Low geriatric nutritional risk index predicts poor prognosis in patients with cirrhosis: a retrospective study
- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, The Jikei University School of Medicine, Tokyo, Japan
- 2Division of Gastroenterology, Department of Internal Medicine, Fuji City General Hospital, Shizuoka, Japan
- 3Division of Gastroenterology and Hepatology, Department of Internal Medicine, The Jikei University Daisan Hospital, Tokyo, Japan
- 4Project Research Units, Research Center for Medical Science, The Jikei University School of Medicine, Tokyo, Japan
Aim: Malnutrition, which increases the risk of liver disease-related events and mortality, is a serious complication in cirrhosis. This study aimed to investigate whether the geriatric nutritional risk index (GNRI) could predict the long-term prognosis in patients with cirrhosis.
Methods: We retrospectively evaluated 266 patients with cirrhosis and classified them into two groups based on baseline GNRI scores: risk (≤98, n = 104) and no-risk groups (>98, n = 162). The cumulative survival rates were compared between the two groups in patients with compensated and decompensated cirrhosis, respectively. Cox proportional hazards regression analysis was used to identify significant and independent factors associated with mortality.
Results: The median observation period was 54.9 (33.6–61.7) months and 65 (24.4%) liver disease-related deaths occurred during the follow-up period. The GNRI scores significantly and inversely correlated with Child-Pugh score (r = −0.579), model for end-stage liver disease score (r = −0.286), and Mac-2 binding protein glycosylation isomer (r = −0.494). Multivariate analysis identified low GNRI as a significant and independent factor associated with mortality [overall cohort: hazard ratio (HR), 0.926; p < 0.001; compensated cirrhosis: HR, 0.947; p = 0.003; decompensated cirrhosis: HR, 0.923; p < 0.001]. The risk group demonstrated significantly lower cumulative survival rates than the no-risk group in overall cohort, and patients with compensated and decompensated cirrhosis (p < 0.001, <0.001, and = 0.013, respectively).
Conclusion: Low GNRI was associated with poor long-term prognosis in both patients with compensated and decompensated cirrhosis. Therefore, the GNRI is a simple and useful tool for predicting prognosis and modifying the nutritional status in patients with cirrhosis.
1. Introduction
The liver is an essential organ in the metabolism of nutrients such as carbohydrates, lipids, proteins, vitamins, and minerals (1). Patients with cirrhosis have reduced liver functional reserve frequently complicated by malnutrition due to altered metabolism of these nutrients, as well as hypermetabolic state, malabsorption, decreased nutrients intake, hormonal imbalance, and systemic inflammation (1–4). Malnutrition is associated with adverse clinical outcomes, including portal hypertension, encephalopathy, variceal bleeding, hepatorenal syndrome, hepatic spontaneous bacterial peritonitis, sarcopenia, reduced quality of life, and mortality (5–8). Patients with cirrhosis may achieve improved survival rates through appropriate multidisciplinary nutritional intervention (9). Therefore, simple and practical methods for assessing nutritional status and predicting clinical outcomes are required to promptly initiate treatment and improve patient prognosis in a clinical setting.
The geriatric nutritional risk index (GNRI), which is calculated based on actual/ideal body weight [or body mass index (BMI)] and serum albumin levels, was proposed to estimate the risk of malnutrition-related complications in older adults (10). This scoring system categorizes individuals into the four nutrition-related risk groups, and lower GNRI scores indicate higher morbidity and mortality risks. The GNRI demonstrated superior 1-year prognostic predictability over the global leadership initiative on malnutrition (GLIM) criteria and mini nutritional assessment-short form (MNA-SF) in hospitalized Japanese older adults (11). Several studies have revealed the usefulness of GNRI in predicting the prognosis of malignancies, including esophageal, gastric, hepatic, pancreatic, and colorectal cancers (12–16). Reportedly, low GNRI is associated with poor prognosis in non-malignant diseases, including heart failure, stroke, and chronic kidney disease (17–19). Our recent study of patients with cirrhosis discovered low GNRI as an independent risk factor for sarcopenia, which has been reported to be associated with malnutrition and poor prognosis (20, 21). Therefore, the GNRI may be a simple and useful indicator of nutritional status and prognosis in both malignant and non-malignant diseases. However, no study has reported the association between GNRI and prognosis in patients with cirrhosis.
This study aimed to determine the usefulness of the GNRI in predicting the long-term prognosis of patients with cirrhosis.
2. Materials and methods
2.1. Study participants
This retrospective study enrolled 266 consecutive patients with cirrhosis who presented to the Jikei University School of Medicine (Tokyo, Japan) and Fuji City General Hospital (Shizuoka, Japan) between 2017 and 2020. The study cohort included 182 patients analyzed in our previous report (20). The inclusion criteria were (i) patient age ≥ 20 years and (ii) presence of cirrhosis diagnosed based on noninvasive alternatives to liver biopsy, such as laboratory tests and imaging/endoscopic findings, as described elsewhere (20). The exclusion criteria were (i) pre-existing malignancies other than hepatocellular carcinoma (HCC); (ii) massive and uncontrollable ascites; (iii) HCC beyond the Milan criteria (22); (iv) acute liver failure; (v) liver transplantation history; and (vi) undergoing hemodialysis. Serum total bilirubin, albumin, creatinine, sodium, Mac-2 binding protein glycosylation isomer (M2BPGi, which is a hepatic fibrosis marker), and prothrombin time (PT) were measured using standard methods. The liver functional reserve was assessed with the Child-Pugh (CP) classification and model for end-stage liver disease (MELD) (23, 24). Alcohol-related cirrhosis was diagnosed based on past and/or current history of excessive alcohol consumption (> 60 g/day) and exclusion of other etiologies (25). Metabolic dysfunction-associated steatotic liver disease-related cirrhosis was diagnosed based on the multi-society Delphi consensus statement on new fatty liver disease nomenclature (26). Decompensated cirrhosis was defined as cirrhosis complicated by encephalopathy, variceal bleeding, ascites, and/or jaundice (27). The endpoint in this study was liver disease-related death. Patients who underwent liver transplantation during the study period were considered as death and censored cases. This study protocol was approved by the ethics committees of the Jikei University School of Medicine (approval number: 34-021) and Fuji City General Hospital (approval number: 279) and complied with the 2013 Declaration of Helsinki.
2.2. Patient classification based on GNRI score
The GNRI was calculated based on actual/ideal body weight and serum albumin levels using the following formula: GNRI = [14.89 × albumin (g/dL)] + [41.7 × (actual body weight/ideal body weight)] (10). This scoring system categorizes individuals into four risk groups: no-risk (>98); low-risk (92 to ≤98); moderate-risk (82 to <92); and major-risk (<82) groups. The present study classified the participants into two groups, referring to the original GNRI classification (10) and previous reports (14, 28): risk group (with nutrition-related risk; GNRI ≤98.0) and no-risk group (without nutrition-related risk; GNRI >98.0).
2.3. Statistical analysis
Continuous variables were presented as medians (interquartile ranges), and between-group differences were compared using the Mann–Whitney U test or the Kruskal–Wallis test, as appropriate. Categorical variables were presented as numbers (percentages), and between-group differences were compared using the chi-squared test. The trend of proportions or continuous variable levels among multiple groups was analyzed using the Cochran-Armitage trend test or the Jonckheere-Terpstra trend test, respectively. Correlations between GNRI scores and continuous variables were analyzed using Spearman’s rank correlation test. The cumulative survival rates were estimated using the Kaplan–Meier method, and between-group differences were compared using the log-rank test, followed by the Bonferroni multiple-comparison method, or the log-rank trend test, as appropriate. Univariate analysis was initially performed to identify mortality-related variables that achieved p < 0.10. Multicollinearity among these variables was evaluated using variance inflation factor (VIF). Variables with VIF > 5, which was considered as high multicollinearity, were excluded from the analysis. Subsequently, multivariate Cox proportional hazards regression analysis with forward stepwise selection was performed to identify significant and independent factors associated with mortality. Nomograms were constructed to visualize the prognostic strengths of the significant and independent factors in predicting survival. All statistical analyses were performed using SPSS Statistics version 27 (IBM Japan, Tokyo, Japan) and R software (version 4.3.1). Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Patient characteristics
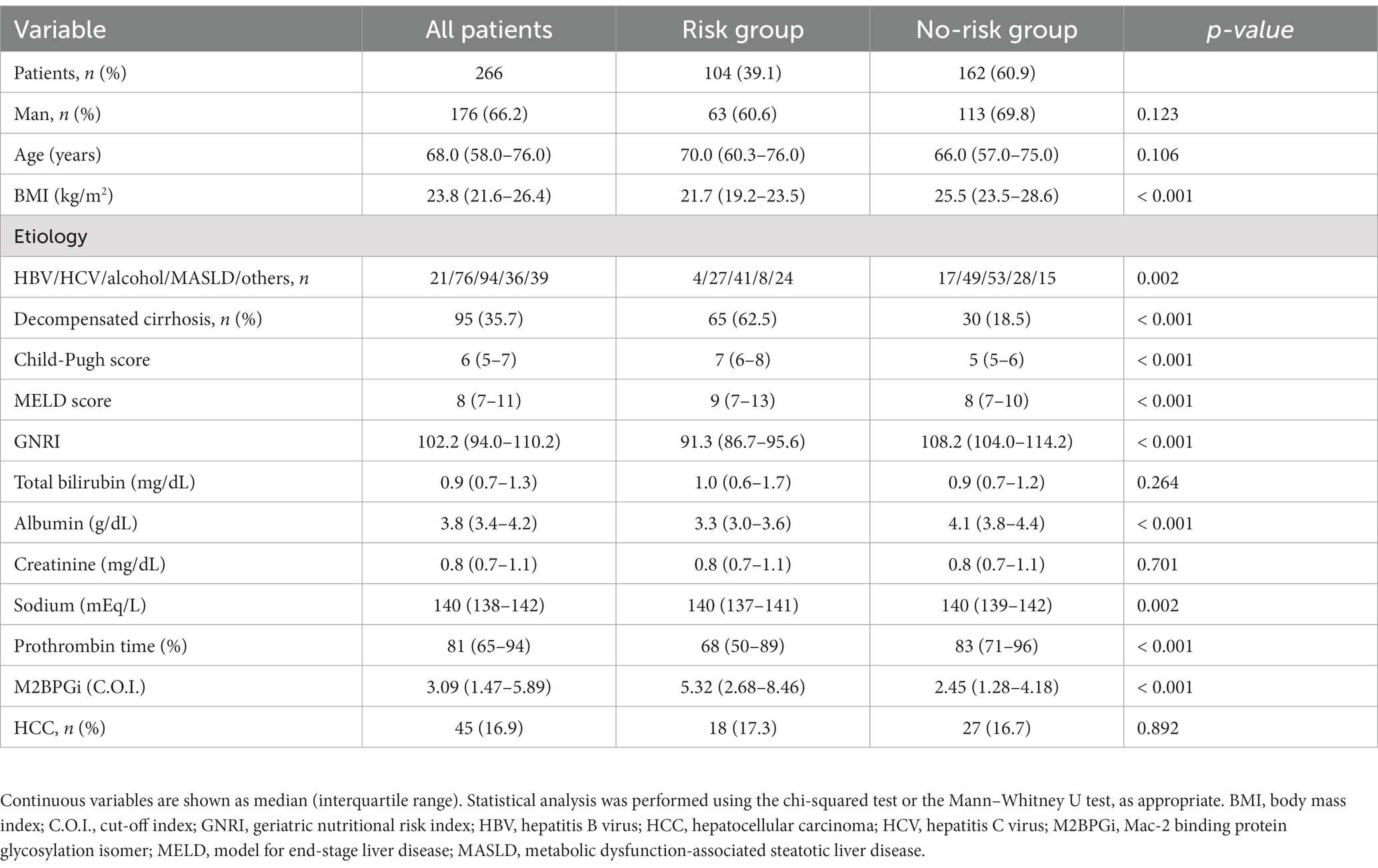
A flow diagram of patient selection in this study is presented in Supplementary Figure S1. A total of 318 patients with cirrhosis were initially screened for eligibility. Of them, 52 met the exclusion criteria, and the remaining 266 patients were enrolled. The baseline patient characteristics are summarized in Table 1. The median age was 68 (58.0–76.0) years, and 176 (66.2%) patients were men. The median CP and MELD scores were 6 (5–7) and 8 (7–11), respectively. The prevalence of decompensated cirrhosis and HCC were 35.7% (95/266) and 16.9% (45/266), respectively. The median GNRI score was 102.2 (94.0–110.2). Patients with decompensated cirrhosis demonstrated significantly lower GNRI scores than those with compensated cirrhosis (median, 94.0 vs. 107.3; p < 0.001; Supplementary Figure S2).
3.2. Clinical characteristics of the risk and no-risk groups
The proportions of the risk and no-risk groups were 39.1% (104/266) and 60.9% (162/266), respectively (Table 1). Hepatitis B virus and metabolic dysfunction-associated steatotic liver disease were significantly more frequent in the no-risk group, whereas the others were significantly more frequent in the risk group (p = 0.002). The risk group demonstrated significantly higher decompensated cirrhosis prevalence, CP and MELD scores, and M2BPGi levels (p < 0.001 for all), and lower sodium and PT levels (p = 0.002 and < 0.001, respectively) than the no-risk group.
3.3. Correlations between GNRI and liver functional reserve and fibrosis marker
The correlations between GNRI and CP scores, MELD scores, and M2BPGi were investigated using Spearman’s rank correlation test (Figure 1). The GNRI significantly and inversely correlated with CP score (r = −0.579), MELD score (r = −0.286), and M2BPGi (r = −0.494) (p < 0.001 for all).
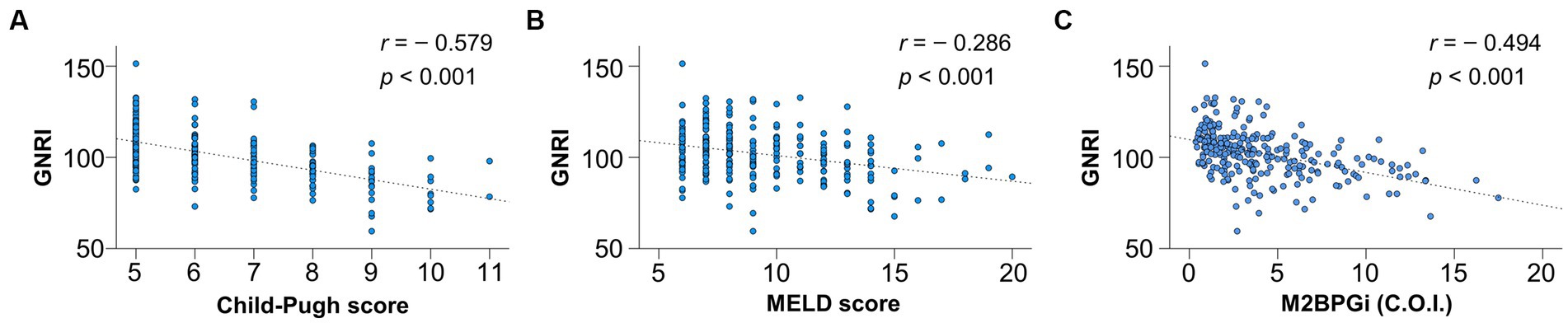
Figure 1. Correlation of geriatric nutritional risk index (GNRI) with liver functional reserve-related scores and fibrosis marker. GNRI significantly correlated with (A) Child-Pugh score, (B) model for end-stage liver disease (MELD) score, and (C) Mac-2 binding protein glycosylation isomer (M2BPGi) (p < 0.001 for all).
3.4. Comparison of cumulative survival rates between the risk and no-risk groups
The median follow-up period was 54.9 (33.6–61.7) months. During the observation period, 65 (24.4%) liver disease-related deaths occurred: liver failure (n = 42), HCC (n = 10), rupture of esophageal varices (n = 8), and liver transplantation (n = 5) (Supplementary Figure S1). The 1-, 3-, and 5-year cumulative survival rates were 94.2%, 65.0%, and 49.2% in the risk group and 98.7%, 94.1%, and 88.1% in the no-risk group, respectively (Figure 2). The risk group demonstrated significantly lower cumulative survival rates than the no-risk group (p < 0.001).
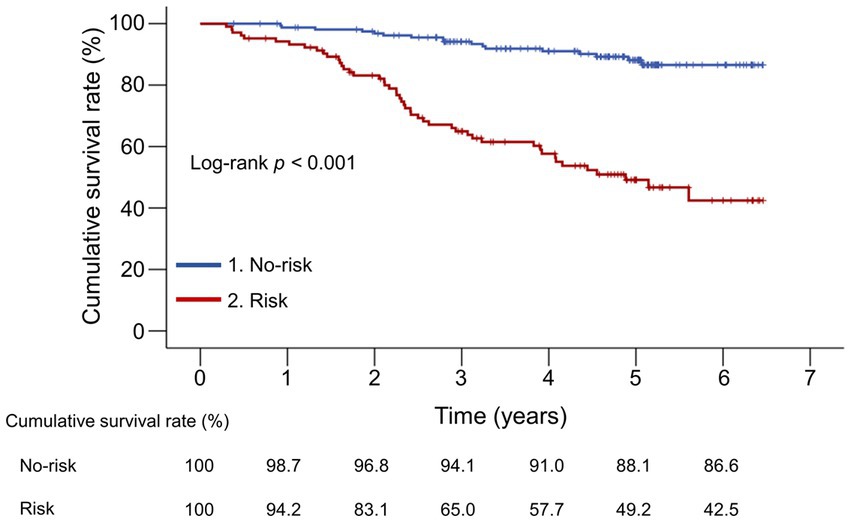
Figure 2. Comparison of the cumulative survival rates between the risk and no-risk groups. The cumulative survival rates were significantly lower in the risk group than in the no-risk group (p < 0.001).
Furthermore, we investigated whether the cumulative survival rates differed following the original GNRI classification. Age, decompensated cirrhosis prevalence, CP and MELD scores, and M2BPGi levels significantly increased stepwise, whereas BMI, albumin, and PT levels significantly decreased stepwise from the no-risk group to the low-, moderate-, and major-risk groups (Supplementary Table S1). The cumulative survival rates significantly decreased stepwise with increasing GNRI risk (p < 0.001; Supplementary Figure S3). The no-risk group demonstrated the highest cumulative survival rates than any other group (p < 0.001 for all), whereas the major-risk group had significantly lower survival rates than the no-risk and low-risk groups (p < 0.001 for both). Therefore, the combined group had significantly lower cumulative survival rates than the no- and low-risk groups when the major- and moderate-risk groups were combined into one group (p < 0.001 and = 0.012, respectively; Supplementary Figure S4).
Death from various non-liver-related causes was recorded in 13 patients who were considered censored cases (Supplementary Table S2). Even when they were included in the endpoint cases, similar results were obtained as above: i.e., the no-risk group demonstrated the highest cumulative survival rates than any other group, whereas the major-risk group had significantly lower survival rates than the no- and low-risk groups (Supplementary Figure S5). The cumulative survival rates for the major-risk group were identical to the above-described results, indicating that the major-risk group did not include non-liver-related deaths.
3.5. Comparison of cumulative survival rates between the risk and no-risk groups in subgroup populations
We compared the cumulative survival rates between the risk and no-risk groups in patients with compensated and decompensated cirrhosis, respectively (Figure 3). The 1-, 3-, and 5-year cumulative survival rates were 97.4% vs. 98.5%, 74.9% vs. 96.0%, and 61.6% vs. 90.7% in the risk and no-risk groups, respectively, in patients with compensated cirrhosis, indicating significant differences between the two groups (p < 0.001; Figure 3A). These were 92.3% vs. 100%, 59.4% vs. 85.7%, and 42.2% vs. 77.0% in the risk and no-risk groups, respectively, in patients with decompensated cirrhosis, indicating significant differences between the two groups (p = 0.013; Figure 3B).
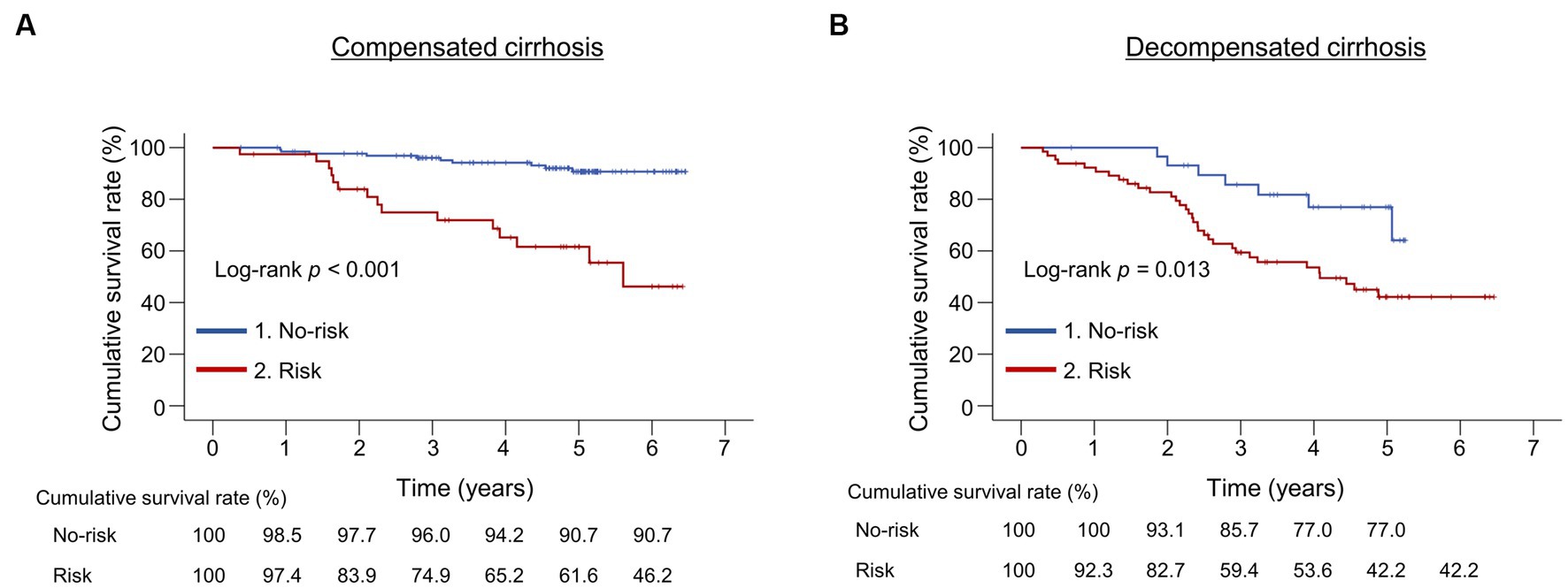
Figure 3. Comparison of the cumulative survival rates between the risk and no-risk groups in patients with compensated or decompensated cirrhosis. (A,B) The cumulative survival rates were significantly lower in the risk group than in the no-risk group in both patients with compensated and decompensated cirrhosis (p < 0.001 and = 0.013, respectively).
We compared the cumulative survival rates between the risk and no-risk groups in patients with and without HCC, respectively (Supplementary Figure S6). In patients without HCC, the cumulative survival rates in the risk group were significantly lower than those in the no-risk group (p < 0.001; Supplementary Figure S6A). In patients with HCC, a marginally significant difference was found in the cumulative survival rates between the risk and no-risk groups (p = 0.065; Supplementary Figure S6B).
3.6. Significant factors associated with mortality in patients with cirrhosis
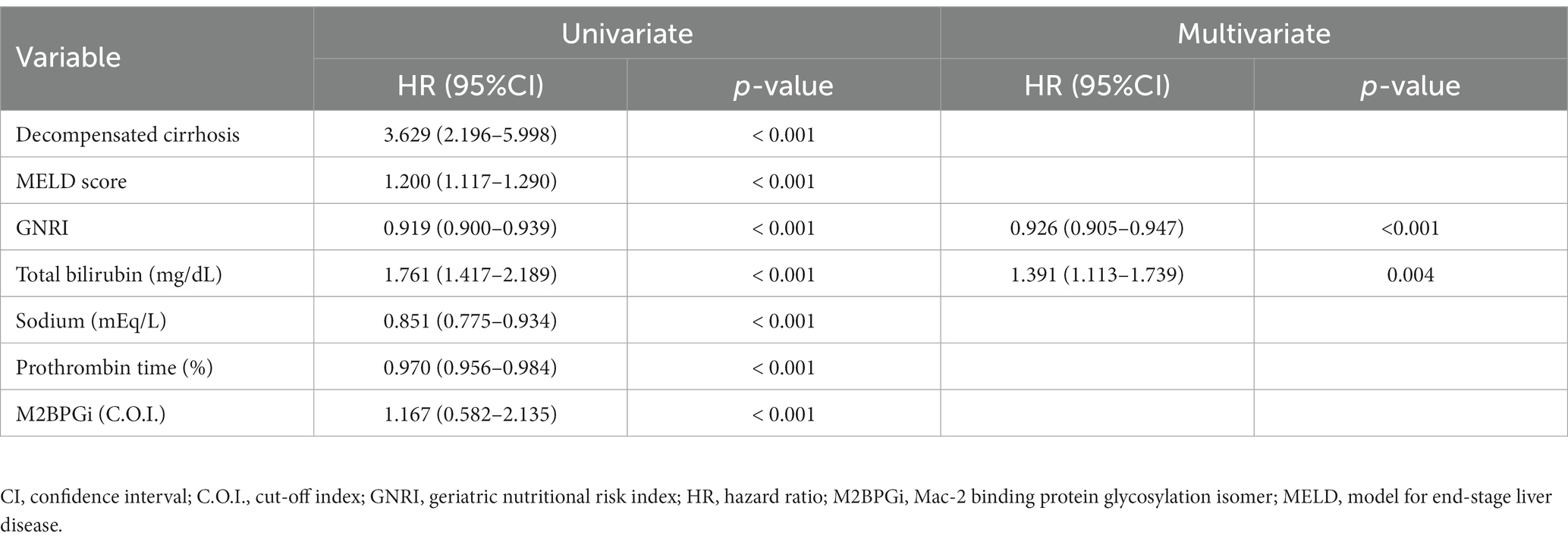
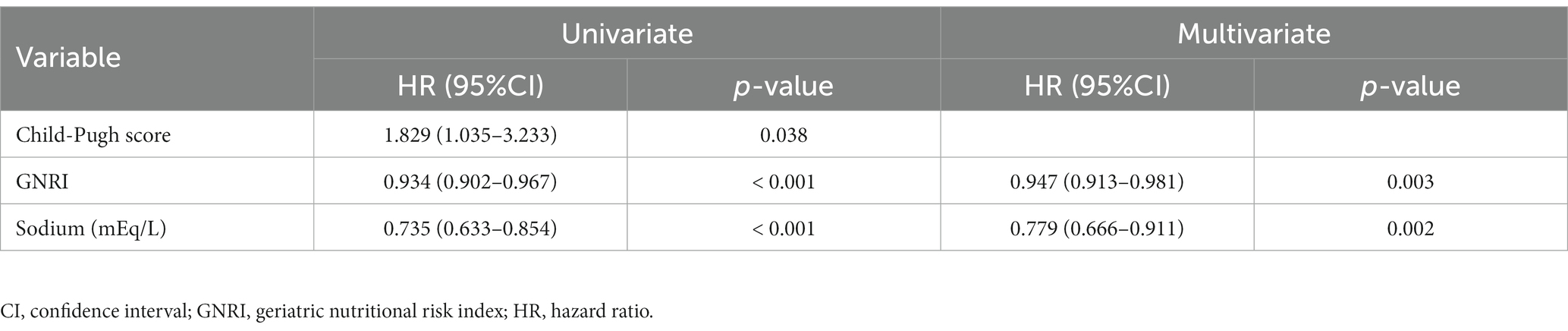
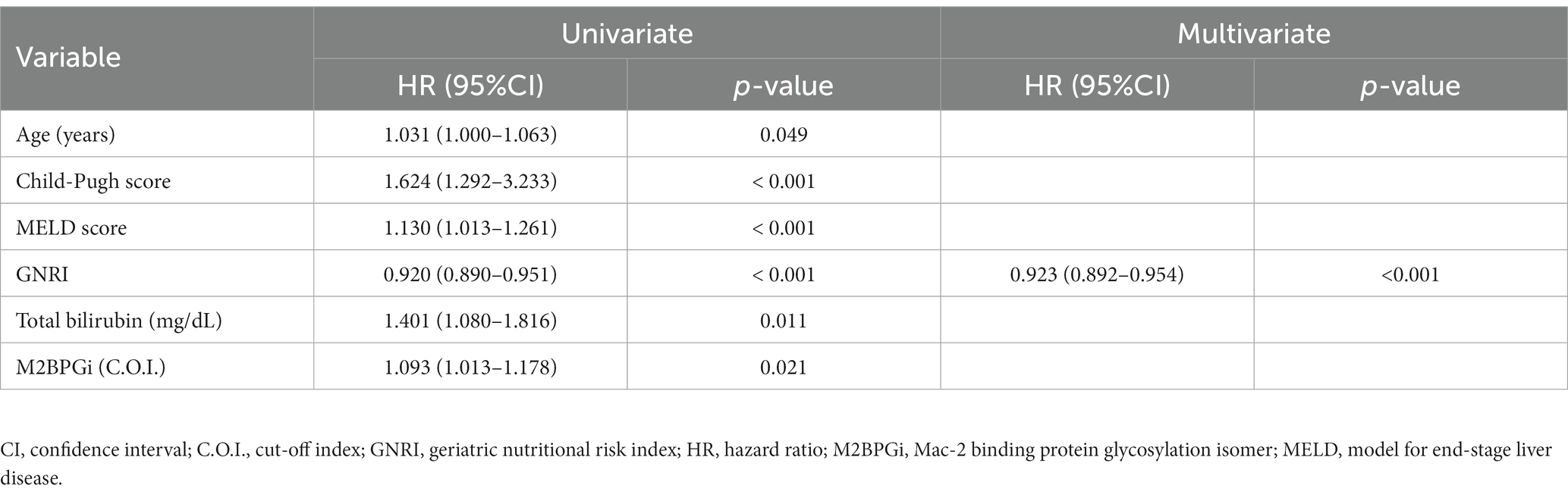
Univariate analysis revealed that the following variables were significantly associated with mortality: decompensated cirrhosis, CP score, MELD score, GNRI, total bilirubin, sodium, PT, and M2BPGi in all patients (Supplementary Table S3); CP score, GNRI, and sodium in patients with compensated cirrhosis (Supplementary Table S4); and age, CP score, MELD score, GNRI, total bilirubin, and M2BPGi in patients with decompensated cirrhosis (Supplementary Table S5). Among them, only CP score of all patients had a VIF > 5 and was excluded from further analysis (Supplementary Table S6). Cox proportional hazards regression analysis identified the following variables as significant and independent prognostic factors: low GNRI [hazard ratio (HR), 0.926; 95% confidence interval (CI), 0.905–0.947; p < 0.001] and high total bilirubin levels (HR, 1.391; 95% CI, 1.113–1.739; p = 0.004) in all patients (Table 2); low GNRI (HR, 0.947; 95% CI, 0.913–0.981; p = 0.003) and low sodium levels (HR, 0.779; 95% CI, 0.666–0.911; p = 0.002) in patients with compensated cirrhosis (Table 3); and low GNRI (HR, 0.923; 95% CI, 0.892–0.954; p < 0.001) in patients with decompensated cirrhosis (Table 4).
3.7. Prognostic nomograms for survival prediction
Prognostic nomograms of the independent factors identified in multivariate analysis were constructed to visually estimate 1-, 3-, and 5-year survival probabilities in all patients and those with compensated and decompensated cirrhosis (Figure 4; Supplementary Figures S7, S8, respectively). Points assigned to GNRI, total bilirubin, and sodium, respectively, were determined by drawing a vertical line from the corresponding value to the point scale (top in each figure). The total point was the sum of the points for these factors (middle in each figure). Finally, each survival probability was a value that matched the total point on the corresponding scale (lower in each figure).
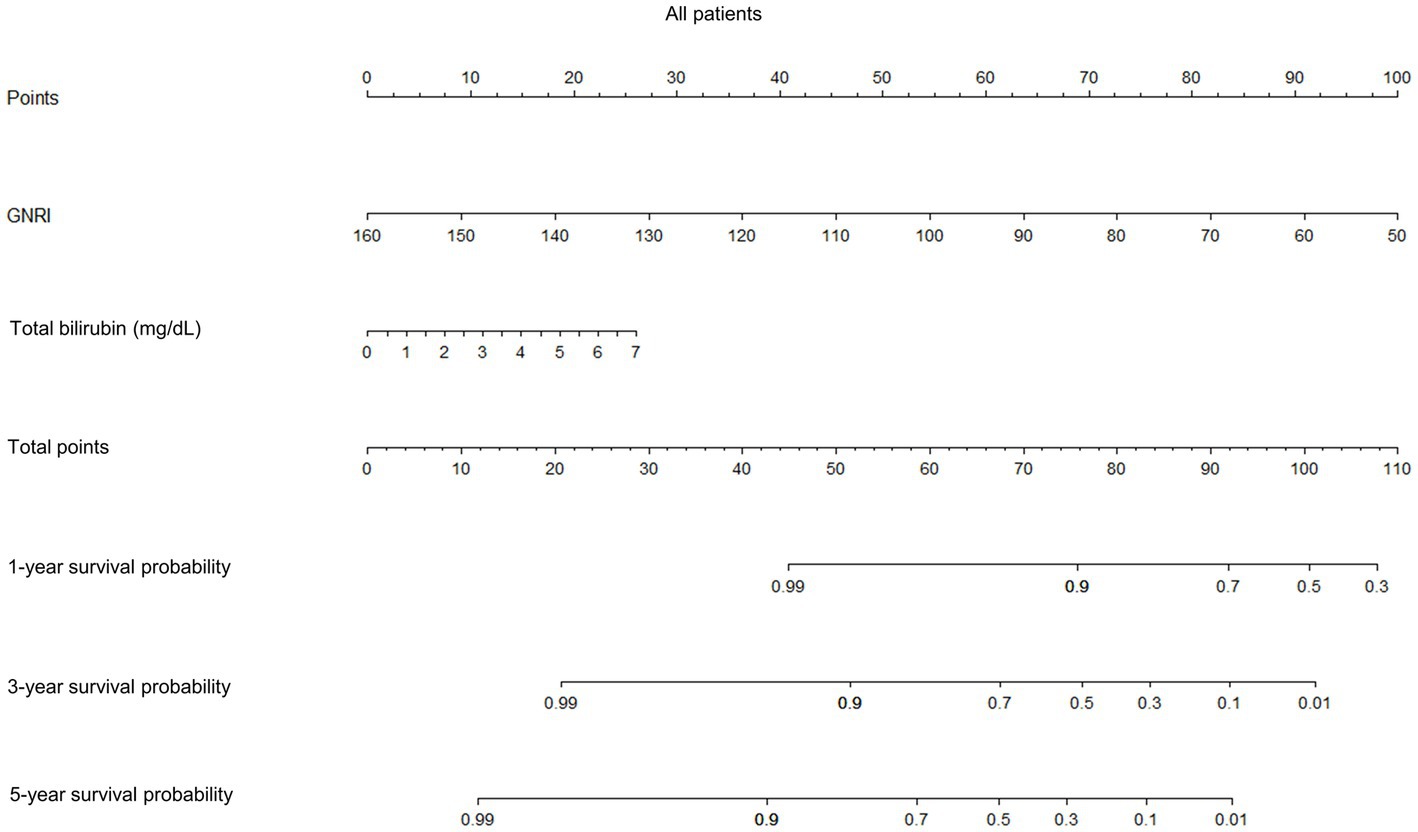
Figure 4. Prognostic nomograms for 1-, 3-, and 5-year survival probabilities of all patients. GNRI, geriatric nutritional risk index.
4. Discussion
Malnutrition is a serious complication that negatively affects the prognosis of patients with cirrhosis (7, 8). Significant changes in body composition, such as body water, body fat, and body cell mass, occur in patients with cirrhosis: a marked loss of body fat in the early stages of cirrhosis (i.e., CP class A) and an accelerated loss of body cell mass in the advanced stages of cirrhosis (i.e., CP class B or C) (29). Therefore, early nutritional status assessment is crucial in managing cirrhosis from a prognostic standpoint. Nevertheless, appropriate nutritional screening and assessment strategies remain to be determined due to the diversity of malnutrition definitions, lack of validated rapid screening methods, and difficulties in interpreting body composition and laboratory data in real-world clinical settings (30).
The GNRI is a simple and objective nutritional status assessment tool that can predict prognosis in patients with malignant (mainly digestive system cancers) and non-malignant diseases (such as heart failure, stroke, and chronic kidney disease) (12–19). However, no study has reported on the usefulness of GNRI in patients with cirrhosis. Hence, the present study is the first to report the association between GNRI and long-term prognosis in patients with cirrhosis. This study revealed that GNRI scores negatively correlated with CP and MELD scores and were the strongest independent prognostic factor, especially in patients with decompensated cirrhosis. The cumulative survival rates were determinately lower in the risk group and decreased stepwise with increasing risk in the original GNRI classification. One study of patients who underwent hepatic resection for HCC (CP class A/B: 96.8%/3.2%) revealed lower recurrence-free survival and overall survival (OS) rates in the low-GNRI (GNRI ≤98) group than the high-GNRI (GNRI >98) group and low GNRI as an independent prognostic factor for both rates (14). Another study of patients with CP class A and hepatitis B virus-related HCC revealed the major- and moderate-risk GNRI groups as independent risk factors for postoperative severe complications and OS (31). Another study of patients who underwent transarterial chemoembolization for HCC (CP class A/B: 45.9%/54.1%) revealed GNRI <98, tumor number ≥ 2, tumor size ≥5 cm, TACE times <3, and alpha-fetoprotein ≥400 as independent risk factors for poor prognosis (32). Furthermore, our recent study of patients with HCC treated with lenvatinib (CP class A/B: 85.2%/14.8%) revealed that low GNRI was independently associated with treatment discontinuation, and that the low-GNRI (GNRI ≤98) group had worse progression-free survival than the high-GNRI (GNRI >98) group (33). The present study revealed lower cumulative survival rates in the risk group than the no-risk group in both patients with and without HCC. These findings indicate that low GNRI is associated with poor prognosis, irrespective of HCC and liver functional reserve, and that the GNRI may be useful for predicting prognosis in patients with cirrhosis.
Various criteria for assessing malnutrition have been reported as useful for predicting prognosis in patients with cirrhosis. The European Society for Clinical Nutrition and Metabolism guidelines recommend the Royal Free Hospital-Nutritional Prioritizing Tool (RFH-NPT) to screen patients with liver disease for malnutrition risk (34). This screening tool comprises three major steps. First, the presence of acute alcoholic hepatitis or tube feeding is evaluated. Second, fluid overload (i.e., edema or ascites) and its impacts on food intake and weight loss are assessed. BMI, unplanned weight loss, and daily nutritional intake are assessed if patients do not have fluid overload. Third, the scores are calculated and patients are classified into the corresponding risk group (35, 36). Reportedly, the RFH-NPT was associated with CP and MELD scores and clinical complications (ascites, hepatorenal syndrome, and encephalopathy), and it was an independent predictor of MELD score deterioration and transplant-free survival (35). In addition, RFH-NPT amelioration caused an improvement in survival time. Furthermore, the RFH-NPT had superior prognostic predictability compared to the Nutritional Risk Screening 2002, including nutritional score (BMI, weight loss, and dietary intake), disease severity score, and age score (36). Meanwhile, the GLIM criteria, which includes unplanned weight loss, low BMI, reduced muscle mass, reduced dietary intake, and the presence of inflammation or disease burden, could predict mortality in patients with chronic liver disease, independent of liver functional reserve and HCC (8). However, a recent study of hospitalized Japanese older adults revealed that the GNRI was superior to the MNA-SF, which includes dietary intake, weight loss, mobility, the presence of psychological stress or acute disease, neuropsychological problem, and BMI, and GLIM criteria in predicting 1-year mortality (11). Muscle mass assessment included in the GLIM phenotypic criteria requires specialized equipment (such as dual-energy X-ray absorptiometry, computed tomography, and bioelectrical impedance analysis) and is not easy to perform in daily medical practice. In addition, the cutoff values for reduced muscle mass are not clearly indicated in the GLIM criteria and vary among races or different diagnostic criteria. Furthermore, the GLIM etiologic criteria components, such as reduced food intake, digestion or absorption, and chronic disease-related inflammation, are difficult to assess objectively. Meanwhile, the GNRI can be easily calculated from body weight and height and serum albumin levels, both of which are objective test items evaluated routinely in outpatient clinics. Thus, the GNRI is a simpler, more objective and convenient, and universal assessment tool for predicting prognosis, compared with the GLIM criteria.
The GNRI may reflect the chronic inflammatory condition. One study of chronic kidney disease revealed higher systemic inflammatory marker levels, such as C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor-α, in patients with low GNRI scores than those with high GNRI scores (37). Plasma IL-6 levels were independently and negatively associated with GNRI scores. Another study of older inpatients revealed the association of a higher GNRI risk with increased CRP levels and low lymphocyte counts (38). Another study of patients with cirrhosis revealed serum IL-6 and CRP levels as independent predictors of mortality and liver transplantation (39). The GNRI is a simple and easy-to-use malnutrition screening tool for predicting prognosis, although the most suitable scoring system for evaluating malnutrition in patients with cirrhosis remains to be determined. In the future, the GNRI must be compared with other assessment tools to determine the method appropriate for which patient, the anthropometric assessment to be included, and the more detailed or simplified assessment methods.
This study has some limitations. First, the presence of ascites may overestimate BMI and GNRI scores. However, this influence was reduced by excluding patients with massive ascites. Nutritional assessment tools, including BMI as an evaluation item, may optimistically interpret the nutrition status in patients with hepatic edema and ascites. Second, the inflammatory markers, which may influence the GNRI scores, were not assessed. Finally, this was a retrospective, two-center study; therefore, prospective, multicenter studies are needed to validate the aforementioned findings.
5. Conclusion
In conclusion, the GNRI score was the strongest factor associated with long-term prognosis in patients with cirrhosis. The GNRI is a simple, objective, and useful tool for predicting prognosis in patients with cirrhosis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committees of the Jikei University School of Medicine (approval number: 34-021) and Fuji City General Hospital (approval number: 279). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The requirement for written informed consent from participants was waived by the ethics committee of the Jikei University School of Medicine and Fuji City General Hospital due to the retrospective nature of the study.
Author contributions
HK: Data curation, Writing – original draft. CS: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. AK: Conceptualization, Supervision, Writing – review & editing. CN: Data curation, Writing – review & editing. TK: Data curation, Writing – review & editing. KU: Data curation, Writing – review & editing. MN: Data curation, Writing – review & editing. TO: Data curation, Writing – review & editing. YT: Data curation, Writing – review & editing. MS: Supervision, Writing – review & editing. AT: Formal analysis, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1269399/full#supplementary-material
References
1. Traub, J, Reiss, L, Aliwa, B, and Stadlbauer, V. Malnutrition in patients with liver cirrhosis. Nutrients. (2021) 13:540. doi: 10.3390/nu13020540
2. Bunchorntavakul, C, and Reddy, KR. Review article: malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment Pharmacol Ther. (2020) 51:64–77. doi: 10.1111/apt.15571
3. Lai, JC, Tandon, P, Bernal, W, Tapper, EB, Ekong, U, Dasarathy, S, et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. (2021) 74:1611–44. doi: 10.1002/hep.32049
4. Meyer, F, Bannert, K, Wiese, M, Esau, S, Sautter, LF, Ehlers, L, et al. Molecular mechanism contributing to malnutrition and sarcopenia in patients with liver cirrhosis. Int J Mol Sci. (2020) 21:5357. doi: 10.3390/ijms21155357
5. Periyalwar, P, and Dasarathy, S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. (2012) 16:95–131. doi: 10.1016/j.cld.2011.12.009
6. Alvares-da-Silva, MR, and Reverbel da Silveira, T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. (2005) 21:113–7. doi: 10.1016/j.nut.2004.02.002
7. Maharshi, S, Sharma, BC, and Srivastava, S. Malnutrition in cirrhosis increases morbidity and mortality. J Gastroenterol Hepatol. (2015) 30:1507–13. doi: 10.1111/jgh.12999
8. Miwa, T, Hanai, T, Nishimura, K, Unome, S, Maeda, T, Ogiso, Y, et al. Usefulness of the global leadership initiative on malnutrition criteria to predict sarcopenia and mortality in patients with chronic liver disease. Hepatol Res. (2022) 52:928–36. doi: 10.1111/hepr.13816
9. Iwasa, M, Iwata, K, Hara, N, Hattori, A, Ishidome, M, Sekoguchi-Fujikawa, N, et al. Nutrition therapy using a multidisciplinary team improves survival rates in patients with liver cirrhosis. Nutrition. (2013) 29:1418–21. doi: 10.1016/j.nut.2013.05.016
10. Bouillanne, O, Morineau, G, Dupont, C, Coulombel, I, Vincent, JP, Nicolis, I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. (2005) 82:777–83. doi: 10.1093/ajcn/82.4.777
11. Hiraike, T, Momoki, C, and Habu, D. Comparison of the adequacy of geriatric nutritional risk index with that of the mini nutritional assessment-short form and global leadership initiative on malnutrition criteria in assessing nutritional status to predict the 1-year prognosis of hospitalized Japanese older adults: a single-institutional cohort study. BMC Geriatr. (2023) 23:35. doi: 10.1186/s12877-023-03740-5
12. Zhou, J, Fang, P, Li, X, Luan, S, Xiao, X, Gu, Y, et al. Prognostic value of geriatric nutritional risk index in esophageal carcinoma: a systematic review and meta-analysis. Front Nutr. (2022) 9:831283. doi: 10.3389/fnut.2022.831283
13. He, L, Li, Y, Qu, L, and Zhang, F. Prognostic and clinicopathological value of the geriatric nutritional risk index in gastric cancer: a meta-analysis of 5,834 patients. Front Surg. (2023) 9:1087298. doi: 10.3389/fsurg.2022.1087298
14. Kanno, H, Goto, Y, Sasaki, S, Fukutomi, S, Hisaka, T, Fujita, F, et al. Geriatric nutritional risk index predicts prognosis in hepatocellular carcinoma after hepatectomy: a propensity score matching analysis. Sci Rep. (2021) 11:9038. doi: 10.1038/s41598-021-88254-z
15. Li, L, and He, J. Prognostic role of geriatric nutritional risk index in patients with pancreatic cancer: a meta-analysis. Nutr Cancer. (2023) 75:1531–40. doi: 10.1080/01635581.2023.2209345
16. Zhao, H, Xu, L, Tang, P, and Guo, R. Geriatric nutritional risk index and survival of patients with colorectal cancer: a meta-analysis. Front Oncol. (2022) 12:906711. doi: 10.3389/fonc.2022.906711
17. Li, H, Cen, K, Sun, W, and Feng, B. Prognostic value of geriatric nutritional risk index in elderly patients with heart failure: a meta-analysis. Aging Clin Exp Res. (2021) 33:1477–86. doi: 10.1007/s40520-020-01656-3
18. Hu, J, Chen, T, Wang, Z, Chen, X, Lin, K, Zhang, G, et al. Geriatric nutritional risk index and the prognosis of patients with stroke: a meta-analysis. Horm Metab Res. (2022) 54:736–46. doi: 10.1055/a-1886-4276
19. Nakagawa, N, Maruyama, K, and Hasebe, N. Utility of geriatric nutritional risk index in patients with chronic kidney disease: a mini-review. Nutrients. (2021) 13:3688. doi: 10.3390/nu13113688
20. Saeki, C, Kinoshita, A, Kanai, T, Ueda, K, Nakano, M, Oikawa, T, et al. The geriatric nutritional risk index predicts sarcopenia in patients with cirrhosis. Sci Rep. (2023) 13:3888. doi: 10.1038/s41598-023-31065-1
21. Tantai, X, Liu, Y, Yeo, YH, Praktiknjo, M, Mauro, E, Hamaguchi, Y, et al. Effect of sarcopenia on survival in patients with cirrhosis: a meta-analysis. J Hepatol. (2022) 76:588–99. doi: 10.1016/j.jhep.2021.11.006
22. Mazzaferro, V, Regalia, E, Doci, R, Andreola, S, Pulvirenti, A, Bozzetti, F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. (1996) 334:693–700. doi: 10.1056/NEJM199603143341104
23. Pugh, RN, Murray-Lyon, IM, Dawson, JL, Pietroni, MC, and Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. (1973) 60:646–9. doi: 10.1002/bjs.1800600817
24. Malinchoc, M, Kamath, PS, Gordon, FD, Peine, CJ, Rank, J, and ter Borg, PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. (2000) 31:864–71. doi: 10.1053/he.2000.5852
25. Saeki, C, Kanai, T, Nakano, M, Oikawa, T, Torisu, Y, Saruta, M, et al. Clinical characteristics of sarcopenia in patients with alcoholic liver cirrhosis. JGH Open. (2021) 5:763–9. doi: 10.1002/jgh3.12582
26. Rinella, ME, Lazarus, JV, Ratziu, V, Francque, SM, Sanyal, AJ, Kanwal, F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. (2023) S0168-8278(23)00418-X. doi: 10.1016/j.jhep.2023.06.003 [Epub ahead of print].
27. European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. (2018) 69:406–60. doi: 10.1016/j.jhep.2018.03.024
28. Sasaki, M, Miyoshi, N, Fujino, S, Ogino, T, Takahashi, H, Uemura, M, et al. The geriatric nutritional risk index predicts postoperative complications and prognosis in elderly patients with colorectal cancer after curative surgery. Sci Rep. (2020) 10:10744. doi: 10.1038/s41598-020-67285-y
29. Figueiredo, FA, De Mello, PR, and Kondo, M. Effect of liver cirrhosis on body composition: evidence of significant depletion even in mild disease. J Gastroenterol Hepatol. (2005) 20:209–16. doi: 10.1111/j.1440-1746.2004.03544.x
30. Tandon, P, Raman, M, Mourtzakis, M, and Merli, M. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology. (2017) 65:1044–57. doi: 10.1002/hep.29003
31. Li, L, Wang, H, Yang, J, Jiang, L, Yang, J, Wu, H, et al. Geriatric nutritional risk index predicts prognosis after hepatectomy in elderly patients with hepatitis B virus-related hepatocellular carcinoma. Sci Rep. (2018) 8:12561. doi: 10.1038/s41598-018-30906-8
32. Si, Y, Xu, P, Xu, A, Wang, P, and Zhao, K. Geriatric nutritional risk index as a prognostic factor in patients with hepatocellular carcinoma following transarterial chemoembolization: a retrospective study. Medicine (Baltimore). (2022) 101:e32322. doi: 10.1097/MD.0000000000032322
33. Kinoshita, A, Hagiwara, N, Osawa, A, Akasu, T, Matsumoto, Y, Ueda, K, et al. The geriatric nutritional risk index predicts tolerability of lenvatinib in patients with hepatocellular carcinoma. In Vivo. (2022) 36:865–73. doi: 10.21873/invivo.12775
34. Bischoff, SC, Bernal, W, Dasarathy, S, Merli, M, Plank, LD, Schütz, T, et al. ESPEN practical guideline: clinical nutrition in liver disease. Clin Nutr. (2020) 39:3533–62. doi: 10.1016/j.clnu.2020.09.001
35. Borhofen, SM, Gerner, C, Lehmann, J, Fimmers, R, Görtzen, J, Hey, B, et al. The Royal Free Hospital-Nutritional Prioritizing Tool is an independent predictor of deterioration of liver function and survival in cirrhosis. Dig Dis Sci. (2016) 61:1735–43. doi: 10.1007/s10620-015-4015-z
36. Wu, Y, Zhu, Y, Feng, Y, Wang, R, Yao, N, Zhang, M, et al. Royal Free Hospital-Nutritional Prioritizing Tool improves the prediction of malnutrition risk outcomes in liver cirrhosis patients compared with nutritional risk screening 2002. Br J Nutr. (2020) 124:1293–302. doi: 10.1017/S0007114520002366
37. Lin, TY, and Hung, SC. Geriatric nutritional risk index is associated with unique health conditions and clinical outcomes in chronic kidney disease patients. Nutrients. (2019) 11:2769. doi: 10.3390/nu11112769
38. Gärtner, S, Kraft, M, Krüger, J, Vogt, LJ, Fiene, M, Mayerle, J, et al. Geriatric nutritional risk index correlates with length of hospital stay and inflammatory markers in older inpatients. Clin Nutr. (2017) 36:1048–53. doi: 10.1016/j.clnu.2016.06.019
Keywords: cirrhosis, malnutrition, nutritional assessment tool, geriatric nutritional risk index, prognosis
Citation: Kamioka H, Saeki C, Kinoshita A, Nakagawa C, Kanai T, Ueda K, Nakano M, Oikawa T, Torisu Y, Saruta M and Tsubota A (2023) Low geriatric nutritional risk index predicts poor prognosis in patients with cirrhosis: a retrospective study. Front. Nutr. 10:1269399. doi: 10.3389/fnut.2023.1269399
Edited by:
Sabrina Alves Fernandes, Federal University of Health Sciences of Porto Alegre, BrazilReviewed by:
Zihan Yu, Tianjin Medical University, ChinaMasatsugu Ohara, Hokkaido University Graduate School of Medicine, Japan
Copyright © 2023 Kamioka, Saeki, Kinoshita, Nakagawa, Kanai, Ueda, Nakano, Oikawa, Torisu, Saruta and Tsubota. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chisato Saeki, chisato@jikei.ac.jp; Akihito Tsubota, atsubo@jikei.ac.jp
 Hiroshi Kamioka
Hiroshi Kamioka Chisato Saeki
Chisato Saeki Akiyoshi Kinoshita
Akiyoshi Kinoshita Chika Nakagawa1
Chika Nakagawa1  Masanori Nakano
Masanori Nakano Tsunekazu Oikawa
Tsunekazu Oikawa Yuichi Torisu
Yuichi Torisu Akihito Tsubota
Akihito Tsubota