Article biodiversity inside bottles: animals, fungi, and plants in traditional alcoholic drinks
- 1Unidad Académica de Trabajo Social y Ciencias para el Desarrollo Humano, Universidad Autónoma de Tamaulipas, Ciudad Victoria, Tamaulipas, Mexico
- 2Facultad de Ingeniería y Ciencias, Universidad Autónoma de Tamaulipas, Ciudad Victoria, Tamaulipas, Mexico
- 3Unidad Académica Multidisciplinaria Mante Centro, Universidad Autónoma de Tamaulipas, Ciudad Mante, Tamaulipas, Mexico
- 4Instituto de Ecología Aplicada, Universidad Autónoma de Tamaulipas, Ciudad Victoria, Tamaulipas, Mexico
The use of animals, fungi, and plants as a source of bioactive compounds has been widely practiced in diverse cultures throughout the world, particularly in alcoholic drinks. The nature of the biological material, method of preparation and alcohol concentration play a predominant role in the extraction of bioactive compounds and the achievement of desired results. However, certain aspects must be considered to guarantee the innocuity of these drinks and reduce the risk of intoxication, infections and allergic reactions, aspects which are sometimes overlooked. In addition, the implications of using threatened or protected species must be considered to reduce the negative impact on their populations. The authors recommend the establishment of production systems which guarantee products with adequate quality controls and ensure the benefits to the consumer.
1 Introduction
Thousands of bottles containing alcoholic drinks have withstood the passage of time and diverse events (shipwrecks, migrations, changes in political and cultural power, among others) preserving within them their chemical composition and the resources used in their production, which together serve as unique chemical fingerprints revealing their past and potential future. The use of biotic resources to meet nutritional and health needs throughout human history has given rise to specific methods of preparation and consumption linked to social, cultural, economic, and political development and in distinct geographical spaces and times (1). Alcoholic preparations to which bioactive properties are attributed have been part of traditions and cultural identity for centuries, although their functional principles (antioxidant, relaxant, anti-inflammatory, analgesic, anticancer, neuroactive) and the characterization of the organisms used in their preparation, for the most part, remain to be explored. In this sense, traditional alcoholic drinks which are fortified with plants, animals, and fungi—usually used as part of the culinary, medical, and cultural repertoire—provide benefits to the consumer which, in many cases, has limited the use of these organisms to commercial attraction, that is, a marketing strategy (2). Although for the most of these alcoholic drinks, no clinical evidence has demonstrated such bioactive benefits, their consumption has been kept through the time. Nevertheless, the incorporation of these organisms can have greater significance due to their health benefits, contribution to food security, benefits to rural tourism, social and religious value as well as their role in the preservation and transmission of cultural heritage (3). All the above and consumer literacy of this knowledge can expand the use of these traditional drinks beyond solely recreational purposes. However, it is important to ensure product quality and the benefits to the consumer and consider regulations regarding their consumption and the sustainable use of species which become part of these alcoholic drinks rich in bioculture and potential.
2 Diversity in preparations
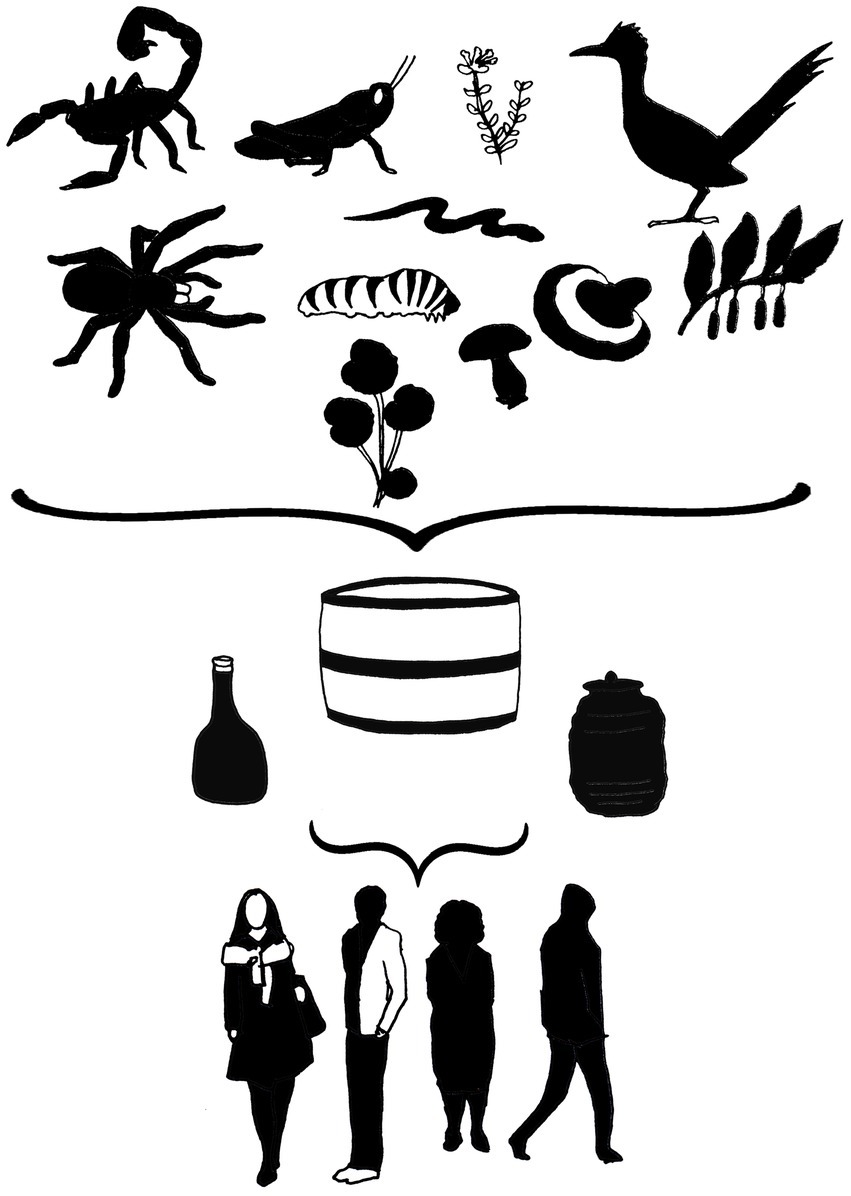
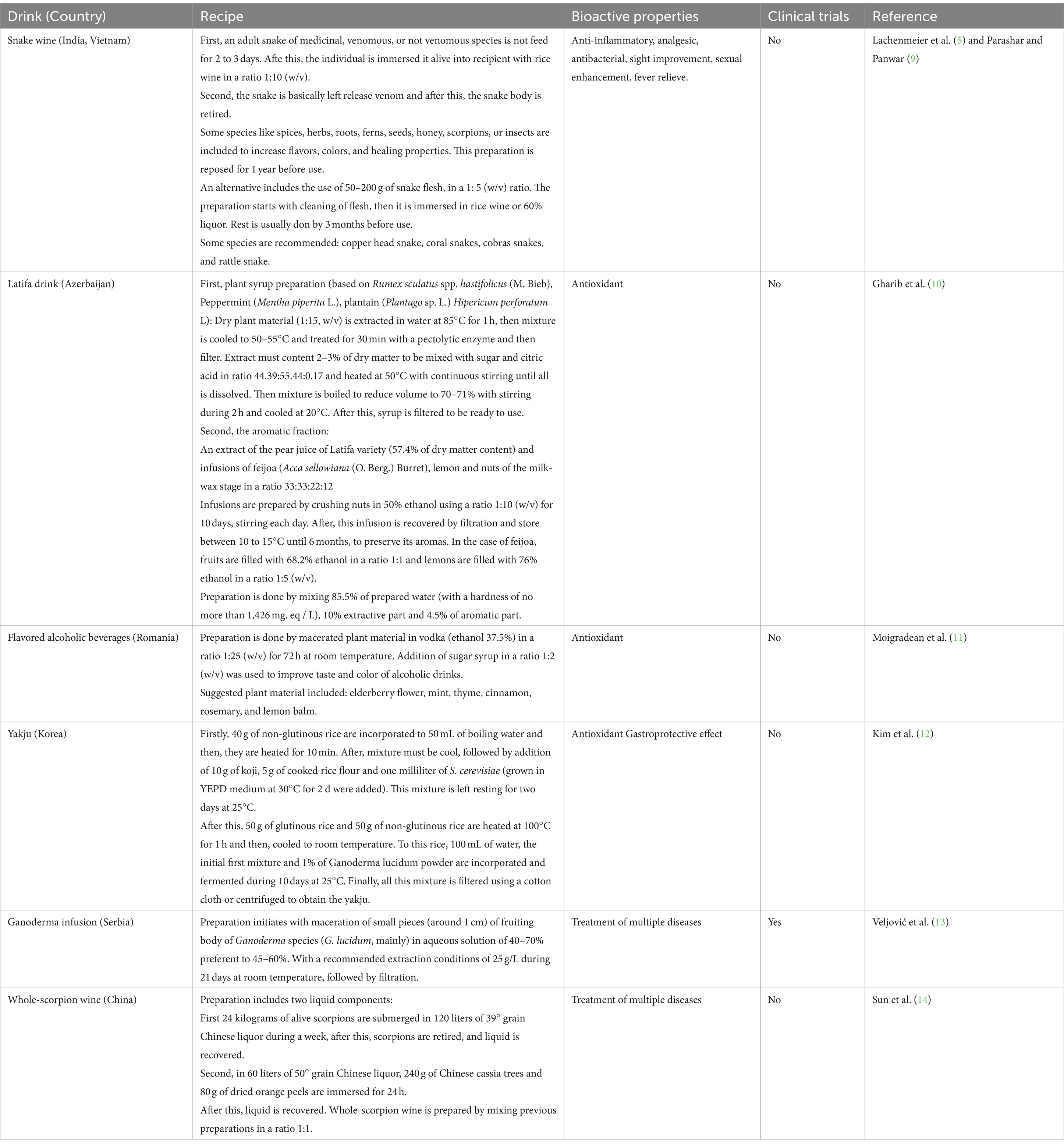
The solvents most often used in the preparation of alcoholic drinks are ethanol solutions in varying concentrations. In some such drinks, the percentage of alcohol varies between 30 and 40% according to the general content for most spirits, although concentrations of some may be as high as 75%, while some are only 14% (4–6). Preparation time is variable, and the surrounding operations are diverse; Armor Tail Scorpion Vodka from the Thai brand Unique®, for example, is infused with a scorpion (3 to 4 cm in length) for several months (Thailand Unique Trademark). The maguey worm (Comadia redtenbacheri Hammerschmidt) is incorporated into bottles of mezcal (distilled form Mexican agave), a simple process which has been diversified. One way is to submerge C. redtenbacheri in water at 80° C before adding them to white vinegar and 40% ethyl alcohol at a ratio of 1:1 for 24 h. The worms are then put in 40% alcohol for 7d before being incorporated into the bottles of mezcal (7), where they can remain for months and even years. In Korea, fermentation is a favored technique in alcohol production for the addition of plant species. Some 50% of drinks are prepared in specific seasons depending on the availability of ingredients (flowers, fruits, roots, and leaves) and for several purposes associated with celebrations and religious practices (8). The above illustrates the wide variability of base drinks and the procedures for preparing traditional liquors in different parts of the world (Table 1).
3 Drinks with arthropods and reptiles
The use of arachnids (mainly tarantulas and scorpions) in alcoholic drinks is to increase the visual impact on the consumer, and the practice has been broadly developed in a variety of traditional drinks, such as rice wine in the case of the Thai brand, Unique®, for scorpion wine in Vietnam, which is a product widely recognized in Asia (15, 16). The elaboration of mezcal has been part of Mexican culture since the 17th century and one of the most popular mezcals is tequila, designated with this name based on the use of Agave tequilana Weber. However, it was not until the 1940s and 50s that the mezcal worm was incorporated and became identified as the tequila worm, increasing public interest in mezcals, especially in Asia, Europe, and the United States (17). Many myths have arisen around the addition of C. redtenbacheri to mezcals, such as its aphrodisiac properties; however, since there is no evidence to support the claim, it is considered more marketing than functionality. Nevertheless, the addition has driven the incorporation of other arthropods, such as scorpions. Scorpions are used based on their availability and distribution in the region and their incidence in the area where the mezcal is prepared. For example, mezcal produced in the state of Durango, Mexico includes scorpions from the genus Centruroides spp., while some Oaxacan mezcals include species from the genera Centruroides and Vaejovis, giving resinous notes to the drink (18). While the above speaks to recreational use, the incorporation of arthropods to mezcal for medicinal purposes is practiced by ethnic groups in Central Mexico, such as in the case of centipedes from the genus Scolopendra, which are used as a traditional remedy against poisonous animals (19). The addition of insects to increase antioxidant levels in alcoholic drinks and provide them with sensory attributes in a controlled way is an innovative contribution to generate products with attainable quality criteria, as indicated with the incorporation of Pterophylla beltrani Bolívar and Bolívar., Schistocerca piceifrons Walker and Tenebrio molitor L. (6, 20). More extravagant is the introduction of snakes, geckos, birds, and other animals into some alcoholic mixtures, ostensibly for visual impact and health benefits. Snake wine, from Vietnam, is perhaps the best known and most striking exotic drink, with the use of venomous species including cobras. Meanwhile, in Mexico, the incorporation of rattlesnake into sotol (a distilled beverage from the fermentation of the stem of Dasylirion spp.) has been reported (21, 22). A wide range of practices and production processes are carried out in the different regions of origin of these resources (plants and animals) and production. Within each region, the incorporation of different animal groups shows the convergence between culture and biodiversity, that is, the bioculture surrounding the traditional alcoholic drinks recognized as icons of the state and country’s tradition.
4 Drinks with fungi
Fungi are widely recognized around the world as important sources of antioxidant and neuroactive compounds. They are associated with benefits such as analgesic and neuroprotective properties, in the treatment of nervous, circulatory, and digestive diseases, cancer and diabetes (23); their use to fortify alcoholic drinks with medicinal attributes is common and highly represented in Chinese medicine (13), and they have earned certain popularity in other parts of the world. The most popular fungi used in the elaboration of this kind of drink is the Agaricomycete group, which has shown numerous beneficial effects associated with its bioactive compounds (24–26). The fungus Lignosus rhinocerus (Cooke) Ryvarden has been indicated to treat wounds, asthma, fever, cancers and food poisoning; L. rhinoceros powder is also mixed with Chinese rice wine and, surprisingly, can be applied topically to treat lumps, sores and boils (27). On the other hand, the fungus Hericium erinaceus (Bull.: Fr.), known as lion’s mane, is associated with a decrease in neurodegenerative diseases. Commonly mixed with a made wine, it is highly recommended for its regenerative effects on the nervous system; in fact, an alcoholic preparation of H. erinaceus is sold by Fungi Perfecti Co. (28). Perhaps the most popular fungus as a source of antioxidants and used medicinally by infusing in alcohol is Ganoderma lucidum (Curtis) P. Kart., which owes its antioxidant and antiaging properties to its metabolites, mainly terpenoid and phenolic compounds, and much information exists related to the technical aspects of its production, handling, and preparation, as well as its bioactive effects, in fact, it is the only fungus which an alcoholic preparation has been submitted to clinical trials (14, 29). The fungus Cordyceps sinensis (Berk.) Sacc., belonging to the Ascomycetes, has been widely used for its medicinal and invigorating properties, but has its origins in traditional Chinese medicine. Its consumption as a supplement in alcoholic drinks has mainly been as a tonic or an ingredient mixed with local alcoholic drinks which are left to rest for around an hour before consuming (30). The use of fungi in the preparation of alcoholic drinks is versatile and can be used in various contexts (medicinal, recreational, ceremonial), which determines the production method and technical degree involved, from traditional to more elaborate and well-researched methods to achieve mass production. Cordyceps strains have been evaluated in human studies as capsules treatment and results have shown health and fitness improvement; however, no alcoholic drinks have been reported with clinical trials (31–33).
5 Drinks with plants
Alcoholic drinks with medicinal purposes which incorporate plants are very diverse and, as in the previous cases, their fortification will depend on the cultural practices and prevailing vegetation in the ecosystem, and in some cases, seasonality, and festivities (8). Wu Jia Pi wine is perhaps one of the most famous medicinal liquors and has its origin in traditional Chinese medicine. The drink is attributed with properties which aid in blood circulation, invigorate the body, and reduce fatigue. Its preparation includes nine species of medicinal plants containing alkaloids, antioxidants, and essential oils, notably Gardenia jasminoides J. Ellis, Polygonatum odoratum (Mill.) Druce, Cortex acanthopanacis (Acanthopanax gracilistylus W. W. Smith), Vladimirae Radix root (Vladimiria souliei (Franch.) Ling), Fructus lycii (Lycium chinense Mill.), Cinnamomum cassia (L.) J. Presl, Angelica sinensis (Oliv.) Diels., Fructus amomi (Amomum vilosum Lour.), and the spice Syzygium aromaticum ((L.) Merr. and L. M. Perry). The liquor has even been produced industrially, sustained by the beneficial effects that have been promoted traditionally for centuries (34). Another example is the consumption of damiana liqueur (Turnera diffusa Willd. ex Schult.), which is related to toning and relaxing, as well as hypoglycemic and supposedly aphrodisiac effects brought on by compounds such as flavonoids, which are highly soluble in the hydroalcoholic mixture. The plant is widely used across much of Latin America; in Mexico, it is mixed with tequila or mezcal to prepare relaxing and sexually stimulating drinks (35). Traditionally, the consumption of alcoholic drinks as part of the gastronomy and customs in southern Europe and the Mediterranean involves a variety of aromatic and medicinal species, giving rise to diverse flavors, aromas and biofunctional compounds. The diversity of plants recorded as raw materials for the fortification of functional alcoholic drinks includes mainly members of the Lamiaceae, Asteraceae, Rosaceae, Rutaceae, and Apiaceae families. Their preparation involves procedures such as maceration and codistillation, and they can be consumed in a variety of ways, as recreational or accompanying drinks or as traditional medicines (36). In particular, the flavonoids as a conspicuous phytochemical group, are present in many plants-derived alcoholic drinks, and although no strong clinical evidence about benefic effects of alcoholic drinks themselves, flavonoids have been associated with beneficial health effects like anticancer, promotion of cognitive functions, prevention of hypertension and antidiabetic activities by clinical trials (37–40), which suggests the need for research on beneficial effects promoted by plant-derived alcoholic drink consumption. Clearly, flora as a natural resource base to produce fortified drinks is associated with traditional practices that are closely linked to the ecosystem and resource availability, and which have been used for centuries and are part of our cultural repertoire. However, it is important to consider the technical issues regarding the preparations between regions or for formulas developed for home use or on an industrial level, to preserve the properties which benefit the consumer.
6 Points to consider
The consumption of the traditional alcoholic drinks is a common practice, which, depending on the case, can be traced back for centuries, an indication that these products are deeply rooted in the culture of the people. However, the inherent risks associated with their preparation and consumption cannot be ignored (36). Products which originate from official establishments with a tradition of handling such drinks may be the best option, although the effects claimed in most cases remain to be confirmed. Risks have reported regarding the authenticity, origin and harmlessness of the specimens used to prepare some of these drinks, as in the case of snake wine, which incorporates snakes of uncertain origin (21); or the scorpions added to drinks in Mexico, which are collected in the wild with no guarantee their safety or composition (18). It is also worth mentioning that plants and fungi must be correctly identified to avoid possible intoxications, as well as to ensure the desired effect. Alcohol content is another important point which, depending on the material to be incorporated into the base preparation, should be high enough to promote extraction, reduce microbial growth and adequate to avoid intoxication if consumed in excess (5). As many of these products are traditional in nature, the majority lack registration, labelling or content specifications of bioactive substances or raw materials, suggesting risks for consumers and an urgent issue to consider by health authorities. The consumption of traditional alcoholic drinks of this kind would appear common and, to a point, harmless and recreational. Nevertheless, serious health situations can occur, such as intoxication, allergy and even death, as reported in the consumption of snake wine, which caused severe coagulopathy (41), or anaphylaxis on consuming the fungus H. erinaceus (42) or the high level of fluoride that accumulates in black tea and could lead to excess fluoride in the human body if consumed in excess (43). Above all, it is vitally important that the production of such drinks considers the vulnerability of the natural populations of the species collected, to avoid threatening the biodiversity and promote their permanence over time.
7 Conclusion
Traditional alcoholic drinks have maintained the same time-honored ingredients and characteristic preparation methods and techniques which give them identity and reflect their bioculture with specific properties that distinguish them from others. They are an important part of cultural heritage, upholding an historic-cultural legacy linked to biotic resources from their region of origin/distribution. The incorporation of various biotic components from the ecosystem promotes both their existence and the value of their chemical composition, which for decades, even centuries, has facilitated the inclusion of bioactive compounds in the human diet. However, gaps in knowledge, a lack of health regulations and the unsustainable use of some of the species used, explain the urgency to introduce sustainable practices for the use and preservation of all their elements (plants, animals, and fungi), including the bioculture relevance of those alcoholic drinks prepared using traditional knowledge. More research is needed to ensure the quality, quantity, bioavailability, and clinical support for benefits of their consumption.
Author contributions
MJ-A: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. YM-R: Investigation, Validation, Writing – original draft, Writing – review & editing. RT-A: Writing – original draft, Writing – review & editing. JT-C: Conceptualization, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This was supported by the Research budget from Universidad Autónoma de Tamaulipas – Project: UAT/SIP/INV/2023/058.
Acknowledgments
Authors thank to the Project UAT/SIP/INV/2023/058- Prospección del aprovechamiento y conservación de ciertos elementos del componente biótico de la Reserva de la Biosfera EL Cielo.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Katerinopoulou, K, Kontogeorgos, A, Salmas, CE, Patakas, A, and Ladavos, A. Geographical origin authentication of Agri-food products: a review. Food Secur. (2020) 9:489. doi: 10.3390/foods9040489
2. Palomo-Ligas, L, Flores, SDN, García-Ortiz, JD, Pérez-Juárez, CM, Flores-Gallegos, AC, Rodríguez-Herrera, R, et al. Molecular effects of bioactive compounds from semi-desert plants and their uses as potential ingredient in food products In: R Campos-Vega and B Dave-Oomah, editors. Molecular mechanisms of functional food. Hoboken, New Jersey, USA: John Wiley & Sons, Incorporated (2022). 142–92.
3. Keskin, B, and Güneş, E. Social and cultural aspects of traditional drinks: a review on traditional Turkish drinks. Int J Gastron Food Sci. (2021) 25:100382. doi: 10.1016/j.ijgfs.2021.100382
4. Lee, HK, Choi, YM, Noh, DO, and Suh, HJ. Antioxidant effect of Korean traditional lotus liquor (Yunyupju). Int J Food Sci Technol. (2005) 40:709–15. doi: 10.1111/j.1365-2621.2005.00990.x
5. Lachenmeier, DW, Anh, PTH, Popova, S, and Rehm, J. The quality of alcohol products in Vietnam and its implications for public health. Int J Environ Res Public Health. (2009) 6:2090–101. doi: 10.3390/ijerph6082090
6. García-García, LD, Torres-Acosta, RI, Torres-delos Santos, R, Niño-García, N, and Torres-Castillo, JA. From the forest to the bottle: entomochemicals from Pterophylla beltrani (bolivar and bolivar) for the beverage industry. Southwest Entomol. (2019) 44:449–55. doi: 10.3958/059.044.0210
7. Millán-Mercado, E, Llanderal-Cazáres, C, Valdez-Carrasco, J, and Vigueras, AL. Conservación del color y de la turgencia del gusano rojo Comadia redtenbacheri para mezcal embotellado. Southwest Entomol. (2016) 41:751–60. doi: 10.3958/059.041.0317
8. Koehler, R . Korean wines & spirits: drinks that warm the soul. Jongno-gu, Seoul, South Korea: Seoul Selection-Korean Publishers Association (2016). 100 p.
9. Parashar, GK, and Panwar, N. Snake wine-an innovative method of wine preparation. Just Agric. (2023) 3:302–5.
10. Gharib, HS, and Gharib, HR. Development of recipes for health drinks based on vegetable raw materials of Azerbaijan In: R Voegeli , editor. Proceedings of the 4th international scientific conference reviews of modern science, vol. 121. Zürich, Switzerland: Agency Switzerland (2023)
11. Moigradean, D, Poiana, MA, Baloi, LN, Alda, LM, and Bordean, DM. Characterization of some flavored alcoholic beverages. J Agroaliment Processes Technol. (2021) 27:399–402.
12. Kim, JH, Lee, DH, Lee, SH, Choi, SY, and Lee, JS. Effect of Ganoderma lucidum on the quality and functionality of Korean traditional rice wine, yakju. J Biosci Bioeng. (2004) 97:24–8. doi: 10.1016/S1389-1723(04)70160-7
13. Veljović, S, Nikićević, N, and Nikšić, M. Medicinal fungus Ganoderma lucidum as raw material for alcohol beverage production In: A Grumezescu and AM Holban, editors. Alcoholic drinks: The science of drinks. United Kingdom: Woodhead Publishing (2019). 161–97.
14. Sun, DH . CN1030229C-Method for making whole-scorpion wine. China: Inventor; Mengyin County brewery Shandong Prov., assignee (1995).
16. Snake-Wine.com . Laos snake wine medium size liquor. (2023). Available at: https://www.snake-wine.com/product/smallest-snake-liquor-bottle/ (Accessed November 12, 2023).
17. Kawahara, AY, Martinez, JI, Plotkin, D, Markee, A, Butterwort, V, Couch, CD, et al. Mezcal worm in a bottle: DNA evidence suggests a single moth species. PeerJ. (2023) 11:e14948. doi: 10.7717/peerj.14948
18. Cupul-Magaña, FGFrancke OF . Tres especies de alacranes (Scorpiones: Buthidae y Vaejovidae) embotellados en mezcal mexicano. Rev Iber Aracnol. (2013) 22:123–4.
19. Pagaza-Calderón, EM, González-Insuasti, MS, Pacheco-Olvera, R, and Pulido, MT. Importancia cultural, en función del uso, de cinco especies de artrópodos en Tlacuilotepec, Puebla, México. Sitientibus SCB. (2006) 6:65–71. doi: 10.13102/scb8149
20. Torres-Acosta, RI, Moreno-Ramírez, YR, García-García, LD, Espinoza-Sánchez, EA, Juárez-Aragón, MC, Torres Santos, R, et al. Insects with phenolics and antioxidant activities to supplement mezcal: Tenebrio molitor L. and Schistocerca piceifrons Walker. Southwest Entomol. (2021) 46:625–36. doi: 10.3958/059.046.0304
21. Somaweera, R, and Somaweera, N. Serpents in jars: the snake wine industry in Vietnam. J Threat Taxa. (2010) 2:1251–60. doi: 10.11609/JoTT.o2361.1251-60
23. Purewal, SS, Kaur, P, Garg, G, Sandhu, KS, and Salar, RK. Antioxidant, anti-cancer, and debittering potential of edible fungi (aspergillus oryzae) for bioactive ingredient in personalized foods. Biocatal Agric Biotechnol. (2022) 43:102406. doi: 10.1016/j.bcab.2022.102406
24. Dong, Y, Ma, H, Zhou, C, Moses, GK, Ye, X, Zhang, H, et al. Review of advances in bioactive low-molecular-weight compounds, extracts, and biology of Phellinus sensu lato mushrooms (Agaricomycetes) from 2011 to 2017. Int J Med Mushrooms. (2019) 21:875–94. doi: 10.1615/IntJMedMushrooms.2019031652
25. Kıvrak, I, Kivrak, S, and Karababa, E. Assessment of bioactive compounds and antioxidant activity of Turkey tail medicinal mushroom Trametes versicolor (Agaricomycetes). Int J Med Mushrooms. (2020) 22:559–71. doi: 10.1615/IntJMedMushrooms.2020035027
26. Amiri-Sadeghan, A, Aftabi, Y, Arvanaghi, HR, Shokri, E, Khalili, M, Seyedrezazadeh, E, et al. A review of substrates for solid-state fermentation of lingzhi or reishi medicinal mushroom, Ganoderma lucidum (Agaricomycetes), for basidiome production and effect on bioactive compounds. Int J Med Mushrooms. (2022) 24:15–29. doi: 10.1615/IntJMedMushrooms.2022043192
27. Hui-Yeng, YY, Ariffeen-Rosli, MF, Soon-Hao, T, Boon-Hong, K, and Shin-Yee, F. The wound healing potential of Lignosus rhinocerus and other ethno-myco wound healing agents. Mycobiology. (2023) 51:1–15. doi: 10.1080/12298093.2022.2164641
28. Wong, KH, Naidu, M, David, RP, Bakar, R, and Sabaratnam, V. Neuroregenerative potential of lion's mane mushroom, Hericium erinaceus (bull.: Fr.) Pers. (higher basidiomycetes), in the treatment of peripheral nerve injury. Int J Med Mushrooms. (2012) 14:427–46. doi: 10.1615/IntJMedMushr.v14.i5.10
29. Wu, YL, Han, F, Luan, SS, Ai, R, Zhang, P, Li, H, et al. Triterpenoids from Ganoderma lucidum and their potential anti-inflammatory effects. J Agric Food Chem. (2019) 67:5147–58. doi: 10.1021/acs.jafc.9b01195
30. Panda, AK, and Swain, KC. Traditional uses and medicinal potential of Cordyceps sinensis of Sikkim. J Ayurveda Integr Med. (2011) 2:9–13. doi: 10.4103/0975-9476.78183
31. Chen, S, Li, Z, Krochmal, R, Abrazado, M, Kim, W, and Cooper, CB. Effect of Cs-4® (Cordyceps sinensis) on exercise performance in healthy older subjects: a double-blind, placebo-controlled trial. J Altern Complement Med. (2010) 16:585–90. doi: 10.1089/acm.2009.0226
32. Jung, SJ, Jung, ES, Choi, EK, Sin, HS, Ha, KC, and Chae, SW. Immunomodulatory effects of a mycelium extract of Cordyceps (Paecilomyces haepiali; CBG-CS-2): a randomized and double-blind clinical trial. BMC Complement Med Ther. (2019) 19:1–8. doi: 10.1186/s12906-019-2483-y
33. Savioli, FP, Zogaib, P, Franco, E, de Salles, FCA, Giorelli, GV, and Andreoli, CV. Effects of Cordyceps sinensis supplementation during 12 weeks in amateur marathoners: a randomized, double-blind placebo-controlled trial. J Herb Med. (2022) 34:100570. doi: 10.1016/j.hermed.2022.100570
34. Ma, L, Gao, W, Chen, F, and Meng, Q. HS-SPME and SDE combined with GC–MS and GC-O for characterization of flavor compounds in Zhizhonghe wujiapi medicinal liquor. Food Res Int. (2020) 137:109590. doi: 10.1016/j.foodres.2020.109590
35. Willer, J, Jöhrer, K, Greil, R, Zidorn, C, and Çiçek, SS. Cytotoxic properties of Damiana (Turnera diffusa) extracts and constituents and a validated quantitative UHPLC-DAD assay. Molecules. (2019) 24:855. doi: 10.3390/molecules24050855
36. Martinez-Frances, V, Rivera, D, Obon, C, Alcaraz, F, and Ríos, S. Medicinal plants in traditional herbal wines and liquors in the east of Spain and the Balearic Islands. Front Pharmacol. (2021) 12:713414. doi: 10.3389/fphar.2021.713414
37. Lajous, M, Rossignol, E, Fagherazzi, G, Perquier, F, Scalbert, A, Clavel-Chapelon, F, et al. Flavonoid intake and incident hypertension in women. Am J Clin Nutr. (2016) 103:1091–8. doi: 10.3945/ajcn.115.109249
38. Fernandes, I, Pérez-Gregorio, R, Soares, S, Mateus, N, and De Freitas, V. Wine flavonoids in health and disease prevention. Molecules. (2017) 22:292. doi: 10.3390/molecules22020292
39. Bisol, Â, de Campos, PS, and Lamers, ML. Flavonoids as anticancer therapies: a systematic review of clinical trials. Phytother Res. (2020) 34:568–82. doi: 10.1002/ptr.6551
40. Hussain, T, Tan, B, Murtaza, G, Liu, G, Rahu, N, Kalhoro, MS, et al. Flavonoids and type 2 diabetes: evidence of efficacy in clinical and animal studies and delivery strategies to enhance their therapeutic efficacy. Pharmacol Res. (2020) 152:104629. doi: 10.1016/j.phrs.2020.104629
41. Moon, JM, and Chun, BJ. Severe coagulopathy after ingestion of “Snake wine”. J Emerg Med. (2016) 50:848–51. doi: 10.1016/j.jemermed.2015.11.037
42. Watson, C, and Kobernick, A. Dangers at the dinner table– a report of anaphylaxis to lion's mane mushroom. Ann Allergy Asthma Immunol. (2022) 129:S147. doi: 10.1016/j.anai.2022.08.931
Keywords: drinks, plant extract, bioactive compounds, antioxidants, native foods
Citation: Juárez-Aragón MC, Moreno-Ramírez YR, Torres-Acosta RI and Torres-Castillo JA (2024) Article biodiversity inside bottles: animals, fungi, and plants in traditional alcoholic drinks. Front. Nutr. 11:1368110. doi: 10.3389/fnut.2024.1368110
Edited by:
Smail Aazza, Sidi Mohamed Ben Abdellah University, MoroccoReviewed by:
Marcin Szymanski, Adam Mickiewicz University, PolandCopyright © 2024 Juárez-Aragón, Moreno-Ramírez, Torres-Acosta and Torres-Castillo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jorge Ariel Torres-Castillo, joatorres@docentes.uat.edu.mx
 María Cruz Juárez-Aragón1
María Cruz Juárez-Aragón1  Jorge Ariel Torres-Castillo
Jorge Ariel Torres-Castillo