- Laboratory of Nucleic Acids Biochemistry, Institute of Chemical Biology and Fundamental Medicine SB RAS, Novosibirsk, Russia
Small double-stranded RNAs with certain sequence motifs are able to interact with pattern-recognition receptors and activate the innate immune system. Recently, we identified a set of short double-stranded 19-bp RNA molecules with 3-nucleotide 3′-overhangs that exhibited pronounced antiproliferative activity against cancer cells in vitro, and antitumor and antimetastatic activities in mouse models in vivo. The main objectives of this study were to identify the pattern recognition receptors that mediate the antiproliferative action of immunostimulating RNA (isRNA). Two cell lines, epidermoid carcinoma KB-3-1 cells and lung cancer A549 cells, were used in the study. These lines respond to the action of isRNA by a decrease in the growth rate, and in the case of A549 cells, also by a secretion of IL-6. Two sets of cell lines with selectively silenced genes encoding potential sensors and signal transducers of isRNA action were obtained on the basis of KB-3-1 and A549 cells. It was found that the selective silencing of PKR and RIG-I genes blocked the antiproliferative effect of isRNA, both in KB-3-1 and A549 cells, whereas the expression of MDA5 and IRF3 was not required for the antiproliferative action of isRNA. It was shown that, along with PKR and RIG-I genes, the expression of IRF3 also plays a role in isRNA mediated IL-6 synthesis in A549 cells. Thus, PKR and RIG-I sensors play a major role in the anti-proliferative signaling triggered by isRNA.
Introduction
Oncological diseases are among the greatest challenges of modern medicine. Current approaches to cancer treatment, including surgical removal of tumor mass followed by chemotherapy or radiotherapy, are not always sufficiently effective due to incomplete removal of cancer cells resulting in disease recurrence and a high incidence of side effects. Thus, the development of new agents for antitumor therapy, combining low toxicity, and high efficiency, is required.
Carcinogenesis is often accompanied by genetic and epigenetic alterations, resulting in the expression of tumor antigens. Normally, the immune system can recognize these tumor antigens as foreign, but tumors escape from immunosurveillance by exploiting various mechanisms and suppressing the immune response. Immunotherapy is a highly potent approach for the therapy of neoplastic diseases, which allow the activation of the host immune system and elimination of tumor cells.
Long and short dsRNAs, depending on their sequence and structure, can activate the innate immune system through pattern recognition receptors (PRRs). These PRRs include Toll like receptors (TLRs), such as TLR3/7/8 (1–4), retinoic acid inducible gene-I (RIG-I), RIG-I-like helicase MDA5 (5, 6), dsRNA dependent protein kinase R (PKR) (7, 8), and NOD-like receptors such as NLRP3 and NOD2 (9, 10). After activation, these receptors, acting through signal transduction pathways, can induce apoptosis, proliferation blockage and induction of secretion of type I interferons and/or inflammatory cytokines by immune cells. Thus, these agents can have both a direct effect on the tumor and an indirect effect by activating the immune system.
The spectrum of biological effects induced by an immunostimulating agent in certain cells may differ depending on the receptors recognizing the agent and the activity of the signaling pathways determined by the pattern of gene expression of the cell (11, 12). Preferential secretion of type I interferons will have a pronounced antitumor effect; however, if immunostimulation is accompanied by a significant enhancement of pro-inflammatory cytokine synthesis, severe side effects will occur preventing the use of such agents for therapy. In particular, upregulation of TNFα synthesis leads to fever, apoptotic death of normal cells, cachexia, and inflammation. A high level of IL-6, another pro-inflammatory cytokine, causes inflammatory, and auto-immune processes in normal tissues (13).
Currently, immunostimulatory nucleic acids, such as long dsRNAs (14, 15), CpG-containing oligodeoxyribonucleotides (16–19), siRNAs with immunostimulatory motifs (20–24), oligoribonucleotides with 5′-terminal triphosphates (5, 25, 26), and chimeric molecules containing an immunostimulatory domain (27) are being investigated as potential adjuvants in antitumor immunotherapy. Previously, we identified a short 19-bp dsRNA with 3-nt 3′-overhangs, which possesses immunostimulatory properties (here and after the Immunostimulatory RNA is expressed as isRNA). The isRNA did not have any substantial homology with any human or mouse mRNA and, thus, does not change gene expression via an RNA interference mechanism. We have shown that isRNA displays pronounced antiproliferative activity with respect to tumor cells, induces interferon-α synthesis by PBMC (28, 29) and exhibits antitumor and antimetastatic effects in vivo in animal models of tumor progression (30, 31). isRNA is low in toxicity in vivo, because it does not stimulate a notable production of pro-inflammatory cytokines, including TNF-α (29).
The main objective of this study was to identify the PRRs that are responsible for isRNA recognition and that mediate its antiproliferative action with respect to KB-3-1 and A549 cells and the induction of IL-6 synthesis in A549 cells. We evaluated the effects of selective silencing of genes that encode PRRs on the antiproliferative activity of isRNA in KB-3-1 and A549 cell lines. The data obtained reveal that isRNA implements antiproliferative activity and induction of IL-6 synthesis by acting through PKR and RIG-I sensors.
Materials and Methods
Synthesis of isRNA
Oligoribonucleotides (strand 1: 5′-GUGUCAGGCUUUCAGAUUUUUU-3′; strand 2: 5′-AAAUCUGAAAGCCUGACACUUA-3′) were synthesized on an automated DNA/RNA synthesizer ASM-800 (Biosset, Russia) using ribo-β-cyanoethyl phosphite amides (GlenResearch, United States) and the published protocols (32) optimized for this device. After complete deblocking by standard methods, target products were isolated by electrophoresis in 12% PAAM/ 8M urea gel (acrylamide:N,N′-methylenebisacrylamide 30:0.5), using 0.1 M TBE as the running buffer at 30 V/cm. Bands corresponding to the target products were detected in the gel by UV shadow and excised; the oligonucleotides were eluted with 0.3 M NaAc (pH 5.2) and precipitated with ethanol as sodium salts. The purified oligoribonucleotides were characterized by MALDI-TOF-MS. isRNA was prepared by annealing strands 1 and 2 at 100 μM each in a buffer containing 15 mM HEPES/KOH (pH 7.4), 50 mM potassium acetate, 1 mM magnesium acetate, and subsequently stored at −20°C.
Cell Cultures
Human epidermoid carcinoma KB-3-1 cells, lung carcinoma A549 cells and embryonic kidney HEK293T cells were purchased from the bank of cell cultures of the Institute of Cytology, Russian Academy of Sciences (St. Petersburg, Russia). A549 and KB-3-1 cells do not produce type I IFN, A549 is able to produce IL-6 after stimulation. The cells were cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 0.25 μg/ml amphotericin at 37°C in a humidified atmosphere with 5% CO2 (standard conditions).
RNA Isolation and RT-qPCR
Total RNA was isolated from the cells using the standard Trizol method (33). The reverse transcription (RT) reaction was carried out using the RT2 First Strand Kit (QIAGEN, Germany) according to the manufacturer's protocol. The resulting cDNA was amplified in a reaction mixture of 20 μl, containing 1 μl of cDNA, 0.25 μM specific primers (Table 1) and Biomaster HS-qPCR SYBR Blue (2×) master mix (BioLab Mix, Russia) containing SYBR-Green fluorescent dye and hot start DNA polymerase. Real-time PCR was carried out using an iQ5 amplifier (Bio-Rad, USA) according to the following scheme: one cycle−3 min, 95°C; 40 cycles−30 s, 95°C, 30 s−58°C; and 30 s−72°C. The mRNA level of the specific genes was normalized to the level of GAPDH mRNA. All measurements were done in triplicates.
Generation of shRNA-Expressing Lentiviral Vectors and Transduction of Target Cells
The lentiviral vectors were constructed by cloning synthetic insertions, encoding specific shRNA, into the pGPV vector (Evrogen, Russia) under the H1 promoter, between 4,605 bp and 4,614 bp (BamHI and EcoRI restriction sites, respectively) according to standard genetic engineering techniques. The lentiviral vector encoded the reporter fluorescence protein copGFP to visualize the transduced cells. The sequences of insertions, encoding shRNA, were selected using an RNAi Design Tool program (34) (Table 2).

Table 2. Sequences of insertions encoding shRNA, targeting IRF3/7, TLR7/8, NOD2, RIG-I, MDA5, and PKR genes, and shRNA with scrambled sequence.
The transient co-transfection of HEK293 T cells with shRNA-expressing lentiviral plasmid and packaging plasmids (Invitrogen, USA) was carried out using Lipofectamin 2000 (Invitrogen) according to the manufacturer's protocol. Viral stocks were collected 3 days after transfection. Obtained viruses were used for the transduction of KB-3-1 and A549 cells using polybrene at a final concentration of 10 μg/ml. Three days after transduction the cells were subjected to FACS analysis and sorting.
FACS
Transduced cells were isolated using a FACSAria (BD, USA) in the single cell mode at the appropriate sort rate (e.g., below 100 cells per second). Cell sorting was performed using fluorescent protein copGFP expressed in the target cells as a reporter. Parental cell lines were used to determine the background fluorescence in such a way that <1% of non-fluorescent cells were included in the sort gate.
Cell Proliferation Assay
The relative number of living cells (normalized cell index) was monitored in real time using the xCELLigence RTCA DP Analyzer (ACEA Bioscience, USA). One day before transfection, the cells were plated in a 16-well E-Plate (ACEA Bioscience, USA) (at densities of 1 × 103 cells per well for KB-3-1 or 1.5 × 103 cells per well for A549 in 100 μl), and allowed to adhere overnight under standard conditions. On the day of the experiment the cells were transfected with isRNA using cationic liposomes 2X3-DOPE (35). Complexes of cationic liposomes with isRNA were preformed in a serum-free Opti-MEM medium by mixing equal volumes of solutions of 2X3-DOPE and isRNA, followed by incubation for 20 min at room temperature.
isRNA/2X3-DOPE complexes used for KB-3-1 cells were prepared using N/P = 1/4, with a final concentration in the well of 2.2 μM for 2X3-DOPE and 50 nM for isRNA. isRNA/2X3-DOPE complexes using A549 cells were prepared using N/P = 1/6 with a final concentration in the well of 6.6 μM for 2X3-DOPE and 100 nM for isRNA. The resulting complexes, in a volume of 50 μL, were added to the cells growing in 100 μl of medium. Cells were incubated with the complexes under standard conditions for 4 h. Then, the culture medium was replaced by fresh medium. The cells were allowed to grow under standard conditions for 96 h. Data were recorded every 4 h for 96 h post transfection, and were expressed as mean values of measurements in four wells ± SD.
IL-6 Level Measurement
An ELISA was used to measure the IL-6 level in culture medium containing A549 cells stimulated with isRNA. One day before transfection, A549 cells were plated in a 24-well plate (at densities of 5 × 104 cells per well) and allowed to adhere overnight under standard conditions. On the day of the experiment, cells were transfected with isRNA as described above. The cells were incubated under standard conditions for 20 h. The IL-6 level in culture medium was measured by ELISA using Interleukin-6-EIA-BEST (Vector-Best, Russia), according to the manufacturer's protocol, and the data were expressed as mean values of measurements in two wells ± SD.
Cell Cycle Analysis
For the analysis of cell cycle progression, KB-3-1 cells were plated in a 24-well plate (at densities of 2 × 104 cells per well) and allowed to adhere overnight under standard conditions. On the day of the experiment, cells were transfected with isRNA as described above. The cells were incubated under standard conditions for 48 h. Then cells were stained using Hoechst 33342 Ready Flow™ Reagent (R37165, Thermo Fisher Scientific, USA), according to the manufacturer's protocol. Stained cells were analyzed utilizing flow cytometer NovoCyte 3000 (ACEA Bioscience, USA) and NovoExpress Software. A total of 10,000 cells were analyzed from each sample. The described above apoptosis and cell cycle analyses were carried out two times in independent experiments, and the data were expressed as mean values ± SD.
Statistical Analysis
The statistical significance of the differences in experiments was determined using the two-tailed Student's t-test (data are expressed as means ± SD). Differences were considered statistically significant for p < 0.05.
Results
Selection of Potential Mediators of isRNA Antiproliferative Action and Cell Models
Two cell lines, epidermoid carcinoma KB-3-1 cells and lung cancer A549 cells were used to identify sensors mediating isRNA antiproliferative activity in this study, because, as highlighted above, it has been shown that isRNA inhibits proliferation of these cells (28, 29). Moreover, A549 cells also secreted IL-6 in response to isRNA and, therefore, A549 cells can be used to evaluate both the direct antiproliferative effect and the antiproliferative effects mediated by cytokine secretion.
As a first step, we selected cytoplasmic receptors RIG-I, MDA5, NOD2, and PKR, and the interferon regulatory transcription factor IRF3/7 as potential mediators or sensors of isRNA antiproliferative activity based on data in the literature (5, 7–10, 36, 37) and estimated the expression levels of the genes encoding potential mediators of isRNA action in the KB-3-1 and A549 cell lines to assess the possibility of their participation in the signal transduction in these lines.
Relative levels of mRNA encoded potential isRNA sensors and signal transducers were measured in KB-3-1 and A549 cells by qRT-PCR with specific primers (Table 3). It can be seen that KB-3-1 cells had a high level of PKR mRNA and average levels of IRF3, MDA5, RIG-I mRNA. Expression of TLR3/7/8, NOD2, and IRF7 was not detected in these cells. A549 cells also had a high level of PKR, average levels of IRF3, MDA5, RIG-I, and a very low level of TLR3 mRNA. Levels of TLR7/8, NOD2 and IRF7 mRNA were below the detection limit. It should be noted that the relative levels of the studied mRNA in KB-3-1 normalized to GAPDH mRNA were 2–6 fold higher than those in A549 cells.
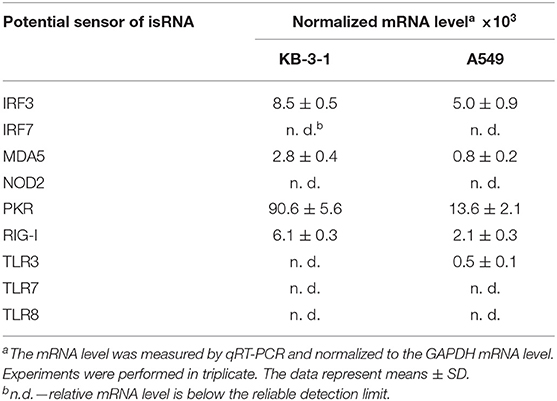
Table 3. Relative mRNA level of potential isRNA sensors and signal transducers in KB-3-1 and A549 cells.
Thus, based on mRNA levels, PKR, IRF3, MDA5, and RIG-I proteins can be present in the cells and participate in the recognition of isRNA or/and isRNA signal transduction in both cell lines. Therefore, these mRNAs were chosen as targets for RNAi to elucidate the role of the corresponding proteins in the antiproliferative process.
Silencing of Genes Encoding Potential Mediators of isRNA Antiproliferative Action by shRNA
The proteins considered as participants in the signaling cascades have different stability and half-lifetimes, therefore, in order to reliably reduce their level in the studied cells during the experiment, it is necessary to achieve a sustained reduction in the level of the corresponding mRNA. For this purpose, we used short hairpin RNA (shRNA) instead of traditional siRNA and obtained cell sublines stably expressing particular shRNA. Recombinant lentiviruses were used as a vector for delivery of shRNA-encoding sequences (Table 2) to the cell genome. A vector containing shRNA with a scrambled sequence was used as a control of specificity.
A549 and KB-3-1 cells were transduced by shRNA-expressing lentiviruses with the transduction level, according to the expression of reporter fluorescent protein copGFP, of at least 70–90%. Then, cell sorting was performed in such a way that <1% of non-fluorescent cells were present in the final population. The relative mRNA level of silenced genes in the obtained cell sublines was measured by qRT-PCR. It is seen (Table 4) that, in the sublines, expression of the silenced genes is significantly reduced (from 55 to 94% in comparison with parent cells). Maximum inhibition levels were observed for MDA5 and RIG-I in A549 (93 and 94% respectively). Moreover, the inhibition of the studied genes in A549 cells was higher than those in KB-3-1 cells, which may be explained by the fact that the initial expression levels of the corresponding mRNAs were lower in these cells. It should be noted that suppression of gene expression was observed only under specific shRNA, expression of other target genes in the individual cell lines expressing shRNA, directed to one of the target genes, did not change. PKR, RIG-I, MDA5 silencing in A549 sublines at the protein level was shown by us previously by western blot analysis (38). Thus, we obtained A549 and KB-3-1 cell sublines with selectively silenced IRF3, MDA5, PKR, and RIG-I genes to study the participation of proteins encoded by inhibited genes in signaling pathways activated by isRNA.
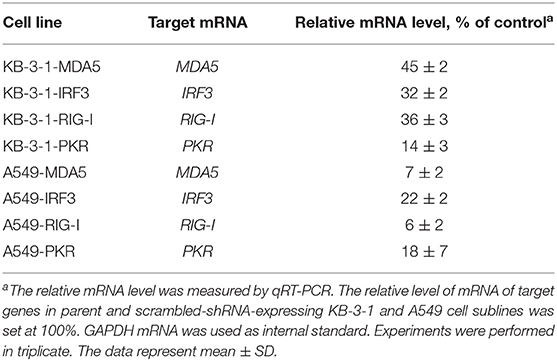
Table 4. Inhibition of the expression of PRRs and transcription factors by shRNA in transduced KB-3-1 and A549 cell lines.
The Effect of Specific Gene Silencing on the Antiproliferative Activity of isRNA in KB-3-1 and A549 Cell Sublines Stably Expressing shRNAs
To identify the sensors mediating the antiproliferative activity of isRNA on the cancer cells, we investigated the effect of PRR gene silencing on the antiproliferative activity of isRNA using the obtained KB-3-1 and A549 sublines. The relative number of living cells (normalized cell index) in real time was monitored every 4 h using the xCELLigence RTCA DP Analyzer, starting 4 h after transfection with isRNA (Figure 1). The data (Figure 1; Supplementary Figure S1) are displayed as time dependence of Normalized Cell Index (the relative number of living cells, normalized to the number of living cells 4 h after transfection in the same cell sublines) of cells, treated with isRNA/2X3-DOPE lipoplexes and 2X3-DOPE only. It is seen (Figure 1) that isRNA significantly inhibits proliferation of parental KB-3-1 cells. The decrease in the number of living cells under isRNA treatment of the KB-3-1-Scr was comparable with those in the parental cell line. The cells with selectively inhibited MDA5 and IRF3 (KB-3-1-MDA5, KB-3-1-IRF3) were also as sensitive to the antiproliferative action of isRNA as the parent cell line. On the contrary, KB-3-1-RIG-I and KB-3-1-PKR cells with downregulated PKR and RIG-I, respectively, did not respond to isRNA, and the growth rate of these cells did not differ reliably from the proliferation rate of the cells treated with 2X3-DOPE only.
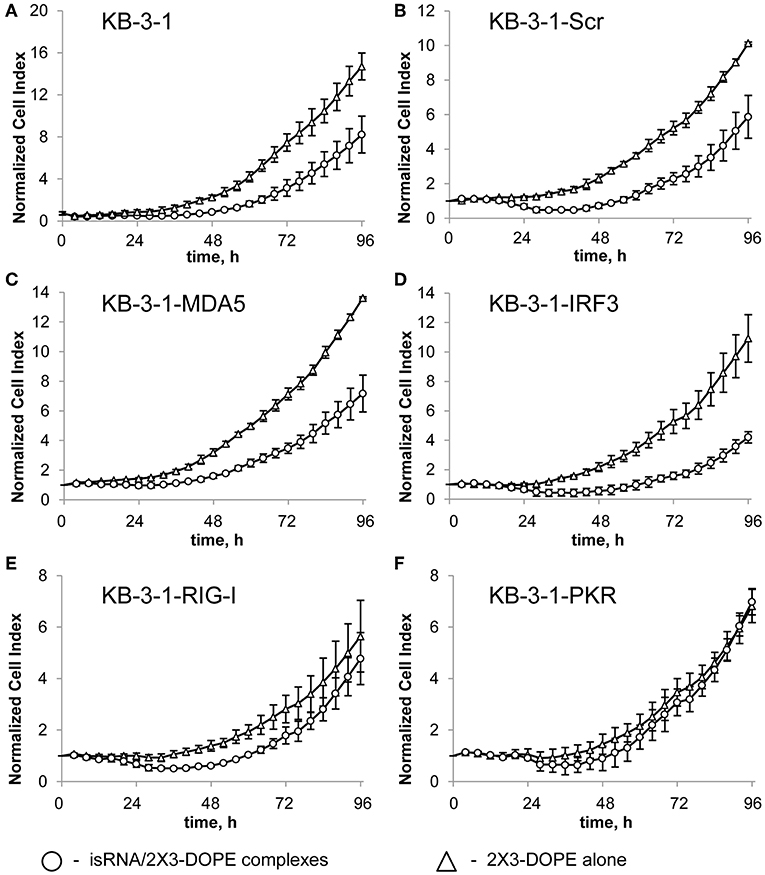
Figure 1. The effect of isRNA/2X3-DOPE complexes (◦) or 2X3-DOPE alone (Δ) on the proliferation of parent KB-3-1 cells (A), and sublines KB-3-1-Scr (B), KB-3-1-MDA5 (C), KB-3-1-IRF3 (D), KB-3-1-RIG-I (E), and KB-3-1-PKR (F). After transfection, the relative number of living cells was measured every 4 h for 96 h. The number of living cells 4 h after transfection was set at 1. Experiments were performed in four repeats. The data represent means ± SD.
An analysis of the action of isRNA on A549 cell sublines (Supplementary Figure S1; Table 5) showed similar dependences. In particular, cell treatment with isRNA resulted in a decrease in cell proliferation in parental A549 cells, A549-Scr, A549-MDA5, and A549-IRF3 cells, but did not reliably reduce the proliferation rate of A549-RIG-I and A549-PKR cells. A quantitative comparison of the antiproliferative effect of isRNA—the decrease in the number of living cells 96 h after transfection with isRNA/2X3-DOPE compared to mock-treated cells—was performed. The relative number of cells treated with 2X3-DOPE alone was set at 100%. It was shown (Table 5, Figure 1, and Supplementary Figure S1) that isRNA effectively inhibited proliferation in parental KB-3-1 and A549 cells to 44 ± 11% and 47 ± 7%, respectively. The antiproliferative effects of isRNA on the cells expressing Scr-shRNA were comparable with the effects of isRNA on parental cell lines (41 ± 11% and 62 ± 5% for KB-3-1-Scr and A549-Scr, respectively). The cells with selectively inhibited MDA5 and IRF3 (KB-3-1-MDA5, KB-3-1-IRF3, A549-MDA5, and A549-IRF3), were as sensitive to the antiproliferative effects of isRNA as parent cell lines (47 ± 7, 61 ± 5, 68 ± 11, and 63 ± 11%, respectively). On the contrary, silencing of PKR and RIG-I genes significantly reduces the antiproliferative effect of isRNA. The growth rate of KB-3-1-RIG-I, KB-3-1-PKR, A549-RIG-I, and A549-PKR cells after isRNA treatment did not differ reliably from the proliferation rate of the cells treated with 2X3:DOPE only. It is worth mentioning that both in KB-3-1 and A549 cell sublines, the effects of isRNA were similar (Table 5).
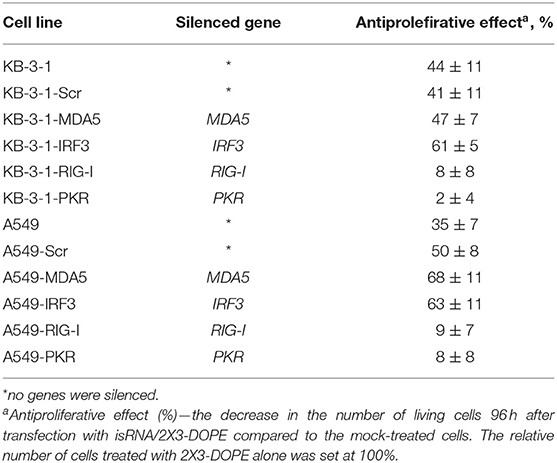
Table 5. The effect of PRRs gene silencing by shRNA on the antiproliferative activity of isRNA in KB-3-1 and A549 cell lines and sublines.
It should be noted (Tables 4, 5) that the inhibition level of RIG-I in KB-3-1-RIG-I was lower than the inhibition level of PKR in KB-3-1-PKR (64 and 86%, respectively), and the inhibition level of RIG-I in A549-RIG-I was higher than the inhibition level of PKR in A549-PKR (94 and 82%, respectively). However, for all of these sublines comparable levels of antiproliferative effects mediated by isRNA were observed: 8, 2, 9, and 8% for KB-3-1-RIG-I, KB-3-1-PKR, A549-RIG-I, and A549-PKR, correspondingly showing no sensitivity toward isRNA.
We assume that proteins whose genes were silenced in the cellular sublines, which retained sensitivity to the anti-proliferative action of isRNA, do not interact with or do not participate in the signal transduction from isRNA, leading to a significant decrease in their proliferation rate. On the contrary, the inhibited proteins in the sublines which have lost sensitivity to isRNA are mediators of isRNA antiproliferative activity. Thus, the obtained data show that isRNA implements its antiproliferative activity though PKR and RIG-I sensors.
The Effect of Gene Silencing on the Cytokine-Inducing Activity of isRNA in A549 Cell Sublines
We investigated the effect of silencing particular genes on the production of IL-6 in response to isRNA in A549 sublines, to determine the PRR responsible for the cytokine-inducing activity of isRNA. The cells were transfected with isRNA/2X3-DOPE lipoplexes (35), and levels of IL-6, produced by different A549 sublines in response to isRNA, were measured by Interleukin-6-EIA-BEST 24 h after transfection. Parent A549 cells produced IL-6 in response to isRNA treatment (Figure 2); the IL-6 level in the medium of treated cells was 3 times higher than the IL-6 level produced by untreated cells (27 ± 1.4 pg/ml and 10 ± 1.3 pg/ml for treated and untreated cells, respectively). The activation of IL-6 production by isRNA in A549-Scr and A549-MDA5 cells was similar to the IL-6 level in isRNA-treated parental cells (26 ± 0.06 pg/ml and 27 ± 0.09 pg/ml, respectively). Silencing of IRF3 resulted in a less pronounced activation by isRNA of IL-6 synthesis in A549-IRF3 cells (19 ± 0.67 pg/ml) in comparison with controls (A549, A549-Scr) and A549-MDA5 cells.
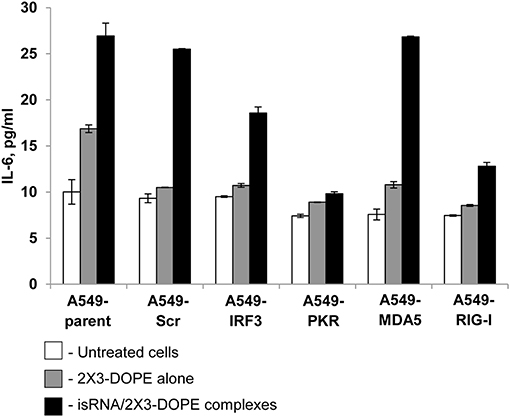
Figure 2. IL-6 levels in A549 cell sublines after transfection with isRNA/2X3-DOPE complexes (black bars) or 2X3-DOPE alone (gray bars) compared to untreated cells (white bars). The IL-6 level was determined by ELISA 24 h after transfection. Experiments were performed in duplicate. The data represent means ± SD.
Silencing of RIG-I and PKR by shRNA blocked the activation of IL-6 synthesis by isRNA. IL-6 levels in A549-RIG-I and A549-PKR cells were 13 ± 0.41 and 10 ± 0.23 pg/ml, respectively, which was similar to the IL6 level in the untreated or mock-treated cells (Figure 2). The data obtained show that RIG-I and PKR receptors play a major role in the induction of IL-6 synthesis in A549 cells in response to isRNA. Also, IRF3 could participate in signal transduction from isRNA, triggering activation of IL6 synthesis.
The Effect of Gene Silencing on the isRNA Induced Retardation of Cell Cycle Progression
We previously showed that isRNAs (100 nM) delivered to the cells by Lipofectamine 2000 induce cell growth arrest in KB-3-1 cell line rather than cell death by apoptosis (29). We investigated the effect of particular genes silencing on the isRNA induced retardation of cell cycle progression in KB-3-1 sublines, to determine the PRR responsible for the antiproliferative activity of isRNA. The cells were transfected with isRNA/2X3-DOPE lipoplexes (35), and the distribution of cells between cell cycle stages were measured by flow cytometry after staining with Hoechst 33342 Ready Flow™ Reagent, 48 h after transfection. isRNA concentration 50 nM was used, with no observed toxicity or cell death. The use of a lower concentration for studying the antiproliferative effect was required due to the application of a more efficient transfection agent (2 × 3-DOPE) in the present study. All cells grown in the experimental wells were collected, including floating cells. It was found (Figure 3) that the number of cells in the subG1 population in all sublines was insignificant, which does not indicate the induction of apoptosis when using isRNA at a concentration of 50 nM. The obtained data show, that the number of parental KB-3-1 cells in G1-phase does not alter significantly in the presence of isRNA/2X3-DOPE lipoplexes, while the number of cells in S-phase increases, simultaneously the number of cells in G2-phase decreases, which indicates the blocking of cell division in cells under the action of isRNA. Similar changes in the distribution of cells over the phases of the cell cycle were also observed for subline cells with the MDA5 and IFR3 genes silencing, in which there was a decrease in the number of cells in the G2 phase under isRNA action. Another situation was observed for a cell line with inhibited PKR: the number of cells in the G2 phase in the cells treated only with a transfection agent was lower than in other sublines, however, under the action of isRNA it did not decrease, but even increased, which confirms what we obtained using the antiproliferation test evidence that blocking PKR gene expression prevents the antiproliferative effect of isRNA in this cell line. The data obtained confirms that PKR sensor play a major role in the antiproliferative effect of isRNA.
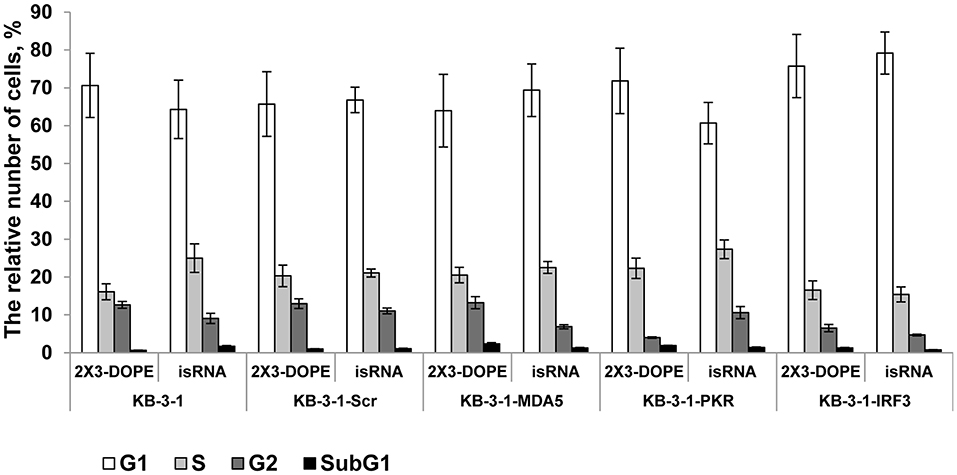
Figure 3. The effect of isRNA/2X3-DOPE complexes (isRNA) or 2X3-DOPE alone (2X3-DOPE) on retardation of cell cycle progression of KB-3-1 cell sublines. The cell cycle distribution was analyzed 48 h after transfection. The data expressed as a relative number of cells from total population related to different cell cycle stages. Experiments were performed in two repeats. The data represent means ± SD.
Discussion
Several groups of PRR can recognize exogenous nucleic acids. The first group of receptors includes TLRs 3/7/8/9 (36), which are localized in the endosomal membranes or on the cell surface. It was shown that TLR9 recognizes unmethylated CpG DNA, while TLR7 and TLR8 (G/U-rich ssRNA) and TLR3 recognize dsRNA. Another group of PRRs is located in the cytoplasm and includes RIG-I, which has been shown to interact with short 5′-triphosphate containing dsRNA (5). In contrast, another member of this group, MDA5, recognizes long dsRNA, while dsRNAs more than 30 b.p. in length are substrates of PKR (37). It was also shown that NOD-like receptors (NLRP3, NOD2) can recognize viral ssRNA (9, 10). The main signal cascades initiated by PRR, and triggered by exogenous nucleic acids, pass through IRF3 or IRF7 proteins. So, the study of the involvement of IRF3/7 in isRNA signaling is important for the identification of pathways involved in isRNA signaling.
It was shown (28), that isRNA without any transfection reagent it does not have an antiproliferative effect on tumor cell lines and does not activate the secretion of cytokines after intravenous administration to mice. This allows us to conclude, that isRNA activate some cytosolic or endosomal sensors. Therefore, we used cationic liposomes as transfection reagent to deliver isRNA inside cell.
It is also should be noted, isRNA recognition in the cell could be similar to the recognition of the vital RNA in case of viral infection resulting in the formation of the antiviral state. During binding of virus to the cell, viral envelope proteins interact with cell membrane, but viral RNA does not. After fusion, viral double-stranded RNA is released into the cellular cytoplasm or is synthetized in the cytoplasm depending on the type of the virus. Therefore, viral dsRNA is recognized by cytoplasmic or endosomal receptors and not by cell surface receptors. Similarly, isRNA is also recognized by PRR inside cell and triggers immune reactions (39).
Based on literature data (5, 7–10, 36, 37) we selected RIG-I, MDA5, NOD2, and PKR, and interferon regulatory transcription factors IRF3/7 as potential mediators or sensors of isRNA antiproliferative activity. We show (Table 3), that KB-3-1 and A549 cells have a high level of PKR mRNA and medium levels of IRF3, MDA5, and RIG-I mRNAs. Thus, based on the mRNA levels, PKR, IRF3, MDA5, and RIG-I can participate in the recognition of isRNA or/and isRNA signal transduction in both cell lines. Using silencing of these genes by shRNA, we show that isRNA implements its antiproliferative activity through PKR and RIG-I sensors in KB-3-1 and A549 cells. We also show that RIG-I and PKR receptors play a major role in the induction of IL-6 synthesis in A549 cells in response to isRNA.
It is known that RIG-I recognizes RNA depending on sequence, length, single- or double-stranded structure and the presence of 5′ cap or 5′triphosphate (5′ppp). However, the variety of RNA sequences and structures is large enough that there is not enough comprehensive data allowing the assignment of certain molecules to agonists or antagonists by default. It has been reported that RIG-I signaling is not activated by 5′ capped RNA or RNA without 5′ppp or 5′ diphosphosphate (5′pp) (40–43). In addition, RIG-I signaling can be triggered by panhandle or bulge/loop RNA structures with blunted 5′ppp (44). RIG-I can also recognize synthetic dsRNA, for example poly (I:C) (45). Despite the fact that the studied isRNA did not have 5′ppp or 5′pp, it still activated RIG-I signaling. Apparently, the key elements that are important for activation of signaling are the motives of the sequence of isRNA, as previously we showed that certain sites of the sequence are important for the antiproliferative effect of isRNA and the substitutions introduced therein block the antiproliferative effect (29). It is also known that effective activation of RIG-I requires dsRNA from 10 to 300 b.p. in length, containing no mismatches near the blunt ends (5). In terms of length, isRNA fits well with the known RIG-I substrates, because isRNA has 22 b.p. Possible scenarios for the activation of cellular cascades under the action of isRNA, according to the obtained data and data known from the literature, are shown in Figure 4.
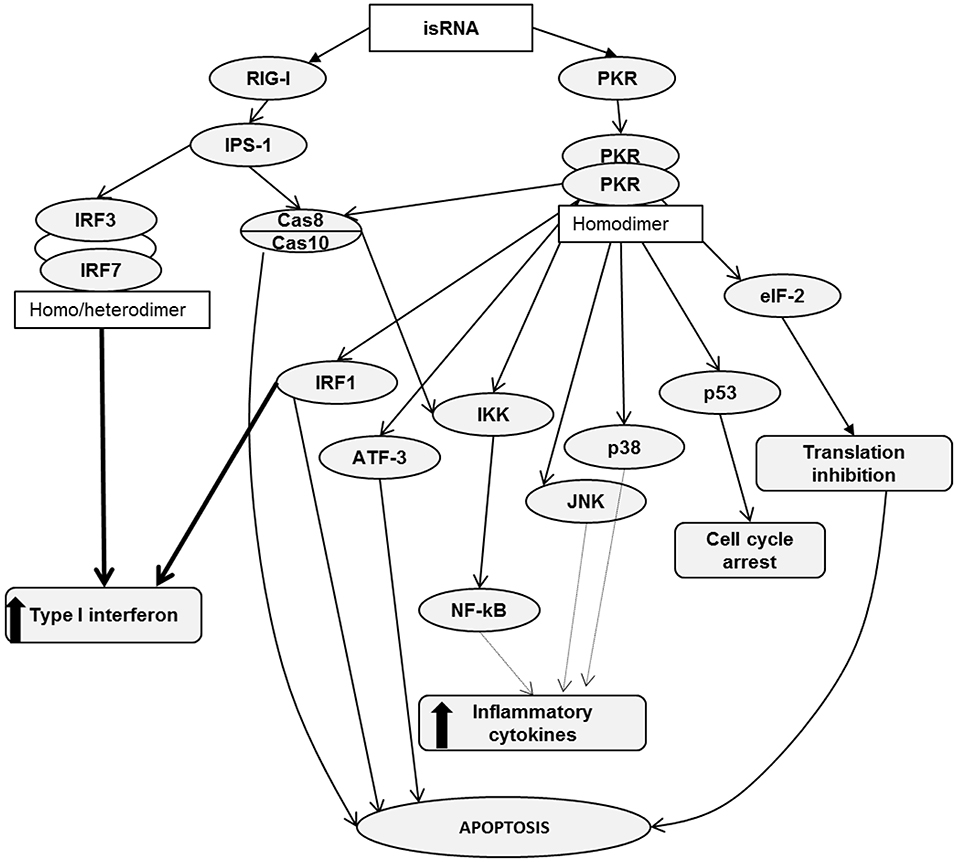
Figure 4. RIG-I and PKR signaling pathways. Activation of RIG-I/PKR signaling by RNA ligands in cancer cells induces different mechanisms, leading to apoptosis, cell cycle arrest, type I interferon and inflammatory cytokine production.
RIG-I consists of two N-terminal caspase activation and recruitment domains (CARDs), a central DExD/H box RNA helicase ATP-hydrolyzing domain and a C-terminal domain (CTD) (46). Based on literature data, it can be assumed that binding of isRNA to RIG-I leads to a conformational change in CARDs, which facilitates the association with an adaptor protein IPS-1 (IFNβ promoter stimulator 1, also known as MAVS). Then, through a number of mediators, the signal proceeds to caspase 8/10, which induce apoptosis and, through IKK family serine kinases, activate NF-κB signaling, which triggers the production of type I interferons and inflammatory cytokines. In particular, A549 cells secrete IL-6. Another possible pathway leading to type I interferon production passes through interferon regulatory transcription factors 3/7, IRF3/7. We did not find noticeable levels of IRF7 expression in KB-3-1 and A549 cells. We also showed that IRF3 silencing does not affect the antiproliferative effects of isRNA in KB-3-1 and A549 cells, but decreased the IL-6 inducing activity of isRNA in A549 cells. Perhaps the low level or lack of expression of this regulator in the studied cell lines is a reason why this line, unlike PBMC, does not secrete type I interferon in response to the action of isRNA. Thus, it seems likely that IRF3/IRF7 is not directly involved in the antiproliferative action but plays a role in isRNA signaling in these cells, which triggers the synthesis and secretion of interferons and cytokines.
PKR is a serine-threonine kinase that consists of N-terminal dsRNA binding domains (dsRBD) and a C-terminal kinase domain containing major phosphorylation sites (47, 48). PKR recognizes long dsRNA in a non-sequence specific manner, and activation of PKR by dsRNA does not require a 5′-ppp (49). A number of authors have shown that PKR recognizes dsRNA not shorter than 30 b.p. (50–53), but activation of PKR by dsRNA containing 19–21 b.p. has also been reported (7, 54). We showed that isRNA does activate PKR, because PKR silencing entirely abolished both the antiproliferative and IL-6 inducing activities of this molecule. It should be noted that blunt end dsRNAs activate PKR less potently than those with protruding ends (7) and, importantly, isRNA has 3-nucleotide 3′-overhangs. Previously, we have shown that shortening of the protruding ends of isRNA or nucleotide replacement of A/U by G/C blocks the antiproliferative effect of isRNA (29).
We can assume that PKR binding with isRNA leads to its homodimerization and rapid autophosphorylation by analogy with similar molecules (55, 56). Then, activated PKR phosphorylates translation initiation factor eIF2α, and phosphorylated eIF2α prevents the formation of the translation initiation complex eIF2-GTP-Met-tRNAi. Such inhibition of protein synthesis limits cell proliferation and induces apoptosis in cancer cells (12, 57, 58). PKR can also act through several other signal transduction pathways, including Cas8/10 (59), IRF1 (60), and p53 (61). PKR activation either mediates apoptosis or interferon production or both, and thereby reduces the proliferation rate. PKR is able to activate the transcription factor ATF-3, which is involved in apoptosis (62). Activation of NF-κB, JNK, and p38 by PKR leads to the production of inflammatory cytokines (63–67). Thus, there are many PKR-triggered pathways that can lead to IL-6 production and inhibition of cancer cell proliferation. It was shown (68), that the function of PKR, independently of translation inhibition, is critical for type I interferon production downstream of MDA5 and upstream of MAVS. It has also been shown (68) that after viral infection, PKR co-immunoprecipitates with RIG-I and MDA5 independently on RNA binding. It also should be noted that RIG-I and PKR sensors are upstream proteins in these signal transduction pathways. Previously, we showed that penetration into the cell is necessary for the antiproliferative effect of the isRNA. This indicates that the sensors that trigger the signaling pathways leading to a decrease in the rate of cell growth are localized in the cytoplasm rather than on the cell membrane (29).
PKR- and RIG-I-triggered pathways intersect at two points: the first one is Cas8/10, the activation of which leads to apoptosis and IKK activation, and the second IKK itself, because IKK can be activated by RIG-I through Cas8/10 only, but PKR activates IKK both in a Cas8/10-dependent or Cas8/10-independent manner. IKK activation by RIG-I or PKR leads to the synthesis of proinflammatory cytokines through the mediator NF-kB. This comparison of RIG-I and PKR functions is in a good agreement with the data obtained in this study and data previously obtained on the in vitro and in vivo effects of isRNA (28–30, 69).
Previously, we have demonstrated that the antiproliferative effect of isRNA on KB-3-1 cells is associated with a decrease in the growth rate, and not with the induction of apoptosis (29). It was found that isRNA only slightly increases the number of apoptotic and dead cells in comparison with controls, but increases the number of cells in G0/G1 phase and reduces the number of cells in G2/M phase (29). Considering the results obtained in this study, we can conclude that signal transduction pathways triggered by isRNA/RIG-I or isRNA/PKR interactions lead mostly to type I interferon synthesis, translation inhibition and cell cycle arrest, but not to the induction of apoptosis, or synthesis of proinflammatory cytokines.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
EC conceived and designed the experiments. MIZ performed the experiments. MIZ and EC analyzed the data. VV and EC contributed reagents, materials, and analysis tools. MAZ and EC wrote the paper.
Funding
This work was supported by Russian Scientific Foundation grants 16-15-10105 and 19-74-30011.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. M. A. Maslov (Lomonosov Moscow State University of Fine Chemical Technologies) for the preparation of 2X3-DOPE, Dr. Tatyana O. Kabilova for help with xCELLigence experiments and Mrs. Albina V. Vladimirova (Institute of Chemical Biology and Fundamental Medicine SB RAS) for cell maintenance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.01454/full#supplementary-material
References
1. Karikó K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. (2004) 279:12542–50. doi: 10.1074/jbc.M310175200
2. Reynolds A, Anderson EM, Vermeulen A, Fedorov Y, Robinson K, Leake D, et al. Induction of the interferon response by siRNA is cell type- and duplex length-dependent. RNA. (2006) 12:988–93. doi: 10.1261/rna.2340906
3. Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, et al. Sequence-specific potent induction of IFN-α by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. (2005) 11:263–70. doi: 10.1038/nm1191
4. Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. (2005) 23:457–62. doi: 10.1038/nbt1081
5. Poeck H, Besch R, Maihoefer C, Renn M, Tormo D, Morskaya SS, et al. 5′-triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma. Nat Med. (2008) 14:1256–63. doi: 10.1038/nm.1887
6. Kang D, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, Fisher PB. mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci USA. (2002) 99:637–42. doi: 10.1073/pnas.022637199
7. Puthenveetil S, Whitby L, Ren J, Kelnar K, Krebs JF, Beal PA. Controlling activation of the RNA-dependent protein kinase by siRNAs using site-specific chemical modification. Nucleic Acids Res. (2006) 34:4900–11. doi: 10.1093/nar/gkl464
8. Zhang Z, Weinschenk T, Guo K, Schluesener HJ. siRNA binding proteins of microglial cells: PKR is an unanticipated ligand. J Cell Biochem. (2006) 97:1217–29. doi: 10.1002/jcb.20716
9. Olejniczak M, Galka-Marciniak P, Polak K, Fligier A, Krzyzosiak WJ. RNAimmuno: a database of the nonspecific immunological effects of RNA interference and microRNA reagents. RNA. (2012) 18:930–5. doi: 10.1261/rna.025627.110
10. Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol. (2012) 12:479–91. doi: 10.1038/nri3247
11. Bhattarai D, Worku T, Dad R, Rehman ZU, Gong X, Zhang S. Mechanism of pattern recognition receptors (PRRs) and host pathogen interplay in bovine mastitis. Microb Pathog. (2018) 120:64–70. doi: 10.1016/j.micpath.2018.04.010
12. Jagus R, Joshi B, Barber GN. PKR, apoptosis and cancer. Int J Biochem Cell Biol. (1999) 31:123–38. doi: 10.1016/S1357-2725(98)00136-8
13. Mansoori B, Mohammadi A, Shir Jang S, Baradaran B. Mechanisms of immune system activation in mammalians by small interfering RNA (siRNA). Artif Cells, Nanomedicine Biotechnol. (2016) 44:1589–96. doi: 10.3109/21691401.2015.1102738
14. Chang CIl, Lee TY, Dua P, Kim S, Li CJ, Lee D. Long double-stranded RNA-mediated RNA interference and immunostimulation: long interfering double-stranded RNA as a potent anticancer therapeutics. Nucleic Acid Ther. (2011) 21:149–55. doi: 10.1089/nat.2011.0296
15. Sajeesh S, Lee TY, Hong SW, Dua P, Choe JY, Kang A, et al. Long dsRNA-mediated RNA interference and immunostimulation: a targeted delivery approach using polyethyleneimine based nano-carriers. Mol Pharm. (2014) 11:872–84. doi: 10.1021/mp400541z
16. Du HY, Dong LH, Zhao BJ, Fu J, Wang QQ, Chen F, et al. Immunostimulatory and anti-neoplasm effects of a novel palindrome CpG oligodeoxynucleotide in mice. Acta Pharmacol Sin. (2012) 33:1047–54. doi: 10.1038/aps.2012.54
17. Kortylewski M, Swiderski P, Herrmann A, Wang L, Kowolik C, Kujawski M, et al. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol. (2009) 27:925–32. doi: 10.1038/nbt.1564
18. Nechaev S, Gao C, Moreira D, Swiderski P, Jozwiak A, Kowolik CM, et al. Intracellular processing of immunostimulatory CpG-siRNA: toll-like receptor 9 facilitates siRNA dicing and endosomal escape. J Control Release. (2013) 170:307–15. doi: 10.1016/j.jconrel.2013.06.007
19. Zhang Q, Hossain DM, Nechaev S, Kozlowska A, Zhang W, Liu Y, et al. TLR9-mediated siRNA delivery for targeting of normal and malignant human hematopoietic cells in vivo. Blood. (2013) 121:1304–15. doi: 10.1182/blood-2012-07-442590
20. Hashimoto Y, Abu Lila AS, Shimizu T, Ishida T, Kiwada H. B cell-intrinsic toll-like receptor 7 is responsible for the enhanced anti-PEG IgM production following injection of siRNA-containing PEGylated lipoplex in mice. J Control Release. (2014) 184:1–8. doi: 10.1016/j.jconrel.2014.04.003
21. Khairuddin N, Gantier MP, Blake SJ, Wu SY, Behlke MA, Williams BR, et al. SiRNA-induced immunostimulation through TLR7 promotes antitumoral activity against HPV-driven tumors in vivo. Immunol Cell Biol. (2012) 90:187–96. doi: 10.1038/icb.2011.19
22. Khairuddin N, Blake SJ, Firdaus F, Steptoe RJ, Behlke MA, Hertzog PJ, et al. In vivo comparison of local versus systemic delivery of immunostimulating siRNA in HPV-driven tumours. Immunol Cell Biol. (2014) 92:156–63. doi: 10.1038/icb.2013.75
23. Anz D, Koelzer VH, Moder S, Thaler R, Schwerd T, Lahl K, et al. Immunostimulatory RNA blocks suppression by regulatory T cells. J Immunol. (2010) 184:939–46. doi: 10.4049/jimmunol.0901245
24. Stewart CR, Karpala AJ, Lowther S, Lowenthal JW, Bean AG. Immunostimulatory motifs enhance antiviral siRNAs targeting highly pathogenic avian influenza H5N1. PLoS ONE. (2011) 6:e21552. doi: 10.1371/journal.pone.0021552
25. Wang K, Chen X, Yan F, Xing Y, Yang X, Tu J, et al. 5′-triphosphate-siRNA against survivin gene induces interferon production and inhibits proliferation of lung cancer cells in vitro. J Immunother. (2013) 36:294–304. doi: 10.1097/CJI.0b013e318294183b
26. Chen X, Qian Y, Yan F, Tu J, Yang X, Xing Y, et al. 5′-Triphosphate-siRNA activates RIG-I-dependent type i interferon production and enhances inhibition of hepatitis B virus replication in HepG2.2.15 cells. Eur J Pharmacol. (2013) 721:86–95. doi: 10.1016/j.ejphar.2013.09.050
27. Prins RM, Craft N, Bruhn KW, Khan-Farooqi H, Koya RC, Stripecke R, et al. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol. (2006) 176:157–64. doi: 10.4049/jimmunol.176.1.157
28. Kabilova TO, Vladimirova AV, Zenkova MA, Chernolovskaya EL, Vlassov VV. Antiproliferative and interferon-inducing activities of unique short double-stranded RNA. Dokl Biochem Biophys. (2011) 436:8–11. doi: 10.1134/S1607672911010042
29. Kabilova TO, Meschaninova MI, Venyaminova AG, Nikolin VP, Zenkova MA, Vlassov VV, et al. Short double-stranded RNA with immunostimulatory activity: sequence dependence. Nucleic Acid Ther. (2012) 22:196–204. doi: 10.1089/nat.2011.0328
30. Kabilova TO, Kovtonyuk LV, Zonov EV, Ryabchikova EI, Popova NA, Nikolin VP, et al. Immunotherapy of hepatocellular carcinoma with small double-stranded RNA. BMC Cancer. (2014) 14:338. doi: 10.1186/1471-2407-14-338
31. Kabilova TO, Sen'kova AV, Nikolin VP, Popova NA, Zenkova MA, Vlassov VV, et al. Antitumor and antimetastatic effect of small immunostimulatory RNA against B16 melanoma in mice. PLoS ONE. (2016) 11:e0150751. doi: 10.1371/journal.pone.0150751
32. Bellon L. Oligoribonucleotides with 2′-O-(tert-butyldimethylsilyl) groups. Curr Protoc Nucleic Acid Chem. (2001) 1:3.6.1–13. doi: 10.1002/0471142700.nc0306s01
33. Rio DC, Ares M, Hannon GJ, Nilsen TW, Ares M Jr, Hannon GJ, et al. Preparation of cytoplasmic and nuclear RNA from tissue culture cells. Cold Spring Harb Protoc. (2010) 2010:pdb.prot5441. doi: 10.1101/pdb.prot5441
34. Predesigned Dicer-Substrate siRNA (DsiRNA) IDT. Available online at: eu.idtdna.com/site/order/designtool/index/DSIRNA_PREDESIGN
35. Maslov MA, Kabilova TO, Petukhov IA, Morozova NG, Serebrennikova GA, Vlassov VV, et al. Novel cholesterol spermine conjugates provide efficient cellular delivery of plasmid DNA and small interfering RNA. J Control Release. (2012) 160:182–93. doi: 10.1016/j.jconrel.2011.11.023
36. Gantier MP, Williams BRG. siRNA delivery not Toll-free. Nat Biotechnol. (2009) 27:911–2. doi: 10.1038/nbt1009-911
37. Marques JT, Devosse T, Wang D, Zamanian-Daryoush M, Serbinowski P, Hartmann R, et al. A structural basis for discriminating between self and nonself double-stranded RNAs in mammalian cells. Nat Biotechnol. (2006) 24:559–65. doi: 10.1038/nbt1205
38. Stepanov G, Zhuravlev E, Shender V, Nushtaeva A, Balakhonova E, Mozhaeva E, et al. Nucleotide modifications decrease innate immune response induced by synthetic analogs of snRNAs and snoRNAs. Genes. (2018) 9:1–18. doi: 10.3390/genes9110531
39. Pawlotsky JM, Chevaliez S, McHutchison JG. The hepatitis C virus life cycle as a target for new antiviral therapies. Gastroenterology. (2007) 132:1979–98. doi: 10.1053/j.gastro.2007.03.116
40. Hornung V, Ellegast J, Kim S, Brzózka K, Jung A, Kato H, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. (2006) 314:994–7. doi: 10.1126/science.1132505
41. Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. (2006) 441:101–5. doi: 10.1038/nature04734
42. Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, et al. RIG-I - mediated antiviral responses to single-stranded RNA bearing 5′-pshophates. Science. (2006) 314:997–1001. doi: 10.1126/science.1132998
43. Schmidt A, Schwerd T, Hamm W, Hellmuth JC, Cui S, Wenzel M, et al. 5′-triphosphate RNA requires base-paired structures to activate antiviral signaling via RIG-I. Proc Natl Acad Sci USA. (2009) 106:12067–72. doi: 10.1073/pnas.0900971106
44. Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid–inducible gene-I and melanoma differentiation–associated gene 5. J Exp Med. (2008) 205:1601–10. doi: 10.1084/jem.20080091
45. Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. (2004) 5:730–7. doi: 10.1038/ni1087
46. Bruns AM, Horvath CM. LGP2 synergy with MDA5 in RLR-mediated RNA recognition and antiviral signaling. Cytokine. (2015) 74:198–206. doi: 10.1016/j.cyto.2015.02.010
47. Cole JL. Activation of PKR: an open and shut case? Trends Biochem Sci. (2007) 32:57–62. doi: 10.1016/j.tibs.2006.12.003
48. Sadler AJ. Orchestration of the activation of protein kinase R by the RNA-binding motif. J Interferon Cytokine Res. (2010) 30:195–204. doi: 10.1089/jir.2010.0005
49. Nallagatla SR, Bevilacqua PC. Nucleoside modifications modulate activation of the protein kinase PKR in an RNA structure-specific manner. RNA. (2008) 14:1201–13. doi: 10.1261/rna.1007408
50. Bevilacqua PC, Cech TR. Minor-groove recognition of double-stranded RNA by the double-stranded RNA-binding domain from the RNA-activated protein kinase PKR. Biochemistry. (1996) 35:9983–94. doi: 10.1021/bi9607259
51. Ucci JW, Kobayashi Y, Choi G, Alexandrescu AT, Cole JL. Mechanism of interaction of the double-stranded RNA (dsRNA) binding domain of protein kinase R with short dsRNA sequences. Biochemistry. (2007) 46:55–65. doi: 10.1021/bi061531o
52. Zheng X, Bevilacqua PC. Activation of the protein kinase PKR by short double-stranded RNAs with single-stranded tails. RNA. (2004) 10:1934–45. doi: 10.1261/rna.7150804
53. Kim DH, Behlke MA, Rose SD, Chang MS, Choi S, Rossi JJ. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat Biotechnol. (2005) 23:222–6. doi: 10.1038/nbt1051
54. Sledz CA, Holko M, De Veer MJ, Silverman RH, Williams BRG. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol. (2003) 5:834–9. doi: 10.1038/ncb1038
55. Zhang F, Romano PR, Nagamura-Inoue T, Tian B, Dever TE, Mathews MB, et al. Binding of double-stranded RNA to protein kinase PKR is required for dimerization and promotes critical autophosphorylation events in the activation loop. J Biol Chem. (2001) 276:24946–58. doi: 10.1074/jbc.M102108200
56. Dey M, Cao C, Dar AC, Tamura T, Ozato K, Sicheri F, et al. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2α substrate recognition. Cell. (2005) 122:901–13. doi: 10.1016/j.cell.2005.06.041
57. Balachandran S, Kim CN, Yeh WC, Mak TW, Bhalla K, Barber GN. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J. (1998) 17:6888–902. doi: 10.1093/emboj/17.23.6888
58. Balachandran S, Roberts PC, Brown LE, Truong H, Pattnaik AK, Archer DR, et al. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity. (2000) 13:129–41. doi: 10.1016/S1074-7613(00)00014-5
59. Garcia-Ortega M, Lopez G, Jimenez G, Garcia-Garcia J, Conde V, Boulaiz H, et al. Clinical and therapeutic potential of protein kinase PKR in cancer and metabolism. Expert Rev Mol Med. (2017) 19:e9. doi: 10.1017/erm.2017.11
60. Kirchhoff S, Koromilas AE, Schaper F, Grashoff M, Sonenberg N, Hauser H. IRF-1 induced cell growth inhibition and interferon induction requires the activity of the protein kinase PKR. Oncogene. (1995) 11:439–45.
61. Cuddihy AR, Wong AHT, Nancy Wai Ning T, Suiyang L, Koromilas AE. The double-stranded RNA activated protein kinase PKR physically associates with the tumor suppressor p53 protein and phosphorylates human p53 on serine 392 in vitro. Oncogene. (1999) 18:2690–702. doi: 10.1038/sj.onc.1202620
62. Garcia MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, et al. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev. (2006) 70:1032–60. doi: 10.1128/MMBR.00027-06
63. Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. (1995) 9:2723–35. doi: 10.1101/gad.9.22.2723
64. Roff M, Thompson J, Rodriguez MS, Jacque JM, Baleux F, Arenzana-Seisdedos F, et al. Role of IκBα ubiquitination in signal-induced activation of NF-κB in vivo. J Biol Chem. (1996) 271:7844–50. doi: 10.1074/jbc.271.13.7844
65. Goh KC, DeVeer MJ, Williams BR. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. (2000) 19:4292–7. doi: 10.1093/emboj/19.16.4292
66. Chu WM, Ostertag D, Li ZW, Chang L, Chen Y, Hu Y, et al. JNK2 and IKKβ are required for activating the innate response to viral infection. Immunity. (1999) 11:721–31. doi: 10.1016/S1074-7613(00)80146-6
67. Visvanathan KV, Goodbourn S. Double-stranded RNA activates binding of NF-kappa B to an inducible element in the human beta-interferon promoter. EMBO J. (1989) 8:1129–38. doi: 10.1002/j.1460-2075.1989.tb03483.x
68. Pham AM, Santa Maria FG, Lahiri T, Friedman E, Marié IJ, Levy DE. PKR transduces MDA5-dependent signals for Type I IFN induction. PLoS Pathog. (2016) 12:1–27. doi: 10.1371/journal.ppat.1005489
Keywords: immunostimulating dsRNA, antiproliferative activity, gene silencing, shRNA, pattern recognition receptors, PKR, RIG-I, IL-6
Citation: Zharkov MI, Zenkova MA, Vlassov VV and Chernolovskaya EL (2019) Molecular Mechanism of the Antiproliferative Activity of Short Immunostimulating dsRNA. Front. Oncol. 9:1454. doi: 10.3389/fonc.2019.01454
Received: 16 July 2019; Accepted: 05 December 2019;
Published: 20 December 2019.
Edited by:
Albrecht Reichle, University Medical Center Regensburg, GermanyReviewed by:
Tsukasa Seya, Hokkaido University School of Medicine, JapanBryan R. G. Williams, Hudson Institute of Medical Research, Australia
Copyright © 2019 Zharkov, Zenkova, Vlassov and Chernolovskaya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena L. Chernolovskaya, elena_ch@niboch.nsc.ru
 Mikhail I. Zharkov
Mikhail I. Zharkov Marina A. Zenkova
Marina A. Zenkova Valentin V. Vlassov
Valentin V. Vlassov Elena L. Chernolovskaya
Elena L. Chernolovskaya