- 1Department of Surgical Oncology, Paoli Calmettes Institute, Marseille, France
- 2CRCM, CNRS, INSERM, Aix Marseille Université, Marseille, France
Background: Several studies reported the feasibility and safety of robotic-NSM (R-NSM). The aim of our prospective study was to compare R-NSM and conventional-NSM (C-NSM).
Methods: We analyzed patients who were operated on with and without robotic assistance (R-NSM or C-NSM) and who received immediate breast reconstruction (IBR) with implant or latissimus dorsi-flap (LDF). The main objective was complication rate and secondary aims were post-operative length of hospitalization (POLH), duration of surgery, and cost.
Results: We analyzed 87 R-NSM and 142 C-NSM with implant-IBR in 50 and 135 patients, with LDF-IBR in 37 and 7 patients, respectively. Higher durations of surgery and costs were observed for R-NSM, without a difference in POLH and interval time to adjuvant therapy between R-NSM and C-NSM. In the multivariate analysis, R-NSM was not associated with a higher breast complication rate (OR=0.608) and significant factors were breast cup-size, LDF combined with implant-IBR, tobacco and inversed-T incision. Grade 2-3 breast complications rate were 13% for R-NSM and 17.3% for C-NSM, significantly higher for LDF combined with implant-IBR, areolar/radial incisions and BMI>=30. A predictive score was calculated (AUC=0.754). In logistic regression, patient’s satisfaction between C-NSM and R-NSM were not significantly different, with unfavorable results for BMI >=25 (OR=2.139), NSM for recurrence (OR=5.371) and primary breast cancer with radiotherapy (OR=4.533). A predictive score was calculated. In conclusion, our study confirms the comparable clinical outcome between C- NSM and R-NSM, in the price of longer surgery and higher cost for R-NSM. Predictive scores of breast complications and satisfaction were significantly associated with factors known in the pre-operative period.
Introduction
Despite an increase in breast conservative surgery, a total mastectomy is still necessary in 12% to 30% of patients (1–3) in cases of extended ductal carcinoma in-situ (DCIS), invasive breast cancer (BC) with an extensive DCIS component, multifocal disease, large BC according to breast size without indication of neoadjuvant chemotherapy (NAC), prophylactic mastectomies, ipsilateral BC local recurrence (ILBCLR), non in-sano initial resection, and patient’s wishes. Immediate breast reconstruction (IBR) rate increased progressively in relation with patient’s wishes and better quality of life (4, 5).
Nipplesparing mastectomy (NSM) is associated with better aesthetic results and better quality of life than skin-sparing mastectomy (6, 7). Consequently, NSM is the procedure of choice when this technique is possible, mainly in relation to the tumor nipple-areolar-complex (NACx) distance on radiologic exams. Several studies reported a few cases of robotic-NSM (R-NSM) to evaluate feasibility, reproducibility, and safety (8–27). Recently, a technical robotic surgical consensual NSM procedure was reported (28). However, comparison between R-NSM and conventional- NSM (C-NSM) has been recently reported in only one retrospective study with a small sample size and for procedures realized by only one surgeon (29). Moreover, a recent US FDA safety communication (30), in February 2019 (US) does not validate this technique which required more data before new evaluation.
The aim of our prospective study was to compare R-NSM and C-NSM in terms of breast complication rate as a main objective, and hospital stay, duration of surgery, cost evaluation, and patient’s satisfaction as secondary objectives.
Methods
Patients
Robotic NSM and IBR were performed by two surgeons over 51 months (from the first procedure in November 2016 to March 2020). All patients were informed of robotic assistance surgery. Our institutional ethical committee approved robotic breast surgery procedures and data were collected in institutional breast database.
After the preliminary experience of R-NSM with 27 R-NSM (27), we determined a standardized technique with dissection with non-robotic scissors after sub cutaneous infiltration with adrenaline serum and then robotic dissection through a mono-trocar insert in axillary or lateral small incision (26, 27). This technique was reported as the consensus (28). In this prospective study, we analyzed patients operated on between March 2018 and March 2020, with and without robotic assistance (R-NSM and C-NSM) and IBR with implant breast or latissimus dorsiflap (LDF) with or without association with implant. The choice between R-NSM and C-NSM was determined by surgeons. The main objective was complication rate for R-NSM versus C-NSM and 240 patients were planned to achieve this comparison since March 2018, with a first hypothesis of 80 RNSM and 160 C-NSM. Secondary aims were: post-operative length of hospitalization (POLH), duration of surgical procedure, cost evaluation, and patient’s satisfaction according the two groups, R-NSM and C-NSM.
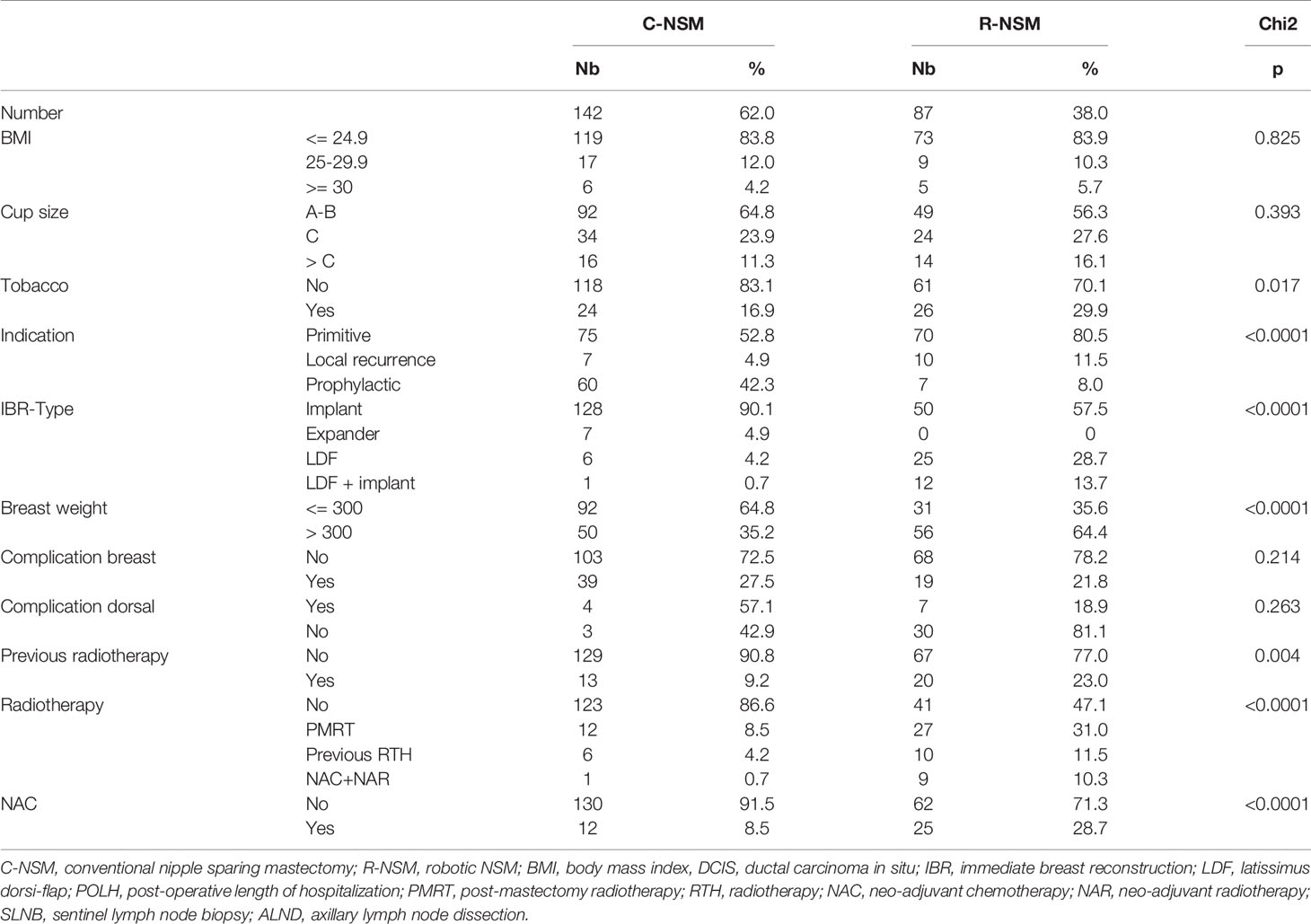
We determined the characteristics of patients (age, body mass index (BMI), tobacco use, diabetes, ASA status, breast cup-size), previous treatment for BC (sentinel lymph node biopsy, axillary lymph node dissection (ALND), neo-adjuvant chemotherapy, previous breast radiotherapy), indications of NSM (primary BC or local recurrence, reconstruction with robotic latissimus dorsi-flap (RLDF) and or breast implant). Interval times between surgery and adjuvant chemotherapy (AC) or post-mastectomy radiotherapy (PMRT) for patients without AC were recorded.
Surgical techniques with a type of Da Vinci system, number of trocars, skin incision, and duration of anesthesia and surgery were reported according to period of treatment and association of surgical procedures (mastectomy, breast implant, robotic-LDF (R-LDF), ALND, and contra-lateral breast surgery). Complication rate was determined by Clavien-Dindo grading (31) during a post-operative period of 30 days. Re-operation rate, type of complication, and number of POLH days were analyzed. During this period of study, we did not use an enhanced recovery program.
Indications of NSM
NSM was proposed to patients for prophylactic mastectomy, for local recurrence when a second breast conservative treatment was not possible or not the patient’s choice and for primary BC with indication of total mastectomy. NSM was realized when the tumor-nipple distance was 1 centimeter or more on radiologic exams. A retro nipple-areolar complex biopsy was systematically performed with definitive pathologic exam without per-operative analysis. Incisions were determined by surgeons according to breast characteristics and usual practice of surgeons. Inversed-T incisions with C-NSM were used for high breast volume with ptotic breast (breast cup-size >C and nipple areolar complex under infra mammary fold). A pre-operative flap thickness was assessed by surgeons during a clinical exam and a digital mammogram.
C-NSM Procedure
Depending on the surgeon’s habits, there was either infiltration with adrenalin serum, or just serum, or no infiltration at all. For superficial dissection, two techniques were used: scissors, or monopolar coagulation with electric usual coagulation or peak plasma blade. The exposition was done using retractors.
R-NSM procedure was reported previously (26, 27, 32). In summary, after superficial adrenal infiltration, dissection between skin and breast gland was conducted with scissors. Then, through a Gel point mono-trocar device disposed in axillar or external incision, two robotic trocars were inserted for the robotic camera (inferior part of Gel point) and one robotic instrument (superior part of Gel point). Another trocar was inserted at the inferior external part of breast (at the infra-mammary fold level). Complementary superficial dissection, periphery, and deep dissections were realized with robotic instruments (scissors with monopolar coagulation), using a low insufflation pressure (7mm Hg). When the implant was disposed under the pectoral muscle, the pocket was also performed with robotic instruments. Cost evaluation has been analyzed for all patients and for the following sub-populations: R-NSM and C-NSM, R-NSM with implant-IBR, and C-NSM with implant-IBR. Cost evaluation, expressed in Euros, was performed with cost of duration of anesthesia (length of operative room occupation), length of hospitalization (day number), cost of robotic instrumentation and other devices used, and cost of breast implant. We did not include purchase and maintenance costs of Da Vinci systems which are in relation with number of procedures per-year for breast surgery and others indications of robotic procedures for urologic, gynecologic and digestive tumors with a total of 862 procedures during the study period (98 patients for breast robotic surgery and 764 patients for others indications). In France, all fees for breast reconstruction are reimbursed by national insurance and cost of robotic procedure was supported by the institution. Patients did not have to pay out-of-pocket. Patient satisfaction was assessed by asking, orally by surgeons, at the consultation 6 to 12 months after surgery if satisfaction was very good, good, medium, fair, or unsatisfied.
Statistics
Main characteristics were reported with median, mean, and confident interval 95% (CI 95) for quantitative criteria. Comparisons were performed using Chi2, t-test, and binary logistic regression adjusted to significant univariate variables, with SPSS 16.0 (SPSS Inc., Chicago, Illinois). Predictive scores were calculated using Odds Ratios (OR) determined by logistic regression and evaluated by calculation of area under the ROC curves (AUC).
Results
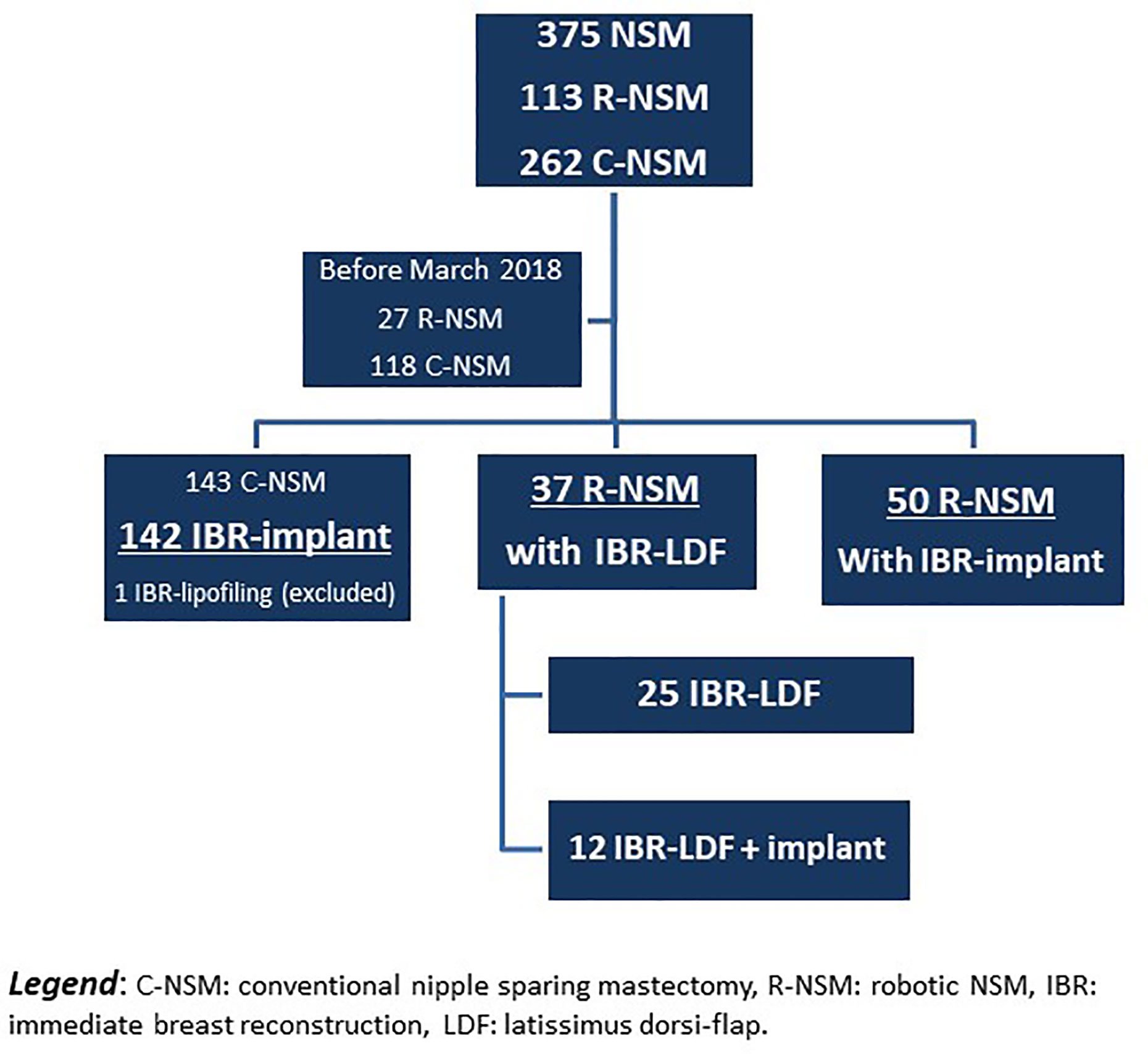
Out of 375 NSM performed since January 2016, 145 NSM were realized before March 2018, with 27 R-NSM reported in the preliminary experience of R-NSM. The present study analyzed 229 patients operated on from March 2018 to March 2020 (breast robotic surgery stopped on 2020-03-15 due to COVID-19 pandemic) with the exclusion of 1 patient with C-NSM and exclusive IBR-lipofilling (Figure 1). R-NSM were performed by two surgeons (82 and 5, respectively) and C-NSM (n=142) were performed by 8 surgeons (6 to 53 C-NSM). Characteristics of patients are reported in Table 1 and Supplemental Data File 1 according to technique used for NSM, R-NSM or C-NSM, and type of IBR (implant or expander, latissimus dorsi-flap). R-NSM with R-LDF-IBR was performed in 37 cases, with associated implant breast in 12 cases. All others R-NSM-IBR were realized with definitive breast implant (n=50) in 7 cases with pre-pectoral implant (3 prophylactic NSM and 4 for primary BC).
Several significant differences were reported between two groups with higher risk factor rates in RNSM group for tobacco use, histology, primary BC and local recurrence, breast weight, and oncologic treatments (Table 1, Supplemental Data Files 2). Breast cancer treatment: In the R-NSM group, higher rates of adjuvant chemotherapy, neo-adjuvant chemotherapy, and endocrine therapy were observed in comparison with the C-NSM group. More PMRT, previous radiotherapy, and neo-adjuvant chemotherapy with neo-adjuvant radiotherapy were also reported (Table 1, Supplemental Data Files 1).
Durations of surgery included all procedures and several installations from skin incision to the end of skin suture for R-NSM with R-LDF-IBR. Mean duration of surgery for all patients was 174 minutes. Median and mean durations are reported in Supplemental Data Files 2, for all patients and according to surgical procedures with higher values for robotic procedures. Duration of surgery >180mn was significantly associated in univariate analysis with R-NSM (p<0.0001), type of incision (p=0.003), implant-IBR or LDF-IBR or LDF-IBR with implant (p<0.001), age (p<0.0001), axillary surgery type (p<0.0001), breast cup-size (p=0.002), previous radiotherapy (p<0.0001), neo-adjuvant chemotherapy (p<0.0001), ASA status (p=0.001), year of surgery (p=0.005), indication for prophylactic surgery or local recurrence or primary BC (p=0.021), and surgeons (p<0.0001). In binary logistic regression, duration of surgery >180mn was significantly associated with R-NSM (OR: 101, CI95% 6.59-1548).
Outcome
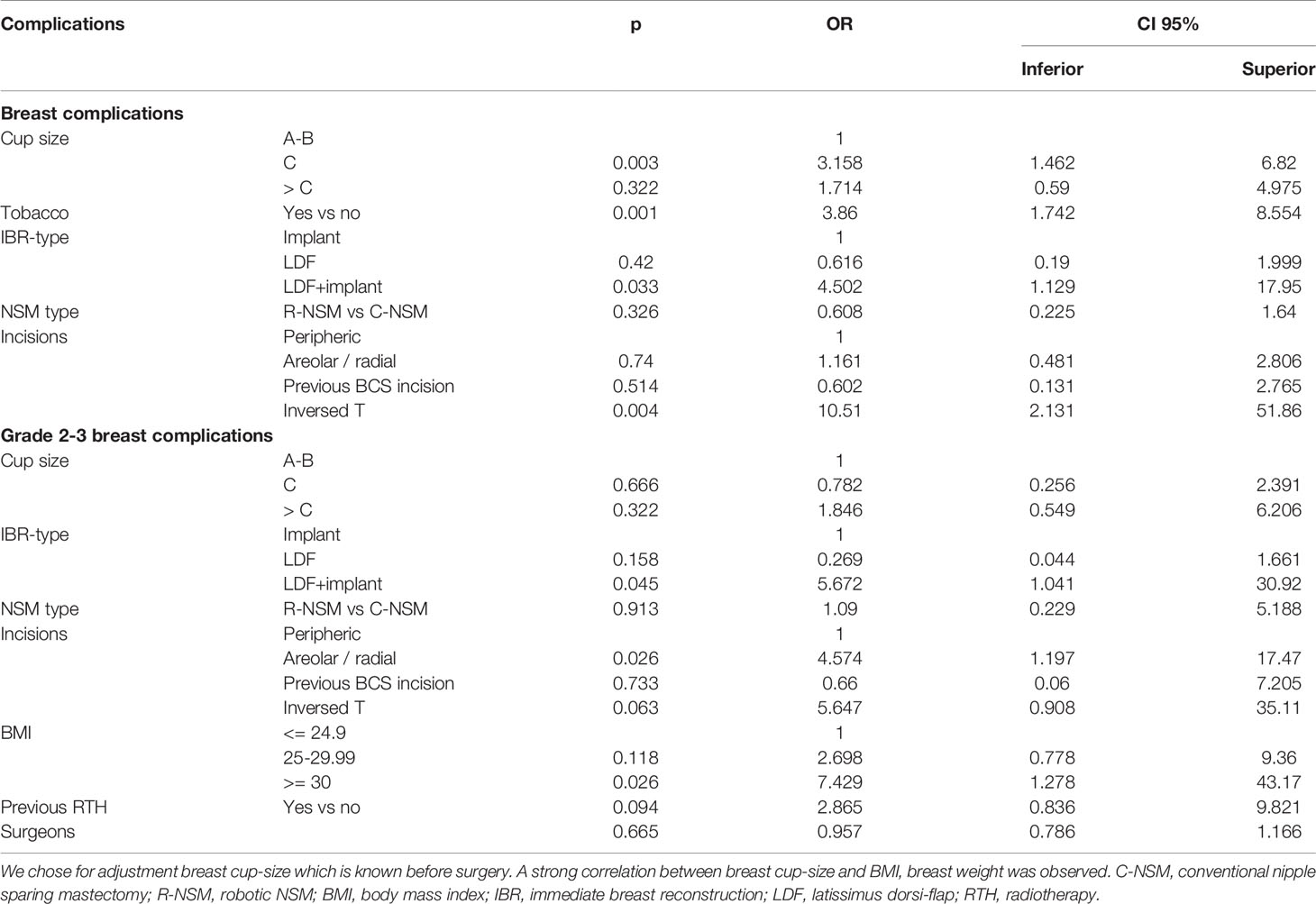
Median POLH was 2 days: 3 days for R-NSM and 2 days for C-NSM without significant difference between C-NSM-implant and R-NSM-implant, and between C-NSM-LDF and R-NSM-LDF (Supplemental Data Files 2). Overall breast complication crude rate was 25.3%: 21.8% (CI95% 13.2-30.5) for R-NSM and 27.5% (CI95% 20.1-34.8) for C-NSM (p=0.214). For patients with R-NSM combined with LDF-IBR, overall complication rate was 23.3%, 19.0% for breast complications, and 70.0% for dorsal complications (85.7% Grade 1 dorsal complications: dorsal seroma) in comparison with 13.8% breast complications for R-NSM with implant-IBR. Overall breast complications rates according to type of incisions were significantly different with a higher complication rate for inversed-T incision and areolar/radial incisions (Table 2). Inversed-T incisions were significantly associated with high breast volume: breast cup-size (p=0.003), breast weight (p=0.001) and BMI (p=0.017). In regression analysis adjusted on significant factors in univariate analysis, R-NSM was not associated with a higher breast complication rate in comparison with C-NSM (OR: 0.608, CI95% 0.225-1.64, p=0.326). Significant factors of breast complications were breast cup-size, LDF-IBR with implant, tobacco, and inversed-T incision (Table 3).
Grade 2-3 breast complications rate was 13.5%, 13% for R-NSM (CI95% 4.8-18.2) and 17.3% for CNSM (CI95% 8.95-20.63) (Table 2). Grade 2-3 breast complications rates according to type of incisions were significantly different with higher complication rates for inversed-T incision and areolar/radial incisions (Table 2). Re-operation rates were not significantly different between two groups of NSM (p=0.583).
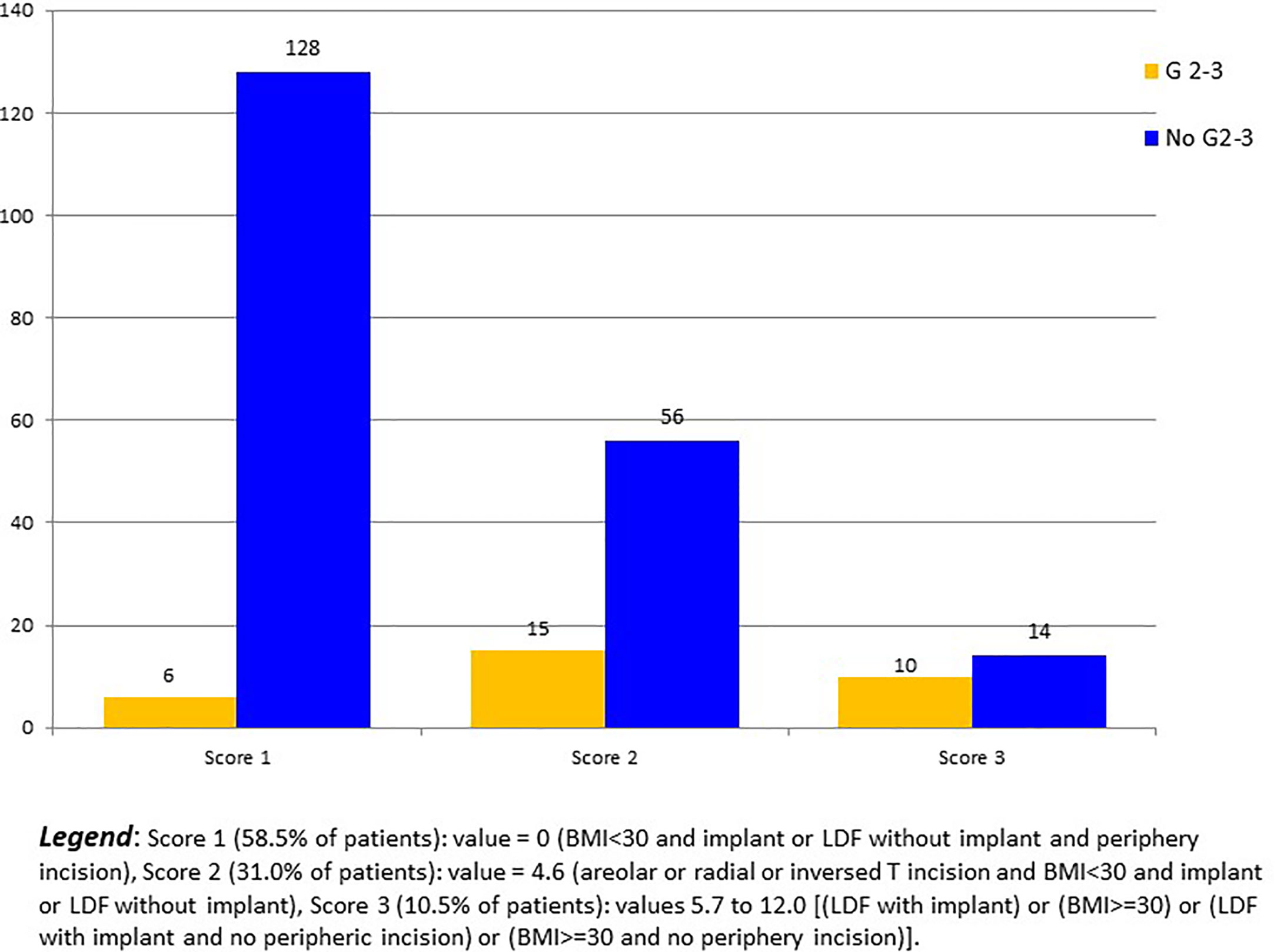
In regression analysis, R-NSM was not associated with a higher Grade 2-3 breast complication rate in comparison with C-NSM (OR: 1.09, CI95% 0.229-5.188, p=0.913). Significant factors of Grade 2-3 breast complications were LDF-IBR with implant, areolar/radial incisions, and BMI >=30 (Table 3). Predictive score of breast complication Grade 2-3 was calculated (BMI >=30: 7.4 versus 0, LDF with implant: 5.7 versus 0, areolar or radial or inversed T incisions: 4.6 versus 0) with breast complications Grade 2-3 rates of 4.5% (6/134), 21.1% (15/71), 50.0% (6/12), 28.6% (2/7), 0% (0/1) and 50.0% (2/4) for score values 0, 4.6, 5.7, 7.4, 10.3 and 12.0, respectively (p<0.0001) (Figure 2). AUC of ROC curve was 0.754 (CI95% 0.660-0.847).
Implant loss rate was 7.8%: 10.2% for R-NSM (CI95% 2.5-17.9) (2.1% for R-NSM-implant and 41.7% for R-NSM-LDF-implant: 5/12) and 6.7% for C-NSM (CI95% 2.2-11.1) (p=0.293). High BMI (4 patients >24.9), high breast cup-size (8>=C), and high mastectomy weight (9>470gr) were observed among the twelve patients with R-NSM-LDF-implant.
Types of complications according to Grade of complication are reported in Supplemental Data Files 3. The more frequent complications were NACx or skin-flap suffering or necrosis (56.9%) and hematomas. Types of complications were not significantly different between C-NSM and R-NSM (p=0.770).
Interval Time Between Surgery to Adjuvant Therapy
There was no difference between C-NSM and RNSM for interval time <= or >60 days (p=0.530) and median interval time (Supplemental Data Files 2). For adjuvant chemotherapy and PMRT, median interval times were 48 days (mean 51, CI95% 41.9-60.2) and 60 days (mean 67.8, CI95% 53.5-82.1) respectively (p=0.042), 23.5% >60 days for chemotherapy and 46.7% for PMRT.
Cost Evaluation
Significantly higher cost was observed for R-NSM versus C-NSM. Mean cost was higher (+34.7%: 1749 Euros) for R-NSM-implant versus C-NSM-implant and higher (+30%: 2357 Euros) for RNSM-LDF versus C-NSM-LDF (Supplemental Data Files 2).
Patient’s Satisfaction
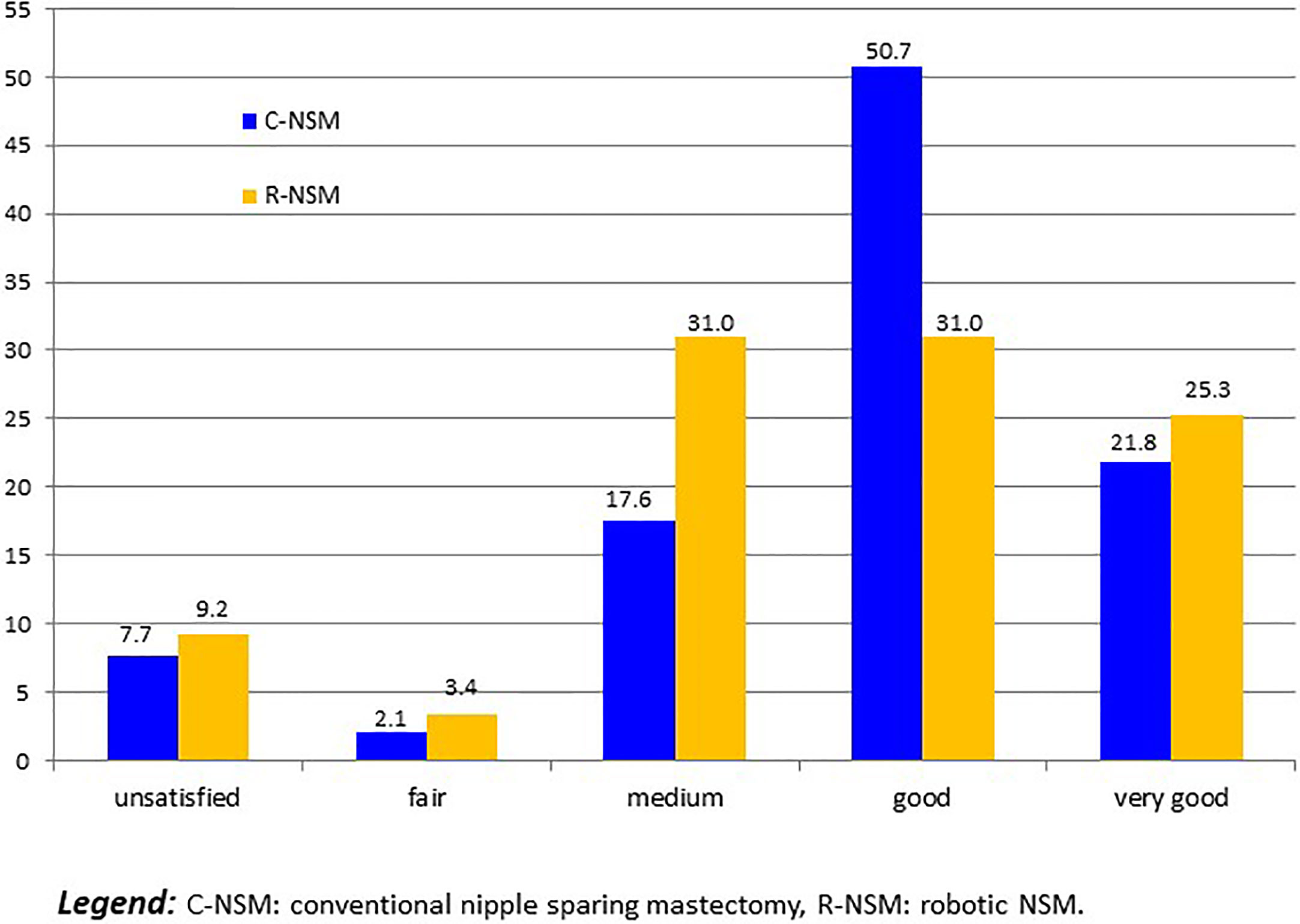
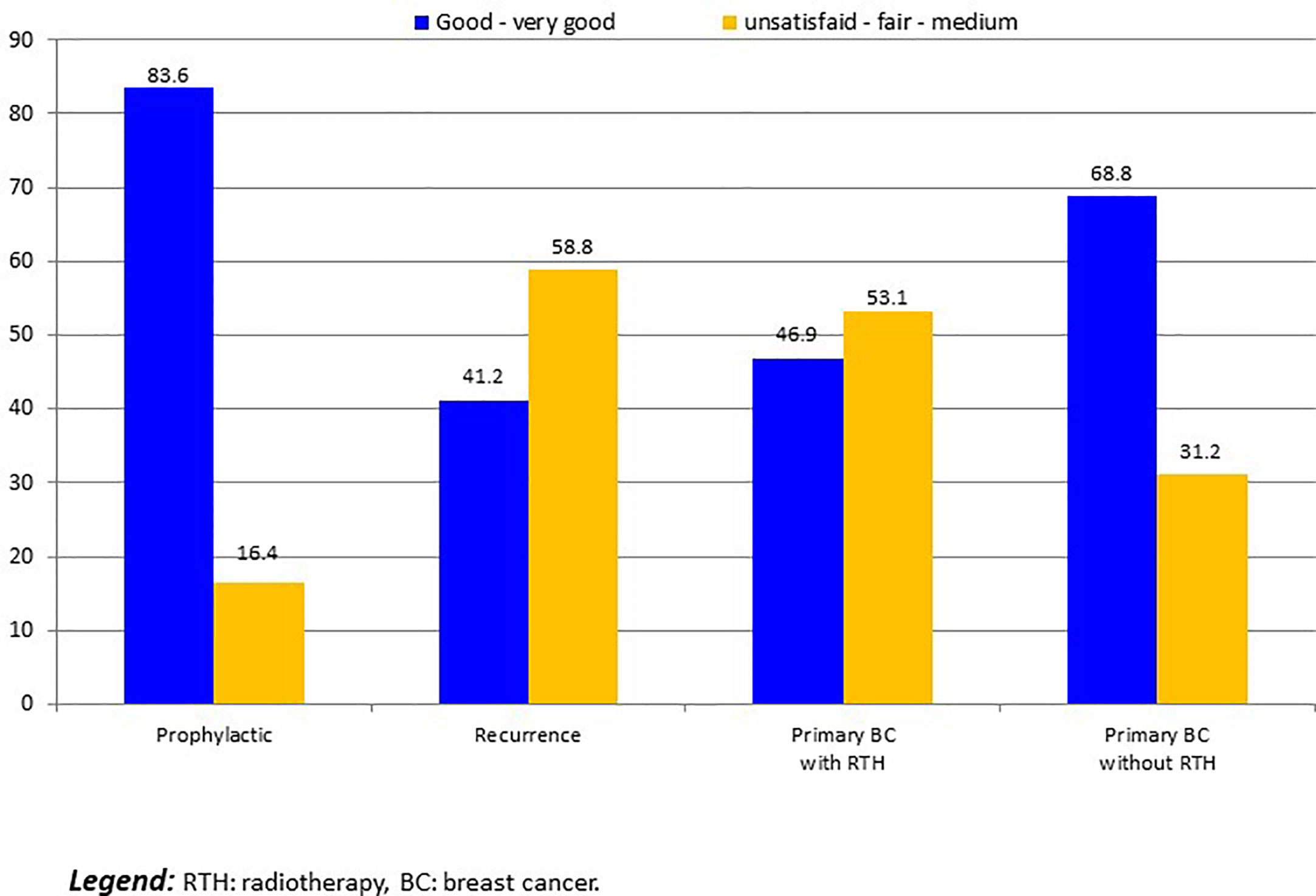
For 14 patients with implant loss, we considered that patients were unsatisfied. Five others patients required delayed explantation and were classified as unsatisfied: 2 R-NSM-implant for secondary complication with interval between IBR and explantation of more than 1 year, and 3 C-NSM-implant for local recurrence in 2 cases and patient’s choice in 1 case. Satisfaction was: 19 unsatisfied (8.3%:19/229), 6 fair (2.6%), 52 medium (22.7%), 99 good (43.2%) and 53 very good (23.1%). Satisfactions according to groups C-NSM and R-NSM were better for C-NSM (p=0.042) (Figure 3). In univariate analysis, satisfaction with 2 groups (>= or < good) were associated with BMI (p=0.012), tobacco (p=0.013), indication and radiotherapy (p=0.001) (Figure 4) and C-NSM or R-NSM (p=0.009). In binary logistic regression, adjusted on tobacco, indication and radiotherapy, BMI, C-NSM, and R-NSM were not significantly different (OR: 1.151, CI95% 0.591-2.241, p=0.679) with unfavorable results for BMI >=25 (OR: 2.139, CI95% 0.996-4.595, p=0.051), NSM for recurrence (OR: 5.371, CI95% 1.560-18.49, p=0.008) and primary BC with radiotherapy (OR: 4.533, CI95% 1.728-11.89, p=0.002) (non-significant: tobacco and primary BC without radiotherapy in comparison with prophylactic NSM). Predictive score of satisfaction good or very good (versus others) was calculated (BMI >=25: 2 versus 0, recurrence: 5.0 and primary BC with radiotherapy: 4.5 (versus 0 for prophylactic and primary BC without radiotherapy)) with unsatisfied or bad or medium results in 24.8%, 27.3%, 46.2%, 41.7%, 80.0% and 100% for score values of 0 (141 patients: 61.6%), 2.0 (22 patients: 9.6%), 4.5 (39 patients: 17.0%), 5.0 (12 patients: 5.2%), 6.5 (10 patients: 5.2%) and 7.0 (5 patients: 2.2%), respectively (p<0.0001). AUC of ROC curve was 0.651 (CI95% 0.572-0.730).
Discussion
Despite several significant higher rates of risk factors for complications in the R-NSM group in comparison with C-NSM group, we reported no significant difference for breast complications and Grade 2-3 breast complications in univariate and multivariate analysis between the two groups. There was also no significant difference for POLH and interval time before adjuvant treatments. However, significant higher durations of surgery and costs were observed for the R-NSM group. Since the first publications, R-NSM were reported in several studies to determine the feasibility and technique, including 1 to 94 procedures (8–27). Despite these reports, FDA safety communication in February 2019 underlined caution when using robotically-assisted surgical devices for mastectomy (30).
In a recent study by Lai et al. (29), a comparison between R-NSM and C-NSM with implant IBR was reported to determine complication rates, duration of surgery, and costs. During a period of 99 months, authors reported 54 R-NSM and 62 C-NSM. In our study, we reported higher numbers of patients in each group, 87 R-NSM and 142 C-NSM, during a shorter period of 25 months. A lower RNSM rate of 38.0% in our study was reported in comparison with 46.5% in the Lai et al. study.
Complication rates were not significantly different between two groups (29) as we observed. Complication rates were 41% and 46.8% in the Lai et al. study (29), 21.8% and 27.5% in our study, for RNSM and C-NSM groups, respectively. The rate of reoperation was 4.3% in the Toesca et al. study among 73 women who underwent 94 R-NSM (10), lesser than our results of 9.2% in each groups. Breast complications Grade 2-3 could be predicted using our score with a good accuracy (AUC: 0.754), with low rates of Grade 2-3 breast complications for patients with BMI<30 and periphery incision and implant-IBR or LDF-IBR without implant (4.48%) and for patients with BMI <30 and implant-IBR or LDF-IBR without implant and no periphery incision (21.1%) in comparison with a rate of 41.7% for other patients. However, this score needs validation in another independent study.
Hospital stays were higher in the Lai et al. study (29) for all patients and the two groups in comparison with our practice: 6 days versus 2 days in our study. This might be correlated with the Enhanced Recovery after Surgery program, set up in our institute in 2017, initially for gynecologic, digestive, and urologic surgery. Another important difference was mean mastectomy weights which were 293gm and 386gm for R-NSM, 321 and 280gm for C-NSM, in the Lai et al. study (29) and in our study, respectively.
As we reported, higher durations of surgery were observed (29) for R-NSM with a difference of 27mn between the two groups, corresponding to increase of 12% of time for R-NSM versus C-NSM. We reported higher differences: an increase of 41.5% for R-NSM-implant versus C-NSM-implant and 28% for R-NSM-LDF versus C-NSM-LDF. However, the mean durations of surgeries were comparable: 224 and 184mn for R-NSM-implant, 197 and 130mn for C-NSM in the Lai et al. study (29) versus our study respectively.
Cost evaluation in the Lai et al. study (29) differed between two groups with higher total costs for R-NSM versus C-NSM: 90.7% more expensive for R-NSM in comparison with C-NSM. In our study, R-NSM was also more expensive but with a difference of 34.7% for R-NSM-implant and 30% for R-NSM-LDF. However, the method of costs evaluations differed between the two studies. The overall satisfaction rate was higher in the R-NSM group versus C-NSM group in study of Lai et al. (29) mainly attributed with periphery breast scar location for R-NSM. In our study, there was no significant difference between C-NSM and R-NSM in multivariate analysis and unfavorable satisfaction results could be predicted using our score with intermediate accuracy (AUC=0.651). However, good and very good results were reported in 74.8% of patients with score value <=2 (BMI <25 without other pejorative factor) which represented 71.2% of patients and in 54.9% of patients with score value 4.5 or 5.0 (primary BC with radiotherapy or recurrence and BMI <25) which represented 22.2% of patients.
We determined several situations with factors known in the pre-operative period associated with high rates of breast complications (tobacco use, IBR with LDF and implant, cup-size >= C, BMI >=30 and incisions with inversed-T or areolar/radial) and a predictive pre-operative score of Grade 2-3 complications with a contributive accuracy (AUC=0.754). In these situations, information for patients should be completed before the patient’s choice for IBR or no IBR. Despite a greater number of patients in comparison with the first reported study comparing C-NSM and R-NSM (29), our study is limited in its small sample size and satisfaction evaluation performed at different intervals between surgery and last follow-up, without use of a validated questionnaire and without aesthetic assessment using photography before and after breast reconstruction.
Selection bias between two groups, C-NSM versus R-NSM, was compassed by multivariate analysis. Oncological results cannot be analyzed due to the very short follow-up period. In conclusion, our study confirms comparable clinical outcomes between conventional NSM and robotic NSM, with a greater number of patients than the previous study, at the cost of longer surgery and higher cost for robotic surgery. Interestingly, predictive scores of breast complications and satisfaction were significantly associated with factors known in the pre-operative period.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Paoli Calmettes Institute review board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Authors who made substantial contributions to conception and design, and/or acquisition of data, and/or analysis and interpretation of data: GH, JB, CJ, MC, and MBa. Authors who participated in drafting the article or revising it critically for important intellectual content: GH, JB, MC, and MBa. Authors who gave final approval of the version to be published: GH, JB, CJ, SR, LS, AV, MBu, GB, EL, MC, and MBa. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.637049/full#supplementary-material
References
1. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinellymph- node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol (2010) 11(10):927−33. doi: 10.1016/S1470-2045(10)70207-2
2. Houvenaeghel G, Cohen M, Raro P, De Troyer J, de Lara CT, Gimbergues P, et al. Overview of the pathological results and treatment characteristics in the first 1000 patients randomized in the SERC trial: axillary dissection versus no axillary dissection in patients with involved sentinel node. BMC Cancer (2018) 18(1):1153. doi: 10.1186/s12885-018-5053-7
3. Houvenaeghel G, Lambaudie E, Cohen M, Classe J-M, Reyal F, Garbay J-R, et al. Therapeutic escalation - De-escalation: Data from 15.508 early breast cancer treated with upfront surgery and sentinel lymph node biopsy (SLNB). Breast (2017) 34:24−33. doi: 10.1016/j.breast.2017.04.008
4. Nègre G, Balcaen T, Dast S, Sinna R, Chazard E. Breast reconstruction in France, observational study of 140,904 cases of mastectomy for breast cancer. Ann Chir Plast Esthet (2020) 65(1):36−43. doi: 10.1016/j.anplas.2019.07.014
5. Dauplat J, Kwiatkowski F, Rouanet P, Delay E, Clough K, Verhaeghe JL, et al. Quality of life after mastectomy with or without immediate breast reconstruction. Br J Surg (2017) 104(9):1197−206. doi: 10.1002/bjs.10537
6. Wei CH, Scott AM, Price AN, Miller HC, Klassen AF, Jhanwar SM, et al. Psychosocial and Sexual Well-Being Following Nipple-Sparing Mastectomy and Reconstruction. Breast J (2016) 22(1):10−7. doi: 10.1111/tbj.12542
7. Mota BS, Riera R, Ricci MD, Barrett J, de Castria TB, Atallah ÁN, et al. Nipple- and areolasparing mastectomy for the treatment of breast cancer. Cochrane Database Syst Rev (2016) 11:CD008932. doi: 10.1002/14651858.CD008932.pub3
8. Toesca A, Peradze N, Manconi A, Galimberti V, Intra M, Colleoni M, et al. Robotic nipple-sparing mastectomy for the treatment of breast cancer: Feasibility and safety study. Breast (2017) 31:51–6. doi: 10.1016/j.breast.2016.10.009
9. Toesca A, Peradze N, Galimberti V, Manconi A, Intra M, Gentilini O, et al. Robotic Nipple-sparing Mastectomy and Immediate Breast Reconstruction with Implant: First Report of Surgical Technique. Ann Surg (2017) 266(2):e28–30. doi: 10.1097/SLA.0000000000001397
10. Toesca A, Invento A, Massari G, Girardi A, Peradze N, Lissidini G, et al. Update on the Feasibility and Progress on Robotic Breast Surgery. Ann Surg Oncol (2019) 26(10):3046−51. doi: 10.1245/s10434-019-07590-7
11. Ahn SJ, Song SY, Park HS, Park SH, Lew DH, Roh TS, et al. Early experiences with robotassisted prosthetic breast reconstruction. Arch Plast Surg (2019) 46(1):79−83. doi: 10.5999/aps.2018.00052
12. Tukenmez M, Ozden BC, Agcaoglu O, Kecer M, Ozmen V, Muslumanoglu M, et al. Videoendoscopic single-port nipple-sparing mastectomy and immediate reconstruction. J Laparoendosc Adv Surg Tech A (2014) 24(2):77−82. doi: 10.1089/lap.2013.0172
13. Kitamura K, Ishida M, Inoue H, Kinoshita J, Hashizume M, Sugimachi K. Early results of an endoscope-assisted subcutaneous mastectomy and reconstruction for breast cancer. Surgery (2002) 131(1 Suppl):S324–329. doi: 10.1067/msy.2002.120120
14. Lai HW. Robotic Nipple-Sparing Mastectomy and Immediate Breast Reconstruction with Gel Implant. Ann Surg Oncol (2019) 26(1):53–4. doi: 10.1245/s10434-018-6711-3
15. Selber JC. Robotic Nipple-Sparing Mastectomy: The Next Step in the Evolution of Minimally Invasive Breast Surgery. Ann Surg Oncol (2019) 26(1):10–1. doi: 10.1245/s10434-018-6936-1
16. Sarfati B, Honart JF, Leymarie N, Rimareix F, Al Khashnam H, Kolb F. Robotic da Vinci Xi assisted nipple-sparing mastectomy: First clinical report. Breast J (2018) 24(3):373–6. doi: 10.1111/tbj.12937
17. Sarfati B, Struk S, Leymarie N, Honart JF, Alkhashnam H, Kolb F, et al. Robotic Nipple-Sparing Mastectomy with Immediate Prosthetic Breast Reconstruction: Surgical Technique. Plast Reconstr Surg (2018) 142(3):624–7. doi: 10.1097/PRS.0000000000004703
18. Lai HW, Lin SL, Chen ST, Chen SL, Lin YL, Chen DR, et al. Robotic Nipple-sparing Mastectomy and Immediate Breast Reconstruction with Gel Implant. Plast Reconstr Surg Glob Open (2018) 6:e1828. doi: 10.1097/GOX.0000000000001828
19. Lai HW, Chen ST, Lin SL, Chen CJ, Lin YL, Pai SH, et al. Robotic Nipple- Sparing Mastectomy and Immediate Breast Reconstruction with Gel Implant: Technique, Preliminary Results and Patient-Reported Cosmetic Outcome. Ann Surg Oncol (2019) 26(1):42–52. doi: 10.1245/s10434-018-6704-2
20. Sarfati B, Struk S, Leymarie N, Honart JF, Alkhashnam H, Tran de Fremicourt K, et al. Robotic Prophylactic Nipple-Sparing Mastectomy with Immediate Prosthetic Breast Reconstruction: A Prospective Study. Ann Surg Oncol (2018) 25(9):2579–86. doi: 10.1245/s10434-018-6555-x
21. Lai HW, Wang CC, Lai YC, Chen CJ, Lin SL, Chen ST, et al. The learning curve of robotic nipple sparing mastectomy for breast cancer: An analysis of consecutive 39 procedures with cumulative sum plot. Eur J Surg Oncol (2019) 45(2):125–33. doi: 10.1016/j.ejso.2018.09.021
22. Lai HW, Lin SL, Chen ST, Lin YL, Chen DR, Pai SS, et al. Robotic nipple sparing mastectomy and immediate breast reconstruction with robotic latissimus dorsi flap harvest - Technique and preliminary results. J Plast Reconstr Aesthet Surg (2018) 71(10):e59–61. doi: 10.1016/j.bjps.2018.07.006
23. Park HS, Kim JH, Lee DW, Song SY, Park S, Kim SI, et al. Gasless Robot- Assisted Nipple-Sparing Mastectomy: A Case Report. J Breast Cancer (2018) 21(3):334–8. doi: 10.4048/jbc.2018.21.e45
24. Rajappa SK, Kumar R, Garg S, Ram D. Robotic nipple-sparing mastectomy: The first experience from Indian subcontinent. Breast J (2018) 24(6):1114–5. doi: 10.1111/tbj.13146
25. Mittal AK, Dubey M, Arora M, Bhagat S, Bhargava AK. Anaesthetic consideration for robotic nipple sparing mastectomy. Indian J Anaesthesia (2017) 61(6):519–21. doi: 10.4103/ija.IJA_130_17
26. Houvenaeghel G, Bannier M, Rua S, Barrou J, Heinemann M, Knight S, et al. Robotic breast and reconstructive surgery: 100 procedures in 2-years for 80 patients. Surg Oncol (2019) 31:38–45. doi: 10.1016/j.suronc.2019.09.005
27. Houvenaeghel G, Bannier M, Rua S, Barrou J, Heinemann M, Van Troy A, et al. Breast cancer robotic nipple sparing mastectomy: evaluation of several surgical procedures and learning curve. World J Surg Oncol (2019) 17(1):27. doi: 10.1186/s12957-019-1567-y
28. Lai H-W, Toesca A, Sarfati B, Park HS, Houvenaeghel G, Selber JC, et al. Consensus Statement on Robotic Mastectomy—Expert Panel From International Endoscopic and Robotic Breast Surgery Symposium (IERBS) 2019. Ann Surg (2020) 271(6):1005–12. doi: 10.1097/SLA.0000000000003789
29. Lai HW, Chen ST, Mok CW, Lin YJ, Wu HK, Lin SL, et al. Robotic versus conventional nipple sparing mastectomy and immediate gel implant breast reconstruction in the management of breast cancer- A case control comparison study with analysis of clinical outcome, medical cost, and patient-reported cosmetic results. J Plast Reconstr Aesthet Surg (2020) 73(8):1514–25. doi: 10.1016/j.bjps.2020.02.021
30. US Food and Drug Administration (FDA). Caution When Using Robotically-Assisted Surgical Devices in Women’s Health including Mastectomy and Other Cancer-Related Surgeries: FDA Safety Communication Febuary. (2019).
31. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240(2):205−13. doi: 10.1097/01.sla.0000133083.54934.ae
Keywords: nipple sparing mastectomy, robotic surgery, breast reconstruction, predictive score, breast cancer
Citation: Houvenaeghel G, Barrou J, Jauffret C, Rua S, Sabiani L, Van Troy A, Buttarelli M, Blache G, Lambaudie E, Cohen M and Bannier M (2021) Robotic Versus Conventional Nipple-Sparing Mastectomy With Immediate Breast Reconstruction. Front. Oncol. 11:637049. doi: 10.3389/fonc.2021.637049
Received: 02 December 2020; Accepted: 25 January 2021;
Published: 04 March 2021.
Edited by:
Gianluca Vanni, University of Rome Tor Vergata, ItalyReviewed by:
Gianluca Franceschini, Catholic University of the Sacred Heart, ItalyAlejandro Martin Sanchez, Fondazione Policlinico Universitario A. Gemelli IRCCS, Italy
Copyright © 2021 Houvenaeghel, Barrou, Jauffret, Rua, Sabiani, Van Troy, Buttarelli, Blache, Lambaudie, Cohen and Bannier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gilles Houvenaeghel, houvenaeghelg@ipc.unicancer.fr
 Gilles Houvenaeghel
Gilles Houvenaeghel Julien Barrou
Julien Barrou Camille Jauffret1
Camille Jauffret1 Guillaume Blache
Guillaume Blache