Exosomes: The Link between GPCR Activation and Metastatic Potential?
- 1Cancer and Ageing Research Program, Translational Research Institute, Queensland University of Technology, Brisbane, QLD, Australia
- 2Eph Receptor Biology Group, Translational Research Institute, Queensland University of Technology, Brisbane, QLD, Australia
The role of exosomes in cancer development has become the focus of much research, due to the many emerging roles possessed by exosomes. These micro-vesicles that are ubiquitously released in to the extracellular milieu, have been found to regulate immune system function, particularly in tumorigenesis, as well as conditioning future metastatic sites for the attachment and growth of tumor tissue. Through an interaction with a range of host tissue, exosomes are able to generate a pro-tumor environment that is essential for carcinogenesis. Herein, we discuss the contents of exosomes and their contribution to tumorigenesis, as well as their role in chemotherapeutic resistance and the development of novel cancer treatments and the identification of cancer biomarkers.
Introduction
There is no doubt that identifying novel methods for improving diagnostic and prognostic monitoring of cancer patients, as well as designing novel cancer therapies has proved more challenging than anticipated. Despite decades of intensive investigation, treatments resulting in a consistent and permanent reversal from an oncogenic cell state have remained elusive, and patient outcomes have failed to considerably improve. Although there are numerous factors contributing to cancer patient outcomes, two ever-critical problems remain, the design of more effective treatment regimes and the identification of biomarkers for patient diagnosis and prognosis. With the increasing heterogeneity and complexity observed in cancers, the need for specific and accurate diagnosis of disease state, as well as molecular monitoring of disease progression have become more important than ever. Fortunately, the intensive search for cancer biomarkers, and potential novel therapeutic targets, has revealed a factor with significant potential, the exosome. Exosomes are nanovesicles released from many cell types, but are secreted in substantially higher concentrations from cancer cells (1–3). Microvesiculation, the process of exosome production, results in the formation of small membrane-derived vesicles up 100 nm in diameter (and of a homogenous shape and density) that are released into the cellular environment by the process of exocytosis (4). They possess complex lipid membranes that contain integral proteins, while the interior cargo is comprised of an assortment of proteins and nucleic acid. These nanovesicles can travel to distant tissues where they fuse with cell membrane of target cells and induce an array of changes. In recent years, these once-neglected particles have been analyzed to reveal roles in many cellular functions, particularly cancer (5–8). This review serves to discuss the recent research on the multifunctional way exosomes affect the cellular microenvironment, and the relevance of these processes in tumorigenesis and metastasis.
Originally designated as a mechanism for the cellular release of waste and toxins, there is now substantial data demonstrating exosomes as important mediators of extracellular signaling, via the membrane-protected transfer of cellular material. Exosomes derived from both normal and malignant cells, have now been recognized as important in tumorigenesis, apoptosis, and chemotherapeutic resistance. At this stage, the contribution of exosomes to tumorigenesis primarily from two complementary processes; the modulation and restructuring of the cellular microenvironment to generate the metastatic niche (9, 10), combined with the attenuation/modulation of tumor immune responses (11, 12). In these cases, exosomes from a range of cells, induce microenvironmental changes in tissue that facilitate tumor formation, while simultaneously disarming anti-tumor immune responses, allowing cancerous cells to migrate, avoid immune detection, attach to secondary sites within the patient, and establish metastatic growth (Figure 1). Studies have revealed the significant clinical potential of exosomal signaling, both as a point of intervention or biological target in the treatment of carcinoma and prevention of chemotherapeutic resistance, as well as a potential biomarker for cancer diagnosis and prognosis. This potential has not gone unnoticed and has resulted in a substantial body of work investigating tumor-derived exosome signaling; research which is paramount in improving patient outcomes.
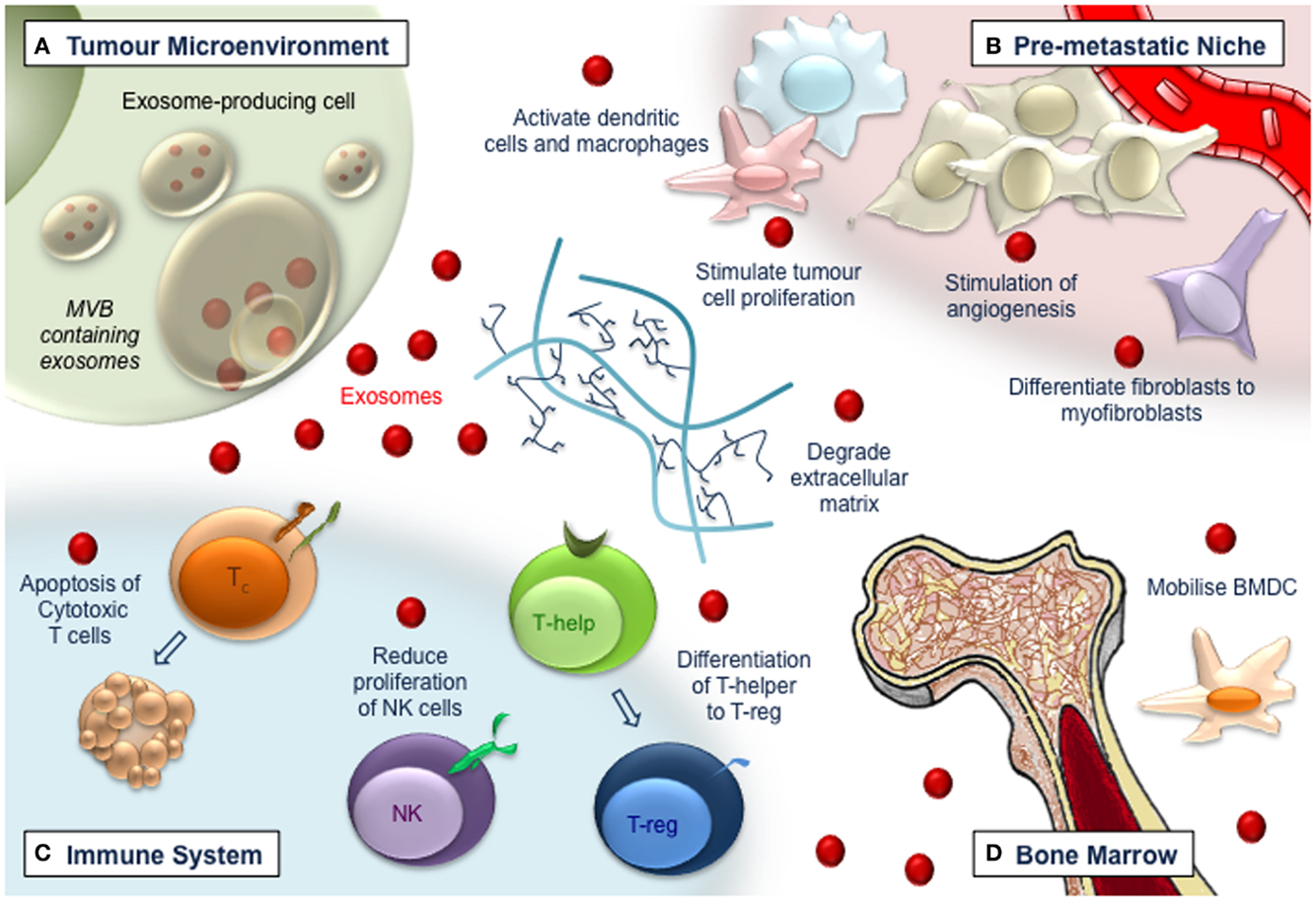
Figure 1. Exosomes (red dots) have multiple roles in tumorigenesis. (A) Exosomes released from tumor cells affect the local tumor microenvironment, remodeling extracellular matrix, and promoting vasculogenesis and tumor cell proliferation. (B) Exosomes travel to distant sites to promote the generation of the pre-metastatic niche. Vascularization is augmented and endothelial and stromal cell differentiation is induced, leading to a pro-tumor environment. (C) Immune responses become deregulated in a manner that impedes tumor recognition and anti-tumor immune functions. Cytotoxic T-cells are induced to apoptose, while NK cell proliferation is impaired and T-helper cells differentiate toward a T-regulatory cell phenotype. (D) Bone-marrow-derived cells are recruited to tumor and pre-tumor tissue where they contribute to cancer development.
The Exosomal Protein Cargo
Proteins trafficked by exosomes from both patient samples and in vitro cell lines have been studied in considerable detail. Studies show exosomes contain a vast array of proteins including membrane trafficking and cytoskeletal proteins, major histocompatibility complexes, signal transducers, heat-shock proteins, as well as many others (13). To date over 4,000 different proteins have been identified from purified exosomes (14). Some of these proteins are universal to most exosomes, including Tumor suppressor gene 101 (Tsg101), Heat-shock protein 70 (Hsp70), and the tetraspanins CD9, CD63, and CD81, while others seem to be specific to cell and tissue type. Some cargos suggest a role for exosomes in transformation with the identification of factors involved in cell cycle regulation, p53, epidermal growth factor (EGF), and fibroblastic growth factor (FGF) signaling, as well as angiogenesis.
The cargo of exosomes is particularly interesting as exosomes excreted from one cell are known to be able to fuse with surrounding cells, and thus have the potential to initiate signaling responses (15). This is of particular relevance in tumorigenesis, as both surrounding and distant tissues are known to adopt characteristics of the primary tumor. Many studies have now demonstrated the pertinence of exosomal proteins in cell function with particular interest paid to the role of these proteins in cancer. These proteins include the MET oncoprotein, mutant KRAS, and Tissue Factor, which are known to promote proliferation and coagulation, important processes in tumor formation. (16–18). Interestingly, the tumor environment itself may promote exosome up-take by cells. The double-layer lipid membrane structure of exosomes can change composition and rigidity in response to decreasing pH, a characteristic common to the hypoxic tumor microenvironment (15). This change in lipid composition was predicted to increase the ability of exosomes to fuse with neighboring cells. These data provide the intriguing possibility that exosomes are critical components of our signaling networks that function in the disease state to negatively affect the surrounding cellular environment.
Nucleic Acid Trafficking via Exosomes
Tumor-derived exosomes have been shown to contain a wide range of nucleic acids, with most studies investigating exosomal microRNA or messenger RNA (mRNA). Exosomes containing these nucleic acids have been shown to be able to transfer their cargo to the recipient cells and to induce phenotypic changes (19–21). Research has revealed that tumor-derived exosomes contain distinct microRNA profiles in many cancers, including prostate (22), lung (23), breast (24), and ovarian (1). Several studies have also demonstrated the transfer of onco-microRNAs to target cells, with these microRNAs capable of modulating target pathways in host tissue (25–28). These studies have shown that oncogenic functions of microRNA may arise from the expression of both pro- and anti-tumor microRNAs. Combined with the plethora of recent work on microRNAs, it is almost certain that exosomes are fundamentally required for microRNA transport and signaling, a process particularly important in cancer.
The functional transfer of mRNA and DNA in nanovesicles has also been observed experimentally. Analysis revealed exosomes contained mutated mRNA transcripts and DNA fragments, which may contribute to growth and proliferation of many primary and metastatic cancers (29–32). A body of research has now demonstrated that tumor-derived exosomes contain a range of nucleic acids that can induce cellular responses in recipient transformed or untransformed cells. Although studies have demonstrated that many cancers express distinct RNA profiles within exosomes and that these profiles often reflect disease state, the subtle and collaborative effects of microRNA signaling and function become complicated when combined with diverse signaling potential of the other factors contained within those exosomes.
Metastatic Niche Formation Requires Exosomes
The metastatic spread of tumor cells from the primary site, to other tissues within the body, is the primary cause of cancer mortality, but remains the most poorly understood aspect of carcinogenesis. Continuing to build on Paget’s (33) “seed and soil hypothesis,” the concept of metastatic niche formation at secondary sites is well established, thanks to an important body of work demonstrating distinct oncogenic changes including extracellular matrix restructuring and the recruitment of pro-tumorigenic factors at future metastatic sites (33). Normal cells within the microenvironment of the tumor are known to influence metastatic behavior, and there is evidence demonstrating that the successful formation of a metastatic deposit depends on the prior priming of the site for future metastatic growth (34).
It is now well accepted that primary tumor cells release a variety of cytokines and growth factors that firstly mobilize bone-marrow-derived cells and then recruit them to the site of future metastasis, creating the permissive environment for incoming tumor cells, that is called the pre-metastatic niche (10, 34). In addition to the release of soluble mediators as individual molecules, tumor cells release exosomes containing complex mixtures of these molecules, including many known effectors of tumorigenesis (13). Several studies suggest that exosomes educate bone-marrow-derived cells recruited to the pre-metastatic niche, to induce a phenotype that supports tumor cell metastasis. It was recently reported that MET receptor positive exosomes from highly metastatic melanomas could reprogram BMDCs in lung to a pro-vasculogenic phenotype (35). Early events in pre-metastatic niche formation have also been associated with exosomes, including enhanced lung endothelial permeability, proliferation, and angiogenesis, which are known contributors to tumor formation (36–38).
In vivo tracking of labeled melanoma exosomes injected intravenously into mice has shown that circulating exosomes are rapidly taken up from the systemic circulation into tissues, including the liver, lung, kidney, and spleen (39). Exosomes derived from renal cancer stem cells were found to contain a range of pro-tumor factors, and mice injected with these exosomes had significantly increase levels of lung cancer metastases (40). These studies therefore support a hypothesis that in addition to the influence of exosomes and their cargoes on endothelial cells within the pre-metastatic niche, other resident cells including fibroblasts and immune cells may also be stimulated to create the environment required for successful tumor cell metastasis.
Fibroblasts are a cell-type involved in the host-tumor cell interaction and more recently the activation of fibroblasts to a myofibroblastic phenotype has been associated with exosomes and has been described as a key event in the formation of the pre-metastatic niche (41–43). Myofibroblasts are often enriched in the altered stromal environment of many solid cancers, where they are known to support tumor growth, vascularization and metastasis. Modulation of the extracellular matrix has also been attributed directly to the cargo of exosomes and this may augment tumor cell invasion within the stroma of the pre-metastatic niche. Several studies have reported the deregulation of the extracellular matrix by cancer exosomes carrying factors such as glycoproteins and metalloproteinases (44–48). Exosomes that contribute to development of the pre-metastatic niche may also originate from healthy cells within the niche itself, prior to arrival of tumor cells and/or tumor-derived exosomes (49). Although the complex interplay that dictates the generation of the in vivo metastatic niche is yet to be fully elucidated, there is no doubt that exosomes, secreted from both healthy and cancerous cells, are necessary mediators of niche formation. Much data now suggests that exosomes contribute to niche formation through deregulation of host immune responses targeted toward the tumors.
Exosomes Regulate Tumor Immune Responses
As well as conditioning the metastatic niche through interaction with the stromal and matrix microenvironment, exosomes display the ability to regulate immune responses targeted toward tumor cells. A significant collection of studies has explored exosome interactions with, and production by, immune cells (50, 51). Exosomes possess the ability to disable the cytotoxic arm of immune response by inducing apoptosis in cytotoxic T-cells and reducing proliferation of Natural Killer (NK) cells (12, 52). Data also indicated that tumor-derived exosome-induced differentiation of T-helper cells to regulatory T-cells, indicating a possible mechanism of evading immune surveillance, as regulatory T-cells mediate immune tolerance to tumors, by regulating tolerance of self-antigens. It has also been demonstrated that NK cells were unable to be activated, in response to Interleukin 2-mediated blocking by tumor-derived exosomes, and that tumor-derived exosomes suppress T-cells via induction of adenosine (53, 54). Apoptosis of T-cells was also found to be inducible with exosomes from melanosomes containing Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand (55, 56). Additionally, tumor-derived exosomes prepared from the ascites of ovarian adenocarcinoma were found to have immunosuppressive properties, through the down-regulation of the expression of T-cell activation signaling components (57).
Exosomes have also been shown to generate anti-tumor immune responses. NK cells release exosomes containing perforin and CD56, as well as granzyme B that have been shown to inhibit tumor growth (58, 59). Due to the contradictory tumor-promoting and anti-tumor responses more research is required to determine if exosome stimulation skews the balance toward a tumor immune escape mechanism. The most probable cause for discrepancies in studies of exosomes and immune responses is due to the inherent differences between in vitro and in vivo analyses, as accounting for the complex interplay between tumor and immune responses over the course of disease has always proved problematic. It is likely that disease progression encompasses stages where exosomes induce either/both immunostimulatory and – inhibitory functions toward tumor cells. The balance between these two responses is one of the most important factors dictating disease progression, and may also provide a mechanism by which exosomal function could be regulated for the generation of novel therapies.
Exosomes in Cancer Therapy
The use of exosomes as a vehicle for the administration of anti-tumor compounds, either as cell-derived material or therapeutic drug, has garnered considerable attention of late. Exosomes represent bioavailable vehicles that are well tolerated, bioavailable, targetable to specific tissues, resistant to metabolic processes, and membrane-permeable. This makes exosomes ideal candidates for delivery of drugs, proteins, microRNA/silent interfering RNA (siRNA), and other molecules, that would otherwise be rapidly degraded. The potential therapeutic targeting of exosomes could take a number forms, some targeting, or modulating the intrinsic effect of exosomes in the prevention of tumorigenesis or metastasis, via interactions with tumor cells, stromal tissue, and immune cells. Other strategies aim to use exosomes to generate therapeutic effects, through their use as a vehicle for the delivery of anti-tumor agents, or priming of immune responses.
The removal of exosomes from the circulatory system is an attractive therapeutic option in mitigating the metastatic effect of exosomes. Data has shown that prevention of exosome production can inhibit tumorigenesis and a range of methods have been suggested for the inhibition of exosome production, including the targeting of microtubule assembly and stability, endosomal sorting pathways, and the use of proton pump inhibitors (6, 15, 60–62). It has also been suggested that the use of extracorporeal purification already utilized to reduce viral titers in patients may be useful as a mechanism for removal of exosomes from circulation (61). Although these processes have been shown to be effective, therapies based on modulating levels or the removal of exosomes face technical and financial challenges and are yet to be implemented clinically.
Another therapeutic avenue involves the use of exosomes to efficiently deliver cargo such as drugs, microRNA’s, and antigens to target recipient cells in order to treat tumorigenesis or metastasis. The potential use of exosomes to deliver targeted chemotherapeutics has been investigated in a number of studies (63, 64). Exosomes may also provide an opportunity to deliver tissue-targeted siRNA and microRNA’s to regulate gene expression within target cells (65, 66). siRNA containing exosomes have also been shown to cross the blood brain barrier, targeting neuronal cells and knocking down their target protein by more than 60% with little toxicity (67).
Investigation has also revealed that exosomes may be an effective option for the delivery of tumor-derived antigens, to elicit an immune response (65–67). The immunostimulatory potential of exosomes was first revealed 15 years ago, when exosomes secreted from dendritic cells were found to contain functional major histocompatibility complexes that could present tumor antigen to T-cells and induce anti-tumor immune responses in mice (68). These promising results, led to Phase I clinical trials that have demonstrated that anti-tumor immune responses can be induced using dendritic-cell-derived exosomes (69–71). This has been further extended using peptide-loaded dexosomes (dendritic-cell-derived exosomes) as a cancer vaccine and is now in Phase II clinical trials.
Exosomes as Cancer Biomarkers
The need for accurate novel biomarkers is of primary importance in the detection, diagnosis, and prognosis of patients, and the burgeoning area of exosomal biomarkers shows significant potential, particularly in cancer. Exosomes may provide an excellent biomarker to monitor the emergence, progression, and prognosis of cancer, as well as the efficacy of treatment regimes. Although few, studies have revealed exosomes can be readily detected in tumor tissue and many bodily fluids, and can be found in higher concentrations, both in tumor tissue, and the serum and plasma of cancer patients (1, 72, 73). The fact that exosomes display individualized expression, that are often reflective of disease state, and can be easily detected in bodily fluids, even after extended cryo-storage, make these small nanovesicles an ideal candidate for a non-invasive biomarker of tumor progression. Along with many studies characterizing possible exosome biomarkers in cell lines, there are several reports that have identified potential exosomal biomarkers in patient samples.
The presence of nucleic acid in exosomes has been described as a biomarker in glioblastoma patients via the identification of the disease-specific EGF receptor transcript (29). In an analysis of ovarian cancer patients, eight microRNAs have been identified that can be used to distinguish between benign and malignant disease, while in melanoma patients exosomes contain high levels of the proteins Caveolin-1 and CD63 (1, 74). Other studies have described markers in exosomes from prostate cancer patients (75) and non-small cell lung cancer patients (76). Interestingly, analysis of the lipids composition of prostate cancer exosomes revealed certain lipid signatures that may also serve as candidate biomarkers (77). Though the analysis of exosomes for cancer biomarkers has revealed many possible candidates (particularly microRNA), none have demonstrated enough promise to be implemented clinically. Further investigation of the dynamic expressional profile of exosomal contents throughout tumor development and treatment, combined with improved collection methods, is needed before the clinical implementation of exosomes as a biomarker for either diagnosis or prognosis.
Exosomes and Chemotherapeutic Resistance
The acquisition of chemotherapeutic resistance is the major contributing factor to cancer mortality. While treatment regimes can be effective in the short term, the development of metastases that are refractory to radio- and chemotherapeutic treatments are common. Although this is characteristic of a wide range of cancers, the exact mechanisms by which the tumor can evade the therapeutic induction of apoptosis and develop resistance to anticancer drugs and therapies remain unclear. Data indicates that chemoresistance is likely due to a combination of processes, including increased detoxification of the drugs within the cellular environment, an increased efflux and a reduced accumulation of chemotherapeutic drugs within the cell and an increased tolerance to or repair of DNA lesions and resistance to pro-apoptotic signals (78, 79). However, the role of exosomes and nanovesicles in chemoresistance revolves around the sequestration, transport and expulsion of chemotherapeutic drugs within/from tumor cells (80, 81). Additionally, exosomes have been shown to deliver hepatic enzymes throughout the body, and given the ability of endothelial cells to up-take circulating exosomes, this may aid in the extra-hepatic detoxification of xenobiotic drugs (82).
Drug expulsion is a likely contributor to exosomal chemotherapeutic resistance. Analyses of vesicle shedding in response to drug treatment in numerous cancer cell lines revealed a consistent correlation between vesicle shedding-related gene expression, vesicle shedding rates, and drug sensitivity (83). Furthermore, vesicles isolated from these cell lines were shown to contain doxorubicin, a clinical chemotherapeutic to which tumors often develop resistance. Exosomes released from tumor cells have also been shown to contain the platinum-based chemotherapeutic Cisplatin, potentially redirecting the drug away from the nucleus where it would normally cause DNA damage, cell cycle arrest, and apoptosis (80, 81, 84).
Exosomes have also been shown to confer chemotherapeutic resistance to non-resistant cells. P-glycoprotein is an ATP-binding cassette transporter involved in multi-drug resistance in cancer (85), and has been observed transferring from drug-resistant cancer cells to recipient cells was via microparticles (86). Interestingly, Docetaxel resistance in hormone refractory prostate cancer cells can be acquired by non-invasive cell lines via exosomes. Cells then become prone to mobilization, invasion, proliferation, and anchorage dependent growth (87). Interestingly, analysis of exosomes from patient serum before and after undergoing treatment with Docetaxel showed correlation between cellular response to Docetaxel and patient response to treatment. A recent study identified another method by which exosomes may contribute to chemotherapeutic resistance. It was observed that exosomes released from cancer cells might impede antibody and drug therapies by expressing cancer derived cell surface proteins that sequester the compound away from the target cell (88, 89). Furthermore, exosomes have been shown to reduce antibody dependent cell cytotoxicity by binding to tumor reactive antibodies (90).
Concluding Remarks
The vast repertoire of proteins and nucleic acid that can be packaged within exosomes appears to reflect the extensive, diverse, and complex signaling potential of these nanovesicles. Only now are scientists beginning to unravel the complex roles of exosomes, and although both in vitro and in vivo data clearly demonstrate the tumor-modulating potential of exosomes, the extent to which these signaling pathways dictate tumorigenesis in patients is far from being fully understood. Regardless of the contribution, the driving force behind exosome research (the significant potential of exosomes as a non-invasive biomarker, as well as a method of drug delivery and chemotherapeutic sensitization) has lead to a substantial body of work investigating these messengers. In spite of this, there are still many technical challenges that need to be overcome before the provision of exosomes as a biomarker, biological target, or drug delivery vehicle. Exosome concentrations, though increased in cancer, are relatively tiny, and methods of exosome isolation tend to be time-consuming and can be expensive while yielding samples that require further downstream purification (though these tendencies will wane with the improving technologies that yield enriched samples via affinity capture methods). Furthermore, purification techniques may serve to enrich exosomes subpopulations, often by their surface-expressed antigens or by density, which will significantly affect results. This problem may be confounded by discrepancies commonly observed in “exosome” studies, as isolation methods and terminology can differ considerably. Perhaps the greatest challenge in the investigation of exosome function is understanding the balance between healthy and oncogenic exosomal signaling, the degree to which cancer exosomes corrupt or ablate healthy exosome signaling, and the extent to which these interactions dictate metastasis over the course of disease. What makes this particularly challenging is the complex and multifunctional nature of exosomal signaling. The ability of exosomes to concurrently/concomitantly and simultaneously signal via many forms of cellular material (proteins, RNA, and lipids) makes functional analysis difficult, and this signaling method is the primary difference between exosomal signaling and other pathways (via the secretion or regulated transport individual moieties). Again, this is complicated by the fact that exosomes have been shown to possess both anti- and pro-tumor effects. To fully appreciate the signaling potential of exosomes, studies will need to investigate the co-contribution of proteins, nucleic acids, and lipids to the observed phenotype. Dissecting and revealing the contributions of each exosomal component, and modifying this intricate signaling pathway to elicit the required therapeutic response, will undoubtedly prove the most significant challenge in the utilization of exosomes as biomarkers and drug targets. Nonetheless, these significant challenges are being undertaken by many groups showing keen scientific interest in these tumor-derived exosomes and their multiple roles and functions. It is for these reasons, and the many diverse reasons discussed in this article, that exosomes may prove to be the most useful biological effector so far identified in cancer, and may finally provide viable treatment options and biomarkers.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol (2008) 110(10):13–21. doi: 10.1016/j.ygyno.2008.04.033
2. Rabinowits G, Gerçel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer (2009) 10(1):42–6. doi:10.3816/CLC.2009.n.006
3. Tavoosidana G, Ronquist G, Darmanis S, Yan J, Carlsson L, Wu D, et al. Multiple recognition assay reveals prostasomes as promising plasma biomarkers for prostate cancer. Proc Natl Acad Sci U S A (2011) 108(21):8809–14. doi:10.1073/pnas.1019330108
4. Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol (2002) 2(8):569–79. doi:10.1038/nri855
5. Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett (2006) 107(2):102–8. doi:10.1016/j.imlet.2006.09.005
6. Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ (2007) 15(1):80–8. doi:10.1038/sj.cdd.4402237
7. Yang C, Robbins PD. The roles of tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol (2011) 2011:1–11. doi:10.1155/2011/842849
8. Azorsa DO. The genomic and proteomic content of cancer cell-derived exosomes. Front Oncol (2012) 2:38. doi:10.3389/fonc.2012.00038/abstract
9. Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer (2009) 9(4):285–93. doi:10.1038/nrc2621
10. Sceneay J, Smyth MJ, Moller A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev (2013) 32(3–4):449–64. doi:10.1007/s10555-013-9420-1
11. Zhang H-G, Grizzle WE. Exosomes and cancer: a newly described pathway of immune suppression. Clin Cancer Res (2011) 17(5):959–64. doi:10.1158/1078-0432.ccr-10-1489
12. Whiteside TL. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes). Biochem Soc Trans (2013) 41(1):245–51. doi:10.1042/bst20120265
13. Kharaziha P, Ceder S, Li Q, Panaretakis T. Tumor cell-derived exosomes: a message in a bottle. Biochim Biophys Acta (2012) 1826(1):103–11. doi:10.1016/j.bbcan.2012.03.006
14. Mathivanan S, Simpson RJ. ExoCarta: A Compendium of Exosomal Proteins and RNA. (2011). [cited 2013 Feb 16]. Available from: http://exocarta.org/index.html
15. Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem (2009) 284(49):34211–22. doi:10.1074/jbc.M109.041152
16. Park JA, Sharif AS, Tschumperlin DJ, Lau L, Limbrey R, Howarth P, et al. Tissue factor-bearing exosome secretion from human mechanically stimulated bronchial epithelial cells in vitro and in vivo. J Allergy Clin Immunol (2012) 130(6):1375–83. doi:10.1016/j.jaci.2012.05.031
17. Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics (2013) 12(2):343–55. doi:10.1074/mcp.M112.022806
18. Ji H, Greening DW, Barnes TW, Lim JW, Tauro BJ, Rai A, et al. Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics (2013) 13(10–11):1672–86. doi:10.1002/pmic.201200562
19. Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia (2006) 20(5):847–56. doi:10.1038/sj.leu.2404132
20. Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood (2007) 110(7):2440–8. doi:10.1182/blood-2007-03-078709
21. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol (2007) 9(6):654–9. doi:10.1038/ncb1596
22. Hessvik NP, Phuyal S, Brech A, Sandvig K, Llorente A. Profiling of microRNAs in exosomes released from PC-3 prostate cancer cells. Biochim Biophys Acta (2012) 1819(11–12):1154–63. doi:10.1016/j.bbagrm.2012.08.016
23. Rosell R, Wei J, Taron M. Circulating microRNA signatures of tumor-derived exosomes for early diagnosis of non-small-cell lung cancer. Clin Lung Cancer (2009) 10(1):8–9. doi:10.3816/CLC.2009.n.001
24. Corcoran C, Friel AM, Duffy MJ, Crown J, O’Driscoll L. Intracellular and extracellular microRNAs in breast cancer. Clin Chem (2011) 57(1):18–32. doi:10.1373/clinchem.2010.150730
25. Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One (2010) 5(10):e13247. doi:10.1371/journal.pone.0013247.t001
26. Fernandez-Medarde A, Santos E. Ras in cancer and developmental diseases. Genes Cancer (2011) 2(3):344–58. doi:10.1177/1947601911411084
27. Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology (2011) 54(4):1237–48. doi:10.1002/hep.24504
28. Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest (2013) 123(4):1542–55. doi:10.1172/jci66517
29. Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Curry WT, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol (2008) 10(12):1470–6. doi:10.1038/ncb1800
30. Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A (2009) 106(10):3794–9. doi:10.1073/pnas.0804543106
31. Dvorakova M, Karafiat V, Pajer P, Kluzakova E, Jarkovska K, Pekova S, et al. DNA released by leukemic cells contributes to the disruption of the bone marrow microenvironment. Oncogene (2012) 32(44):5201–9. doi:10.1038/onc.2012.553
32. Huan J, Hornick NI, Shurtleff MJ, Skinner AM, Goloviznina NA, Roberts CT Jr, et al. RNA trafficking by acute myelogenous leukemia exosomes. Cancer Res (2013) 73(2):918–29. doi:10.1158/0008-5472.can-12-2184
33. Paget S. The distribution of secondary growths in cancer of the breast. Lancet (1889) 133(3421):571–3. doi:10.1016/S0140-6736(00)49915-0
34. Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature (2005) 438(7069):820–7. doi:10.1038/nature04186
35. Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med (2012) 18(6):883–91. doi:10.1038/nm.2753
36. Hong B, Cho J-H, Kim H, Choi E-J, Rho S, Kim J, et al. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics (2009) 10:556. doi:10.1186/1471-2164-10-556
37. Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CE, et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood (2010) 116(13):2385–94. doi:10.1182/blood-2009-08-239228
38. Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res (2011) 71(11):3792–801. doi:10.1158/0008-5472.can-10-4455
39. Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol (2013) 165(2):77–84. doi:10.1016/j.jbiotec.2013.03.013
40. Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res (2011) 71(15):5346–56. doi:10.1158/0008-5472.can-11-0241
41. De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer (2008) 123(10):2229–38. doi:10.1002/ijc.23925
42. Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res (2010) 70(23):9621–30. doi:10.1158/0008-5472.CAN-10-1722
43. Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol (2012) 40(1):130–8. doi:10.3892/ijo.2011.1193
44. Stoeck A, Keller S, Riedle S, Sanderson MP, Runz S, Le Naour F, et al. A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD44. Biochem J (2006) 393(Pt 3):609–18. doi:10.1042/bj20051013
45. Runz S, Keller S, Rupp C, Stoeck A, Issa Y, Koensgen D, et al. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol (2007) 107(3):563–71. doi:10.1016/j.ygyno.2007.08.064
46. Jung T, Castellana D, Klingbeil P, Cuesta Hernandez I, Vitacolonna M, Orlicky DJ, et al. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia (2009) 11(10):1093–105. doi:10.1593/neo.09822
47. Nieuwland R, van der Post JA, Lok CA, Kenter G, Sturk A. Microparticles and exosomes in gynecologic neoplasias. Semin Thromb Hemost (2010) 36(8):925–9. doi:10.1055/s-0030-1267046
48. Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang Q, et al. Activated T cell exosomes promote tumor invasion via Fas signaling pathway. J Immunol (2012) 188(12):5954–61. doi:10.4049/jimmunol.1103466
49. Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell (2012) 151(7):1542–56. doi:10.1016/j.cell.2012.11.024
50. Filipazzi P, Burdek M, Villa A, Rivoltini L, Huber V. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol (2012) 22(4):342–9. doi:10.1016/j.semcancer.2012.02.005
51. Gutierrez-Vazquez C, Villarroya-Beltri C, Mittelbrunn M, Sanchez-Madrid F. Transfer of extracellular vesicles during immune cell-cell interactions. Immunol Rev (2013) 251(1):125–42. doi:10.1111/imr.12013
52. Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol (2009) 183(6):3720–30. doi:10.4049/jimmunol.0900970
53. Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol (2006) 176(3):1375–85. doi:10.4049/jimmunol.176.3.1375
54. Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol (2011) 187(2):676–83. doi:10.4049/jimmunol.1003884
55. Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med (2002) 195(10):1303–16. doi:10.1084/jem.20011624
56. Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology (2005) 128(7):1796–804. doi:10.1053/j.gastro.2005.03.045
57. Taylor DD, Gercel-Taylor C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer (2005) 92(2):305–11. doi:10.1038/sj.bjc.6602316
58. Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res (2005) 65(12):5238–47. doi:10.1158/0008-5472.can-04-3804
59. Lugini L, Cecchetti S, Huber V, Luciani F, Macchia G, Spadaro F, et al. Immune surveillance properties of human NK cell-derived exosomes. J Immunol (2012) 189(6):2833–42. doi:10.4049/jimmunol.1101988
60. Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res (2012) 72(19):4920–30. doi:10.1158/0008-5472.can-12-0925
61. Marleau AM, Chen CS, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med (2012) 10:134. doi:10.1186/1479-5876-10-134
62. Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev (2013) 32(3–4):623–42. doi:10.1007/s10555-013-9441-9
63. Tan A, Rajadas J, Seifalian AM. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv Drug Deliv Rev (2013) 65(3):357–67. doi:10.1016/j.addr.2012.06.014
64. Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev (2013) 65(3):336–41. doi:10.1016/j.addr.2012.07.001
65. Wahlgren J, De LKT, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res (2012) 40(17):e130. doi:10.1093/nar/gks463
66. el-Andaloussi S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov (2013) 12(5):347–57. doi:10.1038/nrd3978
67. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol (2011) 29(4):341–5. doi:10.1038/nbt.1807
68. Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med (1998) 4(5):594–600. doi:10.1038/nm0598-594
69. Escudier B, Dorval T, Chaput N, André F, Caby M-P, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med (2005) 3(1):1–13. doi:10.1186/1479-5876-3-10
70. Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med (2005) 3(1):9. doi:10.1186/1479-5876-3-9
71. Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther (2008) 16(4):782–90. doi:10.1038/mt.2008.1
72. Chen H-Y, Yu S-L, Chen C-H, Chang G-C, Chen C-Y, Yuan A, et al. A five-gene signature and clinical outcome in non-small-cell lung cancer. N Eng J Med (2007) 356(1):11–20. doi:10.1056/NEJMoa060096
73. Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol (2009) 20(2):363–79. doi:10.1681/ASN.2008040406
74. Logozzi M, De Milito A, Lugini L, Borghi M, Calabro L, Spada M, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One (2009) 4(4):e5219. doi:10.1371/journal.pone.0005219
75. Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, et al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer (2009) 100(10):1603–7. doi:10.1038/sj.bjc.6605058
76. Li Y, Zhang Y, Qiu F, Qiu Z. Proteomic identification of exosomal LRG1: a potential urinary biomarker for detecting NSCLC. Electrophoresis (2011) 32(15):1976–83. doi:10.1002/elps.201000598
77. Llorente A, Skotland T, Sylvanne T, Kauhanen D, Rog T, Orlowski A, et al. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta (2013) 1831(7):1302–9. doi:10.1016/j.bbalip.2013.04.011
78. Gatti L, Zunino F. Overview of tumor cell chemoresistance mechanisms. Methods Mol Med (2005) 111:127–48. doi:10.1385/1-59259-889-7:127
79. Fodale V, Pierobon M, Liotta L, Petricoin E. Mechanism of cell adaptation: when and how do cancer cells develop chemoresistance? Cancer J (2011) 17(2):89–95. doi:10.1097/PPO.0b013e318212dd3d
80. Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, et al. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther (2005) 4(10):1595–604. doi:10.1158/1535-7163.mct-05-0102
81. Federici C, Petrucci F, Caimi S, Cesolini A, Logozzi M, Borghi M, et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS One (2014) 9(2):e88193. doi:10.1371/journal.pone.0088193
82. Conde-Vancells J, Gonzalez E, Lu SC, Mato JM, Falcon-Perez JM. Overview of extracellular microvesicles in drug metabolism. Expert Opin Drug Metab Toxicol (2010) 6(5):543–54. doi:10.1517/17425251003614766
83. Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res (2003) 63(15):4331–7.
84. Yin J, Yan X, Yao X, Zhang Y, Shan Y, Mao N, et al. Secretion of annexin A3 from ovarian cancer cells and its association with platinum resistance in ovarian cancer patients. J Cell Mol Med (2012) 16(2):337–48. doi:10.1111/j.1582-4934.2011.01316.x
85. Roninson IB. Molecular mechanism of multidrug resistance in tumor cells. Clin Physiol Biochem (1987) 5(3–4):140–51.
86. Bebawy M, Combes V, Lee E, Jaiswal R, Gong J, Bonhoure A, et al. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia (2009) 23(9):1643–9. doi:10.1038/leu.2009.76
87. Corcoran C, Rani S, O’Brien K, O’Neill A, Prencipe M, Sheikh R, et al. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One (2012) 7(12):e50999. doi:10.1371/journal.pone.0050999
88. Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M, et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci U S A (2011) 108(37):15336–41. doi:10.1073/pnas.1102855108
89. Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol (2012) 227(2):658–67. doi:10.1002/jcp.22773
Keywords: exosome, cancer, metastatic niche, chemotherapeutic resistance, biomarker
Citation: Tickner JA, Urquhart AJ, Stephenson S-A, Richard DJ and O’Byrne KJ (2014) Functions and therapeutic roles of exosomes in cancer. Front. Oncol. 4:127. doi: 10.3389/fonc.2014.00127
Received: 25 March 2014; Accepted: 13 May 2014;
Published online: 27 May 2014.
Edited by:
Carmen Garrido, Institut National de la Santé et de la Recherche Médicale, FranceReviewed by:
Gabriele Multhoff, Technische Universität München, GermanyStefano Fais, Istituto Superiore di Sanità, Italy
Copyright: © 2014 Tickner, Urquhart, Stephenson, Richard and O’Byrne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kenneth J. O’Byrne, Queensland University of Technology, Translational Research Institute, 37 Kent Street, Woolloongabba, Brisbane, QLD 4102, Australia e-mail: k.obyrne@qut.edu.au
 Jacob A. Tickner
Jacob A. Tickner Aaron J. Urquhart
Aaron J. Urquhart Sally-Anne Stephenson
Sally-Anne Stephenson Derek J. Richard
Derek J. Richard Kenneth J. O’Byrne
Kenneth J. O’Byrne