- 1Department of Laboratory Medicine, West China Hospital of Sichuan University, Chengdu, China
- 2Department of Liver Surgery, Liver Transplantation Center, West China Hospital of Sichuan University, Chengdu, China
CD35, an important molecule implicated in inflammation and immunity, is reportedly associated with several cancers. However, very few studies have investigated the relationship between CD35 polymorphisms and hepatocellular carcinoma (HCC). The current study was conducted to investigate the association between tag SNPs in CD35 and HCC susceptibility and postoperative recurrence, in an attempt to elucidate the gene-environment interactions in HCC. A total of 1233 Chinese Han people, including 647 healthy controls and 586 HCC cases, were sampled in this study. Six Tag SNPs (rs10494885, rs2296160, rs3737002, rs3849266, rs669117, and rs7525160) of CD35 were selected using the HaploView 4.2 program and genotyped by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS). Overall, the mutation genotypes CC/CG of CD35 rs7525160 significantly increased the risk of HCC. Stratification analysis indicated that CD35 rs7525160 CC/CG genotypes increased HCC risk in patients younger than 65 years and were closely related to the pathological type of poor prognosis of HCC. Cox proportional hazard ratio model analysis revealed that the rs7525160 CC/CG genotype remains a significant independent risk factor for postoperative recurrence of HCC. In conclusion, CD35 rs7525160 polymorphism may contribute to the susceptibility and prognosis of HCC in the Chinese Han population.
Introduction
Hepatocellular carcinoma (HCC), which accounts for more than 80% of primary liver cancers worldwide, is estimated to be the fourth most common cause of cancer-related deaths, and poses a severe disease burden (1, 2). Liver cancer has long dominated all malignancies in China (3), and the cases from China constituted over half of the liver cancer cases (446,100 cases) and deaths (422,100 cases) worldwide in 2015 (4). A multitude of risk factors, including viral factors (HBV and HCV) and non-viral factors, such as alcohol, smoking, aflatoxin, obesity, diabetes, and non-alcoholic fatty liver disease, have been found to be associated with liver cancer (5–9). However, these known risk factors do not fully explain the overall incidence of HCC. Numerous studies have identified many genetic factors that are correlated with HCC susceptibility (10–13). This evidence indicates the consequential role played by genetic backgrounds in the etiology of HCC. Therefore, a better understanding of the molecular mechanisms underlying the initiation and progression of HCC may lead to earlier diagnoses and efficacious therapeutic strategies (14).
The complement system, which has been primarily viewed as the “first line of defense” against microbial intruders, participates in diverse processes, such as clearance of immune complexes, tissue regeneration, and lipid metabolism (15). However, several studies have indicated that the complement system may also promote tumor development in a diverse manner, including sustained cellular proliferation, decreased apoptosis, enhanced invasion, and metastasis (16–18). The liver is the organ, which plays a predominant role in the functioning of the complement system (19). This, in turn, leads to a variety of liver associated effects, such as liver damage, regeneration, and liver transplantation (20).
Complement regulatory proteins comprise a large family that is essential for activation of the complement system (21). One of its members, termed complement receptor 1 (CR1/CD35; hereafter named CD35), is strikingly significant because it interacts not only with C3b and C4b to promote neutrophil-mediated phagocytosis but also plays a role in T-cell and B-cell mediated immune regulation (22, 23). Due to its role in complement activation, innate immunity, and chronic inflammation, CD35 has become a trending molecule in cancer predisposition studies, as attested to by studies that have been conducted on gallbladder cancer, nasopharyngeal carcinoma, non-small cell lung cancer, and gastric cancer (24–27).
However, very few studies have investigated the relationship between CD35 polymorphisms and HCC predisposition. Therefore, the current case-control study was conducted to investigate the association between tag SNPs in CD35 and susceptibility to and recurrence of HCC, in an attempt to explore the effect of gene-environment interaction on the risk for HCC.
Materials and Methods
This study strictly followed all relevant Chinese laws, regulations and rules, as well as the stipulations of the Declaration of Helsinki of the World Medical Association, and was approved by the Ethics Committee of Sichuan University (Ethics No. 2017-264).
Study Population
Newly diagnosed, untreated HCC patients admitted to the Department of Liver Surgery of the West China Hospital in Sichuan University (Chengdu, China), between May 2017 and March 2019, were enrolled in this study. Diagnosis of HCC was based on histological findings or a combination of elevated serum α-fetoprotein (AFP) and coincident imaging manifestations from two different imaging based diagnostic methods (ultrasonography, computed tomography, or magnetic resonance imaging). Staging criteria for liver cancer were based on Barcelona-Clinic-Liver-Cancer (BCLC) stages (28) and the tumor–node–metastasis (TNM) classification system (29). None of the patients had received radiotherapy or anticancer cytotoxic drug chemotherapy one month prior to blood collection. Patients with previous malignancies or metastasized cancers in other organs, autoimmune diseases, Alzheimer’s disease (AD), and HCC combined pregnancy were excluded. Those who had undergone laparoscopic cholecystectomy for gallstones in the West China Hospital during the same period and had no history of other diseases, tumors, autoimmune diseases or Alzheimer’s disease, were included as healthy controls. All subjects were genetically unrelated Han Chinese, and there were no age or gender restrictions.
Written informed consent was obtained from each participant during recruitment. Baseline characteristics of each subject, such as age, sex, smoking history, drinking history, and family history of HCC, were obtained from the subjects, or their family members, and relevant information on HCC patients, such as tumor size, number of tumors, metastasis, Child–Pugh classification, and detection of microvascular invasion, was further improved by referring to medical records, laboratory tests, and imaging studies.
Selection of Tag SNPs and Genotype Analysis
SNP genotyping of CD35 was downloaded from the Human Genome Project and the International HapMap Project. HaploView 4.2 was used to select candidate tag SNPs of CD35 with an r2 threshold of 0.8, and minor allele frequency (MAF) greater than or equal to 0.05, based on Chinese population data from the HapMap database. Finally, six SNPs (rs10494885, rs2296160, rs3737002, rs3849266, rs669117, and rs7525160) were included in this study (Supplementary Table S1).
Genomic DNA was extracted from whole blood samples using EDTA anticoagulant via a TIANamp Genomic DNA Kit (Tiangen Biotech Co. Ltd., Beijing, China) strictly according to the manufacturer’s instructions. All extracted DNA specimens were diluted to 20 ng/μL in order to obtain a working concentration and stored at -20°C.
Genotyping was carried out using the MassARRAY system (Sequenom, San Diego, CA, USA) via the matrix-assisted laser desorption ionization-time of flight mass spectrometry method (MALDI-TOF), according to the manufacturer’s instructions. The detailed genotyping process is described in another study of ours (12). Primer information for selected tag SNPs is shown (Supplementary Table S2). Genotyping quality control was performed by verifying genotypic consistency in 5% of samples which were randomly selected and duplicated, while approximately another 5% of the samples were further confirmed via direct sequencing.
HCC Surveillance and Follow-Up
All HCC patients who underwent hepatectomy were regularly followed up in the Outpatient Department of Liver Surgery of the West China Hospital in Sichuan University. During the first year after surgery, the patients were followed up every three months, and the patients with no recurrence for at least 2 years following surgery were followed up every six months instead. Abdominal ultrasonography and blood tests, including liver function tests and AFP levels, were performed at each follow-up. Any subject with a positive finding of AFP or abnormal ultrasonography was subjected to enhanced computed tomography (CT) or magnetic resonance imaging (MRI) of the upper abdomen immediately. Recurrence-free survival (RFS) was defined as the time interval from the date of hepatectomy to the date of recurrence or the deadline for the last follow-up. Overall survival (OS) was defined as the time interval from the date of hepatectomy to the date of death or the last follow-up.
Statistical Analysis
Statistical analysis was performed using SPSS (version 24.0; IBM Corp., Armonk, NY, USA). All tests were two-tailed, and statistical significance was set at P<0.05. Each SNP frequency in controls was analyzed for deviation from the Hardy-Weinberg equilibrium (HWE) using the chi-squared goodness-of-fit test. Mann-Whitney U test (for continuous variables) and chi-square tests with Fisher’s exact test (for categorical variables) were used to compare differences in the distributions of demographic characteristics between the HCC and control groups. The association between HCC risk and CD35 tag SNPs was estimated as odds ratios (OR) and 95% confidence intervals (CI) using a logistic regression model, adjusted by sex, HBV status, smoking, and drinking status where it was appropriate. The relationship between RFS/OS and CD35 tag SNPs was analyzed using univariate survival analysis and the Cox proportional hazard ratio model. Family history of liver cancer is defined as family members within three generations of patients with liver cancer. Ever/current smoking is defined as continuous/cumulative smoking for 6 months or more in a lifetime, and consumption of at least 100 cigarettes. Those who had consumed 12 standard drinking amounts (1 standard drinking amount is equal to 10 g of alcohol, approximately 350 ml of regular beer, or about 148 ml of wine, or about 45 ml of 40% white wine) in the past 12 months were defined as ever/current drinkers (30).
Results
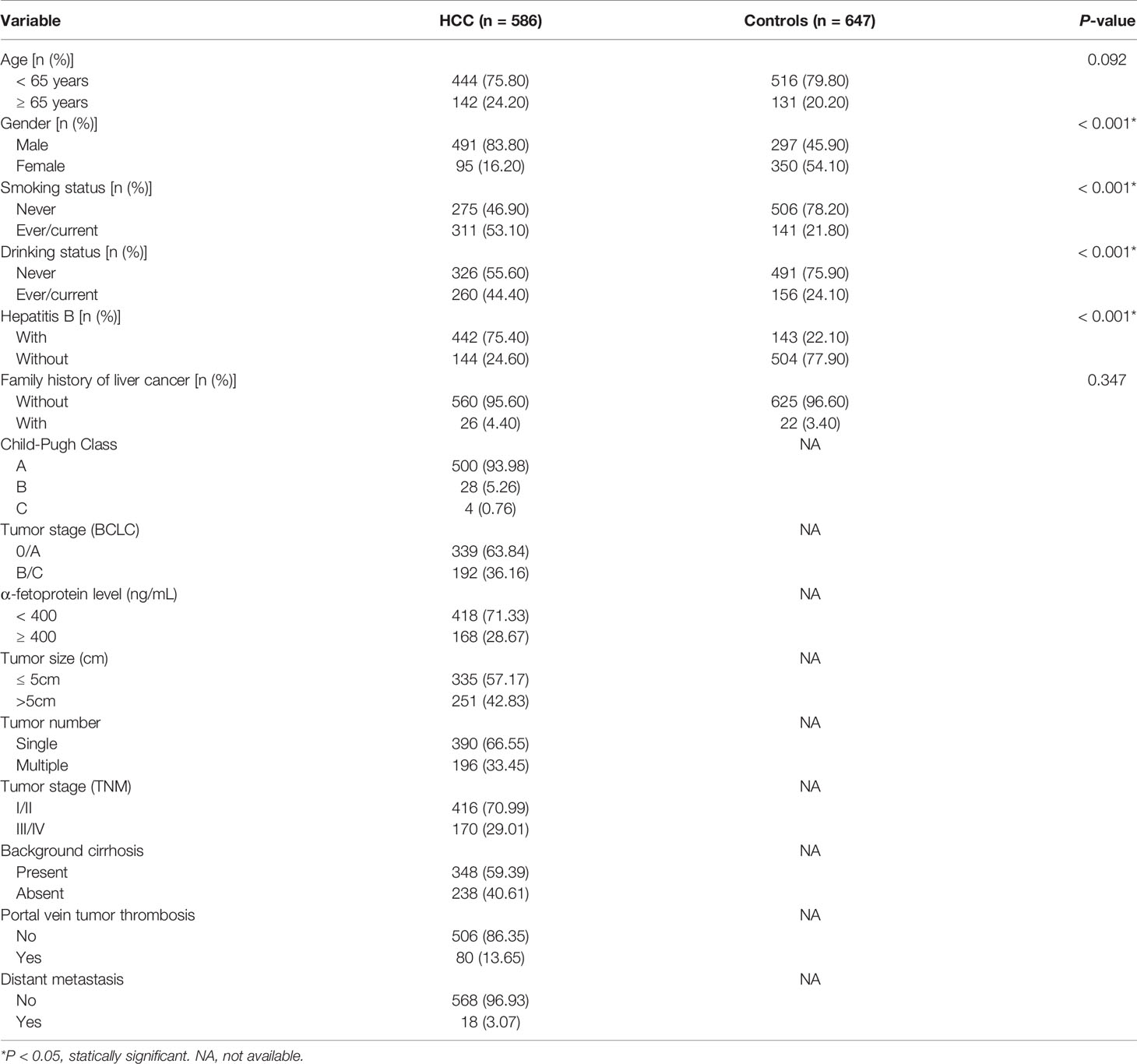
Patient Characteristics
Initially, 600 HCC cases and 650 cancer-free controls were included in this study. Due to unsuccessful genotyping of 14 HCC cases and 3 controls, a total of 1233 participants consisting of 586 HCC patients (491 men and 95 women) and 647 controls (297 men and 350 women) remained. Baseline characteristics of the HCC cases and controls are summarized (Table 1). While there were no significant differences in age (stratified by 65 years) and family history of liver cancer, there were significant differences between gender, hepatitis B, smoking, and drinking status of the two groups (P<0.05). A total of 299 HCC patients underwent hepatectomy treatment and were followed up to December 2019, the maximum follow-up time being 30.7 months and a medium follow-up time of 18.0 months. The characteristics and clinical features of HCC patients treated with hepatectomy are summarized (Supplementary Table S3).
Genotype Frequency and Effects of CD35 on HCC Risk
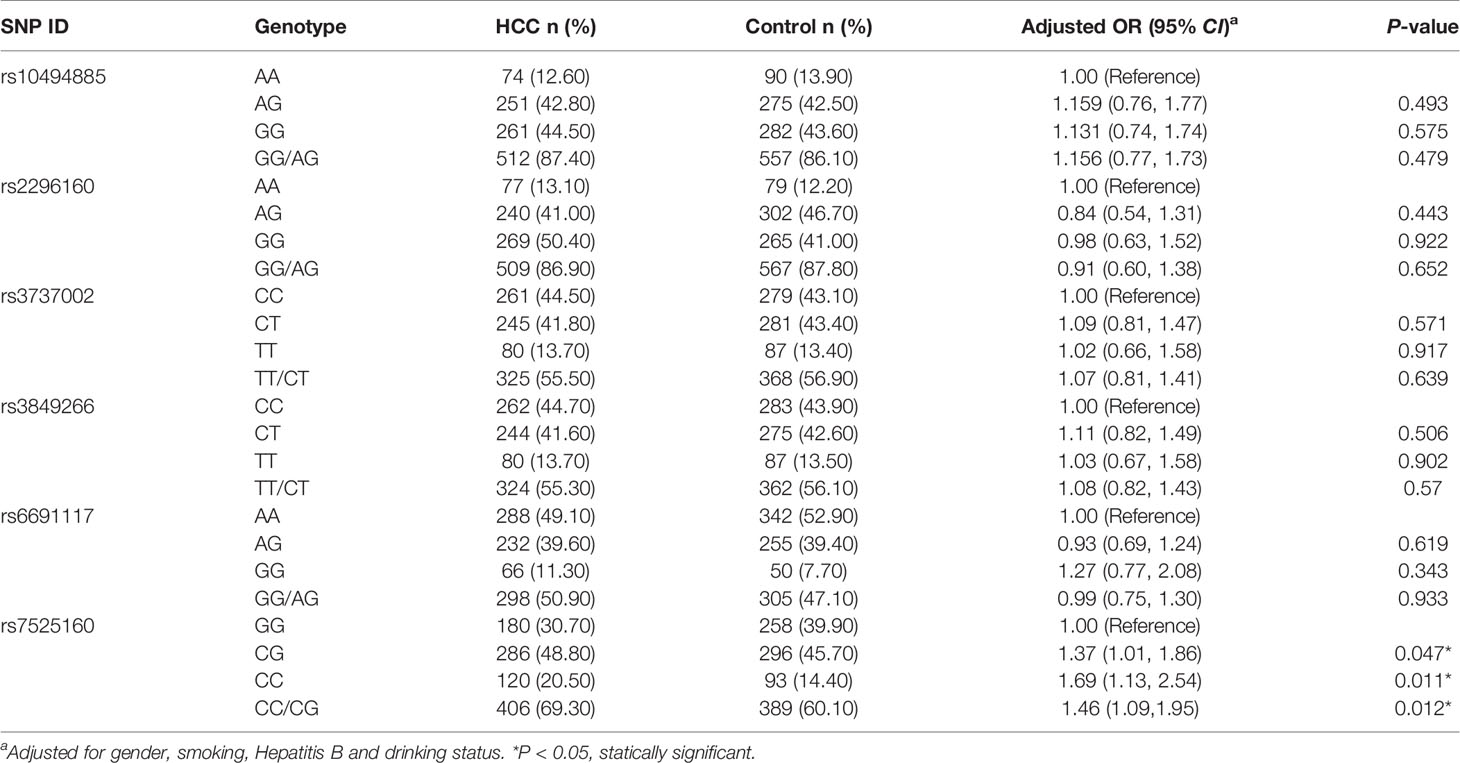
The genotype distribution of CD35 in controls followed Hardy-Weinberg equilibrium predictions (Supplementary Table S1; P>0.05). Among the six SNPs of CD35, only the frequencies of GG, CG, and CC genotypes of rs7525160 in HCC and control groups were statistically significant (P<0.05; Table 2). After adjusting for sex, smoking, hepatitis B, and drinking status, the risk of HCC was 1.46-times higher in individuals with at least one mutant allele C (CG+CC) than in individuals with the GG genotype (adjusted OR=1.46; 95% CI [1.09-1.95]; P=0.012); (Table 2). No significant differences were observed between the distribution of rs10494885, rs2296160, rs3737002, rs3849266, and rs6691117 genotypes of the HCC and control groups (P>0.05; Table 2). The three genotypes of rs7525160 polymorphisms detected by MALDI-TOF and the sequencing map for CD35 rs7525160 GG and CG genotypes are summarized (Supplementary Figure S1).
Stratified Analysis of Demographic Characteristics and Environmental Factors
We analyzed the polymorphism of CD35 rs7525160 and HCC risk, based on factors including demographic characteristics (gender, age) and environmental factors (hepatitis B, family history of liver cancer, drinking, and smoking status); (Supplementary Table S4). Stratified analysis indicated that the CD35 rs7525160 CC/CG genotype increased the risk of HCC in patients younger than 65 years (P for interaction=0.042; adjusted OR=1.85; 95% CI [1.30–2.62]; P=0.001).
Genotype Frequency and Effects on Tumor Clinicopathological Types
Further stratified analysis of the clinical characteristics of HCC patients revealed that the CD35 rs7525160 CC/CG genotype may increase the risk of different clinicopathological types of HCC, especially among patients with AFP ≥ 400 ng/ml [adjusted OR=1.94; 95% CI (1.24–3.04); P=0.004], tumor size > 5 cm [adjusted OR=1.75; 95% CI (1.19–2.56); P=0.004], TNM stage III/IV [adjusted OR=1.64; 95% CI (1.07–2.52); P=0.024], background cirrhosis [adjusted OR=1.50; 95% CI (1.05–2.15); P=0.026], or portal vein tumor thrombosis [adjusted OR=2.30; 95% CI (1.24–4.27); P=0.008] (Supplementary Table S5).
CD35 Genetic Variation on Postoperative Recurrence of HCC and OS Analysis
As mentioned above, CD35 rs7525160 resulted in increased HCC risk, especially in advanced stage or big tumors, suggesting its potential predictive value in prognosis. To evaluate this, we detected the effects of six CD35 SNP genotypes on the recurrence rate and mean recurrence-free survival (MRFS) of 299 HCC patients who underwent curative hepatectomy. Patients who were treated with transcatheter arterial chemoembolization (TACE), radiofrequency ablation, or drugs only were excluded to eliminate the influence of different treatments on the prognosis of HCC. Kaplan-Meier analyses showed that the MRFS was 17.82 months with 95% CI of 16.104–19.544 months for CD35 rs7525160 CC/CG individuals, which was shorter than that for individuals carrying the GG genotype (MRFS 22.05 months; 95% CI [19.819–24.289 months]; P=0.0073); (Figure 1).
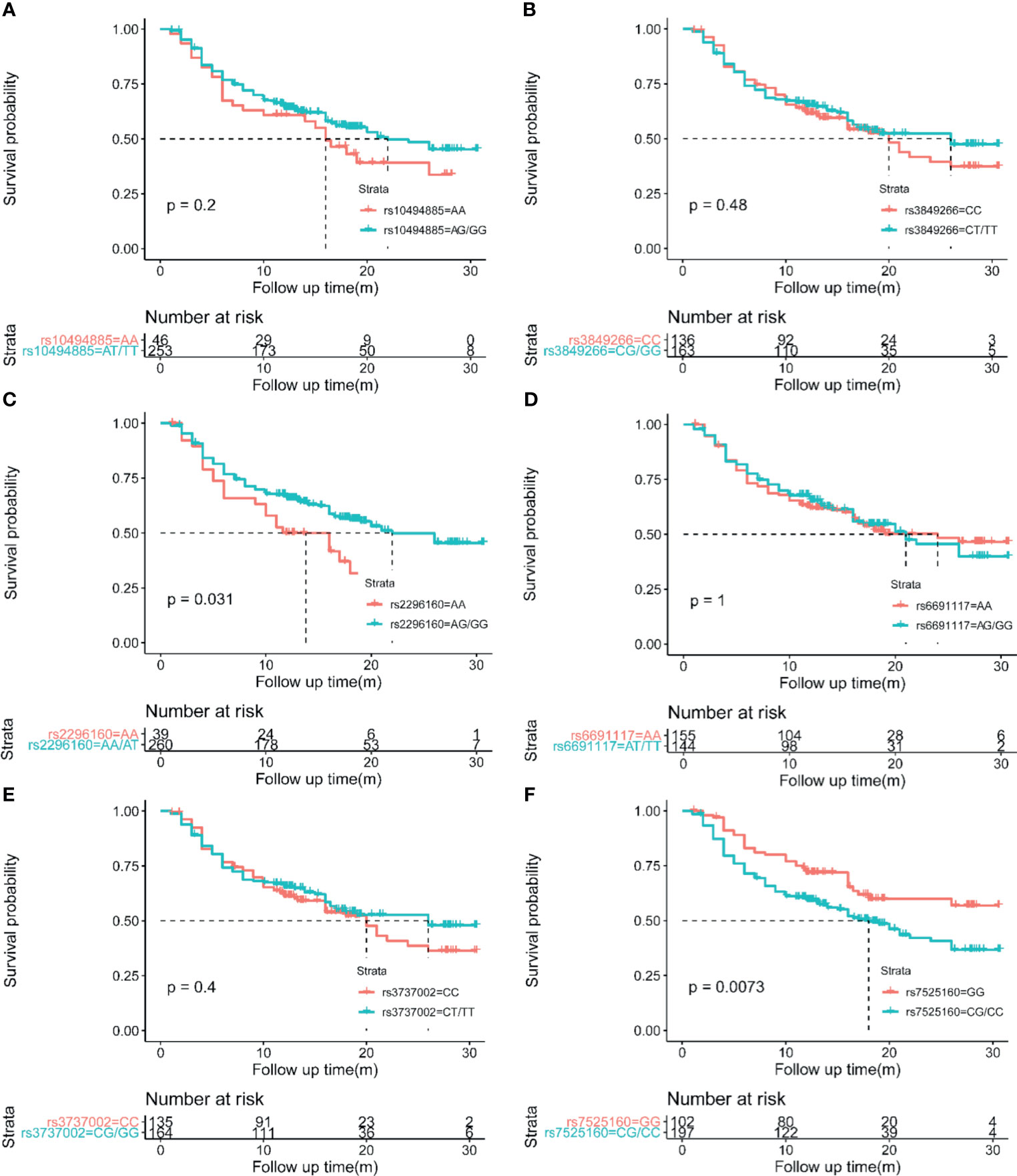
Figure 1 Kaplan-Meier analyses of CD35 genetic variation on postoperative HCC recurrence. (A) CD35 rs10494885. (B) CD35 rs3849266. (C) CD35 rs2296160. (D) CD35 rs6691117. (E) CD35 rs3737002. (F) CD35 rs7525160.
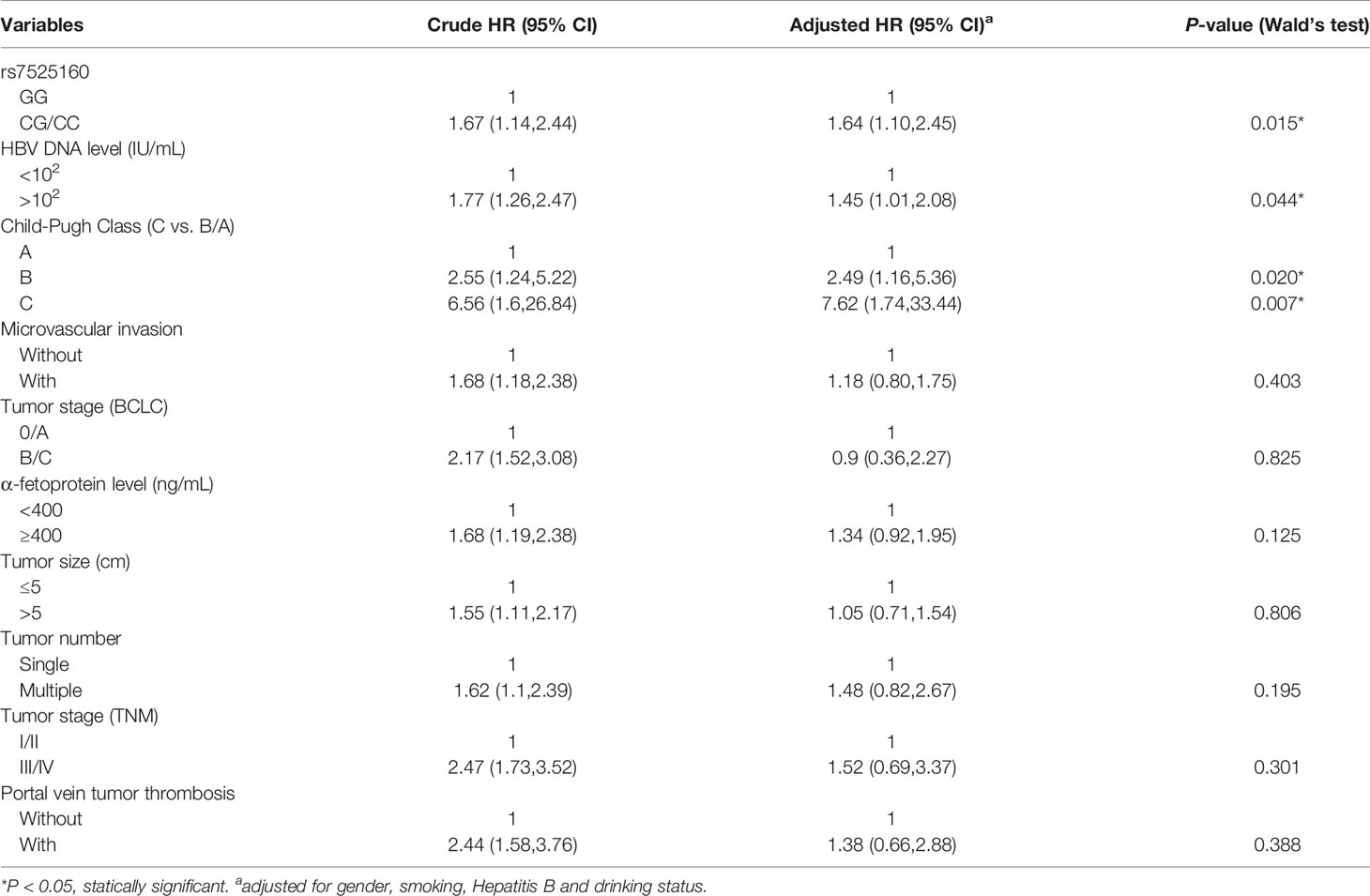
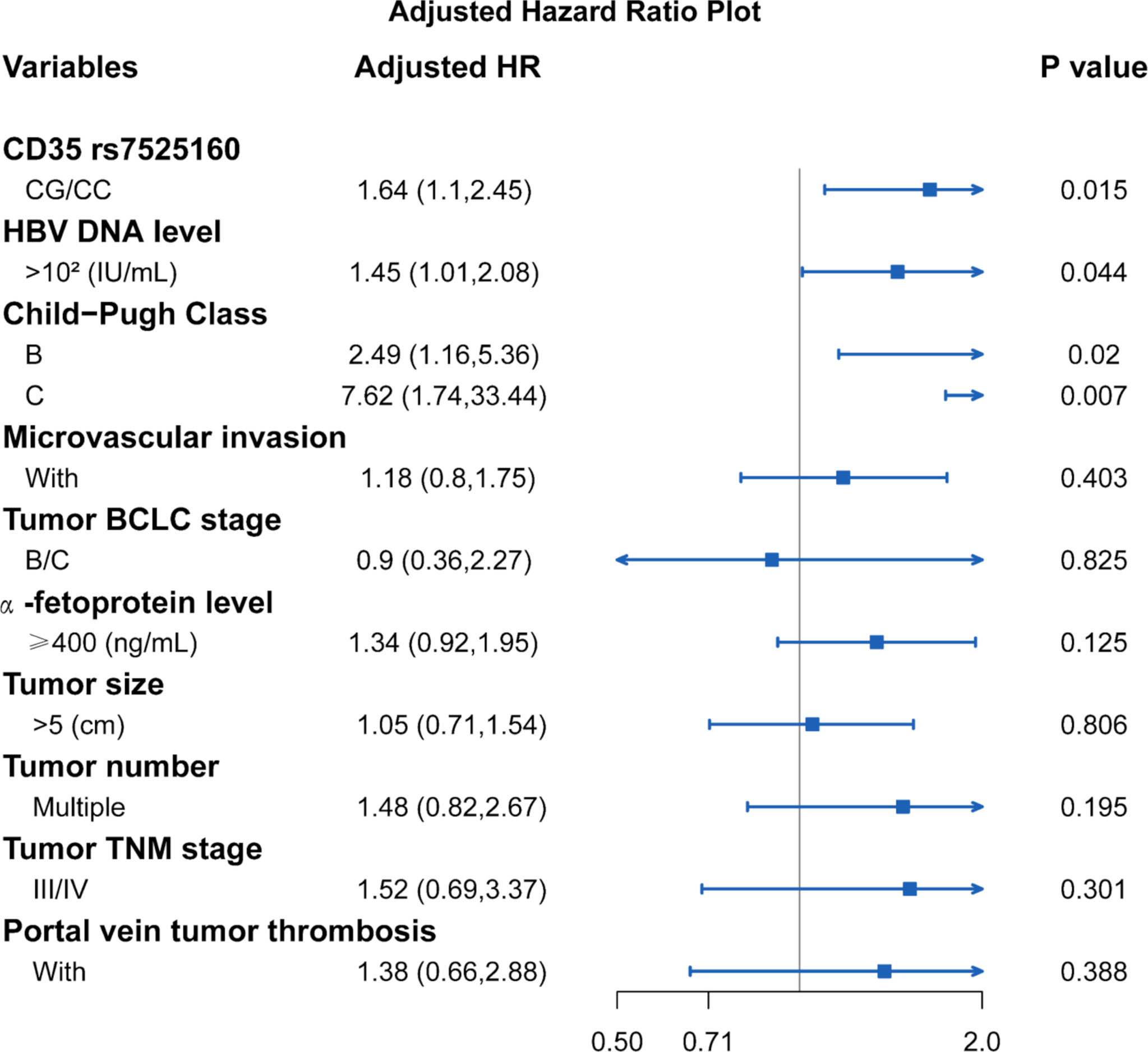
Considering that the recurrence of HCC is related to many clinical factors, we performed univariate survival analysis to identify possible factors affecting the recurrence of HCC in hepatectomy patients. Our result showed that HBV DNA level ≥102 IU/mL [HR=1.77; 95% CI (1.26,2.47); P=0.011], Child-Pugh Class B [HR=2.55; 95% CI (1.24,5.22); P=0.011], Child-Pugh Class C [HR=6.56; 95% CI (1.60,26.84); P=0.009], microvascular invasion [HR=1.68; 95% CI (1.18,2.38); P=0.004], tumor BCLC stage B/C [HR=2.17; 95% CI (1.52,3.08); P<0.001], AFP level ≥ 400 ng/mL [HR=1.68; 95% CI (1.19,2.38); P=0.003], tumor size > 5 cm [HR=1.55; 95% CI (1.11,2.17); P=0.014], multiple tumor number [HR=1.62; 95% CI (1.10,2.39); P=0.014], TNM tumor stage III/IV [HR=2.47; 95% CI (1.73,3.52); P<0.001], and portal vein tumor thrombosis [HR=2.44; 95% CI (1.58,3.76); P<0.001] were factors that were possibly associated with HCC recurrence (Table 3). The Cox proportional hazard ratio model was used to analyze the effects of the CD35 rs7525160 genotype on HCC recurrence in patients undergoing hepatectomy. The analysis indicated that CD35 rs7525160 remained a significant independent risk factor for postoperative recurrence of HCC (adjusted HR=1.64; 95% CI [1.10–2.45]; P=0.015); (Table 4 and Figure 2). With regard to OS analysis, 27 of 299 patients (9.0%) had died between the median follow-up of 18.0 months and maximum follow-up time of 30.7 months. Univariate analyses of overall survival are summarized (Supplementary Table S6). No statistically significant association was found between the six Tag SNPs in CD35 and OS.
Discussion
Chronic inflammation is a hallmark of cancer (31), while the complement cascade, which is implicated in immune and inflammatory responses, plays a pivotal role in tumorigenesis (24). CD35, an important component of innate immunity, not only plays a key role in the clearance of circulating immune complexes (32), but also inhibits the activity of C3 and C5 convertases in classical and alternative pathways and promotes their degradation to regulate complement cascade activation and inhibit the inflammatory response. Therefore, it is reasonable to postulate that genetic variants of CD35 in the complement system may confer susceptibility to cancer.
Several studies have confirmed that the CD35 gene polymorphisms are closely related to a variety of malignant tumors, such as nasopharyngeal carcinoma, gallbladder cancer, bladder cancer, non-Hodgkin’s lymphoma, small cell lung cancer, stomach cancer, and ovarian cancer (24–26, 33). However, few studies have investigated the relationship between CD35 polymorphisms and HCC. In our study, we demonstrated, for the first time, that one SNP (rs7525160) of six tag SNPs of CD35 was associated with the risk of HCC in the Chinese Han population. Notably, compared with the GG genotype, the CD35 rs7525160 CC/CG genotype was associated with an increased risk for developing HCC.
Diseases caused by genetic mutations usually occur at a relatively younger age; this is attributed to the vulnerability of youth to genetic effects rather than environmental exposure, as evidenced by several studies on young patients (34–36), indicating that gene-environmental interaction may enhance susceptibility to malignant tumors. Consistent with these data, our results also demonstrated that the CD35 rs7525160 CC/CG genotype was a risk factor for HCC in patients younger than 65 years.
Although the recurrence and fatality rates of HCC have decreased with the popularization of screening among high-risk groups and the improvement of treatment regimens (7), HCC remains by far the second most common cause of cancer-related deaths worldwide (37). Numerous predictors related to the prognosis of liver cancer, such as AFP levels, tumor number, and tumor size (38), have been reported, but no effective indicators have been found to date. Studies have shown that SNPs may be useful for predicting the prognosis of many cancers (39). Further, our subgroup analysis revealed that genotype frequency of the C allele of CD35 rs7525160 in HCC patients was significantly different from that in controls, especially in patients with AFP ≥ 400 ng/ml, tumor diameter of > 5 cm, TNM stage III/IV, cirrhosis, and portal vein thrombosis, which suggested that CD rs7525160 CC/CG genotype may be related to the type of HCC with poor prognosis. To eliminate the effect of different treatments on the prognosis for HCC, we only studied the 299 untreated patients who had undergone hepatectomy to analyze the association between CD35 and RFS/OS. We found that the CD35 rs7525160 CC/CG genotypes were significantly associated with a shorter mean RFS for HCC patients, and rs7525160 was identified as an independent predictor of RFS in HCC patients by Cox analysis. However, no relationship between CD35 polymorphism and OS was found in this study, which may be due to OS and postoperative recurrence of HCC being affected by many factors, including the choice of treatment following recurrence and the economic status of patients, among others.
This study was beset by several limitations that need to be addressed. First, our results are based only on the Chinese Han population. However, the allele frequency patterns of CD35 polymorphisms vary greatly between different ethnic groups. Therefore, our results cannot be applied to other populations, and data pertaining to multi-ethnic and multi-region populations of non-Chinese Han populations are felt to be required. Secondly, our study focused only on CD35 polymorphism, HCC susceptibility and prognosis at the genetic level, and therefore, further functional verification needs to be considered. Thirdly, a larger sample size is needed, especially when investigating the association between genetic polymorphism and HCC prognosis.
Conclusion
In summary, we found that CD35 rs7525160 may be associated with increased HCC susceptibility in the Chinese Han population, especially in individuals younger than 65 years. In addition, our results indicated that the CD35 rs7525160 CC/CG genotype is closely related to a pathological type associated with poor prognoses for HCC and acts as an independent risk factor for a shorter RFS time following hepatectomy. Our results also suggest that CD35 rs7525160 may be used as a biomarker for screening as well as for prognostic prediction of HCC. Additional well-designed studies with larger sample sizes and long-term follow-up periods, that encompass different ethnic groups, may be required to better elucidate the causal relationship between CD35 polymorphism and HCC risk and prognosis.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Sichuan University (Ethics No. 2017-264). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LmL and QL performed the research and wrote the manuscript. LmL and ZS were responsible for the conceptualization, investigation, and validation of the study. QL and ZS analyzed the data. LxL and BC contributed to the sample collection. YP and YB reviewed and edited the manuscript. FL was in charge of supervision, conceptualization, and project administration. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China [grant numbers 81602910, 81702002], and the Sichuan Science and Technology Program [grant numbers 2019YFS0284, 2019YFS0287, 2019YFS0370].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.700711/full#supplementary-material
Supplementary Figure 1 | MALDI-TOF genotyping and direct sequencing map for CD35 rs7525160. (A) The three genotypes of CD35 rs7525160 polymorphism detected by MALDI-TOF. (B) Sequencing map for genotypes of CD35 rs7525160 GG genotype. (C) Sequencing map for genotypes of CD35 rs7525160 CG genotype.
Abbreviations
HCC, hepatocellular carcinoma; MALDI-TOF-MS, matrix assisted laser desorption ionization time of flight mass spectrometry method; AFP, α-fetoprotein; BCLC, Barcelona-Clinic-Liver-Cancer; TNM, tumor–node–metastasis; AD, Alzheimer’s disease; MAF, minor allele frequency; CT, computed tomography; MRI, magnetic resonance imaging; RFS, Recurrence Free Survival; OS, Overall Survival; HWE, Hardy‐Weinberg equilibrium; OR, odds ratios; CI, confidence intervals; MRFS, mean recurrence-free survival; TACE, Transcatheter Arterial Chemoembolization; SNP, Single Nucleotide Polymorphism; Tag SNP, Tag Single Nucleotide Polymorphism; HBV, Hepatitis B Virus; HCV, Hepatitis C Virus.
References
1. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A Global View of Hepatocellular Carcinoma: Trends, Risk, Prevention and Management. Nat Rev Gastroenterol Hepatol (2019) 16(10):589–604. doi: 10.1038/s41575-019-0186-y
2. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol (2017) 3(4):524–48. doi: 10.1001/jamaoncol.2016.5688
3. Ding C, Fu X, Zhou Y, Liu X, Wu J, Huang C, et al. Disease Burden of Liver Cancer in China From 1997 to 2016: An Observational Study Based on the Global Burden of Diseases. BMJ Open (2019) 9(4):e025613. doi: 10.1136/bmjopen-2018-025613
4. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
5. Yang JD, Mohamed HA, Cvinar JL, Gores GJ, Roberts LR, Kim WR. Diabetes Mellitus Heightens the Risk of Hepatocellular Carcinoma Except in Patients With Hepatitis C Cirrhosis. Am J Gastroenterol (2016) 111(11):1573–80. doi: 10.1038/ajg.2016.330
6. Chayanupatkul M, Omino R, Mittal S, Kramer JR, Richardson P, Thrift AP, et al. Hepatocellular Carcinoma in the Absence of Cirrhosis in Patients With Chronic Hepatitis B Virus Infection. J Hepatol (2017) 66(2):355–62. doi: 10.1016/j.jhep.2016.09.013
7. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology (2016) 64(1):73–84. doi: 10.1002/hep.28431
8. El-Serag HB, Hampel H, Javadi F. The Association Between Diabetes and Hepatocellular Carcinoma: A Systematic Review of Epidemiologic Evidence. Clin Gastroenterol Hepatol (2006) 4(3):369–80. doi: 10.1016/j.cgh.2005.12.007
9. Huang SF, Chang IC, Hong CC, Yen TC, Chen CL, Wu CC, et al. Metabolic Risk Factors Are Associated With Non-Hepatitis B Non-Hepatitis C Hepatocellular Carcinoma in Taiwan, an Endemic Area of Chronic Hepatitis B. Hepatol Commun (2018) 2(6):747–59. doi: 10.1002/hep4.1182
10. Zhang C, Ye Z, Zhang Z, Zheng J, Tang Y, Hou E, et al. A Comprehensive Evaluation of Single Nucleotide Polymorphisms Associated With Hepatocellular Carcinoma Risk in Asian Populations: A Systematic Review and Network Meta-Analysis. Gene (2020) 735:144365. doi: 10.1016/j.gene.2020.144365
11. Clifford RJ, Zhang J, Meerzaman DM, Lyu MS, Hu Y, Cultraro CM, et al. Genetic Variations at Loci Involved in the Immune Response Are Risk Factors for Hepatocellular Carcinoma. Hepatology (2010) 52(6):2034–43. doi: 10.1002/hep.23943
12. Liu F, Li F, Luo L, Yang H, Wei Y, Wang W, et al. Genetic Variants in Cell Death Pathway Genes and HBV-Related Hepatocellular Carcinoma Among a Chinese Han Population. Apoptosis (2017) 22(8):1035–47. doi: 10.1007/s10495-017-1385-z
13. Shen FM, Lee MK, Gong HM, Cai XQ, King MC. Complex Segregation Analysis of Primary Hepatocellular Carcinoma in Chinese Families: Interaction of Inherited Susceptibility and Hepatitis B Viral Infection. Am J Hum Genet (1991) 49(1):88–93.
14. An P, Xu J, Yu Y, Winkler CA. Host and Viral Genetic Variation in HBV-Related Hepatocellular Carcinoma. Front Genet (2018) 9:261. doi: 10.3389/fgene.2018.00261
15. Sunyer JO, Zarkadis IK, Lambris JD. Complement Diversity: A Mechanism for Generating Immune Diversity? Immunol Today (1998) 19(11):519–23. doi: 10.1016/s0167-5699(98)01341-3
16. Rutkowski MJ, Sughrue ME, Kane AJ, Mills SA, Parsa AT. Cancer and the Complement Cascade. Mol Cancer Res (2010) 8(11):1453–65. doi: 10.1158/1541-7786.Mcr-10-0225
17. Markiewski MM, Lambris JD. Is Complement Good or Bad for Cancer Patients? A New Perspective on an Old Dilemma. Trends Immunol (2009) 30(6):286–92. doi: 10.1016/j.it.2009.04.002
18. Reis ES, Mastellos DC, Ricklin D, Mantovani A, Lambris JD. Complement in Cancer: Untangling an Intricate Relationship. Nat Rev Immunol (2018) 18(1):5–18. doi: 10.1038/nri.2017.97
20. Thorgersen EB, Barratt-Due A, Haugaa H, Harboe M, Pischke SE, Nilsson PH, et al. The Role of Complement in Liver Injury, Regeneration, and Transplantation. Hepatology (2019) 70(2):725–36. doi: 10.1002/hep.30508
21. Noris M, Remuzzi G. Overview of Complement Activation and Regulation. Semin Nephrol (2013) 33(6):479–92. doi: 10.1016/j.semnephrol.2013.08.001
22. Krych-Goldberg M, Atkinson JP. Structure-Function Relationships of Complement Receptor Type 1. Immunol Rev (2001) 180:112–22. doi: 10.1034/j.1600-065x.2001.1800110.x
23. Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: A Key System for Immune Surveillance and Homeostasis. Nat Immunol (2010) 11(9):785–97. doi: 10.1038/ni.1923
24. Yu X, Rao J, Lin J, Zhang Z, Cao L, Zhang X. Tag SNPs in Complement Receptor-1 Contribute to the Susceptibility to Non-Small Cell Lung Cancer. Mol Cancer (2014) 13:56. doi: 10.1186/1476-4598-13-56
25. He JR, Xi J, Ren ZF, Qin H, Zhang Y, Zeng YX, et al. Complement Receptor 1 Expression in Peripheral Blood Mononuclear Cells and the Association With Clinicopathological Features and Prognosis of Nasopharyngeal Carcinoma. Asian Pac J Cancer Prev (2012) 13(12):6527–31. doi: 10.7314/apjcp.2012.13.12.6527
26. Srivastava A, Mittal B. Complement Receptor 1 (A3650G RsaI and Intron 27 HindIII) Polymorphisms and Risk of Gallbladder Cancer in North Indian Population. Scand J Immunol (2009) 70(6):614–20. doi: 10.1111/j.1365-3083.2009.02329.x
27. Zhao L, Zhang Z, Lin J, Cao L, He B, Han S, et al. Complement Receptor 1 Genetic Variants Contribute to the Susceptibility to Gastric Cancer in Chinese Population. J Cancer (2015) 6(6):525–30. doi: 10.7150/jca.10749
28. Liver EAftSot. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
29. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th Edition. New york: Springer (2010).
30. Hassan MM, Spitz MR, Thomas MB, El-Deeb AS, Glover KY, Nguyen NT, et al. Effect of Different Types of Smoking and Synergism With Hepatitis C Virus on Risk of Hepatocellular Carcinoma in American Men and Women: Case-Control Study. Int J Cancer (2008) 123(8):1883–91. doi: 10.1002/ijc.23730
31. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-Related Inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205
32. Katyal M, Tiwari SC, Kumar A, Dinda AK, Arora V, Kumar R, et al. Association of Complement Receptor 1 (CR1, CD35, C3b/C4b Receptor) Density Polymorphism With Glomerulonephritis in Indian Subjects. Mol Immunol (2004) 40(18):1325–32. doi: 10.1016/j.molimm.2003.12.003
33. Chaszczewska-Markowska M, Kosacka M, Chryplewicz A, Dyła T, Brzecka A, Bogunia-Kubik K. ECCR1 and NFKB2 Polymorphisms as Potential Biomarkers of Non-Small Cell Lung Cancer in a Polish Population. Anticancer Res (2019) 39(6):3269–72. doi: 10.21873/anticanres.13469
34. Wang W, Li Z, Wang J, Du M, Li B, Zhang L, et al. A Functional Polymorphism in TFF1 Promoter Is Associated With the Risk and Prognosis of Gastric Cancer. Int J Cancer (2018) 142(9):1805–16. doi: 10.1002/ijc.31197
35. Chen YL, Tseng HS, Kuo WH, Yang SF, Chen DR, Tsai HT. Glutathione S-Transferase P1 (GSTP1) Gene Polymorphism Increases Age-Related Susceptibility to Hepatocellular Carcinoma. BMC Med Genet (2010) 11:46. doi: 10.1186/1471-2350-11-46
36. Qu K, Liu SS, Wang ZX, Huang ZC, Liu SN, Chang HL, et al. Polymorphisms of Glutathione S-Transferase Genes and Survival of Resected Hepatocellular Carcinoma Patients. World J Gastroenterol (2015) 21(14):4310–22. doi: 10.3748/wjg.v21.i14.4310
37. Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific Clinical Practice Guidelines on the Management of Hepatocellular Carcinoma: A 2017 Update. Hepatol Int (2017) 11(4):317–70. doi: 10.1007/s12072-017-9799-9
38. Lee SD, Park SJ, Han SS, Kim SH, Kim YK, Lee SA, et al. Clinicopathological Features and Prognosis of Combined Hepatocellular Carcinoma and Cholangiocarcinoma After Surgery. Hepatobiliary Pancreat Dis Int (2014) 13(6):594–601. doi: 10.1016/s1499-3872(14)60275-7
Keywords: CD35, genetic variation, hepatocellular carcinoma, prognosis, susceptibility
Citation: Luo L, Li Q, Su Z, Li L, Cai B, Peng Y, Bai Y and Liu F (2021) Genetic Polymorphisms in CD35 Gene Contribute to the Susceptibility and Prognosis of Hepatocellular Carcinoma. Front. Oncol. 11:700711. doi: 10.3389/fonc.2021.700711
Received: 03 May 2021; Accepted: 19 July 2021;
Published: 05 August 2021.
Edited by:
Xia Li, Shenzhen Institutes of Advanced Technology (CAS), ChinaReviewed by:
Kiran Kumar Mudnakudu N., JSS Academy of Higher Education and Research, IndiaHamed Mirzaei, Kashan University of Medical Sciences, Iran
Peifeng Li, Qingdao University, China
Copyright © 2021 Luo, Li, Su, Li, Cai, Peng, Bai and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Liu, liufei8306@163.com
 Limei Luo
Limei Luo Qin Li
Qin Li Zhenzhen Su
Zhenzhen Su Lixin Li
Lixin Li Bei Cai
Bei Cai Yufu Peng
Yufu Peng Yangjuan Bai
Yangjuan Bai Fei Liu
Fei Liu