- 1Division of Cancer Medicine, Medical Oncology Fellowship, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 2Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
Bruton tyrosine kinase (BTK) is a validated target for treatment of B-cell malignancies, and oral inhibitors of BTK have emerged as a standard of care for these diseases. Acalabrutinib is a second generation, highly selective, potent, covalent BTK inhibitor that exhibits minimal off-target activity in in vitro assays, providing the potential to improve tolerability over the first-in-class BTK inhibitor, ibrutinib. Acalabrutinib was approved for the treatment of relapsed/refractory mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL) in the US in 2017 and 2019, respectively. Acalabrutinib is also undergoing trials for other B-cell malignancies, both as monotherapy and in combinations. In this review, we discuss results from clinical trials evaluating the efficacy and safety of acalabrutinib in patients with CLL, MCL, and Waldenstrom’s macroglobulinemia. Recent phase 3 data showed that acalabrutinib improved progression-free survival (PFS) compared with rituximab plus idelalisib or rituximab plus bendamustine in patients with relapsed/refractory CLL, and acalabrutinib with or without obinutuzumab improved PFS compared with chlorambucil plus obinutuzumab in patients with treatment-naïve CLL. Overall, acalabrutinib had a tolerable safety profile, with most adverse events being grade 1/2 severity (most commonly headache and diarrhea) and a low rate of discontinuation due to adverse events.
Introduction to Bruton Tyrosine Kinase Inhibitors
Non-Hodgkin lymphoma (NHL) is the most common hematological malignancy, accounting for approximately 4% of all cancers in the US (1). B-cell malignancies, such as diffuse large B-cell lymphoma (DLBCL) and chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), account for most NHL cases (2). Bruton tyrosine kinase (BTK), an essential part of the B-cell receptor signaling pathway, is required for the survival and proliferation of normal (3, 4) and malignant (5) B cells. Accordingly, BTK emerged as an important therapeutic target in B-cell malignancies (6, 7).
The first developed BTK inhibitor, ibrutinib, was approved by the US Food and Drug Administration (FDA) in 2013 for patients with relapsed/refractory mantle cell lymphoma (MCL) who had received ≥1 prior therapy. Ibrutinib was subsequently approved for patients with CLL/SLL (as monotherapy or combined with obinutuzumab), Waldenstrom’s macroglobulinemia (WM), marginal zone lymphoma (MZL; who require systemic therapy and had received ≥1 prior anti-CD20 therapy), and chronic graft versus host disease (cGVHD; after failure of ≥1 systemic therapies for GVHD) (8). Ibrutinib monotherapy achieved very high response rates but few, if any, complete remissions and required continuous treatment until disease progression or unacceptable toxicity.
Ibrutinib treatment yielded high response rates (68% in relapsed/refractory MCL; 93% in untreated CLL) and was a major therapeutic advance, with less associated toxicity than seen with chemotherapy (9, 10). Phase 3 trial data showed superior progression-free survival (PFS) versus chemoimmunotherapy in patients with CLL who were ≥65 years (87 versus 74% with PFS after 2 years) and ≤70 years (89 versus 73% PFS after 3 years) (10, 11). Improved overall survival (OS) was reported in two trials evaluating ibrutinib versus chlorambucil monotherapy (RESONATE-2) and versus a chemoimmunotherapeutic regimen of fludarabine, cyclophosphamide, and rituximab (ECOG E1912) (11, 12).
Ibrutinib-associated adverse events (AEs) included atrial fibrillation and hemorrhage (13, 14). In recent meta-analyses, investigators reported risk ratios of 4.69 [95% confidence interval (CI): 2.17–7.64] for atrial fibrillation and 2.82 (95% CI: 1.52–5.23) for hypertension with ibrutinib treatment (15), plus a higher relative risk of overall bleeding in patients receiving ibrutinib (2.72, 95% CI: 1.62–4.58) versus alternative therapy (13). AEs associated with ibrutinib have led to treatment interruption and long-term discontinuation. Some treatment-associated toxicities may be explained by inhibition of kinases other than BTK, including Tec, epidermal growth factor (EGF) receptor, and interleukin-2-inducible T-cell kinase (16). In clinical practice, 24% of patients discontinued ibrutinib by 4 years on treatment, owing to intolerance (17), leaving an unmet need for BTK inhibitors with improved safety and tolerability. Second-generation BTK inhibitors with improved selectivity may address some of the associated toxicity issues. Additionally, mechanisms of resistance via mutations downstream of BTK indicate a need for new treatments (18).
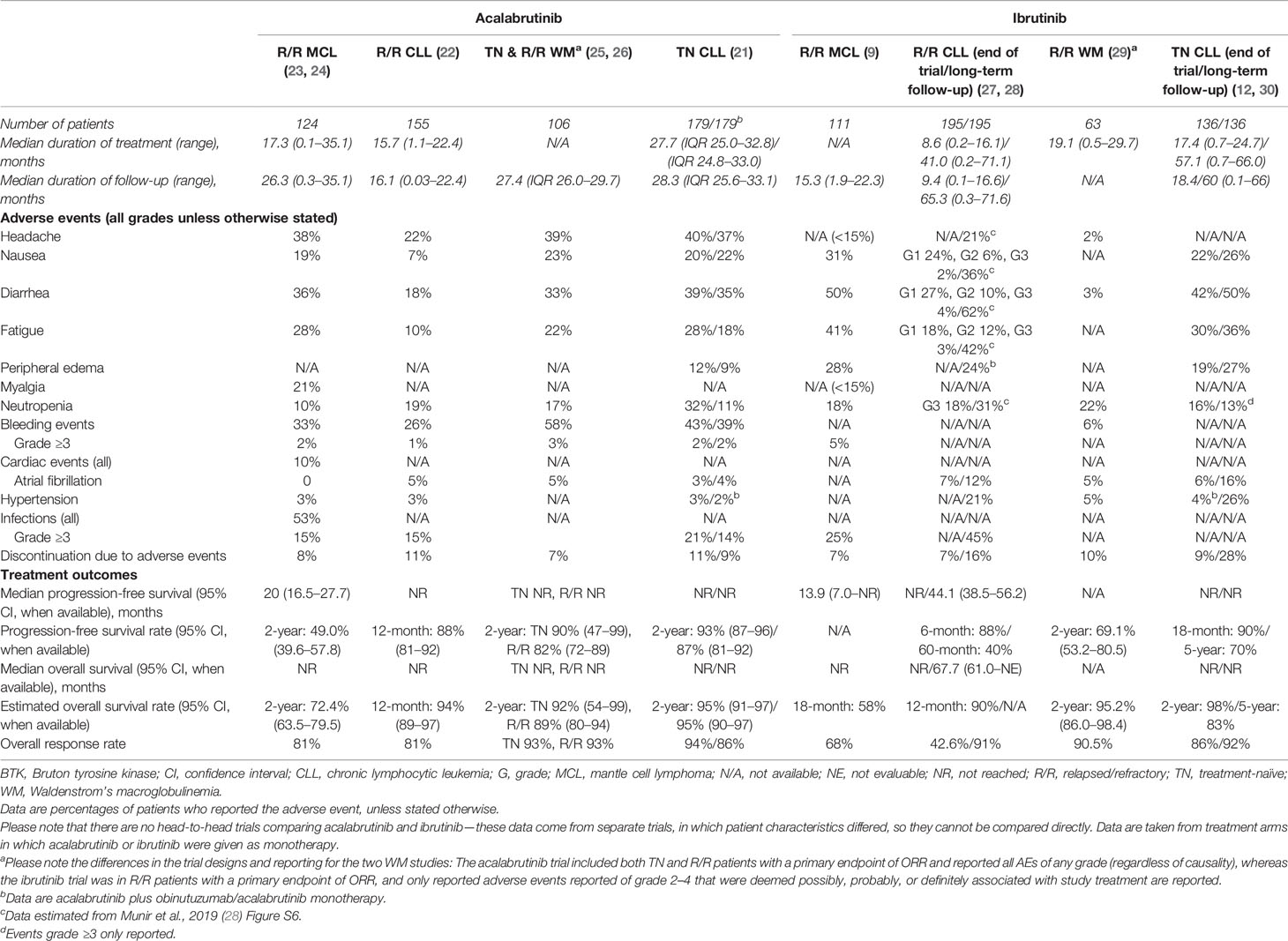
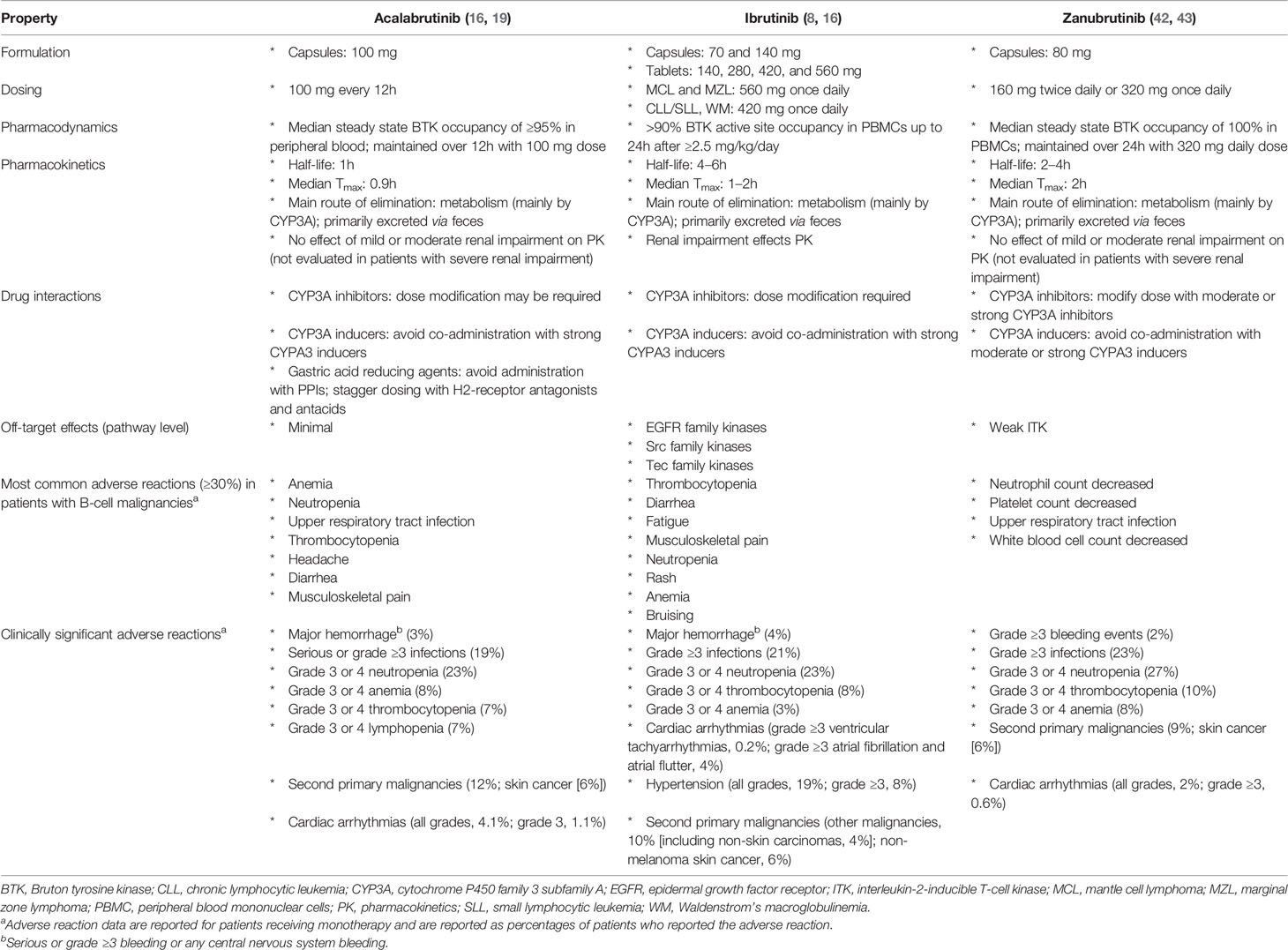
Acalabrutinib received FDA accelerated approval in 2017 for the treatment of patients with MCL who had received ≥1 prior therapy (19) and was recently approved for adults with previously untreated or relapsed/refractory CLL based on ELEVATE-TN and ASCEND trials (20–22). Outcomes and safety for acalabrutinib and ibrutinib in patients with MCL, CLL, and WM are listed in Table 1; results from direct comparisons of these two agents are currently unavailable. Other covalent BTK inhibitors are in development. Zanubrutinib (BGB-3111) has greater BTK selectivity than ibrutinib with minimal off-target in vitro inhibition (31). Zanubrutinib was efficacious in clinical trials of patients with MCL and CLL/SLL, was recently FDA approved for MCL treatment (32), and is being investigated in patients with WM (33–36). Another BTK inhibitor, spebrutinib (CC-292), impaired CLL cell proliferation and improved control of CLL progression when given concurrent with bendamustine in preclinical models (37). However, in a phase 1 study, spebrutinib monotherapy had a shorter duration of response than those reported for ibrutinib or acalabrutinib, although it was well tolerated (38–40). Tirabrutinib (ONO-4059/GS-4059) was investigated in a phase 2 trial for patients with relapsed/refractory CLL combined with idelalisib, entospletinib, and obinutuzumab (41). Basic properties of approved covalent BTK inhibitors are listed in Table 2. Third-generation BTK inhibitors, involving noncovalent and reversible inhibitory mechanisms, are in development and include pirtobrutinib (LOXO-305) (44). In this article, we focus on clinical data obtained for acalabrutinib.
Acalabrutinib Mechanism of Action and Pharmacokinetics
Acalabrutinib is a highly selective, potent, covalent inhibitor of BTK with minimal off-target activity (16), having a narrower spectrum of kinase inhibition on kinome analysis than observed with ibrutinib. It has a 2-pyridylbenzamide moiety and an electrophilic 2-butynamide moiety that are involved in covalent binding to the cysteine (C)481 of BTK (16). Acalabrutinib had higher in vitro kinase selectivity than ibrutinib, zanubrutinib, and spebrutinib, and similar selectivity to tirabrutinib (45). In biochemical assays, ibrutinib inhibited Src family kinases, whereas acalabrutinib and tirabrutinib did not inhibit EGF-induced EGF receptor activation or T-cell receptor-mediated T-cell activation in cellular assays at physiologically relevant concentrations (45). The improved selectivity of acalabrutinib compared with ibrutinib is expected to reduce the occurrence of some ibrutinib-associated AEs. As an example, studies showed reduced platelet dysfunction with acalabrutinib compared with ibrutinib, which may be due to inhibition of Src family kinases by ibrutinib but not by acalabrutinib (46, 47); however, whether this results in fewer bleeding AEs in the clinic is unclear.
In healthy volunteers, acalabrutinib showed rapid absorption (time to maximum plasma concentration, 0.5–1.0 h across dose cohorts up to 100 mg) and fast elimination (half-life, 0.88–2.1 h) (16). Twice-daily dosing of acalabrutinib 100 mg resulted in a median BTK occupancy of 97% before and 99% 4 h after dosing at steady state (40). Reduced absorption and plasma levels may be seen with concurrent proton pump inhibitors (which are therefore contraindicated with acalabrutinib); staggered dosing with antacids and H2-receptor antagonists is recommended (19).
Clinical Results
MCL
The approval of acalabrutinib for patients with MCL was based on the ACE-LY-004 open-label, phase 2, single-arm trial conducted in 124 patients with relapsed/refractory MCL. Median age of patients was 68 years, 80% were male, and 93% had an Eastern Cooperative Oncology Group (ECOG) score ≤1 (23). After a median follow-up of 15.2 months, acalabrutinib monotherapy showed a durable high response rate and a favorable safety profile (23). With longer-term follow-up (median, 26 months; range, 0.3–35.1 months), the investigator-assessed overall response rate (ORR) was 81%; 43% of patients achieved complete remission (CR). The median duration of response (DOR) was 26 months, and the median PFS was 20 months (24). Discontinuations were mainly due to disease progression (44%). Minimal residual disease (MRD) was evaluated in 29 of 124 patients, eight of whom achieved CR with undetectable MRD (sensitivity 5 × 10−6) on acalabrutinib monotherapy (24).
The most common AEs (any grade) were headache (38%), diarrhea (36%), fatigue (28%), cough (22%), and myalgia (21%). Headaches typically occurred during the first month of treatment, diarrhea generally was limited to the first 6 months, and these AEs usually were grade 1/2. Cardiac AEs occurred in 10% of patients. Four patients (3%) had grade 3/4 events; one of these (acute coronary syndrome) was considered treatment related. There were no cases of new-onset atrial fibrillation. Hypertension occurred in four patients. Bleeding events occurred in 33% of patients [grade 3, 2% (n = 3)]. Grade 3/4 infections occurred in 15% of patients; pneumonia was most common (6%) (24). No new safety signals were observed during long-term follow-up.
CLL
The open-label, multicenter, phase 1/2 ACE-CL-001 trial evaluated acalabrutinib in 61 patients [median age, 62 years; median three prior therapies; 31% del(17p); 75% unmutated immunoglobulin heavy-chain variable gene (IGHV)] with relapsed/refractory CLL (40). After a median of 14.3 months of follow-up (range, 0.5–20 months), 87% of patients remained on acalabrutinib. Discontinuations occurred owing to the investigators’ or patients’ decision (n = 8), active autoimmune hemolytic anemia (n = 2), fatal pneumonia (n = 1), AEs (n = 3: diarrhea, gastritis, dyspnea), or disease progression (n = 1) (40). The ORR was 95% [partial response (PR), 85%; PR with lymphocytosis, 10%]; no patients achieved CR. In an expansion of this study that included 134 patients, responses were similar after a median follow-up of 41 months (range, 0.2–58 months); 56% of patients remained on treatment, with 21% and 11% of patients discontinuing because of progressive disease or AEs, respectively. CR was achieved in 4% of patients, and Richter transformation occurred in three patients (48).
ACE-CL-001 included a treatment-naïve cohort (n = 99; median age, 64 years; 10% del(17p); 62% unmutated IGHV) (49). After 42 months of follow-up (range, 1–48 months), 89% of patients remained on acalabrutinib. Discontinuations were due to AEs (5%: second malignancies, sepsis, urinary tract infection), disease progression (2%), withdrawal of consent (2%), pregnancy (1%), and initiation of subsequent cancer treatment (1%) (49). The ORR was 97%; 5% achieved CR and 92% achieved PR. Median DOR was not reached; the 36-month DOR rate was 98%. Median PFS was not reached; the 36-month PFS rate was 97%. Responses were consistent with longer follow-up (median, 53 months; range, 1–59 months) (50).
Across both cohorts (relapsed/refractory and treatment-naïve), most AEs were grade 1/2 and resolved (48, 49). Diarrhea (relapsed/refractory, 52%; treatment-naïve, 49%), headache (51%; 44%), and upper respiratory tract infection (37%; 40%) were most common. Atrial fibrillation (none led to discontinuation) occurred in 7 and 6% and severe bleeding in 5 and 2% of relapsed/refractory and treatment-naïve patients, respectively (48, 49).
Results from two global, randomized, multicenter, open-label phase 3 trials were recently published. ASCEND (ACE-CL-309) was conducted in 310 patients with relapsed/refractory CLL comparing acalabrutinib versus investigator’s choice (idelalisib plus rituximab or bendamustine plus rituximab) (22). At the median follow-up of 16.1 months (range, 0.03–22.4 months), median PFS was not reached on acalabrutinib but was significantly longer than that of the comparator group [16.5 months (range, 14.0–17.1 months); hazard ratio (HR), 0.31; 95% CI: 0.20–0.49; p < 0.0001]. Most patients in the comparator arm received targeted therapy, and PFS outcomes appeared similar between comparator options. Twelve-month PFS rates were 88% for acalabrutinib and 68% for comparator; respective ORRs were 81 and 75%. PFS was superior for acalabrutinib versus comparator across high-risk subgroups, including del(17p) + mutated TP53 and advanced Rai stage. Outcomes for patients treated with idelalisib plus rituximab were markedly worse in this study compared with previous studies; this was attributed to a lower treatment exposure that was mainly the result of a high rate of discontinuation because of AEs. Mean duration of exposure was 15.7 months for acalabrutinib monotherapy and 11.5 months for idelalisib plus rituximab. AEs most common with acalabrutinib were headache (22%), neutropenia (19%), and diarrhea (18%). Atrial fibrillation occurred in 5% of patients receiving acalabrutinib, and bleeding events occurred in 26% (major hemorrhage, 1%) (22). There were much lower rates of grade 3/4 AEs and AEs leading to discontinuation in the acalabrutinib monotherapy group (45% and 11%, respectively) than in the idelalisib plus rituximab group (86% and 47%, respectively). Safety results for the bendamustine plus rituximab group were similar to those for acalabrutinib monotherapy.
The ELEVATE-TN (ACE-CL-007) study enrolled 535 patients who were randomly assigned (1:1:1) to acalabrutinib plus the anti-CD20 antibody obinutuzumab, acalabrutinib monotherapy, or obinutuzumab plus chlorambucil (21). At median follow-up (28.3 months), the median PFS was not reached with acalabrutinib-obinutuzumab, compared with obinutuzumab-chlorambucil (22.6 months; HR, 0.10; 95% CI: 0.06–0.17; p < 0.0001). Acalabrutinib monotherapy was also associated with a superior PFS (median not reached) versus obinutuzumab–chlorambucil (HR, 0.20; 95% CI: 0.13–0.30; p < 0.0001). The estimated 24-month PFS rates with acalabrutinib–obinutuzumab, acalabrutinib monotherapy, and obinutuzumab–chlorambucil were 93%, 87%, and 47%, respectively. The superior PFS rates of both acalabrutinib-containing arms versus obinutuzumab–chlorambucil were consistent across high-risk subgroups, including del(17p). At this time, median OS was not reached in any arm. The median treatment duration was 27.7 months for acalabrutinib-obinutuzumab and acalabrutinib monotherapy and 5.6 months for obinutuzumab–chlorambucil. AEs were similar in the acalabrutinib-containing arms; AEs of interest (acalabrutinib–obinutuzumab or acalabrutinib monotherapy versus obinutuzumab–chlorambucil) were atrial fibrillation (any grade: 3% or 4 versus 1%), bleeding (any grade/grade ≥3: 43%/2% or 39%/2% versus 12%/0%), and hypertension (grade ≥3: 3% or 2 versus 3%) (21). Patients in both acalabrutinib-containing arms were treated with a BTK inhibitor until disease progression. Debate continues regarding the contribution of obinutuzumab to the overall outcome in this setting. Currently, the authors would not routinely add CD20 monoclonal antibody (mAb) treatment to a BTK inhibitor-based treatment, either in first-line or the relapsed setting, when a BTK inhibitor is administered until disease progression in patients with CLL.
The phase 1b/2 ACE-CL-003 study also evaluated treatment with acalabrutinib plus obinutuzumab and was conducted in patients with relapsed/refractory (n = 26) or treatment-naïve (≥65 years of age; n = 19) CLL (51). The ORR was 95% (6% CR) in treatment-naïve patients (median follow-up, 39 months; range, 1–45 months) and 92% (2% CR) in those with relapsed/refractory CLL (median follow-up, 42 months; range, 20–49 months). At 36 months, 94% of treatment-naïve and 88% of relapsed/refractory patients were progression-free. Treatment was discontinued in 11% of treatment-naïve patients [owing to an AE of metastatic squamous cell carcinoma (n = 1) and Richter transformation (n = 1)] and in 30% of relapsed/refractory patients [owing to AEs (n = 4), Richter transformation (n = 2), progressive disease (n = 1), and death (n = 1)]. The most common AEs were upper respiratory tract infection (71%), weight gain (71%), maculopapular rash (67%), cough (64%), diarrhea (62%), headache (56%), nausea (51%), arthralgia (47%), and dizziness (47%). Two relapsed/refractory patients had grade 3 bleeding events (one hematuria and one muscle hemorrhage). One patient experienced grade 3 atrial fibrillation, which did not result in treatment discontinuation (51).
Patients With CLL Who Were Intolerant to Ibrutinib
An additional cohort was added to ACE-CL-001 to evaluate patients who were intolerant to ibrutinib due to severe AEs (52). The most common ibrutinib-related AEs reported at trial entry were rash (24%), arthralgia (18%), diarrhea (15%), fatigue (12%), and hemorrhage (12%). Most of the 33 patients had high-risk disease, including Rai stage III or IV (27%), bulky lymph nodes (31%), del(17p) (38%), del(11q) (22%), and unmutated IGHV (81%); additionally, patients were heavily pretreated (median of four prior therapies). After a median of 19.0 months of treatment (range, 0.7–30.6 months), 23 patients remained on acalabrutinib. Ten patients discontinued treatment owing to progressive disease (n = 4), AEs (n = 3), and physician decision (n = 3). The ORR was 76%. Among 25 responders, median DOR and PFS were not reached; 1-year PFS was 83.4%. Acalabrutinib was well tolerated; 72% of the 61 ibrutinib-related AEs associated with intolerance did not recur with acalabrutinib treatment, and 13% recurred at a lower grade (52). This study indicated tolerability and safety for acalabrutinib in ibrutinib-intolerant patients, allowing these patients to continue to receive a BTK inhibitor. Following this, a phase 2 study (ACE-CL-208) was conducted in 60 patients with relapsed/refractory CLL who were ibrutinib-intolerant due to grade 3/4 AEs (including atrial fibrillation/flutter, diarrhea, arthralgia, and rash) after a median treatment duration of 6 months (range, <1–55 months). At the median follow-up of 23 months, 37 patients remained on acalabrutinib. The ORR was 72% (5% CR). Of the 23 patients who discontinued treatment, seven (12%) discontinued due to AEs (53). In the authors’ experience, a lower proportion of patients with CLL require dose reduction or need to stop treatment when on acalabrutinib, compared with ibrutinib. The ability to maintain on-target treatment may improve overall long-term disease control and outcomes. Objective evidence for this hypothesis will require a randomized comparison assessment, which is currently ongoing in a phase 3 study (NCT02477696).
Waldenstrom’s Macroglobulinemia
The efficacy and safety of acalabrutinib were investigated in patients with treatment-naïve (n = 14) or relapsed/refractory (n = 92) WM in a phase 2 trial (ACE-WM-001) (25). Acalabrutinib was highly effective, with an ORR of 93% (median follow-up, 27.4 months; range, 26.0–29.7 months). The 2-year DOR was 90% (95% CI: 47–99%) for treatment-naïve and 82% (95% CI: 72–89%) for relapsed/refractory patients; the respective 2-year PFS rates were 90% (47–99%) and 82% (72–89%), and the respective 2-year OS rates were 92% (95% CI: 54–99%) and 89% (95% CI: 80–94%). Overall, 50% of treatment-naïve and 25% of relapsed/refractory patients discontinued treatment. The most common AEs were headache (39%) and diarrhea (31%). Five patients had atrial fibrillation (grade 3/4, n = 1), and three had grade 3/4 bleeding. One treatment-related death (intracranial hematoma) was reported (25).
Acalabrutinib Combination Therapy in B-Cell Malignancies
Acalabrutinib combined with the phosphatidyl-3-kinase delta (PI3Kδ) inhibitor ACP-319 was investigated in 40 patients with relapsed/refractory B-cell malignancies. The combined treatment was tolerated with manageable AEs; however, patients with CLL/SLL were switched to acalabrutinib monotherapy, owing to toxicities and limited benefit with added ACP-319 (54). The most common AEs were increased levels of aspartate aminotransferase (48%) and alanine aminotransferase (52%), diarrhea (52%), fatigue (40%), and rash (40%). Twenty-two patients discontinued treatment (5 of 7 owing to AEs with acalabrutinib/ACP-319). No deaths were due to AEs (54).
A single-arm phase 1/2 trial of acalabrutinib combined with the PD-1 mAb pembrolizumab was conducted in 61 patients with relapsed/refractory DLBCL. The ORR was 26% and was similar for germinal center B-cell-like (GCB) DLBCL and non-GCB DLBCL. The median DOR was 6.9 months, with just six patients remaining on study therapy; however, exceptional responses were observed in two patients (DOR, >24 months) (55).
Safety of Acalabrutinib
In a pooled safety analysis of seven trials of patients (n = 610) receiving acalabrutinib monotherapy (701.5 patient–years of exposure), the most common reported AEs were headache (42.3% any cause, 29.2% treatment-related, 1.3% grade ≥3), diarrhea (38.4%, 16.6%, 2.1%), fatigue (23.4%, 7.4%, 1.5%), nausea (23.1%, 9.3%, 1.5%), and contusion (21.6%, 13.4%, 0%), and 35.7% of patients had serious AEs (treatment-related, 9.5%) (56). Grade 5 AEs were reported in 3.6% of patients; pneumonia was the most frequent. Atrial fibrillation occurred in 2.3% of patients (1.0% grade 3), mostly in those with known risk factors. Infections were reported in 61.0% of patients (16.2% grade ≥3); pneumonia was the most frequent.
Many acalabrutinib-associated side effects were grade 1/2 and were easily managed (57). Ibrutinib treatment was associated with an increase in bleeding events through platelet inhibitory mechanisms, partly due to the off-target Tec kinase inhibition (13, 58). Analysis of platelet aggregation showed that acalabrutinib did not result in the platelet dysfunction observed with ibrutinib (46, 47). However, bleeding events were reported with acalabrutinib (Table 1). Although no results from head-to-head trials comparing acalabrutinib with ibrutinib or any other BTK inhibitor are available, a summary of the safety profile of each agent in MCL, CLL, and WM is presented in Table 1. Findings of a matching-adjusted indirect comparison suggested significantly lower rates of atrial fibrillation and thrombocytopenia with acalabrutinib versus ibrutinib (59).
Mechanism of Acalabrutinib Therapeutic Resistance
A primary reported mechanism of acalabrutinib resistance in CLL is BTK mutation (60). In 103 patients with CLL treated with acalabrutinib and routinely screened for BTK mutation, mutations developed in 22 (median time from acalabrutinib initiation to detection, 31.6 months). Sixteen patients had progression of CLL; 11 of these patients (69%) had BTK C481 mutations (C481S in ten patients, C481R and C481Y in one). Four patients with the BTK C481S mutation had coexisting mutations of either BTK T474I (n = 1), BTK C481R (n = 1), or PLCG2 (n = 2); these mutations previously were associated with ibrutinib resistance. These data indicate a similar mechanism of resistance for acalabrutinib and ibrutinib.
Future Studies
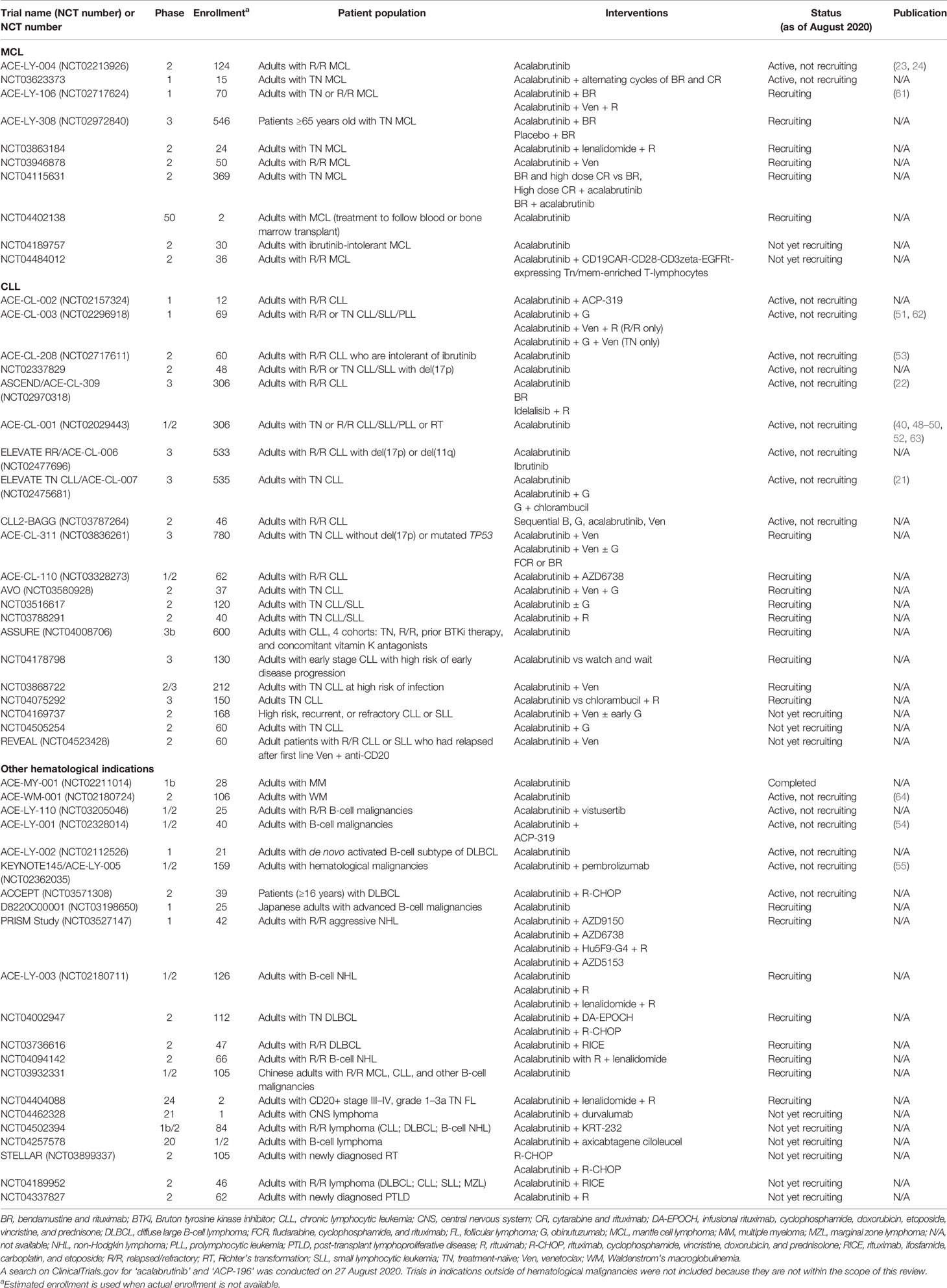
Table 3 summarizes registered clinical trials of patients with hematological malignancies that include acalabrutinib. The ELEVATE RR/ACE-CL-006 randomized, phase 3, open-label study of acalabrutinib versus ibrutinib in 533 previously treated patients with high-risk relapsed/refractory CLL has recently completed (NCT02477696). It has been reported that the trial met the primary endpoint of non-inferiority while demonstrating a significantly reduced rate of atrial fibrillation in patients treated with acalabrutinib versus those treated with ibrutinib (65).
B-cell lymphoma-2 (BCL-2) proteins regulate the intrinsic mitochondrial apoptotic pathway and represent another therapeutic target in B-cell malignancies (66). Venetoclax is an approved, highly selective BCL-2 inhibitor used in treatment of patients with CLL. In ACE-CL-311, an ongoing, randomized, open-label phase 3 study with a target enrollment of 780 patients with treatment-naïve CLL without del(17p) or mutated TP53, acalabrutinib in combination with the BCL-2 inhibitor venetoclax with and without obinutuzumab versus chemotherapy will be evaluated (expected completion in 2024) (67). A randomized, phase 3, double-blind trial is recruiting patients with MCL (ACE-LY-308; NCT02972840) to investigate bendamustine/rituximab alone or combined with acalabrutinib in treatment-naïve patients (expected completion in 2023).
Encouraging safety and efficacy results have been demonstrated in an ongoing phase 2 study of patients with CLL given combined acalabrutinib, venetoclax, and obinutuzumab (AVO) (NCT03580928). Of 36 patients with at least 16 months of follow-up at the interim analysis, the ORR was 100%; 78% of patients—in a population comprising nearly 40% of patients with TP53 mutations—had bone marrow-undetectable MRD by 15 months of time-limited therapy (68). In a phase 1b study of relapsed/refractory or treatment-naïve CLL patients (NCT02296918) (n = 12 per cohort), 92% and 83% of patients, respectively, remained on a modified AVO combination treatment (acalabrutinib plus obinutuzumab/rituximab plus venetoclax) at the median follow-up time of 23.2 and 22.0 months. AEs of the combined treatment were similar to those of the individual agents. After 16 treatment cycles, the ORR for relapsed/refractory patients was 92% and for treatment-naïve patients was 100% (69).
Discussion
Results of clinical trials in patients with relapsed/refractory MCL, relapsed/refractory and treatment-naïve CLL, or WM demonstrate that acalabrutinib is well tolerated and effective. Acalabrutinib is also well tolerated in high-risk patients with CLL, including those with del(17p) and mutated TP53 (21, 45) and those who are intolerant to ibrutinib (52). The improved selectivity profile of acalabrutinib, compared with ibrutinib, provides the potential for a reduced risk of toxicity, which may result in improved treatment outcomes and decreased rates of discontinuation.
The treatment landscape for patients with CLL and other B-cell malignancies is rapidly evolving away from chemoimmunotherapy-based treatment to targeted treatment and combinations. For example, BTK has been shown to modulate PD-1 expression in DLBCL cells in vitro, which raises the possibility that combinations of BTK inhibitors with immune checkpoint inhibitors may increase efficacy (70). A clinical trial assessing avelumab (anti-PDL1) combined with ibrutinib for patients with relapsed/refractory DLBCL or MCL is currently underway (NCT03440567).
New targeted therapies are available for the treatment of B-cell malignancies, including BTK inhibitors, the BCL-2 inhibitor venetoclax, anti-CD20 mAbs (obinutuzumab, ofatumumab), immunomodulatory agents (e.g. lenalidomide), and PI3K inhibitors (e.g. idelalisib). Additionally, trials of combined targeted therapy with agents aiming toward fixed-duration treatment to achieve deep and durable unmaintained remission in patients with CLL are underway (71). Recent results showed targeted therapies are more effective than traditional chemoimmunotherapy, changing the standard of care in B-cell malignancies. For example, the ALLIANCE phase 3 trial showed superior PFS with ibrutinib and ibrutinib plus rituximab compared with bendamustine plus rituximab in older treatment-naïve patients with CLL; the addition of rituximab to ibrutinib did not provide additional PFS benefit compared to ibrutinib alone (10). Similarly, the E1912 phase 3 trial showed that ibrutinib plus rituximab was superior to fludarabine, cyclophosphamide, and rituximab, with respect to PFS and OS in treatment-naïve patients with CLL (11). Results of iLLUMINATE showed that ibrutinib plus obinutuzumab resulted in superior PFS and OS compared with chlorambucil plus obinutuzumab in treatment-naïve patients with CLL (72). Additionally, findings of ELEVATE-TN indicated superior PFS rates of both acalabrutinib-containing arms compared with obinutuzumab–chlorambucil (21). With venetoclax, PFS and OS were superior to bendamustine plus rituximab in patients with relapsed/refractory CLL in the phase 3 MURANO trial (73). Results of the phase 3 CLL14 trial indicated that patients with treatment-naïve CLL receiving venetoclax plus obinutuzumab had a significantly longer PFS than did patients on chlorambucil plus obinutuzumab (HR, 0.31; 95% CI: 0.22–0.44; p < 0.0001) (74). Interim results from an ongoing phase 2 trial evaluating a time-limited combination of acalabrutinib, venetoclax, and obinutuzumab in treatment-naïve CLL (NCT03580928) showed a 100% response rate (75% PR; 25% CR) (75).
Results of a recent network meta-analysis comparing the outcomes of acalabrutinib plus obinutuzumab (ELEVATE-TN), ibrutinib plus obinutuzumab (iLLUMINATE), and venetoclax plus obinutuzumab (CLL14) in treatment-naïve patients with CLL suggested that PFS improvement was greatest in patients treated with acalabrutinib plus obinutuzumab (76). A head-to-head comparison of acalabrutinib versus ibrutinib is ongoing. It will be interesting to see results of ongoing phase 3 trials with acalabrutinib and whether the improved tolerability translates to a real-world clinical benefit.
Conclusion
BTK inhibitors have remarkably advanced treatment options for patients with B-cell malignancies; hence, BTK is a proven critical therapeutic target. Factors driving further development of BTK inhibitors have been the toxicities and tolerability issues noted with the first-in-class compound, ibrutinib, and the patterns and factors associated with resistance, most notably mutations in C481 of BTK, the binding site of the covalent inhibitors. Off-target kinase inhibition is thought to contribute to the toxicity profile of individual agents, so focusing on compounds with narrower kinome profiles of inhibition may reduce toxicity. Tolerability and toxicities can heavily impact the ability to deliver an intended effective dose and maintain patients on treatment. The covalent BTK inhibitors—particularly ibrutinib and acalabrutinib—have some toxicities in common, and each has circumstances and conditions that might limit use. The experience of the authors has been that fewer patients require dose adjustment or treatment discontinuation with acalabrutinib compared with ibrutinib. Head-to-head randomized trials, such as the recently completed ELEVATE RR/ACE-CL-006 trial (NCT02477696), are needed to confirm differences in tolerability. The efficacies of the covalent BTK inhibitors appear to be similar; however, long-term differences may be revealed related to tolerability. Acalabrutinib was tolerated in patients who had discontinued ibrutinib because of side effects, importantly allowing for longer on-target treatment (77). Further, acalabrutinib had reported activity in patients with CLL who progressed on ibrutinib (52).
Given that treatment with BTK inhibitors continues until disease progression, and as these agents are highly effective—providing many years of disease control—consideration must be given for longer exposure time and follow-up with ibrutinib-based studies compared with those of other BTK inhibitors. Reversible third-generation inhibitors of BTK are in development, with early reports indicating activity and excellent tolerability in patients who are refractory to a covalent BTK inhibitor.
Finally, fixed durations of treatment, with long remissions off-treatment, have been made possible by the very deep remissions observed with venetoclax-based treatment. The authors believe that fixed-duration targeted therapy, including BTK and BCL-2 inhibitors, will be the next standard of care.
Author Contributions
WW and HA contributed to the writing and editing of this manuscript. Both authors also reviewed and approved the final draft for submission and take responsibility for all the content.
Funding
Medical writing support was provided by Adele Buss, PhD, of Oxford PharmaGenesis, Oxford, UK, and was funded by AstraZeneca LP.
Conflict of Interest
WW has received research funding from AbbVie, Acerta Pharma, Emergent, Genentech, Gilead, GlaxoSmithKline/Novartis, Janssen, Juno, Karyopharm, Kite, and Pharmacyclics.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Astra Zeneca LP. The funder had the following involvement in the study: medical writing support.
Abbreviations
AE, adverse event; BTK, Bruton tyrosine kinase; cGVHD; chronic graft versus host disease; CI, confidence interval; CLL, chronic lymphocytic leukemia; CR, complete response; DLBCL, diffuse large B-cell lymphoma; DOR, duration of response; ECOG, Eastern Cooperative Oncology Group; EGF, epidermal growth factor; FDA, Food and Drug Administration; GCB, germinal center B-cell; IGHV, immunoglobulin heavy-chain variable; MCL, mantle cell lymphoma; NHL, non-Hodgkin lymphoma; ORR, overall response rate; OS, overall survival; PD-1, programmed cell death protein 1; PFS, progression-free survival; PI3Kδ, phosphatidyl-3-kinase delta; PK, pharmacokinetic; PR, partial response; SLL, small lymphocytic leukemia; WM, Waldenstrom’s macroglobulinemia.
References
1. National Cancer Institute, Surveillance, Epidemiology, and End Results Program (SEER). Cancer Stat Facts: non-Hodgkin Lymphoma. Available at: https://seer.cancer.gov/statfacts/html/nhl.html (Accessed January 22, 2021).
2. Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non-Hodgkin Lymphoma Subtype Distribution, Geodemographic Patterns, and Survival in the US: A Longitudinal Analysis of the National Cancer Data Base From 1998 to 2011. Am J Hematol (2015) 90:790–5. doi: 10.1002/ajh.24086
3. Vetrie D, Vořechovský I, Sideras P, Holland J, Davies A, Flinter F, et al. The Gene Involved in X-linked Agammaglobulinaemia is a Member of the Src Family of Protein-Tyrosine Kinases. Nature (1993) 361:226–33. doi: 10.1038/361226a0
4. Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, et al. Deficient Expression of a B Cell Cytoplasmic Tyrosine Kinase in Human X-linked Agammaglobulinemia. Cell (1993) 72:279–90. doi: 10.1016/0092-8674(93)90667-f
5. Gururajan M, Jennings CD, Bondada S. Cutting Edge: Constitutive B Cell Receptor Signaling is Critical for Basal Growth of B Lymphoma. J Immunol (2006) 176:5715–9. doi: 10.4049/jimmunol.176.10.5715
6. AstraZeneca. US FDA Approves Astrazeneca’s Calquence (Acalabrutinib) for Adult Patients With Previously-Treated Mantle Cell Lymphoma [Press Release] (2017). Available at: https://www.astrazeneca.com/media-centre/press-releases/2017/us-fda-approves-astrazenecas-calquence-acalabrutinib-for-adult-patients-with-previously-treated-mantle-cell-lymphoma-24102017.html# (Accessed January 22, 2021).
7. de Claro RA, McGinn KM, Verdun N, Lee SL, Chiu HJ, Saber H, et al. FDA Approval: Ibrutinib for Patients With Previously Treated Mantle Cell Lymphoma and Previously Treated Chronic Lymphocytic Leukemia. Clin Cancer Res (2015) 21:3586–90. doi: 10.1158/1078-0432.CCR-14-2225
8. Janssen. Imbruvica (Ibrutinib) [Prescribing Information]. Sunnyvale, CA: Pharmacyclics LLC (2020).
9. Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK With Ibrutinib in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med (2013) 369:507–16. doi: 10.1056/NEJMoa1306220
10. Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib Regimens Versus Chemoimmunotherapy in Older Patients With Untreated CLL. N Engl J Med (2018) 379:2517–28. doi: 10.1056/NEJMoa1812836
11. Shanafelt TD, Wang XV, Kay NE, Hanson CA, O’Brien S, Barrientos J, et al. Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N Engl J Med (2019) 381:432–43. doi: 10.1056/NEJMoa1817073
12. Burger JA, Barr PM, Robak T, Owen C, Ghia P, Tedeschi A, et al. Long-Term Efficacy and Safety of First-Line Ibrutinib Treatment for Patients With CLL/SLL: 5 Years of Follow-Up From the Phase 3 RESONATE-2 Study. Leukemia (2020) 34:787–98. doi: 10.1038/s41375-019-0602-x
13. Caron F, Leong DP, Hillis C, Fraser G, Siegal D. Current Understanding of Bleeding With Ibrutinib Use: A Systematic Review and Meta-Analysis. Blood Adv (2017) 1:772–8. doi: 10.1182/bloodadvances.2016001883
14. Ganatra S, Sharma A, Shah S, Chaudhry GM, Martin DT, Neilan TG, et al. Ibrutinib-Associated Atrial Fibrillation. JACC Clin Electrophysiol (2018) 4:1491–500. doi: 10.1016/j.jacep.2018.06.004
15. Caldeira D, Alves D, Costa J, Ferreira JJ, Pinto FJ. Ibrutinib Increases the Risk of Hypertension and Atrial Fibrillation: Systematic Review and Meta-Analysis. PloS One (2019) 14:e0211228. doi: 10.1371/journal.pone.0211228
16. Barf T, Covey T, Izumi R, van de Kar B, Gulrajani M, van Lith B, et al. Acalabrutinib (ACP-196): A Covalent Bruton Tyrosine Kinase Inhibitor With a Differentiated Selectivity and In Vivo Potency Profile. J Pharmacol Exp Ther (2017) 363:240–52. doi: 10.1124/jpet.117.242909
17. Woyach JA, Ruppert AS, Guinn D, Lehman A, Blachly JS, Lozanski A, et al. BTKC481S-Mediated Resistance to Ibrutinib in Chronic Lymphocytic Leukemia. J Clin Oncol (2017) 35:1437–43. doi: 10.1200/JCO.2016.70.2282
18. Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS, et al. Resistance Mechanisms for the Bruton’s Tyrosine Kinase Inhibitor Ibrutinib. N Engl J Med (2014) 370:2286–94. doi: 10.1056/NEJMoa1400029
19. AstraZeneca. Calquence (Acalabrutinib) [Prescribing Information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP (2019).
20. U.S. Food and Drug Administration. FDA Takes Second Action Under International Collaboration, Approves New Treatment Option for Patients With Chronic Lymphocytic Leukemia [FDA News Release] (2019). Available at: https://www.fda.gov/news-events/press-announcements/fda-takes-second-action-under-international-collaboration-approves-new-treatment-option-patients (Accessed January 22, 2021).
21. Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Acalabrutinib With or Without Obinutuzumab Versus Chlorambucil and Obinutuzmab for Treatment-Naive Chronic Lymphocytic Leukaemia (ELEVATE TN): A Randomised, Controlled, Phase 3 Trial. Lancet (2020) 395:1278–91. doi: 10.1016/S0140-6736(20)30262-2
22. Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, et al. Ascend: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. J Clin Oncol (2020) 38:2849–61. doi: 10.1200/JCO.19.03355
23. Wang M, Rule S, Zinzani PL, Goy A, Casasnovas O, Smith SD, et al. Acalabrutinib in Relapsed or Refractory Mantle Cell Lymphoma (ACE-LY-004): A Single-Arm, Multicentre, Phase 2 Trial. Lancet (2018) 391:659–67. doi: 10.1016/S0140-6736(17)33108-2
24. Wang M, Rule S, Zinzani PL, Goy A, Casasnovas O, Smith SD, et al. Durable Response With Single-Agent Acalabrutinib in Patients With Relapsed or Refractory Mantle Cell Lymphoma. Leukemia (2019) 33:2762–6. doi: 10.1038/s41375-019-0575-9
25. Owen RG, McCarthy H, Rule S, D’Sa S, Thomas SK, Tournilhac O, et al. Acalabrutinib Monotherapy in Patients With Waldenstrom Macroglobulinemia: A Single-Arm, Multicentre, Phase 2 Study. Lancet Haematol (2020) 7:e112–e21. doi: 10.1016/S2352-3026(19)30210-8
26. Owen RG, McCarthy H, Rule S, D’Sa S, Thomas S, Foroni F, et al. Acalabrutinib in Patients (Pts) With Waldenström Macroglobulinemia (WM) [Abstract]. J Clin Oncol (2018) 36(15 Suppl):Abstract 7501. doi: 10.1200/JCO.2018.36.15_suppl.7501
27. Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib Versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia. N Engl J Med (2014) 371:213–23. doi: 10.1056/NEJMoa1400376
28. Munir T, Brown JR, O’Brien S, Barrientos JC, Barr PM, Reddy NM, et al. Final Analysis From RESONATE: Up to Six Years of Follow-Up on Ibrutinib in Patients With Previously Treated Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma. Am J Hematol (2019) 94:1353–63. doi: 10.1002/ajh.25638
29. Treon SP, Tripsas CK, Meid K, Warren D, Varma G, Green R, et al. Ibrutinib in Previously Treated Waldenstrom’s Macroglobulinemia. N Engl J Med (2015) 372:1430–40. doi: 10.1056/NEJMoa1501548
30. Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as Initial Therapy for Patients With Chronic Lymphocytic Leukemia. N Engl J Med (2015) 373:2425–37. doi: 10.1056/NEJMoa1509388
31. Li N, Sun Z, Liu Y, Guo M, Zhang Y, Zhou D, et al. Bgb-3111 is a Novel and Highly Selective Bruton’s Tyrosine Kinase (BTK) Inhibitor [Abstract]. Cancer Res (2015) 75(15 Suppl):Abstract 2597. doi: 10.1158/1538-7445.AM2015-2597
32. U.S. Food and Drug Administration. FDA Grants Accelerated Approval to Zanubrutinib for Mantle Cell Lymphoma (2019). Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-zanubrutinib-mantle-cell-lymphoma (Accessed January 22, 2021).
33. Tam CS, LeBlond V, Novotny W, Owen RG, Tedeschi A, Atwal S, et al. A Head-to-Head Phase III Study Comparing Zanubrutinib Versus Ibrutinib in Patients With Waldenstrom Macroglobulinemia. Future Oncol (2018) 14:2229–37. doi: 10.2217/fon-2018-0163
34. Cull G, Simpson D, Opat S, Burger JA, Trotman J, Marlton P, et al. Treatment With the Bruton Tyrosine Kinase Inhibitor Zanubrutinib (BGB-3111) Demonstrates High Overall Response Rate and Durable Responses in Patients With Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL): Updated Results From a Phase 1/2 Trial [Abstract]. Blood 134(Suppl 1):500. doi: 10.1182/blood-2019-125483
35. Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, et al. Phase 1 Study of the Selective BTK Inhibitor Zanubrutinib in B-cell Malignancies and Safety and Efficacy Evaluation in CLL. Blood (2019) 134:851–9. doi: 10.1182/blood.2019001160
36. Tam CS, Opat S, D’Sa S, Jurczak W, Lee HP, Cull G, et al. A Randomized Phase 3 Trial of Zanubrutinib vs Ibrutinib in Symptomatic Waldenstrom Macroglobulinemia: The ASPEN Study. Blood (2020) 136:2038–50. doi: 10.1182/blood.2020006844
37. Lee-Verges E, Hanna BS, Yazdanparast H, Rodriguez V, Rodriguez ML, Giro A, et al. Selective BTK Inhibition Improves Bendamustine Therapy Response and Normalizes Immune Effector Functions in Chronic Lymphocytic Leukemia. Int J Cancer (2019) 144:2762–73. doi: 10.1002/ijc.32010
38. Brown JR, Harb WA, Hill BT, Gabrilove J, Sharman JP, Schreeder MT, et al. Phase I Study of Single-Agent CC-292, a Highly Selective Bruton’s Tyrosine Kinase Inhibitor, in Relapsed/Refractory Chronic Lymphocytic Leukemia. Haematologica (2016) 101:e295–8. doi: 10.3324/haematol.2015.140806
39. Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-Year Follow-Up of Treatment-Naive and Previously Treated Patients With CLL and SLL Receiving Single-Agent Ibrutinib. Blood (2015) 125:2497–506. doi: 10.1182/blood-2014-10-606038
40. Byrd JC, Harrington B, O’Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med (2016) 374:323–32. doi: 10.1056/NEJMoa1509981
41. Kutsch N, Pallasch C, Decker T, Hebart H, Chow KU, Graeven U, et al. A Prospective, Open-Label, Multicenter, Phase 2 Trial to Evaluate the Safety and Efficacy of the Combination of Tirabrutinib (ONO/GS-4059) and Idelalisib With and Without Obinutuzumab in Patients With Relapsed/Refractory Chronic Lymphocytic Leukemia (CLL) [Abstract]. Blood (2019) 134(Suppl 1):3047. doi: 10.1182/blood-2019-131025
43. Flinsenberg TWH, Tromedjo CC, Hu N, Liu Y, Guo Y, Thia KYT, et al. Differential Effects of BTK Inhibitors Ibrutinib and Zanubrutinib on NK-cell Effector Function in Patients With Mantle Cell Lymphoma. Haematologica (2020) 105:e76–e9. doi: 10.3324/haematol.2019.220590
44. Michot JM, Ribrag V. Pirtobrutinib Shows Evidence to Inaugurate a Third Generation of BTK Inhibitors. Lancet (2021) 397:855–7. doi: 10.1016/S0140-6736(21)00235-X
45. Kaptein A, de Bruin G, Emmelot-van Hoek M, van de Kar B, de Jong A, Gulrajani M, et al. Potency and Selectivity of BTK Inhibitors in Clinical Development for B-cell Malignancies [Abstract]. Blood (2018) 132(Suppl 1):1871. doi: 10.1182/blood-2018-99-109973
46. Bye AP, Unsworth AJ, Desborough MJ, Hildyard CAT, Appleby N, Bruce D, et al. Severe Platelet Dysfunction in NHL Patients Receiving Ibrutinib is Absent in Patients Receiving Acalabrutinib. Blood Adv (2017) 1:2610–23. doi: 10.1182/bloodadvances.2017011999
47. Series J, Garcia C, Levade M, Viaud J, Sié P, Ysebaert L, et al. Differences and Similarities in the Effects of Ibrutinib and Acalabrutinib on Platelet Functions. Haematologica (2019) 104:2292–9. doi: 10.3324/haematol.2018.207183
48. Byrd JC, Wierda WG, Schuh A, Devereux S, Chaves JM, Brown JR, et al. Acalabrutinib Monotherapy in Patients With Relapsed/Refractory Chronic Lymphocytic Leukemia: Updated Phase 2 Results. Blood (2020) 135:1204–13. doi: 10.1182/blood.2018884940
49. Byrd JC, Woyach J, Furman RR, Martin P, SM OB, Brown JR, et al. Acalabrutinib in Treatment-Naive (TN) Chronic Lymphocytic Leukemia (CLL): Updated Results From the Phase 1/2 ACE-CL-001 Study [Abstract]. Blood (2018) 132(Suppl 1):692. doi: 10.1182/blood-2018-99-110451
50. Byrd JC, Woyach JA, Furman RR, Martin P, O’Brien SM, Brown JR, et al. Acalabrutinib in Treatment-Naïve Chronic Lymphocytic Leukemia: Mature Results From Phase II Study Demonstrating Durable Remissions and Long-Term Tolerability [Abstract]. J Clin Oncol (2020) 38(15 Suppl):Abstract 8024. doi: 10.1200/JCO.2020.38.15_suppl.8024
51. Woyach JA, Rogers KA, Bhat SA, Blachly JS, Jianfar M, Frigault MM, et al. Acalabrutinib With Obinutuzumab (Ob) in Treatment-Naive (TN) and Relapsed/Refractory (R/R) Chronic Lymphocytic Leukemia (CLL): Three-year Follow-Up [Abstract]. J Clin Oncol (2019) 37(15 Suppl):Abstract 7500. doi: 10.1200/JCO.2019.37.15_suppl.7500
52. Awan FT, Schuh A, Brown JR, Furman RR, Pagel JM, Hillmen P, et al. Acalabrutinib Monotherapy in Patients With Chronic Lymphocytic Leukemia Who are Intolerant to Ibrutinib. Blood Adv (2019) 3:1553–62. doi: 10.1182/bloodadvances.2018030007
53. Rogers KA, Thompson PA, Allan JN, Coleman M, Sharman JP, Cheson BD, et al. Phase 2 Study of Acalabrutinib in Ibrutinib (IBR)-Intolerant Patients (Pts) With Relapsed/Refractory (R/R) Chronic Lymphocytic Leukemia (CLL) [Abstract]. J Clin Oncol (2019) 37(15 Suppl):Abstract 7530. doi: 10.1200/JCO.2019.37.15_suppl.7530
54. Barr P, Smith S, Roschewski M, O’Brien S, Sharman JP, Melear J, et al. Acalabrutinib Combined With PI3Kδ Inhibitor ACP-319 in Patients (Pts) With Relapsed/Refractory (R/R) B-cell Malignancies [Abstract]. J Clin Oncol (2018) 36(15 Suppl):Abstract 7518. doi: 10.1200/JCO.2018.36.15_suppl.7518
55. Witzig T, Maddocks KJ, de Vos S, Lyons R, Edenfield W, Sharman J, et al. Phase 1/2 Trial of Acalabrutinib Plus Pembrolizumab (Pem) in Relapsed/Refractory (R/R) Diffuse Large B-cell Lymphoma (DLBCL) [Abstract]. J Clin Oncol (2019) 37(15 Suppl):Abstract 7519. doi: 10.1200/JCO.2019.37.15_suppl.7519
56. Byrd JC, Owen RG, O’Brien SM, Brown JR, Hillmen P, Bitman B, et al. Pooled Analysis of Safety Data From Clinical Trials Evaluating Acalabrutinib Monotherapy in Hematologic Malignancies [Abstract]. Blood (2017) 130(Suppl 1):4326. doi: 10.1182/blood.V130.Suppl_1.4326.4326
57. Badillo M, Nava D, Dela Rosa M, Chen W, Wang M. Management of Adherence and Adverse Events in Patients With Mantle Cell Lymphoma Treated With Acalabrutinib: The MD Anderson Cancer Center Experience(2018). Available at: https://www.eventscribe.com//2018/posters/PPLC/SplitViewer.asp?PID=MjQ3MDE3ODAyMjQ%0D# (Accessed January 22, 2021).
58. Shatzel JJ, Olson SR, Tao DL, McCarty OJT, Danilov AV, DeLoughery TG. Ibrutinib-Associated Bleeding: Pathogenesis, Management and Risk Reduction Strategies. J Thromb Haemost (2017) 15:835–47. doi: 10.1111/jth.13651
59. Telford C, Kabadi SM, Abhyankar S, Song J, Signorovitch J, Zhao J, et al. Matching-Adjusted Indirect Comparisons of the Efficacy and Safety of Acalabrutinib Versus Other Targeted Therapies in Relapsed/Refractory Mantle Cell Lymphoma. Clin Ther (2019) 41:2357–79.e1. doi: 10.1016/j.clinthera.2019.09.012
60. Woyach J, Huang Y, Rogers K, Bhat SA, Grever MR, Lozanski A, et al. Resistance to Acalabrutinib in CLL is Mediated Primarily by BTK Mutations [Abstract]. Blood (2019) 134(Suppl 1):504. doi: 10.1182/blood-2019-127674
61. Phillips TJ, Smith SD, Jurczak W, Robak T, Stevens DA, Farber CM, et al. Safety and Efficacy of Acalabrutinib Plus Bendamustine and Rituximab (BR) in Patients With Treatment-Naive (TN) or Relapsed/Refractory (R/R) Mantle Cell Lymphoma (MCL) [Abstract]. Blood (2018) 132(Suppl 1):4144. doi: 10.1182/blood-2018-99-110617
62. Woyach JA, Awan FT, Jianfar M, Rogers KA, Jones J, Covey T, et al. Acalabrutinib With Obinutuzumab in Relapsed/Refractory and Treatment-Naive Patients With Chronic Lymphocytic Leukemia: The Phase 1b/2 ACE-CL-003 Study. Blood (2017) 130(Suppl 1):432. doi: 10.1182/blood.V130.Suppl_1.432.432
63. Byrd JC, Wierda WG, Schuh A, Devereux S, Chaves JM, Brown JR, et al. Acalabrutinib Monotherapy in Patients With Relapsed/Refractory Chronic Lymphocytic Leukemia: Updated Results From the Phase 1/2 ACE-CL-001 Study. Blood (2017) 130(Suppl 1):498. doi: 10.1182/blood.V130.Suppl_1.498.498
64. Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton Tyrosine Kinase Inhibitor Ibrutinib (PCI-32765) has Significant Activity in Patients With Relapsed/Refractory B-cell Malignancies. J Clin Oncol (2013) 31:88–94. doi: 10.1200/JCO.2012.42.7906
65. Byrd JC, Hillmen P, Ghia P, Kater AP, Chanan-Khan A, Furman RR, et al. First Results of a Head-to-Head Trial of Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia [Abstract]. Am Soc Clin Oncol Annu Meeting (Forthcoming 2021).
66. Kapoor I, Bodo J, Hill BT, Hsi ED, Almasan A. Targeting BCL-2 in B-cell Malignancies and Overcoming Therapeutic Resistance. Cell Death Dis (2020) 11:941. doi: 10.1038/s41419-020-03144-y
67. Brown JR, Eichhorst BF, Ghia P, Kater AP, Li J, Khurana S, et al. A Phase 3 Trial Comparing the Efficacy and Safety of Acalabrutinib in Combination With Venetoclax With or Without Obinutuzumab, Compared With Investigator’s Choice of Chemoimmunotherapy in Patients With Previously Untreated Chronic Lymphocytic Leukemia (CLL) Without Del(17p) or TP53 Mutation [Abstract]. Blood (2019) 134(Suppl 1):4318. doi: 10.1182/blood-2019-123057
68. Davids MS, Lampson BL, Tyekucheva S, Crombie JL, Ng S, Kim AI, et al. Updated Safety and Efficacy Results From a Phase 2 Study of Acalabrutinib, Ventoclax and Obinutuzumab (AVO) for Frontline Treatment of Chronic Lymphocytic Leukemia (CLL) [Abstract]. In: 62nd ASH Annual Meeting and Exposition. Blood (2020) 136:20–1. doi: 10.1182/blood-2020-139864
69. Woyach JA, Blachly JS, Rogers KA, Bhat SA, Grever MR, Kittai AS, et al. Acalabrutinib in Combination With Venetoclax and Obinutuzumab or Rituximab in Patients With Treatment-Naïve or Relapsed/Refractory Chronic Lymphocytic Leukemia [Abstract]. In: 62nd ASH Annual Meeting and Exposition. Blood (2020) 136:16–8. doi: 10.1182/blood-2020-136317
70. Li L, Zhang J, Chen J, Xu-Monette ZY, Miao Y, Xiao M, et al. B-Cell Receptor-Mediated NFATc1 Activation Induces IL-10/STAT3/PD-L1 Signaling in Diffuse Large B-cell Lymphoma. Blood (2018) 132:1805–17. doi: 10.1182/blood-2018-03-841015
71. Khan M, Siddiqi T. Targeted Therapies in CLL: Monotherapy Versus Combination Approaches. Curr Hematol Malig Rep (2018) 13:525–33. doi: 10.1007/s11899-018-0481-7
72. Moreno C, Greil R, Demirkan F, Tedeschi A, Anz B, Larratt L, et al. Ibrutinib Plus Obinutuzumab Versus Chlorambucil Plus Obinutuzumab in First-Line Treatment of Chronic Lymphocytic Leukaemia (iLLUMINATE): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2019) 20:43–56. doi: 10.1016/S1470-2045(18)30788-5
73. Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D’Rozario J, Assouline S, et al. Murano Trial Establishes Feasibility of Time-Limited Venetoclax-Rituximab (VenR) Combination Therapy in Relapsed/Refractory (R/R) Chronic Lymphocytic Leukemia (CLL). Blood (2018) 132(Suppl 1):184. doi: 10.1182/blood-2018-184
74. Al-Sawaf O, Zhang C, Tandon M, Sinha A, Fink AM, Robrecht S, et al. Venetoclax Plus Obinutuzumab Versus Chlorambucil Plus Obinutuzumab for Previously Untreated Chronic Lymphocytic Leukaemia (CLL14): Follow-Up Results From a Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol (2020) 21:1188–200. doi: 10.1016/S1470-2045(20)30443-5
75. Lampson BL, Tyekucheva S, Crombie JL, Kim AI, Merryman RW, Lowney J, et al. Preliminary Safety and Efficacy Results From a Phase 2 Study of Acalabrutinib, Venetoclax and Obinutuzumab in Patients With Previously Untreated Chronic Lymphocytic Leukemia (CLL) [Abstract]. Blood (2019) 134(Suppl 1):32. doi: 10.1182/blood-2019-127506
76. Sheng Z, Song S, Yu M, Zhu H, Gao A, Gao W, et al. Comparison of Acalabrutinib Plus Obinutuzumab, Ibrutinib Plus Obinutuzumab and Venetoclax Plus Obinutuzumab for Untreated CLL: A Network Meta-Analysis. Leuk Lymphoma (2020) 61:3432–9. doi: 10.1080/10428194.2020.1811271
Keywords: acalabrutinib, Bruton tyrosine kinase inhibitor, B-cell malignancies, chronic lymphocytic leukemia, mantle cell lymphoma, Waldenstrom’s macroglobulinemia
Citation: Abbas HA and Wierda WG (2021) Acalabrutinib: A Selective Bruton Tyrosine Kinase Inhibitor for the Treatment of B-Cell Malignancies. Front. Oncol. 11:668162. doi: 10.3389/fonc.2021.668162
Received: 15 February 2021; Accepted: 19 April 2021;
Published: 14 May 2021.
Edited by:
Alessandro Isidori, AORMN Hospital, ItalyReviewed by:
Lydia Scarfò, Vita-Salute San Raffaele University, ItalyNarendranath Epperla, The Ohio State University, United States
Copyright © 2021 Abbas and Wierda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: William G. Wierda, wwierda@mdanderson.org
 Hussein A. Abbas
Hussein A. Abbas William G. Wierda
William G. Wierda