Modulation effect of sulfated polysaccharide from Sargassum fusiforme on gut microbiota and their metabolites in vitro fermentation
- SKL of Marine Food Processing and Safety Control, National Engineering Research Center of Seafood, Collaborative Innovation Center of Seafood Deep Processing, National and Local Joint Engineering Laboratory for Marine Bioactive Polysaccharide Development and Application, Liaoning Key Laboratory of Food Nutrition and Health, School of Food Science and Technology, Dalian Polytechnic University, Dalian, China
The present study demonstrated the digestion behavior and fermentation characteristics of a sulfated polysaccharide from Sargassum fusiforme (SFSP) in the simulated digestion tract environment. The results showed that the molecular weight of two components in SFSP could not be changed by simulated digestion, and no free monosaccharide was produced. This indicates that most of SFSP can reach the colon as prototypes. During the fermentation with human intestinal flora in vitro, the higher-molecular-weight component of SFSP was utilized, the total sugar content decreased by 16%, the reducing sugar content increased, and the galactose content in monosaccharide composition decreased relatively. This indicates that SFSP can be selectively utilized by human intestinal flora. At the same time, SFSP also changed the structure of intestinal flora. Compared with the blank group, SFSP significantly increased the abundance of Bacteroidetes and decreased the abundance of Firmicutes. At the genus level, the abundances of Bacteroides and Megamonas increased, while the abundances of Shigella, Klebsiella, and Collinsella decreased. Moreover, the concentrations of total short-chain fatty acids (SCFAs), acetic, propionic and n-butyric acids significantly increased compared to the blank group. SFSP could down-regulate the contents of trimethylamine, piperidone and secondary bile acid in fermentation broth. The contents of nicotinic acid, pantothenic acid and other organic acids were increased. Therefore, SFSP shows significant potential to regulate gut microbiota and promote human health.
1 Introduction
Bioactive polysaccharides derived from various natural resources have been proven to have prebiotic properties (1–3). In recent years, the fermentation of polysaccharides by gut microbiota has received increasing attention due to its benefits on host health, such as preventing cancer (4), improving lipid metabolism (5), and affecting gut microbiota (6). As is well known, the biological activity of bioactive components is closely related to the degradation and absorption in the digestive system. Some reports have shown that bioactive polysaccharides can go through the gastrointestinal tract and reach the distal end of the gastrointestinal tract. It has been well documented that various of indigestible polysaccharides could be degraded by gut microbes, such as species from Bacteroides (7). Moreover, sugars released from indigestible polysaccharides in the fermentation by some intestinal bacteria often support microbial growth and survival in the gut, and further regulate the gut microbial metabolism (8). Notably, the metabolites of gut microbiota play an important role in improving colon health, and further benefit the host health. In addition to short chain fatty acids (1, 5, 9), other microbial metabolites in gut could also regulate the host physiological state, such as trimethylamine (10) and secondary bile acids (11). Thus, these indigestible polysaccharides can alter the structure and metabolism of gut microbiota to promote the health of host through gut-brain axis, gut-liver axis, gut-lung axis, etc.
Sargassum fusiforme is a brown alga belonging to the Sargasaceae family and it is used as a drug in traditional Chinese medicine to treat diseases, such as tumor, scrofula, edema, beriberi, and chronic bronchitis (12, 13). Nowadays, large-scale aquaculture of S. fusiforme has been carried out in the coastal areas of Zhejiang and Fujian in China, with an annual output of nearly 30 thousand tons. The chemical composition of sulfated polysaccharides from S. fusiforme (SFSP) extracted by different methods varies slightly, but they mainly contain three monosaccharides, fucose, galactose, and mannose. In addition, it also contains a certain amount of uronic acid and sulfate groups, the sulfation mainly occurred at C2 or C4 of the fucose residue and C2, C4 or C6 of the galactose residue (14–16). Through methylation analysis and NMR analysis of purified components, it was shown that SFSP had the following repeating units of →2)-α-D-Man-(1 → 4)-β-D-GlcA-(1 → , → 3)-β-L-Fuc-(1 → 3,4)-β-L-Fuc-(1 → 3,4)-β-L-Fuc-(1 → and →3,4)-β-L-GlcA-(1→, →4)-β-L-Xyl-(1→, →4)-β-L-Gal-(1→, →3,6)–β-L-Manp-(1→ (14, 17, 18). Modern pharmacological research revealed that SFSPhas multiple bioactivities. SFSP could promote the immune responses in macrophages via inducing the CD14/IKK/NF-κB and P38/NF-κB signaling pathways (19), and it could have the ability to resist oxidative stress damage induced by lipopolysaccharides in cells and inhibit tumor angiogenesis (4). Moreover, Cheng reported that SFSP could alleviate HFD-induced early fasting hypoglycemia and regulate the structure of gut microbiota (20). However, so far, there has been relatively little research on the digestion and fermentation characteristics of sulfated polysaccharides from Sargassum fusiforme.
The present study aimed to reveal the digestion behavior and fermentation characteristics of SFSP in the digestion tract. It demonstrated the digestion behavior of SFSP by monitoring the change of molecular weight and the release of free monosaccharides in simulated digestion. Then the sugar content, monosaccharide composition and the production of short chain fatty acids were monitored in the fermentation of SFSP by human gut microbiota. Moreover, the regulation effect of SFSP microbiota composition was evaluated and the changes in metabolites of gut microbiota were also determined by ultra performance liquid chromatography-time of flight mass spectrometry (UPLC-TOF-MS). The findings of the present study could promote the exploration and utilization of SFSP and S. fusiforme in industries of foods and pharmaceuticals.
2 Materials and methods
2.1 Materials and chemicals
Brown seaweed Sargassum fusiforme was collected in July 2019 from Wenzhou, China. The dextrans (1, 5, 25, 50, 41 kDa) and monosaccharides (D-glucose, D-galactose, D-mannose, D-rhamnose, D-xylose, L-fucose, D-arabinose, D-glucuronic acid, D-galactose acid, D-Galactosamine, D-Glucosamine) used as standards for High performance liquid chromatography (HPLC) were purchased from sigma Chemical Co., Ltd. (St. Louis, MO, United States). The SCFAS (Acetic acid, propionic acid, butyric acid), digestive enzymes (Gastric lipase, pepsin, pancreatin, and trypsin) and ammonium acetate were purchased from Aladdin Industrial Inc. (Shanghai, China). High performance liquid chromatography grade solvents including acetonitrile, chloroform, methyl alcohol and formic acid were purchased from Spectrum Chemical (New Brunswick, Canada). Other analytical grade chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2 Preparation of SFSP
The preparation of SFSP was performed according to the previously reported method with some modifications (5). Briefly, the seaweed Sargassum fusiforme was washed, air-dried, smashed and mixed with deionized-water at 50°C for 30 min and the ratio of solid to liquid was 1: 20. The enzymatic hydrolysis process was implemented by using 1% cellulase, pectinase and papain (4: 1: 1, 620 U/g) at 50°C for 4 h. Then the solution was heated to 98°C to inactivate enzymes. After the centrifugation at 3000 rpm/min for 10 min, the supernatant was collected and concentrated to 1/10 of the original volume. Then a four-step ethanol precipitation was conducted to separate SFSP. Briefly, Firstly, ethanol was added to achieve a final proportion of 20%, and after standing for a night, the precipitate which mainly contained alginate was removed by configuration (10,000 rpm, 15 min, 4°C); Secondly, then the supernatant was collected and mixed with ethanol to achieve a final ethanol proportion of 60%, and the precipitate was collected by standing for a night and configuration (10,000 rpm, 15 min, 4°C); Thirdly, the precipitate was dissolved in hot water in a ratio of 1:10 (m/v), and then ethanol precipitation method was conducted again with an ethanol proportion of 30% to remove the residue alginate Fourthly, more ethanol was added to achieve a final proportion of 70%, and the precipitate was collected after configuration (10,000 rpm, 15 min, 4°C). The precipitate obtained by the four-step ethanol precipitation was dissolved in water (1.5%, m/v) and mixed with 2% CTAB solution (4:1, v/v). Then the resulting precipitate was dissolved in 3 M KCl, and SFSP was precipitated by adding ethanol with the final ethanol proportion of 70%. Finally, the resulting precipitate was dissolved in the deionized-water, dialyzed for 3 days to remove the salt, and lyophilized to obtain SFSP powder.
2.3 Characterization of SFSP
Total carbohydrate and uronic acid content in SFSP were measured using the phenol-sulphuric method and m-hydroxydiphenyl method (21, 22). Quantitative analysis of protein in SFSP was carried out according to the Bradford method (23). The sulfate group was quantified by the BaCl2-gelatin turbidimetric assay (24). The content of reducing sugar was measured by the method of DNS (25).
Fourier transform infrared spectroscopy (FTIR) assay of SFSP was determined via the Fourier transform infrared reflection (FTIR) Spectrometer (Perkin Elmer, Norwalk, United States) after pressed potassium bromide into tablet (2 mg of SFSP in 100 mg of KBr).
2.4 Simulated digestion
The fresh saliva was provided by four donors who had not been treated with antibiotic in the past 3 months. Then the saliva was mixed and centrifuged at 2500 g for 20 min and the supernatant was collected and used as oral digestion juices. 4 mL SFSP solution (2 mg/mL) was mixed with equal volume of oral juice for digestion in the water bath at 37°C for 2 h. The mixture of 4 mL deionized water and 4 mL oral digestive juice was used as blank control. Samples were collected at 0 h, 0.5 h, 1 h and 2 h and the enzyme was inactivated with a boiling water bath for 10 min for further analysis.
For simulated gastric and intestinal digestion, the simulated gastric juice and intestinal juice were prepared as previously described (26). The mixtures of 6 mL SFSP solution (4 mg/mL) and 6 mL gastric juice, and 6 mL deionized water and 6 mL gastric juice were subjected to simulated gastric digestion at 37°C and 150 rpm for 6 h in a constant temperature shaker. Samples were collected at 0 h, 1 h, 2 h, 4 h and 6 h and the enzyme was eliminated with a boiling water bath for 10 min. After gastric digestion, 4 mL solution was collected, neutralized with 1 M NaHCO3, and mixed with 4 mL intestinal digestive juice. Then the simulated intestinal digestion was performed with the constant temperature shaker for 6 h at 37°C and 150 rpm. Finally, samples of intestinal digestion were collected at 0 h, 1 h, 2 h, 4 h and 6 h and the enzyme was inactivated with boiling water for 10 min bath treatment. The molecular weight distribution and the released monosaccharides of samples at different times were measured according to the HPLC methods as we previously described (7).
2.5 In vitro fermentation of polysaccharides
Fecal samples were collected from 4 healthy volunteers (two males and two females, 18 ~ 24 years old) who had no history of intestinal diseases during last 3 months. Four equal fecal samples were mixed and dissolved in the saline solution containing 0.5 g/L cysteine-HCl (10%, w/v). 300 mL of the basal growth medium for in vitro fermentation was prepared by adding 0.6 g peptone, 0.6 g yeast extract, 0.006 g hemin, 0.15 g L-cysteine, 0.15 g bile salts, 0.03 g NaCl, 0.012 g K2HPO4, 0.012 g KH2PO4, 0.003 g MgSO4, 0.003 g CaCl2, 0.6 g NaHCO3, 0.3 mL resazurin solution (1%, w/v), 0.6 mL Tween-80, and 3 μL vitamin K. 500 mg SFSP was dissolved in 50 mL the basal growth medium and autoclaved for the next in vitro fermentation. 1.5 mL of fecal slurry was added to the culture medium containing polysaccharides and the basic culture medium as SFSP group and blank control group (CON), respectively. Each group had triple parallel experiment. Then, SFSP and CON group were transferred to an anaerobic incubator and fermented at 37°C for 48 h. The fermentation products of 0 h, 12 h, 24 h, and 48 h were collected and put into ice water for 5 min. Then these samples were centrifuged at 8000 g for 10 min and the supernatants were used for the further study.
2.6 Determination of pH and short chain fatty acids (SCFAs)
The pH values of supernatant samples were measured by a pH meter (S21 Seven compact, Mettler-Toledo instrument Co., Ltd., Shanghai, China). The contents of SCFAs were determined by a reported HPLC method with some modifications. In brief, 30 μL 10% sulfuric acid was added into 0.5 mL of the filtrate samples, and 2 mL ethyl ether was then added into the mixture to extract SCFAS for 15 min. After centrifugation (3,500 rpm, 10 min), the supernatant was collected and alkalized by adding 500 μL 1 M NaOH. Then the resulting aqueous phase was acidified with 10% sulfuric acid again. The aqueous samples were analyzed on HPLC system (e2695, Wasters, Milford, United States) equipped with a Silgreen ODS C-18 column (250 × 4.6 mm, 0.5 μm) and a photodiode array detector (PDA). The operating parameters of HPLC were as follows: column oven temperature, 30°C; mobile phase A, phoric acid solution (0.025%, v/v); mobile phase B, acetonitrile; the mobile phase ratio, 95: 5; flow rate, 1.0 mL/min; detector wavelength, 205 nm; and injection volume, 10 μL.
2.7 Analysis of gut microbiota
After in vitro fermentation of 48 h, bacterium cells of SFSP and CON groups were separated by centrifugation at 8000 g for 10 min from fermentation broth. The total bacterial DNA in each group was extracted by the Power Fecal DNA Isolation Kit (MO BIO, Carlsbad, USA). Agarose gel electrophoresis method was used to evaluate the DNA quality. The V3 regions of 16S rRNA was amplified with universal primers 515F and 806R by PCR assay. The QIAquick Gel Extraction Kit was used for purification of PCR products and then sequenced by the Illumina HiSeq 2,500 platform. Bioinformatics classification of species in the different levels were based on the operational taxonomic units (OTUS) and the sequences with similarity of ≥97% were classified into an OTU unit individually performed by RDP classifier Bayesian algorithm at Usearch (version 7.0 http://drive5.com/uparse/). The biological classification information of each operational taxonomic unit (OUT) unit was obtained by matching the Silva database (http://www.arb-silva.de).
2.8 Analysis of microbial metabolites
Micromolecules produced by microbial fermentation were extracted by adding acetonitrile to 70%. The precipitate was removed by centrifugation (10,000 g, 10 min) and the supernatant cultivated with 0.22 μm filter was analyzed for metabolites. Microbial metabolites detection was performed by using UPLC- MS. Separation was carried out on an UPLC system (Nexera LC-30A, Kyoto, Japan) equipped with an Xselect HSS T3 column (100 × 2.1 mm, 2.5 μm, Waters). Mobile phases A and B were water/acetonitrile (95:5, v/v) with 0.1% formic acid and acetonitrile, respectively. The gradient elution was as follows: 0 min 15% B; 0 ~ 5 min 20% B; 5 ~ 7 min 20% B; 7 ~ 14 min 100% B; 14 ~ 16 min 100% B; 16 ~ 16.1 min 20% B; 16.1 ~ 21 min 20% B. Untargeted metabolites mass detection was performed on a Triple TOF 5600 (AB SCIEX, Milwaukee, USA) in the electrospray ionization (ESI) (+) mode. The dry gas was 10 L/min at 350°C. The declustering potential, the collision energy, and the capillary voltage were 40 V, 10 V, and 5,500 V. Information dependent acquisition (IDA) Experiment mode was selected and the cycle time was 1.0011 s for 1,259 cycles during 21.007 min. Tuning Mix solution (AB SCIEX, Milwaukee, United States) was applied to the instrument calibration and formate solution of 10 mM sodium was used for auto internal calibration.
2.9 Statistical analysis
The data were reported as mean ± standard error of mean (SEM) using Student’s t-test for comparison or one-way ANOVA tests for multiple comparisons by SPSS version 9.0 software. The principal component analysis (PCA) was performed by MetaboAnalyst 4.0.
3 Results and discussion
3.1 Characterization of SFSP
SFSP was obtained from Sargassum fusiforme by enzymatic hydrolysis and stepwise ethanol precipitation followed by the quaternary ammonium salt precipitation. The total carbohydrate and uronic acid contents of SFSP were 39.25 ± 1.01% and 13.72 ± 0.30%, respectively. SFSP had a significantly higher sulfate content (34.39 ± 2.01%) than previously reported (14, 17) which could be attributed to the application of CTAB which could selectively adsorb acidic polysaccharides. The monosaccharide composition analysis showed that SFSP mainly contained fucose, galactose, mannose, rhamnose, glucuronic acid, glucose and xylose in a ratio of 55.39: 24.01: 8.09: 1.23: 9.07: 0.7: 1.51. The proportions of fucose and galactose in SFSP were similar to those of the polysaccharide extracted from Sargassum fusiforme previously by Cheng et al. (20), but the contents of glucuronic acid and the other neutral monosaccharides were different from Cheng’s report. Moreover, a small amount of protein (0.88 ± 0.05%) was detected in SFSP. From the high-performance gel permeation chromatography (HPGPC) chromatogram of SFSP in Figure 1A, two peaks were observed except for the salt peak. Based on the dextran standards, the molecular weights (Mw) for fractions I and II were calculated to be 526.9 kDa and 106 kDa. In addition, The FTIR spectrum (Figure 1B) showed the typical absorption peaks of polysaccharides. The signals at 1654 cm−1 were assigned to symmetric stretching vibration of C=O due to the uronic acid in SFSP. The strong signals around 1,250 cm−1 were attributed to the O=S=O asymmetric stretching vibration from sulfate indicated SFSP was a highly sulfated polysaccharide (27).
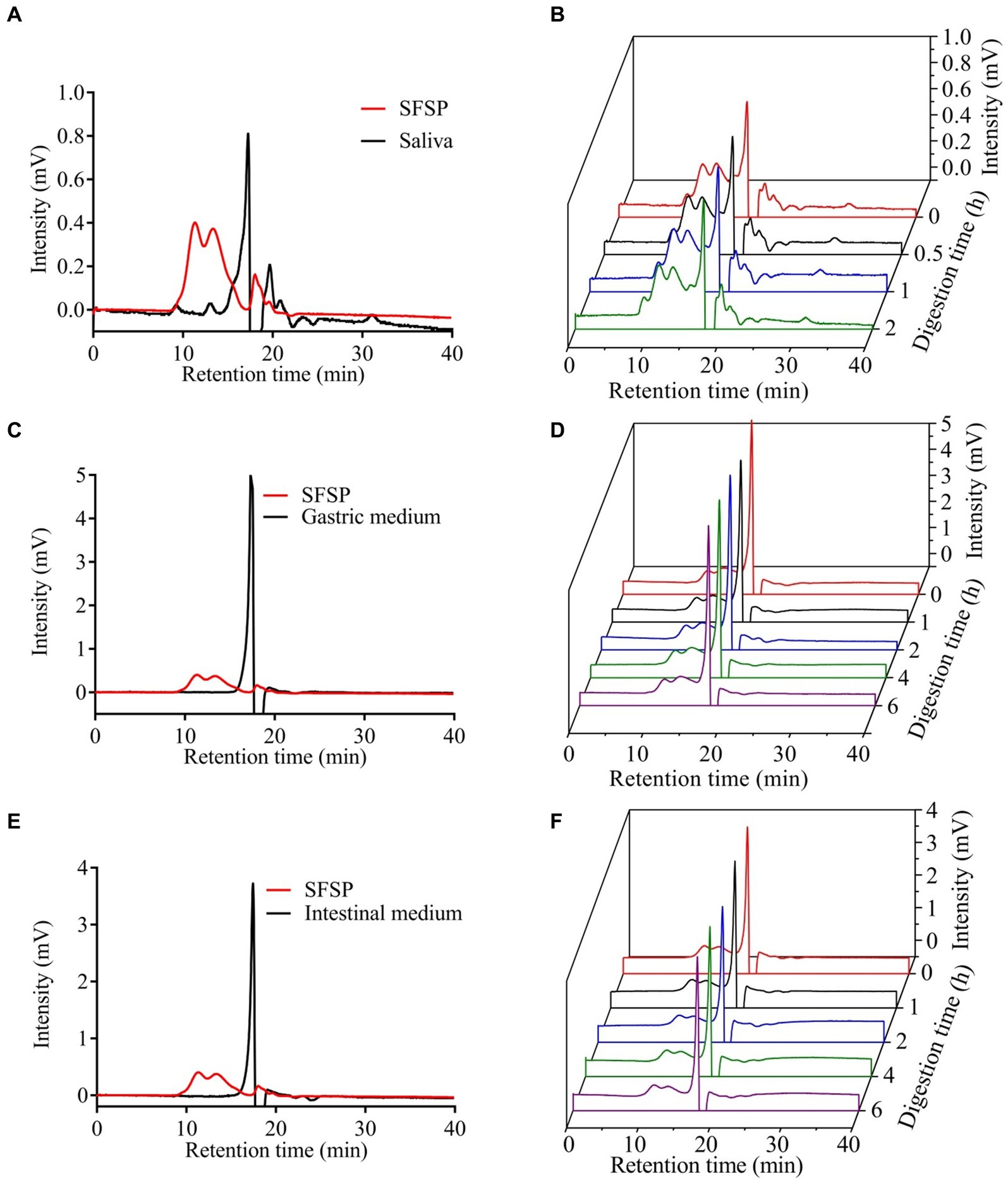
Figure 1. The retention times of saliva (A), gastric medium (C), intestinal medium (E) and the change of the Mw of SFSP at different times during simulated mouth (B), stomach (D) and small intestine (F) digestion.
3.2 Possible change of SFSP during in vitro digestion
The upper digestive system including saliva, gastric and small intestinal digestion with enzymes and acidic environment has the hydrolysis ability for some carbohydrates (28). The simulated digestion process of SFSP was monitored by detecting the changes of Mw, reducing sugars and free monosaccharides of digested samples. As shown in Figures 1A,B, the retention times of fractions I and II in HPGPC were not changed during digestion. This indicated that the treatment of saliva had no effect on SFSP. Furthermore, the gastrointestinal digestion was performed subsequently by the simulated physiological environment and a mixed enzyme system in our study. As shown by the chromatogram peaks in Figures 1C–F, the two major fractions of SFSP remain unchanged under both gastric and small intestinal digestion conditions. Moreover, no free monosaccharide was detected after saliva, gastric and small intestinal digestion (Figure 2). These results all indicated that SFSP could not be degraded in the upper digestive system. Sulfated polysaccharides from Ascophyllum nodosum, another kind of brown seaweed, also exhibit resistance to digestion as reported in previous study (29). But a report on research from Laminaria japonica sulfated polysaccharides showed that, it could resist the digestion of saliva and simulated gastric juice, but it was degraded in simulated small intestine juice (30).
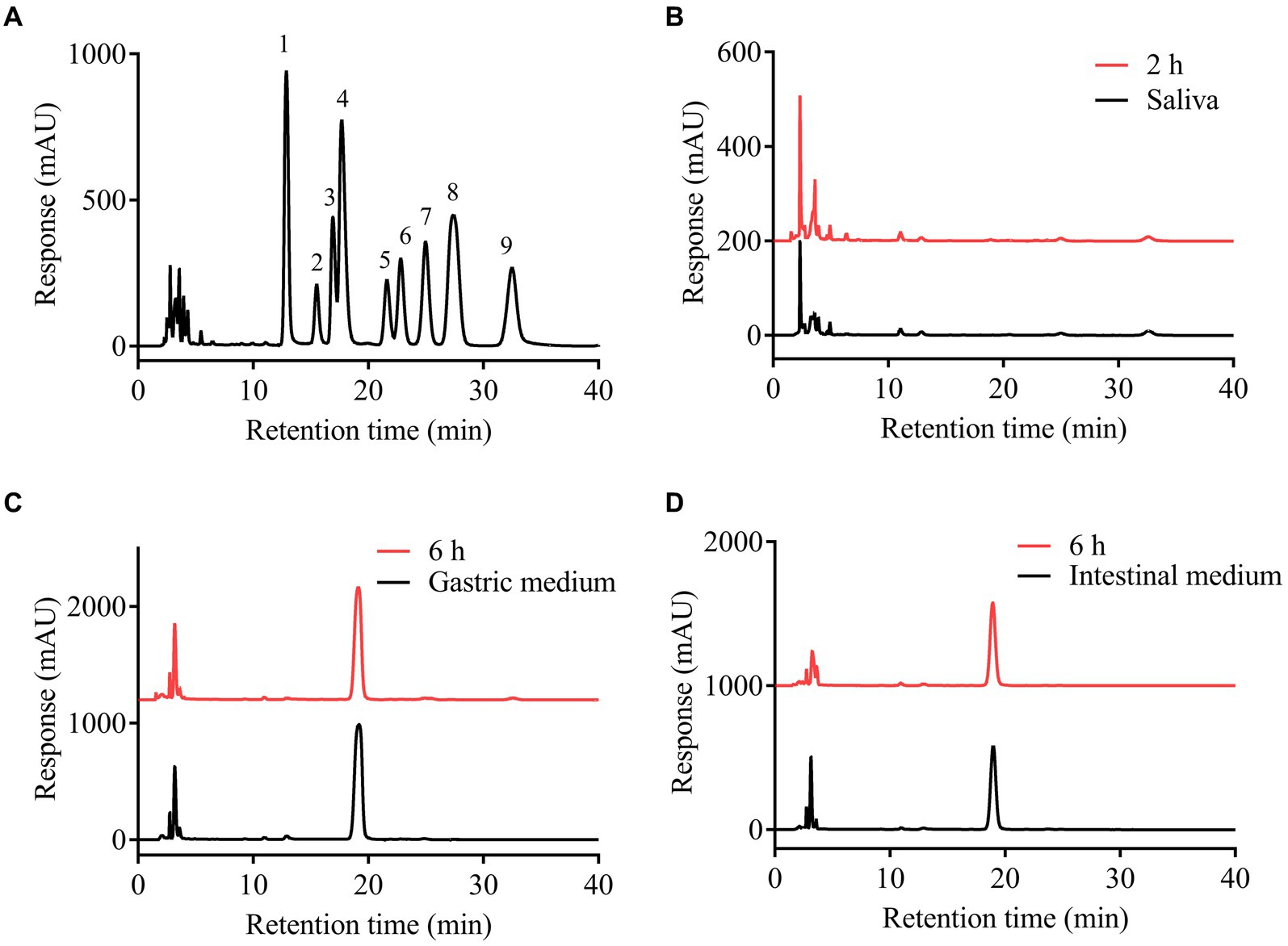
Figure 2. High performance liquid chromatograms of PMP derivatives of the mixed standard monosaccharides (A) and the analysis of the free monosaccharides released from SFSP at different times during simulated mouth (B), stomach (C) and small intestine (D) digestion. 1, mannose; 2, rhamnose; 3, glucuronic acid; 4, galacturonic acid; 5, glucose; 6, galactose; 7, xylose; 8, arabinose; 9, fucose.
3.3 Utilization of SFSP by human gut microbiota during in vitro fermentation
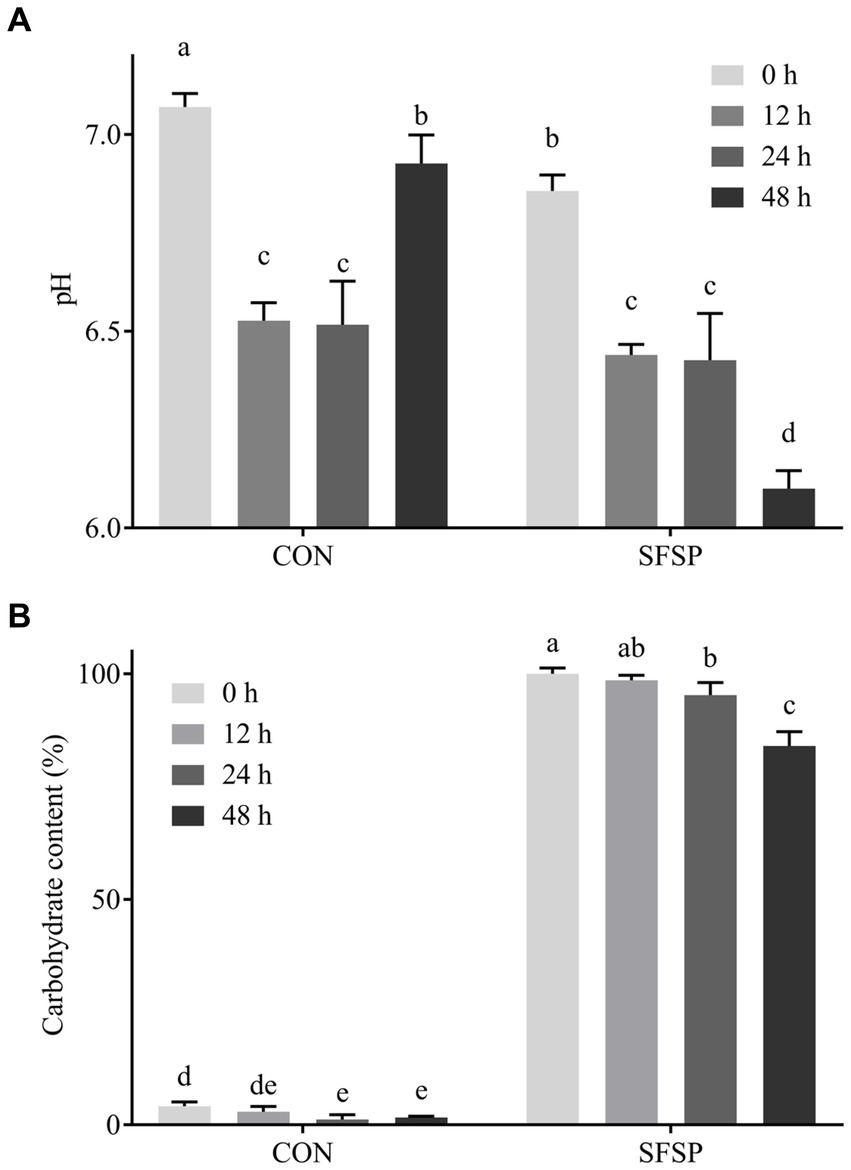
Undigested polysaccharides are possibly utilized by gut microbiota, and the SCFAs were considered as their major metabolites (8). So carbohydrate content, pH value, and SCFA contents of SFSP fermentation broth were monitored. As shown in Figure 3B, the total carbohydrate content of SFSP group decreased gradually, and after 48 h of in vitro fermentation, 16% of total carbohydrate was consumed in SFSP group. The pH value changes as a fundamental feature of fermentation processes were monitored at different times (Figure 3A). The initial pH values of SFSP and CON were 7.07 ± 0.03 and 6.86 ± 0.03, respectively, and the difference may be attributed to the uronic acid in SFSP structure (28). After fermentation for 12 h, the pH values of SFSP and CON evidently decreased to 6.53 ± 0.03 and 6.44 ± 0.02, respectively. Thereafter, the pH of SFSP and CON groups had tended to be stable during 12–24 h fermentation, but then the pH value of SFSP group began to decrease and reached to 6.09 ± 0.04 at 48 h. As shown in Table 1, SCFA contents were consistent with pH values observed at these time points, which confirmed that SFSP were utilized by gut microbiota to produce SCFAs. The decrease in pH value and the increase in short chain fatty acid content may be a common characteristic of the fermentation of sulfated polysaccharides by gut microbiota, and these results were also observed in the fermentation of sulfated polysaccharides Sargassum thunbergii and Laminaria japonica (31, 32).
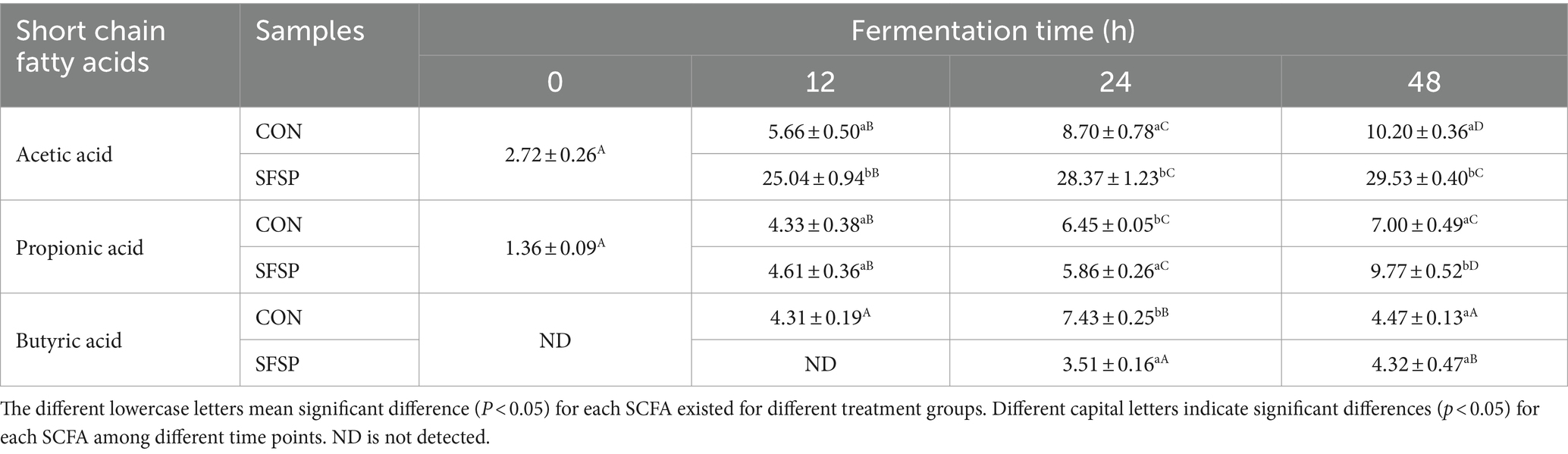
Table 1. Concentrations of SCFAs in fermentation solutions at different time points of fermentation in vitro.
It has been reported that different fractions of dietary polysaccharides might be selectively utilized by gut microbiota in fermentation (28). In addition, the fermentation characteristics of polysaccharides were greatly related to their component monosaccharides (33). Therefore, in order to reveal the utilization preferences of the gut microbiota for SFSP fractions, HPGPC profiles and monosaccharide compositions of the polysaccharides in the fermentation solutions were measured in the present study. The molecular weight distribution of SFSP at 0 and 48 h in vitro fermentation were shown by HPGPC in Figure 4A. Interestingly, the peak of fraction I disappeared after 48 h fermentation while peak of fraction II did not change obviously. Furthermore, the monosaccharide compositions of the polysaccharides in the fermentation solutions at 0 h and 48 h were measured and compared (Figure 4B). Compared to that of original SFSP (0 h), after 48 h fermentation, the molar ratio of galactose to fucose of residual polysaccharides decreased from 1: 2.31 to 1: 5.27 while the ratios of other monosaccharides (manmose and glucuronic acid) to fucose almost remained unchanged. These results indicated that galactose in SFSP was prone to be utilized or degraded by human gut bacteria. It could be inferred that Fraction I, which was utilized in fermentation, was mainly composed of galactose, and it was the effective prebiotics in SFSP for gut microbiota. Fraction II containing Fuc as the major monosaccharide component was resistant to the fermentation, and this phenomenon has also been found for some fucoidan and galactofucan from brown algae (34).
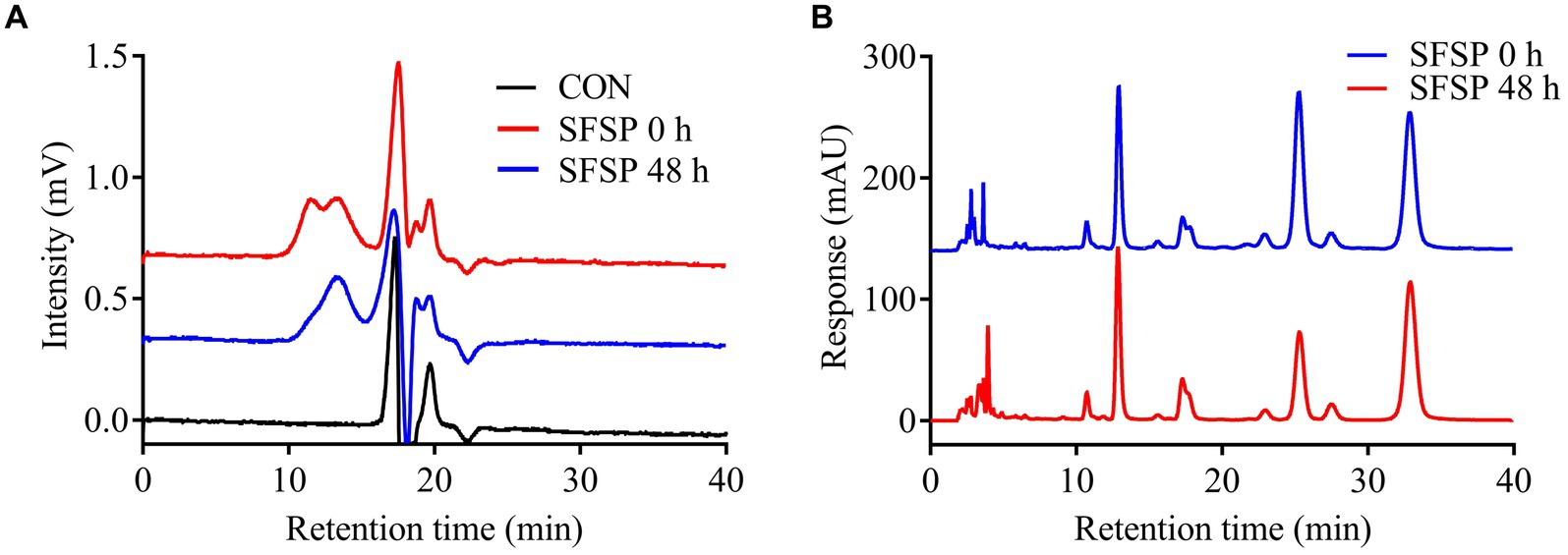
Figure 4. Analysis of the Mw of SFSP (A) and monosaccharides released from SFSP (B) after 48 h in vitro fermentation.
3.4 Effects of SFSP fermentation in vitro on gut microbiota
To reveal the regulation effect of SFSP on the gut microbial community, the bacterial 16S rRNA V3 regions amplicon sequencing was performed to compare the CON and SFSP groups after 48 h in vitro fermentation (5). As shown in Figure 5A, at the phylum level, all the samples mainly consisted of Firmicutes, Bacteroidetes, Proteobacteria, Fusobacteria, and Actinobacteria, but the hierarchical cluster analysis using unweighted pair-group method with arithmetic means (UPGMA) showed the distinction of microbial community composition between the two groups. Furthermore, as shown in Figures 5B,C, compared with the CON group, SFSP significantly reduced the abundance of Firmicutes and promoted the growth of Bacteroidetes (p < 0.05), resulting in the significantly heighted ratio of Firmicutes phylum to Bacteroidetes phylum (F/B) in SFSP group (p < 0.05) in Figure 5D. Our results are similar to those of a recent study on the fermentation of sulfated polysaccharides from Laminaria japonica, which the high molecular weight component of sulfated polysaccharides from Laminaria japonica significantly down regulated the value of F/B (35). This suggests that molecular weight may be an important factor in regulating gut microbiota structure. It has been well documented that obese individuals generally have higher F/B ratios (36), while the restoration of the F/B ratio by total fecal microbiota transplantation or dietary fiber intervention could prevent weight gain (37, 38). Our findings also indicate that SFSP could also prevent obesity by decreasing the F/B ratio.
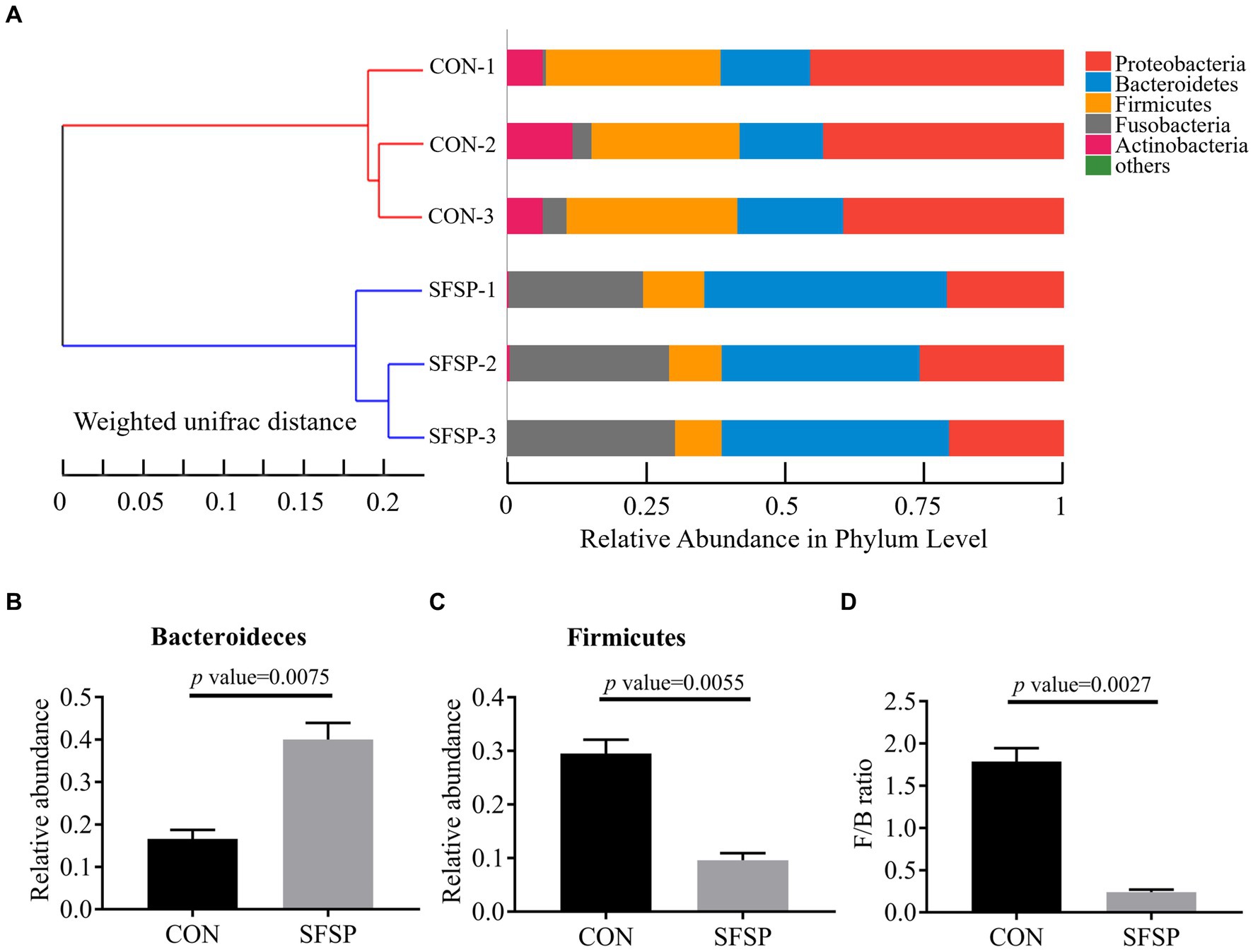
Figure 5. UPGMA analysis (A), relative abundance of Bacteroidetes (B), relative abundance of firmicutes (C), F/B ratio (D). Data were presented as mean ± SEM (n = 3).
Top 25 genera of bacteria found in the samples were shown in Figure 6B, and Escherichia_Shigella, Bacteroides, Fusobacterium, Megamonas, Morganella, Proteus, Enterobacteriaceae, Bilophila, Phasolarctobacterium, Bifidobacterium, Collinsella, Klebsiella, Lachnoclostridium (%) were the main genera after in vitro fermentation. In order to identify the altered genus attributed to the microbiota composition, linear discriminant analysis effect size (LEfSe) was carried out between CON and SFSP groups from the Phylum level to genus level as shown in Figure 6C. It was obvious that SFSP showed inhibitory effect on many genera from Clostridia, and greatly promote the growth of Bacteroides resulting an obviously increase of the Bacteroidetes phylum. On the basis of the Linear Discriminant Analysis (LDA) score (higher than 3) in Figure 6A, the addition of SFSP resulted in an increase in a total of 5 genera (Bacteroides, Fusobacterium, Megamonas, Morganella, Proteu) and lowered the abundances of 19 genera mainly including Escherichia_Shigella, Lachnoclostridium, Bifidobacterium, Klebsiella, and Collinsella.
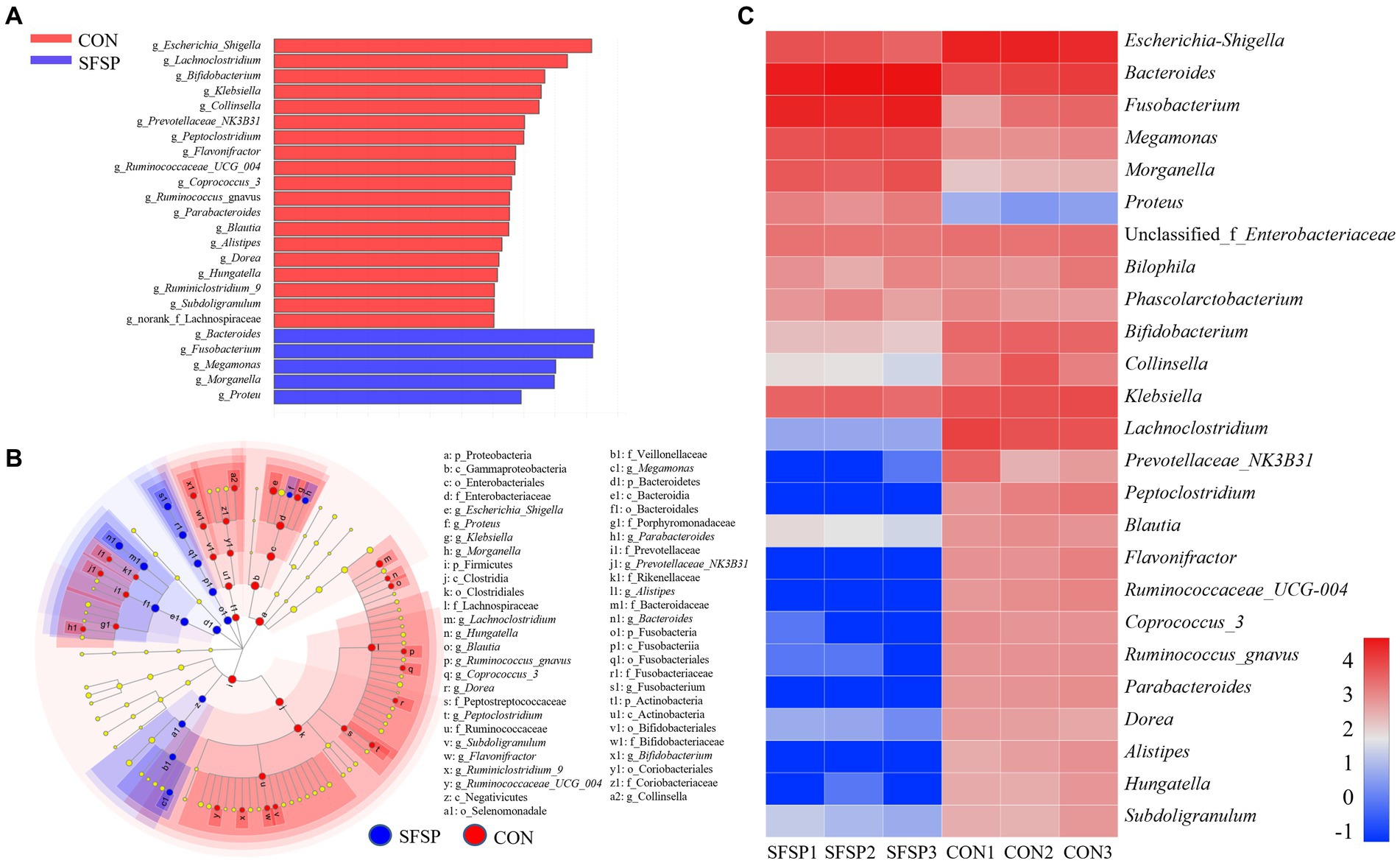
Figure 6. LDA score (A), heatmap of top 25 dominant genera (B) and LEfSe analyses (C) between SFSP group and CON group (n = 3, LDA score > 3).
It was obvious that SFSP feeding could significantly promote the proliferation of Bacteroides. Previous studies have shown that many species of Bacteroides contain a variety of hydrolases to degrade polysaccharides, such as B. thetaiotaomicron and B. uniformis, and they could synthesize glucosidases for fructan and agarose fermentation (39, 40). In addition, recent studies have shown that the increase of some Bacteroides, such as B. fragilis, could prevent the chronic colitis and colon tumors (41). Fusobacterium, eg. F. gonidiaformans and F. varium, could produce butyric acid by the utilization of protein in gut on the basis of the lysine pathway (42). Megamonas, eg. M. rupellensis, was the acetic acid and propionic acid producer (43). These two kinds of genera might regulate host health as the SCFAs producers. Escherichia_Shigella, eg. E. coli, was considered as opportunistic pathogen might cause an intestinal infection (44). Lachnoclostridium spp. had been reported to be significantly enriched in adenoma and DSS-induced colitis model (45). Collinsella had been described as a beneficial genus in some studies (46). But more reports showed that Collinsella had a strong correlation with a variety of diseases, such as nonalcoholic steatohepatitis, type 2 diabetics and atherosclerosis (47, 48). Klebsiella was regarded as typical opportunistic pathogenic bacteria, such as Klebsiella pneumonia, and it was the major pathogen in human pneumonia. It had been reported that Klebsiella pneumonia could induce fatty liver in mice by producing excessive alcohol (49). These results all illustrated that SFSP could be utilize by the Bacteroides and inhibited the growth of intestinal pathogens.
3.5 UPLC-MS/MS reveals the effect of SFSP on the gut microbiota metabolite signatures
A UPLC-MS/MS-based method was performed to investigate the small molecules produced by the gut microbiota using an in vitro fermentation modal. Principal component analysis (PCA) of metabolites revealed the substrate-specific clustering for both CON and SFSP samples (Figure 7A). At the starting point of fermentation, all these samples were close to each other in the direction of PC1 or PC2, indicating that the addition of SFSP had no effect on the small molecular components of the medium. After 48 h of fermentation, the gut microbiota metabolites of CON group changed significantly in PC1 while that of the SFSP changed mainly in PC2. Based on the intensities of the detected features in samples, the differentiation between gut microbiota metabolic capacity was also analyzed by hierarchical clustering (Figure 7B). A total of 945 differentiated metabolites were detected in the two groups at 0 h and 48 h. (Figure 7C) These results indicated that small molecular metabolites produced by microbiota were significantly changed with the utilization of SFSP.
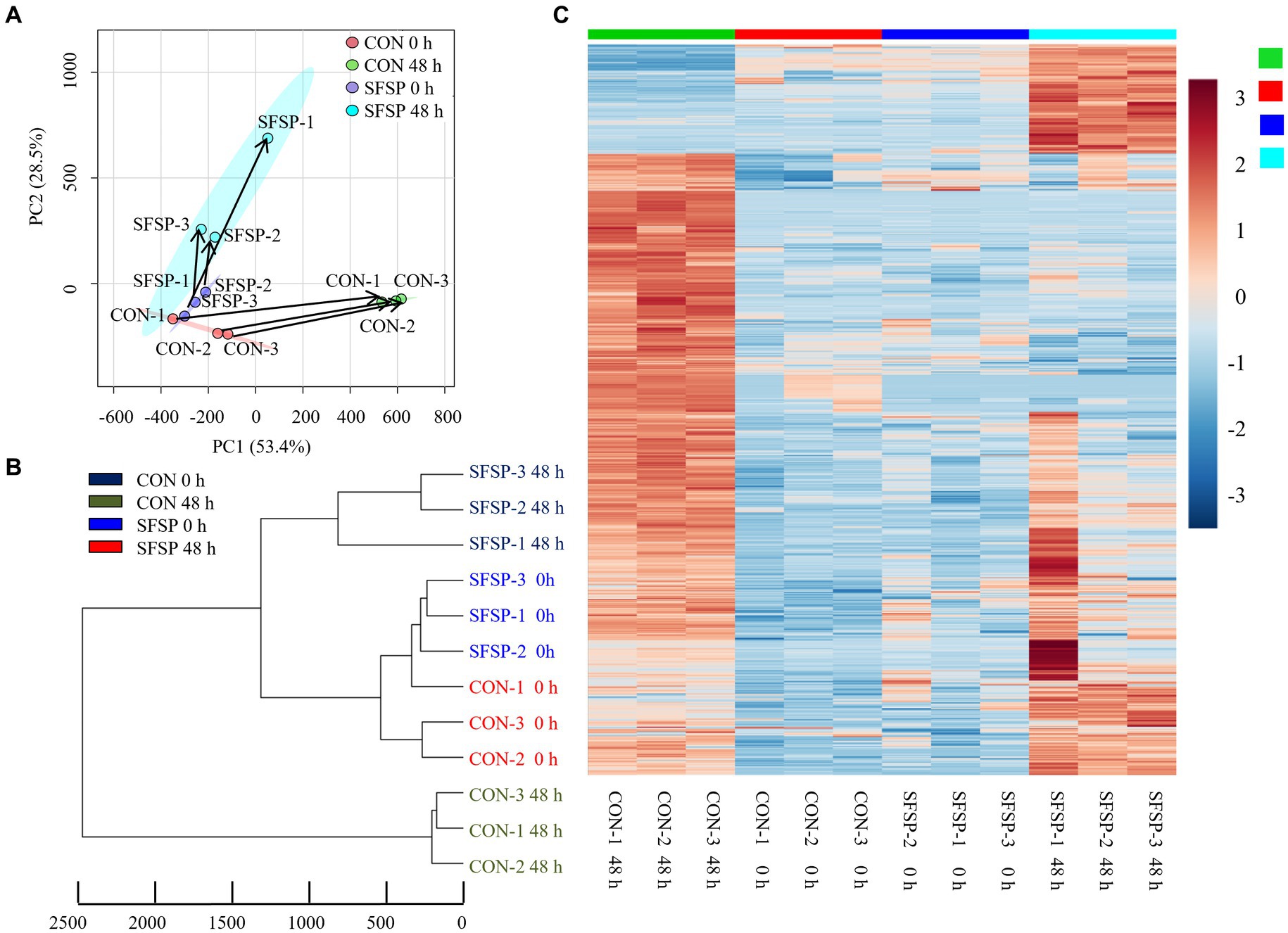
Figure 7. PCA (A), UPGMA (B) analysis and heatmaps (C) of the differential metabolite profiles between SFSP group and CON group\with fold change >2 or < 0.5. Data are expressed as mean ± SEM (n = 3) and p < 0.05 by the Student’s t-test.
Confirming the chemical identity of the detected m/z values is the major obstacle in metabolomic studies because there are few databases and kinds of metabolites that can be used to characterize metabolites. Therefore, we selected a metabolite database which contains MS, MS/MS and isotopic molecular information of more than 500 metabolites. We matched the information of 945 small molecule compounds produced by microorganisms with the compounds in the database, and 11 marker metabolites were identified as shown in Table 2 and chemical structure were drawn in Figure 2A. Among them, 7 compounds increased significantly and 4 compounds including trimethylamine and 7α-hydroxy-3-oxo-5β-cholanoic acid decreased significantly. These compounds are involved in the pathways of amino acid, lipid and secondary bile acid metabolism.
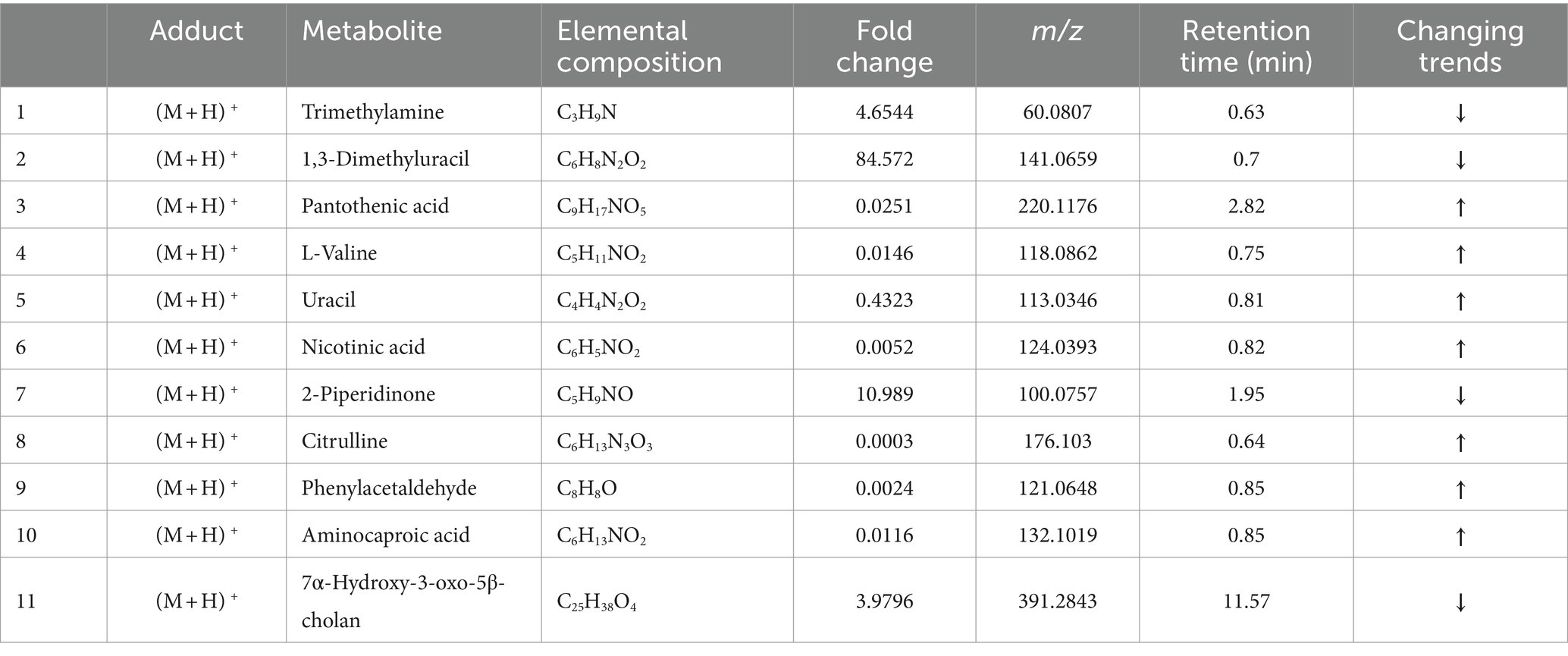
Table 2. Identification results of differential metabolites between the CON group and the SFSP group.
Trimethylamine is produced by the choline utilization cluster (cutC) (50) of gut bacteria from choline, and it could be absorbed into the enterohepatic circulation where it could be converted to trimethylamine oxide by oxidases in the liver. Trimethylamine oxide has been proved to be related to arteriosclerosis (48). It has been reported that trimethylamine oxidation is related to the ratio of Bacteroidetes and Firmicutes which is consistent with the results of the present study (51). Klebsiella has been proven to produce choline lyase cutC (52) and in Section 3.4, we have stated that SFSP can reduce the abundance of Klebsiella. It can be speculated that dietary SFSP may regulate trimethylamine by changing the ratio of Bacteroides and Firmicutes as well as reducing the abundance of the cutC enzyme producing bacterium Klebsiella. 7α-hydroxy-3-oxo-5β- cholanoic acid is a secondary bile acid whose biological function is constantly being discovered, such as regulating serum glucose and triglyceride (53). Nicotinic acid and pantothenic acid belong to the vitamin B family and can regulate a variety of physiological metabolism. The gut microbiota has been proven to be a widespread provider of vitamins (54) Studies have shown that supplementing with vitamin B can reshape the gut microbiota structure of obese individuals and have anti-obesity effects (55). It can be inferred that SFSP induces the production of more vitamin B in the gut microbiota, which may help alter the microbiota structure and prevent obesity. Phenylacetaldehyde is an aromatic compound that is often detected in human urine. Prebiotics can up-regulate the content of phenylacetaldehyde in vitro (56). Uracil and its precursor 1.3-dimethyluracil belong to base compounds. Previous studies have shown that uracil could stimulate the differentiation of intestinal epithelial cells, but may also lead to excessive immune production of reactive oxygen species (57). Citrulline, a precursor of arginine, is considered to have the same activity as arginine. It has been reported that citrulline supplementation can maintain the integrity of intestinal epithelial cells, and it can also alleviate the intestinal disorders and insulin resistance (58, 59). In addition, we conducted a correlation analysis between differential microbial communities and differential metabolites (Figure 2B). The content of pantothenic acid in fermentation broth is positively correlated with Proteus, Bacteroides, Megamonas, Fusobacterium, and Morganella. There is a positive correlation between the content of 7α-Hydroxy-3-oxo-5β-cholan and phenylacetaldehyde. The content of aminocaproic acid and nicotinic acid is positively correlated with the abundance of Flavonifractor, Prevotellaceae-NK3B31u, Coprococcus-3, and Lachnochlostrium. Trimethylamine, the pathogenic factor of atherosclerosis, is also positively correlated with the abundance of Blautia, Klebsiella, Ruminococcus_gnavus, Flavonifractor, Peptoclostridium and Ruminococcae_UCG-004. Klebsiella and trimethylamine showed a strong correlation, which confirms the previous speculation that SFSP may improve human health by inhibiting pathogenic bacteria.
4 Conclusion
The present study demonstrated the digestion behavior and fermentation characteristics of SFSP in the simulated digestion tract environment. The results suggested that SFSP could not be digested and could reach the colon as prototypes. During the fermentation with human intestinal flora, the higher-molecular-weight fraction of SFSP was utilized, and the galactose content in monosaccharide composition decreased relatively, which indicates that SFSP fractions can be selectively utilized by human gut microbiota. At the same time, SFSP also changed the composition of intestinal microbiota such as increasing the abundance of Bacteroides and decreasing the abundance of Firmicutes. SFSP promoted the production of acetic, propionic and n-butyric acids significantly. Moreover, SFSP could down-regulate the contents of trimethylamine, piperidone and secondary bile acid and increase nicotinic acid, pantothenic acid and other organic acids. The characteristics of SFSP in the fermentation with human intestinal flora suggests its potential application as nutritional supplement to benefit human health.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. CS: Validation, Writing – review & editing. CA: Methodology, Writing – review & editing. CW: Methodology, Writing – review & editing. SS: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Natural Science Foundation of Liaoning Province (No. 2023-MS-278).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1400063/full#supplementary-material
References
1. Xu, B, Song, S, Yao, L, Wang, H, Sun, M, Zhuang, H, et al. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human gut microbiota of polysaccharides from Ficus carica Linn. Food Hydrocoll. (2024) 146:109204. doi: 10.1016/j.foodhyd.2023.109204
2. Zhang, C, Yu, L, Zhai, Q, Zhao, R, Zhao, J, Zhang, H, et al. In vitro fermentation of heparin by the human gut microbiota: changes in the microbiota community and metabolic functions. Food Chem. (2023) 406:135010. doi: 10.1016/j.foodchem.2022.135010
3. Li, Y, Liu, S, Ding, Y, Li, S, Sang, X, Li, T, et al. Structure, in vitro digestive characteristics and effect on gut microbiota of sea cucumber polysaccharide fermented by Bacillus subtilis Natto. Food Res Int. (2023) 169:112872. doi: 10.1016/j.foodres.2023.112872
4. Chen, H, Zhang, L, Long, X, Li, P, Chen, S, Kuang, W, et al. Sargassum fusiforme polysaccharides inhibit VEGF-A-related angiogenesis and proliferation of lung cancer in vitro and in vivo. Biomed Express. (2017) 85:22–7. doi: 10.1016/j.biopha.2016.11.131
5. Zhu, Z, Zhu, B, Sun, Y, Ai, C, Wang, L, Wen, C, et al. Sulfated polysaccharide from sea cucumber and its depolymerized derivative prevent obesity in association with modification of gut microbiota in high-fat diet-fed mice. Mol Nutr Food Res. (2018) 62:e1800446. doi: 10.1002/mnfr.201800446
6. Li, Y, Gong, T, Lu, H, Ma, S, and Liu, X. In vitro fermentation characteristics of oxidized konjac glucomannan and its modulation effects on gut microbiota. Food Hydrocoll. (2023) 141:108693. doi: 10.1016/j.foodhyd.2023.108693
7. Ai, C, Jiang, P, Liu, Y, Duan, M, Sun, X, Luo, T, et al. The specific use of alginate from Laminaria japonica by Bacteroides species determined its modulation of the Bacteroides community. Food Funct. (2019) 10:4304–14. doi: 10.1039/C9FO00289H
8. Donohoe, DR, Garge, N, Zhang, X, Sun, W, O’Connell, TM, and Bunger, MK. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. (2011) 13:517–26. doi: 10.1016/j.cmet.2011.02.018
9. Inokuma, K, Sasaki, D, Kurata, K, Ichikawa, M, Otsuka, Y, and Kondo, A. Sulfated and non-sulfated chondroitin affect the composition and metabolism of human colonic microbiota simulated in an in vitro fermentation system. Sci Rep. (2023) 13:12313. doi: 10.1038/s41598-023-38849-5
10. Koeth, RA, Wang, Z, Levison, BS, Buffa, JA, Org, E, Sheehy, BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. (2013) 19:576–85. doi: 10.1038/nm.3145
11. Funabashi, M, Grove, TL, Wang, M, Varma, Y, McFadden, ME, Brown, LC, et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature. (2020) 582:566–70. doi: 10.1038/s41586-020-2396-4
12. Liu, J, Luthuli, S, Yang, Y, Cheng, Y, Zhang, Y, Wu, M, et al. Therapeutic and nutraceutical potentials of a brown seaweed Sargassum fusiforme. Food Sci Nutr. (2020) 8:5195–205. doi: 10.1002/fsn3.1835
13. Liu, L, Heinrich, M, Myers, S, and Dworjanyn, SA. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in traditional Chinese medicine: a phytochemical and pharmacological review. J Ethnopharmacol. (2012) 142:591–619. doi: 10.1016/j.jep.2012.05.046
14. Li, B, Wei, X, Sun, J, and Xu, S. Structural investigation of a fucoidan containing a fucose-free core from the brown seaweed. Hizikia fusiforme Carbohydrate Res. (2006) 341:1135–46. doi: 10.1016/j.carres.2006.03.035
15. Wang, P, Zhao, X, Lv, Y, Liu, Y, Lang, Y, Wu, J, et al. Analysis of structural heterogeneity of fucoidan from Hizikia fusiforme by ES-CID-MS/MS. Carbohydr Polym. (2012) 90:602–7. doi: 10.1016/j.carbpol.2012.05.084
16. Jin, W, Zhang, W, Wang, J, Ren, S, Song, N, Duan, D, et al. Characterization of laminaran and a highly sulfated polysaccharide from Sargassum fusiforme. Carbohydr Res. (2014) 385:58–64. doi: 10.1016/j.carres.2013.12.009
17. Hu, P, Xue, R, Li, Z, Chen, M, Sun, Z, Jiang, J, et al. Structural investigation and immunological activity of a heteropolysaccharide from Sargassum fusiforme. Carbohydr Res. (2014) 390:28–32. doi: 10.1016/j.carres.2014.02.027
18. Hu, P, Li, Z, Chen, M, Sun, Z, Ling, Y, Jiang, J, et al. Structural elucidation and protective role of a polysaccharide from Sargassum fusiforme on ameliorating learning and memory deficiencies in mice. Carbohydr Polym. (2016) 139:150–8. doi: 10.1016/j.carbpol.2015.12.019
19. Chen, L, Chen, P, Liu, J, Hu, C, Yang, S, He, D, et al. Sargassum fusiforme polysaccharide SFP-F2 activates the NF-κB signaling pathway via CD14/IKK and P 38 axes in RAW264. 7 cells. Mar Drugs. (2018) 16:264. doi: 10.3390/md16080264
20. Cheng, Y, Sibusiso, L, Hou, L, Jiang, H, Chen, P, Zhang, X, et al. Sargassum fusiforme fucoidan modifies the gut microbiota during alleviation of streptozotocin-induced hyperglycemia in mice. Int J Biol Macromol. (2019) 131:1162–70. doi: 10.1016/j.ijbiomac.2019.04.040
21. Dubois, M, Gilles, K, Hamilton, J, Rebers, P, and Smith, F. Colorimetric method for determination of sugars and related substances. Anal Biochem. (1956) 28:350–6. doi: 10.1021/ac60111a017
22. Blumenkrantz, N, and Asboe, HG. New method for quantitative determination of uranic acids. Anal Biochem. (1973) 54:484–9. doi: 10.1016/0003-2697(73)90377-1
23. Bradford, MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. (1976) 72:248–54. doi: 10.1016/0003-2697(76)90527-3
24. Silvestri, LJ, Hurst, RE, Simpson, L, and Settine, JM. Analysis of sulfate in complex carbohydrates. Anal Biochem. (1982) 123:303–9. doi: 10.1016/0003-2697(82)90450-X
25. Miller, GL . Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. (1959) 31:426–8. doi: 10.1021/ac60147a030
26. Chen, G, Xie, M, Wan, P, Chen, D, Ye, H, Chen, L, et al. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. (2017) 244:331–9. doi: 10.1016/j.foodchem.2017.10.074
27. Rupérez, P, Ahrazem, O, and Leal, JA. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J Agric Food Chem. (2002) 50:840–5. doi: 10.1021/jf010908o
28. Zhou, W, Yan, Y, Mi, J, Zhang, H, Lu, L, Luo, Q, et al. Simulated digestion and fermentation in vitro by human gut microbiota of polysaccharides from bee collected pollen of Chinese wolfberry. J Agric Food Chem. (2018) 66:898–907. doi: 10.1021/acs.jafc.7b05546
29. Chen, L, Xu, W, Chen, D, Chen, G, Liu, J, Zeng, X, et al. Digestibility of sulfated polysaccharide from the brown seaweed Ascophyllum nodosum and its effect on the human gut microbiota in vitro. Int J Biol Macromol. (2018) 112:1055–61. doi: 10.1016/j.ijbiomac.2018.01.183
30. Gao, J, Lin, L, Chen, Z, Cai, Y, Xiao, C, Zhou, F, et al. In vitro digestion and fermentation of three polysaccharide fractions from Laminaria japonica and their impact on lipid metabolism-associated human gut microbiota. J Agric Food Chem. (2019) 67:7496–505. doi: 10.1021/acs.jafc.9b00970
31. Fu, X, Cao, C, Ren, B, Zhang, B, Huang, Q, and Li, C. Structural characterization and in vitro fermentation of a novel polysaccharide from Sargassum thunbergii and its impact on gut microbiota. Carbohydr Polym. (2018) 183:230–9. doi: 10.1016/j.carbpol.2017.12.048
32. Kong, Q, Dong, S, Gao, J, and Jiang, C. In vitro fermentation of sulfated polysaccharides from E. Prolifera and L. japonica by human fecal microbiota. Int J Biol Macromol. (2016) 91:867–71. doi: 10.1016/j.ijbiomac.2016.06.036
33. Lee, H, Jo, E, Song, J, Min, J, Song, Y, Lee, H, et al. Correlation between monosaccharide, oligosaccharide, and microbial community profile changes in traditional soybean brick (Meju) fermentation. Food Res Int. (2024) 184:114233. doi: 10.1016/j.foodres.2024.114233
34. Zhang, T, Wu, S, Ai, C, Wen, C, Liu, Z, and Wang, L. Galactofucan from Laminaria japonica is not degraded by the human digestive system but inhibits pancreatic lipase and modifies the intestinal microbiota. Int J Biol Macromol. (2020) 166:611–20. doi: 10.1016/j.ijbiomac.2020.10.219
35. Lu, X, Xu, H, Fang, F, Liu, J, Wu, K, Zhang, Y, et al. In vitro effects of two polysaccharide fractions from Laminaria japonica on gut microbiota and metabolome. Food Funct. (2023) 14:3379–90. doi: 10.1039/D2FO04085A
36. Abdallah, IN, Ragab, SH, Abd, EA, Shoeib, AR, Alhosry, Y, and Fekry, D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch Med Sci. (2011) 3:501–7. doi: 10.5114/aoms.2011.23418
37. Zhou, D, Pan, Q, Shen, F, Cao, H, and Fan, J. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. (2017) 7:1529. doi: 10.1038/s41598-017-01751-y
38. Chang, C, Lin, C, Lu, C, Martel, J, Ko, Y, Ojcius, DM, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. (2015) 6:7489. doi: 10.1038/ncomms8489
39. Signe, A, Katrin, T, Heiki, V, Marju, P, Natalja, K, Triinu, V, et al. Degradation of fructans and production of propionic acid by Bacteroides thetaiotaomicron are enhanced by the shortage of amino acids. Front Nutr. (2014) 1:1. doi: 10.3389/fnut.2014.00021
40. Li, M, Shang, Q, Li, G, Wang, X, and Yu, G. Degradation of marine algae-derived carbohydrates by Bacteroidetes isolated from human gut microbiota. Mar Drugs. (2017) 15:92. doi: 10.3390/md15040092
41. Chan, JL, Wu, S, Geis, AL, Chan, GV, Gomes, T, Beck, SE, et al. Non-toxigenic Bacteroides fragilis (NTBF) administration reduces bacteria-driven chronic colitis and tumor development independent of polysaccharide A. Mucosal immunology. (2019). 12:164–177. doi: 10.1038/s41385-018-0085-5
42. Vital, M, Howe, C, and Tiedje, M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta) genomic data. MBio. (2014) 5:1–11. doi: 10.1128/mBio.00889-14
43. Martín-Núñez, GM, Cornejo-Pareja, I, Coin-Aragüez, L, Roca-Rodríguez, MDM, Muñoz-Garach, A, Clemente-Postigo, M, et al. H. pylori eradication with antibiotic treatment causes changes in glucose homeostasis related to modifications in the gut microbiota. PLoS One. (2019) 14:e0213548. doi: 10.1371/journal.pone.0213548
44. Packey, CD, and Sartor, RB. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis. (2009) 22:292–301. doi: 10.1097/QCO.0b013e32832a8a5d
45. Wang, C, Li, W, Wang, H, Ma, Y, Zhao, X, Zhang, X, et al. Saccharomyces boulardii alleviates ulcerative colitis carcinogenesis in mice by reducing TNF-α and IL-6 levels and functions and by rebalancing intestinal microbiota. BMC Microbiol. (2019) 19:1–12. doi: 10.1186/s12866-019-1610-8
46. Qin, P, Zou, Y, Dai, Y, Luo, G, Zhang, X, and Xiao, L. Characterization a novel butyric acid-producing bacterium Collinsella aerofaciens Subsp. Shenzhenensis Subsp Nov. Microorganisms. (2019) 7. doi: 10.3390/microorganisms7030078
47. Frost, F, Storck, LJ, Kacprowski, T, Gärtner, S, Rühlemann, M, Bang, C, et al. A structured weight loss program increases gut microbiota phylogenetic diversity and reduces levels of Collinsella in obese type 2 diabetics: a pilot study. PLoS One. (2019) 14:e0219489. doi: 10.1371/journal.pone.0219489
48. Karlsson, FH, Fåk, F, Nookaew, I, Tremaroli, V, Fagerberg, B, Petranovic, D, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. (2012) 3:1245. doi: 10.1038/ncomms2266
49. Yuan, J, Chen, C, Cui, J, Lu, J, Yan, C, Wei, X, et al. Fatty liver disease caused by high-alcohol-producing Klebsiella pneumoniae. Cell Metab. (2019) 30:1172. doi: 10.1016/j.cmet.2019.11.006
50. Martínez-del Campo, A, Bodea, S, Hamer, HA, Marks, JA, Haiser, HJ, Turnbaugh, PJ, et al. Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut bacteria. MBio. (2015) 6:10–1128. doi: 10.1128/mbio.00042-15
51. Cho, CE, Taesuwan, S, Malysheva, OV, Bender, E, Tulchinsky, NF, Yan, J, et al. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res. (2017) 61:1600324. doi: 10.1002/mnfr.201600324
52. Kalnins, G, Kuka, J, Grinberga, S, Makrecka-Kuka, M, Liepinsh, E, Dambrova, M, et al. Structure and function of cut C choline lyase from human microbiota bacterium Klebsiella pneumoniae. J Biol Chem. (2015) 290:21732–40. doi: 10.1074/jbc.M115.670471
53. Kuno, T, Hirayama-Kurogi, M, Ito, S, and Ohtsuki, S. Reduction in hepatic secondary bile acids caused by short-term antibiotic-induced dysbiosis decreases mouse serum glucose and triglyceride levels. Sci Rep. (2018) 8:1253. doi: 10.1038/s41598-018-19545-1
54. LeBlanc, JG, Milani, C, De Giori, GS, Sesma, F, Van Sinderen, D, et al. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. (2013) 24:160–8. doi: 10.1016/j.copbio.2012.08.005
55. Jiang, S, Zhu, Q, Mai, M, Yang, W, and Du, G. Vitamin B and vitamin D as modulators of gut microbiota in overweight individuals. Int J Food Sci Nutr. (2020) 71:1001–9. doi: 10.1080/09637486.2020.1748580
56. Vitali, B, Ndagijimana, M, Maccaferri, S, Biagi, E, Guerzoni, ME, and Brigidi, P. An in vitro evaluation of the effect of probiotics and prebiotics on the metabolic profile of human microbiota. Anaerobe. (2012) 18:386–91. doi: 10.1016/j.anaerobe.2012.04.014
57. López-Sámano, M, Beltrán, LF, Sánchez-Thomas, R, Dávalos, A, Villaseñor, T, García-García, JD, et al. A novel way to synthesize pantothenate in bacteria involves β-alanine synthase present in uracil degradation pathway. Microbiology. (2020) 9:e1006. doi: 10.1002/mbo3.1006
58. Ho, SW, El-Nezami, H, and Shah, NP. Effects of supplementation of citrulline and Lactobacillus helveticus ASCC 511 on intestinal epithelial cell integrity. J Funct Foods. (2020) 64:103571. doi: 10.1016/j.jff.2019.103571
Keywords: Sargassum fusiforme, sulfated polysaccharide, fermentation, Bacteroides, gut microbiota
Citation: Jiang L, Song C, Ai C, Wen C and Song S (2024) Modulation effect of sulfated polysaccharide from Sargassum fusiforme on gut microbiota and their metabolites in vitro fermentation. Front. Nutr. 11:1400063. doi: 10.3389/fnut.2024.1400063
Edited by:
Han Sun, Shenzhen University, ChinaReviewed by:
Juncai Tu, Shenzhen University, ChinaXue Sang, Dalian Ocean University, China
Changliang Zhu, Ocean University of China, China
Copyright © 2024 Jiang, Song, Ai, Wen and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuang Song, songs1008@163.com
 Long Jiang
Long Jiang  Chunqing Ai
Chunqing Ai Chengrong Wen
Chengrong Wen Shuang Song
Shuang Song