- Department of Medical Oncology, Changzheng Hospital, Naval Medical University, Shanghai, China
Currently, genomic characterization has become standard of care for tumor types such as non-small cell lung cancer, breast cancer, melanoma, and colorectal cancer. A deep understanding of genomic alterations in different tumor types would help identify potentially actionable genomic changes which occur across a wide variety of tumor types. A basket trial is a new type of clinical trial for which eligibility is based on the presence of a specific genomic alteration, irrespective of histology. Basket trials are phase II screening trials for the off-label use of a targeted drug in patients with the same genomic alterations for which it was approved. Intractable cancer refers to a type or condition of cancer which is unresponsive or resistant to treatment; intractable cancers may be classified into five subtypes as follows: hard-to-treat condition of common advanced cancer after multiple-line therapy, rare cancer in which no standard of care has been recommended, advanced cancer in which standard of care does not work well, cancer accompanied with organ dysfunction, and cancers in older or younger cancer patients. Previous studies have demonstrated that in basket trials, genomic-guided therapy yields clinical benefits in intractable cancer, thereby providing novel insights into the optimal clinical management of such cancers. In this review, we describe a novel way to classify intractable cancer, and summarize the current knowledge on such cancers. We additionally provide information on the role of basket trials in intractable cancer.
The landscape of genome-driven oncology was established based on the understanding of the detailed genetic profiles of tumors and attempts to target gene alterations. Advancements in sequencing technologies as well as innovations in the development of drugs that target molecular alterations have brought forth the promise of genome-driven oncology care (1). Basket trials have been formulated to investigate the efficacy of molecular-targeted therapy for oncogene-defined subsets of cancers across different tumor histologies (2). While basket trials might be an useful design regardless of the setting, the use of basket trials might be especially useful when the cancer is intractable (3, 4). Therefore, we will provide a review of intractable cancer settings with some specific examples to illustrate the need for improved therapy in these cases.
Intractable Cancer
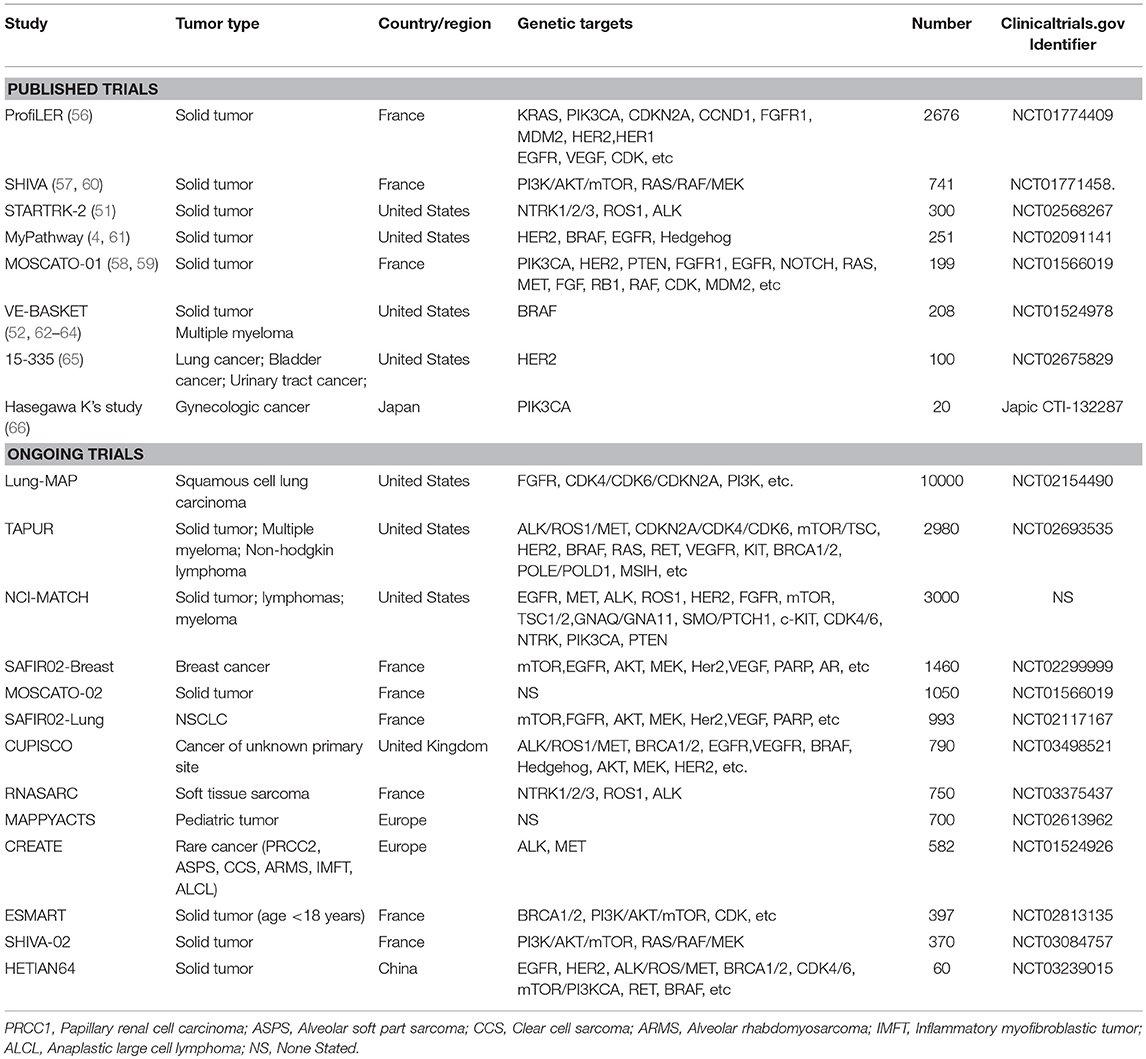
Intractable cancer refers to a “hard-to-treat” cancer or condition of cancer that does not respond to/is resistant to cancer treatment, or for which standard of care treatment has not been defined. Common characteristics of intractable cancer include: (1) no standard treatment for that cancer type or condition; (2) low response to standard treatment; and (3) lack of highly effective and low-toxicity regimens. Based on these characteristics, we classified intractable cancers into the following five subtypes (Figure 1): (a) hard-to-treat condition of common advanced cancer after multiple-line therapy; (b) rare cancer in which no standard of care has been recommended; (c) advanced cancer in which standard of care does not work well; (d) cancer accompanied by organ dysfunction; and (e) cancers in older (>75 years) or younger (<18 years) patients.
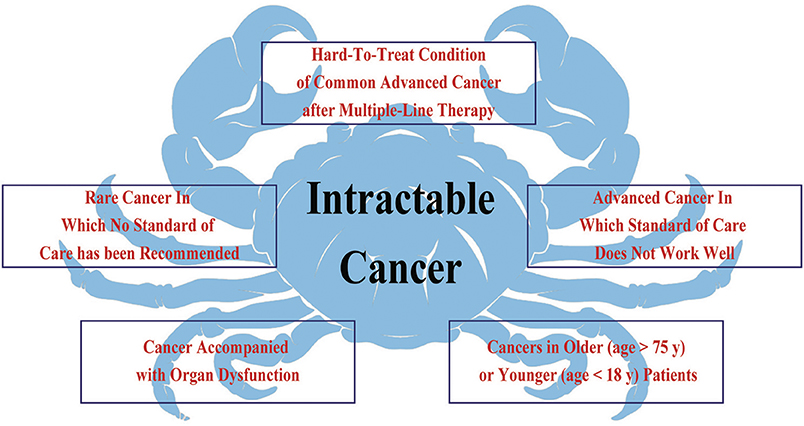
Figure 1. Intractable cancer refers to a type of cancer or condition of cancer that does not respond to/is resistant to cancer treatment. The common characteristics include, no standard treatment for that cancer type and status, standard treatment that has not worked well, and lack of highly effective and low-toxicity regimens. Therefore, intractable cancer could be divided into five subtypes.
Hard-to-Treat Condition of Common Advanced Cancer After Multiple-Line Therapy
Hard-to-treat conditions in patients with common advanced solid tumors who have experienced failure of multi-line standard treatment are a common subtype of intractable cancer. Advancements in modern oncology treatments have significantly prolonged disease control and consequently improved quality of life of patients. Patients who fail standard treatment often have a good performance status, and can be offered additional lines of treatment. In addition, due to the availability of targeted therapies that are highly effective with low toxicity, patients with advanced solid tumors often have the chance to receive third- and further-line therapy. However, lack of appropriate treatment regimens affects the quality of care, even in patients with a good performance status. The paucity of approved agents for third-line therapy and beyond for patients with advanced cancer constitutes an important unmet medical need. There are a number of well-documented treatment options for first- and second-line therapy for common cancers, but there are less data on the beneficial effects of third-line systemic therapy. Following the second chemotherapy regimen, the likelihood of an objective tumor response and disease control generally decreases with each subsequent line of chemotherapy. For example, the overall response rate (ORR) of docetaxel regimens as first- or second-line therapy was 20.9 and 16.3%, respectively, but was only 2.3% for third-line or further-line therapy in non-small cell lung cancer (NSCLC) (5).
An increasing number of clinical studies have attempted to confirm the effects of chemotherapy in patients who are heavily treated, but are still in good clinical condition. However, the survival advantages are no longer present because of drug resistance and/or a lack of agents with any real efficacy. For example, the objective response rate (ORR) for third-line treatment in lung cancer was only ~6–23% (6). The ORRs for the most common chemotherapy regimens used in third-line therapy for metastatic colorectal cancer vary from 5 to 36% (7).
Due to the low efficacy of chemotherapy in further-line treatment, targeted agent therapies have been considered as ideal regimens with greater efficacy and less toxicity than traditional non-targeted cytotoxic drugs such as anlotinib for NSCLC and regorafenib for colorectal cancer. In the ALTER 0303 trial, anlotinib as third-line therapy or further treatment prolonged the median overall survival (mOS) by only 3.3 months with an elevated ORR (9.2 vs. 0.7%) compared to the placebo group in patients with advanced NSCLC (8). Similarly, in the CORRECT trial, regorafenib prolonged the mOS by only 1.4 months (6.4 vs. 5.0 months) and the median progression-free survival (PFS) by 0.2 months (1.9 vs. 1.7 months), with an elevated ORR of 0.6% (1.0 vs. 0.4%) in metastatic colorectal cancer after standard therapies (9). Anlotinib or regorafenib now constitute the recommended standard for third-line therapies even with the small improvement in prognosis associated with their use; this further confirms the intractability of this subtype and the high demand for novel treatment strategies.
Rare Cancer in Which No Standard of Care Has Been Recommended
Rare tumors refer to those with an incidence of 6–15 per 100,000 cases per year (10). Epidemiological data have estimated that all subtypes of rare cancers account for 22–25% of all adult tumors, although they are individually uncommon (6, 11). In the United States, rare cancers are believed to be the fourth leading cause of cancer-related death each year, contributing to 25% of cancer mortality cases (12). These numbers are expected to rise as genomic-based classification becomes more prevalent, resulting in the increased identification of rarer, molecularly-defined subgroups of cancer (12). Hence, the overall burden of rare tumors is significant. For example, rare cancers accounted for 24% of all cancers diagnosed in Europe during 2000 to 2007, with an annual rise in incidence of 0.5%. The 5-year relative survival rate for all rare cancers is 48.5% compared to 63.4% for common cancers, only increasing by 2.9% from 1999–2001 to 2007–2009 (13). Rare cancers pose challenges for diagnosis, treatments, and clinical decision making (14).
The core challenge and difficulty may be the rarity of such cancer types. Clinical management of rare malignancies is challenging due to lack of information about diagnosis as well as a shortage of approved therapeutic options (15). Rare cancers are also scientifically challenging to study, because most reports on these cancer types tend to be case studies rather than phase III clinical trials (16). Thus, there is a paucity of standard recommended regimens with supporting high-quality evidence for the treatment of rare cancers. Notably, advances in cancer biology and genomic technology have also led to the in-depth understanding of the biology of rare tumors. For example, a previous study reported that 92.5% of rare patients had ≥1 actionable target, and that the outcome could be improved when the patient received matched targeted therapy (17). This customized precision strategy has provided insights into the development of novel treatment regimens for rare cancers. While clinical decision-making in rare cancers has historically been difficult due to the low numbers of cases, new treatment strategies are currently being explored due to improved identification of these rarer entities; flexibility and innovation in clinical trial design has also allowed for testing of multiple drugs in multiple rare cancer patients.
Advanced Cancer in Which Standard of Care Does Not Work Well
In clinical practice, for several cancer types, recommended standard of care has been established, but these standard regimens do not work well or elicit a week response; examples of such cancers include hepatocellular carcinoma (HCC), biliary tract cancer, pancreatic cancer, and large-cell neuroendocrine carcinoma. Thus, these cancers are considered hard-to-treat due to the lack of highly effective regimens. Sorafenib is an effective first-line treatment for unresectable HCC, and leads to disease stabilization which can prolong OS by 2–3 months; however, the ORRs are very low at 2–5% (18, 19). Targeted therapy for HCC in the past 10 years has been marked by several failed global phase III trials which did not show non-inferiority or superiority to sorafenib (20–23). Before the REFLECT study, no approved first-line systemic treatments were available for advanced HCC other than sorafenib, although sorafenib treatment was associated with a low ORR. The REFLECT study is the first trial to show positive results for sorafenib therapy in HCC (24). Although lenvatinib has shown statistically significant clinically meaningful improvement in PFS (7.3 months) or ORR (24.1%) for HCC treatment, its efficacy as first-line treatment is not satisfactory. With the emergence of innovative molecular-targeted agents and novel therapeutic strategies (e.g., immunotherapy), a combination of different targeted agents or immunotherapy has been a popular topic of research (25).
With the exception of HCC, biliary tract cancer is another intractable cancer type in which first-line treatment does not work well. Two randomized trials (ABC-02 and BT22) provided supporting evidence for the use of gemcitabine combined with cisplatin as standard first-line treatment for biliary tract cancer, but the effectiveness of this regimen needs to be enhanced in the first-line setting (26–28). The median OS of standard first-line therapy is modestly approaching 1 year with an ORR of 19.5–26.1%; however, almost 70% of patients who receive this regimen develop grade 3 or 4 toxicity. This standard of care is representative of a regimen with low efficacy and high toxicity. In addition, no other established standard regimens in the second-line setting are available when gemcitabine regimens fail (29). Standard treatments for pancreatic cancer and large-cell neuroendocrine carcinoma also have low efficacy (30, 31).
Cancer Accompanied With Organ Dysfunction
Organ dysfunction in cancer patients is a common occurrence, and is associated with an increased risk of mortality. A recent study found that hyperlactacidemia, number of dysfunctional organs, and liver dysfunction were independent risk factors for mortality (32). Thus, comprehensive support therapy is vital to improve this type of intractable cancer. On the other hand, organ dysfunction (especially hepatic and renal dysfunction) affects the metabolism and excretion of anti-tumor agents, further influencing the efficacy and/or safety of such agents in cancer patients; this often leads to dose reductions. However, most dose adjustments are empiric, and balancing the adjustment of certain anti-tumor agents to prevent excessive toxicity with the risk of undertreating the disease remains a concern (33). Meanwhile, the recommendations for dosing adjustments are based on studies in small cohorts or on case studies without high-quality evidence (34, 35). Therefore, personalized dose adjustments and regimens with low organ toxicity may improve efficacy and safety of treatments for these hard-to-treat intractable tumors.
Cancers in Older (Age > 75 Years) or Younger (Age < 18 Years) Patients
In the coming years, the number of elderly patients with cancer will considerably increase, for example, the incidence of cancer is 11-fold higher after the age of 65 years compared to that at 65 years of age and younger (36). However, despite the high incidence of cancer in older patients, administering treatment is challenging. Most elderly cancer patients (age >75 years) have been excluded from clinical trials because of age limits or comorbidities. A recent survey from 25 European Organization for Research and Treatment of Cancer randomized trials involving more than 6,024 patients only included 9% of patients aged 70 years or older (37). Therefore, solid data regarding the most appropriate approach and optimal treatment for older cancer patients are lacking due to their underrepresentation in prospective clinical trials. The results of several studies analyzing treatment in the elderly have revealed the overall diminished use of chemotherapy despite significant benefits for those who can receive therapy with equal or at least manageable toxicity (38–40). These data suggest that elderly patients may be a vulnerable population, and as such, anti-tumor therapy should sometimes be withheld in such populations. In fact, the decision-making process is much more complicated for elderly cancer patients. Older cancer patients are highly heterogeneous in all domains of physical and psychological function; thus, to treat hard-to-treat cancers in this group, we need a higher participation of elderly patients in clinical trials and more applicable data pertaining to patient characteristics, treatment responsiveness, and the toxicity profile in such patients. The most important outcomes of treatment in elderly cancer patients, namely PFS, time to treatment failure, and even OS, may be less important than the preservation of independence, good performance status, and quality of life (41, 42). Less toxic and personalized therapy should be considered for this type of intractable cancer.
Some organs and body systems can still be growing and developing in younger cancer patients, which can make such patients more sensitive to treatments such as chemotherapy and radiation therapy. However, specific tumor characteristics as well as the diversity of physical and psychological functions render younger cancer patients “hard-to-treat.” Cancer in younger patients (age <18 years) can be classified into two subtypes. One subtype is solid tumors in younger patients which are also common in adults, such as breast and colorectal cancers. In these cases, similar to elderly cancer patients, most therapeutic trials in oncology do not admit younger cancer patients. These younger patients are more likely to receive treatments recommended for adults. Although younger patients are generally better able to recover from higher doses of chemotherapy than are adult patients, there is no standard of care. Meanwhile, failure after multiple-line therapy makes these patients “hard-to-treat,” similar to adult patients. The other subtype is cancer that is common only in younger patients, such as soft tissue sarcoma (STS). The relative rarity of cancer among younger patients limits the familiarity of oncologists with the diagnostic and therapeutic aspects (43). For example, STS is a biologically heterogeneous malignancy with more than 50 subtypes. Traditional cytotoxic agents have limited clinical benefit beyond the first-line setting. Across all high-grade STS subtypes, median OS remains ~12–18 months for advanced metastatic disease (43). After resistance to first-line regimens, highly effective second-line agents which can be used in younger cancer patients are lacking. Further progress in the management of this type of intractable cancer will rely on novel trial design, subtype-specific therapies, and validation of biomarkers to tailor therapy.
Basket Trials
The initial proof-of-concept of genome-driven oncology was the development of imatinib for chronic myelogenous leukemia in patients harboring the BCR-ABL translocation (44). Subsequently, agents targeting human epidermal growth factor receptor 2 (HER-2)-overexpressing breast or gastric cancer, BRAF V600E-mutation melanoma, and EGFR-, ALK-, and ROS1-mutation lung cancer enables patients with those molecular alterations to have a significantly prolonged life expectancy. These early successes in identifying and targeting cancer-driving genomic alterations have propelled a paradigm of choosing therapy strategies guided by an individual tumor's genomic profile (45). Genomic characterization has become the standard of care for some cancer types such as lung cancer, breast cancer, melanoma, and colorectal cancer. With the deep understanding of genomic alterations in different tumor types, recognition that potentially actionable genomic changes could occur across a wide variety of tumor types have been proposed, although with low frequency in any individual tumor type (46). Could drugs that target specific gene alterations cause responses across a wide variety of diseases? Could drugs work based on genomics rather than the site of origin of the cancer? To address these questions, a novel clinical design called a “basket trial” was developed. This is a new clinical trial paradigm that determines eligibility based on the presence of a specific genomic alteration, irrespective of histology (1, 47, 48). Unlike traditional clinical trials which focus on patients with a single cancer histology, the core organizing principle of basket trials focused on a specific genomic alteration found in the tumor, regardless of where the cancer originated (Figure 2). Basket trial is virtually phase II screening trial for the off-label use of a targeted drug in patients with the same genomic alterations, and there are several potential advantages in the precision oncology era.
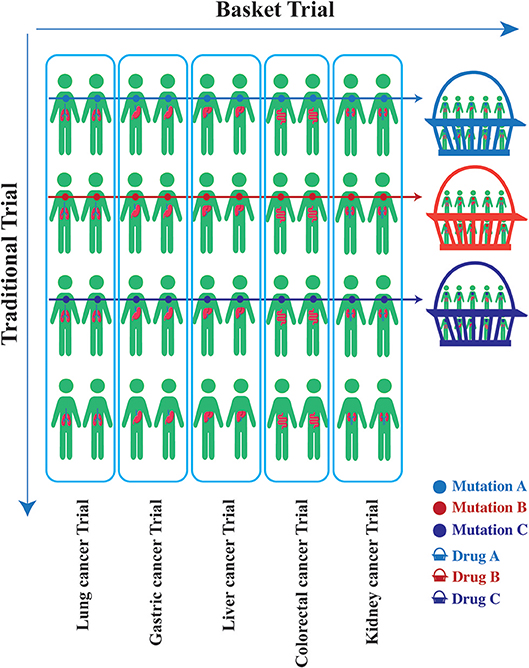
Figure 2. Unlike traditional clinical trials which focus on patients with a single cancer histology, the core organizing principle of basket trials is concentrated on a specific genomic alteration found in the tumor, regardless of where the cancer originated. A basket trial tests the effect of one drug (Drug A/B/C) that targets the same genomic alteration (Mutation A/B/C) across a variety of tumor types.
First, a basket trial may provide initial proof-of-principle evidence for the clinical validation of a newly discovered tumor-driven target, especially for uncommon, low frequency, or orphan genomic alterations. Since all participating patients share a genomic alteration that drives cancer progression across tumor types, a high sensitivity to targeted therapy is theoretically possible. In addition, the low incidence of these genomic segments makes randomized trials challenging, and thus basket trials are optimal for investigating the efficacy of target therapies. NTRK (NTRK-1, 2, 3) translocations have been observed in <1% of cancers and in more than 20 cancer types. Drilon et al. used a non-comparative, genomic-driven, single-arm basket trial in 55 patients with TRK translocations across 12 different tumor types, and found that the ORR of larotrectinib (LOXO-101, a TRK inhibitor) was 80%, and 71% of the responses were still ongoing at 1 year (49). This study has made a significant impact on clinical practice in cancer patients harboring the TRK translocation, and also illustrates the potential for future advancements in drug development and clinical trials of cancers harboring rare genomic alterations (50).
Second, a basket trial may allow screening for potential efficacy across multiple tumor types in order to guide more traditional, disease-specific, follow-up studies. The goal of some basket trials is to obtain an overall assessment of the drug with pooled histologies; in such trials, subsequent analyses are straightforward, and are comparable to those in standard phase II trials. Some basket trials aim to evaluate the possibility that the efficacy of targeted therapy depends on the histology; several such trials have been unable to find evidence of trans-tumor efficacy of the targeted therapy being studied. In these cases, the targeted drug regimen can be evaluated separately for each histology by increasing the sample size to conduct separate two-stage phase II trials for each histological type (48). If the ORRs appear heterogeneous, a two-stage phase II design is conducted separately for each histology. In the basket trial of vemerafinib in patients with BRAF V600E mutations, the drug was active in NSCLC and several other histologies, but not in colorectal cancer. Therefore, follow-up studies were conducted separately in the NSCLC cohort.
Third, a basket trial can determine if new drugs effectively inhibit their intended targets, by evaluating them in optimal candidates based on genomic selection. In drug development, pre-clinical trials assess the inhibition of targets both in vitro and in vivo. After achieving satisfactory results on safety and toxicity, the inhibitory activity of targeted agents in tumor patients can be tested. Basket trials can identify patients with identical mutations across different tumor types to achieve clinical confirmation. For example, the STARTRK-2 study is a basket study that assessed the efficacy of RXDX-101 (entrectinib) for the treatment of patients with solid tumors harboring the NTRK1/2/3, ROS1, or ALK gene fusions. A total of 54 patients carrying the NTRK gene fusion had an ORR of 57.4% with a median OS of 20.9 months, and 32 patients with ROS-1 fusion had an ORR of 78% with a median PFS of 29.6 months, suggesting that entrectinib exhibits highly effective NTRK or ROS1 inhibitory activity (51).
Fourth, a basket trial can generate pivotal data that support new standards of care. In clinical practice, some cancer types such as Erdheim-Chester disease and Langerhans cell histiocytosis (LCH) lack a recommended standard of care. In the basket trial of vemurafenib in patients with BRAF V600E mutations, patients with Erdheim-Chester disease or LCH had significant clinical benefits from vemurafenib (52). Thus, this basket trial established a standard of care for these rare cancers with BRAF mutation. Another example is basket trial testing of programmed cell death protein 1 (PD-1) blockade in patients with deficient mismatch repair (dMMR)/microsatellite instability-high (MSI-H) tumors, which confirmed that dMMR can predict the response of solid tumors to PD-1 blockade; this trial has led to the regulatory approval of pembrolizumab for dMMR patients, regardless of cancer origin (53). It generated a novel standard of care for cancer patients with dMMR/MSI-H.
The Role of Basket Trials in Intractable Cancer
Initially, the critical use of a basket trial is to determine whether a drug already approved to target a genomic alteration in one cancer type is efficacious against the identical mutation in other cancer types. Theoretically, a druggable genomic alteration is also vital to the initiation and progression of cancer, and drugs targeting tumor-driving alterations could block cancer cell metastasis, providing clinical benefits to patients with intractable cancer. Given the increasing number of drugs that target genomic alterations in a tumor-specific context and the rapid development and popularity of tumor genome sequencing, the potential for broadening the utility of genome-driven oncology though basket trials has been explored in intractable cancer. To achieve the treatment value of basket trials in intractable cancer, two questions must be answered: (a) do these intractable cancer patients harbor a druggable molecular alteration; and (b) is targeting specific alterations still efficacious in intractable cancer patients carrying identical mutations.
Frequency of Druggable Molecular Alterations in Intractable Cancer
Genome-driven oncology has helped establish the effectiveness of the application of clinical genomics to treatment (54). In certain cancers like those of the lung, it has become standard practice to profile tumors for targetable mutations. The application of next-generation sequencing (NGS) has enabled the marked expansion of molecular profiling in cancer patients. In 2017, the Memorial Sloan Kettering Cancer Center (MSKCC) revealed the mutational landscape of metastatic cancer using the MSK-IMPACT NGS panel in 10,336 patients with advanced disease; these patients were frequently heavily treated. The data included details of more than 300 tumor types. The results revealed that a total of 36.7% of patients (N = 3792) harbored at least one actionable alteration (55). Many druggable targets were shared across these patients with intractable cancer (Table 1).
Table 1. The frequency of common molecular alterations in pan-cancer based on the data from MSK-IMPACT sequencing(%).
ProfiLER is another molecular profiling clinical trial that explored cancer cell genomic alterations in 2,676 patients with 16 refractory cancer types to guide treatment. A total of 1004/1944 (52%) patients had at least one actionable mutation: 609 patients had one actionable mutation, and 394 had two or more actionable mutations. Molecular-target therapy was also recommended in 676/1944 (35%) patients with refractory cancer (56). Similarly, the SHIVA study assessed the off-label use of molecularly targeted agents in 741 patients with metastatic solid tumors refractory to the standard of care, and found that 293 (40%) refractory cancer patients had at least one druggable molecular alteration (57). Besides, the MOSCATO-01 study demonstrated that an actionable molecular alteration could be identified in 411/843 (49%) patients with advanced hard-to-treat cancers (58). This was also the first trial to investigate characterized genomic alterations in recurrent or refractory solid tumors in pediatric patients (59). Successful molecular analyses in 69 pediatric patients revealed that 60.9% (42/69) of patients have actionable alterations in various oncogenic pathways. Among these, 26% had more than one actionable alteration, 42.4% harbored a target detected by copy-number analysis, 33.3% had an actionable mutation, and 14.5% had both. With regard to rare cancers, Kato et al. reported 37 patients (92.5%) had at least one potentially actionable target among 40 patients who received genomic and protein analyses (17).
Based on the data from these studies, it is evident that the frequency of druggable molecular alterations in intractable cancer is not low; this allows clinicians or physicians to conduct genomically-guided clinical trials to evaluate the efficacy of approved and investigational molecularly targeted therapies across distinct tumor types with shared genetic features in those with intractable cancer.
Effects of Targeted Therapy on Intractable Cancers Carrying Identical Mutations
Previous studies have provided validation for the use of genomics in clinical practice, and have shown that it could very well guide treatment choices for patients with intractable cancer (Table 2). In 2010, Von Hoff conducted a pilot study using molecular profiling to identify potential targets and select treatments for refractory cancers (67). A molecular target was detected in 84 of 86 patients with refractory metastatic cancer, and 18 of 66 patients (27%) had a longer PFS (67). In this trial, Von Hoff also proposed an endpoint called PFS2/PFS1 (PFS on genomic-guided targeted therapy/PFS on prior therapy), and concluded that a ratio > 1.3 would indicate a treatment benefit (67); such a benefit is often considered a clinical benefit in basket trials.
Subsequently, Hyman et al. conducted one of the most famous basket trials (52). A total of 120 patients with refractory cancer who harbored BRAF mutation received vemurafenib. In NSCLC patients after multiple-line therapy, the ORRs were 42% with a disease control rate (DCR) of 84%. This rate compares favorably with the 7% ORR reported for standard second-line docetaxel in molecularly unselected patients (68, 69). The DCR was 62% in a cholangiocarcinoma cohort, which was also better than second-line chemotherapy (49.5%), although there are no standard second-line regimens for this refractory cancer (29). This study demonstrated that histology-independent, biomarker-selected basket studies are feasible and can serve as tools for developing molecular-targeted cancer therapies for intractable cancer. Meanwhile, multiple basket studies have also been conducted in intractable cancers. The MyPathway study included 251 patients with 35 different tumor types who had advanced refractory solid tumors harboring molecular alterations in HER-2, EGFR, BRAF, and Hedgehog (4). Patients with refractory, metastatic HER2-amplified/overexpressing colorectal cancer accounted for the largest treatment group in this study. A total of 37 colorectal cancer patients with HER-2 amplification/overexpression had an ORR of 38% and a median duration of response of 11 months, which compares favorably to the ORR of other drugs that were recently approved for refractory colorectal cancer. HER-2 targeted therapy is also efficacious in other refractory cancers such as biliary tract cancer (ORR, 29%) and bladder cancer (ORR, 33%). In particular, HER-2 targeted therapy has an ORR of 80% in rare salivary duct carcinomas.
For rare cancers and younger cancer patients, basket trials have also shown improvements in prognosis. For example, imatinib has been used in diverse rare cancers known to express imatinib-sensitive tyrosine kinases. A basket trial showed responses among multiple rare malignancies including dermatofibrosarcoma protuberans, hypereosinophilic syndrome, myeloproliferative disorders, and systemic mastocytosis, which facilitated the FDA approval of imatinib for these rare and ultra-rare disease conditions (70). Furthermore, another basket trial assessed 21 rare cancer patients who received matched therapy for an actionable alteration; 52.4% (11/21) attained stable disease (SD) > 6 months, partial responses (PR), or complete response (CR) (14.3% had SD > 6 months; 28.6% had PR; 9.5% had CR) with a median PFS of 19.6 months. Moreover, the matched therapy approach resulted in a statistically significant improvement in PFS compared with the last prior unmatched therapy (17). Genomic-guided targeted therapy also renders clinical benefits for younger cancer patents. In the pediatric subgroup of the MOSCATO-01 study, 14 pediatric patients with genomic alterations received a “matching” target agent including clinical trial agents and registered agents, and 5 (35.7%) experienced an objective tumor response. This outcome is ideal for pediatric patients who have received median two (ranged from 1 to 8) prior lines of treatment. For other refractory cancers, basket trials in patients with cancers of unknown origin were also conducted. The CUPISCO study is a phase II, randomized, multi-center study that aims to compare the efficacy and safety of molecular-guided therapy vs. standard platinum-containing chemotherapy in patients with cancer of unknown primary site who achieved disease control after three cycles of first-line platinum doublet induction chemotherapy (NCT03498521, Table 2). This trial is currently recruiting patients.
Basket trials have also provided an efficient method for patients with intractable cancer harboring actionable germline alterations. Study 42 is an open-label non-comparative single-arm phase II study that examined olaparib monotherapy in germline BRCA1/2-associated cancers, regardless of tumor type (71). A prolonged tumor response was seen across a spectrum of malignancies. Specifically, 31.3, 12.9, 21.7, and 50.0% of patients with ovarian, breast, pancreatic, and prostate cancers, respectively, who were heavily treated with a mean number of 4.6 prior chemotherapy regimens in the metastatic setting, achieved a response. In this study, the potential efficacy of poly (ADP-ribose) polymerase (PARP) inhibitors in pancreatic, breast, and prostate cancers was preliminarily validated. This was also the first study to support the efficacy of PARP inhibitors in platinum-resistant or platinum-refractory ovarian cancer. Based on data from this basket trial, subsequent studies, such as the SOLO1 and SOLO2 were performed, and revealed marked antitumor activity in patients with advanced ovarian cancer harboring the gBRCA1/2 mutation. The germline status of BRCA1/2 in individuals with cancer defines a target population in whom PARP inhibitors seem beneficial, supporting the hypothesis that therapy directed against a genetically defined target has activity regardless of anatomic organ of origin.
Besides, the MOSCATO-01 and ProfiLER studies also demonstrated that molecular-targeted cancer therapy could bring survival benefit for intractable caner. For example, in the MOSCATO-01 study, 199 patients were treated with a targeted therapy matched to a genomic alteration, and a PFS2/PFS1 ratio > 1.3 was observed in 33% of patients (63/193). Objective responses were observed in 22 of 194 patients, and the mOS was 11.9 months. Also, the ProfiLER study showed that a total of 53.7% of 143 patients who received the recommended targeted therapy were alive at 3 years, compared with 46.1% of 502 patients who did not receive that therapy. The 5-year survival rate was also higher for patients who received targeted therapy (34.8 vs. 28.1%) (56).
However, there have also been some controversial findings regarding the role of basket trials in intractable caner. For example, the SHIVA study is a two-arm control trial which aims to investigate the efficacy of molecular-targeted therapy in refractory cancer. One group received molecularly targeted agents (MTAs) base on tumor molecular profiling, while the other group received treatment based on the physician's choice (TPC). The SHIVA study demonstrated that the use of MTA outside their approved indications did not improve PFS compared with TPC in heavily treated cancer patients. In the MTA group, the median PFS was 2.3 months compared with 2.0 months in the TPC group, without significant statistical difference. Subsequent crossover analyses revealed that the proportion of patients with a PFS ratio >1.3 was 37 and 61% in those who crossed-over from the TPC to MTA, or from MTA to TPC, respectively. Indeed, a substantial proportion of patients could also get clinical benefit from the treatment algorithm evaluated in the SHIVA trial (60).
Whether the use of genomic-guided therapy can improve outcome in intractable cancer patients is still a controversial topic, and there are several evidences which can help resolve this issue. First, targeted agents can affect the results of a basket trial. It is well-established that only therapies that hit the target with high bioactivity can improve outcome. For example, vemurafenib dramatically improves the outcome of BRAFV600-mutant melanoma, whereas sorafenib, a weak BRAF inhibitor, does not have this effect (72). In the MOSCATO trial, 75% of patients received last-generation targeted therapies in the phase I/II trial, and thus, a positive outcome was obtained in this basket trial. Second, extensive molecular analysis can identify specific genomic contexts that may condition the response to targeted therapy. A recent multihistology basket trial of an AKT inhibitor (AZD5363) in advanced solid tumor refractory to standard therapy with AKT1 E17K mutation illustrated the utility of comprehensive tumor biomarker analysis. Overall, the median PFS for these patients who received a median of five lines of prior therapy was 4.2–6.6 months. In exploratory biomarker analyses, imbalance of the AKT1 E17K–mutant allele as well as the presence of coincident phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) pathway hotspot mutations was associated with a longer PFS (73). If the outcome determination relied solely on the presence or absence of a single molecular alteration in basket trials, we could overlook additional factors that inform optimal patient selection. Therefore, to better understand the underlying biology of this heterogeneity in response, basket trials should evolve to more routinely incorporate comprehensive genomic analyses. Third, tumor location is important in assessing the outcome. For example, in a recent basket study evaluating the efficacy of a pan-HER inhibition (neratinib) in refractory tumors harboring activating mutations in HER2/3, neratinib activity was influenced by both tumor lineage and mutation type (74). Single-agent neratinib showed activity in breast, biliary, and cervical cancers and not in colorectal and bladder cancers. Missense mutations appeared more sensitive compared with exon 20 insertions. Coincidentally, in the MyPathway study, all seven uterine cancer patients with HER-2 amplification/overexpression had no response to HER-2 targeted therapy. Although a common set of driver mutations exist in each cancer type, the combination of drivers within a cancer type and their distribution within the founding clone and subclones vary for individual patients. This suggests that knowing the clonal architecture of each patient's tumor will be crucial for optimizing treatment (46). Meanwhile, the investigators should be very cautious about over-interpretation of results from basket trial. Especially, there is only little or modest improvement in prognosis. In this setting, basket trials cannot distinguish whether the effect is due to improved prognostic outcomes in patients with the genetic biomarker identified, or due to the treatment itself. Besides, with a basket trial across a number of histologies, and a small number of patients within any one histology, it is possible that variation in outcomes might be due to chance differences, and not due to a true treatment effect. Therefore, the effect of basket trial in intractable cancer required to be further confirmed in the next-step.
Perspective
In the era of genome-driven oncology, understanding the genomic profile of individual cancer patients has become necessary for delivering a selection of regimens for intractable cancer. In the real-world setting, previous targeted therapy studies have yielded disappointing genomic match rates of about 4%−13% (75). One possible explanation for this is the clinician's limited ability to interpret genomic testing results. The other possibility is that gene panels might not cover the full spectrum of genes, or that gene panels might not adequately detect mutations. Therefore, basket trials for intractable cancer may be conducted efficiently only with an in-depth understanding of annotated genomic data in conjunction with their functional and therapeutic implications. An increasing number of large basket trials have been conducted such as TAPUR and NCI-MATCH (Table 2). Multiple basket trials have assessed whether specific targeted therapies could benefit more patients and have led to more personalized therapies in advanced cancer patients without standard treatment options. We believe that these trials will further illustrate the potential scope of this novel therapeutic strategy. Besides, it remains unclear whether basket trial is feasible in cancer patients with organ dysfunction due to less relevant reports. Theoretically, intractable cancer patients with organ dysfunction could receive this novel therapeutic regimen because targeted therapy had less organ toxicity than chemotherapy. In the future, more and more studies are required to confirm the feasibility of basket trial in intractable cancer patients with organ dysfunction, and this also will be an important direction of basket trial. Basket trials represent an important research tool for the efficient generation of knowledge needed to deliver clinically valuable therapies. Therefore, improvements in the selection of molecular alterations, genomic knowledge-base integration, as well as patient-matching are needed to translate genomic knowledge into clinically meaningful outcomes and to improve the clinical management of these intractable cancers.
Author Contributions
YW, JL, XH, ZW, and W-XQ were responsible for data analysis, preparation of figures and tables. B-DQ, X-DJ, KL, and Y-SZ made substantial contributions to conception and design of the review, and analysis and interpretation of articles. All authors have been involved in drafting the manuscript or revising it critically for important intellectual content. B-DQ, X-DJ, KL, and Y-SZ have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This study was supported by the Shanghai Sailing Program [grant number 17YF1425200, 2017]; Chinese National Natural Science Funding [grant number 81702249, 2017]; Science and Technology Commission of Shanghai Municipality [grant number 17511103403, 2017]; The funder has no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the experts from Refractory Malignancy Society of Chinese Medical Education Association for the consultation.
References
1. Tao JJ, Schram AM, Hyman DM. Basket studies: redefining clinical trials in the era of genome-driven oncology. Annu Rev Med. (2018) 69:319–31. doi: 10.1146/annurev-med-062016-050343
2. Mcneil C. NCI-MATCH launch highlights new trial design in precision-medicine era. J Natl Cancer Inst. (2015) 107:djv193. doi: 10.1093/jnci/djv193
3. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. (2011) 364:2507–16. doi: 10.1056/NEJMoa1103782
4. Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J Clin Oncol. (2018) 36:536–42. doi: 10.1200/JCO.2017.75.3780
5. Massarelli E, Andre F, Liu DD, Lee JJ, Wolf M, Fandi A, et al. A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer. Lung Cancer. (2003) 39:55–61. doi: 10.1016/S0169-5002(02)00308-2
6. Genestreti G, Grossi F, Genova C, Burgio MA, Bongiovanni A, Gavelli G, et al. Third- and further-line therapy in advanced non-small-cell lung cancer patients: an overview. Future Oncol. (2014) 10:2081–96. doi: 10.2217/fon.14.96
7. Gundgaard MG, Soerensen JB, Ehrnrooth E. Third-line therapy for metastatic colorectal cancer. Cancer Chemother Pharmacol. (2008) 61:1–13. doi: 10.1007/s00280-007-0573-x
8. Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. (2018) 4:1569–75. doi: 10.1001/jamaoncol.2018.3039
9. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. (2013) 381:303–12. doi: 10.1016/S0140-6736(12)61900-X
10. Gatta G, Van Der Zwan JM, Casali PG, Siesling S, Dei Tos AP, Kunkler I, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. (2011) 47:2493–511. doi: 10.1016/j.ejca.2011.08.008
11. Bogaerts J, Sydes MR, Keat N, Mcconnell A, Benson A, Ho A, et al. Clinical trial designs for rare diseases: studies developed and discussed by the International Rare Cancers Initiative. Eur J Cancer. (2015) 51:271–81. doi: 10.1016/j.ejca.2014.10.027
12. Boyd N, Dancey JE, Gilks CB, Huntsman DG. Rare cancers: a sea of opportunity. Lancet Oncol. (2016) 17:e52–61. doi: 10.1016/S1470-2045(15)00386-1
13. Gatta G, Capocaccia R, Botta L, Mallone S, De Angelis R, Ardanaz E, et al. Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet-a population-based study. Lancet Oncol. (2017) 18:1022–39. doi: 10.1016/S1470-2045(17)30445-X
14. Qin BD, Jiao XD, Yuan LY, Liu K, Zang YS. Adenosquamous carcinoma of the bile duct: a population-based study. Cancer Manag Res. (2018) 10:439–46. doi: 10.2147/CMAR.S144850
15. Qin BD, Jiao XD, Zang YS. Primary pulmonary leiomyosarcoma: a population-based study. Lung Cancer. (2018) 116:67–72. doi: 10.1016/j.lungcan.2017.12.015
16. Qin BD, Jiao XD, Liu K, Wu Y, He X, Liu J, et al. Clinical, pathological and treatment factors associated with the survival of patients with primary pulmonary salivary gland-type tumors. Lung Cancer. (2018) 126:174–81. doi: 10.1016/j.lungcan.2018.11.010
17. Kato S, Kurasaki K, Ikeda S, Kurzrock R. Rare tumor clinic: the University of California San Diego moores cancer center experience with a precision therapy approach. Oncologist. (2018) 23:171–8. doi: 10.1634/theoncologist.2017-0199
18. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. (2008) 359:378–90. doi: 10.1056/NEJMoa0708857
19. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. (2009) 10:25–34. doi: 10.1016/S1470-2045(08)70285-7
20. Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. (2013) 31:4067–75. doi: 10.1200/JCO.2012.45.8372
21. Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. (2013) 31:3517–24. doi: 10.1200/JCO.2012.48.4410
22. Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. (2015) 33:172–9. doi: 10.1200/JCO.2013.54.3298
23. Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. (2015) 33:559–66. doi: 10.1200/JCO.2013.53.7746
24. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
25. Xie F, Feng S, Sun L, Mao Y. The first-line treatment for unresectable hepatocellular carcinoma patients: lenvatinib versus sorafenib, or beyond? Hepatobiliary Surg Nutr. (2018) 7:221–4. doi: 10.21037/hbsn.2018.06.06
26. Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. (2010) 103:469–74. doi: 10.1038/sj.bjc.6605779
27. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. (2010) 362:1273–81. doi: 10.1056/NEJMoa0908721
28. Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. (2014) 25:391–8. doi: 10.1093/annonc/mdt540
29. Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. (2014) 25:2328–38. doi: 10.1093/annonc/mdu162
30. Macchini M, Chiaravalli M, Zanon S, Peretti U, Mazza E, Gianni L, et al. Chemotherapy in elderly patients with pancreatic cancer: efficacy, feasibility and future perspectives. Cancer Treat Rev. (2018) 72:1–6. doi: 10.1016/j.ctrv.2018.10.013
31. Zhou F, Hou L, Ding T, Song Q, Chen X, Su C, et al. Distinct clinicopathologic features, genomic characteristics and survival of central and peripheral pulmonary large cell neuroendocrine carcinoma: from different origin cells? Lung Cancer. (2018) 116:30–7. doi: 10.1016/j.lungcan.2017.12.009
32. Pereira FM, Dias SAJ, Guerreiro IM, Silva MJ, Afonso A, Mergulhão P, et al. Impact of sepsis and organ dysfunction on cancer patients' mortality. Annal Oncol. (2016) 27:1454P. doi: 10.1093/annonc/mdw390.22
33. Superfin D, Iannucci AA, Davies AM. Commentary: oncologic drugs in patients with organ dysfunction: a summary. Oncologist. (2007) 12:1070–83. doi: 10.1634/theoncologist.12-9-1070
34. Fleming GF, Schilsky RL, Schumm LP, Meyerson A, Hong AM, Vogelzang NJ, et al. Phase I and pharmacokinetic study of 24-hour infusion 5-fluorouracil and leucovorin in patients with organ dysfunction. Ann Oncol. (2003) 14:1142–7. doi: 10.1093/annonc/mdg302
35. Mita AC, Sweeney CJ, Baker SD, Goetz A, Hammond LA, Patnaik A, et al. Phase I and pharmacokinetic study of pemetrexed administered every 3 weeks to advanced cancer patients with normal and impaired renal function. J Clin Oncol. (2006) 24:552–62. doi: 10.1200/JCO.2004.00.9720
36. Pallis AG, Fortpied C, Wedding U, Van Nes MC, Penninckx B, Ring A, et al. EORTC elderly task force position paper: approach to the older cancer patient. Eur J Cancer. (2010) 46:1502–13. doi: 10.1016/j.ejca.2010.02.022
37. Quinten C, Coens C, Ghislain I, Zikos E, Sprangers MA, Ringash J, et al. The effects of age on health-related quality of life in cancer populations: a pooled analysis of randomized controlled trials using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 involving 6024 cancer patients. Eur J Cancer. (2015) 51:2808–19. doi: 10.1016/j.ejca.2015.08.027
38. Hutchins LF, Unger JM, Crowley JJ, Coltman CAJr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. (1999) 341:2061–7. doi: 10.1056/NEJM199912303412706
39. Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. (2001) 345:1091–7. doi: 10.1056/NEJMoa010957
40. Hurria A, Hurria A, Zuckerman E, Panageas KS, Fornier M, D'andrea G, et al. A prospective, longitudinal study of the functional status and quality of life of older patients with breast cancer receiving adjuvant chemotherapy. J Am Geriatr Soc. (2006) 54:1119–24. doi: 10.1111/j.1532-5415.2006.00789.x
41. Marosi C, Koller M. Challenge of cancer in the elderly. ESMO Open. (2016) 1:e000020. doi: 10.1136/esmoopen-2015-000020
42. Ogino H, Hanibuchi M, Sakaguchi S, Toyoda Y, Tezuka T, Kawano H, et al. The clinical features of older patients with lung cancer in comparison with their younger counterparts. Respir Investig. (2018) 57:40–8. doi: 10.1016/j.resinv.2018.10.003
43. Rotz SJ, Nagarajan R, Sorger JI, Pressey JG. Challenges in the treatment of sarcomas of adolescents and young adults. J Adolesc Young Adult Oncol. (2017) 6:406–13. doi: 10.1089/jayao.2017.0007
44. Druker BJ, Guilhot F, O'brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. (2006) 355:2408–17. doi: 10.1056/NEJMoa062867
45. Hyman DM, Taylor BS, Baselga J. Implementing genome-driven oncology. Cell. (2017) 168:584–99. doi: 10.1016/j.cell.2016.12.015
46. Kandoth C, Mclellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. (2013) 502:333–9. doi: 10.1038/nature12634
47. Astsaturov I. Future clinical trials: genetically driven trials. Surg Oncol Clin N Am. (2017) 26:791–7. doi: 10.1016/j.soc.2017.05.014
48. Simon R. Critical review of umbrella, basket, and platform designs for oncology clinical trials. Clin Pharmacol Ther. (2017) 102:934–41. doi: 10.1002/cpt.814
49. Drilon A, Laetsch TW, Kummar S, Dubois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. (2018) 378:731–9. doi: 10.1056/NEJMoa1714448
50. Andre F. Developing anticancer drugs in orphan molecular entities - A paradigm under construction. N Engl J Med. (2018) 378:763–5. doi: 10.1056/NEJMe1716821
51. Demetri GD, Paz-Ares L, Farago AF. LBA17-Efficacy and safety of entrectinib in patients with NTRK fusion-positive (NTRK-fp) tumors: pooled analysis of STARTRK-2, STARTRK-1 and ALKA-372–A-001, ESMO, Germany (2018).
52. Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. (2015) 373:726–36. doi: 10.1056/NEJMoa1502309
53. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. (2017) 357:409–13. doi: 10.1126/science.aan6733
54. Garraway LA. Genomics-driven oncology: framework for an emerging paradigm. J Clin Oncol. (2013) 31:1806–14. doi: 10.1200/JCO.2012.46.8934
55. Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. (2017) 23:703–13. doi: 10.1038/nm.4333
56. Tredan O, Corset V, Wang Q, Varnier R, Pacaud C, Torroja A, et al. Routine molecular screening of advanced refractory cancer patients: an analysis of the first 2490 patients of the ProfiLER study. J Clin Oncol. (2017). 35(18_Suppl.):LBA100. doi: 10.1200/JCO.2017.35.18_suppl.LBA100
57. Le Tourneau C, Delord JP, Goncalves A, Gavoille C, Dubot C, Isambert N, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. (2015) 16:1324–34. doi: 10.1016/S1470-2045(15)00188-6
58. Massard C, Michiels S, Ferte C, Le Deley MC, Lacroix L, Hollebecque A, et al. High-throughput genomics and clinical outcome in hard-to-treat advanced cancers: results of the MOSCATO 01 trial. Cancer Discov. (2017) 7:586–95. doi: 10.1158/2159-8290.CD-16-1396
59. Harttrampf AC, Lacroix L, Deloger M, Deschamps F, Puget S, Auger N, et al. Molecular screening for cancer treatment optimization (MOSCATO-01) in pediatric patients: a single-institutional prospective molecular stratification trial. Clin Cancer Res. (2017) 23:6101–12. doi: 10.1158/1078-0432.CCR-17-0381
60. Belin L, Kamal M, Mauborgne C, Plancher C, Mulot F, Delord JP, et al. Randomized phase II trial comparing molecularly targeted therapy based on tumor molecular profiling versus conventional therapy in patients with refractory cancer: cross-over analysis from the SHIVA trial. Ann Oncol. (2017) 28:590–6. doi: 10.1093/annonc/mdw666
61. Meric-Bernstam F, Hurwitz H, Raghav KPS, Mcwilliams RR, Fakih M, Vanderwalde A, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. (2019). doi: 10.1016/S1470-2045(18)30904-5
62. Cohn AL, Day BM, Abhyankar S, Mckenna E, Riehl T, Puzanov I. BRAF(V600) mutations in solid tumors, other than metastatic melanoma and papillary thyroid cancer, or multiple myeloma: a screening study. Onco Targets Ther. (2017) 10:965–71. doi: 10.2147/OTT.S120440
63. Diamond EL, Subbiah V, Lockhart AC, Blay JY, Puzanov I, Chau I, et al. Vemurafenib for BRAF V600-mutant erdheim-chester disease and langerhans cell histiocytosis: analysis of data from the histology-independent, Phase 2, Open-label VE-BASKET Study. JAMA Oncol. (2018) 4:384–8. doi: 10.1001/jamaoncol.2017.5029
64. Kaley T, Touat M, Subbiah V, Hollebecque A, Rodon J, Lockhart AC, et al. BRAF Inhibition in BRAF(V600)-Mutant Gliomas: Results From the VE-BASKET Study. J Clin Oncol. (2018) 36:JCO2018789990. doi: 10.1200/JCO.2018.78.9990
65. Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J Clin Oncol. (2018) 36:2532–7. doi: 10.1200/JCO.2018.77.9777
66. Hasegawa K, Kagabu M, Mizuno M, Oda K, Aoki D, Mabuchi S, et al. Phase II basket trial of perifosine monotherapy for recurrent gynecologic cancer with or without PIK3CA mutations. Invest New Drugs. (2017) 35:800–12. doi: 10.1007/s10637-017-0504-6
67. Von Hoff DD, Stephenson JJ Jr, Rosen P, Loesch DM, Borad MJ, Anthony S, et al. Pilot study using molecular profiling of patients' tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol. (2010) 28:4877–83. doi: 10.1200/JCO.2009.26.5983
68. Fossella FV, Devore R, Kerr RN, Crawford J, Natale RR, Dunphy F, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. (2000) 18:2354–62. doi: 10.1200/JCO.2000.18.12.2354
69. Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. (2000) 18:2095–103. doi: 10.1200/JCO.2000.18.10.2095
70. Heinrich MC, Joensuu H, Demetri GD, Corless CL, Apperley J, Fletcher JA, et al. Phase II, open-label study evaluating the activity of imatinib in treating life-threatening malignancies known to be associated with imatinib-sensitive tyrosine kinases. Clin Cancer Res. (2008) 14:2717–25. doi: 10.1158/1078-0432.CCR-07-4575
71. Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmana J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. (2015) 33:244–50. doi: 10.1200/JCO.2014.56.2728
72. Flaherty KT, Puzanov I, Kim KB, Ribas A, Mcarthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. (2010) 363:809–19. doi: 10.1056/NEJMoa1002011
73. Hyman DM, Smyth LM, Donoghue MTA, Westin SN, Bedard PL, Dean EJ, et al. AKT inhibition in solid tumors with AKT1 mutations. J Clin Oncol. (2017) 35:2251–9. doi: 10.1200/JCO.2017.73.0143
74. Hyman DM, Piha-Paul SA, Rodon J. Neratinib in HER2- or HER3-mutant solid tumors: SUMMIT,a global, multi-histology, open-label, phase 2 “basket” study. Presented at Annual Meeting American Association for Cancer Research. Chicago, IL. Abstr. CT001 (2017).
Keywords: basket trial, intractable cancer, molecular alteration, personalized precision therapy, genome-driven oncology, refractory cancer
Citation: Qin B-D, Jiao X-D, Liu K, Wu Y, He X, Liu J, Qin W-X, Wang Z and Zang Y-S (2019) Basket Trials for Intractable Cancer. Front. Oncol. 9:229. doi: 10.3389/fonc.2019.00229
Received: 14 January 2019; Accepted: 14 March 2019;
Published: 12 April 2019.
Edited by:
Kuzhuvelil B. Harikumar, Rajiv Gandhi Centre for Biotechnology, IndiaCopyright © 2019 Qin, Jiao, Liu, Wu, He, Liu, Qin, Wang and Zang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan-Sheng Zang, doctorzangys@163.com
†These authors have contributed equally to this work
 Bao-Dong Qin
Bao-Dong Qin Xiao-Dong Jiao†
Xiao-Dong Jiao† Ke Liu
Ke Liu Xi He
Xi He