allo-SCT and continued until day +100 if no adverse events occurred during the observation period. If LET was co-administered with cyclosporine, the dosage of LET has been decreased to 240 mg once daily. The intravenous (IV) formulation of LET was not available in Italy. All patients continued herpesvirus prophylaxis as per standard practice.
A high sensitivity and high specificity serologic test was used to detect CMV-IgG before transplant and real-time PCR assay was used for post allo-SCT CMV monitoring (18). DNAemia was determined at least once a week in the first three months, and every other week in the second three-month period. Pre-emptive treatment was initiated when the CMV-DNAemia level was >1,000 copies/mL in plasma or 10,000 copies/mL in whole blood, in two consecutive assessments (18, 19). Patients were evaluated up to day +100 for the primary efficacy endpoint, after which follow-up continued through week 24 post-transplant.
Conditioning intensity was classified according to Working Group definitions (20). Donor selection, conditioning, graft-versus-host disease (GVHD) prophylaxis, and supportive care followed standard institutional operating procedures (21).
Patients were considered at high risk of CSC if one or more of the following criteria were present: human leukocyte antigen (HLA)-related (sibling) donor with at least one mismatch at HLA-A, -B or -DR loci; haploidentical donor; unrelated donor with at least one mismatch at HLA-A, -B, -C or -DRB; use of umbilical cord blood as stem cell source; use of ex vivo T-cell-depleted grafts; ≥grade 2 GVHD requiring systemic corticosteroids. Given the retrospective design (i.e., the non-interventional nature) of the study, no sample size calculation was performed.
Endpoints
The primary endpoint was the incidence of CSCI leading to pre-emptive treatment (22) at day +100 (14 weeks) post allo-SCT, and the time to CSCI. The secondary endpoint was the incidence of CSCI at day +168 (24 weeks). Follow-up time was calculated from the transplant date to the first positive CMV DNAemia or its last measurement during the study period, whichever occurred first. Censoring time was the last date of the positive CMV test or the date of death if <14 days from the last negative test. Since endpoints were evaluated at day +100 and day +168, no data on CMV tests were collected after day +168.
Statistical Analysis
The follow-up period (spanning from January 2019 to June 2020) was calculated as the time (in days) spanning from the transplant date to the first positive CMV DNAemia (i.e., the achievement of the study endpoint) or the last observation coinciding with 168 days or the date of death or lost to follow-up. Data were summarized as median and interquartile range, or absolute number and percentage. To identify the demographic and clinical correlates of CMV infection and drug discontinuation we did not use a face-to-face comparison of patients’ characteristics but the univariate logistic analysis, a method that specifically allows to assess the strength of the risk factor-study outcomes links. Bonferroni’s correction was used to minimize the possibility of false-positive findings due to multiple testing. Kaplan-Meier survival analysis was performed for time to infection, overall-survival (OS), and non-relapse mortality (NRM). NRM was defined as death without recurrent or progressive disease after allo-SCT. Probabilities of NRM were estimated with the use of cumulative incidence curves, with relapse viewed as a competing risk. Gray’s method was used to evaluate the differences between groups (23). If no competitive risk was found, a standard Kaplan-Meier analysis was applied. Data were analysed with STATA/IC 13.1 for Windows (College Station, TX) and RStudio-1.2.5033.1.
Compliance With Ethical Standards
This multicenter, retrospective, cohort study was approved by the Ethics Committee of the coordinating centre, Grande OspedaleMetropolitano “Bianchi-Melacrino-Morelli” of Reggio Calabria, Italy, and by those of the other participating centres. All procedures were performed according to the principles laid down in the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients signed the informed consent.
Results
Overall, 230 patients who underwent an allograft in the participating Italian centres were enrolled; 26 were excluded from the analysis because of incomplete data. Median age of the 204 patients in the study cohort was 52 years, and 53.9% were males (Table 1). Acute myeloid leukaemia (AML) was diagnosed in 53.4%. Overall, 28.9% underwent a haploidentical, 32.4% a matched related (MRD), and 27.5% a matched unrelated donor (MUD) transplant. In the majority of patients, stem cell source was G-CSF mobilized peripheral blood (65.7%). 65.7% of patients were considered at high risk of CMV and 65.2% had a CMV seropositive donor. The first patient was enrolled in January 2019 and the last one in June 2020.
Incidence of CMV Infection and Discontinuation
The cumulative incidence of CSCI was 5.4% at day +100 and 18.1% at day +168 after transplant (Table 2). Overall, from day +100 to day +168, the cumulative incidence of CSCI was 12.7%. Twenty patients discontinued the trial in the first 100 days, the majority (13 patients) because of death. The percentage of discontinuation was 28.4% at 168 days. Thirteen (6.4%) patients died before day +100 day and 15 (7.3%) before day +168. The Kaplan-Meier event rate of CSCI through 24 weeks was 23.3% (95% CI: 16.3-29.7), 5.8% at day +100 (95% CI: 2.4-9.1). Of note, starting at week +19, the incidence of CMV infection rapidly increased (Figure 1A). Given that no competitive risk from drug discontinuation was found on incidence rate by baseline CMV risk categories, low versus high, (competitive risk: 14 weeks, p=0.15; 24 weeks, p=0.84), a standard Kaplan-Meier analysis showed substantially similar curves (Figure 1B).
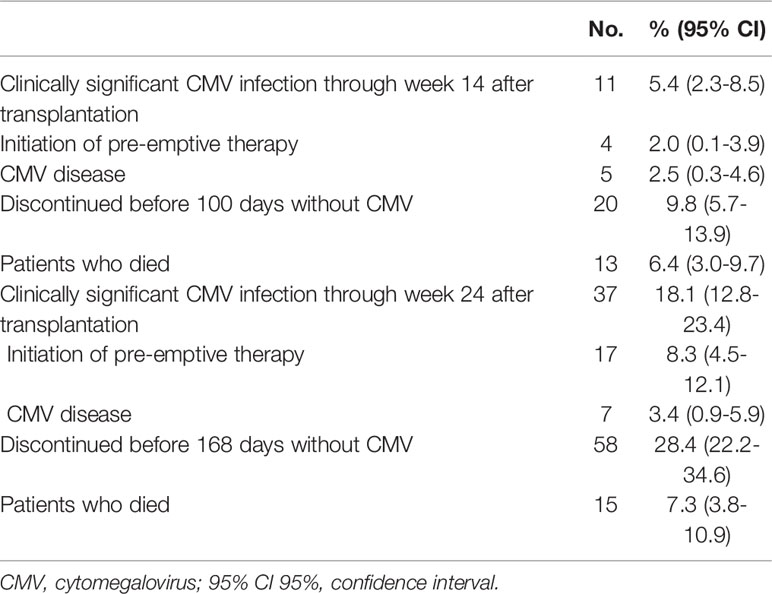
Table 2 Cumulative incidence of CMV infection and discontinuation at 100 days and at 168 days in 204 patients.
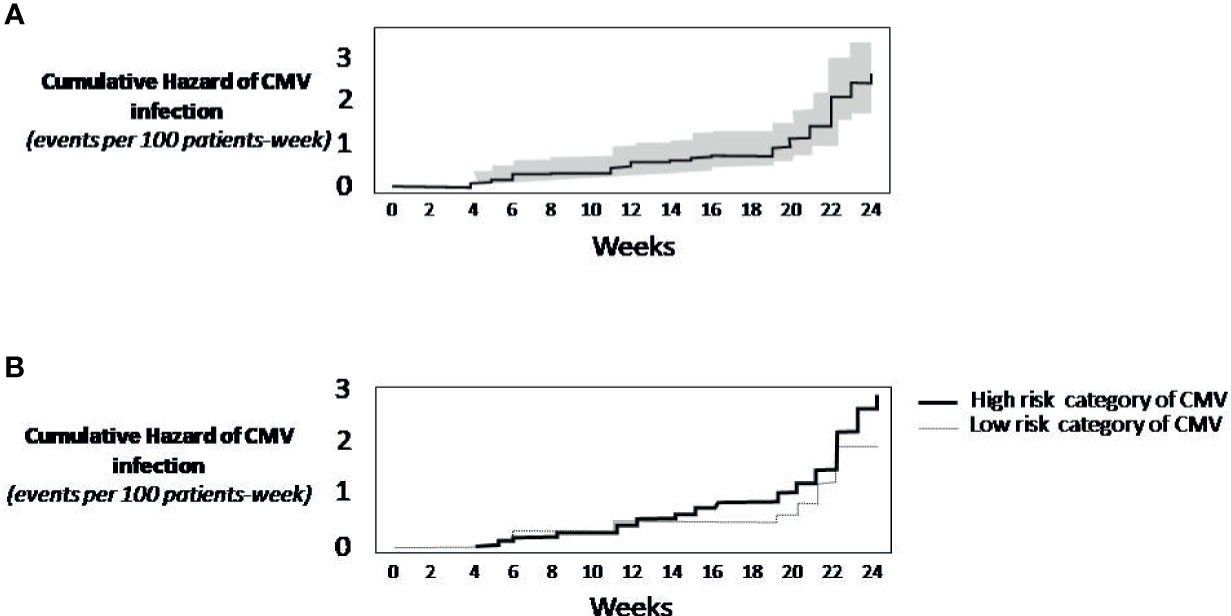
Figure 1 Kaplan-Meier Survival Analysis. (A) Cumulative rate of CMV infection (continuous line). The grey area around the continuous line represents the 95% confidence intervals. (B) Cumulative incidence of CMV infection by risk category of CMV. The analysis did not consider the competitive risk of mortality. When not considering the competing mortality risk, the practical implication is that the analysis of CMV infection censors patients who die. As this censoring is assumed to be uninformative, the resulting prognosis should be interpreted as the risk of CMV infection in a hypothetical setting in which patients do not die.
Adverse Events
Over 100 days of treatment, 40.7% of patients treated with LET experienced adverse events (AEs) (Table 3). Gastrointestinal AEs were the most frequent (36.3%), followed by skin rash (10.3%). Six cases were CMV-positive; four were CMV negative and died before 100 days. LET was discontinued in 20 patients before 100 days.
Correlates of Time to Drug Discontinuation and CMV Infection
After Bonferroni’s correction, no baseline characteristics were significantly associated with time to drug discontinuation or CMV infection at day +100 and at day +168 (Tables 4 and 5).

Table 4 Univariate logistic regression analysis of treatment discontinuation through first 100 days and through 168 days by baseline characteristics.
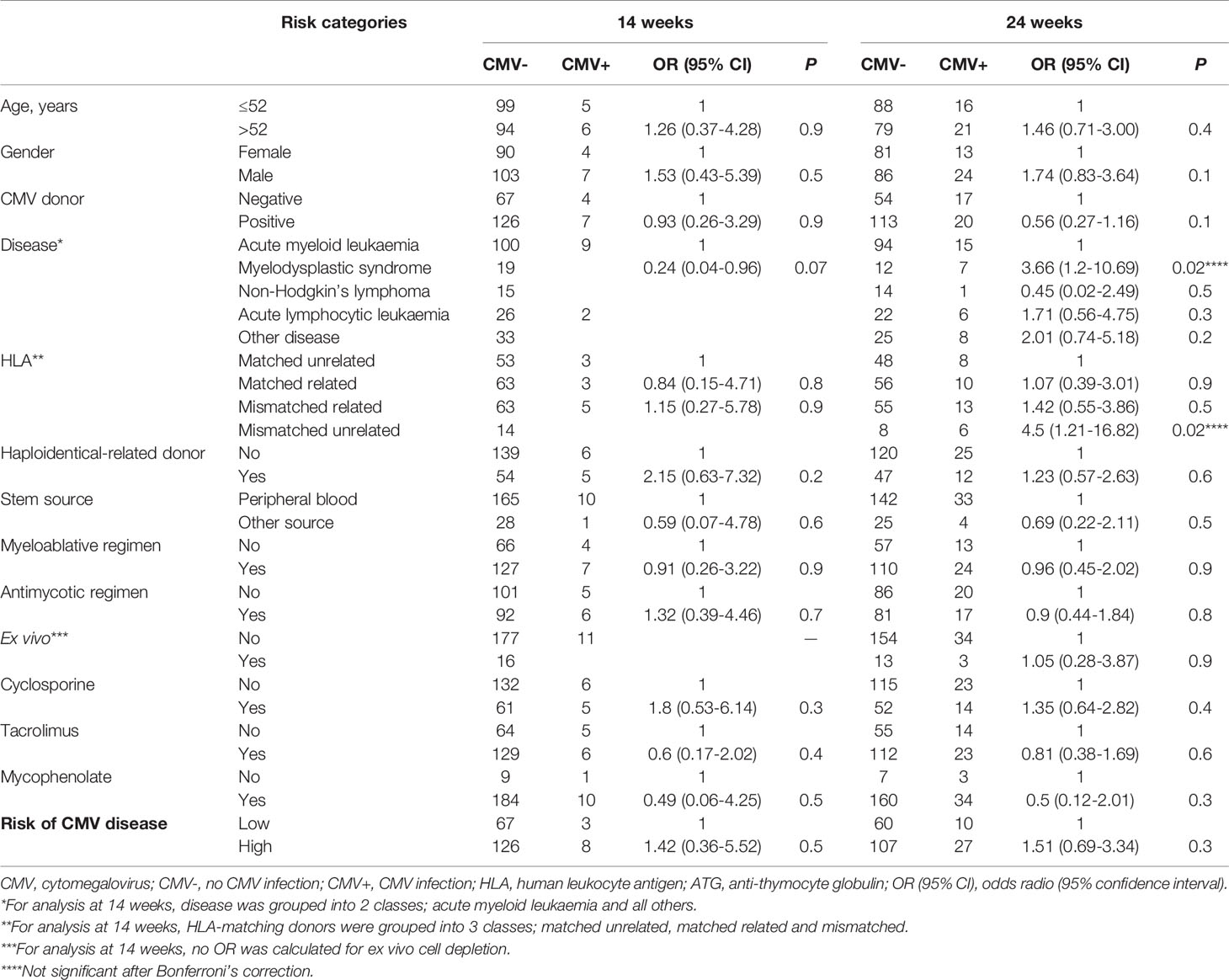
Table 5 Univariate logistic regression analysis of CMV infection through first 100 days and through 168 days by baseline characteristics.
GVHD
Incidences of acute GvHD grades II-IV by 168 days was 2% (4 patients) (Table 1). No patients experienced chronic GvHD during the period of follow-up
Survival
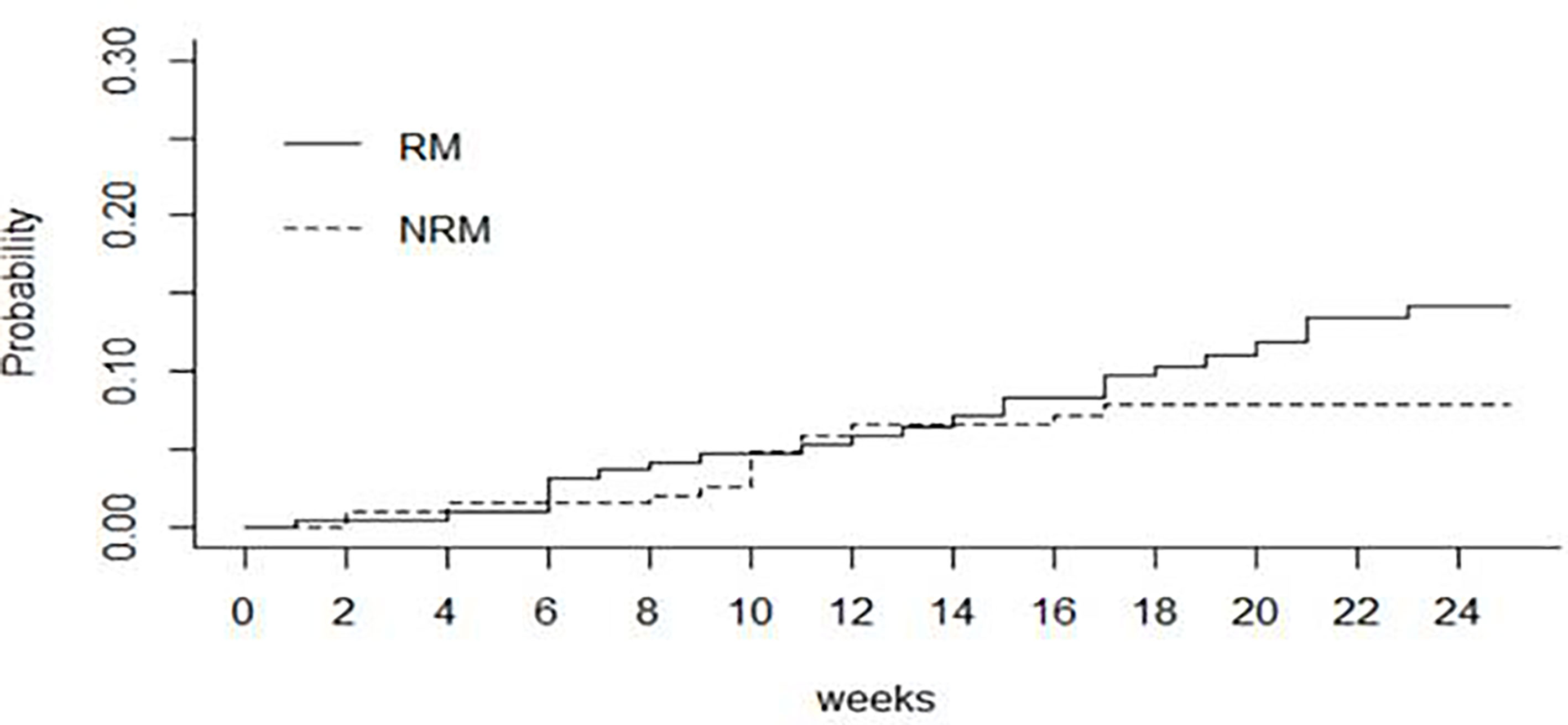
4 patients with incomplete data set for OS and EFS have been excluded from the analysis; 200 patients were eligible for survival analysis. The cumulative incidence of death was 9.9% (95% CI 5.4-14.1) at day +100 and 14.3% (95% CI 8.7-19.5) at day +168 after transplant, respectively. Among patients who died (n=24 patients),10 had relapse. Figure 2 shows the cumulative incidence of relapse mortality (RM) and non-relapse mortality (NRM), taking into account the competing risk of relapse. Overall, the cumulative incidences of NRM through the first 14 and 24 weeks were 7% (95% CI 4.0-11.0) and 8% (95% CI 4.0-12.0) respectively. The cumulative incidences of RM were 7% (95% CI 4.0-12.0) through the first 14 weeks and 14% (95% CI 9.0-20.0) through 24 weeks.
Discussion
This multicenter, retrospective, cohort study shows that the cumulative incidence of CSCI was 5.4% and 18.1% at day +100 and at day +168 after transplant, respectively. Twenty patients discontinued the trial in the first 100 days, the majority (13 patients) because of death.
CSCI has been associated with increased NRM in transplant patients (2–6). Up until the introduction of LET, no antiviral prophylaxis had proven capable of preventing CSCI in seropositive patients. In randomized studies, prophylaxis with IV ganciclovir reduced the risk of CSCI without improving survival, while high doses of aciclovir or valaciclovir reduced the risk of CMV viremia reactivation but not of CMV disease (24–28). No differences in the risk of CMV disease or in patient survival were observed between prophylaxis with ganciclovir and valaciclovir (29), or between ganciclovir prophylaxis and pre-emptive therapy (9). Prophylaxis with foscarnet has only been used in uncontrolled trials, and its prolonged use is commonly limited by toxicity (30, 31).
In a phase 3 trial, maribavir at 100 mg BID did not prevent CMV disease (32). In another phase 3 trial, brincidofovir did not reduce CSCI at week 24 and was associated with significant gastrointestinal toxicity (33). Two systematic reviews focused on the effects of antiviral prophylaxis in allo-SCT recipients (11, 12). In both analyses, none of the drugs previously described showed reduction in all-cause mortality. Moreover, IV immunoglobulins or CMV-specific immunoglobulins are not recommended for prophylaxis of CSCI (34).
In 2017, the FDA approved LET to prevent CSCI in adult allo-SCT recipients (35). In the registration trial, prophylaxis with LET was started a median of 9 days after allo-SCT and administered through week 14. It was significantly associated with lower all-cause mortality than placebo through week 24 after allo-SCT (15). Patients considered at high risk of CSCI benefitted the most from antiviral prophylaxis. However, multiple CYP3A- and OATP1B1/3-mediated drug interactions may occur, especially when LET is co-administered with cyclosporine. Interestingly, LET does not appear to have significant hematologic or extra-hematologic toxicity.
Prospective randomized clinical trials are the statistical “gold standard” to evaluate the safety and efficacy of novel therapeutic agents. However, inclusion criteria are often very stringent, and the reproducibility of their results in real-world practice remains to be confirmed. Real-world studies have become increasingly important in their role of providing evidence of safety and efficacy in larger and more representative patient populations (36). Overall, they provide physicians with important clinical findings outside the context of clinical trials. Moreover, more rigorous methodology has greatly enhanced their quality, to the point that regulatory agencies such as the FDA and EMA currently recognize their potential value (37). Agencies underline the importance of real-life research in assessing marketed products and their life cycle, including development/monitoring and regulatory decision-making.
Within the setting of CMV prophylaxis, transplant programs will have to determine whether prophylaxis with LET is associated with survival benefits that offset the risk of toxicity and justify costs. In the present study, we described the most extensive real-world experience to date of prophylaxis with LET in allo-SCT patients, highlighting the reproducibility of the safety and efficacy of this commercially available antiviral agent. Although a stringent comparison of our findings with the registration trial is not possible, it is of note that only 40% of the enrolled patients experienced low to mild AEs that were easily managed. In our real-world experience, the efficacy of prophylaxis with LET was confirmed. Despite differences from the registration study in terms of baseline patient characteristics, none were significantly associated with treatment discontinuation or CSCI. Of note, the cumulative incidence of CSCI did not differ between our study and that of the registration trial in both observation periods, i.e. 5.4% versus 7.7% within day +100, and 18.1% versus 17.5% within day +168, respectively. In particular, we did not observe an increased frequency of CSCI in patients considered at high risk of this event at baseline versus those at low risk, suggesting that prophylaxis abrogated the impact of this variable on the study endpoint. Of note, the cumulative incidence of mortality at day +100 and at day +168 was higher than that reported in the registration trial (6.4 versus 1.5% and 7.4 versus 1.8%, respectively). This was probably due to differences in baseline prognostic characteristics and/or inclusion criteria between our study and the registration trial.
Other real-world experiences have also been published. An Italian study compared 45 patients undergoing prophylaxis with LET with a retrospective cohort that did not receive prophylaxis (38). Results showed that prophylaxis was highly effective and safe in reducing the incidence of CSCI when administered from day 0 to day +100. The incidence of CSCI at day +100 was significantly lower in patients who received prophylaxis than in those who did not (8% versus 44%, respectively). In another retrospective real-world study on 80 patients, prophylaxis was started after neutrophil engraftment, around the third week after allo-SCT (39). The incidence of CSCI at day +100 was 14%, lower than in the retrospective cohort not administered LET (41%).
The strength of the present study are the sample size, that is probably the largest one published so far, assessing the real word experience of LET prophylaxis, and the multicenter characteristic, comprising 17 centers in Italy. Nevertheless, this study has some limitations: first, the absence of a control group to make a comparison led to difficult interpretation of results; second, the results confirm what has been reported in other real-life studies, without adding new clinical information; and lastly, today we know that the presence of circulating infectious CMV particles is determined by virus isolation and degradation of free-floating viral DNA. For this reason, during LET prophylaxis the clinical relevance of CMV DNAemia should be critically considered, since the presence of DNAemia during letermovir prophylaxis may not represent a real CMV reactivation, but just an abortive infection (40). We don’t have this data in the few patients with DNAemia within day 100
There are still several unanswered questions on prophylaxis with LET, including potential benefits of its extension beyond day +100. Our real-life study showed that, after discontinuation of prophylaxis at day +101, some 13% of patients experienced CMV reactivation, supporting the prolongation of its administration. Moreover, a notable increase in reactivation was observed after week +19. An ongoing phase 3 clinical trial is currently evaluating the safety and efficacy of prophylaxis with LET beyond day +100 (41), focusing on the hypothesis that prolonged prophylaxis until day +200 is superior to placebo in preventing long-term CMV reactivation.
Moreover, the use of LET can reduce NRM. In a posthoc analysis performed to investigate the effects of LET on all-cause mortality, the incidence of all-cause mortality in the LET group was similar in patients with or without clinically significant CMV infection (42). In contrast, in the placebo group, all-cause mortality was higher in patients with versus those without clinically significant CMV infection, despite the use of pre-emptive therapy for CMV infection. These results suggest that there may be a benefit to avoiding clinically significant CMV infection and potentially toxic antivirals such as ganciclovir.
There are currently scanty data on the cost-effectiveness of prophylaxis with LET (43). However, in a recent study, prophylaxis in adult patients compared with no-prophylaxis showed favourable cost-effectiveness for the Italian National Health Service (44).
In conclusion, our real-world analysis reports similar efficacy findings to those of the registration trial. However, the costs of CMV prophylaxis may be prohibitive in countries with socioeconomic healthcare issues.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Grande Ospedale Metropolitano “Bianchi-Melacrino-Morelli” of Reggio Calabria, Italy. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Study concepts: MMa, AMC, and CC. Quality control of data and algorithms: MMa, APit, GP, GT, MG, and CC. Data analysis and interpretation: MMa, APit, GP, GT, MG, and CC. Statistical analysis: APit, GT, and MG. Manuscript preparation: MMa, APit, GP, GT, MG, and CC. Manuscript editing: MMa, BB, and CC. Critical revision of the article: MMa, APit, BB, GP, GT, MG, CC, GMa and GMu. Data collection: AC, VF, APic, ST, CI, PC, DP, GMu, APa, AV, BS, GS, NM, SL, MC, LP, BL, AF, FC, EM, LG, AB, MMu, RS, NR, SM, PMa, PMu, IA, CS, FAC, and MP. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the medical and nursing staff who cared for the patients at the GITMO (Gruppo Italiano TrapiantoMidollo Osseo) Centers and provided information on the patients.
Abbreviations
AE, adverse event; allo-SCT, allogeneic stem cell transplant; AML, acute myeloid leukaemia; CMV, cytomegalovirus; CSCI, clinically significant CMV infection; GVHD, graft-versus-host disease; HLA, human leukocyte antigen; LET, letermovir; MRD, matched related; MUD, matched unrelated donor; NRM, non-relapse mortality.
References
1. Cho SY, Lee DG, Kim HJ. Cytomegalovirus Infections After Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci (2019) 20(11):2666. doi: 10.3390/ijms20112666
2. Maffini E, Busca A, Costa C, Giaccone L, Cerrano M, Curtoni A, et al. An Update on the Treatment of Cytomegalovirus Infection After Allogeneic Hematopoietic Stem Cell Transplantation. Expert Rev Hematol (2019) 12(11):937–45. doi: 10.1080/17474086.2019.1657399
3. Maffini E, Giaccone L, Festuccia M, Brunello L, Busca A, Bruno B. Treatment of CMV Infection After Allogeneic Hematopoietic Stem Cell Transplantation. Expert Rev Hematol (2016) 9(6):585–96. doi: 10.1080/17474086.2016.1174571
4. Maertens J, Lyon S. Current and Future Current and Future Options for Cytomegalovirus Reactivation in Hematopoietic Cell Transplantation Patients. Future Microbiol (2017) 12:839–42. doi: 10.2217/fmb-2017-0095
5. Jeon S, Lee WK, Lee Y, Lee DG, Lee JW. Risk Factors for Cytomegalovirus Retinitis in Patients With Cytomegalovirus Viremia After Hematopoietic Stem Cell Transplantation. Ophthalmology (2012) 119(9):1892–8. doi: 10.1016/j.ophtha.2012.03.032
6. Schmidt-Hieber M, Labopin M, Beelen D, Volin L, Ehninger G, Finke J, et al. CMV Serostatus Still has an Important Prognostic Impact in De Novo Acute Leukemia Patients After Allogeneic Stem Cell Transplantation: A Report From the Acute Leukemia Working Party of EBMT. Blood (2013) 122(19):3359–64. doi: 10.1182/blood-2013-05-499830
7. Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, et al. Early Cytomegalovirus Reactivation Remains Associated With Increased Transplant-Related Mortality in the Current Era: A CIBMTR Analysis. Blood (2016) 127(20):2427–38. doi: 10.1182/blood-2015-11-679639
8. Green ML, Leisenring W, Xie H, Mast TC, Cui Y, Sandmaier BM, et al. Cytomegalovirus Viral Load and Mortality After Haemopoietic Stem Cell Transplantation in the Era of Pre-Emptive Therapy: A Retrospective Cohort Study. Lancet Haematol (2016) 3(3):e119–27. doi: 10.1016/S2352-3026(15)00289-6
9. Boeckh M, Nichols WG. The Impact of Cytomegalovirus Serostatus of Donor and Recipient Before Hematopoietic Stem Cell Transplantation in the Era of Antiviral Prophylaxis and Preemptive Therapy. Blood (2004) 103(6):2003–8. doi: 10.1182/blood-2003-10-3616
10. Ljungman P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA, et al. Guidelines for the Management of Cytomegalovirus Infection in Patients With Haematological Malignancies and After Stem Cell Transplantation From the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis (2019) 19(8):e260–72. doi: 10.1016/S1473-3099(19)30107-0
11. Chen K, Cheng MP, Hammond SP, Einsele H, Marty FM. Antiviral Prophylaxis for Cytomegalovirus Infection in Allogeneic Hematopoietic Cell Transplantation. Blood Adv (2018) 2(16):2159–75. doi: 10.1182/bloodadvances.2018016493
12. Gagelmann N, Ljungman P, Styczynski J, Kroger N. Comparative Efficacy and Safety of Different Antiviral Agents for Cytomegalovirus Prophylaxis in Allogeneic Hematopoietic Cell Transplantation: A Systematic Review and Meta-Analysis. Biol Blood Marrow Transplant (2018) 24(10):2101–9. doi: 10.1016/j.bbmt.2018.05.017
13. Reefschlaeger J, Bender W, Hallenberger S, Weber O, Eckenberg P, Goldmann S, et al. Novel Non-Nucleoside Inhibitors of Cytomegaloviruses (BAY 38-4766): In Vitro and In Vivo Antiviral Activity and Mechanism of Action. J Antimicrob Chemother (2001) 48(6):757–67. doi: 10.1093/jac/48.6.757
14. Weber O, Bender W, Eckenberg P, Goldmann S, Haerter M, Hallenberger S, et al. Inhibition of Murine Cytomegalovirus and Human Cytomegalovirus by a Novel Non-Nucleosidic Compound In Vivo. Antiviral Res (2001) 49(3):179–89. doi: 10.1016/s0166-3542(01)00127-9
15. Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med (2017) 377(25):2433–44. doi: 10.1056/NEJMoa1706640
16. Baynes R. FDA Approves Letermovir for CMV Prophylaxis Post-Transplantation. November 9, 2017. Available at: https://www.onclive.com/view/fda-approves-letermovir-for-cmv-prophylaxis-posttransplantation.
17. U.S. Food and Drug Administration (FDA). Advancing Health Trough Innovation. New Drug Therapy Approvals 2017 (2018). Available at: https://www.fda.gov/files/about%20fda/published/2017-New-Drug-Therapy-Approvals-Report.pdf.
18. Girmenia C, Lazzarotto T, Bonifazi F, Patriarca F, Irrera G, Ciceri F, et al. Assessment and Prevention of Cytomegalovirus Infection in Allogeneic Hematopoietic Stem Cell Transplant and in Solid Organ Transplant: A Multidisciplinary Consensus Conference by the Italian GITMO, SITO, and AMCLI Societies. Clin Transplant (2019) 33(10):e13666. doi: 10.1111/ctr.13666
19. Sidoti F, Piralla A, Costa C, Scarasciulli ML, Calvario A, Conaldi PG, et al. Collaborative National Multicenter for the Identification of Conversion Factors From Copies/mL to Internationalunits/mL for the Normalization of HCMV DNA Load. Diagn Microbiol Infect Dis (2019) 95(2):152–8. doi: 10.1016/j.diagmicrobio.2019.05.012
20. Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the Intensity of Conditioning Regimens: Working Definitions. Biol Blood Marrow Transplant (2009) 15(12):1628–33. doi: 10.1016/j.bbmt.2009.07.004
21. Gratwohl A, Brand R, McGrath E, van Biezen A, Sureda A, Ljungman P, et al. Use of the Quality Management System “JACIE” and Outcome After Hematopoietic Stem Cell Transplantation. Haematologica (2014) 99(5):908–15. doi: 10.3324/haematol.2013.096461
22. Ljungman P, Griffiths P, Paya C. Definitions of Cytomegalovirus Infection and Disease in Transplant Recipients. Clin Infect Dis (2002) 34(8):1094–7. doi: 10.1086/339329
23. Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Statist (1988) 16(3):1141–54. doi: 10.1214/aos/1176350951
24. Prentice HG, Gluckman E, Powles RL, Ljungman P, Milpied N, Fernandez Rañada JM, et al. Impact of Long-Term Acyclovir on Cytomegalovirus Infection and Survival After Allogeneic Bone Marrow Transplantation. European Acyclovir for CMV Prophylaxis Study Group. Lancet (1994) 343(8900):749–53. doi: 10.1016/s0140-6736(94)91835-x
25. Ljungman P, de la Camara R, Milpied N, Volin L, Russell CA, Crisp A, et al. Randomized Study of Valacyclovir as Prophylaxis Against Cytomegalovirus Reactivation in Recipients of Allogeneic Bone Marrow Transplants. Blood (2002) 99(8):3050–6. doi: 10.1182/blood.v99.8.3050
26. Goodrich JM, Bowden RA, Fisher L, Keller C, Schoch G, Meyers JD. Ganciclovir Prophylaxis to Prevent Cytomegalovirus Disease After Allogeneic Marrow Transplant. Ann Intern Med (1993) 118(3):173–8. doi: 10.7326/0003-4819-118-3-199302010-00003
27. Winston DJ, Ho WG, Bartoni K, Du Mond C, Ebeling DF, Buhles WC, et al. Ganciclovir Prophylaxis of Cytomegalovirus Infection and Disease in Allogeneic Bone Marrow Transplant Recipients. Results of a Placebo-Controlled, Double-Blind Trial. Ann Intern Med (1993) 118(3):179–84. doi: 10.7326/0003-4819-118-3-199302010-00004
28. Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus Pp65 Antigenemia-Guided Early Treatment With Ganciclovir Versus Ganciclovir at Engraftment After Allogeneic Marrow Transplantation: A Randomized Double-Blind Study. Blood (1996) 88(10):4063–71. doi: 10.1182/blood.V88.10.4063.bloodjournal88104063
29. Winston DJ, Yeager AM, Chandrasekar PH, Snydman DR, Petersen FB, Territo MC, et al. Randomized Comparison of Oral Valacyclovir and Intravenous Ganciclovir for Prevention of Cytomegalovirus Disease After Allogeneic Bone Marrow Transplantation. Clin Infect Dis (2003) 36(6):749–58. doi: 10.1086/367836
30. Bacigalupo A, Tedone E, Van Lint MT, Trespi G, Lonngren M, Sanna MA, et al. CMV Prophylaxis With Foscarnet in Allogeneic Bone Marrow Transplant Recipients at High Risk of Developing CMV Infections. Bone Marrow Transplant (1994) 13(6):783–8.
31. Bregante S, Bertilson S, Tedone E, Van Lint MT, Trespi G, Mordini N, et al. Foscarnet Prophylaxis of Cytomegalovirus Infections in Patients Undergoing Allogeneic Bone Marrow Transplantation (BMT): A Dose-Finding Study. Bone Marrow Transplant (2000) 26(1):23–9. doi: 10.1038/sj.bmt.1702450
32. Marty FM, Ljungman P, Papanicolaou GA, Winston DJ, Chemaly RF, Strasfeld L, et al. Maribavir Prophylaxis for Prevention of Cytomegalovirus Disease in Recipients of Allogeneic Stem-Cell Transplants: A Phase 3, Double-Blind, Placebo-Controlled, Randomised Trial. Lancet Infect Dis (2011) 11(4):284–92. doi: 10.1016/S1473-3099(11)70024-X
33. Marty FM, Winston DJ, Chemaly RF, Mullane KM, Shore TB, Papanicolaou GA, et al. A Randomized, Double-Blind, Placebo-Controlled Phase 3 Trial of Oral Brincidofovir for Cytomegalovirus Prophylaxis in Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant (2019) 25(2):369–81. doi: 10.1016/j.bbmt.2018.09.038
34. Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. Immunoglobulin Prophylaxis in Hematopoietic Stem Cell Transplantation: Systematic Review and Meta-Analysis. J Clin Oncol (2009) 27(5):770–81. doi: 10.1200/JCO.2008.16.8450
35. U.S. Food & Drug Administration. PREVYMIS (Letermovir) Tablets and PREVYMIS (Letermovir) Injection. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209939Orig1s000,209940Orig1s000TOC.cfm.
36. Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and Impact of Real-World Clinical Data for the Practicing Clinician. Adv Ther (2018) 35(11):1763–74. doi: 10.1007/s12325-018-0805-y
37. U.S. Food & Drug Administration. Real-World Data (RWD) and Real-World Evidence (RWE) Are Playing an Increasing Role in Health Care Decisions. Available at: https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence.
38. Malagola M, Pollara C, Polverelli N, Zollner T, Bettoni D, Gandolfi L, et al. Advances in CMV Management: A Single Center Real-Life Experience. Front Cell Dev Biol (2020) 8:534268. doi: 10.3389/fcell.2020.534268
39. Derigs P, Radujkovic A, Schubert ML, Schnitzler P, Schöning T, Müller-Tidow C, et al. Letermovir Prophylaxis Is Effective in Preventing Cytomegalovirus Reactivation After Allogeneic Hematopoietic Cell Transplantation: Single-Center Real-World Data. Ann Hematol (2021) 100(8):2087–93. doi: 10.1007/s00277-020-04362-2
40. Cassaniti I, Colombo AA, Bernasconi P, Malagola M, Russo D, Iori AP, et al. Positive HCMV DNAemia in Stem Cell Recipients Undergoing Letermovir Prophylaxis Is Expression of Abortive Infection. Am J Transplant (2021) 21(4):1622–8. doi: 10.1111/ajt.16450
41. Clinical trial. Extension of Letermovir (LET) From Day 100 to Day 200 Post-Transplant for the Prevention of Cytomegalovirus (CMV) Infection in Hematopoietic Stem Cell Transplant (HSCT) Participants (MK-8228-040). Available at: https://clinicaltrials.gov/ct2/show/NCT03930615.
42. Ljungman P, Schmitt M, Marty FM, Maertens J, Chemaly RF, Kartsonis NA, et al. A Mortality Analysis of Letermovir Prophylaxis for Cytomegalovirus (CMV) in CMV-Seropositive Recipients of Allogeneic Hematopoietic Cell Transplantation. Clin Infect Dis (2020) 70(8):1525–33. doi: 10.1093/cid/ciz490
43. Chan TS, Cheng SS, Chen WT, Hsu DC, Chau RW, Kang SH, et al. Cost-Effectiveness of Letermovir as Cytomegalovirus Prophylaxis in Adult Recipients of Allogeneic Hematopoietic Stem Cell Transplantation in Hong Kong. J Med Econ (2020) 23(12):1485–92. doi: 10.1080/13696998.2020.1843321
44. Restelli U, Croce D, Pacelli V, Ciceri F, Girmenia C. Cost-Effectiveness Analysis of the Use of Letermovir for the Prophylaxis of Cytomegalovirus in Adult Cytomegalovirus Seropositive Recipients Undergoing Allogenic Hematopoietic Stem Cell Transplantation in Italy. Infect Drug Resist (2019) 12:1127–38. doi: 10.2147/IDR.S196282
Keywords: cytomegalovirus infection, allogeneic stem cell transplantation, prophylaxis, real-world data, Letermovir
Citation: Martino M, Pitino A, Gori M, Bruno B, Crescimanno A, Federico V, Picardi A, Tringali S, Ingrosso C, Carluccio P, Pastore D, Musuraca G, Paviglianiti A, Vacca A, Serio B, Storti G, Mordini N, Leotta S, Cimminiello M, Prezioso L, Loteta B, Ferreri A, Colasante F, Merla E, Giaccone L, Busca A, Musso M, Scalone R, Di Renzo N, Marotta S, Mazza P, Musto P, Attolico I, Selleri C, Canale FA, Pugliese M, Tripepi G, Porto G, Martinelli G, Carella AM Jr. and Cerchione C (2021) Letermovir Prophylaxis for Cytomegalovirus Infection in Allogeneic Stem Cell Transplantation: A Real-World Experience. Front. Oncol. 11:740079. doi: 10.3389/fonc.2021.740079
Received: 12 July 2021; Accepted: 13 August 2021;
Published: 06 September 2021.
Edited by:
Massimo Gentile, Health Agency of Cosenza, ItalyReviewed by:
Shin Mineishi, Penn State Milton S. Hershey Medical Center, United StatesJacopo Mariotti, Humanitas Research Hospital, Italy
Copyright © 2021 Martino, Pitino, Gori, Bruno, Crescimanno, Federico, Picardi, Tringali, Ingrosso, Carluccio, Pastore, Musuraca, Paviglianiti, Vacca, Serio, Storti, Mordini, Leotta, Cimminiello, Prezioso, Loteta, Ferreri, Colasante, Merla, Giaccone, Busca, Musso, Scalone, Di Renzo, Marotta, Mazza, Musto, Attolico, Selleri, Canale, Pugliese, Tripepi, Porto, Martinelli, Carella and Cerchione. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimo Martino, massimo.martino@ospedalerc.it; Claudio Cerchione, claudio.cerchione@irst.emr.it
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Massimo Martino
Massimo Martino Annalisa Pitino2†
Annalisa Pitino2† Benedetto Bruno
Benedetto Bruno Alessandra Picardi
Alessandra Picardi Stefania Tringali
Stefania Tringali Gerardo Musuraca
Gerardo Musuraca Salvatore Leotta
Salvatore Leotta Barbara Loteta
Barbara Loteta Luisa Giaccone
Luisa Giaccone Alessandro Busca
Alessandro Busca Nicola Di Renzo
Nicola Di Renzo Pellegrino Musto
Pellegrino Musto Carmine Selleri
Carmine Selleri Filippo Antonio Canale
Filippo Antonio Canale Giovanni Tripepi
Giovanni Tripepi Giovanni Martinelli
Giovanni Martinelli Claudio Cerchione
Claudio Cerchione