- 1National Institute of Integrative Medicine (NIIM), Melbourne, VIC, Australia
- 2Department of Health, Torrens University, Melbourne, VIC, Australia
- 3Discipline of General Practice, The University of Adelaide, Adelaide, SA, Australia
The current screening-test for prostate cancer, affecting 10% of men worldwide, has a high false negative rate and a low true positive rate. A more reliable screening test is needed. Circulating-Tumor-Cells (CTC) provide a biomarker for early carcinogenesis, cancer progression and treatment effectiveness. The cytology-based ISET®-CTC Test is a clinically validated blood test with high sensitivity and specificity. This study aimed to evaluate the ISET®-CTC test combined with prostate-specific-marker staining as a screening test for the detection of prostate cancer. We selected a group of 47 men from our ongoing CTC screening study involving 2,000 patient-tests from Sep-2014 to July-2019, who also underwent standard diagnostic cancer testing before or after CTC testing. While 20 of the 47 men were diagnosed with prostate cancer before the ISET®-CTC test, 27 men underwent screening. We studied the CTC identified in 45 CTC-positive men by Immuno-Cyto-Chemistry (ICC) assays with the prostate-specific-marker PSA. CTC were ICC-PSA-marker positive in all men diagnosed with primary prostate cancer (n = 20). Secondary cancers were detected in 63% (n = 7/11) of men with mixed CTC-population (ICC-PSA-positive/ICC-PSA-negative). Of the 27 men screened, 25 had CTC, and 84% of those (n = 20) were positive for the prostate-specific-PSA-marker. Follow-up testing suggested suspected prostate cancer in 20/20 men by a positive PSMA-PET scan, and biopsies performed in 45% (n = 9/20) men confirmed the diagnosis of early prostate cancer. Kidney cancer or B-cell lymphoma were detected in two men with ICC-PSA-marker negative CTC. Our study suggests that the combination of ISET®-CTC and ICC-PSA-marker-testing has an estimated positive-predictive-value (PPV) of 99% and a negative-predictive-value (NPV) of 97%, providing a more reliable screening test for prostate cancer than the standard PSA-blood-test (PPV = 25%; NPV = 15.5%). Our findings warrant further studies to evaluate the new test's potential for prostate cancer screening on a population level.
Introduction
Prostate cancer is the most common in men, and the second leading cause of cancer deaths (25%) in Australia (1). One in seven men (14%) will be diagnosed with prostate cancer in their lifetime worldwide (1, 2). In 2018, 22.5% men lived with prostate cancer, and >3.7% deaths were attributable to prostate cancer in Australia (1, 2).
The prostate-specific-antigen (PSA) blood test was originally approved by the US-Food-and-Drug-Administration in 1986 to monitor the progression of prostate cancer in men who had already been diagnosed with the disease (2) As a screening-test for men from the age-of-50-years, the PSA-blood-test in conjunction with the digital-rectal-exam has also received support from Medical-Benefit-Schemes and was routinely recommended from 1994 to 2008 (2).
Rising PSA levels are not always associated with prostate cancer, as several non-cancerous conditions, i.e., prostatitis and benign-prostatic-hyperplasia may increase PSA levels in the blood, and can result in false positives (3). In fact, only 25% of men with elevated PSA levels were found to have prostate cancer, leading to unnecessary and potentially harmful follow-up tests such as biopsies in 75% of the men (4). Of these men, 12% experienced bothersome symptoms, including pain and bleeding, or serious infections with 6.9% requiring hospitalization (2, 5).
In addition, a large study with 18,882 men found that routine screening for prostate cancer by the PSA-blood-test and digital-rectal-examination failed to detect prostate cancer in a large proportion (85.5% false-negatives) of screened men with PSA levels below <4.0 ng/ml (3). Three-quarters (78%) of these men had high grade (Gleason ≥ 7) tumors of the prostate (3).
The low-sensitivity (25% true-positives) and low-specificity (14.5% true-negatives) of the PSA-blood-test led to the recommendation against this test as a routine screening-test (6), highlighting the need for a more accurate screening-test for prostate cancer.
To date, modifications of the PSA-blood-test and its interpretation to improve the sensitivity and specificity to better predict cancer have failed (2). Suggested alternative screening methods included free-to-total-PSA-ratio, age-specific-reference-ranges, isoforms-of-PSA, and precursors-of-PSA (2). The PSA-blood-test may however be useful in patients with diagnosed prostate cancer with or without prostatectomy to monitor relapse, indicated by a rising PSA-blood-level over time (7).
Furthermore, current conventional treatment options for prostate cancer, such as surgery and radiation have a high burden of serious complications, including urinary incontinence, bowel and erectile dysfunction, and infection. Specifically, 80% of men diagnosed with prostate cancer undergo a prostatectomy and/or receive radiation therapy, with 60% reporting serious complications (8). Late detection of prostate cancer avoided only one death in 1,000 men (0.1%) (8). Consequently, physicians often recommend a watch-and-wait-approach.
In contrast, our group and others have found that non-invasive blood tests for Circulating-Tumor-Cells (CTC) can help with the early detection of cancer (9, 10), as CTC have been associated with early carcinogenesis and cancer risk (10–12). A meta-analysis on the prognostic role of CTC and prostate cancer specifically involving 33 clinical trials and 4,170 patients suggested a significant association between CTC count and overall survival, biochemical relapse-free survival/disease free survival (HR (95% CI): 2.43 [2.07, 2.86]; 2.15 [1.69, 2.73]; p < 0.001) (13).
Several technologies have been developed to identify CTC, including the Isolation-by-SizE-of-Tumor-Cells (ISET®)-CTC-test (Rarecells-Diagnostics, France) (14). The ISET®-CTC-test is a cytology-based clinically validated blood test with high sensitivity and specificity (15–17).
Cytology is an established technique in the field of cancer diagnostics and has been for over a century, with the first microscopic discovery of cancer cells in 1838, and the first monograph of clinical cytology published in 1960s (18, 19). It is now an invaluable specialty in pathology laboratories worldwide and is routinely used as a first line of investigation in cancer diagnostics.
The cytology-based ISET®-CTC-test can distinguish cancer cells from benign cells, using the same cytological criteria as used in routine cancer diagnostics, including anisonucleosis, enlarged nuclei, high nuclear-cytoplasmic-ratio, and irregular nuclear borders (15, 16). Comprehensive analyses of CTC isolated with the ISET® technique, including spiking experiments and genetic testing, verified that all CTC in a blood sample are captured, and had the features of malignant cancer cells (17).
The cytology-based ISET®-CTC-test is independent of the presence of any tumor-surface-markers on cancer cells, such as the Epithelial-Cell-Adhesion-Molecule (EpCAM) markers, used by most other CTC technologies (20, 21). In fact, the ISET®-CTC-test can detect CTC of all cancer types, including epithelial and non-epithelial tumors (22), can distinguish between single-CTC and CTC-clusters (23, 24), with CTC-clusters having greater metastasing potential associated with shorter overall survival (25).
Marker-based CTC-tests have an implicit high risk of false negative results, as cancer cells undergo frequent changes in protein expression and have the potential to lose surface markers, often in later stages of cancer (16). Marker-based CTC-tests can also lead to false-positive-results, as many tumor-markers can be expressed on non-pathological cells, including EPCAM and other markers (26–29).
While it had been recognized that CTC were better prognostic biomarkers than the PSA blood levels for prostate cancer, the CTC detection rate of 57–62% using the marker-based CTC test CellSearch® was considered non-optimal (30, 31). In contrast, the cytology-based ISET®-CTC-test has proven to be of high sensitivity (17) and high specificity (10, 23, 32, 33), and is therefore superior to marker-based CTC-tests in detecting true CTC (16). The ISET®-CTC-test can find one CTC/10 ml of blood, corresponding to 500 CTC/5L of blood in an adult, which is extremely sensitive (17).
While biopsy is the gold-standard technique in the diagnosis of prostate cancer, advances in imaging technologies have been developed to improve the detection of cancer. Specifically, the Gallium-68-prostate-specific-membrane-antigen-positron-emission-tomography (Ga68-PSMA-PET) scan is a highly sensitive imaging-test, which can detect tumors as small as 2.4 mm, and has shown promise in enhancing the detection and localization of prostate cancer (34, 35).
In a study of men with persistently elevated prostate PSA levels but negative digital rectal examination and negative biopsy, Ga68 PSMA-PET guided biopsy was found to be useful in identifying clinically significant primary prostate cancer (Gleason ≥ 7), with 100% overall sensitivity, and 76–88% specificity (36).
The superior diagnostic accuracy of the PSMA-PET scan compared to MRI and PET imaging alone in the detection of primary prostate cancer, its usefulness in screening, as well as guiding biopsies have been described in several independent studies (37–39).
Continuous screening and monitoring are also relevant for patients previously diagnosed with cancer, as recurrences or relapses of their cancer may be more challenging to treat, and timely detection may assist with recovery. In this context, the accuracy of a screening/diagnostic test for recurrent cancer is as important as for the early detection of primary cancer.
A study of 42 patients concluded that the PSMA-PET scan was helpful in detecting prostate cancer in 83% of patients with suspected recurrent cancer, while false positive lesions were not detected (40). Furthermore, a large Australian study of 431 patients with prostate cancer found the PSMA-PET scan to be a valuable diagnostic tool in the management of patients with prostate cancer, as the uptake of 68GA-labeled PSMA marker allowed the detection of unsuspected disease in the prostate bed, local lymph nodes, and distant metastases, which had not been detected by other imaging techniques. The study found that the PSMA-PET scan reduced the need for bone scintigraphy, CT scans, MRI, and 18F-FDG PET scans (41).
The high sensitivity (near 100%) and high specificity (about 80%) of the PSMA-PET scan demonstrated in several studies, makes it the excellent non-invasive tool for the detection of prostate cancer.
In our previous study with 2,000 patients, CTC were detected in 50% and early cancer in 25%, including prostate cancer (9). The prostate cancer diagnosis was facilitated by the PSMA-PET scan, followed by biopsy in a proportion of men (9).
PSA-markers are found in cells of prostate origin (99% specificity), and are less expressed in kidney, parotid gland and pancreas (42). Prostein (also known as P501S) markers are only in prostatic epithelial cells (100% specificity; 100% sensitivity) (43). A small proportion (3%) of men have no PSA-markers on prostate cells, providing a 97% sensitivity (3, 43, 44).
In this study we combined the ISET®-CTC screening blood test and immuno-cyto-chemistry (ICC) with prostate-specific markers in men with prostate cancer and in men screened for prostate cancer. Screened men with a positive CTC count and prostate-marker positive CTC were followed up with PSMA-PET scan and/or biopsy for prostate-specific diagnostic testing.
Materials and Methods
Study Design and Participants
For this observational study, doctors and health practitioners mainly from two medical clinics in Melbourne, Australia, the National-Institute-of-Integrative-Medicine Clinic, and the Eng-Medical-Center, referred patients to the ISET®-CTC-test between Sep-2014 and Jul-2019. Follow-up of participants was coordinated by the patients' doctors as per usual clinical practice. Routine and follow-up testing included PSA-blood-tests, PSMA-PET scans, MRI, biopsies, and any other diagnostic tests, according to the patient's doctor advice.
The study was approved by the NHMRC-endorsed NIIM Human-Research-Ethics-Committee. Participating patients provided written informed consent. The study has been registered on the Australian-New-Zealand-Clinical-Trial-Registry, ANZCTR 12614001143617.
Inclusion Criteria and Selection of Patient Samples for ICC Prostate-Specific Analysis
For this study, we selected two groups of men from our ongoing CTC study involving 2,000 patients-tests (9), who had also standard cancer diagnostic test results available, either before (group PC) or after (group ED) CTC testing. CTC blood test samples for ICC analysis from the first group of men diagnosed with prostate cancer before CTC analysis were considered as positive control. The second group of men underwent follow-up diagnostic testing after the CTC screening blood test. As negative control for the prostate-specific markers, we undertook ICC-analysis on CTC of two female cancer patients.
Circulating Tumor Cell (CTC) Detection by ISET®-CTC Test
In this study, we used the ISET®-CTC methodology combining blood filtration and microscopic analysis using standard cyto-pathological criteria, validated in the over 70 peer-reviewed publications over 20 years (16, 32). We followed standardized validated protocols described previously (15–17).
Briefly, the ISET® method is a blood filtration-based approach, which enriches rare cells on a polycarbonate membrane with 8 μm pores. Ten milliliter of peripheral blood was collected in buffered EDTA, maintained at room temperature (RT) and processed within 2 h of collection. Blood was then diluted 1:10 with Rarecells® Buffer reagent set (Rarecells Diagnostics, France) containing saponin, salt and bovine serum albumin, agitated for 10 min at RT, and filtered with the ISET® filtration blocks and device (15).
The ISET®-CTC-test can detect CTC of all cancer types, including epithelial and non-epithelial tumors. Human cancer cells are larger than the 8 microns filter pore size (22). In fact, CTC range from 11.7 to 23.8 microns for solid tumor cells, 7.2–10 microns for small-cell type cancers (e.g., small cell lung carcinoma) and 8.9–15.3 microns for blood type cancers.
The dried filter membrane was stained with May-Gruenwald-Giemsa for cytological analysis. A cytologist, with Australian and International cytology certification [CT(ASC), CT(IAC)] and more than 25 years' experience conducted the analysis using a Leica DMLB microscope with 63 × 10 magnification and standard cytological criteria to identify malignant cells.
Circulating malignant cells were defined by the presence of 4 of the following criteria: (a) anisonucleosis (ratio > 0.5), (b) nuclei larger than 1–3 calibrated pore sizes (8 μm) of the membrane (i.e., >8–24 microns), (c) irregular nuclear borders, (d) high nuclear-cytoplasmic ratio, and/or (e) presence of three-dimensional sheets. Cells displaying 1–3 criteria were defined as atypical cells with uncertain malignant potential. Circulating benign cells were characterized by the absence of these criteria (16).
Images of CTC and atypical cells were taken with a digital Leica EC3 camera, reviewed independently by a second cytologist / researcher and any discrepancies were discussed.
Immuno-Cyto-Chemistry (ICC) Staining With Prostate-Specific Antibodies
ICC staining was conducted with the Dako EnVision Flex Mini Kit, high pH, and antibodies (a) PSA (Dako monoclonal mouse anti-human prostate-specific antigen Clone ER-PR8, concentrate), and (b) Prostein/P501S [Dako Flex Monoclonal mouse anti-human Prostein Clone 10E3, Ready-to-Use (RTU)].
For antigen retrieval, ISET® filters were placed into a 50 ml tube and heated in a water bath at 99°C for 40 min in 50 mL of 2% v/v target retrieval solution (high pH (50x): 3 × 30 mL, 50x concentrate Tris/EDTA buffer, pH9; Dako Agilent). Next, the ISET® filters were washed 15x in 0.2 M PBS and incubated with permeabilization buffer (0.1% Triton) for 2 min. After washing the ISET® filter as before, the ISET® filter was incubated for 30 min with peroxidase-blocking reagent (80 μL of H2O2, 15 mmol/L Na3N and detergent), and washed with distilled water 15 times.
Each ISET®-filter spot was incubated overnight at 4°C in 600 μL solution of the primary antibody (a) 1:200 dilution of concentrated PSA antibody or (b) 1:4 dilution of Prostein antibody RTU in a buffer of 1xPBS / 5%BSA in a 1.5 ml Eppendorf tube.
After incubation with the primary antibody, ISET®-filter spots were washed in PBS as before, placed on a clean slide, for incubation with 80 μL of the secondary antibody (Dako Envision Flex Kit goat secondary antibody) for 45 min at RT.
The ISET®-filter spots were washed again, before incubation with 80 μL of chromogen (Dako Liquid DAB + Substrate Chromogen (3,3 diaminobenzidine tetrahydrochloride) on a glass slide in the dark for 10 min. The excess chromogen was removed by washing in 0.2 M PBS. The filter spots were allowed to air-dry, then counterstained with hematoxylin for 2 min. After washing in PBS as before, each air-dried immuno-stained spot was mounted on a clean glass slide for microscopic analysis.
Results
A total of 49 patient samples were selected for this study. These included samples from 47 men and 2 women (Figures 1, 2).
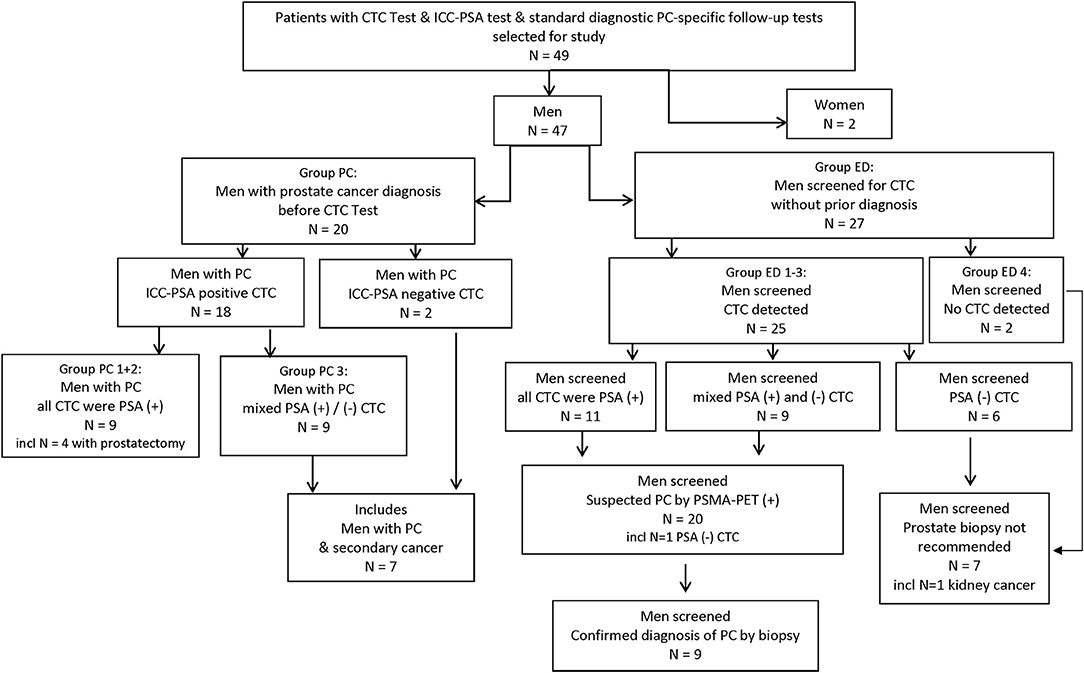
Figure 1. Study flow chart. CTC, circulating tumor cell; ED, early detection; ICC, immuno-cyto-chemistry; incl, including; PC, prostate cancer; PSA, prostate specific Antigen; (+)/(–), positive/negative test result.
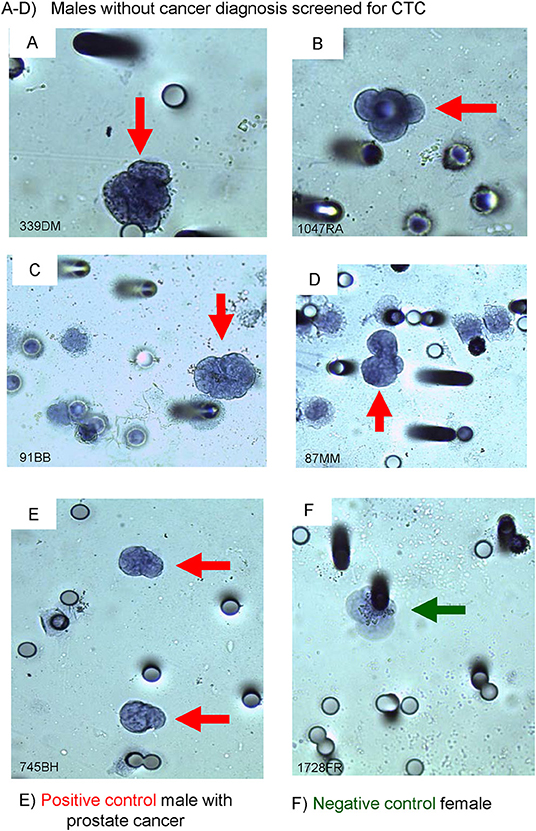
Figure 2. Immuno-cyto-chemistry (ICC) on ISET®-CTC with PSA-antibody. (A–D) screened males with PSA (+) stained CTC, (E) male with PROSTATE CANCER (positive control), and (F) a female breast cancer patient (negative control). The black outline of the Circulating Tumor Cell (CTC) (red arrows) depicts a positive ICC marker stain, no black outline around the CTC depicts a negative ICC marker stain (green arrow).
Prostate Cancer Patients (Group PC)
In a group of 20 men with diagnosed prostate cancer and positive CTC count, we tested CTC cells for ICC-PSA-markers. Of the men aged 50–80 years (mean 65 years), the CTC-count ranged between 1 and 23 CTC/ml (mean 6.5 CTC/ml) at 1 month to 12 years after prostate cancer diagnosis (Table 1).
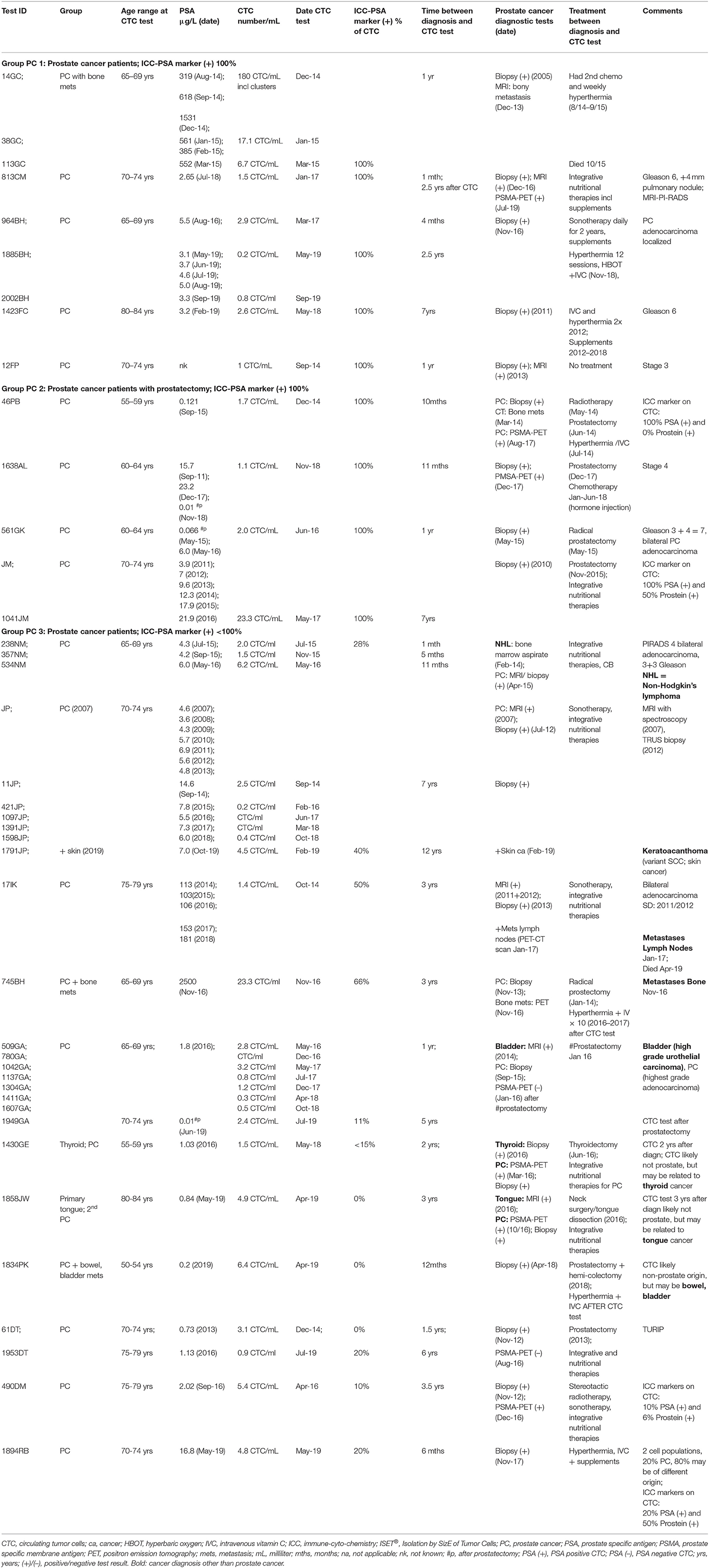
Table 1. ISET®-CTC and ICC prostate marker test results AFTER diagnosis in patients with prostate cancer (group PC).
PSA-blood-test results were ≤ 5 ng/ml in two-thirds of men (65%, 13/20) while 35% (7/20) showed elevated PSA-levels at time of diagnosis or CTC testing (Table 1).
The ICC-PSA-marker test was positive in all men diagnosed with prostate cancer (90%, n = 18), except in two cases with secondary cancers (oral or colon/bladder) and/or prostatectomy.
Forty-five percent of the men with prostate cancer (9/20) demonstrated 100% positivity for PSA-markers, while 55% had a heterogeneous population of cancer cells (ICC-PSA-positive-CTC and ICC-PSA-negative-CTC) (Figure 1, Table 1).
In two-thirds of the cases with mixed CTC populations or ICC-PSA-negative-CTC (66%, 8/12), secondary cancer or metastases were diagnosed within 1-to-12 years after prostate cancer diagnosis, and included bladder, bowel, skin, Non-Hodgkin's-Lymphoma, thyroid, tongue cancer, and lymph node involvement (Table 1).
The ICC-PSA-negative-CTC may represent cells from the secondary cancers in these patients. However, other explanations such as lower expression of PSA antigen on CTC or loss by mutation cannot be ruled out.
Early Detection of Cancer in Screened Patients (Group ED)
The second group of men we selected for this study had also standard cancer diagnostic test results available after CTC screening (group ED). Out of the selected 27 men without prior cancer diagnosis, 25 men were positive for CTC. The majority of these were also positive for ICC-PSA-markers (80%, 20/25). Three-quarters of men screened with CTC (80%, 20/25) had prostate-specific diagnostic follow-up tests, and 20% (5/25) had other follow-up diagnostic testing within 1 month to 3.5 years of the CTC-test (Figure 1, Table 2, group ED1+2).
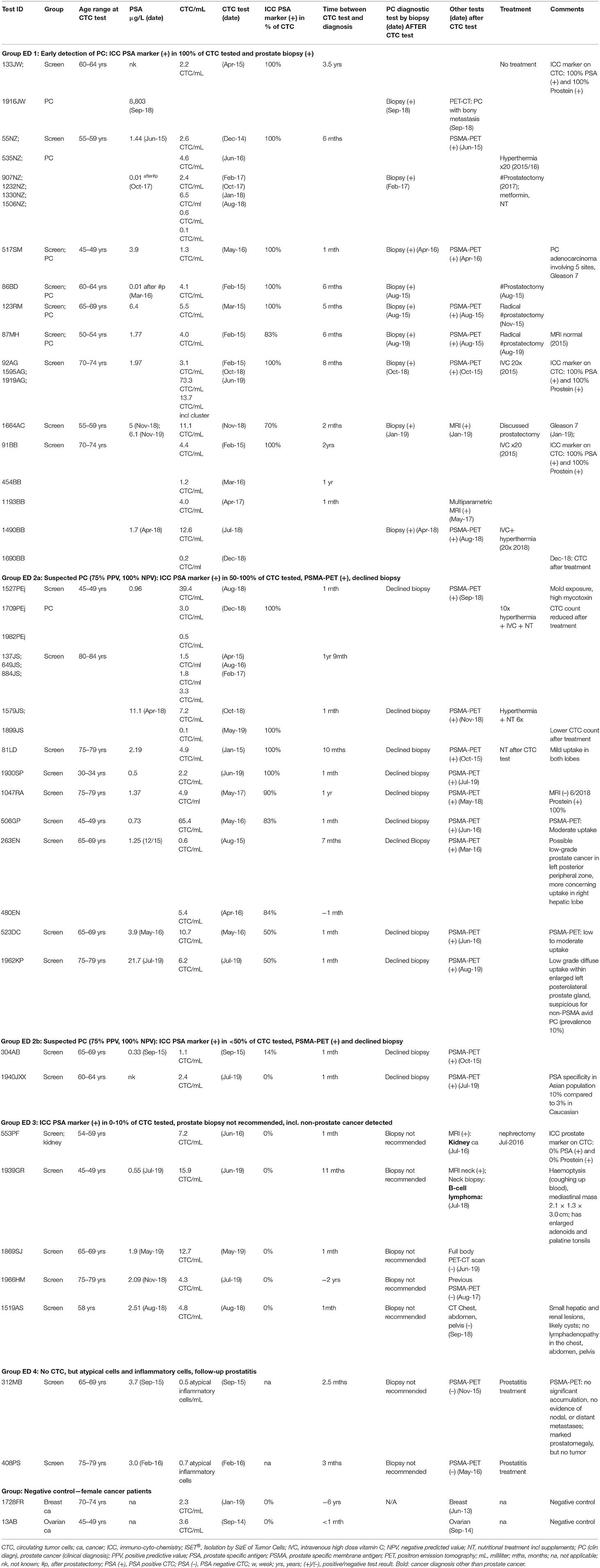
Table 2. ISET®-CTC and ICC-prostate marker test results of men not previously diagnosed with cancer—Group “early detection” (ED).
The average CTC count in the tested samples of men aged 30–83 years (mean = 58 years) was 1–13 CTC/ml (mean = 3.1 CTC/ml), and two patient samples had higher counts of 39 and 65 CTC/ml.
PSA-blood-test results at the time of the CTC-tests were ≤ 5 ng/ml for all but three men (88%, 22/25) (Table 2).
Follow-up prostate-specific diagnostic tests including biopsy were recommended for all men with ICC-PSA-positive-CTC (80%, n = 20/25). This group included a man of Asian background with ICC-PSA-negative-CTC, as the specificity of the PSA-marker is lower (90%) in an Asian population compared to 97% in Caucasians (45).
Positive PSMA-PET scan results suggested suspected prostate cancer in all men with ICC-PSA-positive-CTC (n = 20). Forty-five percent of these men (n = 9/20) agreed to biopsies, which confirmed the diagnosis of early prostate cancer in each one (Figure 1, Table 2).
Prostate-specific biopsy was not recommended in five Caucasian men with ICC-PSA-negative CTC, instead non-prostate specific follow-up tests detected kidney cancer in one patient, and B-cell lymphoma in another, while no other tumors were detected in 3/6 men with ICC-PSA-negative CTC (Table 2, group ED3).
In summary, out of the 27 men in the early detection group (group ED), 25 men had CTC, 2 men had no CTC. Twenty out of the 25 men with CTC had ICC-PSA-positive markers, and all of these 20 (100%) were diagnosed with prostate cancer.
In these subgroups of men the detection of CTC by the cytology-based ISET methodology matched exactly the detection of cancer by standard diagnostic methods. All men with diagnosed prostate cancer before CTC testing had CTC, and screened men with PSA-marker positive CTC had a positive prostate-specific diagnostic follow-up test.
The high accuracy of the ISET-CTC test combined with the 97% sensitivity and 99% specificity of the PSA-marker presence on prostate cancer cells, suggests an estimated positive predictive value (PPV) of 99% and a negative predictive value (NPV) of 97% for this novel screening test.
Prostein (P501S)
ICC staining with Prostein in a subgroup of patients (n = 8) was equivalent to the staining by PSA markers in all except one case (Tables 1, 2).
Inflammatory Prostate Conditions and Negative Controls
No CTC but atypical inflammatory cells were found by the cytology-based ISET®-CTC technology in two out of the 27 men screened for CTC (group ED). These men presented with prostate symptoms and slightly elevated PSA-levels (Table 2, group ED4). The ISET®-CTC-test accurately demonstrated a non-cancerous inflammatory condition in these patients. As a negative control, we undertook PSA-marker-testing on two females with breast or ovarian cancer, and the result was negative as expected (Table 2, negative control).
Long-Term Follow-Up
For a subset of patients in this study, long-term data was available which included regular CTC-testing results, treatment details, and disease progression.
ISET®-CTC-test results before and after prostate cancer diagnosis were available for 6 men in the early detection group (Table 2), with PSA-marker-positive-CTC apparent 6 months-to-3.5 years before prostate cancer diagnosis. Five of these patients received advice and treatments of integrative therapies, and one underwent prostatectomy. CTC-count reduced over-time in those following treatment, evident by repeated ISET®-CTC-tests, suggesting a decreased malignant potential. One of the six patients however, followed the watch-and-wait approach, not receiving advice on preventative therapies, and unfortunately developed metastatic-bone-lesions 3.5 years after the initial CTC-test, with PSA(+) CTC (2.2 CTC/ml) (Table 2).
In this small number of patients where long-term monitoring by repeated CTC-testing was available, CTC-count by ISET® was consistent with cancer progression and treatment effectiveness (Table 2).
Discussion
Our study suggests the combination of ISET®-CTC and ICC-prostate-marker testing to be a highly sensitive and accurate screening test for prostate cancer, with 97% sensitivity and 99% specificity. All patients with confirmed prostate cancer tested positive for prostate-specific CTC, and all screened males with prostate-specific CTC had an affirmative PSMA-PET scan result, highly suggestive of prostate cancer. Half of the screened men with positive PSMA-PET scan results underwent biopsies, confirming the diagnosis of prostate cancer in each case.
Our findings are in line with a recently published study using a similar filtration-based isolation of CTC method, the Parsortix system (ANGLE Guildford, UK; which combines CTC and ICC) to screen men for the likelihood of prostate cancer (46). The study concluded that the combination of CTC and a 12-gene panel screening test was able to predict the presence of prostate cancer in subsequent biopsies with over 90% accuracy (46).
In contrast, the PSA-blood-test failed to match the abnormal CTC test results in 83% of study participants, including 73% of men with prostate cancer (group PC), and 88% of the men screened (group ED) with positive PSA-marker-CTC and early prostate cancer diagnostic results.
Importantly, the detection of ICC-PSA-negative CTC in screened men allowed the early detection of other types of cancer, namely renal cancer, or B-cell lymphoma. Additionally, the new combination of CTC and ICC testing provided evidence of the presence of relapses or secondary cancers such as bladder, bowel, thyroid, tongue cancer, and metastasis in bone and lymph nodes in men with prostate cancer.
The findings are in line with earlier studies, whereby the CTC count was directly related to the risk of cancer detection and cancer status (9, 10, 12).
The early detection of cancer is paramount to enable medical interventions that will reduce the risk of cancer morbidity and mortality. Early detection allows more time and treatment options in addition to active surveillance, before surgery and/or radiation therapy.
There is evidence that preventative integrative and nutritional therapies can reduce the CTC count and therefore cancer risk, outlined elsewhere in more detail (9). Hyperthermia treatment has also shown to improve treatment effectiveness of other therapies e.g., chemotherapy, by an average of 40–80% (47, 48), and may be considered for patients with higher CTC-counts.
With its estimated sensitivity of 97% and specificity of 99%, this study's combination of ISET®-CTC and ICC-PSA-marker testing offers a unique opportunity to replace the unsatisfactory PSA-blood-test as a screening test.
Due to the PSA-blood-test's high false negative rate of 85.5%, and the low 25% true positive rate (3), the PSA-blood-test is of limited use for prostate cancer screening. On the other hand the low sensitivity of the PSA-blood-test results in many men have led to unnecessary procedures that carry with them high complication rates, exposure to toxic treatments and debilitating post-surgical complications (2).
In current clinical practice, the limitations of the PSA-blood-test are being addressed by performing multi-parametric magnetic resonance imaging (MRI) before biopsies to decrease the detection of non-significant prostate cancer and to improve the detection of significant prostate cancer (49, 50). However, a more accurate screening test than the PSA-blood-test may improve the prediction of prostate cancer by itself, and would reduce the necessity of MRI imaging before biopsy.
A strength of our study was the use of one of the most sensitive and accurate CTC-screening tests currently available, the cytology-based ISET®-CTC-test (14), which clearly distinguishes cancer cells from atypical inflammatory cells, making it possible to accurately predict a risk of cancer or an inflammatory condition, such as prostatitis (16). Almost every participant with an inflammatory condition in our larger study involving 2,000 patients had no CTC upon screening (9), while marker-based CTC-tests such as CellSearch® cannot distinguish between CTC and atypical inflammatory cells, as both types of cells can have the cell-surface markers, leading to many false positives, and false negatives (20, 21, 51).
Confirmatory prostate cancer diagnosis by gold-standard biopsy was applicable for 40 out of 47 participants and was performed in 73% of those. These included all men with previously diagnosed prostate cancer (n = 20), and a proportion of men screened with positive PSA-marker stained CTC (n = 9/20 men, 45%).
Our study has some limitations. First, not all screened men with positive PSMA-PET scans agreed to biopsies for confirmatory diagnosis of prostate cancer at the time of the study. However, the PSMA-PET scan has been shown to be a highly accurate, less invasive diagnostic tool with high sensitivity and specificity, useful in the detection of prostate cancer, especially in lower risk men reluctant to undergo biopsy (35–37, 39–41).
Second, prostate-specific diagnostic testing was not available for a small number of screened men (n = 5/25) included in this study. However, these men underwent other relevant follow-up testing as advised by their doctor, and in line with their non-prostate specific CTC test results, which enabled the detection of other cancers in two of the five men.
Third, we tested only a small group of patients with the ICC-Prostein-marker, therefore findings are preliminary and warrant further studies.
Despite the limitations, our results indicate that combining the ISET®-CTC-test with other ICC-tumor-cell markers for the early detection of other cancers is promising and warranted. Early detection will be particularly important for aggressive silent cancers, such as ovarian or pancreatic cancer, as time is of the essence for long-term quality of life and survival. Only 15% of ovarian cancers are currently detected in stage 1 with the available screening-tests, and preventive oophorectomy results in premature menopause and aging (52, 53).
Pancreatic cancer is specifically deadly (16% 5-year-survival-rate) when detected late. In contrast, if detected early, treatment by surgery can improve 5-year survival rates substantially to 61% (54). However, only a small percentage of pancreatic cancer is detected early, highlighting the need for better screening tests.
Conclusions
This study provides evidence for the combined ISET®-CTC and ICC-PSA-marker testing to be a more accurate screening test than the PSA-blood-test alone. This novel combination of tests has an estimated positive predictive value of 99% and a negative predictive value of 97%, warranting further studies to evaluate the new test's potential for prostate cancer screening on a population level. The test allows early detection of prostate cancer, as well as other types of cancer, providing the opportunity for early intervention. The new screening test combination also allowed long-term monitoring of patients diagnosed with prostate cancer, providing insight to relapse or metastasing potential.
The ISET®-CTC-test alone allows monitoring of treatment effectiveness in patients with cancer of any type and offers an opportunity for the early detection of cancer risk. The combination of the ISET®-CTC and organ-specific cell markers provides an exciting future for cancer screening.
Data Availability Statement
All datasets generated for this study are included in the article.
Ethics Statement
The studies involving human participants were reviewed and approved by NIIM HREC, National Institute of Integrative Medicine, Melbourne, Australia. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
KR established and oversaw ISET®-CTC-testing and ICC prostate marker testing at the NIIM lab, with assistance from cytologist SM and post-doctoral researcher TT. Medical practitioners PE and AS provided patients, and patient data for the study. KR collated and analyzed the data and wrote the manuscript with contributions from co-authors. All authors read and approved the final version.
Funding
No external funds have been received. The open access publication fee will be carried by NIIM.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the team of technical and research assistants who made CTC-testing at NIIM possible, including Nikolaj Travica and Ranjini Dorairaj. We would also like to thank Dr. Peter Fakler and Prof. Patrizia Paterlini-Brechot for valuable feedback on the manuscript.
References
1. Cancer Australia. Prostate Cancer Statistics. (2019). Available online at: https://prostate-cancer.canceraustralia.gov.au/statistics (accessed March 15, 2020).
2. National Cancer Institute USA. Prostate-Specific-Antigen (PSA) Test Fact Sheet. (2017). Available online at: www.cancer.gov/types/prostate/psa-fact-sheet (accessed March 15, 2020).
3. Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤ 4.0 ng per milliliter. N Eng J Med. (2004) 350:2239–46. doi: 10.1056/NEJMoa031918
4. Barry MJ. Prostate-specific–antigen testing for early diagnosis of prostate cancer. N Engl J Med. (2001) 344:1373–7. doi: 10.1056/NEJM200105033441806
5. Pinkhasov GI, Lin YK, Palmerola R, Smith P, Mahon F, Kaag MG, et al. Complications following prostate needle biopsy requiring hospital admission or emergency department visits–experience from 1000 consecutive cases. BJU Int. (2012) 110:369–74. doi: 10.1111/j.1464-410X.2011.10926.x
6. Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Int Med. (2012) 157:120–34. doi: 10.7326/0003-4819-157-2-201207170-00459
7. Schaefer U, Witt F, Schueller P, Micke O, Willich N. Prostate-specific antigen (PSA) in the monitoring of prostate cancer after radical prostatectomy and external beam radiation. Anticancer Res. (2000) 20:4989–92.
8. Bibbins-Domingo K, Grossman DC, Curry SJ. The US Preventive Services Task Force 2017 draft recommendation statement on screening for prostate cancer: an invitation to review and comment. JAMA. (2017) 317:1949–50. doi: 10.1001/jama.2017.4413
9. Ried K, Eng P, Sali A. Screening for circulating tumour cells allows early detection of cancer and monitoring of treatment effectiveness: an observational study. Asian Pac J Cancer Prev. (2017) 18:2275–85. doi: 10.4172/2472-0429.1000123
10. Ilie M, Hofman V, Long-Mira E, Selva E, Vignaud JM, Padovani B, et al. Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS ONE. (2014). 9:e111597. doi: 10.1371/journal.pone.0111597
11. Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. (2006) 12:4218–24. doi: 10.1158/1078-0432.CCR-05-2821
12. Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. (2004) 351:781–91. doi: 10.1056/NEJMoa040766
13. Ma X, Xiao Z, Li X, Wang F, Zhang J, Zhou R, et al. Prognostic role of circulating tumor cells and disseminated tumor cells in patients with prostate cancer: a systematic review and meta-analysis. Tumor Biol. (2014) 35:5551–60. doi: 10.1007/s13277-014-1731-5
14. Tungland B, Meyer D. Nondigestible oligo-and polysaccharides (dietary fiber): their physiology and role in human health and food. Comprehensive Rev Food Sci food Saf. (2002) 1:90–109. doi: 10.1111/j.1541-4337.2002.tb00009.x
15. Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schutze K, et al. Isolation by size of epithelial tumor cells : a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol. (2000) 156:57–63. doi: 10.1016/S0002-9440(10)64706-2
16. Hofman VJ, Ilie MI, Bonnetaud C, Selva E, Long E, Molina T, et al. Cytopathologic detection of circulating tumor cells using the isolation by size of epithelial tumor cell method: promises and pitfalls. Am J Clin Pathol. (2011) 135:146–56. doi: 10.1309/AJCP9X8OZBEIQVVI
17. Laget S, Broncy L, Hormigos K, Dhingra DM, BenMohamed F, Capiod T, et al. Technical insights into highly sensitive isolation and molecular characterization of fixed and live circulating tumor cells for early detection of tumor invasion. PLoS ONE. (2017) 12:e0169427. doi: 10.1371/journal.pone.0169427
18. Hajdu SI, Ehya H. Foundation of diagnostic cytology. Ann Clin Lab Sci. (2008) 38:296–9. Available online at: http://www.annclinlabsci.org/content/38/3/296.long
20. Paterlini-Bréchot P. Circulating tumor cells: who is the killer? Cancer Microenviron. (2014) 7:161–76. doi: 10.1007/s12307-014-0164-4
21. Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. (2007) 253:180–204. doi: 10.1016/j.canlet.2006.12.014
22. Harouaka RA, Nisic M, Zheng SY. Circulating tumor cell enrichment based on physical properties. J Lab Autom. (2013) 18:455–68. doi: 10.1177/2211068213494391
23. Hofman V, Ilie M, Long E, Guibert N, Selva E, Washetine K, et al. Detection of circulating tumor cells from lung cancer patients in the era of targeted therapy: promises, drawbacks and pitfalls. Curr Mol Med. (2014) 14:440–56. doi: 10.2174/1566524014666140414205455
24. Khoja L, Shenjere P, Hodgson C, Hodgetts J, Clack G, Hughes A, et al. Prevalence and heterogeneity of circulating tumour cells in metastatic cutaneous melanoma. Melanoma Res. (2014) 24:40–6. doi: 10.1097/CMR.0000000000000025
25. Wang C, Mu Z, Chervoneva I, Austin L, Ye Z, Rossi G, et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res Treat. (2016) 161:83–94. doi: 10.1007/s10549-016-4026-2
26. Schmelzer E, Reid LM. EpCAM expression in normal, non-pathological tissues. Front Biosci. (2008) 13:3096–100. doi: 10.2741/2911
27. Fang X, Zheng P, Tang J, Liu Y. CD24: from A to Z. Cell Mol Immunol. (2010) 7:100. doi: 10.1038/cmi.2009.119
28. Jordan AR, Racine RR, Hennig MJ, Lokeshwar VB. The role of CD44 in disease pathophysiology and targeted treatment. Front Immunol. (2015) 6:182. doi: 10.3389/fimmu.2015.00182
29. Bazewicz CG, Dinavahi SS, Schell TD, Robertson GP. Aldehyde dehydrogenase in regulatory T-cell development, immunity and cancer. Immunology. (2019) 156:47–55. doi: 10.1111/imm.13016
30. De Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. (2008) 14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872
31. Small AC, Gong Y, Oh WK, Hall SJ, van Rijn CJ, Galsky MD. The emerging role of circulating tumor cell detection in genitourinary cancer. J Urol. (2012) 188:21–6. doi: 10.1016/j.juro.2012.02.2558
32. Hofman V, Long E, Ilie M, Bonnetaud C, Vignaud J, Flejou J, et al. Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology. (2012) 23:30–8. doi: 10.1111/j.1365-2303.2010.00835.x
33. Hofman V, Ilie MI, Long E, Selva E, Bonnetaud C, Molina T, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch Assay and the isolation by size of epithelial tumor cell method. Int J Cancer. (2011) 129:1651–60. doi: 10.1002/ijc.25819
34. Lapidus RG, Tiffany CW, Isaacs JT, Slusher BS. Prostate-specific membrane antigen (PSMA) enzyme activity is elevated in prostate cancer cells. Prostate. (2000) 45:350–4.3.0.CO;2-U“ target=”_blank"> doi: 10.1002/1097-0045(20001201)45:4&<;350::AID-PROS10&>;3.0.CO;2-U
35. Giesel FL, Fiedler H, Stefanova M, Sterzing F, Rius M, Kopka K, et al. PSMA PET/CT with Glu-urea-Lys-(Ahx)-[68 Ga (HBED-CC)] versus 3D CT volumetric lymph node assessment in recurrent prostate cancer. Eur J Nuclear Med Mol Imaging. (2015) 42:1794–800. doi: 10.1007/s00259-015-3106-6
36. Lopci E, Saita A, Lazzeri M, Lughezzani G, Colombo P, Buffi NM, et al. 68Ga-PSMA positron emission tomography/computerized tomography for primary diagnosis of prostate cancer in men with contraindications to or negative multiparametric magnetic resonance imaging: a prospective observational study. J Urol. (2018). 200:95–103. doi: 10.1016/j.juro.2018.01.079
37. Eiber M, Weirich G, Holzapfel K, Souvatzoglou M, Haller B, Rauscher I, et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur Urol. (2016) 70:829–36. doi: 10.1016/j.eururo.2015.12.053
38. Kumar N, Yadav S, Kumar S, Saurav K, Prasad V, Vasudeva P. Comparison of percentage free PSA, MRI and GaPSMA PET scan for diagnosing cancer prostate in men with PSA between 4 and 20 ng/ml. Indian J Urol. (2019) 35:202–7. doi: 10.4103/iju.IJU_91_19
39. uz Zaman M, Fatima N, Zaman A, Sajid M, Zaman U, Zaman S. Diagnostic challenges in prostate cancer and 68Ga-PSMA PET imaging: a game changer? Asian Pac J Cancer Prevent. (2017) 18:2625–28. doi: 10.22034/APJCP.2017.18.10.2625
40. Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the 68 Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nuclear Med Mol Imaging. (2015) 42:197–209. doi: 10.1007/s00259-014-2949-6
41. Roach PJ, Francis R, Emmett L, Hsiao E, Kneebone A, Hruby G, et al. The impact of 68Ga-PSMA PET/CT on management intent in prostate cancer: results of an Australian prospective multicenter study. J Nuclear Med. (2018) 59:82–8. doi: 10.2967/jnumed.117.197160
42. Alanen K, Kuopio T, Koskinen P, Nevalainen T. Immunohistochemical labelling for prostate specific antigen in non-prostatic tissues. Pathol Res Pract. (1996) 192:233–7. doi: 10.1016/S0344-0338(96)80226-3
43. Chuang A-Y, DeMarzo AM, Veltri RW, Sharma RB, Bieberich CJ, Epstein JI. Immunohistochemical differentiation of high-grade prostate carcinoma from urothelial carcinoma. Am J Surg Pathol. (2007) 31:1246–55. doi: 10.1097/PAS.0b013e31802f5d33
44. Sheridan T, Herawi M, Epstein JI, Illei PB. The role of P501S and PSA in the diagnosis of metastatic adenocarcinoma of the prostate. Am J Surg Pathol. (2007) 31:1351–5. doi: 10.1097/PAS.0b013e3180536678
45. Oh WJ, Chung AM, Kim JS, Han JH, Hong SH, Lee JY, et al. Differential immunohistochemical profiles for distinguishing prostate carcinoma and urothelial carcinoma. J Pathol Transl Med. (2016) 50:345–54. doi: 10.4132/jptm.2016.06.14
46. Xu L, Mao X, Grey A, Scandura G, Guo T, Burke E, et al. Non-invasive detection of clinically significant prostate cancer using circulating tumor cells. J Urol. (2019) 203:73–82. doi: 10.1097/JU.0000000000000475
47. van der Zee J, Vujaskovic Z, Kondo M, Sugahara T. The Kadota Fund International Forum 2004-Clinical group consensus. Int J Hyperthermia. (2008) 24:111–22. doi: 10.1080/02656730801895058
48. Cihoric N, Tsikkinis A, van Rhoon G, Crezee H, Aebersold DM, Bodis S, et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int J Hyperthermia. (2015) 31:609–14. doi: 10.3109/02656736.2015.1040471
49. Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. (2018) 378:1767–77. doi: 10.1056/NEJMoa1801993
50. Ahmed HU, Bosaily AE-S, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. (2017) 389:815–22. doi: 10.1016/S0140-6736(16)32401-1
51. Krebs MG, Hou J-M, Sloane R, Lancashire L, Priest L, Nonaka D, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and-independent approaches. J Thor Oncol. (2012) 7:306–15. doi: 10.1097/JTO.0b013e31823c5c16
52. Healthline. The Outlook for Ovarian Cancer: Prognosis, Life Expectancy, and Survival Rates by Stage. (2019). Available online at: https://www.healthline.com/health/cancer/ovarian-cancer-outlook (accessed March 15, 2020).
53. Das PM, Bast Jr. RC. Early detection of ovarian cancer. Biomark Med. (2008) 2:291–303. doi: 10.2217/17520363.2.3.291
54. Hirshberg Foundation for Pancreatic Cancer Research. (2019). Available online at: http://pancreatic.org/pancreatic-cancer/about-the-pancreas/prognosis/ (accessed March 15, 2020).
Keywords: prostate cancer, circulating tumor cells (CTC), prostate specific antigen (PSA), early detection, cancer screening
Citation: Ried K, Tamanna T, Matthews S, Eng P and Sali A (2020) New Screening Test Improves Detection of Prostate Cancer Using Circulating Tumor Cells and Prostate-Specific Markers. Front. Oncol. 10:582. doi: 10.3389/fonc.2020.00582
Received: 20 September 2019; Accepted: 30 March 2020;
Published: 23 April 2020.
Edited by:
Walter J. Storkus, University of Pittsburgh, United StatesReviewed by:
Felix K. H. Chun, University Medical Center Hamburg-Eppendorf, GermanyZhou Wang, University of Pittsburgh, United States
Copyright © 2020 Ried, Tamanna, Matthews, Eng and Sali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karin Ried, karinried@niim.com.au
 Karin Ried
Karin Ried Tasnuva Tamanna
Tasnuva Tamanna Sonja Matthews1
Sonja Matthews1 Avni Sali
Avni Sali