- 1Department of Radiation Oncology, Qingdao University Medical College Affiliated Yantai Yuhuangding Hospital, Yantai, China
- 2Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, China
- 3Department of Radiation Oncology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, China
- 4Department of Radiation Oncology, Liaocheng People’s Hospital, Liaocheng, China
- 5Department of Radiation Oncology, ZiBo Central Hospital, Zibo, China
- 6Department of Radiation Oncology, Shandong Cancer Hospital and Institute-Shandong Cancer Hospital Affiliated to Shandong University, Jinan, China
Background: Owing to improved systemic therapies, the survival of patients with non-small cell lung cancer (NSCLC) was prolonged, and the risk of brain metastases was consequently increased. This study aims to compare different radiotherapy for brain metastases in patients with NSCLC.
Materials and methods: The patients with NSCLC who were treated with whole brain radiation therapy (WBRT) or stereotactic radiosurgery (SRS) for brain metastases at three medical centers between January 2012 and December 2017 were retrospectively analyzed.
Results: Of the 684 eligible patients, 217 received WBRT plus focal radiation boost (WBRT+boost), 324 received WBRT, and 143 received SRS. Patients with WBRT+boost lived longer than those with WBRT (median overall survival (OS), 22.2 vs 13.7 months, P < 0.001) or SRS (22.2 vs 17.3 months, P = 0.011). In subgroup analyses, the survival advantage of WBRT+boost was more obvious among patients with 1 to 3 brain metastases or who received targeted therapy than did SRS. From pair-wise comparisons of intracranial progression-free survival (iPFS), WBRT+boost was also superior to WBRT (12.9 vs 10.6 months, P = 0.028) and SRS (12.9 vs 9.1 months, P = 0.001).
Conclusions: Patients who were treated with WBRT+boost experienced significantly longer OS and iPFS than those with WBRT or SRS alone. WBRT+boost should be a preferred strategy for brain metastases in NSCLC patients.
Introduction
Brain metastasis is the most common source of central nervous system tumors, and the majority of brain metastases originate from non-small cell lung cancer (NSCLC) (1, 2). The prognosis of NSCLC patients with brain metastasis is poor. With the best supportive care (dexamethasone), the median overall survival (OS) is only 8.5 weeks (3). In 1970 to 1990s, whole brain radiation therapy (WBRT) became the standard treatment for brain metastasis, which doubled the median OS to 16 weeks (4). However, no studies were conducted to identify whether other altered fractionation schemes could obtain a longer survival benefit or less neurotoxicity than the scheme of 30Gy in 10 fractions (5). Since 1990s, the treatment of brain metastasis evolved localized therapies such as surgery, stereotactic radiosurgery (SRS), three-dimensional conformal radiotherapy (3D-CRT), intensity-modulated radiation therapy (IMRT), or combinations of the above treatments, together with systemic therapies, such as chemotherapy, molecular targeted therapy, and immunotherapy (6, 7). With these treatments, median OS has been prolonged to more than 10 months (8, 9). For patients with drug-sensitive gene mutations, tyrosine kinase inhibitor (TKI) therapy could even prolong the median OS to 46 months (10). Owing to various of effective systemic therapies, the risk of brain metastases was also increased because of the prolonged survival. Currently, whether WBRT should be administered or not is still of considerable controversy. In order to decrease the neurotoxicity, some investigators suggested that SRS should/might be a standard treatment for limited brain metastases (up to 4 brain metastases), and even for multiple brain metastases (5–10 brain metastases) (8, 11, 12). In order to improve the intracranial control rate, some investigators proposed the use of WBRT plus focal radiation boost (13, 14). Therefore, it is important to identify a better strategy for brain metastasis in patients with NSCLC.
Materials and Methods
Data Collection
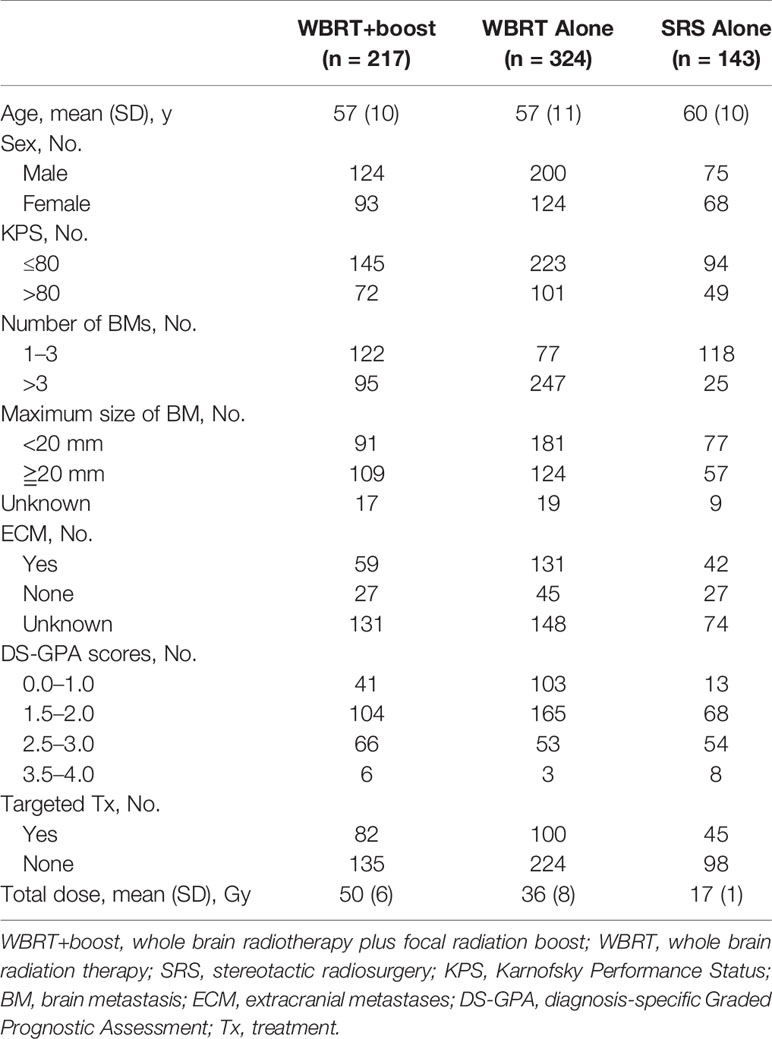
This was a multi-center retrospective study. The NSCLC patients who treated with WBRT or SRS for brain metastases in three medical institutions between January 2012 and December 2017 were reviewed. The eligible criteria included pathologically identified NSCLC and imaging (MRI/CT) identified brain metastases. All patients were treated with WBRT or SRS for brain metastases. Cranial MRI/CT surveillance was performed every 2 to 3 months after brain radiotherapy, then every 5 to 6 months after 3 years. Patients with incomplete medical records or those who did not complete the prescribed treatment were excluded (Figure 1). According to the accepted brain radiotherapy, the remaining 684 patients were divided into three groups: the WBRT plus focal radiation boost group (WBRT+boost, n = 217), WBRT group (n = 324), and SRS group (n = 143). The general characteristics were recorded, including gender, age, Karnofsky Performance Status (KPS), diagnosis-specific Graded Prognostic Assessment (DS-GPA), gene mutations, number of brain metastases, maximum size of brain metastasis, systemic treatment, type of radiotherapy, and extracranial metastases.
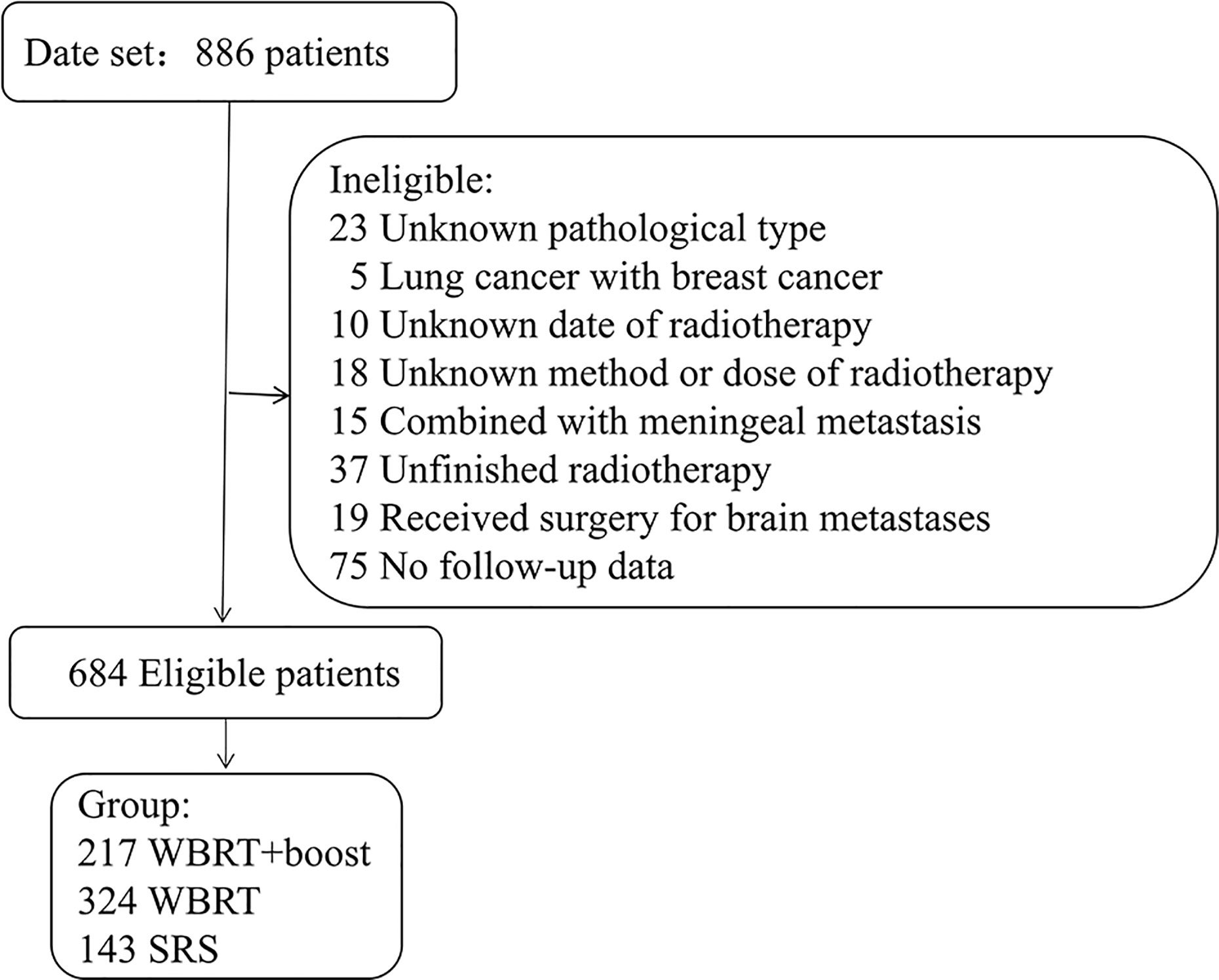
Figure 1 Patients screened and determined to be, eligible or ineligible for inclusion in the study. Abbreviations: WBRT+boost, WBRT plus focal radiation boost.
Study Treatment
WBRT was performed using 3D-CRT or IMRT techniques, and for 19 patients, WBRT was administered under guidance by 2D-CRT. In WBRT, the clinical target volume (CTV) was contoured as the region of the whole brain, and expansion of the CTV by 3 mm was used as the planning tumor volume (PTV). The radiation dose was 25 to 45 Gy in 10 to 20 fractions, 2 to 3 Gy per fraction. The additional radiation boost following WBRT was delivered by linear accelerators, with the total dose of 10 to 30 Gy in 5 to 15 fractions. The gross tumor volume (GTV) was defined as the range of contrast-enhanced tumor, and expansion of the GTV by 2 to 3 mm was used as the PTV. SRS was performed using gamma knife according to the size of the brain metastasis and the location of the tumor in the functional area, with a 40% to 60% isodose line. The prescribed radiation dose of SRS was 13.5 to 21.0 Gy in 1 to 2 fractions, 8.5 to 16.0 Gy per fraction.
Study End Points and Statistical Analysis
The primary end point was OS, and the secondary end point was iPFS (the progression of intracranial metastases or death). Disease progression was assessed according to the Response Evaluation Criteria in Solid Tumors version 1.1 criteria (15). All end points were defined as the time from the start of brain radiotherapy to the respective events, which were censored at the final follow-up (data cutoff was December 31, 2018) if no respective events were observed. The general characteristics of patients were calculated by ANOVA or Chi-square test. The OS and iPFS were evaluated by Kaplan–Meier method, and groups were compared using the Breslow test. For pairwise comparisons of OS, multiplicity adjustment was implemented by the sequentially rejective Bonferroni method (16). Planned subgroup analyses of OS among WBRT+boost and SRS group did not adjust for multiplicity adjustment, so these results should be interpreted as exploratory. Univariate and multivariate analyses based on Cox proportional hazards regression models were performed to assess the impact of prognostic variables (17). A two-sided value of P < 0.05 was considered as statistically significant. All analyses were performed using IBM SPSS version 22.0 (IBM Corp).
Results
Patient Characteristics
Among the 684 included patients, 447 (65.3%) patients had died, 130 (19.0%) patients remained alive, and 107 (15.6%) patients were lost to follow-up. The mean age was 58 years (SD, 10 years); 399 (58.3%) patients were men. The baseline characteristics of patients are presented in Table 1.
Overall Survival
The median follow-up time was 13.0 months. Significant differences in OS were observed among the WBRT+boost, WBRT, and SRS groups (22.2 vs 13.7 vs 17.3 months, P < 0.001, Figure 2). Upon pairwise comparison of the OS rates, WBRT+boost resulted in longer OS than in WBRT (22.2 vs 13.7 months; P < 0.001) or SRS (22.2 vs 17.3 months; P = 0.011). This finding was also supported by univariate analysis (Table 2). Additionally, no significant difference in OS was observed between the WBRT and SRS groups (13.7 vs 17.3 months; P = 0.221, Figure 2).
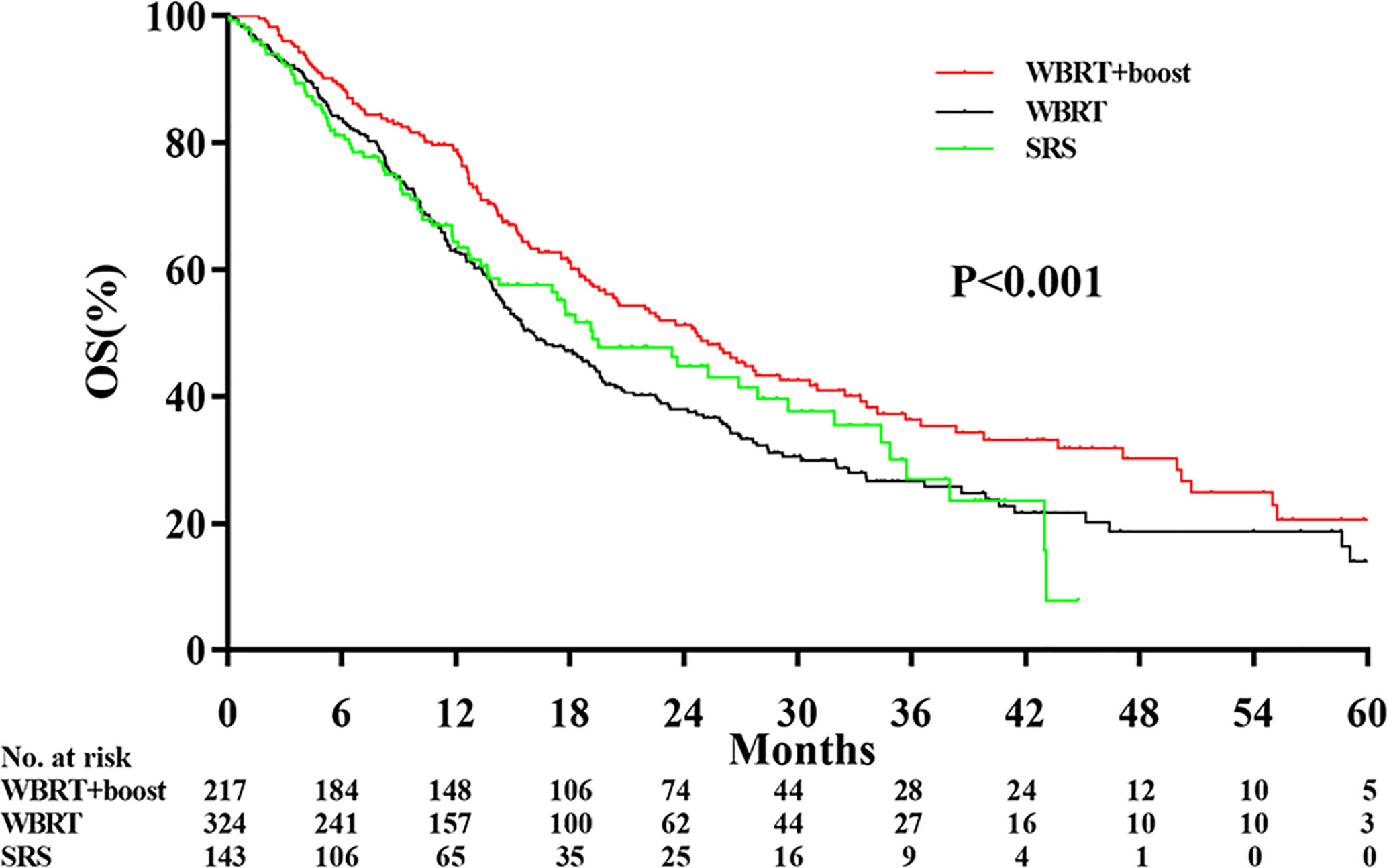
Figure 2 Comparison of overall survival according to the type of adjuvant radiotherapy received. WBRT+boost indicates whole brain radiotherapy plus focal radiation boost; WBRT, whole brain radiotherapy; SRS, stereotactic radiosurgery. There were significant differences in overall survival among the WBRT+boost, WBRT, and SRS groups (22.2 vs 13.7 vs 17.3 months, P < 0.001).
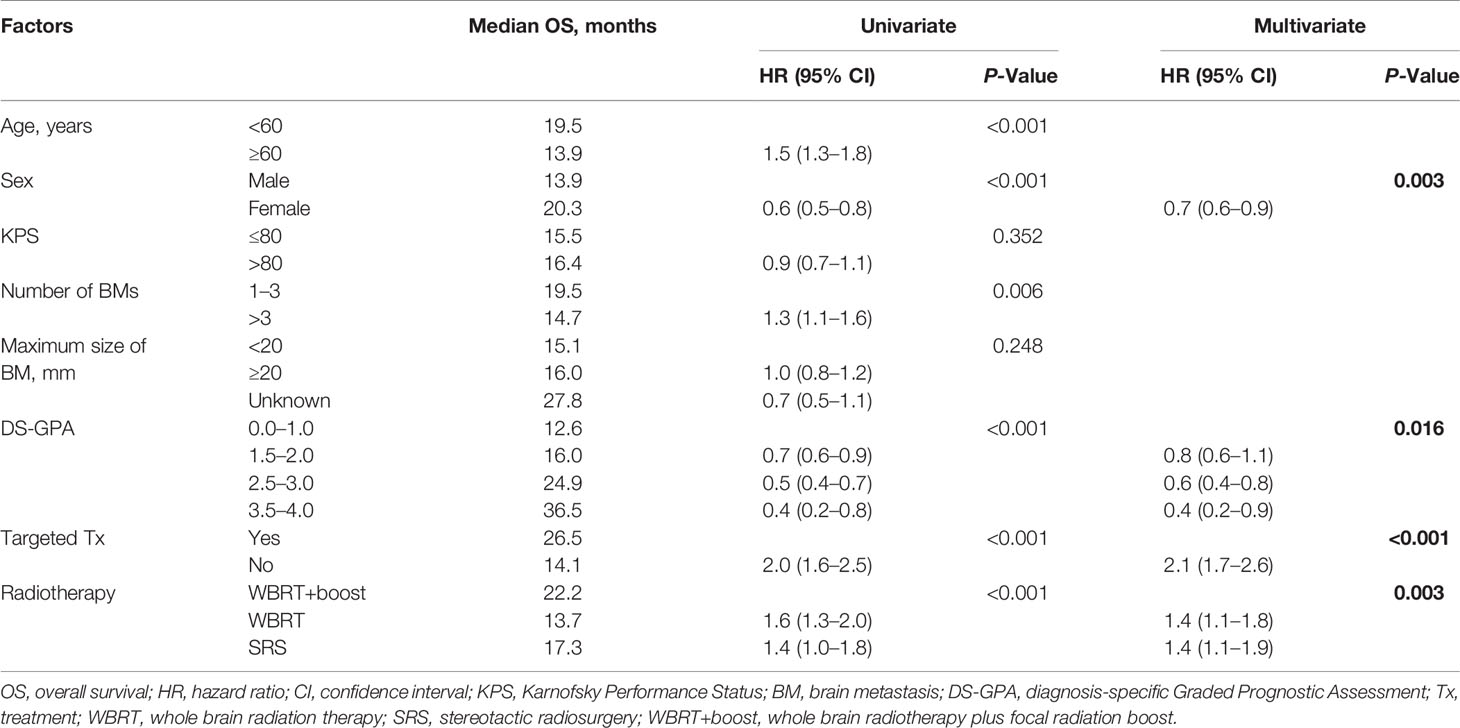
Table 2 Univariate and multivariate analyses of factors influencing OS of NSCLC patients with brain metastases.
Subgroup Analyses
To further analyze the OS between WBRT+boost and SRS group, subgroup analyses were conducted based on number of brain metastases, and use of systemic treatment. Patients with WBRT+boost experienced a longer OS than SRS in patients with 1 to 3 brain metastases (24.6 vs 14.3 months, P = 0.001; eFigure 1A in the Supplement), or in patients who received targeted therapy (31.0 vs 17.3 months, P = 0.026; eFigure 2A in the Supplement). No survival advantage was observed in patients with multiple (>3) brain metastases (19.1 vs 18.3 months, P = 0.649; eFigure 1B in the Supplement) or in patients who did not receive targeted therapy (19.2 vs 18.3 months, P = 0.146; eFigure 2B in the Supplement).
Intracranial Progression-free Survival
Significant differences in the iPFS were observed among the WBRT+boost, WBRT, and SRS groups (12.9 vs 10.6 vs 9.1 months, P = 0.004; Figure 3). From pairwise comparisons of groups, the iPFS of WBRT+boost group was significantly longer than WBRT (12.9 vs 10.6 months, P = 0.028) or SRS (12.9 vs 9.1 months, P = 0.001). No significant difference in iPFS was observed between the WBRT and SRS groups (10.6 vs 9.1 months, P = 0.108; Figure 3).
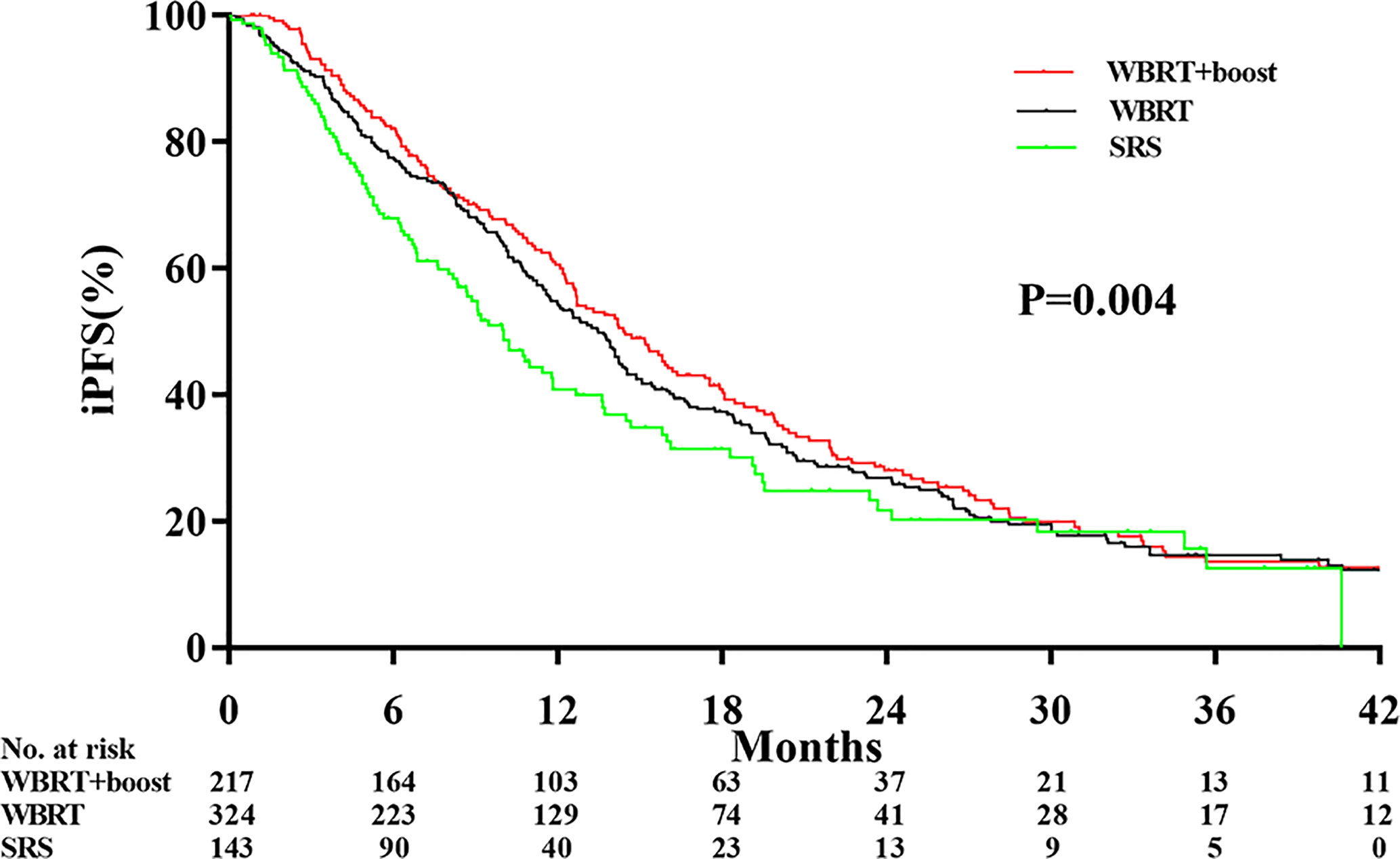
Figure 3 Comparison of intracranial tumor progression-free survival according to the type of adjuvant radiotherapy received. Abbreviations: WBRT+boost indicates whole brain radiotherapy plus focal radiation boost; WBRT, whole brain radiotherapy; SRS, stereotactic radiosurgery. The intracranial tumor progression-free survival time was different among the WBRT+boost, WBRT alone, and SRS alone groups (12.9 vs 10.6 vs 9.1 months, P = 0.004).
Univariate and Multivariate Analyses of Survival
Statistically significant covariates in univariate analysis were further analyzed by multivariate Cox proportional hazards analyses. In multivariate analyses for OS, female sex, high DS-GPA score (1.5–4), targeted therapy, and WBRT+boost were significant protective factors. The mortality rates in patients who were treated with WBRT and SRS alone were about 1.4-fold greater than those with WBRT+boost. KPS scores, the size and number of brain metastases were not significantly associated with OS (Table 2). Additionally, sex, DS-GPA score, systemic therapy, and the type of radiotherapy were also significant prognostic factors for iPFS (Table 3).
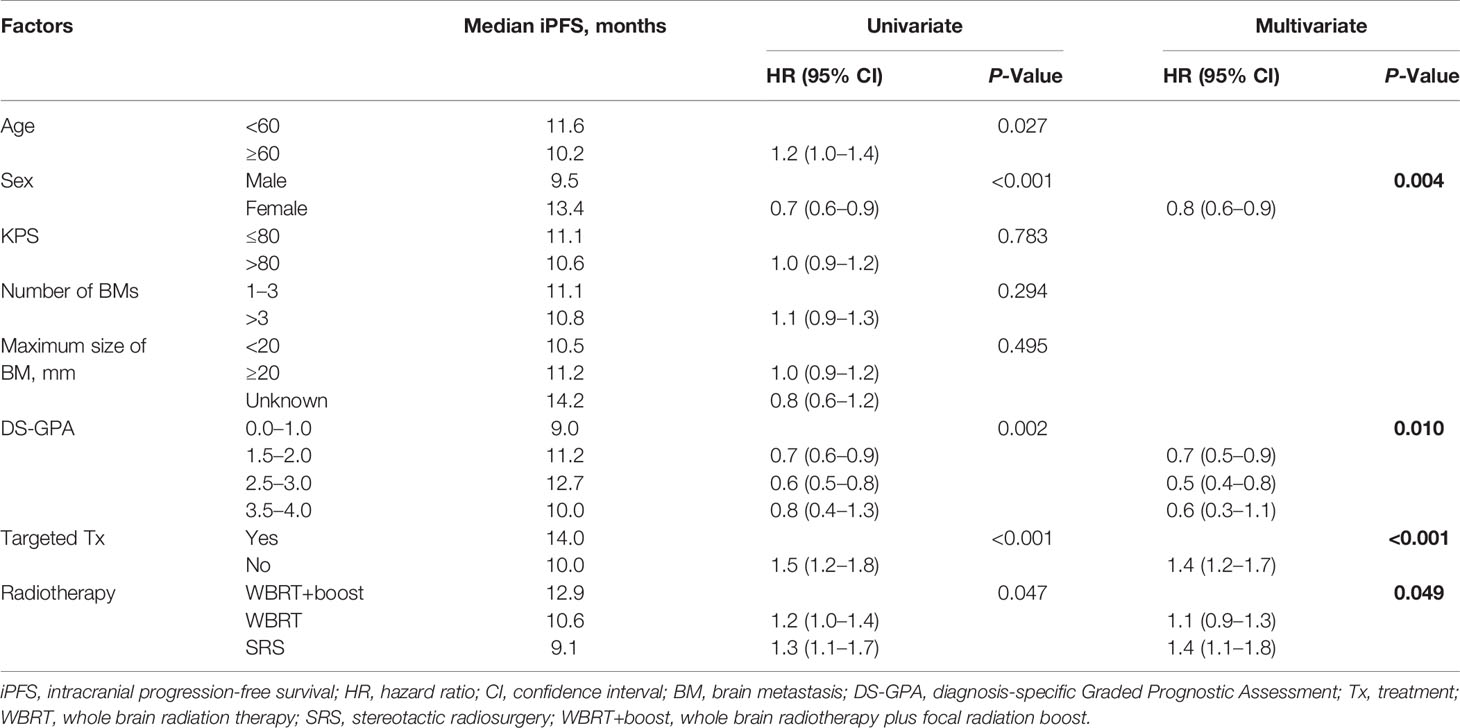
Table 3 Univariate and multivariate analyses of factors influencing iPFS in NSCLC patients with brain metastases.
Discussion
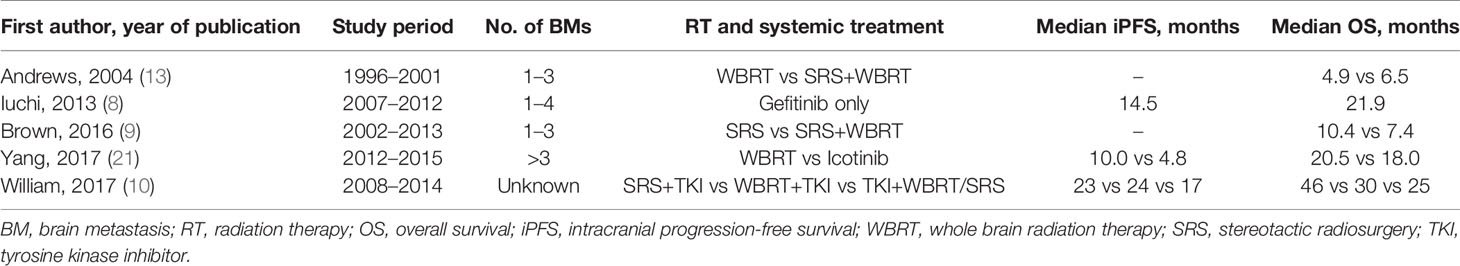
Our data showed that WBRT+boost resulted in significantly longer iPFS and OS than did WBRT or SRS, in agreement with other reported series (9, 18). For iPFS, Rades et al. found that WBRT plus stereotactic boost achieved better iPFS at 1-year than WBRT (71% vs 48%, P = 0.005) (18). Additionally, Brown et al. reported that patients who were treated with WBRT plus SRS was associated with significantly higher 1-year intracranial control rate than SRS alone (90.1% vs 72.8%, P = 0.003) (9). As for OS, some studies observed a survival advantage in WBRT+boost group over WBRT group (13, 19). In the RTOG 9508 randomized clinical trial, Andrews et al. found that compared to WBRT alone, WBRT plus SRS increased the OS in patients with single brain metastasis (6.5 vs 4.9 months, P = 0.039) (13). Sun et al. also found that WBRT plus radiation boost increased OS than WBRT alone in SCLC patients (13.4 vs 8.5 months, P = 0.004) (19). Of note, none of the above studies only included NSCLC patients.
The most important finding in this study was that WBRT+boost resulted in significantly longer iPFS and OS than SRS. To further analyze the survival difference between WBRT+boost and SRS group, several subgroup analyses were performed. Compared to SRS in patients with either 1 to 3 brain metastases, WBRT+boost improved survival (25.3 vs 17.8 months, P < 0.001). Similarly, compared to patients who received targeted therapy, WBRT+boost also improved survival (32.5 vs 23.7 months, P = 0.033). These results challenged the current guidelines for brain metastases. For example, National Comprehensive Cancer Network (NCCN) guidelines version 7·2019 recommend SRS alone for patients with limited brain metastases. WBRT or additional focal boost was totally abandoned in the treatment for these patients. This strong recommendation was originated from Brown’s famous randomized clinical trial published on JAMA 2016. Although they reported that the SRS plus WBRT lead to much lower cumulative incidence of intracranial tumor progression than SRS alone (6.3% vs 24.7% at 3 months, 11.6% vs 35.3% at 6 months, and 15.0% vs 49.5% at 12 months; P < 0.001), there was no significant difference in OS (7.4 vs 10.4 months, P = 0.92). Thus, they concluded that SRS alone were a preferred strategy in patients with limited brain metastases (9). The conclusion was then widely accepted by oncologists because it originated from the multicenter randomized clinical trial, and was therefore cited in NCCN guidelines. WBRT has been abandoned because of the side effects of cognitive impairment (20). Notably, the median OS in our study were much better than those reported by Brown et al. (9). Such improvement in OS could possibly be attributed to the use of multidisciplinary comprehensive treatments, particularly the targeted therapy (8, 21). According to the published articles included in Table 4, the median OS in NSCLC patients with brain metastases has been prolonged by administration of targeted therapy (8–10, 13, 21). Other researches have showed that prolonged survival can also result in higher incidence of intracranial metastasis recurrence, which was always associated with a neurologic deficit (22–24). With the improved extracranial control by effective targeted therapy or other systematic therapy, the high intracranial tumor control rate following WBRT+boost may translate into OS benefit. Therefore, this study suggested that WBRT+boost might be a preferred strategy for brain metastases in NSCLC patients, especially in patients with 1 to 3 brain metastases in the era of effective systematic therapy.
Of course, we did not ignore the cognitive decline caused by WBRT, too. NRG Oncology CC001 trial showed that in the absence of differences in OS and intracranial PFS, hippocampus sparing during WBRT plus memantine significantly reduced the risk of cognitive failure than did WBRT plus memantine (HR, 0.74; 95% CI, 0.58–0.95; P = 0.02) (25). RTOG 0933 trail also showed that hippocampus sparing during WBRT was associated with preservation of neurocognitive function (26). Hippocampus sparing during WBRT plus focal boost may be associated with longer OS and lower neurotoxicity, of course, which needs to be verified by prospective randomized clinical trial.
Our present study also has several limitations. Firstly, the inherent characteristics of retrospective research and patient heterogeneity may have led to bias in the results. Second, the average prescription dose in this study was lower than RTOG prescription isodose (27), which may bias the results. Thirdly, this retrospective study did not evaluate radiation-related neurotoxicity.
Conclusions
Our data showed that for NSCLC patients who experienced brain metastases, WBRT+boost is associated with better OS and iPFS than WBRT or SRS alone. The additional focal radiation boost is not accompanied by increased neurotoxicity, the adjuvant WBRT is always associated with better intracranial local control. Therefore, WBRT+boost should be considered for NSCLC patients with brain metastases.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee of Shandong Cancer Hospital, The Ethics Committee of Liaocheng People's Hospital, The Ethics Committee of The Affiliated Hospital of Xuzhou Medical University . The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SY, JY, and MN contributed conception and design of the study. MN, WL, AJ, YoW, YS, HZ, NL, LL, YQ, and YuW organized the database. MN, WL, AJ, YoW, and YQ performed the statistical analysis. MN, WL, AJ, and LL wrote the first draft of the manuscript. YoW, YS, HZ, and NL wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Natural Science Foundation of China (NSFC81872475, NSFC81372413), Shandong Key Research and Development Plan (2017CXGC1209, 2017GSF18164) and the Outstanding Youth Natural Science Foundation of Shandong Province (JQ201423), Jinan Clinical Medicine Science and Technology Innovation Plan (201704095), National Key Research and Development Program of China (2016YFC0904700).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.576700/full#supplementary-material
Supplementary Figure 1 | Overall survival impact of number of brain metastases. Abbreviations: WBRT+boost indicates whole brain radiotherapy plus focal radiation boost; SRS, stereotactic radiosurgery. The plots showed that compared to SRS alone, a significant survival benefit of WBRT+boost in patients with 1 to 3 brain metastases (A) (24.6 vs 14.3 months, P = 0.001).
Supplementary Figure 2 | Comparison of overall survival among subgroups based on the administration of systemic treatment. Abbreviations: WBRT+boost indicates whole brain radiotherapy plus focal radiation boost; SRS, stereotactic radiosurgery. The plots showed a significant difference in OS was observed in patients who received targeted therapy (A) in the comparison of groups (31.0 vs 17.3 months, P = 0.026).
References
1. Smedby KE, Brandt L, Backlund ML, Blomqvist P. Brain metastases admissions in Sweden between 1987 and 2006. Br J Cancer (2009) 101(11):1919–24. doi: 10.1038/sj.bjc.6605373
2. Ostrom QT, Wright CH, Barnholtz-Sloan JS. Brain metastases: epidemiology. Handb Clin Neurol (2018) 149:27–42. doi: 10.1016/B978-0-12-811161-1.00002-5
3. Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet (2016) 388(10055):2004–14. doi: 10.1016/S0140-6736(16)30825-X
4. Borgelt B, Gelber R, Kramer S, Brady LW, Chang CH, Davis LW, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys (1980) 6(1):1–9. doi: 10.1016/0360-3016(80)90195-9
5. Tsao MN, Xu W, Wong RK, Lloyd N, Laperriere N, Sahgal A, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev (2018) 1:CD003869. doi: 10.1002/14651858.CD003869.pub4
6. Goldberg SB, Contessa JN, Omay SB, Chiang V. Lung Cancer Brain Metastases. Cancer J (2015) 21(5):398–403. doi: 10.1097/PPO.0000000000000146
7. Tsao MN. Brain metastases: advances over the decades. Ann Palliat Med (2015) 4(4):225–32. doi: 10.3978/j.issn.2224-5820.2015.09.01
8. Iuchi T, Shingyoji M, Sakaida T, Hatano K, Nagano O, Itakura M, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer (2013) 82(2):282–7. doi: 10.1016/j.lungcan.2013.08.016
9. Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA (2016) 316(4):401–9. doi: 10.1001/jama.2016.9839
10. Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, Gerber NK, et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naive Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J Clin Oncol (2017) 35(10):1070–7. doi: 10.1200/JCO.2016.69.7144
11. Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol (2014) 15(4):387–95. doi: 10.1016/S1470-2045(14)70061-0
12. Kraft J, Zindler J, Minniti G, Guckenberger M, Andratschke N. Stereotactic Radiosurgery for Multiple Brain Metastases. Curr Treat Options Neurol (2019) 21(2):6. doi: 10.1007/s11940-019-0548-3
13. Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet (2004) 363(9422):1665–72. doi: 10.1016/S0140-6736(04)16250-8
14. Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. Whole Brain Radiation Therapy Plus Stereotactic Radiosurgery in the Treatment of Brain Metastases Leading to Improved Survival in Patients With Favorable Prognostic Factors. Front Oncol (2019) 9:205. doi: 10.3389/fonc.2019.00205
15. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
16. Proschan MA, Waclawiw MA. Practical guidelines for multiplicity adjustment in clinical trials. Control Clin Trials (2000) 21(6):527–39. doi: 10.1016/s0197-2456(00)00106-9
17. Gill RD. Multistate life-tables and regression models. Math Popul Stud (1992) 3(4):259–76. doi: 10.1080/08898489209525345
18. Rades D, Janssen S, Bajrovic A, Khoa MT, Veninga T. Schild SE. A matched-pair analysis comparing whole-brain radiotherapy with and without a stereotactic boost for intracerebral control and overall survival in patients with one to three cerebral metastases. Radiat Oncol (2017) 12(1):69. doi: 10.1186/s13014-017-0804-1
19. Sun H, Xu L, Wang Y, Zhao J, Xu K, Qi J, et al. Additional radiation boost to whole brain radiation therapy may improve the survival of patients with brain metastases in small cell lung cancer. Radiat Oncol (2018) 13(1):250. doi: 10.1186/s13014-018-1198-4
20. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol (2009) 10(11):1037–44. doi: 10.1016/S1470-2045(09)70263-3
21. Yang JJ, Zhou C, Huang Y, Feng J, Lu S, Song Y, et al. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med (2017) 5(9):707–16. doi: 10.1016/S2213-2600(17)30262-X
22. Pechoux CL, Dhermain F, Besse B. Whole brain radiotherapy in patients with NSCLC and brain metastases. Lancet (2016) 388(10055):1960–2. doi: 10.1016/S0140-6736(16)31391-5
23. Regine WF, Huhn JL, Patchell RA, St Clair WH, Strottmann J, Meigooni A, et al. Risk of symptomatic brain tumor recurrence and neurologic deficit after radiosurgery alone in patients with newly diagnosed brain metastases: results and implications. Int J Radiat Oncol Biol Phys (2002) 52(2):333–8. doi: 10.1016/s0360-3016(01)02645-1
24. Regine WF, Scott C, Murray K, Curran W. Neurocognitive outcome in brain metastases patients treated with accelerated-fractionation vs. accelerated-hyperfractionated radiotherapy: an analysis from Radiation Therapy Oncology Group Study 91-04. Int J Radiat Oncol Biol Phys (2001) 51(3):711–7. doi: 10.1016/s0360-3016(01)01676-5
25. Brown PD, Gondi V, Pugh S, Tome WA, Wefel JS, Armstrong TS, et al. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients With Brain Metastases: Phase III Trial NRG Oncology CC001. J Clin Oncol (2020) 38(10):1019–29. doi: 10.1200/JCO.19.02767
26. Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol (2014) 32(34):3810–6. doi: 10.1200/JCO.2014.57.2909
27. Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys (2000) 47(2):291–8. doi: 10.1016/s0360-3016(99)00507-6
Keywords: brain metastases, non-small cell lung cancer, whole brain radiation therapy, whole brain radiation therapy plus focal boost, stereotactic radiosurgery
Citation: Ni M, Liu W, Jiang A, Wang Y, Sheng Y, Zeng H, Liu N, Li L, Qi Y, Wang Y, Yu J and Yuan S (2020) Whole Brain Radiation Therapy Plus Focal Radiation Boost May Generate Better Survival Benefit for Brain Metastases From Non-small Cell Lung Cancer. Front. Oncol. 10:576700. doi: 10.3389/fonc.2020.576700
Received: 26 June 2020; Accepted: 28 September 2020;
Published: 20 October 2020.
Edited by:
Gene A. Cardarelli, Brown University, United StatesReviewed by:
Minesh P. Mehta, Baptist Health South Florida, United StatesMichael Chan, Wake Forest University, United States
Copyright © 2020 Ni, Liu, Jiang, Wang, Sheng, Zeng, Liu, Li, Qi, Wang, Yu and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuanghu Yuan, yuanshuanghu@sina.com
 Meng Ni1
Meng Ni1 Jinming Yu
Jinming Yu Shuanghu Yuan
Shuanghu Yuan