- 1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Thoracic Medical Oncology, Peking University Cancer Hospital & Institute, Beijing, China
- 2Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, China
- 3Department of Thoracic and Cardiovascular Surgery, the University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 4Department of Thoracic/Head and Neck Medical Oncology, the University of Texas MD Anderson Cancer Center, Houston, TX, United States
Purpose: We aimed to assess the survival benefit of surgery for patients with stage IA–IIB small cell lung cancer (SCLC) and construct a nomogram for predicting overall survival (OS).
Methods: Patients who had been diagnosed with stage IA–IIB SCLC between 2004 and 2014 and who had received active treatment were selected from the Surveillance, Epidemiology, and End Results database. The primary endpoint was OS. Cox proportional hazards models and propensity score (PS) analyses were used to compare the associations between surgery and OS. The probability of 1- and 3-year OS was predicted using a nomogram.
Results: We reviewed 2,246 patients. The median OS of the surgery and non-surgery groups was 35 months and 19 months, respectively. Multivariable Cox proportional hazards models showed a survival benefit in the surgery group (hazards ratio [HR], 0.642; 95% confidence interval [CI], 0.557–0.740; P < 0.001). To balance the between-group measurable confounders, the impact of surgery on OS was assessed using PS matching. After PS matching, OS analysis still favored surgical resection. The PS-stratification, PS-weighting, and PS-adjustment models showed similar results to demonstrate a statistically significant benefit for surgery. Further, the nomogram was well calibrated and had good discriminative ability (Harrell's C-index = 0.645).
Conclusion: Our analysis suggests that surgery is a viable option for patients with early-stage SCLC. Our nomogram is a viable tool for quantifying treatment trade-off assumptions and may assist clinicians in decision-making. Future work is needed to validate our results and improve our tools.
Introduction
The most commonly diagnosed cancer worldwide is lung cancer; it is the leading cause of cancer-related death worldwide, with 2018 recording ~2.1 million new cases and 1.8 million deaths (1). Small cell lung cancer (SCLC) is differentiated from non-SCLC (NSCLC) by its high growth fraction, rapid doubling time, and the early development of widespread metastases (2). SCLC involves 13~20% of all lung cancers. While its incidence in developed countries has declined in recent years, its frequency remains higher in developing countries, possibly due to variations in smoking practices and cigarette composition (3).
Most SCLC is sensitive to initial treatment, either platinum-based chemotherapy alone in advanced/metastatic disease, or platinum-based chemotherapy in combination with radiation in earlier stage disease, limited to a single hemithorax (i.e., limited stage). However, virtually all patients will eventually experience recurrent disease due to early dissemination and acquired drug resistance (4). At diagnosis, most patients with SCLC already have hematogenous metastases (i.e., extensive-stage disease). Consequently, locally advanced or distant metastatic disease (stage III/IV) will be diagnosed in >90% of patients (5), which contributes to the high mortality from the disease (6). Therefore, the 5-year survival rate remains low at <7% overall; most patients survive for only <1 year after diagnosis (7). There had been few improvements in SCLC standard of care management in the decades prior to the IMpower133 results, which demonstrated survival benefit with the addition of the anti–PD-L1 agent atezolizumab to platinum-based chemotherapy for patients with extensive-stage SCLC (8).
Surgical resection was once a mainstay of treatment for SCLC; however, survival outcomes were very poor. Subsequent studies demonstrating dramatic initial responses to chemotherapy and/or radiotherapy eventually shifted the SCLC standard of care away from surgical resection. Although previous research indicated the positive role of surgery in early-stage SCLC, the results remain controversial and inconsistent (9–12).
Therefore, we aimed to explore the value of surgery for the survival benefit of patients with stage IA–IIB SCLC and to construct a comprehensive, integrated nomogram to provide clinicians with a quantitative tool for estimating the overall survival (OS) of a patient with early-stage SCLC.
Materials and Methods
Study Participants
We obtained the study population from the Surveillance, Epidemiology, and End Results (SEER) program records of the National Cancer Institute. We selected patients diagnosed with SCLC as their original primary malignancy between 2004 and 2014 from the SEER database. The participants included patients with the following histologic codes (8002, 8041–8045) and site codes (C34.0–C34.3, C34.8–C34.9). We identified a total of 46,238 patients with SCLC in the SEER database. Only patients with early-stage SCLC (6th edition of American Joint Committee on Cancer [AJCC] staging I–II) were selected for further analysis. Patients were excluded if they had: (1) been diagnosed at autopsy or by death certificate only; (2) 990 or 996–999 tumor size code; (3) 950, 980, or 999 tumor extent code; and (4) lymph node involvement.
The surgery group comprised patients who received cancer-directed surgery. The non-surgery group comprised patients who did not receive cancer-directed surgery but who received radiotherapy and/or chemotherapy. We excluded patients with absence of active treatment codes (e.g., cancer-directed surgery, radiotherapy, or chemotherapy). We considered in our analysis 2,246 eligible patients who met our inclusion and exclusion criteria (Supplementary Figure 1).
The study was exempted from the Peking University Cancer Hospital Medical Ethics Committee. The data agreement was obtained and we downloaded the database directly from the SEER website in keeping with SEER requirements.
Statistical Analysis
Categorical variables are reported as frequencies and proportions. Continuous variables are reported as means (standard deviation, SD) and medians (interquartile ranges, IQR). For comparing the baseline characteristics between the surgery and non-surgery groups, we used Student's t-test for normally distributed variables, the chi-square test or Fisher's exact test for categorical variables, and the Mann–Whitney U-test for all other continuous variables. We demonstrated and compared the crude survival differences between two groups using Kalan–Meier curves and log-rank tests. We examined the proportional hazards (PH) assumption and linearity assumption in continuous variables using restricted cubic splines (13, 14).
We transformed continuous variables to adequate form for fitting the PH and linearity assumptions. We assessed the impact of surgery on OS by Cox proportional hazard models with and without risk adjustment for age at diagnosis, sex, marital status, race, primary tumor location, anatomic sites, year of diagnosis, tumor grading, lymph node involvement, tumor size, AJCC stage, and extent of tumor. We estimated hazard ratios (HRs) and 95% confidence intervals (CI).
We performed propensity score (PS) analyses to reduce the potential confounders between the surgery and non-surgery groups (15–17). First, treatment assignment was predicted using multivariable logistic regression based on various confounders, including age at diagnosis, sex, marital status, ethnicity, laterality, anatomic sites, tumor grading, year of diagnosis, AJCC stage, tumor size, extent of tumor, and lymph node involvement. PS, which represented the probability of receiving surgery, was then calculated for each patient (18). Next, patients were matched 1:1 into surgery and non-surgery groups. We used the nearest neighbor method, with a logit SD caliper width of 0.2. The balances of matched covariates were measured by the standardized difference, and a difference between −0.1 and 0.1 was generally considered negligible (19). Additionally, three alternative approaches were used to adjust for the patients' non-random assignment: (1) Adjustment: The regression model included the PS as a covariate. (2) Weighting: Each patient was weighted by the PS by the inverse probability of being in the surgery versus non-surgery group with the aim of balancing the characteristics between the two groups (20). (3) Stratification: The patients were divided into five strata based on the quintile of the PS. The relationship between surgery and survival within each stratum was examined using Cox proportional hazards models; the five HRs of each stratum were joined into an overall HR using inverse variance weights under the fixed model (21, 22).
Based on the predictive model with the identified prognostic factors, we constructed a nomogram for predicting the 1- and 3-year OS probability. Backward stepwise selection with the Akaike information criterion (AIC) was used to select variables into the multivariate Cox proportional hazards regression model, and the predictors' coefficients were calculated. The performance of the nomogram included its discrimination; calibration was tested using internal validation. Discrimination is a model's ability to separate subject outcomes, indicated by Harrell's C index (23). We evaluated calibration, which compares predicted and actual survival, using a calibration plot. In a well-calibrated model, the predictions should fall on a 45-degree diagonal line. The goodness of fit was assessed using the Greenwood-Nam-D'Agostino goodness-of-fit test (24). The final reduced model-predicted probability of OS was compared with the observed OS at 1 and 3 years. The developed model underwent internal validation using bootstrapping technique based on 1,000 resamples.
All statistical analysis was performed with R software (version 3.3.3; http://www.r-project.org). We used the R packages “MatchIt” and “rms” were used for PS analysis and nomogram building. The reported significance levels were all two-sided; statistical significance was set at 0.05.
Results
Patient Characteristics and Factors Associated With Surgery
A total of 2,246 patients fulfilled the eligibility criteria. The median follow-up time was 23 months, and 1,467 deaths (65.3%) were observed. Table 1 presents the patients' baseline characteristics. The majority were female (53%), white (87%), and had N0 lymph node involvement (71%). The median age at diagnosis was 68 years (IQR, 61–75 years). The median tumor size was 3.0 cm (IQR, 2.0–4.6 cm). Overall, 618 patients (27.5%) had cancer-directed surgery and 1,628 patients (72.5%) received radiotherapy and/or chemotherapy without surgical resection. In line with previous studies, the patients without cancer-directed surgery were elderly, non-white, with larger tumor sizes, and higher N stages.
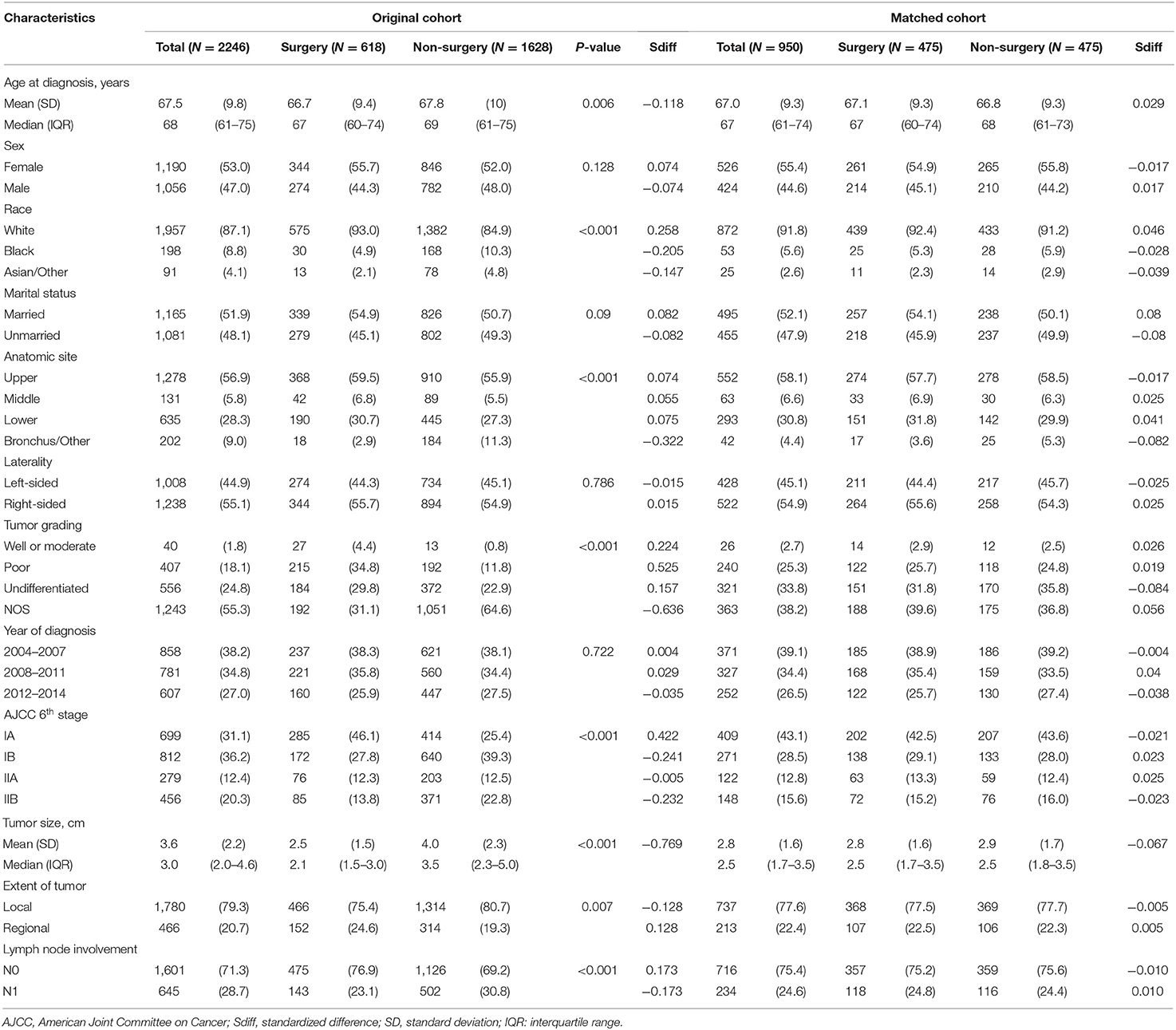
Table 1. Comparison of baseline characteristics between surgery and non-surgery groups among patients with stage I–II SCLC in the original and matched data sets.
Survival Analysis
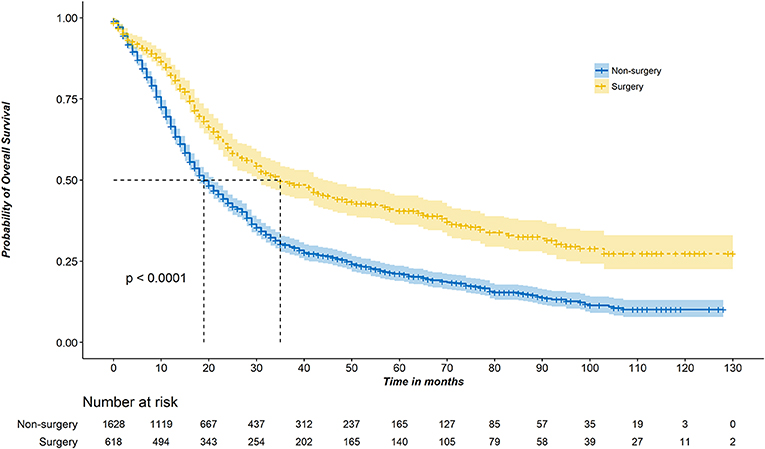
Figure 1 shows the Kaplan–Meier survival curve. The median OS of the surgery group was 35 months (95% CI, 31–44 months) compared with 19 months (95% CI, 18–21 months) in the non-surgery group (P < 0.001). The unadjusted 3-year survival probability was 30.0% (95% CI, 27.6–32.6%) for patients in the non-surgery groups vs. 49.4% (95% CI, 45.3–54.0%) for those in the surgery groups. Controlling for demographic and clinical characteristics in the multivariable Cox regression model revealed a significant difference in OS between the surgery and non-surgery patients (HR, 0.642; 95% CI, 0.557–0.740; P < 0.001) (Table 2).
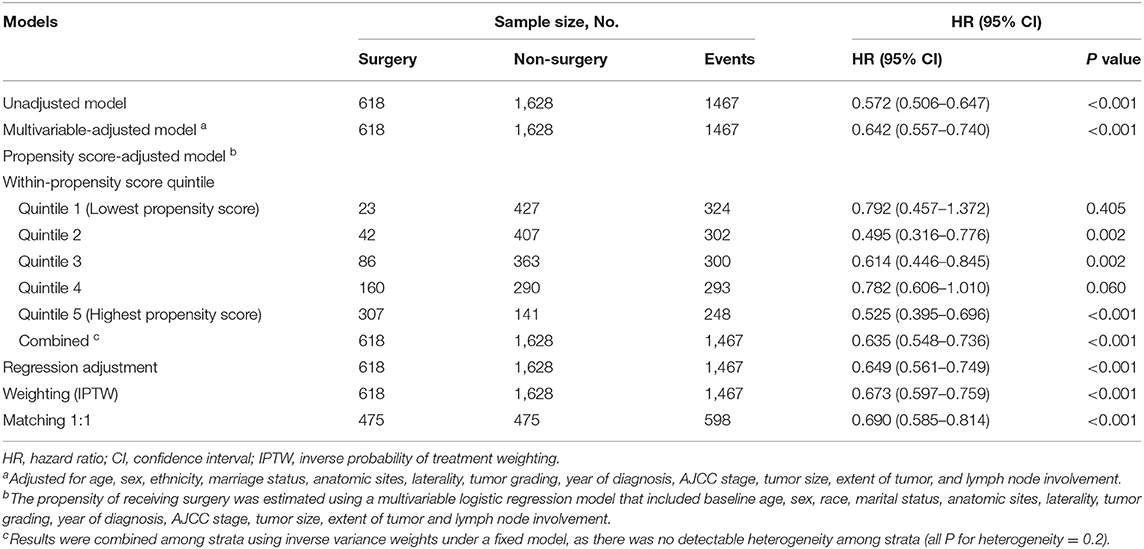
PS analyses were performed to balance measurable confounders between the groups and to evaluate whether surgery has a favorable prognostic impact on survival. We stratified patients into quintiles based on the PS, and assessed the effect of surgery on survival. In each of the five strata, the surgical patients had better outcome compared with non-surgical patients (Supplementary Figure 2). As there was no detectable heterogeneity across strata, we used inverse variance weights under the fixed model to combine the five HRs estimated from each stratum, and obtained the overall HR for the whole cohort (HR, 0.635; 95% CI, 0.548–0.736; P < 0.001) (Table 2). Apart from PS stratification method, we also used other alternative methods, including matching, weighting, and regression adjustment.
Before matching, the surgical patients had a PS of 0.482 ± 0.250 compared to 0.178 ± 0.250 in the non-surgery group (P < 0.001), indicating a strong and clinically relevant bias regarding the two groups' observed demographic and clinical characteristics. When PS matching was used, the surgery and non-surgery groups were well-matched (475 patients each), with no significant differences in clinical and pathologic factors (Table 1, Supplementary Figure 3). Among the PS-matched pairs, surgical patients showed better OS (3-year OS rate, 47.6%) than non-surgical patients (3-year OS rate, 33.9%; Supplementary Figure 4). The multivariable Cox proportional hazards models also showed a significant benefit in OS for the surgery group (HR, 0.690; 95% CI, 0.585-0.814; P < 0.001) (Table 2). Inverse probability weighted Cox models or PS adjustment strategy also showed consistent results that demonstrated favorable evidence supporting the advantage of surgery treatment in patients with stage IA–IIB SCLC (Table 2).
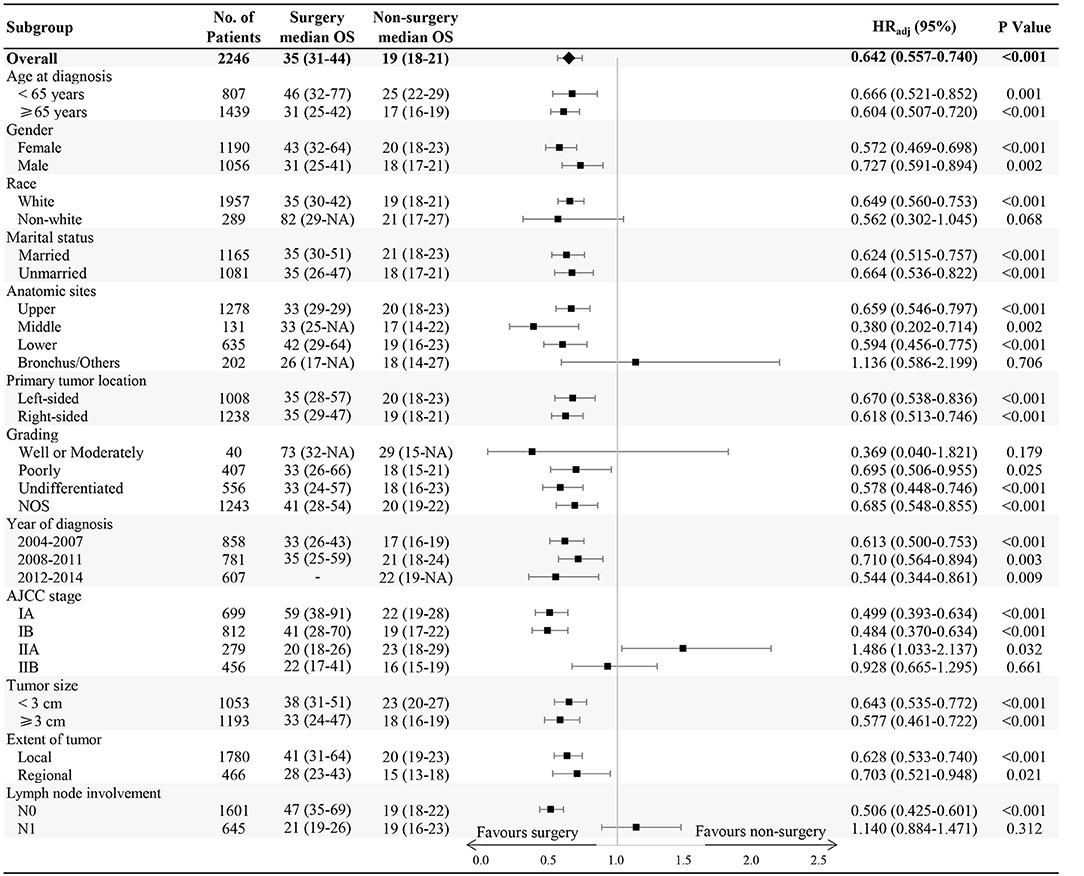
In subgroup analysis, OS benefit was observed across all subgroups in surgical patients compared with those in the non-surgery group, except for non-white race, bronchus and other anatomic sites, stage IIA or IIB, well or moderately differentiated grading, and N1 lymph node involvement (Figure 2).
Nomogram and Model Performance
Restricted cubic splines were used to explore the effects of continuous variables. Tumor size and age had nonlinear effects on the log of HR of OS (Supplementary Figure 5). Seven variables that were the most associated with OS were identified based on backward stepwise procedure: tumor size, age, extent of tumor, N stage, surgery, radiotherapy, and chemotherapy. In addition, significant interactions were noted between surgery and radiotherapy, and surgery and N stage; that is, the effect size of surgery varied by nodal status and radiotherapy treatment (P < 0.001, Supplementary Table 1).
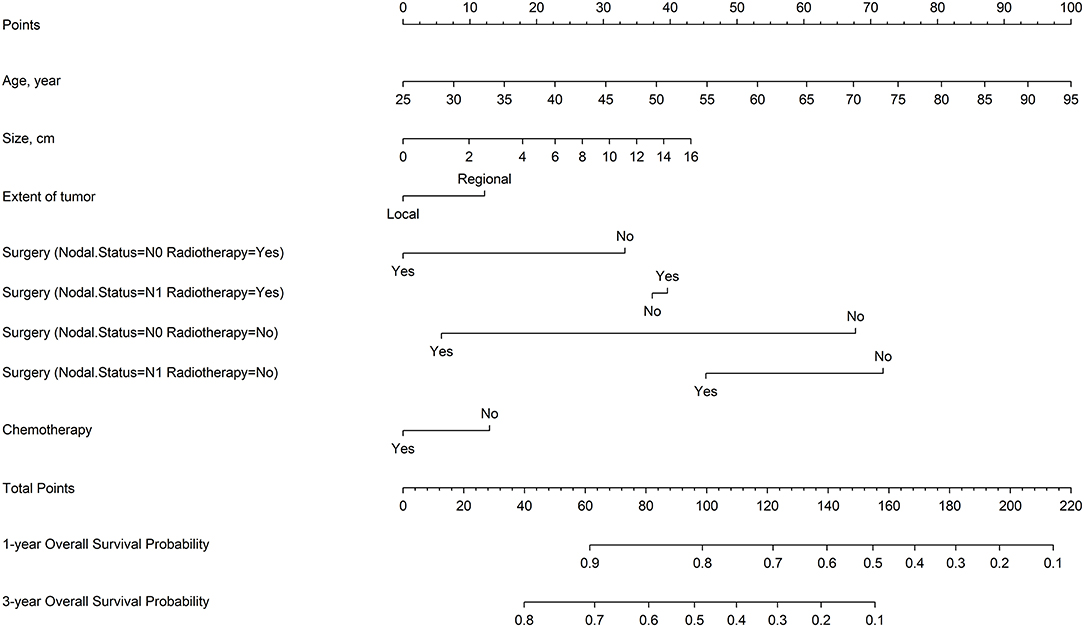
Figure 3 shows a nomogram model for predicting the 1- and 3-year OS of patients with stage IA–IIB SCLC with active treatment. The Harrell's C-index for the established nomogram (0.645; 95% CI, 0.630–0.662) was significantly higher than the tumor-node-metastasis (TNM) staging system (0.549; 95% CI, 0.529–0.565; P < 0.001). The Hosmer-Lemeshow goodness-of-fit test showed that the nomogram had adequate calibration (P = 0.728). Figure 4 presents the nomogram's 1000-sample bootstrapped calibration plot for predicting the 1- and 3-year OS. The calibration plots revealed good predictive accuracy of the nomogram.
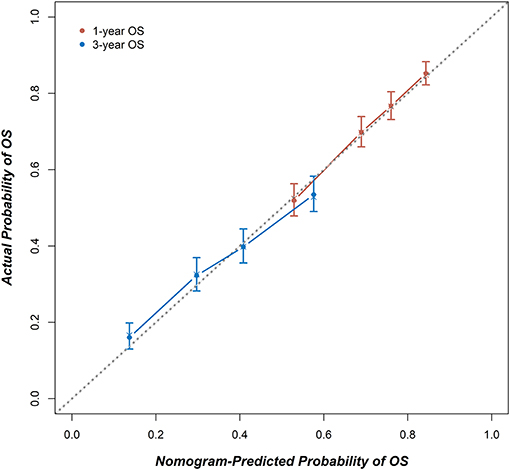
Figure 4. The calibration curves for predicting 1- and 3-year OS. X-axis: The OS probability predicted by the nomogram; y-axis: the actual OS probability. A plot along the 45-degree dotted line indicates a perfect calibration model, where the predicted probabilities and actual outcomes are identical.
Discussion
In this retrospective cohort study, we compared, via the SEER database, the survival outcomes for patients with early-stage SCLC who were treated with or without cancer-directed surgery. By using multivariable regression and PS analyses to reduce confounding, our results consistently demonstrate that treatment with surgery is associated with statistically significantly improved median OS in patients with stage I and II SCLC. Additionally, we present a nomogram for estimating the OS for patients with early-stage SCLC. It can be used to quantify assumptions about treatment trade-off and to guide the management of patients with SCLC.
Although NSCLC remains the most common lung cancer, SCLC nevertheless constitutes a major percentage of advanced-stage lung cancers (25). With the advances in chemotherapy and radiation treatment modalities, surgical resection of even early-stage SCLC tumors had fallen out of favor. However, several recent studies have suggested that, for a select group of patients with early-stage disease, surgery may be a reasonable, and in fact, superior SCLC treatment modality. Unfortunately, these data were based primarily on retrospective analyses, and few prospective data are available on which to base the consideration for surgical resection. Previous retrospective studies have found patients with stage I and II SCLC have prolonged survival following surgical resection as compared to non-surgical treatment models (26–31).
In the present study, we found that treatment with surgery was associated with statistically significantly improved median survival among patients with early-stage SCLC. Stratified analysis showed OS benefit across all subgroups in the surgical patients compared with non-surgery group, with the following exceptions: non-white race, well or moderately differentiated grading, stage IIA or IIB, bronchus and other anatomic sites, and N1 lymph node involvement. The number of patients reviewed who were non-white (289/2246), had bronchus and other anatomic site involvement (202/2246), and well or moderately differentiated grading (40/2246) was relatively low. Considering the relatively small sample size, the result is not statistically significant (P > 0.05). It is possible that among non-white patients with SCLC, cultural differences underlie patient preferences for (or against) surgical resection. As for tumors located in or invading the bronchus, this anatomical site may increase the difficulty of the surgical approach, which may limit R0 resections and lead to increased post-operative complications. Histologically, SCLC is usually poor or undifferentiated; while in the present study 40 patients were diagnosed as having well or moderately differentiated grading. The pathological characteristic might identify patients who respond better to chemotherapy or radiotherapy and lower malignant/metastatic potential. The present study included patients with stage IIA or IIB disease; we found the survival was not significantly prolonged in surgical group compared with those with stage I disease. N1 lymph node involvement is also an indicator of decreased benefit from surgical resection. Although the total population showed survival benefit in the surgery group, subgroup analysis showed that patients with stage II and N1 lymph node metastasis did not benefit from surgery. This result confirmed that surgery should be included in popular multimodality treatment for patients with stage I SCLC as one of the most important therapy.
Multivariate Cox proportional hazards regression analysis showed that with or without PS analysis, the HR still showed benefit in the surgery group. While other studies have suggested that the poor efficacy of current treatment options for disease progression is related to the lack of benefit of early diagnosis, as such subjects do not usually have the option of surgery, as systemic spread and paraneoplastic syndrome typically impede the therapeutic potential of resection (32). Improved or favorable survival rates in patients with resected SCLC have been described in recent retrospective analyses (33, 34), indicating that surgery is associated with improved survival for certain patients with early-stage SCLC (35). The results of the present study are almost identical to that of these previous studies (36).
Nowadays, SCLC in 80–90% of cases is still being diagnosed as TNM stage III–IV (37), and the Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions have confirmed the prognostic value of clinical and pathological TNM staging in patients with SCLC and recommend continued usage for SCLC in the 8th edition of the TNM classification for lung cancer (37, 38). In our nomogram, seven variables were identified as most closely associated with OS: age, chemotherapy, extent of tumor, tumor size, surgery, N stage, and radiotherapy. Additionally, significant interactions were observed between surgery and radiotherapy/N stage, which demonstrated that the effect of the surgery modality varied with different nodal status as well as radiotherapy treatment. The established nomogram model for predicting 1- and 3-year OS of patients with IA–IIB SCLC had a significantly higher Harrell's C-index than the TNM staging system. This indicates that only TNM staging alone is insufficient for defining the prognosis of patients with early-stage SCLC. Further studies with external samples are needed to validate our predictive model.
Our study has several apparent limitations that need to be addressed. First, the SEER database lacks essential clinical details, such as baseline lung function, specific chemotherapy regimen, the treatment sequence following disease progression, information on gene mutation, and tobacco usage, which may be associated with surgery selection, survival, or both. Our estimation of the effect of surgery might be biased by these unmeasurable potential confounders. The results therefore should be interpreted with caution. Second, our ability to identify the patients who would benefit more from treatment with surgery was limited by the comparatively small sample, especially in subgroup analysis. Third, internal validation was used to evaluate model performance via the bootstrap approach. Although good performance was demonstrated, we still require independent cohort–based external validation to assess the accuracy of the model.
In summary, our study demonstrates that treatment with surgery is associated with statistically significantly improved median OS among patients with early-stage SCLC. We have also established a nomogram for predicting the 1- and 3-year OS probability. Our nomogram showed relatively good performance and could become useful personalized predictive tool for prognosis in SCLC patients. Future work with more detailed clinical information might improve our tool, and our results need to validate with large-scale prospective cohorts.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: Data are available from www.seer.cancer.gov.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Peking University Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZW and CG conceived the study, undertook project leadership and are guarantors of this work. YW and QZ wrote the first draft of the manuscript. QZ, YW, and BJ analyzed data and interpreted the data. TA, JZha, MW, MZ, JL, JZho, HC, XY, YC, ZD, BS, and JZhan were responsible for data acquisition and interpretation. All authors contributed to the drafting and critical revision of the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation Young Scholars Program (grant number 81401914), the Beijing Municipal Administration of Hospitals' Young Program (grant number QML20161112), the scholarship of Young backbone teachers' overseas study program under the State Scholarship Fund (File No. 201806015049), the Science Foundation of Peking University Cancer Hospital (grant number 18-02), and Beijing Municipal Science & Technology Commission (grant number Z181100001718104).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the efforts of the SEER Program tumor registries in providing high-quality open resources for researchers.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00626/full#supplementary-material
Abbreviations
SCLC, small cell lung cancer; NSCLC, non-small-cell lung carcinoma; OS, overall survival; SEER, Surveillance, Epidemiology, and End Results; AJCC, American Joint Committee on Cancer; PH, proportional hazard; HR, hazard ratio; CI, confidence interval; PS, propensity score; IPTW, inverse probability of treatment weighting; AIC, Akaike information criterion; IQR, interquartile range; TNM, tumor-node-metastasis.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: gLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Zamay TN, Zamay GS, Kolovskaya OS, Zukov RA, Petrova MM, Gargaun A, et al. Current and prospective protein biomarkers of lung cancer. Cancers. (2017) 9:E155. doi: 10.3390/cancers9110155
3. Bunn PA Jr, Minna JD, Augustyn A, Gazdar AF, Ouadah Y, Krasnow MA, et al. Small cell lung cancer: can recent advances in biology and molecular biology be translated into improved outcomes? J Thor Oncol. (2016) 11:453–74. doi: 10.1016/j.jtho.2016.01.012
4. Kalemkerian GP, Akerley W, Bogner P, Borghaei H, Chow LQ, Downey RJ, et al. Small cell lung cancer. J Natl Compreh Cancer Netw. (2013) 11:78–98. doi: 10.6004/jnccn.2013.0011
5. Walters S, Maringe C, Coleman MP, Peake MD, Butler J, Young N, et al. Lung cancer survival and stage at diagnosis in australia, canada, denmark, norway, sweden and the uK: a population-based study, 2004-2007. Thorax. (2013) 68:551–64. doi: 10.1136/thoraxjnl-2012-202297
6. Fiorentino FP, Macaluso M, Miranda F, Montanari M, Russo A, Bagella L, et al. CTCF and bORIS regulate rb2/p130 gene transcription: a novel mechanism and a new paradigm for understanding the biology of lung cancer. Mol Cancer Res. (2011) 9:225–33. doi: 10.1158/1541-7786.MCR-10-0493
7. Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. (2015) 121:664–72. doi: 10.1002/cncr.29098
8. Pan Y, Kong FW, Wang H, Wang X, Zhang H, Wu WB, et al. A recurrence-free survivor with chemotherapy-refractory small cell lung cancer after pneumonectomy: a case report and review of the literature. Medicine. (2017) 96:e8922. doi: 10.1097/MD.0000000000008922
9. Yang CJ, Chan DY, Shah SA, Yerokun BA, Wang XF, D'Amico TA, et al. Long-term survival after surgery compared with concurrent chemoradiation for node-negative small cell lung cancer. Annals of surgery. (2018) 268:1105–1112. doi: 10.1097/SLA.0000000000002287
10. Inoue M, Miyoshi S, Yasumitsu T, Mori T, Iuchi K, Maeda H, et al. Surgical results for small cell lung cancer based on the new tNM staging system. Thoracic Surgery Study Group of Osaka University, Osaka, Japan. Ann Thor Surg. (2000) 70:1615–9. doi: 10.1016/S0003-4975(00)01401-6
11. Brock MV, Hooker CM, Syphard JE, Westra W, Xu L, Alberg AJ, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: its time has come. J Thor Cardiovasc Surg. (2005) 129:64–72. doi: 10.1016/j.jtcvs.2004.08.022
12. Chandra V, Allen MS, Nichols FC 3rd, Deschamps C, Cassivi SD, Pairolero PC. The role of pulmonary resection in small cell lung cancer. Mayo Clin Proc. (2006) 81:619–24. doi: 10.4065/81.5.619
13. Hess KR. Assessing time-by-covariate interactions in proportional hazards regression models using cubic spline functions. Stat Med. (1994) 13:1045–62. doi: 10.1002/sim.4780131007
14. Harrell FE Jr. Regression Modeling Strategies with Application to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer (2001). doi: 10.1007/978-1-4757-3462-1
15. Little RJ, Rubin DB. Causal effects in clinical and epidemiological studies via potential outcomes: concepts and analytical approaches. Ann Rev Public Health. (2000) 21:121–45. doi: 10.1146/annurev.publhealth.21.1.121
16. D'Agostino RB Jr, D'Agostino RB Sr. Estimating treatment effects using observational data. JAMA. (2007). 297:314–6. doi: 10.1001/jama.297.3.314
17. Zhu J, Sharma DB, Gray SW, Chen AB, Weeks JC, Schrag D. Carboplatin and paclitaxel with vs without bevacizumab in older patients with advanced non-small cell lung cancer. JAMA. (2012) 307:1593–601. doi: 10.1001/jama.2012.454
18. Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. (1984) 79:516–24. doi: 10.1080/01621459.1984.10478078
19. Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on aMI survival using propensity score and instrumental variable methods. JAMA. (2007) 297:278–85. doi: 10.1001/jama.297.3.278
20. Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. (2004) 23:2937–60. doi: 10.1002/sim.1903
21. Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Int Med. (1997) 127:757–63. doi: 10.7326/0003-4819-127-8_Part_2-199710151-00064
22. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. (1998) 17:2265–81. doi: 10.1002/(SICI)1097-0258(19981015)17:19<2265::AID-SIM918>3.0.CO;2-B
23. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. (1996) 15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4
24. Demler OV, Paynter NP, Cook NR. Tests of calibration and goodness-of-fit in the survival setting. Stat Med. (2015) 34:1659–80. doi: 10.1002/sim.6428
25. Silva M, Galeone C, Sverzellati N, Marchianò A, Calareso G, Sestini S, et al. Screening with low-Dose computed tomography does not improve survival of small cell lung cancer. J Thor Oncol. (2016) 11:187–93. doi: 10.1016/j.jtho.2015.10.014
26. Lim E, Belcher E, Yap YK, Nicholson AG, Goldstraw P. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thor Oncol. (2008) 3:1267–71. doi: 10.1097/JTO.0b013e318189a860
27. Ploenes T, Osei-Agyemang T, Krohn A, Kayser G, Burger M, Passlick B. Surgical treatment of early stage small cell lung cancer. As Cardiovasc Thor Ann. (2012) 20:694–8. doi: 10.1177/0218492312453348
28. Iwata T, Nishiyama N, Nagano K, Izumi N, Mizuguchi S, Tsukioka T, et al. Role of pulmonary resection in the diagnosis and treatment of limited-stage small cell lung cancer: revision of clinical diagnosis based on findings of resected specimen and its influence on survival. Gen Thor Cardiovasc Surg. (2012) 60:43–52. doi: 10.1007/s11748-011-0847-4
29. Takei H, Kondo H, Miyaoka E, Asamura H, Yoshino I, Date H, et al. Surgery for small cell lung cancer: a retrospective analysis of 243 patients from japanese lung cancer registry in 2004. J Thor Oncol. (2014) 9:1140–5. doi: 10.1097/JTO.0000000000000226
30. Luchtenborg M, Riaz SP, Lim E, Page R, Baldwin DR, Jakobsen E, et al. Survival of patients with small cell lung cancer undergoing lung resection in england, 1998-2009. Thorax. (2014) 69:269–73. doi: 10.1136/thoraxjnl-2013-203884
31. Turner SR, Butts CA, Debenham BJ, Stewart KC. Is lobectomy superior to sublobar resection for early-stage small-cell lung cancer discovered intraoperatively? Int Cardiovasc Thor Surg. (2019) 28:41–4. doi: 10.1093/icvts/ivy223
32. Van Hoef ME. Limited small cell lung cancer, an early stage cancer that should receive the attention of experts. Tumori. (2015) 101:e34. doi: 10.5301/tj.5000262
33. Yu JB, Decker RH, Detterbeck FC, Wilson LD. Surveillance epidemiology and end results evaluation of the role of surgery for stage i small cell lung cancer. J Thor Oncol. (2010) 5:215–219. doi: 10.1097/JTO.0b013e3181cd3208
34. Schreiber D, Rineer J, Weedon J, Vongtama D, Wortham A, Kim A, et al. Survival outcomes with the use of surgery in limited-stage small cell lung cancer: should its role be re-evaluated? Cancer. (2010) 116:1350–7. doi: 10.1002/cncr.24853
35. Koizumi T, Fukushima T, Hamanaka K, Shiina T, Yoshida K, Kondo R, et al. Surgical outcomes in patients with small cell lung cancer: comparative analysis of computed tomograpy-detected patients with others. World J Surg Oncol. (2013) 11:61. doi: 10.1186/1477-7819-11-61
36. Vallieres E, Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, et al. The iASLC lung cancer staging project: proposals regarding the relevance of tNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the tNM classification for lung cancer. J Thor Oncol. (2009) 4:1049–59. doi: 10.1097/JTO.0b013e3181b27799
37. Aberle DR, Berg CD, Black WC, Church TR, Fagerstrom RM, Galen B, et al. The national lung screening trial: overview and study design. Radiology. (2011) 258:243–53. doi: 10.1148/radiol.10091808
38. Nicholson AG, Chansky K, Crowley J, Beyruti R, Kubota K, Turrisi A, et al. The international association for the study of lung cancer lung cancer staging project: proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the tNM classification for lung cancer. J Thor Oncol. (2016) 11:300–11. doi: 10.1016/j.jtho.2015.10.008
Keywords: early-stage SCLC, surgery, prognosis, nomogram, propensity score analysis
Citation: Wang Y, Zheng Q, Jia B, An T, Zhao J, Wu M, Zhuo M, Li J, Zhong J, Chen H, Yang X, Chi Y, Dong Z, Sepesi B, Zhang J, Gay CM and Wang Z (2020) Effects of Surgery on Survival of Early-Stage Patients With SCLC: Propensity Score Analysis and Nomogram Construction in SEER Database. Front. Oncol. 10:626. doi: 10.3389/fonc.2020.00626
Received: 26 November 2019; Accepted: 03 April 2020;
Published: 24 April 2020.
Edited by:
Alfredo Addeo, Geneva University Hospitals (HUG), SwitzerlandReviewed by:
Ming Li, Fudan University, ChinaJessica Desiree Menis, Istituto Oncologico Veneto (IRCCS), Italy
Copyright © 2020 Wang, Zheng, Jia, An, Zhao, Wu, Zhuo, Li, Zhong, Chen, Yang, Chi, Dong, Sepesi, Zhang, Gay and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carl M. Gay, CGay@mdanderson.org; Ziping Wang, wangzp2007@126.com
†These authors have contributed equally to this work
 Yuyan Wang
Yuyan Wang Qiwen Zheng
Qiwen Zheng Bo Jia
Bo Jia Tongtong An1
Tongtong An1 Jun Zhao
Jun Zhao